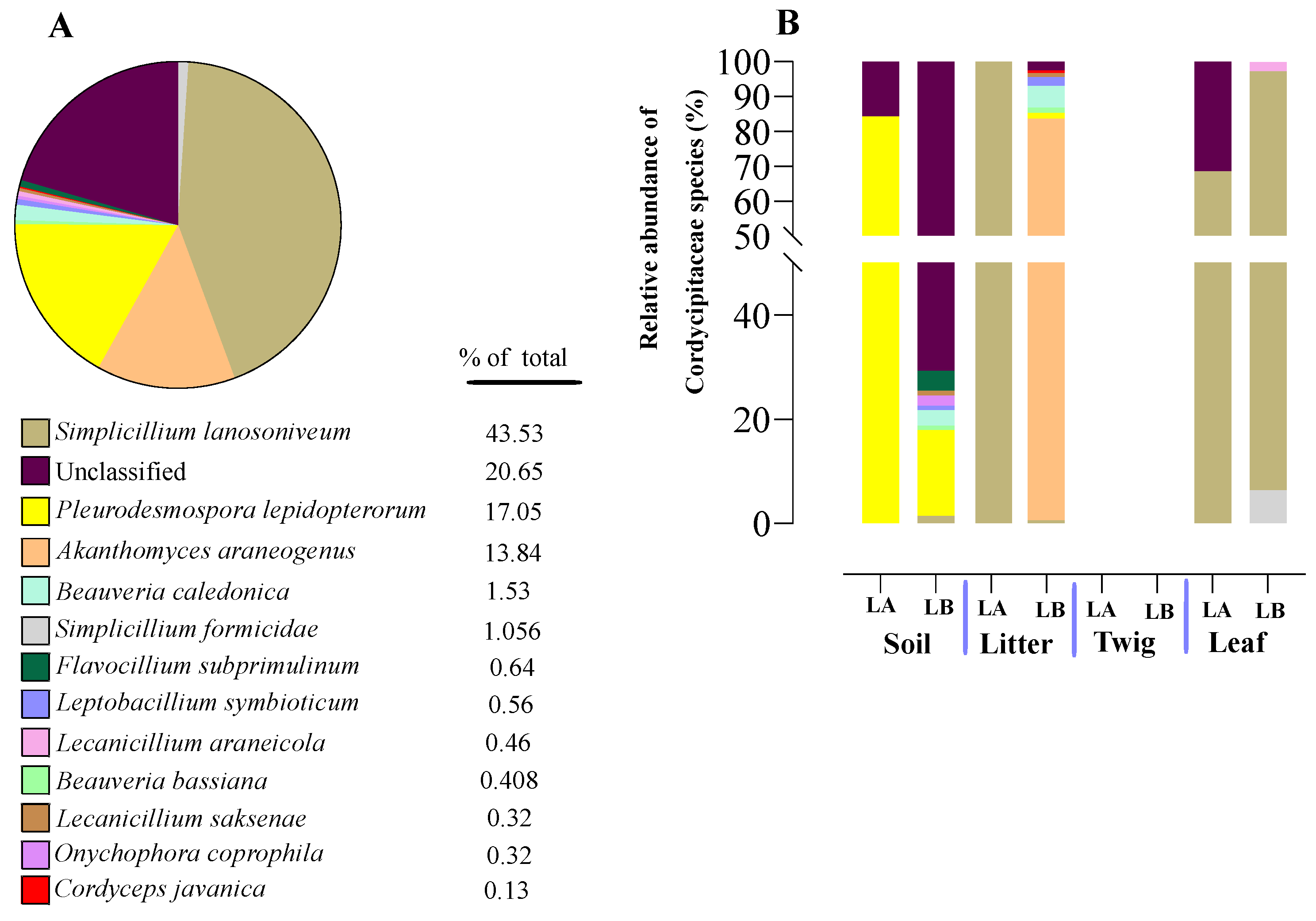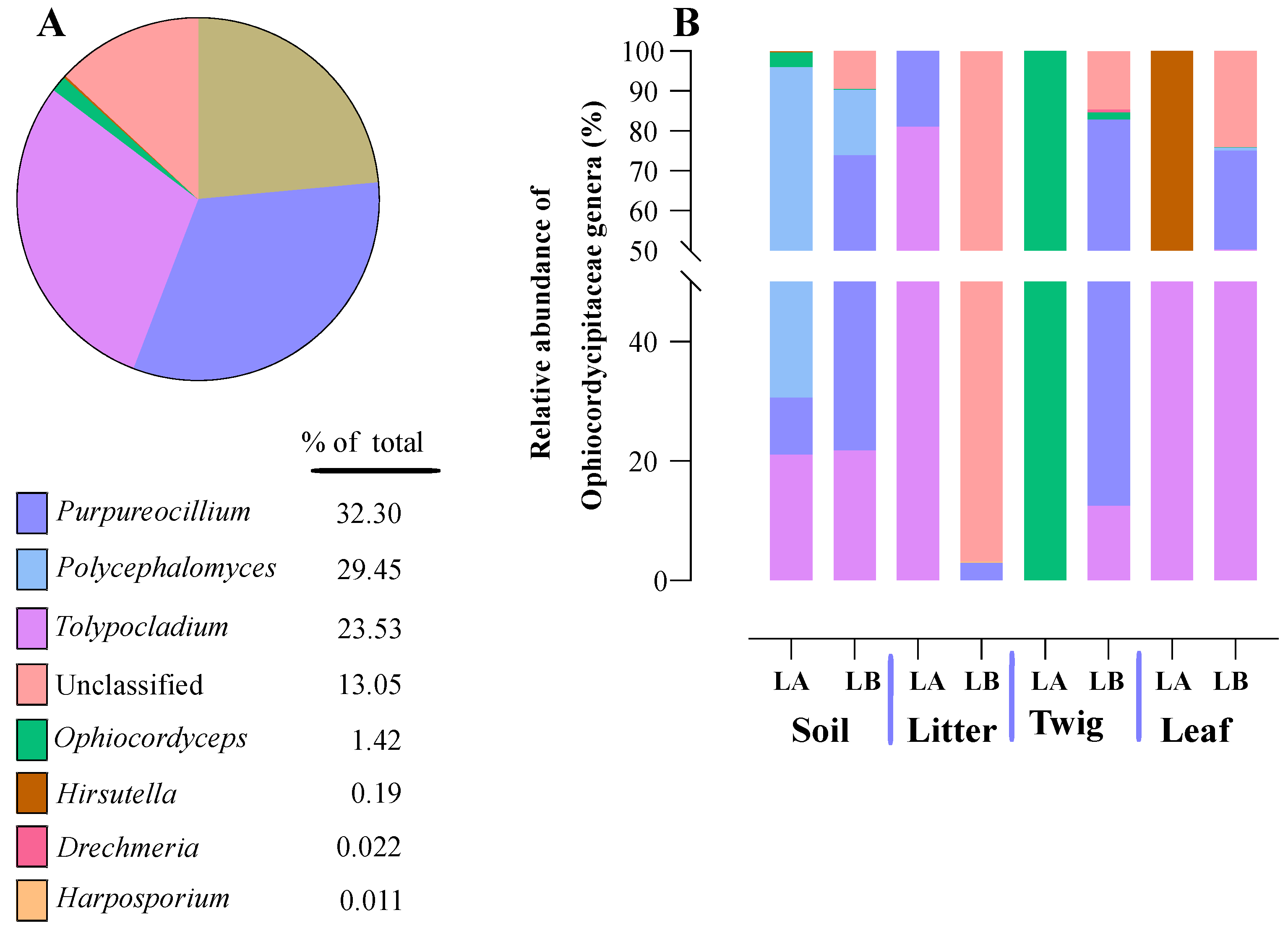1. Introduction
Spiders (Arachnida: Araneae) are highly diverse predatory arthropods characteristic for their use of silk and venom to capture and immobilize prey [
1,
2,
3]. Occupying a wide range of ecosystems, these organisms play critical ecological roles by feeding on species of insects, fish, reptiles, and even birds and mammals [
4,
5,
6]. Similar to insects, a major driver of spider mortality appears to be infection by entomopathogenic fungi, including species in the Cordycipitaceae, Ophiocordycipitaceae, and Clavicipitaceae families [
7,
8,
9,
10,
11]. Among spider-infecting fungi,
Gibellula (Cordycipitaceae), is a genus of specialized obligate spider-infecting pathogens with ~31 species described thus far, mainly from tropical and temperate environments [
12,
13,
14,
15,
16,
17,
18]. These fungi are often characterized by causing its spider hosts to attach themselves to the undersides of leaves during the infection process, where the fungus kills it, covering the cadaver in a thick layer of mycelia and sprouting synnemata which produce conidia that are released to “rain” down on new unsuspecting victims [
19]. Thus far,
Gibellula species have been described to infect over 15 different spider families, although identifying spider hosts can be challenging as the fungus envelops the spider body, obscuring morphological features of the infected organism [
13]. Despite the apparent global distribution of this genus, knowledge concerning the diversity of
Gibellula species in many geographic regions are lacking with most described species isolated from tropical regions of either Southeast Asia or South America [
13,
14,
15,
20,
21,
22,
23]. Indeed, to date, only two
Gibellula species have been reported from North America,
G. leiopus and
G. pulchra, primarily from infected spiders of the Trachelidae and Salticidae families [
13,
24].
Aside from a lack of lack of knowledge concerning
Gibellula species diversity in many areas of the world, almost nothing is known concerning their environmental prevalence and/or the fungal community dynamics surrounding infection sites. Some genera of entomopathogenic fungi, particularly generalist facultative fungi of the Cordycipitaceae and Clavicipitaceae families, such as
Beauveria and
Metarhizium sp. which have broad host ranges readily grow in vitro on mycological media, and can be found outside of their insect hosts in environmental reservoirs such as soil and/or can be associated in the plant rhizosphere or even as plant epi-/endophytes [
25,
26,
27,
28]. In contrast, specialized insect pathogenic fungi of the order Entomophthorales, e.g.,
Massospora cicadina,
Arthrophaga myriapodina, and
Entomophthora muscae, are obligate insect pathogens and are not readily culturable (if at all) in vitro [
29,
30,
31]. Some of these latter fungi, e.g.,
Massospora sp., produce resting spores that can persist in the soil [
32,
33,
34].
Gibellula sp. present an in between phenotype, where they can be cultured
in vitro, i.e., can grow saprophytically, but have narrow, specialized host ranges. Furthermore, though poorly examined, Gibellula infection apparently includes some level of manipulation of host behavior, as part of its extended phenotype is to cause their hosts to become fixed to the underside of leaves and other plant material, often at an elevated position above the ground [
35,
36]. This presumably facilitates the spread spores onto the surrounding area in order to infect other hosts. However, to our knowledge, to date there are no reports attempting to detect
Gibellula outside of their spider hosts and/or to examine the fungal community surrounding infected hosts. Such studies are important as almost nothing is known concerning any potential reservoir for host-specific insect fungal pathogens and/or the fungal community dynamics that may affect infection, persistence, and/or transmission/spread of the fungus.
In the present study, we found the cadavers of five fungal infected spiders in two forested locations surrounding the University of Florida in Gainesville, Florida: Lake Alice Natural Area and Loblolly Woods Nature Park. Based on visible morphological characteristics, all spider hosts appeared to be of a single genus, Trachelas, and all were found to have fixed their bodies in similar positions and on similar substrates; to bark on the undersides of tree branches and trunks one to two meters above the ground. These specimens were collected, and cultures were obtained from three of the five cadavers. Through phylogenetic characterization of cultured isolates using ITS, LSU, SSU, and TEF1a loci as well as morphological characterization, one isolate was confirmed as a new Gibellula species, herein described as Gibellula floridensis and the other two isolates as Parengyodontium album (Cordycipitaceae). The morphology of these isolates was characterized on different growth media and the characters of the Gibellula isolate are described. Gibellula floridensis is most closely related to G. leiopus, but differs by the appearance of their synnemata, as well as the length of their conidiophores, metulae, and conidia. To determine the presence and prevalence of these isolates outside of their spider host, a systematic survey of the fungal communities of samples derived from four trophic levels, namely, soil, leaf litter, leaves, and twigs, from areas surrounding the location of the spider cadavers was performed via amplicon sequencing of the ITS1 region. These areas were found to contain high abundances and diversity of entomopathogenic fungi, particularly in soil samples, with differential distribution between the trophic levels examined. However, sequences corresponding to the Gibellula or Parengyodontium isolated in our survey, nor, surprisingly any other Gibellula or Parengyodontium species were detected in these samples. Potential explanations for failure to detect the characterized isolates are discussed. This study adds to the known diversity of Gibellula species in North America and opens questions of the presence, prevalence, and distribution of Gibellula and other entomopathogenic fungi outside of their arthropod hosts.
2. Materials and Methods
Infected Spider Location and Collection
Spiders infected by fungal pathogens were located and collected from the Lake Alice and Loblolly natural areas in Gainesville, Florida, United States over the course of several months between March and August 2023. During each collection, the trunks, branches, twigs, and leaves of trees were carefully examined for the presence of spider cadavers. Once located, cadavers were photographed in situ prior to removal. Cadavers were collected from locations on tree trunks and branches using a knife to remove a small amount of the bark to which the spiders had become firmly attached. Collected samples were stored in Falcon tubes or sealed Petri dishes until they could be returned to the lab for further investigation. Once in the lab, samples were stored in a cool dark environment for further photographic documentation and culturing of the infecting fungi.
Environmental Sampling
Soil samples were collected at the locations shown (
Supplementary Figure S1), and the soil sampling sites were assigned coordinates via the Global Positioning System (GPS). The two areas sampled where infected spiders were found were: (1) Lake Alice, (LA) (29° 38′ 29.99′′ N, -82° 21′ 23.99 ′′ W) and (2) Loblolly Woods Nature Park (LB) (42°14′.084′′ N, 117°08′.124′′ E) both in Gainesville, Florida. The LA region was treated as a single site, from which 3 soil, 3 plant litter, 2 twig, and 2 leaf samples were collected. The LB region was divided into four sites, from which three soil and three plant litter samples were collected from each site, along with one twig and one leaf sample per site. From the LB region, a total of 12 soil samples, 12 plant litter samples, 4 twig samples, and 4 leaf samples were collected. In total, 42 samples were collected from both regions. Sample collection followed a standard protocol [
26]. Briefly, at each location, soil sampling involved marking out five transects spaced ~100 meters apart. Along each line, the soil was sampled at five points, each 10 meters apart, starting 50 meters from the beginning of the line. Samples were taken from the topsoil down to a depth of 30–50 cm and had a diameter of 30 cm. These subsamples were collected using clean shovels and combined in a plastic bag to create a single mixed sample per line. Efforts were made to remove large roots and gravel bigger than 0.25 inches from the samples. The shovels were rinsed with 70% alcohol and dried before moving to the next location. Subsamples of the mixed soil were then packed into individually labeled zip-lock bags (10 × 12 cm), and for each site, five of these bagged samples were stored in a large, sealable polyethylene bag and kept cool in a cooler. The schematic for the soil sampling is presented in
Supplementary Figure S1.
For isolation of the microbial communities, samples from the plant litter, leaves, and twigs were entirely submerged in 500 ml distilled water and agitated for three hours at 140 revolutions per minute (rpm) to solubilize the microbial communities from the environmental sample surfaces into the water. Suspensions were filtered through a 0.22 μm pore size mixed cellulose esters (MCE) membrane filter (MF-Millipore™, Tullagreen, Carrigtwohill, Ireland) to collect the microbial community. Filters were immediately frozen and stored at –80 °C.
DNA Extraction, PCR, and Sequencing
To extract DNA from cultured isolates, a small plug of actively growing hyphae at the edge of a culture plate was removed and placed into a flask containing 50 mL of potato dextrose broth (PDB) to initiate liquid cultures. Liquid cultures were grown at 25°C with 250 rpm orbital shaking for 7 d, at which point small balls of hyphae could be noted at the bottom of the culture medium. Hyphae was separated from culture medium by filtration through a 0.7 μm filter and washed with sterile distilled water to remove any residual culture medium. Filtered hyphae was then collected into 2 ml tubes along with a sterile glass bead and lyophilized overnight until all water had been removed from the tissues. Lyophilized tissue was powdered by bead beating using an MP FastPrep-24 (MP Biomedicals, Irvine, California) at 4.0 m/s for 60 s. DNA was extracted from powdered tissues using the Norgen Biotek Plant/Fungi DNA Isolation Kit (Norgen Biotek, Ontario, Canada) according to manufacturer’s instructions. Concentration and purity of extracted DNA was assessed using an NP80 nanophotometer (Implemen, Munich, Germany). To amplify loci for downstream sequencing and phylogenetic analysis, the primer sets ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′)/ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (ITS region), LROR-F (5′-ACCCGCTGAACTTAAGC-3′) /LR5-R (5′-TCCTGA GGGAAACTTCG-3′) (LSU region), NS1-F (5′-GTAGTCATATGCTTGTCTC-3′)/NS4-R (5′-CTTCCGTCAATTCCTTTAAG-3′) (SSU region), and EF1-728F (5′-CATCGAGAAGTTCGAGAAGG-3′)/TEF1LLErev (5′-AACTTGCAGGCAATGTGG-3′) (TEF1a region) were used in conjunction with taq 5x master mix (New England Biosciences, Ipswich, Massachusetts) according to manufacturer instructions. The success of all polymerase chain reactions (PCRs) were verified by gel electrophoresis to visually confirm the presence of a single amplified band. PCR products were purified using GeneJET PCR Purification Kit (Thermo Scientific, Waltham, Massachusetts) according to manufacturer instructions and the concentration was verified by nanophotometer as above prior to sending samples for sequencing. PCR products were sequenced using Oxford Nanopore sequencing technology (Plasmidsaurus, Eugene, Oregon). Consensus sequences received from nanopore sequencing results were assessed by BLAST searching and examining CDS features to determine sequence quality, strandedness, and the presence of introns. Sequences for each isolate were deposited in Genbank under the following accession numbers; UFSI_3: PP915745 (ITS), PP915999 (LSU), PP916007 (SSU); UFSI_4: PP915746 (ITS), PP915746 (LSU), PP916008 (SSU); UFSI_5: PP915747 (ITS), PP916001 (LSU), PP916009 (SSU), PP938454 (TEF1a).
For environmental samples (soil, leaves, leaf litter, and twigs), total DNA from soil and those from samples collected on 0.22 μm filters (as above) were extracted using the DNeasy® PowerSoil® Pro kit (Qiagen, GmbH, Germany) or DNeasy® PowerWater® kit (Qiagen, GmbH, Germany), respectively, according to the manufacturer’s instructions. Genomic DNA purity and integrity were determined by using NanoPhotometer® NP80 (IMPLEN, Munich, Germany). DNA was diluted to 1 ng/μl using sterile Mili-Q ddH2O, based on the concentration. Amplification of the DNA samples was performed using ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2R (5′-GCTGCGTTCTTCATCGATGC-3′) primers, which target ~500 bp of the fungal rRNA gene internal transcribed spacer (ITS) region. PCR cycling conditions were: 94°C for 1 min as initial denaturation, 35 cycles of 94°C for 30 s, 52°C for 30 s, and 68°C for 30 s, and a 10 min incubation at 68°C. The PCR products were purified using GeneJET™ Gel Extraction Kit (Thermo Fisher Scientific, Waltman, MA, United States) and quantified by Nanodrop 2000 (Thermo Fisher Scientific). Amplicon sequencing was performed at the University of Illinois at Chicago (UIC) genome research core for next-generation Illumina Miseq sequencing (paired-ended 2x300 base sequencing reads). The raw sequences produced in the current study can be accessed through the National Center for Biotechnology (NCBI) under BioProject PRJNA1119211, accession SUB14498584.
Phylogenetic Analysis
Phylogenetic analyses of multiple loci were carried out in order to determine the taxonomic identity of the three cultured isolates from spider cadavers. Sequence data generated from loci from the cultured isolates, as well as sequences obtained from Genbank encompassing representative genera in the family Cordycipitaceae, and the outgroup genus
Trichoderma, were organized such that sequences for each locus were grouped together into lists of FASTA sequences. Multiple sequence alignments were generated for sequences of each locus individually using the program MAFFT [
37] with default parameters set. Following alignment, sequences were trimmed using the program GBLOCKS [
38] with a minimum block length of 3. Following trimming, sequences from the different loci were concatenated using the concat function in the Seqkit program [
39]. Concatenated sequences were used to generate a phylogeny using the program RAxML [
40] with four partitions in the dataset, GTRGAMMA as the model, a rapid bootstrap random number seed of 6669, a parsimony random seed of 2539, and 2000 bootstrap replicates. The hypocrealean fungus,
Trichoderma harzianum, was used as an outgroup for this tree. To determine how distinct isolate UFSI_5 was from other sequenced
Gibellula isolates, a second tree was generated using the UFSI_5 ITS sequence and ITS sequences from all available
Gibellula isolates deposited in genbank. For this tree, sequences were aligned in MAFFT, with no trimming with GBLOCKS to prevent the removal of excessive sequence from the alignment. RAxML was performed as above but without any partitioning of the single locus. For this tree,
Metarrhizium anisopliae was used as an outgroup. Both trees were visualized using FigTree [
41]. Bootstrap values greater than 70 are displayed on the nodes.
Amplicon Sequence Variants (ASVs) Cluster and Sequence Analyses
Sequences were provided by UIC as fastq files, which were processed through the DADA2 pipeline in the DADA2 R package to assign reads to ASVs using the Majorbio Cloud platform (
https://cloud.majorbio.com/) for high-throughput omics data [
42]. This process includes filtering and trimming reads with quality scores < 30, constructing an ASV table, removing chimeras, and assigning taxonomy [
43]. ASVs were assigned a taxonomic group using the curated fungal database (UNITE) (
https://unite.ut.ee/) [
44], and any ASV with fewer than 2 occurrences was removed [
45]. The sampling effectiveness was evaluated with a rarefaction analysis of data subsets using the rank abundance curve (
Supplementary Figure S2). Since entomopathogenic fungi (EFs) were considered in this study, only three families of Hypocreales order and those with the potential of being specific spider pathogens were chosen to generate the abundance plots, and the rest of the sequences were removed.
Microscopy and Whole Organism Photography
Following collection, whole infected spiders were imaged using a Zeiss Stemi 305 dissecting microscope and a Keyence VHX digital microscope. On the Keyence VHX digital microscope, automated focus stacking was performed to optimal focus and resolution across the entire surface of spider cadavers. This microscope was also used to image colony growth on petri dishes. For higher magnification imaging, a Zeiss LSM 800 confocal microscope, a Motic BA310E with a Moticam X3 camera attached, and a Keyence BZX 800 were used. To image conidiophores, spores, and hyphae from infected spiders, tissue was carefully removed using a scalpel and forceps and placed onto a drop of water on a microscope slide. A small amount of lactophenol cotton blue was then added to this drop and the tissue was allowed to sit in the stain for one minute, after which time the liquid was wicked away using a Kim Wipe and a fresh drop of water was added. A cover slip was then added and the sample was imaged for identifying features using a Keyence BZX 800 and Motic BA310E. To image morphological features from cultures, a small amount of tissue was removed from plates and wet mounts were prepared as above, but without lactophenol blue staining. Tissue samples were imaged using a Zeiss LSM 800 confocal microscope to image features such as spores, conidiophores, and hyphae. Morphological features were measured using ImageJ.js [
46].
4. Discussion
Thus far, only a relatively small number of spider (Araneae) fungal pathogens have been characterized relative to their insect cousins. Most spider pathogenic fungi characterized to data are found in the Ascomycota, Hypocreales order [
8]. A few generalist,
i.e., broad host range, insect fungal pathogens from several genera, e.g.,
Beauveria,
Isaria,
Hirsutella, have described species isolated from infected spiders, although the extent to which these represent specialized spider pathogens remains unclear. Members of the
Akanthomyces genera are typically generalists, however,
A. araneogenus, appears to be spider specific. The most widely characterized apparent more specific Araneae fungal pathogens are found in the
Gibellula (sexual morph
Torrubiella) genera of which up to 60 species (~30
Gibellula, ~28
Torrubiella) have been described capable of infecting at least 25 different spider families.
Here, we identified five spider cadavers apparently infected with fungi in an area of north central Florida. This region’s climate is defined as humid subtropical, with tropical-like summers, warm to hot shoulder seasons, and mild winters. The area around the sampled regions includes a range of wetlands and aquatic vegetation; grasses, mosses, and other moisture-loving plants, as well as live oaks and palm trees (Lake Alice). The plant canopy in the Loblolly state park zone is mixed hardwood, including loblolly pine (Pinus taeda), slash pine (Pinus elliottii), laurel oak (Quercus laurifolia), water oak (Quercus nigra), red maple (Acer rubrum), Carolina laurel cherry (Prunus caroliniana), sweetgum (Liquidambar styraciflua), wax myrtle (Morella cerifera), swamp tupelo (Nyssa biflora), and sugarberry (Celtis laevigata). Based on morphological features and consistent with their range/habitats, the spiders appeared to belong to the genus Trachelas. Spider cadavers were found fixed to bark on the underside of branches and trunks, facing downwards. This is consistent with reports of infection mechanisms of above ground spiders, which normally forage or live close to the ground or soil, by fungal pathogens which result in summit disease, i.e., elevation seeking/negative gravitropism, as the spider host dies. Such behavioral manipulation of the host is considered to maximize spore discharge from the dead spider from heights that would allow for dispersal of infectious spores over a wide area to hosts found in lower trophic levels.
Of the five fungal infected spider cadavers collected, and all had preliminary morphological features consistent with
Gibellula, however, fungi could only be cultured from three. It is possible that either the fungi on the two specimen from which we could not recovered active fungi were either no longer viable or else fastidious and could not be readily cultured. Of the three fungal isolates that could be cultured and were subsequently single spore purified, two were found to likely represent the same species, and closely matched
Parengyodontium album NRRL28022 (LSU/SSU 100% match UFSI_3, 98.28% match UFSI_4) and
Parengyodontium album UTHSCSA_R-4523 (ITS/LSU 99.70% match UFSI_3 and UFSI_4) . The third grouped within
Gibellula, with closest match to
G. leiopus EBSL13 (80% query cover, 93.54% identity) and what has only been listed as
Gibellula sp. CA02 (ITS, 97% query cover 91.64% identity), albeit both multilocus and greater depth ITS phylogenetic reconstruction indicated the isolate to be distinct. We therefore describe this as a new species, namely,
G. floridensis based on combined morphological (Supplementary table S3) and multilocus molecular phylogenetic characterization. To the best of our knowledge, this is the first description of
Gibellula from Florida and the broader southeastern United States. Members of the
Gibellula genera are well known pathogens of a range of spiders [
13,
14,
15,
20], and
Gibellula leiopus/
Gibellula sp. have been found infecting members of the
Anyphaenidae,
Araneidae,
Corinnidae,
Linyphiidae,
Pholcidae,
Salticidae,
Sparassidae,
Theridiidae,
Thomisidae, and
Zodariidae families, but not
Trachelidae as reported here. For the
Gibellula sp. isolate that grouped most closely with our isolate, this was found in California and the host spider identity was not listed [
36,
48], and hese regions are significantly separated from the southeastern US. In addition, multilocus data for
G. leiopus separated it from
G. floridensis further than what was seen in the ITS tree, indicating our isolate remains distinct at both the molecular and morphological levels.
P. album has a curious distribution and reporting. Originally,
Tritirachium album, then
Beauveria alba,
Engyodontium album, it now has its own genera (
Parengyodontium) with
P. album as its sole described species, although it is unclear the extent to which various
P. album isolates have been confirmed via molecular phylogenetic analyses. It is reported to be globally distributed, including from geographically diverse deteriorated (cultural heritage) sites, potentially adapted to salty, moist environments, as well as being an opportunistic mammalian pathogen [
49,
50,
51,
52,
53,
54,
55,
56,
57]. An isolate found on floating plastic debris in the North Pacific Subtropical Gyre has been shown to be able to mineralize plastic [
50,
58],.
P. album has been isolated from spider cadavers [
59,
60,
61], but it has not been definitively proven to be a pathogen and not a saprotroph or secondary infection in these cases. Similarly, we cannot make any definitive conclusions concerning whether our isolate is a spider pathogen, and the fact that we isolated
P. album by performing single spore isolation from the synnemata and conidiophores from two of the collected spiders was surprising but might point to some kind of secondary association between
P. album and spider cadavers, or even
P. album and
Gibellula species. Additional sampling and/or infection assays are needed to examine this point and/or complete Koch’s postulate.
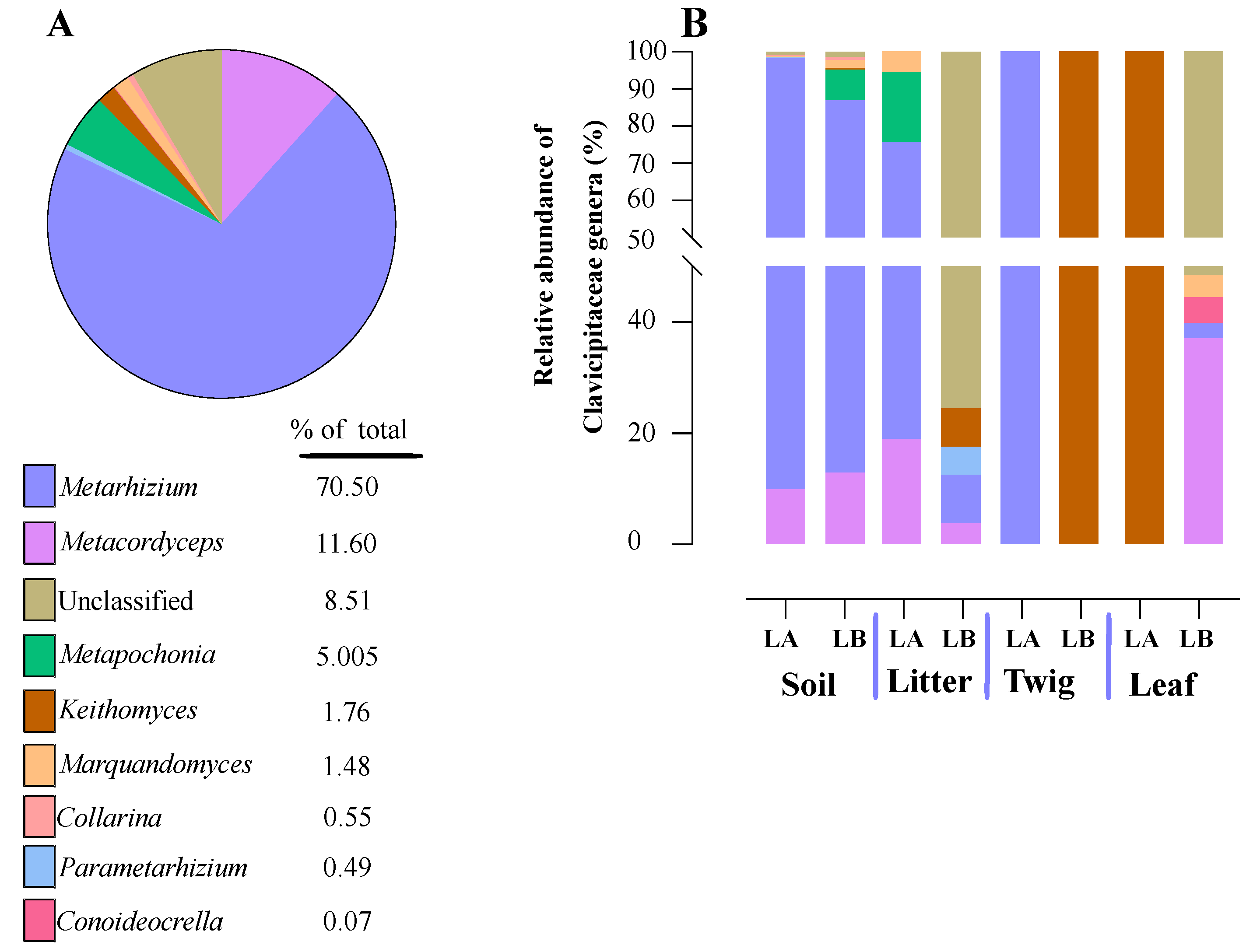
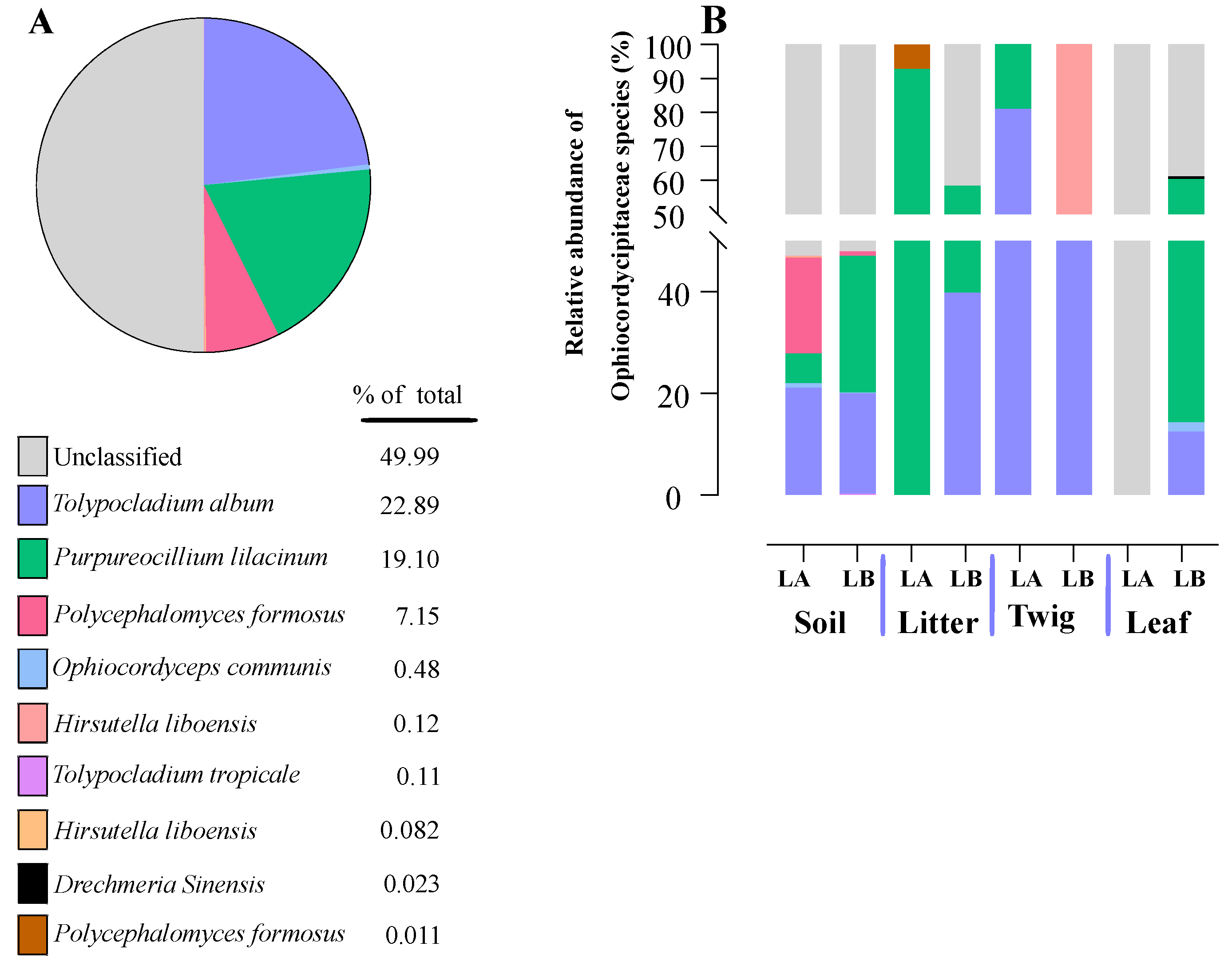
An open question concerning facultative, yet specialized insect/arachnid fungal pathogens concerns their environmental distribution (aside from on the host), and at which trophic levels in the environment they can be found. To address this, we performed a systematic sampling of the areas surrounding the spider cadavers, separating our collection into four trophic levels, namely: soil, leaf litter, leaves, and twigs. Sampling was performed following a grid like pattern that extended the scope to an area of ~10x10 m, with multiple sample points for each level that were pooled and subjected to genomic DNA extraction and subsequent amplicon ITS sequencing. Entomopathogenic fungi were found to represent ~ 1.92% of the total reads generated, and within the three major families, Clavicipitaceae predominated (64%), followed by Ophiocordyceps (29%) and Cordycipitaceae (7%). Important differences between trophic levels were seen, with soil showing the highest diversity, followed by some variation between twigs, leaves, and leaf litter as well as site (Lake Alice vs Loblolly nature park). Of note, Cordycipitaceae was found to represent a greater proportion than the others at the leaf trophic level, whereas Clavicipitaceae predominated at the soil and twig levels. For leaf litter, Ophiocordycipitaceae predominated at the Lake Alice site, whereas Cordycipitaceae was found more prevalent in the leaf litter of the Loblolly site. Important differences were also seen at the genera level across the trophic levels and sites, with Akanthomyces, Metarhizium, Purpueocillium, Tolypocladium, and Polycephalomyces highly abundant. At the species level: Simplicillium lanosoniveum, Pleurodesmonspora lepiopterorum, and A. araneogenus (Cordycipitaceae), Metarhizium indigoticum and Metacordyceps chlamydosporia (Clavicipitaceae), and Tolypocladium album, Purpueocillium lilacinum, and Polycephalus formosus could be identified as highly prevalent. However, it should be noted that a significant portion (9-20%) of our ITS dataset, while categorized at the family level, could not be placed in any described genera, and of those that could be placed into genera, 15-50% could not be assigned at the species level. This indicates significant room for discovery and addition to available databases.
Surprisingly, despite obtaining >1.5 million ITS reads, direct BLAST analysis with the ITS sequences obtained from
G. floridensis and
P. album, no hits were obtained. Furthermore, on a broader level, we could not detect any
Gibellula or
Parengyodontium even at the genera level in our dataset. There are several potential explanations for these results. First, it cannot be excluded that we did not sample enough, despite examining both areas where the spider cadavers were found and looking at the surrounding soil, leaf litter, leaves, and twigs. Second, our collection excluded insects or other animals in the samples. In tropical areas where spiders are active year-round, it is possible for direct spider to spider transmission of
Gibellula to account for the full life cycle of this fungus. However in more temperate regions where many species of spider and other arthropod go dormant during the winter, other strategies may be necessary in order to survive this period, including potentially reducing pathogenicity in order to overwinter with infected individuals or surviving outside of their host in environmental reservoirs [
62]. As Florida is subtropical, the latter condition may prevail, in which case
Gibellula may be at below the detection limit of our sampling. Third, persistence/survival of
Gibellula or
Parengyodontium in the sampled environments may be low. As the cadavers were found after full infection and likely dispersal of any spores, if persistence is low (days to weeks time-frame), the fungi could have been gone by the time we sampled the surrounding environment. We could however, detect two fungal spider pathogens,
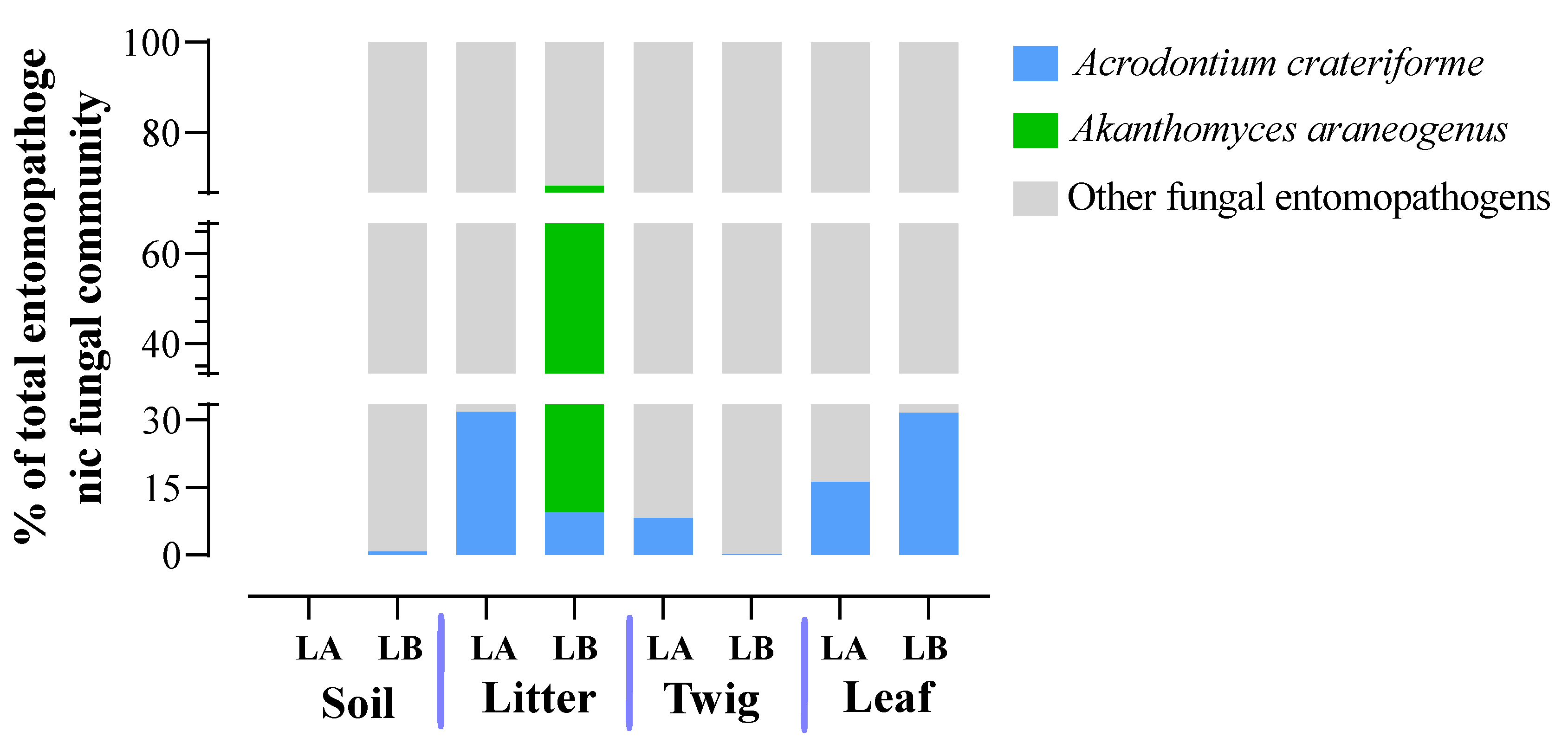
A. crateriforme and
A. araneogenus [
47]. The latter was found only in the plant litter at the Loblolly but at a high proportion (59%) of the total entomopathogenic fungal community.
A. crateriforme was widely distributed in all environmental habitats examined except for Lake Alice soil. Wed did not detect any other major spider fungal pathogens described in North America [
47]. These data indicate the prevalence of a small subset of specific fungal spider pathogens in the environment examined. However, some caution should be taken in interpreting any results, and as more species are described and the (ITS) database expands, additional members may be identified.
Figure 1.
In situ photographs of spider cadavers. (A) Spider cadaver S1. (B) Spider cadaver S2. (C) Spider cadaver S3. (D) Spider cadaver S4. (E) Spider cadaver S5. Cultivatable fungi were able to be isolated and single spore purified from cadavers S3-S5 (D-E).
Figure 1.
In situ photographs of spider cadavers. (A) Spider cadaver S1. (B) Spider cadaver S2. (C) Spider cadaver S3. (D) Spider cadaver S4. (E) Spider cadaver S5. Cultivatable fungi were able to be isolated and single spore purified from cadavers S3-S5 (D-E).
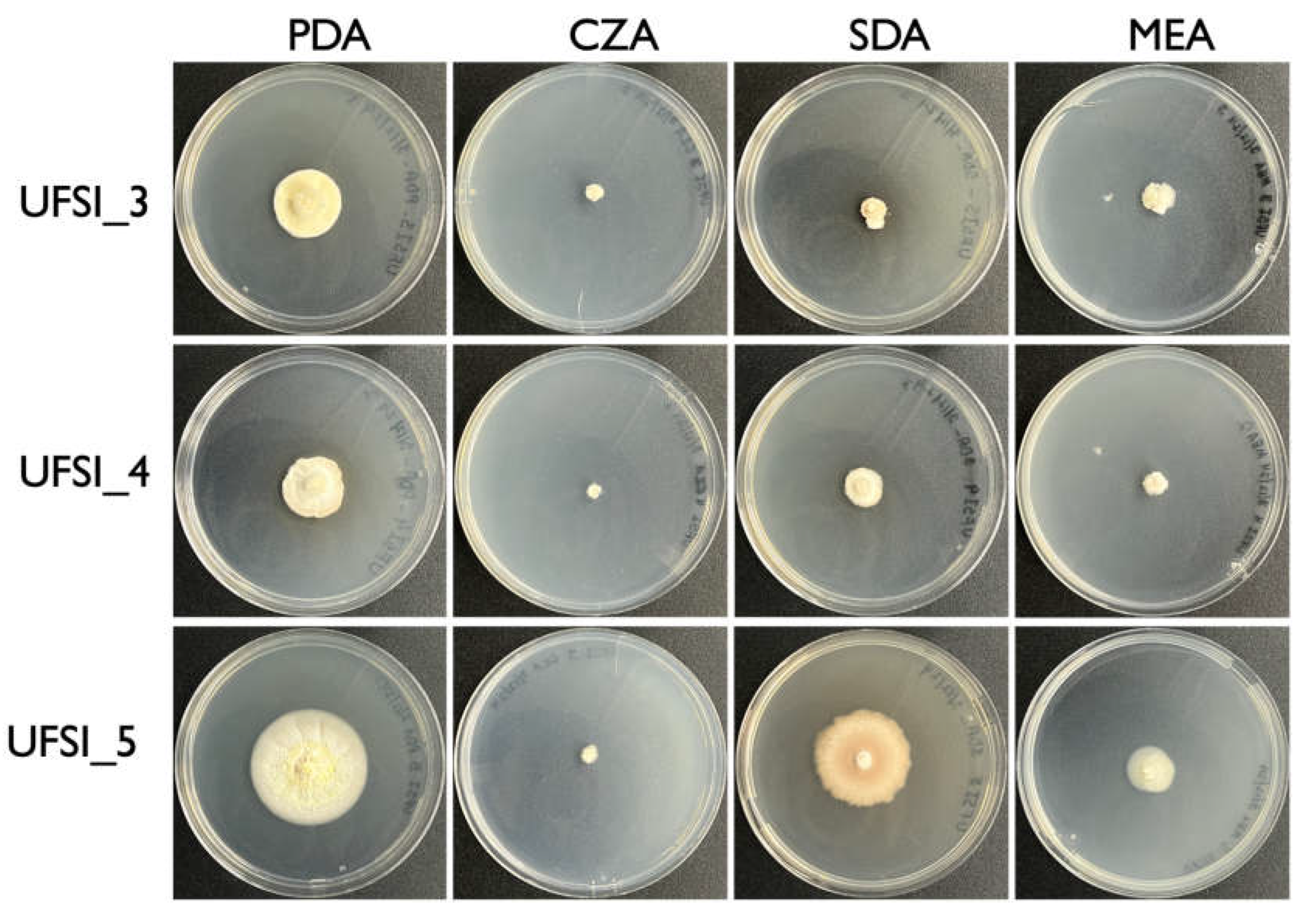
Figure 2.
Colony morphology of fungal isolates (UFSI_3, UFSI_4, and UFSI_5) grown on potato dextrose agar (PDA), Czapek-Dox agar (CZA), malt extract agar (MEA), and Sabouraud dextrose agar (SDA) for 30 d at 25oC.
Figure 2.
Colony morphology of fungal isolates (UFSI_3, UFSI_4, and UFSI_5) grown on potato dextrose agar (PDA), Czapek-Dox agar (CZA), malt extract agar (MEA), and Sabouraud dextrose agar (SDA) for 30 d at 25oC.
Figure 3.
Macroscopic and microscopic morphological characters of Gibellula isolate on spider cadavers and in culture. (A-B) Appearance of spiders (assigned to Trachelas) infected by fungal (Gibellula) isolates S4 and S5, respectively. (C) Synnemata emerging from infected spider cadavers. (D) Synnemata head showing purple-pigmented conidiophores. (E) Lactophenol blue staining of conidiophore from synnemata showing aspergillus-like morphology. (F) Close up of conidiophore head displaying vesicle, metulae, phialides, and conidia. (G) Lactophenol blue stained Granulomanus-like asexual morph conidiophore. (H) Conidia originating from aspergillus-like asexual morph conidiophores. (I) Front and back appearance of Gibellula isolate in culture on PDA. (J) Gibellula isolate in culture on PDA showing synnema. (K) Closeup of synnema produced in culture showing purple-pigmented conidiophores forming at the end. (L) Conidiophore forming on synnema produced in culture. (M) Closeup of conidiophore produced on synnema in culture. (N) Possible Granulomanus-like conidiophore produced in culture. Scale bars in images are as follows: (A-B) 500µm, (C) 200µm, (D) 200µm, (E) 25µm, (F) 10µm, (G) 25µm, (H) 5µm, (J) 2000µm, (K) 200µm, (L) 20µm, (M) 5µm, (N) 5µm.
Figure 3.
Macroscopic and microscopic morphological characters of Gibellula isolate on spider cadavers and in culture. (A-B) Appearance of spiders (assigned to Trachelas) infected by fungal (Gibellula) isolates S4 and S5, respectively. (C) Synnemata emerging from infected spider cadavers. (D) Synnemata head showing purple-pigmented conidiophores. (E) Lactophenol blue staining of conidiophore from synnemata showing aspergillus-like morphology. (F) Close up of conidiophore head displaying vesicle, metulae, phialides, and conidia. (G) Lactophenol blue stained Granulomanus-like asexual morph conidiophore. (H) Conidia originating from aspergillus-like asexual morph conidiophores. (I) Front and back appearance of Gibellula isolate in culture on PDA. (J) Gibellula isolate in culture on PDA showing synnema. (K) Closeup of synnema produced in culture showing purple-pigmented conidiophores forming at the end. (L) Conidiophore forming on synnema produced in culture. (M) Closeup of conidiophore produced on synnema in culture. (N) Possible Granulomanus-like conidiophore produced in culture. Scale bars in images are as follows: (A-B) 500µm, (C) 200µm, (D) 200µm, (E) 25µm, (F) 10µm, (G) 25µm, (H) 5µm, (J) 2000µm, (K) 200µm, (L) 20µm, (M) 5µm, (N) 5µm.
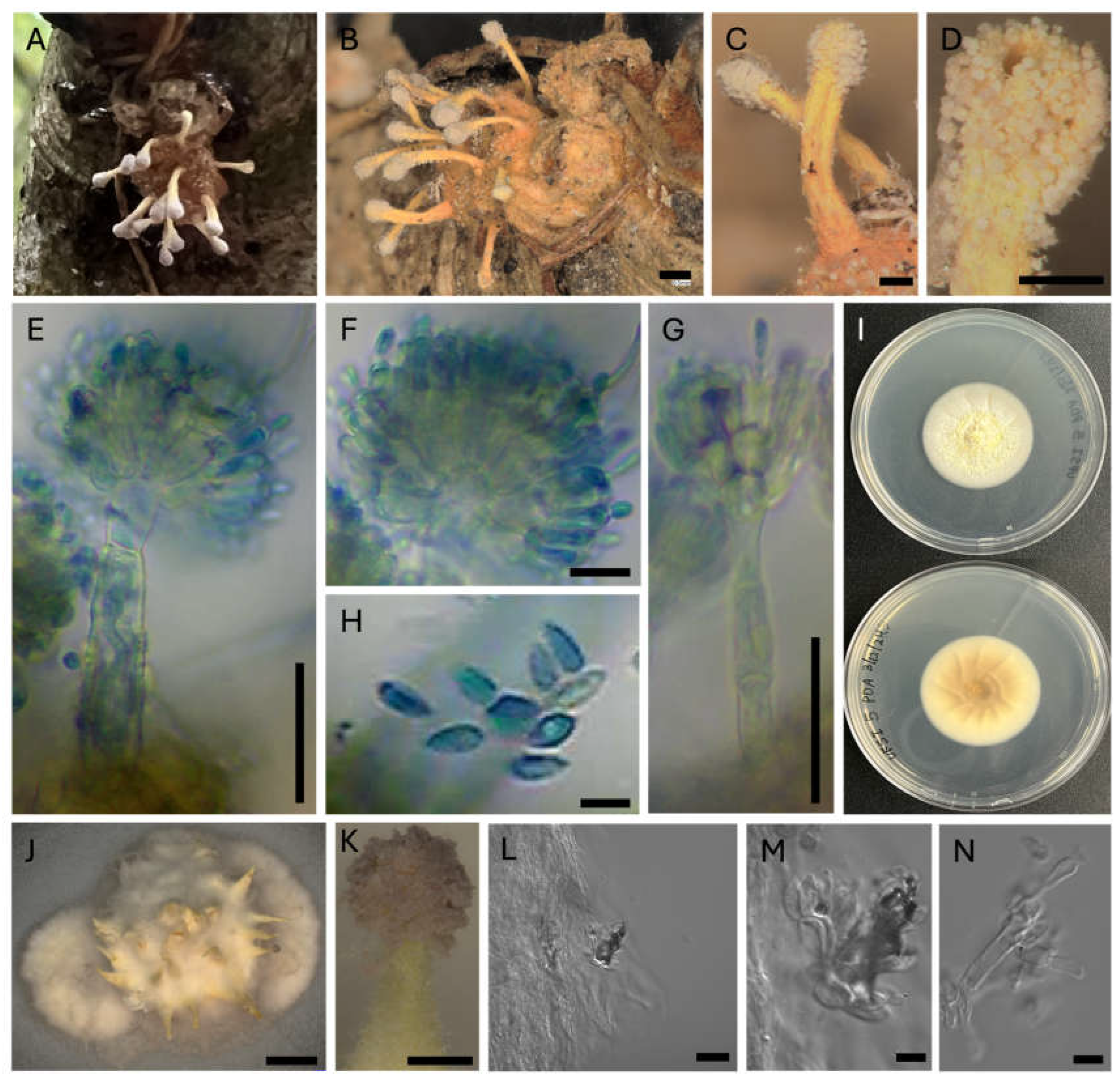
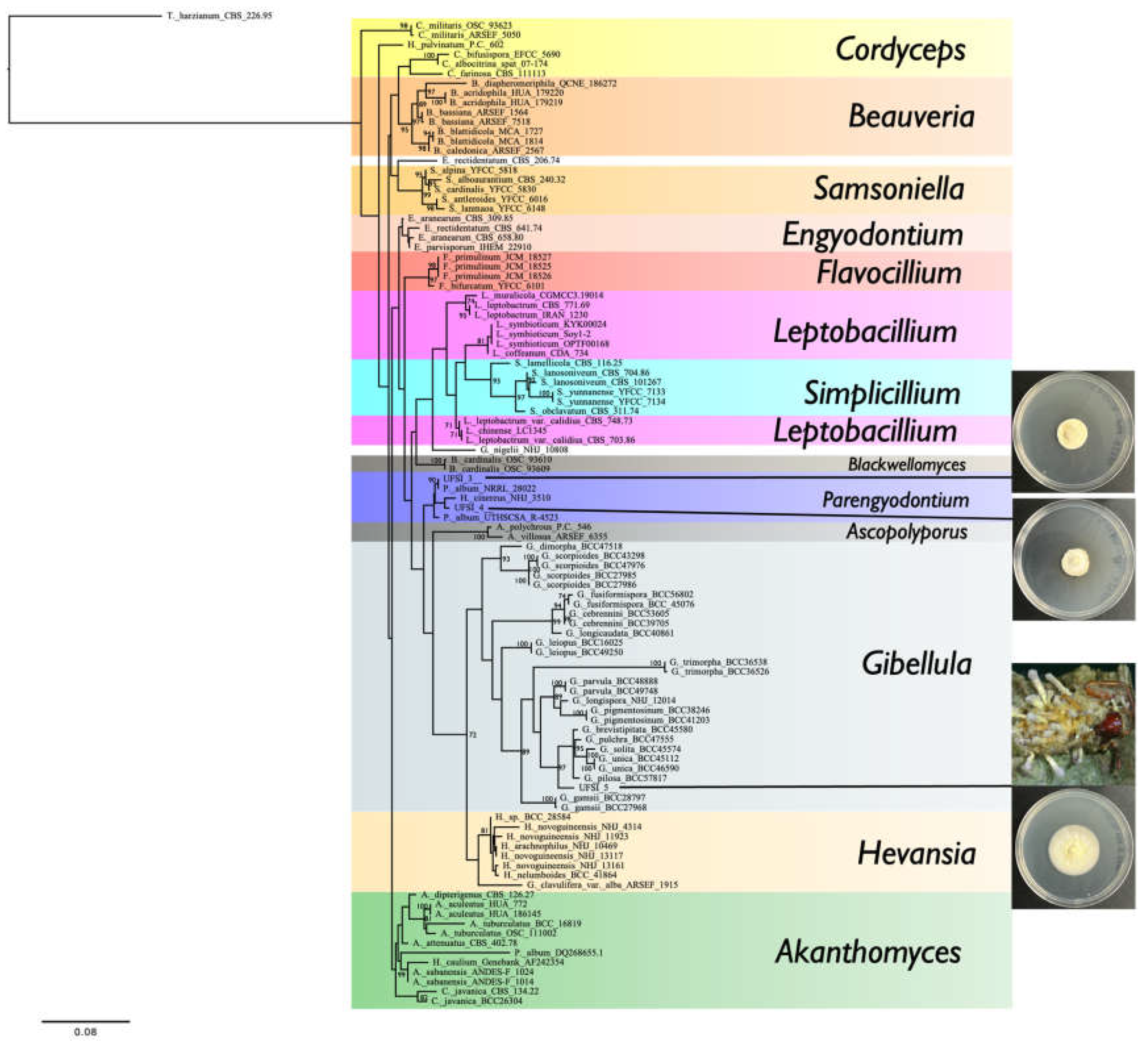
Figure 4.
Multilocus maximum likelihood tree of the Cordycipitaceae family generated by RAxML with 2000 bootstrap replicates and containing concatenated ITS, LSU, SSU, and TEF1a sequences to determine the phylogenetic placement of isolates UFSI_3, UFSI_4, and UFSI_5. Bootstrap support values greater than or equal to 70 are displayed on tree nodes.
Figure 4.
Multilocus maximum likelihood tree of the Cordycipitaceae family generated by RAxML with 2000 bootstrap replicates and containing concatenated ITS, LSU, SSU, and TEF1a sequences to determine the phylogenetic placement of isolates UFSI_3, UFSI_4, and UFSI_5. Bootstrap support values greater than or equal to 70 are displayed on tree nodes.
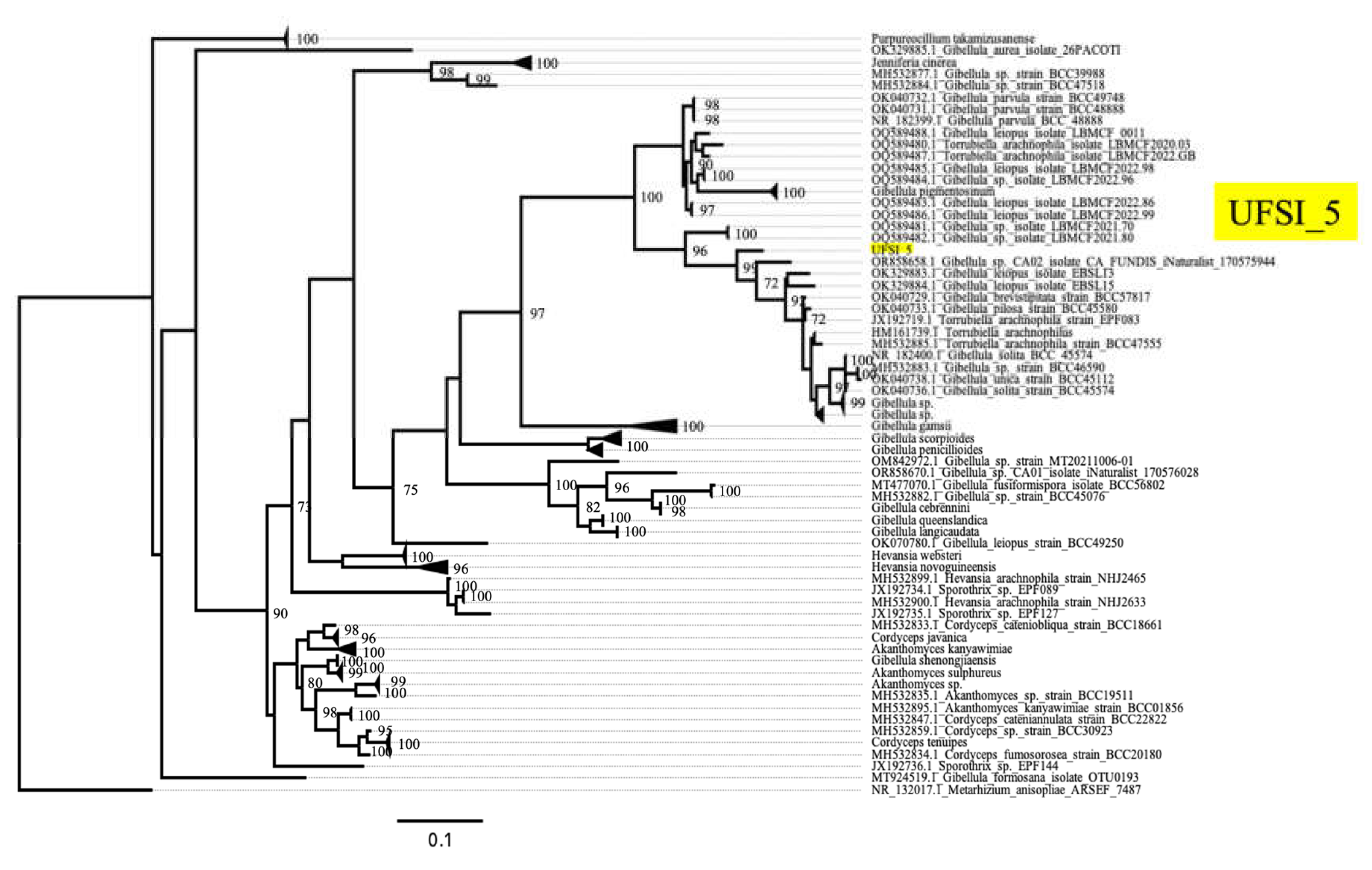
Figure 5.
Maximum likelihood ITS tree of UFSI_5 and Gibellula sequences downloaded from Genbank. This tree was generated using RAxML with 2000 bootstrap replicates. Bootstrap support values greater than or equal to 70 are displayed on the nodes of the tree. Clades containing many isolates of the same species are collapsed and appear as triangles at the tips of the tree. Isolate UFSI_5 is highlighted in yellow to emphasize its phylogenetic placement among other Gibellula species.
Figure 5.
Maximum likelihood ITS tree of UFSI_5 and Gibellula sequences downloaded from Genbank. This tree was generated using RAxML with 2000 bootstrap replicates. Bootstrap support values greater than or equal to 70 are displayed on the nodes of the tree. Clades containing many isolates of the same species are collapsed and appear as triangles at the tips of the tree. Isolate UFSI_5 is highlighted in yellow to emphasize its phylogenetic placement among other Gibellula species.
Figure 6.
Relative taxonomic abundance of soil-inhabiting entomopathogenic fungi. (A) Total percentages of Ophiocordycipitaceae, Clavicipitaceae, and Cordycipitaceae from environmental samples. (B) Relative abundances of the three families were detected by amplicon sequencing within each environmental sample type at the two different sampling sites Lake Alice and Loblolly (LA and LB).
Figure 6.
Relative taxonomic abundance of soil-inhabiting entomopathogenic fungi. (A) Total percentages of Ophiocordycipitaceae, Clavicipitaceae, and Cordycipitaceae from environmental samples. (B) Relative abundances of the three families were detected by amplicon sequencing within each environmental sample type at the two different sampling sites Lake Alice and Loblolly (LA and LB).
Figure 7.
Relative abundance of entomopathogenic fungi in the Cordycipitaceae family from environmental samples. (A) Total percentages of Cordycipitaceae genera among all samples. (B) Relative abundances of Cordycipitaceae genera from the different environmental samples at both sampling sites (LA and LB).
Figure 7.
Relative abundance of entomopathogenic fungi in the Cordycipitaceae family from environmental samples. (A) Total percentages of Cordycipitaceae genera among all samples. (B) Relative abundances of Cordycipitaceae genera from the different environmental samples at both sampling sites (LA and LB).
Figure 8.
Relative abundance of entomopathogenic fungal species in the family Cordcypitaceae from environmental samples. (A) Total percentages of species within the Corcycipitaceae. (B) Relative abundances of species within the Cordycipitaceae within environmental sample types and across sample locations (LA and LB).
Figure 8.
Relative abundance of entomopathogenic fungal species in the family Cordcypitaceae from environmental samples. (A) Total percentages of species within the Corcycipitaceae. (B) Relative abundances of species within the Cordycipitaceae within environmental sample types and across sample locations (LA and LB).
Figure 9.
Relative abundance of entomopathogenic fungi in the Clavicipitaceae family from environmental samples. (A) Total percentage of Clavicipitaceae genera within all environmental samples. (B) Relative abundance of Clavicipitaceae genera for each of the environmental sample types and at the two different sampling locations (LA and LB).
Figure 9.
Relative abundance of entomopathogenic fungi in the Clavicipitaceae family from environmental samples. (A) Total percentage of Clavicipitaceae genera within all environmental samples. (B) Relative abundance of Clavicipitaceae genera for each of the environmental sample types and at the two different sampling locations (LA and LB).
Figure 10.
Relative abundances of entomopathogenic fungal species in the Clavicipitaceae from environmental samples. (A) Total percentages of Clavicipitaceae fungal species detected in environmental samples. (B) Relative abundance of fungal species in the Clavicipitaceae at each environmental sample type and across the two sampling sites (LA and LB).
Figure 10.
Relative abundances of entomopathogenic fungal species in the Clavicipitaceae from environmental samples. (A) Total percentages of Clavicipitaceae fungal species detected in environmental samples. (B) Relative abundance of fungal species in the Clavicipitaceae at each environmental sample type and across the two sampling sites (LA and LB).
Figure 11.
Relative abundances of entomopathogenic fungal genera in the Ophiocordycipitaceae family detected in environmental samples. (A) Total percentages of Ophiocordycipitaceae genera detected from environmental samples. (B) Relative abundance of Ophiocordycipitaceae genera detected at each environmental sample type from each of the two sampling locations (LA and LB).
Figure 11.
Relative abundances of entomopathogenic fungal genera in the Ophiocordycipitaceae family detected in environmental samples. (A) Total percentages of Ophiocordycipitaceae genera detected from environmental samples. (B) Relative abundance of Ophiocordycipitaceae genera detected at each environmental sample type from each of the two sampling locations (LA and LB).
Figure 12.
Relative abundances of entomopathogenic fungal species in the Ophiocordycipitaceae detected from environmental samples. (A) Total percentages of fungal species in the Ophiocordycipitaceae detected across all environmental samples. (B) Relative abundance of fungal species in the Ophiocordycipitaceae detected at each environmental sample type and across the two sampling locations (LA and LB).
Figure 12.
Relative abundances of entomopathogenic fungal species in the Ophiocordycipitaceae detected from environmental samples. (A) Total percentages of fungal species in the Ophiocordycipitaceae detected across all environmental samples. (B) Relative abundance of fungal species in the Ophiocordycipitaceae detected at each environmental sample type and across the two sampling locations (LA and LB).
Figure 13.
The total percentages of two specific spider pathogens within the entomopathogenic fungal communities were identified across all environmental sample types and both sampling locations (LA and LB).
Figure 13.
The total percentages of two specific spider pathogens within the entomopathogenic fungal communities were identified across all environmental sample types and both sampling locations (LA and LB).