Submitted:
03 September 2024
Posted:
04 September 2024
You are already at the latest version
Abstract
Keywords:
Introduction
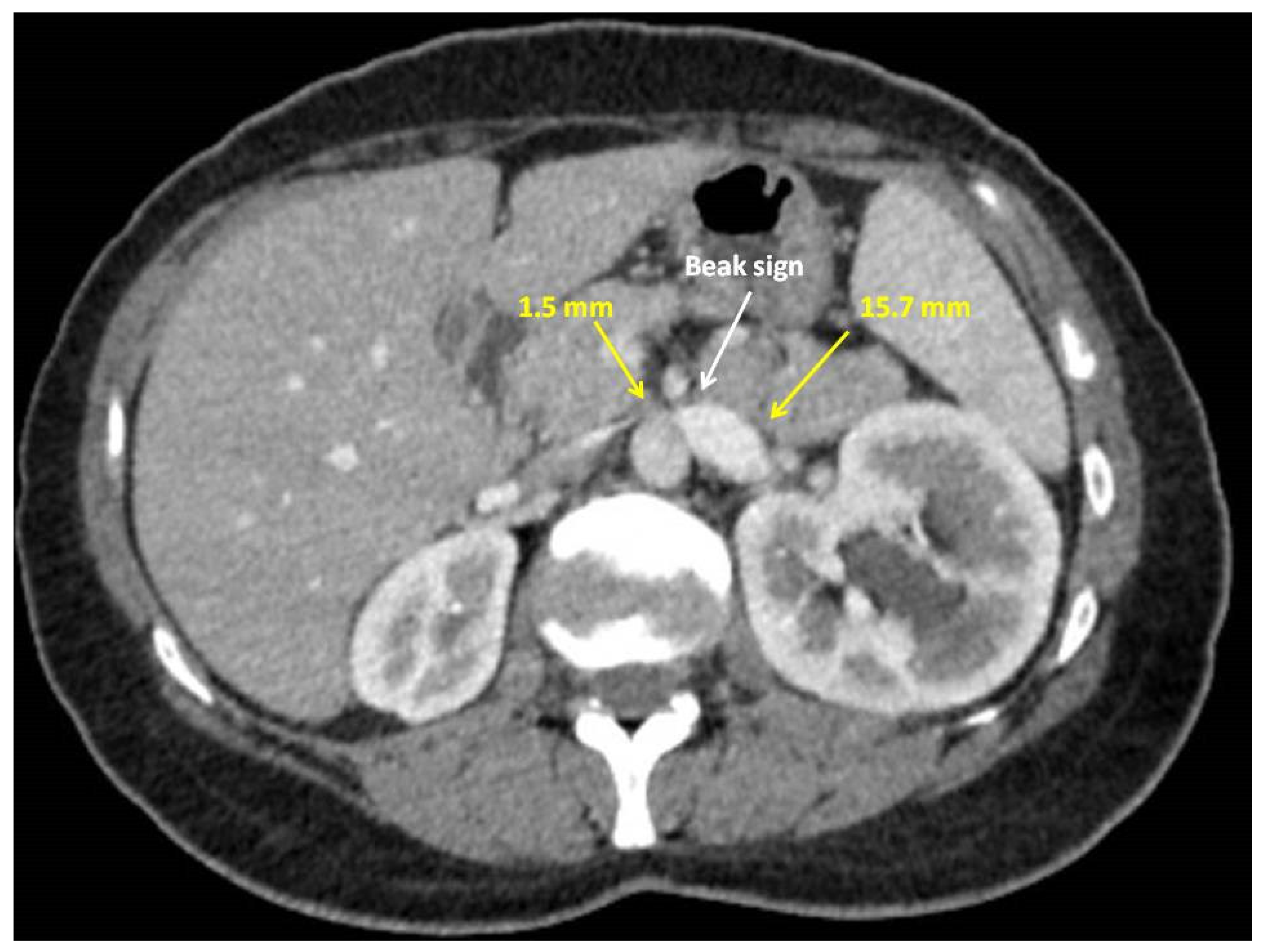
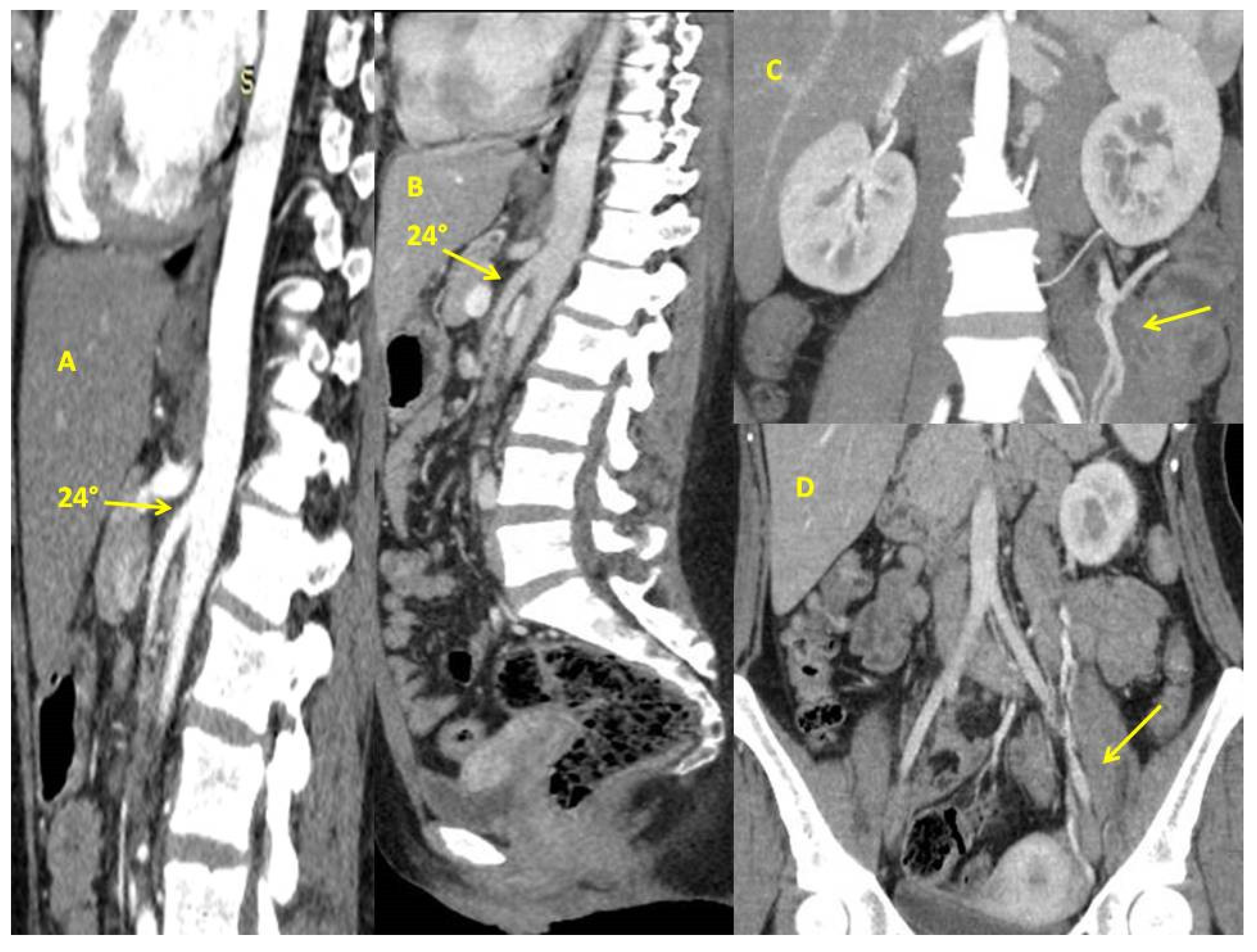
Case Report
Discussion
Conclusion
Financial Disclosure
Informed Consent Statement
Conflict of Interest
References
- de Los Reyes T, Keefe DT, Rickard M, Lorenzo AJ (2021) Diagnosis and therapeutic strategies for nutcracker syndrome. Curr Opin Urol 31:135–137. https://doi.org/10.1097/MOU.0000000000000831. [CrossRef]
- Dieleman F, Hamming JF, Erben Y, Van Der Vorst JR (2023) Nutcracker Syndrome: Challenges in Diagnosis and Surgical Treatment. Annals of Vascular Surgery 94:178–185. https://doi.org/10.1016/j.avsg.2023.03.030. [CrossRef]
- Kurklinsky AK, Rooke TW (2010) Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc 85:552–559. https://doi.org/10.4065/mcp.2009.0586. [CrossRef]
- Bookwalter CA, VanBuren WM, Neisen MJ, Bjarnason H (2019) Imaging Appearance and Nonsurgical Management of Pelvic Venous Congestion Syndrome. RadioGraphics 39:596–608. https://doi.org/10.1148/rg.2019180159. [CrossRef]
- Chait J, Sen I, Kalra M (2021) Nutcracker Syndrome: How to Diagnose It and When/How Should It Be Treated in the Pelvic Venous Disease Population. Tech Vasc Interv Radiol 24:100734. https://doi.org/10.1016/j.tvir.2021.100734. [CrossRef]
- Ananthan K, Onida S, Davies AH (2017) Nutcracker Syndrome: An Update on Current Diagnostic Criteria and Management Guidelines. Eur J Vasc Endovasc Surg 53:886–894. https://doi.org/10.1016/j.ejvs.2017.02.015. [CrossRef]
- Wang R-F, Zhou C-Z, Fu Y-Q, Lv W-F (2021) Nutcracker syndrome accompanied by hypertension: a case report and literature review. J Int Med Res 49:300060520985733. https://doi.org/10.1177/0300060520985733. [CrossRef]
- Ismailoglu T (2022) The Nutcracker Syndrome. J Radiol Case Rep 16:17–23. https://doi.org/10.3941/jrcr.v16i5.4339. [CrossRef]
- Granata A, Distefano G, Sturiale A, Figuera M, Foti PV, Palmucci S, Basile A (2021) From Nutcracker Phenomenon to Nutcracker Syndrome: A Pictorial Review. Diagnostics (Basel) 11:101. https://doi.org/10.3390/diagnostics11010101. [CrossRef]
- Kaur R, Airey D (2022) Nutcracker syndrome: A case report and review of the literature. Front Surg 9:984500. https://doi.org/10.3389/fsurg.2022.984500. [CrossRef]
- Berthelot J-M, Douane F, Maugars Y, Frampas E (2017) Nutcracker syndrome: A rare cause of left flank pain that can also manifest as unexplained pelvic pain. Joint Bone Spine 84:557–562. https://doi.org/10.1016/j.jbspin.2016.10.006. [CrossRef]
- Kolber MK, Cui Z, Chen CK, Habibollahi P, Kalva SP (2021) Nutcracker syndrome: diagnosis and therapy. Cardiovasc Diagn Ther 11:1140–1149. https://doi.org/10.21037/cdt-20-160. [CrossRef]
- Nakashima T, Sahashi Y, Kanamori H, Ohno Y, Okura H (2020) Localized solitary left renal vein thrombus complicating nutcracker syndrome: a case report and review of the literature. CEN Case Rep 9:252–256. https://doi.org/10.1007/s13730-020-00467-9. [CrossRef]
- Muheilan M, Walsh A, O’Brien F, Tuite D (2022) Nutcracker syndrome, conservative approach: a case report. J Surg Case Rep 2022:rjac423. https://doi.org/10.1093/jscr/rjac423. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




