1. Introduction
The bovine gut is a complex ecosystem that plays a crucial role in the health and well-being of cattle [
1]. Understanding the complex dynamics of host-pathogen interactions during Shiga toxin-producing
Escherichia coli (STEC) colonization of the bovine gut is pivotal for understanding the epidemiology and pathogenesis of this important foodborne pathogen. While there are more than 470 STEC serogroups, serogroup O157, and other six non-O157 serotypes popularly known as
“Big Six
” namely O26, O45, O103, O111, O121, and O145, are considered major foodborne pathogens because of their ability to induce a spectrum of severe gastrointestinal illnesses in humans upon foodborne transmission. These illnesses range from relatively mild diarrhea to life-threatening diseases, such as hemorrhagic colitis and hemolytic uremic syndrome (HUS) [
2]. Cattle asymptotically carry these bacteria within the gastrointestinal tract and have been identified as a principal reservoir for STEC. This asymptomatic carriage is a double-edged sword; while it spares cattle from illness, it allows for continuously shedding these pathogens into the environment. This shedding facilitates zoonotic transmission of STEC to humans, primarily through the consumption of contaminated food and water, underscoring the agricultural and environmental aspects of STEC management [
3].
According to the Centers for Disease Control and Prevention (CDC), STEC remains a significant public health concern and causes an estimated 265,000 illnesses each year in the United States alone, leading to approximately 3,600 hospitalizations and 30 deaths [
4]. Globally, the World Health Organization (WHO) highlights that STEC infections are a leading cause of diarrhea, which is a major cause of morbidity and mortality in children under five years of age in low-income countries [
5]. In particular, STEC O157:H7, the most well-characterized serotype, has been responsible for numerous outbreaks associated with contaminated food and food products, including leafy greens, raw milk, and undercooked meat [
6]. The economic impact is also significant, with costs associated with healthcare, lost productivity, and food recalls running into millions of dollars annually [
7].
The study of host-pathogen interactions in the context of STEC colonization in cattle is essential for several reasons. First, it sheds light on the mechanisms by which STEC establishes and maintains colonization within the bovine gut, which is fundamental to its persistence and spread within cattle. Understanding these mechanisms can help identify intervention strategies to reduce STEC shedding in cattle, thereby decreasing food and water contamination and the risk of human exposure and infection. Second, exploring the host response to STEC colonization provides insights into the factors contributing to the asymptomatic carrier state in cattle, which is crucial to understanding STEC epidemiology and implementing control strategies. Thirdly, elucidating the complex interplay between STEC, bovine hosts, and gut microbiota opens avenues for developing novel preventative and therapeutic approaches [
8]. Understanding host-pathogen interactions at the molecular level can also contribute to developing rapid diagnostic tools and targeted treatments for STEC infections in humans. It is paramount to advance our knowledge of STEC pathogenesis, improve public health interventions, and enhance food safety. The STEC colonization of the bovine gut can lead to a range of health problems, including foodborne illnesses in humans [
9,
10,
11,
12]. This review aims to provide a comprehensive overview of the current state of knowledge on the host-pathogen interaction during STEC colonization of the bovine gut, including the key factors that influence host-pathogen interactions, its effects on the human host, and to identify areas where further research is needed.
2. STEC Colonization of the Bovine Gut
2.1. STEC Prevalence in the Bovine Gut
STEC is a frequent colonizer of the bovine gut, with young calves being particularly susceptible to colonization and frequent shedding compared to adult cattle [
13]. Early exposure to STEC via horizontal or vertical transmission contributes to its high prevalence among cattle worldwide [
12,
14]. Studies have indicated that significant fecal shedding in young calves is influenced by factors such as age and gut microflora composition [
10,
15,
16]. Shiga toxin (Stx) variants, particularly Stx2, correlate with younger calves, highlighting age-related differences in STEC colonization and shedding dynamics [
17]. Beef calves shed more STEC during their first six months of age, decreasing as they mature. This shedding pattern also correlates with a lower diversity of gut microbiota in younger animals, which increases as cattle mature [
10]. In addition to age-related shedding patterns, factors such as breed, sex, and weight gain also influence STEC shedding in cattle [
18].
Among various STEC serotypes, O157:H7 is one of the most studied. It has been implicated in numerous outbreaks of foodborne illnesses worldwide, leading to severe symptoms, such as bloody diarrhea and hemolytic uremic syndrome (HUS) [
19]. In addition to STEC O157:H7, several other non-O157 STEC serogroups, including O26, O45, O103, O111, O121, and O145, are also considered major foodborne pathogens, with cattle serving as reservoirs and posing a risk of contamination of food and water sources, subsequently causing disease in humans [
20,
21].
Table 1 lists the major STEC serogroups in the bovine gut, key virulence factors, and types of illness inflicted in humans. The prevalence of STEC in bovine gut varies depending on factors such as geographic location, farming practices, and environmental conditions [
22,
23].
2.2. Modes of Transmission from Bovine to Human and the Potential Risks to Human Health
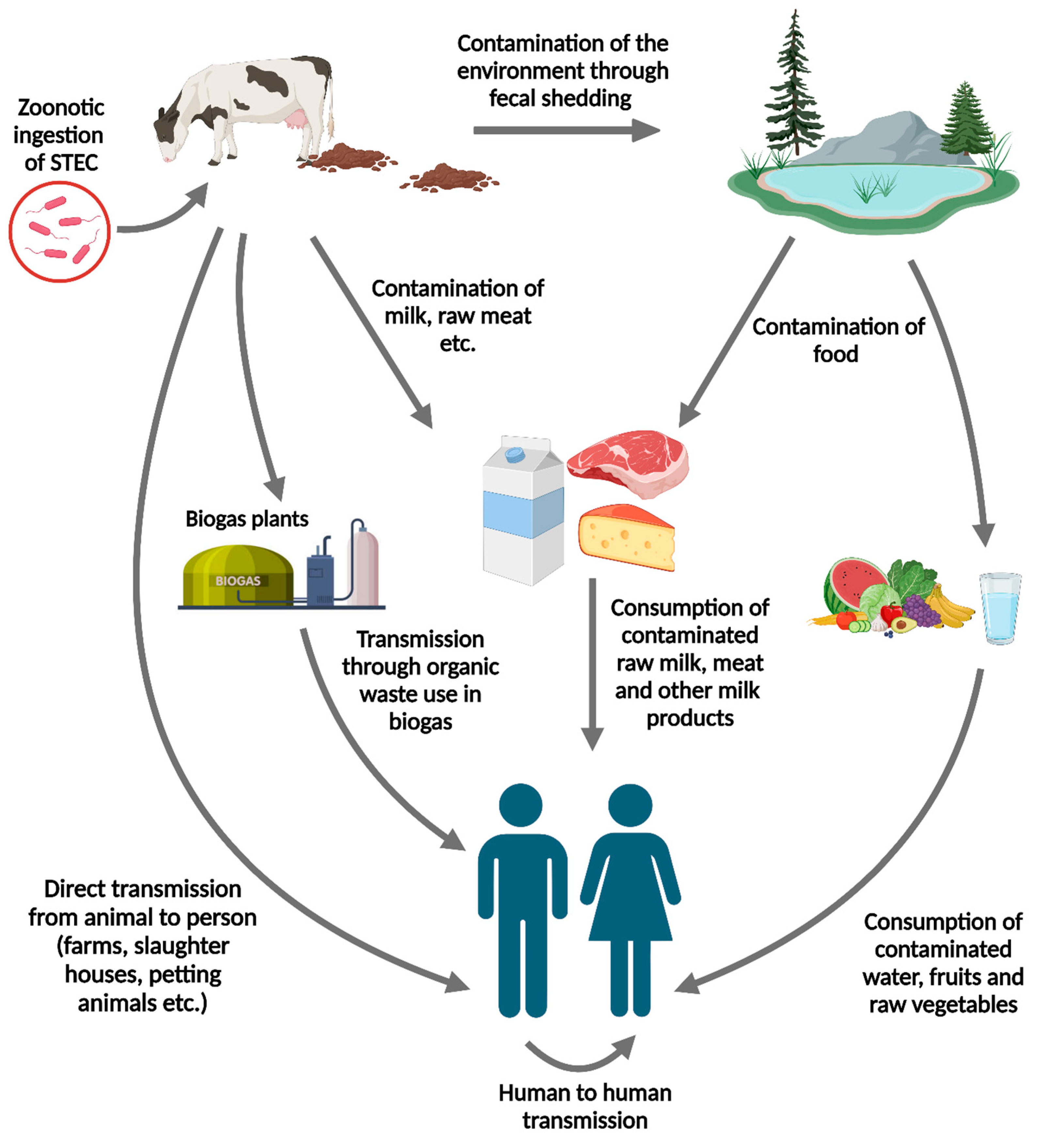
STEC transmission from cattle to humans occurs via various pathways (
Figure 1). The primary mode of transmission from bovine sources to humans is consuming contaminated raw or undercooked milk and meat contaminated with cattle feces during farming and production practices [
34,
35]. The infectious dose of STEC is low, with approximately 100 bacteria, making person-to-person transmission possible and leading to secondary cases in contact with infected individuals [
36]. Direct animal-to-human contact is another crucial pathway for STEC transmission from handling cattle. Visiting farms or venues where the public interacts with farm animals can increase the risk of infection, emphasizing the importance of maintaining hygienic practices in these environments [
37]. Waterborne transmission has also been reported, highlighting the need for safe water practices to prevent STEC outbreaks [
6].
The risks associated with STEC shedding from bovine sources are substantial and can lead to severe health complications in humans. Symptoms of STEC infection include abdominal cramps, diarrhea, and, in more severe cases, hemorrhagic colitis and HUS. HUS is a life-threatening condition characterized by acute renal failure, hemolytic anemia, and thrombocytopenia, and up to 10% of patients with STEC develop this syndrome [
38]. Children under ten years of age are particularly vulnerable to serious STEC infections, with approximately 15% of the children with STEC diarrhea progressing to HUS [
39].
3. Bovine Host Factors Influencing STEC Colonization
3.1. Characteristics of Bovine Gastrointestinal Tract
The unique characteristics of the bovine gastrointestinal tract (GIT), especially at the rectoanal junction (RAJ), play a crucial role in STEC colonization. The RAJ, situated at the terminal end of the ruminant GIT, transitions from columnar epithelial cells in the distal colon to stratified squamous epithelial cells towards the anus. This site is particularly conducive to STEC colonization and persistence and plays a major role in the super-shedding of STEC in cattle feces. Dense lymphoid follicles with different cell types, such as follicle-associated epithelium (FAE) and RAJ squamous epithelium (RSE), play distinct roles in bacterial adherence. STEC promotes adherence to RSE via a specialized adherence strategy that is not absent in commensal bacteria [
40,
41]. STEC can colonize various sites within the bovine GIT beyond RAJ. Other parts of the intestine, such as the ileum, jejunum, cecum, colon, and rectum, offer favorable microenvironments for STEC adherence and persistence, emphasizing the ability of bacteria to navigate and exploit different niches within the bovine GIT for survival and multiplication [
42].
The pH levels throughout the bovine GIT create regions that are favorable for the survival and proliferation of STEC. While the rumen has a relatively neutral pH favorable to diverse microbiota, the pH in other parts of the GIT, such as the small and large intestines, can vary, creating niches that STEC can uniquely exploit [
43]. STEC strains are adapted to survive under acidic conditions that may kill other bacteria, allowing STEC to effectively colonize downstream of the rumen, especially the colon, where they can attach to the intestinal epithelium [
44].
3.2. Immune Responses and Other Host Factors That Influence STEC Colonization of the Bovine Gut
STEC has evolved mechanisms to evade immune detection and establish colonization in cattle. Studies have shown that calves infected with STEC O157 strains produce antibodies against O157 lipopolysaccharide (LPS) but may suppress cellular immune responses, especially when infected with Stx producing strains. This suppression is significant because it allows the pathogen to persist in the host and continue to be shed in feces. Stx targets bovine lymphocytes, particularly CD8+ T cells and B cells, such as intraepithelial lymphocytes (IEL), located between epithelial cells adjacent to the basement membrane in the intestines of cattle. The toxin binds to receptors on Gb3/CD77-positive lymphocytes at the early activation stages, which inhibits lymphocyte proliferation. This suppression affects the immune system’s ability to mount an effective cellular response against the pathogen, and the inflammatory response is less pronounced, facilitating the persistence of STEC in the gut [
12,
45].
STEC effector proteins such as NleE and NleB can inhibit the activation of NF-κB, a central regulator of inflammation. NleE modifies the host ubiquitination machinery and prevents the degradation of IκB, an inhibitor of NF-κB, thereby maintaining NF-κB in an inactive state. In contrast, NleB is a glycosylated death domain-containing protein involved in NF-κB signaling, thereby blocking NF-κB signalling pathway. By keeping NF-κB inactive, these effectors inhibit the expression of pro-inflammatory cytokines such as IL-2, TNF-α, and IFN-α, dampening the host immune response and allowing the bacteria to evade detection and destruction [
24,
46]. The Mitogen-Activated Protein Kinase (MAPK) pathway is crucial for producing inflammatory cytokines, the key signaling molecules of the host immune response. T3SS effector proteins, such as NleC, NleD, and OspF, can inhibit the MAPK pathway by interfering with its signaling components, thereby blocking the activation of transcription factors, including AP-1, responsible for cytokine production. This inhibition weakens the host inflammatory response, making it harder for the immune system to detect and eliminate bacteria, thus facilitating bacterial survival and colonization. Both mechanisms do not induce lymphocyte death. Instead, they prevent the proliferation and response of lymphocytes to antigens by blocking the activation of their response to mitogens, the substances that stimulate cell division [
47,
48,
49,
50]. This blockage indicates that even if the immune system recognizes the pathogen, it cannot effectively activate the cells needed to fight the infection. Thus, the lymphocytes remain viable but are functionally impaired, increasing host susceptibility to pathogen infection and colonization. Furthermore, the ability of STEC to adhere to the gut epithelium through fimbriae and other adhesins facilitates intimate interactions promoting colonization [
12].
Mucosal-associated mechanisms are believed to facilitate the adherence of STEC to the intestinal surface. Various receptors on the mucosal epithelium or in the mucus bind to bacterial adherence factors, including fimbriae, intimin, and other adhesins. The number and affinity of these receptors differ between neonatal and adult animals, leading to age-related differences in susceptibility to STEC intestinal colonization and subsequent excretion, which are higher in neonatal animals [
51]. Additionally, factors such as attaching and effacing lesions formed by STEC alter the gut environment, promoting STEC colonization [
9]. The diet also has a significant impact on STEC intestinal colonization. Cattle-fed forage-based diets showed more shedding than those fed rich-grain diets because volatile fatty acid (VFA) concentrations in grain cattle feed can inhibit the adherence of STEC to the mucosal surface. Younger cattle, especially pre-ruminant calves, are more susceptible to STEC colonization because of their immature immune system, lower levels of specific antibodies, and more affinity mucosal surface receptors. Pre-weaned calves also have lower VFA concentrations and higher lactate levels in their gut, creating a more favorable environment for STEC colonization [
51].
4. Role of STEC Virulence Factors in Bovine Colonization
STEC utilizes a complex array of virulence factors, including Stx, adhesins, autotransporters, type III secretion system effectors, fimbriae, and pili proteins, to colonize the bovine gut successfully while overcoming host defenses.
Table 2 summarizes the key aspects of STEC virulence factors, including their roles in colonization. The interplay between STEC virulence factors during bovine colonization is a coordinated process that allows bacteria to establish infection without causing disease, evade host defenses, and persist within the gut environment [
52].
4.1. Initial Adherence through Adhesins and Fimbriae/Pili
Hair-like structures such as fimbriae and pili on the surface of STEC play crucial roles in bacterial adherence, colonization, and interaction with host cells. These structures are composed of protein subunits and can be categorized into several types, including Type 1 Fimbriae, F9 Fimbriae, Curli Fimbriae, Long Polar Fimbriae (LPF), and E. coli YcbQ Laminin-Binding Fimbriae (ELF) based on their function and the genes that encode them. These structures serve as the primary factors for the initial attachment of STEC to the intestinal epithelium leading to colonization [
53]. Fimbriae and pili bind to specific receptors on the surface of the bovine intestinal epithelial cells, typically glycoprotein or glycolipid present on the mucosal surfaces. The tip of the fimbriae or pili contains a specialized protein called adhesins, such as IrgA homolog adhesin (Iha), and STEC autoagglutinating adhesin (Saa), which recognize and bind to host receptors with high affinity [
65,
67]. Intimin, encoded by the eae gene of STEC, is another key adhesin that enables the formation of attaching and effacing (A/E) lesions on cattle intestinal epithelial cells. It is a well-studied non-fimbrial adhesin that plays a critical role in tight adhesion to host cells after initial contact mediated by fimbriae/pili [
53]. This type of attachment enabled by adhesins, along with fimbriae and pili, is crucial for establishing a foothold in the gut and overcoming mechanical defense mechanisms such as mucus flow [
52]. Different types of fimbriae and pili recognize different host receptors, contributing to tissue specificity and tropism. This specificity determines the sites within the gastrointestinal tract where STEC can effectively colonize [
52,
53,
54,
55].
4.2. Manipulation of Host Cells via Type III Secretion System (T3SS) Effectors
Once STEC is attached, it releases an effector protein called Tir (translocated intimin receptor) into the host gut epithelial cell membrane through T3SS. Once inside the host cell membrane, Tir acts as a strong receptor for intimin and creates a stable, intimate attachment between the bacterium and host cell, anchoring bacteria firmly to the gut epithelial surface and facilitating colonization [
46]. By adhering tightly to host cells, STEC can resist phagocytosis by immune cells and avoid being cleared by the host’s innate immune defenses. In addition to this, STEC utilizes T3SS to inject a group of other effector proteins such as EspF, Tir (Translocated intimin receptor, Map (Mitochondrial associated protein), EspG, EspG2, NleB (Non-LEE-encoded effector B), NleE, NleH, etc (
Table 2) into the host epithelial cells [
56]. These effectors manipulate host cell processes, such as altering the actin cytoskeleton to form pedestal-like structures that secure bacteria more firmly to the cell surface. Additionally, T3SS effectors can suppress host immune responses, helping STEC evade detection and destruction by the immune system [
46,
56,
57,
58].
4.3. Damage and Evasion through Shiga Toxins (Stx)
Stx, such as Stx1 and Stx2, are potent cytotoxins prominently associated with pathogenesis in humans; however, they play a complex role in the colonization and adherence of STEC in the bovine gut. Stx toxins are AB5 toxins with a single A subunit and five B subunits. B subunits bind to globotriaosylceramide (Gb3) receptors on the surface of host cells and are internalized via receptor-mediated endocytosis [
30,
59]. Inside the cell, Stx undergoes retrograde transport from the Golgi apparatus to the endoplasmic reticulum (ER). In the ER, the A subunit, which possesses RNA N-glycosidase activity, cleaves a specific adenine base from the 28S rRNA of the 60S ribosomal subunit. This action inactivates ribosomes, halts protein synthesis, and leads to localized epithelial damage. It exposes deeper layers of the mucosa, providing new binding sites for STEC and enhancing its ability to persist within the host. The Gb3 receptor distribution and expression level vary between humans and cattle. In humans, particularly children, Gb3 is highly expressed in the kidneys, which explains the prevalence of HUS following STEC infection [
38]. However, cattle, natural reservoirs of STEC, have lower Gb3 expression levels in their intestines and typically do not develop HUS, allowing them to carry bacteria asymptomatically [
3]. Additionally, the presence of Stx might modulate the host immune response, reducing the effectiveness of immune defenses and inflammatory response, which allows bacteria to establish more robust colonization, as discussed in the previous section.
4.4. Tissue Penetration and Immune Evasion via Autotransporters
Autotransporters are also crucial in facilitating colonization of the bovine gut. Some notable autotransporters included OmpA, EspP, IcsA (VirG), Hbp, Pet, Ag43, Tsh, and EhaA-B-J (
Table 1). The autotransporter protein EspP has proteolytic activity and degrades host proteins in the extracellular matrix and mucus layer, allowing STEC to penetrate deeper into the intestinal tissue. This degradation exposes some receptors on the epithelial surface, promoting STEC binding via other adherence factors, such as fimbriae and adhesins [
60]. Another important autotransporter in STEC is the serine protease autotransporter of the Enterobacteriaceae (SPATE) family, which includes proteins Pet and Sat. These proteases can cleave host substrates, including mucins and immune components, weakening the host defense mechanisms and promoting a more conducive environment for bacterial colonization [
61]. Variability in the expression of autotransporters and host immune system recognition can lead to host-specific interactions. In bovines and all other hosts, biofilm formation by STEC is also crucial for their persistent colonization of the intestines and environmental survival. Autotransporters contribute to biofilm stability by providing structural components or mediating adhesion to surfaces or other cells [
53]. In cattle, where host-pathogen interactions have co-evolved, the immune response to these autotransporters may be more controlled, often resulting in an asymptomatic carriage rather than disease. For instance, cattle may express different receptor patterns or immune defenses that affect the outcome of STEC autotransporter activity compared to humans. Autotransporters may also influence the degree of STEC shedding in cattle, affecting the likelihood of its transmission to humans [
3,
62,
63,
64].
Table 2.
Major STEC virulence factors and their role in bovine gut colonization.
Table 2.
Major STEC virulence factors and their role in bovine gut colonization.
| Virulence Factor |
Role in Bovine Gut Colonization |
References |
| Shiga Toxins |
| Stx1 |
Modulates the local immune response and contributes to the persistence of bacteria by dampening inflammatory signals and immune cell activation. |
[12] |
| Stx2 |
Restricts epithelial cell proliferation within bovine crypts without inducing cell death and helps the bacteria maintain its niche within the gut, facilitating long-term colonization and shedding in cattle. |
[12] |
| Adhesins |
| Eae (Intimin) |
Essential for strong adherence and colonization of the bovine gut epithelium by forming attaching and effacing (A/E) lesions. |
[53] |
| Iha (IrgA homolog adhesin) |
Supports adhesion and persistence under iron-limited conditions in the bovine gut. |
[65] |
| Saa (STEC Agglutinating adhesin) |
Promotes strong adhesion to epithelial cells through autoagglutination, thereby facilitating the persistence and establishment of the bacteria within the host intestine. |
[66,67] |
| Efa1/LifA (E. coli factor for adherence) |
Enhances colonization by inhibiting the bovine immune response and aiding in bacterial adherence. |
[64] |
| Type 1 Fimbriae |
Promotes initial attachment and colonization of the bovine gut by adhering to mannose-containing receptors on host cells. |
[68] |
| F9 Fimbriae |
Contributes to colonization and persistence in the bovine gut by facilitating adhesion. |
[3] |
| Long Polar Fimbriae (LPF) |
Serves as a key factor in maintaining long-term colonization and persistence in the bovine gut. |
[69] |
| Curli Fimbriae |
Enhances environmental persistence and colonization through biofilm formation in the bovine gut. |
[70] |
|
E. coli YcbQ Laminin-Binding Fimbriae (ELF) |
Supports colonization by adhering to laminin in the bovine extracellular matrix. |
[71] |
| Autotransporters |
| OmpA (Outer Membrane Protein A) |
Modulates adherence of the bacteria to the recto-anal junction squamous epithelial cells, facilitating the interaction of other adhesins involved in the colonization process. |
[72] |
| EspP (E. coli Secreted Protein P) |
Enhances colonization by degrading bovine host defenses through proteolytic activity. |
[60] |
| EhaA-B-J |
Contributes to stable colonization through adherence and biofilm formation in the bovine gut. |
[53] |
| Type III Secretion System Effectors |
| EspF |
Disrupts tight junctions and alters the host cell cytoskeleton, which facilitate bacterial adherence and invasion, and evasion of the host immune response, ultimately promoting bacterial survival and persistence within the bovine intestine. |
[56] |
| Tir (Translocated intimin receptor) |
Serves as a receptor for intimin, facilitating intimate adherence and the formation of attaching and effacing lesions in the bovine intestinal epithelium. |
[3,46] |
| Map (Mitochondrial-associated protein) |
Enhances bacterial survival and colonization by modulating host cell responses in the bovine gut. |
[73] |
| EspG and EspG2 |
Disrupt the host cell microtubule network, leading to cytoskeletal alterations that enhance bacterial adherence and persistence. |
[57] |
| NleB (Non-LEE-encoded effector B) |
Is involved in initial adherence and plays a significant role in enhancing bacterial survival and persistence within the bovine gut by modulating host cell processes, such as inhibition of apoptosis, thus promoting long-term colonization. |
[46] |
| EspA, EspB, EspD |
Form translocon pores and inject other effector proteins into the host cells, leading to the formation of attaching and effacing (A/E) lesions, which are essential for stable bacterial attachment and colonization in the bovine intestine. |
[74] |
| NleH1 and NleH2 (Non-LEE-encoded effector1 and 2) |
Inhibits the NF-κB signaling pathway, which dampens the pro-inflammatory cytokine production, thereby creating a more favorable environment for STEC colonization and persistence. |
[58] |
5. Molecular Insights into STEC-Bovine Host Interactions
Recent molecular studies and advancements have significantly deepened our understanding of interactions between STEC and the host. These interactions are complex, involving several bacterial factors and host cellular processes. The use of transcriptomics, proteomics, and genomics has revealed the detailed mechanisms of STEC pathogenicity, offering new perspectives for mitigating STEC foodborne illness.
Transcriptomic analyses revealed that STEC responds to the GIT environment by adjusting gene expression to enhance survival and colonization. For instance, studies have shown that STEC upregulates genes involved in iron acquisition, stress response, and toxin production upon entry into the host gastrointestinal tract. Molecular insights into the early stages of STEC colonization in cattle are critical to understanding and controlling its spread. A study compared transcriptomic profiles between nonpathogenic E. coli strain MG1655 and STEC (O26, O111, or O157) to identify the factors used by STEC during colonization of the bovine GIT. STEC demonstrated a numerically, though not significantly, higher level of adherence to cattle colonic explants compared to the nonpathogenic E. coli strain, suggesting a role of these upregulated STEC factors in early colonization. In particular, this study observed a significant upregulation of the flagellin gene (fliC) in O157 STEC and the Lon protease gene (lon) in all STEC strains when incubated with colonic explants. The upregulation of H7 fliC suggests that it serves as an adhesin in the bovine GIT. The collective upregulation of lon in STEC strains compared to that of nonpathogenic E. coli suggests its involvement in the stress response and possibly in regulating other virulence factors critical for colonization [
75].
We recently examined variations in the gene expression of STEC O157:H7 when exposed to RAJ cells and human colonic epithelial cells (CCD CoN 841). This study aimed to understand how bacteria adjust their gene expression to adapt to the distinct environments of different hosts during initial attachment. STEC O157:H7 exhibited two different transcriptional profiles when attached to human and bovine intestinal cells, suggesting that STEC employ different strategies for colonization and survival in each host. In human cells, enrichment of genes related to LPS polysaccharide, and lipid biosynthesis indicates strategic manipulation by STEC to enhance adherence and possibly establish a niche within the host environment. STEC adapt to the host cell environment by regulating genes associated with metal ion homeostasis, which is crucial for its survival and colonization. Downregulation of pathways related to antibiotic resistance, drug metabolism, and secondary metabolism in bovine cells suggests that STEC prioritizes immediate colonization over secondary metabolic functions in cattle reservoirs. STEC upregulated genes involved in iron transport and utilization in human cells, highlighting the critical role of iron in bacterial growth and pathogenesis. This is in contrast to the downregulation of iron-related genes in bovine cells, indicating a different approach to iron usage in cattle. The study also identified the upregulation of specific virulence genes, such as those involved in heme utilization and the Type III secretion system (espW), demonstrating STE
C’s pathogenic mechanisms of STEC during early host interactions. Overexpression of genes related to capsular polysaccharides and outer membrane proteins in the bovine host suggests strategies to evade immune responses and establish colonization. The upregulation of genes involved in colanic acid biosynthesis and extracellular polysaccharide production indicates the importance of biofilm formation for STEC survival and colonization in both human and bovine hosts. [
76]. These findings elucidate the complex transcriptional adaptations of STEC O157:H7 in response to different host environments during initial attachment, providing insights into pathogen colonization strategies, virulence mechanisms, and potential targets for interventions to prevent STEC infection.
Like genomics studies, proteomics on STEC colonization in cattle also underscored the critical role of metabolic and phenotypic traits in determining adherence and colonization mechanisms of different STEC strains. Persistently colonizing STEC strains (STECper) and sporadically colonizing strains (STECspo) exhibit distinct metabolic profiles, particularly in their ability to utilize specific carbon and sulfur sources. Notably, the metabolism of substrates such as glyoxylic acid and l-rhamnose were different between STECper and STECspo. Proteomic analyses have also identified mutations or disruptions in genes involved in the glyoxylate metabolism and rhamnose utilization pathways, which correlate with the colonization patterns observed in these strains. Additionally, the ability of STEC to form biofilms, a key proteomic trait, was found to be influenced by temperature, with STECspo producing more biofilms at lower temperatures as compared to STECper, suggesting an adaptation to environmental conditions [
43]. These findings highlight the importance of metabolic versatility and ecological adaptability during STEC adherence and colonization of the bovine gut.
Genome sequence analysis of STEC strains isolated from cattle has provided insight into genetic diversity, colonization factors, and evolution of STEC of bovine origin. Whole-genome sequencing and DNA microarray analysis have revealed that STECper isolates tend to possess a specific set of accessory genes absent in STECspo isolates. These accessory genes, including virulence-associated genes such as eae, nleA, nleB, and nleC, contribute to STEC adherence to the host intestinal epithelium, evasion of host immune response, and establishment of persistent infection. The presence of these genes and their variants correlates with the genetic background of STEC, as determined by multilocus sequence typing (MLST), with STECper clustering into separte phylogenetic groups. Moreover, the genomic plasticity of STEC allows for the horizontal transfer of these accessory genes, enabling the evolution of new virulent strains that can pose significant public health risks. These genomic differences between STECper and STECspo strains suggest formulating different mitigation strategies for different STEC genotypes to reduce STEC colonization of the bovine host [
77].
Genome-wide association studies were conducted on a diverse collection of STEC isolates from various hosts, including cattle. These studies revealed that certain accessory genes were strongly associated with cattle-specific isolates, suggesting that these genes play a crucial role in the bovine host. Genes involved in iron acquisition, such as the iroBCDEN gene cluster, were found to be prevalent in cattle-associated
STEC. These genes enhance the ability of bacteria to thrive in the bovine gut by effectively scavenging iron, a critical nutrient in the host environment. Additionally, the study identified variants of omptin proteins, such as OmpP, which are associated with cattle isolates and may contribute to the ability of bacteria to degrade antimicrobial peptides, thus aiding immune evasion and persistence within the bovine host [
78]. These genomic insights underscore the complex interplay of genetic factors that enable STEC to adapt to and colonize the bovine gut, which might have implications for STEC mitigation approaches.
6. Bovine Gut Microbiota and STEC Colonization
The bovine gut microbiota too plays a crucial role in shaping colonization dynamics of STEC [
72]. The gut microbiota comprises a diverse community of microorganisms that coexist within the GIT, aiding host nutrition, metabolism, and immune system development. In the context of STEC colonization, the gut microbiota acts as both a barrier and a facilitator. On the one hand, a healthy and diverse gut microbiota can prevent pathogen colonization through competitive exclusion and the production of antimicrobial compounds [
79,
80,
81]. Competitive exclusion refers to the mechanism by which resident microbiota outcompete potential pathogens for nutrients and attachment sites, thereby inhibiting colonization. Gut microbiota can also modulate the host immune response to enhance defense mechanisms against pathogens like STEC. [
81,
82].
The role of gut microbiota in regulating STEC colonization and in several therapeutic strategies has been proposed. Administration of beneficial microbes (probiotics) or substrates that selectively stimulate their growth (prebiotics) can help restore or maintain a healthy gut microbiome, potentially reducing STEC colonization [
81]. Specific probiotic strains (Lactobacillus plantarum and Lactobacillus fermentum) that inhibit STEC adhesion and toxin production have been identified [
83]. Another potential intervention strategy, Fecal Microbiota Transplantation (FMT), which involves the transfer of fecal material from a healthy donor to the gastrointestinal tract of a recipient to restore a healthy microbial community, has also been explored [
84]. This approach is particularly beneficial for managing recurrent or severe STEC infections because it aids in outcompeting pathogens by introducing healthy microbiota. Bacteriophage therapy utilizing bacteriophages that specifically target STEC can selectively reduce pathogen levels without disrupting the overall microbiota composition [
85,
86]. STEC-specific bacteriophages have both therapeutic and prophylactic uses against STEC colonization. Modification of host diet to promote the growth of beneficial microbes and inhibit the growth of pathogens can also be exploited to reduce STEC colonization of the bovine gut. In this context, dietary components that enhance the production of SCFAs or other antimicrobial metabolites by gut microbiota might be particularly valuable [
87].
7. Future Directions and Challenges
The study of host-pathogen interactions, mainly STEC colonization in bovine intestine, has advanced significantly. However, numerous gaps and challenges remain, necessitating a focused direction for future research. Although significant efforts have been made to understand STEC adherence and colonization mechanisms in cattle, the detailed strategies employed by STEC to adapt, survive, and thrive within the diverse environment of gut microbiota have not been fully elucidated. Understanding these adaptive mechanisms at a molecular level remains a critical challenge. The complex interplay between STEC, the gut microbiota, and the host immune system is not fully understood. Specifically, the role of non-bacterial components of the gut microbiota, such as fungi and viruses, in influencing STEC colonization and disease progression requires further exploration. Factors contributing to variability in bovine susceptibility to STEC colonization, including genetic, dietary, and environmental influences, require further investigation.
7.1. Challenges and Limitations
Studying STEC colonization in the bovine gut presents several challenges and limitations that complicate our understanding of pathogen behavior and the development of effective control strategies. One major challenge is the inherent genetic diversity among STEC strains [
88], which leads to varying colonization patterns and virulence profiles, making it difficult to generalize the findings across different strains. Additionally, the complex and dynamic nature of the bovine gut microbiome, influenced by factors such as diet, age, and environmental conditions, can affect the consistency and reproducibility of colonization studies. The use of animal models, although necessary for in vivo studies, also introduces limitations, as experimental conditions may not fully replicate the natural environment of commercial cattle herds. Furthermore, ethical considerations and logistical difficulties in conducting long-term studies on large animal populations can limit the scope and scale of research. Finally, it is challenging to translate findings from laboratory settings to practical on-farm interventions, as the controlled experimental conditions often do not account for the variability and complexity of the real-world farming environment. These challenges highlight the need for more refined methodologies and a multidisciplinary approach to better understand and mitigate STEC colonization in cattle.
7.2. Future Directions
Leveraging advanced omics technologies and bioinformatics tools to profile host-pathogen-microbiota interactions comprehensively is crucial. These approaches encompass single-cell sequencing, which can elucidate heterogeneity in the responses of STEC in human and bovine hosts during infection. CRISPR-Cas systems to dissect gene functions in STEC and the host is another approach to gain deeper insights into virulence mechanisms, host defense strategies, and potential therapeutic targets. Exploring novel prebiotics, probiotics, and dietary interventions to modulate gut microbiota composition could offer opportunities to mitigate STEC colonization and infection. A transdisciplinary approach to address the gaps and provide new insights while overcoming the challenges will significantly advance our understanding of STEC colonization, host-pathogen interactions, and the role of gut microbiota in the bovine host. Ultimately, this knowledge will contribute to developing innovative and effective strategies to combat STEC infections and improve public health.
Funding
ITK was supported by the USDA-ARS CRIS project 5030-32000-225-00D. SK was supported by the College of Veterinary Medicine, University of Florida.
Data Availability Statement
No data were created or analysed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Disclaimer: All opinions expressed in this paper are the authors’ and do not necessarily reflect the policies and views of USDA-ARS. The mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply a recommendation or endorsement by the USDA. The USDA is an equal opportunity provider and employer.
References
- Pennsylvania State University Extension. Gut Health in Cattle. Available online: https://extension.psu.edu/gut-health-in-cattle (accessed on 24 March 2024).
- Smith, J.L.; Fratamico, P.M.; Gunther, N.W. Shiga Toxin-Producing Escherichia coli. Adv. Appl. Microbiol. 2014, 86, 145–197. [Google Scholar] [CrossRef] [PubMed]
- Sapountzis, P.; Segura, A.; Desvaux, M.; Forano, E. An Overview of the Elusive Passenger in the Gastrointestinal Tract of Cattle: The Shiga Toxin-Producing Escherichia coli. Microorganisms 2020, 8, 877. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). National Shiga Toxin-Producing Escherichia coli (STEC) Surveillance Overview. Atlanta, Georgia: US Department of Health and Human Services, CDC, 2012.
- World Health Organization. Diarrhoeal Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 23 March 2024).
- Terajima, J.; Izumiya, H.; Hara-Kudo, Y.; Ohnishi, M. Shiga Toxin (Verotoxin)-Producing Escherichia coli and Foodborne Disease: A Review. Food Saf. 2017, 5, 35–53. [Google Scholar] [CrossRef]
- Frenzen, P.D.; Drake, A.; Angulo, F.J.; Emerging Infections Program FoodNet Working Group. Economic Cost of Illness Due to Escherichia coli O157 Infections in the United States. J. Food Prot. 2005, 68, 2623–2630. [Google Scholar] [CrossRef]
- Lee, K.S.; Jeong, Y.J.; Lee, M.S. Escherichia coli Shiga Toxins and Gut Microbiota Interactions. Toxins 2021, 13, 416. [Google Scholar] [CrossRef]
- Vasco, K.; Nohomovich, B.; Singh, P.; Venegas-Vargas, C.; Mosci, R.E.; Rust, S.; Bartlett, P.; Norby, B.; Grooms, D.; Zhang, L.; Manning, S.D. Characterizing the Cattle Gut Microbiome in Farms with a High and Low Prevalence of Shiga Toxin-Producing Escherichia coli. Microorganisms 2021, 9, 1737. [Google Scholar] [CrossRef]
- Mir, R.A.; Weppelmann, T.A.; Elzo, M.; Ahn, S.; Driver, J.D.; Jeong, K.C. Colonization of Beef Cattle by Shiga Toxin-Producing Escherichia coli During the First Year of Life: A Cohort Study. PLoS ONE 2016, 11, e0148518. [Google Scholar] [CrossRef]
- Cáceres, M.E.; Etcheverría, A.I.; Fernández, D.; Rodríguez, E.M.; Padola, N.L. Variation in the Distribution of Putative Virulence and Colonization Factors in Shiga Toxin-Producing Escherichia coli Isolated from Different Categories of Cattle. Front. Cell. Infect. Microbiol. 2017, 7, 147. [Google Scholar] [CrossRef]
- Menge, C. The Role of Escherichia coli Shiga Toxins in STEC Colonization of Cattle. Toxins 2020, 12, 607. [Google Scholar] [CrossRef]
- Hussein, H.S.; Sakuma, T. Prevalence of Shiga Toxin-Producing Escherichia coli in Dairy Cattle and Their Products. J. Dairy Sci. 2005, 88, 450–465. [Google Scholar] [CrossRef]
- Sajeena, T.A.M.; Kalyanikutty, S. Pathogenic Factors of Shiga Toxigenic Escherichia coli. J. Pure Appl. Microbiol. 2024, 18, 46–63. [Google Scholar] [CrossRef]
- Auvray, F.; Bièche-Terrier, C.; Um, M.M.; Dupouy, V.; Nzuzi, N.; David, L.; Allais, L.; Drouet, M.; Oswald, E.; Bibbal, D.; Brugère, H. Prevalence and Characterization of the Seven Major Serotypes of Shiga Toxin-Producing Escherichia coli (STEC) in Veal Calves Slaughtered in France. Vet. Microbiol. 2023, 282, 109754. [Google Scholar] [CrossRef]
- Zhao, L.; Tyler, P.J.; Starnes, J.; Bratcher, C.L.; Rankins, D.; McCaskey, T.A.; Wang, L. Correlation Analysis of Shiga Toxin-Producing Escherichia coli Shedding and Faecal Bacterial Composition in Beef Cattle. J. Appl. Microbiol. 2013, 115, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Baines, D.; Erb, S. Characterization of Shiga Toxin-Producing Escherichia coli Infections in Beef Feeder Calves and the Effectiveness of a Prebiotic in Alleviating Shiga Toxin-Producing Escherichia coli Infections. Ir. Vet. J. 2013, 66, 17. [Google Scholar] [CrossRef]
- Cáceres, M.E.; Etcheverría, A.I.; Fernández, D.; Rodríguez, E.M.; Padola, N.L. Variation in the Distribution of Putative Virulence and Colonization Factors in Shiga Toxin-Producing Escherichia coli Isolated from Different Categories of Cattle. Front. Cell. Infect. Microbiol. 2017, 7, 262141. [Google Scholar] [CrossRef]
- Lim, J.Y.; Yoon, J.; Hovde, C.J. A Brief Overview of Escherichia coli O157 and Its Plasmid O157. J. Microbiol. Biotechnol. 2010, 20, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Free, A.L.; Duoss, H.A.; Bergeron, L.V.; Shields-Menard, S.A.; Ward, E.; Callaway, T.R.; Carroll, J.A.; Schmidt, T.B.; Donaldson, J.R. Survival of O157 and Non-O157 Serogroups of Escherichia coli in Bovine Rumen Fluid and Bile Salts. Foodborne Pathog. Dis. 2012, 9, 1010–1014. [Google Scholar] [CrossRef]
- Ekiri, A.B.; Landblom, D.; Doetkott, D.; Olet, S.; Shelver, W.L.; Khaitsa, M.L. Isolation and Characterization of Shiga Toxin-Producing Escherichia coli Serogroups O26, O45, O103, O111, O113, O121, O145, and O157 Shed from Range and Feedlot Cattle from Postweaning to Slaughter. J. Food Prot. 2014, 77, 1052–1061. [Google Scholar] [CrossRef]
- Venegas-Vargas, C.; Henderson, S.; Khare, A.; Mosci, R.E.; Lehnert, J.D.; Singh, P.; Ouellette, L.M.; Norby, B.; Funk, J.A.; Rust, S.; Bartlett, P.C.; Grooms, D.; Manning, S.D. Factors Associated with Shiga Toxin-Producing Escherichia coli Shedding by Dairy and Beef Cattle. Appl. Environ. Microbiol. 2016, 82, 5049–5056. [Google Scholar] [CrossRef]
- Blankenship, H.M.; Carbonell, S.; Mosci, R.E.; McWilliams, K.; Pietrzen, K.; Benko, S.; Gatesy, T.; Grooms, D.; Manning, S.D. Genetic and Phenotypic Factors Associated with Persistent Shedding of Shiga Toxin-Producing Escherichia coli by Beef Cattle. Appl. Environ. Microbiol. 2020, 86, e01292–20. [Google Scholar] [CrossRef]
- Newton, H.J.; Pearson, J.S.; Badea, L.; Kelly, M.; Lucas, M.; Holloway, G.; Wagstaff, K.M.; Dunstone, M.A.; Sloan, J.; Whisstock, J.C.; Kaper, J.B.; Robins-Browne, R.M.; Jans, D.A.; Frankel, G.; Phillips, A.D.; Coulson, B.S.; Hartland, E.L. The Type III Effectors NleE and NleB from Enteropathogenic Escherichia coli and OspZ from Shigella Block Nuclear Translocation of NF-κB p65. PLoS Pathog. 2010, 6, e1000898. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Annual Epidemiological Report for 2021: Shiga Toxin-Producing Escherichia coli (STEC) Infections. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER%20STEC%20-%202021.pdf (accessed on 11 August 2024).
- Ballem, A.; Gonçalves, S.; Garcia-Meniño, I.; Flament-Simon, S.C.; Blanco, J.E.; Fernandes, C.; Saavedra, M.J.; Pinto, C.; Oliveira, H.; Blanco, J.; Almeida, G.; Almeida, C. Prevalence and Serotypes of Shiga Toxin-Producing Escherichia coli (STEC) in Dairy Cattle from Northern Portugal. PLoS ONE 2020, 15, e0244713. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, D.V.; Keith, M.; Williamson, H.; Macleod, K.; Mathie, H.; Handel, I.; Currie, C.; Holmes, A.; Allison, L.; McLean, R.; Callaby, R.; Porphyre, T.; Tongue, S.C.; Henry, M.K.; Evans, J.; Gunn, G.J.; Gally, D.L.; Silva, N.; Chase-Topping, M.E. Prevalence and Epidemiology of Non-O157 Escherichia coli Serogroups O26, O103, O111, and O145 and Shiga Toxin Gene Carriage in Scottish Cattle, 2014–2015. Appl. Environ. Microbiol. 2021, 87, e03142–20. [Google Scholar] [CrossRef]
- dos Santos, L.F.; Gonçalves, E.M.; Vaz, T.M.; Irino, K.; Guth, B.E. Distinct Pathotypes of O113 Escherichia coli Strains Isolated from Humans and Animals in Brazil. J. Clin. Microbiol. 2007, 45, 2028–2030. [Google Scholar] [CrossRef]
- Elmonir, W.; Shalaan, S.; Tahoun, A. Prevalence, Antimicrobial Resistance, and Genotyping of Shiga Toxin-Producing Escherichia coli in Foods of Cattle Origin, Diarrheic Cattle, and Diarrheic Humans in Egypt. Gut Pathog. 2021, 13, 8. [Google Scholar] [CrossRef]
- Capps, K.M.; Ludwig, J.B.; Shridhar, P.B.; Shi, X.; Roberts, E.; DebRoy, C.; Cernicchiaro, N.; Phebus, R.K.; Bai, J.; Nagaraja, T.G. Identification, Shiga Toxin Subtypes and Prevalence of Minor Serogroups of Shiga Toxin-Producing Escherichia coli in Feedlot Cattle Feces. Sci. Rep. 2021, 11, 8601. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, M.S.; Kim, J.H. Recent Updates on Outbreaks of Shiga Toxin-Producing Escherichia coli and Its Potential Reservoirs. Front. Cell Infect. Microbiol. 2020, 10, 273. [Google Scholar] [CrossRef]
- Conrad, C.; Stanford, K.; McAllister, T.; Thomas, J.; Reuter, T. Shiga Toxin-Producing Escherichia coli and Current Trends in Diagnostics. Anim. Front. 2016, 6, 37–43. [Google Scholar] [CrossRef]
- Díaz, L.; Gutierrez, S.; Moreno-Switt, A.I.; Hervé, L.P.; Hamilton-West, C.; Padola, N.L.; Navarrete, P.; Reyes-Jara, A.; Meng, J.; González-Escalona, N.; Toro, M. Diversity of Non-O157 Shiga Toxin-Producing Escherichia coli Isolated from Cattle from Central and Southern Chile. Animals 2021, 11, 2388. [Google Scholar] [CrossRef]
- Ray, R.; Singh, P. Prevalence and Implications of Shiga Toxin-Producing E. coli in Farm and Wild Ruminants. Pathogens 2022, 11, 1332. [Google Scholar] [CrossRef]
- World Health Organization. E. coli. Available online: https://www.who.int/news-room/fact-sheets/detail/e-coli (accessed on 12 March 2024).
- Hunt, J.M. Shiga Toxin-Producing Escherichia coli (STEC). Clin. Lab. Med. 2010, 30, 21–45. [Google Scholar] [CrossRef]
- Vachon, M.S.; Khalid, M.; Tarr, G.A.M.; Hedberg, C.; Brown, J.A. Farm Animal Contact Is Associated with Progression to Hemolytic Uremic Syndrome in Patients with Shiga Toxin-Producing Escherichia coli - Indiana, 2012-2018. One Health 2020, 11, 100175. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Cointe, A.; Mariani Kurkdjian, P.; Rafat, C.; Hertig, A. Shiga Toxin-Associated Hemolytic Uremic Syndrome: A Narrative Review. Toxins 2020, 12, 67. [Google Scholar] [CrossRef]
- Liu, Y.; Thaker, H.; Wang, C.; Xu, Z.; Dong, M. Diagnosis and Treatment for Shiga Toxin-Producing Escherichia coli Associated Hemolytic Uremic Syndrome. Toxins 2023, 15, 10. [Google Scholar] [CrossRef]
- Kudva, I.T.; Biernbaum, E.N.; Cassmann, E.D.; Palmer, M.V. Bovine Rectoanal Junction In Vitro Organ Culture Model System to Study Shiga Toxin-Producing Escherichia coli Adherence. Microorganisms 2023, 11, 1289. [Google Scholar] [CrossRef]
- Kudva, I.T.; Dean-Nystrom, E.A. Bovine Recto-Anal Junction Squamous Epithelial (RSE) Cell Adhesion Assay for Studying Escherichia coli O157 Adherence. J. Appl. Microbiol. 2011, 111, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Segura, A.; Bertin, Y.; Durand, A. ; Transcriptional Analysis Reveals Specific Niche Factors and Response to Environmental Stresses of Enterohemorrhagic Escherichia coli O157 in Bovine Digestive Contents. BMC Microbiol. 2021, 21, 284. [Google Scholar] [CrossRef]
- Barth, S.A.; Weber, M.; Schaufler, K.; Berens, C.; Geue, L.; Menge, C. Metabolic Traits of Bovine Shiga Toxin-Producing Escherichia coli (STEC) Strains with Different Colonization Properties. Toxins 2020, 12, 414. [Google Scholar] [CrossRef]
- Castro, V.S.; Ngo, S.; Stanford, K. Influence of Temperature and pH on Induction of Shiga Toxin Stx1a in Escherichia coli. Front. Microbiol. 2023, 14, 1181027. [Google Scholar] [CrossRef]
- Hoffman, M.A.; Menge, C.; Casey, T.A.; Laegreid, W.; Bosworth, B.T.; Dean-Nystrom, E.A. Bovine Immune Response to Shiga-Toxigenic Escherichia coli O157. Clin. Vaccine Immunol. 2006, 13, 1322–1327. [Google Scholar] [CrossRef]
- Misyurina, O.; Asper, D.J.; Deng, W.; Finlay, B.B.; Rogan, D.; Potter, A.A. The Role of Tir, EspA, and NleB in the Colonization of Cattle by Shiga Toxin Producing Escherichia coli O26. Can. J. Microbiol. 2010, 56, 739–747. [Google Scholar] [CrossRef]
- Gaytán, M.O.; Martínez-Santos, V.I.; Soto, E.; González-Pedrajo, B. Type Three Secretion System in Attaching and Effacing Pathogens. Front. Cell Infect. Microbiol. 2016, 6, 129. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, Z.; Duan, Q.; Hardwidge, P.R.; Zhu, G. Escherichia coli Type III Secretion System 2: A New Kind of T3SS? Vet. Res. 2014, 45, 32. [Google Scholar] [CrossRef] [PubMed]
- Coburn, B.; Sekirov, I.; Finlay, B.B. Type III Secretion Systems and Disease. Clin. Microbiol. Rev. 2007, 20, 535–549. [Google Scholar] [CrossRef]
- Hotinger, J.A.; Pendergrass, H.A.; May, A.E. Molecular Targets and Strategies for Inhibition of the Bacterial Type III Secretion System (T3SS); Inhibitors Directly Binding to T3SS Components. Biomolecules 2021, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Cobbold, R.N.; Desmarchelier, P.M. In Vitro Studies on the Colonization of Bovine Colonic Mucosa by Shiga-Toxigenic Escherichia coli (STEC). Epidemiol. Infect. 2004, 132, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.; Nataro, J.; Mobley, H. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Farfan, M.J.; Torres, A.G. Molecular Mechanisms That Mediate Colonization of Shiga Toxin-Producing Escherichia coli Strains. Infect. Immun. 2012, 80. [Google Scholar] [CrossRef]
- McWilliams, B.D.; Torres, A.G. EHEC Adhesins. Microbiol. Spectr. 2014, 2, EHEC00032013. [Google Scholar] [CrossRef]
- Fedorchuk, C.; Kudva, I.T.; Kariyawasam, S. The Escherichia coli O157 Carbon Starvation-Inducible Lipoprotein Slp Contributes to Initial Adherence In Vitro via the Human Polymeric Immunoglobulin Receptor. PLoS ONE 2019, 14, e0216791. [Google Scholar] [CrossRef]
- Ritchie, J.M.; Brady, M.J.; Riley, K.N.; Ho, T.D.; Campellone, K.G.; Herman, I.M.; Donohue-Rolfe, A.; Tzipori, S.; Waldor, M.K.; Leong, J.M. EspFU, a Type III-Translocated Effector of Actin Assembly, Fosters Epithelial Association and Late-Stage Intestinal Colonization by Escherichia coli O157. Cell. Microbiol. 2008, 10, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.K.; Smollett, K.; Cleary, J.; Garmendia, J.; Straatman-Iwanowska, A.; Frankel, G.; Knutton, S. Enteropathogenic Escherichia coli Type III Effectors EspG and EspG2 Disrupt the Microtubule Network of Intestinal Epithelial Cells. Infect. Immun. 2005, 73, 4385–4390. [Google Scholar] [CrossRef]
- Royan, S.V.; Jones, R.M.; Koutsouris, A.; Roxas, J.L.; Falzari, K.; Weflen, A.W.; Kim, A.; Bellmeyer, A.; Turner, J.R.; Neish, A.S.; Rhee, K.J.; Viswanathan, V.K.; Hecht, G.A. Enteropathogenic Escherichia coli Non-LEE Encoded Effectors NleH1 and NleH2 Attenuate NF-κB Activation. Mol. Microbiol. 2010, 78, 1232–1245. [Google Scholar] [CrossRef]
- Kavaliauskiene, S.; Dyve Lingelem, A.B.; Skotland, T.; Sandvig, K. Protection Against Shiga Toxins. Toxins 2017, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Dziva, F.; Mahajan, A.; Cameron, P.; Currie, C.; McKendrick, I.J.; Wallis, T.S.; Smith, D.G.E.; Stevens, M.P. EspP, a Type V-Secreted Serine Protease of Enterohaemorrhagic Escherichia coli O157, Influences Intestinal Colonization of Calves and Adherence to Bovine Primary Intestinal Epithelial Cells. FEMS Microbiol. Lett. 2007, 271, 258–264. [Google Scholar] [CrossRef]
- Dautin, N. Serine Protease Autotransporters of Enterobacteriaceae (SPATEs): Biogenesis and Function. Toxins 2010, 2, 1179–1206. [Google Scholar] [CrossRef] [PubMed]
- Ta, A.; Vasudevan, S.O.; Wright, S.S.; Kumari, P.; Havira, M.S.; Surendran Nair, M.; Rathinam, V.A.; Vanaja, S.K. A Bacterial Autotransporter Impairs Innate Immune Responses by Targeting the Transcription Factor TFE3. Nat. Commun. 2023, 14, 1–17. [Google Scholar] [CrossRef]
- Xing, Y.; Clark, J.R.; Chang, J.D.; Chirman, D.M.; Green, S.; Zulk, J.J.; Jelinski, J.; Patras, K.A.; Maresso, A.W. Broad Protective Vaccination Against Systemic Escherichia coli with Autotransporter Antigens. PLoS Pathog. 2023, 19, e1011082. [Google Scholar] [CrossRef]
- Stevens, M.P.; van Diemen, P.M.; Frankel, G.; Phillips, A.D.; Wallis, T.S. Efa1 Influences Colonization of the Bovine Intestine by Shiga Toxin-Producing Escherichia coli Serotypes O5 and O111. Infect. Immun. 2002, 70, 5158–5166. [Google Scholar] [CrossRef]
- Rashid, R.A.; Tarr, P.I.; Moseley, S.L. Expression of the Escherichia coli IrgA Homolog Adhesin Is Regulated by the Ferric Uptake Regulation Protein. Microb. Pathog. 2006, 41, 207–217. [Google Scholar] [CrossRef]
- Padola, N.L.; Etcheverría, A.I. Shiga Toxin-Producing Escherichia coli in Human, Cattle, and Foods. Strategies for Detection and Control. Front. Cell. Infect. Microbiol. 2014, 4, 89. [Google Scholar] [CrossRef] [PubMed]
- Paton, A.W.; Srimanote, P.; Woodrow, M.C.; Paton, J.C. Characterization of Saa, a Novel Autoagglutinating Adhesin Produced by Locus of Enterocyte Effacement-Negative Shiga-Toxigenic Escherichia coli Strains That Are Virulent for Humans. Infect. Immun. 2001, 69, 6999–7009. [Google Scholar] [CrossRef]
- Katani, R.; Kudva, I.T.; Srinivasan, S.; Stasko, J.B.; Schilling, M.; Li, L.; Cote, R.; DebRoy, C.; Arthur, T.M.; Sokurenko, E.V.; Kapur, V. Strain and Host-Cell Type Dependent Role of Type 1 Fimbriae Genes in the Adherence Phenotype of Super-Shedder Strains of Escherichia coli O157. Int. J. Med. Microbiol. 2021, Article ID 151511, 11 pages. [CrossRef]
- Galli, L.; Torres, A.G.; Rivas, M. Identification of the Long Polar Fimbriae Gene Variants in the Locus of Enterocyte Effacement-Negative Shiga Toxin-Producing Escherichia coli Strains Isolated from Humans and Cattle in Argentina. FEMS Microbiol. Lett. 2010, 308, 123–129. [Google Scholar] [CrossRef]
- Sheng, H.; Xue, Y.; Zhao, W.; Hovde, C.J.; Minnich, S.A. Escherichia coli O157 Curli Fimbriae Promote Biofilm Formation, Epithelial Cell Invasion, and Persistence in Cattle. Microorganisms 2020, 8, 580. [Google Scholar] [CrossRef]
- Samadder, P.; Xicohtencatl-Cortes, J.; Saldaña, Z.; Jordan, D.; Tarr, P.I.; Kaper, J.B.; Girón, J.A. The Escherichia coli ycbQRST Operon Encodes Fimbriae with Laminin-Binding and Epithelial Cell Adherence Properties in Shiga-Toxigenic Escherichia coli O157. Environ. Microbiol. 2009, 11, 1815–1826. [Google Scholar] [CrossRef]
- Kudva, I.T.; Krastins, B.; Torres, A.G.; Griffin, R.W.; Sheng, H.; Sarracino, D.A.; Hovde, C.J.; Calderwood, S.B.; John, M. The Escherichia coli O157 Cattle Immunoproteome Includes Outer Membrane Protein A (OmpA), a Modulator of Adherence to Bovine Rectoanal Junction Squamous Epithelial (RSE) Cells. Proteomics 2015, 15, 1829–1842. [Google Scholar] [CrossRef]
- Wong, A.R.; Pearson, J.S.; Bright, M.D.; Munera, D.; Robinson, K.S.; Lee, S.F.; Frankel, G.; Hartland, E.L. Enteropathogenic and Enterohemorrhagic Escherichia coli: Even More Subversive Elements. Mol. Microbiol. 2011, 80, 1420–1438. [Google Scholar] [CrossRef]
- Tristão, L.C.; Gonzalez, A.G.; Coutinho, C.A.; Cerqueira, A.M.; Gomes, M.J.; Irino, K.; Guth, B.E.; Andrade, J.R. Virulence Markers and Genetic Relationships of Shiga Toxin-Producing Escherichia coli Strains from Serogroup O111 Isolated from Cattle. Vet. Microbiol. 2007, 119, 358–365. [Google Scholar] [CrossRef]
- Stromberg, Z.R.; Masonbrink, R.E.; Mellata, M. Transcriptomic Analysis of Shiga Toxin-Producing Escherichia coli During Initial Contact with Cattle Colonic Explants. Microorganisms 2020, 8, 1662. [Google Scholar] [CrossRef]
- Edison, L.K.; Kudva, I.T.; Kariyawasam, S. Comparative Transcriptome Analysis of Shiga Toxin-Producing Escherichia coli O157 on Bovine Rectoanal Junction Cells and Human Colonic Epithelial Cells During Initial Adherence. Microorganisms 2023, 11, 2562. [Google Scholar] [CrossRef]
- Tiwari, S.K.; van der Putten, B.C.L.; Fuchs, T.M.; Vinh, T.N.; Bootsma, M.; Oldenkamp, R.; La Ragione, R.; Matamoros, S.; Hoa, N.T.; Berens, C.; Leng, J.; Álvarez, J.; Ferrandis-Vila, M.; Ritchie, J.M.; Fruth, A.; Schwarz, S.; Domínguez, L.; Ugarte-Ruiz, M.; Bethe, A.; Huber, C.; Johanns, V.; Stamm, I.; Wieler, L.H.; Ewers, C.; Fivian-Hughes, A.; Schmidt, H.; Menge, C.; Semmler, T.; Schultsz, C. Genome-Wide Association Reveals Host-Specific Genomic Traits in Escherichia coli. BMC Biol. 2023, 21, 76. [Google Scholar] [CrossRef] [PubMed]
- Barth, S.A.; Menge, C.; Eichhorn, I.; Semmler, T.; Wieler, L.H.; Pickard, D.; Belka, A.; Berens, C.; Geue, L. The Accessory Genome of Shiga Toxin-Producing Escherichia coli Defines a Persistent Colonization Type in Cattle. Appl. Environ. Microbiol. 2016, 82, 5455–5464. [Google Scholar] [CrossRef] [PubMed]
- Vasco, K.; Nohomovich, B.; Singh, P.; Venegas-Vargas, C.; Mosci, R.E.; Rust, S.; Bartlett, P.; Norby, B.; Grooms, D.; Zhang, L.; Manning, S.D. Characterizing the Cattle Gut Microbiome in Farms with a High and Low Prevalence of Shiga Toxin-Producing Escherichia coli. Microorganisms 2021, 9, 1737. [Google Scholar] [CrossRef] [PubMed]
- Ducarmon, Q.R.; Zwittink, R.D.; Hornung, B.V.H.; van Schaik, W.; Young, V.B.; Kuijper, E.J. Gut Microbiota and Colonization Resistance Against Bacterial Enteric Infection. Microbiol. Mol. Biol. Rev. 2019, 83, e00007–19. [Google Scholar] [CrossRef]
- Khan, I.; Bai, Y.; Zha, L.; Ullah, N.; Ullah, H.; Shah, S.R.; Sun, H.; Zhang, C. Mechanism of the Gut Microbiota Colonization Resistance and Enteric Pathogen Infection. Front. Cell. Infect. Microbiol. 2021, 11, 716299. [Google Scholar] [CrossRef]
- Chang, P.V. Chemical Mechanisms of Colonization Resistance by the Gut Microbial Metabolome. ACS Chem. Biol. 2020, 15, 1119–1126. [Google Scholar] [CrossRef]
- Giordano, M.; Baldassarre, M.E.; Palmieri, V.; Torres, D.D.; Carbone, V.; Santangelo, L.; Gentile, F.; Panza, R.; Di Mauro, F.; Capozza, M.; Di Mauro, A.; Laforgia, N. Management of STEC Gastroenteritis: Is There a Role for Probiotics? Int. J. Environ. Res. Public Health 2019, 16, 1649. [Google Scholar] [CrossRef]
- Gupta, S.; Allen-Vercoe, E.; Petrof, E.O. Fecal Microbiota Transplantation: In Perspective. Ther. Adv. Gastroenterol. 2016, 9, 229–239. [Google Scholar] [CrossRef]
- Hyla, K.; Dusza, I.; Skaradzińska, A. Recent Advances in the Application of Bacteriophages Against Common Foodborne Pathogens. Antibiotics 2022, 11, 1536. [Google Scholar] [CrossRef]
- Emencheta, S.C.; Olovo, C.V.; Eze, O.C.; Kalu, C.F.; Berebon, D.P.; Onuigbo, E.B.; Vila, M.M.D.C.; Balcão, V.M.; Attama, A.A. The Role of Bacteriophages in the Gut Microbiota: Implications for Human Health. Pharmaceutics 2023, 15, 2416. [Google Scholar] [CrossRef]
- Mazhar, M.; Zhu, Y.; Qin, L. The Interplay of Dietary Fibers and Intestinal Microbiota Affects Type 2 Diabetes by Generating Short-Chain Fatty Acids. Foods 2023, 12, 1023. [Google Scholar] [CrossRef] [PubMed]
- Galarce, N.; Sánchez, F.; Escobar, B.; Lapierre, L.; Cornejo, J.; Alegría-Morán, R.; Neira, V.; Martínez, V.; Johnson, T.; Fuentes-Castillo, D.; Sano, E.; Lincopan, N. Genomic Epidemiology of Shiga Toxin-Producing Escherichia coli Isolated from the Livestock-Food-Human Interface in South America. Animals 2021, 11, 1845. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).