Introduction
In forest ecosystem, litter decomposition play important roles in nutrient cycling, linking biogeochemical processes above and below the ground (Prescott and Vesterdal, 2021; Latterini et al., 2023). Leaf and other organic matter, including twigs, stems and propagative structures, account for 70% and 30% of above-ground litterfall, respectively (Robertson and Paul, 2000). As litter decomposes, soluble compounds leach into the soil, converting organic matter into accessible nutrients for plants (Krishna and Mohan, 2017), thereby maintain a balance between soil carbon storage and atmospheric CO2 release (van der Wal et al., 2013). This process significantly influences forest soil fertility and nutrient cycling, especially microbial nutrient absorption and utilization (Guo et al., 2006; Keller and Phillips, 2019).
Litter decomposition is a dynamic process (Jing and Wang, 2020) and influenced by environmental conditions, litter quality, and microbial communities involved in decomposition (Dechaine et al., 2005; Krishna and Mohan, 2017). Bacteria may dominate the microbial decomposer community at the early stage due to the large population (Bray et al., 2012; Purahong et al., 2016), while fungi communities play a more significant role at the latter stage (Aerts et al., 2006). Different forest types influence decomposition rate through variations in litter quality. In subtropical forests, the litter decomposition rate rank follows: pure Cinnamomum camphora forest > mixed C. camphora and Pinus massoniana forest > pure P. massoniana forest (Farooq et al., 2022). Studies have shown notable variations in decomposition rate among different forest types. For instance, Populus davidiana-dominated forests exhibited a higher decomposition rate than Quercus liaotungensis-dominated climax forests, primarily due to lower litter quality (Bai et al., 2023). Additionally, research indicates that broadleaf forests potentially facilitated litter to decompose faster compared to coniferous forests in British Columbia (Prescott et al., 2000).
Fungi are primary decomposer, breaking down organic polymers through the release of hydrolytic and oxidative enzymes (Baldrian and Valásková, 2008; Floudas et al., 2012; Chávez-Vergara et al., 2016). These fungi frequently encode enzymes such as acidic phosphatase, β-glucosidase and N-acetylglucosaminidase, while enzymes like hemicellulases or laccase are less common (Eichlerová et al., 2015). Saprotrophic fungi can directly modify or decompose recalcitrant lignin (Osono et al., 2011; Eastwood et al., 2011; Floudas et al., 2012). Additionally, other functional fungi, including mycorrhizal fungi, pathogens, endophytes, can metabolize organic matter (van der Wal et al., 2013; Sun et al., 2021).
During decomposition, fungal community composition undergoes substratum succession (Osono, 2007). These changes correspond to evolving catabolic abilities required at different stage of decomposition, influenced by continuous variations in litter quality (Frankland, 1998; Dilly et al., 2001; Osono, 2006). Research indicates that active fungi species richness peaks near the end of the degradation, with ectomycorrhizal fungi becoming the dominant group (Rajala et al., 2011). Similar microbial succession patterns have been detected in the fungal community during the decomposing of beech leaf litter, where Basidiomycetes dynamically replace Ascomycetes (Purahong et al., 2016). A 24-month study on decaying Q. petraea leaves also confirmed an increase in abundance of Basidiomycetes over time and enhanced fungal diversity in the fourth month (Voříšková and Baldrian, 2013).
Research suggests a strong link between shifts in forest vegetation and changes in fungal communities. The composition of fungal communities in both litter and soil is profoundly modulated by the prevailing tree species, underscoring their significant ecological influence (Urbanová et al., 2015; Sun et al., 2016). Conifer-broadleaf forests exhibit greater diversity in saprotrophs and other functional groups associated with carbon and nitrogen cycling compared to pure conifer forest (Bai et al., 2023). Changes in dominant tree species in mixed boreal forests lead to shifts in soil fungal communities (Nagati et al., 2018), with diverse litter types shaping the saprotrophic fungi group (Treseder et al., 2014; Foudyl-Bey et al., 2016). These findings underscore the significance of understanding shifts in fungal communities that correlate with dominant tree species within forest ecosystems.
The original conifer forests are located in Zijin Mountain, Nanjing, China, and have undergone significant successions due to pine wilt disease(Qu et al., 2020). Currently, the forest types include mixed broadleaved and coniferous forests dominated by P. massoniana, P. thunbergii, Quercus variabilis, and Liquidamabar formosana (Chen et al., 2018). Additionally, there are deciduous broadleaved forests dominated by L. formosana, Q. acutissima, and Q. fabri (Deng et al., 2019). The transition from pure conifer to mixed conifer and broadleaved forest, and eventually to broadleaved forest, is still ongoing (Chen et al., 2018; Liu et al., 2023).
To better understand how fungal communities respond to decomposition time and changes in dominant tree species, we selected a pure broadleaved forest dominated by L. formosana, as well as a mixed forest equally dominated by L. formosana and P. thunbergii. This study tests two hypotheses: (1) decomposition time and changes in dominant tree species influence enzyme activities and fungal community structures during decomposition; and (2) correlations exist between decomposition rate, enzyme activities, and fungal community composition. The findings offer a unique perspective on fungal communities in litter, as most studies focus on microbial communities within the soil.
Materials and Methods
Study Sites, Litter Bag Embedding and Sample Collection
The study site (32 °16’15” N, 118 °48’00” W) is situated in the Linggu Temple Scenic Area at the south-eastern foot of the Zijin Mountain in Nanjing, Jiangsu, China. The Zijin mountain rises 448.9 meters and covers an area of 2970 hm2. This area has a subtropical monsoon climate, with an annual precipitation of 1000-1050 mm and an average temperature of 15.4 ℃(Chen et al., 2020; Jiang et al., 2018).
In this study, we selected two sites: one mixed forest dominated by L. formosana and P. thunbergii, and one pure broadleaved forest dominated by L. formosana, situated more than 500 meters apart. In order to avoid self-correlation, we selected three subplots (50 ×80 m) in every forest, located at a distance of 50 meters away from each other. In each subplot, we randomly chose three L. formosana trees which were good in health status as the sampling trees, therefore generating 9 sample trees in the mixed forest and 9 sample trees in the pure forest. We assessed the litter degradation by using the traditional mesh bag method (Anderson, 1973), the detailed process are as followed. In October 2017, we set the nylon mesh on the forest floor near each sampling tree to gather the fresh falling L. formosana litter for three weeks.
The collected litter was dried at 65 ℃ until it reached a stable weight. Subsequently, 20 grams of L. formosana leaves or 7 grams of L. formosana twigs were placed into each nylon mesh bag (25 × 25 cm, with 2 mm mesh size). In November 2017, six bags of leaves and six bags of twigs were buried beneath the humus layer around each sample tree, after removing the existing litter near these trees. This setup resulted in a total of 216 litter bags, with 108 bags placed in the pure forest and 108 bags in the mixed forest.
After one, two, and three years of litter decomposition, two bags of leaves and two bags of twigs were taken to the laboratory in ice boxes. The litter from one leaf bag and one twig bag was dried at 65℃ until the weight stayed stable to determine the decomposition rate, which is the ratio of weight loss in the current year and the original weight. The first-year decomposition rate was calculated by
while the second-year decomposition rate was calculated by
and the third-year decomposition rate was calculated by
The R stands for decomposition rate, while the W1, W2 and W3 represent the litter constant weight after drying process for the first, second and third year respectively. The W0 is the original weight, 20 grams for the leaves and 7 grams for the twigs. The litter from the other two mesh bags was used for enzyme activity measurements and DNA extraction.
DNA Extraction, Amplification of ITS2 Region and Sequencing
The plant genomic DNA kits (Tiangen Biotech Company, Beijing, China) were used to extracte the genomic DNA from each litter sample according to the manufacturer’s protocol (Ma et al., 2020). Then the extracted DNA was tested on the NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) for DNA quality and concentration. The fungal internal transcribed spacer 2 region (ITS2) was amplified by polymerase chain reaction (PCR) using the forward primer ITS1F (5’ -CTTGGTCATTTAGAGGAAGTAA -3’) and the reverse primer ITS2 (5’-GCTGCGTTCTTCATCGATGC -3’) (Smith and Peay, 2014). The triplicate 50 μl reaction mixture, utilized for the PCR assays, comprises 25 μl of 2× Premix Taq, 1 μl of forward primer at a concentration of 10 μM, 1 μl of reverse primer at an equivalent concentration, 10 ng of template DNA, and an appropriate volume of nuclease-free water to reach the final reaction volume. A PCR control with sterile water instead of DNA was used to check for contamination. The PCR program included: 3 min at 95°C for denaturation, followed by 36 cycles of 30 s at 95°C, 30 s at 55°C for annealing, 45 s at 72°C for elongation, and a final extension at 72°C for 10 min. The product was analyzed using 2% agarose gel electrophoresis, then purified and quantified with a spectrophotometer. The amplicons underwent sequencing on the Illumina MiSeq platform (PE=300) at Majorbio Company (Shanghai, China). The resulting raw sequence data have been deposited in the National Center for Biotechnology Information (
https://www.ncbi.nlm.nih.gov/) Sequence Read Archive under the project accession number
PRJNA1056890.
Enzyme Activity Measurements
The activities of eight enzymes involved in carbon degradation—namely β-xylosidase (XYL), β-d-glucuronidase (GLR), β-cellobiosidase (CEL), β-glucosidase (GLS), and laccase, along with enzymes implicated in nitrogen degradation (N-acetyl-glucosamidase, NAG), organic phosphorus degradation (phosphatase, PHO), and organic sulfur hydrolysis (sulfatase, SUL) were systematically measured and quantified. The enzyme activities were quantified fluorometrically using 4-methylumbelliferone-linked (4-MUB) substrates (Sinsabaugh et al., 2003). For each sample, 0.45 g of litter was placed into three filter tubes and centrifuged following incubation in 600 μl of culture solution. The reaction was initiated by adding 200 μL of suspension containing 50 μL of the substrate to each well. The fluid was transferred to 5 mL tubes and adjusted to a volume of 3.5-4 mL with culture buffers to create enzyme-active samples. A negative control group was prepared by heating 1 mL of the sample fluid. Following the addition of fluorogenic substrate working solutions, the plates were incubated in the dark at 22 °C with shaking at 160 RPM for 15 minutes (for GLS and NAG) or 30 minutes (for all other enzymes except laccase). To terminate the reactions, 10 µL of 1 M NaOH was added. Fluorescence was measured using a Thermo Scientific Multiskan SkyHigh, with excitation and emission filters set to 365 nm and 450 nm, respectively (Xu et al. 2020). Enzyme activity was calculated based on the amount of MUB released from the litter samples over a specified period, expressed as nmol·g⁻¹·h⁻¹. For laccase measurements, ABTS solution was added to the sample plates, which were then incubated in the dark at 22 °C with shaking at 160 RPM for 60 minutes. The emission wavelength for laccase was set to 420 nm.
Quantitative Microbial Element Cycling
Quantitative Microbial Element Cycling (QMEC) employs a high-throughput quantitative PCR-based chip to assess and quantify the genetic potential of microbiota in transforming essential nutrients, including carbon, nitrogen, phosphorus, and sulfur.(Zheng et al., 2018). The chip comprises 72 primer pairs targeting 64 microbial functional genes involved in carbon, nitrogen, phosphorus, and sulfur cycling, as well as methane metabolism. Twig litter from the third year of decomposition was selected for analysis. QMEC was conducted using the WaferGen Smart Chip Real-time PCR system at Majorbio Company. (Shanghai, China). Each primer set was run in triplicate, with a non-template negative control included in each run. The qPCR process included the first denaturation step at 95°C for 5 minutes, followed by 40 cycles of denaturation at 95°C for 30 seconds, annealing at 58°C for 30 seconds, and extension at 72°C for 30 seconds. Data quality control was performed using the Ct values for each gene, as analyzed with Canco Software (Zheng et al., 2018).
Sequence Data Processing and Statistical Analysis
Sequence processing was conducted on Mothur (v. 1.39.5) referring to the operating procedures described previously(Schloss et al., 2009). Initially, paired-end sequences were merged into contigs, followed by error correction using the chimera and PCR.seq commands. The corrected sequences were then assigned to operational taxonomic units (OTUs) with a 97% similarity threshold, employing the neighbor-joining algorithm (Tedersoo et al., 2010). Representative sequences from each OTU were classified using the UNITE database version 8.0, applying a bootstrap cutoff value of 80 (Abarenkov et al., 2010). Non-fungal sequences were eliminated using the ‘remove.lineage’ command. The sequence information after sequence denoising and quality filtering was shown in the supplementary table (
Table S1). For data normalization, the smallest sample sizes with sequence number of were randomly subsampled for calculating the diversity indices and further analysis of fungal community structure. The OTUs were subsequently classified into ecological guilds- Saprotroph, Symbiotroph and Pathotroph, based on FUNGuild, a functional annotation tool which uses a curated database to assign fungal taxa to guilds (Nguyen et al., 2016).
Across different decomposition years, one-way analysis of variance was utilized to assess significant differences in litter weight loss rates, enzyme activities, fungal community diversity, and fungal functional groups within the same litter type in SPSS v26.0 (Chicago, IL, USA). To compare these variables between different forests, the Kruskal-Wallis H test was utilized in SPSS v26.0. Spearman correlation analysis was utilized to test the correlation between diversity indices and enzyme activities in SPSS v26.0. Graphpad Prism was utilized to visualize the results and to calculate R-square values and F-statistics within the framework of linear regression analysis. Principal Coordinate Analysis (PCoA)and PERMANOVA were employed to detect the significant differences in fungal community structure and fungal functional structure over three years, as well as the fungal functional gene community structure within twig litter from the third year, using Primer 7 software (Anderson, 2008).
Results
Decomposition Rate and Enzyme Activities during Litter Decomposition
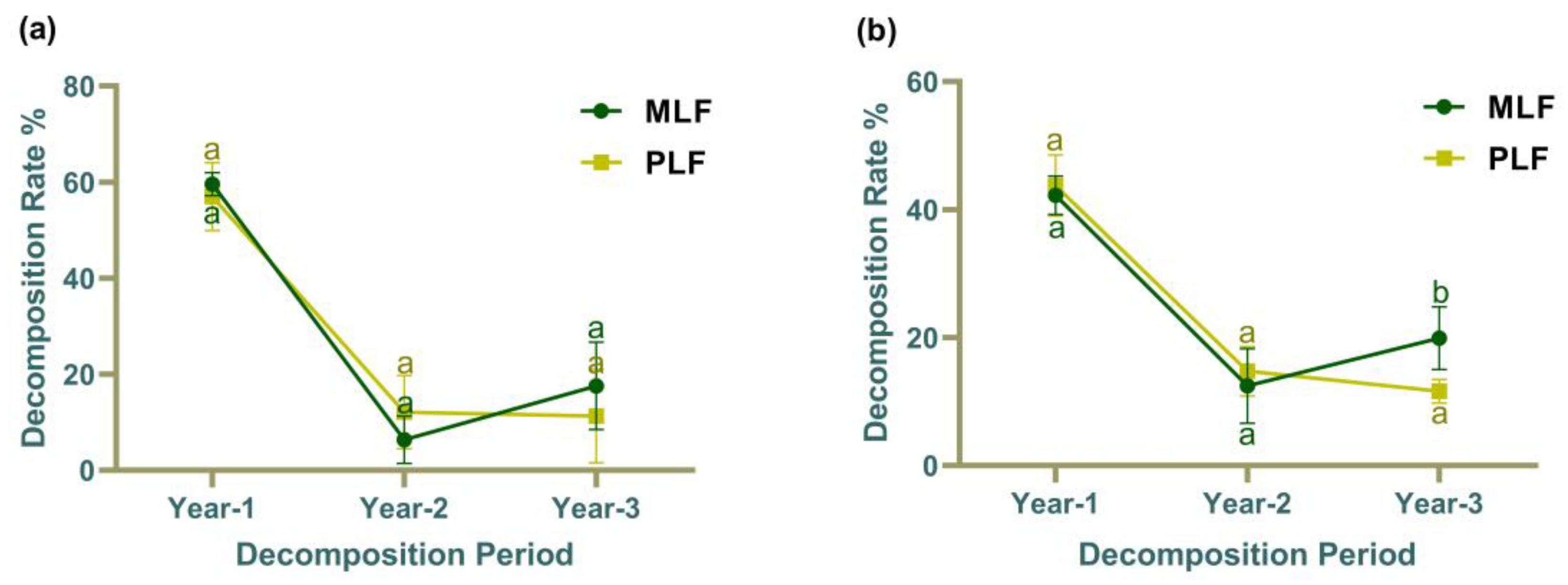
The decomposition rate of leaf litter did not exhibit significant differences between pure and mixed forest stands over the three-year period (
Figure 1a). However, in the third year, the decomposition rate in twig litter was significantly higher in the mixed forest compared to the pure forest (
P < 0.05) (
Figure 1b). Both leaf and twig litters showed similar trend over the 3-year period, with the highest decomposition rate in the first year (
P < 0.05) and the lowest in the second year. Moreover, the leaf litter was decomposed faster than twig litter in the first year (
P < 0.05), but no difference in decomposition rates was observed between leaf and twig litter in the second and third years.
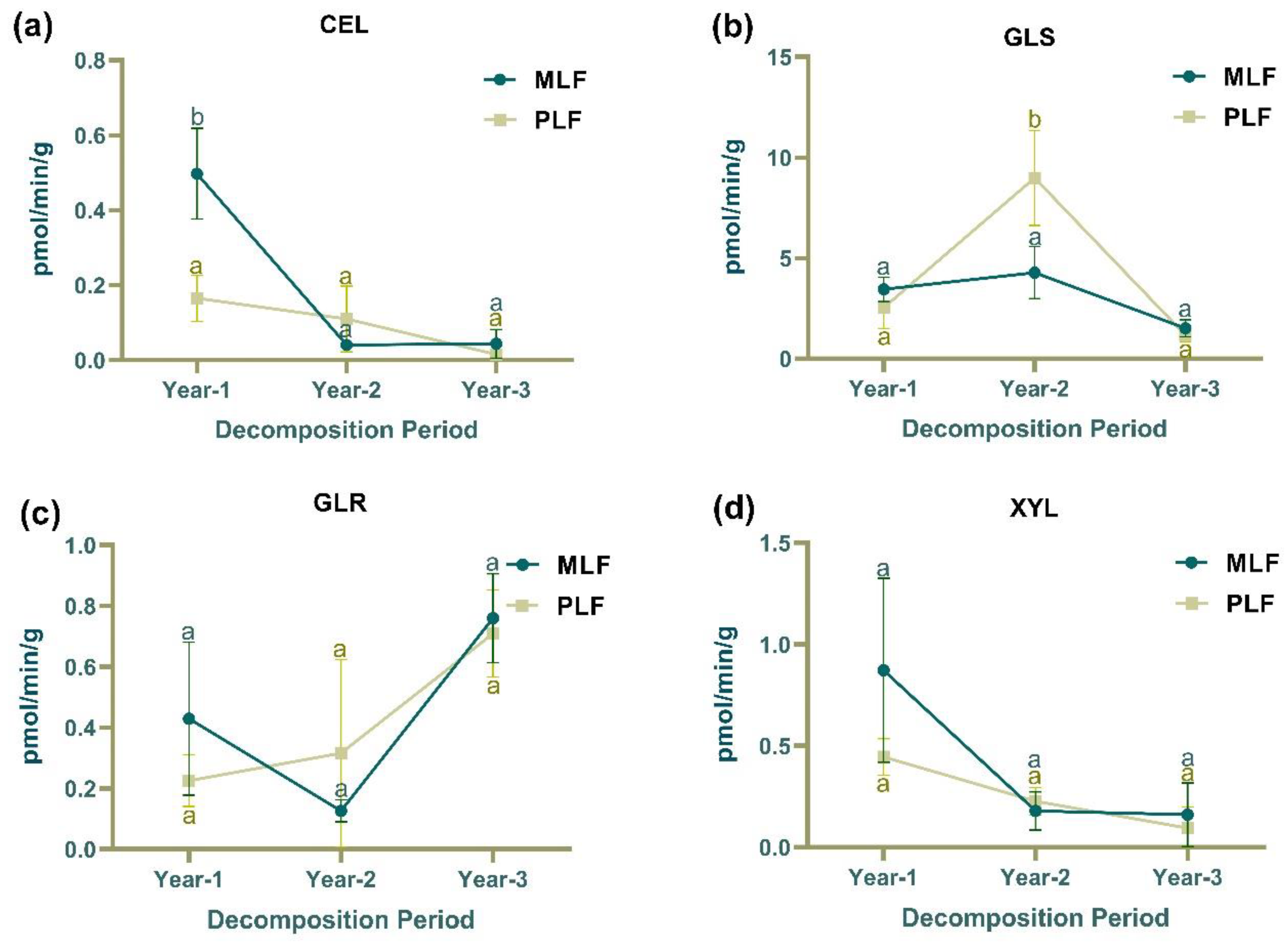
In the first year of leaf decomposition, the activity of β-cellobiosidase (CEL) was significantly higher in the mixed forest compared to the pure forest, whereas laccase activity was significantly higher in the pure forest (
P < 0.05) (
Figure 2a). During the second-year, glucosidase (GLS) and phosphatase (PHO) activity in leaf litter were higher in the pure forest than in the mixed forest (
P < 0.05) (
Figure 2b & 2g). In the second year of twig decomposition, laccase and N-acetylglucosaminidase (NAG) activities were higher in the mixed forest while sulfase (SUL) activity was higher in the pure forest (
P < 0.05) (
Figure 2E, 2F & 2H). In the third year of twig decomposition, CEL and SUL activity were higher in the mixed forest, while xylanase (XYL) activity was higher in the pure forest (
P < 0.05) (
Figure 2A & 2H).
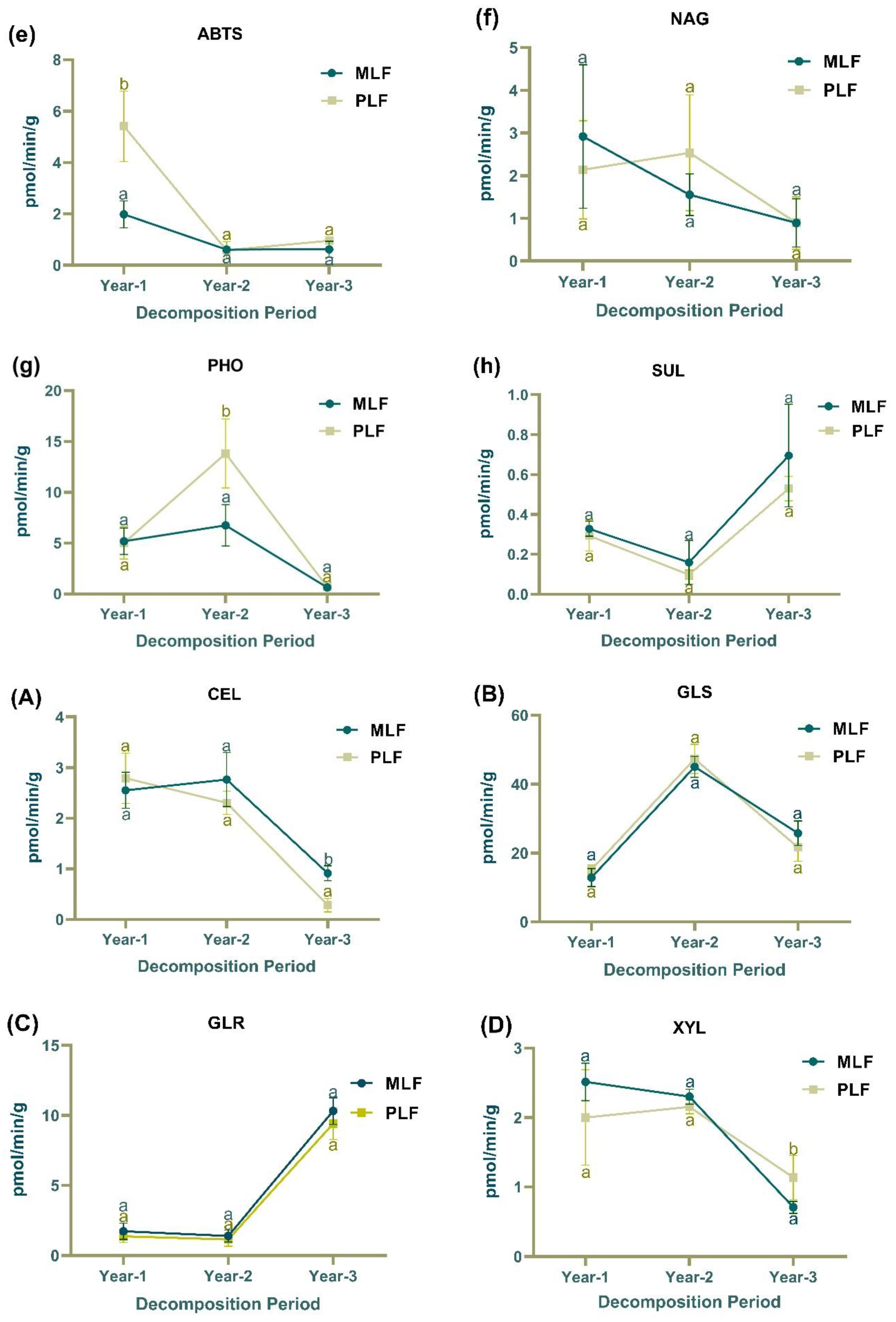
In the mixed forest, the activities of CEL, XYL and NAG in leaf litter decreased over the decomposition period (R
2 =0.69 ,0.52 and 0.47, F=15.64, 7.54 and 6.16 respectively) (
Figure 2a, 2b & 2f). In leaf litter, GLS and PHO activities increased in the second year and decreased in the third year, while SUL activity demonstrated the opposite pattern (
P < 0.05) (
Figure 2b, 2g & 2h). In twig litter, the activities of CEL, XYL and NAG in the mixed forest also showed decreasing trends over time (R
2 =0.57, 0.81 and 0.87, F=9.42, 30.11 and 47.09, respectively), as did CEL and NAG activity in the pure forest (R
2 =0.84 and 0.90, F=36.43 and 63.34, respectively) (
Figure 2A, 2D & 2F). GLS, laccase, and PHO activities increased in the second year and decreased in the third-year twig decomposition (
P < 0.05) (
Figure 2B, 2E & 2G).
In the first-year decomposition, laccase activity was higher in leaf litter compared to twig litter, while all other enzymes, except for SUL, showed higher activities in twig litter (P < 0.05). In the second and third years, all tested enzymes showed higher activities in twig litter compared to leaf litter (P < 0.05).
The decomposition rate of leaf litter was positively correlated with the activities of CEL, XYL, and laccase (
P < 0.01) (
Table S2), while the decomposition rate of twig litter was positively correlated with CEL and NAG activities (
P < 0.05) but negatively correlated with GLS and laccase activities (
P < 0.01) (
Table S3).
Fungal Composition at Taxonomic Level during Litter Decomposition
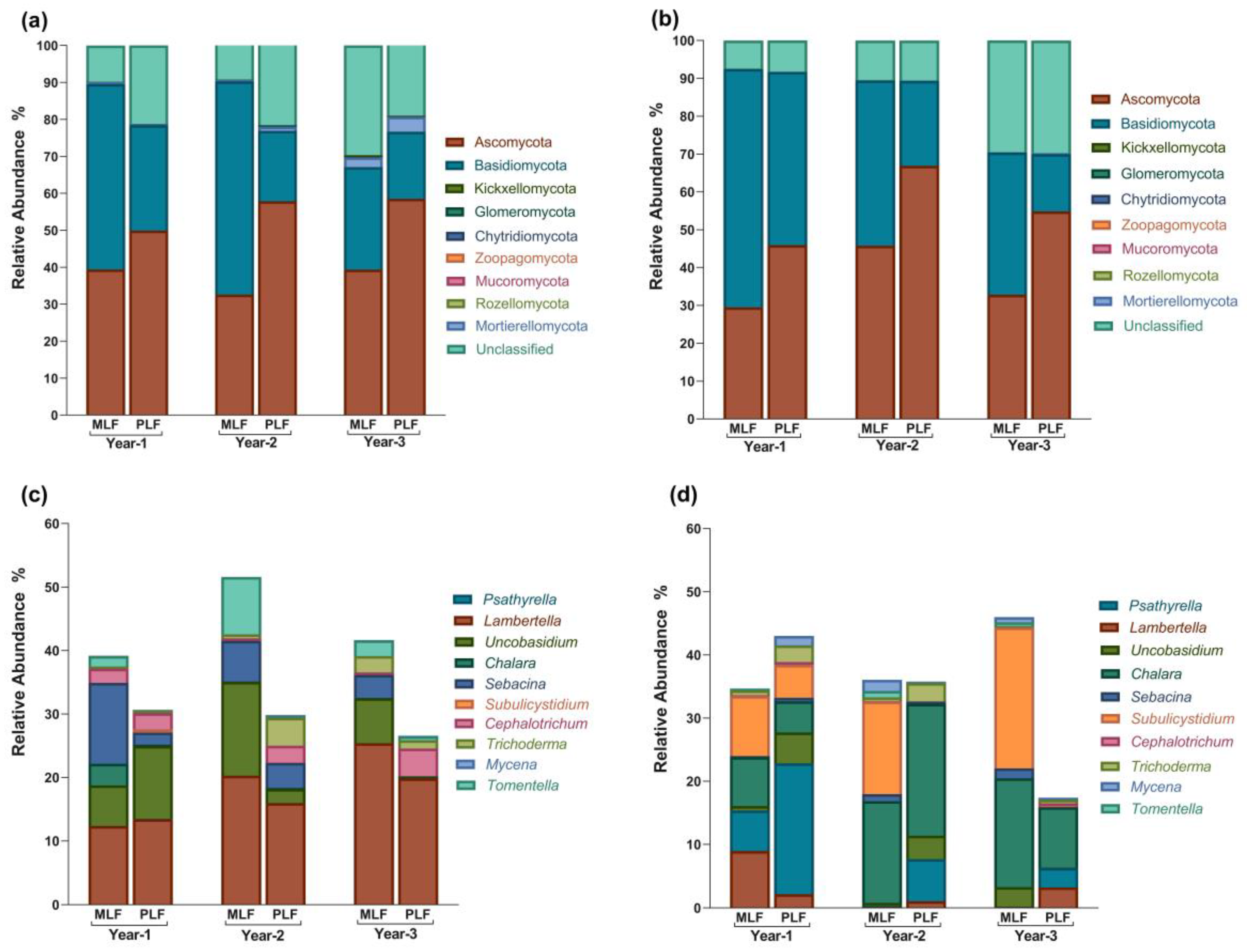
Ascomycota (48.28%) and Basidiomycota (38.94%) were the two most abundant phyla out of the nine detected phyla across the three-year decomposition, followed by Mortierellomycota (0.83%), Kickxellomycota (0.08%) and Glomeromycota (0.06%). Within leaf litter, the abundance of Ascomycota was higher in the pure forest compared to the mixed forest in the second and third year of decomposition (
P < 0.05), while Basidiomycota had a higher abundance in the mixed forest during the first and second year (
P < 0.05) (
Figure 4a).
At the genus level, 359 genera were classified based on the identified OTU over three years.
Lambertella and
Chalara were the most abundant genera in leaf and twig litter respectively. During leaf litter decomposition,
Chalara and
Tomentella were more abundant in the mixed forest compared to the pure forest in the first year and the first two years, respectively (
P < 0.05) (
Figure 4c).
Tomentella and
Sebacina were found to be richer in the second year and third year of twig decomposition in the mixed forest than in the pure forest (
P < 0.05) (
Figure 4c). Along leaf decomposition, the relative abundance of
Chalara and
Uncobasidium dropped in the second year (
P < 0.05).
Lambertella,
Sebacina,
Tomentella,
Uncobasidium and
Cephalotrichum were richer in abundance in leaf, while
Chalara,
Subulicystidium, and
Psathyrella held higher abundance in twig litter (
P < 0.05).
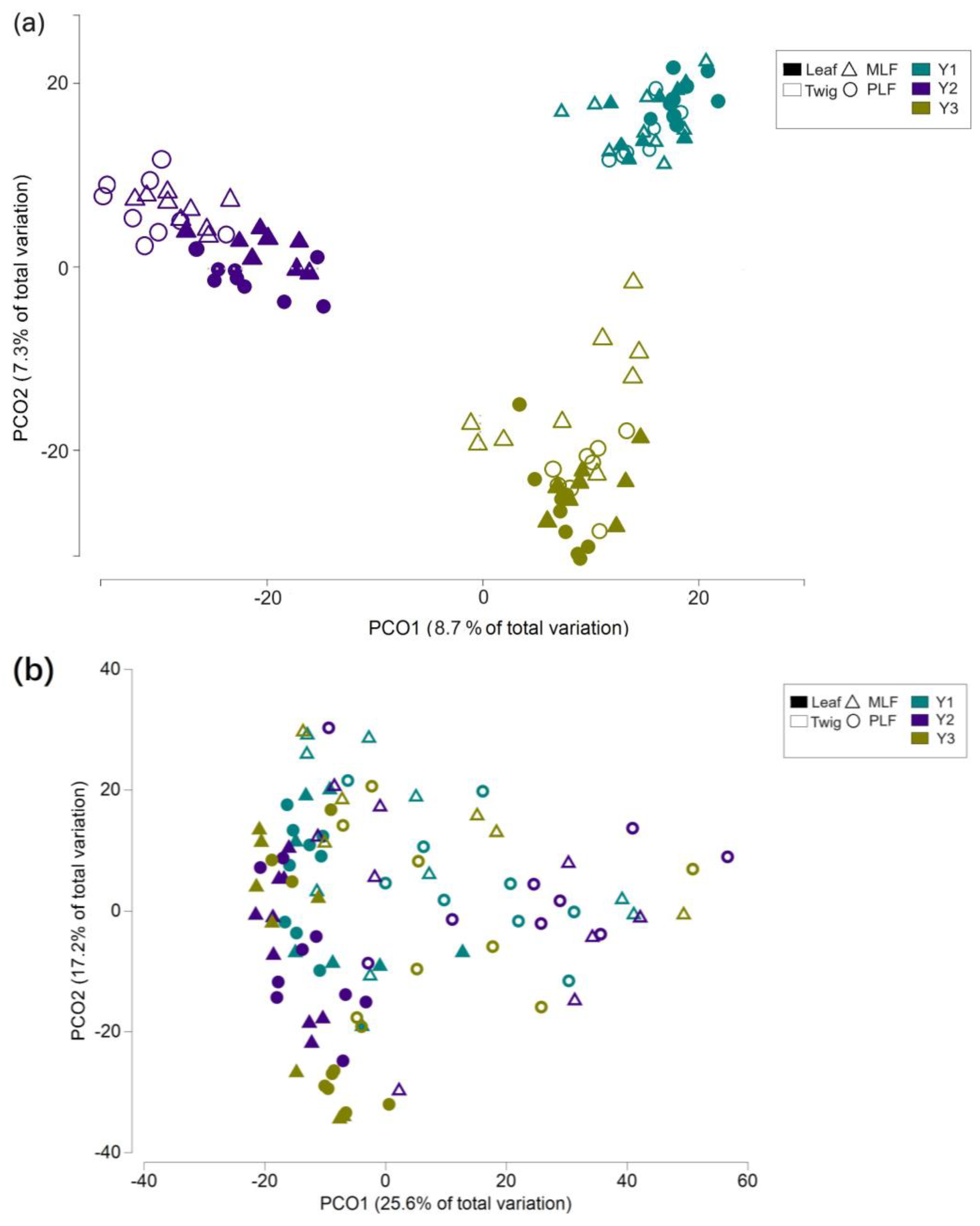
The principal coordinate analysis (PCoA) constructed on the basis of fungal OTU abundance indicated that the fungal community structures during the decomposition differed between the two forests within the same litter, and between the two litters within the same forest (
Figure 5a). The separation of fungal community structures was observed between different decomposition year (
Figure 5a). These differences among community structures were subsequently confirmed by PERMANOVA results (
P < 0.05 for each pair).
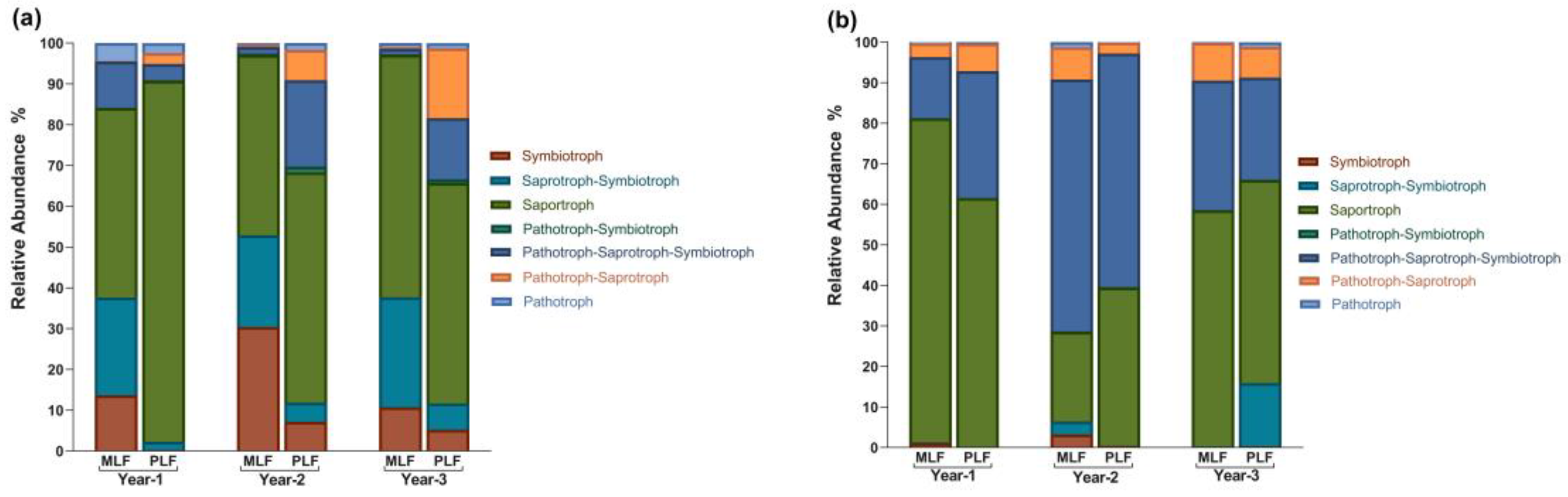
Fungal Functional Structure during Litter Decomposition
In FUNGuild analysis, 3983 OTUs (39.47%) were classified into seven trophic modes. Saprotroph was the most abundant group, covering 55.00% of the assigned OTUs, followed by Pathotroph-Saprotroph-Symbiotroph (23.15%), Saprotroph-Symbiotroph (8.82%), Symbiotroph (6.05%), Pathotroph-Saprotroph (5.51%), Pathotroph (1.20%), and Pathotroph-Symbiotroph (0.26%) (
Figure 6).
Within leaf litter, Saprotroph-Symbiotroph and Symbiotroph had a higher abundance in the mixed forest during the decomposition period, while the pure forest had higher abundance of Saprotroph, Pathotroph-Symbiotroph, and Pathotroph-Saprotroph (
P < 0.05) (
Figure S1a). Within twig litter, the abundance of Saprotroph in the mixed forest was higher in the first year, but lower in the second year compared to the pure forest. Additionally, Symbiotroph had higher abundance in the mixed forest (
P < 0.05) (
Figure S1b).
Within leaf litter, the relative abundance of Symbiotroph, Pathotroph- Symbiotroph and Pathotroph-Saprotroph increased significantly in the second year, while Saprotroph and Pathotroph decreased simultaneously (
P < 0.05) (
Figure S1a). In the third year of leaf decomposition, the relative abundance of Pathotroph- Symbiotroph and Symbiotroph dropped, while Saprotroph increased (
P < 0.05) (
Figure S1a). During twig decomposition, Symbiotroph, Pathotroph- Symbiotroph, and Pathotroph- Symbiotroph- Saprotroph all experienced increase followed by decrease, peaking in the second year (
P < 0.05) (
Figure S1b). The relative abundance of Saprotroph, however, decreased and reached the lowest value in the second year of twig decomposition in both forests (
P < 0.05) (
Figure S1b).
In both forests, Symbiotroph, Saprotroph-Symbiotroph, and Pathotroph- Symbiotroph were more abundant within leaf litter during decomposition compared to twig litter, while Pathotroph- Symbiotroph- Saprotroph exhibited higher abundance within twig litter (P < 0.05).
PCoA based on the functional trophic modes showed distinct fungal trophic structures between the mixed and pure forests in each decomposition period, between the leaf and twig litters in each forest, and between the first-year decomposition and subsequent period (
Figure 5b). The differences in fungal trophic structures were later proved by PERMANOVA (
P < 0.05 between all pairs).
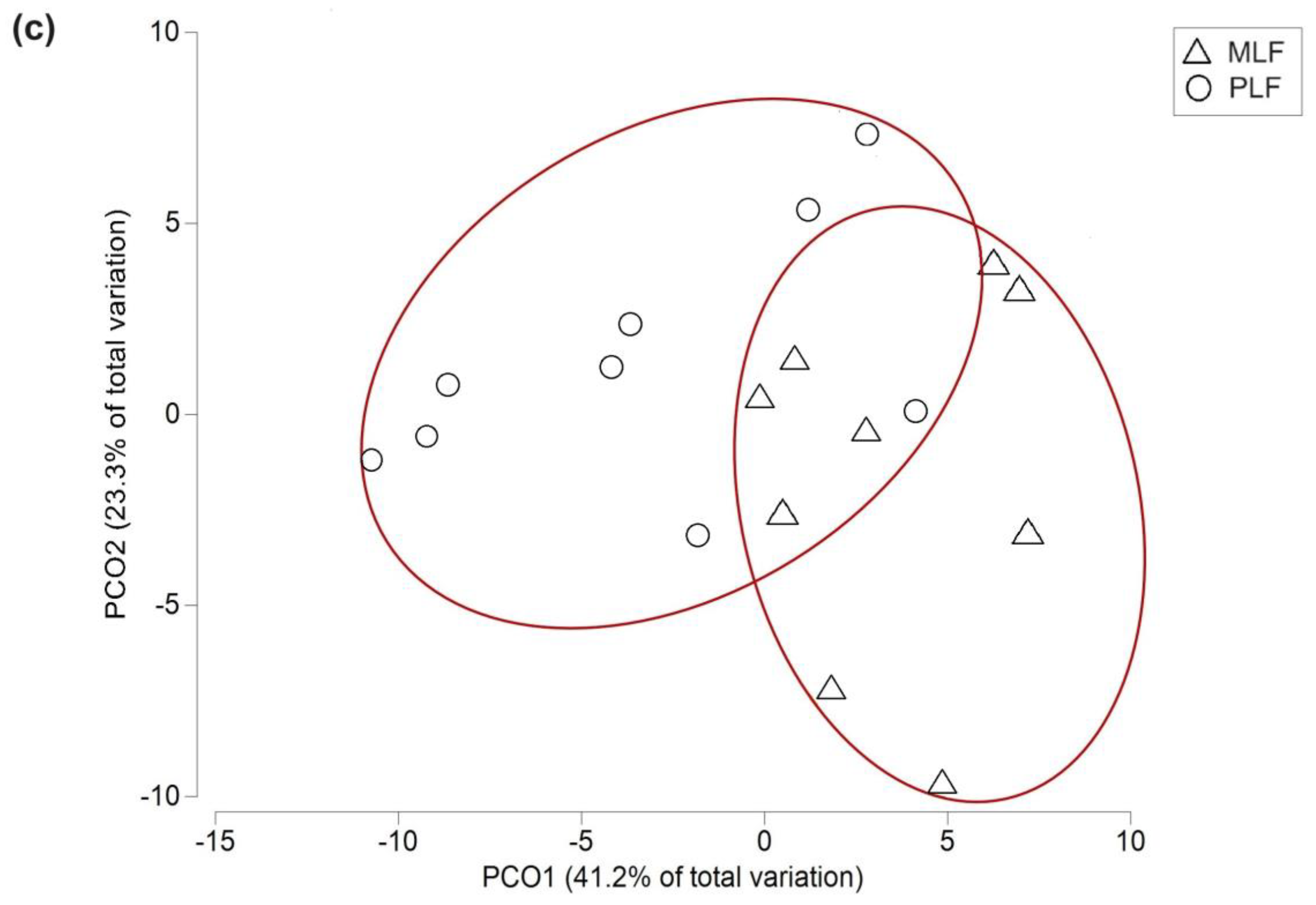
Fungal Function Genes Structure in Twig Litter in the Third-Year Decomposition
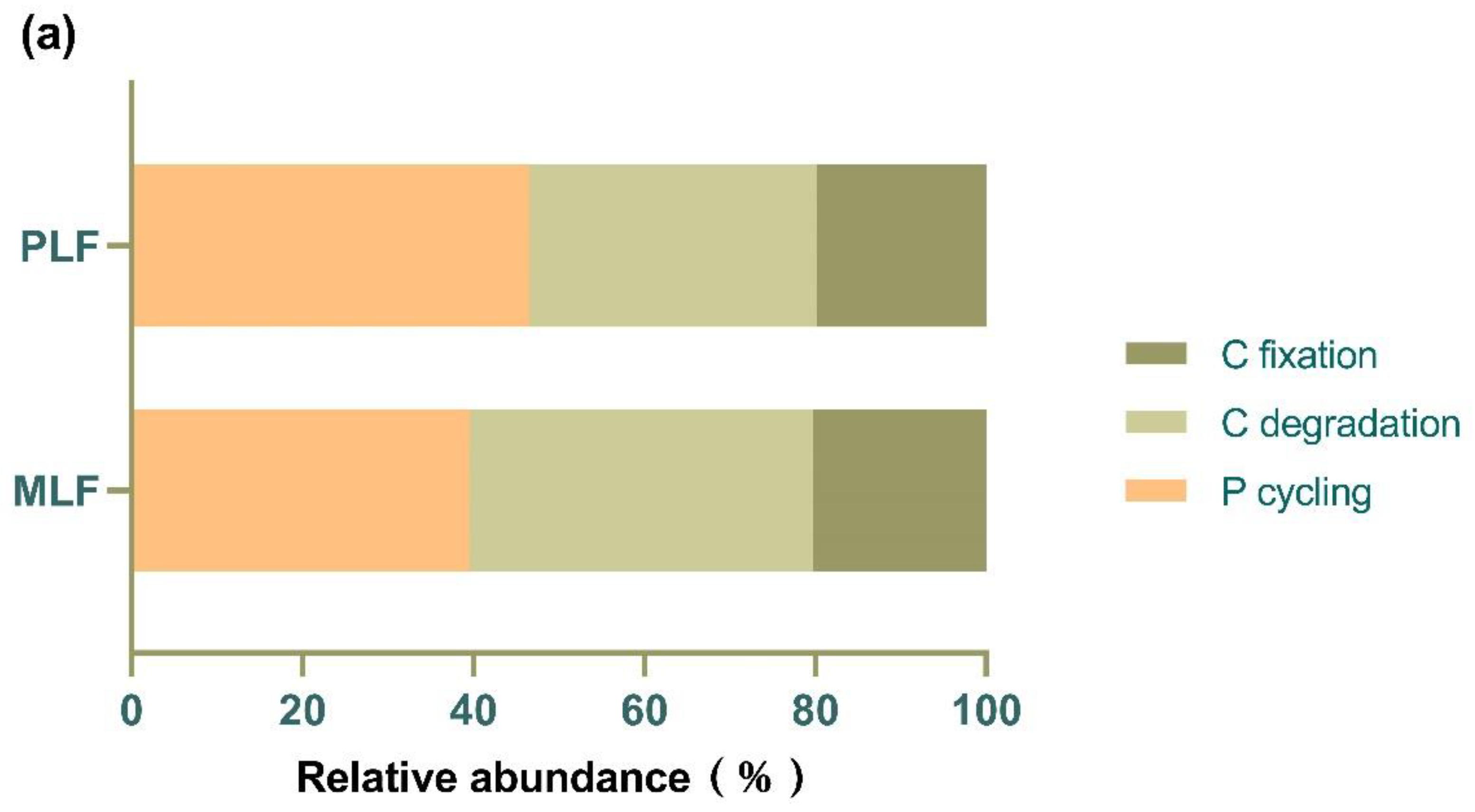
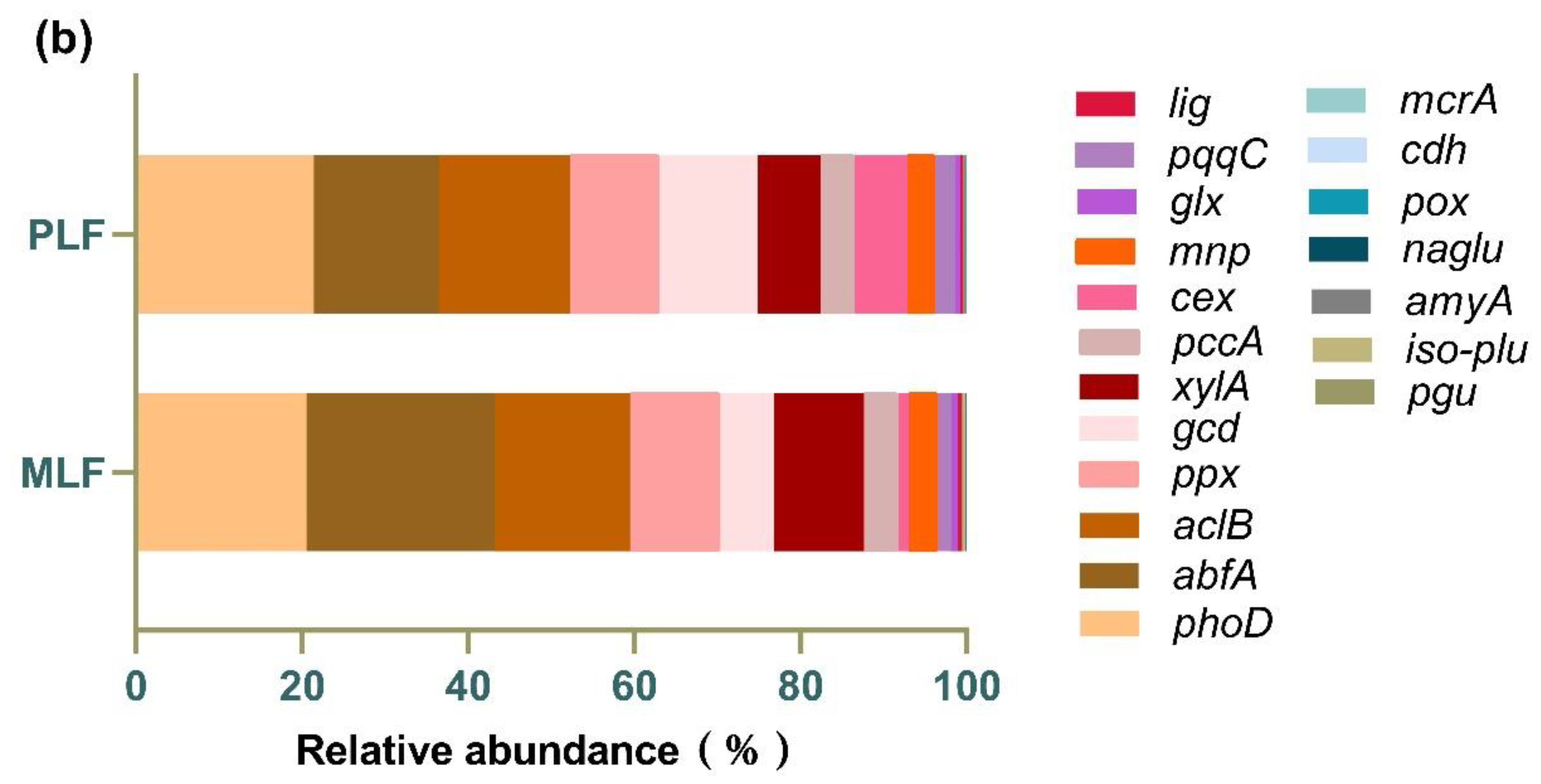
A total of 19 functional genes associated with carbon degradation, carbon fixation and phosphorus cycling were found in the twig litter. Among the two forests, genes participating in P cycling (43.22%) were the most abundant, followed by those involved in C degradation (36.76%) and C fixation (20.02%) (
Figure 7a). Among individual genes, the top three were
phoD (20.99%),
abfA (18.72%), and
aclB (16.01%), involving in carbon degradation, carbon fixation and phosphorus cycling respectively (
Figure 7b).
The relative abundance of the gene cex involved in carbon degradation, and the gene gcd nvolved in phosphorus cycling, were both higher in the pure forest than those in the mixed forest (P < 0.05).
PCoA showed differences in the fungal functional gene structure in the twig litter between the mixed and pure forests, which was further proved by PERMANOVA analysis (
P < 0.001) (
Figure 5b).
Discussion
The mixed forest exhibited a higher twig litter weight loss rate compared to the pure forest. This difference can be attributed to variations in the decomposer communities present in the two forests, as local decomposer communities significantly influence litter degradation (Jackrel et al., 2019). Tree species impact litter decomposition not only through litter qualities, but also by the specific conditions and decomposer communities in their forest floor(Laganière et al., 2010). Dominant plant species significantly affect both abiotic and biotic soil properties (Kamau et al., 2020; Wang et al., 2019), which subsequently shapes the litter decomposition by altering the decomposer assembly (Yang et al., 2021). The litter decomposition rate decreased significantly from the first to the second year, reflecting the common temporal dynamic that the decomposition rate declines over time and stabilizes around a critical value (Yue et al., 2016). Initially, microorganisms decompose small, readily available substances, while more complex macromolecular persist until specific microorganisms capable of degrading them at later stage (Bray et al., 2012; Gołębiewski et al., 2019). The higher decomposition rate of leaf litter compared to twig in the first year suggests difference in the initial qualities of readily degradable substances between leaf and twig litter.
In both litter and twig litter, the mixed forest exhibited elevated β-cellobiosidase, N-acetyl-glucosamidase activities, whereas the pure forest showed higher activities of phosphatase, β-glucosidase and β-xylosidase. These differences suggest that forest type does influence enzyme activity. Forests with specific tree species compositions impact soil properties through leaf litter and root exudates deposition (Cheng et al., 2013). Consequently, variations in tree species attributes lead to distinct soil environments (Ayres et al., 2009), significantly affecting microbial activities through altering soil physical and chemical properties(Bauhus et al., 1998; Aciego Pietri and Brookes, 2009). Enzyme activities, including β-cellobiosidase, β-xylosidase, and N-acetyl-glucosamidase, showed decreasing trends throughout decomposition in both litter types, consistent with the overall decline in decomposition rate. The activities of laccase within twig litter peaked in the second year, aligning with the previous research indicating high ligninolytic enzymes activity after 24 months of decomposition (Šnajdr et al., 2011). Laccases target aromatic moieties in lignin, with a preference for phenolic structures (Steffen et al., 2007). The peak in laccase activities suggests that significant decomposition of twig lignin occurred during the second year, indicating lignin removal following the decomposition of other components(Berg and McClaugherty, 2020; Šnajdr et al., 2011). Higher enzyme activities were observed within twig litter compared to leaf, likely due to the presence of more refractory substances such as polyphenol, nitrogen and lignin in twigs (Lehmann et al., 1995). These substances attract fungal community with higher enzyme production abilities. Strong positive correlations were found between leaf litter decomposition rate and the activities of β-xylosidase, β-cellobiosidase, and laccase. β-xylosidase and β-cellobiosidase are extracellular hydrolytic enzymes related to hemicellulose and cellulose decomposition, respectively (Cai et al., 1999; Kähkönen et al., 2008), breaking down the long chains of xylans and cellulose (Steffen et al., 2007). Thus, our results suggest that the loss of cellulose, hemicellulose, and lignin constitutes a significant proportion of the leaf litter mass loss in this study.
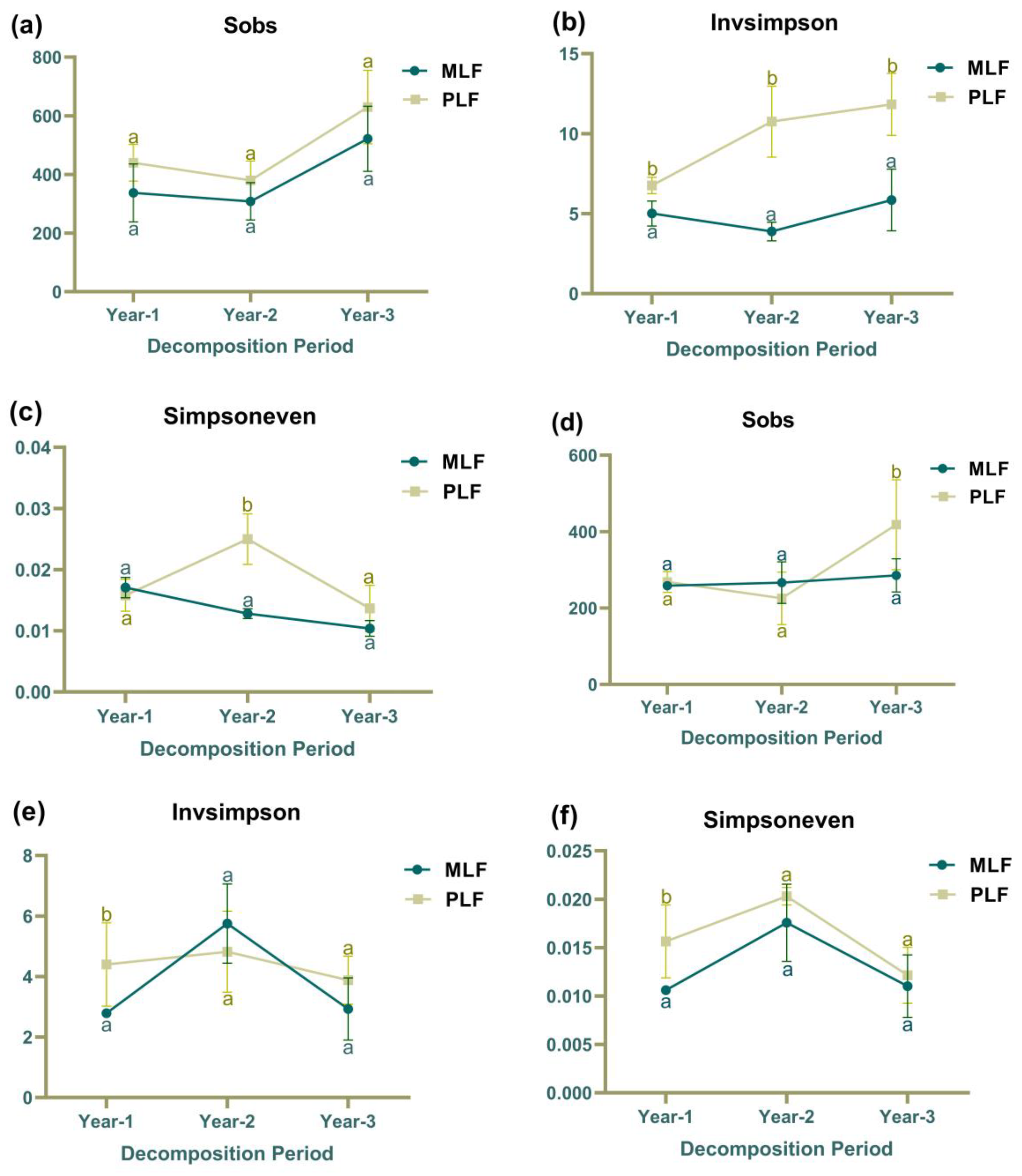
All three fungal α-diversity indices were higher in the pure forest. This suggests that tree species identity significantly influence the diversity and composition of microbial community in organic soil(Chen et al., 2019), with conifer litter providing a poor substrate for microbial growth (Xu et al., 2015). Consequently, the pure broadleaf forest likely offers a more favorable decomposing environment, assembling a decomposers community with higher diversity. Although more diverse microbe communities are hypothesized to enhance decomposition due to their ability to target various substrates and potentially include strong decomposers (Loreau, 2001; Loreau and Hector, 2001), increased diversity can also lead to competition or inhibition among fungal species (LeBauer, 2010). This could explain the inhibited twig decomposition rate in the pure forest, attributed to adverse interactions within a diverse fungal community. The observed increase in fungal community species richness in the final year aligns with the peak richness of active fungi in logs at an advanced stage of decay(Rajala et al., 2011). Fungal community composition tends to shift rapidly with changing litter quality, driven by factors such as nutrient availability, other nutritional requirements, and the competitive abilities of individual taxa (Voříšková and Baldrian, 2013). Competition likely diminishes as decomposition reaches its final phase, when nutrients become less available, leading to higher fungal species richness in the third year. Fungal species richness was positively correlated with β-d-glucuronidase and sulfatase activities, indicating that fungi vary in their enzyme production capabilities (Novotný et al., 2004). Community with more species tend to harbor higher enzyme activities, as a diverse community is more likely to include fungi that produce a broader range of enzymes targeting different components.
Within leaf litter, the pure forest exhibited a higher abundance of Ascomycota, while the mixed forest had more abundant Basidiomycota. Basidiomycota can synthesize enzymes essential for decomposition complex polymers(Baldrian, 2008) and cause the loss of lignin and carbohydrates in variable proportions (Geethanjali and Jayashankar, 2016), making Basidiomycetes well-suited for the more complex litter input in the mixed forest. In contrast, Ascomycota selectively decompose cellulose over lignin (Voříšková and Baldrian, 2013). As the main wood decay fungi (Lundell et al., 2014), Basidiomycota decreased in abundance along twig decomposition as the decomposition process slowed. Lambertella, the most abundant genus in leaf litter, is known as a plant parasitic fungus capable of secreting lignin-degrading enzymes, including Mn-peroxidase and laccase(Hirose et al., 2014; Becarelli et al., 2019). Species of the genus Chalara predominantly thrive as litter saprotrophs, with a notable prevalence on coniferous litter (Koukol, 2011), explaining its higher abundance in the mixed forest. Tomentella, an ectomycorrhizal genus, can secrete extracellular enzymes that facilitate the decomposition of proteins, polysaccharides, and organic phosphorus compounds through root tips (Tedersoo et al., 2012; Fernandez et al., 2020). Another ectomycorrhizal genus, Sebacina, (Tedersoo et al., 2014), was also more abundant in the mixed forest. The higher abundance of Tomentella and Sebacina in the mixed forest may be attributed to their ectomycorrhizal characteristics, allowing them to form mutualistic symbioses with various tree species(Anderson and Cairney, 2007).
Symbiotroph and ectomycorrhizal fungi showed higher abundance in the mixed forest, while saprotroph did not show a clear preference for either forest types. In forest ecosystems, nutrients constraints in soil have driven tree species to engage in mutualistic relationships with mycorrhizal fungi, which play a significant role in plant symbiosis (Strullu-Derrien et al., 2018). High concentrations of polyphenols in conifer trees (Popescu (Stegarus) et al., 2024) potentially enhance the diversity of symbiotrophic fungi (Simon et al., 2018), leading to the prevalence in mixed forest. Evolutionarily, symbiotrophic fungi, compared to saprotrophs, have shifted from producing degrading enzymes to fulfilling new symbiotic functions, though they still retain some saprotrophic abilities such as producing cellobiohydrolases, ligninolytic MnP-ESD peroxidases, proteases, and laccases (Kusuda et al., 2008; Kohler et al., 2015; Miyauchi et al., 2020; Looney et al., 2021). Ectomycorrhiza build associations with trees as the primary symbiosis (Lebreton et al., 2021), and use mycorrhizal hyphae extending from tree roots to reach the upper soil and litter layer and obtain essential nutrients, including inorganic and organic nitrogen and phosphate compounds((Lindahl et al., 2007, Nehls and Plassard, 2018).The increased activity of N-acetyl-glucosamidase, involved in nitrogen cycle and chitin degradation, could partly be due to the richer ectomycorrhiza presence in the mixed forest.
Microorganisms enhance nutrient cycling within ecosystem through diverse functional gene expression pathways (Hug and Co, 2018). After three-year decomposition, the fungal community in the pure forest emphasized more on phosphorus turnover rather than carbon cycling at gene level. This is logical, given phosphorus’s essential and limiting role in soil fertility (Siles et al., 2022). In our study, the most abundant genes in the mixed forest and pure forest were abfA and phod, respectively. The gene abfA encodes α-L-arabinofuranosidase (α-L-AFase), which catabolizes arabinoside and utilizes carbohydrate (Matsumura et al., 2004; Rodionov et al., 2021), while phod encodes alkaline phosphatase, participating in the mineralization of organic phosphorus (Zheng et al., 2018). In addition, the pure forest held higher abundance of cex and gcd genes, which are involved in cellulose hydrolysis (Zhang et al., 2022) and solubilizing inorganic phosphorus, coding for quinoprotein glucose dehydrogenase(Li et al., 2022; Zhou et al., 2022), respectively. The divergence in gene structure between the two forests highlights the distinctiveness of fungal functional community at a deeper level.
Based on OTU abundance, predicted functional modes, and fungal genes, distinct fungal communities and functional structures were formed in different forest types, emphasizing the influence of dominant tree species on litter decomposing microbial community. Previous studies have reported a close association between aboveground plants and belowground fungal communities (Prescott and Grayston, 2013; Xu et al., 2020). As plant characteristics change with the dominant tree species at a site, soil microbial communities can be influenced by microclimate variations, litter production (both above and belowground), herbivore interactions, root exudate production, and symbiotic associations such as mycorrhizal fungi(Prescott and Grayston, 2013). In our study, the drying process removed previously colonized microbes, making the litter microbial community closely associated with the soil microbial community, as soil microbes serve as a primary source of litter-degrading microorganisms (Wu et al., 2023). In the Abitibi region, diverse microbial communities, both functionally and genetically distinct, were also observed in forest stands floor dominated by different tree species(Lamarche et al., 2007). Microbial communities related to litter decomposition are shaped by substrate characteristics, which determine the capacity of different taxa to obtain and utilize distinct structural substrates(Bray et al., 2012; Purahong et al., 2016; Bani et al., 2018). Therefore, the different decomposing conditions provided by various forest types shape the distinct microbial communities. The first-year decomposition exhibited a distinct functional community structure compared to the following two years, corresponding with the dynamic pattern of decomposition rate. This demonstrates the interaction between fungal community structure and litter decomposition patterns. As expected, twig and leaf harbored separate fungal communities, reflecting the sensitivity of fungal communities to litter qualities (Habtewold et al., 2020).
Conclusion
Both litter types had the highest decomposition rate in the first year, which then declined. The mixed L. formosana forest exhibited higher activities of β-cellobiosidase and N-acetyl-glucosamidase, while the pure forest had higher activities of phosphatase, β-xylosidase, and β-glucosidase. Twig litter generally showed higher enzyme activities than leaf litter. Fungal community species richness, diversity and evenness were higher in the pure forest. Basidiomycota was more prevalent in the mixed forest and Ascomycota dominated in the pure forest. Functionally, symbiotroph fungi and ectomycorrhizal fungi were more abundant in the mixed forest. In the third year of twig decomposition, genes associated with phosphorus cycling were the most abundant. The pure forest had higher abundance of cex and gcd genes. Different forest types harbored distinct fungal community structure at community, functional and gene levels. These findings suggest that forest types strongly influence litter decomposition by recruiting different fungal community. Additionally, decomposition time and litter quality affect the decomposition by shifting substrate characteristics.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Funding
The study was supported by the National Key R & D Program of China. (2023YFD1401304), the National Natural Science Foundation of China (31870474) and the funding for Jiangsu Specially Appointed Professorship (project 163010219). The CSC–IT Center for Science (Finland) is gratefully acknowledged for the computational resource support.
Data Availability Statement
The data analyzed in this study are accessible upon request from the corresponding author. Additionally, the raw sequence data have been deposited in the NCBI Sequence Read Archive (SRA) and are available under the project accession number PRJNA1056890.
Competing of Interests
We claim that no conflicts of interest are generated in this study.
References
- Abarenkov, K. Henrik Nilsson, R., Larsson, K.-H., Alexander, I.J., Eberhardt, U., Erland, S., Høiland, K., Kjøller, R., Larsson, E., Pennanen, T., Sen, R., Taylor, A.F.S., Tedersoo, L., Ursing, B.M., Vrålstad, T., Liimatainen, K., Peintner, U., Kõljalg, U., 2010. The UNITE database for molecular identification of fungi--recent updates and future perspectives. New Phytol 186, 281–285. [CrossRef]
- Aciego Pietri, J.C. Brookes, P.C., 2009. Substrate inputs and pH as factors controlling microbial biomass, activity and community structure in an arable soil. Soil Biology and Biochemistry 41, 1396–1405. [CrossRef]
- Aerts, R. Logtestijn, R.S.P. van, Karlsson, P.S., 2006. Nitrogen supply differentially affects litter decomposition rates and nitrogen dynamics of sub-arctic bog species. Oecologia 146, 652–658. [CrossRef]
- Anderson, I.C. Cairney, J.W.G., 2007. Ectomycorrhizal fungi: exploring the mycelial frontier. FEMS Microbiology Reviews 31, 388–406. [CrossRef]
- Anderson, J.M. , 1973. The breakdown and decomposition of sweet chestnut (Castanea sativa Mill.) and beech (Fagus sylvatica L.) leaf litter in two deciduous woodland soils : I. Breakdown, leaching and decomposition. Oecologia 12, 251–274. [CrossRef]
- Anderson, M. , 2008. Permanova+ for primer: Guide to software and statistical methods. Primer-E Limited.
- Ayres, E. , Steltzer, H., Berg, S., Wallenstein, M.D., Simmons, B.L., Wall, D.H., 2009. Tree Species Traits Influence Soil Physical, Chemical, and Biological Properties in High Elevation Forests. PLOS ONE 4, e5964. [CrossRef]
- Bai, Y. , Zhou, Y., Chen, X., An, Z., Zhang, X., Du, J., Chang, S.X., 2023. Tree species composition alters the decomposition of mixed litter and the associated microbial community composition and function in subtropical plantations in China. Forest Ecology and Management 529, 120743. [CrossRef]
- Baldrian, P. , 2008. Chapter 2 Enzymes of saprotrophic basidiomycetes, in: Boddy, L., Frankland, J.C., van West, P. (Eds.), British Mycological Society Symposia Series, Ecology of Saprotrophic Basidiomycetes. Academic Press, pp. 19–41. [CrossRef]
- Baldrian, P. , Valásková, V., 2008. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol Rev 32, 501–521. [CrossRef]
- Bani, A. , Pioli, S., Ventura, M., Panzacchi, P., Borruso, L., Tognetti, R., Tonon, G., Brusetti, L., 2018. The role of microbial community in the decomposition of leaf litter and deadwood. Applied Soil Ecology 126, 75–84. [CrossRef]
- Bauhus, J. , Paré, D., Co ̂té, L., 1998. Effects of tree species, stand age and soil type on soil microbial biomass and its activity in a southern boreal forest. Soil Biology and Biochemistry 30, 1077–1089. [CrossRef]
- Becarelli, S. , Chicca, I., Siracusa, G., La China, S., Gentini, A., Lorenzi, R., Munz, G., Petroni, G., Levin, D.B., Di Gregorio, S., 2019. Hydrocarbonoclastic Ascomycetes to enhance co-composting of total petroleum hydrocarbon (TPH) contaminated dredged sediments and lignocellulosic matrices. N Biotechnol 50, 27–36. [CrossRef]
- Berg, B. , McClaugherty, C., 2020. Decomposition as a Process—some Main Features, in: Berg, B., McClaugherty, C. (Eds.), Plant Litter: Decomposition, Humus Formation, Carbon Sequestration. Springer International Publishing, Cham, pp. 13–43. [CrossRef]
- Bray, S.R. , Kitajima, K., Mack, M.C., 2012. Temporal dynamics of microbial communities on decomposing leaf litter of 10 plant species in relation to decomposition rate. Soil Biology and Biochemistry 49, 30–37. [CrossRef]
- Cai, Y.J. , Chapman, S.J., Buswell, J.A., Chang, S., 1999. Production and Distribution of Endoglucanase, Cellobiohydrolase, and β-Glucosidase Components of the Cellulolytic System of Volvariella volvacea, the Edible Straw Mushroom. Appl Environ Microbiol 65, 553–559.
- Chávez-Vergara, B. , Rosales-Castillo, A., Merino, A., Vázquez-Marrufo, G., Oyama, K., García-Oliva, F., 2016. Quercus species control nutrients dynamics by determining the composition and activity of the forest floor fungal community. Soil Biology and Biochemistry 98, 186–195. [CrossRef]
- Chen, L. , Xiang, W., Wu, H., Ouyang, S., Zhou, B., Zeng, Y., Chen, Y., Kuzyakov, Y., 2019. Tree species identity surpasses richness in affecting soil microbial richness and community composition in subtropical forests. Soil Biology and Biochemistry 130, 113–121. [CrossRef]
- Chen, X. , Yuan, Z., Jin, X., Guan, Q., Zhu, J., Dai, K., Zhao, C., 2020. Characteristics of Coniferous and Broad-leaved Mixed Forest Community on Zijin Mountain. Journal of Central South University of Forestry & Technology 40, 113–119.
- Chen, X. , Yuan, Z., Jin, X., Zhu, J., Xu, H., Zhao, C., Chen, B., Guan, Q., 2018. Spatial distribution pattern and interspecific association of dominant tree species in a broad-leaved mixed forest on Zijin Mountain. JOURNAL OF NANJING FORESTRY UNIVERSITY 61, 84. [CrossRef]
- Cheng, F. , Peng, X., Zhao, P., Yuan, J., Zhong, C., Cheng, Y., Cui, C., Zhang, S., 2013. Soil Microbial Biomass, Basal Respiration and Enzyme Activity of Main Forest Types in the Qinling Mountains. PLOS ONE 8, e67353. [CrossRef]
- Dechaine, J. , Ruan, H., Sanchez-de Leon, Y., Zou, X., 2005. Correlation between earthworms and plant litter decomposition in a tropical wet forest of Puerto Rico. Pedobiologia 49, 601–607. [CrossRef]
- Deng, J. , Yin, Y., Luo, J., Zhu, W., Zhou, Y., 2019. Different revegetation types alter soil physical-chemical characteristics and fungal community in the Baishilazi Nature Reserve. PeerJ 6, e6251. [CrossRef]
- Dilly, O. , Bartsch, S., Rosenbrock, P., Buscot, F., Munch, J.C., 2001. Shifts in physiological capabilities of the microbiota during the decomposition of leaf litter in a black alder (Alnus glutinosa (Gaertn.) L.) forest. Soil Biology and Biochemistry 33, 921–930. [CrossRef]
- Eastwood, D.C. , Floudas, D., Binder, M., Majcherczyk, A., Schneider, P., Aerts, A., Asiegbu, F.O., Baker, S.E., Barry, K., Bendiksby, M., Blumentritt, M., Coutinho, P.M., Cullen, D., de Vries, R.P., Gathman, A., Goodell, B., Henrissat, B., Ihrmark, K., Kauserud, H., Kohler, A., LaButti, K., Lapidus, A., Lavin, J.L., Lee, Y.-H., Lindquist, E., Lilly, W., Lucas, S., Morin, E., Murat, C., Oguiza, J.A., Park, J., Pisabarro, A.G., Riley, R., Rosling, A., Salamov, A., Schmidt, O., Schmutz, J., Skrede, I., Stenlid, J., Wiebenga, A., Xie, X., Kües, U., Hibbett, D.S., Hoffmeister, D., Högberg, N., Martin, F., Grigoriev, I.V., Watkinson, S.C., 2011. The plant cell wall-decomposing machinery underlies the functional diversity of forest fungi. Science 333, 762–765. [CrossRef]
- Eichlerová, I. , Homolka, L., Žifčáková, L., Lisá, L., Dobiášová, P., Baldrian, P., 2015. Enzymatic systems involved in decomposition reflects the ecology and taxonomy of saprotrophic fungi. Fungal Ecology 13, 10–22. [CrossRef]
- Farooq, T.H. , Li, Z., Yan, W., Shakoor, A., Kumar, U., Shabbir, R., Peng, Y., Gayathiri, E., Alotaibi, S.S., Wróbel, J., kalaji, H.M., Chen, X., 2022. Variations in Litterfall Dynamics, C:N:P Stoichiometry and Associated Nutrient Return in Pure and Mixed Stands of Camphor Tree and Masson Pine Forests. Frontiers in Environmental Science 10.
- Fernandez, C.W. , See, C.R., Kennedy, P.G., 2020. Decelerated carbon cycling by ectomycorrhizal fungi is controlled by substrate quality and community composition. New Phytologist 226, 569–582. [CrossRef]
- Floudas, D. , Binder, M., Riley, R., Barry, K., Blanchette, R.A., Henrissat, B., Martínez, A.T., Otillar, R., Spatafora, J.W., Yadav, J.S., Aerts, A., Benoit, I., Boyd, A., Carlson, A., Copeland, A., Coutinho, P.M., de Vries, R.P., Ferreira, P., Findley, K., Foster, B., Gaskell, J., Glotzer, D., Górecki, P., Heitman, J., Hesse, C., Hori, C., Igarashi, K., Jurgens, J.A., Kallen, N., Kersten, P., Kohler, A., Kües, U., Kumar, T.K.A., Kuo, A., LaButti, K., Larrondo, L.F., Lindquist, E., Ling, A., Lombard, V., Lucas, S., Lundell, T., Martin, R., McLaughlin, D.J., Morgenstern, I., Morin, E., Murat, C., Nagy, L.G., Nolan, M., Ohm, R.A., Patyshakuliyeva, A., Rokas, A., Ruiz-Dueñas, F.J., Sabat, G., Salamov, A., Samejima, M., Schmutz, J., Slot, J.C., St. John, F., Stenlid, J., Sun, H., Sun, S., Syed, K., Tsang, A., Wiebenga, A., Young, D., Pisabarro, A., Eastwood, D.C., Martin, F., Cullen, D., Grigoriev, I.V., Hibbett, D.S., 2012. The Paleozoic Origin of Enzymatic Lignin Decomposition Reconstructed from 31 Fungal Genomes. Science 336, 1715–1719. [CrossRef]
- Foudyl-Bey, S. , Brais, S., Drouin, P., 2016. Litter heterogeneity modulates fungal activity, C mineralization and N retention in the boreal forest floor. Soil Biology and Biochemistry 100, 264–275. [CrossRef]
- Frankland, J.C. , 1998. Fungal succession — unravelling the unpredictable. Mycological Research 102, 1–15. [CrossRef]
- Geethanjali, P.A. , Jayashankar, Prof.M., 2016. A Review on Litter Decomposition by Soil Fungal Community. IOSR 11, 01–03. [CrossRef]
- Gołębiewski, M. , Tarasek, A., Sikora, M., Deja-Sikora, E., Tretyn, A., Niklińska, M., 2019. Rapid Microbial Community Changes During Initial Stages of Pine Litter Decomposition. Microb Ecol 77, 56–75. [CrossRef]
- Guo, J. , Yang, Y., Chen, G., Lin, P., Xie, J., 2006. A Review on Litter Decomposition in Forest Ecosystem [WWW Document]. URL http://html.rhhz.net/linyekexue/html/20060417.htm (accessed 10.5.23).
- Habtewold, J.Z. , Helgason, B.L., Yanni, S.F., Janzen, H.H., Ellert, B.H., Gregorich, E.G., 2020. Litter composition has stronger influence on the structure of soil fungal than bacterial communities. European Journal of Soil Biology 98, 103190. [CrossRef]
- Hirose, A. , Kudo, S., Murakami, T., Tanaka, K., Harada, Y., Hashimoto, M., 2014. Lambertellin system, the mechanism for fungal replacement of Monilinia fructigena with Lambertella corni-maris without competitive inhibition on agar media. Bioorganic & Medicinal Chemistry 22, 2489–2495. [CrossRef]
- Hug, L.A. , Co, R., 2018. It Takes a Village: Microbial Communities Thrive through Interactions and Metabolic Handoffs. mSystems 3, e00152-17. [CrossRef]
- Jackrel, S.L. , Gilbert, J.A., Wootton, J.T., 2019. The Origin, Succession, and Predicted Metabolism of Bacterial Communities Associated with Leaf Decomposition. mBio 10, e01703-19. [CrossRef]
- Jiang, A. , Wan, F., Hu, F., 2018. Study on Soil Anti-Erodibility in Different Forests in Spirit Valley of Mount Zijin in Nanjing [WWW Document]. URL https://www.cglhub.com/auto/db/detail.aspx?db=950001&rid=13789143&agfi=0&cls=0&uni=False&cid=0&showgp=False&prec=False&md=76&pd=351&msd=63&psd=207&mdd=76&pdd=351&count=10&reds=%E6%8A%97%E8%9A%80%E6%80%A7 (accessed 11.7.23).
- Jing, H. , Wang, G., 2020. Temporal dynamics of Pinus tabulaeformis litter decomposition under nitrogen addition on the Loess Plateau of China. Forest Ecology and Management 476, 118465. [CrossRef]
- Kähkönen, M.A. , Lankinen, P., Hatakka, A., 2008. Hydrolytic and ligninolytic enzyme activities in the Pb contaminated soil inoculated with litter-decomposing fungi. Chemosphere 72, 708–714. [CrossRef]
- Kamau, S. , Barrios, E., Karanja, N.K., Ayuke, F.O., Lehmann, J., 2020. Dominant tree species and earthworms affect soil aggregation and carbon content along a soil degradation gradient in an agricultural landscape. Geoderma 359, 113983. [CrossRef]
- Keller, A.B. , Phillips, R.P., 2019. Leaf litter decay rates differ between mycorrhizal groups in temperate, but not tropical, forests. New Phytologist 222, 556–564. [CrossRef]
- Kohler, A. , Kuo, A., Nagy, L.G., Morin, E., Barry, K.W., Buscot, F., Canbäck, B., Choi, C., Cichocki, N., Clum, A., Colpaert, J., Copeland, A., Costa, M.D., Doré, J., Floudas, D., Gay, G., Girlanda, M., Henrissat, B., Herrmann, S., Hess, J., Högberg, N., Johansson, T., Khouja, H.-R., LaButti, K., Lahrmann, U., Levasseur, A., Lindquist, E.A., Lipzen, A., Marmeisse, R., Martino, E., Murat, C., Ngan, C.Y., Nehls, U., Plett, J.M., Pringle, A., Ohm, R.A., Perotto, S., Peter, M., Riley, R., Rineau, F., Ruytinx, J., Salamov, A., Shah, F., Sun, H., Tarkka, M., Tritt, A., Veneault-Fourrey, C., Zuccaro, A., Tunlid, A., Grigoriev, I.V., Hibbett, D.S., Martin, F., 2015. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat Genet 47, 410–415. [CrossRef]
- Koukol, O. , 2011. New species of Chalara occupying coniferous needles. Fungal Diversity 49, 75–91. [CrossRef]
- Krishna, M.P. , Mohan, M., 2017. Litter decomposition in forest ecosystems: a review. Energ. Ecol. Environ. 2, 236–249. [CrossRef]
- Kusuda, M. , Ueda, M., Miyatake, K., Terashita, T., 2008. Characterization of the carbohydrase productions of an ectomycorrhizal fungus, Tricholoma matsutake. Mycoscience 49, 291–297. [CrossRef]
- Laganière, J. , Paré, D., Bradley, R.L., 2010. How does a tree species influence litter decomposition? Separating the relative contribution of litter quality, litter mixing, and forest floor conditions. Can. J. For. Res. 40, 465–475. [CrossRef]
- Lamarche, J. , Bradley, R.L., Hooper, E., Shipley, B., Simao Beaunoir, A.-M., Beaulieu, C., 2007. Forest floor bacterial community composition and catabolic profiles in relation to landscape features in Québec’s southern boreal forest. Microb Ecol 54, 10–20. [CrossRef]
- Latterini, F. , Dyderski, M.K., Horodecki, P., Picchio, R., Venanzi, R., Lapin, K., Jagodziński, A.M., 2023. The Effects of Forest Operations and Silvicultural Treatments on Litter Decomposition Rate: a Meta-analysis. Curr. For. Rep. 9, 276–290. [CrossRef]
- LeBauer, D.S. , 2010. Litter degradation rate and β-glucosidase activity increase with fungal diversity. Can. J. For. Res. 40, 1076–1085. [CrossRef]
- Lebreton, A. , Zeng, Q., Miyauchi, S., Kohler, A., Dai, Y.-C., Martin, F.M., 2021. Evolution of the Mode of Nutrition in Symbiotic and Saprotrophic Fungi in Forest Ecosystems. Annual Review of Ecology, Evolution, and Systematics 52, 385–404. [CrossRef]
- Lehmann, J. , Schroth, G., Zech, W., 1995. Decomposition and nutrient release from leaves, twigs and roots of three alley-cropped tree legumes in central Togo. Agroforest Syst 29, 21–36. [CrossRef]
- Li, M. , Hao, Y., Yan, Z., Kang, E., Wang, J., Zhang, K., Li, Y., Wu, H., Kang, X., 2022. Long-term degradation from marshes into meadows shifts microbial functional diversity of soil phosphorus cycling in an alpine wetland of the Tibetan Plateau. Land Degrad Dev 33, 628–637. [CrossRef]
- Lindahl, B.D. , Ihrmark, K., Boberg, J., Trumbore, S.E., Högberg, P., Stenlid, J., Finlay, R.D., 2007. Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytologist 173, 611–620. [CrossRef]
- Liu, K. , Meng, W., Qu, Z., Zhang, Y., Liu, B., Ma, Y., Chang, L., Sun, H., 2023. Changes in bacterial communities and functions associated with litter degradation during forest succession caused by forest disease. Phytobiomes Journal. [CrossRef]
- Looney, B. , Miyauchi, S., Morin, E., Drula, E., Courty, P.-E., Kohler, A., Lindquist, E., Kuo, A., Labutti, K., Pangilinan, J., Lipzen, A., Riley, R., Andreopoulos, W., He, G., Johnson, J., Barry, K., Grigoriev, I., G. Nagy, L., Hibbett, D., Martin, F., 2021. Evolutionary priming and transition to the ectomycorrhizal habit in an iconic lineage of mushroom-forming fungi: is preadaptation a requirement? [CrossRef]
- Loreau, M. , 2001. Microbial diversity, producer–decomposer interactions and ecosystem processes: a theoretical model. Proceedings of the Royal Society of London. Series B: Biological Sciences 268, 303–309. [CrossRef]
- Loreau, M. , Hector, A., 2001. Partitioning selection and complementarity in biodiversity experiments. Nature 412, 72–76. [CrossRef]
- Lundell, T.K. , Mäkelä, M.R., de Vries, R.P., Hildén, K.S., 2014. Chapter Eleven - Genomics, Lifestyles and Future Prospects of Wood-Decay and Litter-Decomposing Basidiomycota, in: Martin, F.M. (Ed.), Advances in Botanical Research, Fungi. Academic Press, pp. 329–370. [CrossRef]
- Ma, Y. , Qu, Z.-L., Liu, B., Tan, J.-J., Asiegbu, F.O., Sun, H., 2020. Bacterial Community Structure of Pinus Thunbergii Naturally Infected by the Nematode Bursaphelenchus Xylophilus. Microorganisms 8, 307. [CrossRef]
- Matsumura, K. , Obata, H., Hata, Y., Kawato, A., Abe, Y., Akita, O., 2004. Isolation and characterization of a novel gene encoding α-L-arabinofuranosidase from Aspergillus oryzae. Journal of Bioscience and Bioengineering 98, 77–84. [CrossRef]
- Nagati, M. , Roy, M., Manzi, S., Richard, F., Desrochers, A., Gardes, M., Bergeron, Y., 2018. Impact of local forest composition on soil fungal communities in a mixed boreal forest. Plant Soil 432, 345–357. [CrossRef]
- Nehls, U. , Plassard, C., 2018. Nitrogen and phosphate metabolism in ectomycorrhizas. New Phytologist 220, 1047–1058. [CrossRef]
- Nguyen, N.H. , Song, Z., Bates, S.T., Branco, S., Tedersoo, L., Menke, J., Schilling, J.S., Kennedy, P.G., 2016. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecology 20, 241–248. [CrossRef]
- Novotný, Č. , Svobodová, K., Erbanová, P., Cajthaml, T., Kasinath, A., Lang, E., Šašek, V., 2004. Ligninolytic fungi in bioremediation: extracellular enzyme production and degradation rate. Soil Biology and Biochemistry, Enzymes in the Environment: Activity, Ecology and Applications 36, 1545–1551. [CrossRef]
- Osono, T. , 2007. Ecology of ligninolytic fungi associated with leaf litter decomposition. Ecol Res 22, 955–974. [CrossRef]
- Osono, T. , 2006. Role of phyllosphere fungi of forest trees in the development of decomposer fungal communities and decomposition processes of leaf litter. Can J Microbiol 52, 701–716. [CrossRef]
- Osono, T. , To-Anun, C., Hagiwara, Y., Hirose, D., 2011. Decomposition of wood, petiole and leaf litter by Xylaria species from northern Thailand. Fungal Ecology 4, 210–218. [CrossRef]
- Popescu (Stegarus), D.I. , Frum, A., Dobrea, C.M., Cristea, R., Gligor, F.G., Vicas, L.G., Ionete, R.E., Sutan, N.A., Georgescu, C., 2024. Comparative Antioxidant and Antimicrobial Activities of Several Conifer Needles and Bark Extracts. Pharmaceutics 16, 52. [CrossRef]
- Prescott, C.E. , Grayston, S.J., 2013. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. Forest Ecology and Management, Influence of tree species on forest soils: New evidence from field studies 309, 19–27. [CrossRef]
- Prescott, C.E. , Vesterdal, L., 2021. Decomposition and transformations along the continuum from litter to soil organic matter in forest soils. Forest Ecology and Management 498, 119522. [CrossRef]
- Prescott, C.E. , Zabek, L.M., Staley, C.L., Kabzems, R., 2000. Decomposition of broadleaf and needle litter in forests of British Columbia: influences of litter type, forest type, and litter mixtures. Can. J. For. Res. 30, 1742–1750. [CrossRef]
- Purahong, W. , Wubet, T., Lentendu, G., Schloter, M., Pecyna, M.J., Kapturska, D., Hofrichter, M., Krüger, D., Buscot, F., 2016. Life in leaf litter: novel insights into community dynamics of bacteria and fungi during litter decomposition. Mol Ecol 25, 4059–4074. [CrossRef]
- Qu, Z.-L. , Liu, B., Ma, Y., Xu, J., Sun, H., 2020. The response of the soil bacterial community and function to forest succession caused by forest disease. Functional Ecology 34, 2548–2559. [CrossRef]
- Rajala, T. , Peltoniemi, M., Hantula, J., Mäkipää, R., Pennanen, T., 2011. RNA reveals a succession of active fungi during the decay of Norway spruce logs. Fungal Ecology, Decomposition in Forest Ecosystems 4, 437–448. [CrossRef]
- Robertson, G.P. , Paul, E.A., 2000. Decomposition and Soil Organic Matter Dynamics, in: Sala, O.E., Jackson, R.B., Mooney, H.A., Howarth, R.W. (Eds.), Methods in Ecosystem Science. Springer, New York, NY, pp. 104–116. [CrossRef]
- Rodionov, D.A. , Rodionova, I.A., Rodionov, V.A., Arzamasov, A.A., Zhang, K., Rubinstein, G.M., Tanwee, T.N.N., Bing, R.G., Crosby, J.R., Nookaew, I., Basen, M., Brown, S.D., Wilson, C.M., Klingeman, D.M., Poole, F.L., Zhang, Y., Kelly, R.M., Adams, M.W.W., 2021. Transcriptional Regulation of Plant Biomass Degradation and Carbohydrate Utilization Genes in the Extreme Thermophile Caldicellulosiruptor bescii. mSystems 6, 10.1128/msystems.01345-20. [CrossRef]
- Schloss, P.D. , Westcott, S.L., Ryabin, T., Hall, J.R., Hartmann, M., Hollister, E.B., Lesniewski, R.A., Oakley, B.B., Parks, D.H., Robinson, C.J., Sahl, J.W., Stres, B., Thallinger, G.G., Van Horn, D.J., Weber, C.F., 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75, 7537–7541. [CrossRef]
- Siles, J.A. , Starke, R., Martinovic, T., Parente Fernandes, M.L., Orgiazzi, A., Bastida, F., 2022. Distribution of phosphorus cycling genes across land uses and microbial taxonomic groups based on metagenome and genome mining. Soil Biology and Biochemistry 174, 108826. [CrossRef]
- Simon, J. , Dörken, V.M., L. -M.-Arnold, A., Adamczyk, B., 2018. Environmental Conditions and Species Identity Drive Metabolite Levels in Green Leaves and Leaf Litter of 14 Temperate Woody Species. Forests 9, 775. [CrossRef]
- Smith, D.P. , Peay, K.G., 2014. Sequence depth, not PCR replication, improves ecological inference from next generation DNA sequencing. PLoS One 9, e90234. [CrossRef]
- Šnajdr, J. , Cajthaml, T., Valášková, V., Merhautová, V., Petránková, M., Spetz, P., Leppänen, K., Baldrian, P., 2011. Transformation of Quercus petraea litter: successive changes in litter chemistry are reflected in differential enzyme activity and changes in the microbial community composition. FEMS Microbiology Ecology 75, 291–303. [CrossRef]
- Steffen, K.T. , Cajthaml, T., Šnajdr, J., Baldrian, P., 2007. Differential degradation of oak (Quercus petraea) leaf litter by litter-decomposing basidiomycetes. Research in Microbiology 158, 447–455. [CrossRef]
- Strullu-Derrien, C. , Selosse, M.-A., Kenrick, P., Martin, F.M., 2018. The origin and evolution of mycorrhizal symbioses: from palaeomycology to phylogenomics. New Phytologist 220, 1012–1030. [CrossRef]
- Sun, H. , Terhonen, E., Kovalchuk, A., Tuovila, H., Chen, H., Oghenekaro, A.O., Heinonsalo, J., Kohler, A., Kasanen, R., Vasander, H., Asiegbu, F.O., 2016. Dominant Tree Species and Soil Type Affect the Fungal Community Structure in a Boreal Peatland Forest. Applied and Environmental Microbiology 82, 2632–2643. [CrossRef]
- Sun, X. , Zheng, Y., Xu, G., Guo, Q., Tan, J., Ding, G., 2021. Fungal diversity within the phyllosphere of Pinus massoniana and the possible involvement of phyllospheric fungi in litter decomposition. Fungal Biology 125, 785–795. [CrossRef]
- Tedersoo, L. , Bahram, M., Ryberg, M., Otsing, E., Kõljalg, U., Abarenkov, K., 2014. Global biogeography of the ectomycorrhizal /sebacina lineage (Fungi, Sebacinales) as revealed from comparative phylogenetic analyses. Molecular Ecology 23, 4168–4183. [CrossRef]
- Tedersoo, L. , Bahram, M., Toots, M., Diédhiou, A.G., Henkel, T.W., Kjøller, R., Morris, M.H., Nara, K., Nouhra, E., Peay, K.G., Põlme, S., Ryberg, M., Smith, M.E., Kõljalg, U., 2012. Towards global patterns in the diversity and community structure of ectomycorrhizal fungi. Mol Ecol 21, 4160–4170. [CrossRef]
- Tedersoo, L. , Nilsson, R.H., Abarenkov, K., Jairus, T., Sadam, A., Saar, I., Bahram, M., Bechem, E., Chuyong, G., Kõljalg, U., 2010. 454 Pyrosequencing and Sanger sequencing of tropical mycorrhizal fungi provide similar results but reveal substantial methodological biases. New Phytologist 188, 291–301. [CrossRef]
- Treseder, K.K. , Bent, E., Borneman, J., McGuire, K.L., 2014. Shifts in fungal communities during decomposition of boreal forest litter. Fungal Ecology, Fungi in a changing world: The role of fungi in ecosystem response to global change 10, 58–69. [CrossRef]
- Urbanová, M. , Šnajdr, J., Baldrian, P., 2015. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biology and Biochemistry 84, 53–64. [CrossRef]
- van der Wal, A. , Geydan, T.D., Kuyper, T.W., de Boer, W., 2013. A thready affair: linking fungal diversity and community dynamics to terrestrial decomposition processes. FEMS Microbiol Rev 37, 477–494. [CrossRef]
- Voříšková, J. , Baldrian, P., 2013. Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J 7, 477–486. [CrossRef]
- Wang, X. , Xiao, S., Yang, X., Liu, Z., Zhou, X., Du, G., Zhang, L., Guo, A., Chen, S., Nielsen, U.N., 2019. Dominant plant species influence nematode richness by moderating understory diversity and microbial assemblages. Soil Biology and Biochemistry 137, 107566. [CrossRef]
- Wu, X. , Shi, Y., Zhu, J., Sun, L., Ma, X., 2023. Impacts of Global Warming on Forest Litters [WWW Document]. URL http://www.sjlyyj.com/article/doi/10.13348/j.cnki.sjlyyj.2022.0079.y (accessed 12.28.23).
- Xu, J. , Liu, B., Qu, Z.-L., Ma, Y., Sun, H., 2020. Age and Species of Eucalyptus Plantations Affect Soil Microbial Biomass and Enzymatic Activities. Microorganisms 8, 811. [CrossRef]
- Xu, Z. , Yu, G., Zhang, X., Ge, J., He, N., Wang, Q., Wang, D., 2015. The variations in soil microbial communities, enzyme activities and their relationships with soil organic matter decomposition along the northern slope of Changbai Mountain. Applied Soil Ecology 86, 19–29. [CrossRef]
- Yang, X. , Wang, X., Xiao, S., Liu, Z., Zhou, X., Du, G., Liu, K., Wang, Y., Chen, S., Nielsen, U.N., 2021. Dominant plants affect litter decomposition mainly through modifications of the soil microbial community. Soil Biology and Biochemistry 161, 108399. [CrossRef]
- Yue, K. , Yang, W., Peng, C., Peng, Y., Zhang, C., Huang, C., Tan, Y., Wu, F., 2016. Foliar litter decomposition in an alpine forest meta-ecosystem on the eastern Tibetan Plateau. Science of The Total Environment 566–567, 279–287. [CrossRef]
- Zhang, Y. , Li, X., Xiao, M., Feng, Z., Yu, Y., Yao, H., 2022. Effects of microplastics on soil carbon dioxide emissions and the microbial functional genes involved in organic carbon decomposition in agricultural soil. Science of The Total Environment 806, 150714. [CrossRef]
- Zheng, B. , Zhu, Y., Sardans, J., Peñuelas, J., Su, J., 2018. QMEC: a tool for high-throughput quantitative assessment of microbial functional potential in C, N, P, and S biogeochemical cycling. Sci. China Life Sci. 61, 1451–1462. [CrossRef]
- Zhou, S. , Li, Y., Wang, Jieying, He, L., Wang, Jun, Guo, Y., Zhao, F., 2022. Contrasting Soil Microbial Functional Potential for Phosphorus Cycling in Subtropical and Temperate Forests. Forests 13, 2002. [CrossRef]
Figure 1.
The litter decomposition rate of leaf (a) and twig (b) in mixed Liquidambar formosana forest (MLF) and pure L. formosana forest (PLF) over a 3-year period. Lowercase letters above the bars indicate statistically significant differences (P < 0.05) between the forests.
Figure 1.
The litter decomposition rate of leaf (a) and twig (b) in mixed Liquidambar formosana forest (MLF) and pure L. formosana forest (PLF) over a 3-year period. Lowercase letters above the bars indicate statistically significant differences (P < 0.05) between the forests.
Figure 2.
The activities of extracellular enzymes associated with carbon degradation, including β-cellobiosidase(CEL), β-glucosidase(GLS), β-D-glucuronidase(GLR), β-xylosidase(XYL), and laccase; nitrogen cycling (chitin decomposition N-acetyl-glucosamidase (NAG)); organic phosphorus decomposition (PHO); and sulfur hydrolysis (SUL) in leaf litter (a-h) and twig litter (A-H), respectively, in mixed Liquidambar formosana forest (MLF), and pure L. formosana forest (PLF) during the 3-year decomposition period. Lowercase letters above the bars indicate statistically significant differences (P < 0.05) between the forests.
Figure 2.
The activities of extracellular enzymes associated with carbon degradation, including β-cellobiosidase(CEL), β-glucosidase(GLS), β-D-glucuronidase(GLR), β-xylosidase(XYL), and laccase; nitrogen cycling (chitin decomposition N-acetyl-glucosamidase (NAG)); organic phosphorus decomposition (PHO); and sulfur hydrolysis (SUL) in leaf litter (a-h) and twig litter (A-H), respectively, in mixed Liquidambar formosana forest (MLF), and pure L. formosana forest (PLF) during the 3-year decomposition period. Lowercase letters above the bars indicate statistically significant differences (P < 0.05) between the forests.
Figure 3.
The fungal community richness (Sobs), fungal community diversity (Invsimpson), fungal community evenness (Simpsoneven) within leaf litter (a, b, and c) and twig litter (d, e, and f) in mixed Liquidambar formosana forest (MLF) and pure L. formosana forest (PLF). Lowercase letters above the bars indicate statistically significant differences (P < 0.05) between the forests.
Figure 3.
The fungal community richness (Sobs), fungal community diversity (Invsimpson), fungal community evenness (Simpsoneven) within leaf litter (a, b, and c) and twig litter (d, e, and f) in mixed Liquidambar formosana forest (MLF) and pure L. formosana forest (PLF). Lowercase letters above the bars indicate statistically significant differences (P < 0.05) between the forests.
Figure 4.
The relative abundance of fungal phyla and the top 10 genera in leaf litter (a, c) and twig litter (b, d) during decomposition in the mixed L. formosana forest (MLF) and pure L. formosana forest (PLF).1
Figure 4.
The relative abundance of fungal phyla and the top 10 genera in leaf litter (a, c) and twig litter (b, d) during decomposition in the mixed L. formosana forest (MLF) and pure L. formosana forest (PLF).1
Figure 5.
Principal coordinate analysis (PCoA) representing the fungal community structure (a), the fungal functional structure (b) within leaf and twig litter during the 3-year decomposition, and fungal genes within twig litter in the third-decomposition year (c) in the mixed Liquidambar formosana forest (MLF), and the pure L. formosana forest (PLF). The red circles represent the separation of fungal genes between forest types.
Figure 5.
Principal coordinate analysis (PCoA) representing the fungal community structure (a), the fungal functional structure (b) within leaf and twig litter during the 3-year decomposition, and fungal genes within twig litter in the third-decomposition year (c) in the mixed Liquidambar formosana forest (MLF), and the pure L. formosana forest (PLF). The red circles represent the separation of fungal genes between forest types.
Figure 6.
The relative abundance of fungal trophic modes based on FUNGuild analysis during the leaf litter decomposition (a) and twig litter decomposition (b).
Figure 6.
The relative abundance of fungal trophic modes based on FUNGuild analysis during the leaf litter decomposition (a) and twig litter decomposition (b).
Figure 7.
The relative abundance of fungal functional genes associated with C and P cycles (a) and individual fungal functional gene (b) in twig litter in the third-year decomposition in the mixed Liquidambar formosana forest (MLF), and the pure L. formosana forest (PLF).
Figure 7.
The relative abundance of fungal functional genes associated with C and P cycles (a) and individual fungal functional gene (b) in twig litter in the third-year decomposition in the mixed Liquidambar formosana forest (MLF), and the pure L. formosana forest (PLF).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).