Submitted:
04 September 2024
Posted:
06 September 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Patient Enrollment and Follow-Up
Neuroimaging
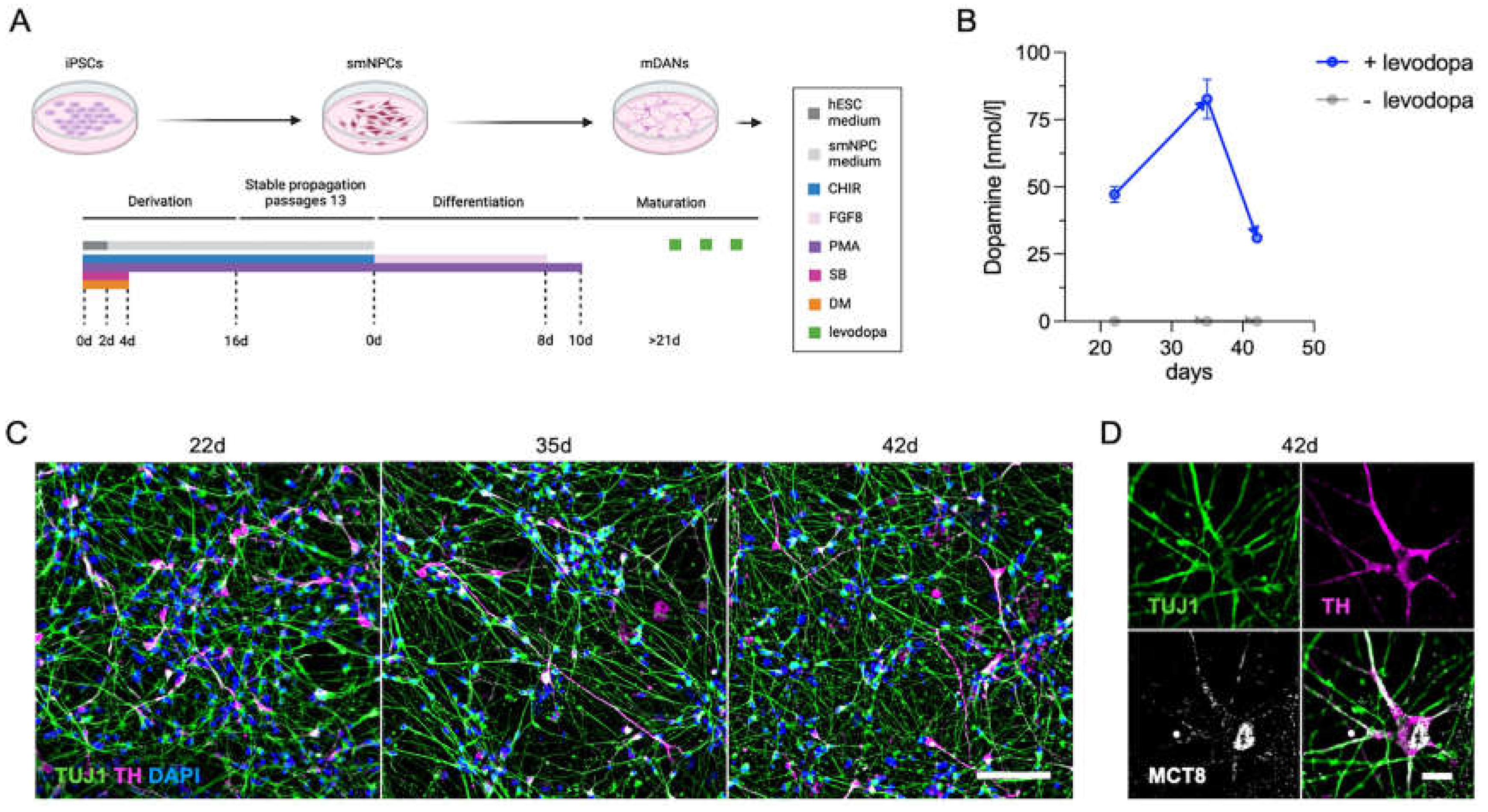
Differentiation of Human Induced Pluripotent Stem Cells (hiPSCs) into Dopaminergic Neurons
Statistical Analysis
Results
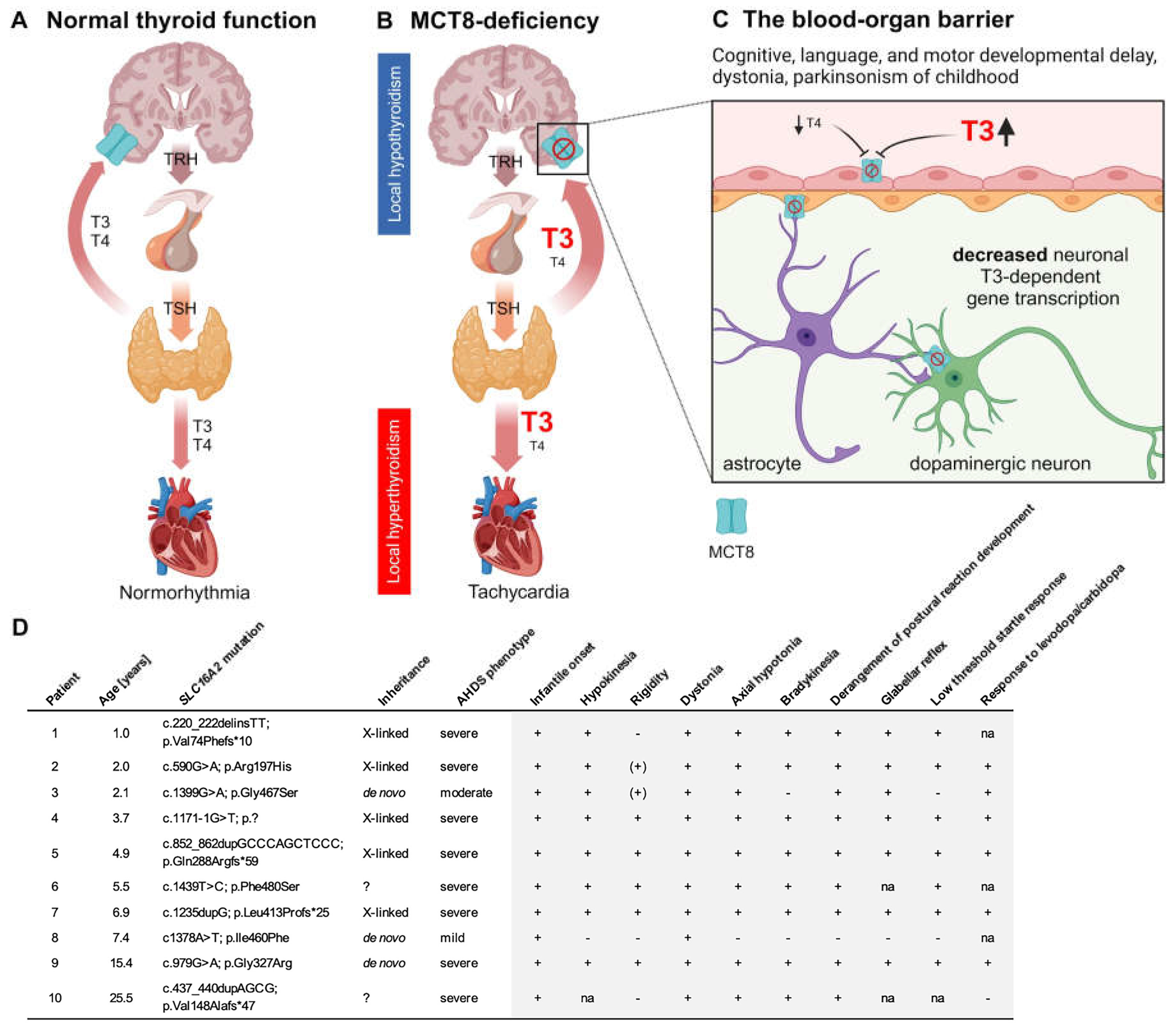
Patients Present with Signs of Parkinsonism in Childhood
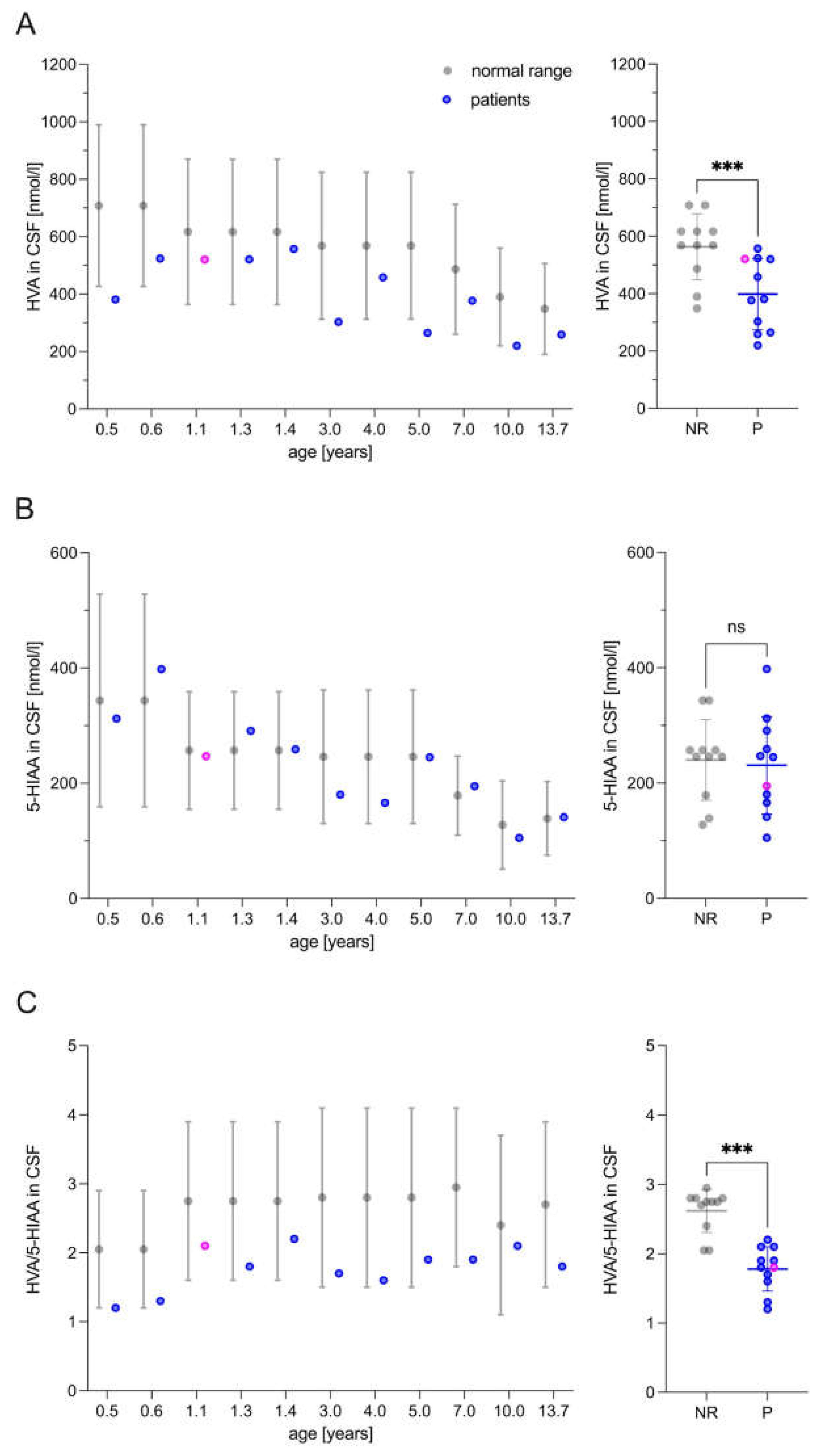
Altered Biogenic Amine Concentrations Indicate an Isolated Dopamine Pathway Impairment
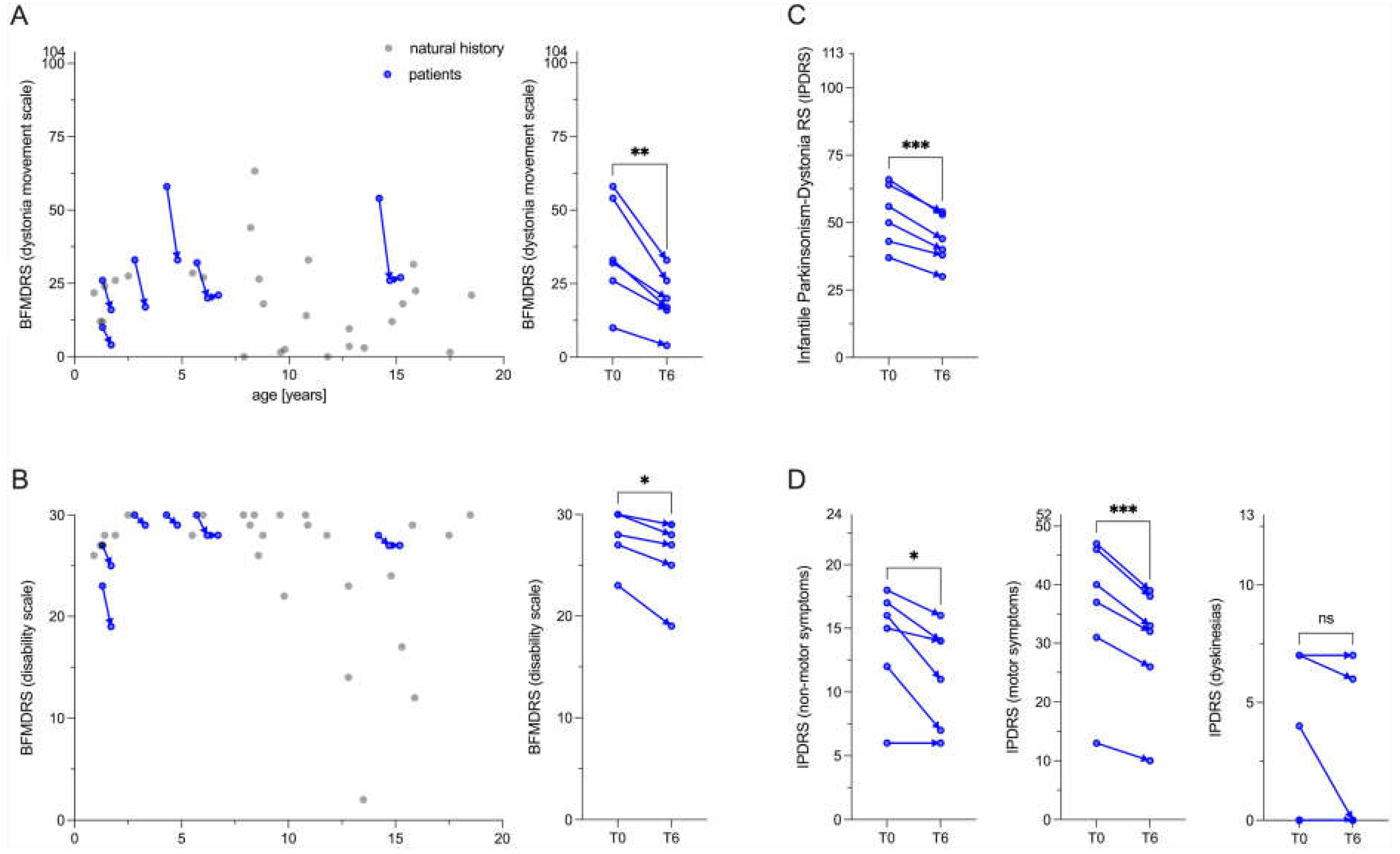
Patients Persistently Respond to Levodopa/Carbidopa Treatment
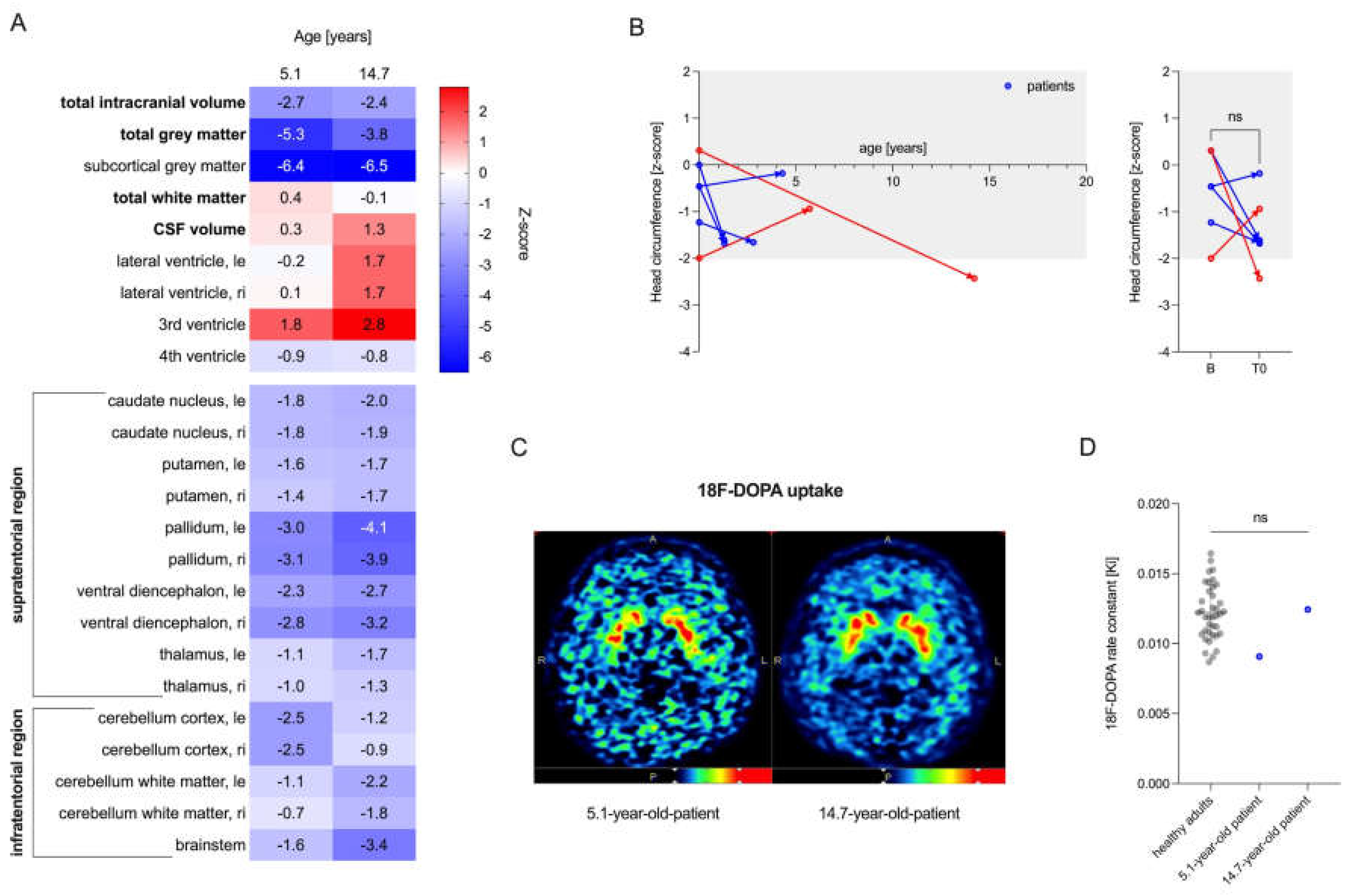
Patients with MCT8 Deficiency Have a Reduced Subcortical Gray Matter Volume
Establishment of a Cell-Based Model to Analyze the Role of MCT8 Deficiency on Dopamine Metabolism
Discussion
Supplementary Materials
Authors' roles
Funding sources
Institutional Review Board Statement
Acknowledgements
Conflicts of interest
References
- Allan W, Herndon, C. Nash, Dudley, Forence C. Some examples of the inheritance of mental deficiency: apparently sex-linked idiocy and microencephaly. Am. J. Ment. Defic. 1944, 48, 325–334. [Google Scholar]
- Dumitrescu, A.M.; Liao, X.H.; Best, T.B.; Brockmann, K.; Refetoff, S. A Novel Syndrome Combining Thyroid and Neurological Abnormalities Is Associated with Mutations in a Monocarboxylate Transporter Gene. Am. J. Hum. Genet. 2004, 74, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Friesema, E.C.; Grueters, A.; Biebermann, H.; Krude, H.; von Moers, A.; Reeser, M.; et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. The Lancet 2004, 364, 1435–1437. [Google Scholar] [CrossRef]
- Vatine, G.D.; Al-Ahmad, A.; Barriga, B.K.; Svendsen, S.; Salim, A.; Garcia, L.; et al. Modeling Psychomotor Retardation using iPSCs from MCT8-Deficient Patients Indicates a Prominent Role for the Blood-Brain Barrier. Cell Stem Cell 2017, 20, 831–843. [Google Scholar] [CrossRef]
- Groeneweg, S.; van Geest, F.S.; Abacı, A.; Alcantud, A.; Ambegaonkar, G.P.; Armour, C.M.; et al. Disease characteristics of MCT8 deficiency: an international, retrospective, multicentre cohort study. Lancet Diabetes Endocrinol. 2020, 8, 594–605. [Google Scholar] [CrossRef]
- Wilpert, N.M.; Thamm, R.; Thamm, M.; Kratzsch, J.; Seelow, D.; Vogel, M.; et al. Normal Values for the fT3/fT4 Ratio: Centile Charts (0–29 Years) and Their Application for the Differential Diagnosis of Children with Developmental Delay. Int. J. Mol. Sci. 2024, 25, 8585. [Google Scholar] [CrossRef]
- Masnada, S.; Sarret, C.; Antonello, C.E.; Fadilah, A.; Krude, H.; Mura, E.; et al. Movement disorders in MCT8 deficiency/Allan-Herndon-Dudley Syndrome. Mol. Genet. Metab. 2022, 135, 109–113. [Google Scholar] [CrossRef]
- Ramon-Gomez, J.L.; Cortés-Rojas, M.C.; Polania-Puentes, M.J.; Guerrero-Ruiz, G.D.P. Movement Disorder Perspectives on Monocarboxylate 8 Deficiency: A Case Series of 3 Colombian Patients with Allan-Herndon-Dudley Syndrome. Mov. Disord. Clin. Pract. 2024, 11, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Tonduti, D.; Vanderver, A.; Berardinelli, A.; Schmidt, J.L.; Collins, C.D.; Novara, F.; et al. MCT8 Deficiency: Extrapyramidal Symptoms and Delayed Myelination as Prominent Features. J. Child Neurol. 2013, 28, 795–800. [Google Scholar] [CrossRef]
- Wilpert, N.M.; Tonduti, D.; Vaia, Y.; Krude, H.; Sarret, C.; Schuelke, M. Establishing Patient-Centered Outcomes for MCT8 Deficiency: Stakeholder Engagement and Systematic Literature Review. Neuropsychiatr. Dis. Treat. 2023, 19, 2195–2216. [Google Scholar] [CrossRef]
- Groeneweg, S.; Peeters, R.P.; Moran, C.; Stoupa, A.; Auriol, F.; Tonduti, D.; et al. Effectiveness and safety of the tri-iodothyronine analogue Triac in children and adults with MCT8 deficiency: an international, single-arm, open-label, phase 2 trial. Lancet Diabetes Endocrinol. 2019, 7, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Verge, C.F.; Konrad, D.; Cohen, M.; Di Cosmo, C.; Dumitrescu, A.M.; Marcinkowski, T.; et al. Diiodothyropropionic Acid (DITPA) in the Treatment of MCT8 Deficiency. J. Clin. Endocrinol. Metab. 2012, 97, 4515–4523. [Google Scholar] [CrossRef]
- Iwayama, H.; Liao, X.H.; Braun, L.; Bárez-López, S.; Kaspar, B.; Weiss, R.E.; et al. Adeno Associated Virus 9–Based Gene Therapy Delivers a Functional Monocarboxylate Transporter 8, Improving Thyroid Hormone Availability to the Brain of Mct8-Deficient Mice. Thyroid 2016, 26, 1311–1319. [Google Scholar] [CrossRef]
- Sundaram, S.M.; Arrulo Pereira, A.; Müller-Fielitz, H.; Köpke, H.; De Angelis, M.; Müller, T.D.; et al. Gene therapy targeting the blood–brain barrier improves neurological symptoms in a model of genetic MCT8 deficiency. Brain 2022, 145, 4264–4274. [Google Scholar] [CrossRef]
- Vanmechelen, I.; Danielsson, A.; Lidbeck, C.; Tedroff, K.; Monbaliu, E.; Krumlinde-Sundholm, L. The Dyskinesia Impairment Scale, Second Edition: Development, construct validity, and reliability. Dev. Med. Child Neurol. 2023, 65, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E.; Fahn, S.; Marsden, C.D.; Bressman, S.B.; Moskowitz, C.; Friedman, J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 1985, 35, 73–77. [Google Scholar] [CrossRef]
- Pons, R.; Pearson, T.; Perez-Dueñas, B.; Garcia-Cazorla, A.; Kurian, M.; Dalibigka, Z.; et al. Validation of a Parkinsonism-dystonia scale for infants and young children. 7th International Symposium on Paediatric Movement Disorders, Barcelona, February 09-11: 2022.
- Bayley, N. Bayley Scales of Infant and Toddler Development. 3rd ed. APA Psyc Tests; 2005.
- Narayanan, U.G.; Fehlings, D.; Weir, S.; Knights, S.; Kiran, S.; Campbell, K. Initial development and validation of the Caregiver Priorities and Child Health Index of Life with Disabilities (CPCHILD). Dev. Med. Child Neurol. 2006, 48, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Hyland, K.; Surtees, R.A.H.; Heales, S.J.R.; Bowron, A.; Howells, D.W.; Smith, I. Cerebrospinal Fluid Concentrations of Pterins and Metabolites of Serotonin and Dopamine in a Pediatric Reference Population. Pediatr. Res. 1993, 34, 10–14. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. NeuroImage 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Lorenz, R.C.; Gleich, T.; Buchert, R.; Schlagenhauf, F.; Kühn, S.; Gallinat, J. Interactions between glutamate, dopamine, and the neuronal signature of response inhibition in the human striatum. Hum. Brain Mapp. 2015, 36, 4031–4040. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, P.; Glatza, M.; Hemmer, K.; Tsytsyura, Y.; Thiel, C.S.; Höing, S.; et al. Derivation and expansion using only small molecules of human neural progenitors for neurodegenerative disease modeling. PloS One 2013, 8, e59252. [Google Scholar] [CrossRef]
- Zink, A.; Lisowski, P.; Prigione, A. Generation of Human iPSC-derived Neural Progenitor Cells (NPCs) as Drug Discovery Model for Neurological and Mitochondrial Disorders. Bio-Protoc. 2021, 11, e3939. [Google Scholar] [CrossRef] [PubMed]
- Mahajani, S.; Raina, A.; Fokken, C.; Kügler, S.; Bähr, M. Homogenous generation of dopaminergic neurons from multiple hiPSC lines by transient expression of transcription factors. Cell Death Dis. 2019, 10, 898. [Google Scholar] [CrossRef]
- Wilpert, N.M.; Krueger, M.; Opitz, R.; Sebinger, D.; Paisdzior, S.; Mages, B.; et al. Spatiotemporal Changes of Cerebral Monocarboxylate Transporter 8 Expression. Thyroid 2020, 30, 1366–1383. [Google Scholar] [CrossRef]
- Remerand, G.; Boespflug-Tanguy, O.; Tonduti, D.; Touraine, R.; Rodriguez, D.; Curie, A.; et al. Expanding the phenotypic spectrum of Allan–Herndon–Dudley syndrome in patients with SLC16A2 mutations. Dev. Med. Child Neurol. 2019, 61, 1439–1447. [Google Scholar] [CrossRef]
- Leuzzi, V.; Nardecchia, F.; Pons, R.; Galosi, S. Parkinsonism in children: Clinical classification and etiological spectrum. Parkinsonism Relat. Disord. 2021, 82, 150–157. [Google Scholar] [CrossRef]
- Willemsen, M.A.; Verbeek, M.M.; Kamsteeg, E.J.; de Rijk-van Andel, J.F.; Aeby, A.; Blau, N.; et al. Tyrosine hydroxylase deficiency: a treatable disorder of brain catecholamine biosynthesis Brain. J. Neurol. 2010, 133, 1810–1822. [Google Scholar] [CrossRef]
- Rutherford, S.; Fraza, C.; Dinga, R.; Kia, S.M.; Wolfers, T.; Zabihi, M.; et al. Charting brain growth and aging at high spatial precision. eLife 2022, 11, e72904. [Google Scholar] [CrossRef]
- Lee, W.T.; Weng, W.C.; Peng, S.F.; Tzen, K.Y. Neuroimaging findings in children with paediatric neurotransmitter diseases. J. Inherit. Metab. Dis. 2009, 32, 361–370. [Google Scholar] [CrossRef]
- Ludwik, K.A.; Opitz, R.; Jyrch, S.; Megges, M.; Weiner, J.; Beule, D.; et al. Generation of iPSC lines with SLC16A2:G401R or SLC16A2 knock out. Stem Cell Res. 2023, 73, 103256. [Google Scholar] [CrossRef]
- Brennenstuhl, H.; Jung-Klawitter, S.; Assmann, B.; Opladen, T. Inherited Disorders of Neurotransmitters: Classification and Practical Approaches for Diagnosis and Treatment. Neuropediatrics 2019, 50, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet Lond. Engl. 2009, 373, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Poewe, W.; Litvan, I.; Lewis, S.; Lang, A.E.; Halliday, G.; et al. Validation of the MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord Off. J. Mov. Disord. Soc. 2018, 33, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Morales-Briceño, H.; Mohammad, S.S.; Post, B.; Fois, A.F.; Dale, R.C.; Tchan, M.; et al. Clinical and neuroimaging phenotypes of genetic parkinsonism from infancy to adolescence. Brain. J. Neurol. 2020, 143, 751–770. [Google Scholar] [CrossRef]
- Niemann, N.; Jankovic, J. Juvenile parkinsonism: Differential diagnosis, genetics, and treatment. Parkinsonism Relat. Disord. 2019, 67, 74–89. [Google Scholar] [CrossRef]
- Pranzatelli, M.R.; Mott, S.H.; Pavlakis, S.G.; Conry, J.A.; Tate, E.D. Clinical spectrum of secondary parkinsonism in childhood: a reversible disorder. Pediatr. Neurol. 1994, 10, 131–140. [Google Scholar] [CrossRef]
- Cao, X.Y.; Jiang, X.M.; Dou, Z.H.; Rakeman, M.A.; Zhang, M.L.; O’Donnell, K.; et al. Timing of Vulnerability of the Brain to Iodine Deficiency in Endemic Cretinism. N. Engl. J. Med. 1994, 331, 1739–1744. [Google Scholar] [CrossRef]
- Korevaar, T.I.M.; Muetzel, R.; Medici, M.; Chaker, L.; Jaddoe, V.W.V.; de Rijke, Y.B.; et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol. 2016, 4, 35–43. [Google Scholar] [CrossRef]
- Zimmermann, M.B. Iodine Deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef]
- Grüters, A.; Krude, H. Detection and treatment of congenital hypothyroidism. Nat. Rev. Endocrinol. 2011, 8, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J. Thyroid hormone regulated genes in cerebral cortex development. J. Endocrinol. 2017, 232, R83–R97. [Google Scholar] [CrossRef] [PubMed]
- Salas-Lucia, F.; Escamilla, S.; Bianco, A.C.; Dumitrescu, A.; Refetoff, S. Impaired T3 uptake and action in MCT8-deficient cerebral organoids underlie Allan-Herndon-Dudley syndrome. JCI Insight 2024, 9, e174645. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.A.; Aly, M.S.; Rahman, T.A.; Shahat, A.S. Impact of experimental hypothyroidism on monoamines level in discrete brain regions and other peripheral tissues of young and adult male rats. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2013, 31, 225–233. [Google Scholar] [CrossRef]
- Gaum, P.M.; Gube, M.; Esser, A.; Schettgen, T.; Quinete, N.; Bertram, J.; et al. Depressive Symptoms After PCB Exposure: Hypotheses for Underlying Pathomechanisms via the Thyroid and Dopamine System. Int. J. Environ. Res. Public. Health 2019, 16, 950. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Montero-Pedrazuela, A.; Prensa, L.; Guadaño-Ferraz, A.; Rausell, E. Thyroid Hormone Transporters MCT8 and OATP1C1 Are Expressed in Projection Neurons and Interneurons of Basal Ganglia and Motor Thalamus in the Adult Human and Macaque Brains. Int. J. Mol. Sci. 2023, 24, 9643. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




