1. Introduction
Bronchopulmonary dysplasia (BPD) remains the most significant respiratory disease among preterm neonates. Despite advances in pre and postnatal care, BPD continues to be associated with increased mortality rates and long-term morbidity, exerting a considerable economic burden on health services [
1,
2,
3].
The etiology of BPD is multifactorial with pre and postnatal factors contributing to its pathogenesis: preterm birth, genetic predisposition, prenatal infection and inflammation, mechanical ventilation, oxygen toxicity, patent ductus arteriosus and postnatal infection [
4,
5].
Since its first description in 1967 by Northway et al, the clinical picture of BPD has changed, due to significant advances in care (eg. antenatal steroids, surfactant) [
6]. In the pre-surfactant era, the so-called “old” BPD was the end result of severe lung injury caused by aggressive mechanical ventilation and high oxygen supplementation in relatively mature preterm infants. BPD was defined by the presence of persistent respiratory signs and symptoms, the need for supplemental oxygen to treat hypoxemia, and an abnormal chest radiograph at 36 weeks postmenstrual age [
7].
After the introduction of surfactant, with improved survival of extremely premature neonates, the clinical picture of BPD changed. The so-called “new” BPD often develops in preterm neonates in the canalicular or saccular stage of lung development, at least in the first postnatal days. An imbalance between pulmonary inflammation and lung repair seems to play a key role in the pathogenesis of the “new” BPD [
8,
9,
10], characterized by abnormal lung development or arrest in lung maturation.
Currently, the diagnostic criteria for BPD include treatment with oxygen above 21% for at least 28 days and an assessment of the type of respiratory support at 36 weeks of postmenstrual age (PMA) to define the severity of BPD [
11].
Despite recent advances in perinatal care (such as the introduction of prenatal steroid use, surfactant treatment, a shift towards non-invasive ventilation as a first-line form of respiratory support, improved nutrition, etc) which have led to increased survival of very preterm babies, the overall incidence of BPD has remained unchanged over the past decade. For babies born before 32 weeks, also known as very preterm infants, the incidence of BPD can vary significantly between different medical centers, ranging from 11 to 50% [
2,
12,
13].
At present, there is no specific and effective treatment for BPD and the current management of neonates at risk of BPD is based on strategies to prevent and/or minimize lung damage (eg preference for non-invasive ventilation, targeting lower oxygen saturation levels, glucocorticoids to allow earlier extubation, vitamin A supplementation) [
9,
14].
Early identification of neonates who will later develop BPD is of paramount importance. In fact, it could enable intervention at an early disease stage, when it may be potentially more effective while avoiding unnecessary exposure to potentially hazardous therapies in infants not at risk. Moreover, it could provide new insights into the pathophysiology of BPD and facilitate the development of new therapies [
15].
In the last years researchers, following the development of “omic” sciences to evaluate metabolites, gene and protein expression, focused their studies on identifying biochemical markers in different matrices to predict infants who will go on to develop BPD [
4].
Some studies have analyzed cytokines and surfactant components in the lung fluid, exhaled breath or tracheal aspirates and exhaled temperature to identify potential biomarkers. Although different biomarkers have been proposed, none have been validated for clinical use.
Exhaled breath condensate (EBC) is a relatively easily sampled biofluid that provides non-invasive identification of lung disease. EBC contains aerosolized airway lining fluid, that is similar to the lining liquid of the airways, and the volatile organic compounds (VOCs) [
16].
VOCs are chemical substances produced during both physiological and pathophysiological processes and can be obtained non-invasively from different matrices (e.g., feces, sweat, exhaled breath condensate, urine, etc.). Numerous studies during the last decades have evaluated the potential role of VOCs in the diagnosis of several metabolic, malignant, infectious and inflammatory diseases [
17].
Several VOCs contained in the EBC derive from hydrocarbons and are the result of metabolic processes that can alter ongoing pathological and inflammatory processes. Therefore, they could potentially be ideal biomarkers of BPD [
18].
VOCs can be analyzed using various technologies, including the electronic nose (e-nose), which is an innovative biomimetic device that simulates the olfactory system. The e-nose has been previously used to evaluate various pulmonary pathologies in adult subjects, such as asthma, chronic obstructive pulmonary disease, respiratory infections, lung cancer, and obstructive sleep apnea syndrome [
19,
20]. The analysis of volatile organic compounds (VOCs) in exhaled breath (EBC) is helpful in diagnosing and monitoring respiratory diseases in children. The changes in resistance of each of the sensors are recorded and collected in an onboard database for generating a distribution (smellprint) that describes the VOCs mixture and that can be used for pattern recognition algorithms. The measurement of metabolomic profiles may have important advantages over detecting single markers.
To the best of our knowledge, this study is one of the very few to report on the use of e-nose in neonates.
The objective of this pilot study is to evaluate whether the e-Nose technology allows early identification of premature infants who will later develop BPD by analyzing VOCs profile in the EBC.
2. Materials and Methods
Study design and participants
The study sample was composed of 14 extremely low gestational age (⩽31 weeks) infants (8 females and 6 males) admitted to the Neonatal Intensive Care Unit, Azienda Ospedaliera Universitaria Integrata of Verona, Italy.
This study was performed in accordance with the Declaration of Helsinki. This human study was approved by “Comitato Etico per la sperimentazione clinica di Verona (Italy)” - approval: CESC37. The study was not registered as a clinical trial because was a pilot study.
All parents, guardians or next of kin provided written informed consent for the minors to participate in this study.
Infants born at a gestational age of ⩽ 31 weeks of either gender and who required intubation and mechanical ventilation were eligible to participate in this study. Exclusion criteria included not intubated infants, congenital heart diseases, respiratory tract congenital malformations/diseases (cystic fibrosis, adenoid cystic pulmonary malformations, esophageal atresia, diaphragmatic hernia), genetic disorders and other major congenital malformations.
Infants diagnosed with BPD, defined as supplemental oxygen treatment for at least 28 days of life and requiring positive pressure support and/or receiving oxygen at 36 weeks postmenstrual age (PMA), were allocated to the BPD group. The remaining infants without BPD [
8] served as a control group. None presented pulmonary hypertension (PH).
Clinical examination and collection of EBC for e-nose VOCs profiling were performed during the first 24 hours of life. In addition, data about mechanical ventilation, supplementary oxygen requirements, postnatal infection/sepsis, medication use and feeding pattern was prospectively collected for each included infant.
Exhaled breath condensate (EBC)
EBC from infants on conventional mechanical ventilation was collected within the first 24 hours of life. EBC samples were collected from the expiratory limb of the ventilator circuit via a valve-less connector. The sample collection adhered to the guidelines set by the European Respiratory Society and the American Thoracic Society (ATS/ERS) [
17], which outline the method for collecting EBC in adult and pediatric patients over 3-4 years of age.
However, no guidelines exist for sample collection in intubated and ventilated newborns. Consequently, we adapted the European guidelines to this particular category of patients.
To ensure the integrity of samples and the standardization of the procedure, the condensate collector was kept inside a temperature-controlled condenser, as illustrated by Hunt [
16]. A specific ventilation circuit (Fisher and Paykel, RT225) was utilized, equipped with a collection chamber for EBC integrated with the expiratory phase of the circuit itself. This setup ensures adequate and constant pressure and oxygenation as imposed by the ventilator. Sample integrity was further preserved by placing the EBC cooling trap in an ice bath, applied it to the container dedicated to EBC collection, as illustrated by Hunt and Kononikhin [
16,
21,
22].
The collection process lasted at least four to five hours for each patient, yielding approximately 5 ml of condensate. Samples were then promptly stored in aliquots within polypropylene tubes at -80°C at the Medical Research Laboratory of the University of Verona, Pediatrics section. The samples were kept in this location until e-nose analysis. For each patient and each e-nose probe, three replicated measurements were obtained.
Electronic nose analysis
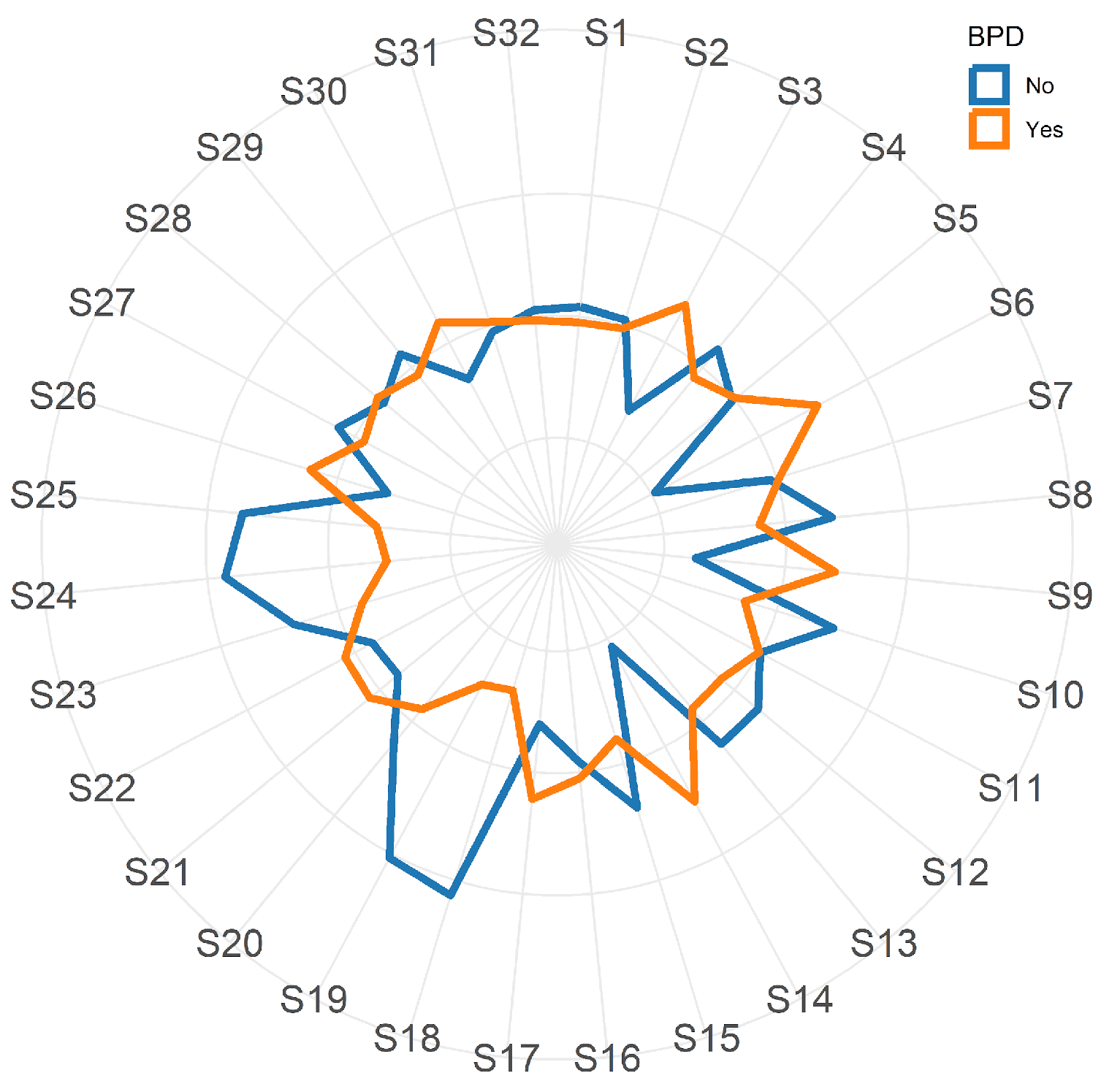
EBC samples were analyzed with a commercial e-nose (Cyranose 320; Smith Detections, Pasadena, CA, USA) with a nanocomposite array of 32 organic polymer sensors. When the sensors are exposed to a mixture of VOCs, the polymers swell, thus inducing a change in their electrical resistance.
The measure was performed by analysis of the VOCs present in the headspace of the tube. To ensure homogeneous study conditions, samples were thawed to room temperature, then maintained in a bath at 37°C for 30 min to increase the concentration of headspace VOCs, and then analyzed in random order. In order to standardize the measurements obtained, we performed a purge of the sensors aimed at removing any remaining VOC. This washout corresponds to 1.5 times (90 seconds) than the analysis time (60s), as the method described by Visser and colleagues [
23]. Moreover, to standardize measurements, we performed three replicates of the analysis for each sample. The values of each probe were standardized, and all the subsequent statistical analyses were performed on the mean values of the standardized replicates.
The changes in resistance of each of the 32 sensors are recorded (and collected) in an onboard database and analyzed by machine learning methods: the distribution pattern generated, or smell-print, describes the VOCs mixture.
Statistical analysis
All subsequent statistical analyses were performed using the means derived from the three observed replicates. The values for each of the 32 probes across the 14 patients were standardized by subtracting the mean and dividing by the standard deviation. Continuous variables were presented as mean ± standard deviation (SD) and the significance of differences between the mean values of two independent samples was assessed using the two-tailed Student’s t-test. Categorical variables were summarized using frequency counts and percentages, with differences between proportions evaluated using Fisher’s exact test. A p-value of <0.05 was considered statistically significant.
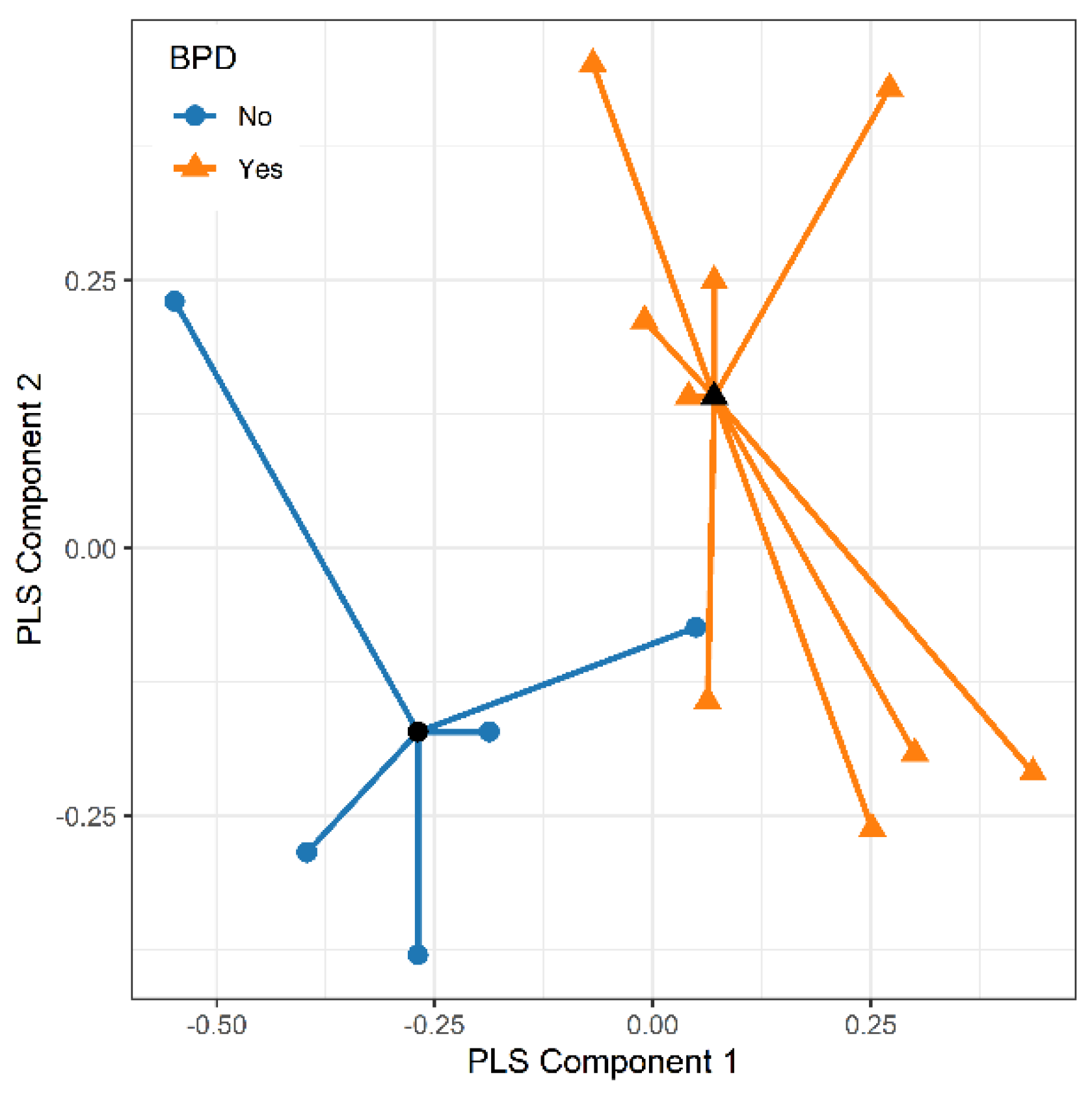
The potential of the 32 e-nose nanosensors to discriminate between the two study groups was initially explored using Partial Least Squares Discriminant Analysis (PLS-DA), a pattern recognition method for classification [
24]. This approach is particularly advantageous when handling datasets with numerous variables and relatively few samples. The predictive accuracy of PLS-DA was evaluated through repeated cross-validation which involves, at each repetition, splitting the data into training and testing sets, building the model on the training sets, and testing its ability to correctly classify the observations in the testing sets.
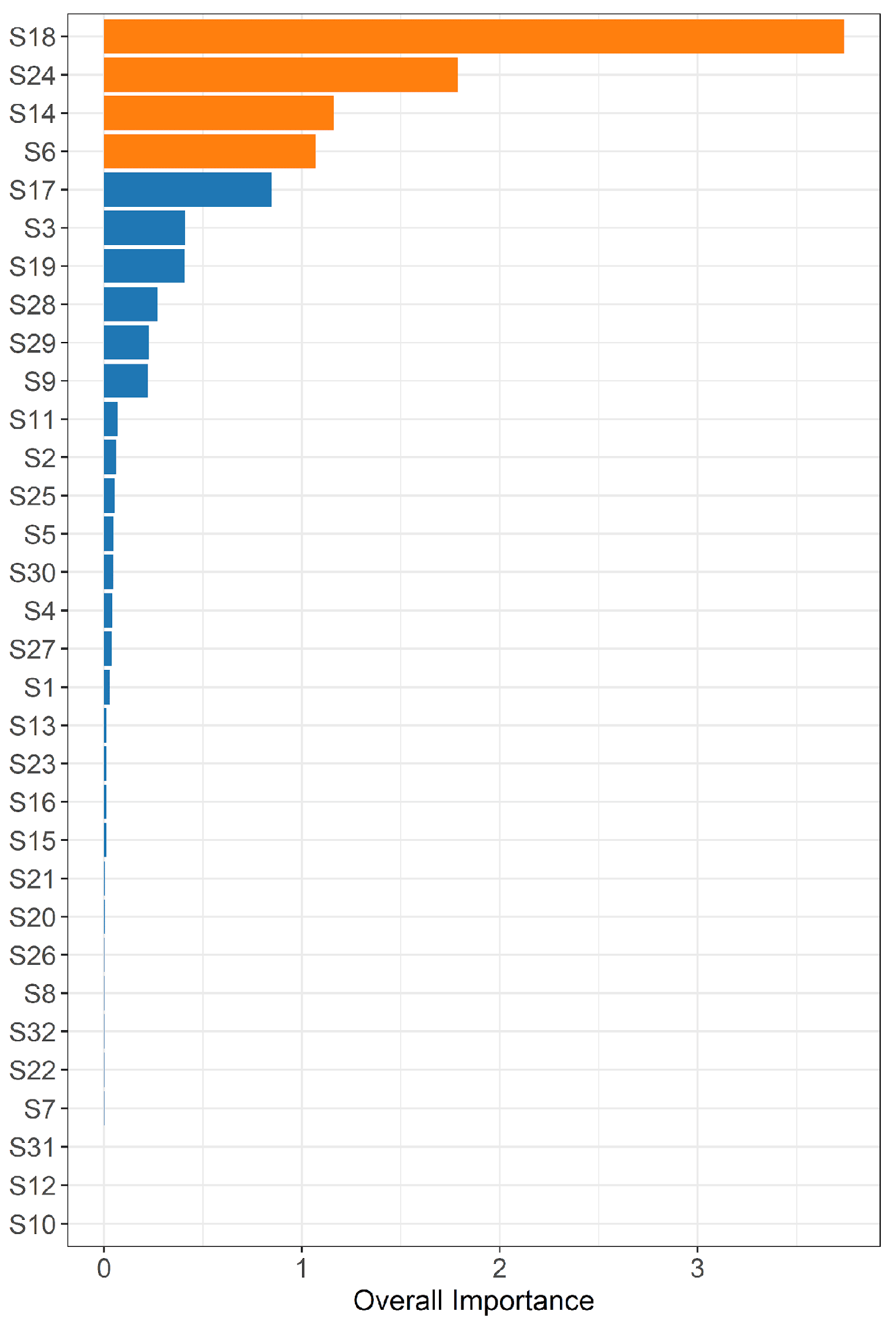
Two different methods were used to evaluate the discriminative ability of each nanoprobe: the estimation of variable importance with the use of a gradient-boosting machine [
25] and the application of exact logistic regression [
26]. The performance of predictive models was evaluated by calculating the area under the receiver operating characteristic curve (AUC).
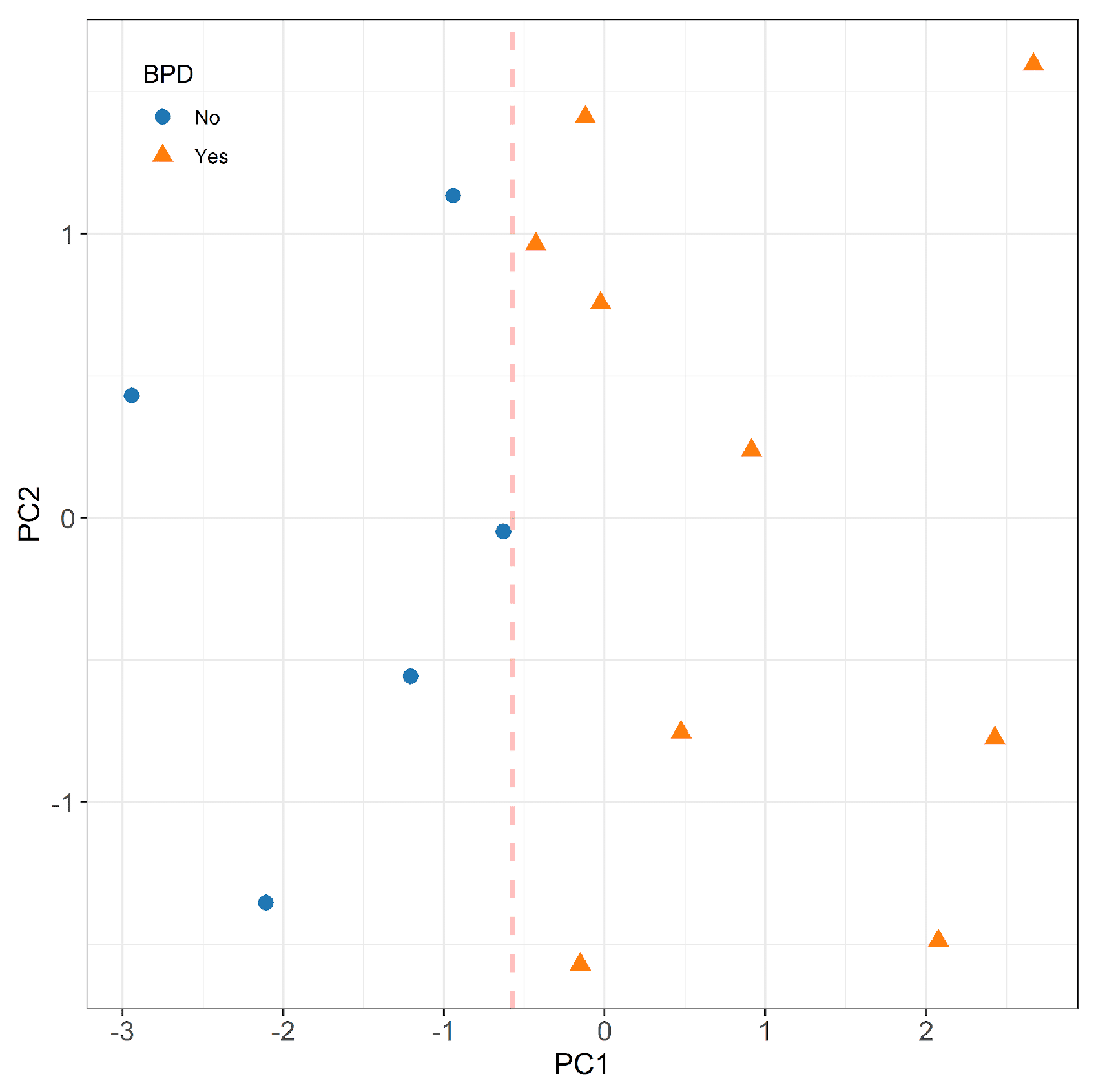
The responses of the most predictive nanosensors were further analyzed using principal component analysis (PCA) with varimax rotation. The e-nose patterns of the study patients were visualized on a plane using the first two principal scores, and a separating line estimated by logistic regression was drawn to discriminate the two groups. Additionally, a classification algorithm based on random forests [
27] was implemented using the selected nanosensors as predictors.
Data were analyzed using Stata software (Stata- Corp. 2017. Stata Statistical Software: Release 16. College Station, TX, USA: StataCorp LLC) and R version 4.1.3 (R Core Team (2021). Vienna, Austria.
https://cran.r-project.org/).
4. Discussion
To the best of our knowledge, this is the first study to assess VOCs profile in EBC using e-nose in a cohort of very preterm mechanically ventilated infants. The aim was to identify early biomarkers that could predict the subsequent development of bronchopulmonary dysplasia
E-nose is an innovative biomimetic technology that simulates the olfactory system to examine VOCs in various organic matrices, including EBC, urine, feces, and blood [
25,
26]. While e-nose has been utilized as an adjunctive diagnostic tool in the adult population, its application in pediatric patients, especially in non-cooperative patients such as preterm neonates, has been limited [
20].
The study of Rogosch et al. describes for the first time the smell prints of VOCS in the tracheal aspirates from preterm with or without bronchopulmonary dysplasia [
20]. Moreover, only a few studies evaluated VOCs in blood or feces as potential biomarkers of future BPD in preterms [
31]
In the current study, we found that VOCs profiles in exhaled breath condensate hold the potential for predicting the subsequent development of BPD. Analysis of the EBC from babies who will develop BPD, compared to those who will not, revealed that the e-nose device enabled to discriminate between the two groups soon after birth. VOCs were analyzed within the first 24 hours of life and four probes of e-nose showed a strong predictive ability of BPD with AUC above 0.8. Currently, the primary BPD prediction tool employed in clinical practice relies solely on clinical variables such as gestational age, birth weight, ethnicity, sex, respiratory support, and fraction of inspired oxygen. It is noteworthy that the predictive performance of this tool improves with advancing postnatal age, with the AUC rising from 0.79 on day 1 to 0.85 on day 28 [
32].
Although our findings are preliminary and require replication in a larger cohort, the analysis using e-nose suggests the potential for improved prediction of BPD starting from the first day of life. Moreover, the non-invasive and cost-effective nature of e-nose analysis positions it as a promising biomarker for later pulmonary disease. Notably, none of the previously studied biomarkers have been effectively implemented in clinical practice to identify children at risk of developing moderate to severe BPD [
33].
We speculate that serial analyses in the first days of life of VOCs profile by e-nose could further increase the predictive ability of BPD: the latter should be evaluated in a future study.
Great interest lies in the identification and validation of early biomarkers that can predict BPD development, since it could offer the potential to reduce health-care costs. Early diagnosis would allow for the initiation of therapies during the early stages of BPD, when there is a therapeutic window of opportunity [
34]. Furthermore, it would help avoid unnecessary treatments and their associated risks in infants who are not at risk of developing BPD [
35,
36].
Our study is subject to certain limitations. Firstly, due to the specific patient population, we conducted a pilot study with a limited sample size. The findings from this exploratory study should be validated in larger cohorts and further linked to inflammatory markers to strengthen their significance. Nonetheless, to mitigate the risk of overfitting, we employed cross-validation across all our data analyses. This statistical tool provides an honest and not overly optimistic assessment of the e-nose's prediction performance. Secondly, the present study did not include the utilization of other chemical analytical techniques, such as gas chromatography-mass spectrometry (GC-MS), to enable the identification of specific VOCs of BPD. This would represent a significant advancement in comprehending the diverse pathways involved in the pathophysiology of lung injury and identifying distinct forms of BPD, often referred to as BPD endotypes. Such understanding would pave the way for the development of tailored and personalized therapeutic strategies [
37].