Introduction
The role of macrovascular and microvascular remodeling in the development of neurodegeneration and dementia of sporadic, aging-related late-onset Alzheimer’s disease (LOAD) as compared to genetic-related early onset Alzheimer’s disease (EOAD) has undergone numerous historic pendulum swings since the early 1900s [
1,
2,
3,
4]. Numerous descriptive hypotheses regarding the causes of LOAD have been considered over the years, which include the aging-related hypothesis, the cholinergic hypothesis, amyloid cascade hypothesis, tau propagation hypothesis, mitochondrial cascade hypothesis, calcium homeostasis hypothesis, neurovascular hypothesis, inflammatory hypothesis, metal ion hypothesis, genetic/environmental hypothesis, vascular hypothesis and lymphatic system hypothesis (Box 1) [
5,
6,
7].
Box.
Major existing and emerging hypotheses for late-onset Alzheimer’s disease (LOAD). In regard to number III. Aβ cascade hypothesis or the amyloid cascade hypothesis, the comment that Aβ may be necessary but not sufficient was introduced by Musiek and Holtzman [6]. Image provided with permission by CC 4.0 [
6]. AD, Alzheimer’s disease; aMt, aberrant mitochondria; aMGCs, aberrant microglia cells; APOE-ε4, apolipoprotein E epsilon 4; BOLD, blood-oxygen-level-dependent imaging; CBF, cerebral blood flow; LOAD, late-onset Alzheimer’s disease; MGC, microglia cell; Mt, mitochondria; PET, positron emission tomography; ROS, reactive oxygen species; T1DM, type 1 diabetes mellitus
Box.
Major existing and emerging hypotheses for late-onset Alzheimer’s disease (LOAD). In regard to number III. Aβ cascade hypothesis or the amyloid cascade hypothesis, the comment that Aβ may be necessary but not sufficient was introduced by Musiek and Holtzman [6]. Image provided with permission by CC 4.0 [
6]. AD, Alzheimer’s disease; aMt, aberrant mitochondria; aMGCs, aberrant microglia cells; APOE-ε4, apolipoprotein E epsilon 4; BOLD, blood-oxygen-level-dependent imaging; CBF, cerebral blood flow; LOAD, late-onset Alzheimer’s disease; MGC, microglia cell; Mt, mitochondria; PET, positron emission tomography; ROS, reactive oxygen species; T1DM, type 1 diabetes mellitus
Initially and for many decades, the aging and vascular hypothesis held strong; however, once the amyloid cascade hypothesis was discovered in 1991 the pendulum rather quickly swung to this hypothesis as the most likely leading cause for LOAD [
8,
9]. Additionally, Swerdlow and Khan importantly proposed a mitochondrial cascade hypothesis with redox stress as a leading cause for sporadic Alzheimer’s disease or LOAD in 2004 [
10]. Notably the amyloid cascade hypothesis may be somewhat flawed, in that, amyloid beta (Aβ) by itself may be necessary but not sufficient to be the primary cause for the development of LOAD [
11].
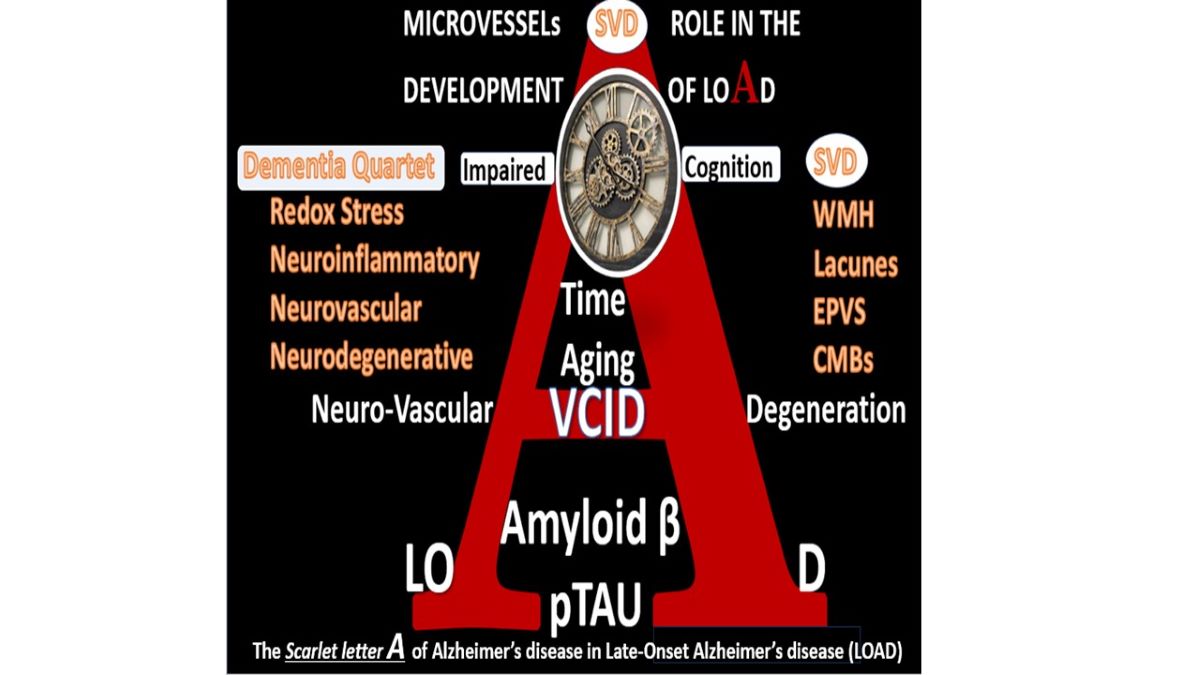
Just as many things in life occur, the ol adage that ‘what goes around comes around’ has taken hold and now the current terminology regarding vascular microvessel remodeling is quite active in the literature and is referred to as the vascular contributions to cognitive impairment and dementia (VCID). VCID encompasses all types of cerebrovascular cardiovascular disease-related conditions associated with impaired cognition and cognitive decline.
Microvessel cerebral small vessel disease (SVD) is considered to be the most important vascular contributor to cognitive decline and dementia [
12]. Importantly, Jacob et al., were able to share that SVD baseline severity and progression were independently associated with an increase in risk of all-cause dementia over a follow-up of 14 years with white matter hyperintensities (WMH) progression predicting incident all-cause dementia [
12].
Because of the proven ability to prevent and treat cardiovascular disease including hypertension, the NIH has recently designated VCID as a critical focus of research [
13]. Moreover, the recent MarkVCID Biomarker Development and Validation Consortium has been designed to better predict, study, and diagnose SVD in the brain and its role in VCID. The National Institutes of Health (NIH) has launched MarkVCID, which is a national consortium designed to accelerate the development of new and existing biomarkers for small vessel VCID. The overall goal of the consortium is to deliver high-quality biomarkers ready for use in clinical trials aimed at generating scientific breakthroughs in our understanding and treatment of VCID [
13].
Brain Microvessels may be defined as those arterioles, precapillary arterioles, capillaries, postcapillary venules and venules that are ≤ 500 μm that may have only one-two layers VSMC in their media or just a single layer of pericyte(s) (Pcs) (
Figure 1) [
1,
2].
Thus, they are structurally designed to function both as resistance and transport microvessels, which allow for the regulation of hydrodynamics and the exchange of nutrients and waste products, oxygen and carbon monoxide, and water within the central nervous system (CNS) [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14].
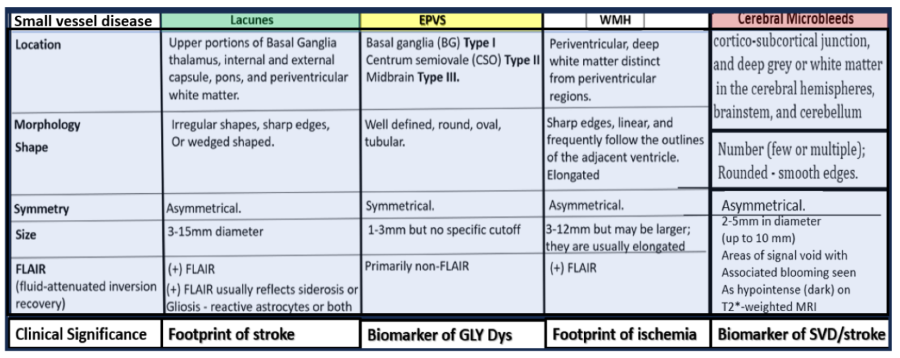
Box 2.
Comparing the four major identifiable structures of cerebral small vessel disease (SVD) by MRI observations. Lacunes (a footprint of stroke); 2. enlarged perivascular spaces (EPVS) (a biomarker of glymphatic system pathway dysfunction (GLY Dys)); 3. white matter hyperintensities (WMH) (footprint of ischemia); 4. cerebral microbleeds (CMBs) (biomarkers of SVD/stroke with hemorrhage or ischemic infarct); 5. recent small subcortical infarcts (MRI representative findings of recent infarction similar to lacune parameters but with greater flair suggesting recent occurrence, not presented in this figure). The location of CMBs has further clinical importance in that lobar/cortical CMBs are CAA-related and deep, basal, infratentorial CMBs are hypertension-related. Modified Figure image provided with permission by CC 4.0 [1ShulyWhy]. CAA, cerebral amyloid angiopathy; mm, millimeter; MRI, magnetic resonance imaging.
Box 2.
Comparing the four major identifiable structures of cerebral small vessel disease (SVD) by MRI observations. Lacunes (a footprint of stroke); 2. enlarged perivascular spaces (EPVS) (a biomarker of glymphatic system pathway dysfunction (GLY Dys)); 3. white matter hyperintensities (WMH) (footprint of ischemia); 4. cerebral microbleeds (CMBs) (biomarkers of SVD/stroke with hemorrhage or ischemic infarct); 5. recent small subcortical infarcts (MRI representative findings of recent infarction similar to lacune parameters but with greater flair suggesting recent occurrence, not presented in this figure). The location of CMBs has further clinical importance in that lobar/cortical CMBs are CAA-related and deep, basal, infratentorial CMBs are hypertension-related. Modified Figure image provided with permission by CC 4.0 [1ShulyWhy]. CAA, cerebral amyloid angiopathy; mm, millimeter; MRI, magnetic resonance imaging.
Microvessel SVD refers to a variety of structural and functional changes involving small perforating arterioles, capillaries, and venules of the brain [
14]. Further, these SVDs are broken down into at least 4 main categories such as WMH, lacunes, enlarged perivascular spaces (EPVS), and cerebral microbleeds (CMBs) (Box 2) [
1,
14].
Indeed, SVD is a group of microvessel diseases that affect small arteries, precapillary arterioles, and postcapillary venules in the brain, and can manifest as lacunes, EPVS, WMH, CMBs capable of promoting the development of neurodegeneration [
14,
15].
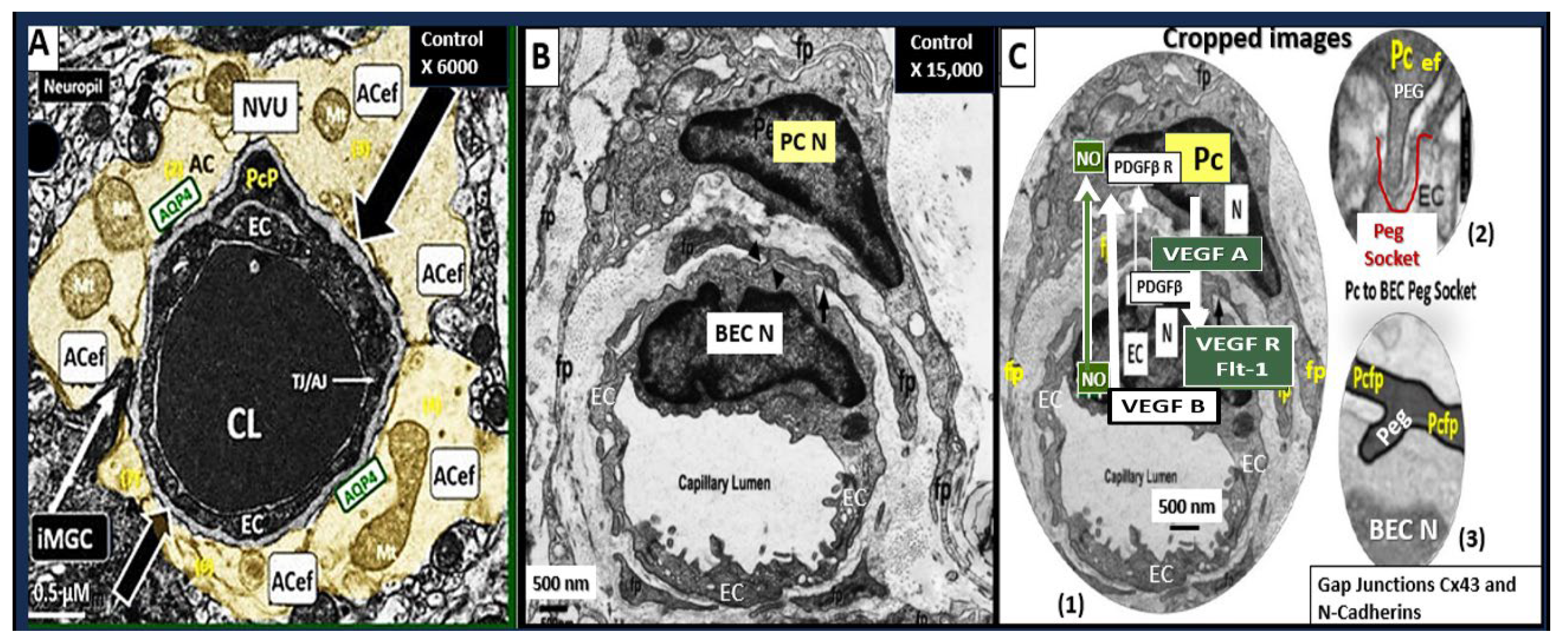
It is important to note here that when utilizing TEM microscopy, one cannot visually observe how the protoplasmic perivascular astrocytes endfeet (pvACef) connect with regional neurons to create neurovascular unit (NVU) coupling. The NVU (
Figure 2) consists of the BEC that line the vascular lumen, the pericytes that are formed within the basement membrane (BM), which supports the BEC and contributes to the synthesis and maintenance of the tight and adherens junctions (TJ/AJ), junctional adhesion molecules (JAMs) and vascular endothelial cadherin(s) (VE-Cads or cluster of differentiation CD144) responsible for creating the blood-brain barrier (BBB), and the adjacent protoplasmic perivascular astrocyte endfeet that adheres tightly to the combined BEC and pericyte BM that communicates via astrocyte cytoplasmic processes to regional neurons, neuronal dendritic synapses, and terminal axon synapses that form a tripartite synapse (
Figure 2) [
16,
17,
18,
19,
20].
Notebly, pvACs with their endfeet are the master connecting cells of the CNS [3, 16,
While this narrative focuses on microvessel remodeling, it is important to briefly discuss the role of macrovessel remodeling in both the extracranial and intracranial macrovessels (≥ 5 μm with at least 2 layers of vascular smooth muscle cell(s) (VSMCs) within their media). Macrovessels are capable of undergoing primarily atherosclerotic and arteriolosclerotic remodeling that result in the development of stroke prone regions of involvement that result in primarily decreased cerebral blood flow (CBF) ischemic strokes via not only decreased CBF but also thromboembolic phenomenon to result in ischemic stroke, which is the number one cause for stroke and impaired remodeling to result in increased redox stress, neuroinflammation, neurodegeneration and impaired cognition of vascular dementia (VaD) or VCID [
22,
23,
24].
Just as LOAD is a multifactorial and heterogenous aging-related neurological disease [
23,
25], neurodegeneration (ND) is also a complicated multifactorial process due to the slow development of CNS neuronal atrophy and loss as a result of programmed cell death that is an important final step in the development of dementia [
26]. In this narrative we will focus on the ND causation due to misfolded proteins, which include the accumulation of toxic oligomers of extracellular Aβ (CNS Aβ (1-42)) as interstitial senile or Aβ plaques (LOAD) and primarily Aβ 1-40 within precapillary arterioles in the interstitial and perivascular adventitia basement membranes and extracellular matrix (ECM) associated cerebral amyloid angiopathy (CAA)). While not a focus, it is important to note that intracellular misfolded tau proteins form misfolded neurofibrillary tangles (NFTs) due to aberrant hyperphosphorylation, which promote neuronal dysfunction, impaired cognition, and neurodegeneration [
5].
Notably, Iturria-Medina et al. (2016) have stated based upon multifactorial data-driven analysis that vascular dysregulation and microvessel remodeling might be the earliest/strongest brain pathologic factor associated with LOAD development that is followed by Aβ deposition, misfolded tau formation with neurofibrillary tangles, glucose metabolism dysregulation, functional impairment, neuronal apoptosis, and grey matter atrophy [
3].
Dementia is an umbrella more generalized term, which consists of four types of dementia including i) Alzheimer’s - LOAD dementia, ii) VaD, co-occurring or mixed dementias (MD), frontal temporal dementia, and Lewy body dementia [
23]. Herein, the discussion will primarily focus on SVD, VaD/VCID, and Alzheimers disease or LOAD. Indeed, sporadic and age-related neurodegenerative disorders such as LOAD are the consequence of aging-associated multifactorial biological dysfunction with a heterogenous background [
3,
4,
27,
28].
When studying chronic age-related diseases such as co-occurring VaD with LOAD, which includes both arterial macrovessel disease and microvessel disease (SVD), it is important to understand structural remodeling [
29]. Microvessel remodeling as a result of chronic peripherally-derived neurotoxic metabolic alterations as it relates to concurrent remodeling of arterioles, precapillary arterioles, the true capillary, the postcapillary venules and veins in development of vascular remodeling and small vessel disease and neurodegeneration is very complicated. Thus, it seems appropriate to pay homage to the microvessels and their component cells consisting of BECs, Pcs, and connecting astrocyte end feet that result in the formation of the NVU of the brain to control CBF providing nourishment and delivery of oxygen to neuronal cells for proper function.
The widely accepted current hypothesis of neurodegeneration in LOAD has previously been widely centered around abnormal misfolded amyloid and tau protein aggregation has been undergoing some changes recently and more are now pointing to the importance of the macro- and microvascular contributions to neurodegeneration [
14,
15,
22,
23,
24,
29,
30,
31,
32,
33]. Recently, emerging evidence suggests that endothelial dysfunction is an early and primary event in AD pathogenesis that may precede abnormal protein aggregation and directly contribute to neurodegeneration and synaptic injury. Importantly, microvessel SVD has been increasingly recognized as a significant contributor to the development of neurodegeneration via Microvessel SVD dysfunction and damage [
14]; WMH Changes and Neurodegeneration [34
Rizvi]; Capillary NVU Blood-Brain Barrier dysfunction [
35]
; Inflammation and Neurodegeneration [
23]; Cerebral Microbleeds and Neurodegeneration [
36].
Neural Oxidative – Redox Stress (OxRS) Including: ROS, Reactive Oxygen, Nitrogen, Sulfur Species (RONSS), Iron Sulfur Clusters (ISCs) of the Reactive Species Interactome (RSI)
LOAD and/or MD may be defined by the accumulation of two types of insoluble misfolded, fibrous proteins, i.e., extracellular amyloid-β peptide deposited in senile Aβ plaques, and intracellular NFTs composed primarily of abnormal and hyperphosphorylated tau protein. In addition to ECM Aβ plaques and intracellular NFT (hyperphosphorylated tau), LOAD may be further pathologically characterized by regional neuronal death and atrophy that is associated with dysfunctional synapses or their loss of synaptic connections [
1,
2,
3,
4,
79]
Redox homeostasis describes the normal physiologic process of reduction and oxidation in order to re-pair unstable, damaging, reduced ROS, which will include the following oxygen free radical’s: superoxide, (O
2●−); (hydrogen peroxide (
H2O2); hydroxyl radical (
●OH), singlet oxygen (
1O2) and reactive nitric oxide (
•NO) along with organic analogues which include reactive nitrogen species (RNS) primarily peroxynitrite (
ONOO-) [
80,
81,
82]. Oxidative Stress implies a loss of redox homeostasis (imbalance) with an excess of ROS by the singular process of oxidation. Both redox and oxidative stress may be associated with an impairment of antioxidant defensive capacity as well as an overproduction of ROS. Oxidative redox stress (OxRS) implies a loss of this unique homeostasis resulting in an excess production of ROS either through the process of oxidation or reduction. Also, reactive iron and sulfur clusters (ISCs) are important regarding OxRS including ROS, RONSS, and the entire reactive species interactome in the brain and are generated by mitochondria as ISCs and mitochondria ROS (mtROS) (
Figure 15) [
83,
84,
85].
Notably, OxRS is known to result in dysfunctional and damaged proteins, lipids, and nucleic acids (RNA and DNA) that result in multiple biomarkers of OxRS in the brain as oxidatively modified proteins such as protein carbonyls and 3-nitrotyrosine; lipids such as 4-hydroxynonenal (HNE); nucleic acids such as 8-hydroxyguanine (8OHG) [
86].
Each of the component cells of the microvascular NVU (BECs, Pcs, pvACef, and MGCs) are capable of generating large amounts of ROS and proinflammatory cytokines/chemokines (
cnsC/C) when they become activated as in BEC
act/dys, or reactive as in rPcs, rACs, or rMGCs, due to various injurious stimuli with the formation of aMt (see figure 8A-E and figure 15 wherein aMt generate not only ROS but also redox reactive prooxidative iron and sulfur species (ISCs) and increased NOX activity [
29,
37]. Additionally, ROS and/or OxRS are known to form a vicious cycle with neuroinflammation and ROS begets ROS (
Figure 16) [
29,
37,
87].
Neurodegeneration
LOAD and/or MD (LOAD plus VaD) are the leading causes of sporadic dementia in the aging population (23, 91, 92). Individuals with LOAD/MD experience clinical symptoms of cognitive impairments, memory loss (especially recent memory loss, and behavioral changes that interfere with activities of daily living [
93,
94]. The dementia in LOAD/MD is associated with neurodegeneration that is characterized initially by synaptic injury, dysfunction and/or loss [
95,
96] followed by neuronal loss [
97]. Further, these findings are also, accompanied by rACs and astrogliosis [
98], rMGC proliferation [
99,
100], and the presence of neurofibrillary tangles composed of dystrophic neurites and hyperphosphorylated tau [
95,
101,
102,
103,
104,
105].
Multiple defective cellular processes such as aMt remodeling as in figure 8A-E with dysfunctional leakage of mtROS, increased OxRS from all cells of the NVU (BECs, Pcs, and reactive ACs and MGCs and neurons, lysosomal dysfunction with decreased disposal of Aβ aggregates and oligomers, and increased inflammation (
cnsCC) with subsequent associated apoptotic loss and neurodegeneration as in figures 8F and 18 [
92].
In regards to neurodegeneration and neuronal death there are four major cellular death pathways including necrosis, apoptosis, excitatory, and autophagic. In MD there would be at least two different neuronal cell death pathways namely apoptosis in LOAD and ischemic necrosis in VaD [
106].
Mitochondria are an important neuronal organelle producing reactive oxygen species (ROS) in addition to plasma membraneous NADPH oxidase (Nox1) in LOAD [
107,
108]. According to Xie et al., leaky aMt could be considered the central pawns in the development of neurodegeneration and loss via neuronal apoptosis and LOAD as illustrated in figure 8E-F. [
108]. Increased and accelerated accumulation of Aβ plaque aggregation and deposition within the CNS ECM plaques in LOAD associate with increased soluble Aβ oligomers as in figure 8 and 9A. Indeed, these findings are result from an imbalance in Aβ production, aggregation and impaired clearance [
92] that associate with the development of SVD and identification of EPVS by MRI. Aβ clearance is mediated by proteolytic enzymes such as neprilysin [
109], chaperone molecules such as ApoE in health [
110] and lysosomal autophagy [
111], and non-lysosomal pathways via the proteasome [
112] in health, which is impaired in LOAD/MD. It is currently thought that soluble Aβ oligomers rather than the fibrils within the Aβ plaques are responsible for the neurotoxic effect on synapses and neurons associated with neurodegeneration [
77,
113,
114].
It is important to note here that SVD is capable of promoting and contributing to the development of neurodegeneration (
Figure 19) [
14,
15].
Further, Crews and Masliah have put for the following sequences for neurogenic development with impaired cognition that is observed in LOAD as follows. Neurotoxic effects of Aβ plaque and its oligomers result in signaling alteration in neuronal neurodegenerative kinases including Fyn, FAK, GSK3β, and CDK5. These alterations result in alterations in cytoskeletal and synaptic proteins resulting in tau-derived neurofibrillary tangles (NFTs). aMt result in leaky neurotoxic mtROS; neuronal oxidative/redox stress; impaired lysosomal uptake and proteolysis and are associated with
cnsCCs, which proceed to neurodegeneration consisting of synaptic injury and dysfunction; defects in neurogenesis that lead to impaired cognition LOAD along with neuronal apoptosis and loss with cerebral atrophy [
92].
Cerebral atrophy is a persistent core marker finding on MRI in LOAD and MD (VaD + LOAD) in both rodent animal models (such as the female diabetic
db/db mouse model at 26-weeks of age) and aging human individuals (
Figure 20) [
114,
115,
116].
Additionally, there is significant dysfunction and/or loss of synaptic transfer of information at degenerative synapses in LOAD and MDs that is a contributory factor in the development of neurodegeneration, impaired cognition and dementia [
119,
120,
121,
122].
Conclusions
While dementia is a more generalized term that describes the loss of the ability to think, remember, and reason to levels that interfere with daily life and activities, it can then be broken down to its specific causes or disease. These specific diseases consist of four types of dementia including i) Alzheimer’s – LOAD-type dementia, ii) VaD or co-occurring LOAD and VaD resulting in mixed dementias) (MDs), iii) frontal temporal dementia, and iv) Lewy body dementia [
23].
Specifically, Dr. Alois Alzheimer described a 55-year-old female individual with dementia and this is typically now referred to as EOAD [
123,
124,
125] in contrast to the global pandemic form of LOAD that has been the main focus of this narrative review. Thus, LOAD is expected to continue to grow nearly exponentially as the growing global population continues to age and experience longer life cycles especially, in the global post-world-war II baby boom generation for the next 1.5 decades with serious costs to the nations and suffering individuals and their family and professional care providers and long-term care facilities [
6].
Importantly, VCID incorporates multiple cardio-cerebrovascular diseases the include cardiac disease, micro and macrovessel disease both within the CNS and extracranial locations such as micro-infarcts/hemorrhages and CMBs, which may be silent; TIAs with brief clinical episodes, small vessel ischemic and hemorrhagic stroke; CAA; macrovessel ischemic and hemorrhagic stroke; cardiac disease (arrhythmia’s such as atrial fibrillation with thromboembolic stroke, decreased cardiac output such as myocardial infarction and congestive heart failure, and cardiac surgeries that are associated with decreased CBF and chronic cerebral hypoxia (CCH) to name a few). This places VCID centrally in the overlapping Venn diagrams (
Figure 21) [
126].
The original amyloid cascade hypothesis has garnered the interest of most researchers, clinicians, and pharmaceutical companies during the past 2 decades [
8,
9]. Zlokovic initially proposed both a 2-hit hypothesis [
23], and a vasculo-neuronal-inflammatory triad model of neurodegenerative disorders in 2011 [
127], which contributes to the development of neurodegeneration as found in LOAD and MD. Herein, author currently proposes an ‘quartet of mechanisms’ that are importantly involved in the development of ND and MD in aging individuals that additionally emphasizes redox stress in addition to neurovascular mechanisms and include: i) oxidative redox stress (OxRS), ii) neuroinflammation, iii) neurovascular, and iv) neurodegenerative mechanisms in the development of LOAD and MD as proposed when figures 17, 19, and 21 are combined (
Figure 22).
Recently, Ter Telgte et al., [
128] have shown that there is a penumbra effect that may result in diffusion defects that associate with SVD phenotypes as observed on MRI images that is comparable to the penumbra effect associated with acute stroke and remodeling [
129,
130]. The post stroke penumbra is the viable tissue around the irreversibly damaged ischemic core as in figure 14C [
130]. Further, they found that these focal effects were capable of spreading to become global effects at remote more distal regions within the CNS from the subcortical white matter regions to the cortical grey matter regions. As a result of their findings, it is now thought that the structural integrity may become dysfunctional and disrupted. This could interrupt the brains network informational highway that is responsible for the information transference and processing of information transfer, which could result in impaired cognition. This suggests that what we observe on MRI studies may only be the tip of the iceberg in regards to the loss of function, impaired cognition, and the aberrant motor skills that may be affected in a concurrent manner as depicted in figures 21 and 22 in the development of neurodegeneration, LOAD, VaD, and MD [
128].
In summary, when studying chronic age-related diseases such as co-occurring VaD (including both arterial macrovessel and microvessel disease SVD), VaD, LOAD, and MD it is important to understand microvessel SVD structural remodeling as a result of chronic peripherally-derived neurotoxic multiple injurious species as it relates to concurrent remodeling of arterioles, precapillary arterioles, the true capillary, the postcapillary venules and veins in development of vascular remodeling and microvessel SVD and neurodegeneration. Thus, it seems appropriate to pay homage to the microvessel SVD and specifically the component NVU cells in SVD. Since the development of both microvessel SVD and LOAD are multifactorial it is felt that it will require a multifactorial approach in order to better understand this association.
The initiation of the microvessel SVD remodeling and neurodegeneration seems to continuously come back and point to the importance of the early development of BEC
act/dys and BBB
dd. The combination of increased BEC inflammatory signaling with the production of
cnsCC, decreased eNOS function with eNOS uncoupling, decreased NO, increased OxRS provided by increased BEC NOX production and mt ROS leakage and eNOS uncoupling, along with the brain endothelial-mediated neurotoxicity proposed by Grammas [
131] as in figure 2 contribute to decreased CBF and neurodegeneration [
55].
This narrative review has examined the background of microvessel SVD development and not only its association to VaD but also LOAD that combines to develop mixed dementia in the introduction. Next the role of microvascular NVU and each of its constituent cells especially, the BECact/dys and BBBdd in section This allows leakage of peripheral neurotoxins into the CNS to result in not only the entry of pCC into the CNS but also the subsequent activation of cnsCC promoting a state of neural OxRS in section 3. This contributes to ongoing neuroinflammation in section 4 and its relationship to neurodegeneration discussed in section 5.
Abbreviations
Aβ, amyloid beta; AC, astrocyte; ACef, astrocyte endfeet; BBB, blood–brain barrier; BEC(s), brain endothelial cell(s); BECact/dys, brain endothelial cell activation/dysfunction; BBBdd, blood-brain barrier dysfunction disruption; BDGF, brain-derived growth factor; bFGF, basic fibroblast growth factor; BM, basement membrane; CAA, cerebral amyloid angiopathy; CBF, cerebral blood flow; CL, capillary lumen; CMB(s), cerebral microbleeds; CNS, central nervous system; cnsCC, central nervous cytokines chemokines; ELOAD, early-onset Alzheimer’s disease; EPVS, enlarged perivascular spaces; ET-1, endothelin 1; GDNF, glia cell-derived growth factor; GS, glymphatic space; ISC, iron sulfur clusters; JAMs, junctional adhesion molecules; LOAD, late-onset Alzheimer’s disease; MCI, mild or minimal cognitive impairment; MD(s), mixed dementias; MGCs, microglia cells; MMP-2,-9, matrix metalloproteinase-2,-9; MRI, magnetic resonance imaging; mtROS, mitochondrial ROS; NADPH Ox, nicotine adenine diphosphate reduced oxidase; ND, neurodegeneration; NGF, nerve growth factor; NIH, National Institute of Health; NGTs, neurofibrillary tangles; NO, nitric oxide; NOX, NADPH oxidase, NVU, neurovascular unit; OxRS, oxidative redox stress; Pc, pericyte; pCC, peripheral cytokines chemokines; Pcfp, pericyte foot process; pvACfp/ef, perivascular astrocyte foot processes/endfeet; PVS, perivascular spaces; PVS/EPVS, perivascular space/enlarged perivascular space; ROS, reactive oxygen species, RONS, reactive oxygen, nitrogen species; RONSS, reactive oxygen, nitrogen, sulfur species; rPVACfp/ef, reactive perivascular astrocyte foot processes/endfeet; rPVMΦ, reactive resident perivascular macrophages; RSI, reactive species interactome; SVD, cerebral small vessel disease; TEM, transmission electron microscopy; TGFβ, transforming growth factor beta; TI/AJs, tight and adherens junctions; TNFα, tumor necrosis factor alpha; VaD, vascular dementia; VCAM-1, vascular cell adhesion molecule-1; VCID, vascular contributions to cognitive impairment and dementia; VEGF A or B, vascular endothelial growth factor A or B; VSMC(s), vascular smooth muscle cells; WMH, white matter hyperintensities.
Figure 1.
Three transition electron microscopy (TEM) cross section images of microvessels from various animal models from layer III in the frontal cortex at various magnifications that represent microvessels. These images are in contrast to those macrovessels that have a diameter of ≥ 5 μm with at least 2 layers of vascular smooth muscle cell (s) (VSMCs) within their media. Magnification 3μm, 0.5 μm, 5 μm (far-left, middle, and far-right respectively). Images provided with permission by CC 4.0 [
1,
2]. AC, astrocytes pseudo-colored gold in; far-left and pseudo-colored blue far-right images. AQP-4, aquaporin 4; AC, perivascular astrocyte; AC1, AC2, astrocyte endfeet numbers 1 and 2 ACef, perivascular astrocyte endfeet; CL, capillary lumen; EC, brain endothelial cell; gs, glymphatic space; lys, lysosome; Mt, mitochondria; N, nucleus; NVU, neurovascular unit; Pc, pericyte; PcN, pericyte nucleus; Pcp, pericyte endfeet processes; PVS, perivascular space; rMGC, interrogating or reactive microglia; rMФ, reactive macrophage; TJ/AJ, tight junctions/adherens junctions.
Figure 1.
Three transition electron microscopy (TEM) cross section images of microvessels from various animal models from layer III in the frontal cortex at various magnifications that represent microvessels. These images are in contrast to those macrovessels that have a diameter of ≥ 5 μm with at least 2 layers of vascular smooth muscle cell (s) (VSMCs) within their media. Magnification 3μm, 0.5 μm, 5 μm (far-left, middle, and far-right respectively). Images provided with permission by CC 4.0 [
1,
2]. AC, astrocytes pseudo-colored gold in; far-left and pseudo-colored blue far-right images. AQP-4, aquaporin 4; AC, perivascular astrocyte; AC1, AC2, astrocyte endfeet numbers 1 and 2 ACef, perivascular astrocyte endfeet; CL, capillary lumen; EC, brain endothelial cell; gs, glymphatic space; lys, lysosome; Mt, mitochondria; N, nucleus; NVU, neurovascular unit; Pc, pericyte; PcN, pericyte nucleus; Pcp, pericyte endfeet processes; PVS, perivascular space; rMGC, interrogating or reactive microglia; rMФ, reactive macrophage; TJ/AJ, tight junctions/adherens junctions.
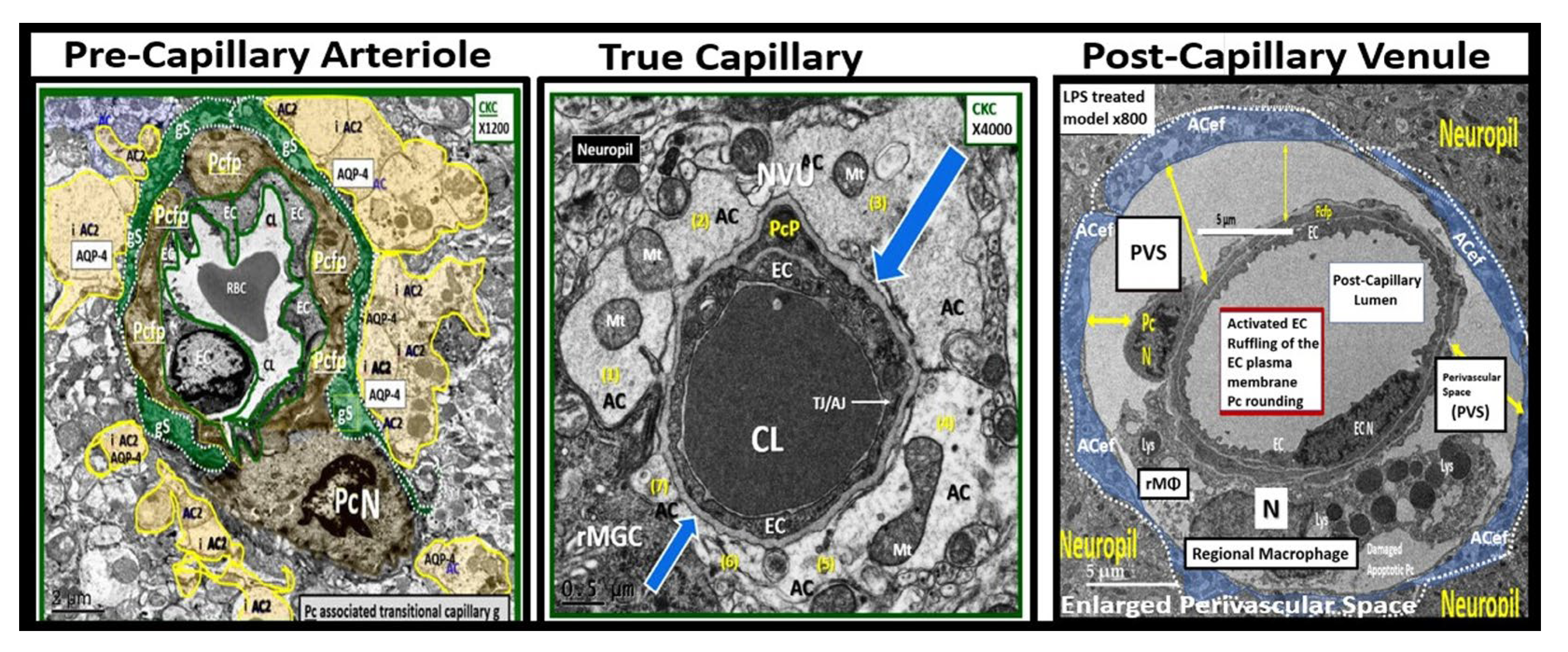
Figure 2.
perivascular astrocyte endfeet (pvACef) provide the connections between the capillary and regional neurons to form the neurovascular unit (NVU). This combined cartoon illustration and transmission electron micrographs allows one to better visualize how the pvACef connect the Mural capillary to the regional neurons (Panel A). Note that in panel A that there is detachment and retraction of pvACef that are noted in the diabetic db/db models. Other pvACef connect with the synapses (Panel B). Images in panel A and B were provided with permission by CC 4.0 [16OK]. AC, astrocyte; ACef, astrocyte endfeet; AQP-4, aquaporin four; BDGF, brain derived growth factor; Ca++, calcium ion; CKC, control model; DBC, diabetic db/db model; DBE, diabetic db/db treated with empagliflozin; GDGF, glioma-derived growth factor; PSD, post synaptic density; S, synapse; TGF-β, transforming growth factor beta.
Figure 2.
perivascular astrocyte endfeet (pvACef) provide the connections between the capillary and regional neurons to form the neurovascular unit (NVU). This combined cartoon illustration and transmission electron micrographs allows one to better visualize how the pvACef connect the Mural capillary to the regional neurons (Panel A). Note that in panel A that there is detachment and retraction of pvACef that are noted in the diabetic db/db models. Other pvACef connect with the synapses (Panel B). Images in panel A and B were provided with permission by CC 4.0 [16OK]. AC, astrocyte; ACef, astrocyte endfeet; AQP-4, aquaporin four; BDGF, brain derived growth factor; Ca++, calcium ion; CKC, control model; DBC, diabetic db/db model; DBE, diabetic db/db treated with empagliflozin; GDGF, glioma-derived growth factor; PSD, post synaptic density; S, synapse; TGF-β, transforming growth factor beta.
Figure 3.
Multiple peripheral-systemic injurious species (neurotoxins) affect the brain endothelial cells (BECs) of the brain. These injurious species active the BECs of the neurovascular unit (NVU) to result in BEC activation and dysfunction (BEC
act/dys) and blood-brain barrier dysfunction/disruption (BBB
dd). BEC
act/dys and BBB
dd are biomarkers for the development of cerebral small vessel disease (SVD). Note the red-dashed line at the top of this image since it demarks the location of the multiple injurious species that are responsible for the initial brain endothelial cell injury in multiple clinical diseases and structural abnormalities, which importantly include SVD. Image provided with permission by CC 4.0 [
37,
39]. Ang II, angiotensin two; BBB, blood–brain barrier; BEC, brain endothelial cell; BECact/dys, brain endothelial cell activation/dysfunction; BH4, tetrahydrobiopterin; CCL2, chemokine (C-C motif) ligand 2; Cox-2, cyclo-oxygenase-2; Cox-2/PGE2 axis, cyclo-oxygenase-2; Prostaglandin E2; ecGCx, endothelial glycocalyx; intercellular adhesion molecule-1; ICAM-1; IL-1β; interleukin-1β; IL-6; interleukin-6; JAMs, junctional adhesion molecules; LDL, low density lipoprotein cholesterol; LPa, lipoprotein little a; MCP-1, monocyte chemotactic protein-1; NO, nitric oxide; Nox2, (NADPH Ox (nicotinamide adenine dinucleotide phosphate oxidase); ONOO, peroxinitrite; pnsCC, peripheral nervous system cytokines and chemokines; peroxinitrite; NVU, neurovascular unit; peroxinitrite; RBC, red blood cell; RONSS, reactive oxygen, nitrogen, sulfur species; ROS, reactive oxygen species; RSI, reactive species interactome; T, transcytosis; TNFα, tumor necro.
Figure 3.
Multiple peripheral-systemic injurious species (neurotoxins) affect the brain endothelial cells (BECs) of the brain. These injurious species active the BECs of the neurovascular unit (NVU) to result in BEC activation and dysfunction (BEC
act/dys) and blood-brain barrier dysfunction/disruption (BBB
dd). BEC
act/dys and BBB
dd are biomarkers for the development of cerebral small vessel disease (SVD). Note the red-dashed line at the top of this image since it demarks the location of the multiple injurious species that are responsible for the initial brain endothelial cell injury in multiple clinical diseases and structural abnormalities, which importantly include SVD. Image provided with permission by CC 4.0 [
37,
39]. Ang II, angiotensin two; BBB, blood–brain barrier; BEC, brain endothelial cell; BECact/dys, brain endothelial cell activation/dysfunction; BH4, tetrahydrobiopterin; CCL2, chemokine (C-C motif) ligand 2; Cox-2, cyclo-oxygenase-2; Cox-2/PGE2 axis, cyclo-oxygenase-2; Prostaglandin E2; ecGCx, endothelial glycocalyx; intercellular adhesion molecule-1; ICAM-1; IL-1β; interleukin-1β; IL-6; interleukin-6; JAMs, junctional adhesion molecules; LDL, low density lipoprotein cholesterol; LPa, lipoprotein little a; MCP-1, monocyte chemotactic protein-1; NO, nitric oxide; Nox2, (NADPH Ox (nicotinamide adenine dinucleotide phosphate oxidase); ONOO, peroxinitrite; pnsCC, peripheral nervous system cytokines and chemokines; peroxinitrite; NVU, neurovascular unit; peroxinitrite; RBC, red blood cell; RONSS, reactive oxygen, nitrogen, sulfur species; ROS, reactive oxygen species; RSI, reactive species interactome; T, transcytosis; TNFα, tumor necro.
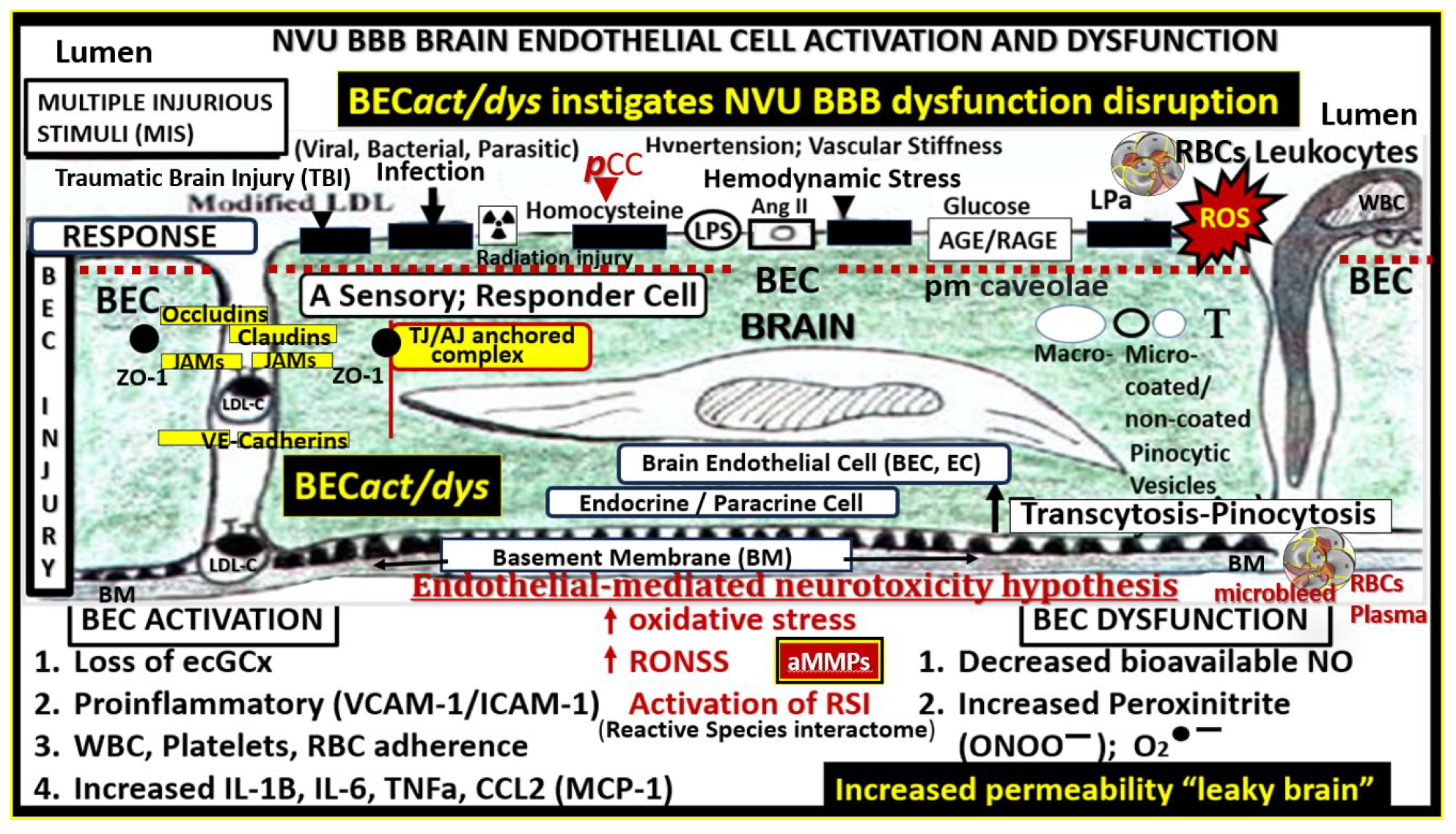
Figure 4.
Brain endothelial cell activation and dysfunction (BEC
act/dys) phenotypes in obese 20-week-old female diabetic
db/db models. Panels A and B demonstrate normal control phenotypes of microvessel neurovascular unit (NVU) BEC from control C57B5J model at 20-weeks in the frontal cortical layer III in cross and longitudinal sections panel A and B respectively. Note that the perivascular astrocytes are pseudo-colored golden with normal appearing electron dense mitochondria and additionally note the cyan-colored line that demarcates the glia limitans in panels A and B. Panel C demonstrates the control normal phenotype in the C57B6J model at 20-weeks. Panel D depicts an activated BEC with marked abrupt swelling of the BEC that is hyperlucent as compared to the adjacent normal thickness of the BEC, which depicts BECact/dys phenotype in the 20-week-old db/db model from the frontal cortex of layer III. Panel E depicts the BM thickening of BECs in panel D and note the vacuole-like structures (V) within the BM. Panels F - I depict the adhesion of monocyte panel F, a lymphocyte panel G, a platelet panel H, and a red blood cell (RBC) panel I. Modified Images provided with permission by CC 4.0 [
2,
39,
40,
41]. AC, astrocyte; ACfp, astrocyte foot process-endfeet; BM, basement membrane; Cap L, capillary lumen; CL, capillary lumen; ECact, brain endothelial cell activation; iMGC, interrogating microglia cell; Mt, mitochondria; Mp, microparticles; Pc, pericyte. .
Figure 4.
Brain endothelial cell activation and dysfunction (BEC
act/dys) phenotypes in obese 20-week-old female diabetic
db/db models. Panels A and B demonstrate normal control phenotypes of microvessel neurovascular unit (NVU) BEC from control C57B5J model at 20-weeks in the frontal cortical layer III in cross and longitudinal sections panel A and B respectively. Note that the perivascular astrocytes are pseudo-colored golden with normal appearing electron dense mitochondria and additionally note the cyan-colored line that demarcates the glia limitans in panels A and B. Panel C demonstrates the control normal phenotype in the C57B6J model at 20-weeks. Panel D depicts an activated BEC with marked abrupt swelling of the BEC that is hyperlucent as compared to the adjacent normal thickness of the BEC, which depicts BECact/dys phenotype in the 20-week-old db/db model from the frontal cortex of layer III. Panel E depicts the BM thickening of BECs in panel D and note the vacuole-like structures (V) within the BM. Panels F - I depict the adhesion of monocyte panel F, a lymphocyte panel G, a platelet panel H, and a red blood cell (RBC) panel I. Modified Images provided with permission by CC 4.0 [
2,
39,
40,
41]. AC, astrocyte; ACfp, astrocyte foot process-endfeet; BM, basement membrane; Cap L, capillary lumen; CL, capillary lumen; ECact, brain endothelial cell activation; iMGC, interrogating microglia cell; Mt, mitochondria; Mp, microparticles; Pc, pericyte. .
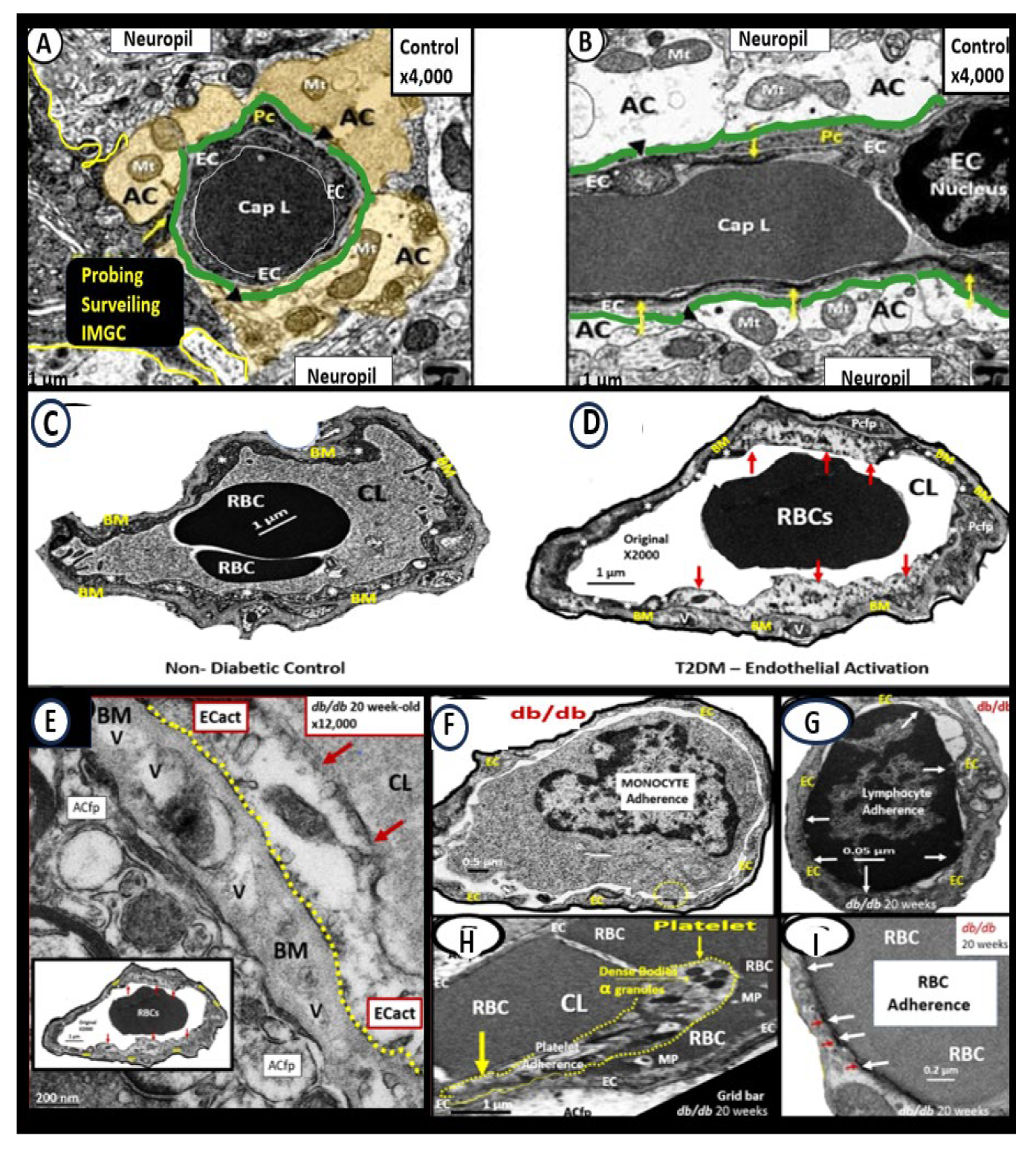
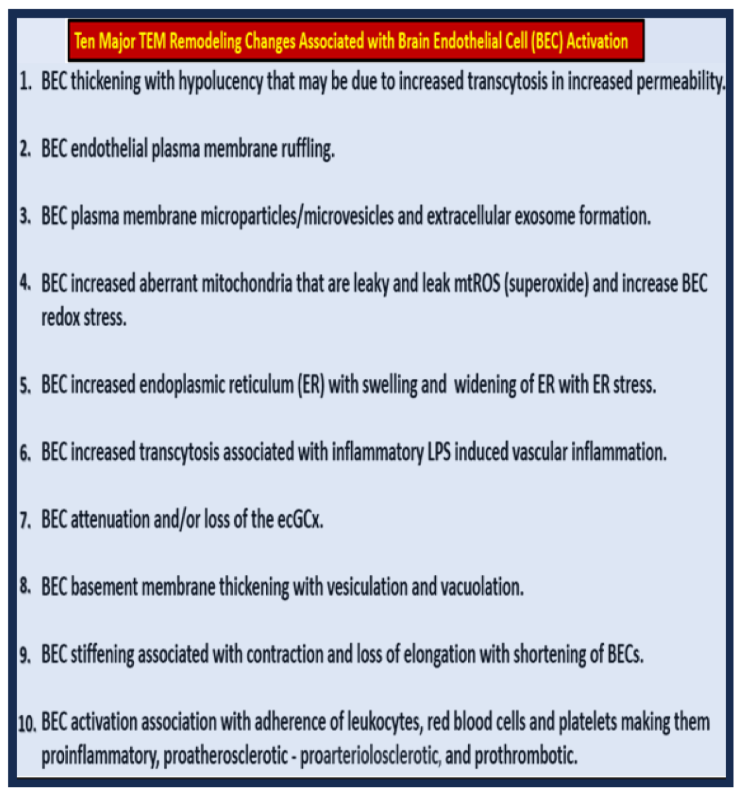
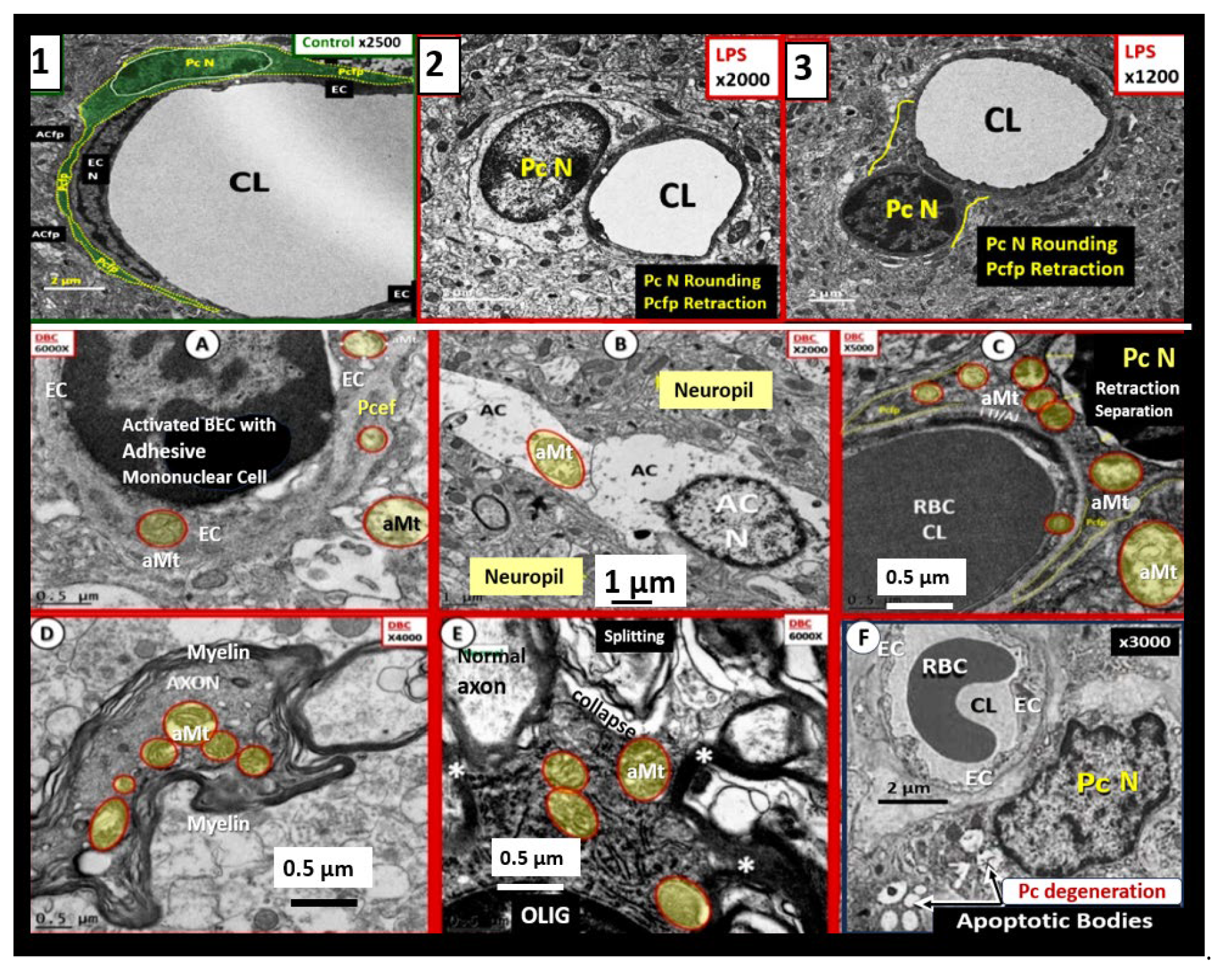
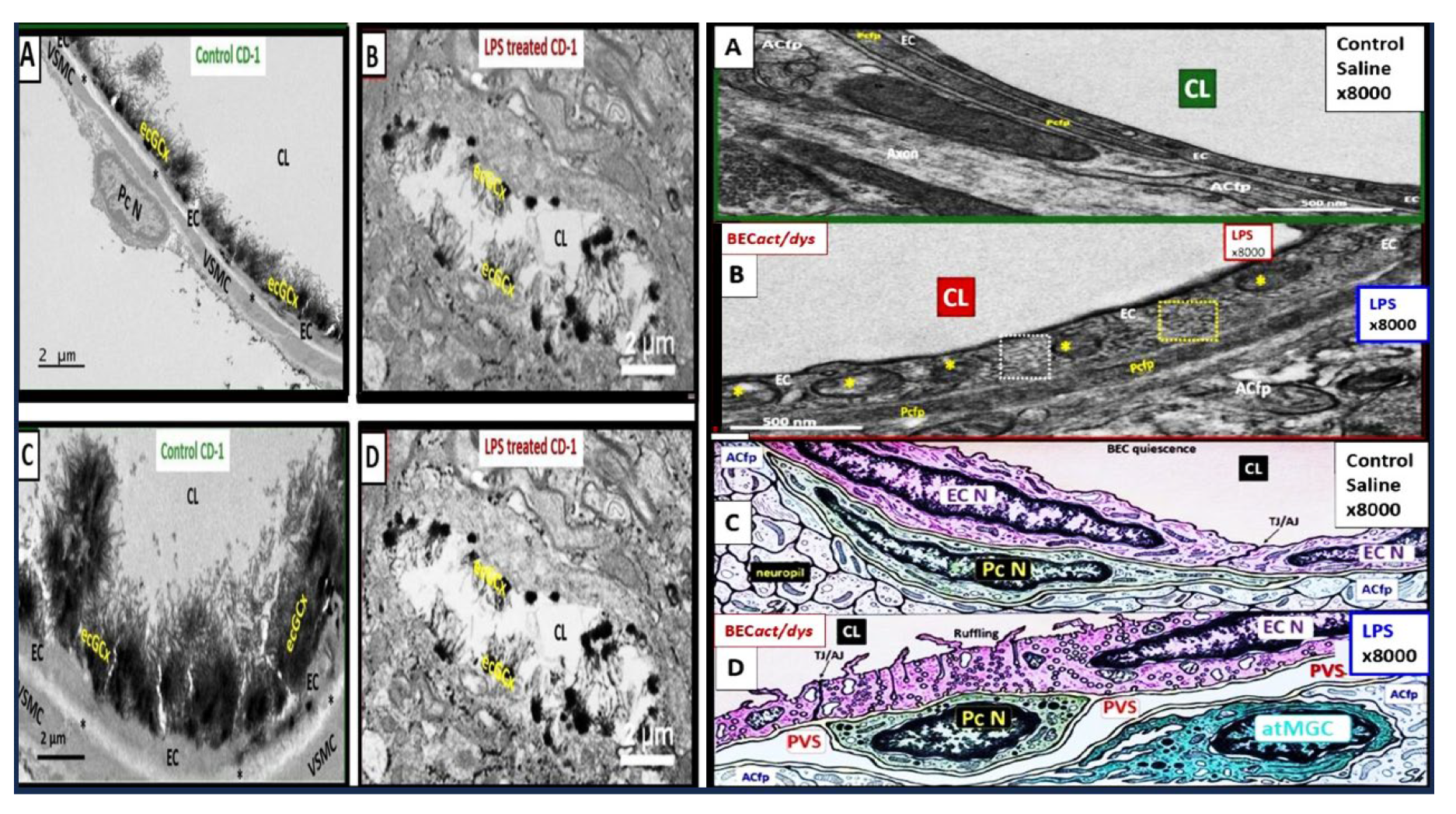
Figure 5.
Proinflammatory LPS results in attenuation and discontinuous endothelial glycocalyx (ecGCx) and increased pinocytosis/transcytosis in activated brain endothelial cell activation/dysfunction (BEC
act/dys). Far-left panels B and D depict an attenuation and discontinuous ecGCx with large naked gaps of BECs as compared to controls with an intact and continuous ecGCx as in panels A and C. Far-right panels B and D depict increased pinocytosis/transcytosis of BEC as compare to control panels A and C. Panels C and D are illustrations to improve and highlight the findings of the TEMs depicted in panels A and D. Images provided with permission by CC 4.0 [
42]. ACfp, astrocyte foot process endfeet; atMGC, attracted microglia cell(s); BECact/dys, brain endothelial cell activation/dysfunction; CL, capillary lumen; ecGCx, brain endothelial cell glycocalyx; EC N, brain endothelial cell nucleus; LPS, lipopolysaccharide; Pc N, pericyte nucleus; PVS, perivascular space;.
Figure 5.
Proinflammatory LPS results in attenuation and discontinuous endothelial glycocalyx (ecGCx) and increased pinocytosis/transcytosis in activated brain endothelial cell activation/dysfunction (BEC
act/dys). Far-left panels B and D depict an attenuation and discontinuous ecGCx with large naked gaps of BECs as compared to controls with an intact and continuous ecGCx as in panels A and C. Far-right panels B and D depict increased pinocytosis/transcytosis of BEC as compare to control panels A and C. Panels C and D are illustrations to improve and highlight the findings of the TEMs depicted in panels A and D. Images provided with permission by CC 4.0 [
42]. ACfp, astrocyte foot process endfeet; atMGC, attracted microglia cell(s); BECact/dys, brain endothelial cell activation/dysfunction; CL, capillary lumen; ecGCx, brain endothelial cell glycocalyx; EC N, brain endothelial cell nucleus; LPS, lipopolysaccharide; Pc N, pericyte nucleus; PVS, perivascular space;.
Figure 6.
Loss and/or disruption of the normal continuous tight and adherence junction(s) (TJ/AJs) paracellular blood–brain barrier (BBB) in male CD-1 streptozotocin-induced (STZ) diabetic preclinical mice models resulting in blood–brain barrier dysfunction and disruption (BBB
dd) protected by the carbonic anhydrase inhibitor (Topiramate a mitochondria antioxidant) in the mid brain as compared to the cerebellum. STZ-induced diabetic mice revealed disruption of the BBB by
14C-sucrose measurements. Panel (A) displays three prominent elongated and continuous highly electron-dense TJ/AJ (white and black arrows). Panel (B) depicts a discontinuous and disrupted TJ/AJ (black arrows) into three distinct segments in the midbrain and note how TJ/AJs tend to form at the BEC-BEC overlap junctions in panels A, B, C. Panel (C) illustrates that treatment with Topiramate (TOP) prevented this disruption in the brain endothelial cell BBB (
yellow and black arrows and yellow dashed line below the intact BBB TJ/AJ) in the midbrain. Revised figure images provided with permission by CC 4.0 [
39,
42]. Magnification ×3000; scale bar = 0.5 μm (
A); ×10,000; scale bar = 0.2 μm in (B, C). BEC, brain endothelial cell; EC, brain endothelial cell; CL, capillary lumen; Pc, pericyte; RBC, red blood cell; Rx, treatment; T1DM, type 1 diabetes mellitus; TOP, topiramate.
Figure 6.
Loss and/or disruption of the normal continuous tight and adherence junction(s) (TJ/AJs) paracellular blood–brain barrier (BBB) in male CD-1 streptozotocin-induced (STZ) diabetic preclinical mice models resulting in blood–brain barrier dysfunction and disruption (BBB
dd) protected by the carbonic anhydrase inhibitor (Topiramate a mitochondria antioxidant) in the mid brain as compared to the cerebellum. STZ-induced diabetic mice revealed disruption of the BBB by
14C-sucrose measurements. Panel (A) displays three prominent elongated and continuous highly electron-dense TJ/AJ (white and black arrows). Panel (B) depicts a discontinuous and disrupted TJ/AJ (black arrows) into three distinct segments in the midbrain and note how TJ/AJs tend to form at the BEC-BEC overlap junctions in panels A, B, C. Panel (C) illustrates that treatment with Topiramate (TOP) prevented this disruption in the brain endothelial cell BBB (
yellow and black arrows and yellow dashed line below the intact BBB TJ/AJ) in the midbrain. Revised figure images provided with permission by CC 4.0 [
39,
42]. Magnification ×3000; scale bar = 0.5 μm (
A); ×10,000; scale bar = 0.2 μm in (B, C). BEC, brain endothelial cell; EC, brain endothelial cell; CL, capillary lumen; Pc, pericyte; RBC, red blood cell; Rx, treatment; T1DM, type 1 diabetes mellitus; TOP, topiramate.
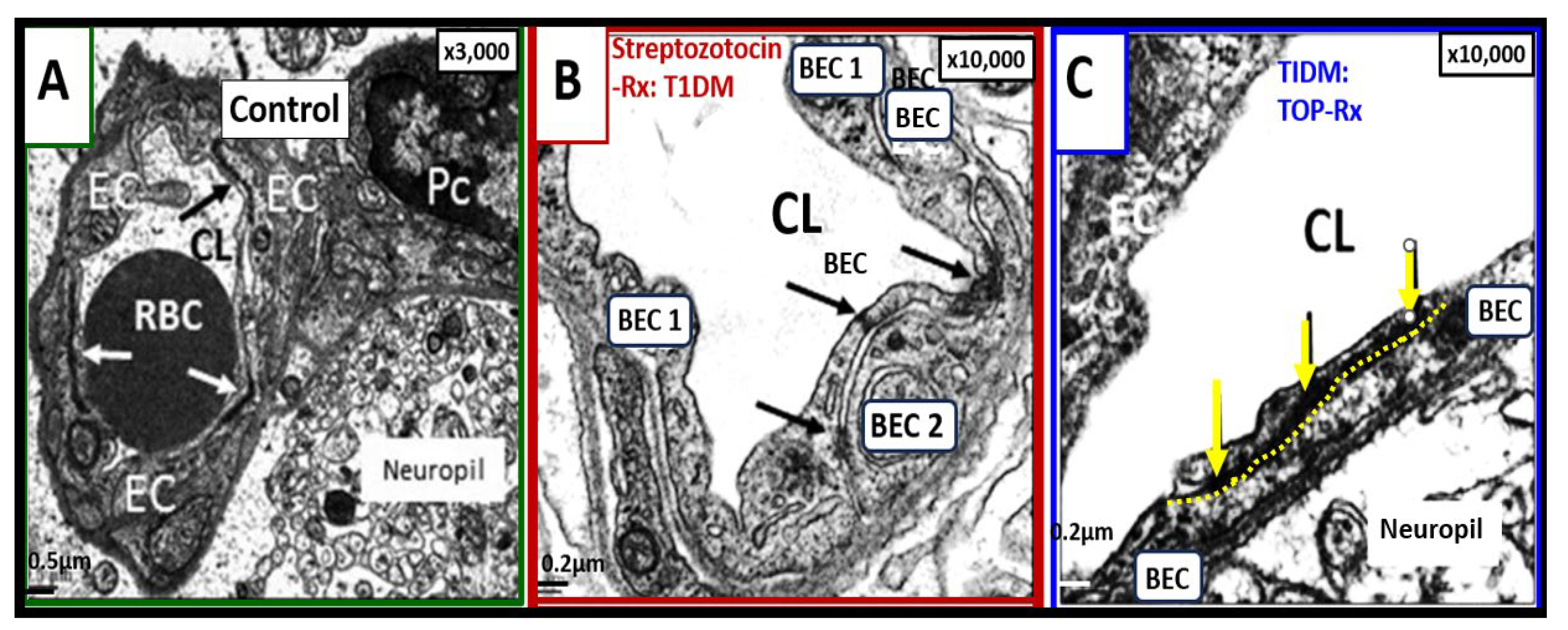
Figure 8.
Neurotoxicity of amyloid beta in close proximity to the neurovascular unit (NVU) brain endothelial cell(s) (BECs) may result in blood-brain barrier dysfunction and disruption (BBBdd). These images are from a nine-month-old 5xFAD male mouse model from an old slide-deck not previously studied or published. Panel A depicts an interstitial extracellular matrix-interstitial amyloid beta (Aβ) (pseudo-colored red) in close proximity to the NVU that appears to nearly touch the outer basement membrane (BM) of the NVU depicted. Importantly, note that this image depicts degenerative neurites (yellow arrows and outlined in yellow dashed-lines) within the adjacent interstitial neuropil. Panel B depicts an exploded image in Microsoft paint with intact scale bar. Insert C is a further exploded image of how Aβ is closely adjacent to the microvessel NVU BEC basement membrane (BM) and appears to actually be in direct contact with the BM. Indeed, this structural arrangement contributes to Aβ being neurotoxic to BECs and creating a vicious cycle of BEC injury by Aβ and BECact/dys that that contributes to a further increase in ROS and damage to the NVU with eventual NVU uncoupling and decrease in regional cerebral blood flow that will ultimately increase neurodegeneration and increased or accelerated amyloid beta deposition. This image has not been previously published. .
Figure 8.
Neurotoxicity of amyloid beta in close proximity to the neurovascular unit (NVU) brain endothelial cell(s) (BECs) may result in blood-brain barrier dysfunction and disruption (BBBdd). These images are from a nine-month-old 5xFAD male mouse model from an old slide-deck not previously studied or published. Panel A depicts an interstitial extracellular matrix-interstitial amyloid beta (Aβ) (pseudo-colored red) in close proximity to the NVU that appears to nearly touch the outer basement membrane (BM) of the NVU depicted. Importantly, note that this image depicts degenerative neurites (yellow arrows and outlined in yellow dashed-lines) within the adjacent interstitial neuropil. Panel B depicts an exploded image in Microsoft paint with intact scale bar. Insert C is a further exploded image of how Aβ is closely adjacent to the microvessel NVU BEC basement membrane (BM) and appears to actually be in direct contact with the BM. Indeed, this structural arrangement contributes to Aβ being neurotoxic to BECs and creating a vicious cycle of BEC injury by Aβ and BECact/dys that that contributes to a further increase in ROS and damage to the NVU with eventual NVU uncoupling and decrease in regional cerebral blood flow that will ultimately increase neurodegeneration and increased or accelerated amyloid beta deposition. This image has not been previously published. .
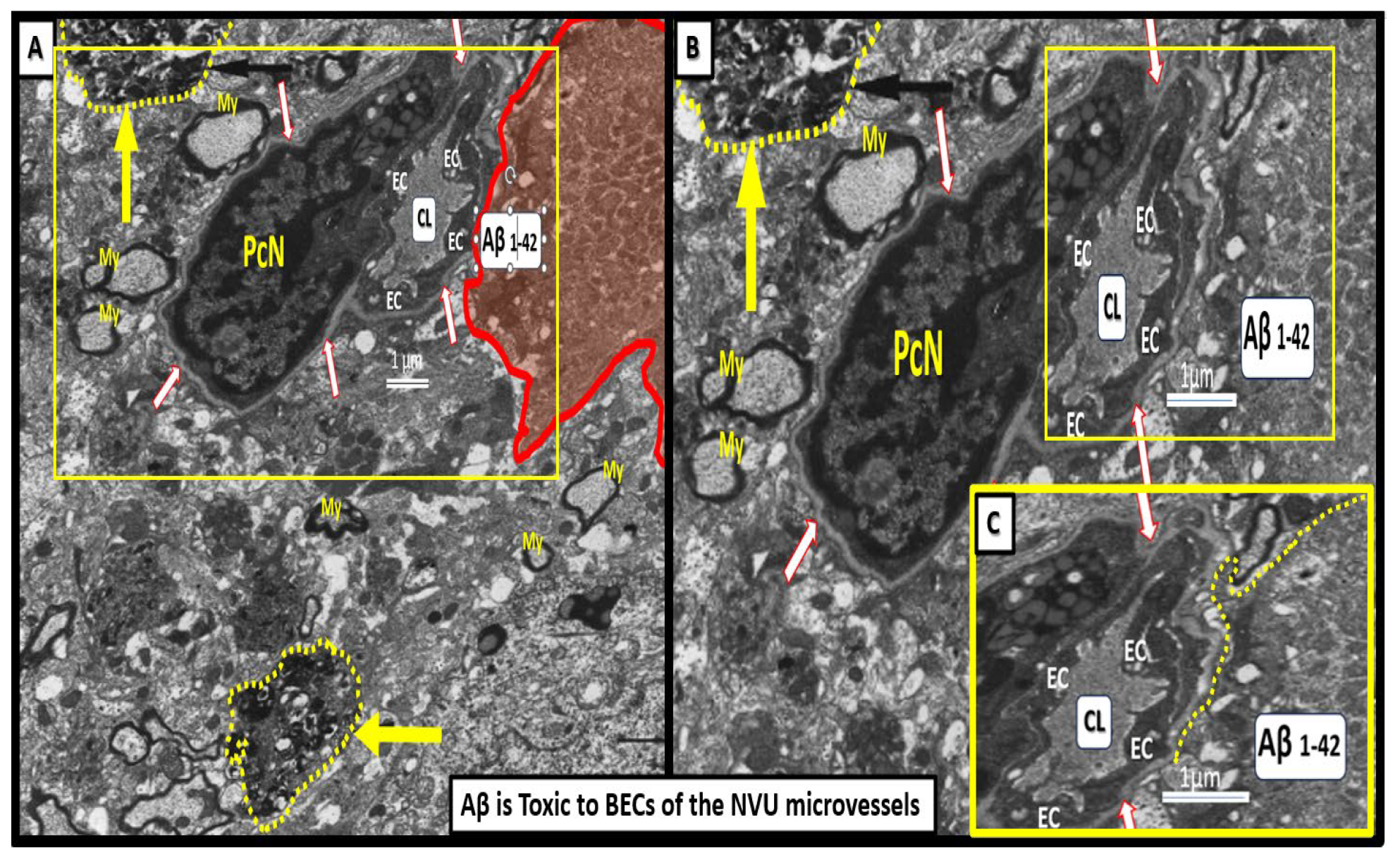
Figure.
Astrocyte(s) (ACs) are the master communication/connecting cell(s) (CCCs) within the brain universe. This collection of illustrations and transmission electron microscopy (TEM) images demonstrates the CCC functions of ACs via their various perivascular, perisynaptic, perineuronal endfeet, and cell-cell junctions (inserts 1-5). Insert 1 demonstrates the important role of perivascular astrocyte endfeet (pvACef; pseudo-colored golden) communication/connection. Note how this communicating/connecting AC allows for neurovascular coupling with regional neurons (insert 3) in frontal cortex layer III in control mice at 20-weeks of age. Insert 2 illustrates the communication/connection of the astrocyte perisynaptic endfeet (psACef) (pseudo-colored yellow). Insert 3 illustrates the communication/connection of ACs to myelinated and unmyelinated neurons. Insert 4 depicts the lost connections between a reactive microglial cell (
rMGC) (pseudo-colored blue with nuclear chromatin condensation) and multiple reactive, detached, and separated ACs (pseudo-colored red) adjacent to a neurovascular unit (NVU) with a single intact non-reactive ACs (pseudo-colored yellow) in diabetic
db/db model cortical layer III at 20-weeks of age. Insert 5 illustrates AC-to-AC connections in cortical layer III in control models (hand-drawn computer-assisted illustration of light microscopic toluidine blue stained images from control C57BL/6J models) via gap junction connexins (Cx43). Inserts 1–4 have scale bars of 0.5 μm, 100 nm, 1 μm, and 2 μm, respectively. The background image represents a hand-drawn computer-assisted image derived from control C57BL/6J toluidine blue stained models and does not have a scale bar. This highly modified image is provided with permission by CC 4.0 [
16]. ACfp, protoplasmic astrocyte endfeet; ACPVef, astrocyte perivascular endfeet; Cap L, capillary lumen; EC, brain endothelial cell; iMGC, interrogating microglial cell; Mt, mitochondria; N, nucleus; Pc, pericyte; PSD, post-synaptic density; PVACef, perivascular astrocyte endfeet; rMGC, reactive microglia cell; psACef, perisynaptic astrocyte endfeet.
Figure.
Astrocyte(s) (ACs) are the master communication/connecting cell(s) (CCCs) within the brain universe. This collection of illustrations and transmission electron microscopy (TEM) images demonstrates the CCC functions of ACs via their various perivascular, perisynaptic, perineuronal endfeet, and cell-cell junctions (inserts 1-5). Insert 1 demonstrates the important role of perivascular astrocyte endfeet (pvACef; pseudo-colored golden) communication/connection. Note how this communicating/connecting AC allows for neurovascular coupling with regional neurons (insert 3) in frontal cortex layer III in control mice at 20-weeks of age. Insert 2 illustrates the communication/connection of the astrocyte perisynaptic endfeet (psACef) (pseudo-colored yellow). Insert 3 illustrates the communication/connection of ACs to myelinated and unmyelinated neurons. Insert 4 depicts the lost connections between a reactive microglial cell (
rMGC) (pseudo-colored blue with nuclear chromatin condensation) and multiple reactive, detached, and separated ACs (pseudo-colored red) adjacent to a neurovascular unit (NVU) with a single intact non-reactive ACs (pseudo-colored yellow) in diabetic
db/db model cortical layer III at 20-weeks of age. Insert 5 illustrates AC-to-AC connections in cortical layer III in control models (hand-drawn computer-assisted illustration of light microscopic toluidine blue stained images from control C57BL/6J models) via gap junction connexins (Cx43). Inserts 1–4 have scale bars of 0.5 μm, 100 nm, 1 μm, and 2 μm, respectively. The background image represents a hand-drawn computer-assisted image derived from control C57BL/6J toluidine blue stained models and does not have a scale bar. This highly modified image is provided with permission by CC 4.0 [
16]. ACfp, protoplasmic astrocyte endfeet; ACPVef, astrocyte perivascular endfeet; Cap L, capillary lumen; EC, brain endothelial cell; iMGC, interrogating microglial cell; Mt, mitochondria; N, nucleus; Pc, pericyte; PSD, post-synaptic density; PVACef, perivascular astrocyte endfeet; rMGC, reactive microglia cell; psACef, perisynaptic astrocyte endfeet.
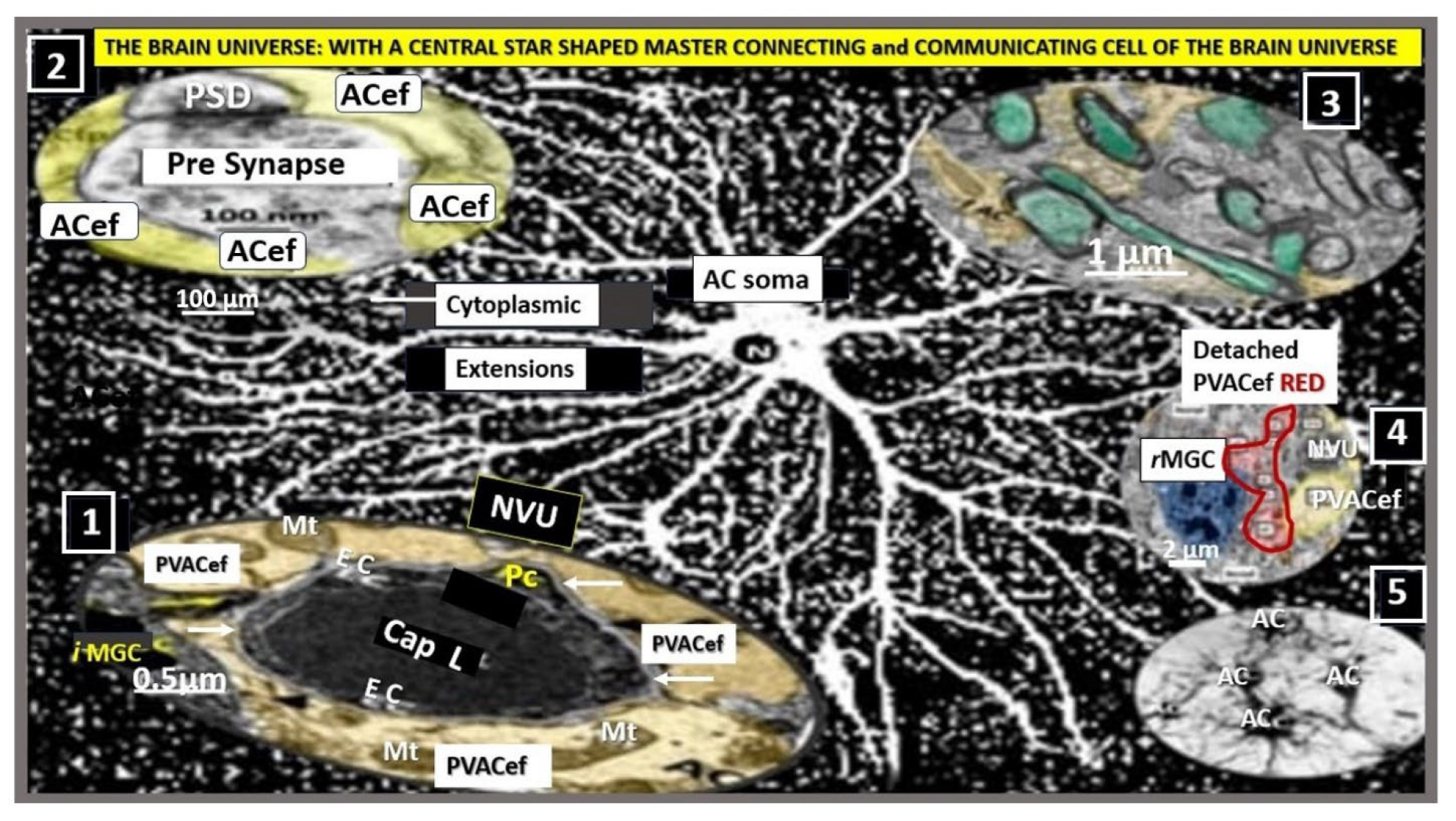
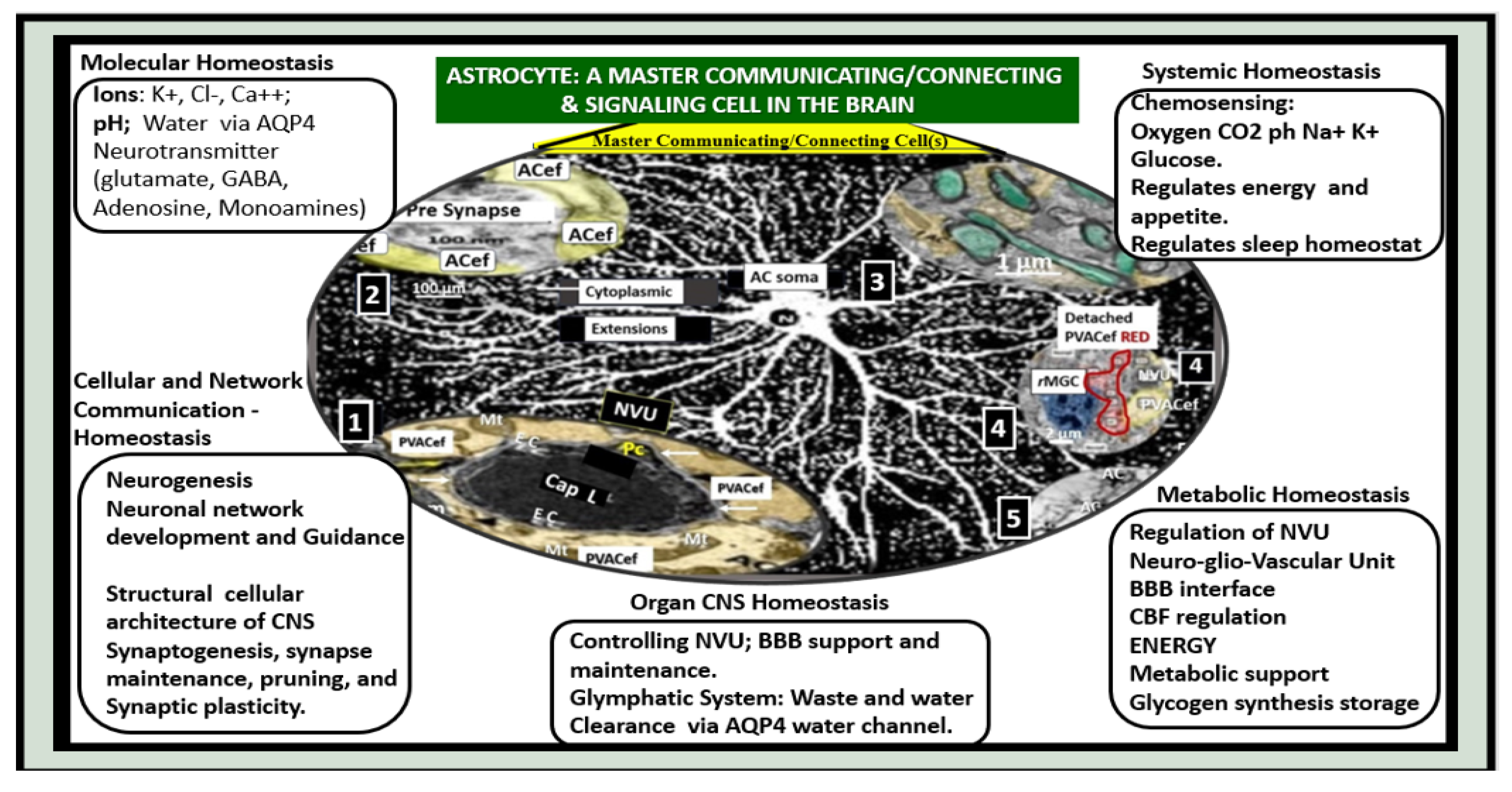
Figure 9.
A Master Communicating, Connecting, and Signaling Cell of the Brain Universe Providing Homeostasis. Astrocytes provide molecular, cellular and network communication, systemic, metabolic and whole organ homeostasis in addition to being neuroprotective. Centrally-located modified image of astrocyte from figure 10 provided with permission by CC 4.0 [
16]. AC, astrocyte; ACef, astrocyte endfeet; AQP4, aquaporin-4; BBB, blood-brain barrier; Ca++, calcium; Cap L, capillary lumen; CBF, cerebral blood flow; Cl-, chloride; CNS, central nervous system; CO2, carbon monoxide; EC, brain endothelial cell; GABA, gamma aminobutyric acid; K+, potassium; Na+, sodium; NVU, neurovascular unit; PVACef, perivascular astrocyte endfeet; rMGC, reactive microglia cell.
Figure 9.
A Master Communicating, Connecting, and Signaling Cell of the Brain Universe Providing Homeostasis. Astrocytes provide molecular, cellular and network communication, systemic, metabolic and whole organ homeostasis in addition to being neuroprotective. Centrally-located modified image of astrocyte from figure 10 provided with permission by CC 4.0 [
16]. AC, astrocyte; ACef, astrocyte endfeet; AQP4, aquaporin-4; BBB, blood-brain barrier; Ca++, calcium; Cap L, capillary lumen; CBF, cerebral blood flow; Cl-, chloride; CNS, central nervous system; CO2, carbon monoxide; EC, brain endothelial cell; GABA, gamma aminobutyric acid; K+, potassium; Na+, sodium; NVU, neurovascular unit; PVACef, perivascular astrocyte endfeet; rMGC, reactive microglia cell.
Figure 12.
Reactive Microglia Cell(s) (rMGCs) and Astrocyte(s) (rACs) Contribute to Decreased Cerebral Blood Flow and Neurodegeneration (ND). Panel A demonstrates a normal neurovascular unit (NVU) with its interrogating MGC (iMGC) (pseudo-colored green) and its intact astrocytes (iAC) (pseudo-colored golden). Panel B depicts a prominent rMGC invading the NVU (pseudo-colored red) and note the aberrant mitochondria (aMt) (pseudo-colored yellow and outlined in red). Notably the normally iAC have become detached and retracted, separated (drAC, pseudo-colored light blue) from the NVU outer basement membrane and results in NVU uncoupling with ensuing decreased cerebral blood flow to regional neurons resulting in hypometabolism, hypoperfusion, regional neuronal hypoxia/ischemia with subsequent neurodegeneration and impaired cognition. Modified images provided with permission by CC 4.0 [
6,
60]. CL, capillary lumen; drAC, detached reactive, retracted astrocyte endfeet; N, nucleus; RBC, red blood cell.
Figure 12.
Reactive Microglia Cell(s) (rMGCs) and Astrocyte(s) (rACs) Contribute to Decreased Cerebral Blood Flow and Neurodegeneration (ND). Panel A demonstrates a normal neurovascular unit (NVU) with its interrogating MGC (iMGC) (pseudo-colored green) and its intact astrocytes (iAC) (pseudo-colored golden). Panel B depicts a prominent rMGC invading the NVU (pseudo-colored red) and note the aberrant mitochondria (aMt) (pseudo-colored yellow and outlined in red). Notably the normally iAC have become detached and retracted, separated (drAC, pseudo-colored light blue) from the NVU outer basement membrane and results in NVU uncoupling with ensuing decreased cerebral blood flow to regional neurons resulting in hypometabolism, hypoperfusion, regional neuronal hypoxia/ischemia with subsequent neurodegeneration and impaired cognition. Modified images provided with permission by CC 4.0 [
6,
60]. CL, capillary lumen; drAC, detached reactive, retracted astrocyte endfeet; N, nucleus; RBC, red blood cell.
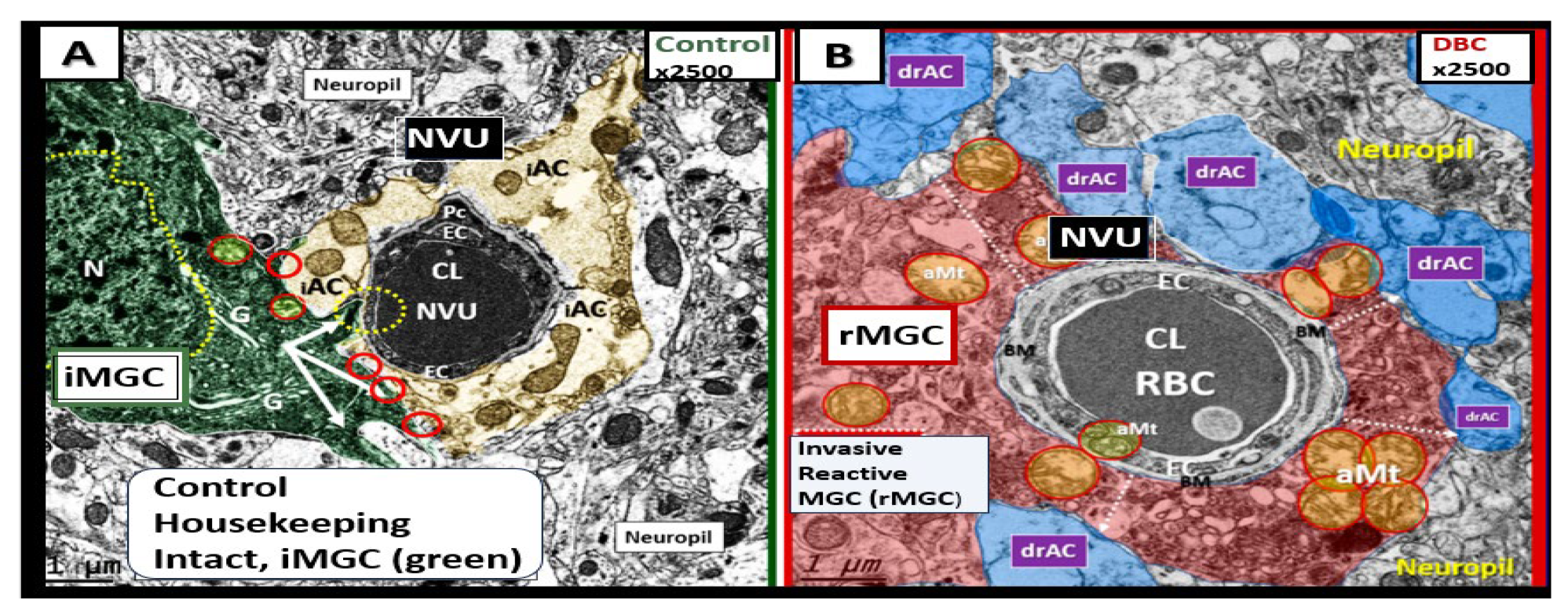
Figure 13.
Enlarged perivascular space (EPVS) and resident-reactive perivascular macrophage (rPVMΦs) in a postcapillary venule compared to a true capillary. Panel A demonstrates a normal true capillary in a 20-week-old female C57B6/J control model and note how the astrocyte endfeet (ACef) tightly abut the shared basement membrane (open black arrows) of the brain endothelial cell (BEC) and pericyte foot process (PcP).
Panel B depicts an EPVS with a prominent rPVMΦ (pseudo-colored red) in a 20-week-old lipopolysaccharide (LPS)-treated CD-1 male model and importantly note how the ACfp are markedly separated from the capillary mural cells (BEC and Pc) (red double arrows). Panel C depicts the rPVMΦs in an exploded image with intimate contact with the Pcfps basal lamina and the rPVMΦ intimate contact with basal lamina of the ACef (outermost boundary of the EPVS abluminal lining) (dashed blue circles). Modified images provided with permission by CC 4.0 [
45]. AQP4, aquaporin 4; Lys, lysosomes; Mt, mitochondria; N, nucleus; NVU, neurovascular unit; V, vacuoles; ves, vesicles.
Figure 13.
Enlarged perivascular space (EPVS) and resident-reactive perivascular macrophage (rPVMΦs) in a postcapillary venule compared to a true capillary. Panel A demonstrates a normal true capillary in a 20-week-old female C57B6/J control model and note how the astrocyte endfeet (ACef) tightly abut the shared basement membrane (open black arrows) of the brain endothelial cell (BEC) and pericyte foot process (PcP).
Panel B depicts an EPVS with a prominent rPVMΦ (pseudo-colored red) in a 20-week-old lipopolysaccharide (LPS)-treated CD-1 male model and importantly note how the ACfp are markedly separated from the capillary mural cells (BEC and Pc) (red double arrows). Panel C depicts the rPVMΦs in an exploded image with intimate contact with the Pcfps basal lamina and the rPVMΦ intimate contact with basal lamina of the ACef (outermost boundary of the EPVS abluminal lining) (dashed blue circles). Modified images provided with permission by CC 4.0 [
45]. AQP4, aquaporin 4; Lys, lysosomes; Mt, mitochondria; N, nucleus; NVU, neurovascular unit; V, vacuoles; ves, vesicles.
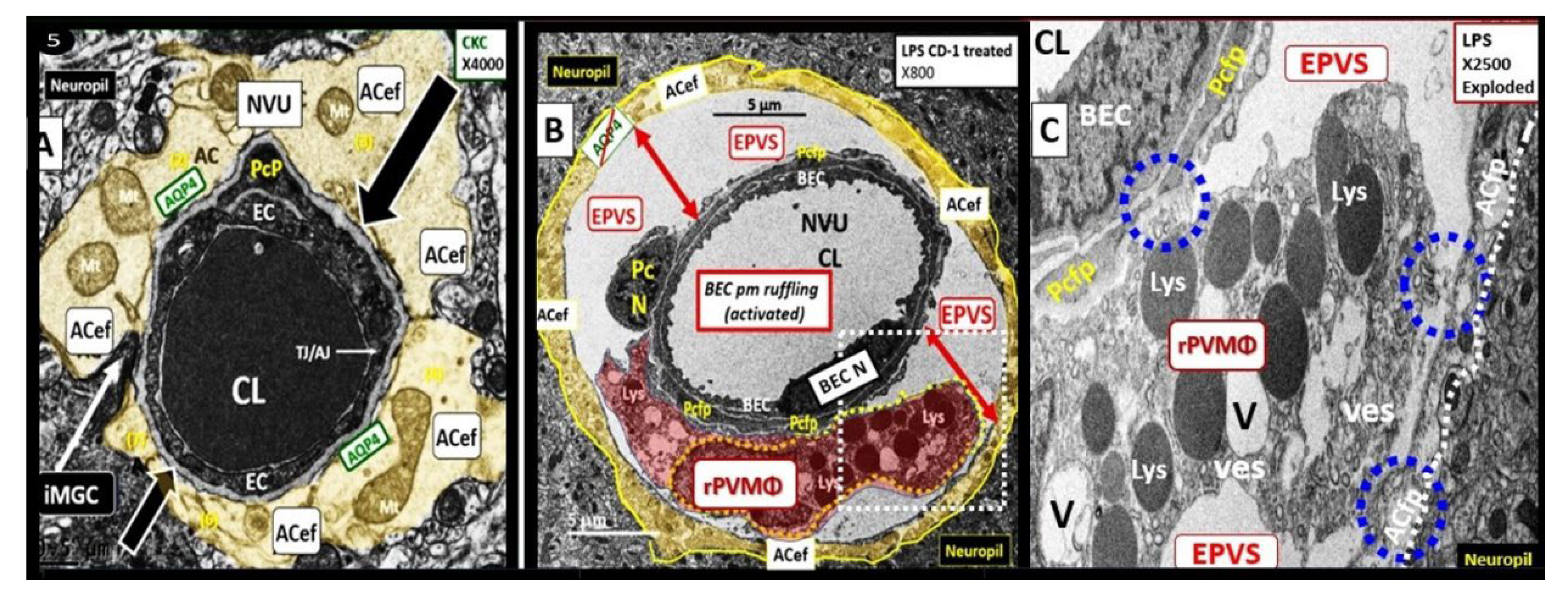
Figure 14.
Multipotential reactive astrocyte (rAC) phenotype remodeling in late-onset alzheimer’s disease (LOAD): From hypertrophy to atrophy. Panel A depicts extracellular matrix interstitial space amyloid beta (Aβ) plaques that are increased in LOAD and co-occur with soluble Aβ oligomers that are actually neurotoxic more than the Aβ mature plaques. Panel B lists the such as ischemic or hemorrhagic stroke with palisading and hypertrophic phenotypes that are in contrast to the rAC phenotype of atrophy that is associated with AC remodeling due to the neurotoxicity of Aβ oligomers and Aβ extracellular plaque aggregation discussed in C2. Modified images of Aβ in panel A are provided with permission by CC 4.0 [
75] and reproduced image in C1 is provided with permission by CC 4.0 [
29,
76]. ApoE, apolipoprotein E; CNS, central nervous system; cnsCC, central nervous system cytokines/chemokines; DNA, deoxyribonucleic acid; GAGS, glycosaminoglycans; LOAD, late-onset Alzheimer’s disease; MMP(s), matrix metalloproteinases; PGN, proteoglycan(s); rAC(s), reactive astrocytes; ROI, region of interest; rMGC(s), reactive microglia cells; ROS, reactive oxygen species; RNA, ribonucleic acid.
Figure 14.
Multipotential reactive astrocyte (rAC) phenotype remodeling in late-onset alzheimer’s disease (LOAD): From hypertrophy to atrophy. Panel A depicts extracellular matrix interstitial space amyloid beta (Aβ) plaques that are increased in LOAD and co-occur with soluble Aβ oligomers that are actually neurotoxic more than the Aβ mature plaques. Panel B lists the such as ischemic or hemorrhagic stroke with palisading and hypertrophic phenotypes that are in contrast to the rAC phenotype of atrophy that is associated with AC remodeling due to the neurotoxicity of Aβ oligomers and Aβ extracellular plaque aggregation discussed in C2. Modified images of Aβ in panel A are provided with permission by CC 4.0 [
75] and reproduced image in C1 is provided with permission by CC 4.0 [
29,
76]. ApoE, apolipoprotein E; CNS, central nervous system; cnsCC, central nervous system cytokines/chemokines; DNA, deoxyribonucleic acid; GAGS, glycosaminoglycans; LOAD, late-onset Alzheimer’s disease; MMP(s), matrix metalloproteinases; PGN, proteoglycan(s); rAC(s), reactive astrocytes; ROI, region of interest; rMGC(s), reactive microglia cells; ROS, reactive oxygen species; RNA, ribonucleic acid.
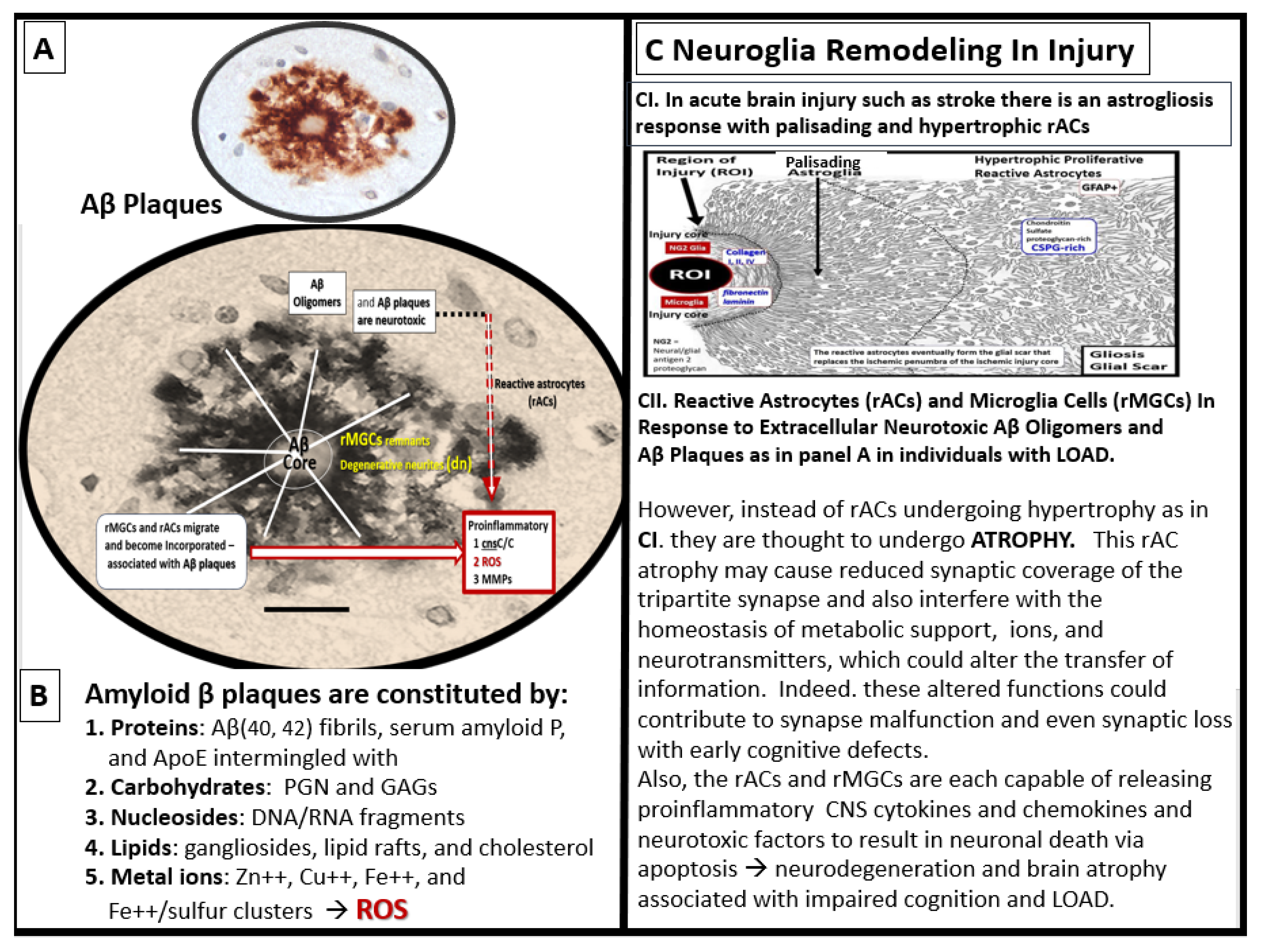
Figure 15.
Aberrant mitochondria (aMt) allow for leaky prooxidative ROS of the reactive species interactome (RSI) and prooxidative iron sulfur cluster(s) (ISCs) to enter the cytosol with dysfunction and damage to the cell and surrounding cells. Note how the aMt remodeling appears as compared to the normal control insert upper left with its hyperlucency, crista fragmentation and loss, loss of electron dense mitochondrial matrix, and permeabilization of the outer mitochondria membrane (asterisk). This permeabilization allows the redox-active and prooxidative mtROS and iron sulfur clusters to escape into the cellular cytosol resulting in cellular dysfunction, damage and even apoptosis. This prooxidant mechanism has an increased impact if the antioxidant reserves of super oxide dismutase (SOD), catalase, and glutathione (GSH) have previously been or are currently depleted. Revised background image of the aMt is provided with permission by CC 4.0 [
61]. aMt, aberrant mitochondria; Cys, cysteine; ETC, electron transport chain; Fe, iron; ISC, iron sulfur cluster(s); MOMP, mitochondrial outer membrane permeabilization; Mt, mitochondria; mtROS, mitochondrial-derived reactive oxygen species; RONSS, reactive oxygen, nitrogen, sulfur species; S, sulfur.
Figure 15.
Aberrant mitochondria (aMt) allow for leaky prooxidative ROS of the reactive species interactome (RSI) and prooxidative iron sulfur cluster(s) (ISCs) to enter the cytosol with dysfunction and damage to the cell and surrounding cells. Note how the aMt remodeling appears as compared to the normal control insert upper left with its hyperlucency, crista fragmentation and loss, loss of electron dense mitochondrial matrix, and permeabilization of the outer mitochondria membrane (asterisk). This permeabilization allows the redox-active and prooxidative mtROS and iron sulfur clusters to escape into the cellular cytosol resulting in cellular dysfunction, damage and even apoptosis. This prooxidant mechanism has an increased impact if the antioxidant reserves of super oxide dismutase (SOD), catalase, and glutathione (GSH) have previously been or are currently depleted. Revised background image of the aMt is provided with permission by CC 4.0 [
61]. aMt, aberrant mitochondria; Cys, cysteine; ETC, electron transport chain; Fe, iron; ISC, iron sulfur cluster(s); MOMP, mitochondrial outer membrane permeabilization; Mt, mitochondria; mtROS, mitochondrial-derived reactive oxygen species; RONSS, reactive oxygen, nitrogen, sulfur species; S, sulfur.
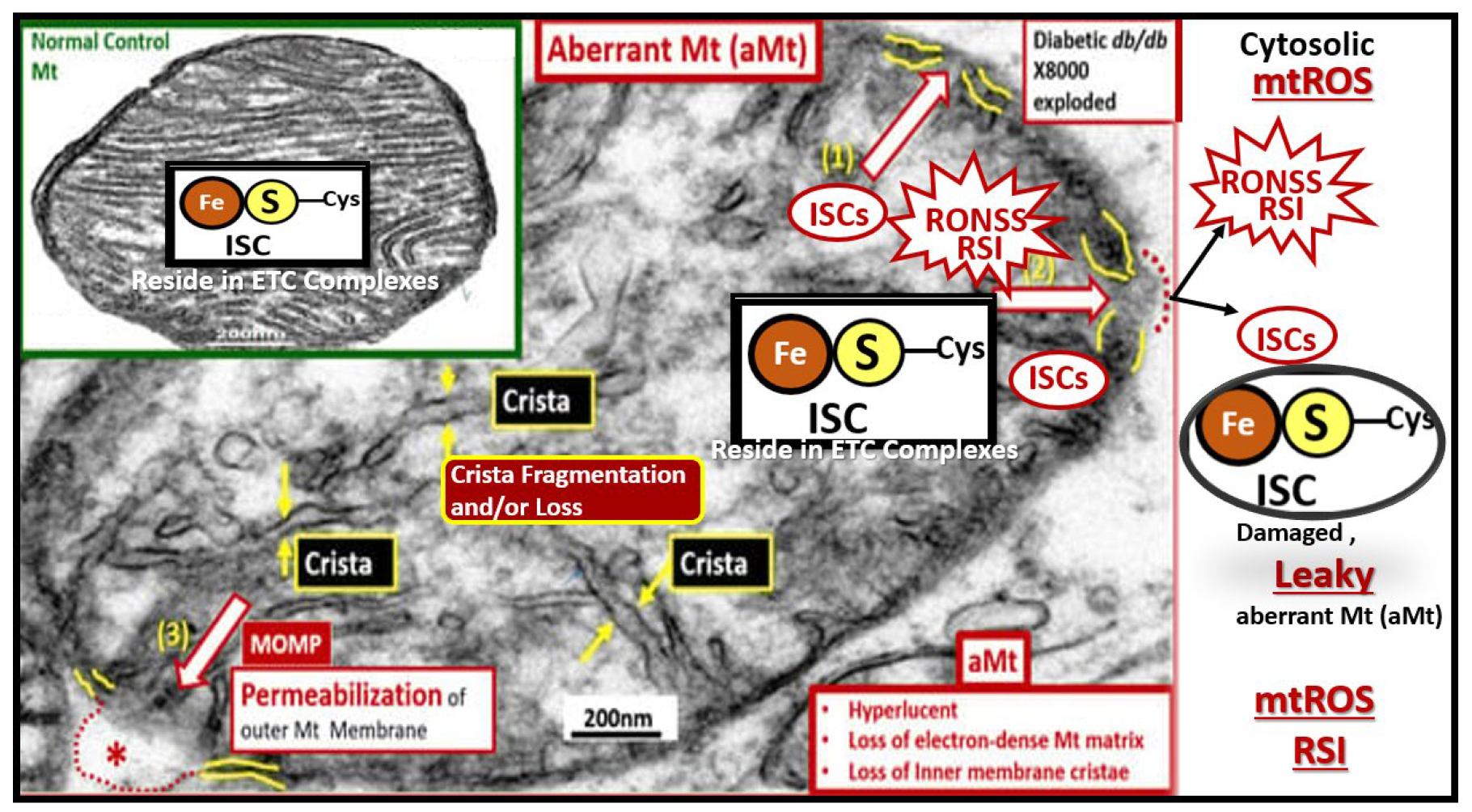
Figure 16.
Reactive oxygen species (ROS) begets ROS and Oxidative-Redox Stress (OxRS) is in a vicious cycle with neuroinflammation wherein this vicious cycle contributes to impaired cognition and neurodegeneration. Metabolic and hormonal excesses in addition to injury-trauma and the vicious cycle of neuroinflammation and ROS – OxRS lead to blood-brain barrier dysfunction and disruption with NVU uncoupling and regional hypometabolism and Ischemia/ischemia reperfusion injury results in a cascade to neurodegeneration and brain atrophy with impaired cognition that is associated with late-onset Alzheimer’s disease (LOAD). Modified figure provided with permission by CC 4.0 [
29,
37,
87]. AGE/RAGE, advanced glycation end products/ receptor for advanced glycation end products; Ang II, angiotensin II; AT1R, angiotensin type 1 receptor; BH4, tetrahydrobiopterin; eNOS, endothelial nitric oxide synthase; FFA, free fatty acids; HPA, hypothalamic pituitary adrenal; I-CAM, intercellular adhesion molecule; MC, mast cell; MGCs, microglia cells; NADPH – NADPH Ox, reduced nicotinamide adenine dinucleotide phosphate oxidase; NVU. neurovascular unit; NF-kB, nuclear factor- kappa B; RAS, renin angiotensin system; RAAS, renin angiotensin aldosterone system; ROS/RNS, reactive oxygen species/reactive nitrogen species. UV, ultraviolet; VE-CAM, vascular endothelial cellular adhesion molecule.
Figure 16.
Reactive oxygen species (ROS) begets ROS and Oxidative-Redox Stress (OxRS) is in a vicious cycle with neuroinflammation wherein this vicious cycle contributes to impaired cognition and neurodegeneration. Metabolic and hormonal excesses in addition to injury-trauma and the vicious cycle of neuroinflammation and ROS – OxRS lead to blood-brain barrier dysfunction and disruption with NVU uncoupling and regional hypometabolism and Ischemia/ischemia reperfusion injury results in a cascade to neurodegeneration and brain atrophy with impaired cognition that is associated with late-onset Alzheimer’s disease (LOAD). Modified figure provided with permission by CC 4.0 [
29,
37,
87]. AGE/RAGE, advanced glycation end products/ receptor for advanced glycation end products; Ang II, angiotensin II; AT1R, angiotensin type 1 receptor; BH4, tetrahydrobiopterin; eNOS, endothelial nitric oxide synthase; FFA, free fatty acids; HPA, hypothalamic pituitary adrenal; I-CAM, intercellular adhesion molecule; MC, mast cell; MGCs, microglia cells; NADPH – NADPH Ox, reduced nicotinamide adenine dinucleotide phosphate oxidase; NVU. neurovascular unit; NF-kB, nuclear factor- kappa B; RAS, renin angiotensin system; RAAS, renin angiotensin aldosterone system; ROS/RNS, reactive oxygen species/reactive nitrogen species. UV, ultraviolet; VE-CAM, vascular endothelial cellular adhesion molecule.
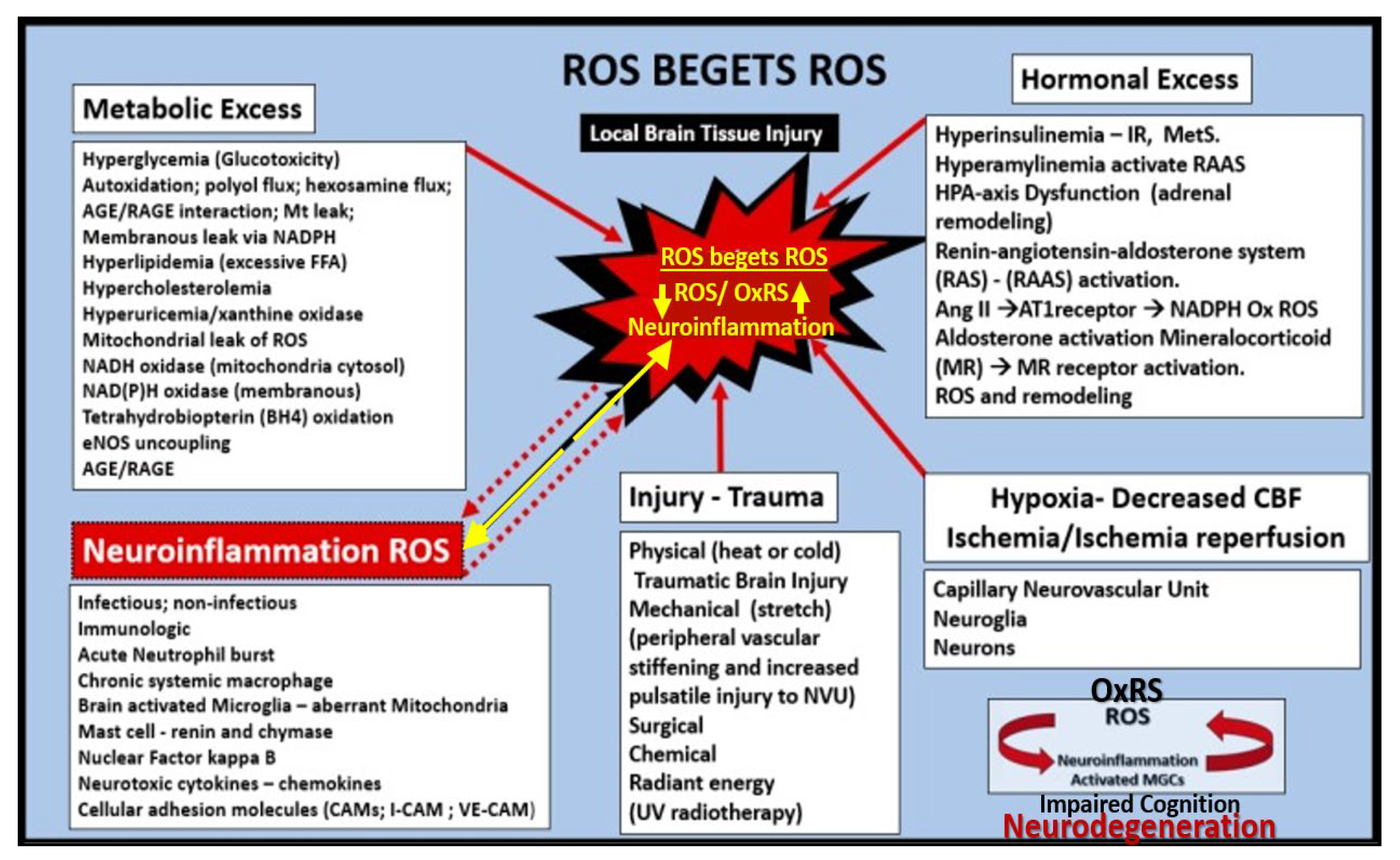
Figure 17.
Vicious cycles and multiple hits combine to result in small vessel disease (SVD), neuronal injury, neurodegeneration, brain atrophy, impaired cognition, and LOAD. Panel A depicts the importance of the vicious cycle between oxidative-redox stress (OxRS) and inflammation that result in microvessel remodeling and cerebral small vessel disease (SVD), which contribute to impaired cognition and the development of neurodegeneration. Panel B depicts the multiple hits that trigger the OxRS and neuroinflammation that are involved in the development of neuronal injury and neurodegeneration. Panel A is reproduced with permission by 4.0 [
37]. Act, activation; BBB, blood-brain barrier; BBB
dd, blood-brain barrier dysfunction and disruption; BEC, brain endothelial cell; CMBs, cerebral microbleeds; cnsCC, central nervous system cytokines/chemokines; dys, dysfunction; EPVS, enlarged perivascular spaces; MMP, matrix metalloproteinases; NFkappaB, nuclear factor kappa beta;
pCC, peripheral cytokines/chemokines; ROS, reactive oxygen species; RONSS, reactive oxygen, nitrogen, sulfur species; RSI, reactive species interactome; TJ/AJ, tight and adherens junctions; VE, vascular endothelial.
Figure 17.
Vicious cycles and multiple hits combine to result in small vessel disease (SVD), neuronal injury, neurodegeneration, brain atrophy, impaired cognition, and LOAD. Panel A depicts the importance of the vicious cycle between oxidative-redox stress (OxRS) and inflammation that result in microvessel remodeling and cerebral small vessel disease (SVD), which contribute to impaired cognition and the development of neurodegeneration. Panel B depicts the multiple hits that trigger the OxRS and neuroinflammation that are involved in the development of neuronal injury and neurodegeneration. Panel A is reproduced with permission by 4.0 [
37]. Act, activation; BBB, blood-brain barrier; BBB
dd, blood-brain barrier dysfunction and disruption; BEC, brain endothelial cell; CMBs, cerebral microbleeds; cnsCC, central nervous system cytokines/chemokines; dys, dysfunction; EPVS, enlarged perivascular spaces; MMP, matrix metalloproteinases; NFkappaB, nuclear factor kappa beta;
pCC, peripheral cytokines/chemokines; ROS, reactive oxygen species; RONSS, reactive oxygen, nitrogen, sulfur species; RSI, reactive species interactome; TJ/AJ, tight and adherens junctions; VE, vascular endothelial.
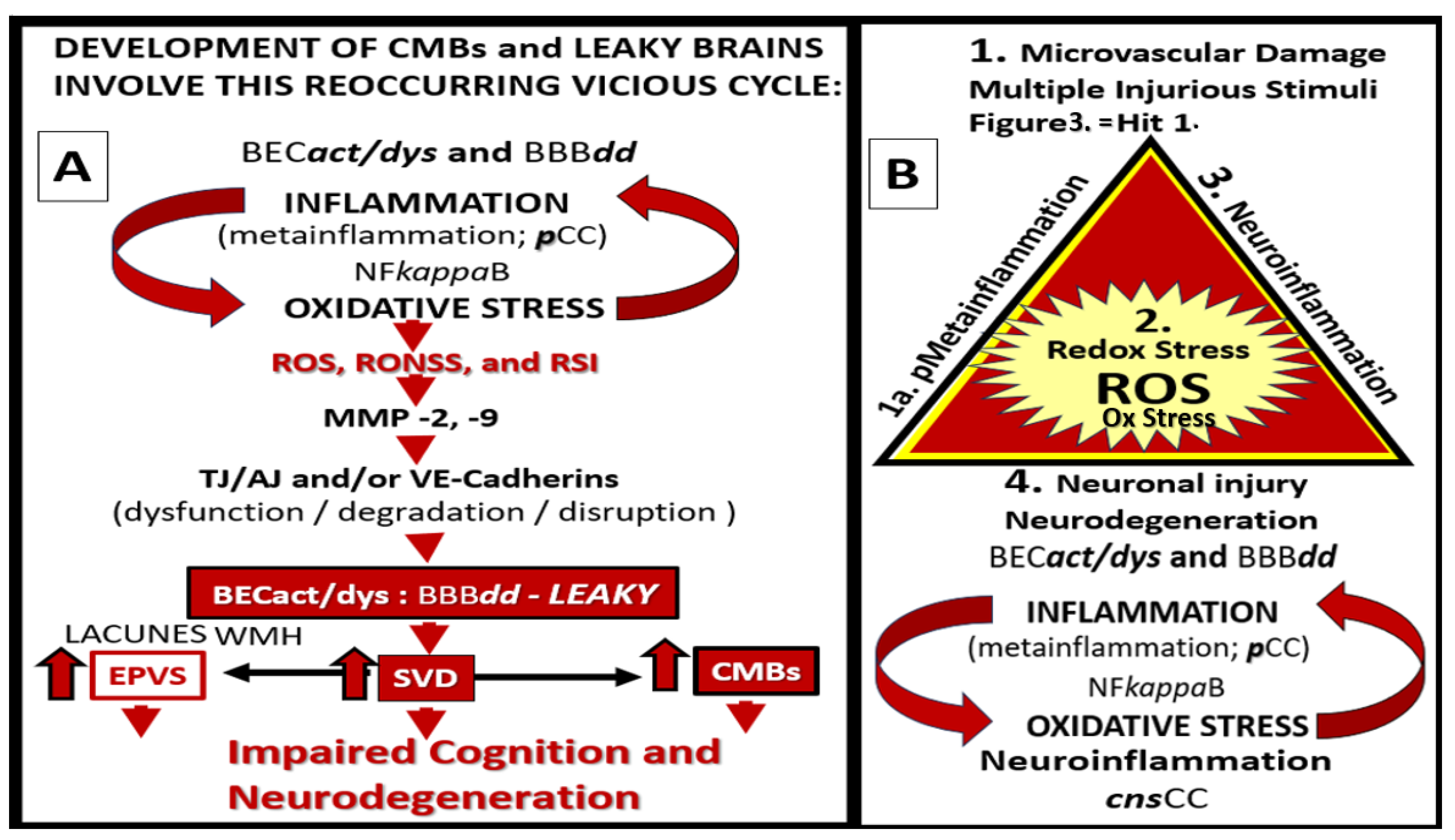
Figure 18.
Neuroinflammation induces neurodegeneration via proinflammatory central nervous system cytokines and chemokines (
cnsCC), amyloid beta (Aβ), and tau. Image A reveals a cleaned transmission electron microscopy (TEM) image of a reactive microglia (rMGC). Image B depicts an illustration of a reactive astrocyte (rAC). Image C represents an illustration of a myelinated neuron. Image D depicts a portion of a myelinated neuronal axon that importantly depicts aberrant mitochondria (aMt) capable of leaking mitochondria reactive oxygen species (mtROS) and splitting of myelin that could impair informational transfer. Image E depicts a TEM image of a dendritic neurite (dn) outlined with yellow-dashed lines. Images F and G depict a TEM image of an Aβ plaque outlined by yellow-dashed lines. Images H and I depict immunohistologic light microscope images of Aβ. Image G scale bar = 5 μm. Image I scale bar = 20μm. Modified images A and D provided with permission by CC 4.0 [
60,
61]. Images H and I provided with permission by CC 4.0 [
90]. aMt, aberrant mitochondria; CC, chromatin condensation; COX, cyclooxygenase; cnsCC, central nervous system cytokines chemokines; dn or dN, dendritic neurite; IL-1, interleukin 1; IL-1β, interleukin 1 beta; IL-6, interleukin 6; ISC, iron sulfur clusters; Lys, lysosome; mtROS, mitochondria reactive oxygen species; N, nucleus; NADPH Ox, nicotine adenine diphosphate oxidase; NFTs, neuro fibrillary tangles; NO, nitric oxide; NOX, NADPH Ox; OxRS, oxidative redox stress; rACs, reactive astrocytes; rMGCs, reactive microglia cells; TNFα, tumor necrosis alpha.
Figure 18.
Neuroinflammation induces neurodegeneration via proinflammatory central nervous system cytokines and chemokines (
cnsCC), amyloid beta (Aβ), and tau. Image A reveals a cleaned transmission electron microscopy (TEM) image of a reactive microglia (rMGC). Image B depicts an illustration of a reactive astrocyte (rAC). Image C represents an illustration of a myelinated neuron. Image D depicts a portion of a myelinated neuronal axon that importantly depicts aberrant mitochondria (aMt) capable of leaking mitochondria reactive oxygen species (mtROS) and splitting of myelin that could impair informational transfer. Image E depicts a TEM image of a dendritic neurite (dn) outlined with yellow-dashed lines. Images F and G depict a TEM image of an Aβ plaque outlined by yellow-dashed lines. Images H and I depict immunohistologic light microscope images of Aβ. Image G scale bar = 5 μm. Image I scale bar = 20μm. Modified images A and D provided with permission by CC 4.0 [
60,
61]. Images H and I provided with permission by CC 4.0 [
90]. aMt, aberrant mitochondria; CC, chromatin condensation; COX, cyclooxygenase; cnsCC, central nervous system cytokines chemokines; dn or dN, dendritic neurite; IL-1, interleukin 1; IL-1β, interleukin 1 beta; IL-6, interleukin 6; ISC, iron sulfur clusters; Lys, lysosome; mtROS, mitochondria reactive oxygen species; N, nucleus; NADPH Ox, nicotine adenine diphosphate oxidase; NFTs, neuro fibrillary tangles; NO, nitric oxide; NOX, NADPH Ox; OxRS, oxidative redox stress; rACs, reactive astrocytes; rMGCs, reactive microglia cells; TNFα, tumor necrosis alpha.
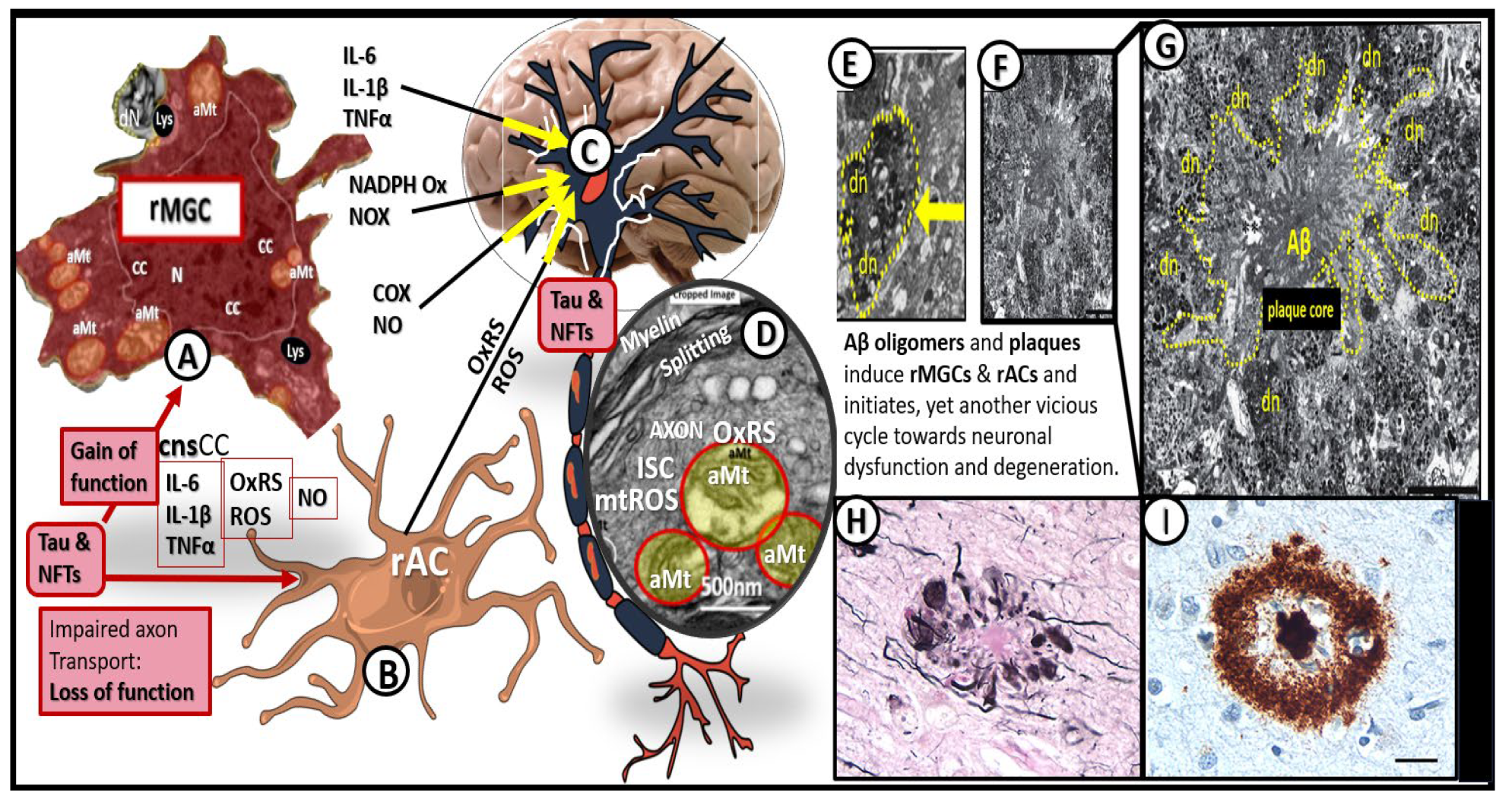
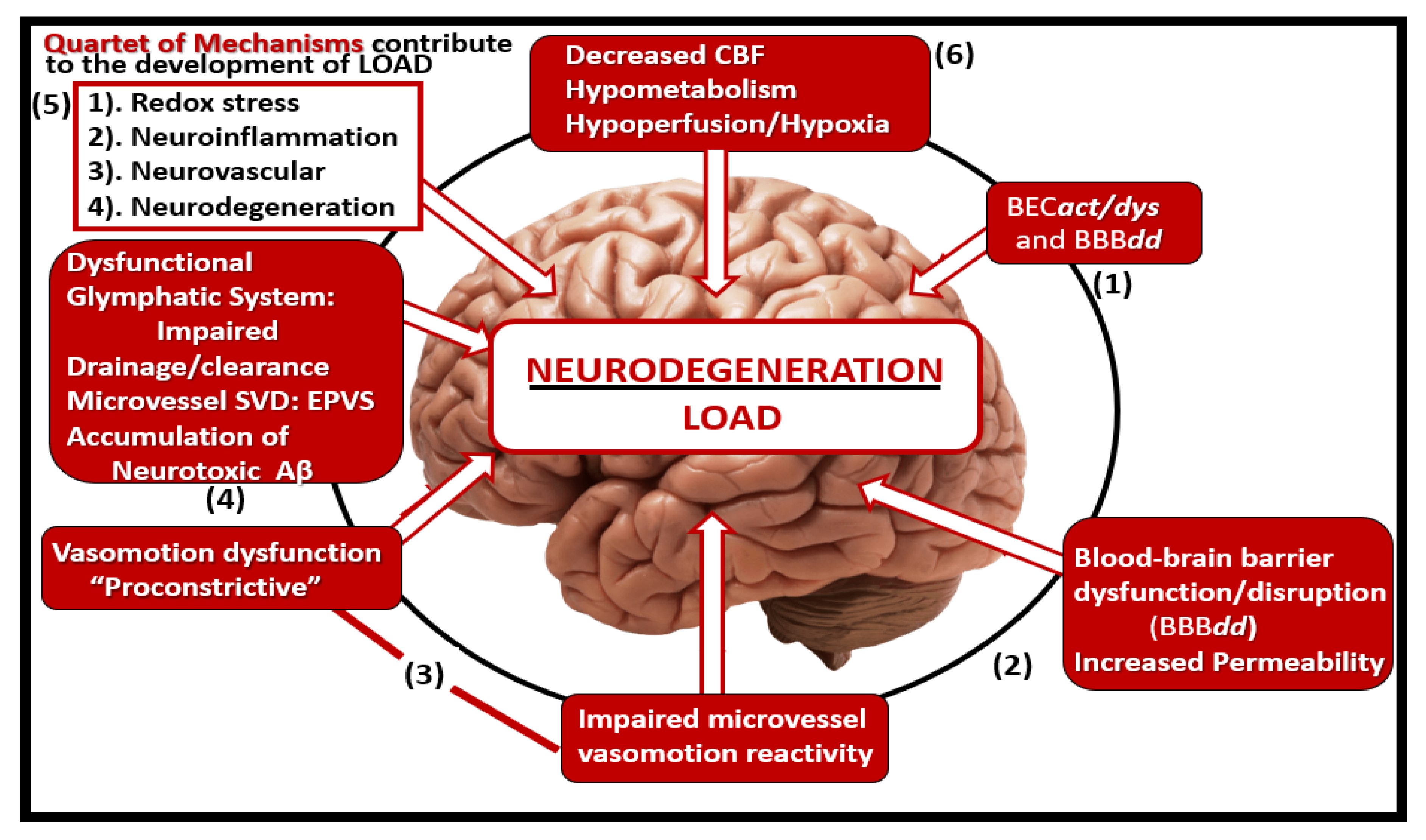
Figure 19.
Microvessel SVD definitely contributes to and are linked to the development of neurodegeneration. At least a half dozen mechanisms are involved. Importantly, note that number 5 points to the quartet of mechanisms involved in this linkage of SVD to neurodegeneration. Importantly these six mechanisms of linage seem to be initiated by brain endothelial cell activation and dysfunction (BECact/dys) with concurrent blood-brain barrier dysfunction and/or disruption (BBBdd). Aβ, amyloid beta; CBF, cerebral blood flow; EPVS, enlarged perivascular spaces, LOAD, late-onset Alzheimer’s disease; SVD, small vessel disease.
Figure 19.
Microvessel SVD definitely contributes to and are linked to the development of neurodegeneration. At least a half dozen mechanisms are involved. Importantly, note that number 5 points to the quartet of mechanisms involved in this linkage of SVD to neurodegeneration. Importantly these six mechanisms of linage seem to be initiated by brain endothelial cell activation and dysfunction (BECact/dys) with concurrent blood-brain barrier dysfunction and/or disruption (BBBdd). Aβ, amyloid beta; CBF, cerebral blood flow; EPVS, enlarged perivascular spaces, LOAD, late-onset Alzheimer’s disease; SVD, small vessel disease.
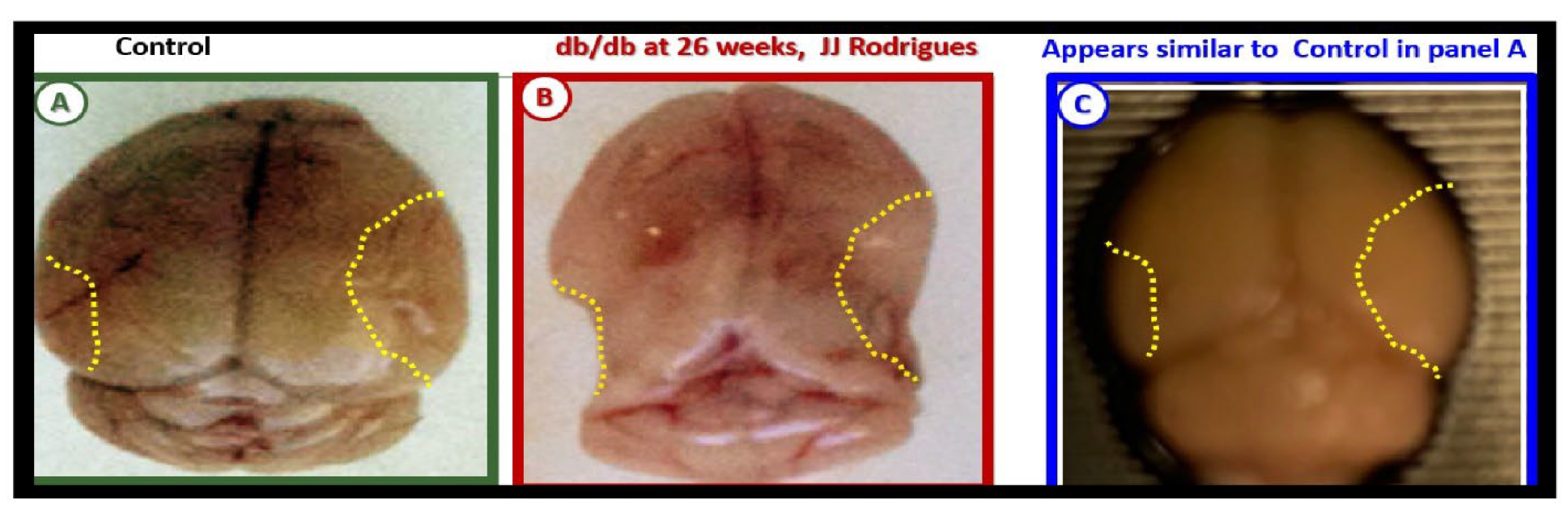
Figure 20.
Gross brain atrophy in the
db/db obese diabetic model but not in the obese diabetic BTBR
ob/ob model or
db/db models treated with empagliflozin. Panel A demonstrates the normal gross brain in the control male model at 20-weeks of age. Panel B depicts marked brain atrophy at the time of surgical removal of the diabetic
db/db models as compared to control models in Panel A. Note the marked atrophy or loss in the cortical-parietal-hippocampal regions (outlined by the yellow dashed lines) in comparison to the control in Panel A at 26-weeks of age and these remodeling changes were associated with a decrease in the brain wet weights upon removal. Panel C demonstrates the absence of remodeling atrophy in the
ob/ob diabetic obese model at 20-weeks of age and also those db/db diabetic models treated with empagliflozin at 20-weeks of age. Modified panels A and B were provided with permission by CC 4.0 [
117,
118].
Figure 20.
Gross brain atrophy in the
db/db obese diabetic model but not in the obese diabetic BTBR
ob/ob model or
db/db models treated with empagliflozin. Panel A demonstrates the normal gross brain in the control male model at 20-weeks of age. Panel B depicts marked brain atrophy at the time of surgical removal of the diabetic
db/db models as compared to control models in Panel A. Note the marked atrophy or loss in the cortical-parietal-hippocampal regions (outlined by the yellow dashed lines) in comparison to the control in Panel A at 26-weeks of age and these remodeling changes were associated with a decrease in the brain wet weights upon removal. Panel C demonstrates the absence of remodeling atrophy in the
ob/ob diabetic obese model at 20-weeks of age and also those db/db diabetic models treated with empagliflozin at 20-weeks of age. Modified panels A and B were provided with permission by CC 4.0 [
117,
118].
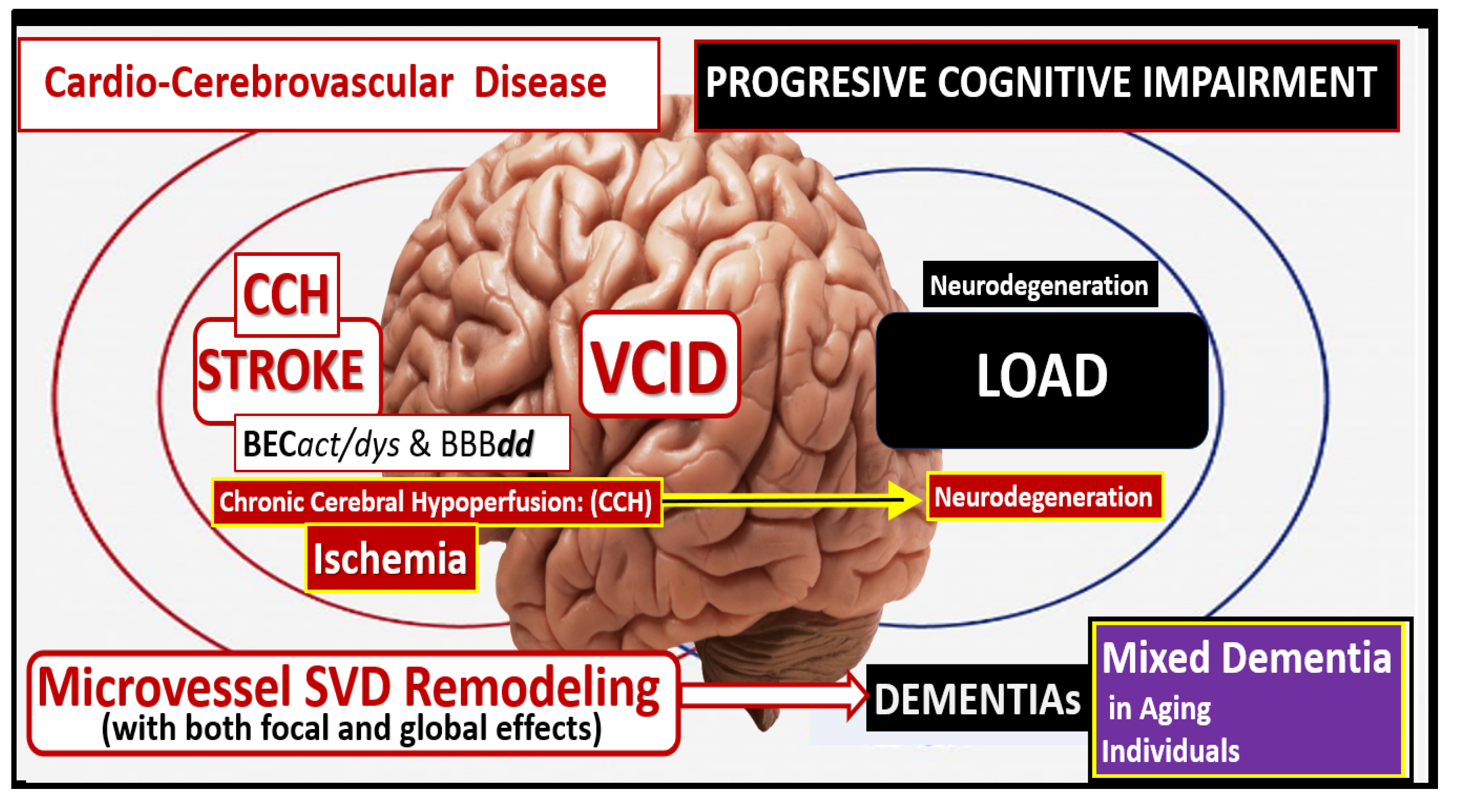
Figure 21.
Vascular contributions to cognitive impairment and dementia (VCID) is central to the development of neurodegeneration and late-onset Alzheimer;s disease (LOAD) and mixed dementia (MD). Cardio-cerebrovascular diseases including stroke (ischemic and hemorrhagic) contribute to brain endothelial cell activation and dysfunction (BECact/dys) with concurrent blood-brain barrier dysfunction and disruption (BBBdd) induce ischemia, hypoxia and chronic cerebral hypoperfusion (CCH) to result in neurodegeneration and dementia in LOAD and MD.
Figure 21.
Vascular contributions to cognitive impairment and dementia (VCID) is central to the development of neurodegeneration and late-onset Alzheimer;s disease (LOAD) and mixed dementia (MD). Cardio-cerebrovascular diseases including stroke (ischemic and hemorrhagic) contribute to brain endothelial cell activation and dysfunction (BECact/dys) with concurrent blood-brain barrier dysfunction and disruption (BBBdd) induce ischemia, hypoxia and chronic cerebral hypoperfusion (CCH) to result in neurodegeneration and dementia in LOAD and MD.
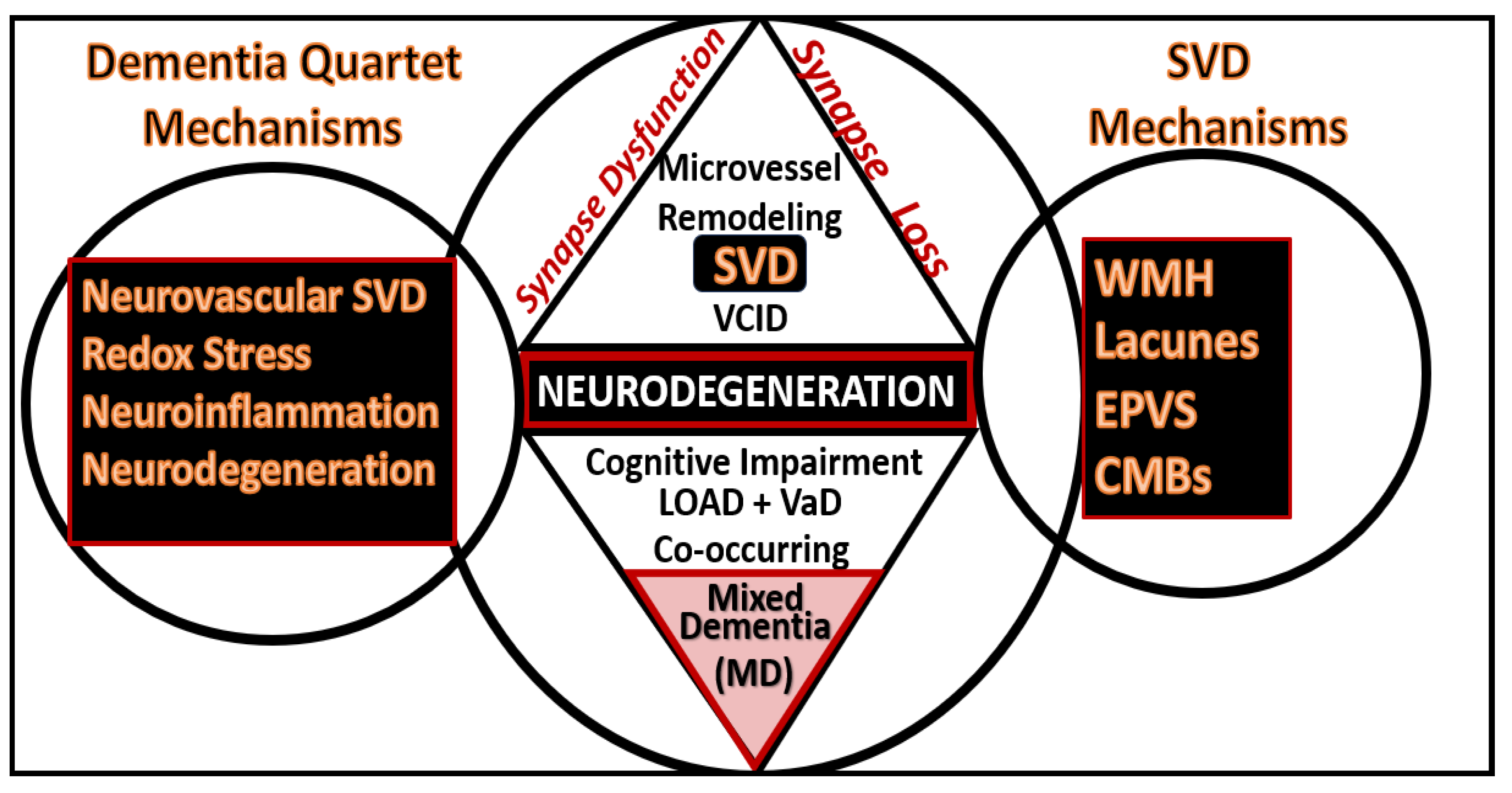
Figure 22.
The dementia quartet mechanisms intersect and contribute to neurodegeneration with neuronal synapse and neuron dysfunction and or loss with regional brain atrophy in LOAD and mixed dementia (MD) in addition to the small vessel disease (SVD) intersection and contribution to Neurodegeneration and MD.
Figure 22.
The dementia quartet mechanisms intersect and contribute to neurodegeneration with neuronal synapse and neuron dysfunction and or loss with regional brain atrophy in LOAD and mixed dementia (MD) in addition to the small vessel disease (SVD) intersection and contribution to Neurodegeneration and MD.
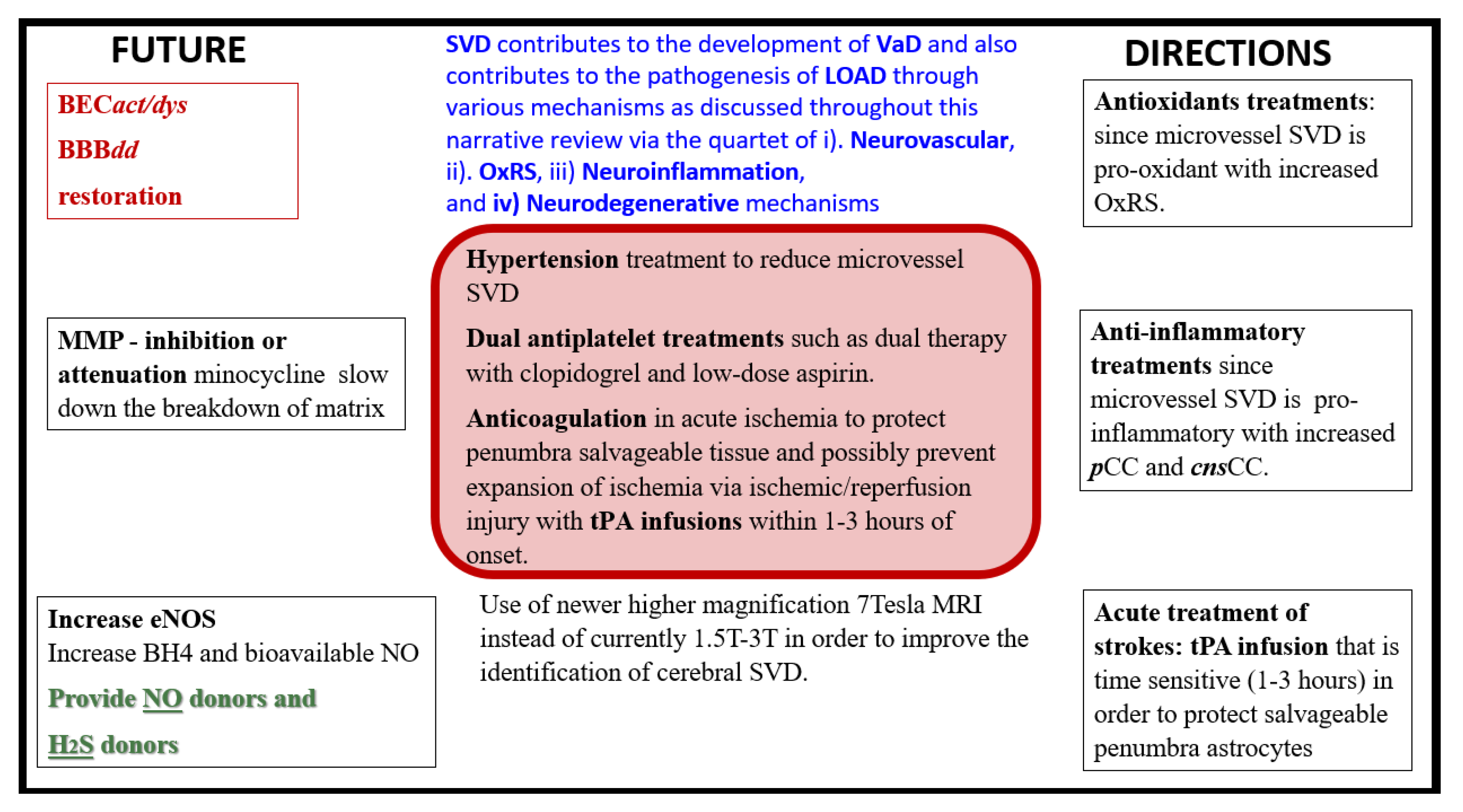
Figure 23.
Future directions in microvessel small vessel disease that contribute to late-onset Alzheimer’s disease. BECact/dys, brain endothelial cell activation and dysfunction; BBBdd, blood-brain barrier dysfunction and disruption; BH4, tetrahydrobiopterin; cnsCC, central nervous system cytokines chemokines; eNOS, endothelial nitric oxide synthase; H2S, hydrogen sulfide; LOAD, late-onset Alzheimer’s disease; MMP, matrix metalloproteinase; NO, nitric oxide; OxRS, oxidative redox stress; pCC, peripheral cytokines chemokines; SVD, small vessel disease; T, tesla; tPA, tissue-type plasminogen activator; VaD/VAD, vascular dementia. .
Figure 23.
Future directions in microvessel small vessel disease that contribute to late-onset Alzheimer’s disease. BECact/dys, brain endothelial cell activation and dysfunction; BBBdd, blood-brain barrier dysfunction and disruption; BH4, tetrahydrobiopterin; cnsCC, central nervous system cytokines chemokines; eNOS, endothelial nitric oxide synthase; H2S, hydrogen sulfide; LOAD, late-onset Alzheimer’s disease; MMP, matrix metalloproteinase; NO, nitric oxide; OxRS, oxidative redox stress; pCC, peripheral cytokines chemokines; SVD, small vessel disease; T, tesla; tPA, tissue-type plasminogen activator; VaD/VAD, vascular dementia. .