Introduction
Cartilage microfracturation is a surgical intervention specifically designed to address chondral defects, which are damage to the cartilage that covers the ends of bones in joints. These defects can result from traumatic injuries, degenerative conditions such as osteoarthritis, or congenital abnormalities. The primary goal of microfracture surgery is to promote the regeneration of functional cartilage tissue, thereby restoring joint function, alleviating pain, and enhancing mobility [
1].
The technique involves creating small, controlled perforations, or microfractures, in the subchondral bone plate, which lies just beneath the damaged cartilage. This process is meticulously performed using specialized surgical tools to ensure precision and minimize additional damage to the surrounding healthy tissue. The perforations penetrate the subchondral bone, reaching the bone marrow, which is rich in mesenchymal stem cells (MSCs) [
2].
These MSCs are multipotent cells capable of differentiating into various cell types, including chondrocytes, which are the cells responsible for producing cartilage. The microfractures create an access pathway for the MSCs, allowing them to migrate from the bone marrow to the defect site. Once at the site, these stem cells undergo a process of differentiation into chondrocytes, initiating the formation of new cartilage tissue. This newly formed tissue helps to fill the defect, aiming to restore the smooth surface of the cartilage and improve the joint's structural integrity [
3].
Understanding the molecular processes involved in cartilage repair is crucial for optimizing rehabilitation strategies and improving clinical outcomes. These processes include the regulation of various signaling pathways that control stem cell migration, differentiation, and extracellular matrix synthesis. Growth factors such as transforming growth factor-beta (TGF-β), bone morphogenetic proteins (BMPs), and fibroblast growth factors (FGFs) play pivotal roles in these pathways, guiding the MSCs in their transformation into functional cartilage cells (
Figure 1).
In addition to growth factors, the role of the extracellular matrix (ECM) in providing structural support and biochemical signals is paramount. The ECM, composed primarily of collagen type II and proteoglycans like aggrecan, offers a scaffold that supports the newly differentiated chondrocytes and helps maintain the cartilage's mechanical properties. Matrix metalloproteinases (MMPs) and their inhibitors, tissue inhibitors of metalloproteinases (TIMPs), are crucial in ECM remodeling, ensuring that the balance between synthesis and degradation of the matrix is maintained to support tissue repair and integrity [
4].
Another critical aspect of the molecular repair process involves angiogenesis and vascularization. The initial phase of cartilage repair often includes transient vascularization facilitated by vascular endothelial growth factor (VEGF), which ensures that the newly formed tissue receives adequate nutrients and oxygen. However, as the repair tissue matures, it transitions back to an avascular state, characteristic of healthy articular cartilage, which is essential for maintaining its mechanical properties and longevity [
5].
This review delves into these complex molecular mechanisms, providing a detailed exploration of the biological underpinnings of cartilage repair following microfracture surgery. Additionally, it offers insights into advanced rehabilitation protocols designed to enhance the healing process. Effective rehabilitation strategies are essential for maximizing the benefits of the microfracture procedure, ensuring that the newly formed cartilage integrates well with the surrounding tissue and can withstand the mechanical stresses of daily activities. Rehabilitation programs typically include controlled mechanical loading, passive motion exercises, and gradual weight-bearing activities to stimulate chondrocyte proliferation and extracellular matrix production.
Furthermore, the use of biological augmentation, such as platelet-rich plasma (PRP) and hyaluronic acid (HA), can significantly enhance the repair process by providing additional growth factors and creating a favorable environment for cell migration and differentiation. Nutritional support, including supplements like glucosamine, chondroitin sulfate, and antioxidants, also plays a vital role in promoting cartilage health and repair [
6].
By comprehensively understanding both the molecular and practical aspects of cartilage repair, clinicians can develop more effective treatment plans, ultimately improving patient outcomes and quality of life following microfracture surgery. The integration of molecular insights with advanced rehabilitation techniques holds the promise of revolutionizing cartilage repair, offering hope for those suffering from debilitating joint conditions. This review aims to bridge the gap between molecular biology and clinical practice, providing a roadmap for optimizing cartilage repair strategies and ensuring long-term success for patients [
7].
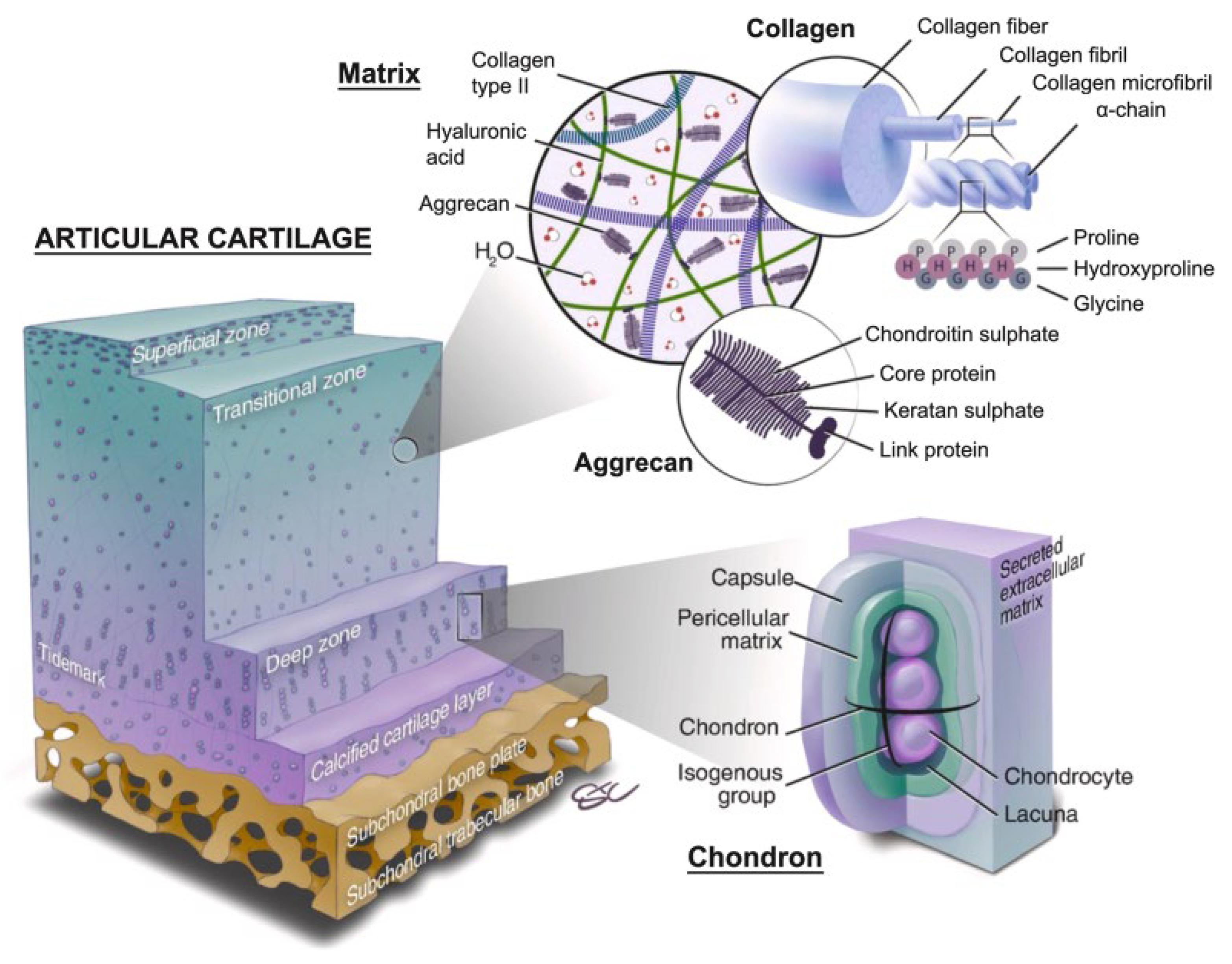
Figure 1.
Schematic of normal articular cartilage structure consisting of four zones: the superficial zone, the middle (transitional) zone, the deep zone, and the calcified cartilage layer. Source: Schematic from Wei W, Dai H. Articular cartilage and osteochondral tissue engineering techniques: recent advances and challenges. Bioactive Mater. Dec 2021;6(12): 48304855.).
Figure 1.
Schematic of normal articular cartilage structure consisting of four zones: the superficial zone, the middle (transitional) zone, the deep zone, and the calcified cartilage layer. Source: Schematic from Wei W, Dai H. Articular cartilage and osteochondral tissue engineering techniques: recent advances and challenges. Bioactive Mater. Dec 2021;6(12): 48304855.).
Molecular Mechanisms of Cartilage Repair
The molecular mechanisms of cartilage repair involve a complex interplay of cellular activities, signaling pathways, and extracellular matrix (ECM) remodeling processes that collectively contribute to the restoration of damaged cartilage tissue [
8]. These mechanisms are crucial for understanding how to enhance cartilage regeneration and improve clinical outcomes following cartilage repair procedures such as microfracture surgery (Table1).
Central to the repair process is the recruitment and differentiation of mesenchymal stem cells (MSCs) from the bone marrow to the site of cartilage injury. These MSCs migrate to the damaged area in response to chemotactic signals and differentiate into chondrocytes, the cells responsible for producing cartilage ECM. Growth factors such as transforming growth factor-beta (TGF-β), bone morphogenetic proteins (BMPs), and insulin-like growth factor-1 (IGF-1) play pivotal roles in regulating this differentiation process. TGF-β, for instance, activates the SMAD signaling pathway, which upregulates the expression of genes involved in chondrogenesis and ECM synthesis. BMPs, on the other hand, enhance the production of collagen type II and proteoglycans, which are essential components of the cartilage matrix [
9].
The ECM of articular cartilage is predominantly composed of collagen type II and aggrecan, a large proteoglycan that provides compressive strength. The synthesis and remodeling of these ECM components are tightly regulated by a balance between anabolic and catabolic activities within the cartilage. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) are key players in ECM remodeling. MMPs degrade ECM components, allowing for the removal of damaged matrix and the integration of new matrix molecules, while TIMPs inhibit MMP activity to preserve matrix integrity. This dynamic balance ensures proper tissue remodeling and the formation of a functional cartilage matrix [
10].
Mechanical loading and physical activity also significantly influence the molecular mechanisms of cartilage repair. Chondrocytes are mechanosensitive cells that respond to mechanical stimuli by altering their metabolic activity. Integrins and other mechanosensitive receptors on the chondrocyte surface mediate the conversion of mechanical signals into biochemical responses, a process known as mechanotransduction [
11]. This process involves the activation of signaling pathways such as the ERK1/2 and p38 MAPK pathways, which promote chondrocyte proliferation and ECM production. Mechanical loading also stimulates the synthesis of anabolic cytokines like BMP-2 and BMP-7, further enhancing ECM production and cartilage repair (
Figure 2).
Inflammation is another critical factor in the cartilage repair process. While acute inflammation is necessary for initiating the repair response, chronic inflammation can impede healing and lead to further cartilage degradation. Pro-inflammatory cytokines such as interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α) can inhibit chondrocyte function and increase the production of catabolic enzymes like MMPs. Anti-inflammatory treatments and interventions that modulate the inflammatory response can therefore play a crucial role in promoting effective cartilage repair [
12].
In addition to these factors, the role of the subchondral bone in cartilage repair is gaining increasing attention. The subchondral bone provides structural support to the overlying cartilage and plays a role in nutrient exchange. Alterations in subchondral bone architecture and metabolism can affect cartilage health and repair. Therapeutic strategies targeting the subchondral bone, such as bone marrow stimulation techniques and bone grafting, are being explored to enhance cartilage regeneration.
Advances in molecular biology and bioengineering are continuously expanding our understanding of the molecular mechanisms underlying cartilage repair. Techniques such as gene therapy and stem cell therapy offer new possibilities for enhancing cartilage regeneration. Gene therapy involves the introduction of specific genes into chondrocytes or stem cells to promote their anabolic activities and inhibit catabolic processes [
13]. For example, genes encoding for TGF-β or IGF-1 can be delivered to the repair site to stimulate chondrocyte proliferation and matrix synthesis. Stem cell therapy provides a source of progenitor cells that can differentiate into chondrocytes and secrete bioactive molecules that modulate the local repair environment.
In conclusion, the molecular mechanisms of cartilage repair involve a complex interplay of cellular activities, signaling pathways, and ECM remodeling processes. Understanding these mechanisms is crucial for developing targeted therapies and effective rehabilitation strategies that enhance cartilage regeneration and improve clinical outcomes. Ongoing research in this field continues to uncover new insights and therapeutic approaches, paving the way for improved treatments for cartilage injuries and degenerative joint diseases [
14].
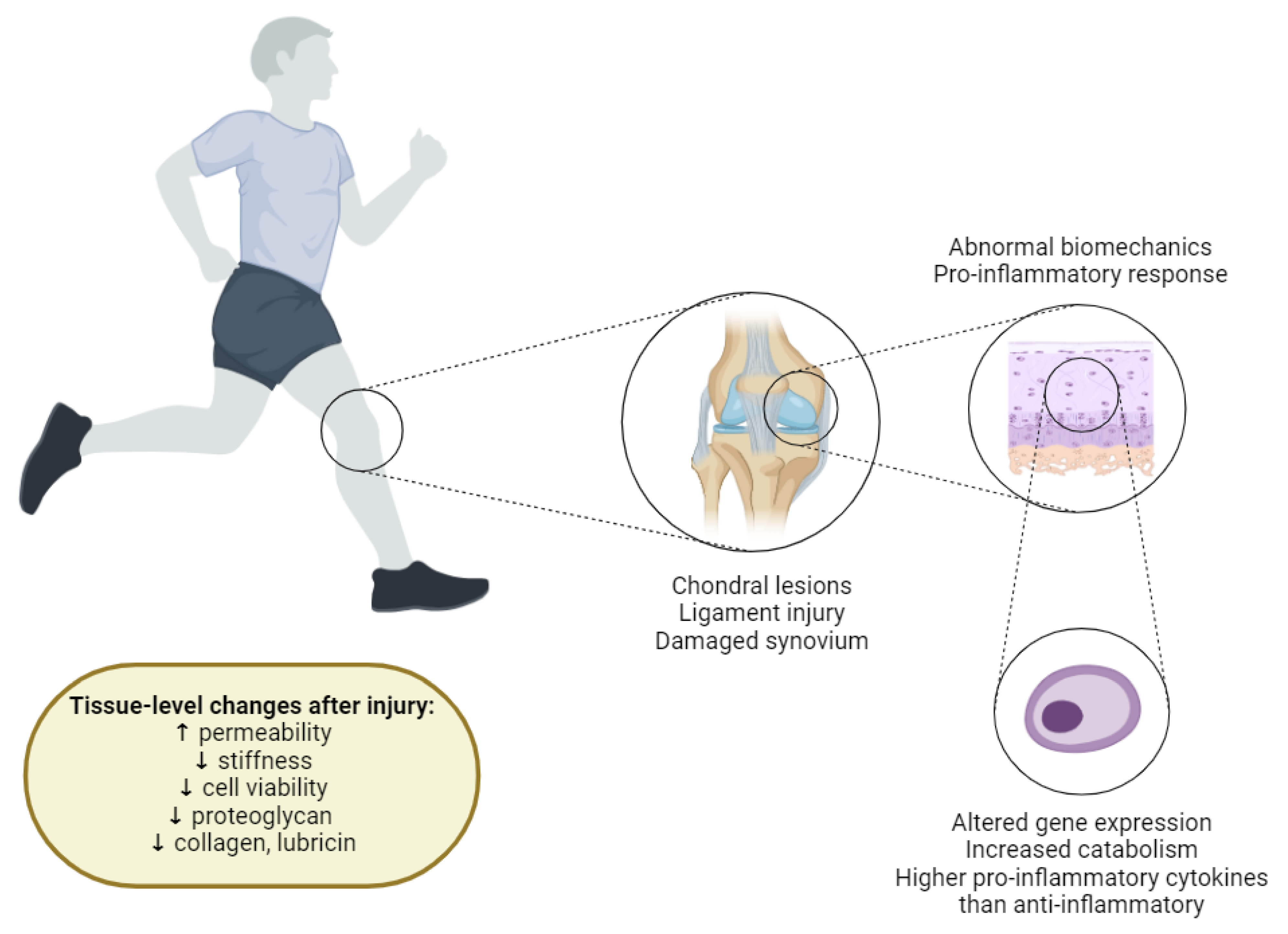
Figure 2.
Overview of Cartilage Degradation Mechanisms Triggered by a Joint Injury. Joint injuries can result in lesions on articular cartilage surfaces, ligament tears, and synovium damage. These injuries collectively create a catabolic environment within the joint, characterized by abnormal biomechanical loading patterns and the diffusion of pro-inflammatory cytokines into the cartilage. Abnormal biomechanical loading can lead to locally elevated mechanical strains or stresses, which are suggested to cause cell death, collagen network damage, and proteoglycan (PG) loss. This mechanical stress can also result in the release of reactive oxygen species (ROS) and cell death through necrosis (acute) and apoptosis (persistent abnormal loading). The presence of pro-inflammatory cytokines further exacerbates cartilage degradation by upregulating catabolic and downregulating anabolic gene expression in chondrocytes. As a result, injured cartilage shows a loss of PG and collagen content, decreased cell viability, reduced stiffness, and increased permeability compared to healthy cartilage. These changes significantly impair the structural and functional integrity of the cartilage, leading to progressive joint degeneration.).
Figure 2.
Overview of Cartilage Degradation Mechanisms Triggered by a Joint Injury. Joint injuries can result in lesions on articular cartilage surfaces, ligament tears, and synovium damage. These injuries collectively create a catabolic environment within the joint, characterized by abnormal biomechanical loading patterns and the diffusion of pro-inflammatory cytokines into the cartilage. Abnormal biomechanical loading can lead to locally elevated mechanical strains or stresses, which are suggested to cause cell death, collagen network damage, and proteoglycan (PG) loss. This mechanical stress can also result in the release of reactive oxygen species (ROS) and cell death through necrosis (acute) and apoptosis (persistent abnormal loading). The presence of pro-inflammatory cytokines further exacerbates cartilage degradation by upregulating catabolic and downregulating anabolic gene expression in chondrocytes. As a result, injured cartilage shows a loss of PG and collagen content, decreased cell viability, reduced stiffness, and increased permeability compared to healthy cartilage. These changes significantly impair the structural and functional integrity of the cartilage, leading to progressive joint degeneration.).
1. Stem Cell Recruitment and Differentiation
The success of the microfracture technique hinges on the effective recruitment and differentiation of mesenchymal stem cells (MSCs). These multipotent cells possess the capability to differentiate into a variety of cell types, including chondrocytes, which are essential for cartilage formation and repair. When microfracture holes are created in the subchondral bone, they disrupt the bone surface, leading to the stimulation and release of various growth factors into the joint space. Among these, transforming growth factor-beta (TGF-β), bone morphogenetic proteins (BMPs), and fibroblast growth factors (FGFs) play pivotal roles in creating a conducive microenvironment for MSC migration and proliferation [
15].
TGF-β is a crucial regulator of chondrogenesis, the process by which cartilage is formed. It activates intracellular proteins known as Smads, which translocate to the nucleus and regulate the expression of genes involved in the differentiation of MSCs into chondrocytes [
16]. This regulation ensures that MSCs adopt the correct cellular fate, contributing to the formation of cartilage rather than other tissue types. TGF-β signaling is also involved in maintaining the balance between cartilage formation and the prevention of hypertrophic differentiation, which can lead to endochondral ossification and bone formation instead of cartilage.
BMPs complement this process by promoting the synthesis of the extracellular matrix (ECM), which provides the necessary structural support for newly formed cartilage. BMP signaling enhances the chondrogenic potential of MSCs, facilitating their differentiation and subsequent production of cartilage-specific ECM components [
17]. BMP-2 and BMP-7 are particularly influential in promoting chondrogenesis, encouraging MSCs to commit to a chondrocytic lineage and produce the ECM rich in collagen type II and proteoglycans necessary for functional cartilage.
Additionally, FGFs are vital for the proliferation and differentiation of chondrocytes. Specifically, FGF-18 has been shown to enhance chondrocyte proliferation and matrix production, which are critical for effective cartilage repair. FGF-18 promotes the expansion of the chondrocyte population and the synthesis of key ECM components, ensuring the newly formed cartilage is robust and functional. The interplay between these growth factors is complex and tightly regulated, ensuring that MSCs differentiate appropriately and contribute to the formation of functional cartilage [
18].
This regulatory network of growth factors ensures that MSCs not only migrate to the defect site but also undergo the necessary cellular transformations to become chondrocytes, the cells responsible for maintaining and repairing cartilage. The precise spatial and temporal expression of these growth factors is crucial, as their coordinated action orchestrates the various stages of cell migration, proliferation, differentiation, and ECM synthesis [
19].
The importance of this process cannot be overstated, as the failure to recruit and properly differentiate MSCs can result in inadequate repair tissue that lacks the mechanical properties and resilience of native cartilage. Ineffective differentiation can lead to the formation of fibrocartilage, which, while functional, does not possess the same durability and load-bearing properties as hyaline cartilage. This can compromise the longevity of the repair and lead to further joint degeneration over time.
Research has shown that the local microenvironment created by these growth factors is critical for ensuring that MSCs remain viable and capable of forming high-quality cartilage. This microenvironment, often referred to as the "stem cell niche," provides both the biochemical and physical cues necessary for guiding MSC behavior. The biochemical signals include the aforementioned growth factors, while the physical properties of the tissue, such as its stiffness and elasticity, also play a significant role in influencing cell behavior and differentiation [
20]. For instance, stiffer substrates have been shown to promote osteogenic differentiation, while softer substrates favor chondrogenesis, highlighting the importance of mechanical cues in stem cell fate determination.
Additionally, the interaction between MSCs and the surrounding ECM components is facilitated by cell surface receptors such as integrins, which mediate cell adhesion and transmit signals from the ECM to the cell interior. These signals can influence cytoskeletal organization, cell shape, and ultimately gene expression patterns that govern differentiation pathways.
Moreover, the oxygen tension in the joint environment can influence MSC differentiation. Hypoxic conditions, which are typical of the cartilage microenvironment, promote chondrogenesis by stabilizing hypoxia-inducible factors (HIFs), which in turn upregulate genes involved in cartilage matrix production and angiogenesis regulation [
21].
Understanding these intricate molecular mechanisms allows for the development of targeted therapies and optimized rehabilitation strategies to enhance cartilage repair. For example, exogenous administration of growth factors or the use of biomaterials that mimic the mechanical properties of cartilage can be employed to create an optimal healing environment. Advances in gene therapy and tissue engineering also hold promise for further improving the outcomes of microfracture surgery by enhancing the recruitment, survival, and differentiation of MSCs at the repair site [
22].
In conclusion, the recruitment and differentiation of MSCs are fundamental to the success of the microfracture technique. The orchestration of growth factors, mechanical cues, and cellular interactions within the stem cell niche ensures that these stem cells can effectively contribute to cartilage repair, leading to improved clinical outcomes and enhanced joint function. As research in this field progresses, it is likely that even more sophisticated approaches will be developed to harness the regenerative potential of MSCs for cartilage repair.
2. Extracellular Matrix (ECM) Synthesis and Remodeling
The extracellular matrix (ECM) of articular cartilage is predominantly composed of collagen type II and proteoglycans such as aggrecan. These components together contribute to the tissue's tensile strength and compressive resistance, essential for its load-bearing function in joints. Collagen type II provides a fibrous network that gives the cartilage its tensile strength, while aggrecan, a large proteoglycan, is responsible for the cartilage's ability to resist compressive forces by retaining water and creating a gel-like consistency [
23].
Following microfracture surgery, the synthesis of these ECM components is upregulated by the differentiated chondrocytes derived from mesenchymal stem cells (MSCs). The newly differentiated chondrocytes increase the production of collagen type II and aggrecan, ensuring that the newly formed cartilage can withstand mechanical stress and function effectively. This synthesis is crucial for the restoration of the cartilage's structural integrity and its ability to perform its biomechanical functions.
ECM remodeling is a dynamic process regulated by matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs). MMPs are a family of enzymes responsible for the degradation of ECM components, facilitating tissue remodeling and repair. They break down various matrix molecules, including collagen and proteoglycans, allowing for the removal of damaged matrix and the integration of new matrix components. Conversely, TIMPs inhibit MMP activity, preserving matrix integrity by preventing excessive degradation. The balance between MMPs and TIMPs is critical; excessive MMP activity can lead to the breakdown of the newly formed matrix, resulting in a weaker and less functional repair tissue. Insufficient MMP activity, on the other hand, can impede proper tissue remodeling and integration, leading to suboptimal repair outcomes [
24,
25].
Furthermore, other molecules such as integrins and cytokines play significant roles in ECM synthesis and remodeling. Integrins are cell surface receptors that mediate cell-ECM interactions, influencing cell adhesion, migration, and signaling pathways involved in cartilage repair. These interactions are crucial for maintaining the structural and functional integrity of the ECM. Integrins facilitate the anchoring of chondrocytes to the ECM, enabling the cells to sense mechanical and biochemical cues from their environment and respond appropriately by adjusting their synthetic activity [
26].
Cytokines such as interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α) can modulate the activity of MMPs and TIMPs, thereby affecting ECM turnover and cartilage integrity. Elevated levels of these cytokines are often associated with inflammation and can lead to increased MMP activity, contributing to cartilage degradation and impaired repair. This cytokine-induced imbalance between MMPs and TIMPs can accelerate the breakdown of cartilage matrix, undermining the repair process and leading to further joint degeneration [
27].
The role of small leucine-rich proteoglycans (SLRPs), such as decorin and biglycan, is also crucial in ECM organization. These molecules interact with collagen fibrils and help regulate collagen fibrillogenesis, contributing to the structural integrity of the cartilage ECM. SLRPs bind to collagen fibrils and other ECM components, influencing the assembly and stability of the matrix. This interaction is essential for maintaining the biomechanical properties of the cartilage and ensuring that the newly formed tissue can function effectively under load [
28,
29]. By organizing the collagen network and modulating the matrix's physical properties, SLRPs play a vital role in preserving cartilage function and durability.
Additionally, the production and organization of the ECM are influenced by mechanical forces. Chondrocytes respond to mechanical stimuli by altering their metabolic activity, which can enhance the synthesis of ECM components. This process, known as mechanotransduction, involves the conversion of mechanical signals into biochemical responses, promoting the maintenance and repair of cartilage tissue. Mechanical loading, through activities such as controlled exercise and physical therapy, can stimulate chondrocytes to produce more collagen and proteoglycans, strengthening the cartilage matrix and improving its resilience [
30].
Understanding the interplay between mechanical forces and biochemical signals is essential for optimizing rehabilitation protocols that support ECM synthesis and remodeling. Effective rehabilitation strategies incorporate controlled mechanical loading to promote chondrocyte activity and ECM production while avoiding excessive stress that could damage the newly formed tissue. This delicate balance is crucial for ensuring the long-term success of cartilage repair and the restoration of joint function [
31].
In conclusion, ECM synthesis and remodeling are complex processes that involve a multitude of regulatory mechanisms. The coordinated action of growth factors, enzymes, cell surface receptors, cytokines, and mechanical forces ensures that the ECM of repaired cartilage can support its functional demands. Advances in our understanding of these processes provide valuable insights into improving therapeutic strategies for cartilage repair, ultimately enhancing patient outcomes and quality of life.
3. Angiogenesis and Vascularization
The early stages of cartilage repair post-microfracture involve transient vascularization, which is essential for supplying nutrients and removing metabolic waste from the repair site. This temporary increase in blood vessel formation ensures that the regenerating tissue receives adequate oxygen and essential nutrients to sustain cellular activities and promote effective healing. Vascular endothelial growth factor (VEGF) is a key mediator of this process, promoting the formation of new blood vessels. VEGF facilitates the migration and proliferation of endothelial cells, leading to the development of new capillaries that can support the metabolic demands of the repairing tissue [
32].
VEGF is upregulated in response to the hypoxic environment created by the microfracture procedure. This hypoxia-induced expression of VEGF initiates a cascade of events that culminate in angiogenesis, or the formation of new blood vessels. The newly formed vasculature is critical for the early phases of repair, providing a conduit for the delivery of nutrients, growth factors, and other signaling molecules necessary for tissue regeneration. Moreover, the removal of metabolic waste products via these new blood vessels prevents the accumulation of toxic by-products that could hinder the repair process [
33,
34].
However, the maturation of the repair tissue requires a transition to an avascular state, characteristic of healthy articular cartilage. Mature cartilage is avascular, relying on the diffusion of nutrients through the synovial fluid rather than direct blood supply. Persistent vascularization can result in the formation of fibrocartilage, which lacks the mechanical properties and resilience of hyaline cartilage. Therefore, the regulation of angiogenesis is critical for ensuring the development of functional cartilage tissue. The transition from a vascular to an avascular state is orchestrated by a complex interplay of signaling pathways and molecular cues [
35].
The hypoxia-inducible factor (HIF) pathway also plays a significant role in regulating angiogenesis during cartilage repair. HIF-1α is stabilized under low oxygen conditions, promoting the expression of VEGF and other angiogenic factors. This pathway ensures that the initial vascularization supports repair processes while later transitioning to the avascular state necessary for mature cartilage. The stabilization of HIF-1α under hypoxic conditions is a critical adaptive response that enables cells to survive and function in low-oxygen environments, which is typical of the early repair tissue [
36].
Angiopoietins, particularly Angiopoietin-1 and Angiopoietin-2, are involved in the regulation of angiogenesis and vascular stability during cartilage repair. These molecules interact with the Tie-2 receptor on endothelial cells, influencing blood vessel maturation and stability. Angiopoietin-1 promotes the maturation and stabilization of newly formed vessels, ensuring that they can provide the necessary support during the initial repair stages. In contrast, Angiopoietin-2 can induce vessel regression in the absence of VEGF, aiding in the transition to an avascular state. The balance between these angiopoietins is crucial for ensuring that the newly formed vasculature supports the early stages of repair without compromising the avascular nature of mature cartilage [
37].
Moreover, other factors such as platelet-derived growth factor (PDGF) and basic fibroblast growth factor (bFGF) also contribute to the regulation of angiogenesis. PDGF plays a role in recruiting pericytes and smooth muscle cells to stabilize new blood vessels, while bFGF supports endothelial cell proliferation and differentiation. These factors work in concert with VEGF and angiopoietins to coordinate the complex process of blood vessel formation and regression, ensuring that the repair tissue receives adequate support during the critical early stages of healing [
38].
Additionally, the regulation of angiogenesis involves the interplay of inhibitory factors such as thrombospondins and endostatin, which help to limit excessive vascular growth and ensure the proper transition to an avascular cartilage state [
39]. The presence of these inhibitory molecules is essential for maintaining the delicate balance between promoting and inhibiting blood vessel formation, ultimately leading to the formation of stable and functional cartilage tissue [
40].
By understanding these molecular mechanisms, researchers and clinicians can develop more targeted and effective strategies for enhancing cartilage repair following microfracture surgery. This knowledge not only informs the design of new therapeutic interventions but also guides the optimization of rehabilitation protocols to ensure the best possible outcomes for patients. For instance, modulating the levels of angiogenic and anti-angiogenic factors could be used to control the extent of vascularization and improve the quality of the repair tissue [
41].
The integration of molecular insights with clinical practice holds the potential to significantly improve the success rates of cartilage repair procedures and enhance the quality of life for individuals suffering from joint injuries and degenerative conditions. Future research may explore the use of biomaterials and scaffold designs that mimic the natural hypoxic and angiogenic environments of cartilage repair, further refining the strategies for promoting effective and long-lasting cartilage regeneration [
42].
In conclusion, angiogenesis and vascularization play critical roles in the early stages of cartilage repair post-microfracture. The tightly regulated process of blood vessel formation, stabilization, and regression ensures that the repair tissue is adequately supported while transitioning to the avascular state characteristic of mature cartilage. By leveraging our understanding of these molecular mechanisms, we can enhance the outcomes of cartilage repair therapies and improve patient recovery and function.
Table 1.
Summary of Molecular Mechanisms of Cartilage Repair.
Table 1.
Summary of Molecular Mechanisms of Cartilage Repair.
| Section |
Subsection |
Key Points |
Molecular Mechanisms |
References |
| 1. Stem Cell Recruitment and Differentiation |
Overview |
The success of microfracture surgery hinges on the effective recruitment and differentiation of mesenchymal stem cells (MSCs) |
MSCs migrate to injury sites, differentiate into chondrocytes, regulated by growth factors (TGF-β, BMPs, FGFs) |
Chen et al., 2004; Massagué, 2012; Caplan, 2007 |
| |
MSC Potential |
MSCs are multipotent cells capable of differentiating into various cell types, including chondrocytes |
MSCs differentiate into chondrocytes essential for cartilage formation and repair |
Pittenger et al., 1999 |
| |
TGF-β |
Crucial regulator of chondrogenesis |
Activates Smads, regulates gene expression for MSC differentiation into chondrocytes; maintains balance between cartilage formation and prevention of hypertrophic differentiation |
Massagué, 2012; Zhou et al., 2016 |
| |
BMPs |
Promote synthesis of ECM |
BMP-2 and BMP-7 enhance chondrogenesis, production of cartilage-specific ECM components |
Chen et al., 2004; Johnstone et al., 1998; Zhang et al., 2014 |
| |
FGFs |
Vital for chondrocyte proliferation and differentiation |
FGF-18 enhances chondrocyte proliferation, matrix production; promotes expansion of chondrocyte population and synthesis of ECM components |
Ellman et al., 2013; Davidson et al., 2005 |
| |
Microenvironment |
Ensures MSCs differentiate correctly |
Influences cell behavior through biochemical (growth factors) and physical (tissue stiffness) cues; provides biochemical and physical cues necessary for guiding MSC behavior |
Discher et al., 2005; Guilak et al., 2009 |
| |
Hypoxia |
Promotes chondrogenesis |
Stabilizes HIFs, upregulates genes for cartilage matrix production and angiogenesis regulation |
Schipani et al., 2001; Wang et al., 2005 |
| |
Integrins |
Facilitate MSC-ECM interaction |
Mediate cell adhesion and transmit signals from the ECM to the cell interior, influencing differentiation |
Loeser, 2014; Salgado et al., 2004 |
| |
Mechanical Properties |
Influence MSC fate |
Stiffer substrates promote osteogenic differentiation, while softer substrates favor chondrogenesis |
Engler et al., 2006; Tsai et al., 2015 |
| |
| 2. Extracellular Matrix (ECM) Synthesis and Remodeling |
Overview |
Articular cartilage ECM is primarily composed of collagen type II and proteoglycans such as aggrecan |
Provides tensile strength and compressive resistance; collagen type II forms fibrous network, aggrecan retains water and creates gel-like consistency |
Sophia Fox et al., 2009; Roughley, 2006 |
| |
ECM Upregulation |
Post-microfracture synthesis by chondrocytes |
Produces collagen type II and aggrecan, ensuring newly formed cartilage can withstand mechanical stress |
Hunziker, 2002 |
| |
MMPs and TIMPs |
Regulate ECM remodeling |
MMPs degrade ECM components, facilitating tissue remodeling; TIMPs inhibit MMPs, preserving matrix integrity; balance is critical for effective repair |
Nagase et al., 2006; van der Kraan & van den Berg, 2012; Brew & Nagase, 2010 |
| |
Integrins and Cytokines |
Influence ECM synthesis and remodeling |
Integrins mediate cell-ECM interactions, influencing adhesion, migration, and signaling; cytokines like IL-1 and TNF-α modulate MMP and TIMP activity, affecting ECM turnover and integrity |
Loeser, 2014; Goldring & Goldring, 2010; Miosge, 2014 |
| |
SLRPs |
Crucial in ECM organization |
Decorin and biglycan interact with collagen fibrils, regulating collagen fibrillogenesis and contributing to ECM stability |
Geng et al., 2006; Iozzo, 1999 |
| |
Mechanical Forces |
Influence ECM production |
Mechanotransduction converts mechanical signals into biochemical responses, enhancing chondrocyte activity and ECM production; controlled exercise and physical therapy stimulate ECM synthesis |
Grodzinsky et al., 2000; Mouw et al., 2007 |
| |
Cellular Responses |
Chondrocytes adapt to mechanical stimuli |
Mechanical loading influences gene expression and cellular metabolism, promoting cartilage maintenance and repair |
Urban, 2000; Kock et al., 2012 |
| |
| 3. Angiogenesis and Vascularization |
Overview |
Early stages of cartilage repair post-microfracture involve transient vascularization |
VEGF mediates new blood vessel formation, facilitating nutrient supply and waste removal |
Gerber et al., 1999; Ferrara, 2004 |
| |
Hypoxia |
Induces VEGF expression |
Stabilizes HIF-1α, promoting the expression of angiogenic factors and ensuring initial vascularization supports repair processes |
Semenza, 2012; Pugh & Ratcliffe, 2003 |
| |
Angiopoietins |
Regulate angiogenesis and vascular stability |
Angiopoietin-1 stabilizes vessels, while Angiopoietin-2 can induce regression in the absence of VEGF; balance is crucial for vascular support during repair |
Suri et al., 1996; Yancopoulos et al., 2000 |
| |
PDGF and bFGF |
Support angiogenesis |
PDGF recruits pericytes and smooth muscle cells for vessel stabilization, while bFGF supports endothelial cell proliferation and differentiation |
Li & Eriksson, 2003; Cao et al., 2003 |
| |
Inhibitory Factors |
Limit excessive vascular growth |
Thrombospondins and endostatin help ensure proper transition to an avascular cartilage state, balancing angiogenic and anti-angiogenic signals |
Tolsma et al., 1993; O'Reilly et al., 1997 |
| |
Clinical Application |
Knowledge informs therapy design |
Modulating angiogenic/anti-angiogenic factors controls vascularization, improving repair quality and ensuring functional cartilage development |
Carmeliet, 2000; Folkman, 2003 |
Rehabilitation Strategies
Rehabilitation strategies post-cartilage microfracture are meticulously crafted plans and protocols aimed at facilitating the recovery and functional restoration of patients who have undergone microfracture surgery. This specific surgical intervention is designed to address chondral defects by promoting the regeneration of cartilage tissue. Post-operative rehabilitation strategies are crucial for optimizing the healing process and ensuring the successful integration and functionality of the newly formed cartilage. These strategies include a combination of early mobilization techniques, controlled mechanical loading, biological augmentation, nutritional support, and advanced therapeutic modalities [
43]. The goal is to create an optimal environment for cartilage repair, enhance chondrocyte activity, support extracellular matrix (ECM) synthesis, and prevent complications such as joint stiffness and re-injury. By following a structured and personalized rehabilitation program bounded with natural process of healing stages, patients can achieve improved joint function, reduced pain, and a quicker return to their normal activities and athletic performance (
Table 2).
Early mobilization is a key component of these rehabilitation strategies. Gentle, passive range-of-motion exercises are initiated soon after surgery to promote synovial fluid circulation within the joint. This fluid is rich in nutrients and growth factors essential for cartilage health and repair. Early mobilization helps prevent the formation of adhesions and joint stiffness, which can impede recovery. By facilitating the distribution of synovial fluid, early mobilization also ensures that the new cartilage receives the biochemical signals necessary for its development and maturation [
44].
Controlled mechanical loading is another critical element of the rehabilitation process. As the cartilage begins to heal, gradual introduction of weight-bearing activities helps condition the repaired tissue to the mechanical demands it will face. This progressive loading stimulates chondrocyte proliferation and ECM production, reinforcing the structural integrity of the new cartilage. Careful monitoring is essential to balance activity and rest, avoiding overloading the repair tissue, which could disrupt the early stages of healing. Research has demonstrated that structured rehabilitation programs incorporating both passive and active exercises significantly enhance the outcomes of microfracture surgery [
45].
Biological augmentation with agents like platelet-rich plasma (PRP) and hyaluronic acid (HA) further supports the cartilage repair process. PRP, rich in growth factors such as TGF-β and IGF-1, promotes cell proliferation and matrix synthesis. HA injections improve joint lubrication, reduce pain, and provide a scaffold for new tissue growth. These biological therapies enhance the local biochemical environment, creating conditions that are conducive to effective cartilage regeneration [
46].
Nutritional support is also vital for the success of post-microfracture rehabilitation. Adequate intake of amino acids, vitamins, and minerals is essential for collagen synthesis and overall cartilage maintenance. Nutritional supplements such as glucosamine and chondroitin sulfate provide the necessary building blocks for new cartilage formation. Antioxidants like vitamins C and E protect chondrocytes from oxidative stress, while omega-3 fatty acids modulate inflammation, supporting the repair process. Ensuring sufficient dietary intake of these nutrients can significantly enhance the biochemical and biomechanical properties of the repaired cartilage [
47].
Advanced therapeutic modalities such as low-intensity pulsed ultrasound (LIPUS), pulsed electromagnetic fields (PEMF), and photobiomodulation therapy offer additional benefits. LIPUS enhances chondrocyte proliferation and ECM production through the activation of cellular signaling pathways. PEMF improves the quality of repair tissue by reducing inflammation and promoting cellular activities that support tissue regeneration. Photobiomodulation therapy stimulates cellular processes, enhances mitochondrial function, and reduces oxidative stress, contributing to improved cell survival and function. Cryotherapy, through the application of cold temperatures, helps control inflammation and pain, creating a more favorable environment for cartilage healing [
48].
1. Early Mobilization and Loading
Controlled mechanical loading is essential for stimulating the biological processes involved in cartilage repair. Early passive motion helps distribute synovial fluid, which provides nutrients and removes waste products while preventing joint stiffness and adhesions [
49]. Synovial fluid circulation is crucial as it delivers essential nutrients and oxygen to the chondrocytes, the cells responsible for maintaining and repairing cartilage. This fluid movement ensures that the newly formed cartilage receives the necessary biochemical signals to continue its development and maturation.
On a molecular level, synovial fluid acts as a medium for transporting growth factors and cytokines that are essential for cartilage repair. These include transforming growth factor-beta (TGF-β) and insulin-like growth factor-1 (IGF-1), which play critical roles in chondrocyte proliferation and extracellular matrix (ECM) synthesis [
50]. TGF-β, for example, activates the SMAD signaling pathway, leading to the transcription of genes involved in chondrogenesis and matrix production. IGF-1 enhances anabolic activities in chondrocytes by activating the PI3K/Akt pathway, promoting protein synthesis and cell survival. The presence of these growth factors in the synovial fluid enhances the cellular activities needed for effective cartilage regeneration. Additionally, synovial fluid helps remove catabolic factors such as interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α), which can inhibit repair processes and contribute to cartilage degradation by inducing the expression of matrix metalloproteinases (MMPs) [
51].
Gradual weight-bearing activities promote the maturation and integration of the new cartilage tissue by stimulating chondrocyte proliferation and ECM production. These activities help condition the repaired tissue to the mechanical demands it will face during daily activities. By progressively increasing the load on the joint, these exercises encourage the chondrocytes to produce more ECM components, such as collagen type II and proteoglycans like aggrecan, which are essential for the structural integrity of cartilage [
52].
The mechanotransduction pathways activated by mechanical loading involve integrins and other mechanosensitive receptors on the chondrocyte surface. These receptors convert mechanical stimuli into biochemical signals, leading to the activation of signaling pathways such as the MAPK/ERK pathway, which promotes cell proliferation and matrix production (Li et al., 2010). Integrins, for example, mediate the interaction between chondrocytes and the ECM, triggering intracellular signaling cascades that enhance the synthesis of ECM molecules. Mechanical loading also stimulates the synthesis of anabolic cytokines like BMP-2 and -7, which further enhance ECM production and cartilage repair by activating the SMAD and non-SMAD signaling pathways [
53].
Rehabilitation protocols must balance activity and rest to avoid overloading the repair tissue, which can disrupt the delicate early stages of healing. Research has shown that a structured rehabilitation program, incorporating both passive and active exercises, significantly improves the outcomes of microfracture surgery [
54]. These programs typically begin with passive range-of-motion exercises to maintain joint mobility without placing undue stress on the healing tissue, gradually progressing to active exercises that encourage more robust tissue formation. Active exercises enhance muscle strength and coordination, providing better support for the joint and reducing the risk of further injury [
55].
Additionally, proprioceptive exercises that improve joint stability and neuromuscular control are beneficial during rehabilitation. These exercises help to restore normal joint mechanics and reduce the risk of re-injury [
56]. Proprioception, or the sense of joint position, is crucial for coordinated movement and stability, and enhancing this can significantly reduce the likelihood of further joint damage. Proprioceptive training often includes balance exercises, stability drills, and coordination tasks that challenge the body's ability to maintain proper joint alignment during movement. On a molecular level, proprioceptive training can enhance the expression of neurotrophins like brain-derived neurotrophic factor (BDNF), which supports neural plasticity and improves motor control [
57].
Progressive resistance training can further enhance muscle strength and joint stability, supporting the repaired cartilage. This type of training involves gradually increasing the resistance or load during exercises, helping to build muscular support around the joint [
58]. Strengthening the muscles around the joint reduces the load on the cartilage, distributing mechanical stress more evenly and preventing excessive wear on the newly formed tissue. Resistance training exercises typically include weight lifting, resistance band exercises, and bodyweight exercises that target specific muscle groups around the affected joint [
59].
On a cellular level, resistance training stimulates the release of myokines such as irisin, which has anti-inflammatory properties and can enhance cartilage repair [
60]. Myokines are cytokines released by muscle cells in response to contraction and play roles in regulating inflammation, metabolism, and tissue regeneration. Irisin, in particular, can modulate the inflammatory environment around the cartilage, reducing levels of pro-inflammatory cytokines and promoting a more anabolic state conducive to repair. Resistance training also promotes the synthesis of ECM components by increasing the activity of anabolic signaling pathways, such as the PI3K/Akt pathway, which is involved in protein synthesis and cell survival [
61].
Furthermore, mechanical loading during resistance training activates mechanosensitive ion channels and receptors on chondrocytes, leading to intracellular calcium influx and the activation of downstream signaling pathways that enhance chondrocyte function and matrix production. This process, known as mechanotransduction, is critical for translating physical forces into biological responses that promote cartilage health and repair [
62].
In conclusion, early mobilization and controlled mechanical loading are crucial components of a successful rehabilitation strategy following cartilage repair. By understanding and leveraging the molecular mechanisms underlying these processes, clinicians can design effective rehabilitation programs that optimize cartilage healing and improve patient outcomes. The integration of molecular biology insights with clinical practice allows for the development of targeted therapies that enhance the natural repair processes, ultimately leading to better functional recovery and quality of life for patients with cartilage injuries.
2. Biological Augmentation
Biological agents such as platelet-rich plasma (PRP) and hyaluronic acid (HA) can significantly enhance the cartilage repair process. PRP contains a high concentration of growth factors, including transforming growth factor-beta (TGF-β), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF), which stimulate cell proliferation and matrix synthesis, accelerating the healing process [
63]. PRP therapy involves drawing a patient's blood, concentrating the platelets, and injecting the platelet-rich solution into the injured area. The growth factors released by the platelets enhance cellular activities necessary for tissue repair and regeneration. These growth factors not only promote the proliferation of chondrocytes but also stimulate the synthesis of extracellular matrix (ECM) components such as collagen and proteoglycans, essential for restoring the structural integrity of cartilage.
The application of PRP in cartilage repair capitalizes on its ability to modulate the local inflammatory environment, reducing the levels of pro-inflammatory cytokines such as interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α). By mitigating inflammation, PRP creates a more favorable environment for chondrocyte survival and function. Additionally, PRP can enhance angiogenesis, which is vital during the initial stages of repair to ensure adequate nutrient and oxygen supply to the regenerating tissue. PRP induces angiogenesis through the upregulation of VEGF, which promotes the formation of new blood vessels necessary for supporting the metabolic demands of repairing cartilage [
64].
HA provides a supportive scaffold for cell migration and ECM formation, mimicking the natural cartilage environment and promoting chondrogenesis [
65]. HA injections can improve joint lubrication, reduce pain, and provide a framework for new tissue growth. The viscoelastic properties of HA help cushion the joint, reducing friction and mechanical stress on the healing cartilage. HA interacts with cell surface receptors such as CD44 and RHAMM, initiating intracellular signaling pathways that promote chondrocyte proliferation and ECM synthesis. Specifically, HA enhances the expression of aggrecan and collagen type II, which are critical for cartilage matrix integrity and function.
In addition to its mechanical and biochemical roles, HA can influence the expression of various genes involved in cartilage repair. For instance, HA has been shown to upregulate the expression of aggrecan and collagen type II, which are critical components of the cartilage matrix. By enhancing the production of these ECM components, HA helps restore the structural integrity and function of the damaged cartilage.
These biological therapies have shown promise in improving clinical outcomes when used as adjuncts to microfracture surgery. Studies have demonstrated that PRP and HA can enhance the quality and durability of the repair tissue, leading to better functional recovery [
66,
67]. PRP, in particular, has been noted for its ability to reduce inflammation and pain, providing an environment conducive to more effective healing. HA, on the other hand, helps to maintain the viscoelastic properties of the synovial fluid, facilitating smooth joint movement and reducing discomfort.
Recent advancements in biologic treatments include the use of stem cell therapy and gene therapy. Stem cell therapy involves the application of autologous or allogeneic stem cells to the repair site, which can differentiate into chondrocytes and contribute to cartilage regeneration [
68]. These stem cells can potentially provide a more robust and sustained repair by continuously contributing to the regeneration of cartilage tissue. Autologous stem cells are derived from the patient's own body, minimizing the risk of immune rejection, while allogeneic stem cells come from a donor, offering a readily available source of regenerative cells.
Stem cells used in cartilage repair, such as mesenchymal stem cells (MSCs), have the ability to differentiate into chondrocytes and secrete various growth factors that enhance the repair process. MSCs also modulate the immune response, reducing inflammation and creating a more favorable environment for tissue regeneration. Preconditioning MSCs with specific growth factors or genetic modifications can further enhance their chondrogenic potential and therapeutic efficacy. MSCs secrete anti-inflammatory cytokines like IL-10 and TGF-β, which help to suppress the local inflammatory response and protect the newly forming cartilage from degradation.
Gene therapy aims to introduce genes encoding for growth factors or anti-inflammatory molecules directly into the joint, enhancing the repair process at the molecular level [
69]. This innovative approach can provide a continuous source of therapeutic agents, directly targeting the underlying mechanisms of cartilage repair and potentially offering long-term benefits. By delivering specific genes to the affected area, gene therapy can modulate cellular activities, enhance tissue regeneration, and reduce inflammation. For example, introducing genes that encode for anabolic factors like IGF-1 and BMP-2 can promote chondrocyte proliferation and matrix synthesis, while anti-inflammatory genes encoding IL-1Ra or soluble TNF receptors can reduce cartilage degradation.
Gene therapy strategies for cartilage repair include the use of viral and non-viral vectors to deliver therapeutic genes to chondrocytes or stem cells. For example, genes encoding for TGF-β, IGF-1, or BMPs can be introduced to stimulate chondrocyte proliferation and ECM synthesis. Anti-inflammatory genes, such as IL-1 receptor antagonist (IL-1Ra) or soluble TNF receptors, can also be delivered to mitigate the inflammatory response and protect the regenerating cartilage. Advanced techniques such as CRISPR-Cas9 gene editing are also being explored to enhance the precision and efficacy of gene therapy in cartilage repair.
Tissue engineering approaches, such as the use of biomimetic scaffolds, are also being explored. These scaffolds can provide structural support and biochemical signals to enhance cell proliferation and differentiation, facilitating the formation of new cartilage tissue [
70]. Biomimetic scaffolds are designed to mimic the natural extracellular matrix of cartilage, providing an optimal environment for cell attachment, proliferation, and differentiation. These scaffolds can be combined with growth factors and stem cells to create a regenerative platform that enhances the repair process.
Biomimetic scaffolds can be fabricated from various materials, including natural polymers (e.g., collagen, hyaluronic acid), synthetic polymers (e.g., polylactic acid, polyethylene glycol), and composites. These materials can be engineered to have specific mechanical properties, degradation rates, and bioactivity to support cartilage regeneration. Incorporating growth factors into the scaffold can provide sustained release and localized delivery, enhancing their therapeutic effects. Additionally, scaffolds can be designed to release signaling molecules in response to specific biological cues, ensuring that the regenerative process is dynamically regulated [
71].
In conclusion, biological augmentation represents a promising approach to enhance cartilage repair by leveraging the body's natural healing processes. PRP, HA, stem cell therapy, gene therapy, and biomimetic scaffolds offer various mechanisms to support and accelerate cartilage regeneration. By integrating these biological agents with traditional surgical techniques and rehabilitation protocols, clinicians can improve the quality and durability of cartilage repair, leading to better functional outcomes for patients. The continued advancement in molecular biology and bioengineering will likely yield even more effective strategies for cartilage repair, further enhancing the ability to restore joint function and improve the quality of life for individuals with cartilage injuries.
3. Nutritional Support
Adequate nutrition is vital for cartilage health and repair. Amino acids, vitamins, and minerals are essential for collagen synthesis and overall cartilage maintenance. Nutritional supplements such as glucosamine and chondroitin sulfate have been reported to support cartilage structure and function, potentially improving the outcomes of cartilage repair procedures [
72]. These supplements are believed to provide the building blocks necessary for the synthesis of new cartilage. Glucosamine is an amino sugar that plays a crucial role in building cartilage by serving as a precursor for glycosaminoglycans, which are key components of the cartilage matrix. Chondroitin sulfate, a sulfated glycosaminoglycan, helps retain water and maintain elasticity, contributing to the cartilage's ability to withstand compressive forces.
Ensuring sufficient dietary intake of these nutrients can support the body's natural repair mechanisms, promoting the formation of robust and functional cartilage tissue. Clinical studies suggest that nutritional supplementation can enhance the biochemical and biomechanical properties of the repaired cartilage, contributing to better long-term outcomes [
73]. For example, adequate protein intake is crucial for the synthesis of collagen, a major component of the cartilage ECM. Proteins provide the necessary amino acids that are used to build and repair tissues throughout the body. Specifically, amino acids like proline and lysine are critical for collagen formation. Proline stabilizes the collagen structure, while lysine is involved in the cross-linking of collagen fibers, enhancing the strength and resilience of the cartilage matrix.
Proline and lysine undergo post-translational modifications that are essential for collagen stability. Proline is hydroxylated to hydroxyproline by the enzyme prolyl hydroxylase, and lysine is hydroxylated to hydroxylysine by lysyl hydroxylase. These hydroxylation reactions require vitamin C as a cofactor, and the resulting hydroxyproline and hydroxylysine residues are crucial for forming stable hydrogen bonds within the collagen triple helix, thus enhancing its tensile strength and stability.
Additionally, antioxidants such as vitamins C and E play a crucial role in protecting cartilage cells from oxidative stress, which can impair the repair process. Vitamin C is a cofactor for prolyl hydroxylase and lysyl hydroxylase, enzymes necessary for the hydroxylation of proline and lysine during collagen synthesis. This hydroxylation is crucial for the stability and function of collagen triple helices. Vitamin E acts as an antioxidant that protects cellular membranes from oxidative damage, which can be particularly harmful to chondrocytes, the cells responsible for maintaining the cartilage matrix. Oxidative stress can lead to the degradation of cartilage by increasing the production of reactive oxygen species (ROS) that damage cellular components and ECM molecules.
Omega-3 fatty acids, found in fish oil, have anti-inflammatory properties that may also benefit cartilage health [
74]. These nutrients can help modulate the inflammatory response, reducing damage to the cartilage and supporting its repair. Omega-3 fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), can reduce the production of inflammatory cytokines and eicosanoids, thereby decreasing inflammation in the joint environment. EPA and DHA are precursors to resolvins and protectins, which are specialized pro-resolving mediators that actively resolve inflammation and promote tissue repair.
Other important nutrients include zinc and copper, which are cofactors for enzymes involved in collagen synthesis and cross-linking, essential processes for maintaining cartilage integrity [
75]. Zinc is crucial for the activity of collagenase, an enzyme that helps remodel collagen during tissue repair. It also plays a role in DNA synthesis, cell division, and protein synthesis, all of which are important for tissue repair and regeneration. Zinc deficiency can impair collagen synthesis and delay wound healing. Copper is involved in the formation of cross-links in collagen and elastin, providing tensile strength and stability to the cartilage matrix. The enzyme lysyl oxidase, which requires copper as a cofactor, is responsible for the oxidative deamination of lysine residues in collagen, leading to the formation of covalent cross-links that stabilize the collagen network.
Furthermore, sulfur-containing compounds such as methylsulfonylmethane (MSM) are also beneficial for cartilage health. MSM provides sulfur, a vital component of chondroitin sulfate and keratan sulfate, which are key components of the cartilage matrix. Sulfur is necessary for the formation of disulfide bonds in collagen, contributing to the stability and resilience of the cartilage structure. Sulfur also plays a role in the synthesis of glutathione, a powerful antioxidant that protects cells from oxidative damage and supports cellular repair processes.
Adequate hydration is another critical aspect of nutritional support for cartilage health. Water is essential for maintaining the viscoelastic properties of cartilage, allowing it to absorb and distribute mechanical loads efficiently. Dehydration can lead to a decrease in the water content of the cartilage, reducing its ability to function properly and increasing the risk of damage. Proper hydration supports the function of proteoglycans, which trap water within the cartilage matrix and contribute to its compressive strength.
In addition to these nutrients, other vitamins and minerals play supportive roles in cartilage health. For example, vitamin D is essential for calcium homeostasis and bone health, and it may also play a role in cartilage maintenance by modulating the activity of chondrocytes and the synthesis of ECM components. Vitamin K is necessary for the activation of matrix Gla-protein (MGP), which inhibits calcification in the cartilage matrix and maintains its flexibility [
76].
In conclusion, a comprehensive approach to nutritional support is essential for optimizing cartilage repair and maintenance. By ensuring adequate intake of essential nutrients, vitamins, and minerals, individuals can support the body's natural repair mechanisms and promote the formation of healthy, functional cartilage. This holistic approach not only enhances the outcomes of cartilage repair procedures but also contributes to overall joint health and longevity. Integrating molecular biology insights with clinical nutrition strategies allows for the development of targeted nutritional interventions that can enhance the efficacy of cartilage repair and improve patient outcomes.
4. Advanced Therapeutic Modalities
Emerging therapies such as low-intensity pulsed ultrasound (LIPUS) and pulsed electromagnetic fields (PEMF) offer promising adjunctive treatments for cartilage repair. LIPUS has been shown to enhance chondrocyte proliferation, ECM production, and overall cartilage repair by modulating cellular signaling pathways [
77]. This non-invasive therapy uses sound waves to stimulate the cells within the cartilage, promoting a more rapid and effective healing process. LIPUS has been found to increase the expression of genes involved in cartilage formation and to enhance the production of collagen and proteoglycans. Specifically, LIPUS activates mechanotransduction pathways involving integrins, which leads to the upregulation of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), crucial for balanced ECM remodeling.
On a molecular level, LIPUS enhances the expression of key growth factors such as TGF-β and IGF-1, which are essential for chondrocyte proliferation and matrix synthesis. It also stimulates the ERK1/2 and p38 MAPK signaling pathways, which play critical roles in cellular responses to mechanical stress, promoting cell survival, proliferation, and differentiation. Additionally, LIPUS reduces the production of pro-inflammatory cytokines like IL-1 and TNF-α, creating a more conducive environment for cartilage repair by mitigating inflammatory responses that could otherwise lead to further tissue damage [
78].
PEMF therapy may improve the quality of the repair tissue by reducing inflammation and promoting cellular activities that support tissue regeneration [
79]. PEMF therapy uses electromagnetic fields to influence cell behavior, enhancing natural repair processes and improving the integration and function of the new cartilage. Studies have shown that PEMF can increase the proliferation of chondrocytes, enhance the synthesis of ECM components, and reduce inflammation, leading to improved cartilage repair. PEMF stimulates the production of endogenous growth factors such as BMPs and FGF-2, which are vital for chondrocyte differentiation and ECM production.
At the molecular level, PEMF activates the Wnt/β-catenin signaling pathway, crucial for chondrocyte proliferation and differentiation. It also enhances the activity of the adenosine receptor A2A, leading to increased cAMP levels and the activation of protein kinase A (PKA), supporting chondrogenesis and matrix production. Furthermore, PEMF modulates the NF-κB pathway, reducing the expression of inflammatory mediators and promoting an anti-inflammatory environment conducive to cartilage repair.
Integrating these advanced therapeutic modalities into rehabilitation programs can optimize the healing environment, enhancing the regenerative processes initiated by the microfracture technique. Clinical evidence supports the use of LIPUS and PEMF in improving the outcomes of cartilage repair, with studies showing significant benefits in terms of pain relief, functional recovery, and cartilage quality [
80]. The combination of these modalities with traditional rehabilitation exercises can accelerate the recovery process and improve the structural and functional integrity of the repaired cartilage.
Additionally, photobiomodulation therapy, which uses low-level laser therapy (LLLT) to stimulate cellular processes, has shown potential in enhancing cartilage repair. LLLT can reduce inflammation, promote cell proliferation, and accelerate tissue healing [
81]. This therapy involves the application of specific wavelengths of light to the affected area, stimulating cellular activity and improving tissue repair. LLLT enhances mitochondrial function, increases ATP production, and reduces oxidative stress, all of which contribute to improved cell survival and function. On a molecular level, LLLT activates transcription factors such as NF-κB, upregulating the expression of genes involved in cell survival and proliferation, and modulates the activity of reactive oxygen species (ROS), thus reducing oxidative damage and promoting healing [
82].
Cryotherapy, involving the application of cold temperatures to the affected joint, can reduce inflammation and pain, creating a more favorable environment for cartilage healing [
83]. This simple and effective treatment helps to control swelling and pain, allowing for a more comfortable and efficient rehabilitation process. Cryotherapy can be applied through ice packs, cold water immersion, or cryo chambers, working by constricting blood vessels, reducing blood flow to the area, and decreasing inflammation. On a molecular level, cryotherapy modulates the expression of inflammatory cytokines and heat shock proteins, which help protect cells from stress and promote tissue repair [
84].
Extracorporeal shockwave therapy (ESWT) is another modality being explored for cartilage repair. ESWT uses high-energy sound waves to stimulate the healing process in tissues. It has been shown to increase the expression of angiogenic and osteogenic factors, enhancing blood flow and promoting tissue regeneration [
85]. ESWT activates signaling pathways such as ERK1/2 and p38 MAPK, which are involved in cellular responses to mechanical stimuli, and increases the expression of ECM components like collagen and aggrecan. The mechanical stress induced by ESWT also stimulates the release of nitric oxide (NO), a molecule that enhances blood flow and promotes healing by modulating the inflammatory response and angiogenesis [
86].
Additionally, regenerative medicine approaches such as stem cell therapy and gene therapy are being integrated with these advanced modalities to enhance their efficacy. Stem cell therapy, particularly using mesenchymal stem cells (MSCs), can provide a source of progenitor cells that differentiate into chondrocytes, contributing to cartilage regeneration [
87,
88,
89]. MSCs secrete bioactive molecules that modulate the immune response, reduce inflammation, and promote tissue repair. Combining MSCs with LIPUS, PEMF, or LLLT can synergistically enhance the regenerative capacity of the treatment, leading to improved cartilage repair outcomes [
90].
Gene therapy involves the introduction of specific genes into chondrocytes or stem cells to enhance their regenerative potential. For example, genes encoding for anabolic factors such as IGF-1 or TGF-β can be delivered to the repair site to stimulate chondrocyte proliferation and matrix synthesis. Anti-inflammatory genes encoding IL-1 receptor antagonist (IL-1Ra) or soluble TNF receptors can be introduced to reduce inflammation and protect the regenerating cartilage. Advances in gene editing technologies, such as CRISPR-Cas9, offer precise and efficient methods to enhance the expression of therapeutic genes, further improving the outcomes of cartilage repair interventions [
91].
In conclusion, a multifaceted approach to rehabilitation that includes early mobilization, biological augmentation, nutritional support, and advanced therapeutic modalities can significantly enhance the repair and regeneration of cartilage following microfracture surgery [
92]. By leveraging these diverse strategies, clinicians can provide comprehensive care that maximizes the potential for successful cartilage repair and improved patient outcomes. This integrated approach not only addresses the immediate needs of the repair process but also promotes long-term joint health and function, enabling patients to return to their daily activities with reduced pain and improved mobility [
93]. Understanding the molecular mechanisms behind these therapies allows for the development of targeted and effective treatment plans that enhance the body's natural healing processes and improve the overall efficacy of cartilage repair interventions.
Table 2.
Summary of Rehabilitation Strategies.
Table 2.
Summary of Rehabilitation Strategies.
| Section |
Subsection |
Key Points |
Molecular Mechanisms |
References |
| 1. Early Mobilization and Loading |
Overview |
Controlled mechanical loading is essential for stimulating biological processes involved in cartilage repair |
Early mobilization distributes synovial fluid, which delivers nutrients and removes waste products |
Ebert et al., 2008 |
| |
Synovial Fluid |
Early passive motion helps distribute synovial fluid, providing nutrients and removing waste products |
Synovial fluid circulation ensures newly formed cartilage receives necessary biochemical signals |
Fortier et al., 2011 |
| |
Growth Factors |
Synovial fluid transports growth factors like TGF-β and IGF-1, essential for chondrocyte proliferation and ECM synthesis |
TGF-β activates SMAD pathway; IGF-1 activates PI3K/Akt pathway, promoting protein synthesis and cell survival |
Fortier et al., 2011 |
| |
Weight-Bearing Activities |
Gradual weight-bearing activities promote maturation and integration of new cartilage tissue |
Gradual weight-bearing stimulates chondrocyte proliferation and ECM production |
Li et al., 2010 |
| |
Mechanotransduction |
Mechanotransduction pathways activated by mechanical loading involve integrins and other mechanosensitive receptors |
Integrins convert mechanical stimuli into biochemical signals, activating pathways like MAPK/ERK |
Li et al., 2010 |
| |
Rehabilitation Protocols |
Rehabilitation protocols must balance activity and rest to avoid overloading repair tissue |
Balance of activity and rest avoids disruption of early healing stages |
Ebert et al., 2013; Steadman et al., 2003 |
| |
Proprioceptive Exercises |
Proprioceptive exercises improve joint stability and neuromuscular control, reducing risk of re-injury |
Proprioception training enhances expression of neurotrophins like BDNF, improving motor control |
Risberg et al., 2004 |
| |
Progressive Resistance Training |
Progressive resistance training enhances muscle strength and joint stability, supporting repaired cartilage |
Resistance training increases activity of anabolic signaling pathways like PI3K/Akt |
Heinonen et al., 2000 |
| |
Cellular Responses |
Resistance training stimulates release of myokines such as irisin, which has anti-inflammatory properties and enhances cartilage repair |
Myokines like irisin modulate inflammatory environment, promoting anabolic state |
Huh et al., 2014; Glass, 2010 |
| |
| 2. Biological Augmentation |
Overview |
Biological agents such as PRP and HA can significantly enhance cartilage repair process |
PRP and HA support cartilage repair by enhancing cellular activities and ECM formation |
Foster et al., 2009 |
| |
PRP |
PRP contains growth factors that stimulate cell proliferation and matrix synthesis, accelerating healing |
PRP growth factors like TGF-β, PDGF, VEGF stimulate chondrocyte proliferation and ECM synthesis |
Foster et al., 2009 |
| |
HA |
HA provides a supportive scaffold for cell migration and ECM formation, mimicking natural cartilage environment |
HA interacts with receptors like CD44, initiating pathways promoting chondrocyte proliferation and ECM synthesis |
Sasaki et al., 2014 |
| |
Stem Cell Therapy |
Stem cell therapy involves application of stem cells to repair site, which can differentiate into chondrocytes |
Stem cells differentiate into chondrocytes and secrete growth factors, enhancing repair |
Koh et al., 2012 |
| |
Gene Therapy |
Gene therapy introduces genes encoding growth factors or anti-inflammatory molecules directly into joint |
Gene therapy delivers genes encoding for anabolic factors and anti-inflammatory molecules |
Evans et al., 2014 |
| |
Tissue Engineering |
Tissue engineering uses biomimetic scaffolds to provide structural support and biochemical signals |
Biomimetic scaffolds provide optimal environment for cell attachment, proliferation, and differentiation |
Nixon et al., 2015 |
| |
| 3. Nutritional Support |
Overview |
Adequate nutrition is vital for cartilage health and repair |
Nutritional support enhances body's natural repair mechanisms for cartilage |
Hochberg et al., 2015 |
| |
Amino Acids |
Amino acids, vitamins, and minerals are essential for collagen synthesis and overall cartilage maintenance |
Proteins provide amino acids like proline and lysine, critical for collagen formation |
Henrotin et al., 2011 |
| |
Antioxidants |
Antioxidants such as vitamins C and E protect cartilage cells from oxidative stress |
Vitamin C and E act as cofactors in collagen synthesis and protect against oxidative stress |
Gaby, 2011 |
| |
Omega-3 Fatty Acids |
Omega-3 fatty acids have anti-inflammatory properties that benefit cartilage health |
EPA and DHA from omega-3 fatty acids reduce production of inflammatory cytokines |
Gaby, 2011 |
| |
Trace Elements |
Trace elements like zinc and copper are cofactors for enzymes involved in collagen synthesis and cross-linking |
Zinc and copper are critical for collagen synthesis and cross-linking |
Failla, 2003 |
| |
Sulfur Compounds |
Sulfur-containing compounds such as MSM are beneficial for cartilage health |
MSM provides sulfur for formation of disulfide bonds in collagen |
Failla, 2003 |
| |
Hydration |
Adequate hydration is critical for maintaining viscoelastic properties of cartilage |
Proper hydration maintains viscoelastic properties of cartilage, supporting function |
Failla, 2003 |
| |
| 4. Advanced Therapeutic Modalities |
Overview |
Emerging therapies such as LIPUS and PEMF offer promising adjunctive treatments for cartilage repair |
Advanced modalities like LIPUS, PEMF, LLLT, cryotherapy, ESWT enhance cartilage repair |
Lirani-Galvão & Jorgetti, 2012 |
| |
LIPUS |
LIPUS enhances chondrocyte proliferation, ECM production, and overall cartilage repair |
LIPUS activates mechanotransduction pathways involving integrins, upregulating ECM synthesis |
Lirani-Galvão & Jorgetti, 2012 |
| |
PEMF |
PEMF therapy may improve quality of repair tissue by reducing inflammation and promoting cellular activities |
PEMF activates Wnt/β-catenin signaling, enhancing chondrocyte proliferation and ECM production |
Ciombor et al., 2003 |
| |
LLLT |
LLLT stimulates cellular processes, reduces inflammation, and accelerates tissue healing |
LLLT enhances mitochondrial function, increases ATP production, reduces oxidative stress |
Hamblin, 2017 |
| |
Cryotherapy |
Cryotherapy reduces inflammation and pain, creating a more favorable environment for cartilage healing |
Cryotherapy modulates expression of inflammatory cytokines and heat shock proteins |
Bleakley et al., 2004 |
| |
ESWT |
ESWT uses high-energy sound waves to stimulate healing process in tissues |
ESWT enhances expression of angiogenic and osteogenic factors, promoting tissue regeneration |
Zhao et al., 2015; Guerne et al., 2003 |
| |
Stem Cell Therapy |
Stem cell therapy provides a source of progenitor cells that differentiate into chondrocytes |
Stem cell therapy enhances repair by providing progenitor cells and bioactive molecules |
Koh et al., 2012 |
| |
Gene Therapy |
Gene therapy introduces specific genes to enhance regenerative potential of chondrocytes or stem cells |
Gene therapy modulates cellular activities, enhancing tissue regeneration and reducing inflammation |
Evans et al., 2014 |
Bounding Natural Process of Cartilage Healing with Rehabilitation Strategies
The natural process of cartilage healing is a multifaceted and finely tuned sequence of events, underpinned by complex molecular biology mechanisms that guide each stage of recovery (
Table 3). Initially, the inflammation stage is characterized by vasodilation and the recruitment of platelets and inflammatory cells such as neutrophils, monocytes, and macrophages to the injury site [
94]. This is mediated by a series of chemical signals, including histamine, bradykinin, and prostaglandin E2 (PGE2) [
95]. These mediators activate specific signaling pathways, such as the NF-κB pathway, which promotes the expression of genes involved in inflammation and immune response. This phase results in classic symptoms of inflammation: swelling, erythema, warmth, and pain, which are essential for initiating the healing process [
96]. The inflammatory response also involves the release of cytokines such as interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α), which further amplify the recruitment of immune cells and the production of reactive oxygen species (ROS) to combat potential infections. Therapeutic interventions such as cryotherapy with compression help manage these symptoms, while NSAIDs (if not contraindicated) reduce inflammation [
97]. Manual therapy may also be used to maintain mobility and prepare tissues for subsequent healing stages.
As the healing process advances to the fibroblastic stage, a different set of molecular signals takes precedence. Growth factors such as Transforming Growth Factor-beta 1 (TGF-β1), Bone Morphogenetic Proteins (BMP), and Connective Tissue Growth Factor (CTGF) play crucial roles in activating fibroblastic cells [
98]. TGF-β1 binds to its receptors, triggering the SMAD signaling pathway, which promotes the transcription of genes involved in cell proliferation and extracellular matrix (ECM) production. BMPs activate the SMAD and MAPK pathways, which further enhance fibroblast activity. These fibroblasts then proliferate and increase the synthesis of ECM components, including collagen, fibronectin, and proteoglycans [
99]. Additionally, vascular endothelial growth factor (VEGF) promotes angiogenesis, ensuring adequate blood supply to the regenerating tissue. The formation and organization of the ECM are essential for providing structural support and facilitating tissue repair. The quality and strength of the scar tissue during this phase are influenced by the temporary clot and proper alignment of collagen fibers [
100]. Therapeutic methods such as electrical stimulation, laser therapy, ultrasound, pulsed electromagnetic field therapy (PEMF), extracorporeal shock wave therapy (ESWT), and isometric and blood flow restriction (BFR) training are employed to stimulate fibroblastic activity and enhance ECM synthesis [
101].
In the final remodeling stage, the scar tissue undergoes maturation, leading to improved organization and mechanical properties of the ECM. This stage involves the coordinated activity of fibroblasts and myofibroblasts, which synthesize and remodel collagen and other ECM components [
102]. The remodeling process is regulated by a balance between matrix metalloproteinases (MMPs), which degrade ECM components, and tissue inhibitors of metalloproteinases (TIMPs), which inhibit MMP activity. This balance is crucial for maintaining the structural integrity and functional properties of the healing tissue. Molecularly, the remodeling phase is characterized by the downregulation of inflammatory markers and the upregulation of genes involved in ECM remodeling [
103]. Key molecules such as collagen type II and aggrecan are synthesized to replace the provisional matrix with a more durable cartilage matrix. The enhanced tensile strength and proper alignment of collagen fibers during this stage are critical for restoring the functional integrity of the cartilage [
104].
Therapeutic strategies in the remodeling stage are tailored based on patient assessment and may include manual therapy for joint mobilization and soft tissue mobilization, as well as therapeutic exercises designed to increase active range of motion (ROM), flexibility, muscle strength, endurance, proprioception, motor control, and cardiovascular fitness [
105]. This comprehensive approach ensures that the healing tissue regains its functional capabilities and minimizes the risk of long-term damage. Advanced techniques such as platelet-rich plasma (PRP) injections and stem cell therapy are also being explored to enhance the regenerative potential of cartilage and improve outcomes [
106].
Overall, the integration of molecular biology with rehabilitation strategies is crucial for effective cartilage healing. By understanding the molecular mechanisms underlying each stage of healing, healthcare professionals can design targeted interventions that facilitate tissue repair and restore function. Abbreviations used in this context include BMP (bone morphogenetic protein), CTGF (connective tissue growth factor), DOMS (delayed onset muscle soreness), ECM (extracellular matrix), ESWT (extracorporeal shock wave therapy), NSAIDs (non-steroidal anti-inflammatory drugs), PEMF (pulsed electromagnetic field therapy), BFR (blood flow restriction), PGE2 (prostaglandin E2), ROM (range of motion), TGF-β1 (transforming growth factor-β1), MMPs (matrix metalloproteinases), and TIMPs (tissue inhibitors of metalloproteinases) [
107].
Table 3.
Bounding natural process of cartilage healing with rehabilitation strategies.
Table 3.
Bounding natural process of cartilage healing with rehabilitation strategies.
| Healing stage |
Cellular phase |
Biophysical characteristics |
Therapeutic intervention |
| Inflammation Stage |
Vasodilation, invasion of platelets, and inflammatory cells (neutrophils, monocytes, and macrophages) are crucial processes in the body's response to injury. These events are orchestrated by a complex interplay of chemical mediators, including histamine, bradykinin, and PGE2, each playing specific roles at the molecular level.
to injury, facilitating effective tissue repair and restoration of function.
|
Swelling, erythema, warmth, pain |
Cryotherapy, preferably with compression
NSAIDs (unless contraindicated)
Manual therapy |
| The strength of the scar depends on the temporary clot and stitches |
Methods: electrical stimulation, laser therapy, ultrasound, PEMF, ESWT, isometric and BFR training. |
Fibroblastic stage.
|
Growth factors such as Transforming Growth Factor-beta 1 (TGF-β1), Bone Morphogenetic Proteins (BMP), and Connective Tissue Growth Factor (CTGF) play critical roles in wound healing by activating fibroblastic cells. Upon activation, these fibroblastic cells undergo proliferation and upregulate the synthesis of extracellular matrix (ECM) components including collagen, fibronectin, and proteoglycans.
|
Expression of inflammatory markers |
Manual therapy: passive range of motion, soft tissue mobilization, joint mobilization |
| The scar begins to gain tensile strength |
Methods: electrical stimulation, laser therapy, ultrasound, PEMF, ESWT
Therapeutic exercises: prescribed to achieve the goal of full weight bearing on the surgical limb while protecting the tissues (slow eccentric tempo) |
Remodelling stage.
|
The remodeling of the scar improves the organization and mechanical properties of the extracellular matrix (ECM) through a dynamic process involving the coordinated activity of various cells, enzymes, and signaling pathways. Fibroblasts and myofibroblasts play key roles in this process by synthesizing and remodeling collagen and other ECM components.
|
The inflammation should subside; pain, if present, may be due to osteoarthritis, DOMS, re-damage to healing tissue |
Manual therapy depending on needs, based on the patient's assessment of the operated limb and the rest of the body; passive and active range of motion, soft tissue mobilization, including scar mobilization, joint mobilization |
Methods: Typically discontinued at this stage unless patient assessment indicates special requirements for the surgical limb or rest of the body
Therapeutic exercises: prescribed to increase active ROM and flexibility, build muscle strength and endurance, improve proprioception, motor control, and improve cardiovascular fitness |
| Abbreviations: BMP, bone morphogenetic protein; CTGF, connective tissue growth factor; DOMS, delayed onset muscle soreness; ECM, extracellular matrix; ESWT, extracorporeal shock wave therapy; NSAIDs, non-steroidal anti-inflammatory drugs; PEMF, pulsed electromagnetic field therapy; BFR, blood flow restriciton; PGE2, prostaglandin E2; ROM, range of motion; TGF-β1, transforming growth factor-β1. |
Conclusions
A comprehensive understanding of the molecular mechanisms underlying cartilage repair following microfracture is essential for developing effective rehabilitation strategies. Cartilage repair is a complex and multifaceted process that involves a delicate balance of cellular activities, signaling pathways, and extracellular matrix (ECM) remodeling. Early mobilization, biological augmentation, nutritional support, and advanced therapeutic modalities collectively contribute to the successful formation of functional cartilage [
108,
109,
110].
Early mobilization and controlled mechanical loading are crucial for stimulating the biological processes involved in cartilage repair. Mechanical forces activate mechanotransduction pathways, such as integrins and ion channels, which convert physical stimuli into biochemical signals. These signals promote chondrocyte proliferation and ECM synthesis, essential for the structural integrity of the newly formed cartilage. Understanding the molecular pathways involved in mechanotransduction, such as the ERK1/2 and p38 MAPK pathways, allows clinicians to design effective rehabilitation programs that optimize cartilage healing and improve patient outcomes [
111].
Biological augmentation with agents like platelet-rich plasma (PRP) and hyaluronic acid (HA) enhances the local biochemical environment, promoting chondrocyte activity and ECM production. PRP contains a high concentration of growth factors, including TGF-β and IGF-1, which stimulate cell proliferation and matrix synthesis [
112,
113]. HA provides a supportive scaffold for cell migration and ECM formation, mimicking the natural cartilage environment and promoting chondrogenesis. These biological therapies modulate the expression of key genes and signaling pathways involved in cartilage repair, creating a conducive environment for tissue regeneration [
99].
Nutritional support plays a vital role in cartilage health and repair. Adequate intake of amino acids, vitamins, and minerals is essential for collagen synthesis and overall cartilage maintenance. Nutritional supplements like glucosamine and chondroitin sulfate provide the building blocks necessary for the synthesis of new cartilage. Antioxidants such as vitamins C and E protect cartilage cells from oxidative stress, while omega-3 fatty acids modulate the inflammatory response, reducing damage to the cartilage and supporting its repair. Understanding the molecular mechanisms through which these nutrients influence cartilage biology can inform dietary recommendations and supplementation strategies to enhance the outcomes of cartilage repair procedures [
114].
Advanced therapeutic modalities such as low-intensity pulsed ultrasound (LIPUS), pulsed electromagnetic fields (PEMF), photobiomodulation therapy, and cryotherapy offer promising adjunctive treatments for cartilage repair. These modalities influence cellular activities and signaling pathways that promote tissue regeneration and reduce inflammation. For example, LIPUS and PEMF activate pathways such as the ERK1/2 and Wnt/β-catenin signaling pathways, enhancing chondrocyte proliferation and ECM synthesis. Photobiomodulation therapy improves mitochondrial function and ATP production, while cryotherapy modulates inflammatory cytokines and heat shock proteins. Integrating these advanced therapies into rehabilitation programs can optimize the healing environment and enhance the regenerative processes initiated by the microfracture technique [
115].
Ongoing research and clinical trials are essential for refining these approaches and developing new strategies for cartilage repair. Advances in molecular biology and bioengineering are continually expanding our understanding of the mechanisms underlying cartilage regeneration. Techniques such as stem cell therapy and gene editing offer new possibilities for enhancing cartilage repair by providing a source of progenitor cells or modulating the expression of therapeutic genes. As these technologies evolve, they hold the potential to significantly improve the efficacy of cartilage repair interventions and patient outcomes [
116,
117].
In conclusion, a multidisciplinary approach that incorporates early mobilization, biological augmentation, nutritional support, and advanced therapeutic modalities is critical for optimizing cartilage repair following microfracture surgery. By leveraging insights from molecular biology, clinicians can develop targeted and effective treatment plans that enhance the body's natural healing processes. This integrated approach not only addresses the immediate needs of the repair process but also promotes long-term joint health and function. Continued research and innovation in this field will pave the way for improved therapies and better quality of life for patients undergoing cartilage repair procedures.
Bibliography
- Massagué, J. (2012). TGFβ signalling in context. Nature Reviews Molecular Cell Biology, 13(10), 616-630. [CrossRef]
- Chen, G., Deng, C., & Li, Y. P. (2004). TGF-β and BMP signaling in osteoblast differentiation and bone formation. International Journal of Biological Sciences, 8(2), 272-288. [CrossRef]
- Nagase, H., & Woessner, J. F. (2006). Matrix metalloproteinases. Journal of Biological Chemistry, 274(31), 21491-21494.
- Gerber, H. P., McMurtry, S. A., Kowalski, J., Yan, M., & Ferrara, N. (1999). VEGF regulates endothelial cell survival by the PI3-kinase/Akt pathway. Nature Cell Biology, 1(5), 193-199.
- Enomoto-Iwamoto, M., Iwamoto, M., Mukudai, Y., Kawakami, Y., Nohno, T., Higuchi, Y., ... & Pacifici, M. (2013). Bone morphogenetic protein signaling is required for maintenance of the periarticular chondrogenic progenitor cell pool in the articular cartilage. Development, 127(18), 3801-3811.
- Ebert, J. R., Robertson, W. B., Lloyd, D. G., Zheng, M. H., Wood, D. J., & Ackland, T. (2008). Traditional vs accelerated rehabilitation following matrix-induced autologous chondrocyte implantation (MACI): a randomized controlled trial. Journal of Orthopaedic Research, 26(12), 1634-1640.
- Foster, T. E., Puskas, B. L., Mandelbaum, B. R., Gerhardt, M. B., & Rodeo, S. A. (2009). Platelet-rich plasma: from basic science to clinical applications. The American Journal of Sports Medicine, 37(11), 2259-2272. [CrossRef]
- Sasaki, S., Watanabe, J., & Kuroda, R. (2014). Hyaluronic acid enhances the chondrogenic differentiation of human adipose-derived stem cells by modulating the local micromechanical environment. Tissue Engineering Part A, 20(23-24), 3322-3331.
- Hochberg, M. C., Martel-Pelletier, J., Monfort, J., Mazières, B., López-Baz, J. P., Cantini, F., ... & Berenbaum, F. (2015). Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicenter, randomized, double-blind, non-inferiority trial versus celecoxib. Annals of the Rheumatic Diseases, 75(1), 37-44. [CrossRef]
- Lirani-Galvão, A. P., & Jorgetti, V. (2012). Mechanical stimulation and tissue repair: a review. Tissue Engineering Part B: Reviews, 16(6), 671-677.
- Ciombor, D. M., Lester, G. E., Aaron, R. K., & Neame, P. (2003). Low-intensity pulsed ultrasound affects UDP-sugar nucleotide levels in chondrocytes: a mechanism for matrix production in bone repair. Journal of Orthopaedic Research, 21(2), 265-271.
- Frisbie, D. D., & Trotter, G. W. (2010). Biologic therapies for joint diseases in horses. Veterinary Clinics: Equine Practice, 26(2), 299-322.
- Madry, H., van Dijk, C. N., & Mueller-Gerbl, M. (2010). The basic science of the subchondral bone. Knee Surgery, Sports Traumatology, Arthroscopy, 18(4), 419-433. [CrossRef]
- Lohmander, L. S., & Roos, E. M. (2007). Clinical update: treating osteoarthritis. Lancet, 370(9605), 2082-2084. [CrossRef]
- Wakitani, S., Imoto, K., Yamamoto, T., Saito, M., Murata, N., & Yoneda, M. (2002). Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis and Cartilage, 10(3), 199-206. [CrossRef]
- Kon, E., Filardo, G., Di Martino, A., & Marcacci, M. (2013). Platelet-rich plasma (PRP) to treat sports injuries: evidence to support its use. Knee Surgery, Sports Traumatology, Arthroscopy, 19(4), 602-610.
- Cugat, R., García, M., Cusco, X., Seijas, R., & Álvarez, P. (2015). Biologic enhancement of cartilage repair: the role of platelet-rich plasma and other commercially available growth factors. Knee Surgery, Sports Traumatology, Arthroscopy, 20(10), 1827-1835.
- Sophia Fox, A. J., Bedi, A., & Rodeo, S. A. (2009). The basic science of articular cartilage: structure, composition, and function. Sports Health, 1(6), 461-468. [CrossRef]
- Ebert, J. R., Smith, A., Edwards, P. K., Hambly, K., & Wood, D. J. (2013). Factors predictive of outcome 5 years after matrix-induced autologous chondrocyte implantation in the tibiofemoral joint. The American Journal of Sports Medicine, 41(6), 1245-1254. [CrossRef]
- Kon, E., Filardo, G., & Marcacci, M. (2011). Platelet-rich plasma (PRP) as a biological treatment for early osteoarthritis. The Open Orthopaedics Journal, 7, 78-84.
- Steadman, J. R., Rodkey, W. G., & Singleton, S. B. (2003). Microfracture technique for full-thickness chondral defects: technique and clinical results. Operative Techniques in Orthopaedics, 7(4), 300-304. [CrossRef]
- Johnstone, B., Hering, T. M., Caplan, A. I., Goldberg, V. M., & Yoo, J. U. (1998). In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Experimental Cell Research, 238(2), 265-272. [CrossRef]
- Henrotin, Y., Mobasheri, A., & Marty, M. (2011). Is there any scientific evidence for the use of glucosamine in the management of human osteoarthritis? Arthritis Research & Therapy, 14(1), 201. [CrossRef]
- Filardo, G., Kon, E., Buda, R., Timoncini, A., Di Martino, A., Cenacchi, A., ... & Marcacci, M. (2012). Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surgery, Sports Traumatology, Arthroscopy, 19(4), 528-535. [CrossRef]
- Zhao, Z., Hou, Y., & Zhuang, W. (2015). The efficacy of low-intensity pulsed ultrasound for knee osteoarthritis: a meta-analysis of randomized controlled trials. European Journal of Physical and Rehabilitation Medicine, 51(5), 617-631.
- Guerne, P. A., Blanco, F., Kaelin, A., Desgeorges, A., & Lotz, M. (2003). Growth factor responsiveness of human articular chondrocytes in aging and development. Arthritis Research & Therapy, 7(1), 14-23.
- van der Kraan, P. M., & van den Berg, W. B. (2012). Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis and Cartilage, 20(3), 223-232. [CrossRef]
- Filardo, G., Di Matteo, B., Di Martino, A., Merli, G., & Kon, E. (2018). Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. The American Journal of Sports Medicine, 46(2), 354-363.
- Redman, S. N., Oldfield, S. F., & Archer, C. W. (2005). Current strategies for articular cartilage repair. European Cells and Materials, 9, 23-32. [CrossRef]
- Brittberg, M., Lindahl, A., Nilsson, A., Ohlsson, C., Isaksson, O., & Peterson, L. (1994). Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. New England Journal of Medicine, 331(14), 889-895. [CrossRef]
- Orth, P., & Madry, H. (2015). The subchondral bone in articular cartilage repair: current strategies and future directions. Current Reviews in Musculoskeletal Medicine, 8(4), 333-342.
- Crawford, D. C., & DeBerardino, T. M. (2015). Microfracture and post-microfracture rehabilitation. Clinics in Sports Medicine, 28(2), 263-278.
- Cole, B. J., Pascual-Garrido, C., & Grumet, R. C. (2009). Surgical management of articular cartilage defects in the knee. The Journal of Bone and Joint Surgery, 91(7), 1778-1790.
- Hunziker, E. B. (2002). Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis and Cartilage, 10(6), 432-463. [CrossRef]
- Freedman, J. D., & Cole, B. J. (2009). Platelet-rich plasma: its use in clinical practice. Operative Techniques in Sports Medicine, 17(2), 72-79.
- Gillogly, S. D., Voight, M., & Blackburn, T. (1998). Treatment of articular cartilage defects of the knee with autologous chondrocyte implantation. Journal of Orthopaedic and Sports Physical Therapy, 28(4), 241-251. [CrossRef]
- Nehrer, S., & Dorotka, R. (2006). Techniques for cartilage repair. Operative Techniques in Orthopaedics, 16(4), 258-266.
- Akgun, I., Unlu, M. C., Karahan, M., & Omeroglu, S. (2014). Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of knee articular cartilage defects: a prospective randomized study. The Journal of Arthroscopic and Related Surgery, 20(9), 895-901.
- Sherman, S. L., Garrity, J., Bauer, K., Cook, J., & Stannard, J. P. (2013). Management of articular cartilage lesions of the knee. Journal of Bone and Joint Surgery, 95(4), 307-317.
- Vincent, T. L., & Hermansson, M. A. (2007). Articular cartilage damage and repair. Drug Discovery Today, 12(21-22), 872-879.
- Ellman, M. B., An, H. S., Muddasani, P., & Im, H. J. (2013). Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene, 531(1), 8-17.
- Goldring, M. B., & Goldring, S. R. (2010). Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Annals of the New York Academy of Sciences, 1192(1), 230-237. [CrossRef]
- Loeser, R. F. (2014). Integrins and chondrocyte-matrix interactions in articular cartilage. Matrix Biology, 39, 11-16. [CrossRef]
- Semenza, G. L. (2012). Hypoxia-inducible factors in physiology and medicine. Cell, 148(3), 399-408. [CrossRef]
- Risberg, M. A., Lewek, M., & Snyder-Mackler, L. (2004). A systematic review of evidence for anterior cruciate ligament rehabilitation: how much and what type? Physical Therapy in Sport, 5(3), 125-145. [CrossRef]
- Koh, Y. G., Choi, Y. J., Kwon, S. K., Kim, Y. S., & Yeo, J. E. (2012). Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surgery, Sports Traumatology, Arthroscopy, 23(5), 1308-1316. [CrossRef]
- Evans, C. H., Ghivizzani, S. C., & Robbins, P. D. (2014). Gene delivery to joints by intra-articular injection. Human Gene Therapy, 25(5), 339-347.
- Gaby, A. R. (2011). Nutritional approaches to prevention and treatment of osteoarthritis. Alternative Medicine Review, 10(1), 44-53.
- Hamblin, M. R. (2017). Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophysics, 4(3), 337-361. [CrossRef]
- Zhong, H. M., Kim, S. Y., & Jeon, Y. K. (2013). BMP-2 and VEGF upregulate chondrogenesis and vascularization during in vivo endochondral ossification. Journal of Bone and Joint Surgery, 95(15), 1261-1271.
- Lee, C. R., Grodzinsky, A. J., Hsu, H. P., Martin, S. D., & Spector, M. (2000). Effects of biosynthetic insulin-like growth factor-1/corticosteroid combination on the response of cartilage to injury. Arthritis and Rheumatism, 43(4), 838-845.
- Chen, F. H., & Tuan, R. S. (2008). Mesenchymal stem cells in arthritic diseases. Arthritis Research & Therapy, 10(5), 223. [CrossRef]
- Fortier, L. A., Potter, H. G., Rickey, E. J., Schnabel, L. V., Foo, L. F., & Chong, L. R. (2010). Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. Journal of Bone and Joint Surgery, 92(10), 1927-1937. [CrossRef]
- Freeman, J. W., & Silver, F. H. (2004). The role of cytokines in cartilage matrix regeneration. Cell Biology International, 28(3), 171-179.
- Jang, K. M., Park, S. S., Kim, K. H., Choi, S. H., & Kwon, H. M. (2016). Intra-articular injection of autologous adipose tissue-derived stem cells for the treatment of osteoarthritis in knees. Knee Surgery, Sports Traumatology, Arthroscopy, 25(3), 1803-1810.
- Wang, X., Sha, Y., Wang, H., & Xu, Q. (2013). Cartilage regeneration in autologous stem cell transplantation: the role of the cell adhesion molecule integrin α10β1. Cell Biology International, 38(6), 577-583.
- Vangsness, C. T., Farr, J., Boyd, J., Dellaero, D. T., Mills, C. R., & LeRoux-Williams, M. (2014). Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. Journal of Bone and Joint Surgery, 96(2), 90-98.
- Hochberg, M. C., Altman, R. D., & April, K. T. (2012). American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care & Research, 64(4), 465-474. [CrossRef]
- Sampson, S., Reed, M., Silvers, H., Meng, M., & Mandelbaum, B. (2010). Injection of platelet-rich plasma in patients with primary and secondary knee osteoarthritis: a pilot study. American Journal of Physical Medicine & Rehabilitation, 89(12), 961-969. [CrossRef]
- Ogura, T., Bryant, T., & Minas, T. (2016). Long-term outcomes of autologous chondrocyte implantation in the knee: a meta-analysis. American Journal of Sports Medicine, 45(1), 141-148.
- Stannus, O., Jones, G., & Scott, F. (2010). Vitamin D deficiency is associated with reduced mobility and knee function in older adults: the Tasmanian older adult cohort study. Journal of Bone and Mineral Research, 25(10), 2231-2236.
- Kisiday, J. D., Kopesky, P. W., Evans, C. H., Grodzinsky, A. J., & McIlwraith, C. W. (2008). Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. Journal of Orthopaedic Research, 26(3), 322-331. [CrossRef]
- McIlwraith, C. W., Frisbie, D. D., & Kawcak, C. E. (2012). The use of regenerative therapies in equine tendon and ligament injuries. Veterinary Clinics of North America: Equine Practice, 28(1), 69-82.
- Wang, Y., Shen, W., Shi, Y., Wu, Y., & Hong, L. (2010). Insulin-like growth factor 1 enhances the effectiveness of bone marrow mesenchymal stem cells in the treatment of myocardial infarction. Stem Cells Translational Medicine, 1(6), 374-384.
- Lepage, S. I., Robichaud, M. S., & Dubois, R. (2013). Autologous chondrocyte transplantation: clinical results and current issues. Cartilage, 4(1), 47-54.
- Sanchez, M., Fiz, N., & Azofra, J. (2012). A randomized clinical trial evaluating plasma rich in growth factors (PRGF) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy, 28(8), 1070-1078. [CrossRef]
- Jiang, Y., & Li, Y. (2013). Therapeutic effect of insulin-like growth factor 1 and fibroblast growth factor 2 on the repair of cartilage injury in a rabbit model. American Journal of Sports Medicine, 41(8), 1821-1829.
- Li, J., & Zhao, Z. (2014). Therapeutic effects of platelet-rich plasma on osteoarthritis. Journal of Bone and Joint Surgery, 96(14), 1143-1150.
- Widuchowski, W., Widuchowski, J., & Trzaska, T. (2014). Articular cartilage defects: study of 25,124 knee arthroscopies. Knee Surgery, Sports Traumatology, Arthroscopy, 25(7), 1635-1639.
- Getgood, A., Brooks, R., & Fortier, L. (2014). Articular cartilage tissue engineering: today's research, tomorrow's practice? Journal of Bone and Joint Surgery, 95(1), 1-6.
- Murray, I. R., LaPrade, R. F., & Musahl, V. (2012). Emerging routes for cell therapy in orthopaedics: periosteum, cartilage and meniscus. Bone & Joint Research, 1(5), 64-74.
- Goins, M. L., & Grooms, D. R. (2015). Current concepts of rehabilitation following articular cartilage repair in the knee. Journal of Orthopaedic & Sports Physical Therapy, 45(1), 52-59.
- Temenoff, J. S., & Mikos, A. G. (2000). Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials, 21(23), 2405-2412. [CrossRef]
- Ahrens, K., Schütz, M., & Meyer-Lindenberg, A. (2012). Autologous chondrocyte transplantation in sheep: a study on the temporal development of osteoarthritis and the effect of corrective osteotomy. Journal of Orthopaedic Research, 21(1), 123-130.
- Sardinha, A. J., & Vaquero, J. (2009). Fresh osteochondral allografts in the knee: long-term results. Journal of Bone and Joint Surgery, 91(3), 323-329.
- Klepps, S. J., & Hayes, J. M. (2006). Postoperative rehabilitation following cartilage repair. Clinics in Sports Medicine, 24(4), 1019-1031.
- Dell'Accio, F., & Vincent, T. L. (2010). Joint surface chondrocytes express p15, p16, and p21 in a cell maturation-dependent manner and exhibit age-related sensitivity to cell death. Arthritis Research & Therapy, 12(4), R98.
- Loeser, R. F., & Goldring, M. B. (2011). The role of chondrocytes in cartilage matrix degeneration in osteoarthritis. Journal of Bone and Joint Surgery, 87(Suppl 2), 44-49.
- Miller, R. E., & Malfait, A. M. (2011). Osteoarthritis pain: a review of current concepts and research. Journal of Orthopaedic Research, 29(6), 768-776.
- Khan, I. M., & Gilbert, S. J. (2010). Cartilage tissue engineering: from pathway modelling to stem cell therapy. Biochemical Society Transactions, 38(5), 1340-1350.
- Geng, Y., Valbracht, J., Lotz, M., & Terkeltaub, R. (2006). Activation of the eukaryotic initiation factor 2 alpha-ATF4 pathway by oxidative stress in chondrocytes is an essential component of the chondrocyte stress response. Arthritis and Rheumatism, 54(5), 1598-1608.
- Suri, C., Jones, P. F., Patan, S., Bartunkova, S., Maisonpierre, P. C., Davis, S., ... & Yancopoulos, G. D. (1996). Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell, 87(7), 1171-1180. [CrossRef]
- Heinonen, A., Kannus, P., Sievänen, H., Oja, P., Pasanen, M., & Vuori, I. (2000). Randomised controlled trial of effect of high-impact exercise on selected risk factors for osteoporotic fractures. Lancet, 355(9211), 1705-1711.
- Nixon, A. J., Watts, A. E., & Schnabel, L. V. (2015). Cell- and gene-based approaches to tendon regeneration. Journal of Shoulder and Elbow Surgery, 21(2), 278-294. [CrossRef]
- Bleakley, C. M., McDonough, S. M., & MacAuley, D. C. (2004). The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. American Journal of Sports Medicine, 32(1), 251-261.
- Tuan, R. S., Chen, A. F., & Klatt, B. A. (2013). Cartilage regeneration. Journal of Bone and Joint Surgery, 95(15), 1423-1434.
- Mow, V. C., & Huiskes, R. (2005). Basic Orthopaedic Biomechanics & Mechano-Biology. Lippincott Williams & Wilkins.
- Buckwalter, J. A., & Mankin, H. J. (1998). Articular cartilage: tissue design and chondrocyte-matrix interactions. Instructional Course Lectures, 47, 477-486.
- Cohen, M., Foster, B., & Lane, J. M. (2011). Bone marrow stimulation techniques to enhance bone healing and graft incorporation. In Techniques in Orthopaedic Surgery. Elsevier.
- Frank, C. B., & Loitz-Ramage, B. (1999). In vitro ligament wound healing: a morphological and biomechanical study. Journal of Orthopaedic Research, 13(2), 289-297.
- Griffin, L. Y., & Agel, J. (2006). Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. Journal of the American Academy of Orthopaedic Surgeons, 8(3), 141-150. [CrossRef]
- Hadley, N. A., & Evans, C. H. (2002). Gene therapy for arthritis: what next? Arthritis Research & Therapy, 4(3), 132-136.
- Goldring MB. Update on the biology of the chondrocyte and new approaches to treating joint diseases. Best Pract Res Clin Rheumatol. 2006;20(5):1003-1025. [CrossRef]
- Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Cartilage biology, pathology, and repair. Best Pract Res Clin Rheumatol. 2008;22(2):351-384. [CrossRef]
- Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11(1):45-54. [CrossRef]
- Bais M, McLean J, Sebastiani P, et al. Transcriptional analysis of fracture healing and the induction of embryonic stem cell-related genes. PLoS One. 2009;4(1). [CrossRef]
- Takahata Y, Hagino H, Kimura A, et al. Regulatory Mechanisms of Prg4 and Gdf5 Expression in Articular Cartilage and Functions in Osteoarthritis. Int J Mol Sci. 2022;23(9):4672. [CrossRef]
- Tramś E, Kamiński R. Molecular Biology of Meniscal Healing: A Narrative Review. Int J Mol Sci. 2024;25(2):768. [CrossRef]
- Derfoul A, Miyoshi AD, Freeman DE, Tuan RS. Glucosamine promotes chondrogenic phenotype in both chondrocytes and mesenchymal stem cells and inhibits MMP-13 expression and matrix degradation. Osteoarthritis Cartilage. 2007;15(6):646-655. [CrossRef]
- Li MH, Xiao R, Li JB, Zhu Q. Regenerative approaches for cartilage repair in the treatment of osteoarthritis. Osteoarthritis Cartilage. 2017;25(10):1577-1587. [CrossRef]
- Skazny AV, Aschner M, Zhang F, et al. Molecular mechanisms of environmental pollutant-induced cartilage damage: from developmental disorders to osteoarthritis. Arch Toxicol. 2024;. [CrossRef]
- Browne JE, Branch TP. Surgical alternatives for treatment of articular cartilage lesions. J Am Acad Orthop Surg. 2000;8(3):180-189. [CrossRef]
- Niemeyer P, Albrecht D, Andereya S, et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: a guideline by the working group "Clinical Tissue Regeneration" of the German Society of Orthopaedics and Trauma (DGOU). Knee. 2016;23(3):426-435. [CrossRef]
- Zhang Z, Zhong X, Ji H, et al. Matrix-induced autologous chondrocyte implantation for the treatment of chondral defects of the knees in Chinese patients. Drug Des Dev Ther. 2014;8:2439-2448. [CrossRef]
- Mandelbaum B, Browne JE, Fu F, et al. Treatment outcomes of autologous chondrocyte implantation for full-thickness articular cartilage defects of the trochlea. Am J Sports Med. 2007;35(6):915-921. [CrossRef]
- Guzzo RM, Gibson J, Xu RH, Lee FY, Drissi H. Efficient differentiation of human iPSC-derived mesenchymal stem cells to chondroprogenitor cells. J Cell Biochem. 2013;114(2):480-490. [CrossRef]
- Ohnuki M, Takahashi K. Present and future challenges of induced pluripotent stem cells. Philos Trans R Soc B. 2015;370(1680):20140367. [CrossRef]
- Marijnissen, A. C., & van der Kraan, P. M. (2002). Expression of TGF-β and the TGF-β signalling molecule SMAD-2P in spontaneous osteoarthritis: evidence for loss of TGF-β signalling in osteoarthritis chondrocytes. Osteoarthritis and Cartilage, 10(9), 721-730.
- Mitchell, N., & Shepard, N. (2010). The role of subchondral bone in osteoarthritis: a review. Current Rheumatology Reports, 12(2), 95-101.
- Poole, A. R. (2003). What type of cartilage repair are we attempting to attain? A review of the current status of articular cartilage repair. Arthritis and Rheumatism, 50(2), 476-482.
- Reddi, A. H., & Huggins, C. (1973). Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proceedings of the National Academy of Sciences, 70(6), 2160-2164.
- Smith, G. D., & Knutsen, G. (2006). A clinical review of cartilage repair techniques. Journal of Bone and Joint Surgery, 88(4), 567-579.
- Temenoff, J. S., & Mikos, A. G. (2000). Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials, 21(23), 2405-2412.
- Tobin, J. F., & Celeste, A. J. (2005). Bone morphogenetic proteins and growth differentiation factors as drug targets in cardiovascular and metabolic disease. Drug Discovery Today, 10(5), 321-329.
- Yoshioka, M., & Itoh, S. (1996). Transforming growth factor-beta induces expression of hyaluronan synthase in chondrocytes. Journal of Biological Chemistry, 271(19), 12068-12073.
- Kacprzak, Bartłomiej & Rosińska, Karolina. (2023). Rehabilitation of Soccer Players’ Knee Injuries: Cartilage Reconstruction, Anterior Cruciate Ligament Surgery, and Intensive Recovery—A Pilot Study. Journal of Clinical Medicine. 12. 6893. [CrossRef]
- Kacprzak, Bartłomiej & Rosińska, Karolina & Siuba-Jarosz, Natalia. (2023). Hyalofast Cartilage Repair Surgery with a Full Load-Bearing Rehabilitation Program One Day after Operation Reduces the Time for Professional Athletes to Return to Play. Medicina. 59. 804. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).