1. Introduction
Bipolar disorder (BD) is a complex, chronic and highly disabling illness characterized by severe and persistent mood fluctuations, during which depressive phases alternate with either manic phases (BD I) or hypomanic phases (BD II) (Carvalho et al, 2020). In the last version of DSM-5TR, BD included BD I, BD II and Cyclothymic disorder (APA, 2022).
Bipolar I disorder is characterized by various symptoms, including expansive mood, verbosity, excessive self-esteem, grandiosity, irritability, significant reduction in the need for sleep, as well as prodigality and extreme disinhibition. Psychotic symptoms such as delusions and hallucinations occur in approximately 3/4 of manic episodes. Bipolar II disorder is primarily characterized by depressive episodes, interspersed with hypomanic episodes that never reach the severity of mania (Carvalho et al, 2020).
Beyond psychopathological symptoms such as anxiety and subthreshold symptoms, BD may be associated with a lack of psychosocial and cognitive functioning, manifested by attention, memory and executive deficits (Szmulewicz et al, 2020). Moreover, global functioning may be compromised even during interepisode periods, causing work, social, and relationship impairment (Tsapekos et al, 2021).
It is estimated that it affects approximately 40 million individuals worldwide (Nierenberg et al, 2023).
Following a trimodal distribution, different ages of onset have been suggested, although the average of BD onset is mainly around the age of 17.3 (Bolton et al, 2021).
Generally, BD presents with a first depressive episode, both for BD I and BD II (Vieta et al, 2018). The depressive episode can have a variable duration but typically lasts much longer than the counterpolar episode. Since BD is also characterized by alternating periods of mood interspersed with intercritical periods of euthimia, it is often misdiagnosed or underdiagnosed (Carvalho et al, 2020).
Over time, manic and depressive episodes, but especially depression, tend to become more frequent and this seems to be associated with greater impairment of global functioning (Grunze and Born, 2020).
Immune system dysfunction has a strong correlation with BD. Empirical research has confirmed that autoimmune diseases (Rosenblat and McIntyre, 2017), chronic stress, cardiovascular disease (Fenton et al, 2006), and metabolic disorders (SayuriYamagata et al, 2017) are among the numerous inflammatory conditions associated with BD (Rantala MJ et al, 2021). For example, studies on cytokines have shown that BD is linked to low-grade chronic inflammation, with pro-inflammatory cytokines being elevated during mood episodes (Goldsmith et al, 2023).
There are multiple mechanisms that account for the reciprocal relationship between immune dysfunction and BD. The hypothalamic-pituitary-adrenal (HPA) axis over-activation, cytokine-induced changes in monoamines, elevated oxidative stress, pathological microglial over-activation, modifications to the microbiome-gut-brain axis, and immunological changes related to sleep are important mechanisms (Goldsmith et al, 2023. When it comes to treating BD, the inflammatory-mood pathway offers several innovative target options. Anti-inflammatory drugs have demonstrated a beneficial effect in a few proof-of-concept clinical trials for the treatment of BD (Rosenblat JD and McIntyre, 2017).
One of the most complex problems related to the management of the DB is suicide. In fact, annually, patients with bipolar disorder present a rates of attempted and completed suicide of the 3.9% and 1.4%, respectively; these rates are significantly higher than those of the general population, which are 0.5% and 0.02% respectively. Bipolar patients experiencing a depressive episode or a mixed state have a higher risk of suicide than those experiencing just mania. Among bipolar patients, risk factors associated with depression for suicide attempts include multiple hospitalizations for depression and the presence of suicidal thoughts during depressive periods. Conversely, a prior suicide attempt has been shown to predict longer durations of depressive episodes, more severe depression, and a greater propensity for suicidal ideation (McIntyre and Calabrese, 2019).
Moreover, among BD patients, females exhibit a greater frequency of suicide attempts, whereas males demonstrate a higher incidence of completed suicides (Hu et al, 2023). The same is true for young people (Miranda-Mendizabal et al, 2019).
Since BD is a complex disorder, international guidelines suggest an integrated management of the illness, in which pharmacological therapy is combined with psychotherapy and psychosocial interventions.
The pharmacotherapy for BD patients, from childhood to adulthood, is managed according to the phase of the illness; in fact, major international guidelines recommend a specific therapy for the manic/mixed state, for the depressive state, or for the maintenance of euthymic states (Tab 1) (Findling et al, 2018).
Table 1.
Pharmacotherapy table for management of the BD different phases.
Table 1.
Pharmacotherapy table for management of the BD different phases.
| Phases |
Acute manic/mixed episodes |
Depressive phase |
Euthymic |
Childhood
|
lithium
+
atypical antipsychotics
(risperidone, olanzapine, quetiapine, aripiprazole, ziprasidone, and asenapine) |
olanzapine and fluoxetine
or
lurasidone (as monotherapy) |
Aripiprazole
Lamotrigine (in adolescents only)
Lithium |
| Adulthood |
Atypical Antipsychotics
(risperidone, olanzapine, quetiapine, aripiprazole, ziprasidone, and asenapine)
+
BDZ
|
Quetiapine
Antidepressant
or SSRI
Lamotrigine
Lithium
|
Lithium
Antidepressant
or SSRI
|
Within the psychotherapy for BD, Cognitive and Behavioral Therapy and Psychoeducational treatments are the most cited and effective interventions (NICE, 2022).
Although approved treatment protocols for BD (both psychopharmacological and psychosocial), already exist and are used routinary, on one hand, pharmacological approaches for BD may not consistently yield desired results and may carry safety concerns, but on the other hand, nonpharmacological interventions (as CBT, psychiatric rehabilitation, psychoeducation etc.) may not always be effective, easily accessible, or scalable (McVoy and Levin, 2023) (Tab 2). Therefore, there is an urgent need for the development of new therapeutic interventions that can be tailored to the specific needs of the patient. For example, neuromodulation, in its various forms, can be considered an important tool to be used within the integrated treatment of BD (Camprodon, 2021).
Table 2.
Psychosocial treatment scheme for management of the BD different phases [CBT: Cognitive and Behavioral Therapy: FFT: Family Focus Therapy; IPSRT: Interpersonal and Social Rhythm Therapy].
Table 2.
Psychosocial treatment scheme for management of the BD different phases [CBT: Cognitive and Behavioral Therapy: FFT: Family Focus Therapy; IPSRT: Interpersonal and Social Rhythm Therapy].
| Effective for management during |
Acute manic/mixed episodes |
Depressive phase |
Euthymic |
Childhood
|
CBT (medication adherence-oriented) |
CBT (on the management of depression symptoms)
|
Psychoeducation (enhanced relapse prevention) individual/group
FFT |
Adulthood
|
CBT (medication adherence-oriented) |
CBT (on the management of depression symptoms) |
Psychoeducation (enhanced relapse prevention) individual/group
FFT
CBT (on the recognition of early signs of crisis)
IPSRT (on the life rhytms) |
2. Aim
Neuromodulation treatments have emerged as promising interventions for different neurological and psychiatric disorders (Oriuwa et al, 2022; Rosson et al, 2022; Guo et al, 2023; Mutz, 2022). As healthcare providers seek to optimize patient care, the process of referring individuals to neuromodulation therapies demands careful consideration and adherence to best practices. This paper aims to i) delineate the major neuromodulation approaches offering updated information on innovative adjunctive treatments for BD, ii) define the essential steps involved in effectively sending patients for neuromodulation treatments, iii) ensuring comprehensive evaluation, appropriate selection, and seamless transition into the therapeutic regimen, aiming to support clinicians in their efforts to enhance functional recovery for patients.
3. Methods
This narrative review aims to described the current neuromodulation strategies used in the clinical practice of Bipolar Disorder treatment. An extensive research study was conducted to identify suitable papers. The references cited in this review were sourced from thorough searches of electronic databases including Embase, PubMed/MEDLINE, EBSCO and APA PsycINFO. The following keywords were used alone and in combination with ″Bipolar Disorder‶. Only studies published until April 2024 in peer-reviewed journals and written in english were included. Priority was given to published reviews and meta-analyses to offer a comprehensive and current summary of research on neuromodulation strategies in patients with BD. When necessary, case reports or open-label study were cited. All information was narratively summarized and the main results were discussed within the dedicated paragraphs.
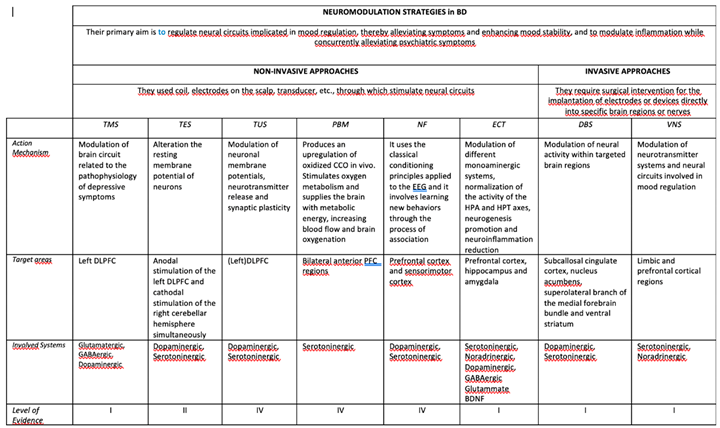
4. Neuromodulation Treatments
Neuromodulation techniques encompass a range of interventions, that has seen rapid development in the last years, aimed at modulating neural activity to treat various neurological and psychiatric disorders, including BD (Rosson et al, 2022; Guo et al, 2023).
These techniques use different devices, and generally they can be broadly categorized into invasive and non-invasive approaches.
Invasive neuromodulation techniques typically involve the implantation of electrodes or devices directly into specific brain regions or nerves. Examples include deep brain stimulation (DBS) and vagus nerve stimulation (VNS). Non-invasive techniques, on the other hand, do not require surgical intervention and can be applied externally to the body. Examples include photobiomodulation (PBM), neurofeedback (NF), electroconvulsive therapy (ECT), and transcranial stimulation with ist different protocols [transcranial Magnetic Stimulation (TMS), tetha-burst stimulation (TBS), transcranial Electrical Stimulation (TES), transcranial Direct Current Stimulation (tDCS), and transcranial Ultrasound Stimulation (TUS)].
The primary goal of neuromodulation in BD is to regulate neural circuits implicated in emotional regulation, thereby alleviating symptoms and enhancing mood stability. Moreover, a mounting body of research has affirmed that these neuromodulation techniques can modulate chronic inflammation affecting BD patients while concurrently alleviating psychiatric symptoms (Geddes and Miklowitz, 2013; Ferrarelli and Phillips, 2021; Iglesias et al, 2017). Neuroplasticity, which encompassess dynamic alterations of neuronal connections in the central nervous system, serves as the foundation for various brain functions, including cognitive processes such as learning and memory. During the last years it became increasinly evident that neuroplasticity dysfunctions are involved in several neuropsychiatric disorders, and many studies show that neuroplasticity can be induced in humans using non-invasive brain stimulation techniques, for example (d’Urso et al, 2023).
The mechanisms of action underlying neuromodulation techniques vary depending on the specific method employed. For example, DBS involves the delivery of electrical impulses to targeted brain regions, modulating neuronal activity and neurotransmitter release. Similarly, VNS modulates neural activity via stimulation of the vagus nerve, which sends projections to different brain regions involved in mood regulation.
However, neuromodulation techniques also offer distinct advantages. They can provide targeted and personalized treatment approaches, particularly for patients who have not responded to traditional pharmacological or psychotherapeutic interventions. Furthermore, these techniques are generally well-tolerated, with very few serious adverse effects reported in clinical trials (Mutz, 2023).
Evidence regarding the efficacy of neuromodulation techniques in BD is still evolving. While some studies have reported significant reductions in depressive and manic symptoms following neuromodulation therapy, others have yielded more mixed results (McIntyre et al, 2020). Further research, including larger randomized controlled trials with longer follow-up periods, is needed to establish the efficacy of neuromodulation as a treatment for BD conclusively (Tab 3).
Table 3.
Differences in the neuromodulation approaches.
Table 3.
Differences in the neuromodulation approaches.
5. Non-Invasive Approaches
-
i.
Transcranial Magnetic Stimulation (TMS)
The past twenty years have marked significant progress in the development of brain stimulation methods.
In a letter to Editor, Barker et al (1985) introduced the TMS as a tool to investigate the functional characteristics oft he motor corticospinal pathways in humans (Barker et al, 1985), and ist use has spread quickly. Although it is used as an investigative tool, the TMS has shown great potential when used to modulate neural circuit activity (Ferrarelli and Phillips, 2021).
In fact, TMS modulates neurotransmitter systems by increasing cortical excitability and synaptic plasticity. It particularly influences the glutamatergic and GABAergic systems, enhancing excitatory and inhibitory balance. Additionally, TMS has been showed to affect the dopaminergic system, potentially increasing dopamine release in targeted areas.
The TMS can be appreciated for ist versatility. The different protocols can vary based on: i) the location of stimulation (i.e. the side of the stimulation: right-sided vs left-sided, bilateral vs unilateral, etc.), ii) duration, iii) stimulus frequency and iv) intensity.
Among these advancements, the establishment of repetitive transcranial magnetic stimulation (rTMS) targeting the left dorsolateral prefrontal cortex (DLPFC) stands out as the most notable, offering a safe and effective treatment for depression with minimal side effects (Carnell et al, 2017).
A standard protocol requires the administration of a high frequency stimulation (10 Hz) for 37.5 min for 5 days per week for at least 6 weeks. However, the accelerated protocols suggesting that the repetitive TMS (rTMS) may be effective anyway using a minor time (e.g. 18.75 min. protocol) or a low-frequency (1-2 Hz) for a 4 week treatment (Fitzgerald et al, 2006; Dell’Osso et al, 2009; Konstantinou et al, 2022). rTMS, which encompasses high-frequency rTMS (HF-rTMS), inducing excitatory effects at ≥ 5 Hz, low-frequency rTMS (LF-rTMS), inducing inhibitory effects ≤ 1 Hz, theta-burst stimulation (TBS) and accelerated intermittent theta-burst stimulation (aiTBS), has been mainly employed in the treatment of Major Depressive Disorder (MDD) (Cai et al, 2023). The stimulation of DLPFC impacts connected neural circuits, including the fronto-striatal and fronto-limbic pathways, which are involved in mood regulation and executive function. The rTMS is the most widely used protocol for the treatment of unipolar depression and, recently, for the treatment of DB too (Desbeaumes et al, 2019; Gold et al, 2019; Cai et al, 2023). Its therapeutic mechanism of action is mainly focused on the remodulation of brain circuits involved in the maintenance of depressive symptoms. In fact, the most research of rTMS is focalized on depressive symptom of BD while little is known about ist effects in mixed or maniac states (Konstantinou et al, 2022).
The other specific rTMS protocol, TBS, employes short bursts (three pulses at 50 Hz) delivered at 5 Hz. Lastly, deep TMS (dTMS) is a particular TMS that uses the H coil held inside a padded helmet to achieve deeper and large brain volumes (Ferrarelli F, Phillips, 2021).
The predictors of response to TMS treatment are different. Some studies suggest that the efficacy of treatment response could be influenced by the type of depressive symptoms declared by the patient (cognitive-affective versus somatic symptoms), some antidepressant or antihistamines or the illness duration (Gold et al, 2019).
Numerous studies confirm the clinical efficacy on depressive symptoms of rTMS in adult BD, moreover, it has shown to be safe, well tolerated (while some studies reported greater energy, insomnia, irritability, anxiety and suicical attempt), and characterized by a low rate of drop-out (Miuli et al, 2021; Zengin et al, 2022; Konstantinou et al, 2022). However, clinical studies are characterized by a small samples size, although randomized control trials are high compared to open-label studies.
In pediatric population, the existing research is very limited, but in the last years, the promising results obtained in the treatment of adults BD, are advocating the use of TMS even among younger patients, especially in cases where traditional treatments are inaccessible, poorly tolerated or ineffective (Jivray et al, 2022).
-
ii.
Transcranial Electrical Stimulation (TES)
Transcranial Electrical Stimulation (TES) includes a range of neuromodulation techniques that involve the application of electrical currents to the scalp with the aim of modulating neural activity in the brain (Antal et al, 2017). These techniques include transcranial direct current stimulation (tDCS), that applies a direct electrical current of low intensity with an anode and a cathode; transcranial pulsed current stimulation (tPCS); transcranial alternating current stimulation (tACS) that applies an ostillating, sinusoidal electrical current of low intensity on specific brain area; and transcranial random noise stimulation (tRNS). Further sub-categories reflect differences in the aspects of electrode montage, wafeform, and outcomes (Bikson et al, 2019; Cho et al, 2022).
TES has gained significant attention in neuroscience research and clinical applications due to its potential to enhance cognitive function, facilitate neuroplasticity, elicit long-lasting alterations of cortical excitability, and treat neuropsychiatric disorders, including BD (Bikson et al, 2019).
The primary purpose of TES in BD is to modulate neural circuits implicated in mood regulation, with the goal of alleviating depressive and manic symptoms and promoting mood stability.
Additionally, TES may induce neuroplastic changes in brain regions associated with mood regulation, such as the prefrontal cortex and limbic system. Cognitive functioning is involved in many psychiatric disorders, the observed impairment can be profound or relatively mild (Robbins, 2011), and its amelioration through TES could lead to improvement in daily life functioning.
The mechanisms underlying the therapeutic effects of TES are not fully understood, but are thought to involve changes in neuronal excitability by altering the resting membrane potential of neurons (Nitsche and Paulus, 2011), and synaptic plasticity. In fact, TES aim to directly modify brain function by passing low or high amplitude electrical currents using an electrode placed on the scalp (Antal et al, 2017).
Within TES techniques, the Transcranial Direct Current Stimulation (tDCS) involves applying a low-intensity electrical current on the scalp via electrodes, with the aim of modulating neuronal activity in the targeted brain regions. tDCS shifts resting neuronal membrane potential toward anodal or cathodal polarization without ever exceeding the depolarization threshold of neurons due tot he weak and constant polarization (Nitsche et al, 2004). Anodal stimulation, characterized by the application of a positive current, is thought to depolarize neurons, thereby increasing their firing rates, whereas cathodal stimulation, involving a negative current, is believed to hyperpolarize neurons, leading to decreased excitability. This current flow between the electrodes stimulates neurons, producing physiological and behavioral modification.
Such protocols simultaneously involve anodal stimulation of the left DLPFC and cathodal stimulation of the right cerebellar hemisphere to improve neuropsychological and neurological functioning (Minichino et al, 2015; Bersani et al, 2017).
These effects can persist beyond the duration of stimulation and may induce long-term changes in synaptic plasticity (Bikson et al, 2016). For example, tDCS is believed to modulate the resting membrane potential of neurons, thereby altering their firing rates and influencing neurotransmitter release. Begemann et al, (2020), in their meta-analysis found a positive and significant effect of tDCS on working memory and also in attention/vigilance in patients affected by neuropsychiatric and psychiatric disorders. Numerous research highlighted significant improvement on BD cognition and sleep quality in euthymic BD patients, too. In particular, prefronto-cerebellar tDCS contributes to improve visuospatial memory, executive functions and neurological soft signs in euthymic adult BD (Bersani et al, 2017). The sleep quality is increased after a prefronto-cerebellar tDCS delivered for 20 min/die for three consecutive weeks in an euthymic adult BD (Minichino et al, 2014).
However, the efficacy of tDCS in BD is still under investigation. Many studies suggest that tDCS has effects on BD depressive phases, although data on tDCS in manic or hypomanic phases are few.
A case report found that tDCS as an add-on pharmacotherapy may improve manic symptoms in patients with BD. More research is needed to assess the potential efficacy oft DCS in patient with hypomanic and/or manic symproms (Mutz et al, 2022).
In the young BD population, tDCS showed promising results, but definitive outcomes are still unknown (Hameed et al, 2017; Muszkat et al, 2016).
Moreover, given the different protocols used in the literature, it is not possible to draw clear conclusions.
In terms of regulatory approval, the U.S. Food and Drug Administration (FDA) has granted clearance for certain tDCS devices and specific indications. However, it is essential to note that the FDA’s regulatory oversight primarily pertains to the safety and efficacy of medical devices rather than specific treatment protocols. As such, there is ongoing research to establish standardized protocols and optimize the efficacy of tDCS for various clinical applications (Brunoni et al, 2012).
Despite its potential benefits, TES has several limitations. One limitation is the variability in treatment response observed among individuals, with some patients experiencing significant symptom improvement while others derive minimal benefit. Additionally, the optimal parameters (e.g., stimulation intensity, duration, and electrode placement) for TES in BD have yet to be established, leading to challenges in standardizing treatment protocols. Moreover, the long-term effects of TES on mood stability and relapse prevention in BD remain unclear (Cho et al, 2022).
However, TES tecniques also offer several advantages. They are non-invasive and relatively well-tolerated, with few serious adverse effects reported in clinical trials. Furthermore, they can be administered as an adjunctive therapy to pharmacological and psychotherapeutic interventions, potentially enhancing treatment outcomes for individuals with BD (Bersani et al, 2017; Dell'Osso et al, 2012).
The efficacy of TES in BD is still being investigated, with mixed findings reported in clinical trials (Brunoni et al, 2016). While some studies have reported significant reductions in depressive and manic symptoms following TES therapy, others have yielded more modest or inconsistent results.
Further research, including large-scale randomized controlled trials with longer follow-up periods, is needed to elucidate the therapeutic efficacy and optimal parameters of TES in BD conclusively (D’Urso et al, 2023).
-
iii.
Transcranial Ultrasound Stimulation (TUS)
Transcranial Ultrasound Stimulation (TUS) is a non-invasive neuromodulation technique that involves the application of low-intensity ultrasound waves to specific regions of the brain, modulating the activity of the primary somatosensory cortex (Bhattacharya et al, 2022). Its use is relatively recent.
For its properties, TUS was first used with neurological patients. The first research study, in 2013, showed improvement of scores at mood and pain evaluation, in chronic pain patients (Sarica et al, 2022).
The mechanism of action of TUS in BD is not fully understood, but it is hypothesized to involve several processes, thanks to its excitatory or inhibitory activity, depending on the sonication parameters applied (Hu et al, 2023).
Ultrasound waves penetrate the skull and reach the brain with a frequency > 20 kHz, where, interactioning with membranes and mechanosensitive channels, they may influence neuronal activity by modulating neuronal membrane potentials, neurotransmitter release, and synaptic plasticity, thereby inducing a neuronal signaling cascade (Osada et al, 2024). In mouse models studies of depression, it has emerged that Low-intensity Transcranial Ultrasound Stimulation (LITUS) of the prefrontal cortex (PFC) or ventromedial prefrontal cortex (vmPFC) weakened depressive-like behaviors. Further evidence for a common BDNF signaling pathway triggered by ultrasound stimulation comes from the observation that LITUS increased BDNF levels in the hippocampal regions of normal mice. Moreover, LITUS increased adult hippocampus neural stem cell proliferation and neurogenesis; the latter is an important mechanism underlying depression and is a side effect of antidepressant medications (Hu et al, 2023). Therefore, TUS may stimulate neurogenesis and enhance neuroplasticity, processes that are disrupted and/or altered, in mood disorders such as BD.
Unlike other transcranial stimulation tecniques, TUS can stimulate both cerebrocortical surface and deep brain structures (Osada et al, 2024).
Despite its potential, TUS has certain limitations. One limitation is the lack of a precise targeting of brain regions, as the therapeutic effects of TUS depend on the accurate delivery of ultrasound waves to specific neural circuits. Additionally, the optimal parameters for TUS therapy in BD, including waveform, intensity, and duration of stimulation, have yet to be fully elucidated. Furthermore, the long-term safety profile of TUS for BD treatment remains to be established.
TUS has garnered interest as a potential therapeutic intervention for various neurological and psychiatric disorders, including BD. The primary objective of TUS in the context of BD is to modulate neural activity in brain regions implicated in mood regulation, with the aim of ameliorating cognitive symptoms and enhancing mood stability.
However, TUS also offers several advantages. It is a non-invasive technique that does not require surgical implantation or anesthesia, making it well-tolerated and suitable for repeated sessions. Moreover, TUS has the potential to modulate neural activity with high spatial resolution, allowing for precise targeting of brain regions implicated in BD pathophysiology (Legon et al, 2014).
The efficacy of TUS in BD is still under investigation. While preclinical studies and small-scale clinical trials have shown promising results in terms of reducing depressive and manic symptoms, larger randomized controlled trials with long-term follow-up are needed to conclusively confirm its efficacy and safety as a treatment for BD.
-
iv.
Photobiomodulation (PBM)
Photobiomodulation (PBM), also known as low-level laser therapy (LLLT) or cold laser therapy, is a non-invasive therapeutic technique that utilizes low-level light sources (usually in the red or near-infrared spectrum) to modulate cellular functions. It has several effects, such as boosting ATP synthesis, repairing damaged hypoxic cells, altering target tissue function, and stimulating mitochondrial metabolism (Salehpour et al, 2018; Montazeri et al, 2022). It is precisely because of these properties that PBM has attracted attention in various medical fields, including neurology and psychiatry. In particular, Transcranial Infrared Laser Stimulation (TILS), based on photobiomodulation, is a new non-invasive form of low-level light therapy which uses an infrared laser within the range of 808 nm and 1064 nm and has been applied for its potential therapeutic effects in mood disorders such as BD (O’Donnel et al, 2022).
The mechanisms of action of PBM involve the absorption of photons by mitochondrial chromophores, leading to increased adenosine triphosphate (ATP) production, modulation of reactive oxygen species (ROS) levels, and upregulation of various cellular signaling pathways. Some studies highlighted the presence of various mitochondrial alterations in psychiatric disease, included BD (O’Donnel et al, 2022; O’Donnel et al, 2023).
Additionally, PBM has been shown to promote neurogenesis, enhance synaptic plasticity, and reduce inflammation, all of which may contribute to its therapeutic effects in BD (Rochkind, 2016).
The primary purpose of PBM in the context of BD is to alleviate depressive and manic symptoms, improve mood regulation, and potentially stabilize mood fluctuations. This is achieved through the modulation of cellular processes, particularly in the brain, which are thought to play a role in the pathophysiology of BD (Montazeri et al, 2022).
In the research study by O’Donnel et al, (2022), the first that examines how TILS improves cognition in BD patients, BD subjects (n=5) receveid 1 minute invisible laser light at 1064 nm and a dim red guiding light of 560 nm alternating every minute between the right and left anterior PFC, for 10 minutes per session. At the end of treatment, they showed an enhancement of cognitive frontal functioning.
Despite its promising potential, PBM also has limitations and challenges. One limitation is the lack of standardized protocols regarding treatment parameters such as wavelength, intensity, and duration of exposure. Additionally, the evidence supporting the efficacy of PBM in BD is still preliminary, with relatively few well-designed clinical studies available (Salehpour et al, 2018).
However, PBM offers several advantages as a therapeutic approach for BD. It is non-invasive, well-tolerated, and associated with minimal side effects, making it suitable for long-term use.
Furthermore, PBM can be easily administered and integrated into existing treatment regimens, including pharmacotherapy and psychotherapy (Hamblin, 2018).
Regarding its efficacy in BD, while preliminary studies have shown promising results in terms of mood improvement and symptom reduction, more rigorous research is needed to establish its effectiveness and determine optimal treatment protocols. Future randomized controlled trials with larger sample sizes and longer follow-up periods are warranted to further elucidate the role of PBM in the management of BD.
-
v.
Neurofeedback (NF)
Neurofeedback (NF) is a form of biofeedback that aims to modulate brain activity by providing real-time feedback on brainwave patterns, typically through electroencephalography (EEG). This technique has gained attention as a potential intervention for various neurological (Lambert-Beaudet et al, 2021) and psychiatric conditions, including BD.
NF involves training individuals to self-regulate their brain activity by presenting them with real-time information about their neural functioning, often in the form of auditory or visual signals. Through repeated sessions, individuals learn to modulate their brainwave patterns, aiming to achieve desired states associated with improved cognitive and emotional functioning (Enriquez-Geppert, et al 2017).
There are several objectives that can be achieved with this treatment: i) symptoms reduction: NF is utilized with the aim of reducing symptoms associated with BD, including mood swings, cognitive impairments, and emotional dysregulation; ii) enhanced self-regulation: the training aims to improve self-regulation of neural activity, potentially leading to better mood stability and overall functioning, including the reduction of impulsivity behaviours (Micoulaud-Franchi et al, 2014); iii) sleep improvement: the training may modulate the amount of sleep (too much or not enough) in different phases of BD (Rosenblat and McIntyre et al, 2017; Lambert-Beaudet et al, 2021).
NF uses different protocols for specific theraputic needs. For example, the therapist may apply from two up twentyone electrodes in different brain areas in reference to 10-20 system; other differences include: i) the frequency band trained [e.g. delta (1-4 Hz); theta (4-8 HZ); alpha (8-12 Hz); sensory-motor rhythm SMR (12-15 Hz); low-beta (13-21 Hz); High-beta (21-35 Hz); gamma (35-45 Hz); theta/beta ratio; etc.)]; ii) the different location of electrodes (Fz, Cz, etc.); iii) the different conditions of the subject (e.g. closed or open eyes, simultaneosly use of a virtual reality headset); iv) the different tasks to be performed (relaxation, problem solving, puzzle, etc).
NF operates on the principle of neuroplasticity, the brain's ability to reorganize and adapt in response to experiences. By providing feedback on brain activity, individuals can learn to modulate their neural patterns, leading to changes in brain functioning over time (Reinhart and Nguyen, 2019).
There are a few limitation to its use. The effects of NF may not generalize well beyond the training setting, limiting its long-term efficacy (generalizability). NF training requires multiple sessions over an extended period, making it resource-intensive and potentially inaccessible to some individuals.
On the other hand, the NF has great advantages. Firstly, NF is non-invasive and does not involve the use of medication, making it a potentially attractive option for individuals seeking non-pharmacological interventions. Secondly, training protocols can be tailored to target specific symptoms or neural networks implicated in BD, allowing for individualized treatment approaches.
Research on the efficacy of neurofeedback in BD is still in its early stages. While some studies have reported promising results in terms of symptom reduction and mood stabilization, larger-scale randomized controlled trials are needed to establish its effectiveness as a standalone or adjunctive treatment for BD (Omejc et al, 2019).
-
vi.
Electroconvulsive Therapy (ECT)
Electroconvulsive Therapy (ECT) is a complex treatment administered by a specialized team within a dedicated hospital setting. It offers potent efficacy and rapid onset of action, even after alternative treatments fail. ECT has an exceptionally broad therapeutic spectrum, with evidence for benefit in unipolar and bipolar disorder (including the acute treatment of depressive and manic episodes, psychotic subtype, as well as relapse prevention), catatonia, schizophrenia, schizoaffective disorder, Parkinson’s disease, epilepsy, status epilepticus, repetitive self-injury in autism, tardive dyskinesia, and neuroleptic malignant syndrome (Rojas et al, 2022). ECT was introduced in 1938s in Italy, its technology and treatment delivery have since seen major improvements. Despite nearly nine decades of use in psychiatry demonstrating its efficacy, the precise mechanisms by which ECT exerts its therapeutic effects and its cognitive side effects are not fully uncovered and are still being studied, in particular the effects of electrical stimulation on the generalized seizure acrivity (Medda et al, 2014).
ECT primarily affects the prefrontal cortex, hippocampus, and subcortical structures such as the amygdala. Although the therapy is believed to modulate the limbic system and frontal lobes, which are crucial in mood regulation and cognitive function (Li et al, 2018).
The mechanisms of action of ECT needs of three different phases: i) the ictal period, characterized by a raising of blood flow, glucose metabolism and oxygen consumption in the cortex; ii) an interictal period and iii) the postictal period, where the therapeutic effect of ECT take place reducing the blood flow and the brain glucose metabolism (Rojas et al, 2022).
ECT influences several neurotransmitter systems. It contibutes to modify the metabolism of monoamines, and normalizes the functions of noradrenaline (NA), serotonin (5-HT), dopamine (DA), glutamate, gamma-aminobutyric acid (GABA), and brain-derived neurotrophic factor (BDNF) (Baldinger et al, 2014).
The practice of ECT involves administering anesthesia, inducing muscle relaxation and the monitoring various vital parameters such as blood pressure, hearth rate, oxygen saturation, etc.
Electrodes are placed on the scalp, either unilaterally or bilaterally, to briefly deliver an electrical charge, which induces a seizure that determines the outcome of the treatment (Abbott et al, 2016). All major international guidelines (APA, CANMAT, RANZCP and WFSBP), recommend ECT as a first, second, third o fourth step in the treatment of several major psychiatric disorders, including BD (Espinoza and Kellner, 2022).
ECT treatment tipically lasts for about 6 to 12 sessions, administered 2 to 3 times per week during the initial phase. After a significant therapaeutic response is achieved, the frequency of treatments can be gradually reduced.
Treatment protocols differ primarily in i) electrode placement (bitemporal, right unilateral or bifrontal), ii) electrical dosage (low, moderate or high) and iii) pulse width (brief or ultra-brief) (Rojas et al, 2022). Despite the overwhelming effectiveness in mania and depressed states of BD as well as maintenance phases of treatment (Elias et al, 2021; Medda et al, 2014), clinicians do not consider ECT earlier in the treatment algorithm.
However, in about 80-year history of ECT, numerous randomized control trial have showed high and rapid rates of improvement or remission for acute mania (Elias et al, 2021).
Bipolar depression has a statistically higher and speeder rate of response than unipolar depression (Rojas et al, 2022). Better understanding of factors that predict higher response to ECT including influence of baseline medication on ECT outcome may lead to better assessment of patients with BD. However, the prognosis may be worse for patients who arrive hostile, agitated, or suspicious (Elias et al, 2021).
While first-line treatments for mood disorders are ineffective for roughly 60% of young patients, the use of ECT is less common in populations of children and adolescents. Even though ECT is not as commonly used in kids and teens, it is still a crucial treatment for kids who have serious illnesses like catatonia, affective psychotic disorders, and depression that put them at high risk of suicide. Additionally, there is growing interest in the use of ECT to treat aggressive and self-harming behavior in individuals with autism spectrum disorder (Becker et al, 2018). Children and adolescents tolerate ECT treatment well. Response rates were primarily 70–82% for depression and 87–90% for mania in studies with a sample higher of thirty subjects (Castaneda-Ramirez et al, 2023). Despite its important role in the treatment of psychopathological symptoms, some patients show a transient cognitive impairment (Kritzer et al, 2023).
The Mini-Mental State Examination was used in seven trials, and none of them showed evidence of any significant cognitive impairment after the treatment with ECT. The majority of adverse effects were mild and transient. In 1.5% of the cases, ECT was stopped early because of side effects. There were no recorded deaths (Castaneda-Ramirez et al, 2023). Typically, the patient's response, both in adults and in children, determines the total number of treatment sessions. After two more sessions, ECT is stopped if the patient's symptoms have improved or not at all (Rojas et al, 2022).
Although there are clear benefits to using ECT, there are still some conditions that require further investigation. For example, in clinical practice, psychopathological status may complicate the performance of ECT when patients are unable to provide informed consent (Elias et al, 2021); furthermore, the possibility of providing ECT in services dedicated to developmental age is rare and is not yet part of the clinical routine.
Another important issue is the stigmatization of ECT by healthcare providers, who still too often do not offer it as a treatment, and by patients or family members who are not sufficiently informed about this treatment.
6. Invasive Approaches
-
i.
Deep Brain Stimulation (DBS)
Deep brain stimulation (DBS) is a neurosurgical procedure involving the implantation of electrodes within specific areas of the brain using stereotactic MRI guidance, followed by the delivery of electrical impulses. Electrodes are connected to a neurostimulator that is implanted near the chest (Krauss al, 2021).
Contact length, number, and shape are different amongst the DBS electrode configurations. More specifically, a larger contact range increases the variety of possible neural targets, whereas a smaller contact range makes it easier to control stimulation more precisely. The type of DBS system being used determines the different stimulation modes. The current flowing from the battery to the contact, or the other way around, is referred to as “unipolar stimulation”. Current flowing between electrode contacts, at least one of which is acting as an anode and the other as a cathode, is indicated by “bipolar stimulation” (Ramasubbu et al, 2018). The term "interleaving stimulation" describes the switching between various stimulation levels (Krauss al, 2021).
Originally developed for the treatment of movement disorders such as Parkinson's disease, DBS has attracted interest as a potential therapeutic option for psychiatric disorders, because it may be considered a viable alternative to ablative neurosurgical procedures in certain debilitating mental disorders where this is the case or in cases that do not respond to treatment, such as several forms of obsessive-compulsive disorder (OCD). In addition, it has also been studied in treatment-resistant depression in major depressive disorder (MDD), which has gained increasing importance as a potential add-on strategy (Ramasubbu et al, 2018; Abraham et al, 2023), as well as in BD (Gippert and et, 2017).
DBS involves the implantation of electrodes in specific brain regions, such as the subthalamic nucleus (STN), globus pallidus internus (GPi), and ventral capsule/ventral striatum (VC/VS) (Cagnan et al, 2019). These areas are part of the basal ganglia-thalamocortical circuits involved in motor control, mood, and reward processing. DBS alters the activity of various neurotransmitter systems depending on the target area. For example, stimulation of the STN can modulate dopaminergic pathways, crucial for its effects on Parkinson's disease. Similarly, DBS in the VC/VS region can influence serotonin and dopamine levels, contributing to its efficacy in treating refractory depression and obsessive-compulsive disorder (OCD) (Ramasubbu et al, 2018; Krauss al, 2021; Bewernick et al, 2012).
The mechanism of action of DBS in BD is complex and not fully understood. However, it is hypothesized to involve the modulation of neural activity, including blood flow, oscillatory synchrony, and synaptic weighting (Bilge et al, 2018), within targeted brain regions, in one or both hemispheres according to the laterality of the symptoms (in movement disorders), such as the subcallosal cingulate cortex (SCC), the ventral striatum, the nucleus aacumbens (NAcc), the supero-lateral branch oft he medial forebrain bundle (sIMFB), and subcallosal cingulate white matter (Cg25WM) (Gippert et al, 2017; Bilge et al, 2018). By modulating neural activity in these regions, DBS may normalize dysfunctional circuits involved in mood regulation and emotional processing, too. The primary aim of DBS in BD is to modulate aberrant neural circuits implicated in mood dysregulation, with the goal of reducing symptom severity and enhancing mood stability (Holtzheimer et al, 2012). An important difference between the DBS application is open or closed loop paradigm. "Open loop" DBS is the current standard in use. The patient receives a single pattern of stimulation to the brain for a few weeks to months after the clinician has examined the medical record and established the stimulation parameters. Indirect behavioral assessment and subjective physician evaluations are the main sources of decision-making in this practice, which heavily relies on trial and error. "Closed loop" DBS identifies a neural biomarker that encapsulates a critical component of disease. After that, the DBS system measures the biomarker directly and makes use of the data to modify the stimulation parameters. Currently manufactured DBS systems can detect local field potentials (LFP) from electrode contacts at the stimulation site. Prediction algorithms can be used to modify stimulation parameters to obtain the desired neurophysiological signature (Bilge et al, 2018).
In their letter to editor, Torres et al (2013) firstly reported on a 78-year-old woman suffering from bipolar depression which was successfully treated with DBS targeting the subcallosal cingulate region. This approach was chosen due to the poor response observed when treating the patient with various mood stabilizers, antipsychotics, and antidepressants, either alone or in combination.
Morevore, several admissions to the hospital for electroconvulsive therapy (a total of 42 sessions) were required, with a limited clinical response. Therefore, she was considered for DBS. From the first month of stimulation, the patient presented a significant and progressive clinical response supported by a significant reduction in her scores on the Hamilton Depression Rating Scale, the Beck Depression Inventory, and the Montgomery-Asberg Depression Scale, and an increase in her Global Assessment Functioning scores, which persisted at the last follow-up at nine months. No relapse of manic symptoms occurred, as reflected in her scores on the Young Mania Scale.
One review (Gippert et al, 2016) identified a few studies with a total of 12 patients who were treated for bipolar depression with DBS, generally presenting improvement in depressive symptomatology. The target sites were subcallosal cingulate, ventral capsule/ventral stiatum, nucleus accumbens, and the supero-lateral branch of the medial forebrain bundle.
The largest study was a 24-week, prospective observational trial (Holtzheimer et al, 2012) in which seven patients with BD II depression and ten with major depressive disorder, received add-on treatment with DBS of subcallosal cingulate white matter. During the study, BD patients were carefully assessed for hypomanic symptoms and no hypomanic effects occurred in BD patients and antidepressant effects in BD patients was comparable to those in patients with unipolar depression. All seven BD patients had previously undergone ECT, which did not produce the desired effects.
A single bipolar patient, who experienced only one previous manic episode, was included in another study (Malone et al, 2009) which investigated the use of DBS of the ventral capsule/ventral striatum for treatment refractory depression. This patient’s clinical course was the most variable. Although the patient had periods of depressive symptom improvement lasting weeks, not previously achieved after aggressive treatment including bilateral ECT and VNS, these were followed by hipomanic episodes. Such episodes ceased rapidly upon discontinuation of stimulation, and it is worth noting that hypomanic symptoms can emerge, in general, as a side effect of DBS even in patients suffering from unipolar depression or movement disorders.
Crowell et al, 2019 published an open-label, long-term follow-up to examine participants enrolled in a clinical trial of subcallosal cingulate DBS for treatment-resistant depression. Among these patients seven were diagnosed with bipolar disorder type II, and one patient, initially in the group of participants with major depression, subsequently was reclassified as bipolar type 2 following an episode of hypomania presented a few years after inclusion in the study. This episode was associated with a change in psychopharmacological therapy and no a change of DBS parameters, so it did not require interruption of stimulation or its modification but only rescheduling of pharmacotherapy.
Among the eight BD patients, five showed a favorable response pattern, and three exhibited limited antidepressant response over time. Four patients in the BD group withdrew from the study, accounting for four of the five participants who withdrew from the study overall.
Most of the above studies, except for the study by Torres et al. (2013), included patients with BD II. Bringing additional knowledge on DBS and BD I, a case series of Graat et al. (2020), presented five patients with OCD in comorbidity with BD I (three patients) and BD II (two patients), who received DBS of the ventral anterior limb of the internal capsule, to examine the impact on depressive symptoms and OCD. DBS effectively treated symptoms of OCD in 4 of 5 patients and significantly reduced depressive symptoms. A focus must be placed on the fact that four of the five patients presented symptoms of hypomania. In three patients (2 with BD I and one with BD II) the symptoms resolved spontaneously, while in the fourth (BD II) the symptoms persisted for a long period and required changes in stimulation. Finally, 2 of the 5 patients presented suicide attempts, which could be due to a side effect of DBS known as DBS-induced impulsivity. Further studies are therefore needed to better define the risk of suicidality following DBS in BD (Malone et al, 2009).
Habenula (Hb) has recently been identified as a new DBS target for treating various psychiatric disorders, including depression (Abraham et al, 2023). Hb is a small bilateral epithalamic structure located adjacent to the posterior commissure in humans, and multiple cortical and cerebellar regions are functionally connected to Hb, which plays a key role in controlling dopaminergic, serotonergic and noradrenergic systems thus regulating a wide range of behavior beyond reward processing and depressive symptoms. Moreover, according to an MRI study of habenula volume in BD patients, it was found that in those untreated for at least, two months, habenula volume was reduced compared with healthy controls (Dougherty, 2018).
Two recent reviews (Germann et al, 2021; Abraham et al, 2023) have attempted to provide an overview of the current status of habenula DBS in human subjects for the treatment of neuropsychiatric disorders. Treated conditions included refractory depression, BD, OCD, schizophrenia, and major depressive disorder. Regarding BD, Zhang and coworkers (Zhang et al, 2019) described the first case of refractory BD successfully treated with habenula DBS. This was a BD I patient who had been suffering from severe depressive symptoms for 4 years and was no longer responding to therapies, including electroconvulsive therapy. Interestingly, after an initial clinical improvement achieved in the first 3 months of high-frequency stimulation, the patient presented with a depressive relapse that improved at 7 months with discontinuation of stimulation. At 8 months, low-frequency stimulation was started, which led to further improvement in quality of life and energy levels. This suggests how low-frequency DBS habenula may be a useful maintenance therapy in bipolar depression. In addition, the patient reported no manic episodes or alterations in cognitive function. In another study, Zhang et al (2021) investigated the habenula's transient responses to deep brain stimulation. Eight deep brain stimulation contacts were used to test four patients (two of whom had schizophrenia and the other two had bipolar disorder). One month following deep brain stimulation surgery, patients underwent transient electrical stimulation examinations. The voltage increased in steps of 1 V, ranging from 0 V to a maximum of 10 V. The pulse width was 60 μs. Two frequencies of stimulation, 60 and 135 Hz, were applied to each patient. Out of the 385 active trials, 221 of them produced effects that were stimulated. Pain, heart rate fluctuations, and numbness were the most frequent temporary side effects. The incidence of pain, involuntary movements, numbness, and changes in heart rate increased as the stimulation voltage increased. All body parts except the scalp experienced numbness due to contralateral stimulation.
In pediatric populations, DBS is currently used for serious neurological disorders such as dystonia, although there are no validated eligibility criteria for DBS in pediatric mental disorders.
Despite its potential, DBS is associated with several limitations. One limitation is the invasiveness of the procedure, as it requires surgical implantation of electrodes within the brain. Additionally, DBS carries risks such as infection, hemorrhage, and device malfunction. Furthermore, response rates to DBS therapy in BD vary among individuals, and not all patients experience significant symptom improvement (Holtzheimer et al, 2012).
However, DBS also offers several advantages. It is a reversible and adjustable treatment option, allowing for fine-tuning of stimulation parameters to optimize therapeutic efficacy. Moreover, DBS may represent a viable treatment option for BD patients who have not responded to traditional pharmacological or psychotherapeutic interventions (Bewernick et al, 2012; Abraham et al, 2023).
Evidence regarding the efficacy of DBS in BD is still limited. While some case reports and small-scale studies have reported significant reductions in depressive and manic symptoms following DBS therapy, larger randomized controlled trials with longer follow-up periods are needed to establish its efficacy conclusively (Bewernick et al, 2012).
-
i.
Vagus Nerve Stimulation (VNS)
Vagus nerve stimulation (VNS), developed as an invasive neuromodulation treatment, is a neuromodulation technique that involves applying electrical impulses to the vagus nerve, a major component of the parasympathetic nervous system. American neurologist James Corning is credited with first developing VNS to treat epilepsy in the 1880s. His theory, which relied on data suggesting increased cerebral blood flow as the cause of seizures, was largely shelved for a few years due to conflicting results but resurfaced in the 20th century. The direct effects of VNS on the central nervous system (CNS) were studied by Bailey and Bremer in the 1930s, while Corning focused on the indirect physiological effects of VNS. The results of these studies suggest that VNS alters electroencephalograms (EEGs). For the rest of the century, VNS studies were conducted in animals, but it was not until the 1990s that they entered in clinical trials. The first report of a VNS device implanted in a human was published in 1988 and the first implantable VNS device for the treatment of refractory epilepsy was approved by the FDA in 1997. The use of VNS for depression, migraines, cluster headaches, and obesity in the abdomen has since been approved by the FDA (Goggins et al, 2022).
The approaches to the targeted treatment of VN must be different depending on the indication. In most cases of VNS, a pulse generator is surgically implanted to stimulate the left cervical VN. To reduce cardiac effects, including bradycardia, the left cervical VN is targeted rather than the right. Most often in the context of heart failure, the right cervical VNS was used.
During the initial experiments, the right cervical VN was connected to a programmable device that was implanted in the chest. By preferentially activating vagal efferent fibers, this device was intended to influence cardiac function. Electrodes placed on the ventral and/or dorsal vagal trunks below the diaphragm can also be used to target the subdiaphragmatic network. This approach has been used to study the treatment of obesity and its effects on food intake.
In contrast to invasive implantable VNS devices, transcutaneous VNS (tVNS) is a non-invasive method. To target the auricular branch of the VN, surface electrodes are typically placed on the outer ear. Only the outer ear is the destination of the auricular vagus nerve, the nerve's peripheral branch (Ruiz, 2024).
The FDA approved a non-invasive VNS (tVNS) device that is applied to the neck for migraine and cluster headaches, as well as an implanted cervical VNS device that includes an external remote control, lead wire, and pulse generator for treating epilepsy and depression, including BD (Cimpianu et al, 2017).
The primary aim of VNS in the context of BD is to modulate neural activity in regions implicated in mood regulation, thereby reducing symptom severity and enhancing mood stability.
One of the main reviews of VNS in BD treatment (Cimpianu et al, 2017), showed the results of 10 weeks to one year of treatment of VNS at 20 - 30 Hz for BD I or II patients in the depressive phase, which had failed at least two medication trials in the current depressive episode.
An observational registry study with a 5-year prospective, open-label, nonrandomized design was carried out at 61 sites in the United States and comprised 795 patients who had gone through four or more unsuccessful attempts at treating their depression, including electroconvulsive therapy (ECT), or who were going through a major depressive episode [unipolar or bipolar depression, 134 subjects with a diagnosis of BD (87 BD I and 47 BD II)], that lasted at least two years. Individuals with rapid cycling bipolar disorder or a history of psychosis were not enrolled. A reduction of at least fifty percent in the initial MontgomeryÅsberg Depression Rating Scale (MADRS) score at any postbaseline visit within the five-year study period was considered the primary efficacy measure, known as response rate. Remission was one type of secondary efficacy measure. A significantly higher 5-year cumulative response rate (67.6% vs. 40.9%) and a significantly higher remission rate (cumulative first-time remitters, 43.3% vs. 25.7%) are among the clinical outcomes that the adjunctive VNS group had over the treatment-as-usual group, according to the registry results. According to a subanalysis, patients in the adjunctive VNS group had a significantly higher 5-year cumulative response rate (71.3% vs 56.9%) than patients in the treatment-as-usual group among patients who had previously responded to ECT. Not responding to ECT showed a comparable substantial response difference. Regarding efficacy results for treatment-resistant depression, this registry is the longest and largest naturalistic study to date (Aaronson et al, 2017).
As Mutz (2023) also reports in his review, VNS has shown promise as a treatment for maintaining symptomatic remission in patients with BD, even when characterized by rapid cycles.
The mechanism of action of VNS in BD is not fully elucidated, but it is thought to involve modulation of neurotransmitter systems and neural circuits involved in mood regulation (Berry et al, 2013). Preclinical studies suggest that VNS may enhance the release of neurotransmitters such as serotonin and norepinephrine, while also modulating activity in limbic and prefrontal cortical regions implicated in mood disorders (Conway et al, 2012).
In addition, the effects of the VNS mechanisms of action can be classified as acute or chronic effects. Using oxygen-15-labeled water PET, acute VNS was shown to alter mean cerebral blood flow in several areas associated with depressive states. The right superior and medial frontal cortex, bilateral orbitofrontal cortex, bilateral anterior cingulate cortex, and bilateral temporal cortex all showed an increase in blood flow, while the bilateral temporal cortex and right parietal area showed a decrease. In addition, using BOLD fMRI to measure blood oxygen levels, the long-term effects of VNS were examined (Conway et al, 2018).
VNS induced activation in the right anterior insular cortex and the right medial prefrontal cortex in the acute state. But after about 30 weeks, this activation gave way to deactivation, which was accompanied by a decrease in depressive symptoms (Goggins et al, 2022).
Despite its potential, VNS has several limitations. One limitation is the invasiveness of the procedure (VNS), as it requires surgical implantation of a device to stimulate the vagus nerve, although the non-invasive procedure can be a good alternative (Ruiz, 2024). Additionally, response rates to VNS therapy in BD vary among individuals, and not all patients experience significant symptom improvement. Moreover, the precise therapeutic mechanisms of VNS in BD remain incompletely understood (Aaronson et al, 2017).
However, VNS also offers several advantages. It is generally well-tolerated, with few serious adverse effects reported in clinical trials. Furthermore, VNS may represent a viable treatment option for BD patients who have not responded to traditional pharmacological or psychotherapeutic interventions. In fact, patients who have not responded to at least four adequate trials of depression treatment, including ECT, are recommended by the APA to consider VNS (Aaronson et al, 2017).
Evidence regarding the efficacy of VNS in BD is still emerging. While some studies have reported significant reductions in depressive and manic symptoms following VNS therapy, others have yielded more mixed results. In the pediatric population, VNG is used to treat neurological conditions, including drug-resistant epilepsy. Further research, including larger randomized controlled trials with longer follow-up periods, is needed to establish the efficacy of VNS as a treatment for BD conclusively.
7. How to Direct Patients to a Neuromodulation Treatment
It is crucial to thoroughly evaluate the patient's medical history, present symptoms, and prior therapies before beginning any neuromodulation therapy. This evaluation should encompass not only the primary neurological or psychiatric condition but also any comorbidities that may influence treatment outcomes or pose contraindications (Iglesias et al, 2017).
Given the complex nature of neuromodulation therapies, a multidisciplinary approach involving neurologists, psychiatrists, psychologists, nurses, pain specialists, and other relevant healthcare professionals is essential. Collaborative discussions allow for comprehensive treatment planning, consideration of alternative options, and mitigation of potential risks (Rossi et al, 2016).
Not all patients may be suitable candidates for neuromodulation treatments. Establishing clear selection criteria based on clinical guidelines, evidence-based research, and individual patient characteristics is imperative. Factors such as symptom severity, treatment responsiveness, and medical history should guide the decision-making process (Mutz, 2023; Bewernick et al, 2012; Abraham et al, 2023).
Prior to referring a patient for neuromodulation treatment, obtaining informed consent is essential. Patients should be provided with detailed information regarding the proposed therapy, including its mechanism of action, potential benefits, associated risks, and alternative treatment options. Additionally, educating patients about the expected outcomes, realistic expectations, and post-treatment care facilitates active participation in decision-making (Rosson et al, 2022).
Neuromodulation treatments often require specialized expertise and infrastructure for proper administration and management. Referring patients to accredited centers with experienced healthcare providers familiar with the specific modality being considered ensures optimal patient outcomes and safety.
Following initiation of neuromodulation treatment, ongoing monitoring and periodic follow-up are essential components of comprehensive care. Healthcare providers should collaborate closely with patients to assess treatment response, address any emerging concerns or side effects, and make necessary adjustments to the therapeutic regimen (Bhattacharya et al, 2022, Rossi et al, 2016).
8. From Research to Practice in the Real-World
Translating neuromodulation techniques for BD from research to clinical practice involves several key steps and considerations. First and foremost, robust empirical evidence from preclinical and clinical studies is essential to establish the safety, efficacy, and mechanisms of action of these techniques in BD. This evidence serves as the foundation for informing clinical guidelines and treatment protocols.
Once the efficacy of neuromodulation techniques for BD has been demonstrated in research settings, the next step is to integrate these techniques into routine clinical practice (Patel and Lieber, 2019). This process requires training healthcare providers in the use of neuromodulation technologies, ensuring that they have the necessary expertise to administer these treatments safely and effectively (Rossi et al, 2016).
Additionally, clinicians need to be educated about the appropriate patient selection criteria, the assessment, treatment protocols, and monitoring procedures associated with neuromodulation therapies.
Collaboration between researchers, clinicians, and regulatory agencies is crucial for navigating the regulatory approval process and ensuring that neuromodulation techniques for BD meet rigorous standards of safety and efficacy. Regulatory approval is necessary to ensure that these therapies are accessible to patients and reimbursable by healthcare payers.
Furthermore, efforts to disseminate knowledge about neuromodulation techniques for BD among patients, families, and caregivers are essential for promoting awareness and acceptance of these treatments within the broader community. This includes providing accurate information about the potential benefits, risks, and side effects of neuromodulation therapies, as well as addressing any misconceptions or stigma associated with these interventions.
Finally, ongoing research and innovation are necessary to refine existing neuromodulation techniques, develop new treatment modalities, and further elucidate the underlying mechanisms of action of these therapies in BD.
In order to define tailored neuromodulation’s treatments there is the need to consider the following essential elements.
The devices used should have the ability to stably record and track individual neurons within neural circuits over time, a capability that not all current technologies have. Another element to consider is the ability to modulate neurons within neural circuits in response to changes in monitored signals; ther ability to implement closed-loop feedback systems, that monitor and modulate individual neurons within neural circuits. Finally, monitoring and modulation of specific neuronal subtypes is also essential (Patel and Lieber, 2019).
Longitudinal studies are needed to assess the long-term safety and effectiveness of neuromodulation interventions, as well as to identify factors that predict treatment response and optimize treatment outcomes.
Another important point to address is the role of the stigma. In fact, reducing the stigma surrounding neuromodulation techniques such as ECT is of paramount importance for their successful translation from research to clinical practice. Stigma can significantly impede patient access to these potentially life-saving treatments and can also influence healthcare professionals' attitudes and willingness to recommend them (Espinoza and Kellner, 2022).
Education and training programs should be implemented to dispel myths and misconceptions about ECT and other neuromodulation techniques. Providing evidence-based information about the safety, efficacy and appropriate use of these treatments can help healthcare providers feel more confident in recommending them to eligible patients.
Similarly, reducing the stigma among the general population is essential for promoting acceptance and accessibility of neuromodulation therapies. Public awareness campaigns, media initiatives, and community outreach efforts can help challenge negative stereotypes and foster a more accurate understanding of these treatments. Personal testimonials from individuals who have benefited from neuromodulation therapies can be particularly impactful in humanizing these interventions and reducing fear and apprehension (Sienaert, 2016).
Furthermore, advocacy efforts aimed at policy-makers and healthcare administrators can help ensure that adequate funding and resources are allocated for the provision of neuromodulation treatments. This includes advocating for insurance coverage and reimbursement policies that enable equitable access to these therapies for all individuals who could benefit from them (Rossi et al, 2016).
By combating stigma and promoting informed understanding of neuromodulation techniques like ECT or VNS, we can create a more supportive and inclusive environment for individuals with BD and other psychiatric conditions. This, in turn, can facilitate their timely access to evidence-based treatments and ultimately improve their quality of life and treatment outcomes.
9. Training for Clinicians
First and foremost, robust empirical evidence from preclinical and clinical studies is essential to establish the safety, efficacy, and mechanisms of action of these techniques in BD. This evidence serves as the foundation for informing clinical guidelines and treatment protocols.
Once the efficacy of neuromodulation techniques for BD has been demonstrated in research settings, the next step is to integrate these techniques into routine clinical practice. This process requires comprehensive training of mental health practitioners in the use of neuromodulation technologies, ensuring they have the necessary expertise to administer these treatments safely and effectively. Providing continuing education opportunities can help practitioners stay updated on the latest advancements in neuromodulation therapies (Rossi et al, 2016).
Additionally, it is crucial to empower patients and the general population with knowledge about neuromodulation techniques for BD. This includes raising awareness about these treatment options, debunking myths and misconceptions, and providing accurate information about their potential benefits, risks and side effects. By empowering patients to make informed decisions about their treatment options, we can promote shared decision-making and improve treatment adherence and outcomes.
Moreover, efforts to disseminate knowledge about neuromodulation techniques among patients, families, and caregivers are essential for promoting acceptance and reducing stigma associated with these interventions. Providing support groups, educational materials, and peer counseling can help individuals navigate their treatment journey and access appropriate care (Sienaert, 2016).
Collaboration between researchers, clinicians, patient advocacy groups, and regulatory agencies is crucial for navigating the regulatory approval process and ensuring that neuromodulation techniques for BD meet rigorous standards of safety and efficacy (Rosson et al, 2022). Regulatory approval is necessary to ensure that these therapies are accessible to patients and reimbursable by healthcare payers.
Finally, ongoing research and innovation are necessary to refine existing neuromodulation techniques, develop new treatment modalities, and further elucidate the underlying mechanisms of action of these therapies in BD. Longitudinal studies are needed to assess the long-term safety and effectiveness of neuromodulation interventions, as well as to identify factors that predict treatment response and optimize treatment outcomes.
10. Limits
Despite their potential, neuromodulation techniques have several limitations. Invasive techniques carry risks associated with surgical implantation, such as infection and device malfunction. Non-invasive techniques may have limited depth of penetration and may be less effective in modulating deeper brain structures. Additionally, individual response to neuromodulation therapy can vary, and not all patients experience significant symptom improvement.
11. Conclusions
Neuromodulation strategies engage complex neural networks and diverse neurotransmitter systems. These therapies offer significant therapeutic benefits for various psychiatric and neurological disorders, including BD, by modulating brain activity in targeted regions.
There are currently no standard neuromodulation treatment methods for the child population, where neuromodulation is often limited to rehabilitation treatment. Therefore, neuromodulation techniques can potentially create new opportunities for both the treatment of psychopathological conditions and neuromodulation to promote brain plasticity (Camprodon, 2023).
Understanding the specific neuroanatomical areas, circuits, and neurotransmitters involved in these treatments is essential for optimizing their efficacy and expanding their clinical applications, above all in lifelong BD (Bhattacharya eta l, 2021; Ferrarelli and Phillips, 2022).
Effective referral of patients to neuromodulation treatments necessitates a systematic approach encompassing comprehensive assessment, multidisciplinary collaboration, adherence to selection criteria, informed consent, referral to specialized centers, and continued monitoring (Rossi et al, 2016). By following these best practices, healthcare providers can optimize patient outcomes and enhance the efficacy of neuromodulation therapies in BD, too (Rosson et al, 2022).
However, more investigation is required to ascertain the clinical usefulness of neuromodulation interventions, in adult and childhood BD. Future clinical trials should aim to determine which specific group(s) of BD patients may benefit from specific neuromodulation treatments (Ferrarelli and Phillips, 2022).
In summary, translating neuromodulation techniques for BD from research to clinical practice requires a multidisciplinary approach, encompassing scientific inquiry, clinical expertise, regulatory oversight, patient education, and ongoing innovation (Camprodon, 2023). By leveraging advances in neuromodulation technology and evidence-based practice, we can improve the quality of care and outcomes for individuals living with BD, ultimately reducing the burden of untreated illness and ineffective treatments.
References
- Aaronson ST, Sears P, Ruvuna F, et al. A 5-Year Observational Study of Patients With Treatment-Resistant Depression Treated With Vagus Nerve Stimulation or Treatment as Usual: Comparison of Response, Remission, and Suicidality. Am J Psychiatry. 2017;174(7):640-648.
- Abraham ME, Ong V, Gendreau J, Brown NJ, Choi EH, Shlobin NA et al. Investigating Deep Brain Stimulation of the habenula: a review of clinical studies. Neuromodulation 2023; 26:292-301. [CrossRef]
- Antal A, Alekseichuk I, Bikson M, Brockmöller J, Brunoni A, Chen R et a l. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin Neuropsysiol 2017;128(9):1774-1809. [CrossRef]
- APA, Diagnostic and Statistical Manual DSM-5-TR. 2022.
- Baldinger P, Lotan A, Frey R, Kasper S, Lerer B, Lanzenberger R. Neurotransmitters and Electroconvulsive Therapy. J. ECT 2014, 30, 116–121. [CrossRef]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985, vol. 325, issue 8437, P1106-1107, May 11. [CrossRef]
- Barrett DW and Gonzales-Lima F (2013). Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans: A randomized controlled trial. Neuroscience, Jan 29 (230), 13-23. [CrossRef]
- Baumeister RF, and Leary MR. Writing Narrative literature Reviews. Review of General Psychology 1997, vol 1, No 3, 311-320. [CrossRef]
- Becker JE, Maley CT, Shultz EKB, Peters TE. Neuromodulation: past, present, and future. Child Adolesc Psychiatr Clin N Am, 2018. [CrossRef]
- Beisteiner R, Hallet M, Lozano AM. Ultrasound neuromodulation as a new brain therapy. Adv Sci 2023 May;10(4):2205634.
- Berry SM, Broglio K, Bunker M, Jayewardene A, Olin B, Rush AJ. A patient-level meta-analysis of studies evaluating vagus nerve stimulation therapy for treatment-resistant depression. Med Devices (Auckl). 2013;6:17-35. [CrossRef]
- Bersani FS, Minichino A, Bernabei L, Spagnoli F, Corrado A, Vergnagni L, Mannarelli D, Pauletti C, Fattapposta F, Biondi M, Delle Chiaie R. Prefronto-cerebellar tDCS enhances neurocognition in euthymic bipolar patients. Findings from a placebo-controlled neuropsychological and psychophysiological investigation. J Affect Dis 2017 Feb; 209:262-269. [CrossRef]
- Bersani FS, Minichino A, Fattapposta F, Bernabei L, Spagnoli F, Mannarelli D, Francesconi M, Pauletti C, Corrado A, Vergnani L et al. Prefrontocerebellar transcranial direct current stimulation increases amplitude and decreases latency of P3b component in patients with euthymic bipolar disorder. Neuropsychiatr. Dis. Treat. 11, 2913–2917.
- Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology. 2012;37(9):1975-1985. [CrossRef]
- Bhattacharya A, MrudulaK, Sreepada SS, Sathyaprabha TN, Pal PK, Chen R, Udupa K. An overview of noninvasive brain stimulation: basic principles and clinical applications. Can J Neurol Sci 2022;49:479-492. [CrossRef]
- Bikson M, Esmaeilpour Z, Adair D, Kronberg G, et al.Transcranial Electrical stimulation nomenclature. Brain Stimul 2019;12(6):1349-1366. [CrossRef]
- Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T et al. (2016). Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimulation, 9(5), 641-661. [CrossRef]
- Bilge MT, Gosai A, Widge AS. Deep brain stimulation in psychiatry: mechanisms, models, and next generation therapies. Psychiatr Clin North Am 2018 Sep;41(3):373-383. [CrossRef]
- Bolton S, Warner J, Harriss E, Geddes J, Saunders KEA. Bipola disorder: trimodal age-at-onset distribution. Bipolar Disorder 2021;23:341-356. [CrossRef]
- Brunoni AR, Nitsche, MA, Bolognini N, Bikson M, Wagner T, Merabet L, Ferrucci R (2012). Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimulation, 5(3), 175-195. [CrossRef]
- Brunoni AR, Moffa AH, Sampaio-Junior B, et al. Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. Br J Psychiatry. 2016;208(6):522-531. [CrossRef]
- Cai D, Qin Z, Lan X, Liu Q, Wang J, Goya-Maldonado R, Huang X, Ungvari G, Ng C, Zheng W, Xiang Y. Accelerated intermittent theta burst stimulationfor major depressive disorder or bipolar depression: a systematic review and meta-analyis. Asian Journal of Psychiatry 2023(85):103618. [CrossRef]
- Camprodon JA. Therapeutic Neuromodulation for Bipolar Disorder – The case for Biomarker-driven treatment development. JAMA Netw Open 2023; 4(3):e211055. [CrossRef]
- Cagnan H, Denison T, McIntyre C, Brown P. Emerging technologies for improved deep brain stimulation. Nature Biotechnology 2019 (37): 1024-1033. [CrossRef]
- Carnell BL, Clarke P, Gill S, Galletly CA. How effective is repetitive transcranial magnetic stimulation for bipolar depression? J Affect Disord 2017(209):270-272. [CrossRef]
- Carvalho AF, Firth J, Vieta E. Bipolar Disorder. N Engl J Med 2020;383:58-66. [CrossRef]
- Castaneda-Ramirez S, Becker TD, Bruges-Boude A, Rice TR. Systematic review: electroconvulsive therapy for treatment-resistant mood disorders in children and adolescents. European Child & Adolescent Psychiatry (2023) 32:1529–1560. [CrossRef]
- Cho H, Razza LB, Borrione L, Bikson M, Charvet L, et al. Transcranial Electrical Stimulation for Psychiatric Disorders in Adults: a primer. Focus 2022;20:19-31. [CrossRef]
- Cimpianu CL, Strube W, Falkai P, Palm U, Hasan A. Vagus nerve stimulation in psychiatry: a systematic review oft he available evidence. J neural Transm 2017,124:145-158. [CrossRef]
- Conway CR, Chibnall JT, Gangwani S, et al. Pretreatment cerebral metabolic activity correlates with antidepressant efficacy of vagus nerve stimulation in treatment-resistant major depression: a potential marker for response? J Affect Disord. 2012;139(3):283-290. [CrossRef]
- Conway, C.R. and Xiong, W. (2018) The mechanism of action of vagus nerve stimulation in treatment-resistant depression: current conceptualizations. Psychiatr. Clin. 41, 395–407. [CrossRef]
- D’Urso G, Toscano E, Barone A, Palermo M, Dell’Osso B, Di Lorenzo G, et al. Transcranial direct current stimulation for bipolar depression: systematic reviews of clinical evidence and biological underpinnings. Prog Neuropsychopharmacol Biol Psychiatry 2023 Mar 8:121:110672. [CrossRef]
- Dean OM, Gliddon E, Van Rheenen T, Giorlando F, Davidson SK, Kaur M, Ngo TT, Williams LJ. An update on adjunctive treatment options for bipolar disorder. Bipolar Disorders 2018; 20:87-96. [CrossRef]
- Dell'Osso B, Mundo E, D'Urso N, et al. Augmentative repetitive navigated transcranial magnetic stimulation (rTMS) in drug-resistant bipolar depression. Bipolar Disord. 2009;11:76-81.
- Dell'Osso B, Zanoni S, Ferrucci R, et al. Transcranial Direct Current Stimulation for the Outpatient Treatment of poor-responder depressed patients. Eur Psychiatry. 2012;27(7):513-7. [CrossRef]
- Desbeaumes Jodoin V, Miron JP, Lespérance P. Safety and efficacy of accelerated repetitive transcranial magnetic stimulation protocol in elderly depressed unipolar and bipolar patients. Am J Geriatr Psychiatry 2019;27:548-558. [CrossRef]
- Dougherty DD. Deep Brain Stimulation. Clinical Applications. Psychiatr Clin North Am 2018 Sep;41(3):385-394. [CrossRef]
- Elias A, Thomas N, Sackeim HA. Electroconvulsive Therapy in Mania: A Review of 80 Years of Clinical Experience. AmJ Psychiatry 2021; 178:229–239. [CrossRef]
- Enriquez-Geppert, S., Huster, R. J., & Herrmann, C. S. (2017). EEG-neurofeedback as a tool to modulate cognition and behavior: a review tutorial. Frontiers in Human Neuroscience, 22:11:51. eCollection 2017. [CrossRef]
- Espinoza RT and Kellner CH. Electroconvulsive therapy. N Engl J Med 2022;386:667-672. [CrossRef]
- Fenton WS, Stover ES. Mood disorders: Cardiovascular and diabetes comorbidity. Curr. Opin. Psychiatry 2006; Jul;19(4):421-427. [CrossRef]
- 2006, 19, 421–427.Ferrarelli F, Phillips ML. Examining and Modulating Neural Circuits in Psychiatric Disorders With Transcranial Magnetic Stimulation and Electroencephalography: Present Practices and Future Developments. Am J Psychiatry. 2021 May 1;178(5):400-413. [CrossRef]
- Findling RL, Stepanova E, Youngstrom EA, Young AS. Progress in diagnosis and treatmetn of bipolar disorder among children and adolescents: an international perspective. Evid Based Mental Health 2018 Nov, 21:4. [CrossRef]
- Fitzgerald PB, Benitez J, de Castella A, Daskalakis ZJ, Brown TL, Kulkarni J. A randomized, controlled trial of sequential bilateral repetitive transcranial magnetic stimulation for treatment-resistant depression. Am J Psychiatry. 2006;163:88-94. 10.1176/appi.ajp.163.1.88.
- Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682. [CrossRef]
- German J, Mameli M, Elias GJ, Loh A, Taha A, Gouveia FV, Lozano AM. Deep brain stimulation oft he habenula: systematic review oft he literature and clinical trial registries. Front Psychiatry 2021;12:730931. [CrossRef]
- Gippert SM, Switala C, Bewernick BH, Kayser S, Brauer A, Coenen VA, Schlaepfer TE. Deep brain stimulation for bipolar disorder – review and outlook. CNS Spectrums 2017(22):254-257. [CrossRef]
- Gold AK, Ornelas AC, Cirillo P, et al. Clinical applications of transcranial magnetic stimulation in bipolar disorder. Brain Behav 2019;9(10):e01419.
- Goldsmith D, Bekhbat M, Mehta ND, Gelger JC. Inflammation-related functional and structural dysconnectivity as a pathway to psychopathology. Biol Psychiatry. 2023 March 01; 93(5): 405–418. [CrossRef]
- Graat I, van Rooijen G, Mocking R, Vulink N, de Koning P, Schhuurman R, Denys D. Is deep brain stimulation effective and safe for patients with obsessive compulsive disorder and comorbid bipolar disorder? J Affect Disord 2020 Mar (1) 264:69-75. [CrossRef]
- Gregory AT, and Denniss AR. An introduction to writing narrative and systematic reviews – tasks, tips and traps for aspiring authors. Heart, Lung and circulation 2018,27:893-898. [CrossRef]
- Grunze H and Born C. The impact of subsyndromal bipolar symptoms on patient’s functionality and quality of life. Front Psychiatry 2020, 11;510. [CrossRef]
- Guo B, Zhang M, Hao W, Wang Y, Zhang T, Liu C. Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression. Translational Psychiatry 2023, 13(5). [CrossRef]
- Hamblin MR (2018). Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS biophysics, 5(4), 337-361. [CrossRef]
- Hameed MQ, Dhamme SC; Gersner R, Kaye HL, Oberman LM, Pascual-Leone A, Rotenberg A. Transcranial magnetic and direct current stimulation in children. Curr Neurol Neurosci Rep 2017 February; 17(2):11. [CrossRef]
- Holtzheimer PE, Husain MM, Lisanby SH, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4(11):839-849. [CrossRef]
- Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry 2012;69(2):150-158. [CrossRef]
- Hu F, Jia Y, Zhao D, Fu X, Zhang W, Tang W, Hu S, Ge M, Du W, Shen W, Chen H. Gender differences in suicide among patients with bipolar disorder: a systematic review and meta-analysis. J Aff Dis 2023, 15(339):601-614. [CrossRef]
- Hu YY, Yang G, Liang XS, Ding XS, Xu DE, Li Z, Ma QH, Chen R, Sun YY. Transcranial low-intensity ultrasound stimulation for treating central nervous system disorders: A promising therapeutic application. Front. Neurol. 2023;14:1117188. [CrossRef]
- Iglesias S, Tomiello S, Schneebeli M, Stephan KE. Models of neuromodulation for computational psychiatry. WIREs Cogn Sci 2017, 8:e1420. [CrossRef]
- Jivrai J, Ameis SH. Is repetitive transcranial magnetic stimulation (rTMS) ready for a clinical use as a treatment tool for mentla health targets in children and youth? J Can Acad Child Adolesc Psychiatry 2022 May;31(2):93-99 PMID: 35614951.
- Konstantinou G, Hui J, Ortiz A, Kaster TS, Downar J, Blumberger DM, Daskalakis ZJ. Repetitive transcranial magnetic stimulation (rTMS) in bipolar disorder: A systematic review. Bipolar Disord. 2022 Feb;24(1):10-26. [CrossRef]
- Krauss JK, Lipsman N, Aziz T, Boutet A, Brown P et al. Technology of deep brain stimulation: current status and future directions. Nat Rev Neurol. 2021 February 01; 17(2): 75–87. [CrossRef]
- Kritzer MD, Peterchev AV, Camprodon JA. Electroconvulsive Therapy: Mechanisms of Action, Clinical Considerations, and Future Directions. Harv Rev Psychiatry. 2023; 31(3): 101–113. [CrossRef]
- Lambert-Beaudet F, Journault WG, Provençal AR, Bastien CH. Neurofeedback for insomnia: current state of research. World J Psychiatry 2021 Oct 19;11(10):897-914. [CrossRef]
- Legon W, Sato TF, Opitz A, et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci. 2014;17(2):322-329. [CrossRef]
- Li BJ, Friston K, Mody M, Wang HN, Lu HB, Hu DW. A brain network model for depression: from symptom understanding to disease intervention. CNS Neuroscience & Therapeutics 2018;24:1004-1019. [CrossRef]
- Malone DA, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry 2009 Feb 15;65(4):267-275.
- Mc Intyre R and Calabrese JR. Bipolar Depression: the clinical characteristics and unmet needs of a comples disorder. Curr Med Res Opinion 2019,35(11):1993-2005. [CrossRef]
- McIntyre RS, Berk M, Brietzke E, Goldstein BI, López-Jaramillo C, et al. Bipolar Disorders. Lancet 2020 Dec 5;396(10265): 1841-1856. [CrossRef]
- McIntyre RS, Alda M, Baldessarini RJ, Bauer M, Berk M, Correll CU, Fagiolini A, et al. The clinical characterization of the adult patient with bipolar disorder aimed at personalization of management. World Psychiatry 2022 Oct; 21(3): 364–387. [CrossRef]
- McVoy M and Levin JB. Update strategies for the management of poor medication adherence in patients with bipolar disorder. Expert Rev Neurotherapeutics 2023,23(4):365-376. [CrossRef]
- Medda P, Toni C, Perugi G. The mood-stabilizing effects of electroconvulsive therapy. J ECT. 2014;30(4):275–282. [CrossRef]
- Micoulaud-Franchi JA, Fovet T. Neurofeedback: time needed for a promising non-pharmacological therapeutic method. Lancet Psychiatry 2016 Sep;3(9):e16. [CrossRef]
- Micoulaud-Franchi JA, McGonigal A, Lopez R, Daudet C, Kotwas I, Bartolomei F. Electroencephalographic neurofeedback: Level of evidence in mental and brain disorders and suggestions for good clinical practice. Neurophysiol Clin 2015 Dec;45(6):423-33. [CrossRef]
- Minichino A, Bersani FS, Spagnoli F, Corrado A, De Michele F, Calò WK, Primavera M, Yang B, Bernabei L, Macrì F, Vergnani L, Biondi M, Delle Chiaie R. Prefronto-cerebellar transcranial direct current stimulation improves sleep quality in euthymic bipolar patients: a brief report. Behav. Neurol. 2014, 876521. [CrossRef]
- Minichino A, Bersani FS, Bernabei L, Spagnoli F, Vergnani L, Corrado A, Taddei I, Biondi M, Delle Chiaie R. Prefronto-cerebellar transcranial direct current stimulation improves visuospatial memory, executive functions, and neurological soft signs in patients with euthymic bipolar disorder. Neuropsychiatr Dis Treat. 2015; 11: 2265–2270. [CrossRef]
- Miranda-Mendizabal A, Castellvı´ P, Pare´s-Badell O, et al. Gender differences in suicidal behavior in adolescents and young adults: systematic review and meta-analysis of longitudinal studies. International Journal of Public Health 2019, 64:265–283. [CrossRef]
- Miuli A, Sepede G, Stigliano G, Mosca A, Di Carlo F, d’Andrea G, Lalli A, Spano MC, Pettoruso M, Martinotti G, di Giannantonio M. Hypomanic/manic switch after transcranial magnetic stimulation in mood disorders: a systematic review and meta-analysis. World J Psychiatry 2021 August 19;11(8):477-490. [CrossRef]
- Montazeri K, Farhadi M, Fekrazad R, Chaibakhsh S, Mahmoudian S. Photobiomodulation therapy in mood disorders: a systematic review. Lasers in Medical Science (2022) 37:3343–3351. [CrossRef]
- Mutz J. Brain stimulation treatment for bipolar disorder. Bipolar Disorder 2023;25:9-24. [CrossRef]
- NICE Guidelines on Bipolar Disorder, 2022 Updated Edition https://www.nice.org.uk/guidance/cg185/evidence/full-guideline-pdf-4840895629.
- Nitsche, M. A., & Paulus, W. (2011). Transcranial direct current stimulation—update 2011. Restorative neurology and neuroscience, 29(6), 463-492.
- Nierenberg AA, Agustini B, Kohler-Forsberg O, Cusin C, Katz D, Sylvia L, Peters A, Berk M. Diagnosis and treatment of Bipolar disorder. A review. JAMA 2023;330(14):1370-1380. [CrossRef]
- O’Donnell CM, Barrett DW, Fink LH, Garcia-Pittman EC, Gonzales-Lima F. Transcranial Infrared Laser Stimulation Improves Cognition in Older Bipolar Patients: Proof of Concept Study. Journal of Geriatric Psychiatry and NeurologyVolume 35, Issue 3, May 2022, Pages 321-332. [CrossRef]
- O'Donnell CM, Barrett DW, O'Connor P, Gonzalez-Lima F. Prefrontal photobiomodulation produces beneficial mitochondrial and oxygenation effects in older adults with bipolar disorder. Front Neurosci. 2023 Oct 31;17:1268955. [CrossRef]
- Oldani L, Altamura CA, Abdelghani M & Young AH. Brain stimulation treatments in bipolar disorder: a review oft he current literature. The World Journal of Biological Psychiatry 2014. [CrossRef]
- Omejc N, Rojc B, Battaglini PP, Marusic U. Review of the therapeutic neurofeedback method using electroencephalography: EEG Neurofeedback. Bosn J Basic Med Sci. 2019;19(3):213-220. [CrossRef]
- Oriuwa C, Mollica A, Feinstein A, Giacobbe P, Lipsman N, Perez DL, Burke MJ. Neuromodulation fort he treatment of functional neurological disorder and somatic symptom disorder: a systematic review. J Neurol Neurosurg Psychiatry 2022 Mar;93(3):280-290. [CrossRef]
- Osada T, Konishi S. Noninvasive intervention by transcranial ultrasound stimulation: Modulation of neural circuits and its clinical perspectives. Psychiatry Clin Neurosci 2024 May;78(5):273-281. [CrossRef]
- Patel Sr and Lieber CM. Precision electronic medicine in the brain. Nature Biotechnology 2019, vol 37 Sep: 1007-1012. [CrossRef]
- Ramasubbu R, Lang S, Kiss ZHT. Dosing of electrical parameters in Deep Brain Stimulation (DBS) for Intractable Depression: a review of clinical studies. Front. Psychiatry 2018, (9):302.
- Rantala MJ, Luoto S, Borràz-Leòn JI, Krams I. Bipolar Disorder: an evolutionary psychoneuroimmunological approach. Neuroscience and Biobehavioral Reviews 122 (2021) 28–37. [CrossRef]
- Reinhart RM & Nguyen JA (2019). Working memory revived in older adults by synchronizing rhythmic brain circuits. Nature Neuroscience, 22(5), 820-827. [CrossRef]
- Rochkind S. Laser photobiomodulation in Neuroscience: from bench to bedside. Photomedicine and laser surgery, 2016;34(12):585-586. [CrossRef]
- Rojas M, Ariza D, Ortega A, Riaño-Garzon ME, et al. Electroconvulsive Therapy in Psychiatric Disorders: A Narrative Review Exploring Neuroendocrine–Immune Therapeutic Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2022, 23, 6918. [CrossRef]
- Rosenblat JD and McIntyre RS. Bipolar Disorder and Immune Dysfunction: Epidemiological Findings, Proposed Pathophysiology and Clinical Implications. Brain Sci. 2017, 7, 144. [CrossRef]
- Rossi PJ, Gunduz A, Judy J, Wilson L, Machado A, et al. Proceedings of the Third annual deep brain stimulation think tank: a review of emerging issues and technologies. Front Neurosci 2016, 10:119. [CrossRef]
- Rosson S, de Filippis R, Croatto G, Collantoni E, Pallottino S, Guinart D, Brunoni AR, Dell’Osso B, Piagato G, Hyde J, Brandt V, Cortese S, Fiedorowicz JG, Petrides G, Correll CU, Solmi M. Brain stimulation and other biological non-pharmacological interentions in mental disoders: an umbrella review. Neuroscience and Biobehav Rev 2022(139):104743. [CrossRef]
- Ruiz AA. Transcutaneous auricolar vagus nerve stimulationto improve emotional state. Biomedicines 2024, 12, 407. [CrossRef]
- Salehpour F, Mahmoudi, J., Kamari, F., Sadigh-Eteghad, S., & Rasta, S. H. (2018). Brain Photobiomodulation Therapy: a Narrative Review. Molecular Neurobiology, 55(8), 6601-6636. [CrossRef]
- Sarica C, Nankoo JF, Fomenko A, Grippe TC, Yamamoto K, Samuel N, et al. Human studies of transcranial ultrsound neuromodulation: a systematic review of effectiveness and safety. Brain stimulation 2022; 15(3):737-746. [CrossRef]
- SayuriYamagata A, Brietzke E, Rosenblat JD, Kakar R, McIntyre RS. Medical comorbidity in bipolar disorder: The link with metabolic-inflammatory systems. J Affect Disord 2017, 211, 99–106. [CrossRef]
- Sienaert O. Based on a true story? The portrayal of ECT in International Movies and television programs. Brain stimulation 2016 Nov-Dec;9(6):882-891. [CrossRef]
- Szmulewicz A, Valerio MP, Martino DJ. Longitudinal analysis of cognitive performances in recent-onset and late-life Bipolar Disorder: A systematic review and meta-analysis. Bipolar Disord. 2020 Feb;22(1):28-37. [CrossRef]
- Torres CV, Ezquiaga E, Navas M, de Sola RG. Deep brain stimulation of the subcallosal cingulate for medication-resistant type I bipolar depression: case report. Bipolar Disorders 2013 (15)6:719-21. [CrossRef]
- Tsapekos D, Strawbridge R, Cella M, Young AH, Wykes T. Does cognitive improvement translate into functional changes? Exploring the transfer mechanisms of cognitive remediation therapy for euthymic people with bipolar disoder. Psychol Med 2021, 1-9. [CrossRef]
- Vieta E, Salagre E, Grande I, Carvalho A, Fernandes BS, Berk M, Birmaher B, Tohen M, Suppes T. Early intervention in Bipolar Disorder. Am J Psychiatry 2018;175:411-426. [CrossRef]
- Yatham LN, Chakrabarty T, Bon DJ, Schaffer A, Beaulieu S, Parikh SV, McIntyre S, Milev RV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) recommendations forthe management of patients with bipolar disorder with mixed presentations. Bipolar Disorders 2021;23:767-788. [CrossRef]
- Zhang C, Kim SG, Li D, Zhang Y, Li Y, Husch A, Hertel F, Yan F, Voon V, Sun B. Habenula deep brain stimulation for refractory bipolar disorder. Brain Stimul 2019 Sep-Oct;12(5):1298-1300. [CrossRef]
- Zhang C, Lai Y, Zhang Y, Xu X, Sun B, Li D. Deep brain stimulation-induced transient effects in the habenula. Front Psychiatry 2021 Jun 23:12:674962. [CrossRef]
- Zengin G, Topak OZ, Atesci O, Atesci FC. The efficacy and safety of transcranial magnetic stimulation in treatment-resistant bipolar depression. Psychiatria Danubina 2022, 34(2):236-244. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).