Submitted:
05 September 2024
Posted:
06 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
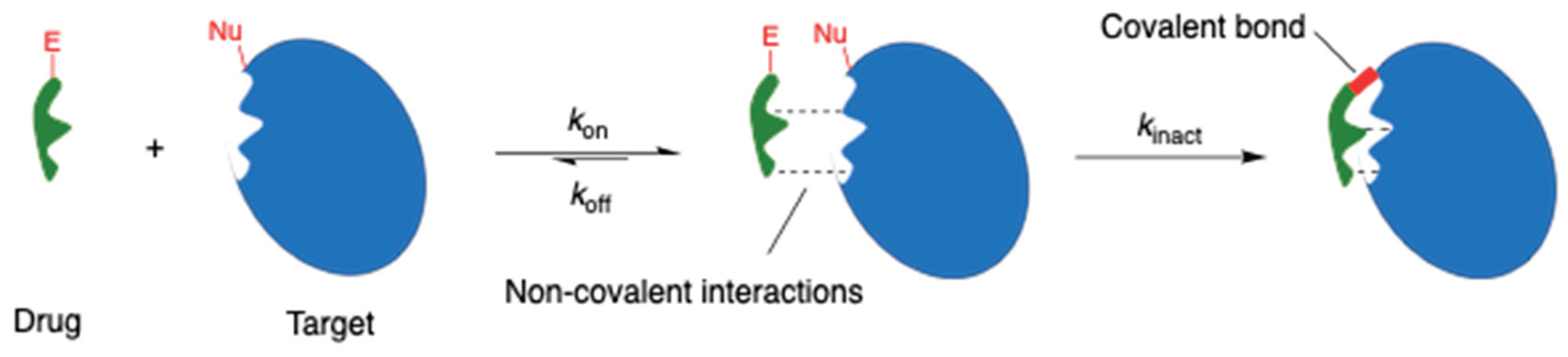
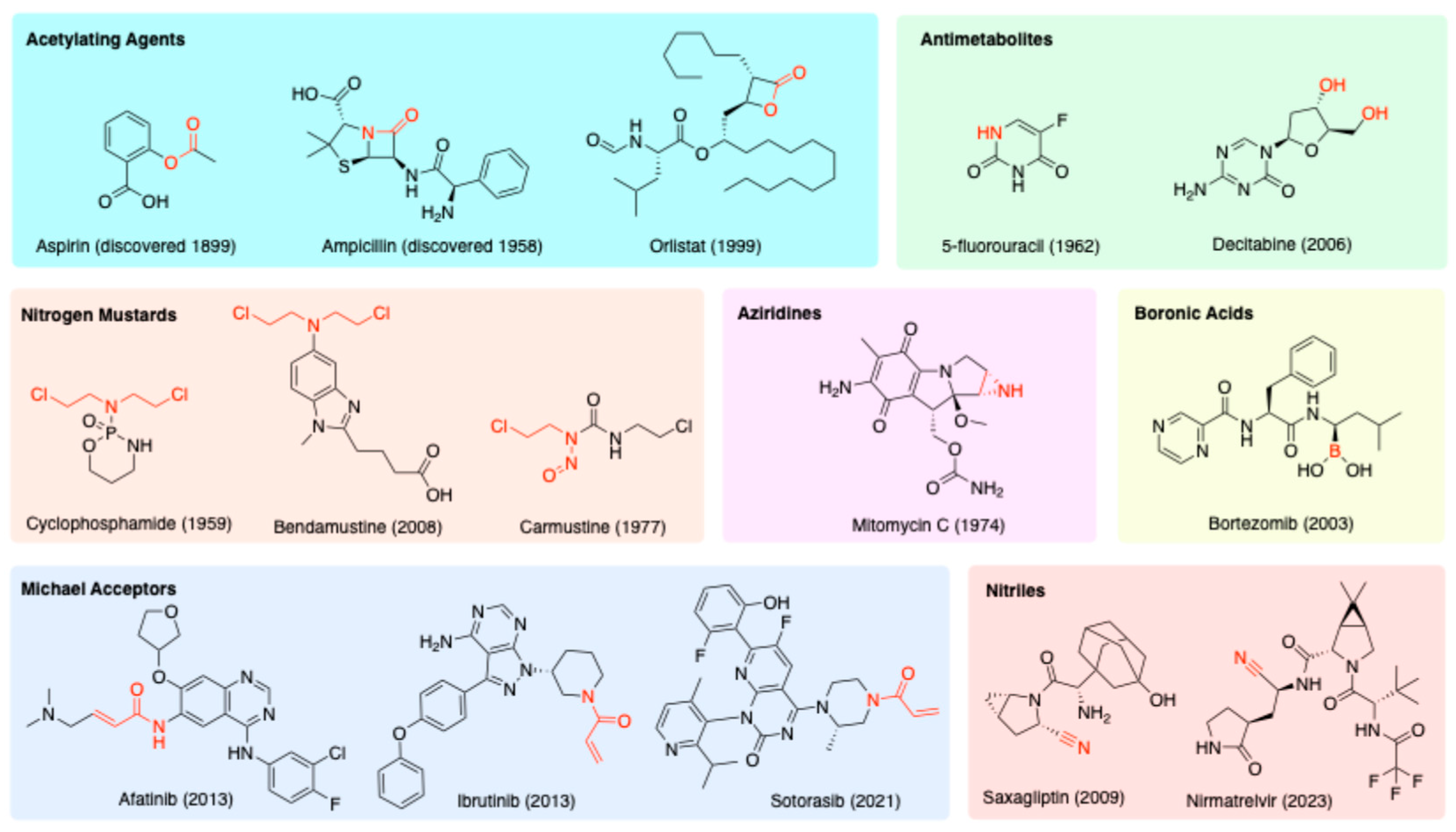
1.1. History of Covalent Drugs
1.2. Disadvantages of Covalent Drugs
1.3. Nanoparticles as a Possible Solution
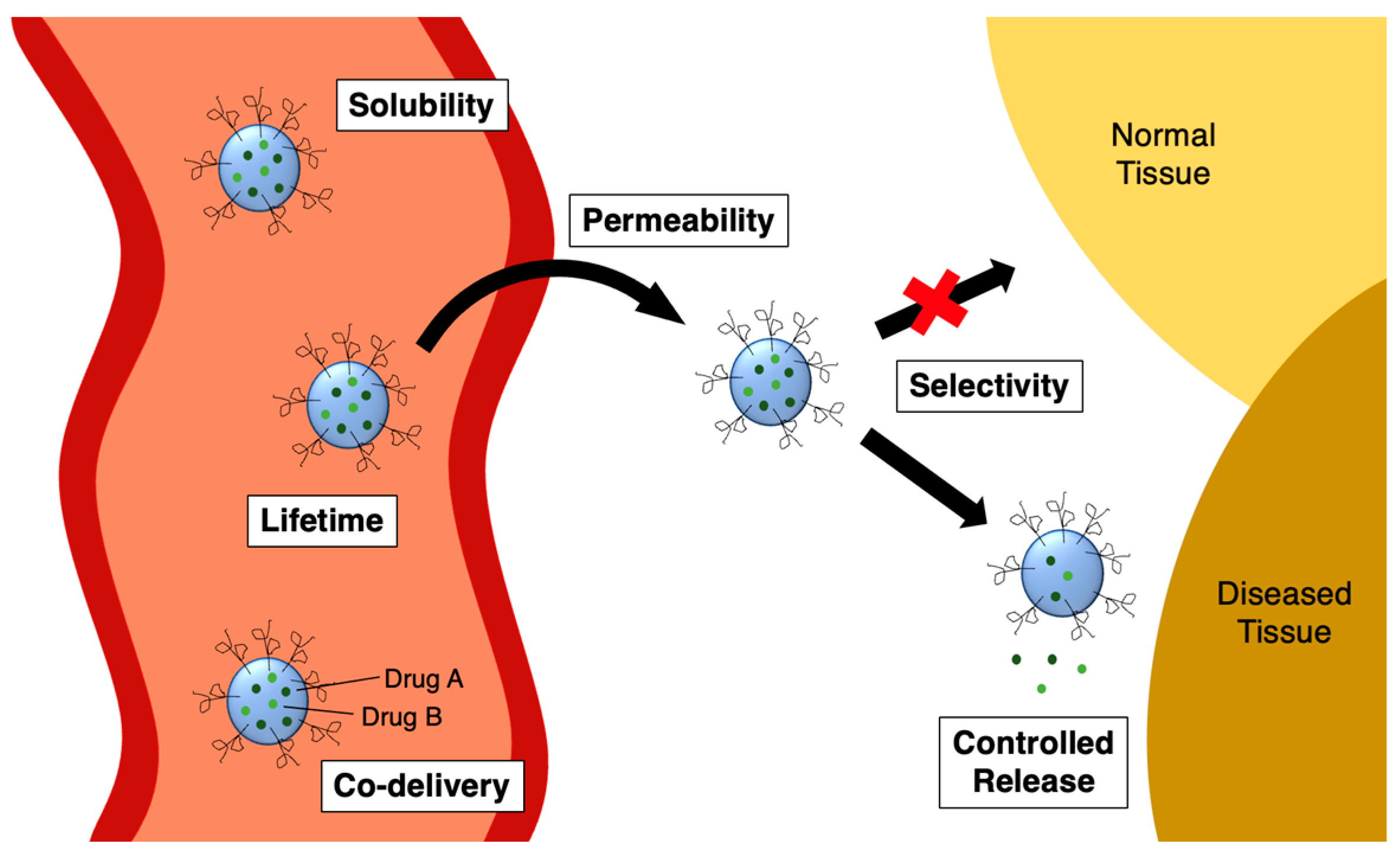
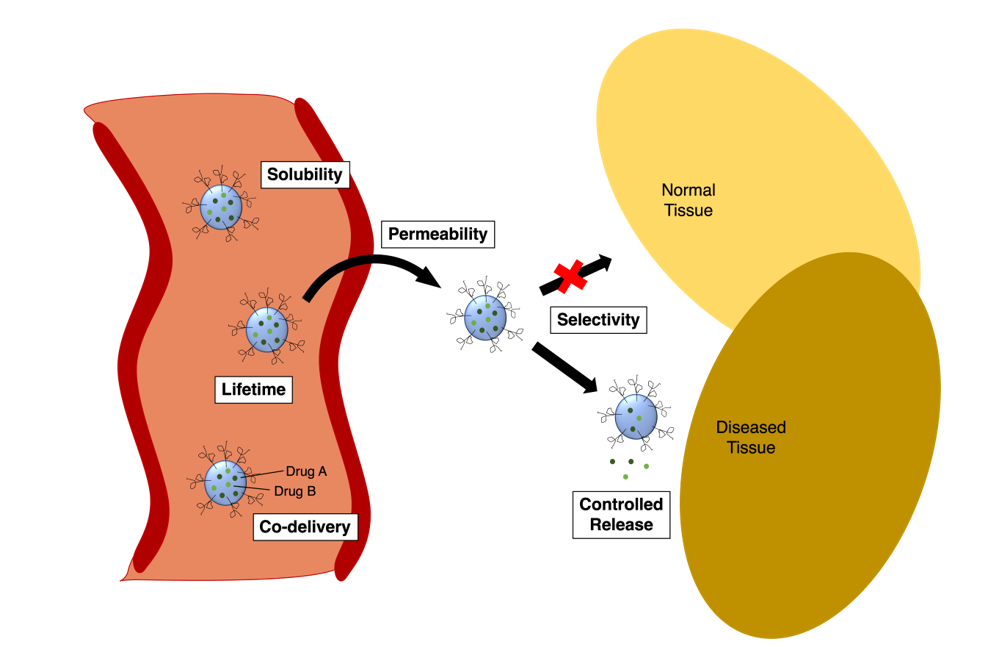
1.4. Scope of This Review
2. Benefits of Nanoparticles
2.1. Solubility
2.2. Permeability
2.3. Lifetime
2.4. Selectivity
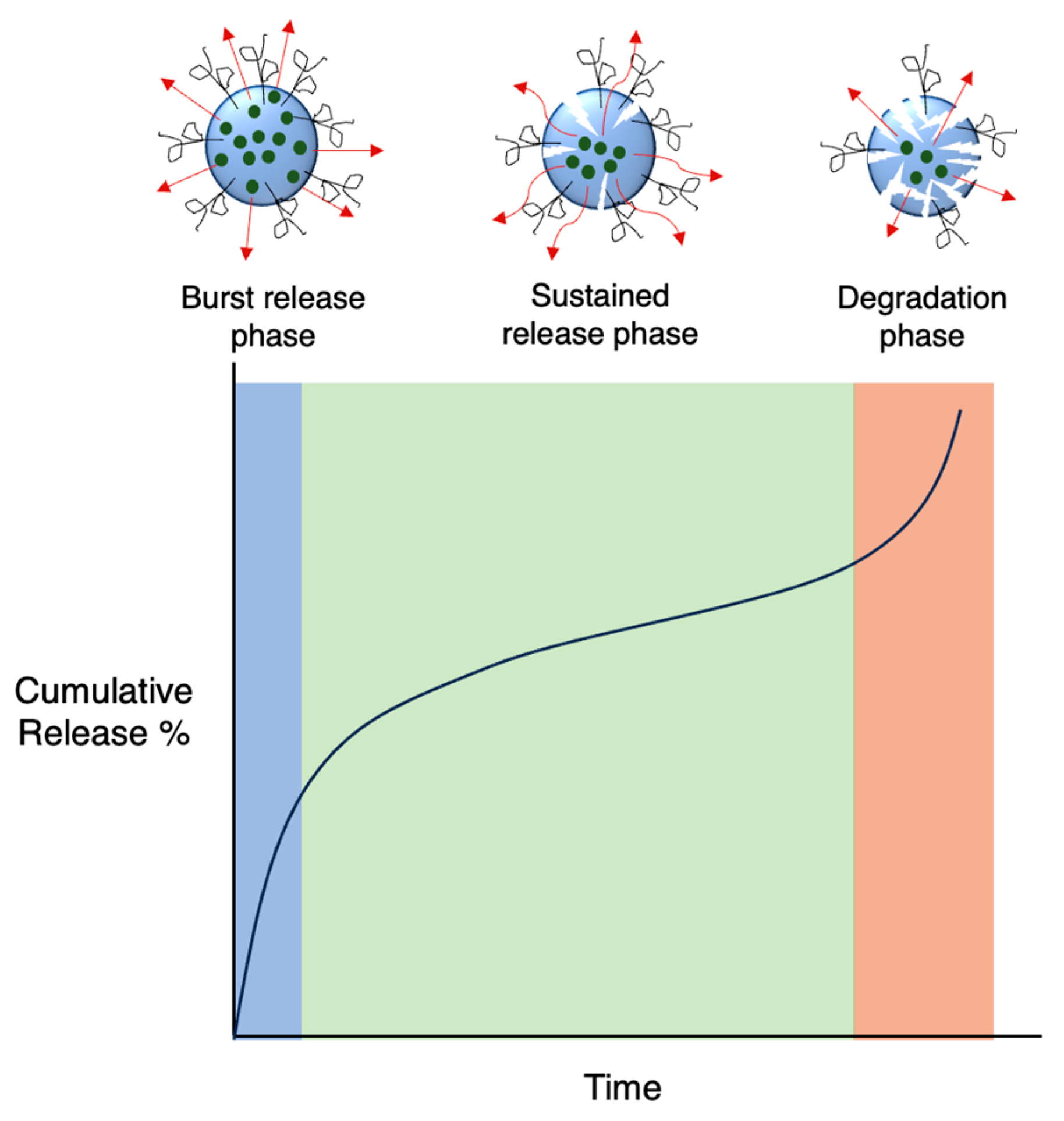
2.5. Controlled Release
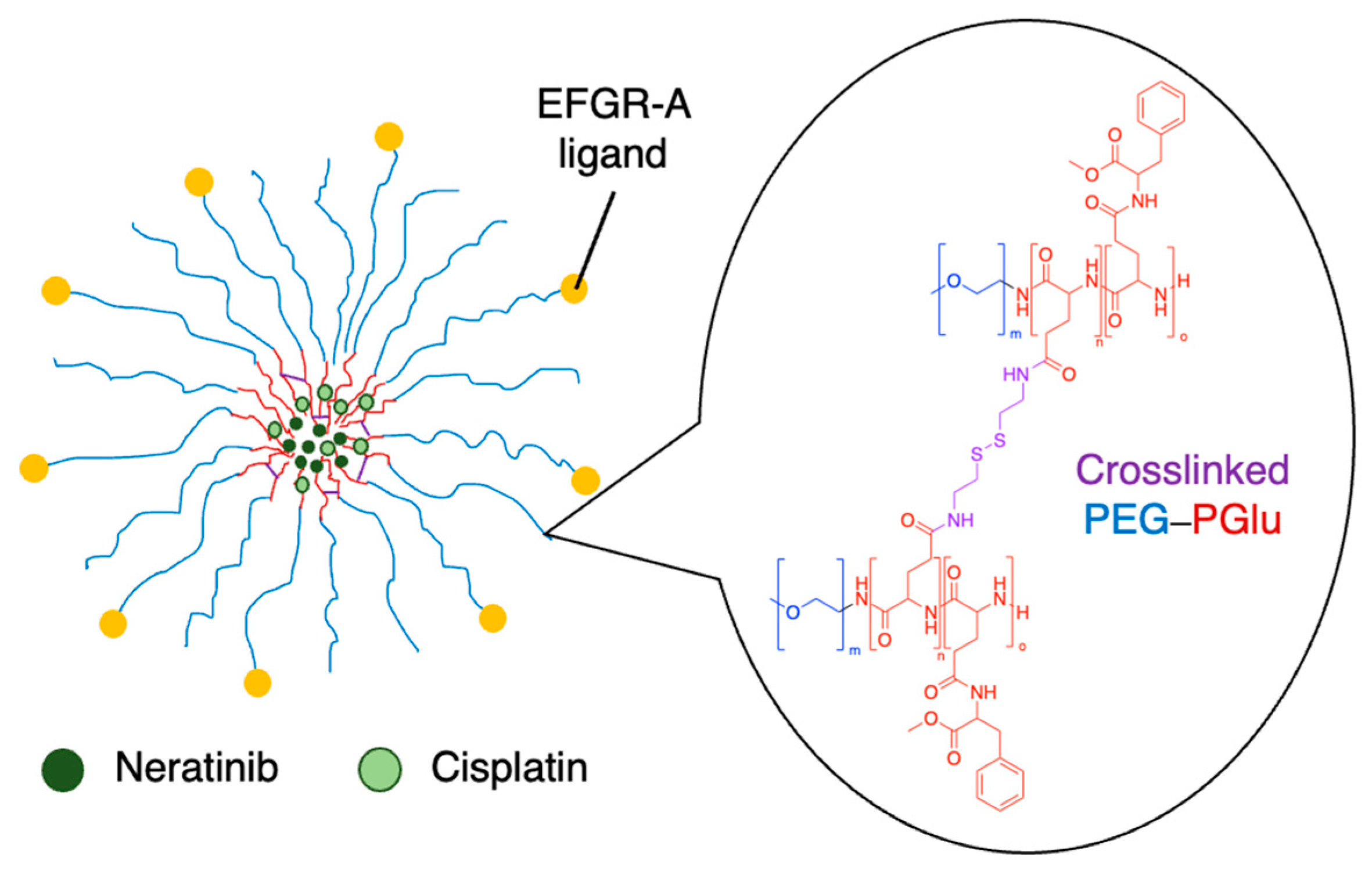
2.6. Co-Delivery of Drugs with Synergistic Abilities
3. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Disclaimer
References
- Wilson, A. J.; Kerns, J. K.; Callahan, J. F.; Moody, C. J. Keap calm, and carry on covalently. J. Med. Chem. 2013, 56, 7463–7476. [Google Scholar] [CrossRef] [PubMed]
- Baillie, T. A. Targeted covalent inhibitors for drug design. Angew. Chem. Int. Ed. 2016, 55, 13408–13421. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, R.; Ward, R. A. Structure-based design of targeted covalent inhibitors. Chem. Soc. Rev. 2018, 47, 3816–3830. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Petter, R. C.; Baillie, T. A.; Whitty, A. The resurgence of covalent drugs. Nat. Rev. Drug Discov. 2011, 10, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Singh, J. The ascension of targeted covalent inhibitors. J. Med. Chem. 2022, 65, 5886–5901. [Google Scholar] [CrossRef]
- Boike, L.; Henning, N. J.; Nomura, D. K. Advances in covalent drug discovery. Nat. Rev. Drug Discov. 2022, 21, 881–898. [Google Scholar] [CrossRef]
- De Vita, E. 10 years into the resurgence of covalent drugs. Future Med. Chem. 2020, 13, 193–210. [Google Scholar] [CrossRef]
- Sutanto, F.; Konstantinidou, M.; Dömling, A. Covalent inhibitors: a rational approach to drug discovery. RSC Med. Chem. 2020, 11, 876–884. [Google Scholar] [CrossRef]
- Gehringer, M. Covalent inhibitors: back on track? Future Med. Chem. 2020, 12, 1363–1368. [Google Scholar] [CrossRef]
- Potashman, M. H.; Duggan, M. E. Covalent modifiers: an orthogonal approach to drug design. J. Med. Chem. 2009, 52, 1231–1246. [Google Scholar] [CrossRef]
- Yang, J.; Tabuchi, Y.; Katsuki, R.; Taki, M. bioTCIs: Middle-to-Macro Biomolecular Targeted Covalent Inhibitors Possessing Both Semi-Permanent Drug Action and Stringent Target Specificity as Potential Antibody Replacements. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A. K.; Samanta, I.; Mondal, A.; Liu, W. R. Covalent inhibition in drug discovery. ChemMedChem 2019, 14, 889–906. [Google Scholar] [CrossRef]
- Vane, J.; Botting, R. The mechanism of action of aspirin. Thromb. Res. 2003, 110, (5–6). [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Muraoka, A.; Bedenbaugh, M.; Childress, B.; Pernot, L.; Wiencek, M.; Peterson, Y. K. The chemical relationship among beta-lactam antibiotics and potential impacts on reactivity and decomposition. Front. Microbiol. 2022, 13, 807955. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P. A. β-Lactams and β-lactamase inhibitors: an overview. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef]
- Parker, W. B. Enzymology of purine and pyrimidine antimetabolites used in the treatment of cancer. Chem. Rev. 2009, 109, 2880–2893. [Google Scholar] [CrossRef]
- Highley, M. S.; Landuyt, B.; Prenen, H.; Harper, P. G.; De Bruijn, E. A. The nitrogen mustards. Pharmacol. Rev. 2022, 74, 552–599. [Google Scholar] [CrossRef] [PubMed]
- Sreerama, L. Alkylating agents. Encyclopedia of Cancer. Springer Berlin Heidelberg 2011, 132–136. [Google Scholar]
- Gandhi, V.; Burger, J. A. Bendamustine in B-cell malignancies: the new 46-year-old kid on the block. Clin. Cancer Res. 2009, 15, 7456–7461. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Gao, J. Targeting biomolecules with reversible covalent chemistry. Curr. Opin. Chem. Biol. 2016, 34, 110–116. [Google Scholar] [CrossRef]
- Parmar, S.; Patel, K.; Pinilla-Ibarz, J. Ibrutinib (imbruvica): a novel targeted therapy for chronic lymphocytic leukemia. Pharm. Ther. 2014, 39. [Google Scholar]
- Nakamura, T.; Nakashima, C.; Komiya, K.; Kitera, K.; Hirai, M.; Kimura, S.; Aragane, N. Mechanisms of acquired resistance to afatinib clarified with liquid biopsy. PLoS One 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Ghate, M. V.; Mallik, S. B.; Lewis, S. A. Amino acid conjugated chitosan nanoparticles for the brain targeting of a model dipeptidyl peptidase-4 inhibitor. Int. J. Pharm. 2018, 547, (1–2). [Google Scholar] [CrossRef] [PubMed]
- Jones, L. H. Design of next-generation covalent inhibitors: Targeting residues beyond cysteine. Annu. Rep. Med. Chem. 2021, 56, 95–134. [Google Scholar] [CrossRef]
- Uetrecht, J.; Naisbitt, D. J. Idiosyncratic adverse drug reactions: current concepts. Pharmacol. Rev. 2013, 65, 779–808. [Google Scholar] [CrossRef]
- Shimada, H.; Kobayashi, Y.; Tanahashi, S.; Kawase, A.; Ogiso, T.; Iwaki, M. Correlation between glucuronidation and covalent adducts formation with proteins of nonsteroidal anti-inflammatory drugs. Eur. J. Pharm. Sci. 2018, 112, 132–138. [Google Scholar] [CrossRef]
- Shibata, Y.; Chiba, M. The role of extrahepatic metabolism in the pharmacokinetics of the targeted covalent inhibitors afatinib, ibrutinib, and neratinib. Drug Metab. Disposition 2015, 43, 375–384. [Google Scholar] [CrossRef]
- Liebler, D. C.; Guengerich, F. P. Elucidating mechanisms of drug-induced toxicity. Nat. Rev. Drug Discov. 2005, 4, 410–420. [Google Scholar] [CrossRef]
- Lipinski, C.; Hopkins, A. Navigating chemical space for biology and medicine. Nature 2004, 432, 855–861. [Google Scholar] [CrossRef]
- Rishton, G. M. Reactive compounds and in vitro false positives in HTS. Drug Discov. Today 1997, 2, 382–384. [Google Scholar] [CrossRef]
- Afzal, O.; Altamimi, A. S.; Nadeem, M. S.; Alzarea, S. I.; Almalki, W. H.; Tariq, A.; Mubeen, B.; Murtaza, B. N.; Iftikhar, S.; Riaz, N. Nanoparticles in drug delivery: From history to therapeutic applications. Nanomater. 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Kohane, D. S. Microparticles and nanoparticles for drug delivery. Biotechnol. Bioeng. 2007, 96, 203–209. [Google Scholar] [CrossRef]
- Patra, J. K.; Das, G.; Fraceto, L. F.; Campos, E. V. R.; Rodriguez-Torres, M. d. P.; Acosta-Torres, L. S.; Diaz-Torres, L. A.; Grillo, R.; Swamy, M. K.; Sharma, S. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnology 2018, 16, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H. F.; Ramadan, Y. N.; Al-Harbi, A. I.; A. Ahmed, E.; Battah, B.; Abd Ellah, N. H.; Zanetti, S.; Donadu, M. G. Nanotechnology as a promising approach to combat multidrug resistant bacteria: a comprehensive review and future perspectives. Biomedicines 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.-H.; Jeong, G.-W.; Nah, J.-W. Preparation and anticancer effect of pegylated-chlorambucil prodrug nanoparticle for enhanced therapeutic efficiency. J. Ind. Eng. Int. 2018, 64, 438–445. [Google Scholar] [CrossRef]
- Pan, J.; Rostamizadeh, K.; Filipczak, N.; Torchilin, V. P. Polymeric co-delivery systems in cancer treatment: an overview on component drugs’ dosage ratio effect. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Beach, M. A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G. K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef]
- Elumalai, K.; Srinivasan, S.; Shanmugam, A. Review of the efficacy of nanoparticle-based drug delivery systems for cancer treatment. Biomed. Technol. 2024, 5, 109–122. [Google Scholar] [CrossRef]
- Gao, W.; Bigham, A.; Ghomi, M.; Zarrabi, A.; Rabiee, N.; Saeb, M. R.; Ertas, Y. N.; Goel, A.; Sharifi, E.; Ashrafizadeh, M. Micelle-engineered nanoplatforms for precision oncology. Chem. Eng. J. 2024, 153438. [Google Scholar] [CrossRef]
- Mitchell, M. J.; Billingsley, M. M.; Haley, R. M.; Wechsler, M. E.; Peppas, N. A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Dorababu, A. Recent Advances in Nanoformulated Chemotherapeutic Drug Delivery (2015-2019). ChemistrySelect 2019, 4, 8731–8744. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Yuhong, J.; Xin, P.; Han, J. L.; Du, Y.; Yu, X.; Zhu, R.; Zhang, M.; Chen, W. Advances in Nanotechnology for Enhancing the Solubility and Bioavailability of Poorly Soluble Drugs. Drug Des. Devel. Ther. 2024, 1469–1495. [Google Scholar] [CrossRef]
- Kim, D. H.; Kim, J. Y.; Kim, R. M.; Maharjan, P.; Ji, Y.-G.; Jang, D.-J.; Min, K. A.; Koo, T.-S.; Cho, K. H. Orlistat-loaded solid SNEDDS for the enhanced solubility, dissolution, and in vivo performance. Int. J. Nanomedicine 2018, 7095–7106. [Google Scholar] [CrossRef]
- Hill, T. K.; Davis, A. L.; Wheeler, F. B.; Kelkar, S. S.; Freund, E. C.; Lowther, W. T.; Kridel, S. J.; Mohs, A. M. Development of a self-assembled nanoparticle formulation of orlistat, nano-ORL, with increased cytotoxicity against human tumor cell lines. Mol. Pharm. 2016, 13, 720–728. [Google Scholar] [CrossRef]
- Qu, Z.; Ren, Y.; Shen, H.; Wang, H.; Shi, L.; Tong, D. Combination therapy of metastatic castration-recurrent prostate cancer: hyaluronic acid decorated, cabazitaxel-prodrug and orlistat co-loaded nano-system. Drug Des. Devel. Ther. 2021, 3605–3616. [Google Scholar] [CrossRef]
- Bhargava-Shah, A.; Foygel, K.; Devulapally, R.; Paulmurugan, R. Orlistat and antisense-miRNA-loaded PLGA-PEG nanoparticles for enhanced triple negative breast cancer therapy. Nanomedicine 2016, 11, 235–247. [Google Scholar] [CrossRef]
- Zhou, X.; Chang, T.-L.; Chen, S.; Liu, T.; Wang, H.; Liang, J. F. Polydopamine-decorated orlistat-loaded hollow capsules with an enhanced cytotoxicity against cancer cell lines. Mol. Pharm. 2019, 16, 2511–2521. [Google Scholar] [CrossRef]
- Rangaraj, N.; Pailla, S. R.; Chowta, P.; Sampathi, S. Fabrication of ibrutinib nanosuspension by quality by design approach: intended for enhanced oral bioavailability and diminished fast fed variability. AAPS PharmSciTech 2019, 20, 1–18. [Google Scholar] [CrossRef]
- Alshetaili, A. S.; Ansari, M. J.; Anwer, M. K.; Ganaie, M. A.; Iqbal, M.; Alshahrani, S. M.; Alalaiwe, A. S.; Alsulays, B. B.; Alshehri, S.; Sultan, A. S. Enhanced oral bioavailability of ibrutinib encapsulated poly (lactic-co-glycolic acid) nanoparticles: Pharmacokinetic evaluation in rats. Curr. Pharm. Anal. 2019, 15, 661–668. [Google Scholar] [CrossRef]
- Zhao, L.; Tang, B.; Tang, P.; Sun, Q.; Suo, Z.; Zhang, M.; Gan, N.; Yang, H.; Li, H. Chitosan/sulfobutylether-β-cyclodextrin nanoparticles for ibrutinib delivery: a potential nanoformulation of novel kinase inhibitor. J. Pharm. Sci. 2020, 109, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S. K.; El-Feky, G. S.; El-Banna, S. T.; Khalil, W. A. Preparation and evaluation of warfarin-β-cyclodextrin loaded chitosan nanoparticles for transdermal delivery. Carbohydr. Polym. 2012, 90, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- d'Angelo, I.; Quaglia, F.; Ungaro, F. PLGA carriers for inhalation: where do we stand, where are we headed? 2015, 6 (10), 1139-1144. [CrossRef]
- Salgueiro, A.; Egea, M.; Espina, M.; Valls, O.; Garcia, M. Stability and ocular tolerance of cyclophosphamide-loaded nanospheres. J. Microencaps. 2004, 21, 213–223. [Google Scholar] [CrossRef]
- Zhao, K.; Xie, Y.; Lin, X.; Xu, W. The mucoadhesive nanoparticle-based delivery system in the development of mucosal vaccines. Int. J. Nanomedicine 2022, 17, 4579. [Google Scholar] [CrossRef]
- Arif, M.; Dong, Q.-J.; Raja, M. A.; Zeenat, S.; Chi, Z.; Liu, C.-G. Development of novel pH-sensitive thiolated chitosan/PMLA nanoparticles for amoxicillin delivery to treat Helicobacter pylori. Mater. Sci. Eng. C 2018, 83, 17–24. [Google Scholar] [CrossRef]
- Sahu, P.; Kashaw, S. K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R. K.; Iyer, A. K. pH responsive 5-fluorouracil loaded biocompatible nanogels for topical chemotherapy of aggressive melanoma. Colloids Surf. B. Biointerfaces 2019, 174, 232–245. [Google Scholar] [CrossRef]
- Elbatanony, R. S.; Parvathaneni, V.; Kulkarni, N. S.; Shukla, S. K.; Chauhan, G.; Kunda, N. K.; Gupta, V. Afatinib-loaded inhalable PLGA nanoparticles for localized therapy of non-small cell lung cancer (NSCLC)—development and in-vitro efficacy. Drug Deliv. Transl. Res. 2021, 11, 927–943. [Google Scholar] [CrossRef]
- Vanza, J. D.; Lalani, J. R.; Patel, R. B.; Patel, M. R. DOE supported optimization of biodegradable polymeric nanoparticles based dry powder inhaler for targeted delivery of afatinib in non-small cell lung cancer. J. Drug Deliv. Sci. Technol. 2023, 84, 104554. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Cheng, S.-J. Brain targeted delivery of carmustine using solid lipid nanoparticles modified with tamoxifen and lactoferrin for antitumor proliferation. Int. J. Pharm. 2016, 499, (1–2). [Google Scholar] [CrossRef]
- Lo, Y.-L.; Lin, H.-C.; Hong, S.-T.; Chang, C.-H.; Wang, C.-S.; Lin, A. M.-Y. Lipid polymeric nanoparticles modified with tight junction-modulating peptides promote afatinib delivery across a blood–brain barrier model. Cancer Nanotechnol. 2021, 12. [Google Scholar] [CrossRef]
- Fahey, R. C.; Hunt, J. S.; Windham, G. C. On the cysteine and cystine content of proteins: differences between intracellular and extracellular proteins. J. Mol. Evol. 1977, 10, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, L.; Kou, L.; Xu, M.; Sun, J.; Wang, Y.; Fu, Q.; Zhang, P.; He, Z. Novel nanostructured enoxaparin sodium-PLGA hybrid carriers overcome tumor multidrug resistance of doxorubicin hydrochloride. Int. J. Pharm. 2016, 513, (1–2). [Google Scholar] [CrossRef]
- Wang, C.; Li, F.; Zhang, T.; Yu, M.; Sun, Y. Recent advances in anti-multidrug resistance for nano-drug delivery system. Drug Deliv. 2022, 29, 1684–1697. [Google Scholar] [CrossRef]
- Manzanares, D.; Ceña, V. Endocytosis: the nanoparticle and submicron nanocompounds gateway into the cell. Pharmaceutics 2020, 12. [Google Scholar] [CrossRef]
- Chithrani, B. D.; Ghazani, A. A.; Chan, W. C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S. C.; Almeida, G. M.; Pereira, M. C.; Santos-Silva, F.; Coelho, M. A. Functionalized gold nanoparticles improve afatinib delivery into cancer cells. Expert Opin. Drug Deliv. 2016, 13, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-T.; Lin, H.; Wang, C.-S.; Chang, C.-H.; Lin, A. M.-Y.; Yang, J. C.-H.; Lo, Y.-L. Improving the anticancer effect of afatinib and microRNA by using lipid polymeric nanoparticles conjugated with dual pH-responsive and targeting peptides. J. Nanobiotechnology 2019, 17, 1–20. [Google Scholar] [CrossRef]
- Wang, Y.; Pi, C.; Feng, X.; Hou, Y.; Zhao, L.; Wei, Y. The influence of nanoparticle properties on oral bioavailability of drugs. Int. J. Nanomedicine 2020, 6295–6310. [Google Scholar] [CrossRef]
- Famta, P.; Shah, S.; Vambhurkar, G.; Srinivasarao, D. A.; Jain, N.; Begum, N.; Sharma, A.; Shahrukh, S.; Kumar, K. C.; Bagasariya, D. Quality by design endorsed fabrication of Ibrutinib-loaded human serum albumin nanoparticles for the management of leukemia. Eur. J. Pharm. Biopharm. 2023, 190, 94–106. [Google Scholar] [CrossRef]
- Yang, Z.; Du, Y.; Lei, L.; Xia, X.; Wang, X.; Tong, F.; Li, Y.; Gao, H. Co-delivery of ibrutinib and hydroxychloroquine by albumin nanoparticles for enhanced chemotherapy of glioma. Int. J. Pharm. 2023, 630, 122436. [Google Scholar] [CrossRef]
- Patel, M.; Desai, A.; Kansara, V.; Vyas, B. Core Shell Lipid-Polymer Hybrid Nanoparticles for Oral Bioavailability Enhancement of Ibrutinib via Lymphatic Uptake. AAPS PharmSciTech 2023, 24. [Google Scholar] [CrossRef]
- Lu, X.; Liu, S.; Han, M.; Yang, X.; Sun, K.; Wang, H.; Mu, H.; Du, Y.; Wang, A.; Ni, L. Afatinib-loaded immunoliposomes functionalized with cetuximab: A novel strategy targeting the epidermal growth factor receptor for treatment of non-small-cell lung cancer. Int. J. Pharm. 2019, 560, 126–135. [Google Scholar] [CrossRef]
- Wang, J.; Su, G.; Yin, X.; Luo, J.; Gu, R.; Wang, S.; Feng, J.; Chen, B. Non-small cell lung cancer-targeted, redox-sensitive lipid-polymer hybrid nanoparticles for the delivery of a second-generation irreversible epidermal growth factor inhibitor—Afatinib: In vitro and in vivo evaluation. Biomed. Pharmacother. 2019, 120, 109493. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Z.; Li, J.; Mo, Z.; Huang, Y.; Ma, C.; Wang, W.; Pan, X.; Wu, C. PLGA porous microspheres dry powders for codelivery of afatinib-loaded solid lipid nanoparticles and paclitaxel: novel therapy for EGFR tyrosine kinase inhibitors resistant nonsmall cell lung cancer. Adv. Healthc. Mater. 2019, 8. [Google Scholar] [CrossRef]
- Qian, L.; Zheng, J.; Wang, K.; Tang, Y.; Zhang, X.; Zhang, H.; Huang, F.; Pei, Y.; Jiang, Y. Cationic core–shell nanoparticles with carmustine contained within O6-benzylguanine shell for glioma therapy. Biomaterials 2013, 34, 8968–8978. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Tahover, E.; Kornev, G.; Patil, Y.; Amitay, Y.; Ohana, P.; Sapir, E.; Zalipsky, S. Development of Promitil®, a lipidic prodrug of mitomycin c in PEGylated liposomes: From bench to bedside. Adv. Drug Del. Rev. 2020, 154, 13–26. [Google Scholar] [CrossRef]
- Wind, S.; Schnell, D.; Ebner, T.; Freiwald, M.; Stopfer, P. Clinical pharmacokinetics and pharmacodynamics of afatinib. Clin. Pharmacokinet. 2017, 56, 235–250. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P. L.; Kobayashi, H. Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer? Bioconj. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.-S.; Chang, J.; Cheng, C.-C.; Luo, T.-Y.; Ho, A.-S.; Wang, C.-C.; Wu, C.-T.; Liu, S.-H. Afatinib and its encapsulated polymeric micelles inhibits HER2-overexpressed colorectal tumor cell growth in vitro and in vivo. Oncotarget 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- de la Puente, P.; Luderer, M. J.; Federico, C.; Jin, A.; Gilson, R. C.; Egbulefu, C.; Alhallak, K.; Shah, S.; Muz, B.; Sun, J. Enhancing proteasome-inhibitory activity and specificity of bortezomib by CD38 targeted nanoparticles in multiple myeloma. J. Control. Release 2018, 270, 158–176. [Google Scholar] [CrossRef] [PubMed]
- Nho, T. D. T.; Ly, H. T.; Vo, T. S.; Nguyen, H. D.; Phung, T. T. H.; Zou, A.; Liu, J. Enhanced anticancer efficacy and tumor targeting through folate-PEG modified nanoliposome loaded with 5-fluorouracil. Adv. Nat. Sci.: Nanosci. 2017, 8. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Yang, X.; Jia, M.; Li, Y.; Huang, Y.; Lin, J.; Wu, S.; Hou, Z. Mitomycin C-soybean phosphatidylcholine complex-loaded self-assembled PEG-lipid-PLA hybrid nanoparticles for targeted drug delivery and dual-controlled drug release. Mol. Pharm. 2014, 11, 2915–2927. [Google Scholar] [CrossRef]
- Wu, X.; Hu, Z.; Nizzero, S.; Zhang, G.; Ramirez, M. R.; Shi, C.; Zhou, J.; Ferrari, M.; Shen, H. Bone-targeting nanoparticle to co-deliver decitabine and arsenic trioxide for effective therapy of myelodysplastic syndrome with low systemic toxicity. J. Control. Release 2017, 268, 92–101. [Google Scholar] [CrossRef]
- Zhang, T.; Lip, H.; He, C.; Cai, P.; Wang, Z.; Henderson, J. T.; Rauth, A. M.; Wu, X. Y. Multitargeted Nanoparticles Deliver Synergistic Drugs across the Blood–Brain Barrier to Brain Metastases of Triple Negative Breast Cancer Cells and Tumor-Associated Macrophages. Adv. Healthc. Mater. 2019, 8. [Google Scholar] [CrossRef]
- Mei, Y.; Qin, X.; Yang, Z.; Song, S.; Liu, X.; Wu, C.; Qian, J.; Huang, X.; Zhang, Y.; He, W. Engineered a dual-targeting HA-TPP/A nanoparticle for combination therapy against KRAS-TP53 co-mutation in gastrointestinal cancers. Bioact. Mater. 2024, 32, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Rajoria, S.; Rani, S.; Chaudhari, D.; Jain, S.; Gupta, U. Glycine-poly-L-lactic acid copolymeric nanoparticles for the efficient delivery of bortezomib. Pharm. Res. 2019, 36, 1–15. [Google Scholar] [CrossRef]
- Kalli, K. R.; Oberg, A. L.; Keeney, G. L.; Christianson, T. J.; Low, P. S.; Knutson, K. L.; Hartmann, L. C. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol. Oncol. 2008, 108, 619–626. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Cai, S.; Mei, H.; He, Y.; Huang, D.; Shi, W.; Li, S.; Cao, J.; He, B. Photo-induced specific intracellular release EGFR inhibitor from enzyme/ROS-dual sensitive nano-platforms for molecular targeted-photodynamic combinational therapy of non-small cell lung cancer. J. Mater. Chem. B 2020, 8, 7931–7940. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O. C. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Pena, E. S.; Graham-Gurysh, E. G.; Bachelder, E. M.; Ainslie, K. M. Design of biopolymer-based interstitial therapies for the treatment of glioblastoma. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Wei, H.; Wang, Q.; Sun, Q.; Zhou, C.; Zhan, C.; Tang, X.; Zhang, Q. New method to prepare mitomycin C loaded PLA-nanoparticles with high drug entrapment efficiency. Nanoscale Res. Lett. 2009, 4, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Li, Y.; Wu, H.; Jia, M.; Yang, X.; Wei, H.; Lin, J.; Wu, S.; Huang, Y.; Hou, Z. Single-step assembly of polymer-lipid hybrid nanoparticles for mitomycin C delivery. Nanoscale Res. Lett. 2014, 9, 1–14. [Google Scholar] [CrossRef]
- Li, Y.; Lin, J.; Wu, H.; Jia, M.; Yuan, C.; Chang, Y.; Hou, Z.; Dai, L. Novel methotrexate prodrug-targeted drug delivery system based on PEG–lipid–PLA hybrid nanoparticles for enhanced anticancer efficacy and reduced toxicity of mitomycin C. J. Mater. Chem. B 2014, 2, 6534–6548. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Warner, S. B.; Wagner, K. T.; Caster, J. M.; Zhang, T.; Ohana, P.; Gabizon, A. A.; Wang, A. Z. Preclinical evaluation of promitil, a radiation-responsive liposomal formulation of mitomycin c prodrug, in chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 547–555. [Google Scholar] [CrossRef]
- Amin, K. W. K.; Abdelghafour, M. M.; Hornok, V.; Kiss, T.; Szabó, D.; Rovó, L.; Janovák, L. Mitomycin loaded self-assembled colloidal prodrug nanoparticles for magnetic drug targeting. J. Drug Deliv. Sci. Technol. 2023, 88, 104948. [Google Scholar] [CrossRef]
- Kiamohammadi, L.; Asadi, L.; Shirvalilou, S.; Khoei, S.; Khoee, S.; Soleymani, M.; Minaei, S. E. Physical and biological properties of 5-fluorouracil polymer-coated magnetite nanographene oxide as a new thermosensitizer for alternative magnetic hyperthermia and a magnetic resonance imaging contrast agent: in vitro and in vivo study. ACS Omega 2021, 6, 20192–20204. [Google Scholar] [CrossRef]
- Gong, H.-Y.; Chen, Y.-G.; Yu, X.-S.; Xiao, H.; Xiao, J.-P.; Wang, Y.; Shuai, X.-T. Co-delivery of doxorubicin and afatinib with pH-responsive polymeric nanovesicle for enhanced lung cancer therapy. Chin. J. Polym. Sci. 2019, 37, 1224–1233. [Google Scholar] [CrossRef]
- Abdelghafour, M. M.; Deák, Á.; Szabó, D.; Dékány, I.; Rovó, L.; Janovák, L. Use of self-assembled colloidal prodrug nanoparticles for controlled drug delivery of anticancer, antifibrotic and antibacterial mitomycin. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Cui, Z.; Zheng, Z.; Lin, L.; Si, J.; Wang, Q.; Peng, X.; Chen, W. Electrospinning and crosslinking of polyvinyl alcohol/chitosan composite nanofiber for transdermal drug delivery. Adv. Polym. Tech. 2018, 37, 1917–1928. [Google Scholar] [CrossRef]
- Fu, D.; Li, C.; Huang, Y. Lipid–Polymer Hybrid Nanoparticle-Based Combination Treatment with Cisplatin and EGFR/HER2 Receptor-Targeting Afatinib to Enhance the Treatment of Nasopharyngeal Carcinoma. Onco Targets Ther. 2021, 2449–2461. [Google Scholar] [CrossRef] [PubMed]
- Morton, S. W.; Lee, M. J.; Deng, Z. J.; Dreaden, E. C.; Siouve, E.; Shopsowitz, K. E.; Shah, N. J.; Yaffe, M. B.; Hammond, P. T. A nanoparticle-based combination chemotherapy delivery system for enhanced tumor killing by dynamic rewiring of signaling pathways. Sci. Signal. 2014, 7, ra44–ra44. [Google Scholar] [CrossRef]
- Xi, X.; Lei, F.; Gao, K.; Li, J.; Liu, R.; Karpf, A. R.; Bronich, T. K. Ligand-installed polymeric nanocarriers for combination chemotherapy of EGFR-positive ovarian cancer. J. Control. Release 2023, 360, 872–887. [Google Scholar] [CrossRef] [PubMed]
- Lee, M. J.; Albert, S. Y.; Gardino, A. K.; Heijink, A. M.; Sorger, P. K.; MacBeath, G.; Yaffe, M. B. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell 2012, 149, 780–794. [Google Scholar] [CrossRef] [PubMed]
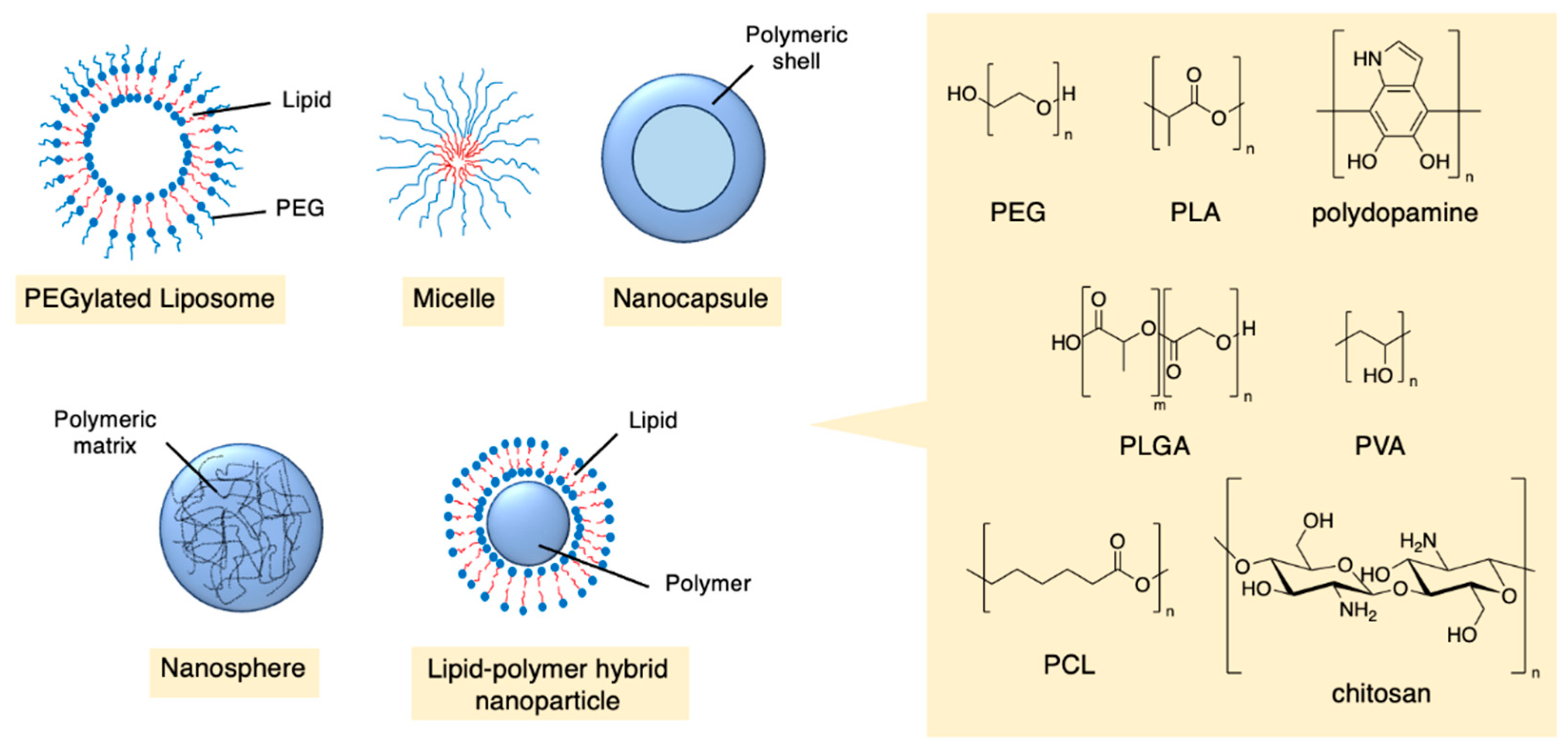
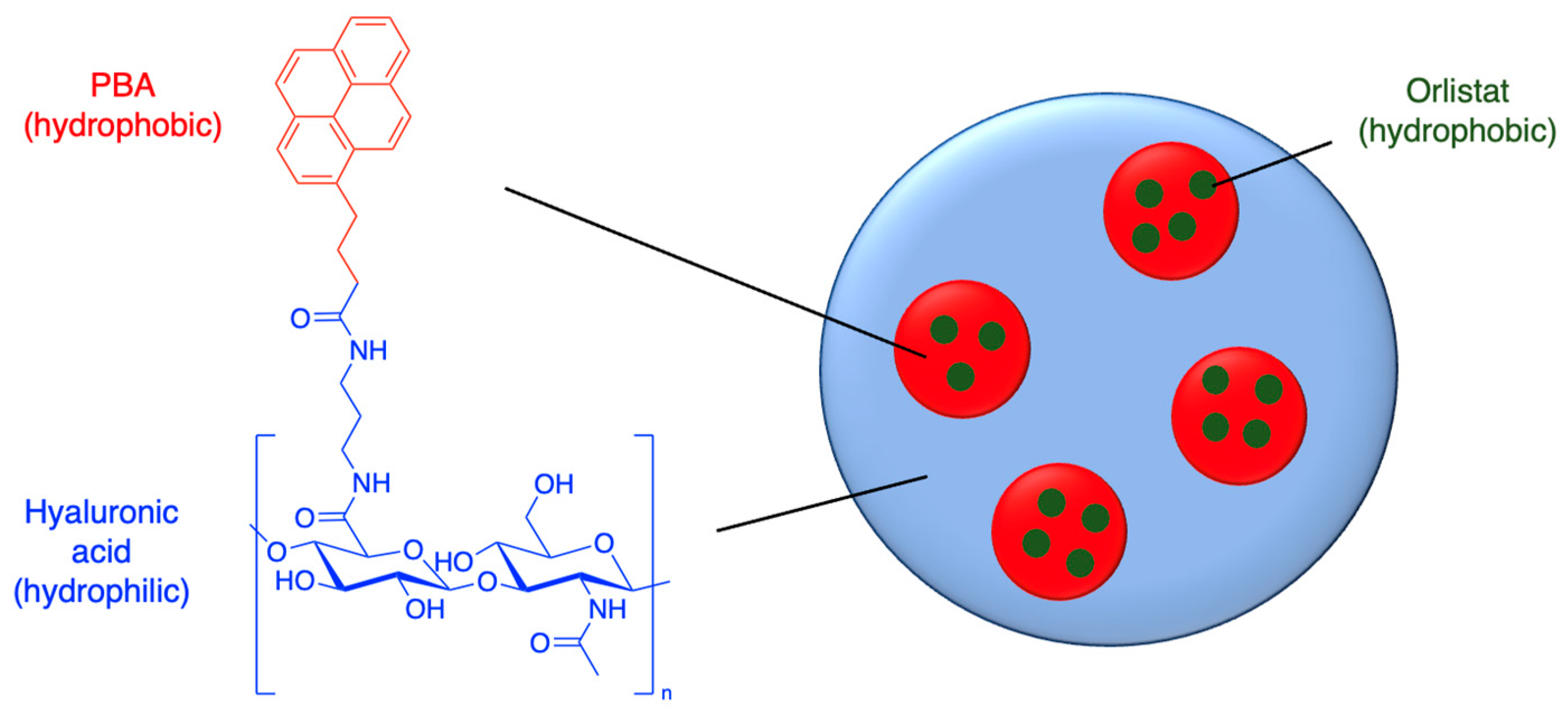
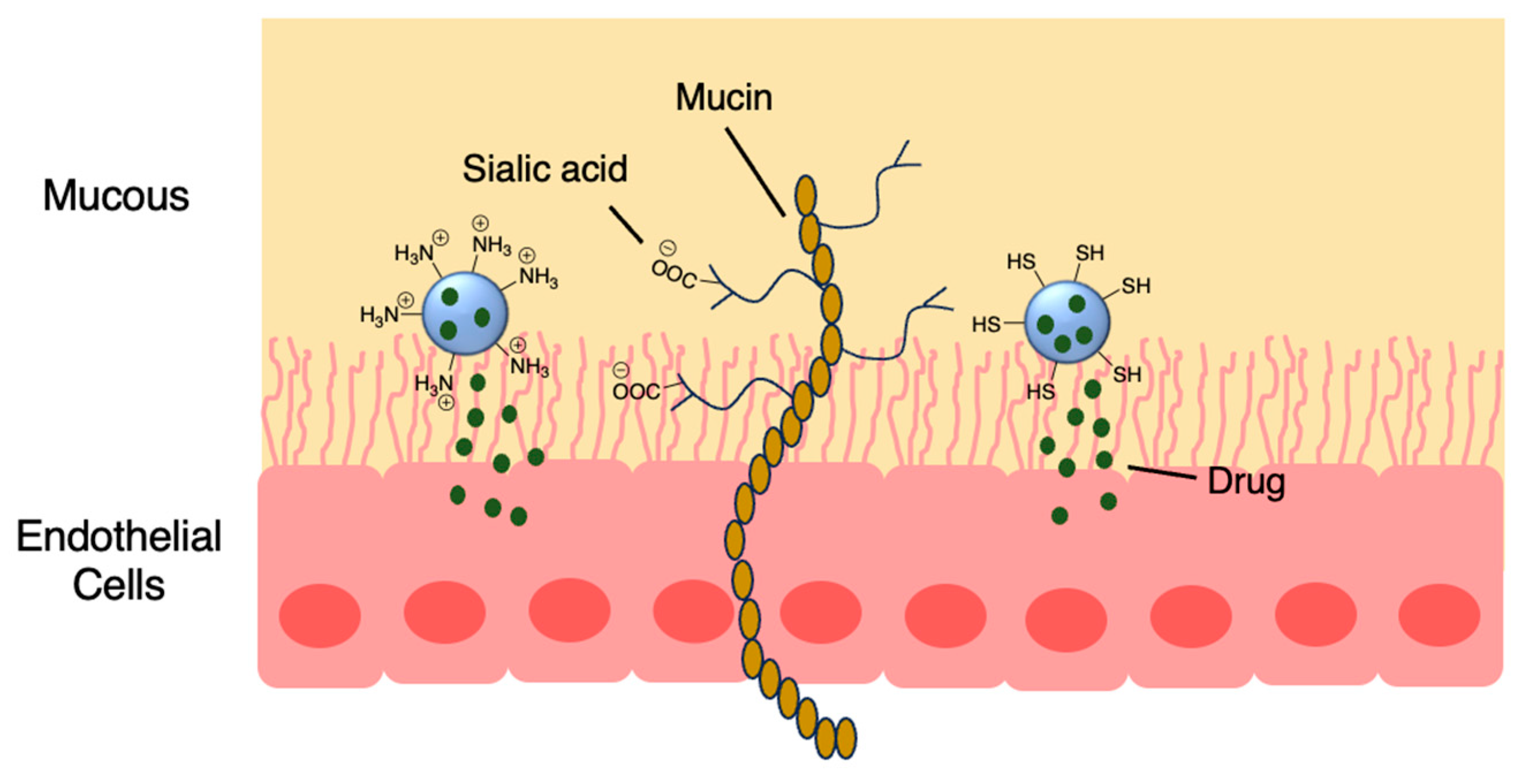
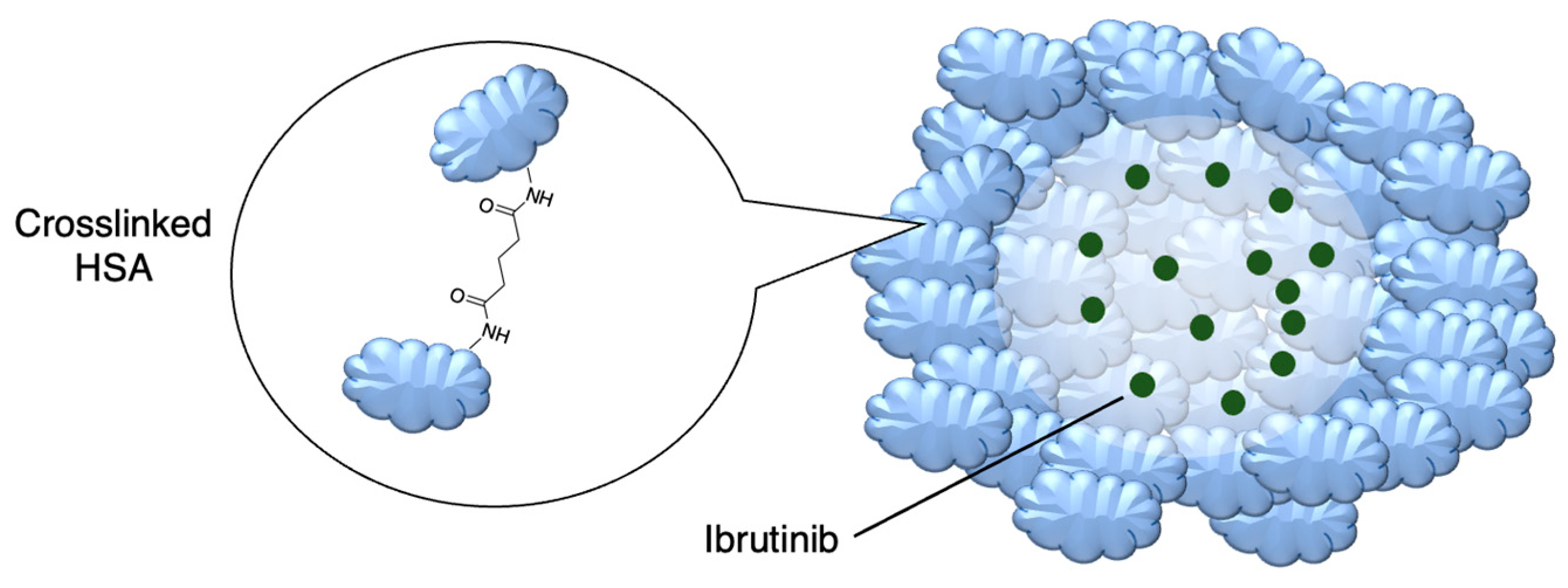
| Entry | Drug | Nanoparticle type | Significant findings | Ref. |
|---|---|---|---|---|
| 1 | Orlistat | PBA-hyaluronic acid nanoparticles | 97% encapsulation efficiency (EE); 19% drug loading capacity (LC) | [46] |
| 2 | Orlistat | Hyaluronic acid-lipid-polymer hybrid nanoparticles | 90% EE; 6% drug LC | [47] |
| 3 | Orlistat | PLGA-PEG nanoparticles | 72% EE; 7% drug LC | [48] |
| 4 | Orlistat | Polydopamine-coated hollow capsules | 91% EE (using Nile Red as proxy drug) | [49] |
| 5 | Ibrutinib | Pluronic-stabilised nanosuspension | 21-fold increase in solubility | [50] |
| 6 | Ibrutinib | Pluronic-stabilised PLGA nanoparticles | 4-fold enhancement of oral bioavailability | [51] |
| 7 | Ibrutinib | Cyclodextrin chitosan nanoparticles | 77% EE; 13% drug LC | [52] |
| Entry | Drug | Nanoparticle type | Biological barrier | Ref. |
|---|---|---|---|---|
| 1 | Amoxicillin | Thiolated chitosan / PMLA nanoparticles | Stomach | [57] |
| 2 | 5-Fluorouracil | Chitosan-pluronic nanogels | Skin | [58] |
| 3 | Cyclophosphamide | Polyalkylcyanoacrylate nanospheres | Eye | [55] |
| 4 | Afatinib | PLGA nanoparticles | Lung | [59] |
| 5 | Afatinib | PLGA nanoparticles | Lung | [60] |
| 6 | Carmustine | Solid lipid nanoparticles conjugated with lactoferrin | BBB | [61] |
| 7 | Saxagliptin | Chitosan nanoparticles with valine | BBB | [23] |
| 8 | Afatinib | Lipid-polymer nanoparticles with tight junction-modulating peptides | BBB | [62] |
| Entry | Drug | Nanoparticle type | T1/2 (h) (free drug vs NP-drug) | Ref. |
|---|---|---|---|---|
| 1 | Ibrutinib | Crosslinked human serum albumin | 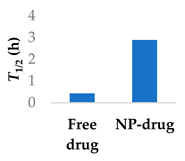 |
[71,72] |
| 2 | Ibrutinib | Lipid-polymer hybrid nanoparticles | 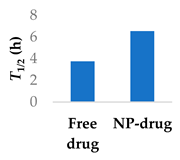 |
[73] |
| 3 | Afatinib | PEGylated liposomes | 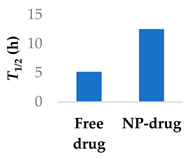 |
[74] |
| 4 | Afatinib | Tf modified redox-sensitive lipid-polymer hybrid nanoparticles | 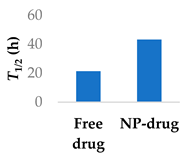 |
[75] |
| 5 | Afatinib | SLN in PLGA large porous particles | 81 h (NP-drug) | [76] |
| 6 | Carmustine | PLGA-chitosan core-shell nanoparticles | 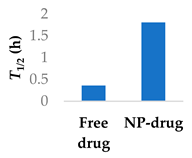 |
[77] |
| Entry | Drug | Nanoparticle type | Targeting moiety | Significant findings | Ref. | |||
|---|---|---|---|---|---|---|---|---|
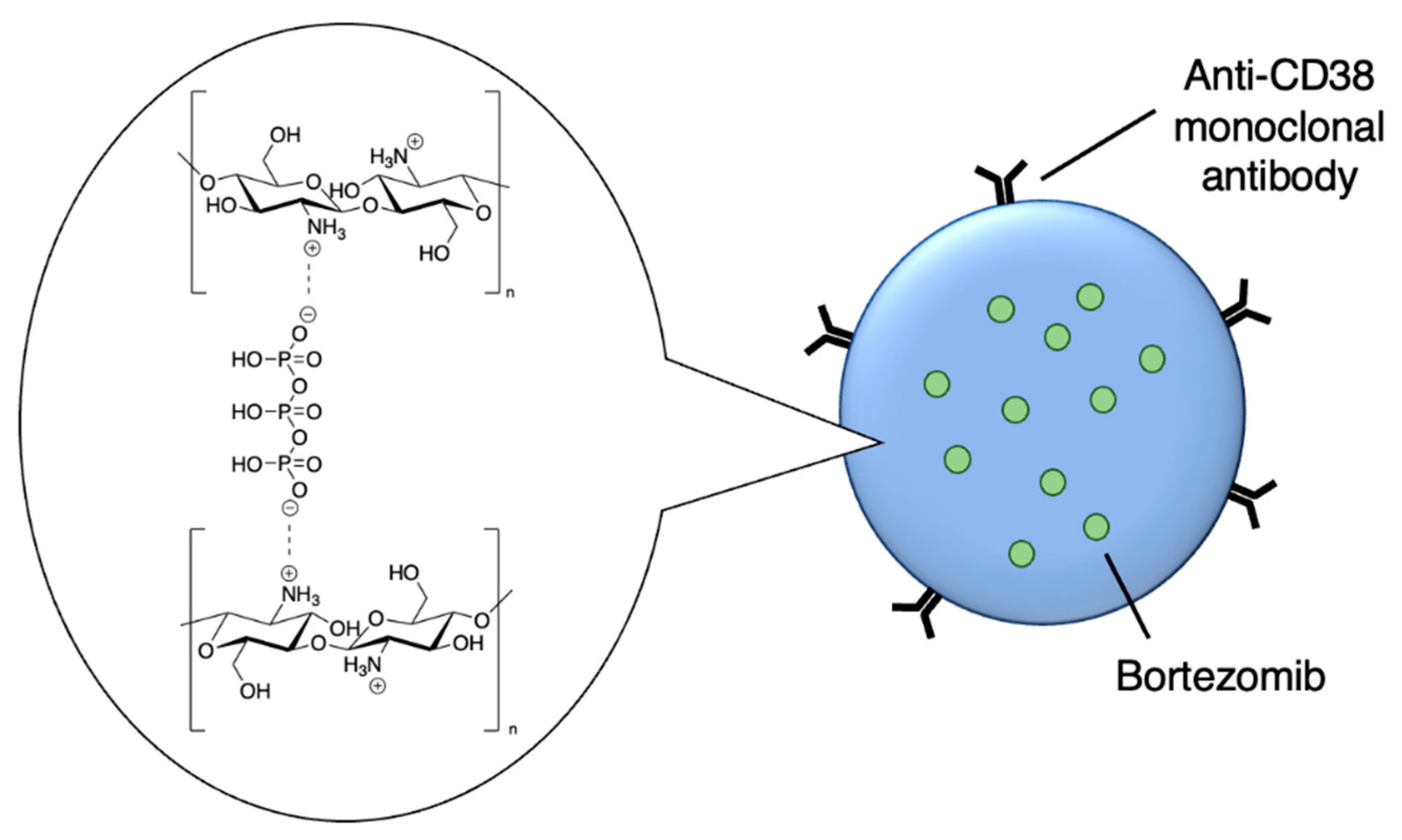
| 1 | Bortezomib | Crosslinked chitosan nanoparticles | CD38-targeting antibody | 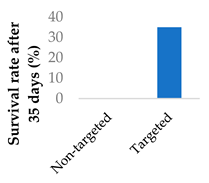 |
[82] | |||
| 2 | 5-Fluorouracil | PEGylated liposomes | Folate | 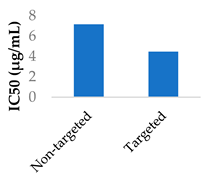 |
[83] | |||
| 3 | Mitomycin C | PEG-lipid-PLA-SPC hybrid nanoparticles | Folate | 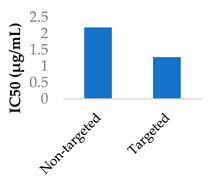 |
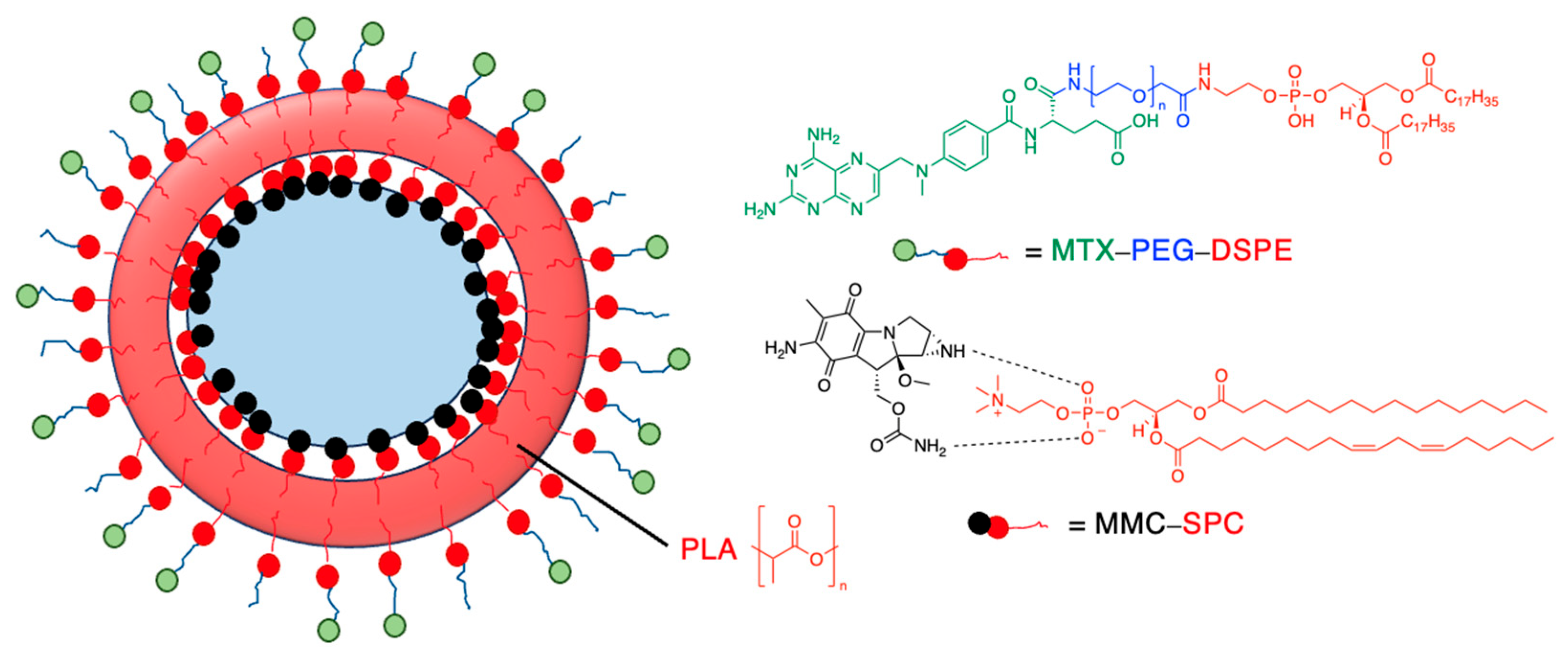
[84] | |||
| 4 | Afatinib | Lipid-polymer hybrid nanoparticles | Transferrin | 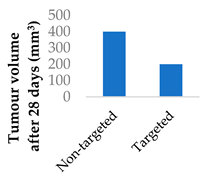 |
[75] | |||
| 5 | Decitabine | Lipid-polymer hybrid nanoparticles | Alendronate | 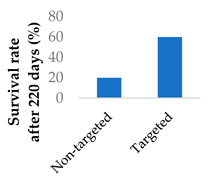 |
[85] | |||
| 6 | Mitomycin C | Terpolymer-lipid hybrid nanoparticles | Peptide iRGD | 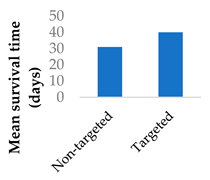 |
[86] | |||
| 7 | Sotorasib | Self-assembled hyaluronic acid-TPP nanoparticles | Hyaluronic acid | Significantly higher killing effect on mutant p53 cells vs normal and non-mutant carcinoma cells | [87] |
| Entry | Drug | Nanoparticle type | Release kinetics | Ref. |
|---|---|---|---|---|
| 1 | Mitomycin C | PLA-SPC nanoparticlesa | 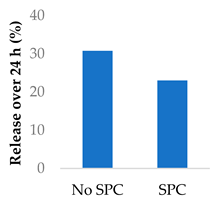 |
[93] |
| 2 | Mitomycin C | PLA-SPC nanoparticles | 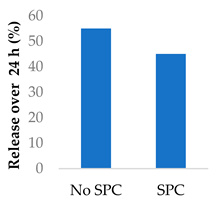 |
[94] |
| 3 | Mitomycin C | PEG-lipid-PLA-SPC hybrid nanoparticles | 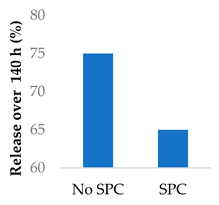 |
[95] |
| 4 | Mitomycin C | PEGylated liposomes | 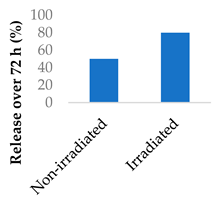 |
[96] |
| 5 | 5-Fluorouracil | Magnetite nanographene oxide PCL nanoparticles | 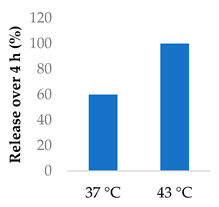 |
[97,98] |
| 6 | Afatinib | PEG-P(Asp(DBA)-co-Phe) polymeric nanovesicles | 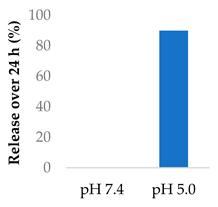 |
[99] |
| 7 | Mitomycin C | Crosslinked PVA-SA nanoparticles | 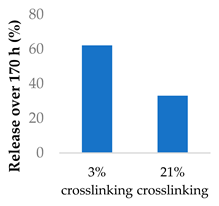 |
[100] |
| 8 | Ampicillin | PVA/chitosan nanofibers | 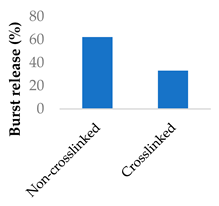 |
[101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





