Submitted:
05 September 2024
Posted:
06 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
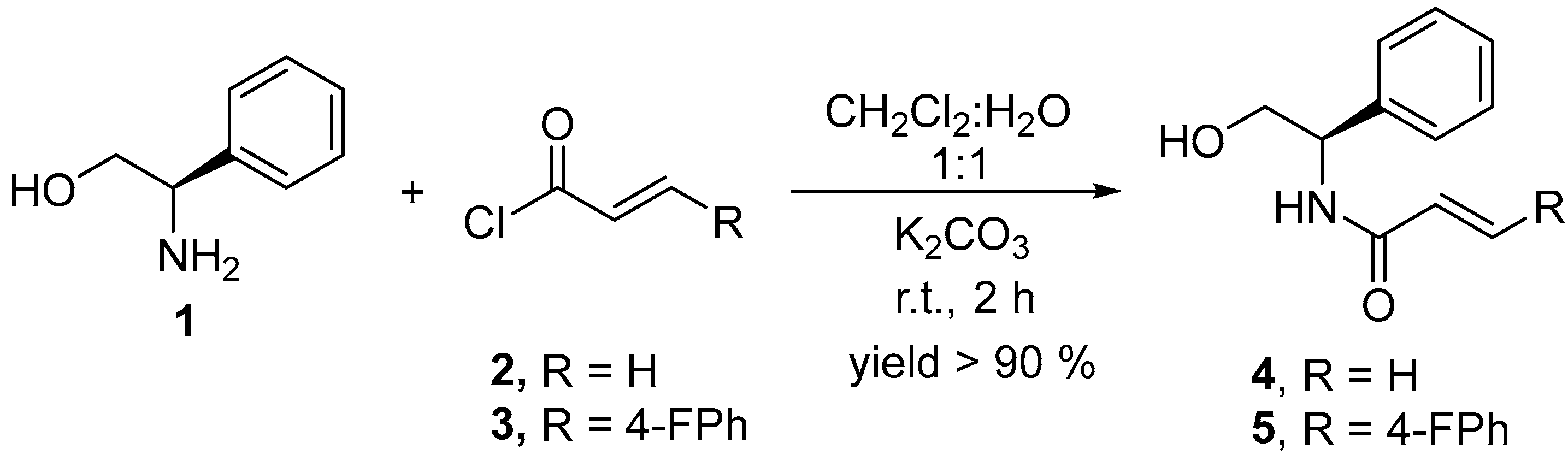
2.1. Synthesis of Chiral Acrylamides
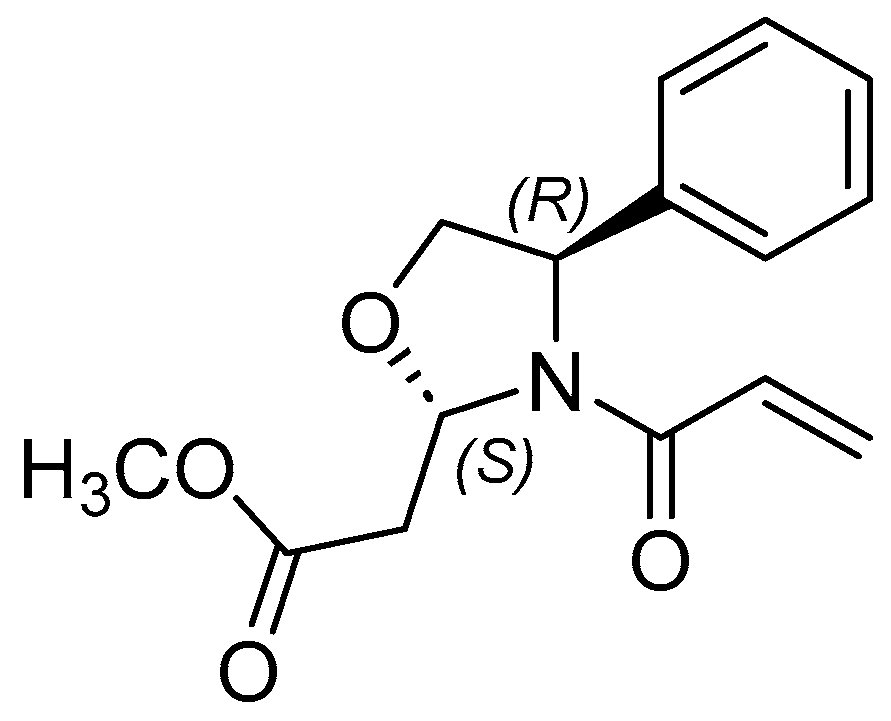
2.2. Synthesis of Methyl 2-(4R)-3-acryloyl-4-phenyloxazolidin-2-yl)acetates, 7 and 8
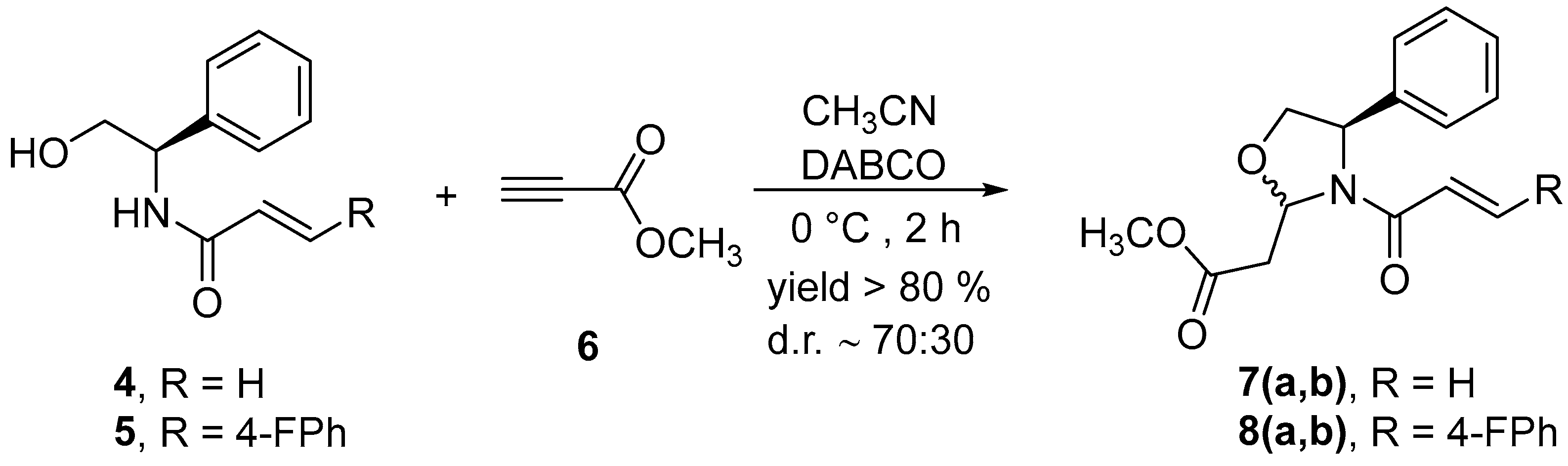
3. Discussion
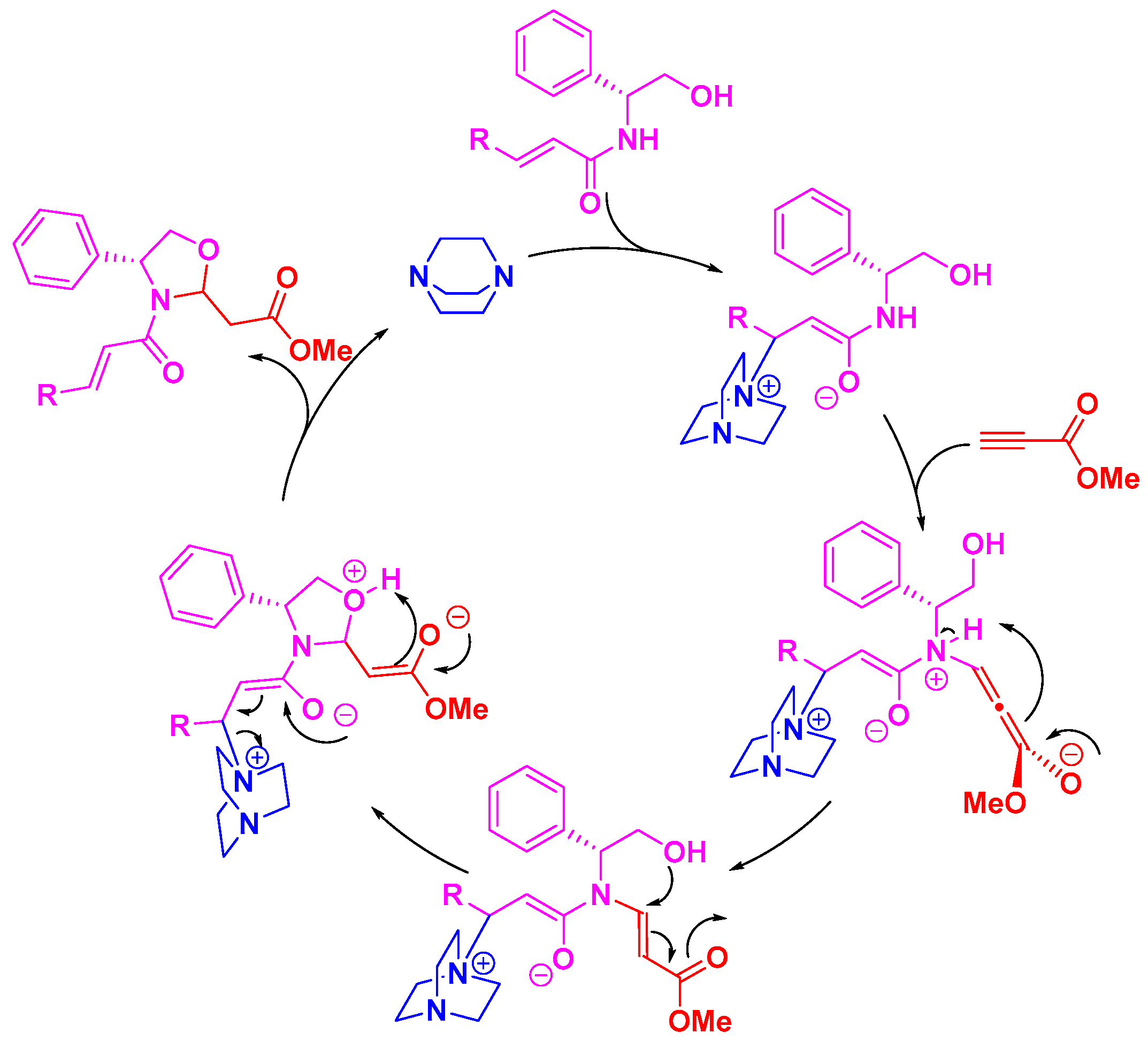
4. Materials and Methods
4.1. General
4.2. Synthesis of Chiral Acrylamides
4.3. Synthesis of Methyl 2-(4R)-3-acryloyl-4-phenyloxazolidin-2-yl)acetates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cordero, F. M.; Giomi, D.; Lascialfari, L. Five-Membered Ring Systems. With O and N Atoms. In Progress in Heterocyclic Chemistry; Elsevier Ltd, 2013; Vol. 25, pp 291–317. [CrossRef]
- Qian, X.; Xu, X.; Li, Z.; Li, Z.; Song, G. Syntheses, Structures and Bioactivities of Fluorine-Containing Phenylimino-Thia(Oxa)Zolidine Derivatives as Agricultural Bioregulators. In Journal of Fluorine Chemistry; Elsevier B.V., 2004; Vol. 125, pp 1609–1620. Morales-Monarca, G.-H.; Gnecco, D.; Terán, J. L. Diastereoselective Functionalization of Chiral N-Acyl-1,3-oxazolidines and Their Applications in the Synthesis of Bioactive Molecules. Eur. J. Org. Chem., 2022, 33. [CrossRef]
- Carbonnelle, A.-C.; Gotta, V.; Roussi, G. b-Amino alcohol-N-oxides as precursors of chiral oxazolidines: synthesis of (R)-(-)-cryptostyline I. Heterocycles 1993, 36, 1763–1769. [Google Scholar]
- Pytkowicz, J.; Stéphany, O.; Marinkovic, S.; Inagaki, S.; Brigaud, T. Straightforward Synthesis of Enantiopure (R)- and (S)-Trifluoroalaninol. Org. Biomol. Chem. 2010, 8, 4540–4542. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y. F.; Wang, R.; Liu, L.; Da, C. S.; Yan, W. J.; Xu, Z. Q. Enantioselective Alkynylation of Aromatic Aldehydes Catalyzed by New Chiral Oxazolidine Ligands. Tetrahedron Lett. 2005, 46, 863–865. [Google Scholar] [CrossRef]
- Pichon-Barré, D.; Zhang, Z.; Cador, A.; Vives, T.; Roisnel, T.; Baslé, O.; Jarrige, L.; Cavallo, L.; Falivene, L.; Mauduit, M. Chiral Oxazolidines Acting as Transient Hydroxyalkyl-Functionalized N-Heterocyclic Carbenes: An Efficient Route to Air Stable Copper and Gold Complexes for Asymmetric Catalysis. Chem. Sci. 2022, 13, 8773–8780. [Google Scholar] [CrossRef] [PubMed]
- Khrapova, A. V.; Saroyants, L. V.; Yushin, M. Y.; Zukhairaeva, A. S.; Velikorodov, A. V. Prospects of Using Pharmacologically Active Compounds for the Creation of Antimycobacterial Drugs. Pharm. Chem. J. 2022, 55, 1108–1114. [Google Scholar] [CrossRef]
- Santos, R. V. C.; Cunha, E. G. C.; de Mello, G. S. V.; Rizzo, J. Â.; de Oliveira, J. F.; de Lima, M. D. C. A.; Pitta, I. D. R.; Pitta, M. G. D. R.; Rêgo, M. J. B. M. New Oxazolidines Inhibit the Secretion of Ifn-γ and Il-17 by Pbmcs from Moderate to Severe Asthmatic Patients. Med Chem (Los Angeles) 2021, 17, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, E. D. The Oxazolidines. 1953. https://pubs.acs.org/sharingguidelines.
- Reyes-Bravo, E.; Gnecco, D.; Juárez, J. R.; Orea, M. L.; Bernès, S.; Aparicio, D. M.; Terán, J. L. Diastereoselective Synthesis of New Zwitterionic Bicyclic Lactams, Scaffolds for Construction of 2-Substituted-4-Hydroxy Piperidine and Its Pipecolic Acid Derivatives. RSC Adv. 2022, 12, 4187–4190. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Buzzetti, L.; Puriņš, M.; Waser, J. Palladium-Catalyzed Trans-Hydroalkoxylation: Counterintuitive Use of an Aryl Iodide Additive to Promote C-H Bond Formation. ACS Catal. 2022, 12, 7565–7570. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zhang, Y.; Zhang, Z.; Chen, F.; Huang, L. Copper-Catalyzed Annulation/A 3 -Coupling Cascade: Diastereodivergent Synthesis of Sterically Hindered Monocyclic Oxazolidines Bearing Multiple Stereocenters. Eur. J. Org. Chem. 2019, 2019, 1931–1939. [Google Scholar] [CrossRef]
- Agami, C.; Dechoux, L.; Hebbe, S. Asymmetric Synthesis of Nitrogen Heterocycles by Reaction of Chiral b-Enaminocarbonyl Substrates with Acrylate Derivatives. Tetrahedron Lett. 2002, 43, 2521–2523. [Google Scholar] [CrossRef]
- Aparicio, D. M.; Gnecco, D.; Juárez, J. R.; Orea, M. L.; Mendoza, A.; Waksman, N.; Salazar, R.; Fores-Alamo, M.; Terán, J. L. Diastereoselective Synthesis of Aryl and Alkyl Trans-Glycidic Amides from Pseudoephedrine-Derived Sulfonium Salt. Chemospecific Exo-Tet Ring Closure for Morpholin-3-Ones. Tetrahedron 2012, 68, 10252–10256. [Google Scholar] [CrossRef]
- Mola, L.; Font, J.; Bosch, L.; Caner, J.; Costa, A. M.; Etxebarría-Jardí, G.; Pineda, O.; De Vicente, D.; Vilarrasa, J. Nucleophile-Catalyzed Additions to Activated Triple Bonds. Protection of Lactams, Imides, and Nucleosides with MocVinyl and Related Groups. J. Org. Chem. 2013, 78, 5832–5842. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, D.; López-Tosco, S.; Cruz-Acosta, F.; Méndez-Abt, G.; García-Tellado, F. Acetylides from Alkyl Propiolates as Building Blocks for C3 Homologation. Angew. Chem. In. Ed. 2009, 48, 2090–2098. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




