Submitted:
07 September 2024
Posted:
09 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Occurrence and Availability of Antimicrobial Proteins and Peptides
| Sources | Sub-category | Approx. Number of AMPs | Examples | Ref. |
|---|---|---|---|---|
| Plants | Bryophyta to Angiosperms | 250 | Plant defensins, cyclotides, 2S albumin, lipid transfer proteins, hevein-like proteins knotins, snakins, purothionins | [9] |
| Animals | Mammals | 373 | Cathelicidins, Defensins, Bactenecin, Indolicidin, LAP, TAP, Dermcidin, Hepcidin 20, LL- 37 | [10] |
| Amphibians | 1179 | Magainins, Cathelicidin AL, Buforin, Bombinin, Fallaxin, Magainin, Palustrin 3a, Ranateurin, Phyllospetin | [11] |
|
| Fish | 146 | Pardaxins, misgurin, C, athelicidin BF, Crotamine, Pelovaterin, Omwaprin | [10] | |
| Reptiles | 52 | Cathelicidin BF, Crotamine, Omwaprin, Pelovaterin | [12] | |
| Birds | 47 | dCATH, AvBD1, chCATH-B1, Fowlicidin 1, CHP2 | [13,14] | |
| Mollusca | 54 | Defensin A, Mytilin-A, Mytilin-G1, Tachyplesin I, Polyphemusin I and II, | [15] | |
| Protozoa | 6 | Discodermin A, Polydiscamide-A, Damicornin | [14] | |
| Arthropoda | 619 | Cecropin A, ceratotoxin, stomoxyn, spinigerenin, thanatin, heliomicin, gallerimycin, termicin, royalisin, drosomycin, drosocin, metchnikowin, formaecin, lebocin, pyrrhocoricintin, attacins, coleoptericin, diptericin | [16] | |
| Bacteria | 383 | Lacticin, Nisin, Lactococcin B, Leucocin A, Enterocin A, Pediocin A,Pediocin F, Pediocin PA-1,Mesentericin Y105, Pediocin AcH, Acidophilin, Acidolin, Lactacin B, Lactacin F, Lactobacillin, Lactobrevin, Reuterin, Plantaricin A, Plantaricin B, Lactolin, Helveticin J | [10,17,18] | |
| Synthetic | 314 | AMP72, AMP126 , AMP2041, BP100, C16, CAMEL0, Dhvar1, Dhvar2, Dhvar4, Dhvar5, FL9, GS14K4, P-Der , Pexiganan , RW BP100, RN7-IN6, WLBU2, WMR-NH2, Pep19-4LF, Guavanin 2 | [19] | |
| Fungi | 29 | defensins, Mytilins, Myticins, and Mytimycin, Dermaseptins | [20] | |
| Predicted | 190 | GL-29, | [21] | |
| Milk | 100 | β-lactoglobulin, αs2-casein, β-casein | [22] |
3. Important Features Considered in Antimicrobial Peptide Design
3.1. Length of Amino Acids within Peptide
3.2. Number of Charged Amino Acids and Their Positions within the Peptide Chain
3.3. Percent Hydrophobicity and Amphipathicity
4. AMPs Derived from Naturally Occurring Proteins
4.1. Identification and Characterization of Numerous Peptide Fragments from Naturally Occurring Host Defense Proteins
4.2. Denovo Approach to the Design of Short AMPs Based on the Sequence pattern of Naturally Occurring Antimicrobial Proteins
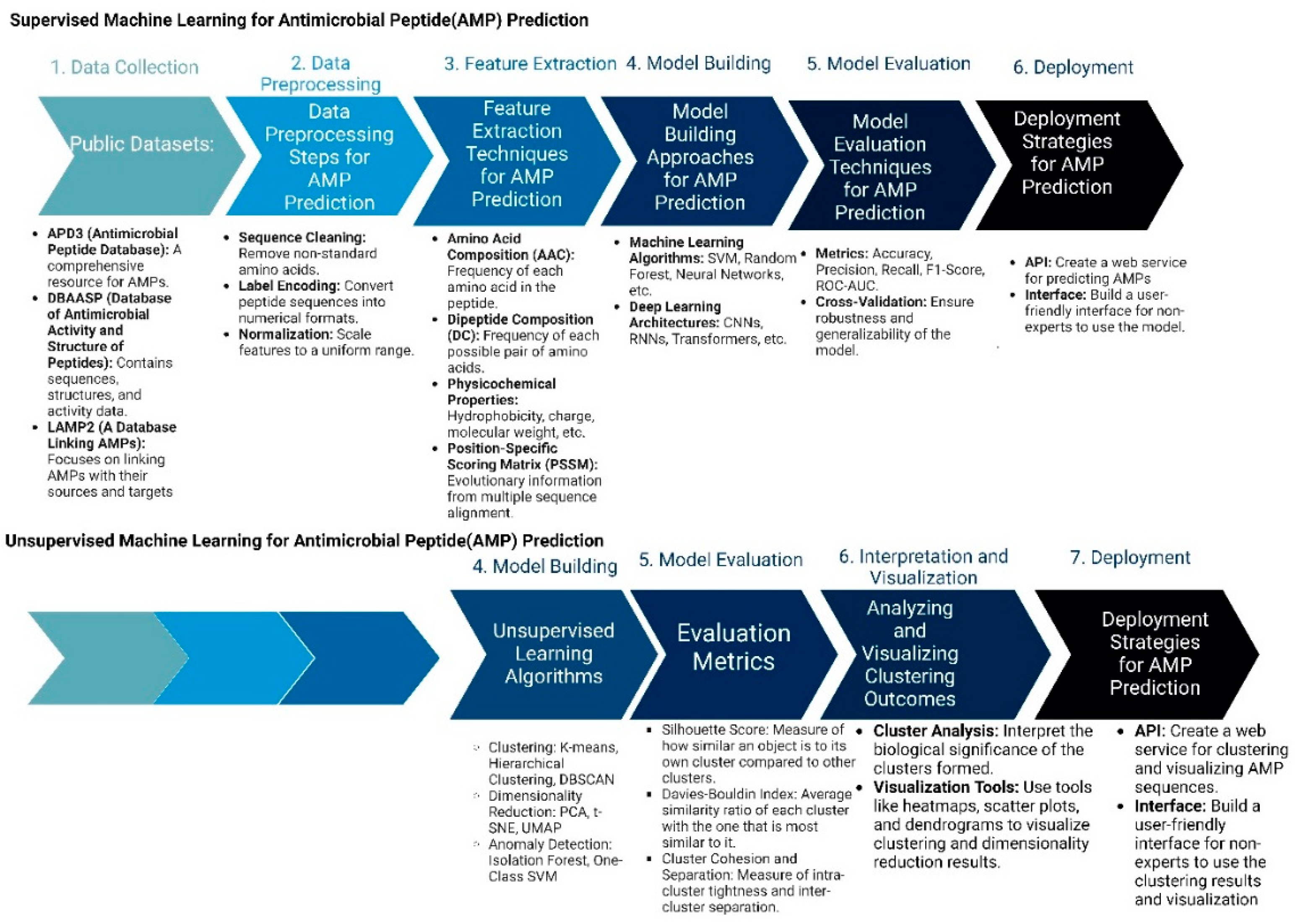
4.3. Designing of AMPs Using Bioinformatics Tools
4.4. ML/AI-Guided Approaches
4.5. Genome Mining Approaches from Natural Environments
5. Engineering of Peptide Fragments to Enhance Their Therapeutic Index
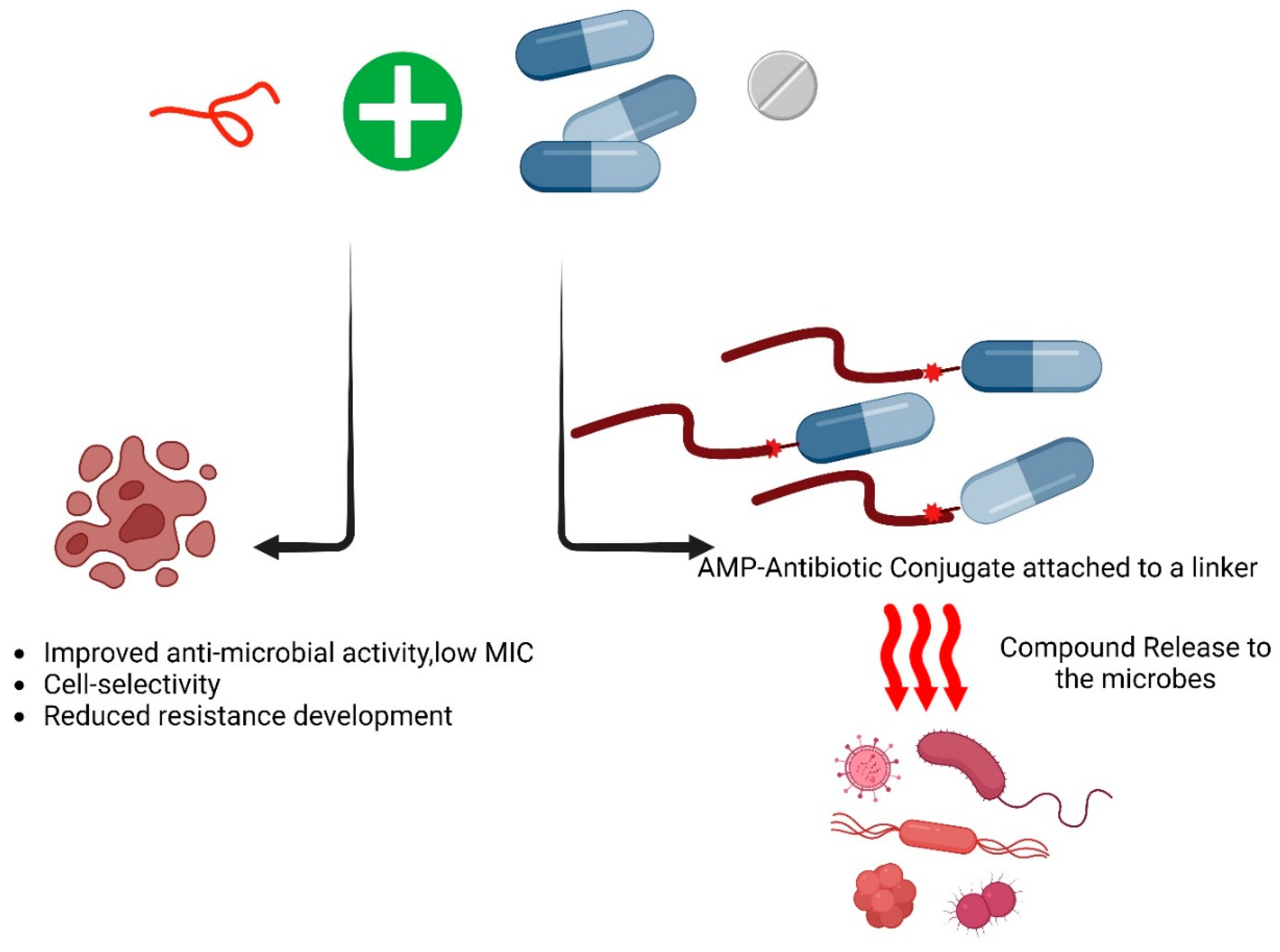
6. Synergistic Approaches to Develop Antimicrobial Peptides with Conventional Antibiotics
7. Amino Acids-Based Conjugated Antimicrobial Agents
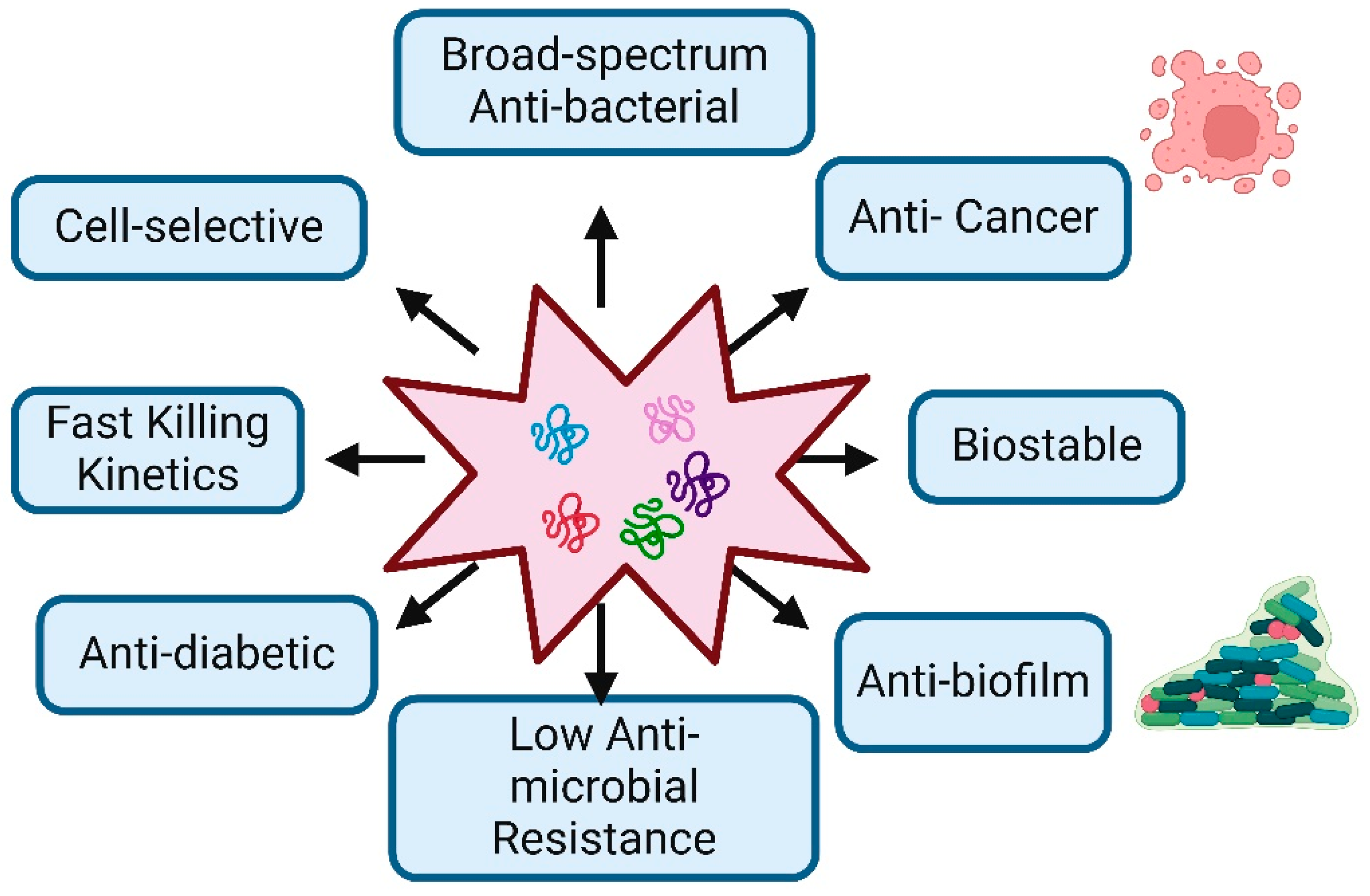
8. Current Status and Application of AMPs
9. Conclusions and Future Prospects
Author Contributions
Conflicts of Interest
References
- Tan, S.Y.; Tatsumura, Y. Alexander Fleming (1881-1955): Discoverer of penicillin. Singapore Med J 2015, 56, 366–367. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals (Basel) 2023, 16. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur J Pharm Sci 2022, 170, 106103. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Diamond, G. The role of cationic antimicrobial peptides in innate host defences. Trends in microbiology 2000, 8, 402–410. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, L.; Dong, C. Antimicrobial Peptides: An Overview of their Structure, Function and Mechanism of Action. Protein Pept Lett 2022, 29, 641–650. [Google Scholar] [CrossRef]
- Mazurkiewicz-Pisarek, A.; Baran, J.; Ciach, T. Antimicrobial Peptides: Challenging Journey to the Pharmaceutical, Biomedical, and Cosmeceutical Use. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Dini, I.; De Biasi, M.G.; Mancusi, A. An Overview of the Potentialities of Antimicrobial Peptides Derived from Natural Sources. Antibiotics (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Bakare, O.O.; Gokul, A.; Fadaka, A.O.; Wu, R.; Niekerk, L.A.; Barker, A.M.; Keyster, M.; Klein, A. Plant Antimicrobial Peptides (PAMPs): Features, Applications, Production, Expression, and Challenges. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front Microbiol 2020, 11, 582779. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial Peptides: An Update on Classifications and Databases. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- van Hoek, M.L. Antimicrobial peptides in reptiles. Pharmaceuticals (Basel) 2014, 7, 723–753. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, P.; Sbarigia, E.; Giannoni, M.F.; Panico, M.A.; Pannone, A. For how long should carotid endarterectomy surveillance be continued? Int Angiol 1994, 13, 190–195. [Google Scholar]
- Nguyen, T.T.T.; Allan, B.; Wheler, C.; Koster, W.; Gerdts, V.; Dar, A. Avian antimicrobial peptides: In vitro and in ovo characterization and protection from early chick mortality caused by yolk sac infection. Sci Rep 2021, 11, 2132. [Google Scholar] [CrossRef]
- Tincu, J.A.; Taylor, S.W. Antimicrobial peptides from marine invertebrates. Antimicrob Agents Chemother 2004, 48, 3645–3654. [Google Scholar] [CrossRef]
- Rai, M.; Pandit, R.; Gaikwad, S.; Kovics, G. Antimicrobial peptides as natural bio-preservative to enhance the shelf-life of food. J Food Sci Technol 2016, 53, 3381–3394. [Google Scholar] [CrossRef]
- McAuliffe, O.; Ryan, M.P.; Ross, R.P.; Hill, C.; Breeuwer, P.; Abee, T. Lacticin 3147, a broad-spectrum bacteriocin which selectively dissipates the membrane potential. Appl Environ Microbiol 1998, 64, 439–445. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am J Transl Res 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Cardoso, M.H.; Orozco, R.Q.; Rezende, S.B.; Rodrigues, G.; Oshiro, K.G.N.; Candido, E.S.; Franco, O.L. Computer-Aided Design of Antimicrobial Peptides: Are We Generating Effective Drug Candidates? Front Microbiol 2019, 10, 3097. [Google Scholar] [CrossRef]
- Fernandez de Ullivarri, M.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal Peptides as Therapeutic Agents. Front Cell Infect Microbiol 2020, 10, 105. [Google Scholar] [CrossRef]
- Yan, J.; Cai, J.; Zhang, B.; Wang, Y.; Wong, D.F.; Siu, S.W.I. Recent Progress in the Discovery and Design of Antimicrobial Peptides Using Traditional Machine Learning and Deep Learning. Antibiotics (Basel) 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Duche, R.T.; Wandhare, A.G.; Sian, J.K.; Singh, B.P.; Sihag, M.K.; Singh, K.S.; Sangwan, V.; Talan, S.; Panwar, H. Milk-Derived Antimicrobial Peptides: Overview, Applications, and Future Perspectives. Probiotics Antimicrob Proteins 2023, 15, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Saebo, I.P.; Bjoras, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the Hemolysis Assay for the Assessment of Cytotoxicity. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Kim, M.K.; Mereuta, L.; Seo, C.H.; Luchian, T.; Park, Y. Mechanism of action of antimicrobial peptide P5 truncations against Pseudomonas aeruginosa and Staphylococcus aureus. AMB Express 2019, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.T.; Hung, W.C.; Chen, F.Y.; Huang, H.W. Mechanism and kinetics of pore formation in membranes by water-soluble amphipathic peptides. Proc Natl Acad Sci U S A 2008, 105, 5087–5092. [Google Scholar] [CrossRef]
- Shai, Y. Mode of action of membrane active antimicrobial peptides. Biopolymers 2002, 66, 236–248. [Google Scholar] [CrossRef]
- Yang, L.; Harroun, T.A.; Weiss, T.M.; Ding, L.; Huang, H.W. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys J 2001, 81, 1475–1485. [Google Scholar] [CrossRef]
- Slezina, M.P.; Istomina, E.A.; Korostyleva, T.V.; Odintsova, T.I. The gamma-Core Motif Peptides of Plant AMPs as Novel Antimicrobials for Medicine and Agriculture. Int J Mol Sci 2022, 24. [Google Scholar] [CrossRef]
- Saxena, R.; Vekariya, U.K.; Kumar, P.; Tripathi, A.K.; Ghosh, J.K.; Tripathi, R.K. HIV-1 Nef CAWLEAQ motif: A regulator of monocytes invasion through ENO1 modulation. Mol Cell Biochem 2018, 447, 151–164. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Kumari, T.; Harioudh, M.K.; Yadav, P.K.; Kathuria, M.; Shukla, P.K.; Mitra, K.; Ghosh, J.K. Identification of GXXXXG motif in Chrysophsin-1 and its implication in the design of analogs with cell-selective antimicrobial and anti-endotoxin activities. Sci Rep 2017, 7, 3384. [Google Scholar] [CrossRef]
- Kumar, A.; Tripathi, A.K.; Kathuria, M.; Shree, S.; Tripathi, J.K.; Purshottam, R.K.; Ramachandran, R.; Mitra, K.; Ghosh, J.K. Single Amino Acid Substitutions at Specific Positions of the Heptad Repeat Sequence of Piscidin-1 Yielded Novel Analogs That Show Low Cytotoxicity and In Vitro and In Vivo Antiendotoxin Activity. Antimicrob Agents Chemother 2016, 60, 3687–3699. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Kumari, T.; Tandon, A.; Sayeed, M.; Afshan, T.; Kathuria, M.; Shukla, P.K.; Mitra, K.; Ghosh, J.K. Selective phenylalanine to proline substitution for improved antimicrobial and anticancer activities of peptides designed on phenylalanine heptad repeat. Acta Biomater 2017, 57, 170–186. [Google Scholar] [CrossRef]
- Srivastava, S.; Kumar, A.; Tripathi, A.K.; Tandon, A.; Ghosh, J.K. Modulation of anti-endotoxin property of Temporin L by minor amino acid substitution in identified phenylalanine zipper sequence. Biochem J 2016, 473, 4045–4062. [Google Scholar] [CrossRef]
- Karnati, P.; Gonuguntala, R.; Barbadikar, K.M.; Mishra, D.; Jha, G.; Prakasham, V.; Chilumula, P.; Shaik, H.; Pesari, M.; Sundaram, R.M.; et al. Performance of Novel Antimicrobial Protein Bg_9562 and In Silico Predictions on Its Properties with Reference to Its Antimicrobial Efficiency against Rhizoctonia solani. Antibiotics (Basel) 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, M.; Lai, R.; Zhang, Z. Chemical modifications to increase the therapeutic potential of antimicrobial peptides. Peptides 2021, 146, 170666. [Google Scholar] [CrossRef]
- Tiwari, A.; Gomez-Alvarez, V.; Siponen, S.; Sarekoski, A.; Hokajärvi, A.-M.; Kauppinen, A.; Torvinen, E.; Miettinen, I.T.; Pitkänen, T. Bacterial Genes Encoding Resistance Against Antibiotics and Metals in Well-Maintained Drinking Water Distribution Systems in Finland. Frontiers in Microbiology 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Singh, J.; Trivedi, R.; Ranade, P. Shaping the Future of Antimicrobial Therapy: Harnessing the Power of Antimicrobial Peptides in Biomedical Applications. J Funct Biomater 2023, 14. [Google Scholar] [CrossRef]
- Bobde, S.S.; Alsaab, F.M.; Wang, G.; Van Hoek, M.L. Ab initio Designed Antimicrobial Peptides Against Gram-Negative Bacteria. Front Microbiol 2021, 12, 715246. [Google Scholar] [CrossRef]
- Chen, Y.; Guarnieri, M.T.; Vasil, A.I.; Vasil, M.L.; Mant, C.T.; Hodges, R.S. Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrob Agents Chemother 2007, 51, 1398–1406. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals (Basel) 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed]
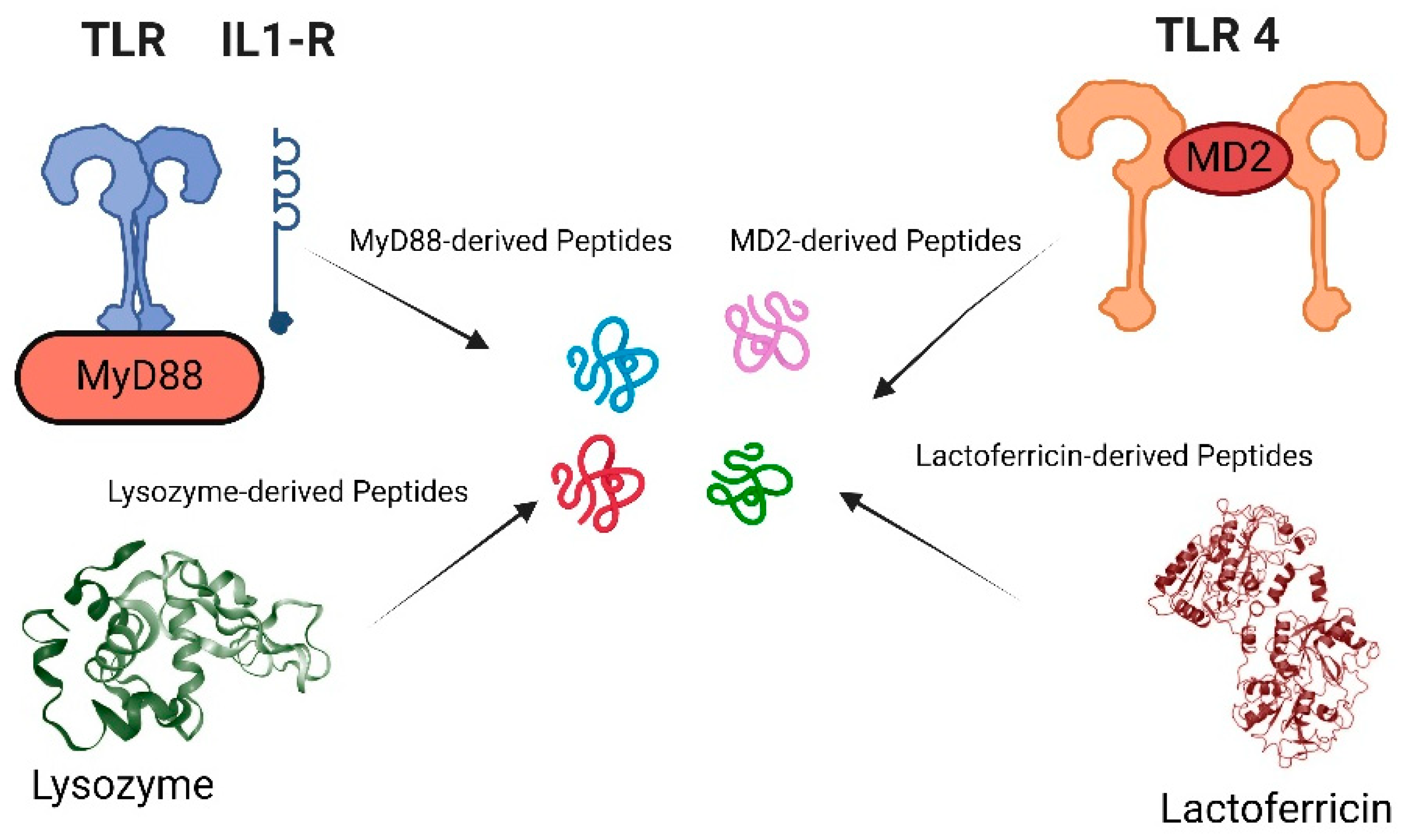
- Kumari, T.; Verma, D.P.; Kuldeep, J.; Dhanabal, V.B.; Verma, N.K.; Sahai, R.; Tripathi, A.K.; Saroj, J.; Ali, M.; Mitra, K.; et al. 10-Residue MyD88-Peptide Adopts beta-Sheet Structure, Self-Assembles, Binds to Lipopolysaccharides, and Rescues Mice from Endotoxin-Mediated Lung-Infection and Death. ACS Chem Biol 2022, 17, 3420–3434. [Google Scholar] [CrossRef] [PubMed]
- Tandon, A.; Harioudh, M.K.; Verma, N.K.; Saroj, J.; Gupta, A.; Pant, G.; Tripathi, J.K.; Kumar, A.; Kumari, T.; Tripathi, A.K.; et al. Characterization of a Myeloid Differentiation Factor 2-Derived Peptide that Facilitates THP-1 Macrophage-Mediated Phagocytosis of Gram-Negative Bacteria. ACS Infect Dis 2024, 10, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Tandon, A.; Harioudh, M.K.; Ishrat, N.; Tripathi, A.K.; Srivastava, S.; Ghosh, J.K. An MD2-derived peptide promotes LPS aggregation, facilitates its internalization in THP-1 cells, and inhibits LPS-induced pro-inflammatory responses. Cell Mol Life Sci 2018, 75, 2431–2446. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Desai, P.P.; Tyagi, A.; Lampe, J.B.; Srivastava, Y.; Donkor, M.; Jones, H.P.; Dzyuba, S.V.; Crossley, E.; Williams, N.S.; et al. Short peptides based on the conserved regions of MIEN1 protein exhibit anticancer activity by targeting the MIEN1 signaling pathway. J Biol Chem 2024, 300, 105680. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Trivedi, R.; Tripathi, A.K.; Nandy, R.R.; Wagner, D.C.; Narra, K.; Chaudhary, P. Higher Expression of Annexin A2 in Metastatic Bladder Urothelial Carcinoma Promotes Migration and Invasion. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Vishwanatha, J.K. Abstract 2184: Short Peptides derived from MIEN1 and their analogs exhibit anti-cancer activity in breast and prostate cancer cells. Cancer Research 2023, 83, 2184. [Google Scholar] [CrossRef]
- Hassan, M.F.; Qutb, A.M.; Dong, W. Prediction and Activity of a Cationic alpha-Helix Antimicrobial Peptide ZM-804 from Maize. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Zhu, X.; Dong, N.; Wang, Z.; Ma, Z.; Zhang, L.; Ma, Q.; Shan, A. Design of imperfectly amphipathic alpha-helical antimicrobial peptides with enhanced cell selectivity. Acta Biomater 2014, 10, 244–257. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Waghu, F.H.; Gopi, L.; Barai, R.S.; Ramteke, P.; Nizami, B.; Idicula-Thomas, S. CAMP: Collection of sequences and structures of antimicrobial peptides. Nucleic Acids Res 2014, 42, D1154–D1158. [Google Scholar] [CrossRef] [PubMed]
- Novkovic, M.; Simunic, J.; Bojovic, V.; Tossi, A.; Juretic, D. DADP: The database of anuran defense peptides. Bioinformatics 2012, 28, 1406–1407. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, P.; Kumar, R.; Bhardwaj, A. dPABBs: A Novel in silico Approach for Predicting and Designing Anti-biofilm Peptides. Sci Rep 2016, 6, 21839. [Google Scholar] [CrossRef]
- Bui Thi Phuong, H.; Doan Ngan, H.; Le Huy, B.; Vu Dinh, H.; Luong Xuan, H. The amphipathic design in helical antimicrobial peptides. ChemMedChem 2024, 19, e202300480. [Google Scholar] [CrossRef]
- Yue, L.; Song, L.; Zhu, S.; Fu, X.; Li, X.; He, C.; Li, J. Machine learning assisted rational design of antimicrobial peptides based on human endogenous proteins and their applications for cosmetic preservative system optimization. Sci Rep 2024, 14, 947. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial intelligence in drug discovery and development. Drug Discov Today 2021, 26, 80–93. [Google Scholar] [CrossRef]
- Muratspahic, E.; Retzl, B.; Duerrauer, L.; Freissmuth, M.; Becker, C.F.W.; Gruber, C.W. Genome Mining-Based Discovery of Blenny Fish-Derived Peptides Targeting the Mouse kappa-Opioid Receptor. Front Pharmacol 2021, 12, 773029. [Google Scholar] [CrossRef]
- Cheung-Lee, W.L.; Link, A.J. Genome mining for lasso peptides: Past, present, and future. J Ind Microbiol Biotechnol 2019, 46, 1371–1379. [Google Scholar] [CrossRef]
- Zhong, Z.; He, B.; Li, J.; Li, Y.X. Challenges and advances in genome mining of ribosomally synthesized and post-translationally modified peptides (RiPPs). Synth Syst Biotechnol 2020, 5, 155–172. [Google Scholar] [CrossRef]
- Liang, W.; Diana, J. Mining the bacterial genome to discover new antimicrobial molecules. EMBO Mol Med 2022, 14, e15409. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Liu, Z.; Liang, Z.; Zhu, C.; Li, D.; Kong, Q.; Mou, H. Development strategies and application of antimicrobial peptides as future alternatives to in-feed antibiotics. Sci Total Environ 2024, 927, 172150. [Google Scholar] [CrossRef]
- Alvares, D.S.; Wilke, N.; Ruggiero Neto, J. Effect of N-terminal acetylation on lytic activity and lipid-packing perturbation induced in model membranes by a mastoparan-like peptide. Biochim Biophys Acta Biomembr 2018, 1860, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Panahi Chegini, P.; Nikokar, I.; Tabarzad, M.; Faezi, S.; Mahboubi, A. Effect of Amino Acid Substitutions on Biological Activity of Antimicrobial Peptide: Design, Recombinant Production, and Biological Activity. Iran J Pharm Res 2019, 18, 157–168. [Google Scholar] [CrossRef]
- Kang, S.J.; Nam, S.H.; Lee, B.J. Engineering Approaches for the Development of Antimicrobial Peptide-Based Antibiotics. Antibiotics (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Dewangan, R.P.; Verma, D.P.; Verma, N.K.; Gupta, A.; Pant, G.; Mitra, K.; Habib, S.; Ghosh, J.K. Spermine-Conjugated Short Proline-Rich Lipopeptides as Broad-Spectrum Intracellular Targeting Antibacterial Agents. J Med Chem 2022, 65, 5433–5448. [Google Scholar] [CrossRef]
- Bellavita, R.; Braccia, S.; Galdiero, S.; Falanga, A. Glycosylation and Lipidation Strategies: Approaches for Improving Antimicrobial Peptide Efficacy. Pharmaceuticals (Basel) 2023, 16. [Google Scholar] [CrossRef] [PubMed]
- Pratap Verma, D.; Ansari, M.M.; Verma, N.K.; Saroj, J.; Akhtar, S.; Pant, G.; Mitra, K.; Singh, B.N.; Ghosh, J.K. Tandem Repeat of a Short Human Chemerin-Derived Peptide and Its Nontoxic d-Lysine-Containing Enantiomer Display Broad-Spectrum Antimicrobial and Antitubercular Activities. J Med Chem 2021, 64, 15349–15366. [Google Scholar] [CrossRef]
- Asaduzzaman, S.M.; Nagao, J.; Iida, H.; Zendo, T.; Nakayama, J.; Sonomoto, K. Nukacin ISK-1, a bacteriostatic lantibiotic. Antimicrob Agents Chemother 2009, 53, 3595–3598. [Google Scholar] [CrossRef]
- Koehbach, J.; Craik, D.J. The Vast Structural Diversity of Antimicrobial Peptides. Trends Pharmacol Sci 2019, 40, 517–528. [Google Scholar] [CrossRef]
- Ma, B.; Niu, C.; Zhou, Y.; Xue, X.; Meng, J.; Luo, X.; Hou, Z. The Disulfide Bond of the Peptide Thanatin Is Dispensible for Its Antimicrobial Activity In Vivo and In Vitro. Antimicrob Agents Chemother 2016, 60, 4283–4289. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hoover, D.M.; Yang, D.; Boulegue, C.; Santamaria, F.; Oppenheim, J.J.; Lubkowski, J.; Lu, W. Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human beta-defensin 3. Proc Natl Acad Sci U S A 2003, 100, 8880–8885. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Shome, A.; Das, D.; Das, P.K. Hydrogelation Through Self-Assembly of Fmoc-Peptide Functionalized Cationic Amphiphiles: Potent Antibacterial Agent. The Journal of Physical Chemistry B 2010, 114, 4407–4415. [Google Scholar] [CrossRef]
- Gahane, A.Y.; Singh, V.; Kumar, A.; Kumar Thakur, A. Development of mechanism-based antibacterial synergy between Fmoc-phenylalanine hydrogel and aztreonam. Biomaterials Science 2020, 8, 1996–2006. [Google Scholar] [CrossRef]
- Shearer, J.; Castro, J.L.; Lawson, A.D.G.; MacCoss, M.; Taylor, R.D. Rings in Clinical Trials and Drugs: Present and Future. J Med Chem 2022, 65, 8699–8712. [Google Scholar] [CrossRef]
- Jia, F.; Zhang, Y.; Wang, J.; Peng, J.; Zhao, P.; Zhang, L.; Yao, H.; Ni, J.; Wang, K. The effect of halogenation on the antimicrobial activity, antibiofilm activity, cytotoxicity and proteolytic stability of the antimicrobial peptide Jelleine-I. Peptides 2019, 112, 56–66. [Google Scholar] [CrossRef]
- Shah, M.B.; Liu, J.; Zhang, Q.; Stout, C.D.; Halpert, J.R. Halogen-pi Interactions in the Cytochrome P450 Active Site: Structural Insights into Human CYP2B6 Substrate Selectivity. ACS Chem Biol 2017, 12, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Borisy, A.A.; Elliott, P.J.; Hurst, N.W.; Lee, M.S.; Lehar, J.; Price, E.R.; Serbedzija, G.; Zimmermann, G.R.; Foley, M.A.; Stockwell, B.R.; et al. Systematic discovery of multicomponent therapeutics. Proc Natl Acad Sci U S A 2003, 100, 7977–7982. [Google Scholar] [CrossRef]
- Lyu, Y.; Yang, Y.; Lyu, X.; Dong, N.; Shan, A. Antimicrobial activity, improved cell selectivity and mode of action of short PMAP-36-derived peptides against bacteria and Candida. Sci Rep 2016, 6, 27258. [Google Scholar] [CrossRef]
- Taheri-Araghi, S. Synergistic action of antimicrobial peptides and antibiotics: Current understanding and future directions. Front Microbiol 2024, 15, 1390765. [Google Scholar] [CrossRef]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.M.; Bechinger, B.; Naas, T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Puttrevu, S.K.; Laxman, T.S.; Tripathi, A.K.; Yadav, A.K.; Verma, S.K.; Mishra, A.; Pradhan, R.; Verma, N.K.; Ghosh, J.K.; Bhatta, R.S. Liquid chromatography-tandem mass spectrometry based method development and validation of S016-1271 (LR8P), a novel cationic antimicrobial peptide for its application to pharmacokinetic studies. J Pharm Biomed Anal 2019, 169, 116–126. [Google Scholar] [CrossRef]
- Arifian, H.; Maharani, R.; Megantara, S.; Gazzali, A.M.; Muchtaridi, M. Amino-Acid-Conjugated Natural Compounds: Aims, Designs and Results. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Vishwanatha, J.K. Role of Anti-Cancer Peptides as Immunomodulatory Agents: Potential and Design Strategy. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef]
- Huang, H.W. DAPTOMYCIN, its membrane-active mechanism vs. that of other antimicrobial peptides. Biochim Biophys Acta Biomembr 2020, 1862, 183395. [Google Scholar] [CrossRef]
- Gupta, S.; Govil, D.; Kakar, P.N.; Prakash, O.; Arora, D.; Das, S.; Govil, P.; Malhotra, A. Colistin and polymyxin B: A re-emergence. Indian J Crit Care Med 2009, 13, 49–53. [Google Scholar] [CrossRef]
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial Mechanisms and Clinical Application Prospects of Antimicrobial Peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef]
- van Groenendael, R.; Beunders, R.; Hemelaar, P.; Hofland, J.; Morshuis, W.J.; van der Hoeven, J.G.; Gerretsen, J.; Wensvoort, G.; Kooistra, E.J.; Claassen, W.J.; et al. Safety and Efficacy of Human Chorionic Gonadotropin Hormone-Derivative EA-230 in Cardiac Surgery Patients: A Randomized Double-Blind Placebo-Controlled Study. Crit Care Med 2021, 49, 790–803. [Google Scholar] [CrossRef]
- Kollef, M.; Pittet, D.; Sanchez Garcia, M.; Chastre, J.; Fagon, J.Y.; Bonten, M.; Hyzy, R.; Fleming, T.R.; Fuchs, H.; Bellm, L.; et al. A randomized double-blind trial of iseganan in prevention of ventilator-associated pneumonia. Am J Respir Crit Care Med 2006, 173, 91–97. [Google Scholar] [CrossRef]
- Yendewa, G.A.; Griffiss, J.M.; Jacobs, M.R.; Fulton, S.A.; O'Riordan, M.A.; Gray, W.A.; Proskin, H.M.; Winkle, P.; Salata, R.A. A two-part phase 1 study to establish and compare the safety and local tolerability of two nasal formulations of XF-73 for decolonisation of Staphylococcus aureus: A previously investigated 0.5mg/g viscosified gel formulation versus a modified formulation. J Glob Antimicrob Resist 2020, 21, 171–180. [Google Scholar] [CrossRef]
- Van Dyke, T.; Paquette, D.; Grossi, S.; Braman, V.; Massaro, J.; D'Agostino, R.; Dibart, S.; Friden, P. Clinical and microbial evaluation of a histatin-containing mouthrinse in humans with experimental gingivitis: A phase-2 multi-center study. J Clin Periodontol 2002, 29, 168–176. [Google Scholar] [CrossRef]
- Niemeyer-van der Kolk, T.; van der Wall, H.; Hogendoorn, G.K.; Rijneveld, R.; Luijten, S.; van Alewijk, D.; van den Munckhof, E.H.A.; de Kam, M.L.; Feiss, G.L.; Prens, E.P.; et al. Pharmacodynamic Effects of Topical Omiganan in Patients With Mild to Moderate Atopic Dermatitis in a Randomized, Placebo-Controlled, Phase II Trial. Clin Transl Sci 2020, 13, 994–1003. [Google Scholar] [CrossRef]
- Nilsson, A.C.; Janson, H.; Wold, H.; Fugelli, A.; Andersson, K.; Håkangård, C.; Olsson, P.; Olsen, W.M. LTX-109 Is a Novel Agent for Nasal Decolonization of Methicillin-Resistant and -Sensitive Staphylococcus aureus. Antimicrobial Agents and Chemotherapy 2015, 59, 145–151. [Google Scholar] [CrossRef]
- Knappe, D.; Adermann, K.; Hoffmann, R. Oncocin Onc72 is efficacious against antibiotic-susceptible Klebsiella pneumoniae ATCC 43816 in a murine thigh infection model. Biopolymers 2015, 104, 707–711. [Google Scholar] [CrossRef]
- de Breij, A.; Riool, M.; Kwakman, P.H.; de Boer, L.; Cordfunke, R.A.; Drijfhout, J.W.; Cohen, O.; Emanuel, N.; Zaat, S.A.; Nibbering, P.H.; et al. Prevention of Staphylococcus aureus biomaterial-associated infections using a polymer-lipid coating containing the antimicrobial peptide OP-145. J Control Release 2016, 222, 1–8. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Zegarra, J.; Bellomo, S.; Carcamo, C.P.; Cam, L.; Castañeda, A.; Villavicencio, A.; Gonzales, J.; Rueda, M.S.; Turin, C.G.; et al. Randomized Controlled Trial of Bovine Lactoferrin for Prevention of Sepsis and Neurodevelopment Impairment in Infants Weighing Less Than 2000 Grams. J Pediatr 2020, 219, 118–125. [Google Scholar] [CrossRef]
- Dale, G.E.; Halabi, A.; Petersen-Sylla, M.; Wach, A.; Zwingelstein, C. Pharmacokinetics, Tolerability, and Safety of Murepavadin, a Novel Antipseudomonal Antibiotic, in Subjects with Mild, Moderate, or Severe Renal Function Impairment. Antimicrobial Agents and Chemotherapy 2018, 62, 10–1128. [Google Scholar] [CrossRef]
- Lee, C.H.; Patino, H.; Stevens, C.; Rege, S.; Chesnel, L.; Louie, T.; Mullane, K.M. Surotomycin versus vancomycin for Clostridium difficile infection: Phase 2, randomized, controlled, double-blind, non-inferiority, multicentre trial. Journal of Antimicrobial Chemotherapy 2016, 71, 2964–2971. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Sidorowicz, A.; Mikosinski, J.; Krzyzanowski, M.; Orleanski, J.; Twardowska-Saucha, K.; Nykaza, A.; Dyaczynski, M.; Belz-Lagoda, B.; Dziwiszek, G.; et al. Evaluation of LL-37 in healing of hard-to-heal venous leg ulcers: A multicentric prospective randomized placebo-controlled clinical trial. Wound Repair Regen 2021, 29, 938–950. [Google Scholar] [CrossRef]
| Modifications | Substitute/Addition moieties | Peptide | Function | Ref. |
|---|---|---|---|---|
| N-Acetylation | Acetyl group | maximin H5 | Increased activity and stability | [63] |
| Amidation of C-terminus | Amide group | maximin H5 | lower levels of hemolysis, | [64] |
| Amino acid conversion | Substitution of amino acids, Lys, His, Ser substitution to Arg amino acid residue, Phe replaced to Trp |
Pexiganan | Improved antimicrobial activity with enhanced cell selectivity | [64] |
| Cyclization | cross-linking constructions with disulfide bonds and cyclization by lactam ring | Baciim, Cubicin | Increased permeability, stability and bioactivity of AMPs | [65] |
| Fatty acid coupling | decanoic acid, lauric acid, myristic acid | C10-PR-Spn, C12-PR-Spn, C14-PR-Spn | Increased antibacterial activity and stability | [66] |
| Glycosylation | glycan moiety/ sugar moiety | Enhances the antimicrobial properties of AMPs, as well as their stability and biological properties, immunomodulation | [67] | |
| Unusual amino acids | Lanthionine, 3-methyllanthionine, and Dehydrobutyrine, D-form of amino acids |
Nukacin ISK-1, Chem-8dK | Antibacterial Gram-positive, bacteria, cell selectivity | [68,69] |
| Disulfide bonds | Bond between two cysteine amino acids | Thanatin, β-defensin 3 | Stabilized structure | [70,71,72] |
| Hydrogel | aztreonam encapsulated Fmoc-F hydrogels | Fmoc-F | Increased efficacy and selectivity against bacteria | [73,74] |
| Halogenation | chlorine, fluorine, bromine, and iodine | Jelleine-I, | Improvement of degradability of therapeutic agents, lipophilicity, catabolic stability, and membrane permeabilization properties | [75,76,77] |
| Antimicrobial peptides (AMPs) | Origin | Synergistic molecule | Antimicrobial properties |
|---|---|---|---|
| PGLa | Frog skin | Magainin 2 | E. coli and S. aureus |
| Ranalexin | -Bullfrog R. Catesbeiana, Staphylococcus simulans |
Endopeptidase lysostaphin |
S. aureus (MRSA) |
| P10 | Ceftazidim/ doripenem |
MDR A. baumannii and colistin-resistant P. aeruginosa |
|
| Tridecaptin M | Mud bacterium | Rifampicin, vancomycin, and ceftazidime |
drug-resistant A. baumannii |
| Dermaseptin | Amphibians skin |
Dermaseptin |
E. coli, P. aeruginosa, S. aureus |
| Lactoferricin | Mammalians milk | Ciprofloxacin, ceftazidime |
P. aeruginosa |
| Nisin | Lactococcus lactis | Colistin |
Pseudomonas biofilms |
| Gad-1 | Fish | Kanamycin, ciprofloxacin |
P. aeruginosa |
| Bactenecin | Lactic acid bacteria |
Bactenecin |
E. coli, P. aeruginosa, S. Typhimurium |
| PMAP-36 | Porcine` | tetracycline | Escherichia coli |
| AA230 | Arenicin-3 | EDTA | Pseudomonas aeruginosa or Escherichia coli |
| Melimine | hybrid peptide of melittin and protamine | ciprofloxacin | P. aeruginosa 37 |
| AMPs | Origin | Clinical Properties | Mechanism of action | Clinical phase | Ref. |
|---|---|---|---|---|---|
| EA-230 | human chorionic gonadotropin | Phase 1/2 | Intravenous | immunomodulatory and renoprotective effects | [88] |
| Iseganan | Protegrin-1 | Phase 2/3 | Topical | Prevention of ventilator-associated pneumonia | [89] |
| XF-73 | Porphyrin | Phase 1 | Nasal gel | Prevention of postoperative S. aureus colonization and infection |
[90] |
| P-113 | Histatin 5 | Phase 2 | Mouth rinse | Reduce gum bleeding, gingivitis and plaque | [91] |
| Omiganan | Indolicidin | Phase 2 | Topical gel | Treatment of mild to moderate atopic dermatitis | [92] |
| LTX-109 | Synthetic peptidomimetic | Phase 1/2 | Topical | Prevention of nasal infections caused by methicillin-sensitive/resistant S. aureus |
[93] |
| Onc72 | Oncocin | Preclinical | Subcutaneous | Treatment of antibiotic-susceptible K. pneumoniae | [94] |
| OP-145 | LL-37 | Preclinical | Implant coating | Prevention of S. aureus-induced biomaterial-associated infections |
[95] |
| Lactoferrin | Not applicable | Phase 4 | Oral | Prevention of neonatal sepsis | [96] |
| Murepavadin | Protegrin-1 | Phase 1 | Intravenous | Treatment of pneumonia caused by P. aeruginosa infection |
[97] |
| Surotomycin | Daptomycin | Phase 2 | Oral | Treatment of C. difficile-associated infection | [98] |
| LL-37 | Not applicable | Phase 2 | Topical | Control of infection of diabetic foot ulcers | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





