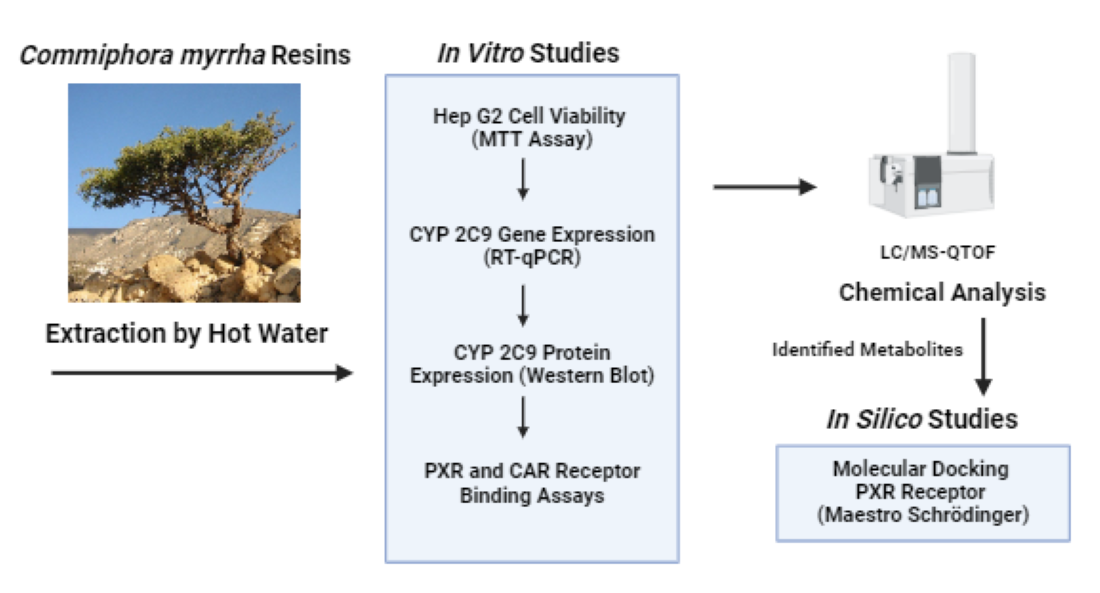
1. Introduction
Myrrha is a traditional medicine consisting of oleo-gum resins obtained from scratching off the bark of
Commiphora myrrha (
C. myrrha) Engl. (or
Commiphora molmol Engl.) tree belonging to the
Burseraceae plant family [
1]. The resins are produced primarily in arid and semi-arid regions, particularly in East Africa and the Arabian Peninsula, and are an important component of several traditional medicines, including Greco-Arabic Unani, Indian Ayurveda, and Chinese alternative medical systems [
2].
C. myrrha resins are a natural health product (NHP) prepared as a decoction, maceration, tincture, or raw resin and used to treat acute and chronic illnesses, including parasitic/fungal infections, asthma, arthritis, diabetes, and cancers [
3]. Consumption of
C. myrrha resins is popular worldwide, especially among patients from Asian populations [
2]. Although scarce, a few studies involving the consumption of
C. myrrha resins in patients have been reported. In Saudi Arabia, a study published that 45% of 51 diabetic patients used
C. myrrha resins [
4]. Another study reported that 90% of 453 cancer patients used complementary medicines, of which approximately 10% of patients consumed
C. myrrha resins [
5]. In Taiwan, a population-based study revealed the high prevalence of traditional medicine use among patients with rheumatoid arthritis, with
C. myrrha resins being among the most frequently consumed medicinal herbs [
6]. Thus, drug interaction studies of consumed
C. myrrha resins, which may affect drug metabolism through modulation of key drug-metabolizing enzyme expression, are necessary.
Herb-drug interaction (HDI) is a clinical situation in which the pharmacological profile of a pharmaceutical drug is altered upon concurrent consumption of a NHP [
7]. Compared to drug-drug interactions, HDI is more common because it contains numerous phytochemicals and NHPs that are more accessible and affordable than modern drugs [
8]. Among the most important HDIs is the alteration of the activity of phase-I cytochrome P-450 (CYP) system, a group of related enzymes primarily located in enterocytes and liver cells, which contribute more than 50% to the overall detoxification and elimination of pharmaceutical drugs [
9]. Alteration of CYP enzyme activity may include its inhibition and induction, which can pose serious health consequences [
10]. The main induction mechanisms involving CYP enzyme modulation occur
via ligand-activated xenobiotic-sensing nuclear receptor transcription factors, including the aryl hydrocarbon receptor, constitutive androstane receptor (CAR) and pregnane X receptor (PXR) [
11]. Among these three nuclear receptors, PXR is described as the major key regulator of drug metabolism and induction of the expression of drug-metabolizing enzymes such as CYP 2C9 [
11].
In a clinical case report, a patient consumed a boiled aqueous extract of
C. myrrha resins concomitantly with warfarin, which resulted in a decrease in its anticoagulant effect due to the resin extract-mediated impaired activity of CYP 2C9, the main CYP isoenzyme involved in warfarin metabolism [
12]. In our previous experimental studies, we observed that boiled aqueous extract of
C. myrrha resins increased the gene and protein expression levels of CYP 2C9 in Hep G2 cells [
13]. In the present study, at low non-toxic doses of boiled
C. myrrha resin aqueous extract, CYP 2C9 enzyme gene and protein expression levels were significantly induced in cultured Hep G2 cells. Further drug interaction investigations included (1) determining whether the boiled
C. myrrha aqueous extract contains agonists of PXR and CAR, well-known CYP 2C9 transcription factors, by performing the human xenobiotic nuclear receptor PXR and CAR binding assays; (2) tentatively identifying the bioactive secondary phytochemical metabolites in the boiled aqueous extract of
C. myrrha resin using liquid chromatography and mass spectrometry/time-of-flight (LC-QTOF); and (3) performing
in silico molecular docking studies involving the identified
C. myrrha-derived metabolites against the crystal structure of the human PXR receptor.
3. Discussion
The production and use of traditional medicines in the world is on the rise [
22]. According to the World Health Organization, approximately 60% of the world population rely on traditional medicines, and 80% of populations in developing countries use traditional medicines as the first-line of treatment [
23]. The high rate of NHP use is expected to continue in developing countries as traditional medicines are widely available and affordable compared to modern medicines. Due to the high consumption of medicinal plants, which contain an array of phytochemical groups, the number of adverse drug reactions due to herb-drug interactions is expected to be higher in these populations. Thus, studies to determine whether traditional folk medicines could modulate the expression of phase I and II metabolic enzymes are urgently needed, especially for CYP enzymes, which are involved in more than 50% of pharmaceutical drug metabolism in humans [
24]. In the current study, we determined the modulation of the expression of the major drug-metabolizing isoenzyme CYP 2C9 by the traditional medicine
C. myrrha resins using a series of
in vitro and
in silico studies, including CYP 2C9 gene and protein expression assays, xenobiotic-sensing nuclear receptor binding assays, and computational molecular docking.
First, we determined the endotoxin contamination levels in the boiled
C. myrrha resin aqueous extract using the quantitative chromogenic endotoxin kit based on the LAL assay because high levels of endotoxin contamination can affect the proliferation rate of numerous cell lines [
25]. Elevated levels of endotoxin contamination were also reported to interfere with CYP isoenzyme expression in humans [
26]. However, in our study, the endotoxin levels measured in the
C. myrrha resin aqueous extract were below the conservative maximum acceptable limit of endotoxin levels of 5.0 EU/mL for
in vitro cell culture conditions [
14]. Thus, an endotoxin removal step for the boiled
C. myrrha resin aqueous extract prior to Hep G2 cell treatment was unnecessary. Once the resin extract was suitable for cell culture, we assessed the viability of Hep G2 cells in response to various boiled
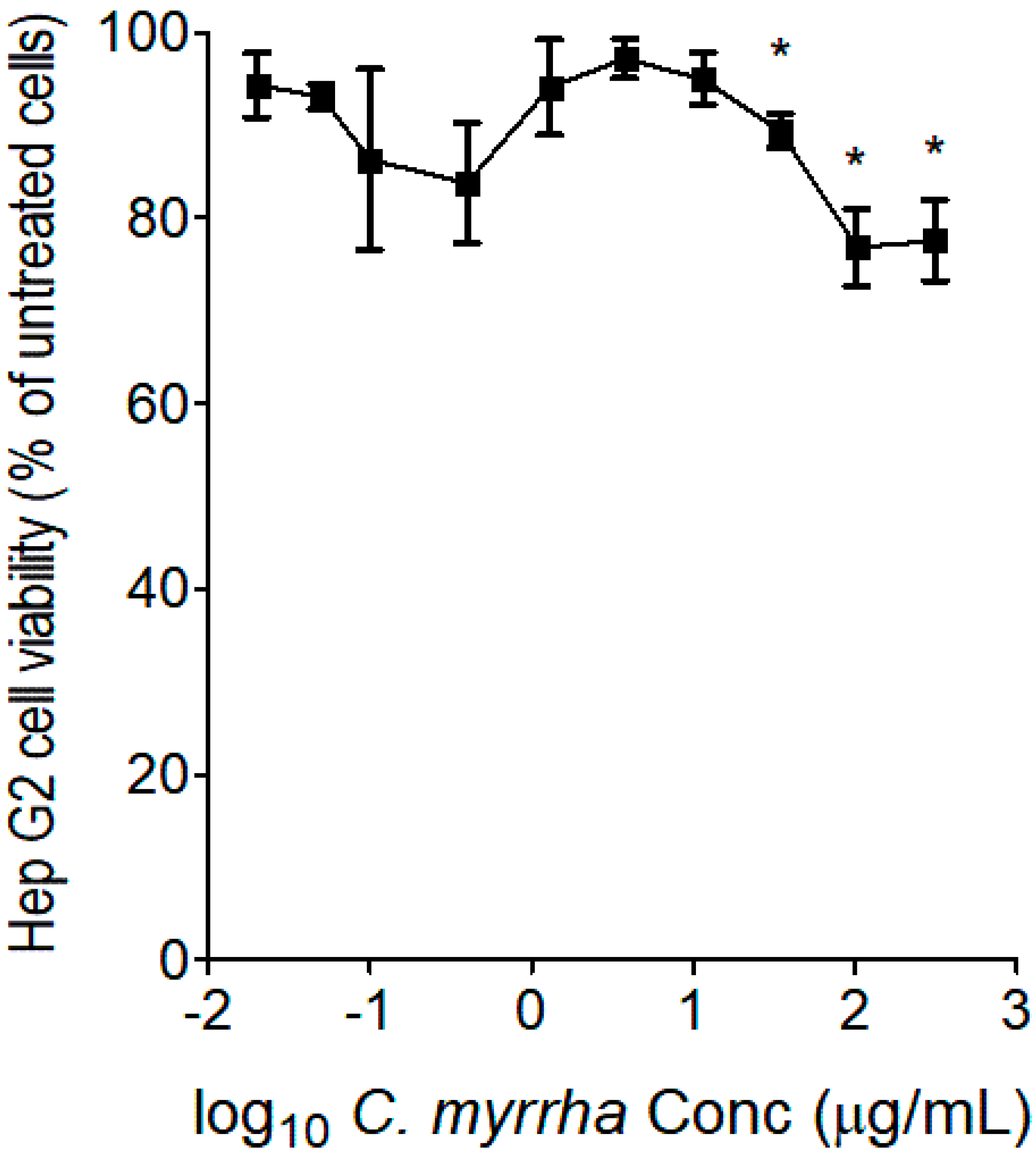
C. myrrha resin aqueous extract concentrations using the MTT assay to select non-toxic resin extract concentrations for subsequent CYP gene and protein expression level evaluations. Based on the concentration-cell viability inhibition slope shown in
Figure 1, we selected concentrations of 10.0 µg/mL of
C. myrrha resin extract and less for subsequent experiments because no inhibition of proliferation was observed at these extract concentrations. Furthermore, the selected non-toxic
C. myrrha resin aqueous extract concentrations were also considered clinically relevant, as the typical dose of consumption by patients was 3-5 g of
C. myrrha resins for 2 to 3 consecutive days [
27].
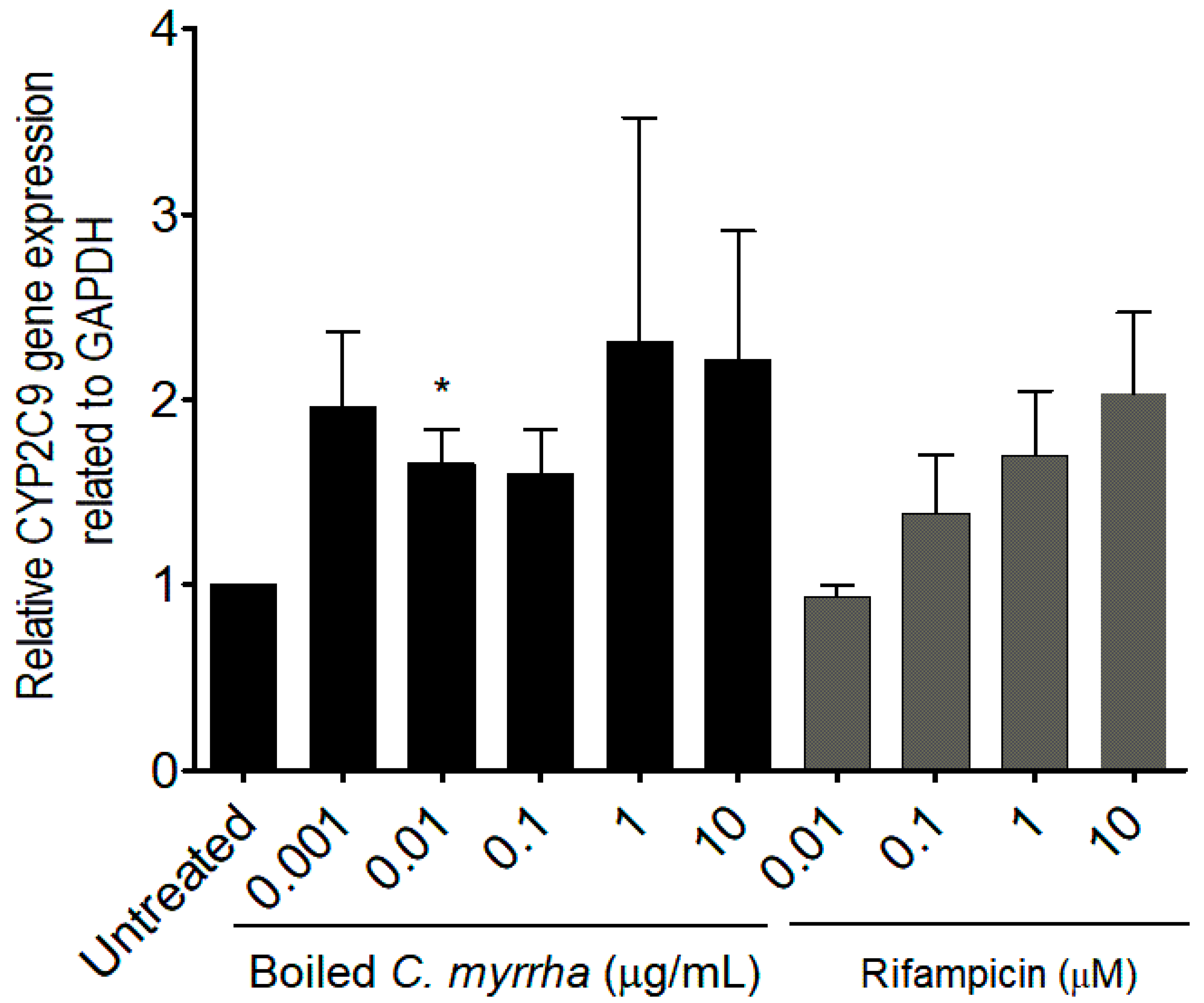
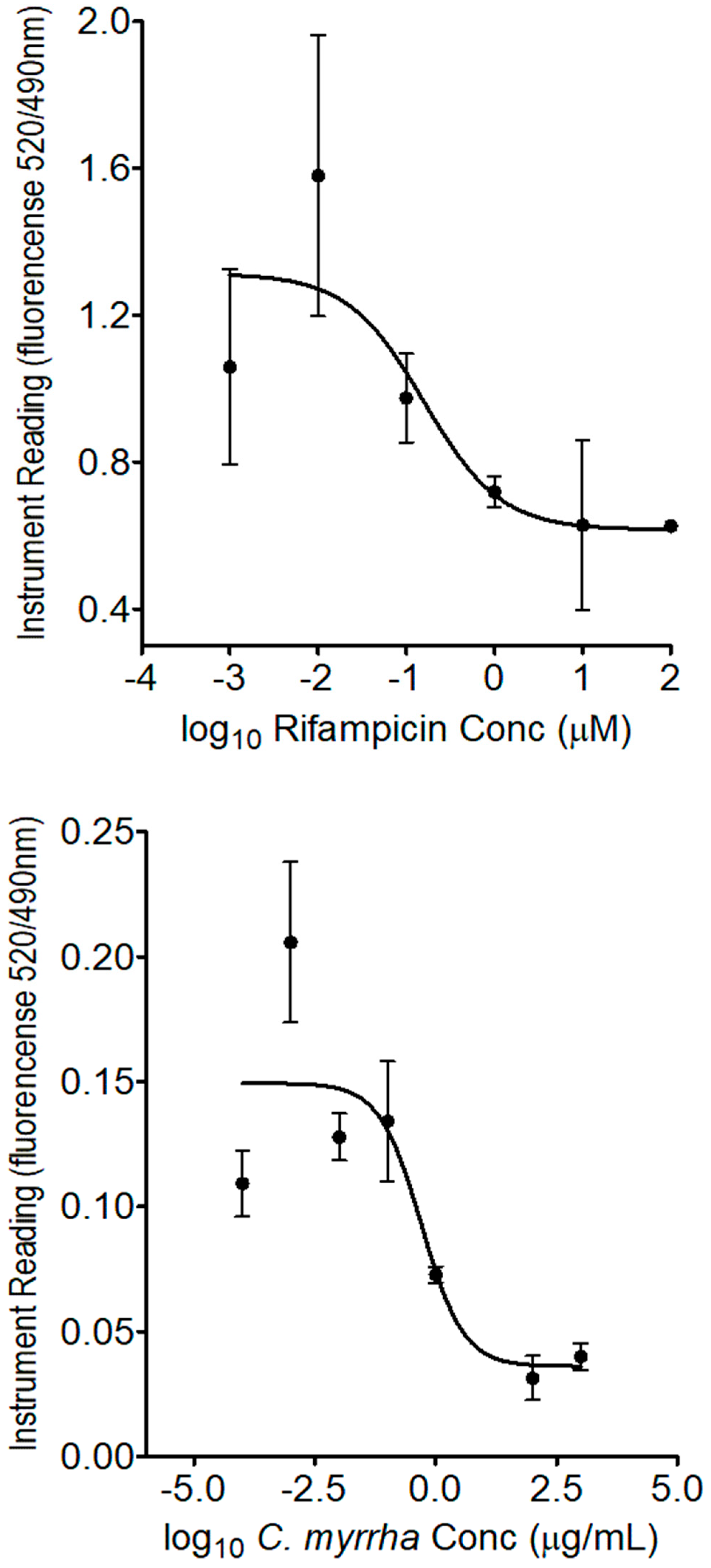
According to the United States Food and Drug Administration (USFDA) guidance for clinical drug interaction studies (
www.fda.gov/regulatory-information/search-fda-guidance-documents/in-vitro-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions), evaluation of CYP gene and protein expression levels must be performed using a negative control (
i.e., untreated control) and a positive control (
i.e., rifampicin) [
28]. The guideline states that the typical
in vitro induction fold change for CYP 2C9 after treatment of cells with rifampicin, at a concentration of 10.0 µM, is approximately 2.0 times the basal CYP 2C9 enzyme gene expression level detected in the negative control [
28]. Based on CYP gene expression monitoring using qPCR, the average induction observed in this study was found to be a 2.0-fold change, indicating that the configuration of the experiment was adapted to the potential detection of CYP 2C9 activity induced by the tested extract. The qPCR results showed an induction of CYP 2C9 isoenzyme in a dose-dependent manner exceeding 2.0-fold change compared to the untreated control. According to USFDA Guidance [
28], the boiled
C. myrrha resin aqueous extract is considered an inducer of CYP 2C9, because the expression in boiled
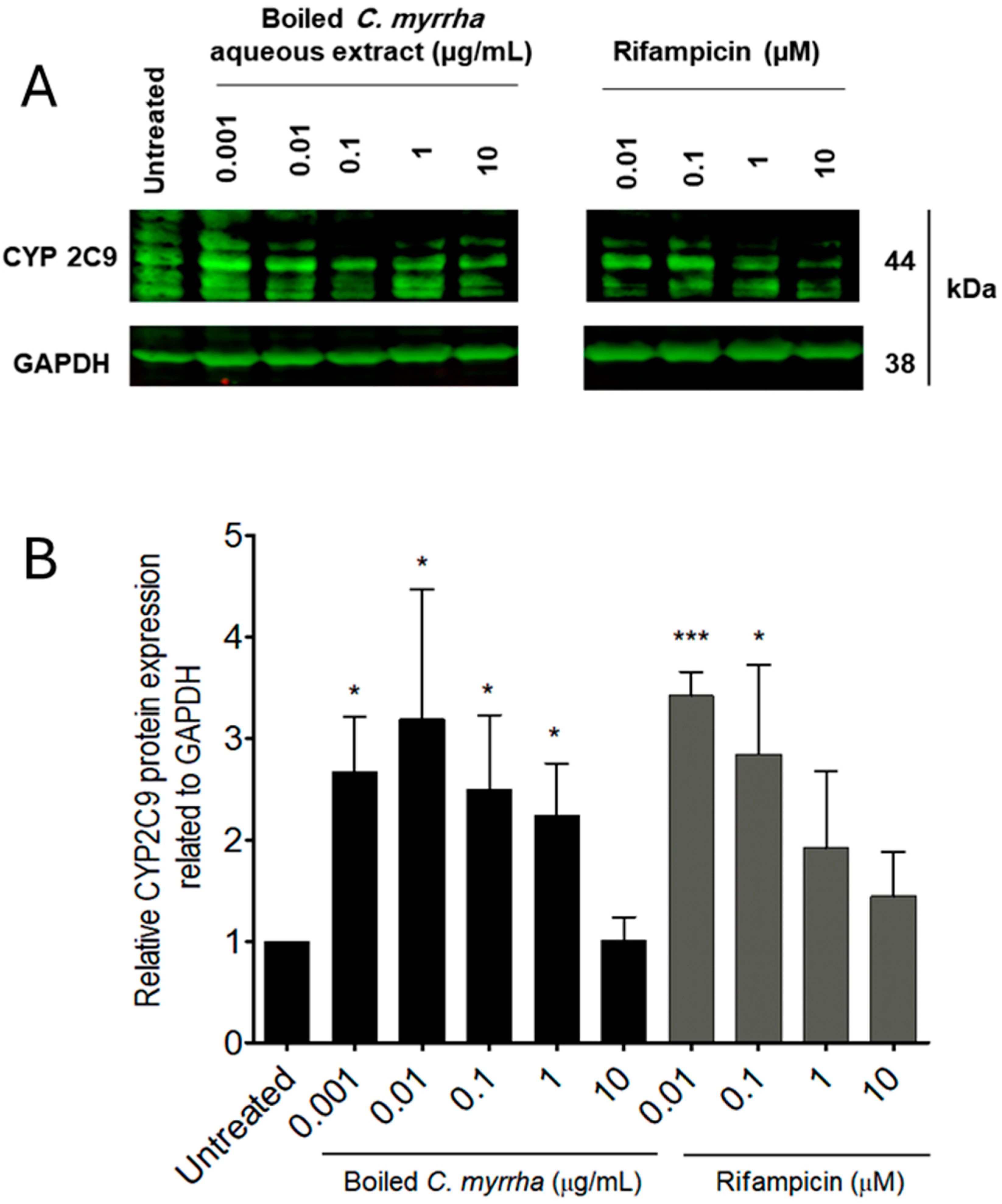
C. myrrha resin aqueous extract-treated cells exceeds the 2.0-fold change cut-off compared to untreated cells. Using Western blot analysis, CYP 2C9 protein expression was shown to be induced in cells treated with
C. myrrha resin aqueous extract, exceeding a 3.0-fold change compared to the basal level detected in untreated cells. Thus, modulation of CYP 2C9 expression level was observed at both transcriptional and translational levels. Once modulation of the CYP 2C9 gene and protein expression was confirmed, we attempted to determine whether the boiled aqueous extract of
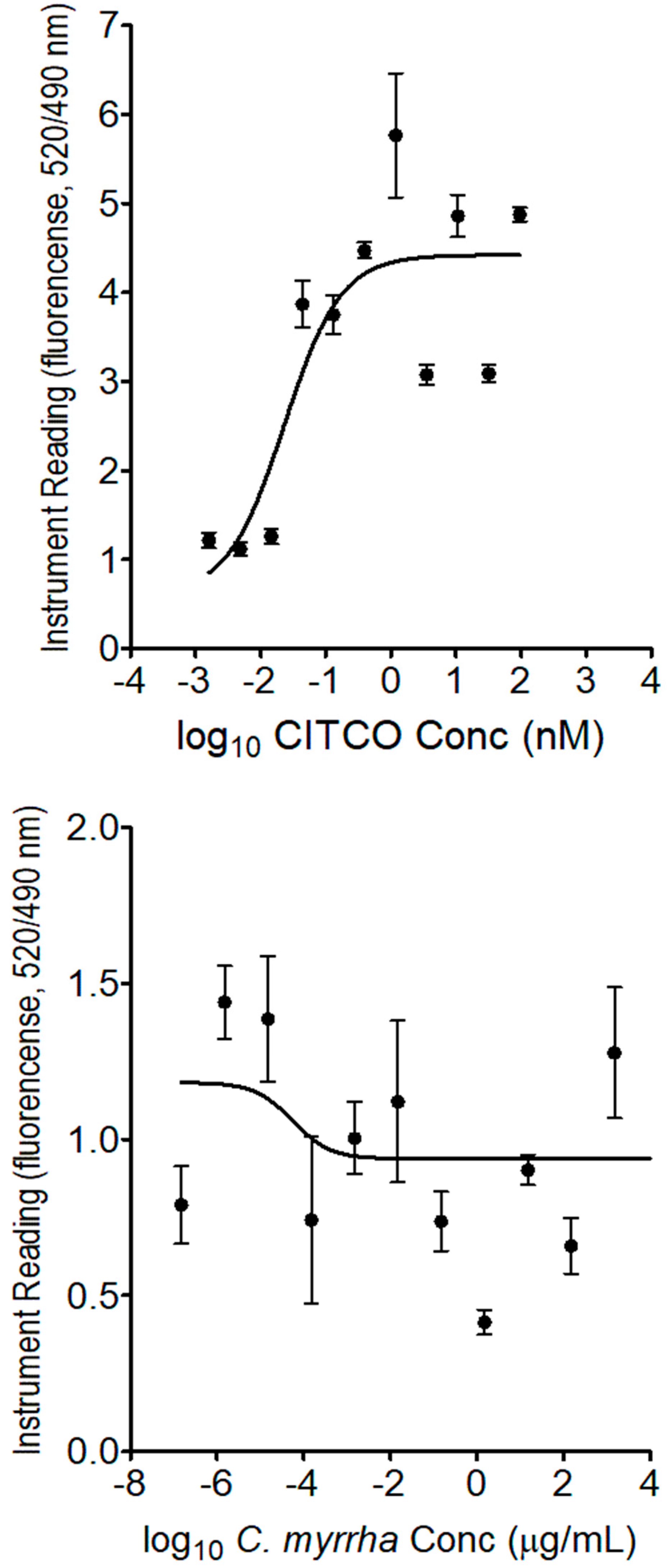
C. myrrha contains ligands for the nuclear receptors PXR and CAR. Both PXR and CAR are considered to be the main nuclear receptors involved in drug metabolism induction mediated by CYP enzymes [
29]. Based on our findings using the receptor binding assays, the
C. myrrha extract does not contain ligands for the nuclear receptor CAR. In contrast, the results for the PXR competitive binding kit indicate that the
C. myrrha extract contained ligand(s) for the nuclear receptor PXR. It is noteworthy that induction of human CYP 2C enzymes, including 2C9, was found to be primarily regulated by PXR activation rather than CAR [
15,
30].
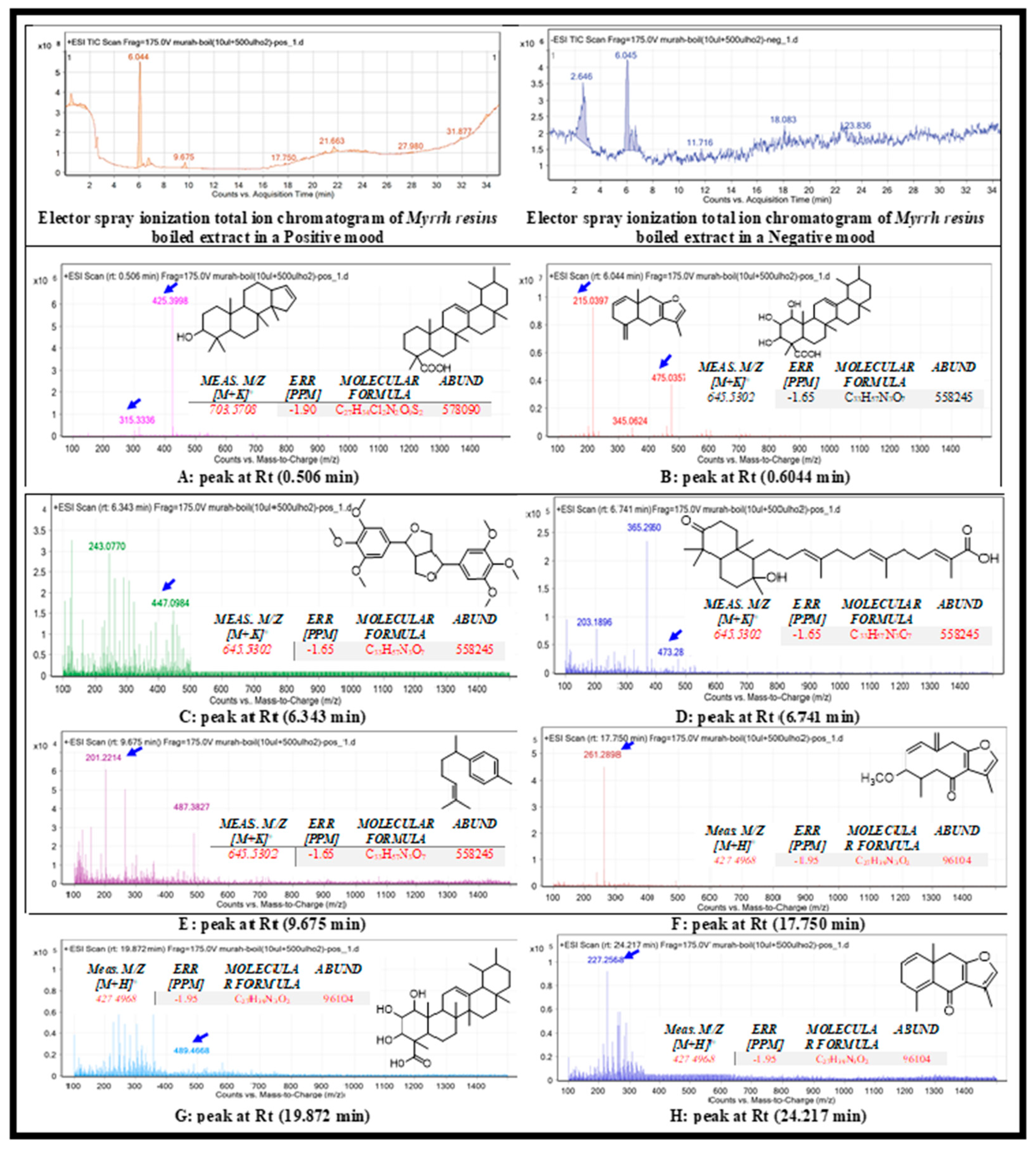
The chemical features of the boiled C. myrrha resin aqueous extract were extracted from the mass spectrometry data using the MFE algorithm and recursive analysis workflow as mentioned above. The following information is detailed for the provisional identification of C. myrrha metabolites as follows:
ElectroSpray Ionisation (ESI) scanning A: The appeared
m/z value at retention time (0.506 min) were correlated with the compound mansumbinol [
17], with
m/z [M + H]+ 315.3336 daltons and molecular formula of [C22H36O]+, in positive ion mode, and [M - H]- with
m/z 313.163 daltons in negative mode, indicating that the compound has a molecular weight of 315.521 g mol-1. The appeared
m/z value at retention time (0.506 min) were correlated with the compound methyl esters of commic acids [
21], with
m/z [M + H]+ 425.3998 daltons and molecular formula of [C29H46O2]+, in positive ion mode, and [M - H]- with
m/z 423.268 daltons in negative mode, indicating that the compound has a molecular weight of 424.350 g mol-1.
ESI scanning B: The appeared
m/z value at retention time (0.6044 min) were correlated with the compound lindestrene [
18], with
m/z [M + H]+ 215.397 daltons and molecular formula of [C15H18O]+, in positive ion mode, and [M - H]- with m/z 214.277 daltons in negative mode, indicating that the compound has a molecular weight of 214.303 g mol-1. The appeared
m/z value at retention time (0.6044 min) were correlated with the compound methyl esters of commic acids [
21], with
m/z [M + H]+ 475.0357 daltons and molecular formula of [C29H46O5]+, in positive ion mode, and [M - H]- with
m/z 473.268 daltons in negative mode, indicating that the compound has a molecular weight of 474.673 g mol-1.
ESI scanning C: The appeared
m/z value at retention time (6.343 min) were correlated with the compound Diayangambin [
19], with
m/z [M + H]+ 447.0984 daltons and molecular formula of [C24H30O8]+, in positive ion mode, and [M - H]- with
m/z 445.357 daltons in negative mode, indicating that the compound has a molecular weight of 446.490 g mol-1.
ESI scanning D: The appeared
m/z value at retention time (6.741 min) were correlated with the compound Myrrhanone B [
19], with
m/z [M + H]+ 473.2819 daltons and molecular formula of [C30H48O4]+, in positive ion mode, and [M - H]- with
m/z 471.517 daltons in negative mode, indicating that the compound has a molecular weight of 472.700 g mol-1.
ESI scanning E: The appeared
m/z value at retention time (9.675 min) were correlated with the compound α-bisabolene [
20], with
m/z [M + H]+ 201.2214 daltons and molecular formula of [C15H22O8]+, in positive ion mode, and [M - H]- with
m/z 199.137 daltons in negative mode, indicating that the compound has a molecular weight of 200.335 g mol-1.
ESI scanning F: The appeared
m/z value at retention time (17.750 min) were correlated with the compound Furanogermacrens [
18], with
m/z [M + H]+ 447.0984 daltons and molecular formula of [C16H20O3]+, in positive ion mode, and [M - H]- with
m/z 259.341 daltons in negative mode, indicating that the compound has a molecular weight of 260.328 g mol-1.
ESI scanning G: The appeared
m/z value at retention time (19.875 min) were correlated with the compound Commic acid E [
21], with
m/z [M + H]+ 488.450 daltons and molecular formula of [C30H48O5]+, in positive ion mode, and [M - H]- with
m/z 487.303 daltons in negative mode, indicating that the compound has a molecular weight of 488.699 g mol-1.
ESI scanning H: The appeared
m/z value at retention time (19.875 min) were correlated with the compound Furanoeudesma-1,4-dien-6-one [
18], with m/z [M + H]+ 226.118 daltons and molecular formula of [C15H16O2]+, in positive ion mode, and [M - H]- with
m/z 225.231 daltons in negative mode, indicating that the compound has a molecular weight of 226.286 g mol-1.
It has been reported that a few metabolites present in
C. myrrha resin could potentially interfere with drug metabolism, resulting in increased or decreased drug concentrations, leading to the adverse drug reactions as mentioned previously [
12,
13,
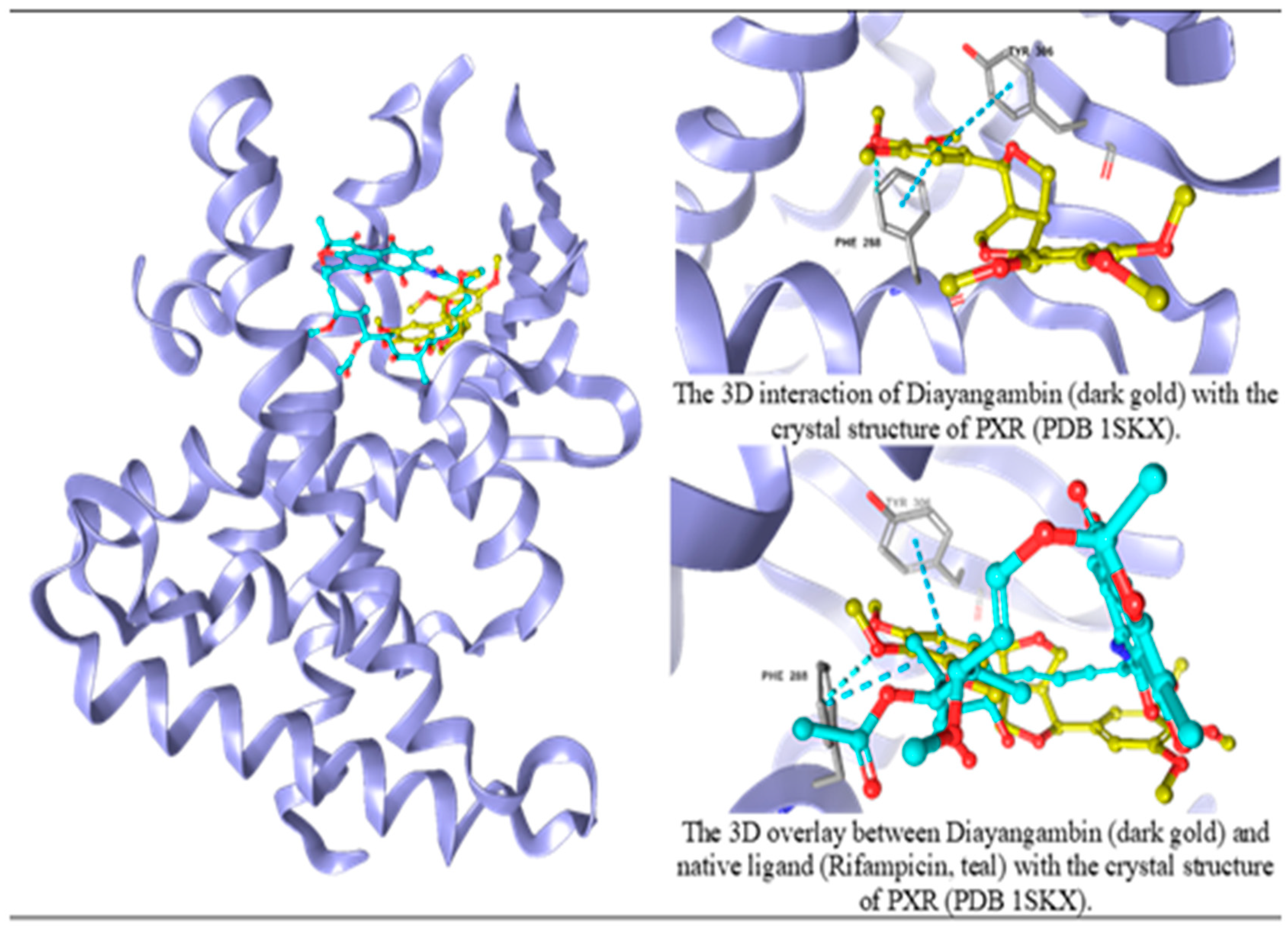
31]. One of the most active secondary metabolites found in
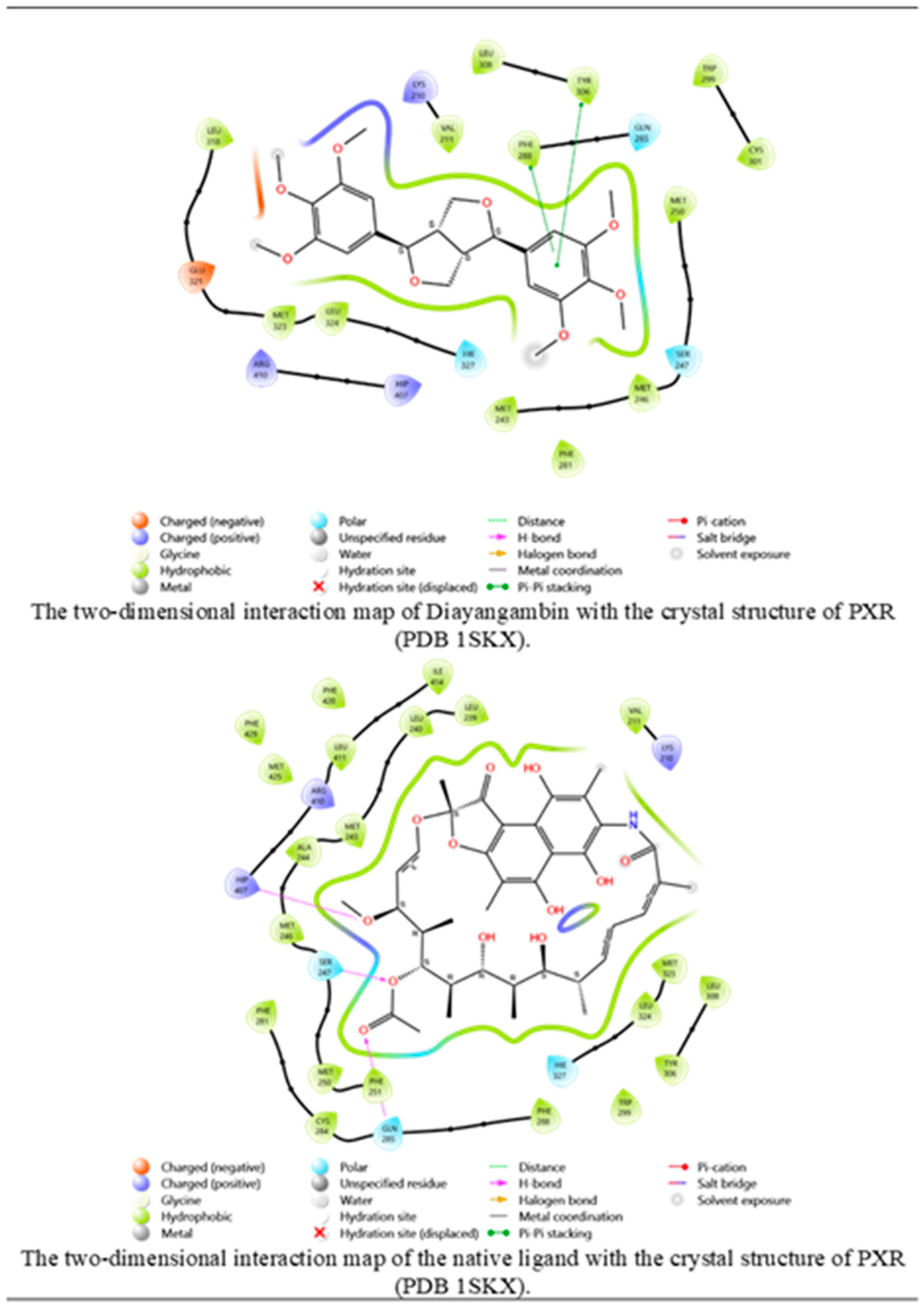
C. myrrha is diayangambin, a furofuran lignin that exhibits anti-inflammatory activity and immunomodulation effects
in vitro and
in vivo [
32]. The present study suggests, for the first time, that diayangambin has the potential to induce CYP 2C9 isoenzyme through modulation of PXR. PXR and CAR binding assays and molecular docking analysis suggest that diayangambin may act as a PXR agonist, thereby leading to upregulation of CYP 2C9, the major drug-metabolizing enzyme. This study extends previously reported findings related to
C. myrrh extract-induced CYP 2C9 expression by providing insights into the potential molecular mechanisms underlying the interaction between
C. myrrh metabolites and the nuclear receptor PXR. Further studies are needed to confirm the
in vivo effects of diayangambin and determine its clinical relevance. However, our results provide a strong foundation for future research in this area. Of note, diayangambin, the main lignan, is distributed in numerous medicinal plant species, including the kawakawa leaves (
Macropiper excelsum Miq.) [
33,
34],
Piper arborescens Roxb. [
35], the Texas Sage (
Leucophyllum frutescens (Berland.) I.M. Johnst.) [
36],
Piper fimbriulatum C.DC. [
37], and
Mitrephora winitii Craib [
38], to name just a few. Thus, caution is recommended in patients consuming diayangambin-rich natural products concomitantly with pharmaceutical drugs mainly metabolized by CYP enzymes, particularly the CYP 2C9 enzyme.
4. Materials and methods
4.1. Chemicals and materials
Dulbecco's modified Eagle medium (DMEM) plus GlutaMax-1 (4.5g/L D-glucose, 25 mM HEPES, pyruvate), fetal bovine serum (FBS), TrypLE™ Express and Dulbecco's phosphate-buffered saline (PBS) were provided from Gibco® (Waltham, MA). Rifampicin (USP grade, ≥ 97%) was obtained from UFC Biotechnology Fine Chemicals (Amherst, NY). NP40 cell lysis buffer was purchased from Invitrogen (Thermo Fisher Scientific, Carlsbad, CA). Purified carbon dioxide (CO2) gas was provided by Saudi Industrial Gas (Dammam, Saudi Arabia). Ultrapure water was produced using a Millipore (Billerica, MA) system with a resistivity reading of 18.2 MΩ·cm at 25°C.
4.2. Source of C. myrrha resins
Resins were purchased from Boswellness (Colchester, VT) under product number 10018 and lot number CM000105. The herb was certified free of organics, pesticides and preservatives as the resins were wildcrafted in the Federal Republic of Somalia by experts. The resins were stored in a cool, dark place until extraction. The remaining resins were kept for future reference if needed.
4.3. Extraction of C. myrrha resins
Prior to the extraction, C. myrrha resins were finely powdered using an electric grinder. Using the boiling water extraction method, approximately 500 mg of resin powder was mixed with 10 mL of ultrapure water and boiled until half of the volume was evaporated. The extracts were filtered using a Sartorius stedim biotech (Göttingen, Germany) ashless quantitative filter paper and dried in a Thermo Scientific Savant SPD2010 SpeedVac Concentrator until completely dry. The dried pellets were weighted and reconstituted with 1 mL of ultrapure water by vortexing until completely dissolved. The reconstituted extracts were stored at a cool temperature in the dark until use.
4.4. Endotoxin Limulus Amebocyte Lysate (LAL) assay
Endotoxin contamination levels in C. myrrha boiled aqueous extracts were measured using the Thermo Scientific Pierce™ Chromogenic Endotoxin Quant kit (#A39553) according to the manufacturer’s instructions.
4.5. Hep G2 cell culture and treatment
The human hepatocarcinoma cell line Hep G2 (#HB-8065), derived from a fifteen-year-old Caucasian adolescent male patient diagnosed with hepatocellular carcinoma, was originally purchased from the American Type Culture Collection (Manassas, VA). Cells were cultured in complete DMEM Glutamax-I medium containing 10% (v/v) heat-inactivated FBS, 1% (v/v) penicillin G (100 IU/mL), and streptomycin (100 µg/mL) and incubated at 37˚C in 5% CO2 and 95% relative humidity. The culture medium was changed every 2 to 3 days, and the cells were subcultured when the cell population reached 70 to 80% confluence. Cells were seeded at different densities depending on each experimental design requirement. For the cell viability assay, C. myrrha boiled aqueous extracts were tested at concentrations ranging from 0.05 to 300 µg dry weight per mL. For gene and protein expression level determination, Hep G2 cells were treated with different concentrations of resin extracts ranging from 0.001 to 10 µg/mL. Rifampicin concentrations ranging from 0.001 to 10 µM were used as a positive control for induction of CYP 2C9 expression.
4.6. Hep G2 cell viability inhibition assay
The biological effect of boiled aqueous extract of C. myrrha resins on Hep G2 cell viability was determined using a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay-based cell growth determination kit (#CGD-1, Sigma-Aldrich, St. Louis, MO). Briefly, previously seeded (1 × 104 cells/well) in 96-well plates, treated and untreated Hep G2 cells were incubated for 48 hrs, then 5% MTT solution was added for 3 hrs at 37°C. The MTT solution was replaced with an equal volume of DMSO. The plates were shaken for 45 min at room temperature. Absorbance was measured at 560 nm using a BioTek Instruments Universal Microplate Reader ELx800 counter (Winooski, VT).
4.7. RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Untreated and treated Hep G2 cells (2 × 10
5 cells/ well) were washed with PBS and harvested using TrypLE™ Express solution. Cells were transferred to a 1.5 mL tube and centrifuged at 15,000 rpm for 5 min to obtain pellets, which were stored at -20°C until use. Whole RNA was extracted from treated and untreated cell pellets using the Qiagen (Hilden, Germany) RNeasy Plus Mini kit (#74106) according to the manufacturer’s instructions. The method used was based on El-Gendy and El-Kadi [
39] with modifications [
13]. Briefly, complementary DNA (cDNA) at 200.0 ng per well was first produced from the whole RNA extract
via reverse transcription using the Applied Biosystems
™ (Waltham, MA) High-Capacity cDNA Reverse Transcription kit (#4368813). Quantitative real-time PCR was performed using a Thermo Fisher Scientific SYBR Green PCR Master Mix kit (#4309155) and carried out on the Applied Biosystems
™ 7900 real-time PCR system. For each analysis, a negative control was prepared using all reagents except the cDNA template. The primer sequences used for CYP 2C9 are as follows: 5ʹ-GACAGAAAGGACAAAGAGGAAGAGG-3ʹ (forward) and 5ʹ-GTTATAGAGAGCTGAGATCACACCACTG-3ʹ (reverse). The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), with primer sequences as follows: 5ʹ-CCAGGGCTGCTTTTAACTCTGG-3ʹ (forward) and 5ʹ-CCAGTGGACTCCACGACGTAC-3ʹ (reverse), was used as an internal control. The pair primers were used at a concentration of 10.0 pmol/µL.
4.8. Protein extraction and Western Blot technology
Hep G2 cells (3 × 10
5 cells) were washed twice with PBS and 80.0 µL of NP40 lysis buffer were added to the cell pellets and incubated for 30 min on ice with repeated vortexing at 5-10 min intervals, resulting in protein extraction. The cell lysate was transferred to a 1.5 mL tube and centrifuged at 13,000 rpm at 4°C for 10 min. The supernatant containing the whole cell lysate was transferred to a 1.5 mL tube and stored at -80°C until use. Protein quantity was measured using the Molecular Probes
™ Life Technologies (Eugene, OR) Qubit Protein Assay kit (#Q33212) as per the manufacturer’s instructions. The method used in this study was based on Alehaideb et al. [
13] with slight modifications. Briefly, protein samples, at 120.0 µg per well, were separated on 12% sodium dodecyl sulfate–polyacrylamide gels and electro-blotted to a 0.45 µm polyvinylidene difluoride (PVDF) transfer membrane (#88518, Thermo Fisher). Primary mouse monoclonal anti-CYP2C9 (#MA5-17064, Invitrogen) and anti-GAPDH (#AM4300, Invitrogen) antibodies were used as probes for staining PVDF membranes. The housekeeping protein GAPDH was used as a loading control. The IRDye
® goat anti-mouse secondary antibody (#926-32210, LI-COR Biosciences, Lincolin, NE) was used for detection on the LI-COR Odyssey imaging system and measurement of CYP 2C9 and GAPDH expressions. In all gels, a LI-COR Chameleon Due Pre-Stained Protein Ladder (#928-60000) was included.
4.9. PXR competitive-binding assay
Binding affinity of C. myrrha resin constituent mixture with PXR was performed using the Thermo Scientific LanthaScreen™ TR-FRET PXR (SXR) Competitive-Binding Assay Kit (# PV4839) according to the manufacturer’s instructions.
4.10. Human CAR Coactivator Assay
Binding of phytochemical(s) from C. myrrha resin aqueous extracts were determined using the LanthaScreen™ TR-FRET Constitutive Androstane Receptor Coactivator Assay kit (# PV4836, Thermo Scientific) as per the manufacturer’s instructions.
4.11. Metabolite identification by liquid chromatography and time-of-flight (LC-QTOF)
The separation method and attempted identification of the boiled
C. myrrha resin aqueous extract-derived metabolites were performed using an Agilent 1260 Infinity high-performance liquid chromatography system coupled to 6530 Q-TOF (Agilent Technologies, Santa Clara, CA) as described in Alehaideb et al. [
40]. Briefly, separation was performed using an Agilent SB-C18 column (4.6 mm × 150 mm, 1.8 μm particle size) with the programmed elution gradient: 0 to 2 min, 5% B; 2 to 17 min, 5 to 100% B; 17 to 21 min, 95% B; 21 to 25 min, 5% B, using mobile-phase A (0.1% formic acid in water) and mobile-phase B (0.1% formic acid in methanol). The injection volume was 10 μL, and the flow rate was set at 250 μL/min. The gas temperature was set at 300℃, the gas flow at 8 L/min, the nebulizer pressure at 35 psi, the sheath gas flow rate at 11 L/min with temperature at 350℃, and the scanning range was set from 50 to 800 mass-to-charge (
m/z). The data was generated by the Agilent MassHunter (version B.06.00) analysis software.
4.12. PXR docking studies
To investigate the potential interference of the boiled C. myrrha aqueous extract-derived secondary metabolites with PXR crystal structure, we obtained the 3D crystal structure of PXR (Protein Data Bank (PDB) 1SKX) from the Research Collaboratory for Structural Bioinformatics PDB. The protein structure was refined, minimized, and optimized using the OPLS4 force field with the Protein Preparation Wizard tool (PrepWizard, Schrödinger Release 2023-2: GLIDE, Schrödinger, LLC, New York, NY). The chemical structures of the identified secondary metabolites were prepared using Schrödinger's LigPrep tool, generating several optimized and minimized conformations for their lowest energy conformation. Molecular docking analysis was performed using the (standard precision) and (extra precision) scoring functions of GLIDE, followed by post-docking analysis for docked poses.
4.13. Data analysis and statistics
Using RT-qPCR assay, gene expression values were normalized to GAPDH and considered positive for induction or suppression if exceeding the 2-fold change relative to untreated cells, the control (
i.e., 2.0 or 0.5-fold cut-off, respectively) (USFDA, 2020). For Western blot analysis, the protein expression level detected in treated cells was measured and then related to the basal protein expression level detected in untreated cells using ImageJ software (
http://rsbweb.nih.gov/ij/index.html). For PXR receptor binding assays, IC
50 values were estimated based on plotting log
10 values of dry extract weight of
C. myrrha versus the time-resolved fluorescence resonance energy transfer (TR-FRET) emission ratio (
i.e., 520/490 nm) using the sigmoidal dose-response model in GraphPad Prism (San Diego, CA) software version 5.04. For the CAR coactivator binding assay, the EC
50 values were estimated based on the plot of log
10 values of
C. myrrha dry extract weight versus TR-FRET emission ratio using the agonist-response (three parameters) model in GraphPad Prism software. The results of this study are expressed as mean ± SD from three independent experiments. A student’s unpaired two-tailed
t-test was employed to determine the statistical significance of treatment compared to untreated controls.
Author Contributions
Conceptualization, A.S. and Z.E.; Methodology, Z.E., A.K, and S.M.; Software, S.G., R.S., S.T., A.S., and B.H.; Validation, A.S., B.H., J.M., and Z.E.; Formal Analysis, A.S., S.T., and Z.E.; Investigation, A.S., Z.E., S.G., R.S., S.T., F.M., B.H., and J.M.; Resources, Z.E., M.B., and S.M.; Data Curation, A.S. and Z.E.; Writing – Original Draft Preparation, A.S., Z.E., and S.M.; Writing – Review & Editing, A.T., M.B., A.K., and S.M.; Visualization, A.S., J.M., and Z.E.; Supervision, Z.E., A.K., M.B., and S.M.; Project Administration, Z.E. and M.B.; Funding Acquisition, Z.E. and M.B.
Abbreviations
CAR, constitutive androstane receptor; cDNA, complementary deoxyribonucleic acid; C. myrrha, Commiphora myrrha; CO2, carbon dioxide; CYP, Cytochrome P-450; DMEM, Dulbecco's modified Eagle medium; EC50, half-maximal effective concentration; ESI, electrospray ionisation; EU, endotoxin unit; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HDI, herb-drug interactions; IC50; half-maximal inhibitory concentration; LAL, limulus amebocyte lysate; LC-QTOF, liquid chromatography-mass spectrometry time-of-flight; log10, logarithm base 10; MFE, molecular feature extraction; MTT, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; m/z, mass to charge; NHP, natural health product; PBS, phosphate-buffered saline; PDB, protein data bank; PVDF, polyvinylidene difluoride; PXR, pregnane X receptor; RCSB, Research Collaboratory for Structural Bioinformatics; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; SD, standard deviation; SP, standard precision; TR-FRET, Time-Resolved Fluorescence Resonance Energy Transfer; USFDA, United States Food and Drug Administration; XP, extra precision