Submitted:
04 September 2024
Posted:
05 September 2024
Read the latest preprint version here
Abstract
Keywords:
Introduction
Interplay of Genetics and Skin Carcinogenesis
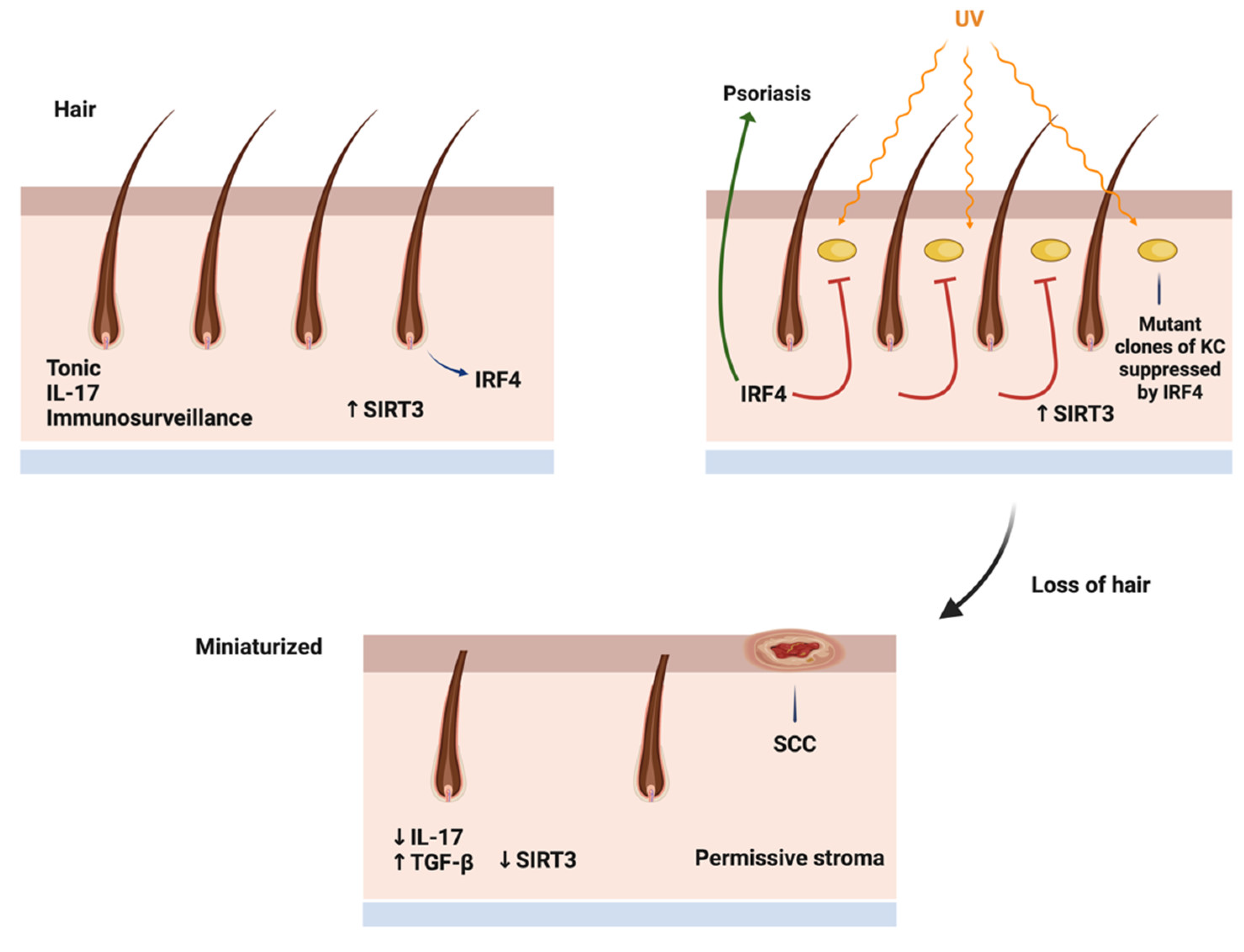
IRF4- the Locus between Hair Growth and Inflammation
Actinic Keratosis and Cutaneous Squamous Cell Carcinoma
Melanoma
Angiosarcoma
IL-17, the Double Edged Sword
Metabolic Parameters of the Aging Scalp
References
- Licata, G.; Scharf, C.; Ronchi, A.; Pellerone, S.; Argenziano, G.; Verolino, P.; Moscarella, E. Diagnosis and Management of Melanoma of the Scalp: A Review of the Literature. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Porto, A. C.; Blumetti, T. P.; Calsavara, V. F.; Torrezan, G. T.; Andrade de Paula, C. A.; Lellis, R.; Duprat Neto, J. P.; Carraro, D. M.; Braga, J. C. T. A Cross-Sectional Study of Clinical, Dermoscopic, Histopathological, and Molecular Patterns of Scalp Melanoma in Patients with or without Androgenetic Alopecia. Sci. Rep. 2022, 12, 15096. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, S. Deciphering the Molecular Mechanisms of Stem Cell Dynamics in Hair Follicle Regeneration. Exp. Mol. Med. 2024, 56, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Westgate, G. E.; Pawlus, A. D.; Sikkink, S. K.; Thornton, M. J. Age-Related Changes in Female Scalp Dermal Sheath and Dermal Fibroblasts: How the Hair Follicle Environment Impacts Hair Aging. J. Invest. Dermatol. 2021, 141, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liang, W.; Yang, Z.; Chen, Z.; Du, Z.; Gong, S. SIRT3-Mediated Deacetylation Protects Inner Hair Cell Synapses in a H2O2-Induced Oxidative Stress Model in Vitro. Exp. Cell Res. 2022, 418, 113280. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, C.; Chen, Z.; Yang, Z.; Liu, K.; Gong, S. Sirtuin-3 Protects Cochlear Hair Cells Against Noise-Induced Damage via the Superoxide Dismutase 2/Reactive Oxygen Species Signaling Pathway. Front. Cell Dev. Biol. 2021, 9, 766512. [Google Scholar] [CrossRef]
- Sennett, R.; Rendl, M. Mesenchymal-Epithelial Interactions During Hair Follicle Morphogenesis and Cycling. Semin. Cell Dev. Biol. 2012, 23, 917–927. [Google Scholar] [CrossRef]
- Sundaresan, N. R.; Bindu, S.; Pillai, V. B.; Samant, S.; Pan, Y.; Huang, J. Y.; Gupta, M.; Nagalingam, R. S.; Wolfgeher, D.; Verdin, E.; et al. SIRT3 Blocks Aging-Associated Tissue Fibrosis in Mice by Deacetylating and Activating Glycogen Synthase Kinase 3β. Mol. Cell Biol. 2015, 36, 678–692. [Google Scholar] [CrossRef]
- Pośpiech, E.; Kukla-Bartoszek, M.; Karłowska-Pik, J.; Zieliński, P.; Woźniak, A.; Boroń, M.; Dąbrowski, M.; Zubańska, M.; Jarosz, A.; Grzybowski, T.; et al. Exploring the Possibility of Predicting Human Head Hair Greying from DNA Using Whole-Exome and Targeted NGS Data. BMC Genomics 2020, 21, 538. [Google Scholar] [CrossRef]
- Mudter, J.; Yu, J.; Zufferey, C.; Brüstle, A.; Wirtz, S.; Weigmann, B.; Hoffman, A.; Schenk, M.; Galle, P.R.; Lehr, H.A.; et al. IRF4 Regulates IL-17A Promoter Activity and Controls RORγt-Dependent Th17 Colitis In Vivo. Inflamm. Bowel Dis. 2011, 17, 1343–1358. [Google Scholar] [CrossRef]
- Ni, A.; Chen, H.; Wu, Y.; Wang, Z. Expression of IRF-4 and IBP in Skin Lesions of Patients with Psoriasis Vulgaris. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2012, 32, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Gaida, M.M.; Ogawa, Y.; Kolios, A.G.A.; Lasitschka, F.; Ashwell, J.D. Counter-Regulation of T Cell Effector Function by Differentially Activated p38. J. Exp. Med. 2014, 211, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Miyagaki, T.; Sugaya, M.; Sato, S. Exacerbated Imiquimod-Induced Psoriasis-Like Skin Inflammation in IRF5-Deficient Mice. Int. J. Mol. Sci. 2020, 21, 3681. [Google Scholar] [CrossRef]
- Thomson, J.; Bewicke-Copley, F.; Anene, C.A.; Gulati, A.; Nagano, A; Purdie, N; Inman, G.J.; Proby, C. M.; Leigh, I.M.; Harwood, C.A.; et al. The Genomic Landscape of Actinic Keratosis. J. Invest. Dermatol. 2021, 141, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Nepote, A.; Avallone, G.; Ribero, S.; Cavallo, F.; Roccuzzo, G.; Mastorino, L.; Conforti, C.; Paruzzo, L.; Poletto, S.; Carnevale Schianca, F.; et al. Current Controversies and Challenges on BRAF V600K-Mutant Cutaneous Melanoma. J. Clin. Med. 2022, 11, 828. [Google Scholar] [CrossRef]
- Bauer, J.; Büttner, P.; Murali, R.; Okamoto, I.; Kolaitis, N. A.; Landi, M. T.; Scolyer, R. A.; Bastian, B. C. BRAF Mutations in Cutaneous Melanoma Are Independently Associated with Age, Anatomic Site of the Primary Tumor, and the Degree of Solar Elastosis at the Primary Tumor Site. Pigment Cell Melanoma Res. 2011, 24, 345–351. [Google Scholar] [CrossRef]
- Goto, K.; Yoshikawa, S.; Takai, T.; Tachibana, K.; Honma, K.; Isei, T.; Kukita, Y.; Oishi, T. Clinicopathologic and Genetic Characterization of Invasive Melanoma with BRAF V600K Mutation: A Study of 16 Cases. J. Cutan. Pathol. 2023, 50, 739–747. [Google Scholar] [CrossRef]
- Tas, F.; Erturk, K. Scalp Melanoma Is Associated with High Mitotic Rate and Is a Poor Prognostic Factor for Recurrence and Outcome. Melanoma Res. 2017, 27, 387–390. [Google Scholar] [CrossRef]
- Dai, J.; Tetzlaff, M. T.; Schuchter, L. M.; Elder, D. E.; Elenitsas, R. Histopathologic and Mutational Analysis of a Case of Blue Nevus-Like Melanoma. J. Cutan. Pathol. 2016, 43, 776–780. [Google Scholar] [CrossRef]
- Patel, S. P.; Kim, D. W.; Lacey, C. L.; Hwu, P. GNA11 Mutation in a Patient With Cutaneous Origin Melanoma: A Case Report. Medicine (Baltimore). 2016, 95, e2336. [Google Scholar] [CrossRef]
- Krauthammer, M.; Kong, Y.; Ha, B. H. , Evans, P.; Bacchiocchi, A.; McCusker, J. P.; Cheng, E.; Davis, M. J.; Goh, G.; Choi, M.; et al. Exome Sequencing Identifies Recurrent Somatic RAC1 Mutations in Melanoma. Nat. Genet. 2012, 44, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Arbiser, J. L.; Bips, M.; Seidler, A.; Bonner, M. Y.; Kovach, C. Combination Therapy of Imiquimod and Gentian Violet for Cutaneous Melanoma Metastases. J. Am. Acad. Dermatol. 2012, 67, e81–e83. [Google Scholar] [CrossRef] [PubMed]
- Painter, C. A.; Jain, E.; Tomson, B. N.; Dunphy, M.; Stoddard, R. E.; Thomas, B. S.; Damon, A. L.; Shah, S.; Kim, D.; Gómez Tejeda Zañudo, J.; et al. The Angiosarcoma Project: Enabling Genomic and Clinical Discoveries in a Rare Cancer through Patient-Partnered Research. Nat. Med. 2020, 26, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Boichard, A.; Wagner, M.J.; Kurzrock, R. Angiosarcoma Heterogeneity and Potential Therapeutic Vulnerability to Immune Checkpoint Blockade: Insights from Genomic Sequencing. Genome Med. 2020, 12, 61. [Google Scholar] [CrossRef]
- Chan, J. Y.; Lim, J. Q.; Yeong, J.; Ravi, V.; Guan, P.; Boot, A.; Tay, T. K. Y.; Selvarajan, S.; Md Nasir, N. D.; Loh, J. H.; et al. Multiomic Analysis and Immunoprofiling Reveal Distinct Subtypes of Human Angiosarcoma. J. Clin. Invest. 2020, 130, 5833–5846. [Google Scholar] [CrossRef]
- Yoshimura, N.; Kariya, R.; Shimada, M.; Tateyama, M.; Matsunaga, H.; Shibata, Y.; Tanimura, S.; Takata, K.; Arima, T.; Kawakami, J.; et al. The IL-17-IL-17RA Axis is Required to Promote Osteosarcoma Progression in Mice. Sci. Rep. 2023, 13, 21572. [Google Scholar] [CrossRef]
- Xu, B.; Guenther, J.F.; Pociask, D.A.; Wang, Y.; Kolls, J.K.; You, Z.; Chandrasekar, B.; Shan, B.; Sullivan, D.E.; Morris, G.F. Promotion of Lung Tumor Growth by Interleukin-17. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 307, L497–L508. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Chen, F.; Wang, C.; Guo, X.; Wang, C.; Fan, Y.; Wang, Y.; Peng, Y.; Li, W. Liposomal Honokiol Promotes Hair Growth via Activating Wnt3a/β-Catenin Signaling Pathway and Down Regulating TGF-β1 in C57BL/6N Mice. Eur. J. Dermatol. 2011, 21, 1012–1014. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. C.; Kang, J. I.; Kim, M. K.; Boo, H. J.; Park, D. B.; Lee, Y. K.; Kang, J. H.; Yoo, E. S.; Kim, Y. H.; Kang, H. K. The Hair Growth Promoting Effect of 4-O-Methylhonokiol. Eur. J. Dermatol. 2011, 21(6), 1012–1014. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, D.; Shan, B.; et al. SIRT3 Enhances Glycolysis and Proliferation in SIRT3-Expressing Gastric Cancer Cells. PLoS One. 2015, 10, e0129834. [Google Scholar] [CrossRef]
- Liu, X.; Flores, A. A.; Situ, L.; Gu, W.; Ding, H.; Christofk, H. R.; Lowry, W. E.; Jung, M. E. Development of Novel Mitochondrial Pyruvate Carrier Inhibitors to Treat Hair Loss. J. Med. Chem. 2021, 64, 2046–2063. [Google Scholar] [CrossRef] [PubMed]
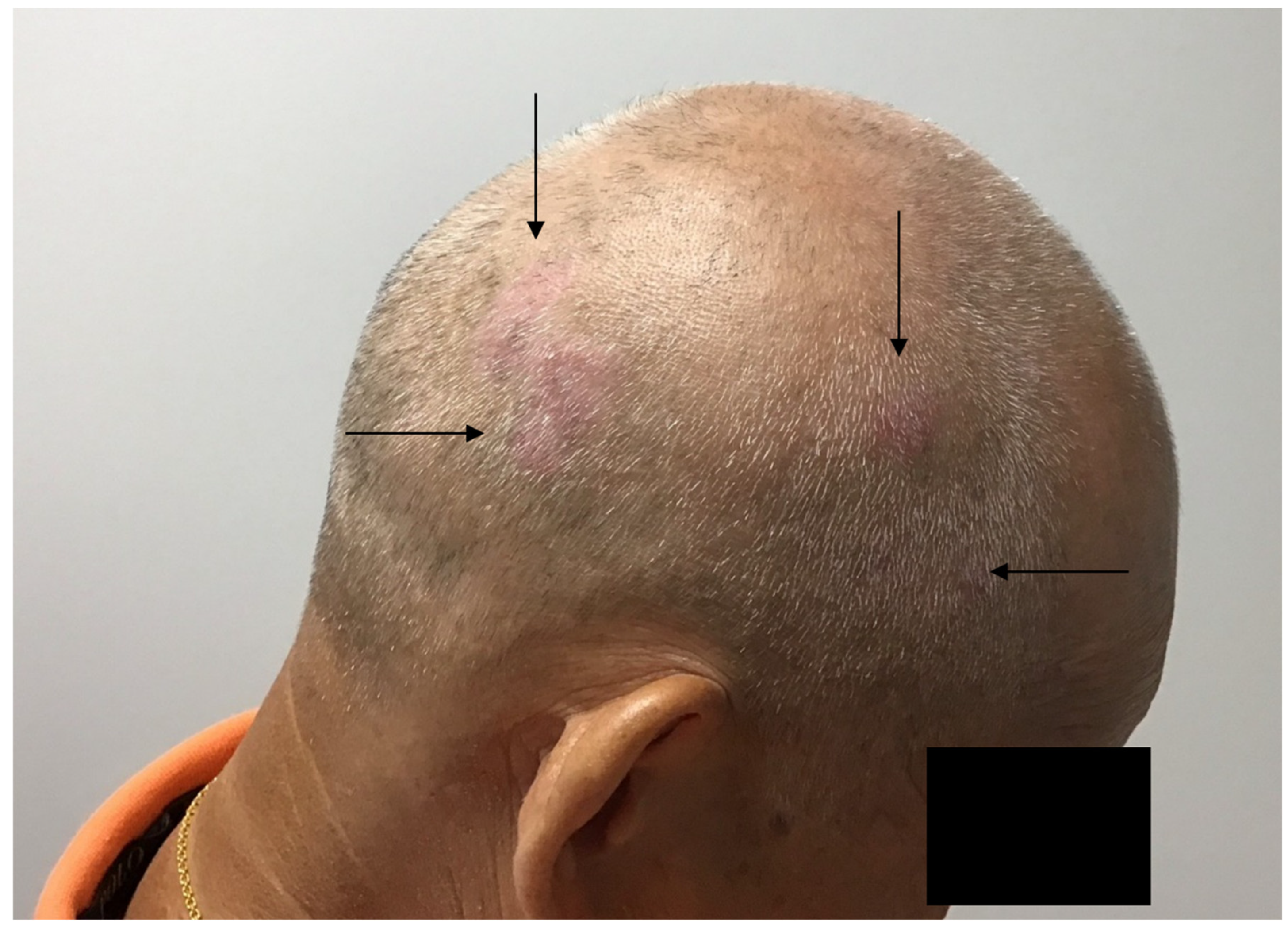
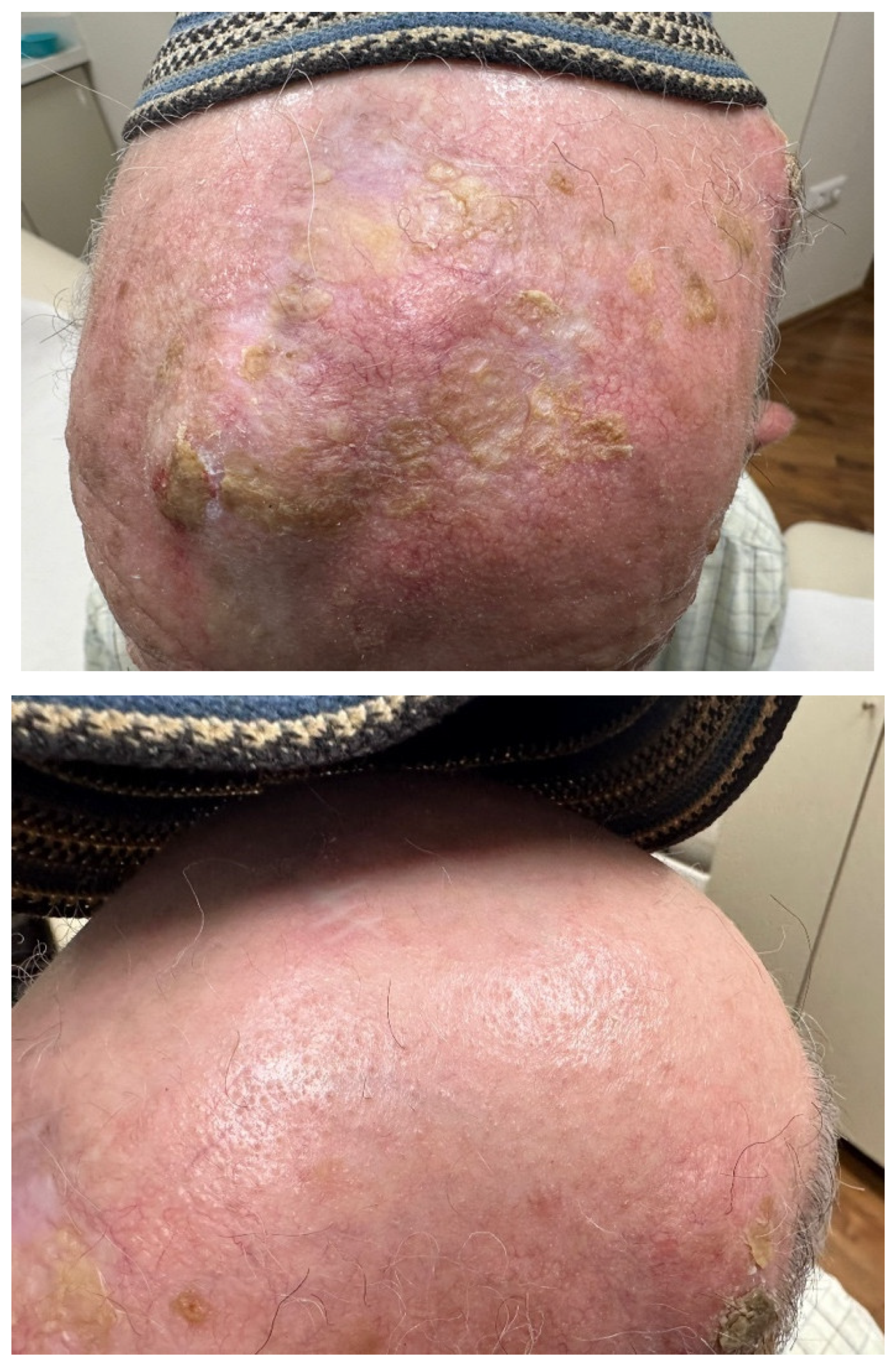
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




