1. Introduction
Clinical trials represent a critical step in the development of new medical treatments, providing essential data on their safety and effectiveness [
1,
2,
3]. As the primary method through which new drugs, devices, and interventions are tested before they reach the general population, clinical trials play a pivotal role in advancing medical science and improving patient care [
4]. The landscape of clinical trials encompasses numerous study designs, objectives, and methodologies, all tailored to answer specific scientific questions [
5].
Over the years, the design and conduct of clinical trials have evolved significantly. Traditional randomized controlled trials (RCTs), long considered the gold standard, are now being complemented by adaptive designs, decentralized trials, and the use of real-world evidence [
3]. These innovations are driven by a need to make trials more efficient, more patient-centered, and better suited to the complexities of modern medicine [
6,
7].
However, clinical trials face numerous challenges that can hinder their effectiveness and efficiency. Ethical considerations related to informed consent and the balance of risk and benefit, are paramount. Additionally, recruiting and retaining participants remains a significant hurdle, particularly in trials involving minority populations, rare diseases, or pediatric and geriatric [
8,
9,
10,
11]. This study provides a comprehensive overview of the current landscape of clinical trials, examining the trends, challenges, and innovations that are shaping the field today.
2. Materials and Methods
To assess the current landscape of clinical trials, we compiled a list of clinical trials and assembled corresponding information for each clinical trial using the ClinicalTrials.gov [
12] database, a publicly accessible database managed by the National Institutes of Health (NIH). The dataset was extracted on July 15, 2024, and included all trials registered up to that date. Each clinical trial was identified by its unique National Clinical Trial (NCT) number, and data were collected on various parameters, including the NCT number, study status, study results, type of intervention, sponsor, collaborators, funder name, funder type, study design, trial phase, enrollment numbers, primary completion days, days to trial completion, available study documents, geographic distribution of trial, country, participants sex, age group and condition/disease for which clinical trial was conducted.
Once the inclusion criteria were established, we added new columns using annotated data using different filter criteria on the search menu bar. First, we calculated total duration of clinical trials subtracting the study start date from the study completion date for each clinical trial that was available in data table extracted from ClinicalTrials.gov webpage. We also added new column days to primary completion using the study start date and primary completion date data. Additionally, wherever available, we also extracted country name information from the location column for each clinical trials and annotated each clinical trial with new country column. For trials with multiple locations, we mined all the country name and annotated with NCT number.
We used the search bar options “condition/disease” to extract clinical trials related to disease/conditions that are listed on the 15 leading causes of death in the United States by the Centers for Disease Control and Prevention (CDC) [
13]. The ranking of the disease list was based on the provisional mortality statistics as of July 15, 2024. We extracted the NCT number for those clinical trials that appeared on the search criteria under “condition/disease” search bar for different disease of query. For the clinical trials that appeared in 2 or more criteria, we counted those clinical trials in diseases name they appeared. We annotated diseases name and added to the master table. The condition/disease search terms and synonyms of conditions or diseases are listed in
Supplementary Table 1.
For the number of trials by year plot (
Figure 1a), we only selected those clinical trials that has complete information on study start date. Data mining, visualization, and statistical analysis were performed using different R packages using R version 3.6.3 [
14] (R Foundation for Statistical Computing, Vienna, Austria) and R Studio. Descriptive statistics were used to summarize the data, and trends were visualized to identify patterns over time. Statistical tests were employed to compare differences between trial characteristics, and temporal trends were analyzed to understand the evolution of clinical trial practices.
The list of clinical trials and information associated with each trail is not exhaustive as list also does not include other clinical trials that are not registered on ClinicalTrials.gov webpage or trials that are registered on webpage after 7am July 15, 2024. Secondly, the list provided by ClinicalTrials.gov contains summaries that are abridged for public information and is not comprehensive in its entirety. Also, the list does not contain new updates to the existing clinical trials information after July 15, 2024.
3. Results
3.1. Overall Trends
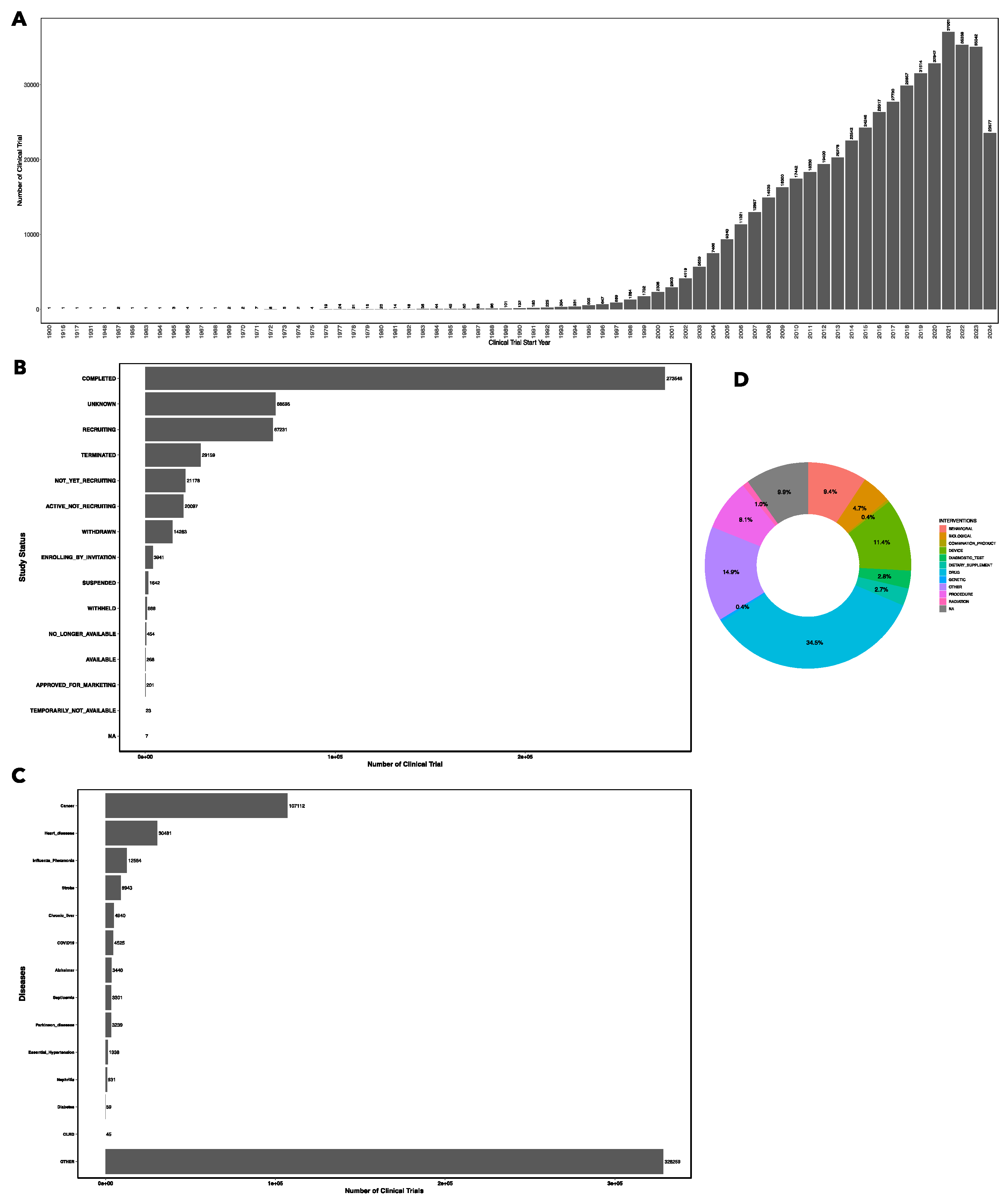
The analysis of data from ClinicalTrials.gov revealed several key trends and patterns in the landscape of clinical trials, spanning over a century of research. On July 15, 2024, the total number of clinical trials available on clinicaltrilas.gov webpage was 501515. The first recorded clinical trial dates back to 1900, and since then, the number of registered trials has grown steadily, with a significant peak in 2021, during which 37,061 trials were initiated followed by 35338 clinical trials initiated in 2022 (
Figure 1A). In this figure, we plot number of clinical trials started in each year up to 2024. Other clinical trials with missing start date and clinical trials with start date beyond 2024 were not shown in
Figure 1A.
3.2. Study Status of Clinical Trials
Among the trials analyzed, the majority (273,548) have been completed, while others are in various stages, including recruiting (67231), terminated (29159), not yet recruiting (21178), enrolling by invitation (3941), available (268) while only 201 clinical trials are approved for marketing (
Figure 1B). This distribution reflects the dynamic nature of clinical research, where trials are continuously starting, progressing, and concluding or terminating.
3.3. Clinical Trials by Disease/Condition
We annotated each clinical trials to 15 leading causes of death in the United States, as reported by the Centers for Disease Control and Prevention (CDC), we found cancer-related trials represent the largest category of studies by disease (107112), reflecting the high burden of this disease and the ongoing efforts to develop effective treatments. Other significant categories include trials focused on heart disease (30481), influenza (12564), stroke (8943) and chronic liver disease (4940). A substantial number of trials address conditions that are not among the leading causes of death, highlighting the broad scope of clinical research (
Figure 1C).
3.4. Types of Intervention/Treatment
Drug-related interventions are the focus of major clinical trials, accounting for 34.5% of all trials. Other types of interventions include device (11.4%), behavioral (9.4%), procedural interventions (8.1%), biological (4.7%), diagnostic test (2.8%), dietary supplements (2.7%) and radiation (1%) as shown in
Figure 1D. The diversity of intervention types illustrates the multifaceted nature of clinical research, which spans pharmacological, behavioral, and technological approaches to treatment.
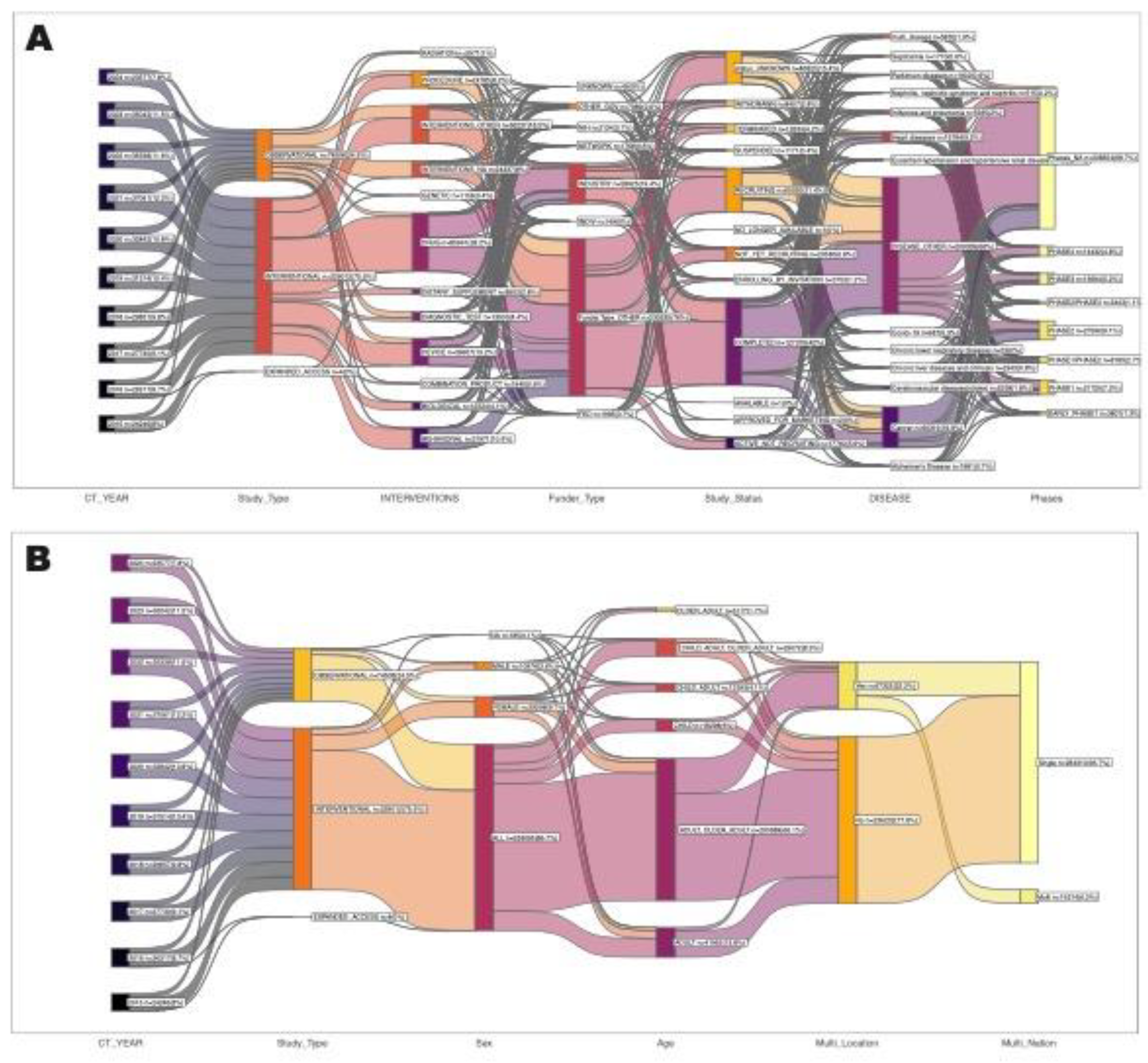
3.5. Nature of Clinical Trials
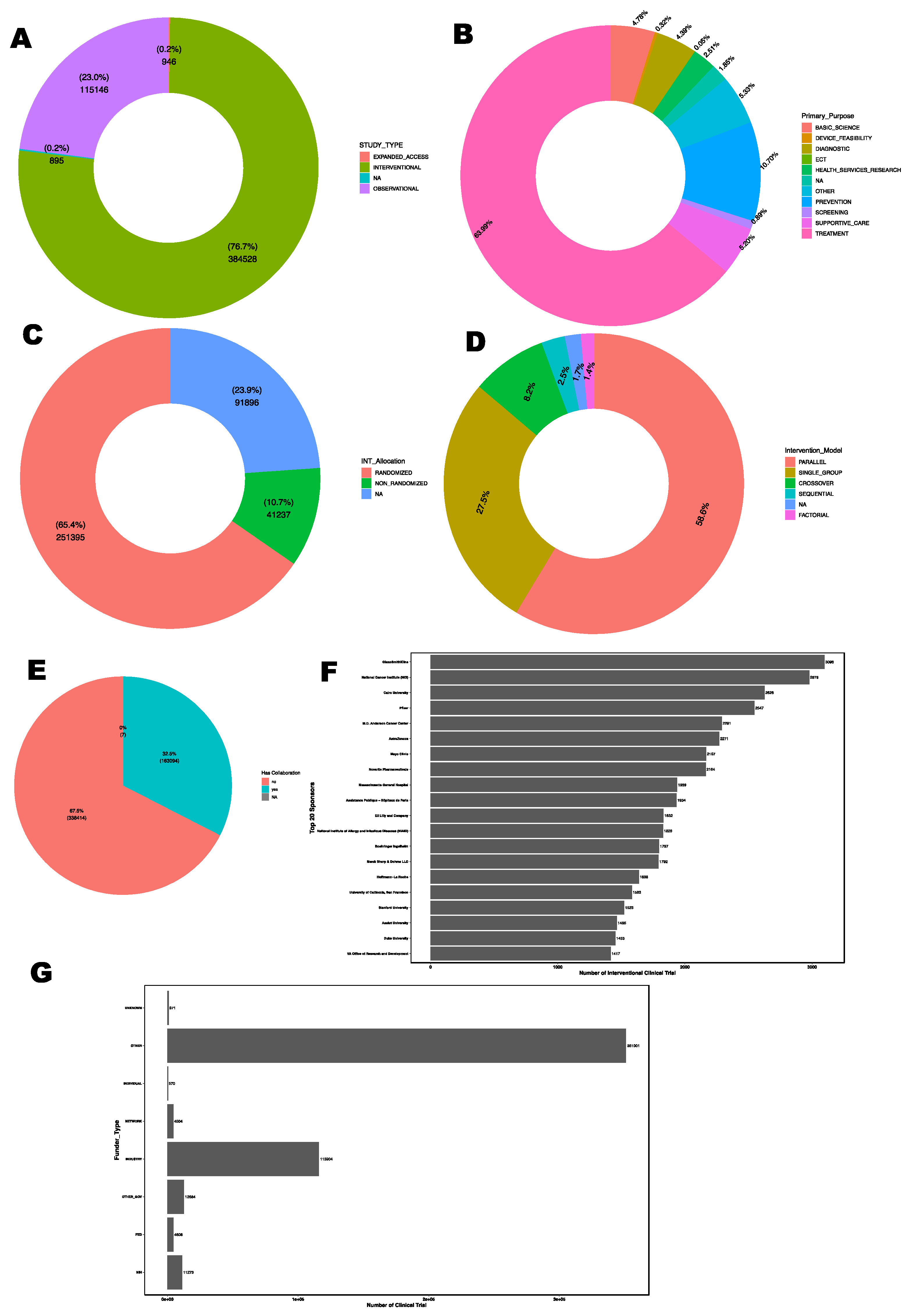
The majority of clinical trials are interventional (76.7%), where participants are assigned to receive specific interventions according to a protocol (
Figure 2A). Observational studies account for 23% of the trials, providing insights into outcomes without manipulating the study environment. Expanded access trials, which allow patients access to experimental treatments outside of a clinical trial, make up a small fraction (0.2%).
3.6. Purpose of Interventional Trials
For interventional type clinical trials, the major primary purpose is treatment (63.9%), followed by prevention (10.7%), supportive care (5.2%), basic science (4.78%), diagnostic (4.39%), health services research (2.51%), screening (0.89%), device feasibility (0.32%), and ECT (0.05%) as shown in
Figure 2B. These proportions highlight the central role of clinical trials in developing new treatments and preventive measures for various health conditions.
3.7. Randomization and Interventional Model
For interventional type clinical trials, randomized controlled trials (RCTs) remain the dominant study design, accounting for 65.4% of interventional trials whereas non-randomized accounted for 10.7% (
Figure 1C). Also, parallel model being the most common study model accounted for 58.6% of the interventional clinical trials followed by single group model (27.5%), and crossover model (8.2%) as shown in
Figure 1D.
3.8. Collaborations, Sponsorship and Funder Type
Approximately 32.5% of all clinical trials involve collaborations between multiple institutions or organizations (
Figure 2E). Industry sponsors, particularly pharmaceutical companies, play a leading role in funding and conducting interventional clinical trials, with GlaxoSmithKline being the most prominent sponsor. Government and academic institutions such as National Cancer Institute (NCI), MD Anderson Cancer Center and Mayo Clinic, also contribute significantly to interventional clinical trial sponsorship. The top 20 sponsors for interventional type clinical trials are summarized in
Figure 2F. In overall, industry sponsorship is the predominant source of funding for clinical trials, reflecting the commercial interest in developing new therapies. The National Institutes of Health (NIH) is a major non-industry sponsor, particularly for trials focused on public health priorities (
Figure 2G).
3.9. Trial Phases and Enrollments
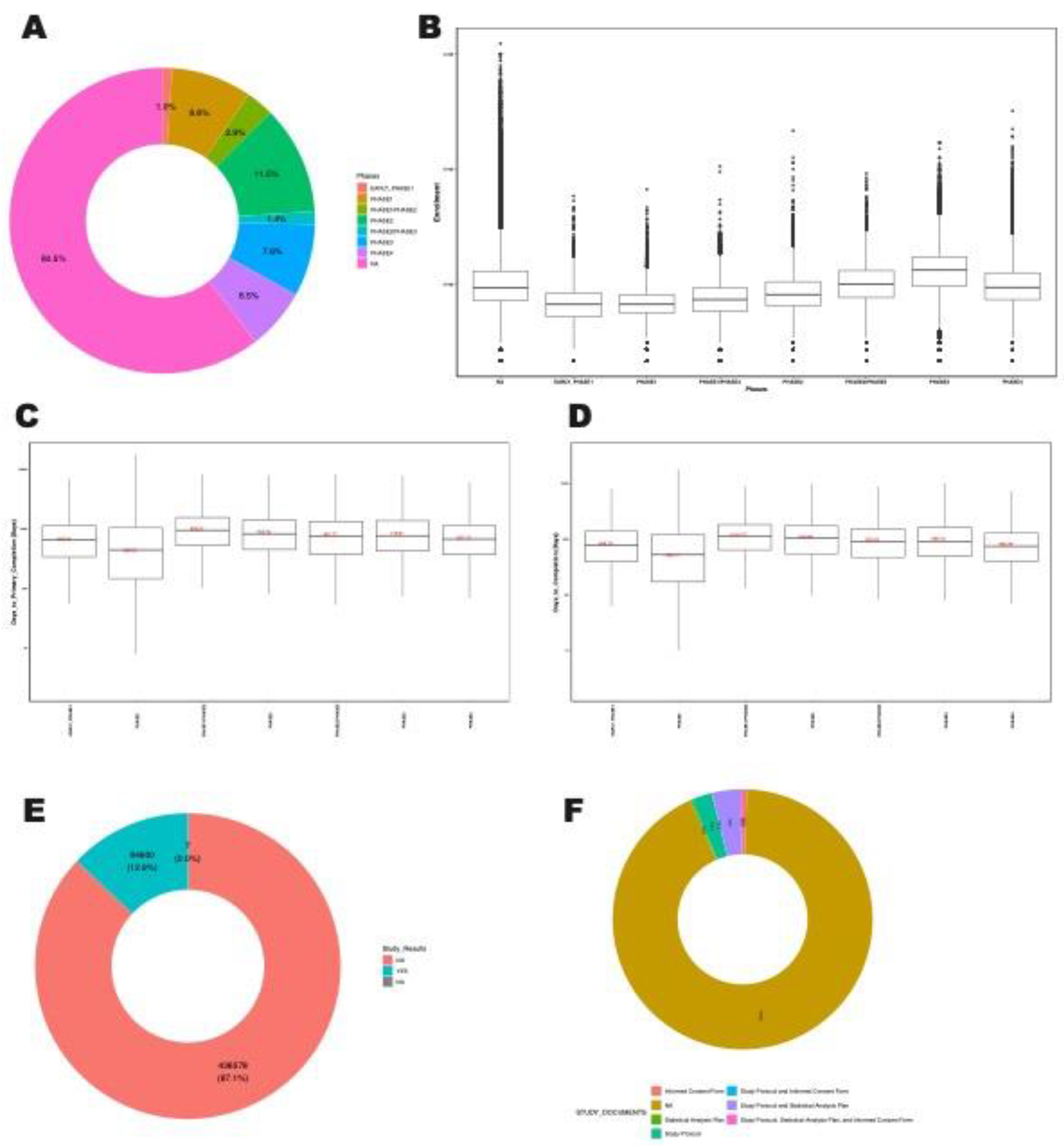
For the clinical trials with phase information, majority of clinical trials are on phase2, followed by phase1, phase3, phase4 and other phases (
Figure 3A). Phase 3 trials, which are critical for determining the efficacy of a treatment before it is approved for general use, have the largest median number of participants (
Figure 3B). This phase is crucial for ensuring that new treatments are safe and effective for a broad population.
3.10. Clinical Trial Duration, Reporting and Documentation
We observed the median time to primary completion varies by trial phase, with phase 1 generally being shorter with 364 days (
Figure 3C). Similarly, median time to completion varies by trial phase, with phase 1 generally being shorter with 434 days (
Figure 3D). Notably, only 12.9% of clinical trials have reported their results on ClinicalTrials.gov (
Figure 3E). Also, only 7.2% of clinical trials has provided study documents either in the form of informed consent form, statistical analysis plan, study protocol or some combinations (
Figure 3F). This indicates a gap in transparency and the dissemination of research findings.
3.11. Participant Demographics and Geographical Distribution
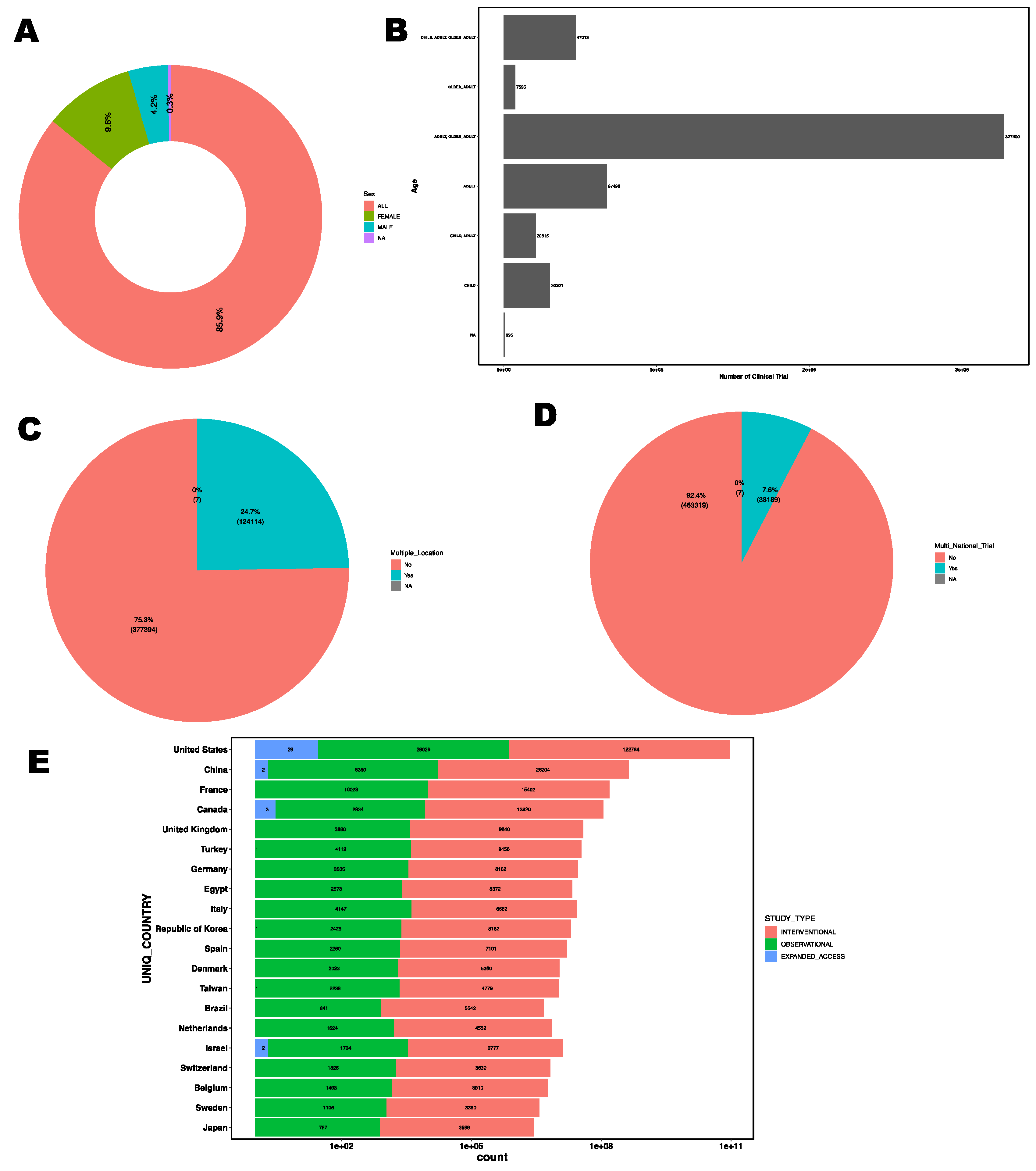
Total of 85.9% of clinical trials include both male and female participants, 9.6% trials were exclusively for female participants and 4.2% trials were exclusively for male participants (
Figure 4A). In relation to age group of the clinical trial participants, there is a significant focus on adult and older adult age group followed by adult only age group (
Figure 4B). Majority of clinical trials were conducted in limited geographical distribution. Only 24.7% of clinical trials were conducted in multiple location (
Figure 4C) and only 7.6% of the total clinical trials were multi-national clinical trials (
Figure 4D) which suggests barriers to international collaboration in clinical research. Notably, the United States hosts the majority of clinical trials, followed by China, France, Canda, and United Kingdom (
Figure 4E).
We were not able to access race and ethnicity distribution of participants. However, there is a noticeable underrepresentation of pediatric and elderly participants, as well as minority populations and low-income countries.
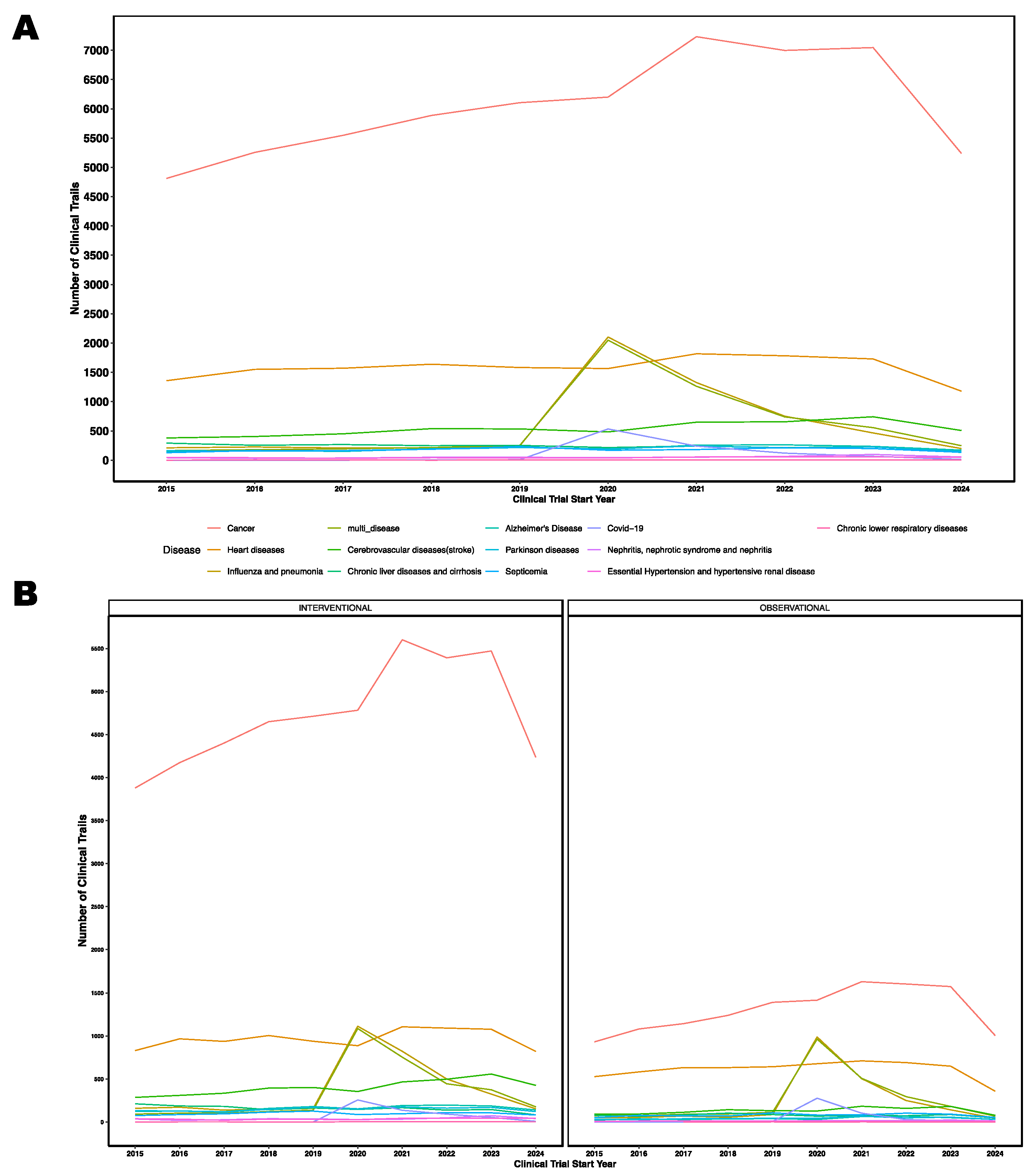
3.12 Trends in Last Decade
Data analysis of clinical trials with start date 2015 to 2024 annotated to disease or conditions revealed a consistent increase in cancer-related trials in last decade, reflecting ongoing efforts to develop new treatments for this complex disease. Additionally, there was a notable surge in influenza trials during the COVID-19 pandemic, underscoring the impact of global health emergencies on clinical research priorities (
Figure 5A). Also, there are more interventional type clinical trials compared to observational clinical trials (
Figure 5B). For the last decade, we also summarized the trends of study type, intervention type, funder type, study status, disease or conditions in
Figure 6A, and participants demographics in
Figure 6B.
4. Discussion
This comprehensive analysis of clinical trials registered on ClinicalTrials.gov provides valuable insights into the trends, challenges, and opportunities in clinical research over the past century. The steady increase in the number of trials reflects the growing complexity of modern medicine and the need for rigorous evaluation of new therapies. The peak in trial registrations in 2021 is particularly noteworthy and can be attributed to the global response to the COVID-19 pandemic, which catalyzed a surge in research activity [
15].
The analysis of trial statuses highlights the dynamic nature of clinical research. While a significant number of trials have been completed, a substantial proportion remains ongoing, with some being terminated or suspended. These findings underscore the challenges inherent in clinical trial conduct, including issues related to participant recruitment, funding, and regulatory approval [
16]. The predominance of cancer-related trials aligns with the high global burden of this disease, but it also raises concerns about the equitable distribution of research resources. Diseases that are less common or less commercially viable may not receive the same level of research attention, potentially leaving significant gaps in medical knowledge and treatment options [
17,
18]. The focus on drug-related interventions and the prevalence of randomized controlled trials reflects the central role of pharmaceuticals and rigorous testing methodologies in clinical research. However, the growing interest in other types of interventions, such as behavioral and procedural, indicates a broadening of research focus to include non-pharmacological approaches to health and disease management.
The analysis of funding and sponsorship reveals the dominance of industry sponsors, which suggests a strong commercial influence on clinical research. While industry sponsorship is crucial for advancing drug development and bringing new therapies to market, it also raises concerns about potential conflicts of interest and the focus of research on commercially viable products rather than public health needs [
19]. Government and academic institutions play a significant role, particularly in funding research that addresses less profitable but equally important health issues, such as rare diseases and public health interventions.
One of the major challenges identified in the analysis is the underrepresentation of certain demographic groups, particularly pediatric, elderly, and lack of data reporting on race and ethnicities. This lack of diversity in clinical trial participants limits the generalizability of research findings and can lead to disparities in treatment outcomes across different population groups. Ensuring that clinical trials are inclusive, and representative is critical for developing therapies that are effective and safe for all segments of the population [
20]. The geographical distribution of clinical trials shows a concentration in high-income countries [
21,
22], particularly the United States. While this reflects the strong research infrastructure and funding available in these regions, it also highlights the barriers to conducting clinical research in low- and middle-income countries. These barriers include limited resources, regulatory challenges, and difficulties in recruiting and retaining participants. Expanding clinical research to these regions is essential for addressing global health challenges and ensuring that treatments are relevant and accessible to diverse populations worldwide [
23]. The trends observed since 2015, particularly the increase in cancer and influenza-related trials, reflect the responsiveness of the clinical research community to emerging health challenges. The surge in influenza trials during the COVID-19 pandemic is a clear example of how global health emergencies can drive research priorities and lead to rapid advancements in the understanding and treatment of infectious diseases [
15].
Despite these advances, there remains a significant gap in the reporting of trial results. The finding that only 12.9% of trials have reported their results on ClinicalTrials.gov and only 7.2% of clinical trials has provided study documents highlights a critical issue in the transparency and dissemination of clinical research findings. This lack of reporting not only undermines the scientific value of the research but also delays the translation of new knowledge into clinical practice [
24]. Improving the reporting and sharing of trial results is essential for maximizing the impact of clinical research and ensuring that findings contribute to the broader body of medical knowledge.
The future of clinical trials will likely be shaped by several key trends and innovations aimed at addressing the current challenges and advancing the field. Some of the most promising directions for future research and development in clinical trials will be towards personalized medicine and precision medicine, decentralized and virtual trials, integration of real-world evidence (RWE), adaptive trial designs, global collaboration and harmonization, ethical and regulatory innovations, and enhanced participant engagement [
25].
5. Conclusions
In conclusion, the landscape of clinical trials is rapidly evolving, driven by advances in technology, a greater emphasis on patient-centered care, and the need to address complex and emerging health challenges. By embracing these future directions, the field of clinical trials can continue to advance, ultimately leading to more effective, safe, and equitable treatments for patients worldwide.
Supplementary Materials
The following supporting information can be downloaded at:
Preprints.org, Table S1: Synonyms of conditions or diseases
Author Contributions
Conceptualization, M.B.; methodology, G.J., S.T., A.A, and H.G.; software, G.J., S.T., A.A, and H.G.; validation, T.K.B., P.J., S.B., S.R.A., A.J., S.K.; formal analysis, G.J., T.K.B., P.J., S.B., S.R.A., A.J., S.K., S.T., A.A, and H.G.; investigation, G.J. and M.B.; resources, G.J. and M.B.; data curation, T.K.B., P.J., S.B., S.R.A., A.J., S.K., S.T., A.A, and H.G.; writing—original draft preparation, G.J., T.K.B, P.J. and S.B.; writing—review and editing, M.B.; visualization, G.J. and M.B.; supervision, M.B.; project administration, G.J., T.K.B. and P.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Learn About Studies | ClinicalTrials.gov. https://clinicaltrials.gov/study-basics/learn-about-studies#q1.
- Schultz, A., Saville, B. R., Marsh, J. A. & Snelling, T. L. An introduction to clinical trial design. Paediatric Respiratory Reviews 32, 30–35 (2019). [CrossRef]
- Introduction and History of Clinical Trial Research. in Introduction to Adaptive Trial Designs and Master Protocols (eds. Mills, E. J., Wathen, J. K. & Park, J. J. H.) 1–20 (Cambridge University Press, Cambridge, 2023).
- Ballman, K. V. Introduction to Clinical Trials, Clinical Trial Designs, and Statistical Terminology Used for Predictive Biomarker Research and Validation. in Predictive Biomarkers in Oncology: Applications in Precision Medicine (eds. Badve, S. & Kumar, G. L.) 19–36 (Springer International Publishing, Cham, 2019). [CrossRef]
- What Are Clinical Trials? - NCI. https://www.cancer.gov/research/participate/clinical-trials/what-are-clinical-trials (2023).
- Adesoye, T., Katz, M. H. G. & Offodile, A. C. Meeting Trial Participants Where They Are: Decentralized Clinical Trials as a Patient-Centered Paradigm for Enhancing Accrual and Diversity in Surgical and Multidisciplinary Trials in Oncology. JCO Oncol Pract 19, 317–321 (2023). [CrossRef]
- Tong, A. et al. Patient-centred clinical trial design. Nat Rev Nephrol 18, 514–523 (2022). [CrossRef]
- Ghadessi, M. et al. Decentralized clinical trials and rare diseases: a Drug Information Association Innovative Design Scientific Working Group (DIA-IDSWG) perspective. Orphanet Journal of Rare Diseases 18, 79 (2023). [CrossRef]
- Jacobson, R. M., Pignolo, R. J. & Lazaridis, K. N. Clinical Trials for Special Populations: Children, Older Adults, and Rare Diseases. Mayo Clinic Proceedings 99, 318–335 (2024). [CrossRef]
- Examination of Clinical Trial Costs and Barriers for Drug Development. ASPE https://aspe.hhs.gov/reports/examination-clinical-trial-costs-barriers-drug-development-0 (2014).
- Mohan, S. V. & Freedman, J. A Review of the Evolving Landscape of Inclusive Research and Improved Clinical Trial Access. Clinical Pharmacology & Therapeutics 113, 518–527 (2023). [CrossRef]
- Home | ClinicalTrials.gov. https://clinicaltrials.gov/.
- CDC WONDER. https://wonder.cdc.gov/.
- R: The R Project for Statistical Computing. https://www.r-project.org/.
- Mullard, A. Shifts in the clinical trial landscape. Nature Reviews Drug Discovery 23, 239–239 (2024). [CrossRef]
- Briel, M. et al. Exploring reasons for recruitment failure in clinical trials: a qualitative study with clinical trial stakeholders in Switzerland, Germany, and Canada. Trials 22, 844 (2021). [CrossRef]
- Hilgers, R.-D., FranzKönig & Senn, G. M. and S. Design and analysis of clinical trials for small rare disease populations. Journal of Rare Diseases Research & Treatment 1, (2016).
- May, M. Rare-disease researchers pioneer a unique approach to clinical trials. Nature Medicine 29, 1884–1886 (2023). [CrossRef]
- Sessler, D. I., Alman, B. A., Treggiari, M. M. & Mont, M. A. Pro-Con Debate: Interdisciplinary Perspectives on Industry-Sponsored Research. The Journal of Arthroplasty 38, 986–991 (2023). [CrossRef]
- Shyr, C., Sulieman, L. & Harris, P. A. Illuminating the landscape of high-level clinical trial opportunities in the All of Us Research Program. Journal of the American Medical Informatics Association ocae062 (2024). [CrossRef]
- Yin, S. et al. Disparities in COVID-19 clinical studies from high-income and low-and middle-income countries. International Journal of Infectious Diseases 132, 9–16 (2023). [CrossRef]
- Ramanan, M., Tong, S. Y. C., Kumar, A. & Venkatesh, B. Geographical Representation of Low- and Middle-Income Countries in Randomized Clinical Trials for COVID-19. JAMA Network Open 5, e220444 (2022). [CrossRef]
- Payedimarri, A. B., Mouhssine, S., Aljadeeah, S., Gaidano, G. & Ravinetto, R. Globalisation of industry-sponsored clinical trials for breast, lung and colon cancer research: trends, threats and opportunities. bmjonc 2, (2023). [CrossRef]
- Sheng, J., Feldhake, E., Zarin, D. A. & Kimmelman, J. Completeness of clinical evidence citation in trial protocols: A cross-sectional analysis. Med 3, 335-343.e6 (2022). [CrossRef]
- Subbiah, V. The next generation of evidence-based medicine. Nat Med 29, 49–58 (2023). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).