Submitted:
05 September 2024
Posted:
06 September 2024
You are already at the latest version
Abstract
Keywords:
Introduction
References
- Watson, J.; Crick, F. Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid. Nature 1953, 171, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, N. The diachronic presence of cancer on Planet Earth. PALAEO-ONCOLOGY: Historical Review of Cancer from Antiquity to the 21st century. Proceedings of the 2nd International Symposium of the European Society for the History of Oncology. Research Centre for the Hellenic and Latin Literature. Academy of Athens, Greece, 2020; pp. 21–33, ISBN 978-960-404-367-5 (In Greek).
- Parisi, F.; Aurisicchio, L.; Pecorari, A.; Poli, A.; Millanta, F. A Preliminary Evaluation of the Prognostic Role of HER-2 and HER-3 Immunohistochemical Expression in Canine Melanomas ANIMALS 2024, 14. [CrossRef]
- Henriques, R.; Borge, L.; Horvath, B.; Magyar, Z. Balancing act: matching growth with environment by the TOR signalling Pathway. J Exp Bot. 2014, 65, 2691–2701. [Google Scholar] [CrossRef] [PubMed]
- Yang Xiong; and Jen Sheen. The role of target of Rapamycin Signalling Networks in Plant Growth and metabolism. Plant Physiology February 2014, 164, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Ghalioungui, P. Malignancy in Ancient Egypt. In Palaeo-Oncology. The Antiquity of Cancer; Retsas, S., Ed.; Farrand Press: London, UK, 1986; pp. 27–33. ISBN 1-85083 006 1. [Google Scholar]
- Fawzi Sweha Boulos. Oncology in Egyptian Papyri. In Palaeo-Oncology. The Antiquity of Cancer. Retsas, S. Ed.; Farrand Press, London, UK, 1986, pp.35-40, ISBN 1-85083 006 1.
- Shan, Jiang. Naming, Explanations, Treatments: Knowledge of Tumour from Chinese Classical Era. Presented at An International Colloquium on PALAEO-ONCOLOGY, The Antiquity of Cancer, organised By the European Society for the History of Oncology and The Hellenic Medical Society – UK. November 16, 2018. Abs. No 8. At the Hellenic Centre, London, U.K. An ESO recommended Event. 16 November 2018.
- Kunzru, K.M.N.; Bhat, R. Tumours in Ancient India: A reading of Suśrutasamhitã. PALAEO-ONCOLOGY: Historical Review of Cancer from Antiquity to the 21st century. Proceedings of the 2nd International Symposium of the European Society for the History of Oncology. Research Centre for the Hellenic and Latin Literature. Academy of Athens, Greece, 2020; pp. 107–122, ISBN 978-960-404-367-5.
- Retsas, S. On the Antiquity of Cancer; from Hippocrates to Galen. In Palaeo-Oncology. The Antiquity of Cancer. Retsas, S. Ed.; Farrand Press, London, UK, 1986, pp.41-53, ISBN 1-85083 006 1.
- Kouzis, A. CANCER AND THE ANCIENT GREEK PHYSICIANS. (O ΚAΡΚΙΝOΣ ΠAΡA ΤOΙΣ AΡΧAΙOΙΣ ΕΛΛHΣΙΝ ΙAΤΡOΙΣ). Athens Medical Society, 1902. (In Greek).
- Retsas, S. Palaeo-Oncology: Diachronic perceptions of the meaning of the term Metastasis. PALAEO-ONCOLOGY: Historical Review of Cancer from Antiquity to the 21st century. Proceedings of the 2nd International Symposium of the European Society for the History of Oncology. Research Centre for the Hellenic and Latin Literature. Academy of Athens, Greece, 2020; pp. 93–106, ISBN 978-960-404-367-5 (in Greek).
- Retsas, S. Cancer and the arts: metastasis - as perceived through the ages. ESMO Open 2017, 2, e000226. [Google Scholar] [CrossRef]
- Ashworth, T.R. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Australian Medical Journal 1869, 14, 146–147. [Google Scholar]
- Gage, H. Cancer of the Colon. Boston Medical and Surgical Journal. 1911, CLXV, 474–479. [Google Scholar] [CrossRef]
- Retsas, S. COVID-19 and cancer: Revisiting "The comfort zone". Hell J Nucl Med. 2020, 23, 65–69. [Google Scholar] [PubMed]
- Ringborg, U. The Radiumhemmet – A Historic Centre for Cancer Research and Treatment. PALAEO-ONCOLOGY: Historical Review of Cancer from Antiquity to the 21st century. Proceedings of the 2nd International Symposium of the European Society for the History of Oncology. Research Centre for the Hellenic and Latin Literature. Academy of Athens, Greece, 2020; pp. 157–168, ISBN 978-960-404-367-5.
- DeVita, V.T., Jr.; Jaffe, E.S.; Hellman, S. Hodgkin’s Disease and the Non-Hodgkin’s Lymphomas. Chapter 44. In Cancer Principles and Practice of Oncology.; DeVita, V.T. Jr., Rosenberg, S.A., Hellman, S. Eds.; J.B. Lippincott Company Philadelphia. 1985. Second Edition, pp 1623-1709. ISBN 0-397-50632-5.
- Chin, A.C. Methotrexate for gestational choriocarcinoma: a paradigm shift in oncology. Nat Rev Endocrinol 2023, 19, 501. [Google Scholar] [CrossRef]
- Powell RG, Weisleder D, Smith CR Jr, Rohwedder WK. Structures of harringtonine, isoharringtonine, and homoharringtonine. Tetrahedron Lett. 1970, 815–818. [CrossRef] [PubMed]
- Monneret, C. Platinum anticancer drugs. From serendipity to rational design. Ann Pharm Fr. 2011, 69, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Sagar SM, Bayliss MA, Chong SL, Retsas, S. Ondansetron; further progress in the prevention of nausea and vomiting induced by anti-cancer chemotherapy. Clin Oncol (R Coll Radiol). 1991, 3, 183. [CrossRef] [PubMed]
- Retsas, S.; Bayliss, M.; Sheikh, N.; Chong, S.L.; Bafaloukos, D. Chemotherapy of malignant melanoma: The European Experience. La Revue de Médicine Interne Mars-Avril 1990 51-58 – Tome XI (Supplément au Numéro 2).
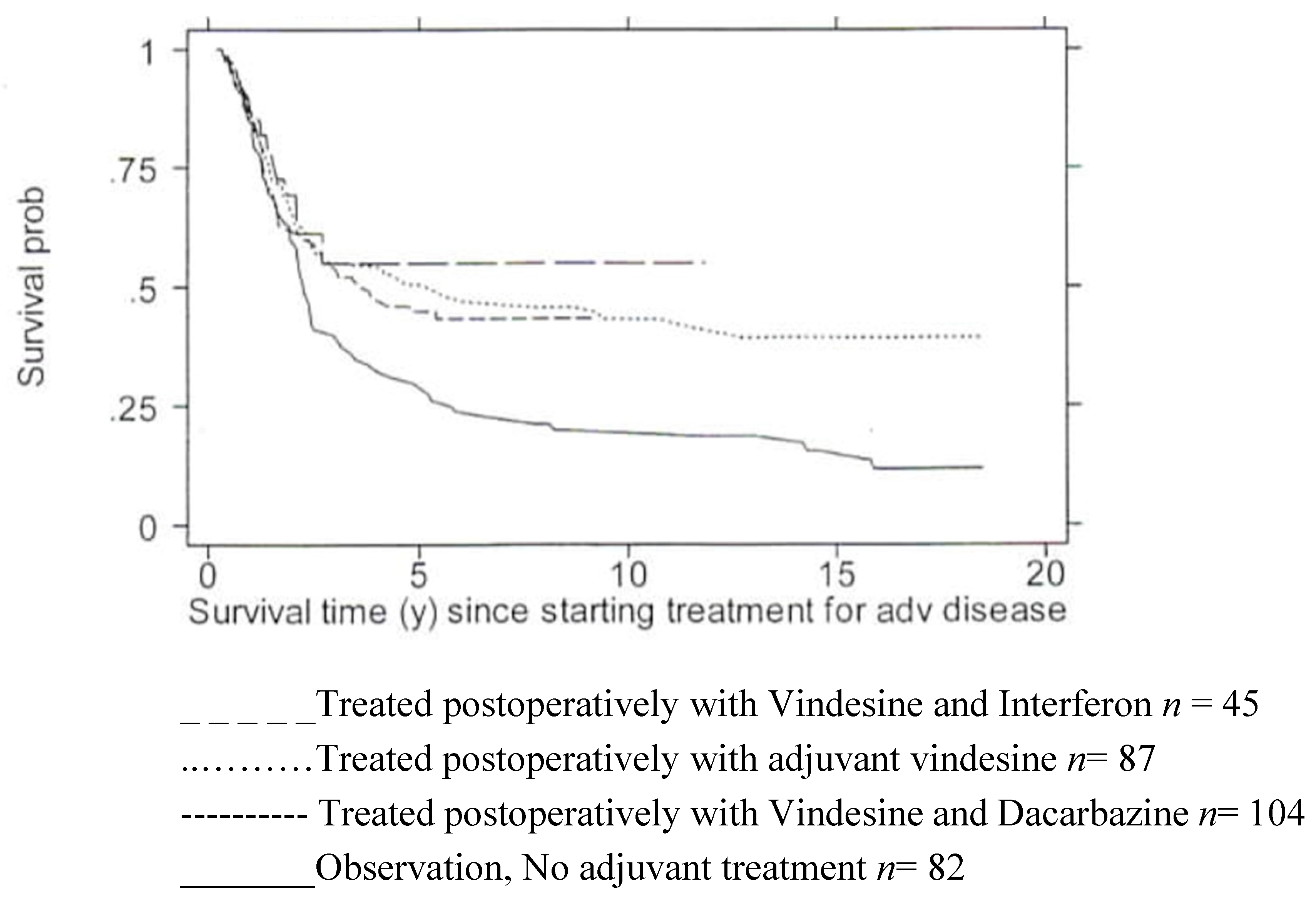
- Retsas, S.; MacRae, K.; Henry, K. Adjuvant Treatment for clinically apparent lymph node metastases (≥N1b) from melanoma: single-institution experience from a cohort of 318 patients. Abstract 26; Melanoma Research – Vol. 12 - 2002, A15.
- Retsas S, Quigley M, Pectasides, D, Macrae, K., Henry, K. Clinical and histologic involvement of regional lymph nodes in malignant melanoma. Adjuvant vindesine improves survival. Cancer 1994, 73, 2119–2130. [CrossRef] [PubMed]
- Retsas, S. Scientific evidence and expert clinical opinion for the investigation and management of stage III malignant melanoma – medical intervention. Chapter 6. In The Effective Management of Malignant Melanoma. MacKie, R.M., Murray, D., Rosin, R.D., Hancock, B., and Miles, A. Eds. Pp 65-82. Uk key advances in clinical practice series 2001. Aesculapius Medical Press.
- Chalabi, M.; Verschoor, Y.L.; Tan, P.B.; Balduzzi, S.; Van Lent, A.U.; Grootscholten, C.; Dokter, S.; Büller, N.V.; Grotenhuis, B.A.; Kuhlmann, K.; et al. Neoadjuvant Immunotherapy in Locally Advanced Mismatch Repair-Deficient Colon Cancer. N Engl J Med. 2024, 390, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Menzies, A.M.; Amaria, R.N.; Rozeman, E.A.; et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat Med. 2021, 27, 301–309. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chen, Y.,; Wright, G.P. Jr; Yost, K.J.; Hyngstrom, J.R.; Hu-Lieskovan, S.; Lao, C.D.; Fecher, L.A.; Truong, T.G. Neoadjuvant-Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N Engl J Med. 2023, 388, 813–823. [CrossRef] [PubMed] [PubMed Central]
- Lo-Coco, F.; Avvisati, G.; Vignetti, M.; Thiede, C.; Orlando, S.M.; Iacobelli, S.; Ferrara, F.; Fazi, P.; Cicconi, L.; Di Bona, E.; et al. Gruppo Italiano Malattie Ematologiche dell'Adulto; German-Austrian Acute Myeloid Leukemia Study Group; Study Alliance Leukemia. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013, 369, 111–121. [Google Scholar] [CrossRef] [PubMed]
- de Thé, H. Lessons taught by acute promyelocytic leukemia cure. Lancet. 2015, 386, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Ernberg, I. From the first curative targeted cancer treatment to the implementation of the most extensive healthcare reform. J Intern Med. 2015, 278, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Retsas, S. Geotherapeutics: the medicinal use of earths, minerals and metals from antiquity to the twenty-first century. In Geology and Medicine: Historical Connections Duffin, C.J., Gardner-Thorpe, C., and Moody, R.T.J. Eds; 2017, 133-139. London Geological Society Special Publication No. 452. 2017. [Google Scholar]
- Yu, D. Li, Y., Wang, M. et al. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer 2022, 21, 56. [Google Scholar] [CrossRef]
- Zoi, V.; Giannakopoulou, M.; Alexiou, G.A.; Bouziotis, P.; Thalasselis, S.; Tzakos, A.G.; Fotopoulos, A.; Papadopoulos, A.N.; Kyritsis, A.P.; Sioka, C. Nuclear Medicine and Cancer Theragnostics: Basic Concepts. Diagnostics (Basel). 2023, 13, 3064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cross, D.; Burmester, J.K. Gene therapy for cancer treatment: past, present and future. Clin Med Res. 2006, 4, 218–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bailey, S.R.; Berger, T.R.; Graham, C.; Larson, R.C.; Maus, M.V. Four challenges to CAR T cells breaking the glass ceiling. Eur J Immunol. 2023, 53, e2250039. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.S.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F.; et al. Plain language summary of the TRANSFORM study primary analysis results: lisocabtagene maraleucel as a second treatment regimen for large B-cell lymphoma following failure of the first treatment regimen. Future Oncol. 2024 Mar 28. [CrossRef] [PubMed]
- CAR T-cell therapy for solid tumours. Editorial. The Lancet Oncology. July 2021. [CrossRef]
- Hamilton, M.P.; Sugio, T.; Noordenbos, T.; Shi, S.; Bulterys, P.L.; Liu, C.L.; Kang, X.; Olsen, M.N.; Good, Z.; Dahiya, S.; et al. Risk of second tumours and T-Cell lymphoma after CAR T-Cell Therapy. N Engl J Med 2024, 390, 2047–2060. [Google Scholar] [CrossRef]
- Lv, J.; Wang, H.; Cheng, X.; Chen, Y.; Wang, D.; Zhang, L.; Cao, Q.; Tang, H.; Hu, S.; Gao, K.; Xun, M.; et al. AAV1-hOTOF gene therapy for autosomal recessive deafness 9: a single-arm trial. Lancet. 2024, 403, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Samadder, N.J.; Giridhar, K.V.; Baffy, N.; Riegert-Johnson, D.; Couch, F.J. Hereditary Cancer Syndromes-A Primer on Diagnosis and Management: Part 1: Breast-Ovarian Cancer Syndromes. Mayo Clin Proc. 2019, 94, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Samadder, N.J.; Baffy, N.; Giridhar, K.V.; Couch, F.J.; Riegert-Johnson, D. Hereditary Cancer Syndromes-A Primer on Diagnosis and Management, Part 2: Gastrointestinal Cancer Syndromes. Mayo Clin Proc. 2019, 94, 1099–1116. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.C.; Knoppers, B.M. Ethical challenges of precision cancer medicine. Seminars in Cancer Biology 2022, 84, 263–270. [Google Scholar] [CrossRef]
- Fidler, I.J. Metastasis: quantitative analysis of distribution and fate of tumor emboli labelled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst 1970, 45, 773–782. [Google Scholar]
- Langley, R.R.; Fidler, I.J. The seed and soil hypothesis revisited – the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer 2011, 128, 2527–2535. [Google Scholar] [CrossRef]
- Jensen, O.A. Malignant melanomas of the human uvea: 25-year follow-up of cases in Denmark, 1943--1952. Acta Ophthalmol (Copenh). 1982, 60, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Kujala, E.; Mäkitie, T.; Kivelä, T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef] [PubMed]
- Editorial. Lung Cancer Treatment: 20 years of progress. www.thelancet.com Vol 403 June 22, 2024; 2663.
- Ringborg, U.; von Braun, J.; Celis, J.; et al. Strategies to decrease inequalities in cancer therapeutics, care and prevention. Mol Oncol 2024, 18, 245–279. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




