1. Introduction
The cause of polycystic ovary syndrome (PCOS) is not fully understood, but it is believed to be a result of a combination of factors such as genetics, hormone imbalances, and external influences [
1]. This complex metabolic and endocrine disease can lead to several long-term health issues, including obesity, insulin resistance, and cardiovascular problems [
2]. PCOS is a major cause of female infertility and its occurrence has been on the rise in recent years [
3,
4]. Numerous studies have highlighted the significant relationship between the gut microbiome and human metabolism [
5]. Furthermore, research has shown that the structure of the intestinal flora plays a crucial role in the development and progression of various hormonal and metabolic disorders. Recent studies have revealed that the composition of gut microbiota in individuals with PCOS differs significantly from healthy women. These studies provide compelling evidence that disruptions in gut flora are closely intertwined with a range of clinical symptoms associated with PCOS, particularly obesity and insulin resistance [
2,
6]. This increased prevalence of obesity in PCOS patients exacerbates metabolic abnormalities, leading to disturbances in glucose and lipid metabolism. Such metabolic dysregulation has detrimental effects on the quality of oocytes and impairs the receptivity of the endometrium, further complicating fertility outcomes for women with PCOS [
3].
The gut microbiota, comprising approximately 10
14 microorganisms and commensals residing within the human intestinal tract, plays a crucial role in maintaining various physiological functions [
7]. Despite its recognized importance, our understanding of the precise mechanisms by which gut microbiota influences patients with PCOS remains incomplete [
2,
8,
9,
10]. One of the key functions of the gut microbiome is the breakdown of dietary fibers into short-chain fatty acids (SCFAs), with acetic acid (AA), propionic acid (PA), butyric acid (BA), and valeric acid (VA) being the primary SCFAs produced [
11,
12]. Among these, BA stands out due to its dual role: it serves as a crucial energy source for intestinal epithelial cells and exhibits significant anti-inflammatory properties [
13]. SCFAs collectively contribute to the maintenance of the intestinal mucosal barrier function and act as vital signaling molecules within the gut ecosystem [
14,
15]. Moreover, SCFAs are integral to metabolic processes that enhance glucose-stimulated insulin secretion, thereby improving insulin sensitivity. This enhancement in insulin sensitivity can be particularly beneficial for individuals with PCOS, as it aids in the regulation of peptide hormones that control appetite and modulates overall metabolic health [
16]. These insights underscore the necessity of further exploration into the gut microbiota’s role in PCOS, opening avenues for potential therapeutic interventions targeting microbial imbalances to ameliorate the condition’s metabolic and inflammatory symptoms.
In this study, we employ a precise and sensitive method using Gas Chromatography with Mass Spectrometry (GC/MS) to quantify SCFAs, specifically AA, PA, BA, and VA, in the fecal and blood samples of women diagnosed with PCOS. The GC/MS technique is renowned for its ability to accurately measure these SCFAs, which are crucial indicators in metabolic studies [
17,
18,
19]. We utilize a highly polar stationary phase column within the GC/MS system, which is particularly effective for the analysis of SCFAs without derivatization. Also, we apply liquid-liquid extraction [
20,
21] and acidification of the samples to ensure that the SCFAs were predominantly in their undissociated form, thereby increasing their hydrophobicity and volatility [
22,
23,
24].
Our primary objective is to elucidate the intricate relationship between the composition of the intestinal microbiota and the progression of PCOS. Furthermore, we aim to assess the efficacy of metformin therapy in modulating SCFA levels and potentially influencing the gut microbiome in women with PCOS. Metformin is well known for its metabolic effects [
25,
26], and exploring its potential impact on the gut microbiota and SCFA production represents a novel aspect of this investigation. By analyzing changes in SCFA profiles following metformin therapy, we aspire to uncover insights into the therapeutic mechanisms and potential benefits of metformin in the context of PCOS. Our approach includes a thorough examination of baseline SCFA levels, followed by a comparative analysis post-therapy to identify any significant shifts in SCFA concentrations and microbial composition.
Through this comprehensive study, we endeavor to contribute to a deeper understanding of the interplay between the gut microbiome, SCFAs, and PCOS pathology. The findings from this research may have significant implications for developing targeted interventions and personalized treatment strategies for women affected by PCOS, potentially leading to more effective and individualized therapeutic options.
2. Results and Discussion
2.1. Study Participants
The study included 69 patients with PCOS aged 25.3±5.9 years. The mean body mass index (BMI) was 24.8±5.2 kg/, with 42/69 (60.9%) patients of normal weight, 14/69 (20.3%) overweight, and 13/69 (18.9%) obese. The control group included 18 women without PCOS (26.6±5.0 years and BMI 24.4±4.8 kg/, ).
Clinical hyperandrogenism was present in 46/69 (66.7%) patients. Seventy-eight percent (53/69) of patients had biochemical hyperandrogenism. Elevated levels of total testosterone and free testosterone were observed in 37/69 (53.6%) and 24/69 (34.8%) patients, respectively, with elevated levels of androstenedione in 28/69 (40.6%) patients. In total, 41/69 (59.4%) patients had sex hormone (SHBG) binding globulin levels less than 50 nmol/L.
An ultrasound examination revealed morphological signs of PCOS in 60/69 (87.0%) patients. On average, the volume of each ovary was 12.7±3.2 , and the number of follicles was 26.8±18.8.
According to the glucose tolerance test (GTT), 8/69 (11.6%) patients had impaired glucose tolerance, with insulin resistance and hyperinsulinemia present in 25/69 (36.2%) and 27/69 (39.1%) patients, respectively. Dyslipidemia was detected in 20/69 (29.0%) cases.
Dual-energy X-ray absorptiometry (DXA) indicated an excess of total fat tissue in 55/69 (79.7%) patients. On average, the total fat tissue was 37.0±7.5%. Among these, 37/55 (67.3%) had excess visceral fat, averaging 540.7±482.9 g.
In patients with PCOS, the average levels of interleukin–6 (IL–6), tumor necrosis factor–alpha (TNF–), and C-reactive protein (CRP) in the blood serum were 1.3 (0.7; 2.0) pg/mL, 0.8 (0.2; 1.2) pg/mL, and 4.8 (3.3; 5.3) mg/L, respectively. These values were notably higher compared to the control group, which exhibited corresponding levels of 0.7 (0.2; 1.2) pg/mL, for IL–6, 0.4 (0.2; 0.9) pg/mL for TNF–, and 3.1 (2.8; 4.6) mg/L for CRP. Increased levels of CRP and IL–6 were observed in 26/69 (37.7%) and 25/69 (37.7%) of patients with PCOS, respectively. Notably, none of the control group participants showed elevated levels of these markers. Additionally, no increase in TNF– levels was detected in any patient, either in the PCOS group or the control group.
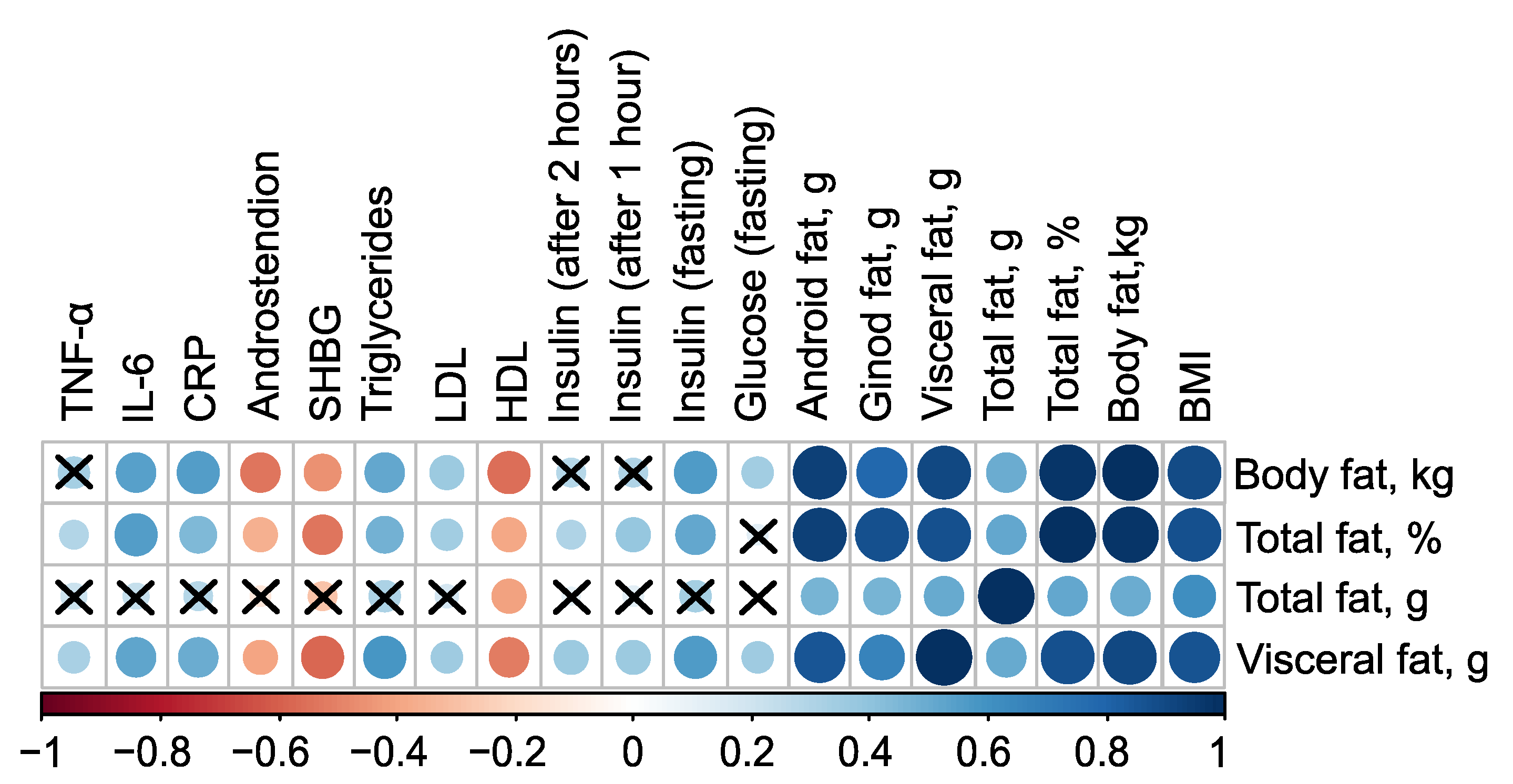
Figure 1 summarizes the statistically significant associations between body composition and clinical parameters.
The mass of body fat, relative proportion of total fat, and mass of visceral fat have very strong direct associations with each other, as well as with the mass of android fat and BMI (
). Additionally, all these parameters have inverse associations with the level of high-density lipoprotein (HDL), indicated by negative correlation coefficients (in case of mass of body fat
,
). Excluding the mass of total fat, these parameters also exhibit negative correlations with the levels of Sex Hormone-Binding Globulin (SHBG) and androstenedione. Furthermore, body fat parameters positively correlate with low-density lipoprotein (LDL), triglycerides, C-reactive protein, and fasting insulin, suggesting a strong link between body fat composition and various metabolic and cardiovascular risk factors (
Figure 1, see
Table S1 in Supplementary Materials).
2.2. PCOS-Induced Disturbance in the Gut Microbiota
Recent scientific investigations have increasingly focused on the relationship between chronic low grade inflammation and metabolic dysfunction, particularly as they relate to disruptions in the gut microbiota composition. The complex interplay between microorganisms and the human immune system, along with the synthesis of biologically active substances by these microorganisms, plays a crucial role in the regulation of various metabolic processes. The gut microbiota is not only vital for digestion, energy balance, and maintaining the intestinal barrier function, but also influences fat storage, vasculature formation, the regulation of nervous and immune systems, and drug metabolism, among other functions [
27,
28]. A stable equilibrium between the gut microbiota and the host is essential for maintaining homeostasis and resistance against a range of pathologies.
The human gastrointestinal tract is home to a diverse community of bacterial species, often numbering in the hundreds. These bacteria share many functional roles, allowing for a degree of compensation through functional redundancy. This redundancy, however, poses challenges in identifying specific microorganisms that may play roles in the onset or progression of diseases like PCOS, which is associated with metabolic dysfunction. Reduced microbial diversity and functional redundancy have been strongly linked to conditions such as obesity, metabolic syndrome, and type 2 diabetes [
29,
30,
31]. Numerous studies have demonstrated a correlation between decreased diversity of gut microbiota, insulin resistance, and obesity [
32,
33].
Alterations in gut microbial composition appear to significantly contribute to the pathogenesis of PCOS. Individuals with PCOS exhibit reduced gut microbiota diversity, and current research indicates that the most prevalent genera in these individuals include Clostridium (phylum Bacillota), Bacteroides (phylum Bacteriodota), and Escherichia (phylum Pseudomonadota). While many members of the genera Lactobacillus and Bifidobacterium are beneficial symbionts that confer health advantages to the host, it is important to note that the genus Clostridium contains both pathogenic species-such as C. tetani, C. botulinum, C. difficile, and C. perfringens-as well as beneficial species, including the SCFA-producing group C. leptum, which encompasses F. prausnitzii, a dominant bacterial species in the large intestine.
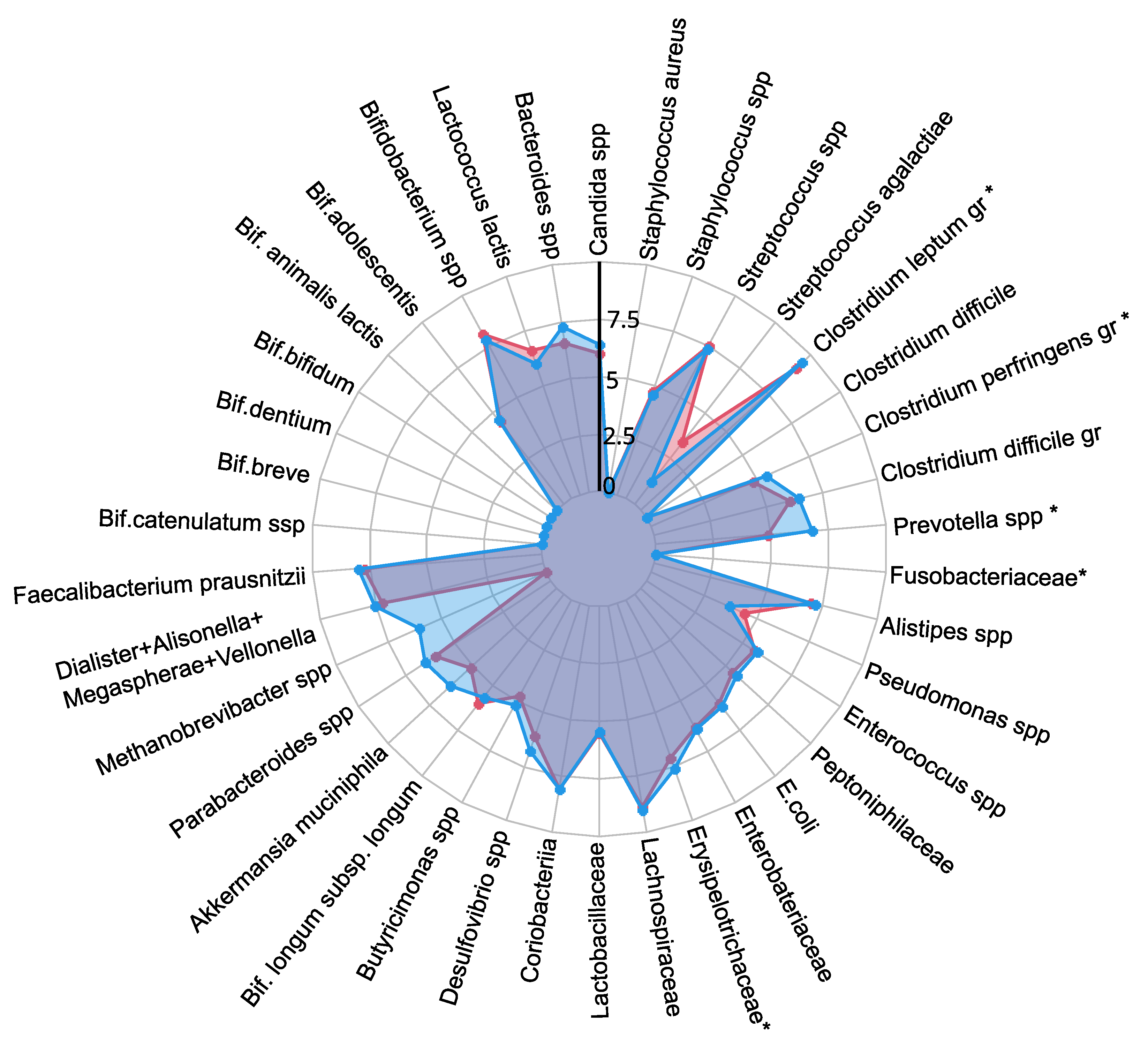
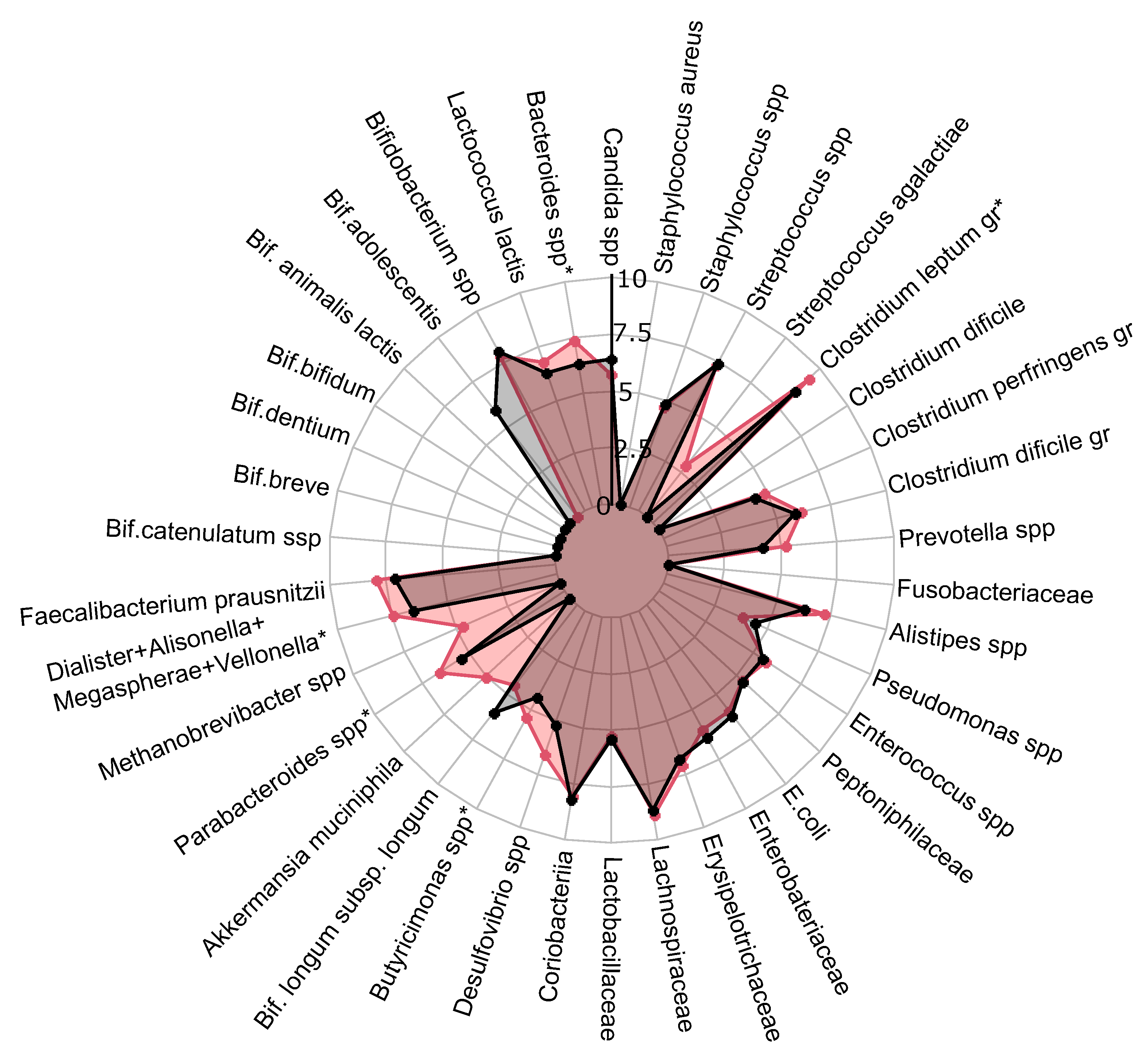
A comprehensive quantitative analysis of gut microbiota composition has revealed significant differences in gastrointestinal tract colonization levels for key microorganisms, especially enzyme and SCFA producers (see
Figure 2). In the PCOS group, a significant reduction in symbiotic bacteria was observed, particularly within the
C. leptum group and
Prevotella spp.. This decline was especially pronounced in individuals experiencing increased body weight. Furthermore, levels of other beneficial microorganisms, including
A. muciniphila,
F. prausnitzii,
Bifidobacterium spp.,
Lactobacillus spp.,
Desulfovibrio spp., and
Bacteroides spp., were generally lower in the PCOS group compared to healthy controls.
The decrease in symbiotic bacteria was accompanied by an overgrowth of opportunistic microorganisms, which can impair tight junction protein expression and increase intestinal permeability. This increased permeability facilitates the translocation of toxins—particularly lipopolysaccharides-into the systemic circulation, which activates the immune system and provokes chronic low grade inflammation [
34]. The heightened presence of opportunistic pathogens, specifically those related to the
C. perfringens group and
Staphylococcus spp., observed in PCOS patients, may further contribute to chronic inflammation. Although weak positive correlations were noted between these opportunistic microorganisms and markers indicative of chronic inflammation, it is important to consider that the inflammation associated with PCOS is multifactorial and influenced by numerous intermediates that stimulate pro-inflammatory cytokine synthesis. Moreover, elevated titers of
C. difficile and
Streptococcus spp. were detected in PCOS patients, although these findings did not achieve statistical significance (
). In conclusion, the gut microbiota in individuals with PCOS is characterized by reduced species richness and a skewed microbial balance, with an increase in opportunistic pathogens and a decline in beneficial symbionts. This microbial imbalance, compounded by genetic and epigenetic factors, may serve as an additional determinant in the development of metabolic disorders and obesity associated with PCOS.
2.3. SCFAs in Feces and Blood Plasma (GC/MS)
Recent advances in the study of SCFAs, particularly AA, PA, BA, and VA, have elucidated their significant effects on various physiological systems at both cellular and molecular levels. Emerging research has demonstrated that the presence or deficiency of SCFAs can influence the pathogenesis of a wide array of diseases, including autoimmune disorders, metabolic syndromes, and neurological conditions [
35].
In patients with PCOS, gut microbiota dysbiosis has been observed, which is characterized by an abnormal composition of microbial metabolites such as SCFAs [
8,
36]. These bacterial-derived molecules play a critical role in regulating food intake, energy expenditure, and maintaining intestinal immune homeostasis. Specifically, BA is predominantly utilized by colonocytes in the gut, whereas AA and PA are transported to the liver via the portal vein. In the liver, propionate is further metabolized by hepatocytes, while acetate often enters the peripheral circulation [
35]. Research has shown that BA can stimulate the secretion of intestinal hormones such as glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), which play crucial roles in reducing blood glucose levels by promoting insulin secretion and inducing a feeling of satiety [
37,
38]. Additionally, BA supports intestinal barrier function [
39], enhances mucin production [
40], and exhibits significant anti-inflammatory effects [
41]. Therefore, a reduction in the population of bacteria that produce BA may contribute to the development of chronic low grade inflammation and insulin resistance. Acetate and PA also significantly impact metabolic processes. It is believed that acetate and propionate influence the function of pancreatic
-cells, enhancing insulin secretion and thus lowering blood glucose levels [
42,
43]. Given their profound roles in metabolic processes, SCFAs have been identified as crucial metabolic biomarkers in the context of PCOS. Alterations in the levels and utilization of these SCFAs could potentially shed light on the underlying mechanisms of PCOS, paving the way for novel diagnostic and therapeutic strategies. The identification of specific SCFA profiles associated with PCOS may lead to targeted interventions aimed at restoring healthy gut microbiota and SCFA production, thereby mitigating the metabolic and inflammatory disturbances characteristic of the condition. This could ultimately improve clinical outcomes for patients suffering from PCOS and related metabolic dysfunctions.
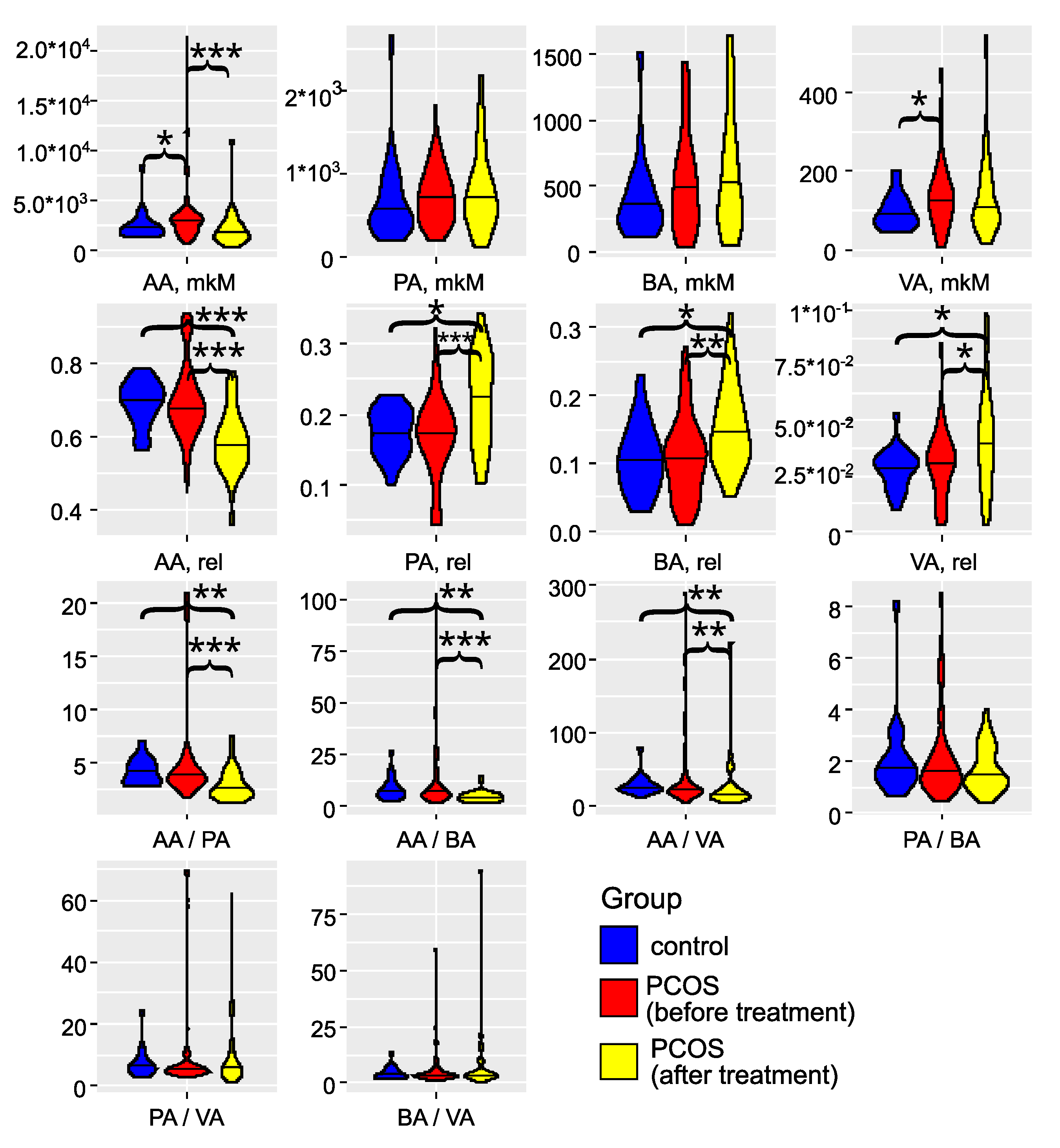
The investigation of gut microbiota metabolites in patients with PCOS has underscored its critical role in the development and progression of this condition (as illustrated in
Figure 3 and detailed in Tables S2 and S3). Specifically, in the PCOS group, the levels of AA and VA were significantly elevated (
). PA and BA also showed an increasing trend, although these changes did not reach statistical significance (
). These findings align with the results reported by Li G. et al. (2022) [
44]. Zhang et al., 2019, reported significantly greater fecal SCFAs in control compared to women with PCOS [
45]. Recent studies have confirmed that an energy-restricted diet significantly reduces the levels of fecal AA and BA in obese and overweight patients with PCOS, thereby improving their serum lipid profile [
46,
47,
48]. Thus, the overproduction or accumulation of AA in the gut may contribute to the development of obesity and PCOS.
In serum, we observed a non-significant (
) trend toward decreased levels of AA, PA, BA, and VA (
Figure S1). The SCFA levels in serum did not correlate with those in feces (
Figure S2), indicating that the concentration of SCFAs in the bloodstream is influenced by a variety of interrelated factors. These factors include the permeability of the large intestine’s epithelial layer, the acidity levels in the colon, and variations in the metabolic activity of immune cells and colonocytes, which are the primary consumers of SCFAs. Additionally, genetic predisposition plays a role, as individual variations in the expression of SCFA transporters, MCT–1 and SMCT–1, by colonocytes can significantly impact SCFA concentrations in the serum [
13]. These elements interact in complex, nonlinear ways to affect SCFA levels. Besides, a portion of the acetate present in the blood is of endogenous origin, being released by various tissues and organs [
49].
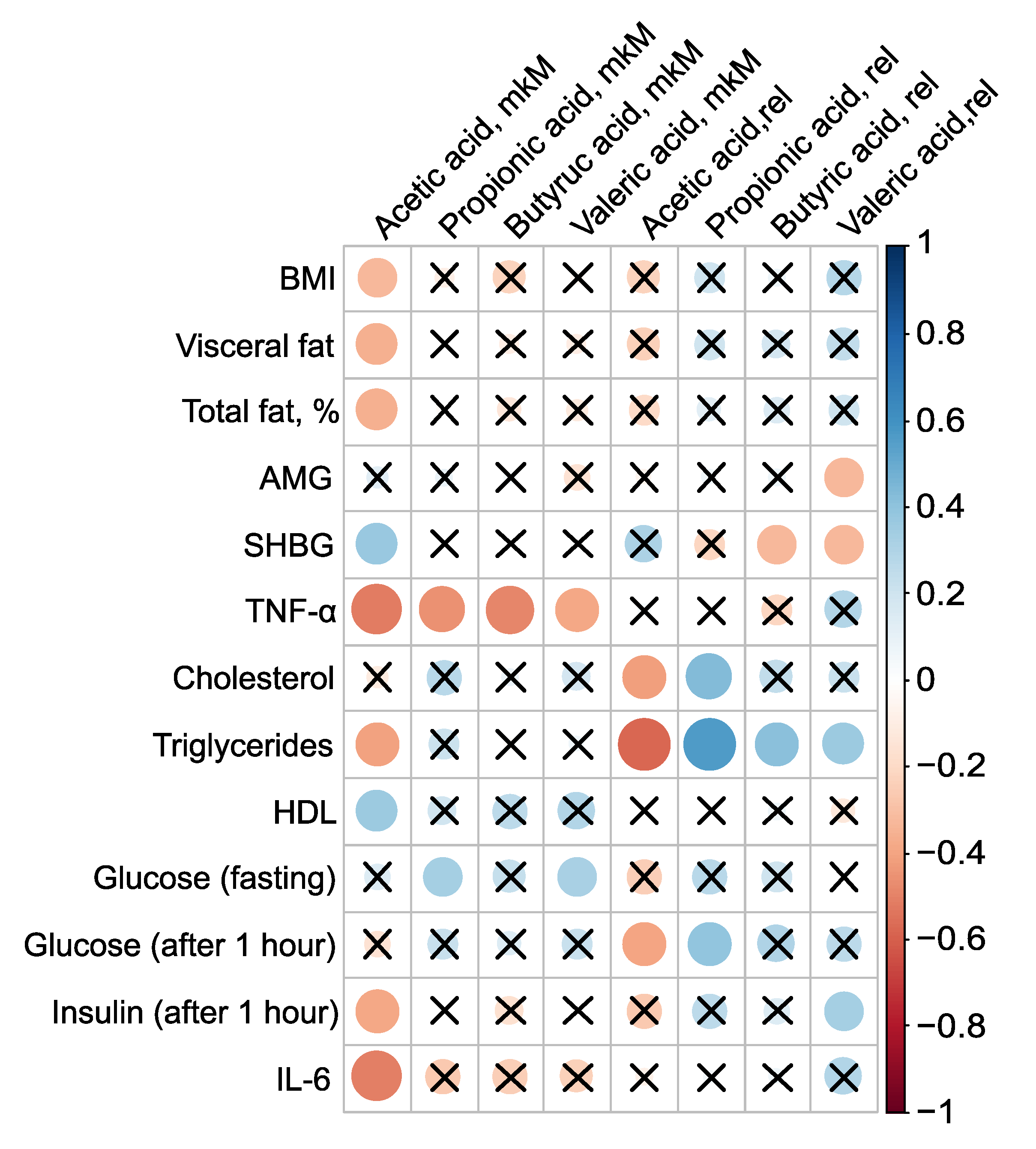
PCOS is associated not only with reproductive disorders but also with metabolic abnormalities, and constitutes a known risk factor for type 2 diabetes and metabolic syndrome. Roughly one-third of patients with PCOS exhibit laboratory evidence of low grade inflammation, such as elevated levels of CRP and/or IL-6. Given that most PCOS patients have excess adipose tissue, an evaluation of the levels of pro-inflammatory markers and adipocytokines in their serum was conducted, examining their relationship to body composition and serum SCFAs (
Figure 4). The absolute concentration of serum AA was inversely associated with BMI, mass of visceral fat, relative proportion of total fat, TNF–
, triglycerides, and insulin levels one hour after glucose loading, as well as IL–6. The strongest correlations for AA were observed with pro-inflammatory TNF–
(
,
) and IL–6 (
,
). Additionally, AA concentration showed a positive association with SHBG (
, p=0.03) and HDL (
,
) (
Figure 4,
Table S4).
Presence of AA in the bloodstream potentially has a protective effect against PCOS. AA inversely correlated with key clinical manifestations of PCOS-including high BMI, insulin resistance, and systemic inflammation, indicating that elevated AA concentrations are associated with a lower severity of these pathological features. This underscores the potential therapeutic value of AA in managing PCOS. Moreover, the strong negative correlation between the primary serum SCFAs AA (
,
), PA (
,
), BA (
,
) and VA (
,
), and TNF–
- a critical player in the inflammatory and metabolic derangements observed in PCOS - confirms the beneficial effect of blood SCFAs in the pathogenesis of PCOS. This relationship highlights how SCFAs can mitigate the inflammatory processes associated with the condition. The primary mechanism through which SCFAs exert their anti-inflammatory effects is by inhibiting the synthesis of pro-inflammatory mediators such as IL-6 and TNF–
[
50].
To elucidate the precise mechanisms by which SCFAs impact PCOS development and progression, extensive and detailed research is required. Future studies should focus on exploring the specific biochemical interactions, cellular responses, and broader physiological effects of SCFAs within the context of PCOS. This will provide clearer insights and potentially reveal novel therapeutic targets for managing this complex condition.
2.4. Prediction of Metformin Therapy’s Success
PCOS is a heterogeneous disease, which complicates the development of optimal treatment regimens. Treatment is symptomatic, and almost all prescribed drugs are used "off-label," which limits the maximum efficacy and often reduces therapy compliance due to adverse events. Although combined oral contraceptives are commonly prescribed, insulin sensitizers, particularly metformin, are also widely used. Metformin’s beneficial effects on glucose metabolism are well-established: it reduces gluconeogenesis in the liver, decreases glucose absorption in the intestine, enhances peripheral glucose utilization, and increases the secretion of GLP–1 [
25]. Additionally, it has been demonstrated that metformin can reduce the severity of chronic inflammation indirectly by improving metabolic parameters and directly due to its anti-inflammatory effects [
26]. Considering identified disturbances in the intestinal microflora, the impact of metformin on the bacterial composition of the gut and the potential for enhancing therapy efficacy by correcting the microbiota with probiotics are of particular interest.
After six months of metformin therapy (n=69), the intermenstrual interval decreased by 64.2±46.1 days, which is statistically significant (). Full restoration of the menstrual cycle was achieved in 16 out of 69 patients (23.2%). A partial improvement, characterized by an increase in the number of menstrual periods, was observed in 23 out of 69 patients (33.3%). However, oligo- or amenorrhea persisted in 30 out of 69 patients (43.5%), indicating no effect from the therapy.
Significant changes in body composition were observed during metformin therapy: the percentage of total adipose tissue decreased from 37.0±7.3% to 35.4±6.6% post-therapy (), and the mass of visceral adipose tissue decreased from 654.4±651.7 g to 382.5±416.2 g (). Among metabolic parameters, fasting insulin levels showed a statistically significant decrease of 27.1%, which was also reflected in a decrease in the HOMA index (). However, lipid profile parameters did not change significantly (). The number of patients with elevated CRP levels decreased by 1.5 times, while the incidence of elevated IL–6 levels decreased by 3.5 times. These results confirm the beneficial effect of metformin in PCOS.
Metformin treatment demonstrates a selective effect on SCFA levels in feces, significantly reducing AA to normal levels, while not substantially affecting other SCFAs. Although the levels of SCFAs in the blood showed a persistent decrease following metformin treatment, this reduction did not reach statistical significance when compared to the control group (). Thus, metformin’s impact on SCFAs may be more pronounced in the gastrointestinal tract than in the bloodstream. Further research is warranted to better understand the mechanisms behind these differential effects and their potential clinical implications in the management of conditions like PCOS.
Given the lack of cycle regulation during metformin therapy in approximately half of the patients (43.5%), the cohort was further divided into groups with a full effect (n=16) and no effect (n=30). Significant differences in gut microbiota composition were observed before treatment between groups that exhibited a full effect from the therapy and those that did not (
Figure 5). In patients who experienced the full effect of the therapy, the abundances of
C. leptum gr.,
Butyricimonas spp.,
Parabacteroides spp.,
Bacteroides spp, and the combined group of
Dialister,
Alisonella,
Megaspherae, and
Vellonella, were significantly higher than in patients who did not respond to the therapy (
). Most of these bacteria are considered beneficial for gut and metabolic health. Notably, the
C. leptum gr. includes
F. prausnitzii, one of the most abundant and important species in the human gut microbiota. A decrease in the number of these symbiotic bacteria may lead to reduced synthesis of SCFAs, which play critical roles in regulating glucose metabolism, fatty acid oxidation, and even appetite. Additionally, these bacteria are involved in the production of bile acids, which are essential for the digestion and emulsification of fats. A reduction in these symbionts could therefore contribute to the development of dyslipidemia and insulin resistance, conditions often associated with PCOS [
51].
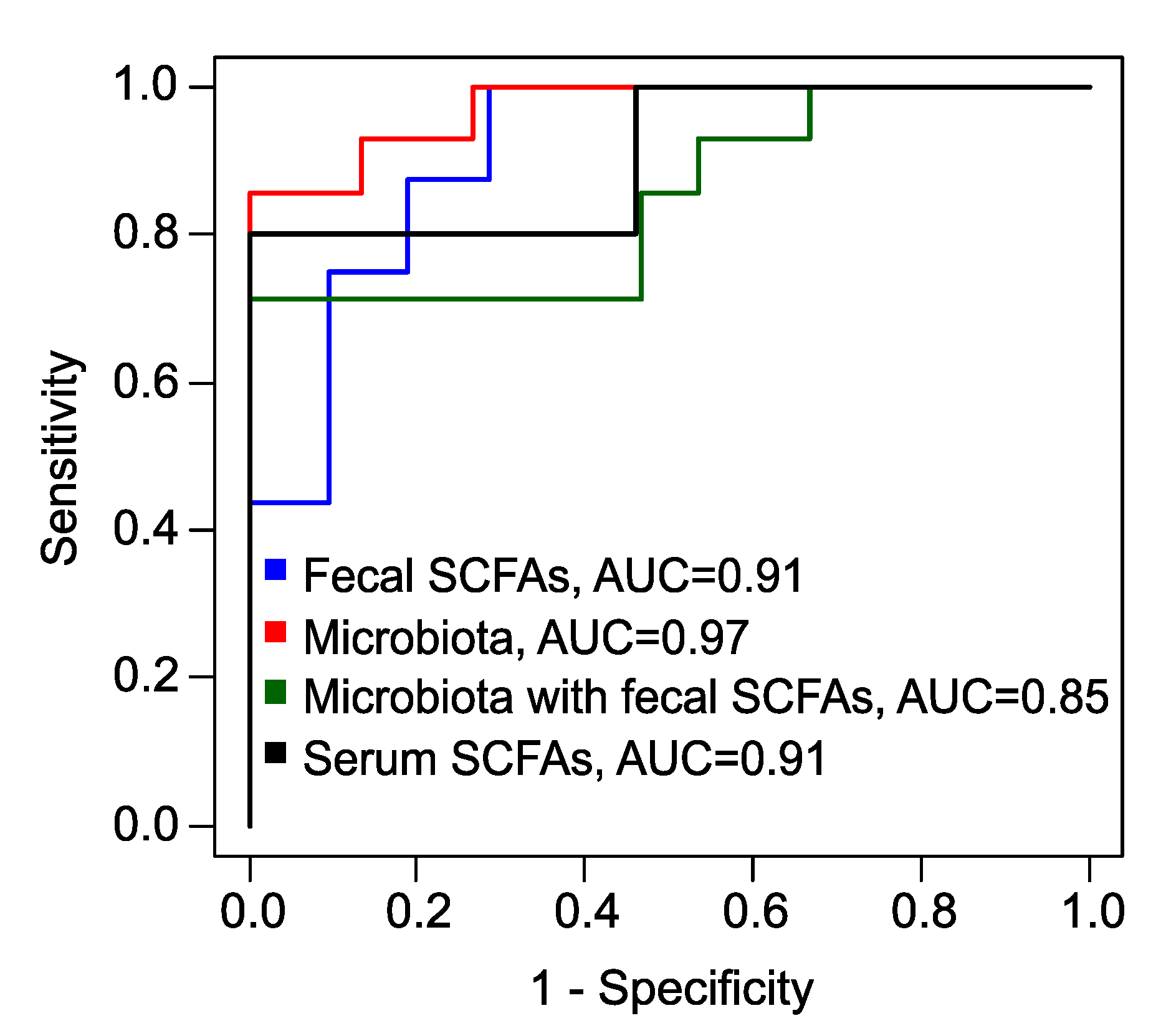
To predict the effectiveness of metformin therapy, specifically the complete restoration of the menstrual cycle as an indicator of treatment success, several eXtreme Gradient Boosting (xGBoost) models were developed. A model, based on the levels of fecal SCFAs, the relative concentration of VA and the absolute concentrations of AA and PA, demonstrated high accuracy (83%), perfect sensitivity (100%), but a comparatively low specificity (71%) (
Figure S3) (
Table 1).
In another xGBoost model that considered various bacterial genera, species, families, and the fungal genus
Candida, performance metrics improved significantly for predicting the full therapeutic effect (
Figure S4). This model achieved an excellent accuracy of 93%, a sensitivity of 86%, and a specificity of 100% (
Table 1).
When an xGBoost model combined levels of fecal SCFAs and microbiota features (
Figure S5a,b), it maintained a perfect sensitivity of 100%, but once again, specificity was lower at 71%.
Finally, utilizing the absolute serum concentrations of butyric acid (BA) and valeric acid (VA), along with their relative concentrations, we developed another xGBoost model (
Figure S6). This model achieved superior performance metrics, boasting a sensitivity of 100%, an accuracy of 91%, and a specificity of 80% (
Table 1,
Figure 6).
Predicting the response to metformin therapy enables the optimization of treatment strategies for patients with PCOS. For individuals with an a priori unfavorable prognosis, it is advisable to consider alternative methods or combination therapies. In the study participants, metformin therapy not only reduced the frequency of insulin resistance and markers of chronic low grade inflammation, but also led to an increase in the abundance of symbiotic bacteria in gut microbiota, such as A. muciniphila, and decrease in opportunistic pathogens such as C. perfringens and C. difficile. The significantly elevated fecal levels of AA observed in the PCOS group normalized after metformin therapy. This suggests a potential relationship between the action of metformin and the correction of both the intestinal microbiota composition and its metabolic byproducts. The normalization of AA levels, alongside changes in microbial populations, indicates that metformin may help restore gut homeostasis, thereby contributing to an overall improvement in metabolic and inflammatory markers among PCOS patients. Restoring the balance of microbial communities likely contributes to a decrease in endotoxemia levels, thereby reducing chronic low grade inflammation and insulin resistance.
3. Materials and Methods
3.1. Study Design
Fecal and serum samples were collected from 87 patients, comprising 69 individuals diagnosed with PCOS and 18 individuals in the control group (
Table 2). All patients with PCOS underwent a 6-month metformin therapy regimen, taking 1500 mg of "Glucophage-long" (Merck Sante, France) daily. The efficacy of the metformin treatment was categorized as follows: a full effect was indicated by the complete restoration of the menstrual cycle, a partial effect was marked by an increase in the frequency of menstrual periods, and no effect was denoted by the persistence of oligo- or amenorrhea (
Table 3).
In alignment with clinical recommendations for the diagnosis of PCOS, a hormonal profile study was conducted on the 2nd or 3rd day of either a spontaneous or progesterone-induced menstrual cycle. Additionally, an ultrasound of the pelvic organs was performed between the 5th and 7th day of the menstrual cycle [
52].
The levels of IL–6 and TNF– in peripheral blood serum samples were quantified using a solid-phase enzyme immunoassay, employing test systems from "Vector-Best" (Russia). The readings were obtained using the Infiniti F50 plate spectrophotometer (TECAN, Switzerland). Additionally, leptin and adiponectin levels were determined through solid-phase enzyme immunoassays utilizing commercial kits: "Leptin ELISA" (DBC, Canada) for leptin, and "Human Adiponectin ELISA" (BioVendor, Czech Republic) for adiponectin. CRP levels were assessed in serum using the turbidimetric method on an automated analyzer (BA-400, Biosystems, Spain), with reagents specifically labeled "C-reactive protein (CRP)" (Biosystems, Spain).
Blood glucose and lipid profile analyses were performed using both photometric and turbidimetric methods on automated analyzer BA-400 (Biosystems, Spain). To diagnose carbohydrate metabolism disorders and assess insulin resistance, a 2-hour oral glucose tolerance test was conducted with a 75 g glucose load. During this test, fasting glucose and immunoreactive insulin levels were measured, followed by subsequent measurements taken at 60 minute intervals throughout the 2 hour duration. The Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) was calculated using the following formula: glucose (mmol/L) × insulin (µIU/mL)/22.5. An index value of HOMA-IR greater than 2.7 was used as a criterion for diagnosing insulin resistance, as established in previous studies [
53]. Impairments in glucose tolerance were identified based on specific thresholds: a post-load glucose level exceeding 7.8 mmol/L indicated impaired glucose tolerance, while fasting glucose levels ranging from 6.1 to 7.0 mmol/L were also indicative of potential glucose metabolism issues.
Body composition was evaluated utilizing DXA with a Lunar 8743 device (GE Medical Systems, Madison, WI, USA). The assessment included the analysis of several key parameters: the percentage of total body fat, total body fat mass, trunk fat mass, and the android-to-gynoid fat ratio. A total body fat percentage of 30% or higher was considered indicative of excess body fat. Additionally, the volume and mass of visceral adipose tissue were determined using the "CoreScan" program by Corescan Pty Ltd (Australia). According to the analysis criteria, an excess of visceral adipose tissue was diagnosed when the measured visceral fat mass exceeded 235 grams [
54].
3.2. Collection and Storage of Samples
Fecal samples were collected in sterile containers with a volume of 8 to 10 cm3 and transported to the laboratory in a bag containing a gas-generating composition (AnaeroGen, ThermoScientific) to create anaerobic conditions, following the provided instructions. Upon arrival at the laboratory, each sample was divided into two parts. The first portion was transferred into plastic Eppendorf tubes with a capacity of 1.5 to 2 cm3 and immediately frozen at 80 °C to maintain the integrity of the SCFAs. The second portion was reserved for microbiological analysis. Serum samples were obtained through venipuncture into vacutainer tubes without anticoagulants. Following collection, the tubes were centrifuged at 4000 rpm for 15 minutes to separate the serum from the blood cells. The resulting supernatant serum was carefully transferred to a labeled screw-cap tube and stored at 80 °C until further analysis.
3.3. GC/MS Quantitative Analysis of SCFAs
3.3.1. Chemicals and Reagents
For the comprehensive GC/MS analysis of SCFAs in plasma and feces samples obtained from women with PCOS and control groups, a variety of high-quality chemicals and reagents were employed, sourced from reputable suppliers such as Sigma-Aldrich, USA. The analysis included reference standards such as AA (≥ 99%), PA (≥ 99.5%), BA (≥ 99.5%), and VA (≥ 99%). Internal standards (IS) were utilized to enhance quantification accuracy: AA–d4 (≥ 99.5%) (AA*) was added for the quantification of serum AA, while BA–1,2–13C2 (≥ 98%) (BA*) was used for the quantification of serum PA, BA, VA, as well as fecal AA, PA, BA, and VA.
Milli-Q water was used for the reconstitution and dilution of samples during fecal sample preparation, while 1.0 M hydrochloric acid (HCl) was applied for sample acidification. Methyl tert-butyl ether (MTBE) served as the solvent for the liquid-liquid extraction (LLE) of SCFAs, ensuring effective extraction of the fatty acids from the biological matrices.
Working solutions of IS at appropriate concentrations were prepared and spiked into the samples to facilitate accurate quantification of SCFAs. All glassware, plasticware, and consumables specific to GC/MS analysis were rigorously cleaned and calibrated to maintain the integrity of the analytical process. To guarantee the accuracy and reliability of the results obtained in this study, all chemicals and reagents were meticulously handled in accordance with standard laboratory protocols.
3.3.2. Preparation of Standard Solutions
Stock solutions of each SCFA were prepared in water. For PA, BA, and VA, the concentrations were set at 100 mM, while for AA, the concentration was 1000 mM. Additionally, working and calibration solutions of SCFAs were also prepared in water. IS solutions were prepared with precise concentrations to facilitate accurate measurements. For BA* IS, the concentrations were as follows: 15,000 µM for AA measurement in fecal samples, 1,500 µM for the assessment of PA, BA, and VA in fecal samples, and 50 µM for determining the levels of PA, BA, and VA in serum. Meanwhile, AA* IS was formulated at a concentration of 500 µM to measure AA in serum accurately.
All solutions were stored at 4 °C. The stability of these solutions was assessed on a weekly basis, with results showing a relative standard deviation of less than 5%.
3.3.3. Sample Preparation
Serum samples were thawed at 4 °C prior to analysis. Fecal samples were reconstituted by adding Milli–Q water at a ratio of 1000 µL per 50 mg of feces immediately before analysis. The fecal samples were then vortexed for 5 minutes to ensure proper mixing, followed by ultrasonic homogenization for 10 minutes and an additional 5 minutes of vortexing. The homogenized feces were centrifuged at 15,000 RPM for 5 minutes to separate the supernatant. The concentration of SCFAs in feces was measured in micromoles (µM) per 50 grams of unprocessed feces.
3.3.4. Extraction Procedure
A volume of 100 µL from standard solutions, serum samples, or supernatants derived from the homogenized feces was transferred into 500 µL plastic tubes. Following this, 10 µL of 1.0 M HCl was added to each sample to acidify the solution. In order to facilitate the quantification of SCFAs, 10 µL of the IS working solution was then spiked into each sample. The resulting sample mixtures were vortexed for 1 minute, centrifuged at 15,000 RPM for 5 minutes to effectively separate the phases.
A volume of 100 µL of the resulting supernatants was transferred into new 500 µL plastic tubes. To initiate LLE, 200 µL of MTBE was added to each tube. The LLE process was activated by vigorously vortexing the mixture for 20 minutes, ensuring thorough and efficient extraction of the target compounds. Then, the tubes were centrifuged at 15,000 RPM for 5 minutes to promote phase separation. A volume of 100 µL from the MTBE phase, containing the extracted SCFAs, was transferred into autosampler vials fitted with glass inserts. Finally, the samples were subjected to analysis using GC/MS for the quantification of SCFAs.
3.3.5. GC/MS Parameters
The sample analysis was conducted using an Agilent 7890B gas chromatograph coupled with an Agilent 5977B GC/MSD single quadrupole mass spectrometer, employing an HP–FFAP column (25 m length, 0.32 mm diameter, 0.5 µm film thickness) for chromatographic separation. Inlet temperature was set to 250 °C, and the injection volume was 1 µL. Two separate GC/MS conditions were utilized for investigating fecal and blood plasma samples. For feces, the system operated in split mode with a ratio of 10:1. In contrast, for plasma, a splitless mode was employed, with a switch to septum purge split mode and a purge flow to split vent of 100 mL/min at 0.1 minutes. Helium gas with a purity of ≥ 99.9999% was used as the carrier gas at a constant flow rate of 1.5 mL/min, with a septum purge of 3 mL/min. The GC oven temperature program was optimized for efficient separation of SCFAs: an initial temperature of 60 °C held for 1.5 minutes, increased by 40 °C/min to 100 °C, held for 1 minute, further increased by 10 °C/min to 158 °C, and finally raised by 100 °C/min to 240 °C. The post-run time was specified at 240 °C. The transfer line, ion source, and quadrupole temperatures were maintained at 250 °C, 230 °C, and 150 °C, respectively. Electron ionization with an energy of 70 eV was employed to ensure efficient ionization of the analytes.
During the development of the separation and identification method, an approach described in a previous study outlined in a recent study published by K.S. Kim et al. (2022) was utilized [
18]. This approach was tailored to accommodate the specific characteristics of the analyzed samples and the available materials and analytical standards. During the optimization of the GC/MS method, MS data acquisition was performed in full scan mode over the m/z range of 39–87. The choice of conditions and compound identification were guided by the injection of chemical standards. Retention times and corresponding mass spectra were compared, ensuring accurate identification of the analytes. The conditions were fine-tuned to maximize the signal-to-noise ratio, effectively separating the peaks of SCFAs from other volatiles present in the samples.
Finally, quantification of analytes was carried out in selected ion monitoring (SIM) mode using target ions (m/z 60.0 — AA, BA, VA; m/z 62.0 — BA*; m/z 63.0 — AA* and m/z 74.0 — PA) and confirmed by confirmative ions (m/z 43.0 — AA; m/z 45.0 — PA; m/z 46.0 — AA* m/z 73.0 — BA, VA and m/z 75.0 — BA*). Integration of compounds was based on specific m/z values. Data acquisition and analysis were conducted using the Masshunter quantitative program, facilitating processing and interpretation of chromatographic and mass spectral data.
3.3.6. Calibration
Two series of calibration solutions were prepared from stock solutions of SCFAs immediately before calibration to quantification of SCFAs in feces. The first series comprised solutions of AA ranging from 100 to 3500 µM, while the second series included solutions of mixtures of PA, BA, and VA, each ranging from 10 to 350 µM. Subsequently, 10 µL of BA∗ IS (at the 1500 µM and 50 µM, respectively) and 10 µL of 1M HCl were added. Seven concentration levels (calibration curves:
Figure S7) of calibration standards and four levels of quality control (QC), diluted in water, were prepared and extracted as described in the extraction procedure. The calibration levels for AA quantification were 100, 500, 1000, 1500, 2000, 2750, 3500 µM. The QC levels for AA quantification were 500, 1500, 2000, 3500 µM. The calibration levels for PA-BA-VA-mixture quantification were 10, 50, 100, 150, 200, 275, 350 µM of each components consequently. The QC levels for for PA-BA-VA-mixture quantification were 50, 150, 200, 350 µM of each components consequently. The example chromatograms for the blank, calibration levels and the samples are represented in
Figure S8.
For the quantification of SCFAs in plasma, solutions were prepared with concentrations ranging from 0.2 to 10 µM for PA, BA, VA, and from 2 to 100 µM for AA. Then, 5 µL of AA* and 5 µL BA* ISs, along with 1M HCl, were added. Eight concentration levels (calibration curves:
Figure S9) of calibration standards and four levels of QC, diluted in water, were prepared and extracted as described in the extraction procedure. The calibration levels were 0.2, 0.6, 1, 2, 4, 6, 8, 10 µM. The QC levels were 0.2, 2, 6, 10 µM. The chromatograms for the blank, calibration levels and the samples are represented in
Figure S10.
The calibration curves were constructed by plotting the peak area ratio of each SCFA to the corresponding IS against the concentration of each SCFA, followed by linear regression. The linearity of the calibration curve for each SCFA was assessed by the coefficient of determination (R2) value exceeding 0.99. The limit of detection (LOD) was calculated as 3.3×SD/b, where SD is the standard deviation of the Y-intercept, and b is the slope of the linear regression curve. The limit of quantification (LOQ) was calculated as 3×LOD.
3.4. Fecal Microbiota Analysis
DNA of intestinal-associated microorganisms (see Supplementary 2) were detected in fecal samples by real-time PCR. For this purpose, DNA extraction was performed using the Proba-Cito reagent kit (DNA-technology LLC, Russia). Subsequent analysis was conducted employing the Enterflor kit (DNA-Technology LLC, Russia), which is specifically designed for the evaluation of the most important members of the gut microbiota.
3.5. Statistical Analysis
SCFAs derived from serum and fecal samples, along with microbiota features, were compared between the polycystic ovary syndrome (PCOS) group and the control group using the Mann-Whitney test, with a significance threshold set at . Signatures exhibiting statistically significant alterations were further assessed for correlation using the Spearman test, also with a significance threshold of .
Clinical parameters were assessed for associations with SCFAs derived from feces and serum, as well as microbiota features, using the Spearman test with a significance level set at . Additionally, body fat mass, total fat mass, relative composition of total fat, and the mass of both visceral fat were evaluated for associations with biochemical parameters. The associations between SCFAs from feces and fecal microbiota were also analyzed using the Spearman test, with a significance level of .
Fecal and serum SCFAs and fecal microbiota data were used to create a diagnostic model for predicting the full success of treatment, specifically the restoration of the menstrual cycle. XGBoost models were developed through an iterative process where features were step-by-step excluded, provided that such exclusion improved model accuracy. Sensitivity and specificity were calculated using leave-one-out cross-validation, with the optimal threshold determined by maximizing the sum of sensitivity and specificity.
Data processing was performed using a laboratory-created script in R version 4.3.2, employing the XGBoost package for model creation.
4. Conclusions
The role of gut microbiota in PCOS has garnered increasing attention, unveiling a complex interplay between intestinal flora and the disorder’s pathogenesis. SCFAs are crucial metabolites produced by gut bacteria, and their levels serve as indicators of microbiota functionality. This study examines SCFAs in fecal and blood samples from women with PCOS compared to healthy controls, using GC/MS for precise measurement. A particular focus is placed on the therapeutic efficacy of metformin treatment. Our objective is to elucidate the potential benefits of metformin in managing PCOS by analyzing how this treatment modulates gut microbiota and SCFA production in affected individuals.
Patients with PCOS exhibited a substantial reduction in beneficial bacteria, including those in the C. leptum group, Prevotella spp., and others vital for gut and metabolic health such as A. muciniphila, F. prausnitzii, Bifidobacterium spp., Lactobacillus spp., Desulfovibrio spp., and Bacteroides spp. Concurrently, there was a notable overgrowth of opportunistic microorganisms (specifically, C. perfringens, C. difficile, Staphylococcus spp., and Streptococcus spp.), contributing to increased intestinal permeability and chronic low-grade inflammation, hallmarks of PCOS pathology. Moreover, an imbalance in SCFA production—particularly the overproduction of AA and VA, aligns with previous studies, suggesting that fecal SCFAs may play a role in the development of obesity and PCOS. Serum SCFA levels showed a non-significant trend towards a decrease, with no correlation between serum and fecal SCFA concentrations, implying complex regulatory mechanisms at play. Nevetherless, AA in the bloodstream may protect against PCOS by decreasing key symptoms like high body mass index and insulin resistance, suggesting a potential therapeutic value. Additionally, serum SCFAs exhibit a strong negative correlation with the inflammatory marker TNF– , indicating their capability to mitigate the inflammatory processes associated with PCOS.
Six month of metformin therapy leads to improvements in endocrine and metabolic parameters, markers of chronic low grade inflammation, and adjustments in the composition of the gut microbiota. Metformin treatment notably reduced fecal AA levels to normal, while other SCFAs remained largely unchanged. Although changes in serum SCFA levels post-treatment were not statistically significant, the normalization of AA levels in the gut indicates that metformin’s effects on SCFAs are more pronounced locally within the gastrointestinal tract. Furthermore, the therapy led to increased abundances of beneficial gut bacteria like A. muciniphila, and a decrease in opportunistic pathogens such as C. perfringens and C. difficile, indicating a restoration of gut microbial balance.
Full restoration of the menstrual cycle was achieved in 23.2% of patients, though 43.5% continued to experience oligo- or amenorrhea, indicating variable responsiveness to the treatment. Patients who responded fully to the therapy showed higher baseline abundances of beneficial bacteria such as those in the C. leptum group and Butyricimonas spp., suggesting that a healthier initial gut microbiota might predict a better therapeutic outcome. A predictive model based on serum concentrations of BA and VA, achieved high sensitivity and accuracy in forecasting the effectiveness of metformin therapy, emphasizing the potential for personalized treatment strategies in PCOS management. For patients with an unfavorable prognosis, alternative or adjunctive therapeutic approaches may be advisable.
In conclusion, our comprehensive study underscores the critical interplay between gut microbiota, SCFAs, and PCOS pathology. The observed microbial imbalances and shifts in SCFA levels provide valuable insights into the metabolic and inflammatory disruptions in PCOS. The beneficial effects of metformin on gut microbiota composition and SCFA normalization highlight its therapeutic potential. These findings pave the way for developing targeted interventions and personalized treatments, ultimately improving clinical outcomes for women with PCOS. Further research is warranted to deepen our understanding of the mechanisms behind these effects and optimize therapeutic strategies for this complex condition.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
Conceptualization, Ev.K., E.K., G.C., T.P. and V.F.; methodology, Ev.K., E.K., N.S., T.P. and V.F.; software, A.T., E.R. and N.S.; validation, E.K. and E.R.; formal analysis, E.K., A.T. and E.R.; investigation, Ev.K., E.K., N.S., T.P. and V.F.; resources, G.C., T.P., V.F. and G.S.; data curation, Ev.K., E.K., A.T., E.R. and N.S.; writing—original draft preparation, Ev.K., E.K., A.T., E.R. and N.S.; writing—review and editing, G.C., T.P., V.F. and G.S.; visualization, Ev.K., A.T., E.R. and N.S.; supervision, G.C., T.P., V.F. and G.S.; project administration, Ev.K., G.C., T.P., V.F. and G.S.; funding acquisition, Ev.K., V.F. and G.S. All authors have read and agreed to the published version of the manuscript
Funding
This research was funded by Russian Science Foundation (RSF) grant number 24-25-00068.
Institutional Review Board Statement
The study was approved by the Ethical Committee of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov (Moscow, Russia, protocol code 8 of 31 October 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Acknowledgments
The authors are grateful to the laboratory for the collection and storage of biological material (Biobank) for providing samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Witchel, S.F.; Oberfield, S.E.; Peña, A.S. Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment With Emphasis on Adolescent Girls. Journal of the Endocrine Society 2019, 3, 1545–1573. [Google Scholar] [CrossRef] [PubMed]
- He, F.f.; Li, Y.m. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. Journal of Ovarian Research 2020, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; He, X.; Huang, J.; Yu, S.; Cui, M.; Gao, M.; Liu, L.; Qian, Y.; Xie, Y.; Hui, M.; Hong, Y.; Nie, X. Short-chain fatty acid-butyric acid ameliorates granulosa cells inflammation through regulating METTL3-mediated N6-methyladenosine modification of FOSL2 in polycystic ovarian syndrome. Clinical Epigenetics 2023, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pang, Y. Metabolic Syndrome and PCOS: Pathogenesis and the Role of Metabolites. Metabolites 2021, 11. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: metabolism of nutrients and other food components. European Journal of Nutrition 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Corrie, L.; Awasthi, A.; Kaur, J.; Vishwas, S.; Gulati, M.; Kaur, I.P.; Gupta, G.; Kommineni, N.; Dua, K.; Singh, S.K. Interplay of Gut Microbiota in Polycystic Ovarian Syndrome: Role of Gut Microbiota, Mechanistic Pathways and Potential Treatment Strategies. Pharmaceuticals 2023, 16. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochemical Journal 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- wei Li, J.; zhi Chen, Y.; Zhang, Y.; hua Zeng, L.; wei Li, K.; zhen Xie, B.; ping Luo, S.; Gao, J. Gut microbiota and risk of polycystic ovary syndrome: Insights from Mendelian randomization. Heliyon 2023, 9, e22155. [Google Scholar] [CrossRef]
- Li, P.; Shuai, P.; Shen, S.; Zheng, H.; Sun, P.; Zhang, R.; Lan, S.; Lan, Z.; Jayawardana, T.; Yang, Y.; Zhao, J.; Liu, Y.; Chen, X.; El-Omar, E.M.; Wan, Z. Perturbations in gut microbiota composition in patients with polycystic ovary syndrome: a systematic review and meta-analysis. BMC Medicine 2023, 21, 302. [Google Scholar] [CrossRef]
- Yu, Z.; Qin, E.; Cheng, S.; Yang, H.; Liu, R.; Xu, T.; Liu, Y.; Yuan, J.; Yu, S.; Yang, J.; Liang, F. Gut microbiome in PCOS associates to serum metabolomics: a cross-sectional study. Scientific Reports 2022, 12, 22184. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Frontiers in Immunology 2019, 10. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiological Reviews 2020, 100, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Shetty, S.S.; Kumari N, S. Role of gut microbiota derived short chain fatty acid metabolites in modulating female reproductive health. Human Nutrition & Metabolism 2024, 36, 200256. [Google Scholar] [CrossRef]
- Roopashree, P.; Shetty, S.S.; Suchetha Kumari, N. Effect of medium chain fatty acid in human health and disease. Journal of Functional Foods 2021, 87, 104724. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; Zhang, S.; Zhu, L. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. International Journal of Molecular Sciences 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Hoving, L.R.; Heijink, M.; van Harmelen, V.; van Dijk, K.W.; Giera, M. GC-MS analysis of short-chain fatty acids in feces, cecum content, and blood samples. Clinical metabolomics: methods and protocols 2018. pp. 247–256. [Google Scholar]
- Kim, K.S.; Lee, Y.; Chae, W.; Cho, J.Y. An Improved Method to Quantify Short-Chain Fatty Acids in Biological Samples Using Gas Chromatography–Mass Spectrometry. Metabolites 2022, 12. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, P.; Xu, H.; Weng, Y.; Tang, Q.; Zhao, H. Analysis of short-chain fatty acids in fecal samples by headspace-gas chromatography. Chromatographia 2018, 81, 1317–1323. [Google Scholar] [CrossRef]
- Ribeiro, W.R.; Vinolo, M.A.R.; Calixto, L.A.; Ferreira, C.M. Use of gas chromatography to quantify short chain fatty acids in the serum, colonic luminal content and feces of mice. Bio-protocol 2018, 8, e3089–e3089. [Google Scholar] [CrossRef]
- Cuervo, A.; Salazar, N.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. Fiber from a regular diet is directly associated with fecal short-chain fatty acid concentrations in the elderly. Nutrition research 2013, 33, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Scortichini, S.; Boarelli, M.C.; Silvi, S.; Fiorini, D. Development and validation of a GC-FID method for the analysis of short chain fatty acids in rat and human faeces and in fermentation fluids. Journal of Chromatography B 2020, 1143, 121972. [Google Scholar] [CrossRef]
- Goossens, D.; Jonkers, D.; Russel, M.; Thijs, A.; Van den Bogaard, A.; Stobberingh, E.; Stockbrügger, R. Survival of the probiotic, L. plantarum 299v and its effects on the faecal bacterial flora, with and without gastric acid inhibition. Digestive and liver disease 2005, 37, 44–50. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Gøbel, R.J.; Michaelsen, K.F.; Forssten, S.D.; Lahtinen, S.J.; Jakobsen, M. Effect of Lactobacillus salivarius Ls-33 on fecal microbiota in obese adolescents. Clinical nutrition 2013, 32, 935–940. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Bharath, L.P.; Nikolajczyk, B.S. The intersection of metformin and inflammation. American Journal of Physiology-Cell Physiology 2021, 320, C873–C879. [Google Scholar] [CrossRef] [PubMed]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; Giglio, M.G.; Hallsworth-Pepin, K.; Lobos, E.A.; Madupu, R.; Magrini, V.; Martin, J.C.; Mitreva, M.; Muzny, D.M.; Sodergren, E.J.; Versalovic, J.; Wollam, A.M.; Worley, K.C.; Wortman, J.R.; Young, S.K.; Zeng, Q.; Aagaard, K.M.; Abolude, O.O.; Allen-Vercoe, E.; Alm, E.J.; Alvarado, L.; Andersen, G.L.; Anderson, S.; Appelbaum, E.; Arachchi, H.M.; Armitage, G.; Arze, C.A.; Ayvaz, T.; Baker, C.C.; Begg, L.; Belachew, T.; Bhonagiri, V.; Bihan, M.; Blaser, M.J.; Bloom, T.; Bonazzi, V.; Paul Brooks, J.; Buck, G.A.; Buhay, C.J.; Busam, D.A.; Campbell, J.L.; Canon, S.R.; Cantarel, B.L.; Chain, P.S.G.; Chen, I.M.A.; Chen, L.; Chhibba, S.; Chu, K.; Ciulla, D.M.; Clemente, J.C.; Clifton, S.W.; Conlan, S.; Crabtree, J.; Cutting, M.A.; Davidovics, N.J.; Davis, C.C.; DeSantis, T.Z.; Deal, C.; Delehaunty, K.D.; Dewhirst, F.E.; Deych, E.; Ding, Y.; Dooling, D.J.; Dugan, S.P.; Michael Dunne, W.; Scott Durkin, A.; Edgar, R.C.; Erlich, R.L.; Farmer, C.N.; Farrell, R.M.; Faust, K.; Feldgarden, M.; Felix, V.M.; Fisher, S.; Fodor, A.A.; Forney, L.J.; Foster, L.; Di Francesco, V.; Friedman, J.; Friedrich, D.C.; Fronick, C.C.; Fulton, L.L.; Gao, H.; Garcia, N.; Giannoukos, G.; Giblin, C.; Giovanni, M.Y.; Goldberg, J.M.; Goll, J.; Gonzalez, A.; Griggs, A.; Gujja, S.; Kinder Haake, S.; Haas, B.J.; Hamilton, H.A.; Harris, E.L.; Hepburn, T.A.; Herter, B.; Hoffmann, D.E.; Holder, M.E.; Howarth, C.; Huang, K.H.; Huse, S.M.; Izard, J.; Jansson, J.K.; Jiang, H.; Jordan, C.; Joshi, V.; Katancik, J.A.; Keitel, W.A.; Kelley, S.T.; Kells, C.; King, N.B.; Knights, D.; Kong, H.H.; Koren, O.; Koren, S.; Kota, K.C.; Kovar, C.L.; Kyrpides, N.C.; La Rosa, P.S.; Lee, S.L.; Lemon, K.P.; Lennon, N.; Lewis, C.M.; Lewis, L.; Ley, R.E.; Li, K.; Liolios, K.; Liu, B.; Liu, Y.; Lo, C.C.; Lozupone, C.A.; Dwayne Lunsford, R.; Madden, T.; Mahurkar, A.A.; Mannon, P.J.; Mardis, E.R.; Markowitz, V.M.; Mavromatis, K.; McCorrison, J.M.; McDonald, D.; McEwen, J.; McGuire, A.L.; McInnes, P.; Mehta, T.; Mihindukulasuriya, K.A.; Miller, J.R.; Minx, P.J.; Newsham, I.; Nusbaum, C.; O’Laughlin, M.; Orvis, J.; Pagani, I.; Palaniappan, K.; Patel, S.M.; Pearson, M.; Peterson, J.; Podar, M.; Pohl, C.; Pollard, K.S.; Pop, M.; Priest, M.E.; Proctor, L.M.; Qin, X.; Raes, J.; Ravel, J.; Reid, J.G.; Rho, M.; Rhodes, R.; Riehle, K.P.; Rivera, M.C.; Rodriguez-Mueller, B.; Rogers, Y.H.; Ross, M.C.; Russ, C.; Sanka, R.K.; Sankar, P.; Fah Sathirapongsasuti, J.; Schloss, J.A.; Schloss, P.D.; Schmidt, T.M.; Scholz, M.; Schriml, L.; Schubert, A.M.; Segata, N.; Segre, J.A.; Shannon, W.D.; Sharp, R.R.; Sharpton, T.J.; Shenoy, N.; Sheth, N.U.; Simone, G.A.; Singh, I.; Smillie, C.S.; Sobel, J.D.; Sommer, D.D.; Spicer, P.; Sutton, G.G.; Sykes, S.M.; Tabbaa, D.G.; Thiagarajan, M.; Tomlinson, C.M.; Torralba, M.; Treangen, T.J.; Truty, R.M.; Vishnivetskaya, T.A.; Walker, J.; Wang, L.; Wang, Z.; Ward, D.V.; Warren, W.; Watson, M.A.; Wellington, C.; Wetterstrand, K.A.; White, J.R.; Wilczek-Boney, K.; Wu, Y.; Wylie, K.M.; Wylie, T.; Yandava, C.; Ye, L.; Ye, Y.; Yooseph, S.; Youmans, B.P.; Zhang, L.; Zhou, Y.; Zhu, Y.; Zoloth, L.; Zucker, J.D.; Birren, B.W.; Gibbs, R.A.; Highlander, S.K.; Methé, B.A.; Nelson, K.E.; Petrosino, J.F.; Weinstock, G.M.; Wilson, R.K.; White, O.; The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S. Human Colonic Microbiota: Ecology, Physiology and Metabolic Potential of Intestinal Bacteria. Scandinavian Journal of Gastroenterology 1997, 32, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; Leonard, P.; Li, J.; Burgdorf, K.; Grarup, N.; Jørgensen, T.; Brandslund, I.; Nielsen, H.B.; Juncker, A.S.; Bertalan, M.; Levenez, F.; Pons, N.; Rasmussen, S.; Sunagawa, S.; Tap, J.; Tims, S.; Zoetendal, E.G.; Brunak, S.; Clément, K.; Doré, J.; Kleerebezem, M.; Kristiansen, K.; Renault, P.; Sicheritz-Ponten, T.; de Vos, W.M.; Zucker, J.D.; Raes, J.; Hansen, T.; Guedon, E.; Delorme, C.; Layec, S.; Khaci, G.; van de Guchte, M.; Vandemeulebrouck, G.; Jamet, A.; Dervyn, R.; Sanchez, N.; Maguin, E.; Haimet, F.; Winogradski, Y.; Cultrone, A.; Leclerc, M.; Juste, C.; Blottière, H.; Pelletier, E.; LePaslier, D.; Artiguenave, F.; Bruls, T.; Weissenbach, J.; Turner, K.; Parkhill, J.; Antolin, M.; Manichanh, C.; Casellas, F.; Boruel, N.; Varela, E.; Torrejon, A.; Guarner, F.; Denariaz, G.; Derrien, M.; van Hylckama Vlieg, J.E.T.; Veiga, P.; Oozeer, R.; Knol, J.; Rescigno, M.; Brechot, C.; M’Rini, C.; Mérieux, A.; Yamada, T.; Bork, P.; Wang, J.; Ehrlich, S.D.; Pedersen, O.; MetaHIT consortium. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Lim, M.Y.; You, H.J.; Yoon, H.S.; Kwon, B.; Lee, J.Y.; Lee, S.; Song, Y.M.; Lee, K.; Sung, J.; Ko, G. The effect of heritability and host genetics on the gut microbiota and metabolic syndrome. Gut 2017, 66, 1031–1038. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Y.; Yan, Y.; Tian, S.; Zheng, D.; Leng, D.; Wang, C.; Jiao, J.; Wang, Z.; Bai, Y. Promising Treatment for Type 2 Diabetes: Fecal Microbiota Transplantation Reverses Insulin Resistance and Impaired Islets. Frontiers in Cellular and Infection Microbiology 2020, 9. [Google Scholar] [CrossRef]
- Chen, Z.; Radjabzadeh, D.; Chen, L.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; Kraaij, R.; Voortman, T. Association of Insulin Resistance and Type 2 Diabetes With Gut Microbial Diversity: A Microbiome-Wide Analysis From Population Studies. JAMA Network Open 2021, 4, e2118811–e2118811, e2118811._eprint:
https://jamanetwork.com/journals/jamanetworkopen/articlepdf/2782527/chen_2021_oi_210559_1630346426.54052.pdf. [Google Scholar] [CrossRef]
- Zouiouich, S.; Loftfield, E.; Huybrechts, I.; Viallon, V.; Louca, P.; Vogtmann, E.; Wells, P.M.; Steves, C.J.; Herzig, K.H.; Menni, C.; Jarvelin, M.R.; Sinha, R.; Gunter, M.J. Markers of metabolic health and gut microbiome diversity: findings from two population-based cohort studies. Diabetologia 2021, 64, 1749–1759. [Google Scholar] [CrossRef]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA) – A novel theory for the development of Polycystic Ovarian Syndrome. Medical Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. Chapter Three - The Role of Short-Chain Fatty Acids in Health and Disease; Academic Press, 2014; Vol. 121, Advances in Immunology; pp. 91–119. [Google Scholar] [CrossRef]
- Sun, J.; Wang, M.; Kan, Z. Causal relationship between gut microbiota and polycystic ovary syndrome: a literature review and Mendelian randomization study. Frontiers in Endocrinology 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. American Journal of Physiology-Gastrointestinal and Liver Physiology 2018, 315, G53–G65. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein–Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Matheus, V.; Monteiro, L.; Oliveira, R.; Maschio, D.; Collares-Buzato, C. Butyrate reduces high-fat diet-induced metabolic alterations, hepatic steatosis and pancreatic beta cell and intestinal barrier dysfunctions in prediabetic mice. Experimental Biology and Medicine 2017, 242, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, L.; Zhou, W.; Yang, C.; Mai, G.; Li, H.; Chen, Y. Gut microbiota-derived butyrate regulates gut mucus barrier repair by activating the macrophage/WNT/ERK signaling pathway. Clinical Science 2022, 136, 291–307. [Google Scholar] [CrossRef]
- Pedersen, S.S.; Prause, M.; Williams, K.; Barrès, R.; Billestrup, N. Butyrate inhibits IL-1β-induced inflammatory gene expression by suppression of NF-κB activity in pancreatic beta cells. Journal of Biological Chemistry 2022, 298, 102312. [Google Scholar] [CrossRef]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; Angriman, I.; Savarino, E.V. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14. [Google Scholar] [CrossRef]
- Blaak, E.; Canfora, E.; Theis, S.; Frost, G.; Groen, A.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; Van Harsselaar, J.; Van Tol, R.; Vaughan, E.; Verbeke, K. Short chain fatty acids in human gut and metabolic health. Beneficial Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Li, G.; Liu, Z.; Ren, F.; Shi, H.; Zhao, Q.; Song, Y.; Fan, X.; Ma, X.; Qin, G. Alterations of Gut Microbiome and Fecal Fatty Acids in Patients With Polycystic Ovary Syndrome in Central China. Frontiers in Microbiology 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Z.; Jiang, S.; Bai, X.; Ma, C.; Peng, Q.; Chen, K.; Chang, H.; Fang, T.; Zhang, H. Probiotic Bifidobacterium lactis V9 Regulates the Secretion of Sex Hormones in Polycystic Ovary Syndrome Patients through the Gut-Brain Axis. mSystems 2019, 4, 10–1128. [Google Scholar] [CrossRef]
- Łagowska, K.; Drzymała-Czyż, S. A low glycemic index, energy-restricted diet but not Lactobacillus rhamnosus supplementation changes fecal short-chain fatty acid and serum lipid concentrations in women with overweight or obesity and polycystic ovary syndrome. European Review for Medical and Pharmacological Sciences 2022, 26, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Farup, P.G.; Valeur, J. Changes in Faecal Short-Chain Fatty Acids after Weight-Loss Interventions in Subjects with Morbid Obesity. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; MICRO-Obes Consortium. ; Dumas, M.E.; Rizkalla, S.W.; Doré, J.; Cani, P.D.; Clément, K. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Knowles, S.E.; Jarrett, I.G.; Filsell, O.H.; Ballard, F.J. Production and utilization of acetate in mammals. Biochemical Journal 1974, 142, 401–411. [Google Scholar] [CrossRef]
- Xiong, R.G.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, Y.; Xi, H.; Chen, L.; Feng, X. Exploration of the Relationship Between Gut Microbiota and Polycystic Ovary Syndrome (PCOS): a Review. Geburtshilfe und Frauenheilkunde 2020, 80, 161–171. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; Norman, R.J.; Mousa, A.; Joham, A.E. Recommendations From the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. The Journal of Clinical Endocrinology & Metabolism 2023, 108, 2447–2469. [Google Scholar] [CrossRef]
- Sumner, A.E.; Cowie, C.C. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis 2008, 196, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Miazgowski, T.; Krzyżanowska-Świniarska, B.; Dziwura-Ogonowska, J.; Widecka, K. The associations between cardiometabolic risk factors and visceral fat measured by a new dual-energy X-ray absorptiometry-derived method in lean healthy Caucasian women. Endocrine 2014, 47, 500–505. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Correlation plot of body composition and clinical parameters.
Figure 1.
Correlation plot of body composition and clinical parameters.
Figure 2.
The influence of PCOS on gut microbiota, presented as a a radar chart (logarithmic scale) with median values of the number of colony-forming units: PCOS (red color, n=69) and control group (blue color, n=18). The data represent the average estimate of the of fecal real-time polymerase chain reaction (PCR) target genetic amplicon copy numbers in 1 gram of feces. Statistically significant alterations () are indicated by an asterisk (*).
Figure 2.
The influence of PCOS on gut microbiota, presented as a a radar chart (logarithmic scale) with median values of the number of colony-forming units: PCOS (red color, n=69) and control group (blue color, n=18). The data represent the average estimate of the of fecal real-time polymerase chain reaction (PCR) target genetic amplicon copy numbers in 1 gram of feces. Statistically significant alterations () are indicated by an asterisk (*).
Figure 3.
Violin plots for SCFAs concentrations, relative abundances, and ratios (feces). Blue color represents the control group (n=18), red color - the group with PCOS before treatment (n=69), and yellow color - the group with PCOS after treatment (n=32). AA stands for acetic acid, VA – valeric acid, BA – butyric acid, and PA – propionic acid. * – , ** – , *** – , according to the Mann-Whitney test.
Figure 3.
Violin plots for SCFAs concentrations, relative abundances, and ratios (feces). Blue color represents the control group (n=18), red color - the group with PCOS before treatment (n=69), and yellow color - the group with PCOS after treatment (n=32). AA stands for acetic acid, VA – valeric acid, BA – butyric acid, and PA – propionic acid. * – , ** – , *** – , according to the Mann-Whitney test.
Figure 4.
Clinically relevant parameters in PCOS group and their statistically significant associations with serum SCFAs.
Figure 4.
Clinically relevant parameters in PCOS group and their statistically significant associations with serum SCFAs.
Figure 5.
Gut microbiota composition before treatment with expected effect from therapy. Red color – radar chart for full effect of therapy, black color – no effect of therapy. Statistically significant alterations () are indicated by an asterisk (*).
Figure 5.
Gut microbiota composition before treatment with expected effect from therapy. Red color – radar chart for full effect of therapy, black color – no effect of therapy. Statistically significant alterations () are indicated by an asterisk (*).
Figure 6.
Receiver operating characteristic (ROC) curves , obtained by leave-one-out cross-validation of XGBoost models to predict the full effect of metformin therapy. Blue – model based on fecal SCFAs, red – based on fecal microbiota, green – combined data of fecal SCFAs and microbiota, black – based on serum SCFAs. AUC – area under the curve.
Figure 6.
Receiver operating characteristic (ROC) curves , obtained by leave-one-out cross-validation of XGBoost models to predict the full effect of metformin therapy. Blue – model based on fecal SCFAs, red – based on fecal microbiota, green – combined data of fecal SCFAs and microbiota, black – based on serum SCFAs. AUC – area under the curve.
Table 1.
Performance metrics of XGBoost models: accuracy, sensitivity, specificity, and optimal threshold.
Table 1.
Performance metrics of XGBoost models: accuracy, sensitivity, specificity, and optimal threshold.
| |
Accuracy, % |
Sensitivity, % |
Specificity, % |
Threshold |
| Fecal SCFAs |
83 |
100 |
71 |
0.45 |
| Gut microbiota |
93 |
86 |
100 |
0.36 |
| Gut microbiota & fecal SCFAs |
86 |
100 |
71 |
0.30 |
| Serum SCFAs |
91 |
100 |
80 |
0.41 |
Table 2.
Number of samples (feces and serum) obtained for the control group (n=18) and PCOS group (n=69).
Table 2.
Number of samples (feces and serum) obtained for the control group (n=18) and PCOS group (n=69).
| Type of sample |
Control (n=18) |
PCOS (n=69) |
| Feces |
18 |
69 |
| Serum |
10 |
38 |
Table 3.
Number of samples (feces and serum) obtained for the PCOS group (n=69) after metformin’s therapy according to the effect achieved.
Table 3.
Number of samples (feces and serum) obtained for the PCOS group (n=69) after metformin’s therapy according to the effect achieved.
| Type of sample |
Full effect (n=16) |
No effect (n=23) |
Partial effect (n=30) |
| Feces |
8 |
7 |
17 |
| Serum |
16 |
23 |
30 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).