1. Introduction
The scalp is an exceedingly common site for basal cell, squamous cell carcinoma and occasional melanomas. Benign lesions, including seborrheic keratoses and blue nevi are also commonly found on the scalp. A major difference between the scalp and other common sun exposed areas for skin cancer is that the scalp is covered with hair during periods of peak sun exposure during youth. Carcinomas on the scalp are more common in men than women, but may become evident in women with sparse hair. Androgenetic alopecia is also common in men, beginning in the 20-30 year old age group, and becoming more evident with age. Women too lose hair, but not usually to the same extent as men. The incidence of scalp carcinomas is also higher in men than in women [
1,
2]. Androgenetic alopecia is thought to be mediated by dihydrotestosterone, and this has led to the use of dihydrotestosterone blockers as a common treatment for alopecia [
3].
Two major differences exist in the scalp of elderly men vs elderly women. The first is the presence of hair. While elderly women experience hair thinning, frank alopecia is uncommon. The second change is the stroma. Stromal changes may be responsible for miniaturization of hair follicles, and stromal changes associated with miniaturization may also favor the development of actinic keratosis and nonmelanoma skin cancer [
3,
4].
Scalp stroma is subject to aging as is every other tissue. A candidate for aging in scalp stroma is Sirt3, a mitochondrial deacetylase which stimulates mitochondrial biogenesis [
5,
6]. TGF beta lowers Sirt3 levels, promotes fibrosis, and is decreased in fibrotic diseases such as scleroderma. Of interest, the scleroderma dermis does not support hair growth, and a similar mechanism of fibrotic stroma may decrease both hair growth and favor the development of cutaneous neoplasia [
7,
8].
2. Interplay of Genetics and Skin Carcinogenesis
Actinic keratoses are exceedingly common lesions on the scalp, and a known precursor to cutaneous squamous cell carcinoma. Genetic studies have implicated mutations in several genes in both actinic keratoses and cutaneous squamous cell carcinomas. These include mutations in p53 resulting in gain of function, activating mutations in phosphoinositol-3 kinase p85, and inactivating mutations in Notch 1 and 2. Genetic predisposition to actinic keratoses has been linked to IRF4, a transcription factor which links immunity to pigmentation, as well as pigmentation associated genes such as tyrosinase [
9,
10,
11,
12,
13]. Current knowledge does not suggest a unique mutational profile of basal cell carcinoma of the scalp, with scalp and non-scalp basal cell carcinomas both having mutations in the Patch/Sonic hedgehog pathway.
3. IRF4- The Locus between Hair Growth and Inflammation
In previous studies, we have shown that normal skin has a tonic IL-12 mediated immunosurveillance, and an intact barrier function leads to a tonic production of IL-12, which suppresses both Th2 and Th17 mediated inflammation. Clinical observations suggest that the presence of hair skews the epidermis to a slight IL-17 predominance, which results in an increased frequency of IL-17 mediated inflammatory disorders, such as psoriasis, hidradenitis suppurativa and seborrheic dermatitis in hair bearing areas [
9,
10,
11].
IRF4 is a transcription factor that plays a role in pigmentation and IL-17 mediated inflammation [
9,
10,
11,
12,
13]. IRF4 has been associated with several human pigmentation traits, including hair graying and hair loss, and IRF4 has also been shown to bind to the MITF (microphthalmia) promoter, which is the master transcriptional switch in melanocytes. The presence of IRF4 in hair may explain in part the presence of scalp psoriasis [
9,
10,
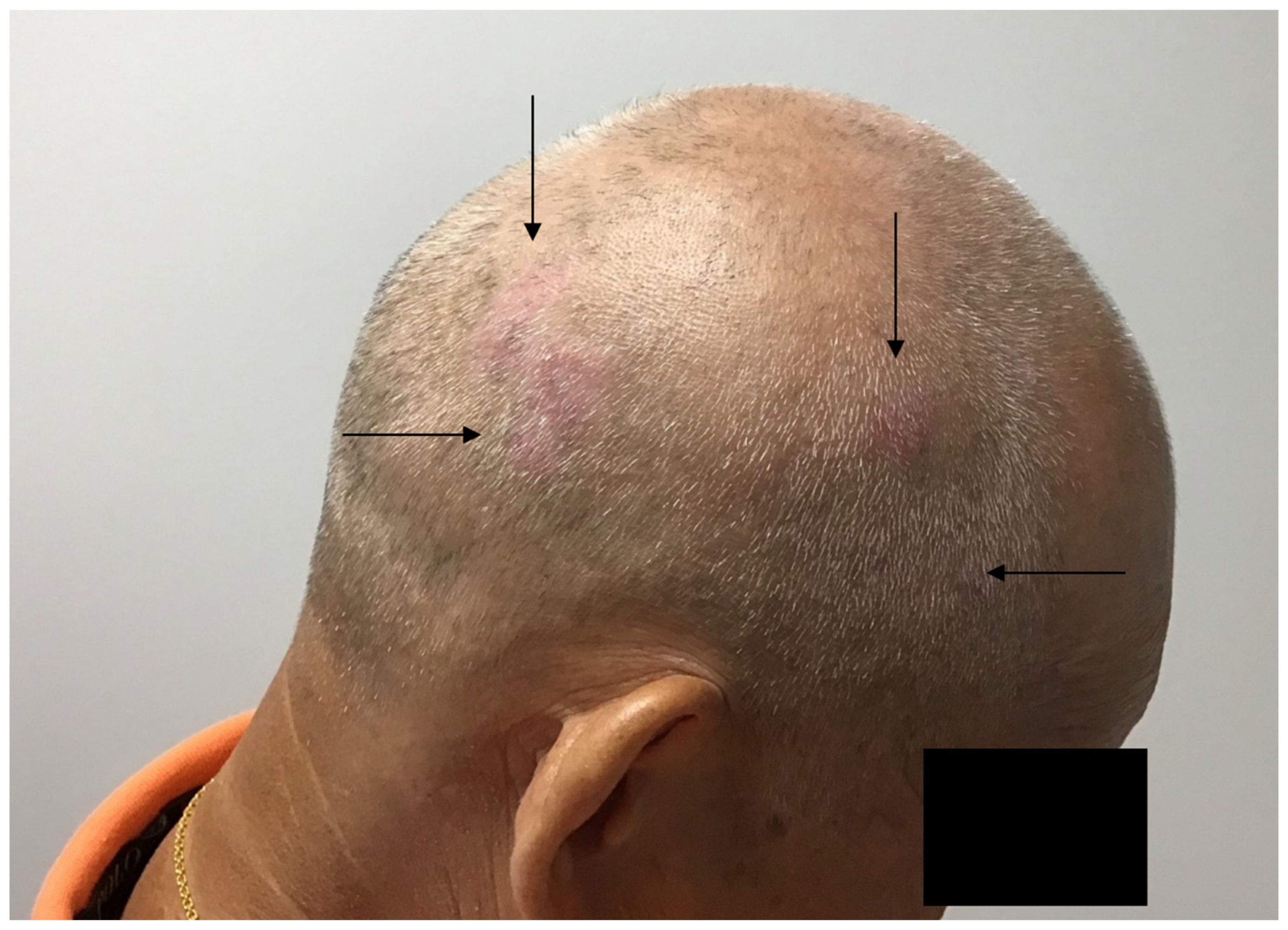
11]. Interestingly, scalp inflammation is often localized to hair bearing areas, and is absent in areas of alopecia (
Figure 1). Additional sun protective measures, such as lifelong wearing of hats, may be protective of UV induced carcinogenesis (
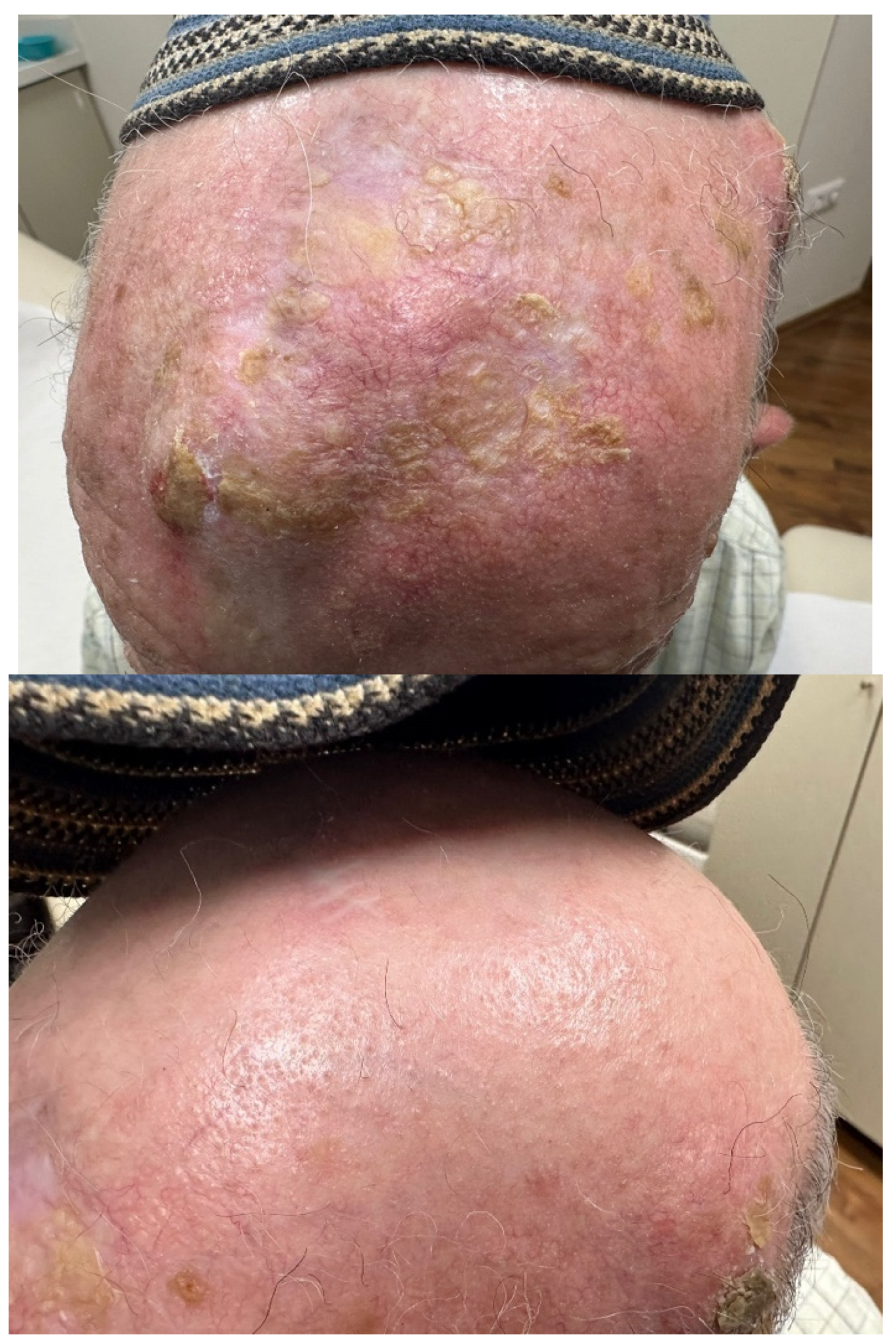
Figure 2).
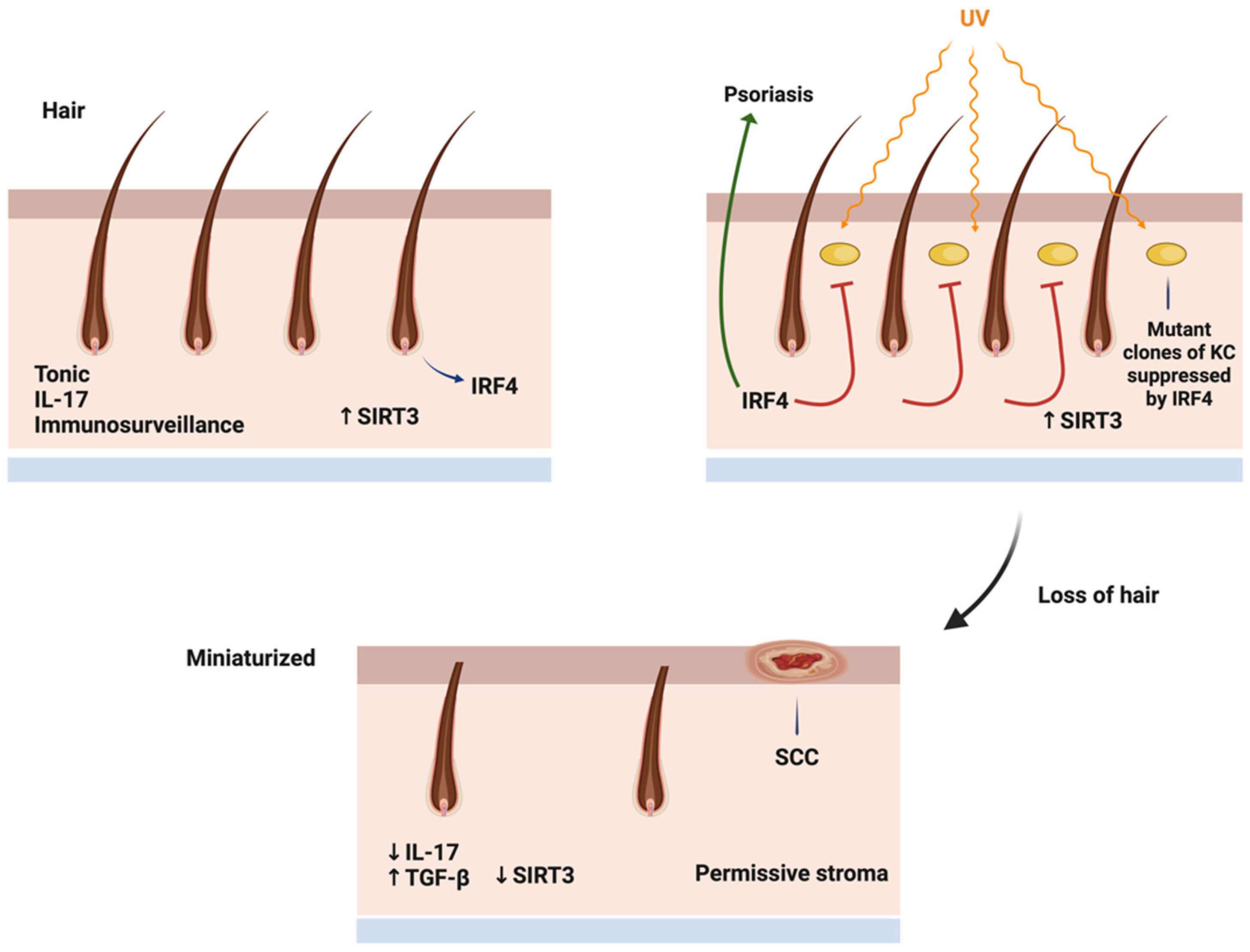
Decreased IRF4 leads to hair graying, which in turn may lead to alopecia and loss of the immune protection against nonmelanoma skin cancer [
3,
7]. Loss of hair likely diminishes the presence of IL-17, resulting in promotion of pre-existing UV mutant cells into clinically evident tumors (
Figure 3).
4. Hair Cycle and Aging
The hair growth cycle consists of three main phases: anagen, catagen, and telogen. Throughout life, hair follicles undergo repeated cycles of growth and rest, which involve changes in the morphology and structure of the dermal papilla (DP), the formation of new hair shafts (HS), and the shedding of old hair. The anagen phase is the active growth period, during which rapid hair growth and complete HS formation occur. This phase begins when quiescent signals from the inner bulge layer and other hair follicle stem cell (HFSC) niches are overridden by a combination of BMP inhibitory and Wnt activation signals. The anagen phase can last between 2 to 6 years, depending on genetic factors. Following this, the catagen phase, lasting about 2 to 3 weeks, serves as a brief transitional stage marked by follicular regression, where apoptosis of hair bulb cells leads to degeneration. During this time, HS growth halts, cell proliferation and differentiation decline, and the DP shrinks. The telogen phase is the resting period, during which proliferative and biochemical activities reach their lowest point. The hair, now referred to as "club hair," separates from the DP and is shed. This phase typically lasts 2 to 3 months, after which DP cells migrate to the bulge, activating stem cells and initiating the next anagen phase [
14].
With aging, the hair cycle undergoes significant changes, resulting in the gradual reduction in hair density, thickness, and pigmentation. The anagen phase shortens, leading to thinner HS, while the interval between hair shedding during telogen and the onset of new growth in anagen lengthens. HFSCs, crucial for hair regeneration, enter a prolonged quiescent state as aging advances. This quiescence, along with changes in the follicle’s extracellular environment, leads to the miniaturization of hair follicles, resulting in thinner and less dense hair. Molecular signaling disruptions, such as increased BMP signaling and decreased Wnt activation, further impair hair regeneration. These accumulated imbalances in cellular signaling compromise HFSC function, resulting in diminished hair production, the emergence of thinner, graying hair, and ultimately, progressive hair loss [
3,
15].
5. Actinic Keratosis and Cutaneous Squamous Cell Carcinoma
Actinic keratosis (AK) and cutaneous squamous cell carcinoma (cSCC) are exceedingly common lesions that occur on the scalp. AK is a known precursor to cSCC, with a small but significant number of AK progressing to cSCC. For this reason, destruction of AK by cryotherapy and treatment with topical agents, such as fluorouracil, are among the most common cause of visits of elderly patients to dermatologists. AK and cSCC have long been known to be caused by UVB, and have a high tumor mutation burden, with mutation of p53 being among the most common. A recent extensive study of Thomson et al [
16] demonstrated additional mutations in tumor suppressor genes, such as Notch1, Notch2, FAT1 KMTC2 and HMCN1. In this study, the only dominant oncogenic mutation was in the phisphoinositol-3-kinase p85B. Our group was the first group to demonstrate that phosphoinositol-3 kinase inhibition in vivo led to decreased tumor growth in angiosarcoma, a tumor also associated with impaired p53 function.
Of note, TGFBR2 is downregulated in the progression from normal skin to AK to cSCC. This may represent an adaptation to scalp stroma, which becomes more rich in TGFb with aging.
6. Melanoma
The scalp is not an uncommon site for melanoma. Melanomas of the scalp have a distinct mutational profile compared to melanomas in other sites. Braf mutation is one of the most common driver mutations in melanoma, but the scalp has a different profile of Braf mutations, with V600K being more common that V600E mutations, and V600K appear to be more associated with chronic skin damage than V600E mutations [
17,
18,
19]. Melanomas also appear to be more common in hair bearing areas than nonmelanoma skin cancers of he scalp, indicting that immunosurveillance may play less of a role for scalp melanoma than for nonmelanoma skin cancer [
1,
2,
20]. Finally, mutations in other drivers are seen in scalp melanomas, including Rac1 and GNA11 [1,21-23]. An exceptional response was observed in a scalp melanoma with metastases in a patient treated with surgical debulking, gentian violet and imiquimod, with no recurrence for over 2 years until the patient died of congestive heart failure [
24]. Scalp melanomas thus appear in two clinical scenarios, one of chronic sun exposure and hair loss, and one of sun protection and retention of hair, occurring at a younger age [
1].
7. Angiosarcoma
Angiosarcoma is a rare malignancy of endothelial cells, and the scalp is the most common site of cutaneous angiosarcoma. This tumor has a high propensity for distant metastases, and the prognosis is poor. Complete excision is often difficult due to large primary size and skip areas. In the largest study of angiosarcoma performed to date, angiosarcoma of the scalp has a high tumor mutation burden and UV signature compared to other sites. Mutation of p53 has also been observed nearly universally in scalp angiosarcoma [
25]. The high tumor mutation burden of scalp angiosarcoma has suggested a role of immunotherapy, and some exceptional responses have been observed [25–27].
8. IL-17, the Double Edged Sword
We have proposed that hair derived IL-17 may play a protective role against the development of skin cancer. Others have shown that IL-17 can promote other cancers [
28,
29]. How do we reconcile these findings. A recent study demonstrated that the presence of wild type p53 is required for the tumor promoting activity of IL-17 [
29]. We have previously shown that tumors with mutant p53 signal differently than tumors with wild-type p53. The majority of basal and squamous cell carcinomas have defects/mutations in p53, while the majority of melanomas have wild type 53. This may explain in part why hair may be more protective against nonmelanoma skin cancer than melanoma.
9. Treatment of Scalp Malignancies
Treatments of skin cancers on the scalp vary and the treatment of choice depends on a multitude of factors including amongst others: cancer type, size, stage and general health status. Treatments may be divided into surgical or non-surgical treatments. Surgical options are usually the treatment of choice and these include: curettage and electrodessication, excisional surgery and Mohs micrographic surgery (MMS). MMs is a surgical technique which allows examination of 100% of the resected margins and achieving high cure rates while at the same time minimizing the unnecessary sacrificing of normal tissue. As such the technique has been accepted as the treatment of choice for the majority of skin cancers on the scalp. Non surgical treatments are usually advocated when surgery is not feasible either due to characteristics of the tumor or underlying illness contraindicating surgery.
Non-surgical treatments include:
Radiation therapy utilizing external beam radiation in which high energy beams target the cancerous area. Additionally, Brachytherapy is a form of radiation therapy in which radioactive material is placed in proximity to tumor. The disadvantage of radiation therapy is the lack of precise treatment of only the tumor itself with indiscriminate treatment of healthy tissue as well. As such the treatment will be prescribed either in cases of inoperable tumors or as an adjuvant treatment following surgical removal. Other disadvantages include the logistics of multiple patient visits to complete a course of radiation therapy.
Cryosurgery- Freezing the cancer cells with liquid nitrogen is a viable treatment for lesions deemed to be pre- cancerous or superficial tumors. Similar to radiation therapy- this treatment is non-precise and often times will not be successful in eradication of all tumor cells. However, it is useful as a palliative measure in frail patients who may be intolerant to more aggressive therapies. Damage is caused by ice crystals disrupting the cell membrane and intracellular organelles.
Topical chemotherapy The most commonly used agent is. 5-Fluorouracil (5-U)- is a topical chemotherapy which enables successful treatment of pre-cancerous or superficial tumors in selective cases. Additionally, topical treatment with Imiquimod cream via stimulating of the immune system is an options for pre-cancerous lesions. Flurouracil targets dividing cells, while Imiquimod is a TLR7 (toll like receptor) agonist.
Targeted therapy, immunotherapy and chemotherapy are utilized in cases of advanced cases when the tumor has spread beyond the scalp but are generally not used for scalp limited disease
The current gold standard for the overwhelming majority of tumors, especially basal cell and squamous cell carcinomas of the scalp has been the surgical removal utilizing the Mohs micrographic technique. As stated previously, the technique enables removing the tissue and immediately evaluating the entirety of the surgical margins. This is in contrast to the standard excision technique where the tissue is processed and examined in what is commonly referred to in a “bread loafing” sectioning. As such the tumor may be missed and lead to a false negative pathology result. Additionally, in general precautionary safety margins are taken in the common excision of tumors leading often to the unnecessary sacrificing of healthy tissue leading to a more complex reconstructive closure. The Mohs technique allows for taking minimal resection borders and thus minimizing the extent of the reconstruction. One has to keep in mind that often times the lack of laxity on the scalp region behooves one to attempt to cause as small a defect as possible and we are able to do so without taking larger than necessary borders. In addition, the more extensive the surgery the greater the risks for increased post-surgical complications such as bleeding and infection with prolonged healing and increased scarring. Patient satisfaction is very high as both the removal of the tumor and reconstruction are all performed on the same day while determining the complete removal of the skin cancer. Only in cases where the tumor is determined to be inoperable, or the patient due to underlying disease is not seen as a candidate for surgery will I opt out for one of non-surgical modalities as described earlier.
10. Metabolic Parameters of the Aging Scalp
The major features of the aging scalp are loss of hair, manifesting in baldness. The aging associated alopecia is primarily a nonscarring alopecia, characterized by miniaturization of hair follicles, often associated with some degree of fibrosis [
3,
7]. The loss of longer hair likely diminishes the tonic IRF-4-IL-17 axis of the skin, allowing for decreased immunosurveillance of UV mutated keratinocytes and melanocytes. Hair requires ATP to maintain its complex, differentiates structure, and this requires optimal metabolism in both hair follicle keratinocytes and stromal support cells. As the scalp ages, there is decreased respiratory capacity in both hair generating cells and stroma, and the IRF-4-IL-17 axis is replaced with a TGF-beta low Sirt3 axis [
8]. Low Sirt3 fibroblasts are associated with tumor stroma, and may play a support role in tumor progression. The clinical observation of increased skin cancers with low hair density reflects aging metabolism. The takeaway lessons from carcinogenesis of the scalp are the following. First, the hair does not provide as good a barrier to UV mutagenesis as commonly assumed. Wearing of hats or other protective measures may prevent scalp carcinogenesis. Second, the hair likely provides an immunological barrier to the development of skin cancer on the scalp, and interventions that increase IL-17 may be immunopreventive of scalp skin cancers. Third, metabolic stromal aging, mediated in part by dihydrotestosterone-reactive oxygen signaling, modifies the scalp into a less supportive environment for hair. At the same time increased TGF-beta, which decreases the mitochondrial enzyme Sirt3, provides a permissive stroma that allows progression of mutated keratinocytes and melanocytes to frank malignancy. Sirt3 has been shown to be protective of cochlear hair cells [
5,
6], and Sirt3 activators like honokiol and methyl-honokiol have been shown to be beneficial in murine models of alopecia [
30,
31]. Pharmacologic interventions which maintain hair may also be preventive of scalp carcinogenesis.
11. Ultraviolet Light and Microbiome
As noted before, hair is an imperfect barrier to UV light. Parisi et al. calculated an ultraviolet protection factor (UPF) that ranged from approximately 5 to 17 in full sun, with the UPF increasing with higher solar zenith angles. Surprisingly, the UPF provided by the shorter hair was generally higher by a range of 2–5 than that provided by the longer hair – this was attributed to more parting of the subjects with long hair, which allowed for a greater surface area for UV exposure [
32]. However, long hair may provide increased IL-17 mediated inflammation, which may balance out the increased UV exposure noted with long hair.
Additional factors that influence UV penetration of the scalp include hair density, thickness and color. De Gálvez et al. demonstrated that hair protects against both UVB and UVA radiation; and as expected, hair density, thickness, and the presence of melanin provide additional protection. White hair offers the least amount of protection [
33]. Eyelashes also provide some degree of protection to the forehead, and similarly, Marro et al. found that light angle and density of hair follicles influenced the degree of protection that the hair provided [
34].
Analysis of the scalp microbiome also provide hypotheses as to a contributory role of the scalp microbiome. The scalp microbiome is influenced by UV diminution, decreased pH and moisture, which favors a different microbiome than sun exposed skin. Lousada et al noted an increased abundance of both
Malassezia restricta and
M. globose has recently been reported in alopecic vertex areas compared with occipital skin and healthy controls [
35]. Of interest, Malzasseisa globosa has been noted to have a tumor promoting activity and is found intratumorally in pancreatic cancer [
36]. Malassezia is controlled by IL-17, and loss of hair derived IL-17 may allow uncontrolled Malassezia growth and promotion of existing mutations towards clinically evident cancer [37-39]. Thus, it is possible that Malassezia species may play a role in scalp carcinogenesis as well. Antifungal treatment of Malassezia should also be considered as a potential chemo preventive measure for scalp carcinogenesis.
Hair and nails are known for their production of anti-microbial peptides [
40]. It is well known that nail dermatophytosis occurs more commonly in the elderly, and perhaps increased Malassezia colonization occurs in the elderly scalp. While it has not been well studied, loss of aging related loss of antimicrobial peptides may be dependent on keratinocytes having sufficient mitochondrial reserve to produce these peptides, which are not essential to the survival of the keratinocyte.
12. Conclusions
The appreciation of the scalp as a distinct immunometabolic site is beginning to be appreciated. A novel candidate for treatment of alopecia, a small molecule mitochondrial pyruvate transport inhibitor, promotes hair growth in mice and is being currently evaluated in human clinical trials (NCT06393452). The proposed mechanism of action of this compound is activation of lactate dehydrogenase (LDH), which activates hair stem cell growth. Of interest, Sirt3 also activates LDH by deacetylation. It is not known whether the activity of mitochondrial pyruvate transport inhibitors are Sirt3 dependent or independent [
41,
42].
Finally, the understanding of scalp carcinogenesis has public health implications. It is generally assumed that the presence of hair is protective against carcinogenesis, and therefore additional measures for sun protection are not needed. The clinical and scientific evidence suggests that hair does not prevent UV induced mutations, but may impede tumor progression until hair is lost. The presence of hair provides tonic IL-17 immunosurveillance, and a stroma which may restrain tumor progression. Thus additional measures to protect the scalp, ie hats, and agetns that prevent hair loss may be preventive of future scalp carcinogenesis.
Acknowledgments
We acknowledge Dr Warren Heymann for helpful suggestions in preparation of this final manuscript.
References
- Licata, G.; Scharf, C.; Ronchi, A.; Pellerone, S.; Argenziano, G.; Verolino, P.; Moscarella, E. Diagnosis and Management of Melanoma of the Scalp: A Review of the Literature. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1435–1447. [Google Scholar] [CrossRef]
- Porto, A. C.; Blumetti, T. P.; Calsavara, V. F.; Torrezan, G. T.; Andrade de Paula, C. A.; Lellis, R.; Duprat Neto, J. P.; Carraro, D. M.; Braga, J. C. T. A Cross-Sectional Study of Clinical, Dermoscopic, Histopathological, and Molecular Patterns of Scalp Melanoma in Patients with or without Androgenetic Alopecia. Sci. Rep. 2022, 12, 15096. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, S. Deciphering the Molecular Mechanisms of Stem Cell Dynamics in Hair Follicle Regeneration. Exp. Mol. Med. 2024, 56, 110–117. [Google Scholar] [CrossRef]
- Williams, R.; Westgate, G. E.; Pawlus, A. D.; Sikkink, S. K.; Thornton, M. J. Age-Related Changes in Female Scalp Dermal Sheath and Dermal Fibroblasts: How the Hair Follicle Environment Impacts Hair Aging. J. Invest. Dermatol. 2021, 141, 1041–1051. [Google Scholar] [CrossRef]
- Zhao, C.; Liang, W.; Yang, Z.; Chen, Z.; Du, Z.; Gong, S. SIRT3-Mediated Deacetylation Protects Inner Hair Cell Synapses in a H2O2-Induced Oxidative Stress Model in Vitro. Exp. Cell Res. 2022, 418, 113280. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, C.; Chen, Z.; Yang, Z.; Liu, K.; Gong, S. Sirtuin-3 Protects Cochlear Hair Cells Against Noise-Induced Damage via the Superoxide Dismutase 2/Reactive Oxygen Species Signaling Pathway. Front. Cell Dev. Biol. 2021, 9, 766512. [Google Scholar] [CrossRef]
- Sennett, R.; Rendl, M. Mesenchymal-Epithelial Interactions During Hair Follicle Morphogenesis and Cycling. Semin. Cell Dev. Biol. 2012, 23, 917–927. [Google Scholar] [CrossRef]
- Sundaresan, N. R.; Bindu, S.; Pillai, V. B.; Samant, S.; Pan, Y.; Huang, J. Y.; Gupta, M.; Nagalingam, R. S.; Wolfgeher, D.; Verdin, E.; et al. SIRT3 Blocks Aging-Associated Tissue Fibrosis in Mice by Deacetylating and Activating Glycogen Synthase Kinase 3β. Mol. Cell Biol. 2015, 36, 678–692. [Google Scholar] [CrossRef]
- Pośpiech, E.; Kukla-Bartoszek, M.; Karłowska-Pik, J.; Zieliński, P.; Woźniak, A.; Boroń, M.; Dąbrowski, M.; Zubańska, M.; Jarosz, A.; Grzybowski, T.; et al. Exploring the Possibility of Predicting Human Head Hair Greying from DNA Using Whole-Exome and Targeted NGS Data. BMC Genomics 2020, 21, 538. [Google Scholar] [CrossRef]
- Mudter, J.; Yu, J.; Zufferey, C.; Brüstle, A.; Wirtz, S.; Weigmann, B.; Hoffman, A.; Schenk, M.; Galle, P.R.; Lehr, H.A.; et al. IRF4 Regulates IL-17A Promoter Activity and Controls RORγt-Dependent Th17 Colitis In Vivo. Inflamm. Bowel Dis. 2011, 17, 1343–1358. [Google Scholar] [CrossRef]
- Ni, A.; Chen, H.; Wu, Y.; Wang, Z. Expression of IRF-4 and IBP in Skin Lesions of Patients with Psoriasis Vulgaris. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2012, 32, 287–290. [Google Scholar] [CrossRef]
- Alam, M.S.; Gaida, M.M.; Ogawa, Y.; Kolios, A.G.A.; Lasitschka, F.; Ashwell, J.D. Counter-Regulation of T Cell Effector Function by Differentially Activated p38. J. Exp. Med. 2014, 211, 1257–1270. [Google Scholar] [CrossRef]
- Nakao, M.; Miyagaki, T.; Sugaya, M.; Sato, S. Exacerbated Imiquimod-Induced Psoriasis-Like Skin Inflammation in IRF5-Deficient Mice. Int. J. Mol. Sci. 2020, 21, 3681. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, L. He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell Dev. Biol 2022, 10, 899095. [Google Scholar] [CrossRef]
- Jang, H. , Jo, Y., Lee, J. H., Choi, S. Aging of hair follicle stem cells and their niches. BMB reports. 2023, 56, 2–9. [Google Scholar] [CrossRef]
- Thomson, J.; Bewicke-Copley, F.; Anene, C.A.; Gulati, A; Nagano, A; Purdie, N; Inman, G.J.; Proby, C. M.; Leigh, I.M.; Harwood, C.A. et al. The Genomic Landscape of Actinic Keratosis. J. Invest. Dermatol. 2021, 141, 1664-1674.e7. [CrossRef]
- Nepote, A.; Avallone, G.; Ribero, S.; Cavallo, F.; Roccuzzo, G.; Mastorino, L.; Conforti, C.; Paruzzo, L.; Poletto, S.; Carnevale Schianca, F.; et al. Current Controversies and Challenges on BRAF V600K-Mutant Cutaneous Melanoma. J. Clin. Med. 2022, 11, 828. [Google Scholar] [CrossRef]
- Bauer, J.; Büttner, P.; Murali, R.; Okamoto, I.; Kolaitis, N. A.; Landi, M. T.; Scolyer, R. A.; Bastian, B. C. BRAF Mutations in Cutaneous Melanoma Are Independently Associated with Age, Anatomic Site of the Primary Tumor, and the Degree of Solar Elastosis at the Primary Tumor Site. Pigment Cell Melanoma Res. 2011, 24, 345–351. [Google Scholar] [CrossRef]
- Goto, K.; Yoshikawa, S.; Takai, T.; Tachibana, K.; Honma, K.; Isei, T.; Kukita, Y.; Oishi, T. Clinicopathologic and Genetic Characterization of Invasive Melanoma with BRAF V600K Mutation: A Study of 16 Cases. J. Cutan. Pathol. 2023, 50, 739–747. [Google Scholar] [CrossRef]
- Tas, F.; Erturk, K. Scalp Melanoma Is Associated with High Mitotic Rate and Is a Poor Prognostic Factor for Recurrence and Outcome. Melanoma Res. 2017, 27, 387–390. [Google Scholar] [CrossRef]
- Dai, J.; Tetzlaff, M. T.; Schuchter, L. M.; Elder, D. E.; Elenitsas, R. Histopathologic and Mutational Analysis of a Case of Blue Nevus-Like Melanoma. J. Cutan. Pathol. 2016, 43, 776–780. [Google Scholar] [CrossRef]
- Patel, S. P.; Kim, D. W.; Lacey, C. L.; Hwu, P. GNA11 Mutation in a Patient With Cutaneous Origin Melanoma: A Case Report. Medicine (Baltimore). 2016, 95, e2336. [Google Scholar] [CrossRef]
- Krauthammer, M.; Kong, Y.; Ha, B. H. , Evans, P.; Bacchiocchi, A.; McCusker, J. P.; Cheng, E.; Davis, M. J.; Goh, G.; Choi, M.; et al. Exome Sequencing Identifies Recurrent Somatic RAC1 Mutations in Melanoma. Nat. Genet. 2012, 44, 1006–1014. [Google Scholar] [CrossRef]
- Arbiser, J. L.; Bips, M.; Seidler, A.; Bonner, M. Y.; Kovach, C. Combination Therapy of Imiquimod and Gentian Violet for Cutaneous Melanoma Metastases. J. Am. Acad. Dermatol. 2012, 67, e81–e83. [Google Scholar] [CrossRef]
- Painter, C. A.; Jain, E.; Tomson, B. N.; Dunphy, M.; Stoddard, R. E.; Thomas, B. S.; Damon, A. L.; Shah, S.; Kim, D.; Gómez Tejeda Zañudo, J.; et al. The Angiosarcoma Project: Enabling Genomic and Clinical Discoveries in a Rare Cancer through Patient-Partnered Research. Nat. Med. 2020, 26, 181–187. [Google Scholar] [CrossRef]
- Boichard, A.; Wagner, M.J.; Kurzrock, R. Angiosarcoma Heterogeneity and Potential Therapeutic Vulnerability to Immune Checkpoint Blockade: Insights from Genomic Sequencing. Genome Med. 2020, 12, 61. [Google Scholar] [CrossRef]
- Chan, J. Y.; Lim, J. Q.; Yeong, J.; Ravi, V.; Guan, P.; Boot, A.; Tay, T. K. Y.; Selvarajan, S.; Md Nasir, N. D.; Loh, J. H.; et al. Multiomic Analysis and Immunoprofiling Reveal Distinct Subtypes of Human Angiosarcoma. J. Clin. Invest. 2020, 130, 5833–5846. [Google Scholar] [CrossRef]
- Yoshimura, N.; Kariya, R.; Shimada, M.; Tateyama, M.; Matsunaga, H.; Shibata, Y.; Tanimura, S.; Takata, K.; Arima, T.; Kawakami, J.; et al. The IL-17-IL-17RA Axis is Required to Promote Osteosarcoma Progression in Mice. Sci. Rep. 2023, 13, 21572. [Google Scholar] [CrossRef]
- Xu, B.; Guenther, J.F.; Pociask, D.A.; Wang, Y.; Kolls, J.K.; You, Z.; Chandrasekar, B.; Shan, B.; Sullivan, D.E.; Morris, G.F. Promotion of Lung Tumor Growth by Interleukin-17. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 307, L497–L508. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Chen, F.; Wang, C.; Guo, X.; Wang, C.; Fan, Y.; Wang, Y.; Peng, Y.; Li, W. Liposomal Honokiol Promotes Hair Growth via Activating Wnt3a/β-Catenin Signaling Pathway and Down Regulating TGF-β1 in C57BL/6N Mice. Eur. J. Dermatol. 2011, 21, 1012–1014. [Google Scholar] [CrossRef]
- Kim, S. C.; Kang, J. I.; Kim, M. K.; Boo, H. J.; Park, D. B.; Lee, Y. K.; Kang, J. H.; Yoo, E. S.; Kim, Y. H.; Kang, H. K. The Hair Growth Promoting Effect of 4-O-Methylhonokiol. Eur. J. Dermatol. 2011, 21, 1012–1014. [Google Scholar] [CrossRef]
- Parisi, A.V.; Smith, D.; Schouten, P.; Turnbull, D.J. Solar ultraviolet protection provided by human head hair. Photochem Photobiol. 2009, 85, 250–4. [Google Scholar] [CrossRef]
- de Gálvez, M.V.; Aguilera, J.; Bernabó, J.L.; Sánchez-Roldán, C.; Herrera-Ceballos, E. Human Hair as a Natural Sun Protection Agent: A Quantitative Study. Photochem Photobiol. 2015, 91, 966–70. [Google Scholar] [CrossRef]
- Marro, M.; Moccozet, L.; Vernez, D. Modeling the protective role of human eyelashes against ultraviolet light exposure. Comput Biol Med. 2022, 141, 105135. [Google Scholar] [CrossRef]
- Lousada, M.B.; Lachnit, T.; Edelkamp, J.; Rouillé, T.; Ajdic, D.; Uchida, Y.; Di Nardo, A.; Bosch, T. C. G.; Paus, R. Exploring the human hair follicle microbiome. Br J Dermatol. 2021, 184, 802–815. [Google Scholar] [CrossRef]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J. I.; Shadaloey, S. A.; Wu, D.; Preiss, P.; Verma, N.; et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 2019, 574, 264–267. [Google Scholar] [CrossRef]
- Tham, K. C.; Lefferdink, R.; Duan, K.; Lim, S. S.; Wong, X. F. C. C.; Ibler, E.; Wu, B.; Abu-Zayed, H.; Rangel, S. M.; Del Duca, E.; et al. Distinct skin microbiome community structures in congenital ichthyosis. The British journal of dermatology. 2022, 187, 557–570. [Google Scholar] [CrossRef]
- Narunsky-Haziza, L.; Sepich-Poore, G. D.; Livyatan, I.; Asraf, O.; Martino, C.; Nejman, D.; Gavert, N.; Stajich, J. E.; Amit, G.; González, A.; et al. Pan-cancer analyses reveal cancer-type-specific fungal ecologies and bacteriome interactions. Cell. 2022, 185, 3789–3806. [Google Scholar] [CrossRef]
- Ruchti, F.; Tuor, M.; Mathew, L.; McCarthy, N. E.; LeibundGut-Landmann, S. γδ T cells respond directly and selectively to the skin commensal yeast Malassezia for IL-17-dependent fungal control. PLoS pathogens. 2024, 20, e1011668. [Google Scholar] [CrossRef]
- Dorschner, R. A.; Lopez-Garcia, B.; Massie, J.; Kim, C.; Gallo, R. L. Innate immune defense of the nail unit by antimicrobial peptides. Journal of the American Academy of Dermatology. 2004, 50, 343–348. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, D.; Shan, B.; et al. SIRT3 Enhances Glycolysis and Proliferation in SIRT3-Expressing Gastric Cancer Cells. PLoS One. 2015, 10, e0129834. [Google Scholar] [CrossRef]
- Liu, X.; Flores, A. A.; Situ, L.; Gu, W.; Ding, H.; Christofk, H. R.; Lowry, W. E.; Jung, M. E. Development of Novel Mitochondrial Pyruvate Carrier Inhibitors to Treat Hair Loss. J. Med. Chem. 2021, 64, 2046–2063. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).