Submitted:
09 September 2024
Posted:
10 September 2024
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
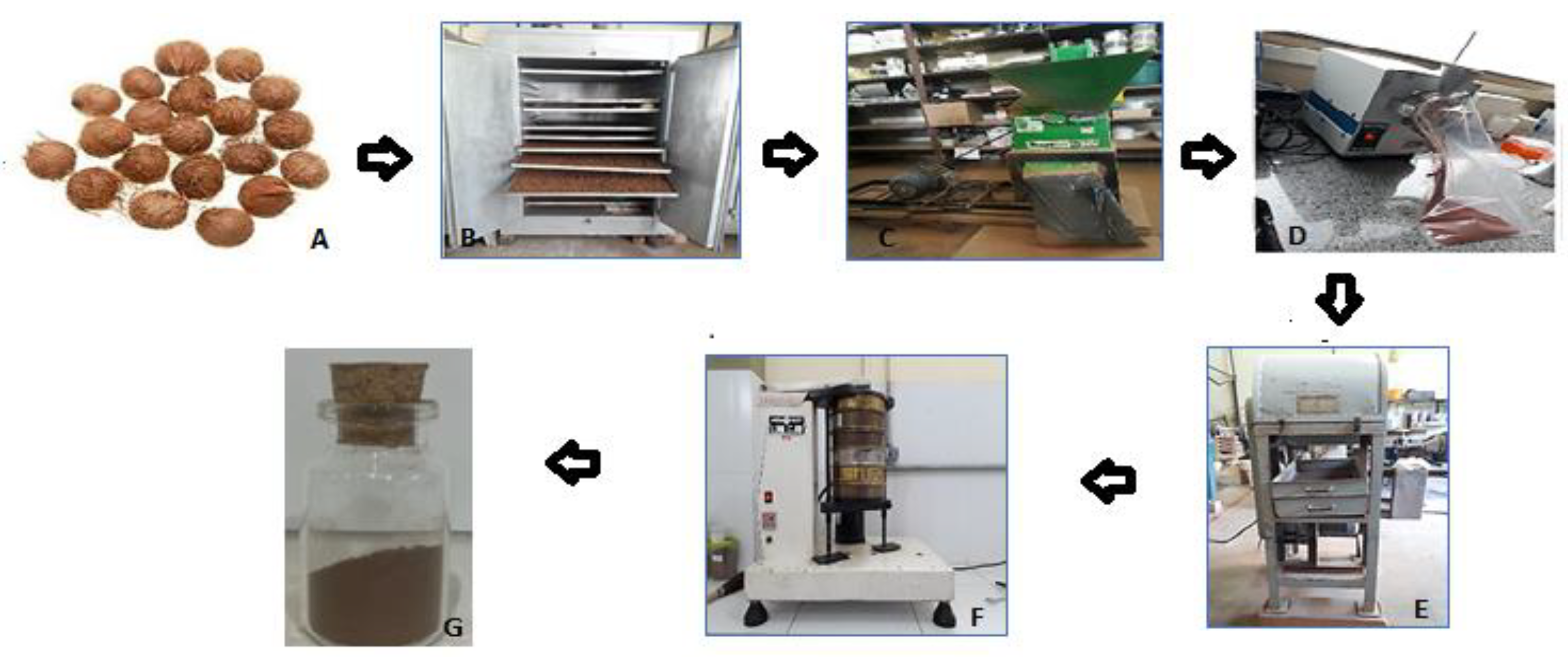
2.2.1. Drying, Grinding, Sieving, and Granulometric Classification of Acai Seeds
2.2.2. Preparation and Characterization of the Toothpaste
2.2.3. Physicochemical Characterization of Acai Toothpaste
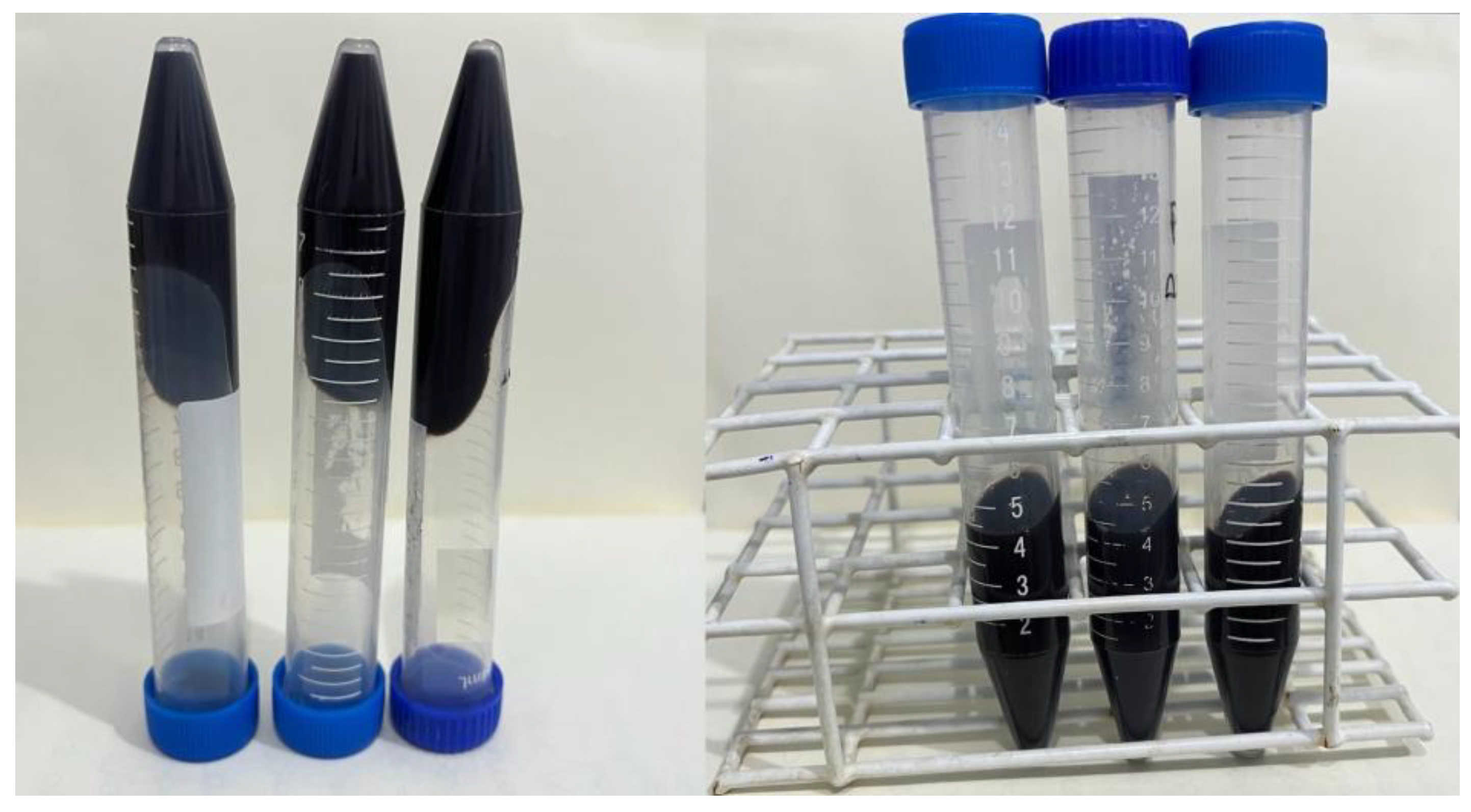
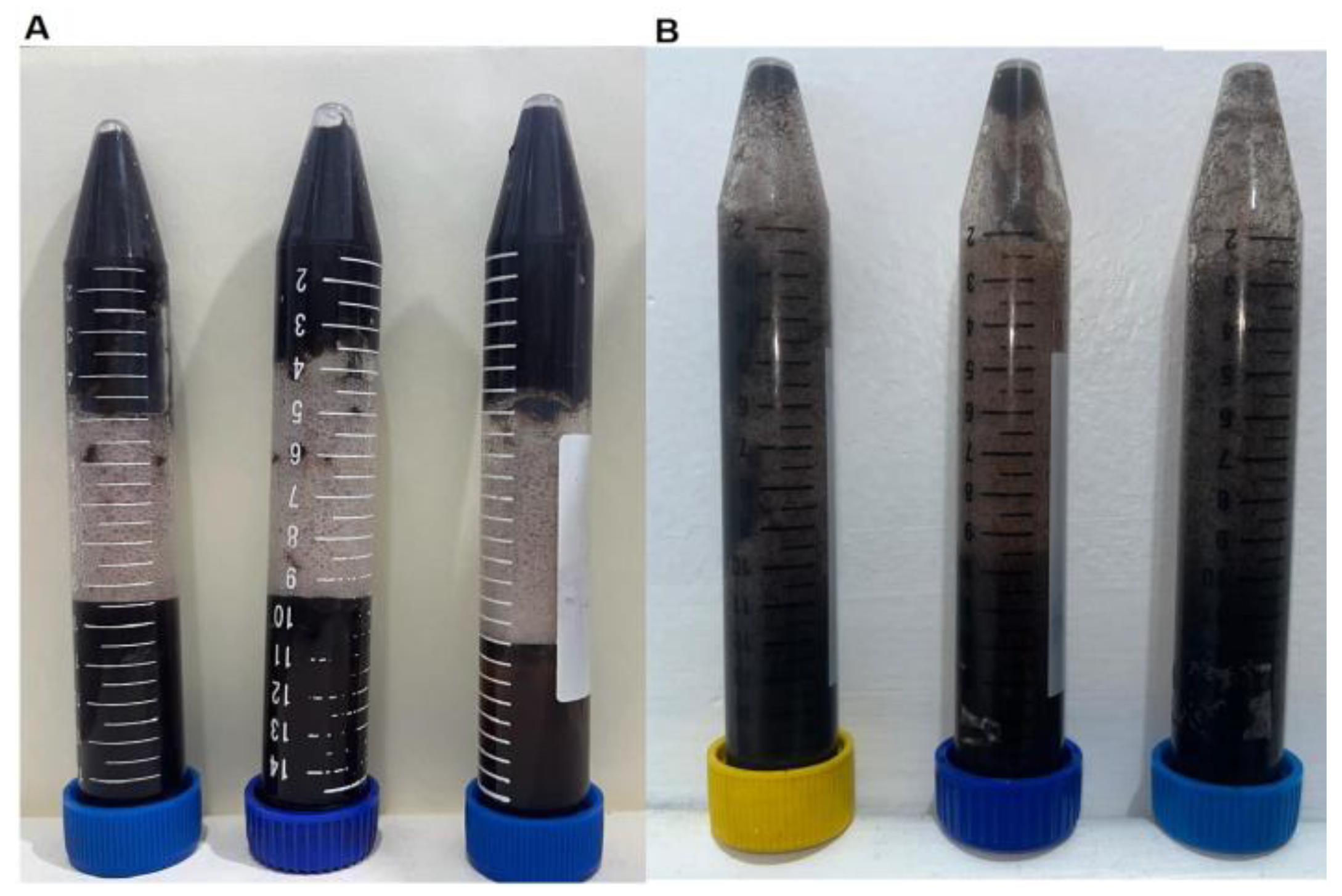
Centrifugation Test
Density Determination
Foam Formation
Acidity Index Analysis
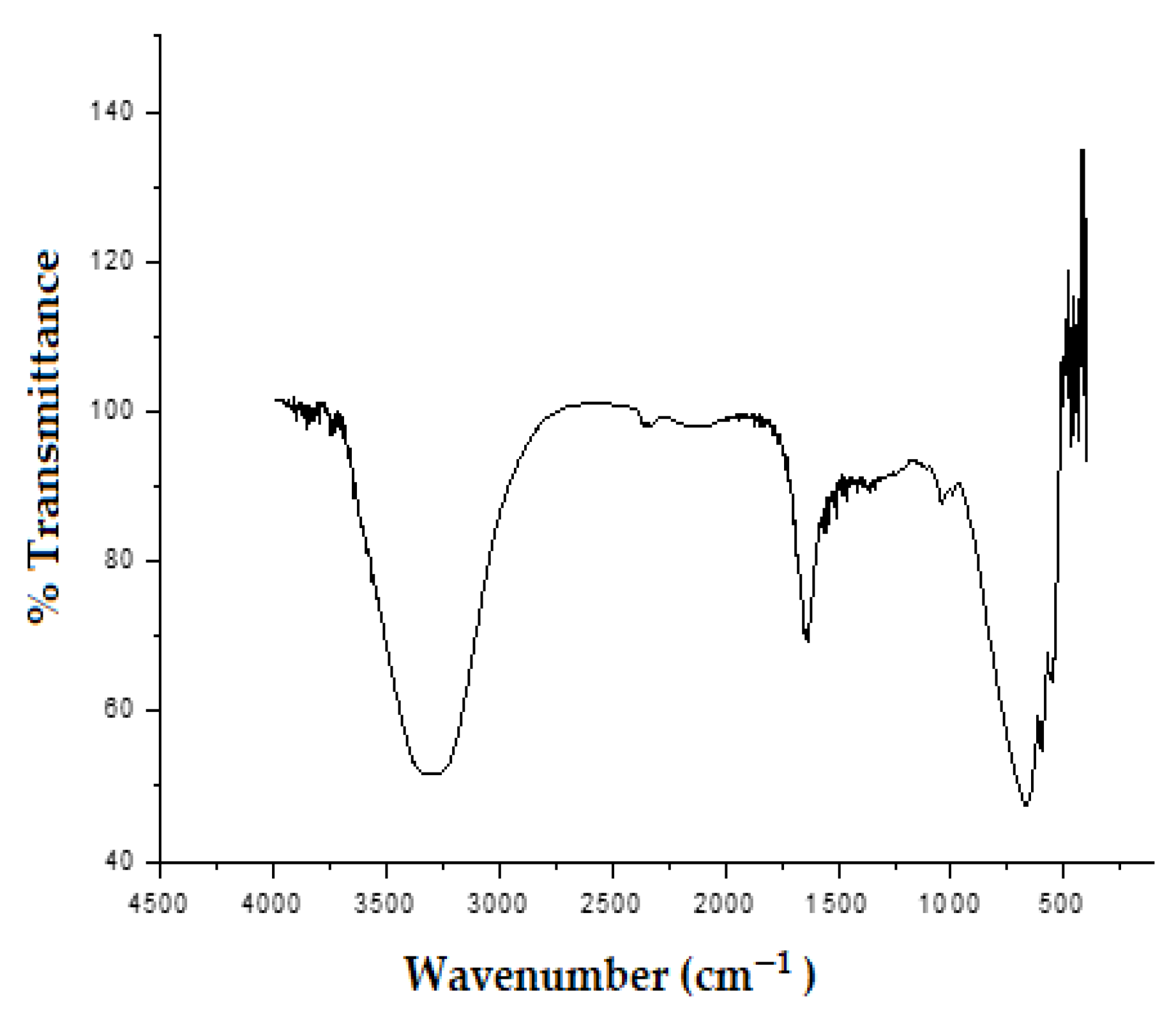
Fourier Transform Infrared Spectroscopy (FTIR)
Thermogravimetric Analysis (TG/DTG)
Rheological Properties
2.3. Formulation Stability Study
3. Results
3.1. Centrifugation Test
3.2. Density Determination
3.3. Foam Formation Test
3.4. Acidity Index Evaluation
3.5. Infrared Spectroscopy Analysis
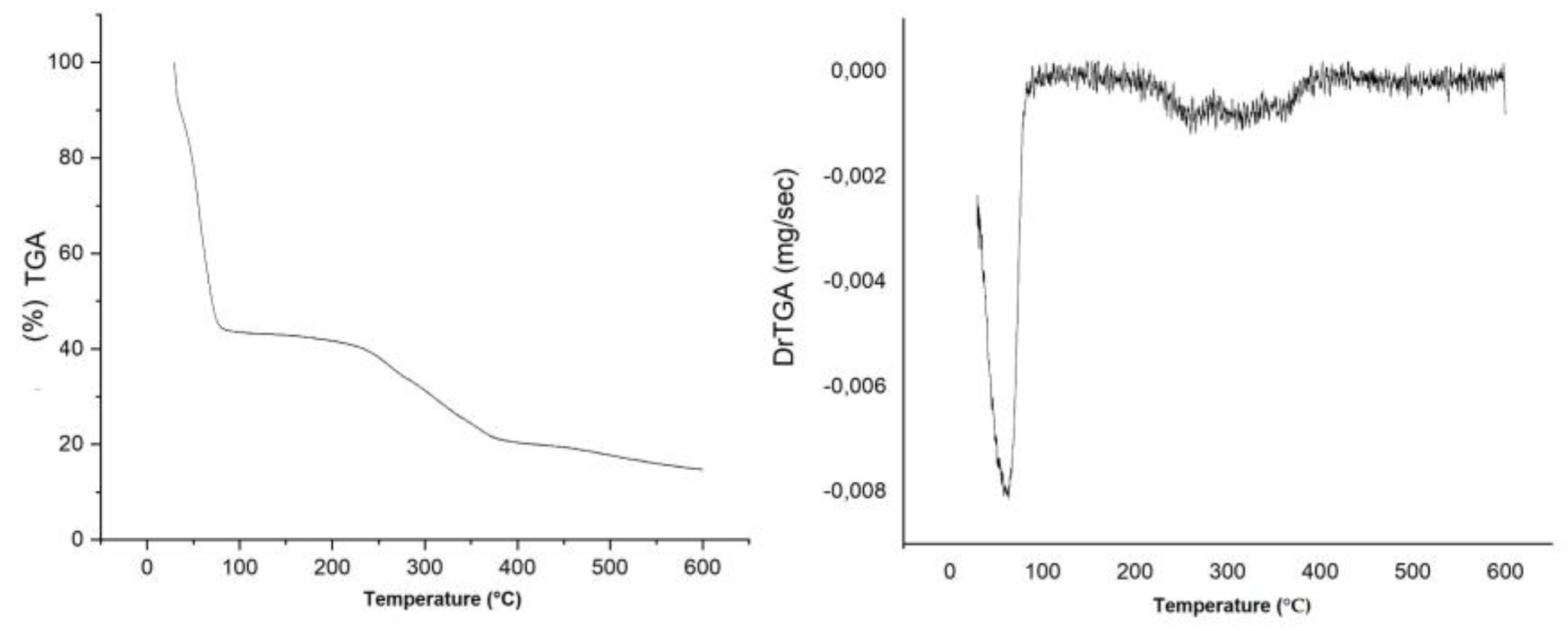
3.6. Thermogravimetric and Derivative Thermogravimetric Analysis
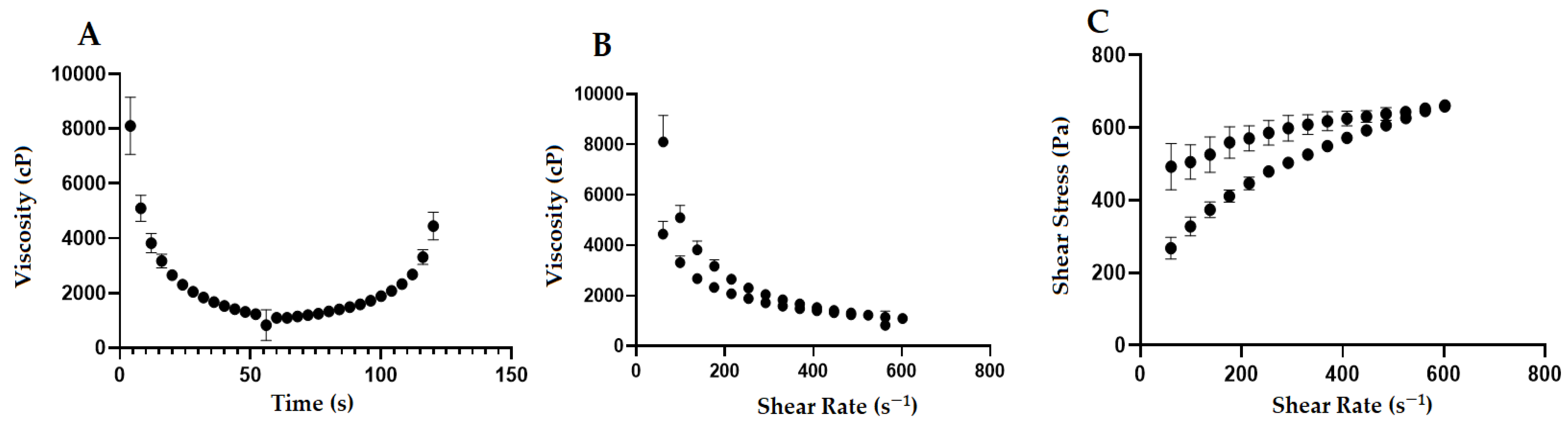
3.7. Rheological Properties Analysis
3.8. Stability Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laurindo, L.F.; Barbalho, S.M.; Araújo, A.C.; Guiguer, E.L.; Mondal, A.; Bachtel, G.; Bishayee, A. Açaí (Euterpe oleracea Mart.) in Health and Disease: A Critical Review. Nutrients 2023, 15, 989. [CrossRef]
- Farias, J.M.; Stamford, C.M.; Resende, A.H.M.; Aguiar, J.S.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Mouthwash containing biosurfactant and chitosan: An eco-sustainable option for controlling cariogenic microorganisms. Int. J. Biol. Macromol. 2019, 129, 853–860. [CrossRef]
- Maia, M. M. M; Silva, B. F.; Andrade, B. V.; Hora, T. A. T.; Soares, T. A. F.; Uma revisão crítica da abrasividade em cremes dentais convencionais e clareadores, Rev. Psic. 2022 V.16, 61, p. 76-87, multidisciplinar. Issn 1981-1179. [CrossRef]
- Filho, D.G.D; Rodrigues, F.A.D.; Ramos, A.M.B.; Teixeira, F.I.S.; Souza, P.A.S.; Revisão de literatura sobre a atividade antioxidante do açaí. Contemporânea–Revista de Ética e Filosofia Política, v. 3, n. 1, 2023. ISSN 2447-0961. [CrossRef]
- Pinheiro, A. dos S.; “Aplicação do pó do caroço do açaí (Euterpe oleracea mart.) como pigmento e carga na composição de tinta à base água, Dissertação de Mestrado, UFPA, Belém, fevereiro de 2020. https://drive.google.com/file/d/1p7HQQhmSGRhHOrHOKAO5xKYiDgAbFB4b/view.
- Veloso, A.B.; Varão, P.S.; Verde, G.M.F.L.; Análise da composição dos dentrifícios clareadores encontrados no mercado de teresina-pI. Brazilian Journal of Implantology and Health SciencesVolume5, Issue5(2023),Page1264-1279. [CrossRef]
- Srivastava, S., Biswal, P. K., Alam, F., Anand, A., & Pal, A. (2023). A review on toothpaste to treat sensitive teeth. [CrossRef]
- Rodrigues, A. R. D. S. P. (2024). Atividade antifúngica do óleo essencial de Mentha piperita (Lamiaceae: Labiatae)–uma revisão. Conexão Ciência (Online), 19(2), 103-114. [CrossRef]
- Moraes, C.F.; Berton, J.; Novak, R.S.; Marins, R.A.; Análises físicoquímicos e parâmetros de qualidade de formulações comerciais de xampus de cetoconazol. Revista Interdisciplinnar de Estudos em Saúde, V1 N 1 2023. [CrossRef]
- Pinheiro, J. C., Araújo, D. M. de, Silva, G. G. da, Silva, L. F. B. da, Lima, J. G. da C., & Leite, R. B. (2020). A utilização do gel de flúor-fosfato acidulado 1,23% como fluorterapia tópica na prevenção da cárie dentária. Revista Saúde e Desenvolvimento, 14(18). DOI:https://www.revistasuninter.com/revistasaude/index.php/saudeDesenvolvimento/article/view/1098.
- Rahman, M. S., Hasan, M. S., Nitai, A. S., Nam, S., Karmakar, A. K., Ahsan, M. S., ... & Ahmed, M. B. (2021). Recent developments of carboxymethyl cellulose. Polymers, 13(8), 1345. [CrossRef]
- Moura, M. S. B., Rezende, M. R. S., Almeida, M. da S., Costa, M. M. S. S. M. da, Gonçalves, N. K. dos S. B., & Costa, J. N. (2024). O papel do flúor no controle, na prevenção e no estágio inicial da lesão cariosa: revisão de literatura. Revista Ibero-Americana De Humanidades, Ciências E Educação, 10(6), 1256–1266. [CrossRef]
- Batista, D.S.; Costa,J.S.S.; Mota, M.D.; Cazedey, E.C.L.; Prospecção de Patentes de Cosméticos com a Presença de Spilanthes acmella e Similares dos anos 2015- 2020, Cadernos de Prospecção, Salvador, v. 16, n. 3, p. 813-830, abril a junho, 2023. [CrossRef]
- Job, M.T.; Ludwig, F.; Óleos essenciais no manejo fitossanitário de gérbera. Revista Cultivando o Saber V. 13 N. 3 2020 DOI: https://cultivandosaber.fag.edu.br/index.php/cultivando/article/view/1012.
- da Saúde, M.; Agência Nacional de Vigilância Sanitária (ANVISA). Formulário Nacional da Farmacopeia Brasileira, 2ª edição, Brasília 2012 Rev. 02.
- Dantas, M.G.B.; Reis, S.A.G.B.; Damasceno, C.M.D.; Rolim, L.A.; Rolim-Neto, P.J.; Carvalho, F.O.; et al. Development and Evaluation of Stability of a Gel Formulation Containing the Monoterpene Borneol. Scient World J. 2016;20. [CrossRef]
- da Saúde, M.; Agência Nacional de Vigilância Sanitária (ANVISA). Farmacopeia Brasileira, 5th ed.; Companhia Editora Nacional: São Paulo, Brazil, 2010; Volume 1.
- INSTITUTO ADOLFO LUTZ. Normas Analíticas do Instituto Adolfo Lutz. Métodos físico-químicos para análises de alimentos. 4ª ed. (1ª Edição digital), 2008. 1020 p.
- Fernandes, N.C.L.; Ferreira, J.B.; Valle, M.L.A.; Lobão, M.S.; Alves, W.F.; Nascimento, L.O.; Marques, J.S.; Óleos naturais no tratamento preservativo de cinco espécies de madeiras amazônicas, Rev. Inst. Flor.,v. 36: e936,2024. [CrossRef]
- Yasin, H.; Al-Taani, B.; Salem, M.; Preparation and characterization of ethylcellulose microspheres for sustained-release of pregabalin. Res Pharm Sci. 2021;16:1. [CrossRef]
- Santos, R. K.S.; Nascimento, B.F.; Araújo, C. M.B.; Cavalcanti,J.V.F.L.;. Bruckmann, F. S.; Rhoden, C. R.B.; Dotto, G.L.; Oliveira, M.L.S.; Silva, L.F.O.; Sobrinho, M.A.M.; Removal of chloroquine from the aqueous solution by adsorption onto açaí-based biochars: Kinetics, thermodynamics, and phytotoxicity, Journal of Molecular Liquids, Volume 383, 2023, 122162, ISSN 0167-7322, . [CrossRef]
- Krishna, M. B., Priya, M. Y. V. N. S., & Padmalatha, K. (2021).A review study on evaluation of dental products. Vol 10, Issue 8, 2021. ISO 9001:2015 World Journal of Pharmaceutical Research . [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; David, L. Bryce., Eds.; Wiley: Sao Paolo, Brazil, 2007; pp. 71–107, ISBN 978-85-216-3637-3.
- Oliveira, L.S.; Silva, A.V.S.; Conconi, C.C.; Gomes, E.B.; Bizzo, W.A.; Cruz, G.; Thermal degradation of açaí seeds and potential application in thermochemical processes, Rev. Prod. Desenvolv.,Rio de Janeiro, v.7: e531, Jan-Dez, 2021, . [CrossRef]
- Oancea, S. (2021). A Review of the Current Knowledge of Thermal Stability of Anthocyanins and Approaches to Their Stabilization to Heat. Antioxidants, 10(9), 1337 . [CrossRef]
- Lima, K. M. de .; Nunes, G. P. T. .; Magno, E. A. A. .; Rivera, J. G. B. .; Quemel, G. K. C. Analysis of organoleptic, physical-chemical characteristics and concentrations of tartrazine yellow dye in tucupi samples sold at markets in Belém-PA. Research, Society and Development, [S. l.], v. 12, n. 10, p. e32121043357, 2023. [CrossRef]
- Santos, A. dos, Miranda, A. da S., Oliveira, I. L. M. de, Barbosa, J. da S., Leite, J. V. C., & Wanderley e Lima, R. B. (2023). Efetividade da ação clareadora dos dentifrícios no clareamento dental: uma Revisão Integrativa. Arquivos Em Odontologia, 59, 30–38. [CrossRef]
- Bispo, L. O. V. .; Messias Neto, F. V. .; Lima, P. M. .; Carvalho, E. de S. P. .; Vieira, I. M. .; Soares, A. F. Effectiveness of whitening toothpastes: A review of the literature. Research, Society and Development, [S. l.], v. 13, n. 3, p. e4613345262, 2024. [CrossRef]
- Mota, M.; Óleo ozonizado de euterpe oleracea mart. Como insumo cosmético para os cuidados labiais: caracterização e atividades biológicas, Tese de doutorado, UFJF, 2023. https://repositorio.ufjf.br/jspui/handle/ufjf/16159.
- Guzzatti, M.F.M.; Avaliação da síntese verde de nanopartículas de ouro e prata com curcumina (Curcuma longa l.) Ou açaí (Euterpe oleracea) no reparo tecidual de ferida palatina em ratos wistar, Tese de Doutorado, Unesp, Criciúma, 2022. http://repositorio.unesc.net/handle/1/10117.
- Azevedo, A.A.; Chagas, F.O.; Júnior, J.H.C.F.; Júnior, E.A.A.; Tapety, C.M.C.; Leite, T.B.; Silva, S.M.S.M.; Araújo, C.S.R.; Medeiros, D.M.; Dantas, T.C.F.B.; Neto, E.M.R.; Physicochemical Evaluation of a Toothpaste Incorporated with Brazilian Red Propolis, J. Adv. Med. Med. Res., vol. 35, no. 20, pp. 259-264, 2023; Article no.JAMMR.105290, . [CrossRef]
- Tokura T, Robinson C, Watson P, Abudiak H, Nakano T, Higashi K, et al. Effect of pH on fluoride penetration into natural human plaque. Pediatric Dental Journal. 2012;22(2012):140-144. [CrossRef]
- Ponpeo, F. T. (2022). Formulação e avaliação de dentifrícios para próteses totais à base de óleos essenciais - características organolépticas, propriedades físico-químicas e efeitos adversos sobre resinas para base protética. Master's Dissertation, Faculdade de Odontologia de Ribeirão Preto, University of São Paulo, Ribeirão Preto. Retrieved 2024-08-29, from www.teses.usp.br. [CrossRef]
- Santos, M.E.S.; Estudo de estabilidade preliminar de formulações cosméticas contendo óleo fixo de açaí e seu complexo em hidroxipropilgama-ciclodextrina, Tese de Doutorado 2023, Rio Grande do Norte, https://repositorio.ufrn.br/handle/123456789/56401.
- Santos, M.M.; Pasolini, F.S.; Costa, A.P.O.; Caracterização físico-química do caroço e da fibra do açaí (Euterpe oleracea mart.) via métodos clássicos e instrumentais, Brazilian Journal of Engeneering, 2023, v 9, p144-160. [CrossRef]
- Santos, A.C.M.; Oliveira, V.C.; Macedo, A.P.; Bastos, J.K.; Ogasawara, M.S.; Watanabe, E.; Chaguri, I.M.; Silva-Lovato, C.G.; Paranhos, H.F.O.; Effectiveness of Oil-Based Denture Dentifrices-Organoleptic Characteristics, Physicochemical Properties and Antimicrobial Action. ANtibiotics (Bsel, Switzerland) vol 10, n. 7, p.813, 2021. [CrossRef]
- Soeteman G.; Valkenburg C.; Van der Weijden G.; Van Loveren C.; Bakker E.; Slot D. Whitening dentifrice and tooth surface discoloration-a systematic review and meta-analysis. Int J Dent Hyg.2018; 16(1):24–35, . [CrossRef]
- Souza, I.R.; Bezerra, K.G.O.; Oliveira, C.L.; Meira, H.M.; Stamford, T.C.M.; Convertti, A.; Sarubbo, S.A.; Ruffino, R.D.; Mouthwash Containing Plant-Derived Biosurfactant and Chitosan Hydrochloride: Assessment of Antimicrobial Activity, Antibiofilm Activity, and Genotoxicity, Assessment of Antimicrobial Activity, Antibiofilm Activity, and Genotoxicity. Appl. Sci. 2024, 14, 6711. [CrossRef]
- Silveira, J. T., da Rosa, A. P. C., de Morais, M. G., Victoria, F. N., & Costa, J. A. V. (2023). An integrative review of Açaí (Euterpe oleracea and Euterpe precatoria): traditional uses, phytochemical composition, market trends, and emerging applications. Food Research International, 113304. Rehbein, P. S.; Mezari, L. da B.; Avaliação da estrutura do esmalte em dentes humanos após o uso de jato de bicarbonato de sódio e pasta profilática; Universidade de Santa Cruz do Sul – UNISC, 2020, . [CrossRef]
- Azevedo, A.; de Matos, P.; Marvila, M.; Sakata, R.; Silvestro, L.; Gleize, P.; Brito, J.d. Rheology, Hydration, and Microstructure of Portland Cement Pastes Produced with Ground Açaí Fibers. Appl. Sci. 2021, 11, 3036. [CrossRef]
- Barros, S.S.; Oliveira, E.S.; Jr, W.A.G.P.; Rosas, A.L.G.; Freitas, A.E.M.; Lira, M.S.F.; Calderaro, F.L.; Saron, C.; Freitas, S.A.; Waste açaí (Euterpe precatoriaMart.) seeds as a new alternative source of cellulose: Extraction and characterization, Research, Society and Development, v. 10, n. 7, e31110716661, 2021(CC BY 4.0) | ISSN 2525-3409, . [CrossRef]
- Silva, T.R.; Matos, P.R.; Júnior, L.U.D.T.; Marvila, M.T.; Azevedo, A.R.T.; A review on the performance of açaí fiber in cementitious composites: Characteristics and application challenges, Journal of Building Engineering, Volume 71, 2023, 106481, ISSN 2352-7102, . [CrossRef]
- Rocha, J.C.B.; Lopes, J.D.; Mascarenhas, M.C.N.; Arellano, D.B.; Guerreiro, L.M.R.; Cunha, R.L. Thermal and rheological properties of organogels formed by sugarcane or candelilla wax in soybean oil. Food Res. Intern. 2013, 50, 318–323, . [CrossRef]
- Neto, J.M.A.C.; Agra, L.A.C.; Luz, M.C.M; Souza, S.V.P.; Santos, J.V.; Mendonça, I.C.G.; Os avanços da odontologia minimamente invasiva nos dias atuais REAS|Vol.13(2), . [CrossRef]
- Remini, H., Dahmoune, F., Sahraoui, Y., Madani, K., Kapranov, V. N., & Kiselev, E. F. (2018). Recent advances on stability of anthocyanins. RUDN Journal of The Journal of Engineering and Exact Sciences –jCEC5Agronomy and Animal Industries, 13(4), 257–286. [CrossRef]
- Shruthi, K. (2023). A Review: Pharmaceutical Gels and Its Types with Prominence Role of Its Drug Delivery Systems, World Journal of Pharmaceutical Research, Vol 12, Issue 9, 2023., . [CrossRef]
- Rossi, I.S.; Costa, J.B.; Nascimento, L.G.L; Carvalho, A.F.; Estabilidade de antocianinas do açaí: uma breve revisão, The Journal of Engineering and Exact Sciences –jCEC, Vol. 08N. 09(2022), . [CrossRef]
- Miranda, P. H. S., Santos, A. C. dos, Freitas, B. C. B. de, Martins, G. A. de S., Vilas Boas, E. V. de B., & Damiani, C. (2021). A scientific approach to extraction methods and stability of pigments from Amazonian fruits. Trends in Food Science & Technology, 113, 335–345, . [CrossRef]
- Costa, Z. G.; Silva, C. de L. O. C. e; Costa, C. M. L.; Faria, L. J. G.; "Estudo da estabilidade de antocianinas extraídas dos frutos de açaí (Euterpea oleracea Mart.)", p. 2177-2182. In: Anais do XI Congresso Brasileiro de Engenharia Química em Iniciação Científica [=Blucher Chemical Engineering Proceedings, v. 1, n.3]. ISSN Impresso: 2446-8711. São Paulo: Blucher, 2015. ISSN 2359-1757, DOI 10.5151/chemeng-cobeqic2015-365-33974-260982, . [CrossRef]
- BRASIL. Ministério da Saúde. Agência Nacional de Vigilância Sanitária (ANVISA). Resolução da Diretoria Colegiada – RDC n° 752, de 19 de setembro de 2022, dispõe sobre a definição, a classificação, os requisitos técnicos para rotulagem e embalagem, os parâmetros para microbiológico, controle bem como os requisitos técnicos e procedimentos para a regularização de produtos de higiene pessoal, perfumes. cosméticos e perfumes.




| Components | % |
|---|---|
| Acai seed powder | 20 |
| Sodium fluoride gel (2%) | 7 |
| Sodium lauryl ether sulfate | 1 |
| Peppermint essential oil | 2 |
| Sodium bicarbonate | 1 |
| Ethylenediaminetetraacetic acid | 0.1 |
| Glycerin | 1.5 |
| Carboxymethylcellulose | 0.9 |
| Water qsf 100g |
| Time (Days) | Room Temperature | Incubator | Refrigerator |
|---|---|---|---|
| 0 | 8.08 | - | - |
| 1 | 7.41 | 7.21 | 7.1 |
| 7 | 7.49 | 7.56 | 7.50 |
| 14 | 7.56 | 7.12 | 7.45 |
| 21 | 7.92 | 7.07 | 7.88 |
| 30 | 7.0 | 6.8 | 7.8 |
| 45 | 6.84 | 6.51 | 7.71 |
| 60 | 6.80 | 6.50 | 7.60 |
| 90 | 7.55 | 6.53 | 7.56 |
| 180 | 6.49 | 6.50 | 6.69 |
| Characteristic | Refrigerator | Room Temperature | Incubator | |
|---|---|---|---|---|
| Initial | Color | D | D | D |
| Odor | C | C | C | |
| Appearance | Ho | Ho | Ho | |
| Final | Color | D | D | W |
| Odor | C | C | C | |
| Appearance | Ho | Ho | He |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





