Abbreviated title: Contribution of SNS to Oxytocin-Elicited Weight Loss in Female Rats
Methods
Adult female Long-Evans rats and C57BL/6J (strain 000664) and DBA/2J (strain 000671) mice were initially obtained from Envigo (Indianapolis, IN-rats) or [The Jackson Laboratory; Bar Harbor, ME (mice)] and maintained for at least 4 months on a HFD prior to study onset. All animals were housed individually in Plexiglas cages in a temperature-controlled room (22±2°C) under a 12:12-h light-dark cycle. All rats and mice were maintained on a 6 a.m. (lights on)/6 p.m. (lights off) light cycle. Rats and mice had ad libitum access to water and a HFD providing 60% kcal from fat [Research Diets, D12492 (rats) or D12492i (mice), New Brunswick, NJ]. The research protocols were approved both by the Institutional Animal Care and Use Committee of the Veterans Affairs Puget Sound Health Care System (VAPSHCS) and the University of Washington in accordance with NIH Guidelines for the Care and Use of Animals.
Fresh solutions of OT acetate salt (Bachem Americas, Inc., Torrance, CA) was prepared the day of each experiment (Study 1). OT was solubilized in sterile water (Study 1). Fresh solutions of OT acetate salt (Bachem Americas, Inc., Torrance, CA) were solubilized in sterile water, loaded into Alzet minipumps (model 2004; DURECT Corporation, Cupertino, CA) and subsequently primed in sterile 0.9% saline at 37º C for approximately 40 hours prior to minipump implantation based on manufacturer’s recommended instructions (Study 3-4). The β3-AR agonist, CL 316243 (Tocris/Bio-Techne Corporation, Minneapolis, MN), was solubilized in sterile water each day of each experiment (Study 1).
A dissecting microscope (Leica M60/M80; Leica Microsystems, Buffalo Grove, IL) was used throughout the procedure. A 1” midline incision was made in the skin dorsally at the level of the thorax and continued rostrally to the base of the skull. Connective tissue was blunt dissected away from the adipose tissue with care to avoid cutting the large thoracodorsal artery that is located medially to both pads. Both left and right fat pads were separated from the mid line. Each fat pad was lifted up and the intercostal nerve bundles were located below. Once the nerves were located, a sharp point forceps was used to pull the nerve bundles straight up while using a 45 degree scissors to cut and remove 3-5 mm of nerves. The interscapular incision was closed with 4-0 non-absorbable monofilament Ethilon (nylon) sutures or with standard metal wound clips. Nerves were similarly identified but not cut for sham operated animals. Rats were treated pre-operatively with the analgesic ketoprofen (2 mg/kg; Fort Dodge Animal Health, Overland Park, KS) prior to the completion of the denervation or sham procedure. This procedure was combined with transponder implantations for studies that involved IBAT temperature measurements in response to acute 4V injections (
Study 4). Animals were allowed to recover for approximately 5-7 days prior to implantation of 4V cannulas. Note that surgical denervation was used in place of chemical denervation because chemical denervation can result in SNS terminal recovery within IBAT [
43,
44] within only 10 days post-chemical denervation [
43].
Animals were implanted with a cannula (P1 Technologies, Roanoke, VA) that was directed towards the 4V as previously described [
45,
46,
47]. Briefly, rats under isoflurane anesthesia were placed in a stereotaxic apparatus apparatus [Digital Lab Standard Stereotaxic, Rat, (Item 51900), Stoelting Co., Wood Dale, IL] with the incisor bar positioned 3.3 mm below the interaural line. A 26-gauge cannula (P1 Technologies) was stereotaxically positioned into the 4V [-3.5 mm caudal to the interaural line; 1.4 mm lateral to the midline, and 6.2 mm ventral to the skull surface [
48]] and secured to the surface of the skull with dental cement and stainless-steel screws.
Briefly, rats were implanted with a cannula within the 4V with a side port that was connected to an osmotic minipump (model 2004, DURECT Corporation) as previously described [
31,
49]. Rats under isoflurane anesthesia were placed in a stereotaxic apparatus [Digital Lab Standard Stereotaxic, Rat, (Item 51900), Stoelting Co.] with the incisor bar positioned 3.3 mm below the interaural line. A 30-gauge cannula (P1 Technologies) was stereotaxically positioned into the 4V [-3.5 mm caudal to the interaural line; 1.4 mm lateral to the midline, and 7.2 mm ventral to the skull surface [
48]] and secured to the surface of the skull with dental cement and stainless steel screws. A 2.4” piece of plastic Tygon
TM Microbore Tubing (0.020" x 0.060"OD; Cole-Parmer) was tunneled subcutaneously along the midline of the back and connected to the 21-gauge sidearm osmotic minipump-cannula assembly. A stainless steel 22-gauge pin plug (Instech Laboratories, Inc.) was temporarily inserted at the end of the tubing during a two week postoperative recovery period, after which it was replaced by an osmotic minipump (DURECT Corporation) containing saline or OT. Rats were treated with the analgesic ketoprofen (2 mg/kg; Fort Dodge Animal Health) and the antibiotic
enrofloxacin (5 mg/kg; Bayer Healthcare LLC., Animal Health Division Shawnee Mission, KS) at the completion of the 4V cannulations and were allowed to recover at least 10 days prior to implantation of osmotic minipumps.
Mice were implanted with a cannula within the 4V with a side port that was connected to an osmotic minipump (model 2004, DURECT Corporation) as previously described [
50]. Mice under isoflurane anesthesia were placed in a stereotaxic apparatus [Digital Just for Mouse Stereotaxic, (Item 51730D), Stoelting Co.] with the incisor bar positioned 4.5 mm below the interaural line. A 30-gauge cannula (P1 Technologies) was stereotaxically positioned into the 4V of either female C57BL/6J mice (-5.9 mm caudal to bregma; 0.4 mm lateral to the midline, and 3.7 mm ventral to the skull surface) [
51] and secured to the surface of the skull with dental cement and stainless steel screws. A 1.2” piece of plastic Tygon
TM Microbore Tubing (0.020" x 0.060"OD; Cole-Parmer) was tunneled subcutaneously along the midline of the back and connected to the 21-gauge sidearm osmotic minipump-cannula assembly. A stainless steel 22-gauge pin plug (Instech Laboratories, Inc.) was temporarily inserted at the end of the tubing during a two week postoperative recovery period, after which it was replaced by an osmotic minipump (DURECT Corporation) containing saline or OT. Mice were treated with the analgesic ketoprofen (5 mg/kg; Fort Dodge Animal Health) and the antibiotic
enrofloxacin (5 mg/kg; Bayer Healthcare LLC., Animal Health Division Shawnee Mission, KS) at the completion of the 4V cannulations and were allowed to recover at least 10 days prior to implantation of osmotic minipumps.
Animals were anesthetized with isoflurane and had the dorsal surface along the upper midline of the back shaved and the area was scrubbed with 70% ethanol followed by betadine swabs. A one-inch incision was made at the midline of the interscapular area. The temperature transponder (14 mm long/2 mm wide) (HTEC IPTT-300; Bio Medic Data Systems, Inc., Seaford, DE) was implanted underneath the left IBAT pad as previously described [
31,
52,
53] and secured in place by suturing it to the brown fat pad with sterile silk suture. The interscapular incision was closed with Nylon sutures (5-0), which were removed in awake animals 10-14 days after surgery.
On an experimental day, animals received either IP (CL 316243 or saline vehicle; 0.1 ml/kg injection volume) or 4V injections (OT or saline vehicle; 1 μL injection volume, 4V) during the early part of the light cycle following 4 hours of food deprivation. Injections were completed in a crossover design over approximately 7-day (CL 316243) or 48-h (OT) intervals such that each animal served as its own control. Animals remained without access to food for an additional 4 h (Study 3-4) during the course of the TIBAT measurements. A handheld reader (DAS-8007-IUS Reader System; Bio Medic Data Systems, Inc) was used to collect measurements of TIBAT. Rats underwent all treatments in a randomized order separated by at least 7-8 days between treatments.
Determinations of lean body mass and fat mass were made on un-anesthetized rats by quantitative magnetic resonance using an EchoMRI 4-in-1-700TM instrument (Echo Medical Systems, Houston, TX) at the VAPSHCS Rodent Metabolic Phenotyping Core. Measurements were taken prior to 4V cannulations and minipump implantations as well as at the end of the infusion period.
Rats were euthanized by rapid conscious decapitation at 8 weeks (
Study 1-2) or 7-12 weeks (
Study 3) post-sham or denervation procedure. Trunk blood and tissues (IBAT, EWAT, IWAT, liver and/or pancreas) were collected from 4-h fasted rats. Tissue was rapidly removed, wrapped in foil and frozen in liquid N2. Samples were stored frozen at -80°C until analysis. Note that anesthesia was not used when collecting tissue for NE content as it can cause the release of NE from SNS terminals within the tissue [
44].
NE content was measured in IBAT, EWAT, IWAT, liver and/or pancreas using previously established techniques [
54]. Successful denervation was noted by ≥60% reduction in IBAT NE content as previously noted [
55]. Experimental animals that did not meet this criterion were excluded from the data analysis.
Study 1: Determine if surgical denervation of IBAT changes the ability of theβ-3R agonist, CL 316243, to increase TIBAT in DIO rats
Rats (N=13 at study onset) from
Study 2 were used in these studies. Animals were fed
ad libitum and maintained on HFD for approximately 4.5 months prior to underdoing sham or SNS denervation procedures and implantation of temperature transponders underneath the left IBAT depot. Rats were subsequently implanted with 4V cannulas approximately 1 week following sham/denervation procedures and implantation of temperature transponders. Rats were allowed to recover for at least 2 weeks during which time they were adapted to a daily 4-h fast, handling and mock injections. On an experimental day, 4-h fasted rats received Cl 316243 (0.1 or 1 mg/kg) or vehicle (sterile water) during the early part of the light cycle in a crossover design at approximately 7-day intervals such that each animal served as its own control (approximately 1-3 weeks post-sham or denervation procedures). T
IBAT was measured at baseline (-2 h; 9:00 a.m.), immediately prior to IP injections (0 h; 9:45-10:00 a.m.), and at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 3, 4, and 24-h post-injection (10:00 a.m.). Food intake and body weight were measured daily. Daily food intake was determined by measuring the difference in weight of the high fat diet pre- vs post-intervention of a 24-h period and converting grams/day to units of energy intake/day (kcal/day; 5.24 kcal/gram [
56]). This dose range was based on doses of CL 316243 found to be effective at reducing food intake and weight gain in rats [
50,
57]. Animals were euthanized by rapid conscious decapitation at 13 weeks post-sham or denervation procedure.
Study 2: Determine the extent to which OT-induced activation of sympathetic outflow to IBAT contributes to its ability to increase TIBAT in DIO rats
Rats (N=16 at study onset) from
Study 1 were used in these studies. On an experimental day, 4-h fasted rats received OT (1 or 5 μg/μl) or vehicle during the early part of the light cycle in order to maximize the effects of OT [
17,
50] during a time when circulating NE levels [
58] and IBAT catecholamine levels are lower [
59]. Injections were completed in a crossover design at approximately 48-h to 72-h intervals such that each animal served as its own control (approximately 4-weeks post-sham or denervation procedures). T
IBAT was measured at baseline (-2 h; 9:00 a.m.), immediately prior to 4V injections (0 h; 9:45-10:00 a.m.), and at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 3, 4, and 24-h post-injection (10:00 a.m.). Food intake and body weight were measured daily. This dose range was based on doses of 4V OT found to be effective at stimulating T
IBAT in male DIO rats in previous studies [
31].
In addition, we examined the impact of a lower dose of OT (0.5 μg/μL) in an identical manner following the completion of the initial studies in Study 2.
Study 3A: Determine the extent to which OT-induced activation of sympathetic outflow to IBAT contributes to its ability to reduce weight gain in female HFD-fed rats
Rats (N=30 at study onset) were used for these studies. Animals were fed
ad libitum and maintained on HFD for approximately 5.25 months prior to receiving implantations of temperature transponders underneath IBAT, 4V cannulas and subcutaneous minipumps to infuse vehicle or OT (16 nmol/day) over 29 days as previously described [
31]. This dose was selected based on a dose of 4V OT found to be effective at reducing body weight in male DIO rats [
31]. Daily food intake and body weight were also tracked for 29 days. Animals were euthanized by rapid conscious decapitation at 7 weeks post-sham or denervation procedure. Trunk blood and tissues [IBAT, epididymal white adipose tissue (EWAT), inguinal white adipose tissue (IWAT), liver and pancreas)] were collected from 4-h fasted rats and tissues were subsequently analyzed for IBAT NE content to confirm success of denervation procedure relative to sham operated animals and other tissues (EWAT, IWAT, liver and pancreas).
Study 3B: Determine the extent to which 4V OT impacts thermogenic gene expression in IBAT and IWAT in female HFD-fed rats
Rats from Study 3A were used for these studies. All rats received chronic infusions of 4V vehicle or OT (16 nmol/day) and were euthanized by rapid conscious decapitation following a 4-h fast.
Study 4A: Determine the effects of chronic 4V OT treatment on body weight, adiposity and energy intake in female HFD-fed C57BL/6J mice
Female mice (N= 20 at study onset) were fed
ad libitum and maintained on HFD for approximately 4.5 months prior to being implanted with temperature transponders underneath IBAT. Mice were allowed up to 1-week post-op recovery prior to receiving 4V cannulas. Mice were allowed up to 2 weeks post-op recovery prior to being implanted with minipumps as previously described [
31]. T
IBAT was measured daily at baseline (-2 h; 9:00 a.m.) and immediately prior to access to food (10:00 a.m.). Daily food intake, body weight and T
IBAT were tracked for 28 days.
Study 4B: Determine the effects of chronic 4V OT treatment on body weight, adiposity and energy intake in female DIO DBA2J mice
Female mice (N= 20 at study onset) were used for these studies. Animals were fed
ad libitum and maintained on HFD for approximately 4.5 months prior to being implanted with temperature transponders underneath IBAT. Mice were allowed up to 2 weeks post-op recovery prior to receiving 4V cannulas. Mice were allowed up to 4 weeks post-op recovery prior to being implanted with minipumps as previously described [
31]. T
IBAT was measured daily at baseline (-2 h; 9:00 a.m.) and immediately prior to access to food (10:00 a.m.). Daily food intake, body weight and T
IBAT were tracked for 28 days.
Study 5: Determine the effects of chronic systemic OT treatment (16 and 50 nmol/day) on body weight, adiposity and energy intake in female DIO DBA/2J mice
Female mice (N= 22 mice at study onset) were fed
ad libitum and maintained on HFD for approximately 4.5 months prior to being implanted with temperature transponders underneath IBAT. Mice were allowed up to 4 weeks post-op recovery prior to being implanted with minipumps as previously described [
31]. T
IBAT was measured daily at baseline (-2 h; 9:00 a.m.) and immediately prior to access to food (10:00 a.m.). Daily food intake, body weight and T
IBAT were tracked for 27 days.
Trunk blood (
Study 1-2) or blood from cardiac stick (
Study 3-5) was collected from 4-h fasted rats or mice within a 2-h window towards the beginning of the light cycle (10:00 a.m.-12:00 p.m.) as previously described in DIO CD
® IGS and Long-Evans rats and C57BL/6J mice [
31,
46]. Treatment groups were counterbalanced at time of euthanasia to avoid time of day bias. Blood samples [up to 1 mL (mice) or 3 mL (rats)] were collected from trunk or via cardiac puncture in chilled K2 EDTA Microtainer Tubes (Becton-Dickinson, Franklin Lakes, NJ). Whole blood was centrifuged at 6,000 rpm for 1.5-min at 4°C; plasma was removed, aliquoted and stored at −80°C for subsequent analysis.
Plasma leptin and insulin were measured using electrochemiluminescence detection [Meso Scale Discovery (MSD
®), Rockville, MD] using established procedures [
31,
60]. Intra-assay coefficient of variation (CV) for leptin was 2.8% and 2.4% for rat and mouse, respectively. Intra-assay CV for insulin was 2.7% and 2.4% for rat and mouse, respectively. The range of detectability for the leptin assay is 0.07-51.9 ng/mL and 0.069-50 ng/mL for insulin. Plasma glucagon (Mercodia, Winston Salem, NC), fibroblast growth factor-21 (FGF-21) (R&D Systems, Minneapolis, MN) and irisin (AdipoGen, San Diego, CA) levels were determined by ELISA. The intra-assay CV for glucagon was 1.6% and 1.9% for rat and mouse, respectively, and the range of detection was 2-182 pmol/L. The intra-assay CV for FGF-21 was 2.7% and 2.3% for rat and mouse, respectively. The intra-assay CV for irisin was 6.9% for mouse (not obtained for rat). The ranges of detectability were 31.3-2000 pg/mL (FGF-21) and 0.078-5 μg/mL (irisin). Plasma adiponectin was also measured using ELISA (Alpco, Salem, NH) using established procedures [
31,
60]. Intra-assay CV for adiponectin was 1.7% and 1.6% for rat and mouse, respectively. The range of detectability for the adiponectin assay is 0.25-10 ng/mL (rat) and 0.025-1 ng/mL (mice). The data were normalized to historical values using a pooled plasma quality control sample that was assayed in each plate.
Blood was collected for glucose measurements by tail vein nick in 4-h fasted rats and measured with a glucometer using the AlphaTRAK 2 blood glucose monitoring system (Abbott Laboratories, Abbott Park, IL) [
31,
61]. Total cholesterol (TC) [Fisher Diagnostics (Middletown, VA)] and free fatty acids (FFAs) [Wako Chemicals USA, Inc., Richmond, VA)] were measured using an enzymatic-based kits. Intra-assay CVs for TC were 3.5% and 3.4% for rat and mouse, respectively. Intra-assay CV for FFA were 1.4% and 2.5% for rat and mouse, respectively. These assay procedures have been validated for rodents [
62].
Adipose tissue depots were collected at the end of the infusion period in DIO rats from
Study 3B (EWAT). EWAT was processed as previously described [
32,
50,
63,
64]. EWAT was dissected and placed in 4% paraformaldehyde-PBS for 24 h and then placed in 70% ethanol (EtOH) prior to paraffin embedding. Sections (5 μm) sampled were obtained using a rotary microtome, slide-mounted using a floatation water bath (37°C), and baked for 30 min at 60°C to give approximately 15-16 slides/fat depot with two sections/slide.
Adipocyte size analysis was performed as previously described [
32,
50,
63,
64]. Analysis was completed on deparaffinized and digitized EWAT sections. The average cell area from two randomized photomicrographs was determined using the built-in particle counting method of ImageJ software (National Institutes of Health, Bethesda, MD). Slides were visualized using bright field on an Olympus BX51 microscope (Olympus Corporation of the Americas; Center Valley, PA) and photographed using a Canon EOS 5D SR DSLR (Canon U.S.A., Inc., Melville, NY) camera at 10X magnification. Values for each tissue within a treatment were averaged to obtain the mean of the treatment group.
Tissue (IBAT and IWAT) was collected from a subset of 4-h (
Study 3B). IBAT and IWAT were collected within a 2-h window towards the start of the light cycle (10:00 a.m.-12:00 p.m.) as previously described in DIO CD
® IGS/Long-Evans rats and C57BL/6J mice [
31,
46,
50]. Tissue was rapidly removed, wrapped in foil and frozen in liquid N2. Samples were stored frozen at -80°C until analysis.
RNA extracted from samples of IBAT and IWAT (
Study 3B) were analyzed using the RNeasy Lipid Mini Kit (Qiagen Sciences Inc, Germantown, MD) followed by reverse transcription into cDNA using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). Quantitative analysis for relative levels of mRNA in the RNA extracts was measured in duplicate by qPCR on an Applied Biosystems 7500 Real-Time PCR system (Thermo Fisher Scientific, Waltham, MA) and normalized to the cycle threshold value of Nono mRNA in each sample. The TaqMan® probes used in the study were Thermo Fisher Scientific Gene Expression Assay probes. The probe for rat
Nono (Rn01418995_g1), uncoupling protein-1 (UCP-1) (
Ucp1; catalog no. Rn00562126_m1), β1-adrenergic receptor (β1-AR) (
Adrb1; catalog no. Rn00824536_s1), β3-adrenergic receptor (β3-AR) (
Adrb3; catalog no. Rn01478698_g1), type 2 deiodinase (D2) (
Dio2; catalog no. Rn00581867_m1), PR domain containing 16 (
Prdm16; catalog no. Rn01516224_m1), G-protein coupled receptor 120 (
Gpr120; catalog no. Rn01759772_m1), cell death-inducing DNA fragmentation factor alpha-like effector A (
Cidea; catalog no. Rn04181355_m1), and peroxisome proliferator-activated receptor gamma coactivator 1 alpha (
Ppargc1a; catalog no. Rn00580241_m1) were acquired from Thermo Fisher Scientific. Relative amounts of target mRNA were determined using the Comparative C
T or 2-
ΔΔCT method [
65] following adjustment for the housekeeping gene, Nono. Specific mRNA levels of all genes of interest were normalized to the cycle threshold value of
Nono mRNA in each sample and expressed as changes normalized to controls (vehicle/sham treatment).
All results are expressed as means ± SE. Comparisons between multiple groups involving between subjects designs were made using one- or two-way ANOVA as appropriate, followed by a post-hoc Fisher's least significant difference test. Comparisons involving within-subjects designs were made using a one-way repeated-measures ANOVA followed by a post-hoc Fisher's least significant difference test. Analyses were performed using the statistical program SYSTAT (Systat Software, Point Richmond, CA). Differences were considered significant at P<0.05, 2-tailed. Non-statistical trends (0.05<P<0.1) have been included in the analysis where appropriate.
Results
Study 1: Determine if surgical denervation of IBAT changes the ability of the beta 3-adrenergic receptor (β3-AR) agonist, CL 316243, to increase TIBAT in DIO rats.
The goal of this study was to confirm there was no functional defect in the ability of IBAT to respond to direct β3-AR stimulation as a result of the denervation procedure relative to sham operated animals. As expected, HFD-fed rats were borderline obese as determined by both body weight (336.8±7.8 g) and adiposity (99.0±6.7 g fat mass; 28.9±1.3% adiposity) after maintenance on the HFD for approximately 4 months prior to sham/denervation procedures.
All IBAT tissues from Study 1/Study 2 animals were analyzed for IBAT NE content and only 1 out of 5 animals was removed on account of having a failed IBAT denervation procedure. IBAT NE content was reduced in denervated rats by 76.9±2.7% in denervated rats relative to sham-operated control rats [(F(1,10) = 18.975, P=0.001). In contrast, NE content was unchanged in IWAT, EWAT, liver or pancreas in denervated rats relative to sham rats (P=NS). There was no significant difference in body weight between sham and denervation groups at the end of the study (P=NS; data not shown).
In sham rats, CL 316243 (1 mg/kg) increased T
IBAT at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 3 and 4-h post-injection. The lowest dose (0.1 mg/kg) also stimulated T
IBAT at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 3 and 4-h post-injection (
P<0.05;
Figure 1A).
Similarly, in denervated rats, CL 316243 (1 mg/kg) increased T
IBAT at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 3 and 4-h post-injection. The lowest dose (0.1 mg/kg) also stimulated T
IBAT at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 3 and 4-h post-injection (
P<0.05;
Figure 1B).
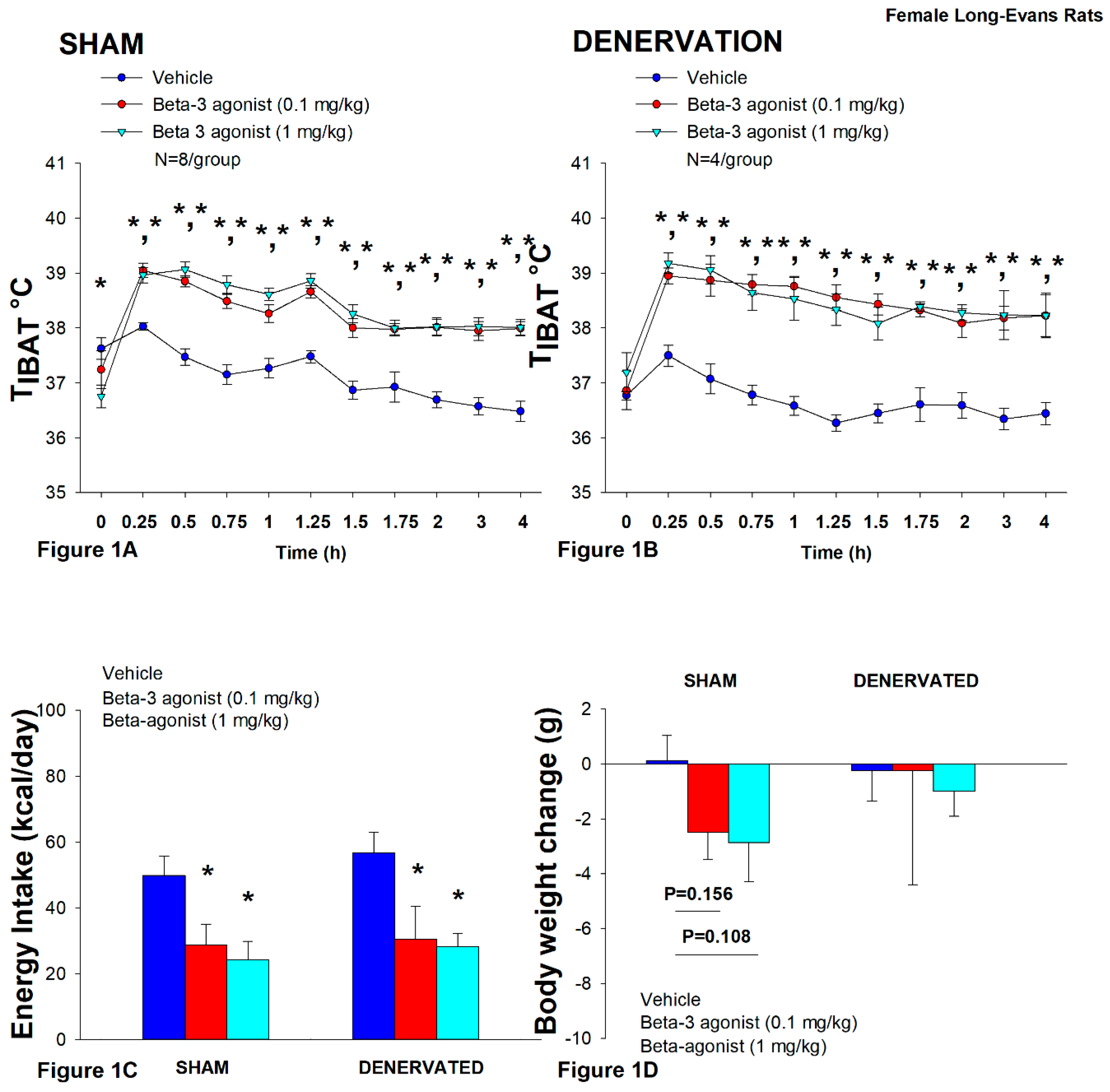
Importantly, there was no difference in the TIBAT response to CL 316243 (0.1 or 1 mg/kg) when the data were averaged over the 1-h or 4-h post-injection period between sham and denervated rats (P=NS).
Overall, these findings indicate that IBAT denervation did not result in a change in the ability of CL 316243 to increase BAT thermogenesis (surrogate measure of EE) in DIO mice relative to sham operated rats.
In sham rats, CL 316243 reduced daily energy intake at both 0.1 and 1 mg/kg by 42.3 and 51.4% (
P<0.05). Similarly, in denervated rats, CL 316243 also reduced daily energy intake at both 0.1 and 1 mg/kg (
P<0.05) by 46.2 and 50.4% relative to vehicle (
Figure 1C).
CL 316243 did not significantly impact body weight or body weight in either group (
P=NS;
Figure 1D). There was a tendency for the high dose (1 mg/kg) to reduce body weight gain in the sham-operated group but this was not significant (
P=0.111).
Importantly, there was no difference in the effectiveness of CL 316243 (0.1 or 1 mg/kg) to reduce food intake between sham and denervated rats (P=NS).
Overall, these findings indicate that IBAT denervation did not result in a significant change in the ability of CL 316243 to reduce food intake in DIO rats relative to sham operated rats.
Study 2: Determine the extent to which OT-induced activation of sympathetic outflow to IBAT contributes to its ability to increase TIBAT in female HFD-fed rats.
After having confirmed there was no functional defect in the ability of IBAT to respond to direct β3-AR stimulation (Study 3), the goal of this study was to determine if OT-elicited elevation of TIBAT requires intact SNS outflow to IBAT. Three of the sixteen rats available at study onset were euthanized during the course of the study and were excluded from the data analysis.
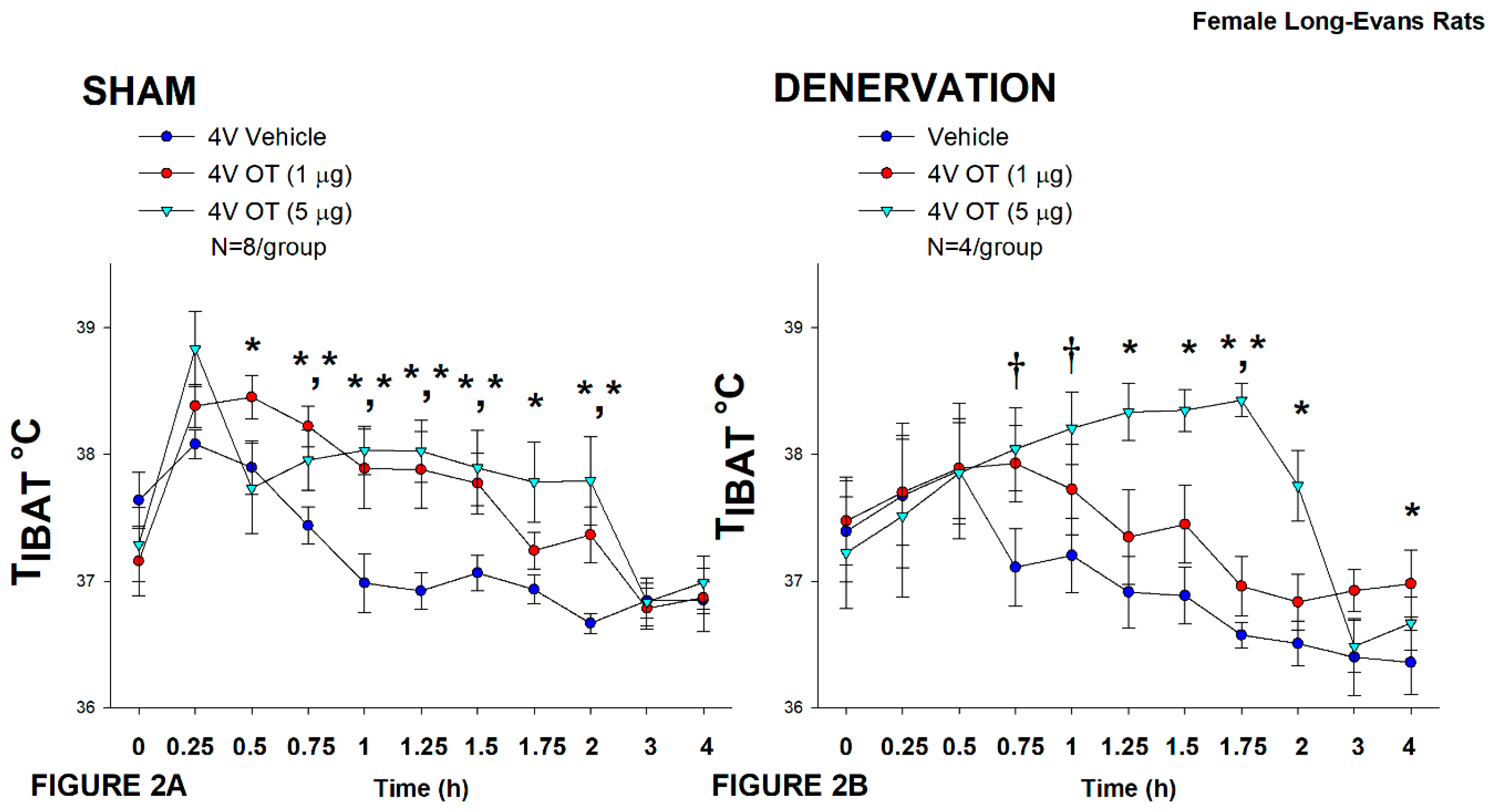
In sham rats, OT (5 μg) increased T
IBAT at 0.75, 1, 1.25, 1.5, 1.75, and 2-h post-injection (
P<0.05). A lower dose (1 μg) also stimulated T
IBAT at 0.5, 0.75, 1, 1.25, and 1.5-h post-injection (
P<0.05;
Figure 2A).
In denervated rats, OT (5 μg) increased T
IBAT at 1.25, 1.5, 1.75, and 2-h post-injection (
P<0.05) and tended to stimulate T
IBAT at 0.75, and 1-h post-injection (0.05<
P<0.1). The low dose (1 μg) stimulated T
IBAT at 1.75 and 4-h post-injection (0.05<
P<0.1;
Figure 2B).
Importantly, there was no difference in the TIBAT response to 4V OT (5 μg) when the when the data were averaged over the 4-h post-injection period between sham and denervated rats (P=NS).
There were, however, seizures, barrel-rolling and unexpected deaths that occurred in three out of the sixteen rats (1 sham, 2 denervated) shortly after 4V administration of OT at the high dose (5 μg). Rinaman also reported that acute ICV administration of a higher dose (10 μg) also resulted in seizure-like activity and barrel-rolling in a subset of adult male Sprague-Dawley rats [
66]. These findings raise the possibility that females may be more sensitive to the effects of acute injections of 4V OT compared to what we have observed previously at similar doses in males in the absence of such effects [
31,
63]. Thus, following the completion of these studies, we also examined the effectiveness of a lower dose of 4V OT (0.5 μg/μL) on T
IBAT in an identical manner.
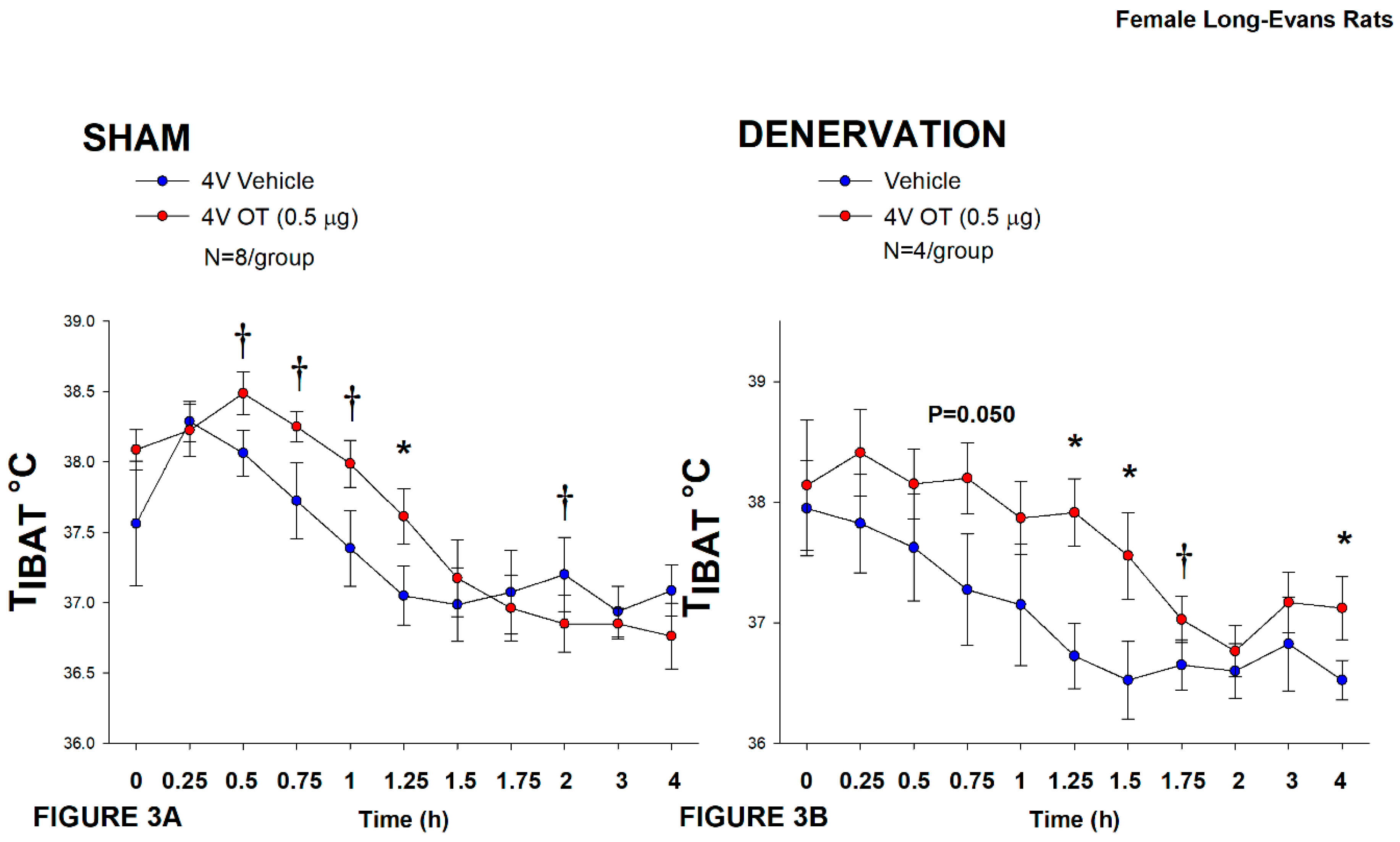
In sham rats, 4V OT (0.5 μg/μL) stimulated T
IBAT 1.25-h post-injection (
P<0.05;
Figure 3A) and tended to stimulate T
IBAT at 0.5, 0.75, and 1-h post-injection (0.05<
P<0.1;
Figure 3A). It also tended to reduce T
IBAT at 2-h post-injection (0.05<
P<0.1;
Figure 3A).
In denervated rats, 4V OT (0.5 μg/μL) stimulated T
IBAT 1.25, 1.5 and 4-h post-injection (
P<0.05;
Figure 3B) and tended to stimulate T
IBAT at 0.75 (
P=0.050) and 1.75-h (
P=0.053) post-injection (
Figure 3B). It also increased T
IBAT at 24-h post-injection (
P<0.05; data not shown).
Importantly, there was no difference in the TIBAT response to 4V OT (0.5 μg) at either 1.25-h post-injection or when the data were averaged over the 1-h post-injection between sham and denervated rats (P=NS).
Overall, these findings indicate that IBAT denervation did not result in a significant change in the ability of 4V OT to increase BAT thermogenesis in denervated rats relative to sham operated rats.
Study 3A: Determine the extent to which OT-induced activation of sympathetic outflow to IBAT contributes to its ability to impact body weight in HFD-fed rats.
The goal of this study was to determine if OT-elicited weight loss requires intact SNS outflow to IBAT. Initially, female rats were lean as defined by body weight (230±2.1 g). Similar to Study 1, HFD-fed rats were borderline obese as determined by both body weight (380±8.3 g) and adiposity (126.4±6.4 g fat mass; 32.8±1.0% adiposity) after maintenance on the HFD for at least 4.5 months prior to sham/denervation procedures.
Note that a subset of rats from Study 3 have been analyzed (9 out of 15) for IBAT NE content and all had successful IBAT denervation procedures. All 15 animals were included in the subsequent analyses. IBAT NE content was reduced in a subset of denervated (9 out of 15) rats by 83.1±3.0% relative to a subset of sham-operated control rats (11 out of 15) [(F(1,18) = 64.663, P=0.000)]. In contrast, NE content was unchanged in IWAT, EWAT, liver or pancreas in denervated rats relative to sham rats (P=NS). There was no significant difference in body weight between sham and denervation groups at the beginning of the study prior to minipump implantation (P=NS; data not shown).
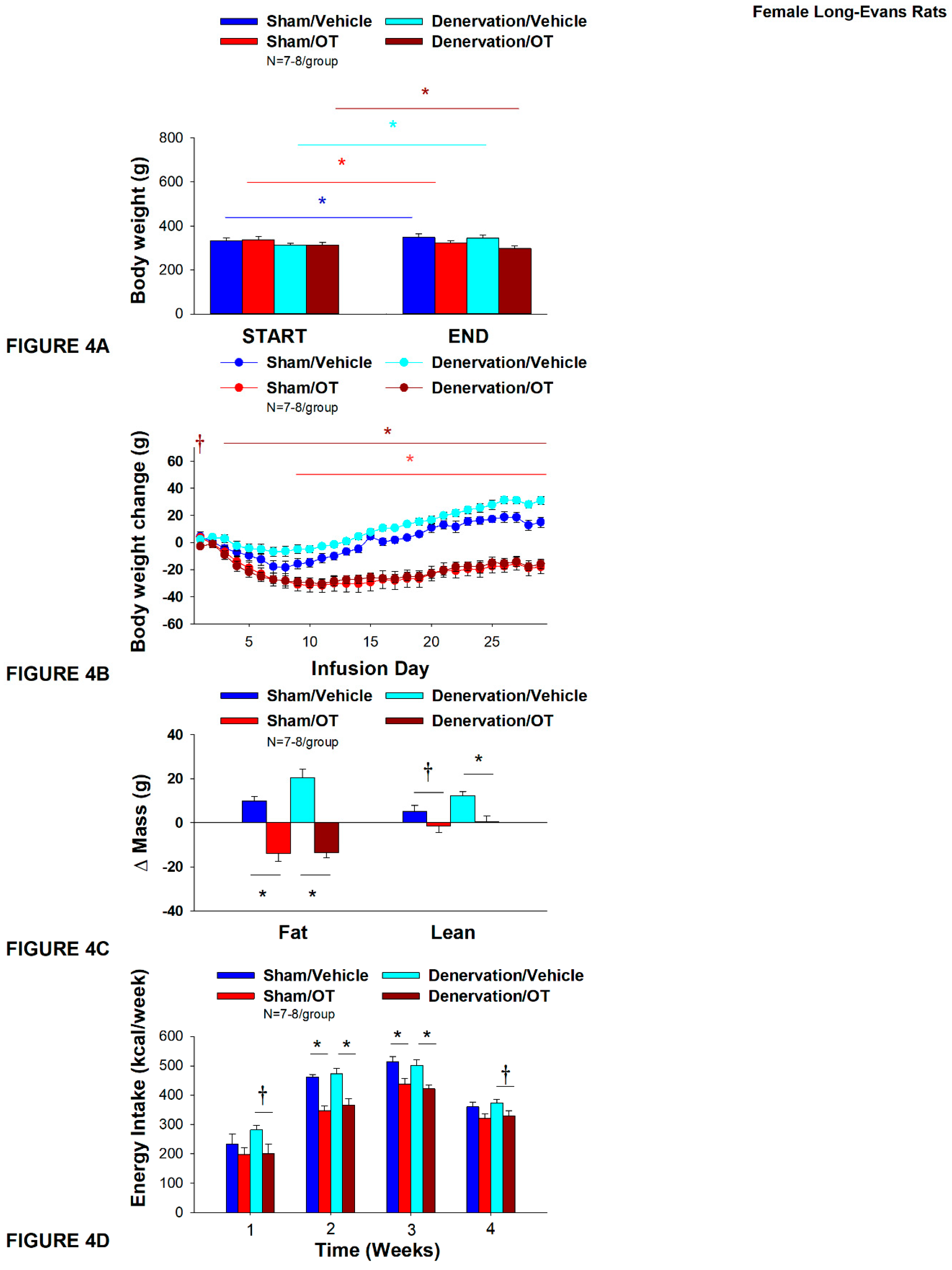
As expected, in sham rats, 4V vehicle resulted in 4.5±1.2% weight gain relative to vehicle pre-treatment [(F(1,6) = 14.125,
P=0.009)]. In addition, 4V OT reduced body weight by 5.5±1.3% relative to 4V OT pre-treatment [(F(1,7) = 8.169,
P=0.024)] (
Figure 4A). In addition, 4V OT treatment reduced weight gain throughout the 29-day infusion period over days 9-29 (
P<0.05;
Figure 4B). By the end of the infusion period (infusion day 29), OT had reduced body weight by -18±5.0 g relative to vehicle treated animals (15.0±3.8 g;
P<0.05). OT reduced relative fat mass (pre- vs post-intervention) (
Figure 4C;
P<0.05), fat mass and relative lean mass (pre- vs post-intervention) but had no effect on total lean body mass (
P=NS). These effects that were mediated, at least in part, by a modest reduction of energy intake that was evident during weeks 2 and 3 of the treatment period (
Figure 4D;
P<0.05).
Similar to what was observed in sham animals, 4V vehicle resulted in 8.9±1.2% weight gain relative to vehicle pre-treatment in denervated rats [(F(1,6) = 65.633,
P=0.000)]. In addition, 4V OT also reduced body weight by 4.1±1.0% relative to 4V OT pre-treatment [(F(1,7) = 13.723,
P=0.008)]. (
Figure 4A). In addition, 4V OT reduced weight gain throughout the 29-day infusion period over days 3-29 (
P<0.05;
Figure 4B). By the end of the infusion period (infusion day 29), OT had reduced body weight by -15.9±3.7 g relative to vehicle treated animals (30±2.9 g;
P<0.05). OT reduced relative fat mass (pre- vs post-intervention) (
Figure 4C;
P<0.05) and fat mass (
P<0.05) but had no effect on total lean body mass (
P=NS). These effects that were mediated, at least in part, by a modest reduction of energy intake that persisted during weeks 2 and 3 of the treatment period (
Figure 4D;
P<0.05). OT also tended to reduce energy intake during week 1 (
P=0.052) and 4 (
P=0.075) of the treatment period. There was also no effect of chronic 4V OT to significant increase kaolin consumption during the treatment period (
P=NS; data not shown).
Importantly, there was no difference in the effectiveness of 4V OT to reduce body weight, energy intake, and relative fat mass or fat mass between sham and denervated rats (P=NS).
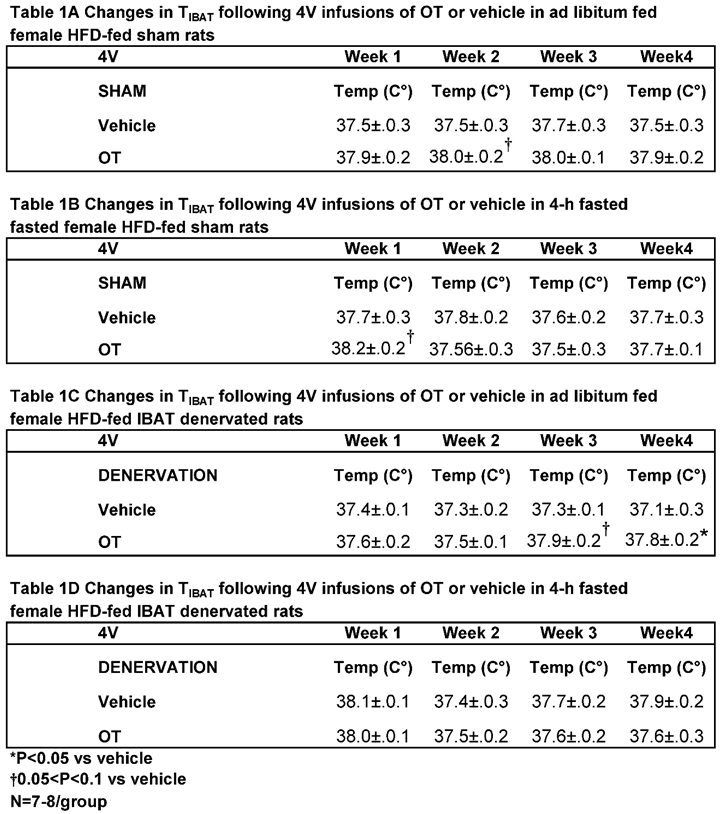
Similar to what we previously observed following chronic 3V [
31] and 4V [
50] administration in male rats, chronic 4V administration of OT appeared to stimulate T
IBAT (at onset of light cycle) relative to vehicle in sham operated rats when the data were averaged over week 2 of the infusion period (
Table 1A;
P=0.081) and throughout the infusion period in ad libitum fed rats on days 6 (
P=0.068), 12 (
P<0.05) and 21 (
P<0.05).
In order to minimize the potential confounding effects of diet-induced thermogenesis,
T
IBAT was also collected 4 hours later from the same sham operated rats following a 4-h fast. Chronic 4V OT elevated T
IBAT when the data were averaged over week 1 of the infusion period (
Table 1A;
P=0.066) and on infusion days 3 (
P<0.05), 5 (
P<0.05), and 7 (
P<0.05).
In addition, chronic 4V administration of OT also appeared to stimulate T
IBAT in denervated rats when the data were averaged over weeks 3 (
Table 1B;
P=0.054) and 4 (
Table 1B;
P<0.05) and throughout the infusion period on days 15 (
P<0.05), 18 (
P=0.063), 20 (
P=0.057), 21 (
P=0.050), 23 (
P<0.05), 24 (
P<0.05) and 26 (
P<0.05.
Based on these collective findings, we conclude that SNS innervation of IBAT does not appear to be a predominant contributor of OT-elicited reduction of weight gain and adiposity.
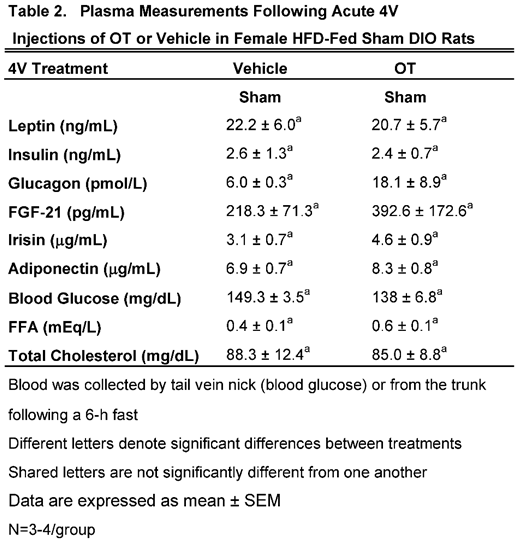
We characterized the endocrine and metabolic effects of acute 4V OT (5 μg/μL) in sham HFD-fed rats (
Table 2). Samples from the denervated HFD-fed rats were excluded due to the lack of samples/group for valid comparisons (N=1-2/group). There were no significant differences in any of the plasma measurements between vehicle and 4V OT-treated rats in the sham-operated groups.
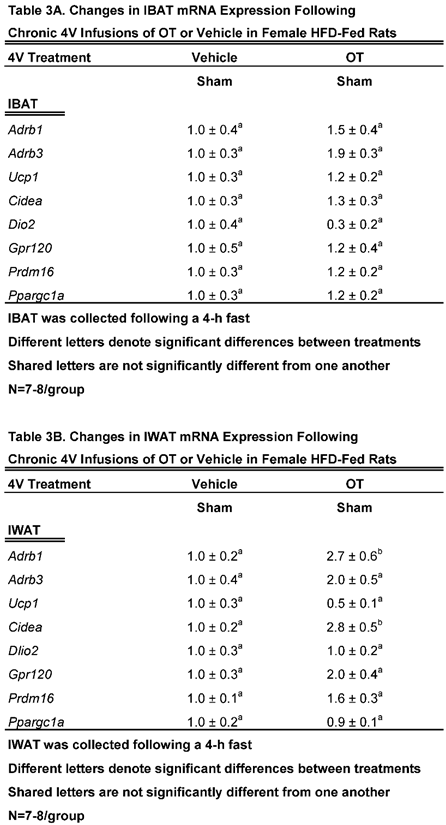
Study 3B: Determine the extent to which 4V OT impacts thermogenic gene expression in IBAT and IWAT in female HFD-fed rats.
The goal of this study was to determine if 4V OT elicits changes in thermogenic gene expression in IBAT and EWAT in sham operated rats.
We found that chronic 4V OT treatment elicited a near significant increase in β3-AR mRNA expression (
Adrb3;
P=0.063) and a near significant reduction of Dio2 (
P=0.077;
Table 3A).
4V OT treatment elicited a significant increase of the thermogenic markers, beta 1 adrenergic receptor (β1-AR) (
Adrb1;
P<0.05;
Table 3B) and Cidea (
P<0.05) mRNA expression. 4V OT treatment also elicited a near significant increase in Gpr120 mRNA expression (
P=0.060) as well as a near significant reduction of Dio2 mRNA expression (
P=0.097) in IWAT from sham-operated rats.
Collectively, these findings raise the possibility that different thermogenic markers in IBAT and IWAT may contribute, in part, to the metabolic effects of 4V OT in sham-operated rats.
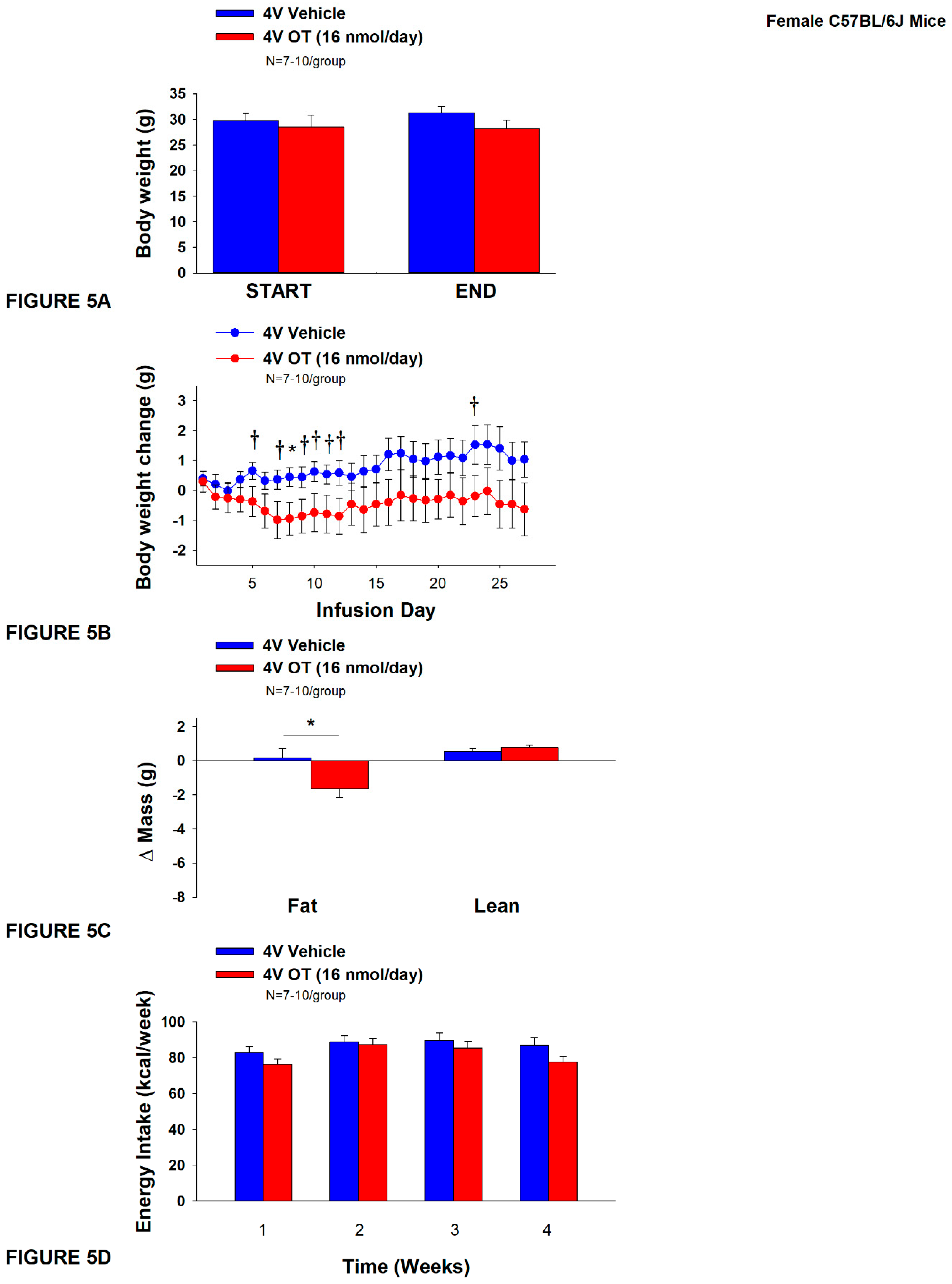
Study 4A: Determine the effects of chronic 4V OT treatment (16 nmol/day) on body weight, adiposity and energy intake in female DIO C57BL/6J mice.
The goal of this study was to determine the susceptibility of female C57BL/6J to DIO and whether the effects of chronic hindbrain (4V) administration to reduce body weight and adiposity could translate to another female rodent model (female C57BL/6J mice). Initially, mice were lean as defined by both body weight (17.4±0.3 g) and adiposity (2.2±0.2 g fat mass; 12.7±0.8% adiposity). HFD-fed C57BL/6J mice became borderline DIO as determined by both body weight (31.2±1.4 g) and adiposity (11.7±1.2 g fat mass; 36±2.2% adiposity) after maintenance on the HFD for at least 4.5 months prior to being implanted with temperature transponders and minipumps as described earlier. There was no significant difference in body weight or adiposity between vehicle and oxytocin treatment groups at the beginning of the study prior to minipump implantation (P=NS; data not shown). Three of the twenty mice available at study onset were euthanized during the course of the study and were excluded from the data analysis (including one whose head cap had become detached).
The mice that received 4V vehicle tended to gain a modest amount of weight relative to vehicle pre-treatment (
P=0.114). While 4V OT treatment failed to result in weight loss (
P=NS;
Figure 5A), it reduced weight gain on treatment day 8 (
P<0.05) and tended to reduce weight gain on treatment days 5, 7, 9-12, and 23 (0.05<
P<0.1;
Figure 5B). OT reduced relative fat mass (pre- vs post-intervention) (
Figure 5C;
P<0.05) and tended to reduce total lean mass (
P=0.131) but had no effect on relative lean mass (pre- vs post-intervention) or total lean mass (
P=NS). These effects were not associated with significant reductions in energy intake (
Figure 5D;
P=NS) or kaolin intake (
P=NS; data not shown) throughout the treatment period.
In contrast to the effects of observed following chronic 4V infusions of OT (16 nmol/day) in male [
64] and female DIO rats, we found that chronic 4V infusions of OT at the same dose (16 nmol/day) in female C57BL/6J mice largely had no effect significant effects on T
IBAT in ad libitum fed mice when the data were averaged over weeks 1, 2, 3 and 4 of the infusion period (data not shown;
P=NS).
While largely similar results were obtained following a 4-h fast over weeks 1-3 (data not shown; P=NS), there was a tendency for chronic 4V OT to reduce TIBAT over week 4 (P=0.076) in 4-h fasted mice. Specifically, chronic 4V OT reduced TIBAT on infusion days 17, 20, and 25 (P<0.05) and tended to reduce TIBAT on infusion days 13, 23, and 24 (0.05<P<0.1).
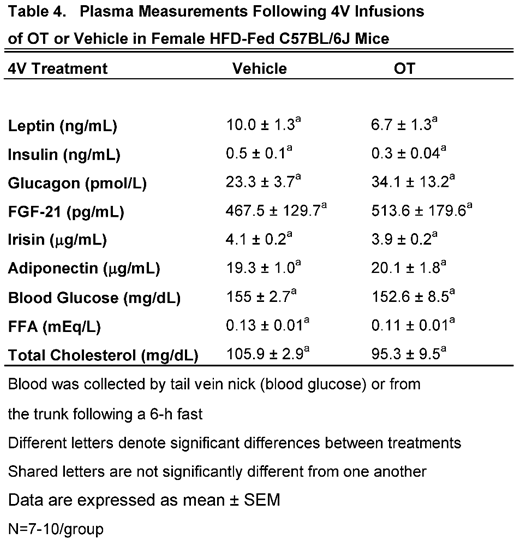
We characterized the endocrine and metabolic effects of chronic 4V OT (16 nmol/day) in female C57BL/6J mice (
Table 4). There were no significant differences in any of the plasma measurements between vehicle and 4V OT in female C57BL/6J mice.
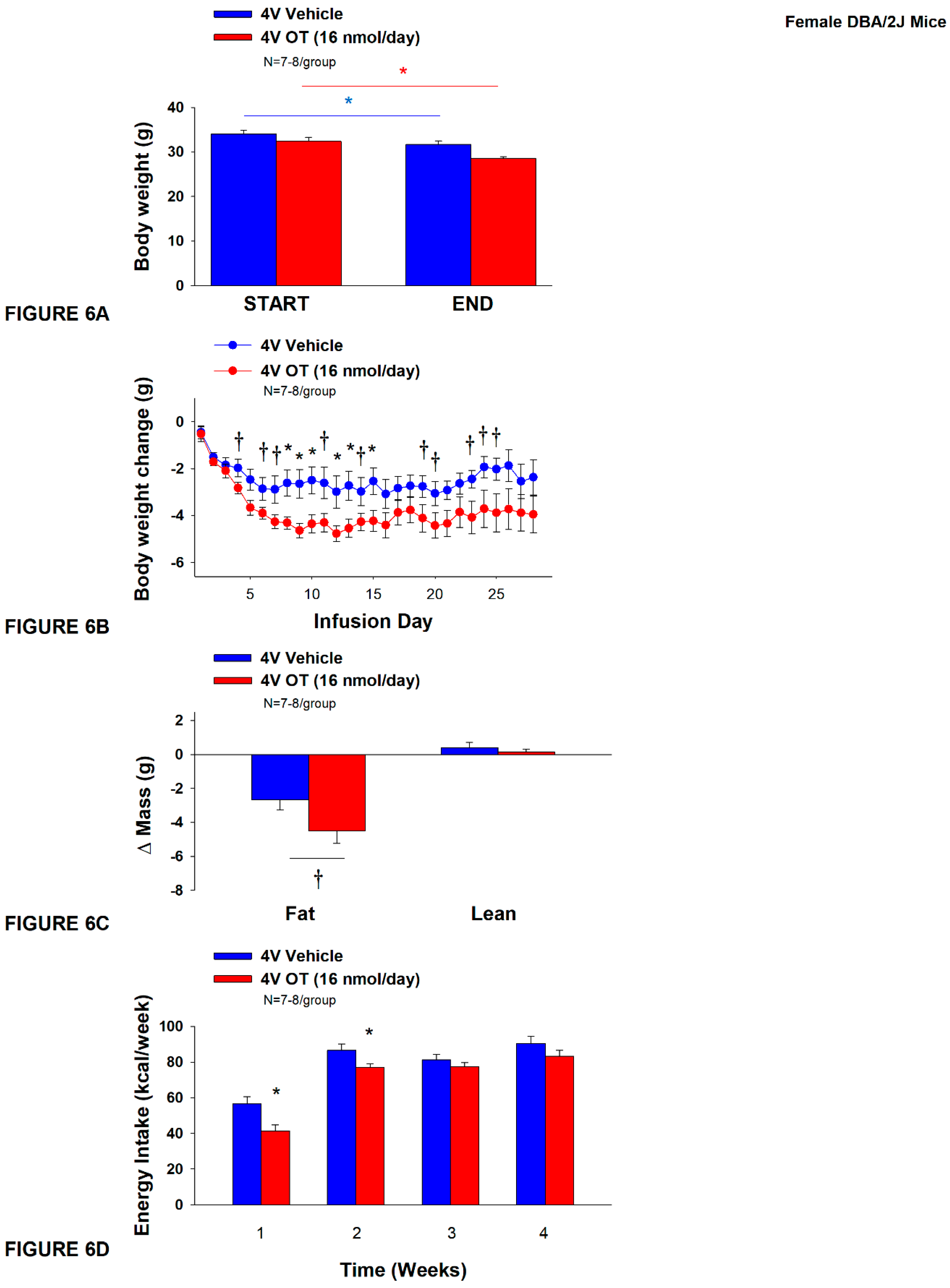
Study 4B: Determine the effects of chronic 4V OT treatment (16 nmol/day) on body weight, adiposity and energy intake in female DIO DBA/2J mice.
The goal of this study was to determine the susceptibility of female DBA/2J to DIO and whether the effects of chronic hindbrain (4V) administration to reduce body weight and adiposity could translate to another rodent model (female DBA/2J mice) that is susceptible to becoming DIO [
67,
68]. Initially, mice were lean as defined by both body weight (20.3±0.3 g) and adiposity (3.4±0.3 g fat mass; 16.8±1.1% adiposity). DBA/2J mice became DIO as determined by both body weight (35.8±0.9 g) and adiposity (15.1±0.8 g fat mass; 41.9±1.2% adiposity) after maintenance on the HFD for at least 4.5 months prior to being implanted with temperature transponders and minipumps as described earlier. There was no significant difference in body weight or adiposity between vehicle and oxytocin treatment groups at the beginning of the study prior to minipump implantation (
P=NS; data not shown). Four of the twenty mice available at study onset were euthanized during the course of the study and were excluded from the data analysis (including two whose head caps had become detached).
Unexpectedly, 4V vehicle resulted in 6.4±2.0% weight loss relative to vehicle pre-treatment [(F(1,7) = 12.781,
P=0.009)] whereas 4V OT reduced body weight by 11.9±2.1% relative to 4V OT pre-treatment [(F(1,6) = 24.802,
P=0.003)] (
Figure 6A). In addition, 4V OT treatment reduced weight gain on treatment days 8-10, 12-13, and 15 (
P<0.05) and tended to reduce weight gain on treatment days 4, 5 (P=0.050), 6-7, 11, 14, 19-21, and 23-25 (0.05<
P<0.1;
Figure 6B). OT tended to reduce relative fat mass (pre- vs post-intervention) (
Figure 6C; 0.05<
P<0.1) and total fat mass (P<0.05) but had no effect on adipocyte size (data not shown) or relative lean mass (pre- vs post-intervention) or total lean mass (
P=NS). These effects that were mediated, at least in part, by a modest reduction of energy intake that was evident during weeks 1 and 2 of the treatment period (
Figure 6D;
P<0.05). There was no effect of chronic 4V OT to significant increase kaolin consumption during the treatment period (
P=NS; data not shown).
We characterized the endocrine and metabolic effects of chronic systemic OT (16 and 50 nmol/day) in female DBA/2J mice (
Table 5). There were no significant differences in any of the plasma measurements between vehicle and 4V OT in female DBA/2J mice.
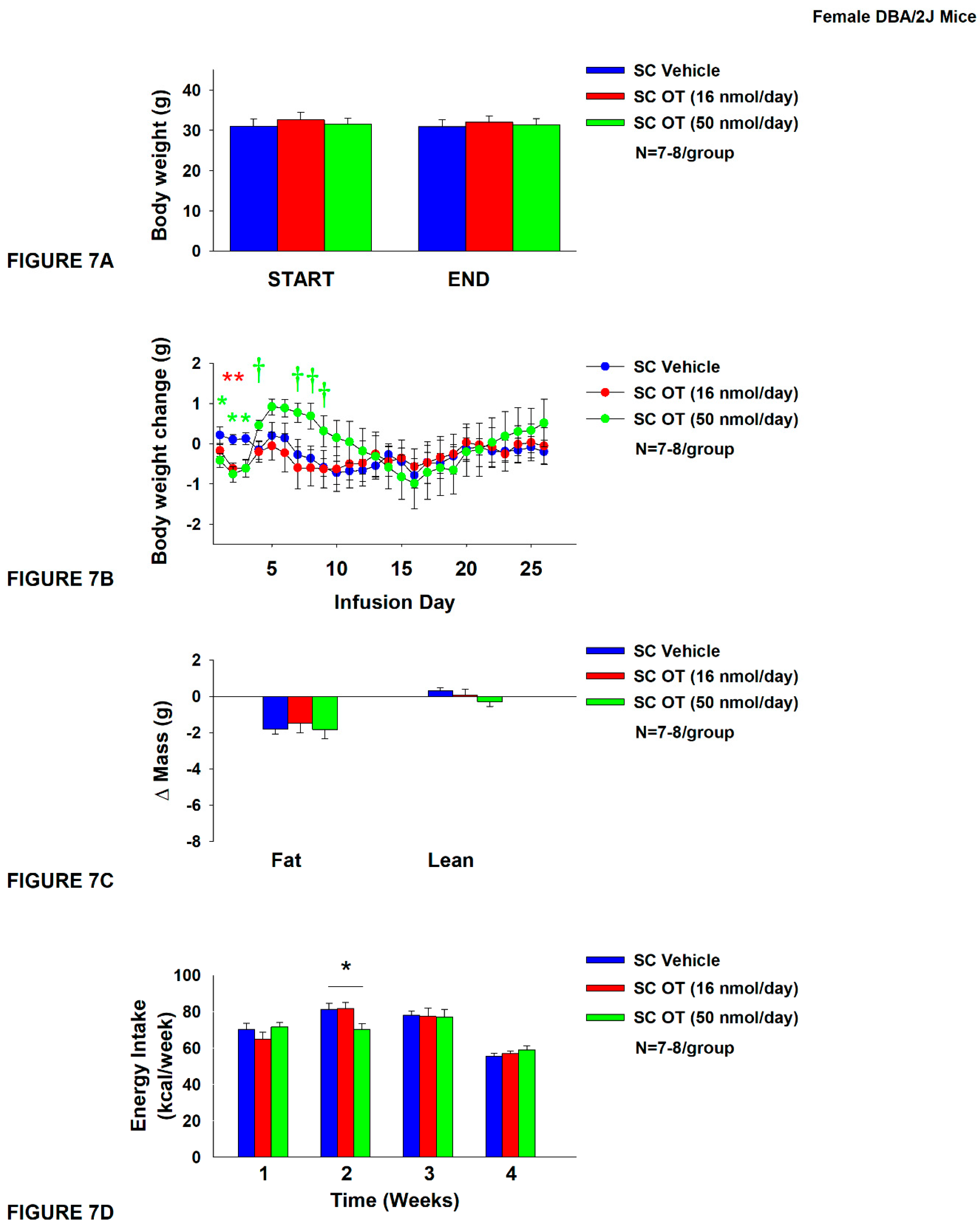
Study 5: Determine the effects of chronic systemic OT treatment (16 and 50 nmol/day) on body weight, adiposity and energy intake in female DIO DBA/2J mice
The goal of this study was to extend the findings from Study 4B and determine the extent to which systemic (subcutaneous) infusion of a centrally effective dose of OT (16 nmol/day) can reduce body weight and adiposity in female DBA/2J mice (same strain used in Study 4B). DBA/2J mice became DIO as determined by both body weight (32.2±0.9 g) and adiposity (12.7±0.7 g fat mass; 38.7±1.2% adiposity) after maintenance on the HFD for at least 4.5 months prior to being implanted with temperature transponders. There was no significant difference in body weight or adiposity between vehicle and oxytocin treatment groups at the beginning of the study prior to minipump implantation (P=NS; data not shown).
In contrast to chronic 4V OT treatment in female DBA/2J mice (
Study 4B), chronic SC OT treatment did not result in a significant reduction of body weight (
Figure 7A). SC OT treatment (16 nmol/day) reduced weight gain on treatment days 2-3 (
P<0.05) and tended to reduce weight gain on treatment day 1 (P=0.138;
Figure 7B). The higher dose (50 nmol/day) also reduced weight gain on treatment days 1-3 and tended to increase body weight gain on treatment days 4 (
P=0.055), 5 (
P=0.106), 7 (
P=0.077), 8 (
P=0.051) and 9 (
P=0.092) (
Figure 7B). There was no effect of SC OT at either dose on relative fat mass or lean mass (pre- vs post-intervention) (
Figure 7C). OT (50 nmol/day) produced a transient reduction of energy intake during week 2 (
Figure7D;
P<0.05) but OT failed to impact energy intake at any other time. There was also no effect of chronic SC OT to significant increase kaolin consumption during weeks 2-4 of the treatment period (
P=NS; data not shown) but a slight reduction of kaolin intake during week 1 in response to the higher dose 50 nmol/day (
P=0.016; data not shown).
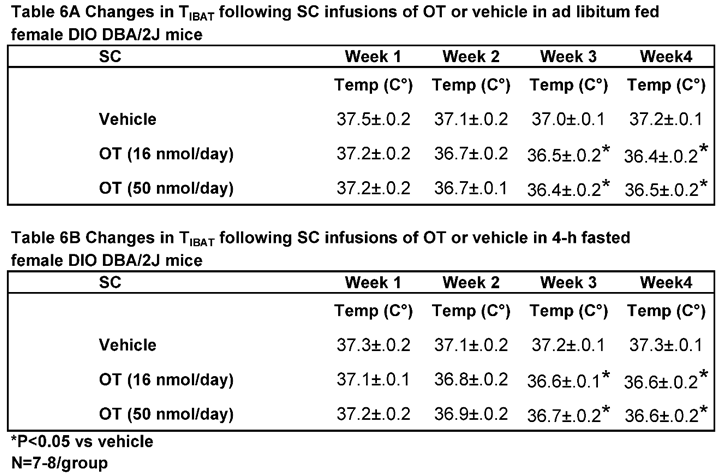
In contrast to chronic 4V administration, chronic systemic administration of OT (16 and 50 nmol/day) reduced T
IBAT relative to vehicle in ad libitum fed mice when the data were averaged over weeks 3 and 4 (
Table 6A;
P<0.05) of the infusion period. Similar results were obtained from the same mice following a 4-h fast over the same period (
Table 6B;
P<0.05).
In addition to the findings in DIO female DBA/2J mice, we found that there appeared to be a very modest effect of chronic systemic OT (16 nmol/day) to reduce TIBAT in 4-h fasted male DIO (C57Bl/6J) mice on infusion day 9 (P<0.05) and tended to reduce TIBAT on days 10 (0.05<P<0.1), 14 (0.05<P<0.1), and 21 (P=0.05) (data not shown; unpublished findings). Likewise, OT (50 nmol/day) tended to reduce TIBAT in 4-h fasted C57Bl/6J mice on infusion day 4 (P<0.05) and tended to reduce TIBAT on infusion day 21 (0.05<P<0.1). In contrast, we found that OT (100 nmol/day) tended to increase TIBAT on infusion day 13 (0.05<P<0.1), but this was only evident in ad libitum fed C57Bl/6J mice but not in 4-h fasted mice.
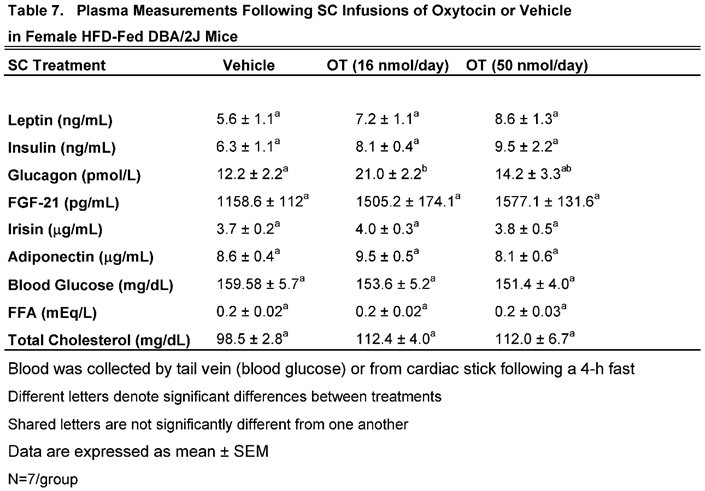
We characterized the endocrine and metabolic effects of chronic systemic OT (16 and 50 nmol/day) in female DBA/2J mice (
Table 7). There was a significant increase in plasma glucagon in response to SC OT (16 nmol/day) in female DBA/2J mice. There tended to be a non-significant increase in total cholesterol in response to systemic infusion of OT at both 16 (
P=0.057) and 50 nmol/day (
P=0.062). In addition, OT (50 nmol/day) also tended to produce a non-significant increase in plasma leptin (
P=0.092). and FGF-21 (P=0.051).
Discussion
The goal of the current studies was to 1) determine if sympathetic innervation of IBAT is required for hindbrain (4V) OT to increase non-shivering BAT thermogenesis and reduce body weight and adiposity in female HFD-fed rats and 2) determine if the ability of hindbrain (4V) infusion of OT to elicit weight loss translates to other rodent species. We determined the effect of disrupting SNS activation of IBAT on OT-elicited stimulation of TIBAT and reduction of body weight in DIO rats. We first measured the impact of bilateral surgical SNS denervation to IBAT on the ability of acute 4V OT (0.5, 1, and 5 µg) to stimulate TIBAT in female HFD-fed rats. We found that the high dose of 4V OT (5 µg) stimulated TIBAT similarly between sham rats and denervated rats. We subsequently determined if OT-elicited reductions of body weight and adiposity require intact SNS outflow to IBAT. To accomplish this, we determined the effect of bilateral surgical or sham denervation of IBAT on the ability of chronic 4V OT (16 nmol/day) or vehicle administration to reduce body weight, adiposity and food intake in female HFD-fed rats. Chronic 4V OT reduced body weight gain (sham: -18.0±4.9 g; denervation: -15.9±3.7 g) and adiposity (sham: -13.9±3.7 g; denervation: -13.6±2.4 g) relative to vehicle treatment and these effects were similar between groups. These effects were attributed, in part, to reduced energy intake evident during weeks 2 and 3. To test whether the effects of 4V OT to elicit weight loss translate to other female rodent species, we also examined the effect of chronic 4V infusion of OT on body weight in two separate strains of female HFD-fed mice. Similar to what we found in the HFD-fed rat model, we also found that chronic 4V OT (16 nmol/day) infusion resulted in reduced body weight gain, adiposity and/or energy intake in female HFD-fed C57BL/6J and DBA/2J mice. Together, these findings support the hypothesis that sympathetic innervation of IBAT is not necessary for OT-elicited increases in BAT thermogenesis and reductions of body weight and adiposity in female HFD-fed rats.
We have now determined that chronic 4V OT-elicited reduction of body weight loss does not require SNS innervation of IBAT in both female HFD-fed rats and in male DIO mice [
32]. These data suggest that 4V administration of OT increases BAT thermogenesis and reduces body weight through a mechanism that does not require SNS innervation of IBAT in both male and female rodent models. As mentioned in [
32], one remaining question is what mechanism is required for 4V OT to stimulate BAT thermogenesis that does not require SNS innervation to IBAT. We have largely ruled out the possibility that, in mice, 4V OT might be leaking into the periphery to act at peripheral OTRs by showing that peripheral administration of OT, at a centrally effective dose was unable to reproduce the effects of 4V OT on weight loss and BAT thermogenesis in DIO mice [
32]. One other possibility that we did not explore in this paper is whether activation of OTRs within the hindbrain and/or spinal cord might stimulate the release of epinephrine from the adrenal gland (adrenal medulla) and activate BAT thermogenesis. We recently found that administration of the β3-AR antagonist, SR 59230A, failed to block the effects of acute 4V OT to increase T
IBAT (unpublished findings) in male DIO Long-Evans rats suggesting that signaling through the β3-AR is not required for OT-elicited BAT thermogenesis. Other potential mediators of 4V-OT elicited BAT thermogenesis include the β1-AR and β2-AR, both of which are expressed in IBAT in both mice [
69] and rats [
70,
71] (for review see [
72]). While we did not find a significant increase in β1-AR mRNA expression in response to 4V OT in IBAT in this study, we did see an increase in β1-AR mRNA expression in IWAT (see discussion below). The β1-AR is important in the control of thermogenesis in mice [
73] and rats [
74] while the β2-AR may be less important in the control of thermogenesis in rodents compared to humans [
75,
76,
77]. In addition, both receptors have nearly equal affinity for L-epinephrine in CHO cells [
78] and epinephrine application to brown adipocytes increases respiration and stimulation of fatty acids [
79]. Although epinephrine deficient mice are still able to maintain body temperature in response to the cold, there is an inability to upregulate the thermogenic genes, UCP-1 and PGC1-alpha, in IBAT, indicating that epinephrine could be important in the control of mitochondrial uncoupling in IBAT [
80]. However, despite the hindbrain and spinal cord being relay sites in multi-synaptic projections to the adrenal gland [
81,
82], only 1% of magnocellular or parvocellular PVN OT neurons are known to have multi-synaptic projections to the adrenal gland [
81]. It will be important to determine the extent to which adrenal demodulation impacts the ability of 4V OT to stimulate BAT thermogenesis and elicit weight loss in DIO rodents.
Our finding that 4V OT treatment elicited an increase in both β1-AR and Cidea mRNA expression in IWAT raises the possibility that WAT browning may also contribute, in part, to the metabolic effects of 4V OT in female rodents. Hindbrain OT might be activating OTRs that could be the recipient of shared and/or distinct neural proejections that originate from the parvocellular PVN (pPVN) and control SNS outflow to IWAT and IBAT [
83]. There are known multi-synaptic relays from parvocellular PVN (pPVN) OT neurons to IWAT [
84,
85], EWAT [
85,
86] and IBAT [
84,
87]. In addition, there appears to be overlap within a subpopulation of pPVN OT neurons that project to both IWAT and IBAT [
84]. Thus, OT neurons within the pPVN are anatomically situated to control both WAT and BAT thermogenesis, respectively. It remains to be determined if these effects are mediated by the same set of pPVN OT neurons or through separate, nonoverlapping pPVN OT neurons that directly innervate the hindbrain (nucleus tractus solitarius [
88,
89]) and/or spinal cord [
89], both of which are regions capable of impacting SNS outflow to IBAT and BAT thermogenesis [
90,
91]. Further studies that determine the extent to which 4V OT treatment 1) elicits more functional changes in IWAT thermogenesis (increased temperature of IWAT) [
83,
92] and 2) reduces body weight and adiposity in animals following IWAT denervation will be helpful in assessing the role of WAT in contributing to the effects of 4V OT to reduce body weight and adiposity.
We recognize the possibility that the effects of 4V OT on BAT thermogenesis could be secondary to increased locomotor activity or spontaneous physical activity-induced thermogenesis [
21] and/or non-shivering and shivering thermogenesis in skeletal muscle [
22]. We recently found that acute injections of OT (5 μg) into the 4V increased T
IBAT, core temperature and gross motor activity in male DIO rats (unpublished observations). However, 4V OT-associated elevations of T
IBAT and core temperature occurred prior to significant increases in gross motor activity suggesting that changes in gross motor activity are not likely a contributing factor to changes in T
IBAT and core temperature at least at the earlier time points. Others have also found that acute CNS administration increase activity in rodents. Sakamoto found that ICV injection of OT (0.5 μg) stimulated activity in mice [
93]. Furthermore, Noble found that ventromedial hypothalamic administration of OT (1 nmol ≈ 1.0072 μg) also increased short-term physical activity in rats [
15]. Thus, our unpublished findings in male DIO rats suggest that locomotor activity does not contribute to the effects of 4V OT on BAT thermogenesis. Whether this holds true in female HFD-fed rats remains to be determined.
We acknowledge the possibility that other BAT depots may contribute to the effects of 4V OT on BAT and weight loss in IBAT denervated rats. The focus of our study was on the specific contribution of IBAT because it contains up to 45% of total UCP-1 [
94] and represents ≥70% of total BAT mass [
95] and this particular depot is the best characterized of BAT depots [
96]. However, other BAT depots [axillary (subscapular), cervical, mediastinal and perirenal depots] show cold-induced elevations of UCP-1 [
97]. In particular, the axillary (subscapular), cervical, periaortic and perirenal BAT depots [
19,
98] may provide up to 50% of total UCP-1 mRNA. Fischer reported that the axillary (subscapular) depot, also showed a significant 2-fold increase of total UCP-1 (UCP-1/scBAT) in response to HFD (diet-induced thermogenesis) in IBAT denervated mice [
94]. There also appeared to be an increase of axillary UCP-1 in response to HFD in sham mice but it was not significant and there were no significant differences in UCP-1 between sham vs denervation groups in response to HFD [
94]. Moreover, beiging of WAT may also contribute to the effects of OT to reduce body weight and increase BAT thermogenesis. Beige depots within WAT may also account for up to 5% of total UCP-1 [
97,
98]. Moreover, Nugyen reported that is potential crosstalk between SNS circuits that innervate IBAT and WAT [
83]. Nguyen found that there is increased NE turnover and IWAT UCP-1 mRNA expression in hamsters following SNS denervation of IBAT [
83]. It will be helpful to develop a model to selectively denervate other BAT and WAT depots in order to more fully understand the extent to which these depots may contribute, in part, to the effects of 4V OT to reduce body weight.
In contrast to the elevation of core temperature and increased IBAT gene expression that Yuan found in response to higher doses of systemic infusions of OT (100 nmol/day) in male HFD-fed mice (C57BL6/J) [
35], we found that systemic infusion (16 and 50 nmol/day) resulted in a reduction of T
IBAT temperature in female DBA/2J mice. Similarly, we and others have found that acute peripheral administration of OT (5 and 10 μg/μL) elicited an initial reduction of T
IBAT prior to a subsequent elevation of T
IBAT. In addition, others have found that an acute
peripheral injection of a higher dose of OT (1 mg/kg) resulted in a profound hypothermic response [
99]
. These hypothermic effects in response to peripheral OT are thought to be mediated, in part, through a arginine vasopressin receptor 1A (AVPR1A) signaling, as pretreatment with a AVPR1A antagonist can reduce OT-mediated hypothermia, while pretreatment with OTR antagonist does not [
100]
. It is not clear whether the differences between our study and Yuan’s study can be explained, in part, by strain, sex, age, length of time that the mice were maintained on the HFD prior to study onset (8 weeks rather than 18 weeks in our study) and/or time of day that the core temperature vs T
IBAT measurements were taken. It will be helpful to include measurements of both core temperature and T
IBAT from the same animals in future studies.
Based on findings from Liu [
10], it is possible that differences in estrus cycle might have impaired the effectiveness of OT to reduce food intake during the measurement period. Liu found that there was an impaired ability of ICV OT to reduce food intake during the pro-estrus stage of the estrus cycle, during which time there is an increase in estrogen [
10]. Despite this, we still found an effect of 4V OT to reduce weight gain suggesting that other mechanisms (i.e. lipolysis, energy expenditure) may also contribute to 4V OT-elicited changes in body weight in female rodents. Future studies, however, should take into account estrus cycle when measuring energy intake in response to OT treatment.
Our findings showing that chronic 4V administration of OT reduced energy intake in female DBA/2J DIO mice recapitulated the effects that Maejima found following chronic systemic administration in female DIO C57BL/6J mice [
101]. However, we failed to find an effect of chronic 4V OT to reduce food intake in female DIO C57BL/6J mice. In addition, we found that systemic OT (16 or 50 nmol/day) produced transient reductions of body weight gain in female DIO DBA/2J mice at doses that Maejima (≈ 27.6 and 55.1 nmol/day) found to reduce body weight in female DIO C57BL/6J mice [
101]. However, Maejima and colleagues study used a different strain of mice (C57BL/6J) that were younger (18 weeks vs 31 weeks at onset of minipump infusions in our study), heavier (34.20 grams vs 31.2±1.4 g in our study) and had been on the HFD diet for a shorter period of time (12 weeks vs 24 weeks at onset of minipump infusions in our study). Thus, there are several differences between studies that might account for the contradictory effects.
In summary, our findings demonstrate that there is no obvious change or functional impairment in the response of the β3-AR agonist, CL 316243, to activate IBAT in female rats with impaired SNS innervation of IBAT in comparison to female sham-operated rats. In addition, we found that acute 4V injections of the low (0.5 µg) and high dose of OT (5 µg) produced comparable increases in TIBAT at in female sham and IBAT denervated rats. We subsequently found that chronic 4V OT (16 nmol/day) produced similar reductions of body weight gain and adiposity in female sham and IBAT denervated rats. Similar to what we found in the HFD-fed rat model, we also found that chronic 4V OT (16 nmol/day) infusion resulted in reduced body weight gain, adiposity and energy intake in female DIO C57BL/6J and DBA/2J mice relative to vehicle treated control mice. Together, these findings suggest that 1) sympathetic innervation of IBAT is not necessary for OT-elicited increases in BAT thermogenesis and weight loss in female HFD-fed rats and 2) the effects of OT to elicit weight loss translate to other mouse models of DIO.
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Figure 1.
A-D. Effect of systemic β3-AR agonist (CL 31643) administration (0.1 and 1 mg/kg) on IBAT temperature (TIBAT), energy intake and body weight post-sham or IBAT denervation in female HFD-fed rats. Rats were maintained on HFD (60% kcal from fat; N=4-8/group) for approximately 4.5 months prior to undergoing a sham or bilateral surgical IBAT denervation and implantation of temperature transponders underneath IBAT. Animals were subsequently adapted to a 4-h fast prior to receiving IP injections of CL 316243 (0.1 or 1 mg/kg, IP) or vehicle (sterile water) where each animal received each treatment at approximately 7-day intervals. A/B, Effect of CL 316243 on TIBAT in A) sham operated or B) IBAT denervated DIO rats; C, Effect of CL 316243 on change in energy intake in sham or IBAT denervated DIO rats; D, Effect of CL 316243 on change in body weight in sham or IBAT denervated DIO rats. Data are expressed as mean ± SEM. *P<0.05 CL 316243 vs. vehicle.
Figure 1.
A-D. Effect of systemic β3-AR agonist (CL 31643) administration (0.1 and 1 mg/kg) on IBAT temperature (TIBAT), energy intake and body weight post-sham or IBAT denervation in female HFD-fed rats. Rats were maintained on HFD (60% kcal from fat; N=4-8/group) for approximately 4.5 months prior to undergoing a sham or bilateral surgical IBAT denervation and implantation of temperature transponders underneath IBAT. Animals were subsequently adapted to a 4-h fast prior to receiving IP injections of CL 316243 (0.1 or 1 mg/kg, IP) or vehicle (sterile water) where each animal received each treatment at approximately 7-day intervals. A/B, Effect of CL 316243 on TIBAT in A) sham operated or B) IBAT denervated DIO rats; C, Effect of CL 316243 on change in energy intake in sham or IBAT denervated DIO rats; D, Effect of CL 316243 on change in body weight in sham or IBAT denervated DIO rats. Data are expressed as mean ± SEM. *P<0.05 CL 316243 vs. vehicle.
Figure 2.
A-B: Effect of acute 4V OT administration (1 and 5 μg) on TIBAT post-sham or IBAT denervation in female HFD-fed rats. Rats were maintained on HFD (60% kcal from fat; N=4-8/group) for approximately 4.5 months prior to undergoing a sham or bilateral surgical IBAT denervation and implantation of temperature transponders underneath IBAT. Rats were subsequently implanted with 4V cannulas and allowed to recover for 2 weeks prior to receiving acute 4V injections of OT or vehicle. Animals were subsequently adapted to a 4-h fast prior to receiving acute 4V injections of OT or vehicle A/B, Effect of acute 4V OT on TIBAT in A) sham operated or B) IBAT denervated DIO rats. Data are expressed as mean ± SEM. *P<0.05, †0.05<P<0.1 OT vs. vehicle.
Figure 2.
A-B: Effect of acute 4V OT administration (1 and 5 μg) on TIBAT post-sham or IBAT denervation in female HFD-fed rats. Rats were maintained on HFD (60% kcal from fat; N=4-8/group) for approximately 4.5 months prior to undergoing a sham or bilateral surgical IBAT denervation and implantation of temperature transponders underneath IBAT. Rats were subsequently implanted with 4V cannulas and allowed to recover for 2 weeks prior to receiving acute 4V injections of OT or vehicle. Animals were subsequently adapted to a 4-h fast prior to receiving acute 4V injections of OT or vehicle A/B, Effect of acute 4V OT on TIBAT in A) sham operated or B) IBAT denervated DIO rats. Data are expressed as mean ± SEM. *P<0.05, †0.05<P<0.1 OT vs. vehicle.
Figure 3.
A-D: Effect of acute 4V OT administration (0.5 μg) on TIBAT post-sham or IBAT denervation in female HFD-fed rats. Rats were maintained on HFD (60% kcal from fat; N=4-8/group) for approximately 4.5 months prior to undergoing a sham or bilateral surgical IBAT denervation and implantation of temperature transponders underneath IBAT. Rats were subsequently implanted with 4V cannulas and allowed to recover for 2 weeks prior to receiving acute 4V injections of OT or vehicle. Animals were subsequently adapted to a 4-h fast prior to receiving acute 4V injections of OT or vehicle A/B, Effect of acute 4V OT on TIBAT in A) sham operated or B) IBAT denervated DIO rats. Data are expressed as mean ± SEM. *P<0.05, †0.05<P<0.1 OT vs. vehicle.
Figure 3.
A-D: Effect of acute 4V OT administration (0.5 μg) on TIBAT post-sham or IBAT denervation in female HFD-fed rats. Rats were maintained on HFD (60% kcal from fat; N=4-8/group) for approximately 4.5 months prior to undergoing a sham or bilateral surgical IBAT denervation and implantation of temperature transponders underneath IBAT. Rats were subsequently implanted with 4V cannulas and allowed to recover for 2 weeks prior to receiving acute 4V injections of OT or vehicle. Animals were subsequently adapted to a 4-h fast prior to receiving acute 4V injections of OT or vehicle A/B, Effect of acute 4V OT on TIBAT in A) sham operated or B) IBAT denervated DIO rats. Data are expressed as mean ± SEM. *P<0.05, †0.05<P<0.1 OT vs. vehicle.
Figure 4.
A-D: Effect of chronic 4V OT infusions (16 nmol/day) on body weight, adiposity and energy intake post-sham or IBAT denervation in female HFD-fed rats.A, Rats were maintained on HFD (60% kcal from fat; N=7-8/group) for approximately 4.75-5.25 months prior to undergoing a sham or bilateral surgical IBAT denervation. Rats were subsequently implanted with 4V cannulas and allowed to recover for 2 weeks prior to being implanted with subcutaneous minipumps that were subsequently attached to the 4V cannula. A, Effect of chronic 4V OT or vehicle on body weight in sham operated or IBAT denervated DIO rats; B, Effect of chronic 4V OT or vehicle on body weight change in sham operated or IBAT denervated DIO rats; C, Effect of chronic 4V OT or vehicle on adiposity in sham operated or IBAT denervated DIO rats; D, Effect of chronic 4V OT or vehicle on adiposity in sham operated or IBAT denervated DIO rats. Data are expressed as mean ± SEM. *P<0.05, †0.05<P<0.1 OT vs. vehicle.
Figure 4.
A-D: Effect of chronic 4V OT infusions (16 nmol/day) on body weight, adiposity and energy intake post-sham or IBAT denervation in female HFD-fed rats.A, Rats were maintained on HFD (60% kcal from fat; N=7-8/group) for approximately 4.75-5.25 months prior to undergoing a sham or bilateral surgical IBAT denervation. Rats were subsequently implanted with 4V cannulas and allowed to recover for 2 weeks prior to being implanted with subcutaneous minipumps that were subsequently attached to the 4V cannula. A, Effect of chronic 4V OT or vehicle on body weight in sham operated or IBAT denervated DIO rats; B, Effect of chronic 4V OT or vehicle on body weight change in sham operated or IBAT denervated DIO rats; C, Effect of chronic 4V OT or vehicle on adiposity in sham operated or IBAT denervated DIO rats; D, Effect of chronic 4V OT or vehicle on adiposity in sham operated or IBAT denervated DIO rats. Data are expressed as mean ± SEM. *P<0.05, †0.05<P<0.1 OT vs. vehicle.
Figure 5.
A-D: Effect of chronic 4V OT infusions (16 nmol/day) on body weight, adiposity and energy intake in female HFD-fed C57BL/6J mice.A, Mice were maintained on HFD (60% kcal from fat; N=7-10/group) for approximately 4.5 months prior to implantation of temperature transponders underneath IBAT. Mice were subsequently implanted with 4V cannulas and allowed to recover for 2 weeks prior to being implanted with subcutaneous minipumps that were subsequently attached to the 4V cannula. A, Effect of chronic 4V OT or vehicle on body weight in female C57BL/6J mice rats; B, Effect of chronic 4V OT or vehicle on body weight change in female C57BL/6J mice; C, Effect of chronic 4V OT or vehicle on adiposity in female C57BL/6J mice; D, Effect of chronic 4V OT or vehicle on adiposity in female C57BL/6J mice. Data are expressed as mean ± SEM. *P<0.05, †0.05<P<0.1 OT vs. vehicle.
Figure 5.
A-D: Effect of chronic 4V OT infusions (16 nmol/day) on body weight, adiposity and energy intake in female HFD-fed C57BL/6J mice.A, Mice were maintained on HFD (60% kcal from fat; N=7-10/group) for approximately 4.5 months prior to implantation of temperature transponders underneath IBAT. Mice were subsequently implanted with 4V cannulas and allowed to recover for 2 weeks prior to being implanted with subcutaneous minipumps that were subsequently attached to the 4V cannula. A, Effect of chronic 4V OT or vehicle on body weight in female C57BL/6J mice rats; B, Effect of chronic 4V OT or vehicle on body weight change in female C57BL/6J mice; C, Effect of chronic 4V OT or vehicle on adiposity in female C57BL/6J mice; D, Effect of chronic 4V OT or vehicle on adiposity in female C57BL/6J mice. Data are expressed as mean ± SEM. *P<0.05, †0.05<P<0.1 OT vs. vehicle.
Figure 6.
A-D: Effect of chronic 4V OT infusions (16 nmol/day) on body weight, adiposity and energy intake in female HFD-fed DBA/2J mice.A, Mice were maintained on HFD (60% kcal from fat; N=7-8/group) for approximately 4.5 months prior to implantation of temperature transponders underneath IBAT. Mice were subsequently implanted with 4V cannulas and allowed to recover for 2 weeks prior to being implanted with subcutaneous minipumps that were subsequently attached to the 4V cannula. A, Effect of chronic 4V OT or vehicle on body weight in female DBA/2J mice; B, Effect of chronic 4V OT or vehicle on body weight change in female DBA/2J mice; C, Effect of chronic 4V OT or vehicle on adiposity in female DBA/2J mice; D, Effect of chronic 4V OT or vehicle on adiposity in female DBA/2J mice. Data are expressed as mean ± SEM. *P<0.05, †0.05<P<0.1 OT vs. vehicle.
Figure 6.
A-D: Effect of chronic 4V OT infusions (16 nmol/day) on body weight, adiposity and energy intake in female HFD-fed DBA/2J mice.A, Mice were maintained on HFD (60% kcal from fat; N=7-8/group) for approximately 4.5 months prior to implantation of temperature transponders underneath IBAT. Mice were subsequently implanted with 4V cannulas and allowed to recover for 2 weeks prior to being implanted with subcutaneous minipumps that were subsequently attached to the 4V cannula. A, Effect of chronic 4V OT or vehicle on body weight in female DBA/2J mice; B, Effect of chronic 4V OT or vehicle on body weight change in female DBA/2J mice; C, Effect of chronic 4V OT or vehicle on adiposity in female DBA/2J mice; D, Effect of chronic 4V OT or vehicle on adiposity in female DBA/2J mice. Data are expressed as mean ± SEM. *P<0.05, †0.05<P<0.1 OT vs. vehicle.
Figure 7.
A-D: Effect of chronic systemic OT infusions (16 and 50 nmol/day) on body weight, adiposity and energy intake in female HFD-fed DBA/2J mice.A, Mice were maintained on HFD (60% kcal from fat; N=7-8/group) for approximately 4.5 months prior to implantation of temperature transponders underneath IBAT. Mice were subsequently implanted with 4V cannulas and allowed to recover for 2 weeks prior to being implanted with subcutaneous minipumps that were subsequently attached to the 4V cannula. A, Effect of chronic 4V OT or vehicle on body weight in female DBA/2J mice; B, Effect of chronic 4V OT or vehicle on body weight change in female DBA/2J mice; C, Effect of chronic 4V OT or vehicle on adiposity in female DBA/2J mice; D, Effect of chronic 4V OT or vehicle on adiposity in female DBA/2J mice. Data are expressed as mean ± SEM. *P<0.05, †0.05<P<0.1 OT vs. vehicle.
Figure 7.
A-D: Effect of chronic systemic OT infusions (16 and 50 nmol/day) on body weight, adiposity and energy intake in female HFD-fed DBA/2J mice.A, Mice were maintained on HFD (60% kcal from fat; N=7-8/group) for approximately 4.5 months prior to implantation of temperature transponders underneath IBAT. Mice were subsequently implanted with 4V cannulas and allowed to recover for 2 weeks prior to being implanted with subcutaneous minipumps that were subsequently attached to the 4V cannula. A, Effect of chronic 4V OT or vehicle on body weight in female DBA/2J mice; B, Effect of chronic 4V OT or vehicle on body weight change in female DBA/2J mice; C, Effect of chronic 4V OT or vehicle on adiposity in female DBA/2J mice; D, Effect of chronic 4V OT or vehicle on adiposity in female DBA/2J mice. Data are expressed as mean ± SEM. *P<0.05, †0.05<P<0.1 OT vs. vehicle.
Table 1.
Plasma measurements following acute injections of 4V OT (5 μg/μL) or vehicle in female sham and IBAT denervated DIO rats. Data are expressed as mean ± SEM. *P<0.05 OT vs. vehicle (N=2-3/group).
Table 1.
Plasma measurements following acute injections of 4V OT (5 μg/μL) or vehicle in female sham and IBAT denervated DIO rats. Data are expressed as mean ± SEM. *P<0.05 OT vs. vehicle (N=2-3/group).
Table 2.
Changes in TIBAT following 4V infusions of OT or vehicle in female sham or IBAT denervated DIO rats. A, Changes in TIBAT following 4V infusions of OT or vehicle in ad libitum fed female sham or IBAT denervated DIO rats; B, Changes in TIBAT following 4V infusions of OT or vehicle in 4-h fasted female sham or IBAT denervated DIO rats. Shared letters are not significantly different from one another. Data are expressed as mean ± SEM. *P<0.05 OT, †0.05<P<0.1 OT vs. vehicle (N=7-8/group).
Table 2.
Changes in TIBAT following 4V infusions of OT or vehicle in female sham or IBAT denervated DIO rats. A, Changes in TIBAT following 4V infusions of OT or vehicle in ad libitum fed female sham or IBAT denervated DIO rats; B, Changes in TIBAT following 4V infusions of OT or vehicle in 4-h fasted female sham or IBAT denervated DIO rats. Shared letters are not significantly different from one another. Data are expressed as mean ± SEM. *P<0.05 OT, †0.05<P<0.1 OT vs. vehicle (N=7-8/group).
Table 3.
A-B. Changes in IBAT and IWAT gene expression following 4V infusions of OT or vehicle in female sham or IBAT denervated DIO rats. A, Changes in IBAT mRNA expression 4V infusions of OT or vehicle in female sham or IBAT denervated DIO rats; B, Changes in IWAT mRNA expression 4V infusions of OT or vehicle in female sham or IBAT denervated DIO rats. Shared letters are not significantly different from one another. Data are expressed as mean ± SEM. *P<0.05 OT, †0.05<P<0.1 OT vs. vehicle (N=7-8/group).
Table 3.
A-B. Changes in IBAT and IWAT gene expression following 4V infusions of OT or vehicle in female sham or IBAT denervated DIO rats. A, Changes in IBAT mRNA expression 4V infusions of OT or vehicle in female sham or IBAT denervated DIO rats; B, Changes in IWAT mRNA expression 4V infusions of OT or vehicle in female sham or IBAT denervated DIO rats. Shared letters are not significantly different from one another. Data are expressed as mean ± SEM. *P<0.05 OT, †0.05<P<0.1 OT vs. vehicle (N=7-8/group).
Table 4.
Plasma measurements following chronic 4V infusions of OT (16 nmol/day) or vehicle in female HFD-fed C57BL/6J mice. Data are expressed as mean ± SEM. *P<0.05 OT vs. vehicle (N=7-10/group).
Table 4.
Plasma measurements following chronic 4V infusions of OT (16 nmol/day) or vehicle in female HFD-fed C57BL/6J mice. Data are expressed as mean ± SEM. *P<0.05 OT vs. vehicle (N=7-10/group).
Table 5.
Plasma measurements following chronic 4V infusions of OT (16 nmol/day) or vehicle in female HFD-fed DBA/2J mice. Data are expressed as mean ± SEM. *P<0.05 OT vs. vehicle (N=7-8/group).
Table 5.
Plasma measurements following chronic 4V infusions of OT (16 nmol/day) or vehicle in female HFD-fed DBA/2J mice. Data are expressed as mean ± SEM. *P<0.05 OT vs. vehicle (N=7-8/group).
Table 6.
Changes in TIBAT following chronic systemic infusions of OT (16 and 50 nmol/day) or vehicle in female DBA/2J mice. A, Changes in TIBAT following chronic systemic infusions of OT (16 and 50 nmol/day) or vehicle in ad libitum fed female DBA/2J mice; B, Changes in TIBAT following chronic systemic infusions of OT (16 and 50 nmol/day) or vehicle in 4-h fasted female DBA/2J mice. Shared letters are not significantly different from one another. Data are expressed as mean ± SEM. *P<0.05 OT, †0.05<P<0.1 OT vs. vehicle (N=7-8/group).
Table 6.
Changes in TIBAT following chronic systemic infusions of OT (16 and 50 nmol/day) or vehicle in female DBA/2J mice. A, Changes in TIBAT following chronic systemic infusions of OT (16 and 50 nmol/day) or vehicle in ad libitum fed female DBA/2J mice; B, Changes in TIBAT following chronic systemic infusions of OT (16 and 50 nmol/day) or vehicle in 4-h fasted female DBA/2J mice. Shared letters are not significantly different from one another. Data are expressed as mean ± SEM. *P<0.05 OT, †0.05<P<0.1 OT vs. vehicle (N=7-8/group).
Table 7.
Plasma measurements following chronic systemic infusions of OT (16 and 50 nmol/day) or vehicle in female HFD-fed DBA/2J mice. Data are expressed as mean ± SEM. *P<0.05 OT vs. vehicle (N=7/group).
Table 7.
Plasma measurements following chronic systemic infusions of OT (16 and 50 nmol/day) or vehicle in female HFD-fed DBA/2J mice. Data are expressed as mean ± SEM. *P<0.05 OT vs. vehicle (N=7/group).