1. Introduction
Salvia miltiorrhiza (SM) is a genus of the
Labiatae family, has been traditionally used in China, Korea, and Japan in Asian countries [
1]. SM contains over 100 active compounds which are classified two main categories: water-soluble phenolic acid components and hydrophobic tanshinones [
2]. The tanshinone group primarily consists of lipophilic phenanthrene-quinone and its derivatives [
3], including dihydrotanshinone I (DHTS), cryptotanshinone (CT), tanshinone I (Tan I), and tanshinone IIA (Tan IIA). These secondary metabolites accumulate mainly in the roots and exhibit various pharmacological effects such as antibacterial, antioxidant, and antitumor properties, making them promising candidates for the treatment of diseases like cardiovascular and cerebrovascular diseases disorders [
4,
5]. Notably, due to their natural origin, tanshinones have been extensively incorporated into various formulations such as tablets, injections, and ointments in combination with modern medicine, providing new therapeutic options and significant industrial value as high-value natural product materials [
6]. However, despite the high value, the extraction yield of tanshinones remains low, and ongoing research to improve their efficient utilization [
7,
8].
The conventional approach to extracting tanshinones involves the use of organic solvents such as ethanol and methanol. However, the application of these solvents in industries like food and pharmaceuticals is limited due to their toxicity and high flammability. Additionally, even when solvents are used, extraction steps are required to remove them at end of the process, which increases production cost. Moreover, residual solvent may inadvertently remain, raising concerns about product quality and safety [
9].
The International Council for Harmonisation (ICH) guidelines set limits for residual solvent in organic solvents specifying a maximum of 30 mg/L for methanol and 50 mg/day for ethanol. While the Food and Agriculture Organization (FAO) does not regulate ethanol, it does recommend a limit 10 mg/kg for methanol in most foods [
10,
11].
Alternative extraction methods include reflux extraction with polar solvents such as CHCl
3 and ethyl acetate [
12], soaking, percolation, and ultrasonic extraction [
13], continuous ultrasound-assisted extraction with high intensity ultrasonic probe (CUAE-HIUP) [
14], supercritical fluid extraction (SFE) [
15], pressurized-liquid extraction [
16], infrared-assisted extraction [
17], and ionic liquid-based ultra-high pressure extraction [
18]. However, the need to develop new, simple, and safe extraction methods remains critical, as existing methods often require expensive equipment or involve complex processes that can increase production costs.
Cloud point extraction (CPE) is a technique in which a surfactant is heated to a temperature to reach its ’cloud point’ where the solution becomes cloudy, leading to the formation of micelles. These micelles separate into a surfactant-rich layer and an aqueous layer, trapping the analytes within the surfactant layer [
19]. The micelles have a hydrophobic core that captures hydrophobic compounds, while the hydrophilic outer layer stabilizes the micelles in the aqueous layer [
20]. CPE is simple, cost-effective, and environmentally friendly method that minimized or eliminates the use of organic solvent [
19,
21]. It has been applied to extract organic compounds from food or heavy metals from water, and bioactive substances from plants [
22,
23,
24]. Nonionic surfactants such as Triton X-100, Triton X-114, and Tween 80 are commonly used in CPE. Previous studies have been used, synthetic surfactants like Genapol X-080 or Triton X-100 to extract tanshinones from SM [
25,
26]. While these surfactants are approved as edible by the U.S. Food and Drug Administration (FDA), they are not naturally derived. In contrast, lecithin, a natural surfactant obtained from sources, like sunflower, soybean, and egg, is widely used in the food, pharmaceutical, and cosmetic industries due to its non-toxic and biocompatible nature. Lecithin is also designated as GRAS (Generally Recognized as Safe) by FDA [
27]. Additionally, it is classified as a food additive in the European Union (EU) under the code E322 and can be used in any quantity [
27,
28]
Given the growing demand for tanshinone derived from Salvia miltiorrhiza (SM), there is an urgent need for efficient extraction methods that maximize yield and preserve bioactivity. This study aims to optimize cloud point extraction (CPE) using lecithin, a natural surfactant, as an alternative to organic solvents for extracting tanshinones, the hydrophobic compounds from SM. The extraction efficiency of the optimized CPE method will be evaluated by comparing it with extracts obtained using conventional solvent-based methods through component analysis.
2. Materials and Methods
2.1. Chemicals
Solid lecithin derived from soybean, along with standards for HPLC analysis, dihydrotanshinone I (DHTS), cryptotanshinone (CT), tanshinone I (Tan I), and tanshinone IIA (Tan IIA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). NaCl, citric acid anhydrous, and methanol were obtained from DAEJUNG (Siheung, Korea). Filter paper (No. 6) was sourced from Whatman International Ltd. (Maidstone, England), and syringe filters (13JP020AN, 13HP020AN, 0.2 ㎛) were purchased from Advantec Mfs. Inc. (Dublin, CA, USA). HPLC-grade acetonitrile and water were supplied by DUKSAN (Ansan, Korea), and acetic acid glacier was purchased from J.T. Baker (Phillipsburg, NJ, USA).
2.2. Sample Pretreatment
The SM samples were provided by the Korean variety ’Dasan’, cultivated and harvested in April 2021 by the SM Specialty Division of the Rural Development Administration in Eumsung, Korea. The samples were dried at 55 °C and ground into fine powder. The powder was then stored at 4 °C until used in the experiments.
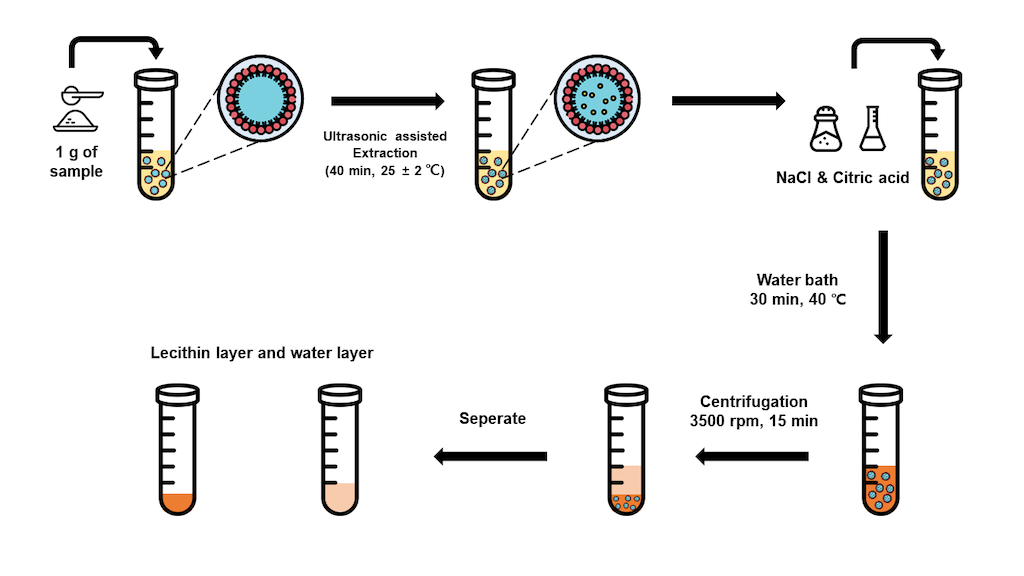
Samples (1 g each) were mixed with water containing 5% (w/v) lecithin, and subjected to ultrasonic-assisted extraction (UAE) at room temperature for 40 minutes. The supernatant was then separated by centrifugation at 3500 rpm for 15 minutes. The supernatant was collected, and as a control, the SM water extract was subjected to the same conditions as the optimized CPE, excluding the CPE process.
2.3. Cloud Point Extraction (CPE) Procedure
The CPE process was optimized by systematically adjusting the solid-to-liquid ratio, surfactant concentration, NaCl concentration, pH, and equilibrium temperature. The CPE method was adopted from Alibade et al. [
27] and Bi et al. [
26]. After the supernatant was separated, 5% (w/v) NaCl was added, and the pH was adjusted to 3 using 1 M citric acid. The mixture was equilibrated in a water bath at 40 °C for 30 minutes, then centrifuged at 3500 rpm for 15 minutes to separate the surfactant layer from water layer. Both layers were freeze-dried, with the water layer diluted with distilled water and the surfactant layer diluted with methanol to a concentration of 10 mg/mL for analysis. The final selection was based on the content of tanshinones (DHTS, CT, Tan I, and Tan IIA), which showed the highest levels in the surfactant layer and the lowest levels in the water layer.
2.4. HPLC Analysis
The constituents in the extract were analyzed using a modified method based on Chen et al. [
29] targeting four components (DHTS, CT, Tan I, Tan IIA). Standards for each tanshinone were prepared at concentrations of 2 ug/mL, 4 ug/mL, 8 ug/mL, 16 ug/mL, and 32 ug/mL. Calibration curves were generated to calculate regression equation and R
2 values to determine the sample’s component content. All samples were analyzed at 10000 ug/mL. High-performance liquid chromatography (HPLC) analysis was conducted using a Shimadzu LC-20AT HPLC system equipped with a YMC-Pack ODS-AM column (250 mm× 4.6 mm I.D., 5 um) maintained at 30 °C. The flow rate was set at 1.0 mL/min, with an injection volume of 10 uL and detection was performed at 280 nm. The mobile phases consisted of 0.8% (v/v) acetic acid in water (A) and 0.8% (v/v) acetic acid in acetonitrile (B) were eluted in a gradient manner as follows: 2-46% B from 0 to 40 min, 46-66% B from 40 to 60 min, 66-48% B from 60 to 70 min, 48-90% B from 70 to 71 min, and 90-90% B from 71 to 80 min.
2.5. Transmission Electron Microscopy (TEM) Analysis
Transmission electron microscopy (TEM; JEM-2100F, JEOL, Akishima, Japan) was used to visually analyze the morphology and size of micelles, as well as the transmittance of the samples based on surfactant concentration. All lyophilized surfactant layer samples were dispersed in distilled water and then dried on filter paper (1002-090, 90 mm, Whatman, Maidstone, UK). Measurements were conducted at an accelerating voltage of 200 kV.
2.6. Statistical Evaluation
Each experiment was performed in triplicate, and the mean value and standard error were calculated. Statistical analysis was conducted using GraphPad Prism 8.0.1 (San Diego, CA, USA) to assess significance at the 1% level of the T-test (p < 0.01).
3. Results and Discussion
3.1. Calibration Curves and Linearity
The standard curves and coefficients of determination for each component are shown in
Table 1. The results confirm the method’s suitability for both quantitative and qualitative analysis, validating its linearity and accuracy.
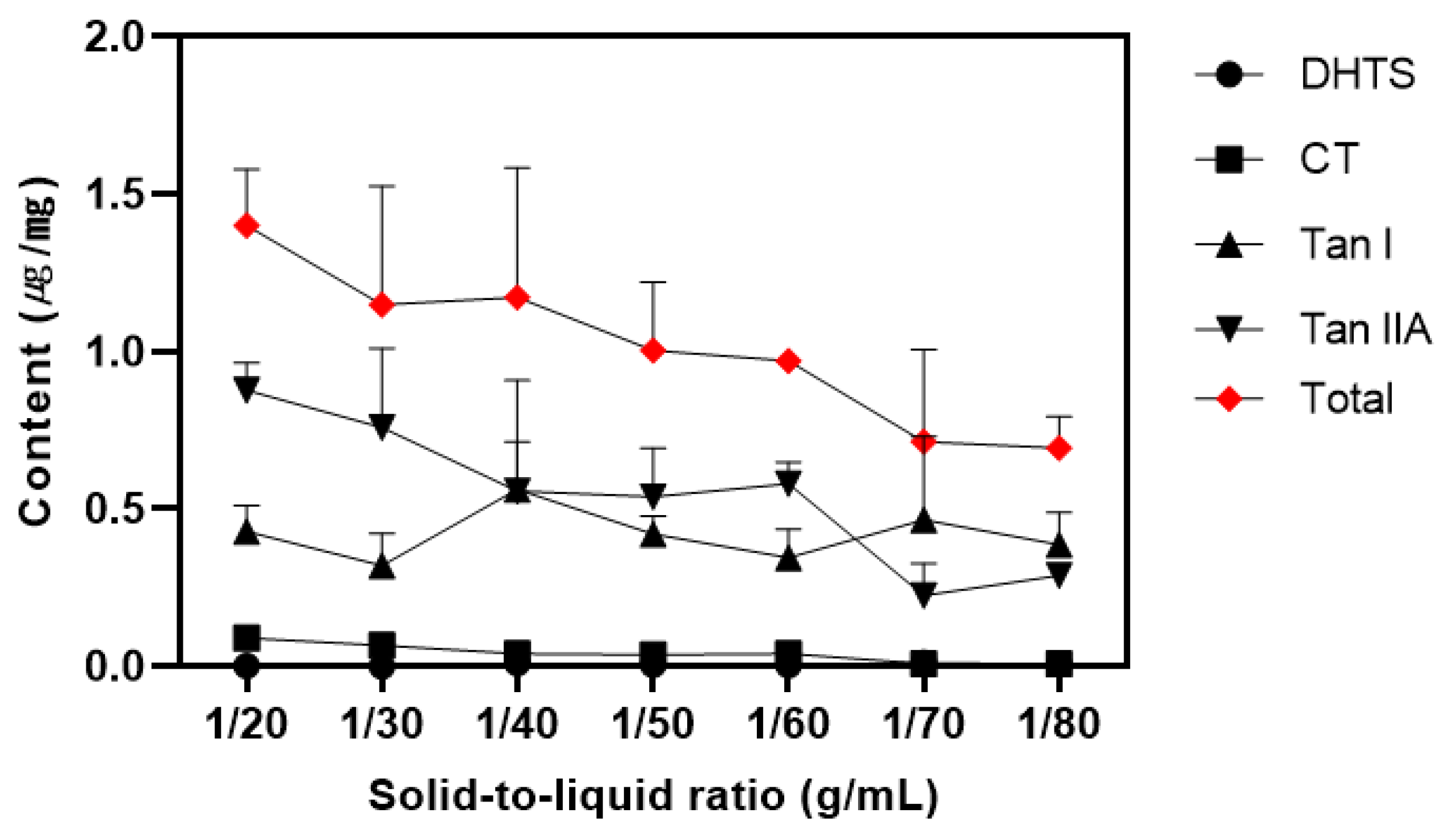
3.2. Effect of Solid-to-Liquid Ratio
The solid-to-liquid ratio is a crucial factor in sample extraction as it influences the concentration gradient between the extraction solvent and the sample surface, thereby affecting the extraction kinetics [
30].
Figure 1 illustrates the impact of varying the extraction solvent ratio from 20 to 80 mL per gram of sample.
For the surfactant layer, CT and Tan IIA showed a gradual decrease in component content as the amounts of extraction solvent increased. Although DHTS displayed statistically significant differences, the practical impact was negligible considering the margin of error. Tan I exhibited high mean values at 40, 50, and 60 mL, but due to the large standard deviation, none of the ratios showed statistically significant differences. Considering the overall content, the extraction ratio of 1 g/20 mL was found to yield the highest content (
Figure 1).
In the water layer, the content of CT, Tan I, and Tan IIA generally increased as the extraction solvent ratio increased (
Table S1). This was attributed to excessive extraction solvent use, which hindered proper layer separation. While a higher solvent ratio can enhance extraction efficiency, an excessive amount actually reduces efficiency and prolongs the concentration time [
31]. A study by Leite et al. [
32] demonstrated that reducing the proportion of non-ionic surfactant solution in the chlorophyll extraction from spinach leaves increased the amount of chlorophyll extracted. Similarly, Shi et al. [
25] found that an extraction ratio of 1 g/20 mL was optimal for extracting tanshinones from SM using micelles and measuring them by HPLC. Therefore, based on the above studies, 1 g/20 mL was concluded to be the optimal extraction ratio.
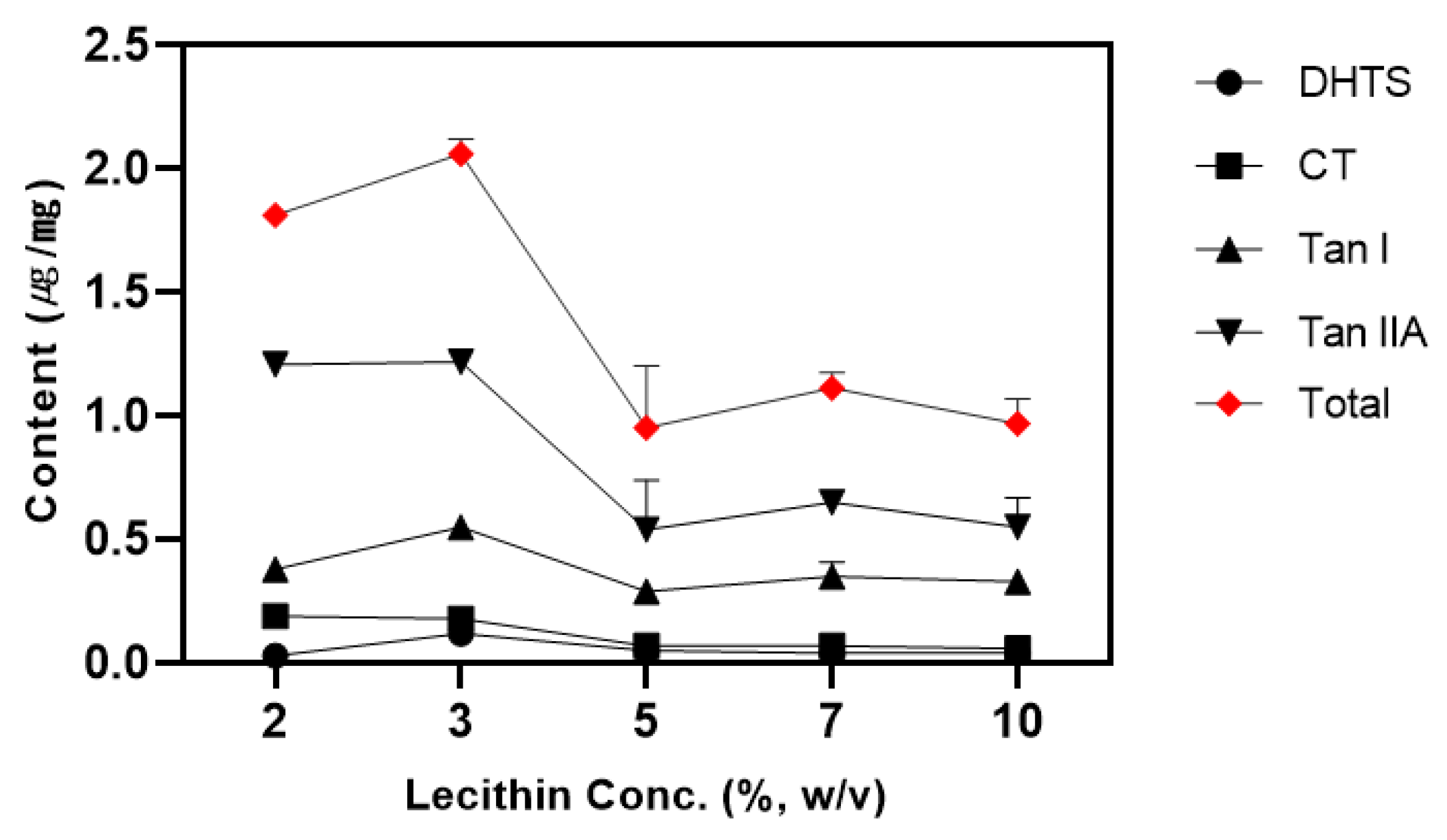
3.3. Effect of Surfactant Concentration
Surfactant concentration is a key factor in optimizing the CPE process and, together with the solid-to-liquid ratio, plays a significant role in determining extraction efficiency. To effectively capture hydrophobic components, sufficient micelle formation above the critical micelle concentration (CMC) is required [
19]. In this study, lecithin concentration was tested within the range of 2% - 10% (w/v) (
Figure 2).
In the surfactant layer, there was a statistically significant difference between 2% and 3% for CT and Tan IIA, while the 3% concentration showed the highest content in DHTS and Tan I. The total tanshinone content increased with lecithin concentrations up to 3%, but showed a decreasing trend thereafter. This is likely due to the effect of surfactant concentration on micelle size, interfacial area, surfactant layer formation, extraction step dilution, and preconcentration factor. High surfactant concentrations can reduce extraction efficiency by decreasing micelle size and causing surfactant saturation in the solution, while low concentrations can result in decreased solubility due to insufficient surfactant layer formation and extraction [
33,
34]. As surfactant concentration decreases, the ratio of the preconcentrated aqueous solution volume to the surfactant layer volume increases, leading to a higher preconcentration factor [
35]. In the water layer, the content of all components increased with rising lecithin concentration, indicating unstable layer separation at higher surfactant concentrations (
Table S2).
Interestingly, the DHTS content in the water layer was relatively high at 2% lecithin concentration, likely due to the lower hydrophobicity of DHTS. Hydrophobicity is usually expressed by the log
P value, based on the octanol/water partition coefficient, with negative values indicating hydrophilicity and positive values indicating hydrophobicity [
36]. Due to the relatively low log
P value (3.904) of DHTS, it likely does not preferentially bind to micelles and is more distributed in the water layer than other tanshione components (
Table 1). According to Fischer et al. [
37], hydrophobic components with a high log
P value are fully soluble in micelles, while hydrophilic components are either evenly distributed between the micelle and aqueous phase or are more present in the aqueous phase. At low lecithin concentrations, micelle formation may be insufficient, resulting in decreased DHTS adsorption into micelles and increased content in the water layer. Based on these results, the optimal lecithin concentration for maximizing extraction efficiency of all components was determined to be 3% (w/v).
Table 2.
Lists of log P values of tanshinones.
Table 2.
Lists of log P values of tanshinones.
| Compounds |
Log P
|
Reference |
| Dihydrotanshinone I |
3.904 |
[38] |
| Cryptotanshinone |
4.931 |
| Tanshinone I |
4.443 |
| Tanshinone IIA |
5.471 |
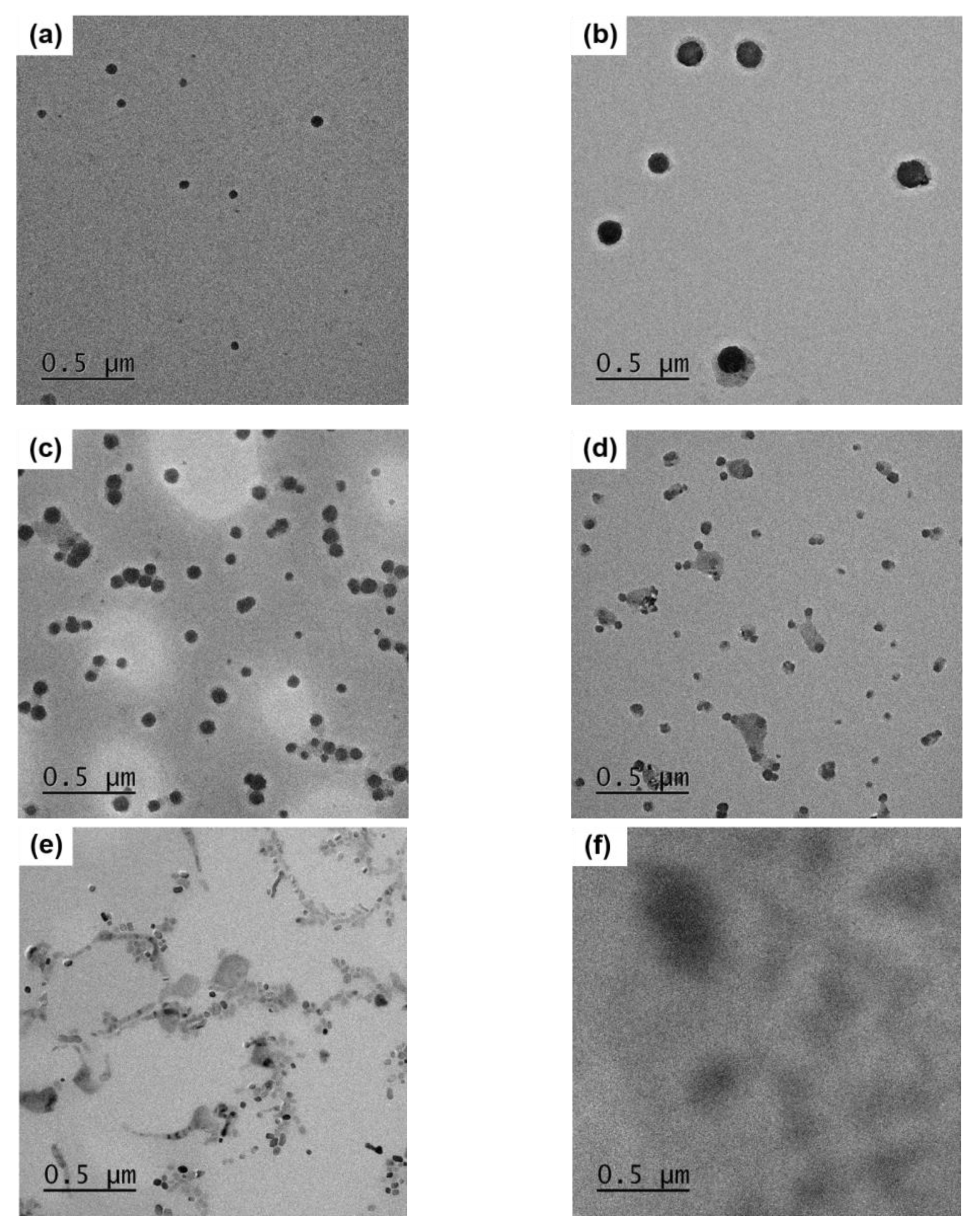
3.4. Morphology of Micelle
The morphology and particle size changes of micelles with the varying lecithin concentration were analyzed using transmission electron microscopy (TEM) and compared to the control sample without lecithin (lecithin 3%) (
Figure 3). The smallest spherical micelles were observed at 2% lecithin concentration with the largest size at 3% lecithin, followed by a gradual decrease in size and increase in particle number with increasing surfactant concentration (
Figure 3a–e). At 10% lecithin, a mixture of spherical and elongated micelles was observed (
Figure 3e).
According to Pisárčik et al. [
39], an increase in cationic surfactant concentration lead to a decrease in micelles size due to stronger charge repulsion. Although lecithin is neutral as a zwitterionic surfactant, under experimental conditions at pH 3, lecithin became positively charged, leading to a decrease in micelle size due to charge repulsion [
40]. This finding is consistent with Pisárčik et al. [
39].
High concentrations of surfactants can cause spherical micelles to transform into worm-like micelles [
41]. Various shapes of micelles were observed at 10% lecithin, considered a transitional concentration where micelle morphology changes at high concentrations.
Comparing micelles with and without SM samples, it was found that micelles in the control group without the sample were relatively arranged compared to micelles with the sample (
Figure 3b,f). This is likely due to the presence of the hydrophobic component, tanshinone, which influenced micelle binding density. The largest micelles capable of effectively entrapping the component were formed at 3% lecithin, corresponding to the most stable concentration for CPE. Correlation with HPLC results also supports this finding.
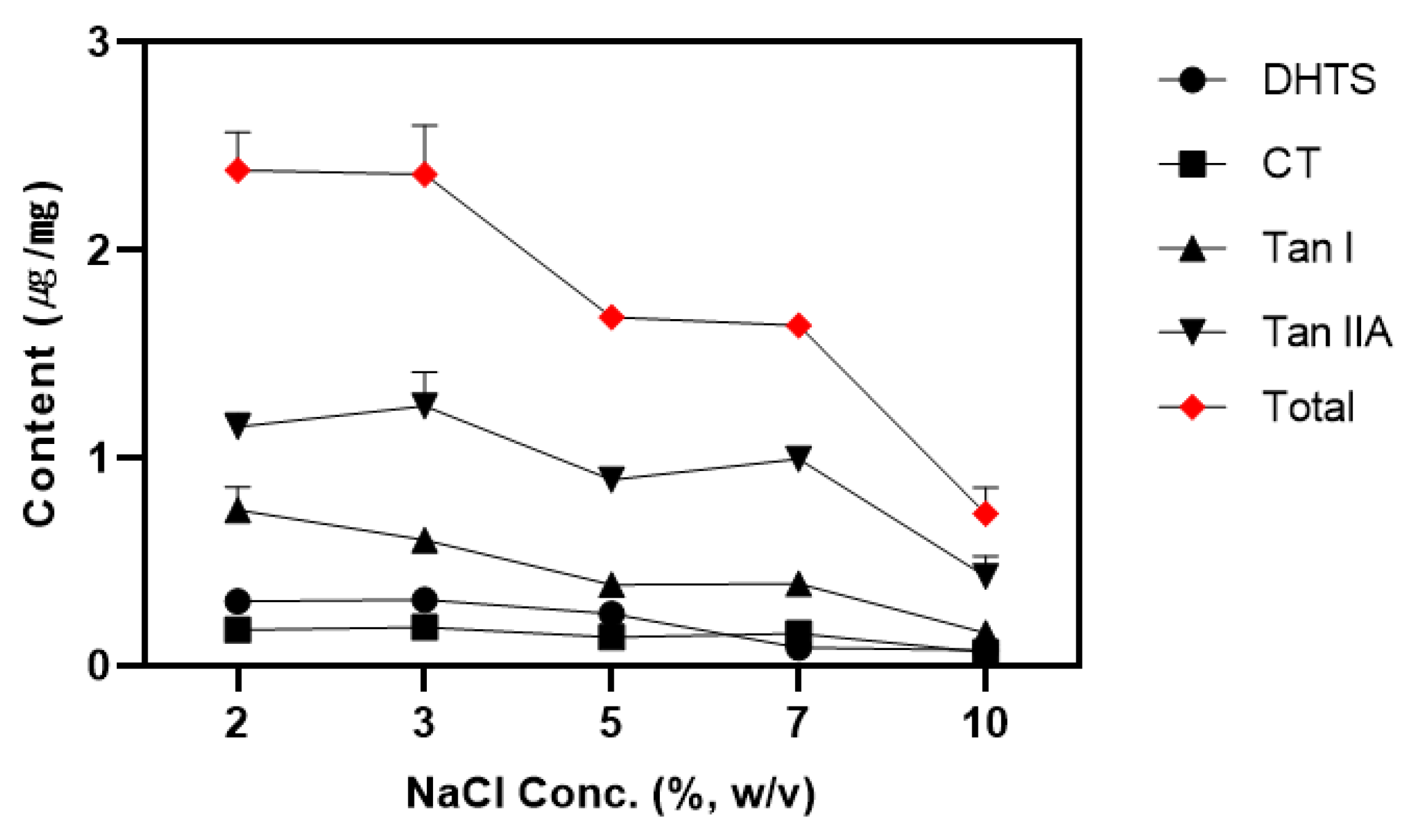
3.5. Effect of NaCl Concentration
Generally, phase separation in CPE is achieved by heating to the cloud point temperature (CPT). However, adding salts such as NaCl and Na
2SO
4 can induce phase separation at lower temperatures to enhance extraction efficiency [
42]. We chose NaCl for its cost-effectiveness and availability and tested concentrations 2%, 3%, 5%, 7%, 10% (w/v). The results are shown in
Figure 4.
The tanshinone content in the surfactant layer was relatively consistent at 2% and 3% NaCl but showed a significant decrease starting at 5%. Tan IIA exhibited the highest content at 3% NaCl, but there was no statistically significant difference between 2% and 3% when considering the total tanshinones content. The other tanshinones showed similar behavior. NaCl induce “salting-out”, where the addition of NaCl increase the attraction between water molecules and salt ions, weakening the interaction between the micelle’s hydrophilic head and water molecules, promoting tanshinone migration into the micelle [
43,
44]. However, when the NaCl concentration exceeds 5%, the tanshinone content tends to decrease because the increased charge on the micelle surface hinders micelle formation [
45]. The content of tanshinones that could not be extracted into micelles and remained in the water layer increased slightly with rising NaCl concentration, but the content was low across the entire range due to stabilization by the surfactant concentration (
Table S3). Considering the economic and environmental factors, the optimal NaCl concentration for tanshinones extraction was determined to be 2%.
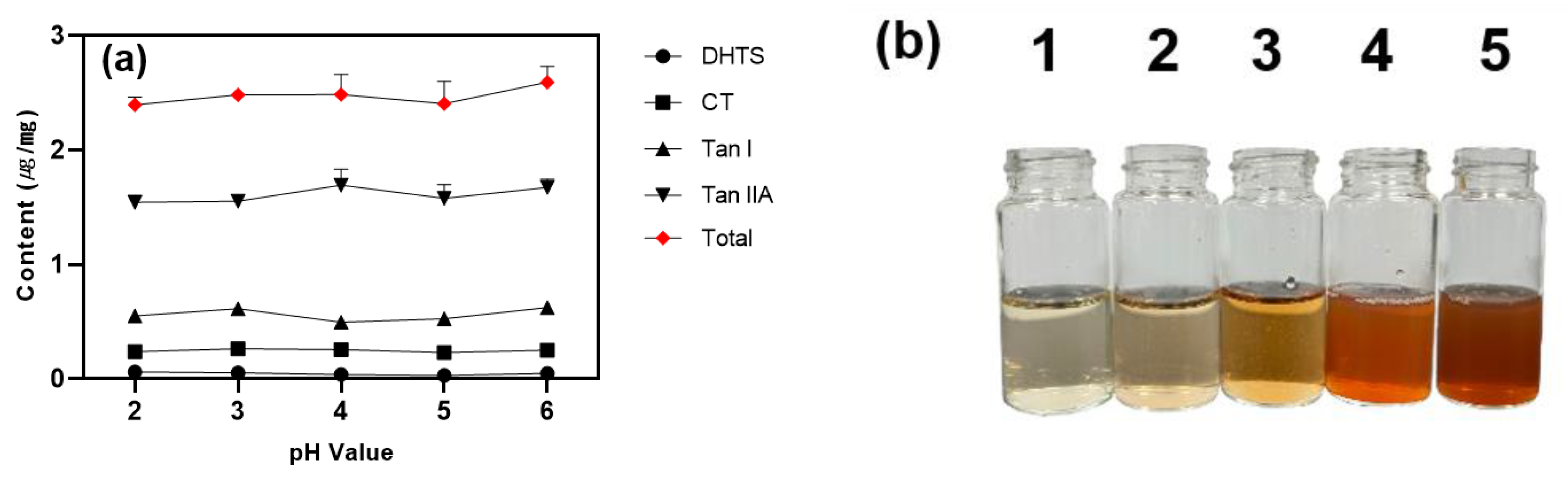
3.6. Effect of pH Value
The pH was adjusted by adding 1 M citric acid in the range of pH 2 to 6 with pH 6 serving as the baseline without citric acid. The extraction efficiency was evaluated and shown in
Figure 4 (pH 6 was without citric acid).
Figure 5.
(a) Effect of pH on tanshinone content in surfactant layer. Conc. denotes concentration; (b) Water layer with surfactant layer removed (1: pH 2; 2: pH 3; 3: pH 4; 4: pH 5; 5: pH 6).
Figure 5.
(a) Effect of pH on tanshinone content in surfactant layer. Conc. denotes concentration; (b) Water layer with surfactant layer removed (1: pH 2; 2: pH 3; 3: pH 4; 4: pH 5; 5: pH 6).
DHTS and CT maintained similar contents at all pH values, with Tan I showing the highest content at pH 3 and Tan IIA at pH 4, though no statistically significant differences were observed. CPE efficiency is generally pH-dependent, influencing complex formation [
46]. For neutral or non-ionic compounds, the impact of pH change is minimal [
47], a finding corroborated by Bi et al. [
26], who isolated CT and Tan I from SM using Triton X-100.
Interestingly, as the pH increased, the color of the water layer darkened to brown, and layer separation became unstable. HPLC analysis confirmed that more tanshinones remained in the water layer at higher pH levels (
Table S4). Tanshinone compounds are known to be sensitive to cations [
48], and lecithin, neutral in aqueous solution, can become positively or negatively charged depending on pH [
40]. At low pH, lecithin is protonated and forms strong bonds with tanshinone, facilitating layer separation. At higher pH, lecithin is deprotonated and becomes neutral or negatively charged, weakening its interaction with tanshinones leading to less separation and more tanshinones remaining in the water layer, darkening its color.
The analysis revealed that adjusting the pH improved the speed and clarity of the layer separation but did not significant impact the tanshinone content in the extract layer. This suggests that effective tanshinone extraction can be achieved without pH adjustment, and the influence of pH on the overall CPE process was deemed insignificant, leading to the elimination of the pH adjustment step.
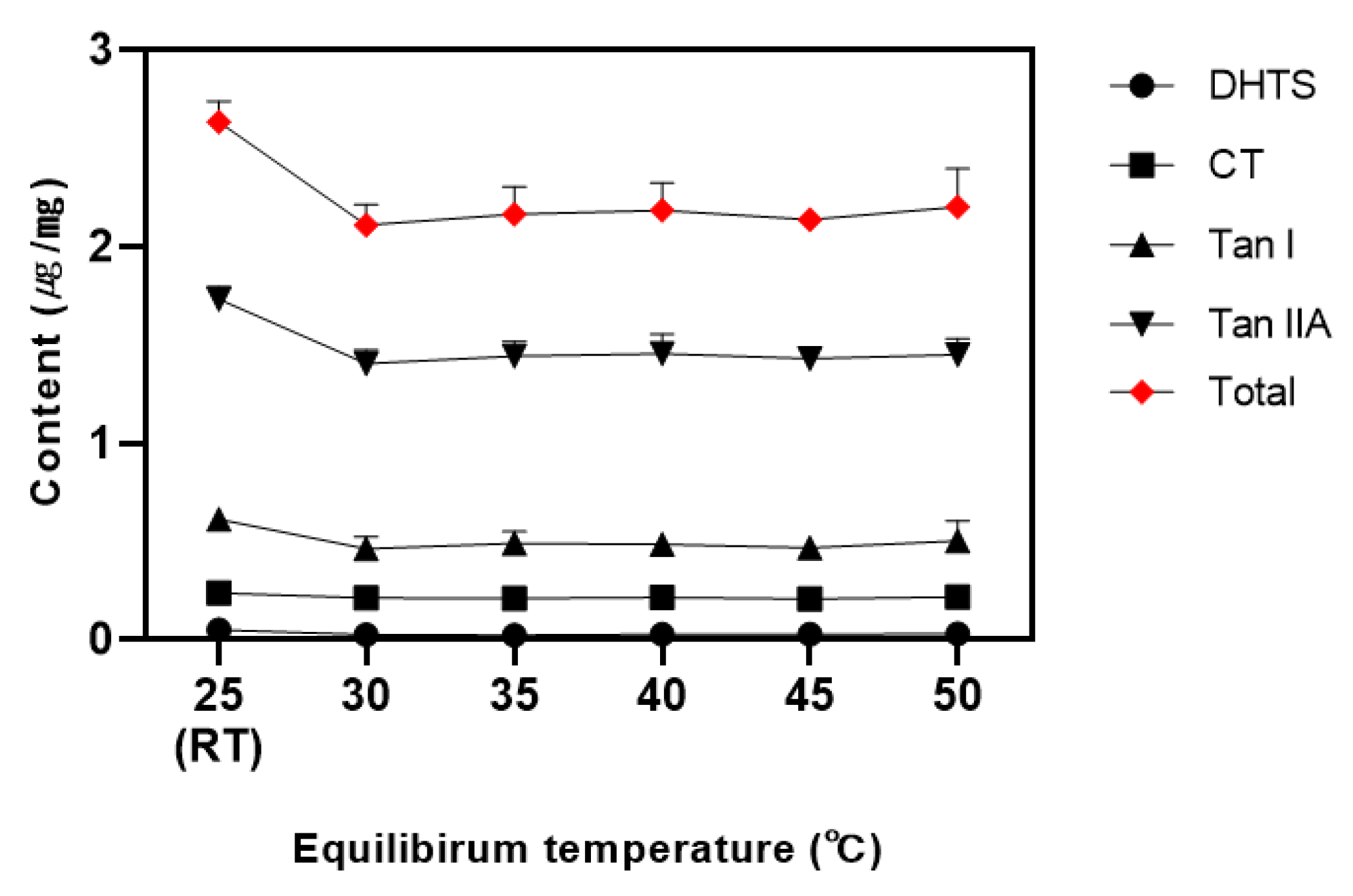
3.7. Effect of Equilibrium Temperature
Lecithin is known for forming micelles at lower equilibrium temperatures than other surfactants, with the cloud point temperature (CPT) further reduced by adding salt [
42]. Leveraging these characteristics of lecithin, tanshinones were extracted at temperatures ranging from 25°C (room temperature) to 50 °C, with phase separation observed (
Figure 6).
The result showed that the sample reacted at room temperature had the highest content of all tanshinones, with no significant difference at other temperature ranges. The content of tanshinones in the aqueous phase was found to be invariant with temperature (
Table S5). These results likely related to lecithin’s low cloud point temperature (CPT). Consistent with previous findings [
19] that certain surfactants exhibit layer separation even at room temperature when salts are added, this study suggests that adding NaCl to lecithin allows effective extraction at lower temperatures. Although low temperature result in slower layer separation, centrifugation achieved the same effective separation as higher temperature. Therefore, 25°C (room temperature) was determined to be the appropriate temperature for the equilibrium reaction.
3.8. Comparison with Other SM Extract
The optimized
Salvia miltiorrhiza (SM) CPE (SM-CPE) conditions were compared with SM water extract (SMW) regarding component content and extraction efficiency. SMW is a common herbal medicine extraction method, was used as the control without CPE. For the comparison of extraction efficiency, the tanshinone content of SM extracted with ethanol (EtOH) supplemented with 5% acetic acid (A.A) (5% A.A+EtOH) was considered the total content. Generally, organic solvents are the easiest and most efficient way to extract tanshinone, and Zhu et al. [
49] reported that adding 5% A.A. to the organic solvent is more effective for tanshinone extraction. Due to solvent limitations in food, EtOH was chosen instead of methanol. The relative extraction efficiency was calculated by comparing the tanshinone content of each extract with the SM 5% A.A+EtOH. The calculation was performed using Equation (1):
where
Cet is the respective tanshinone content in each extract, and
Ttc is the respective tanshinone content in the SM 5% A.A+EtOH.
Table 2.
Extraction efficiency of each SM extracted method.
Table 2.
Extraction efficiency of each SM extracted method.
| Sample |
Extraction Efficiency(%) |
| DHTS |
CT |
Tan I |
Tan IIA |
Total |
| SM-CPE |
8.47±1.34 |
9.57±1.16 |
17.15±2.08 |
7.11±0.57 |
42.30±0.62 |
| SMW |
3.92±0.38 |
1.25±0.19 |
1.38±0.21 |
0.30±0.02 |
6.85±0.14 |
| T-test (p<0.01) |
* |
** |
** |
** |
** |
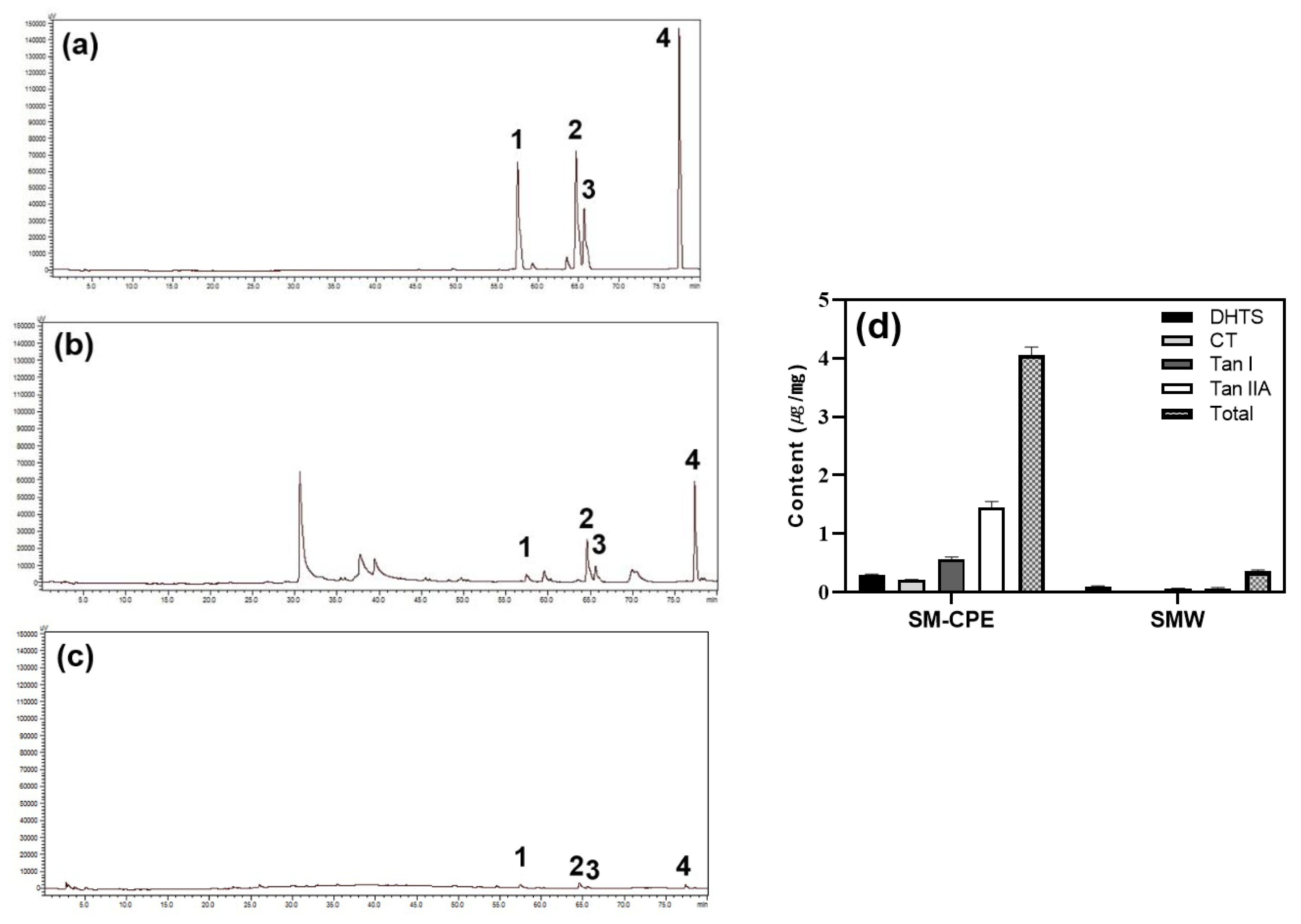
Comparing the two extracts, SMW had significantly lower content than SM-CPE (
Figure 7a–d). For Tan IIA, a representative tanshinone, SM-CPE contained 1.74±0.03 ug/mg, while SMW contained 0.07±0.00 ug/mg, which is about 23.5 times higher. DHTS was about 2.16 times higher, CT was 7.64 times higher, and Tan I was about 12.4 times higher (
Figure 7d). The extraction efficiencies for each tanshinone by different extraction methods were calculated based on 100% content of SM 5% A.A+EtOH and are shown in
Table 2. For all tanshinones, SM-CPE showed higher extraction efficiency than SMW, with difference ranging from 15.77% to 4.55%. Considering the total tanshinone content, SM-CPE recovered about 42.3% of tanshinones compared to 5% A.A+EtOH extract, demonstrating that the optimized CPE conditions were more effective for extracting tanshinones from SM than the conventional water extraction method.
4. Conclusions
In this study, we applied cloud point extraction (CPE) technology to Salvia Miltiorrhiza (SM) to establish optimal extraction conditions for tanshinones, a hydrophobic component, and compared it with conventional water extraction methods. The results showed that 20 mL of solvent, 3% lecithin (w/v), 2% NaCl (w/v), pH 6 (no pH adjustment required), and room temperature (25°C) per 1g of sample yielded the best extraction efficiency. Notably, high extraction efficiency can be achieved without pH adjustment or high temperature reaction, simplifying the process. Additionally, micelle formation promoted by lecithin expected to improve the bioavailability of tansinone. Although the total content is lower than that obtained by organic solvent extraction, this study introduces a new approach to effectively extract tansinones, a hydrophobic component, from aqueous extraction systems. This method is eco-friendly and safe by eliminating the use of organic solvents, making it a promising technology for future natural product extraction.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Effect of solid-to-liquid ratio on the content of tanshinone content in water layer; Table S2: Effect of surfactant concentration on the tanshinone content in water layer, Table S3: Effect of NaCl concentration on the tanshinone content in water layer, Table S4: Effect of pH on the tanshinone content in water layer, Table S5: Effect of equilibrium temperature on the tanshinone content in water layer.
Author Contributions
Conceptualization, Y.R.S.; methodology, Y.R.S. and J.D.L.; formal analysis, M.J.K.; investigation, Y.R.S. and M.J.K.; data curation, B.R.R.; writing—original draft preparation, Y.R.S.; writing—review and editing, Y.R.S. and J.D.L.; supervision, J.D.L.; project administration, B.R.R. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank the Rural Development Administration in Eumsung, Korea, for providing the samples that were essential for the completion of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kum, K.Y.; Kirchhof, R.; Luick, R.; Heinrich, M. Danshen (Salvia Miltiorrhiza) on the Global Market: What Are the Implications for Products’ Quality? Frontiers in Pharmacology 2021, 12. [CrossRef]
- Jia, Q.; Zhu, R.; Tian, Y.; Chen, B.; Li, R.; Li, L.; Wang, L.; Che, Y.; Zhao, D.; Mo, F.; et al. Salvia Miltiorrhiza in Diabetes: A Review of Its Pharmacology, Phytochemistry, and Safety. Phytomedicine 2019, 58, 152871. [CrossRef]
- Zhang, S.; Luo, H.; Sun, S.; Zhang, Y.; Ma, J.; Lin, Y.; Yang, L.; Tan, D.; Fu, C.; Zhong, Z.; et al. Salvia Miltiorrhiza Bge. (Danshen) for Inflammatory Bowel Disease: Clinical Evidence and Network Pharmacology-Based Strategy for Developing Supplementary Medical Application. Frontiers in Pharmacology 2022, 12. [CrossRef]
- Wang. B. Q. Salvia miltiorrhiza: Chemical and pharmacological review of a medicinal plant. J Med Plants Res 2010, 4, 25, 2813-2820.
- Cheng, Y.-C.; Hung, I.-L.; Liao, Y.-N.; Hu, W.-L.; Hung, Y.-C. Salvia Miltiorrhiza Protects Endothelial Dysfunction against Mitochondrial Oxidative Stress. Life 2021, 11, 1257. [CrossRef]
- R, G.; L, L.; J, S.; S, L.; Se, D.; Z, L.; G, F. Pharmacological Activity and Mechanism of Tanshinone IIA in Related Diseases. DOAJ (DOAJ: Directory of Open Access Journals) 2020.
- Wang, X.; Morris-Natschke, S.L.; Lee, K.-H. New Developments in the Chemistry and Biology of the Bioactive Constituents of Tanshen. Medicinal Research Reviews 2006, 27, 133–148. [CrossRef]
- Jiang, Z.; Gao, W.; Huang, L. Tanshinones, Critical Pharmacological Components in Salvia Miltiorrhiza. Frontiers in Pharmacology 2019, 10. [CrossRef]
- Plaskova, A.; Mlcek, J. New Insights of the Application of Water or Ethanol-Water Plant Extract Rich in Active Compounds in Food. Frontiers in Nutrition 2023, 10. [CrossRef]
- Food and Agriculture Organization (FAO). Extraction solvents for foodstuffs regulations. Notice 25 of 1999. p. 1-4. Available online: https://faolex.fao.org/docs/pdf/mlt41702.pdf (accessed on 27 April 1999).
- International Council for Harmonisation (ICH). ICH guideline Q3C (R9) on impurities: guideline for residual solvents. In: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; 2024. Geneva, Switzerland: ICH; p. 1-50. Available online: https://database.ich.org/sites/default/files/ICH_Q3C%28R9%29_Guideline_MinorRevision_2024_2024_Approved.pdf (accessed on 24 January 2024).
- Song, J.-Z.; Li, S.-L.; Zhou, Y.; Qiao, C.-F.; Chen, S.-L.; Xu, H.-X. A Novel Approach to Rapidly Explore Analytical Markers for Quality Control of Radix Salviae Miltiorrhizae Extract Granules by Robust Principal Component Analysis with Ultra-High Performance Liquid Chromatography–Ultraviolet–Quadrupole Time-of-Flight Mass Spectrometry. Journal of Pharmaceutical and Biomedical Analysis 2010, 53, 279–286. [CrossRef]
- Li, Y.-G.; Song, L.; Liu, M.; Hu, Z.-B.; Wang, Z.-T. Advancement in Analysis of Salviae Miltiorrhizae Radix et Rhizoma (Danshen). Journal of Chromatography A 2009, 1216, 1941–1953. [CrossRef]
- Yang, Q.; Zhang, X.-L.; Li, X.-Y.; Tang, W.-K.; Zhang, J.-X.; Fang, C.-X.; Zheng, C.-Y. Coupling Continuous Ultrasound-Assisted Extraction with Ultrasonic Probe, Solid-Phase Extraction and High-Performance Liquid Chromatography for the Determination of Sodium Danshensu and Four Tanshinones in Salvia Miltiorrhiza Bunge. Analytica Chimica Acta 2007, 589, 231–238. [CrossRef]
- Wang, L.; Song, Y.; Cheng, Y.; Liu, X. Orthogonal Array Design for the Optimization of Supercritical Fluid Extraction of Tanshinones from Danshen. Journal of Separation Science 2008, 31, 321–328. [CrossRef]
- Jiang, Y.; David, B.; Tu, P.; Barbin, Y. Recent Analytical Approaches in Quality Control of Traditional Chinese Medicines—A Review. Analytica Chimica Acta 2010, 657, 9–18. [CrossRef]
- Chen, Y.; Duan, G.; Xie, M.; Chen, B.; Li, Y. Infrared-assisted Extraction Coupled with High-performance Liquid Chromatography for Simultaneous Determination of Eight Active Compounds in Radix Salviae Miltiorrhizae. Journal of Separation Science 2010, 33, 2888–2897. [CrossRef]
- Liu, F.; Wang, D.; Liu, W.; Wang, X.; Bai, A.; Huang, L. Ionic Liquid-Based Ultrahigh Pressure Extraction of Five Tanshinones from Salvia Miltiorrhiza Bunge. Separation and Purification Technology 2013, 110, 86–92. [CrossRef]
- Mortada, W.I. Recent Developments and Applications of Cloud Point Extraction: A Critical Review. Microchemical Journal 2020, 157, 105055. [CrossRef]
- El-Helaly, S.N.; Rashad, A.A. Mirtazapine Loaded Polymeric Micelles for Rapid Release Tablet: A Novel Formulation—In Vitro and in Vivo Studies. Drug Delivery and Translational Research 2024, 1-11. [CrossRef]
- More, P.R.; Arya, S.S. A Novel, Green Cloud Point Extraction and Separation of Phenols and Flavonoids from Pomegranate Peel: An Optimization Study Using RCCD. Journal of Environmental Chemical Engineering 2019, 7, 103306. [CrossRef]
- Papaioannou, E.H.; Karabelas, A.J. Lycopene Recovery from Tomato Peel under Mild Conditions Assisted by Enzymatic Pre-Treatment and Non-Ionic Surfactants. Acta Biochimica Polonica 2012, 59. [CrossRef]
- Nekouei, F.; Nekouei, S. Spectrophotometric Determination of Mercury (II) by Simultaneous Micelle Mediated Extraction through Ternary Complex Formation in Water Samples. Oriental Journal of Chemistry 2014, 30, 867–871. [CrossRef]
- Tang, X.; Zhu, D.; Huai, W.; Zhang, W.; Fu, C.; Xie, X.; Quan, S.; Fan, H. Simultaneous Extraction and Separation of Flavonoids and Alkaloids from Crotalaria Sessiliflora L. by Microwave-Assisted Cloud-Point Extraction. Separation and Purification Technology 2017, 175, 266–273. [CrossRef]
- Shi, Z.; He, J.; Chang, W. Micelle-Mediated Extraction of Tanshinones from Salvia Miltiorrhiza Bunge with Analysis by High-Performance Liquid Chromatography. Talanta 2004, 64, 401–407. [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Extraction and Concentration of Tanshinones in Salvia Miltiorrhiza Bunge by Task-Specific Non-Ionic Surfactant Assistance. Food Chemistry 2011, 126, 1985–1990. [CrossRef]
- Alibade, A.; Batra, G.; Bozinou, E.; Salakidou, C.; Lalas, S. Optimization of the Extraction of Antioxidants from Winery Wastes Using Cloud Point Extraction and a Surfactant of Natural Origin (Lecithin). Chemical Papers 2020, 74, 4517–4524. [CrossRef]
- More, P.R.; Arya, S.S. Lecithin-Based Micellar Extraction of Bioactive from Pomegranate Peel and Its Application in Indian Flat Bread (Chapati) as a Bioactive Emulsifier. Food and Humanity 2024, 100346. [CrossRef]
- Chen, X.; Deng, Y.; Xue, Y.; Liang, J. Screening of Bioactive Compounds in Radix Salviae Miltiorrhizae with Liposomes and Cell Membranes Using HPLC. Journal of Pharmaceutical and Biomedical Analysis 2012, 70, 194–201. [CrossRef]
- Tchabo, W.; Ma, Y.; Kwaw, E.; Xiao, L.; Wu, M.; Apaliya, M.T. Impact of Extraction Parameters and Their Optimization on the Nutraceuticals and Antioxidant Properties of Aqueous Extract Mulberry Leaf. International Journal of Food Properties 2018, 21, 717–732. [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chinese Medicine 2018, 13. [CrossRef]
- Leite, A.C.; Ferreira, A.M.; Morais, E.S.; Khan, I.; Freire, M.G.; Coutinho, J. a. P. Cloud Point Extraction of Chlorophylls from Spinach Leaves Using Aqueous Solutions of Nonionic Surfactants. ACS Sustainable Chemistry & Engineering 2017, 6, 590–599. [CrossRef]
- Rasoolzadeh, F.; Hashemi, P.; Serenjeh, F.N. Ionic Liquid-Based Cloud-Point Extraction of Quercetin for Its Sensitive HPLC–UV Determination in Juice Samples. Acta Chromatographica 2017, 29, 493–496. [CrossRef]
- Gouda, A.A.; Sheikh, R.E.; Amin, A.S. Application of Cloud Point Extraction for Separation of Iron in Water, Food and Environmental Samples Perior to Determination by Spectrophotometry. Analytical Chemistry Letters 2016, 6, 296–312. [CrossRef]
- Chen, L.; Zhao, Q.; Jin, H.; Zhang, X.; Xu, Y.; Yu, A.; Zhang, H.; Ding, L. Determination of Xanthohumol in Beer Based on Cloud Point Extraction Coupled with High Performance Liquid Chromatography. Talanta 2010, 81, 692–697. [CrossRef]
- Supriyo, S.; Dilipkumar P. In Encyclopedia of Physical Organic Chemistry; Wang, Z.; Wille, U.; Juaristi, E., Eds.; John Wiley & Sons: Hoboken, NJ, 2017; Volume 6, pp 629 – 651, ISBN 978-1-118-47045-9.
- Fischer, E.; Fieber, W.; Navarro, C.; Sommer, H.; Benczédi, D.; Velazco, M.I.; Schönhoff, M. Partitioning and Localization of Fragrances in Surfactant Mixed Micelles. Journal of Surfactants and Detergents 2008, 12, 73–84. [CrossRef]
- Sutherland, I.A.; Fisher, D. Role of Counter-Current Chromatography in the Modernisation of Chinese Herbal Medicines. Journal of Chromatography A 2009, 1216, 740–753. [CrossRef]
- Pisárčik, M.; Devínsky, F.; Švajdlenka, E. Spherical Dodecyltrimethylammonium Bromide Micelles in the Limit Region of Transition to Rod-like Micelles. A Light Scattering Study. Colloids and Surfaces a Physicochemical and Engineering Aspects 1996, 119, 115–122. [CrossRef]
- Demissie, H.; Duraisamy, R. Effects of Electrolytes on the Surface and Micellar Characteristics of Sodium Dodecyl Sulphate Surfactant Solution. Journal of Scientific and Innovative Research 2016, 5, 208–214. [CrossRef]
- Liu, Y.; Guo, X.; Zhao, M.; Zou, C.; Feng, Y.; Wu, Y.; Dai, C. The Effect and Enhancement Mechanism of Hydrophobic Interaction and Electrostatic Interaction on Zwitterionic Wormlike Micelles. Colloids and Surfaces a Physicochemical and Engineering Aspects 2022, 648, 129424. [CrossRef]
- Arya, S.S.; Kaimal, A.M.; Chib, M.; Sonawane, S.K.; Show, P.L. Novel, Energy Efficient and Green Cloud Point Extraction: Technology and Applications in Food Processing. Journal of Food Science and Technology 2019, 56, 524–534. [CrossRef]
- Godoy, N.V.; Somera, B.F.; Barreto, W.J.; Barreto, S.G.; Tarley, C.R.T. Evaluation of DMIT [4,5-Dimercapto-1,3-Dithyol-2-Thionate] as Chelating Agent in a Cloud Point Extraction Procedure for Pb2+ Determination in Water Samples. Acta Scientiarum. Technology/Acta Scientiarum. Technology 2013, 35. [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Mantiniotou, M.; Kalompatsios, D.; Bozinou, E.; Giovanoudis, I.; Lalas, S.I. Exploring the Feasibility of Cloud-Point Extraction for Bioactive Compound Recovery from Food Byproducts: A Review. Biomass 2023, 3, 306–322. [CrossRef]
- Shokrollahi, A.; Ghaedi, M.; Hossaini, O.; Khanjari, N.; Soylak, M. Cloud Point Extraction and Flame Atomic Absorption Spectrometry Combination for Copper(II) Ion in Environmental and Biological Samples. Journal of Hazardous Materials 2008, 160, 435–440. [CrossRef]
- Dhahir, S.A.; Bakir, S.R. Cloud Point Extraction Spectrophotometric Determination of Copper, Chromium and Cobalt by Salen as Reagent in Wastewater of Iraq. Asian Journal of Chemistry 2014, 26, 5305–5310. [CrossRef]
- Xie, S.; Paau, M.C.; Li, C.F.; Xiao, D.; Choi, M.M.F. Separation and Preconcentration of Persistent Organic Pollutants by Cloud Point Extraction. Journal of Chromatography A 2010, 1217, 2306–2317. [CrossRef]
- Shen, Q.; Wang, H.; Quan, B.; Sun, X.; Wu, G.; Huang, D.; Wang, Q.; Luo, P. Rapid Quantification of Bioactive Compounds in Salvia Miltiorrhiza Bunge Derived Decoction Pieces, Dripping Pill, Injection, and Tablets by Polarity-Switching UPLC-MS/MS. Frontiers in Chemistry 2022, 10. [CrossRef]
- Zhu, T.; Park, H.E.; Row, K.H. Ultrasonic-Assisted Extraction of Tanshinones from Korean Red Ginseng by Using Amino-Modified Monolithic Cartridge. Asian Journal of Chemistry 2013, 25, 7765–7768. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).