1. Introduction
Traditional surgical education, grounded in apprenticeship, didactic instruction, and skill development, has long been recognized as a critical and gradual process [
1]. Each surgical maneuver follows a learning curve that is optimized through continuous understanding and repetition [
2]. The scientific literature emphasizes the substantial time and practice required to master a technical skill, which involves a progression through various stages such as observation, imitation, independent execution, and repeated practice [
3]. However, despite numerous procedural repetitions during residency, intraoperative training faces significant challenges due to variability in exposure and autonomy across different programs, rotations, case volumes, and healthcare system pressures [
1,
4].
The advent of new technologies has significantly contributed to reducing complication risks and enhancing the performance of novice neurosurgeons [
5,
6,
7,
8,
9]. For instance, advanced preoperative imaging has revolutionized the understanding of anatomy and pathology, enabling more precise and targeted surgical planning [
10,
11]. Although the complication rate has steadily declined with these innovations, it remains essential for residents to participate in extra-operative programs to refine their surgical skills and acquire new techniques. Historically, cadaver courses have been the gold standard in surgical training, yet they also present limitations, including inadequate reproduction of surgical pathology, variable cadaver quality, high costs, limited availability, and ethical-legal concerns [
12,
13,
14]. Consequently, there is a growing interest in integrating innovative methods for skill development.
Advanced technologies such as virtual reality (VR), augmented reality (AR), and high-fidelity synthetic models offer promising solutions by providing frequent practice opportunities and enabling objective skill assessments [
15,
16]. Unlike past subjective evaluations, these technologies offer quantifiable indicators of skill progression, facilitating the monitoring of trainee development [
17,
18,
19].
This study aims to evaluate and analyze the learning curve of a cohort of subjects with varying levels of experience in performing lumbar pedicle insertion (Kirschner wire) using a virtual fluoroscopic simulator (DuO - Mediability). The objective is to assess the impact of repeated practice on skill acquisition and performance improvement, and to provide insights into how advanced simulation technologies can be integrated into surgical training programs to enhance skill development and reduce the risk of complications. By exploring these areas, the study seeks to contribute to the optimization of surgical training methodologies and the advancement of surgical education through the use of innovative technologies.
2. Materials and Methods
This study included 18 neurosurgery residents and 2 intern students. Each participant completed a questionnaire to determine their level of expertise in lumbar pedicle K-wire insertion. During the pre-training evaluation, morphometric measurements of the targeted pedicle in a virtual model were recorded. Specifically, the measurements included pedicle height (PH), pedicle width (PW), distance from the entry point into the pedicle to the anterior surface of the vertebral body (D), pedicle area (PA), the angle between the trajectory of the transpedicle screw and the midline of the vertebra (transverse angle - α), and the angle between the trajectory of the transpedicle screw and the anatomical transverse plane of the spine (sagittal angulation - β). These measurements were calculated based on vertebral CT scan analysis provided by the tool.
During the simulation, all participants received the same frontal lecture on the surgical technique, including video sessions and an explanation of the virtual simulator. The number of attempts in lumbar pedicle K-wire insertion continued until each participant reached the "practical learning plateau," defined as non-significant variability (X ± Delta < 25% of results) in performance—measured as a combination of accuracy, timing, and the number of virtual x-ray scans—over the last two attempts with an accuracy grade of 0 or 1. Specifically, accuracy of wire positioning was analyzed by calculating the ideal dimension for pedicle screw insertion, based on the pedicle diameter minus 2 mm (e.g., for a 6 mm pedicle, a 4 mm ideal screw was considered). Accuracy was categorized according to a 2mm system [
20]: Grade 0 (no misplacement, whole screw is in the pedicle), Grade 1 (<2 mm of the screw is out of the pedicle), Grade 2 (between 2 and 4 mm is out of the pedicle), and Grade 3 (>4 mm or the whole screw diameter is out of the pedicle). For each wire insertion, the following data were recorded: total time of wire insertion (from start to end), number of virtual x-ray scans and number of failed attempts/need to restart the wire insertion. After the training session, participants completed a quality and self-evaluation questionnaire with a rating scale from 1 (Bad) to 5 (Excellent) to assess and document their personal perception of the technical skills acquired.
Statistical Analysis
Each parameter analyzed will be recorded and analyzed. Python was used for statistical analysis. To analyze the learning progress of each participant, we applied an exponential model to the data representing the number of attempts and the frequency of failed attempts or restarts during wire insertion. The model used is defined as: y = a•e^{-b•x}, where y is the variable of interest, x is the number of attempts, a is the initial number of failed attempts, and b is the learning rate, which indicates how quickly the number of failed attempts decreases with each additional attempt.
By fitting this exponential model to the data, we estimated the learning rate (b) for each participant for both the total time of wire insertion and the number of virtual x-ray scans. The model was then evaluated using R^2, Akaike Information Criterion (AIC), and Bayesian Information Criterion (BIC). This thorough analysis provides insights into the learning progress and model quality, helping to pinpoint areas where participants may need additional training or support. In addition to R^2, we utilized AIC and BIC to evaluate the model. These criteria balance model fit and complexity by penalizing models with more parameters to prevent overfitting.
Finally, to measure how variables correlate, the Pearson correlation coefficient was calculated to create a table showing the correlation between candidates’ personal questionnaire information and learning variables.
3. Results
The study included 18 participants (10 male; 8 Female) with a mean age of 26,94 ± 2,6 years with an average residency year of 2.8 ± 1.58 (2 students, 2 PGY-1; 4 PGY-2; 6 PGY-3; 2 PGY-4; 3 PGY-5), with most participants not having attended another residency program before (75% - 12/18). Pre-training questionnaire showed a relatively low attendance at cadaver labs and spine simulation courses, with 20% and 40% participation, respectively as well as experience in lumbar pedicle K wire insertion, with most participants having performed fewer than 50 procedures (65% and 45% as First or second operator, respectively). Experience with spinal intraoperative navigation systems is also limited, with 65% having little to no experience.
Table 1 summarizes results provided by pre-training questionnaire.
Based on analysis of the pedicle's morphology and screw trajectory, a relatively medium in size with moderate transverse and sagittal angulation identified in right L1 pedicle on DuO – Mediability simulator was choose as target. The pedicle height was 8 mm with 7 mm width, while the distance from the entry point into the pedicle to the anterior surface of the vertebral body was found to be 35 mm. Additionally, the pedicle area was calculated to be 42 mm². The angle between the trajectory of the transpedicle screw and the midline of the vertebra (transverse angle) was 26 degrees, and the angle between the trajectory of the transpedicle screw and the anatomical transverse plane of the spine (sagittal angulation) was 12 degrees. small pedicle size with moderate transverse and sagittal angulation.
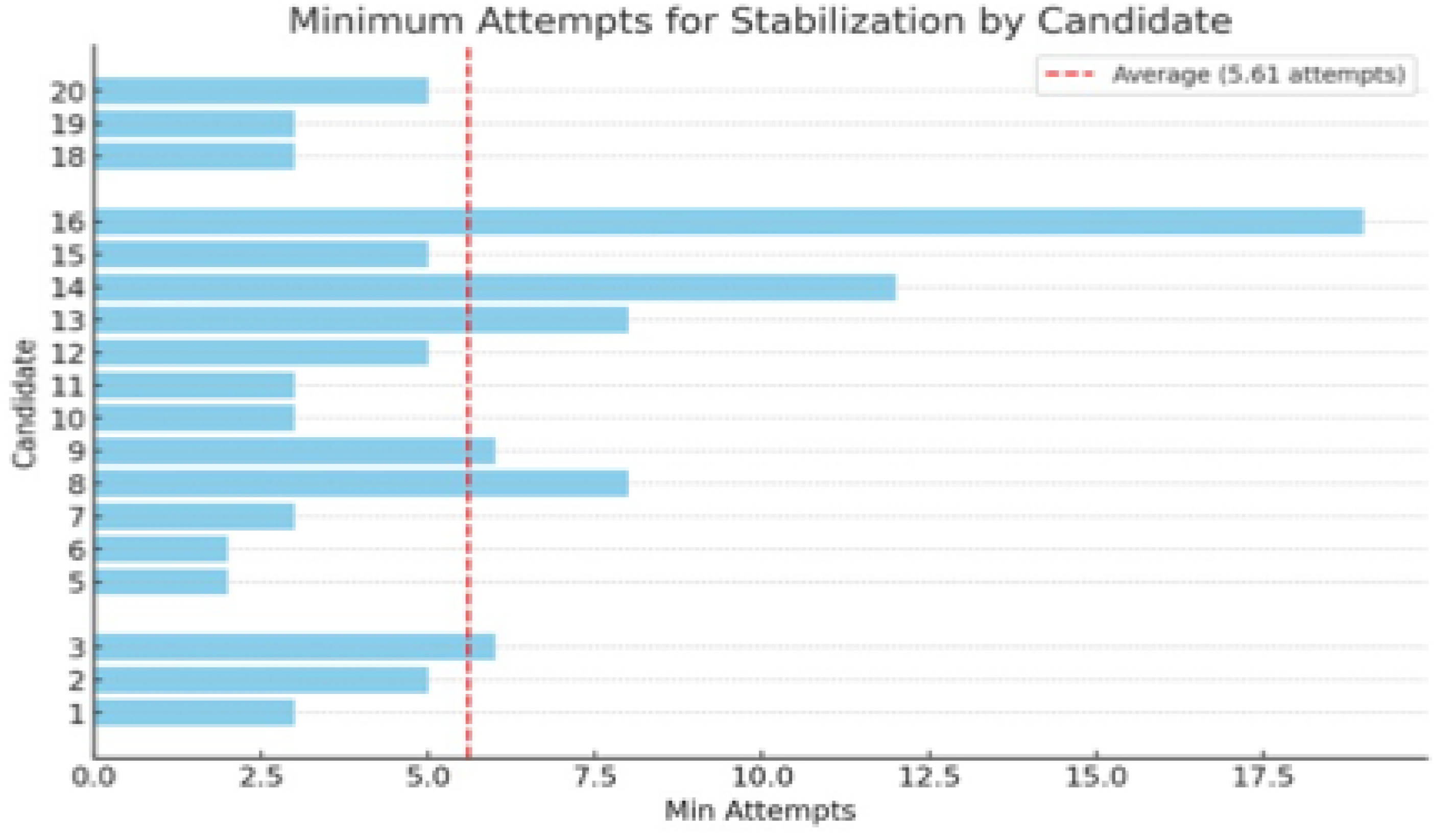
Results of performance analysis indicate that the average minimum number of attempts required for participants to stabilize their performance in lumbar pedicle K-wire insertion was 5,61 (
Figure 1). Two candidates (4 and 17) were excluded due to the impossibility of accomplishing the training program.
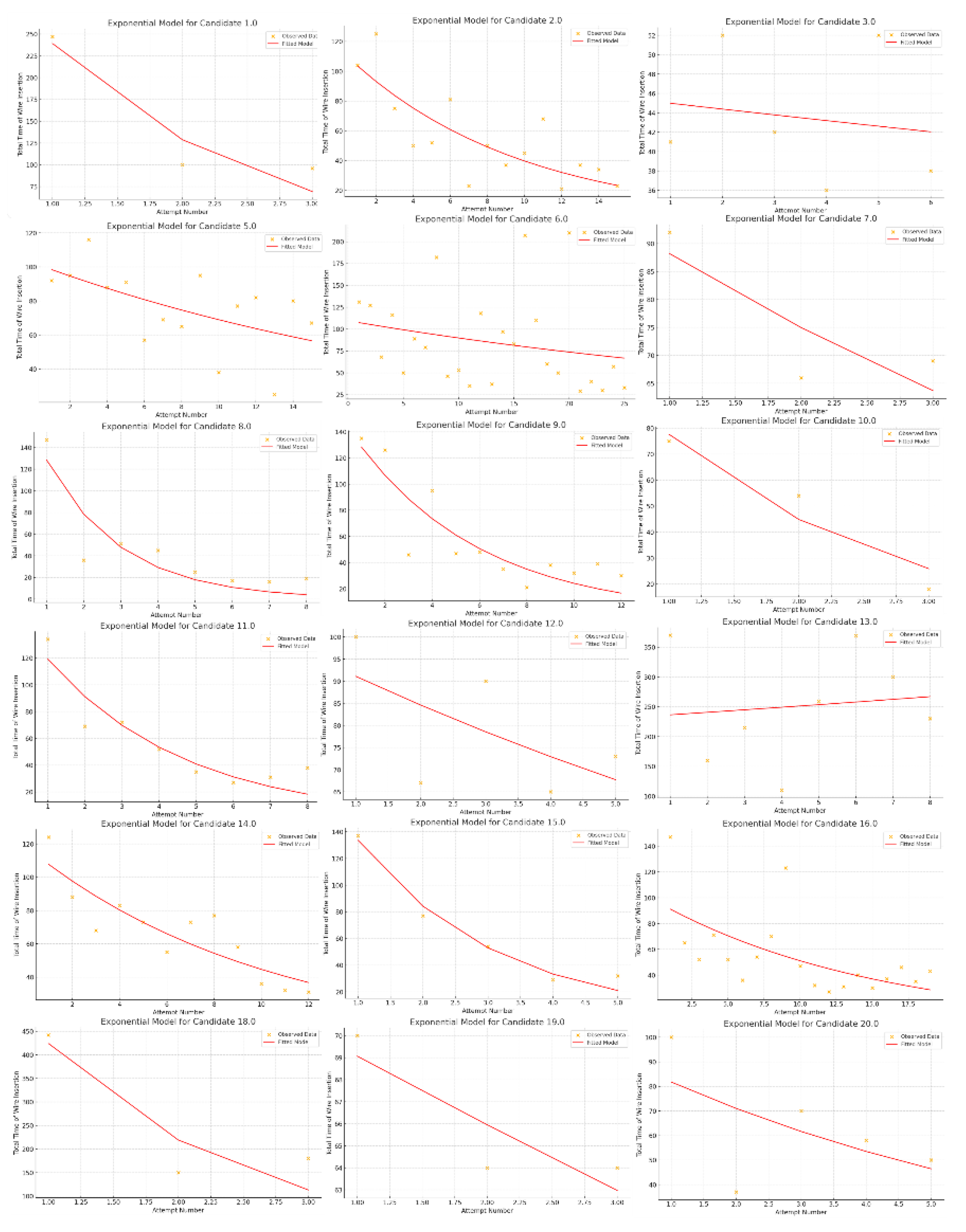
Overall, the total number of attempts recorded across all candidates was 158. The analysis revealed that the average time per attempt was 79,43 ± 63,85 seconds. The analysis showed significant variability among participants with an average insertion time from 257,3 seconds across three attempts to 43,5 seconds over six attempts. Also, the "Actual Initial Times", meant as time to perform the first K-wire insertion, varied significantly among the candidates, ranging from 41 to 442 seconds. Results of "Learning Rates (b)" analysis showed significant differences in the speed of skill acquisition; for example, candidates like 1 (b = 0,45), 10 (b = 0,50), 15 (b = 0,48), and 18 (b = 0,52) demonstrated rapid improvement, while Candidates 3 (b = 0,20), 5 (b = 0,22), 6 (b = 0,18), and 13 (b = 0,19) showed slower progress. Graphic representation of learning curve in wire insertion timing are represented in
Figure 2. Results regarding analysis of wire insertion times are summarized in
Table 2.
The analysis of X-ray usage also provided insights into learning patterns. Overall, for 158 total attempts, an average number of 14,41 ± 4,42 of X-rays per attempt was identified. Even in X-rays usage a significant variability was identified among the candidates with a minimum of 7,75 X-rays per attempt to a maximum of 27,67 X-rays per attempt. "Initial X-rays Actual" varied among candidates, serving as a baseline for assessing initial performance. Interestingly, the learning rate analysis of X-rays usage showed lower improvement among all repetition, demonstrating that despite a progressive reduction in K-wire timing positioning, some candidates did not reduce the amount of X-rays performed. Graphic representation of learning curve in X-rays usage is represented in
Figure 3. Lower AIC and BIC values for candidates with high learning rates further supported the model's adequacy. Results regarding analysis of X-rays usage are summarized in
Table 3.
Among all attempts in 61 cases the K-wire positioning was considered incorrect (2 mm Pedicle scale grade >2) with an overall accuracy across all candidates of 61,39%. Wire placement accuracy varied from a 100% of correct placement rate to rates below 40% of accuracy. The performance analysis showed that candidates with a 100% correct placement rate also demonstrated excellent performance in timing and X-rays usage, while candidates with accuracy below 40% may need additional practice. Repositioning attempts were also analyzed, providing an interesting correlation between candidate with high accuracy and lower number of total repositionings (
Table 4).
Correlations between pre-training questionnaire responses and learning variables (
Table 5) indicated that the year of residency was moderately negatively correlated with learning rates for both X-rays (-0,25) and time (-0,30), but positively correlated with correct placement percentage (0,22). Experience with spinal intraoperative navigation systems was negatively correlated with correct placement percentage (-0,25), but positively correlated with learning rates for X-rays (0,20) and time (0,22). The number of lumbar pedicle K-wire insertions performed as the First operator was positively correlated with learning rates for X-rays (0,18) and time (0,20), but negatively correlated with correct placement percentage (-0,22).
The post-training questionnaire results reflect a generally positive response from users (
Table 6). The model's perceived utility achieved a high average score of 4,55, with most participants assigning a rating of 5, indicating strong usefulness. However, perceived improvement during the test was rated at 4,25 on average, though the variability suggests differing perception on the degree of progress. Pointing perception of anatomical accuracy, the model received an average score of 3,95 highlighting some limitations. Overall, the simulator is viewed favorably, with a strong intent to use it in the future (average score of 4,55) and broad agreement on its integration into training programs (average score of 4,25), though enhancements in anatomical accuracy are needed.
4. Discussion
This study aimed to investigate the learning progress in spine surgery (lumbar pedicle K-wire insertion) using a virtual fluoroscopic simulator among neurosurgical residents and students. The findings reveal significant variability in performance across participants, underscoring the challenges inherent in surgical training and the potential of simulation-based methods to enhance skill acquisition.
One of the primary observations from this study is the considerable range in the number of attempts required for participants to reach a stable performance plateau. On average, participants needed 5.61 attempts to stabilize their performance, with notable differences between individuals. This variability highlights the individual differences in learning rates and suggests that a one-size-fits-all approach to surgical training may be suboptimal. Personalized training programs, tailored to the specific needs and learning paces of each trainee, could be more effective in ensuring that all residents reach the required level of proficiency. For example, by identifying trainees with less efficient learning curves—those who progress more slowly or require more repetitions—educators can tailor the training approach to provide additional practice and targeted feedback for these individuals. This could involve increasing the frequency of simulation sessions or offering supplementary resources to reinforce specific skills. Such a personalized strategy ensures that all trainees, regardless of their initial learning speed, receive the necessary support to achieve competency, ultimately leading to a more effective and efficient training process.
The study also sheds light on the relationship between experience and learning efficiency. Interestingly, more experienced residents did not necessarily achieve faster learning rates. In fact, a negative correlation was observed between the year of residency and the rate of improvement in both X-ray usage and wire insertion time. This finding may suggest that experienced residents, who are likely more accustomed to traditional techniques, might take longer to adapt to new methods or technologies introduced through simulation. However, their experience positively correlated with placement accuracy, indicating that while they may take longer to adjust, their overall skill level remains high. This dichotomy emphasizes the importance of integrating new technologies like virtual simulation early in training, allowing residents to become comfortable with these tools and incorporate them into their skill set.
The use of virtual X-rays during the simulation provided additional insights into the learning process. Participants who showed higher learning rates in reducing X-ray usage also tended to have greater accuracy in wire placement. This correlation suggests that as trainees become more proficient, they rely less on imaging guidance, reflecting increased confidence and precision in their manual skills. Conversely, participants who struggled to reduce their reliance on X-rays also faced challenges in improving their accuracy, indicating a need for targeted interventions to enhance their learning outcomes.
Another significant finding of this study is the variation in wire placement accuracy among participants. This variation could be attributed to differences in prior experience, familiarity with similar procedures, or the ability to adapt to the simulation environment. The lower accuracy rates suggest that additional practice and feedback are essential for these individuals to reach the desired level of competence. The study’s data on repositioning attempts further supports this conclusion, as participants with lower accuracy often required more repositioning, indicating difficulties in achieving the correct placement on the first attempt.
The study also revealed important correlations between pre-training questionnaire responses and performance outcomes. For example, prior experience with spinal intraoperative navigation systems was associated with faster learning rates in both X-ray usage and wire insertion time, though it did not always correlate with higher accuracy. This finding suggests that while familiarity with similar technologies can facilitate quicker adaptation to new tasks, it does not guarantee precision. Therefore, training programs should emphasize not only the speed of task completion but also the accuracy and safety of the procedures.
The post-training evaluations provided further evidence of the simulator's utility. Most participants reported increased confidence in their ability to perform lumbar pedicle K-wire insertion and in their use of fluoroscopy, indicating that the virtual simulation was effective in enhancing their skills. However, some participants noted limitations in the anatomical accuracy of the simulator, suggesting that while virtual simulation is a valuable tool, it may need to be complemented by other training methods, such as cadaveric dissections or augmented reality systems, to provide a more comprehensive learning experience.
The repeatability of virtual simulation presents a clear advantage in neurosurgical training. Unlike traditional methods, where opportunities for practice are often limited by availability and ethical concerns, virtual simulators allow for unlimited repetition of procedures. This capability is particularly important for mastering skills that require precise execution, such as lumbar pedicle K-wire insertion. By allowing trainees to repeatedly practice these skills in a controlled environment, virtual simulators help to reinforce learning, build confidence, and reduce the likelihood of errors in real surgical settings.
The limitations of this study include significant variability in individual learning rates among participants, reflecting natural differences in skill acquisition and potentially affecting the generalizability of the findings. The anatomical accuracy of the virtual simulator, while valuable for practice, was somewhat limited, which may impact the transferability of skills to actual surgical settings. The absence of a control group limits direct comparisons with traditional training methods, a choice made to focus on the simulation's potential. The prior experience of participants with conventional techniques may have influenced their adaptability to the new simulation technologies. Lastly, the study's modest sample size, though sufficient for initial insights, may not fully represent the broader population of neurosurgical residents.
5. Conclusions
In conclusion, this study highlights the value of virtual simulation in neurosurgical training and spine procedures. The results suggest that while there is significant variability in how quickly and accurately trainees learn, simulation-based training offers a powerful tool for improving surgical skills. The ability to repeatedly practice procedures, receive objective feedback, and track progress over time makes virtual simulators an essential component of modern surgical education. Future research should focus on refining these tools and exploring their long-term impact on surgical performance in clinical settings and identifying when and for whom they are more proficient.
Author Contributions
Conceptualization: F.C and E.M; methodology: E.M.; validation: F.R.; formal analysis: E.M and D.R.; investigation: A.F and V.C.; data curation: N.I.; writing—original draft preparation: E.M; writing—review and editing: F.C.; supervision: A.P and F.D.M.; project administration: F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stienen MN, Netuka D, Demetriades AK, et al. Neurosurgical resident education in Europe—results of a multinational survey. Acta Neurochir (Wien). 2016, 158, 3–15.
- Marcus H, Vakharia V, Kirkman MA, et al. Practice makes perfect? The role of simulation-based deliberate practice and script-based mental rehearsal in the acquisition and maintenance of operative neurosurgical skills. Neurosurgery. 2013, 72, A124–A130.
- Ericsson KA, Krampe RT, Tesch-Römer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993, 100, 363–406.
- Encinosa WE, Hellinger FJ. The Impact of Medical Errors on Ninety-Day Costs and Outcomes: An Examination of Surgical Patients. Health Serv Res. 2008, 43, 2067.
- Aggarwal R, Darzi A. Simulation to enhance patient safety: why aren’t we there yet? Chest. 2011, 140, 854–858.
- Issenberg SB, McGaghie WC, Petrusa ER, et al. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005, 27, 10–28.
- Ahlberg G, Enochsson L, Gallagher AG, et al. Proficiency-based virtual reality training significantly reduces the error rate for residents during their first 10 laparoscopic cholecystectomies. Am J Surg. 2007, 193, 797–804.
- Crochet P, Aggarwal R, Dubb SS, et al. Deliberate practice on a virtual reality laparoscopic simulator enhances the quality of surgical technical skills. Ann Surg. 2011, 253, 1216–1222.
- McGaghie WC, Issenberg SB, Cohen ER, et al. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011, 86, 706–711.
- Marcus HJ, Darzi A, Nandi D. Surgical Simulation to Evaluate Surgical Innovation: Preclinical Studies With MARTYN. 2015, 95, 299.
- Marcus HJ, Pratt P, Hughes-Hallett A, et al. Comparative effectiveness and safety of image guidance systems in neurosurgery: a preclinical randomized study. J Neurosurg. 2015, 123, 307–313.
- Tomlinson JE, Yiasemidou M, Watts AL, et al. Cadaveric Spinal Surgery Simulation: A Comparison of Cadaver Types. Glob Spine J. 2016, 6, 357.
- Sundar SJ, Healy AT, Kshettry VR, et al. A pilot study of the utility of a laboratory-based spinal fixation training program for neurosurgical residents. J Neurosurg Spine. 2016, 24, 850–856.
- Cadaver Dissection in Anatomy: The Ethical Aspect.
- Perin A, Galbiati TF, Gambatesa E, et al. Filling the gap between the OR and virtual simulation: a European study on a basic neurosurgical procedure. Acta Neurochir (Wien). 2018, 160, 2087–2097.
- Patel A, Koshy N, Ortega-Barnett J, et al. Neurosurgical tactile discrimination training with haptic-based virtual reality simulation. Neurol Res. 2014, 36, 1035–1039.
- Kirkman MA, Ahmed M, Albert AF, et al. The use of simulation in neurosurgical education and training: A systematic review. J Neurosurg. 2014, 121, 228–246.
- Davids J, Manivannan S, Darzi A, et al. Simulation for skills training in neurosurgery: a systematic review, meta-analysis, and analysis of progressive scholarly acceptance. Neurosurg Rev. 2021, 44, 1853–1867.
- Craven C, Baxter D, Cooke M, et al. Development of a modelled anatomical replica for training young neurosurgeons. Br J Neurosurg. 2014, 28, 707–712.
- Aoude AA, Fortin M, Figueiredo R, et al. Methods to determine pedicle screw placement accuracy in spine surgery: a systematic review. Eur Spine J. 2015, 24, 990–1004.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).