1. Introduction
Gestational diabetes mellitus (GDM) is a type of diabetes that is first recognized during pregnancy. It is characterized by high blood sugar levels that develop during pregnancy in women who did not previously have diabetes. This condition can arise because pregnancy hormones can make the body less effective at using insulin, a hormone that regulates blood sugar levels. GDM diagnosed during pregnancy is present in around 10% in Europe [Dluski 2022], making it the most common medical complications during pregnancy [Tsakiridis I 2021, Visolyi-Kun 2023, Damm P 2016]. Showed an increase in the GDM incidence in last decades. The pooled global standardized prevalence of GDM was 14.0% (95% confidence interval: 13.97-14.04%). The regional standardized prevalence of GDM were 7.1% (7.0-7.2%) in North America and Caribbean, 7.8% (7.2-8.4%) in Europe, 10.4% (10.1-10.7%) in South America and Central America, 14.2% (14.0-14.4%) in Africa, 14.7% (14.7-14.8%) in Western Pacific, 20.8% (20.2-21.4%) in South-East Asia and 27.6% (26.9-28.4%) in Middle East and North Africa. The standardized prevalence of GDM in low-, middle- and high-income countries were 12.7% (11.0-14.6%), 9.2% (9.0-9.3%) and 14.2% (14.1-14.2%), respectively. [Wang 2022 ]. Gestational Diabetes Mellitus (GDM) and obesity are interrelated conditions that pose significant health risks for both mothers and their newborns. The prevalence of maternal obesity is rising rapidly worldwide and constitutes a major obstetric problem. It is as a main risk for GDM is rising significantly on all continents, reflecting broader global trends. Increased rates of obesity among women of childbearing age are contributing to a higher incidence of GDM, mirroring patterns observed throughout Europe. Factors such as urbanization, sedentary lifestyles, and dietary changes are key contributors. This surge presents substantial public health challenges, as both obesity and GDM are linked to adverse outcomes for mothers and infants, including increased risks of metabolic disorders, cardiovascular diseases, and complications during pregnancy and delivery. [Zehravi M 2021, Patham 2015]. Annual reports from the WHO reveal that 43% of women were overweight and 16% of women were obese in 2022. [WHO 2024].
GDM and obesity conditions are influenced by a variety of biochemical and molecular factors, including apelin, leptin, vascular endothelial growth factor (VEGF), and DNA methylation. Understanding the roles of these factors can provide insight into the pathophysiology of GDM and obesity, potentially leading to improved therapeutic strategies.
Apelin
Apelin is an endogenous peptide that plays a key role in the regulation of glucose homeostasis, cardiovascular functions, and angiogenesis. It is the ligand for the G protein-coupled receptor apelin, which is expressed in various tissues, including the heart, adipose tissue, and pancreas. [Antushevich H 2018]. In the context of obesity, apelin levels are generally elevated in serum, possibly as a compensatory mechanism to counteract metabolic dysregulation. Apelin has been shown to enhance glucose uptake in skeletal muscle and adipose tissue, and it may have insulin-sensitizing effects. In GDM, the expression of apelin is also upregulated, potentially as a response to increased metabolic demands and insulin resistance. Elevated serum apelin levels in GDM may contribute to improved glucose tolerance by enhancing insulin sensitivity, though this compensatory mechanism may not always suffice to maintain normal glucose levels. [Antushevich H 2018]. Moreover, apelin is implicated in cardiovascular health, and its dysregulation can exacerbate the risk of cardiovascular complications, which are already heightened in obesity and GDM. [Antushevich H 2018]
Vascular Endothelial Growth Factor (VEGF)
VEGF is a key regulator of angiogenesis, the process of new blood vessel formation, which is crucial for tissue growth and repair. It is particularly important in the context of placental development during pregnancy.[Ballmer-Hofer 2018]. In obese individuals and those with GDM, VEGF serum levels are often elevated. This overexpression can lead to abnormal angiogenesis, contributing to complications such as preeclampsia and fetal growth restriction. In the placenta, excessive VEGF can alter vascular permeability and placental function, potentially leading to adverse pregnancy outcomes.[Bolatai 2022]. The increased expression of VEGF in obesity and GDM is thought to be driven by hypoxic conditions in adipose tissue due to inadequate blood supply. This hypoxia-induced VEGF expression may further exacerbate inflammation and insulin resistance, creating a vicious cycle that aggravates the metabolic and vascular complications associated with these conditions.[Bolatai 2022, Ballmer-Hofer 2018]
Leptin
Leptin is a hormone primarily secreted by adipocytes and is pivotal in regulating energy balance, appetite, and metabolism. It acts on the hypothalamus to suppress appetite and promote energy expenditure. However, in the state of obesity, serum leptin levels are significantly increased, but paradoxically, leptin resistance occurs, where the body's responsiveness to leptin's effects is diminished. [Obradociv 2021]. In GDM, serum leptin levels are also elevated, likely reflecting the increased adiposity and insulin resistance characteristic of the condition. Leptin resistance in GDM can contribute to the dysregulation of energy homeostasis and glucose metabolism. [Obradociv 2021] Additionally, high serum leptin levels may influence placental function and fetal growth, potentially leading to macrosomia (excessive fetal growth), which is a common complication of GDM.[Gaudix 2023]. Leptin is also involved in the regulation of inflammation, and its elevated serum levels in obesity and GDM may contribute to a chronic low-grade inflammatory state. This inflammation can further impair insulin sensitivity and exacerbate metabolic dysregulation. [Obradociv 2021]
DNA Methylation
DNA methylation is an epigenetic modification that involves the addition of a methyl group to the cytosine base of DNA, typically leading to gene repression. This process plays a critical role in regulating gene expression and is influenced by environmental factors, including diet and metabolic status. [Moor 2013]. In the context of obesity and GDM, aberrant DNA methylation patterns have been observed. These changes can affect genes involved in key metabolic pathways, such as those regulating glucose and lipid metabolism, inflammation, and insulin signalling [Heikkinen 2022]. For instance, altered methylation of genes like PPARγ (a regulator of adipogenesis) and IGF2 (involved in fetal growth) has been linked to metabolic disturbances in obesity and GDM.[Horvath 2018, Law 2019]. The changes in DNA methylation in these conditions are not only relevant for the individual but can also have transgenerational effects. Epigenetic modifications influenced by maternal obesity or GDM can impact the health of the offspring, predisposing them to metabolic disorders later in life.[Moor 2013, Heikkinen 2022].
Interactions and Implications
The interplay between apelin, VEGF, leptin, and DNA methylation creates a complex network that influences the development and progression of GDM and obesity. For instance, the inflammatory state driven by leptin and VEGF can alter DNA methylation patterns, which in turn can affect the expression of genes involved in metabolism and vascular function. Apelin’s role in modulating insulin sensitivity and glucose uptake further integrates into this network, potentially offering a protective effect against the metabolic derangements seen in these conditions [Antushevich H 2018]. Understanding these interactions is crucial for developing therapeutic strategies. For example, targeting leptin resistance or VEGF pathways could potentially ameliorate some of the complications associated with GDM and obesity. Similarly, interventions aimed at modifying epigenetic marks, such as through diet or pharmacological agents, hold promise in mitigating the long-term effects of these conditions. Potential of biomarkers in two human deasese that show rather consistent alterations in obesity and glucose imbalance.
4. Discussion
Although there is a rapid increase in the prevalence of diabetes in many parts of the world, such as in Middle East Asia, global estimates and comparisons of the prevalence of diabetes, particularly GDM, are difficult to make due to the wide variation in screening strategies and diagnostic criteria used to identify the disease. Fetal abnormalities, such as cardiac abnormalities, are at least 15 times more common than in the offspring of non-diabetic pregnancies [Damm P 2016]. Aside from the short-term maternal, fetal and neonatal consequences associated with GDM, there are long-term consequences for both mother and their child [Damm P 2016].While studies in adult offspring are limited, several human studies exist in children from different populations, involving a mix of different maternal diabetes types [Fraser A 2014]. The results of these studies are in line with the animal studies, the majority finding increased risk of diabetes and obesity in children exposed to maternal diabetes. [Damm P 2016, Fraser 2024].The prevalence of GDM had been elevated in last decade, because the population BMI is elevated by 12% during fertile ages in women. [Gregory ECW 2022]. In our study we investigated these two high-risk group.
In sonography-based cohort studies of singleton pregnancies, volume and weight of placenta were higher in obese and GDM pregnancies than in normal-weight controls. Our data may be consistent with the report that obese and GDM mothers have larger placentas because vasculogenesis is impaired and edema occurs in these placentas [Musa 2024, Suranyi 2016]. The placental volume were not significantly different in GDM and obes cases.
We observed a negative correlation between 3-D power Doppler indices and maternal BMI in pregnancies with obesity and GDM. Compared to the control group, the indices were reduced, although VI, FI and VFI values overlapped in the GDM and obese groups, but all higher placental volumes were associated with worse vascular indices. Increased placental volume expansion was not only associated with BMI but also with abnormal glycation. Decreases in placental VI and VFI in obesity and GDM were associated with reduced angiogenesis and decreased arteriolar number causing placental edema. However, depressed FI may represent a narrower internal vascular diameter. Th three dimensional ultrasonography did not detect differences between the obese and GDM groups, whereas biomarkers showed characteristic shifts. [Musa 2023]. It has been suggested that the placenta plays an active role in mediating inflammation in women with obesity and GDM. Placental structure and function may be altered in an adaptive response to obesity, and the placenta may act as a target and a source of inflammatory cytokines and biomarkers in pregnancies [Patham 2015].
Obesity and gestational diabetes mellitus (GDM) share several common pathophysiological mechanisms, particularly related to insulin resistance and low-grade inflammation [Patham 2015]. These overlapping pathways contribute to the development and complications of both conditions. Obesity and gestation complicated by GDM by increasing the risk of fetal and placental growth abnormality [Pahtam 2015, Suranyi 2016]. Insulin resistance is a central feature in both obesity and GDM. In obesity the excess adipose tissue, particularly visceral fat, increases the release of free fatty acids and adipokines (hormones produced by fat cells), which can interfere with insulin signalling [Patham 2015, Zehravi 2021]. This leads to reduced glucose uptake by the cells, requiring the pancreas to produce more insulin to maintain normal blood glucose levels. Over time, the pancreas may fail to keep up with this demand, leading to hyperglycemia.[Zehravi 2021]
During pregnancy, hormonal changes naturally increase insulin resistance as a mechanism to ensure more glucose is available to the fetus. In women with pre-existing insulin resistance (often associated with obesity), this additional insulin resistance can overwhelm pancreatic insulin production capacity, leading to elevated blood glucose levels and GDM. [zehravi 2021, Ruppert 2024]. Both obesity and GDM are associated with a state of chronic low-grade inflammation [Patham 2015, Lim 2023, Ruppert 2024]. Inflammation is also characterized by an increased basal metabolic rate due to the focused and rapid response to the insult [Patham 2015].The profile of immune cells favoring a pro-inflammatory environment in tissues such as adipose, liver and pancreas; it is chronically maintained by metabolic cells such as adipocytes without resolution, and it is associated with a reduced metabolic rate [Gregor MF 2011, Ruppert 2024]. For these reasons, the term ‘metaflammation’ or ‘metainflammation’ was coined to describe this particular profile associated with obesity [Gregor MF 2011]. The link between adiposity, inflammation and insulin resistance was first identified when it was observed that levels of the proinflammatory cytokines were increased in adipose tissue of obese individuals, and that antagonism led to increased insulin sensitivity [Ruppert 2024]. Weight-loss is associated with a reduction cytokines and reversal of insulin resistance. It is now evident that, in the obese state, several adipokines, chemokines, and cytokines released from adipose tissue and immune cells may interact in an autocrine and paracrine network, causing impaired insulin sensitivity in the metabolic syndrome [Ruppert 2024]. Adipose tissue thus behaves as one of the largest endocrine organs in the body [Ahima 2000]. Insulin acts by binding to insulin receptors in muscle, liver and adipose tissue, leading to phosphorylation of insulin receptor substrates. The regulatory subunit phosphorylates Akt, resulting in translocation of the glucose transporter in adipose tissue and muscle to the plasma membrane to facilitate glucose uptake [Ruppert 2024]. Insulin-stimulated Akt phosphorylation also activates glycogen synthase and glycogen synthesis. After insulin signaling, mitogen-activated protein kinase plays a role in promoting protein synthesis and cell growth and differentiation [Cildir 2013]. Normal pregnancy is also characterized by a state of insulin resistance, with a 50% reduction in insulin-mediated glucose clearance, and a ~250% increase in insulin production to maintain maternal euglycemia [Catalano 1999].
Pregnancy itself is characterized by an altered inflammatory profile compared to the non-pregnant state. A tightly regulated balance between pro- and anti-inflammatory biosubstrats may be necessary for normal implantation, trophoblast invasion and placentation.[Mor G 2011]
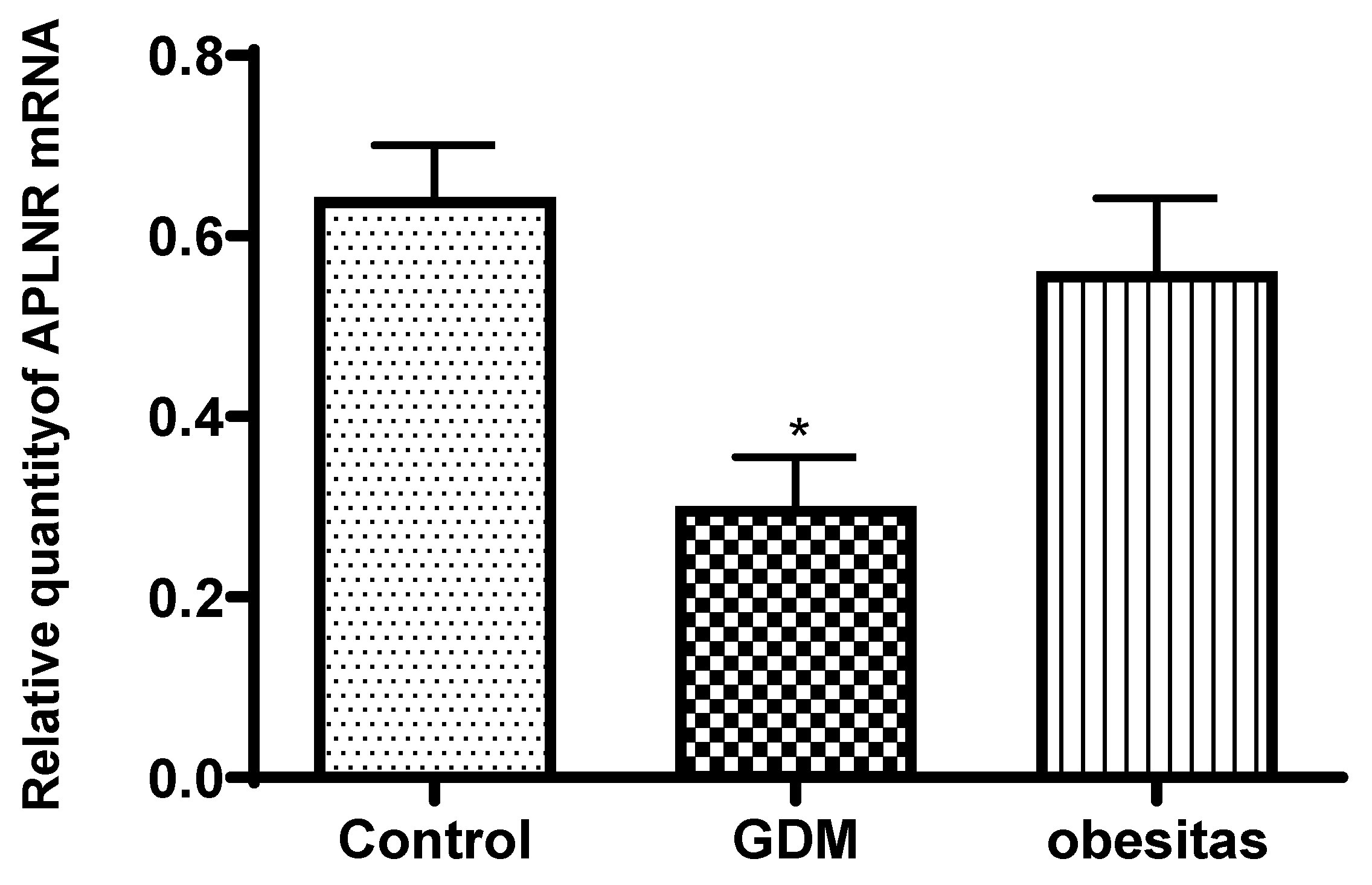
Apelin receptor mRNA expression and leptin concentration was significantly decreased in the placental samples from women with GDM, compare to the normal placenta. The obesity has similar effect, but the depression of leptin and apelin receptor mRNA expression were not significat.
In women with Gestational Diabetes Mellitus (GDM), the expression of apelin receptor mRNA and leptin concentrations are often decreased due to several possible mechanisms [Musa 2023]: In GDM, serum apelin levels are typically elevated, which may contribute to insulin resistance [Zehravi 2021].
Insulin Resistance and Hormonal Dysregulation: GDM is characterized by insulin resistance, which can affect the regulation of various hormones and receptors, including apelin and leptin. Insulin resistance may impair the signaling pathways that regulate the expression of apelin receptors and the secretion of leptin.
Inflammation and Oxidative Stress: GDM is associated with increased inflammation and oxidative stress, which can lead to alterations in the expression of genes and proteins involved in metabolic regulation. These conditions might downregulate apelin receptor expression and leptin production.
Placental Function: The placenta plays a significant role in hormone regulation during pregnancy. In GDM, placental function is often compromised, which can affect the production and regulation of hormones like leptin. Abnormal placental function could also influence the expression of apelin receptors in maternal tissues.
Nutritional and Metabolic Factors: The altered metabolic environment in GDM, including changes in glucose and lipid metabolism, may impact the levels of leptin and the expression of its receptors, including the apelin receptor.
These factors together can lead to the observed decrease in apelin receptor mRNA expression and leptin concentrations in women with GDM compared to those without the condition. (Musa 2023, Musa 2024]. The change of adipokine secretion can lead to the change of glucose homeostasis during pregnancy, and adipokine is also associated with the pathogenesis of GDM and obesity, so adipokines have become a hot spot in GDM and obesity research [Ruppert 2024]. The peripheral blood changes present serum leptin concentration is elevated in obesity and GDM, while the adipokin concentration is depressed in obesity and GDM cases [Xu 2020]. The placental tissue analyses presented adipokin concentration significantly decreased both in the GDM and obes samples [Xu 2022, Ruppert 2024]. The leptin concentration significantly decreased in the GDM samples, while leptin value depressed non significantly in the obese samples. The GDM cause more severe depression in placental tissues, compare to obesity. The serum level of leptin in the early pregnancy is 2–3 times higher than that in the non-pregnancy period, and gradually increases during gestation [Xu 2022]. Therefore, we conclude that the decrease in placental leptin concentration in obese and even more pronounced in GDM patients is a placental compensatory mechanism to maintain serum leptin levels.
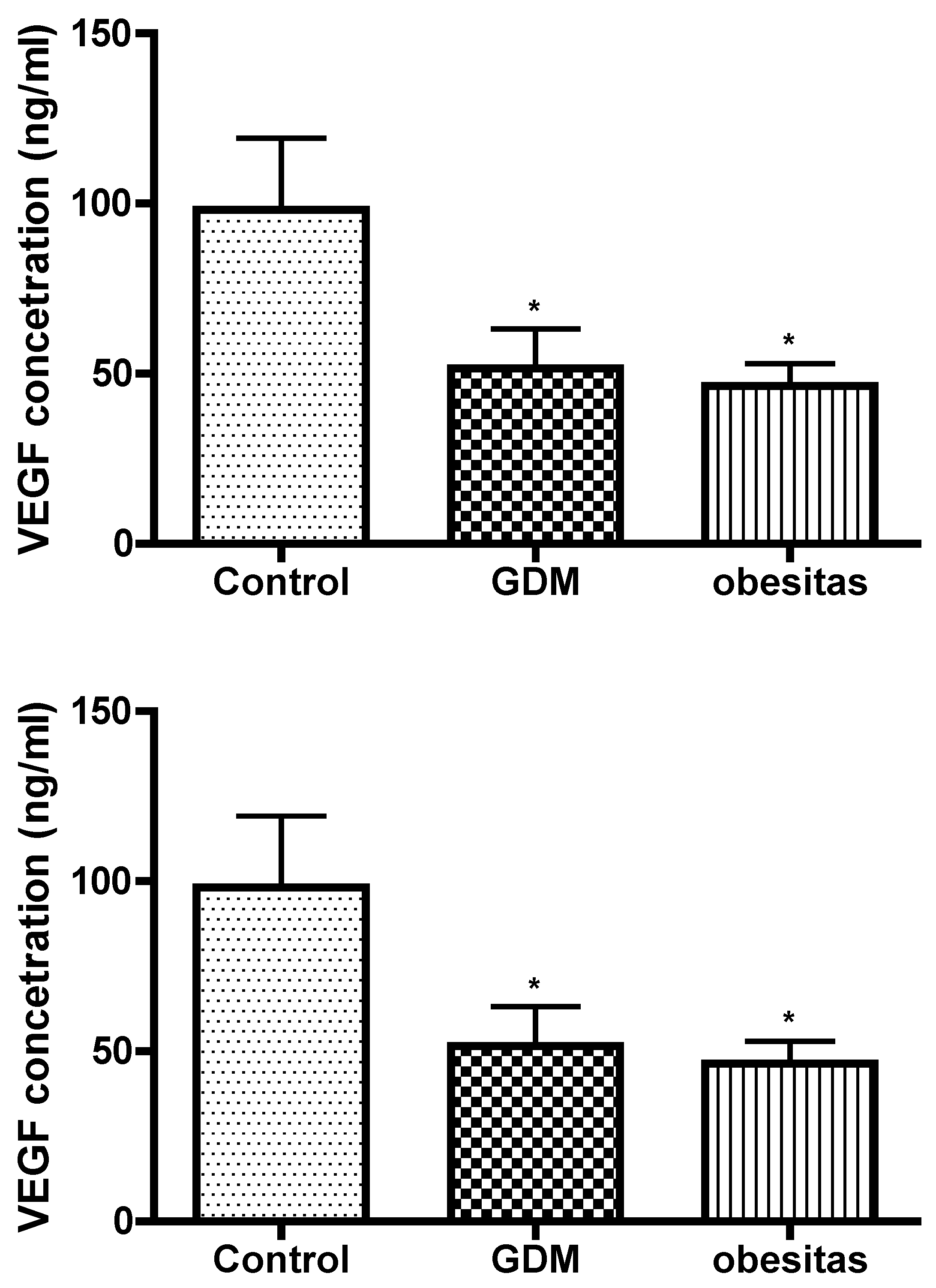
GDM and obesity are dysfunction of the leptin signaling pathways. Leptin appears to be a relevant key hormone that regulates placental transport, and this regulation is altered in pathophysiological conditions such as gestational diabetes and /or obesity. Adaptations in the placental capacity to transport glucose may underlie both under- or overgrowth of the fetus when maternal nutrient and hormone levels are altered due to changes in maternal obesity or metabolic disease. [Gaudix 2023]Leptin can induce phosphorylation of VEGF receptor [Lim PQ 2023] . VEGF results presented the vasculogenesis is damaged in our study. This placental depression may contribute to abnormal placental function and vascular complications, exacerbating both conditions. The effect of obesity is the same as GDM in placental vasculogenesis. Leptin concentration had correlation with VEGF value. In GDM the VEGF depression was 50 percent and Leptin concentration fall down with 64 percent compare to the normal placental tissue value. In obesity the VEGF depression was 53 percent and leptin concentration fall down with 54 percent compare to the normal placental tissue value in our study.
A decreased concentration of Vascular Endothelial Growth Factor (VEGF) in the placenta can have significant implications for pregnancy and fetal development. VEGF is a critical regulator of angiogenesis, the process by which new blood vessels form, and is essential for ensuring adequate blood supply to the developing fetus [Xu 2022].
Key implications of reduced VEGF levels in the placenta include:
Impaired Angiogenesis: VEGF is crucial for the development of the placental vascular network. A reduction in VEGF can lead to insufficient blood vessel formation, resulting in inadequate blood flow to the placenta. This can compromise the delivery of oxygen and nutrients to the fetus, potentially leading to fetal growth restriction or intrauterine growth retardation (IUGR).
Placental Dysfunction: Lower VEGF levels may indicate placental insufficiency, where the placenta cannot adequately support the growing fetus. This condition can increase the risk of pregnancy complications such as preeclampsia, a disorder characterized by high blood pressure and damage to organ systems, which can be life-threatening for both the mother and the baby. Maternal inflammation in obesity and GDM may not always be associated with fetal inflammation. We propose that the placenta ‘senses’ and adapts to the maternal inflammatory environment, and plays a central role as both a target and producer of inflammatory mediators.[Patham 2015]
Hypoxia: Reduced angiogenesis and blood flow can cause a hypoxic environment in the placenta, where there is insufficient oxygen. This can further complicate fetal development and increase the risk of adverse pregnancy outcomes, including preterm birth or stillbirth.
Potential Long-term Effects: Insufficient placental VEGF and the resulting poor placental function can also have long-term health consequences for the child, potentially predisposing them to cardiovascular and metabolic diseases later in life.
The decreased VEGF concentration in the placenta is a critical marker that can indicate underlying issues with placental function and fetal development, necessitating careful monitoring.[Xu 2022, Pantham 2016]
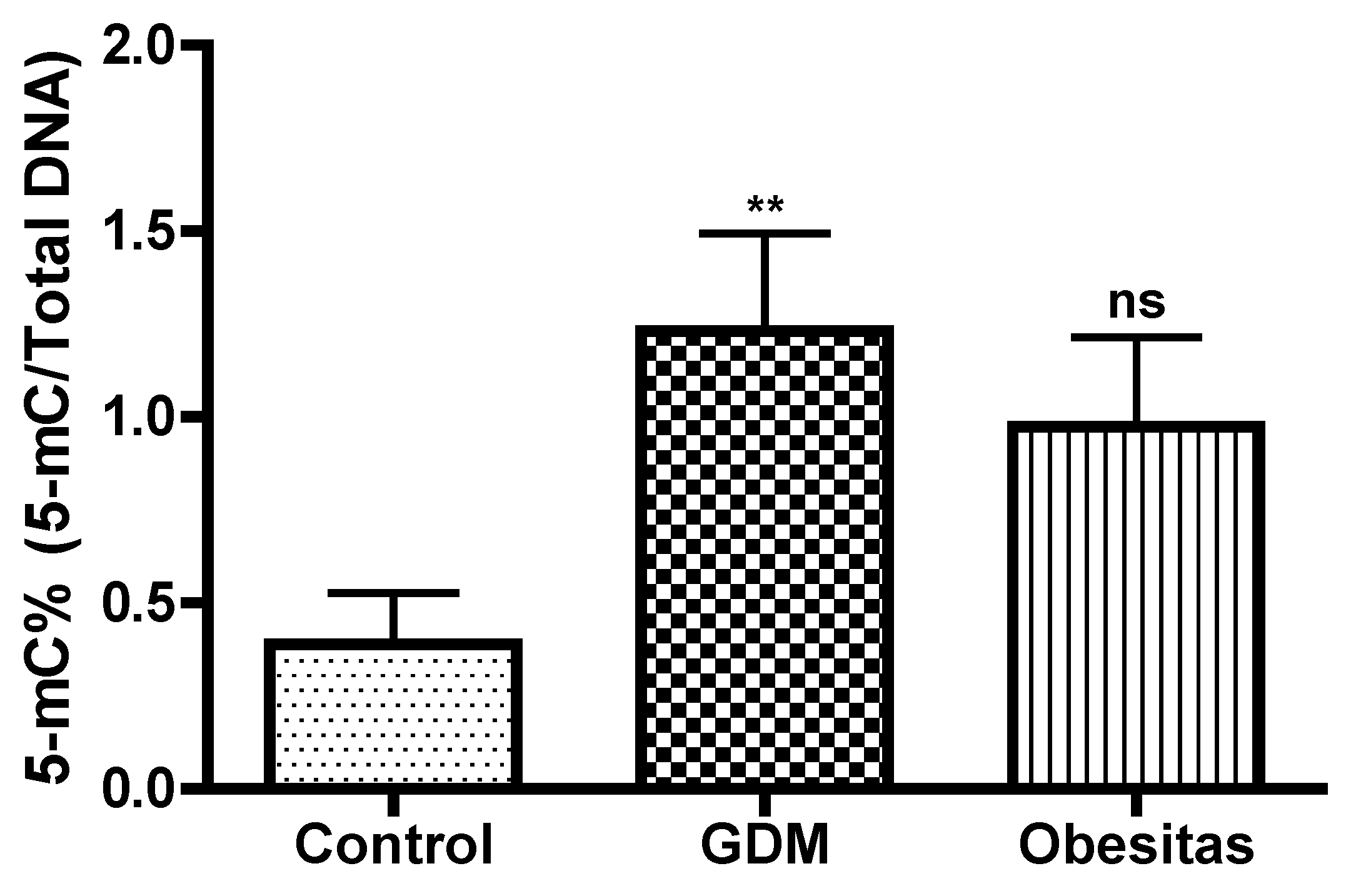
DNA methylation, one of the most common epigenetic modifications, has received considerable attention in last years [Xu P2022]. DNA methylation assays in the placental tissue samples represented elevation both in GDM and obesity. In our study the DNA methylation were significantly higher in GDM, than in obesity. In Gestational Diabetes Mellitus (GDM), the increase in DNA methylation compared to obesity may be due to the unique metabolic and hormonal changes that occur during pregnancy. These changes can influence gene expression differently than in obesity alone. GDM-specific factors, such as altered insulin and glucose levels, hormonal fluctuations, and inflammation, can lead to distinct epigenetic modifications. These modifications can affect genes involved in glucose metabolism, inflammation, and placental function, resulting in more pronounced DNA methylation changes in GDM compared to obesity, which primarily involves chronic metabolic stress. [Xu P 2022]. The placenta in maternal obesity and GDM may represent an adaptation, which could contribute to limit exposure of the fetus to inflammation and oxidative stress. The placental DNA methylation is moderalty higher in GDM compare to obesity in our study. The influence of DNA methylation of leptin, adipokin, insulin molecules and pathway-related genes on gene expression is also closely related to the pathogenesis of GDM.[Xu P 2022]
Systematic follow-up programmes would be ideal to prevent the progression of GDM to diabetes, but unfortunately in most countries such programmes are lacking in routine clinical practice. This study shows possible alternatives for early screening and follow-up in cases of GDM and obesity. If not managed properly, GDM and obesity can lead to complications for both the mother and the baby. For the mother, it can increase the risk of developing high blood pressure and preeclampsia. For the baby, it can lead to excessive birth weight (macrosomia), premature birth, respiratory distress syndrome, and an increased risk of fetal malforamation ( e.g. fetal heart defects) and developing obesity and/or type 2 diabetes later in life.
Limitation of the study was the small sample in study groups. Recruitment was difficult because obesity is a common underlying condition of GDM. It was considered important not to examine cases where obesity was associated with GDM. This allowed us to separate the effects of GDM and obesity as much as possible. Altough the number of GDM with normal BMI is still much lower than in cases with obesity. GDM without obesity is becoming more common, due to bad eating habits [Zehravi 2021]. The ideal dietary habit enjoy three meals a day, while some of us prefer to eat almost continuously throughout the day.
During pregnancy, especially if managing gestational diabetes mellitus (GDM), it is generally recommended to eat small, balanced meals and snacks throughout the day to help maintain stable blood glucose levels. A typical recommendation is: enjoy three meals a day and 2-3 little snacks. This results in eating around 5 to 6 times a day. The goal is to avoid large fluctuations in blood sugar levels, which can occur with irregular eating patterns or large meals. Smaller, more frequent meals can help in managing blood sugar levels effectively.
Monitoring GDM during pregnancy is crucial to manage blood sugar levels and ensure the health of both the mother and the baby. The key components of GDM monitoring is frequent monitoring of blood glucose levels helps to maintain them within a target range and prevent complications. Maintaining a balanced diet with controlled portions of carbohydrates. To monitor the baby's growth and detect any potential complications by ultrasound. Occasionally, to check long-term blood sugar control by HbA1c-test. The HbA1c-test is a useful tool in managing diabetes outside of pregnancy, there are several disadvantages to using the HbA1c test during pregnancy, particularly for diagnosing or monitoring gestational diabetes mellitus (GDM). HbA1c is not sensitive to short-term fluctuations in blood glucose, which are important to monitor in pregnancy to ensure tight glucose control and prevent complications [Stewing 2021] . Pregnant women have increased red blood cell turnover, which can lower HbA1c levels regardless of glucose levels. The increased blood volume during pregnancy can dilute red blood cells, potentially affecting HbA1c values [Nader Rifai:Tiezt 2022]. The HbA1c test reflects average blood glucose over a longer period (2-3 months), which may not capture recent changes in glucose levels, especially if GDM develops later in third trimester of pregnancy. The HbA1c test may not be sensitive enough to detect mild hyperglycemia or to differentiate between normal glucose tolerance and GDM [Nader Rifai:Tiezt 2022]. This can lead to missed diagnoses or a false sense of security if HbA1c levels appear normal while postprandial glucose levels are elevated. There is no universally accepted HbA1c cutoff for diagnosing gestational diabetes. The diagnostic thresholds used for type 2 diabetes do not directly translate to GDM, making it difficult to interpret results in a pregnancy-specific context. HbA1c levels can be influenced by factors such as ethnicity, genetic hemoglobin variants, and certain medical conditions (e.g., anemia, hemoglobinopathies). These factors can further complicate the interpretation of HbA1c results during pregnancy. [Stewing 2021, Culliney 2018].
The underlying pathogenic mechanisms behind the abnormal metabolic risk profile in offspring are unknown, but epigenetic changes induced by exposure to maternal hyperglycaemia during fetal life are implicated. Obesity during pregnancy is a significant risk factor for developing hyperglycemia, which can lead to gestational diabetes mellitus (GDM) or exacerbate pre-existing diabetes. Neither diet, physical activity, nor a combination of both offered significant benefit in preventing GDM in overweight/obese women [Lim 2023] , despite that these measures were all remarkably beneficial for gestational weight gain restriction .
This condition, characterized by elevated blood sugar levels by insulin resistance, increased insulin demand. Adipose tissue, especially in excess, can produce inflammatory cytokines and hormones like leptin and adiponectin, which can interfere with insulin action. This inflammation can exacerbate insulin resistance and contribute to hyperglycemia [Musa 2023 ].
The insulin-blood glucose balance varies from week to week in gestation and a quick response is the key to few complications. To do this, we are researching new biomarkers such as leptin, adipokin, VEGF expression and DNA metilation. Implementing new strategies to modulate placental transport may improve maternal health and prove effective in normalizing placental and fetal growth.
The identification of specific changes in the placenta can help in the development of diagnostic and therapeutic approaches to improve prenatal outcomes, with particularly significant benefits. Concerning prevention in the offspring, our knowledge is still too limited to recommend specific programmes.