1. Introduction
Ovarian cancer (OC) typically presents in an advanced stage and ranks as the second leading cause of death among gynecological malignancies in women following cervical cancer. According to GLOBOCAN, projections indicate that in 2020, there were more than 300,000 newly reported cases of ovarian cancer, with over 200,000 deaths confirmed [
1,
2]. High-grade advanced ovarian, fallopian tube and primary peritoneal cancer are malignancies with similar origin and histological structure; therefore, they are treated as one group of tumors and the treatment regimens are common to them. Ovarian cancer is a cancer in which, apart from surgical treatment, systemic treatment plays an important role. The improvement in treatment results observed for several decades is associated with the introduction of platinum, paclitaxel, bevacizumab, and polyADP-ribose polymerase inhibitors (PARPi).
In 1996, scientists presented the results of the GOG111 study, which proved higher effectiveness of OC treatment with cisplatin and paclitaxel compared to the previously used regimen, i.e., cisplatin combined with cyclophosphamide [
3] in the group of patients with high-grade ovarian cancer (stage III and IV according to FIGO). Further results of the AGO-OVAR3 and GOG158 studies from 2003 revealed that carboplatin has similar effectiveness and a better tolerance profile to cisplatin and improves the quality of life [
4]. The study results on bevacizumab, a recombinant humanized monoclonal antibody produced by recombinant DNA in the primary treatment of ovarian cancer were initially published in 2010 by Burger R. et al. as a result GOG 218 trial [
5]. Bevacizumab binds to endothelial vascular growth factors, thereby inhibiting their binding to receptors on the surface of endothelial cells. The results of the ICON 7 (first time presented 2010 and finally 2015) study made it possible to define the group with the highest risk of disease progression from among the entire study group [
6]. The "high-risk" group was defined as patients with the following characteristics: 1) stage III, undergoing suboptimal surgery (residual disease after surgery > 1 cm); 2) all patients with stage IV; 3) unoperated patients with stage III. However, it was the results of the GOG-0218 trial that formed the basis for the introduction of bevacizumab in Europe [
7]. Median OS for stage IV bevacizumab-concurrent plus maintenance was 42.8
v 32.6 months for stage IV control, but the diffrences were not statistically significant.
Previously, the introduction of a third chemotherapy drug resulted in more side effects while not improving overall response rate (ORR) or prolonging progression-free survival (PFS) and overall survival (OS) [
8,
9,
10]. The introduction of bevacizumab, which in ICON 7 study showed higher ORR and prolongation OS, was not associated with the exposure of patients to aggravating side effects and did not negatively affect the quality of life [
11,
12].
Over the years, treatment of first-line OC has been associated with an increase in the response rate to treatment. Hence, more and more research has been conducted on consolidation and supportive therapy. Neither classic chemotherapy - based on paclitaxel with platinum derivatives, intraperitoneal chemotherapy, nor radio- nor immunotherapy proved sufficiently effective [
13,
14]. A real breakthrough in advanced ovarian, fallopian tube, and primary peritoneal cancer therapy was introduced by PARPis which have revolutionized the treatment landscape for this disease. Introduction of PARPis extended the time to disease progression, and this is the most key element of first-line therapy.
Protein products of genes
BRCA1 and
BRCA2 are key effector of the DNA repair process, involved in the repair of DNA double-strand breaks (DSBs), mainly through the homologous recombination mechanism. Normal cells can repair double-strand breaks through various mechanisms, most commonly the homologous recombination repair pathway. However, cancer cells with homologous recombination deficiency (HRD) (such as BRCA loss-of-function mutations) do not have ability to repair DSBs so blocking the base pair excision repair mechanism with a PARP1 inhibitor results in synthetic lethality [
15].
Changes in the implementation of new therapies are inextricably linked to the introduction of new molecular diagnostic methods. Until recently, patients were tested for founder mutations mostly from bood or saliva. Currently, molecular diagnostics should be a two-stage cascade test. In the first stage, a patient with ovarian cancer is tested for the presence of BRCA1/2 pathogenic or likely-pathogenic variants using Next Generation Sequencing (NGS) from cancer tissue. It is possible to determine not only germline mutation but also a somatic mutation in the tumors. Confirmation of the presence of BRCA1/2 pathogenic or likely-pathogenic variants completes molecular diagnostics at this stage. However, if the patient is not a carrier of BRCA1/2 pathogenic or likely-pathogenic variant, she should be tested for homologous recombination disorders. Testing for a homologous recombination deficiency (HRD) in primary high-grade ovarian cancer is crucial for recommending the appropriate therapy. Patients with a BRCA germline pathogenic or likely-pathogenic variant are defined as harboring an HRD deficiency (30%). However, there is a group of approximately 20% of patients harboring an HRD deficiency based on tumor genomic analyses.
PARPi were initially tested in patients with germline
BRCA1/2 (
gBRCA1/2) pathogenic or likely-pathogenic variant [
16,
17,
18]. In platinum-sensitive recurrent HGSOC, the response rate to PARPi monotherapy was approximately 30–45% higher among
BRCA1/2 pathogenic or likely-pathogenic variant carriers [
19]. Subsequently, clinical trials pivoted their focus from PARPi monotherapy towards maintenance treatment post-platinum chemotherapy response, aiming to prolong the time to progression. In 2018, for the first time, the results of the SOLO 1 study were published in a group of patients with FIGO III and IV ovarian cancer with
gBRCA1/2 pathogenic or likely-pathogenic variant, who achieved a complete or partial response to treatment in the first line of treatment [
20]. The results of the SOLO 1 study were so groundbreaking that the drug olaparib was registered in Europe w 2019. New trials presented at the ESMO 2019 meeting indicate that patients without
BRCA1/2 pathogenic or likely-pathogenic variant also benefit from PARP inhibitors. The PRIMA trial was the first to evaluate the use of the PARPi, niraparib, in patients with advanced OC, regardless of BRCA mutation status and with residual disease. Niraparib, administered as monotherapy following first-line platinum chemotherapy, significantly improved progression-free survival (PFS) [
21]. PAOLA-1 study covered all patients with OC, but the beneficial effect of the use of platinum derivatives with bevacizumab and olaparib was proved only in a group of patients with advanced HGSOC with a damaged homologous recombination system (HRD [
22]. Olaparib, in PAOLA-1 schedule was registered as a first-line treatment for OC in 2019. In November 2020, niraparib was registered in Europe - a new and only maintenance monotherapy after first-line chemotherapy for patients with advanced ovarian cancer, regardless of the the
BRCA1/2 status. However, it was the results of the Chinese PRIME trial that contributed to expanding the indications for the use of niraparib, regardless of residual status after cytoreductive surgery [
23].
The introduction of PARPi into clinical practice has opened a new chapter in the treatment of patients with ovarian cancer. It has offered hope for increasing the rate of long-term survival. The conditions for including patients in drug programs change over time, and the introduction of modern therapy is also accompanied by modern molecular diagnostics. Therefore, the importance of molecular tests should be emphasized.
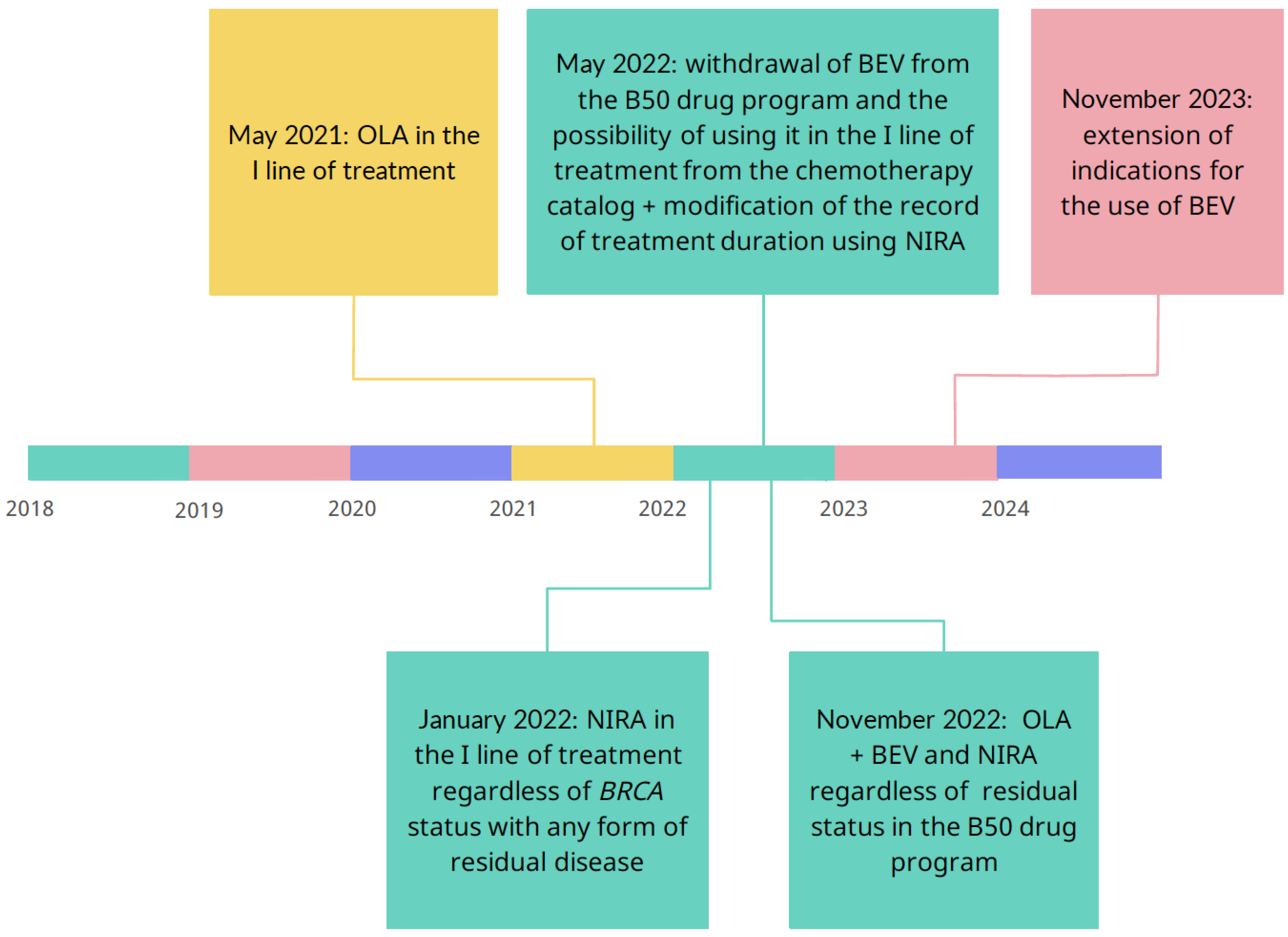
The criteria for inclusion in modern therapies in OC and the availability of them have changed dramatically in recent years, and along with changes in the world, we have witnessed their reflection in Polish conditions. In Poland, modern therapies are implemented as part of the drug program that has been introduced in March 2014 by the Ministry of Health, when was a provision on the possibility of using bevacizumab in the first line of treatment of advanced ovarian, fallopian tube and primary peritoneal cancer [
24]. The drug program was addressed to patients with a high-risk disease progression which group was identified in the ICON7 study and was the only one to achieve a statistically significant benefit from the use of bevacizumab, expressed as prolongation of OS. From September 2016, it was also possible to qualify patients for therapy with olaparib - a PARP inhibitor, in the second line of platinum-sensitive OC after response. The results of the SOLO-1 and PAOLA-1 studies resonated in Polish realities and resulted in an annex to the drug program, and from May 2021, olaparib appeared in the first line of ovarian cancer treatment. Half a year later, from January 2022, patients could use the second PARPi - niraparib - regardless
BRCA status, with any form of residual disease. However, November 2022 brought a real revolution, as the Ministry of Health added a provision in the annex to the therapeutic program about the possibility of combined therapy using bevacizumab and PARPi. The subsequent points marked on the timeline in
Scheme 1 indicate the dynamic changes that have occurred in Polish health care in recent years. It that time Polish program was changed according to the PRIME trial results to use niraparib regardless residual disease.
These modifications prompted the authors to show their impact on patients' access to treatment. The aim of this study is to present a cross-section of patients treated for ovarian, fallopian tube and primary peritoneal cancer, in one center, including the distribution of treatment principles following the most up-to-date guidelines and considering the criteria for inclusion in drug programs.
2. Results
In total, two hundred forty-seven women met the inclusion and exclusion criteria. All patients were taking at least the paclitaxel + platinum derivatives regimen. Of the 247 subjects, 145 patients were treated with bevacizumab, which constitutes 56.4% of the study group. As far as PARPi is concerned, 79 patients used this treatment. Molecular analysis revealed the presence of
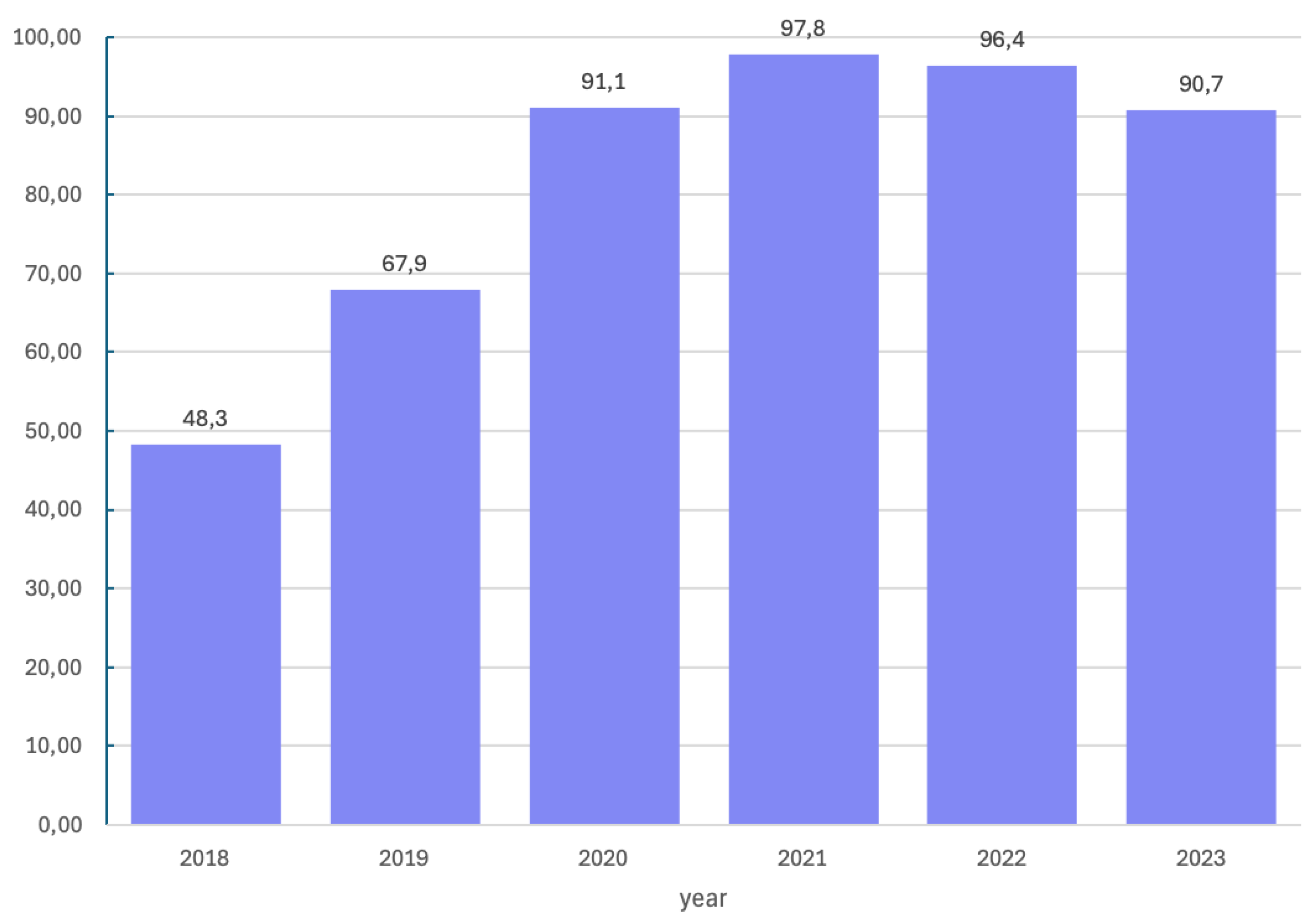
BRCA1/2 pathogenic or likely-pathogenic variant in 70 patients. Patients with a pathogenic or likely-pathogenic variant constituted 27.2% of the study group. Molecular tests were not performed in 14.0%. Over study time, the number of molecular tests performed shows an increasing trend as presented in
Scheme 3. Subsequently, in 2018, when molecular diagnostics were not commonly performed, tests for the
BRCA1/2 pathogenic or likely-pathogenic variant were performed in 48.3% of patients, in 2019 - in 67.9%, in 2020 - in 91.1%, in 2021 - in 97.8%, in 2022 - in 96.4% and in 2023 – in 90.9% of patients.
Scheme 3.
Percentage of molecular tests performed in years 2018 – 2023.
Scheme 3.
Percentage of molecular tests performed in years 2018 – 2023.
Due to the changes in availability of treatment such as PARPi and indications for first-line treatment, the treatment regimens were divided into three periods. Therefore, the first analyzed period is January 2018 - April 2021. In May 2021, the possibility of treatment with olaparib in the first line of chemotherapy was introduced. The second time frame is November 2022 due to the introduction of changes to the list of reimbursed drugs. More patients may use modern treatment for ovarian cancer with combination therapy of PC with bevacizumab and PARPi, regardless of the remnants left during cytoreductive surgery. The reference points are shown in
Scheme 1.
Table 1 shows the distribution of individual chemotherapy protocols used in patients in given periods.
The basic treatment regimen with platinum derivatives (carboplatin or cisplatin) was received by 60 women, constituting less than ¼ of the study group (24.2%). It is noteworthy that 43/60 patients receiving this basic chemotherapy started their treatment between January 2018 and April 2021. Each patient with a BRCA mutation admitted after May 2021 had the opportunity to use more modern therapy. As of May 2021, no BRCA (+) patients received paclitaxel/carboplatin alone. The group of women receiving platinum derivatives with bevacizumab is 100, which constitutes 40.5% of the study group, but taking into account the combination of basic chemotherapy with bevacizumab and PARPi, 145/247 patients (58.7%) achieved it. The introduction of combined treatment with bevacizumab and PARPi into the first line of treatment, regardless of any remnants left during cytoreductive surgery, was introduced in November 2022. Since then, 30% (18/60) of patients (14 with olaparib and 4 with niraparib) have used the above regimen. Previously, in May 2021 - October 2022, it was 27% of patients (13/81 with olaparib and 9/81 with niraparib). Among patients starting treatment in January 2018 - April 2021, only 4.7% (5/106) received the platinum + paclitaxel + olaparib regimen.
A detailed division of chemotherapy used in groups of patients divided according to
BRCA1/2 pathogenic or likely-pathogenic status is shown in
Table 2. The share of PARPi, particularly olaparib, in individual groups and following timeframes is noteworthy. The platinum + paclitaxel + bevacizumab + PARPi regimen in the
BRCA1/2 pathogenic or likely-pathogenic variant carriers was used in 4.7% of patients (5/106) between January 2018 and April 2021 and in 16.0% of women (13/81) admitted between May 2021 and October 2022. Among patients admitted after November 1, 2022, 18/60 patients used the combined treatment of PC + bevacizumab and PARPi, which constitutes 30% of the study group. From this group, 11 were pathogenic or likely-pathogenic
BRCA1/2 variant carriers and additionally, seven women who were not BRCA mutation carriers might benefit from PARP inhibitors.
The following relationship is also noteworthy: in both the BRCAmut and nonBRCAmut patients groups, significantly more patients used PC + PARPi or PC + BEV + PARPi in periods II and III than in period I (p<0.01). Until April 2021, only about 30% of carriers of BRCA1/2 pathogenic or likely-pathogenic variant had access to PARP inhibitors and in the following periods: 92.2% and 85.0% respectively. These changes are also significant in patients from the nonBRCAmut group - in periods II and III, the increase in access to PARPi is 40-fold compared to period I.
We also analyzed patients who had contraindications to bevacizumab. In total, 16 patients could not receive it, which is 6.5% of the study group. Of those patients, the majority of patients (9/16), could not receive bevacizumab due to failure to meet the criteria for inclusion in the drug program in the form of hematological disorders- neutropenia or thrombocytopenia, 3 patients due to poor general clinical condition or disease progression, 3 patients due to a history of pulmonary embolism or cardiac contraindications and 1 patient due to a non-healing postoperative wound.
3. Discussion
The aim of the above work was to show how changes in the availability of new therapeutic options and changing indications for the use of specific drugs affect the qualifications of patients and their use of modern therapy in advanced ovarian, fallopian tube and primary peritoneal cancer. The changes in recommendations and drug usage allowed us to additionally answer the question about the value of molecular diagnostics, which is an indispensable element in introducing subsequent patients to therapeutic programs. Among 247 patients admitted to the University Clinical Hospital to Department of Gyneacological Oncology in Poznań between 2018 and 2023, the number of patients qualified for therapeutic programs that changed over time and had access to the modern therapies increased over time.
Since the Ministry of Health in Poland launched the B50 drug program in March 2014, updates have been systematically appearing in the form of annexes to the drug program. Scheme 4 presents the periods we analyze in relation to changes. the therapeutic program in Poland. Our analysis covers the last six years, so we have been following the patient since 2018. Analyzed period is divided into period I (January 2018 – April 2021); period II (May 2021 – October 2022) and period III (November 2022 – December 2023). A real revolution started with implementing PAPRPis in advanced ovarian, fallopian tube and primary peritoneal cancer. As analysis revealed, only in period I, significantly more patients used PC or the PC + BEV combination, before new therapeutic options became available. In the group of carriers of
BRCA1/2 pathogenic or likely-pathogenic variant, 24% of them received chemotherapy based on platinum derivatives with paclitaxel in period I, and in the following years none of them received chemotherapy alone. This means that every patient with OC FIGO III and IV who is a carrier of
BRCA1/2 pathogenic or likely-pathogenic variant had either PARPi as maintenance treatment or received BEV alone. Patients not receiving bevacizumab most likely underwent radical cytoreductive surgery. However, the group receiving only chemotherapy with bevacizumab in period I (44%) significantly decreased in favor of the introduction of PARP inhibitors, and this shift is visible in
Table 1. In relation to patients with no
BRCA1/2 pathogenic or likely-pathogenic variant, niraparib has been present in therapy since the beginning of period II, which indirectly proves that HRD patients also had the opportunity to use PARPis. The use of a combination of chemotherapy with BEV and PARPi in patients with a
BRCA1/2 pathogenic or likely-pathogenic variant in periods II and III compared to period I (61.9% and 50.0% vs. 17.2%) is statistically significant. The authors assumes that there are no significant differences between periods II and III. The appearance of a provision on the use of niraparib in patients regardless of
BRCA status is reflected in the availability of this drug - in period I, no patients without
BRCA1/2 pathogenic or likely-pathogenic variant received it, while in period II - 18.6% and in period III - 13.3%. Therefore, the introduction of changes in the availability of modern therapy in Poland very quickly changed the structure of patient treatment in our center.
Our study demonstrates a correlation between the changing availability of modern chemotherapy and the increasing number of patients who had access to it. Currently, as of July 2024, the highest usage of the treatment is achieved by patients who took in the first line of treatment - a combination of paclitaxel with platinum derivatives, bevacizumab, and a PARP inhibitor. Our results indicate that in the period until May 2021, 5/106, patients used such therapy, which constitutes 4.7% of patients starting their treatment in January 2018. In the period from May 2021 to October 2022, 22/81, patients took combined therapy, which constituted 27% of patients admitted to the department at that time. Since the official introduction of combination therapy in November 2022, we have observed an even greater increase in the number of patients with access to treatment - they accounted for 30% of the group (18/60). The authors assume that extending the observation period until 2024 might allow for even wider usage of PARPis. Therefore, such analysis and long-term follow-up must be performed to describe the effect of combined therapy on the prolongation of PFS.
Observing the changes that occurred in the BRCAmut group between period II and period III, we can see that by October 2022, 95% of patients had contact with PARPi, and from November 2022 - 85% of patients had. This slight shift may firstly indicate that the patients are autonomous and co-decide about their treatment. Noteworthy, in period I as many as 2/3 of mutation carriers could not have access to PARPi. This means that the introduction of public financing of PARP inhibitors in Poland has dramatically changed the fate of patients, at least those with the BRCA1/2 pathogenic or likely-pathogenic variant.
Our analysis confirmed that for personalized and patient-oriented treatment, genetics diagnostics must be performed as soon as possible. Molecular tests were performed in almost 85% patients in our study. The presence of a
BRCA1/2 pathogenic or likely-pathogenic variant was confirmed in 70 patients, which constitutes 28.3%. Also noteworthy is the fact that the percentage of patients undergoing molecular diagnostics is increasing over the years, as presented in
Scheme 3. It is known that patients who are not
BRCA1/2 pathogenic or likely-pathogenic variant carriers but have a homologous recombination defect (HRD) benefit from additional treatment with PARP inhibitors [
25]. Introducing them to therapy thanks to new indications for reimbursement opened the door and gave hope to many new patients. In Poland, although the need for molecular diagnostics has been paid more in recent years, there is no funding for HRD testing, hence the low percentage of results. Considering current recommendations (as of July 2024), only molecular diagnostics for
BRCA1/2 pathogenic or likely-pathogenic variants in patients diagnosed with OC are covered by reimbursement. According to the data presented in the above work, such diagnostics in patients with a first diagnosis of advanced ovarian, fallopian tube, and primary peritoneal cancer in our center were performed by 85.4% of patients, with an increasing trend over time, but still does not reach 100% coverage of the population. We have not found any data from other foreign centers in the literature that would provide percentage coverage of the population with molecular diagnostics, however other studies also emphasize the need to prioritize clinical trials of innovative treatment methods and improve predictive biomarkers that will enable the selection of patients who would benefit from targeted treatment [
26].
Our work is interesting in that very few centers present therapy regimens for their patients. We believe that sharing such knowledge would be an interesting voice in the discussion. It can be observed that many of our patients use combined therapy. Adding a PARP inhibitor to the combination of carboplatin derivatives + paclitaxel and bevacizumab used during chemotherapy is the most natural treatment as a continuation of the first line. A possible explanation for the molecular synergism of the use of PARP inhibitors with bevacizumab was presented by A.A. Secord. Inhibition of angiogenesis induces hypoxia in the tumor microenvironment, and this reduces the expression of factors involved in homologous recombination. VEGFR3 inhibition is thought to downregulate
BRCA, arrest the cell cycle, and sensitize to chemotherapy, which may benefit
BRCA patients. Inhibition of angiogenesis via VEGFR2 reduces perfusion and increases hypoxia, which reduces the expression of HRR genes, i.e.,
BRCA1, BRCA2, RAD51, which reduces protein production and the ability to repair DNA [
27]. The use of molecular diagnostics is crucial and should be performed as soon as possible after the histopathological diagnosis of the cancer. Thanks to this, information about the molecular structure of cells can directly influence therapeutic decisions and the most personalized treatment.
Still, we do not have direct data on whether PARPi alone is more valuable than PARP inhibitors used together with bevacizumab. Vergote I et al. assumes that patients treated with PARP inhibitors combined with bevacizumab had better results than those treated with niraparib or olaparib alone [
28]. However, it should be remembered that this study is indirect and should be treated with great caution.
The main limitation of our study is a single-center analysis, but we decided to present it to encourage other clinicians to look at their own structure of patient groups and share their experience. Additionally, there is some percentage of patients with missing data, on whom we should focuse on in future. To objectively assess the results of patients' treatment with PARP inhibitors and thus estimate progression-free survival or overall survival, follow-up should be continued for several more years. The clinical data of patients from the current work and their fate will be followed, and we plan to publish the treatment results soon.
This study is the first in Poland to show the profile of patients clearly and reliably with newly diagnosed advanced ovarian, fallopian tube, and primary peritoneal cancer. We assume that this is the first step aimed at sharing knowledge, transparency of the proposed treatment models, and proof that more and more patients are using current therapies following the latest recommendations. We are aware of how comprehensive care oncological patients require, including especially those who have advanced ovarian, fallopian tube, and primary peritoneal cancer. This process is most effective and complete if performed immediately after a cancer diagnosis is obtained. The importance of patient education by health providers but also through social campaigns, should also be emphasized. Patients should be aware of diagnostic and therapeutic options and demand the best treatment for themselves, and we, as healthcare professionals, should treat our patients in accordance with current medical knowledge.
4. Materials and Methods
The study received a positive opinion from the Poznań University of Medical Sciences's Bioethics Committee of the lack of a medical experiment and its retrospective nature. We present a prospective observational study conducted in the University Clinical Hospital in Poznań (former Hospital of the Transfiguration of Lord, former Clinical Hospital named after H. Święcicki), Poland, in 2018-2023.
4.1. Study Design
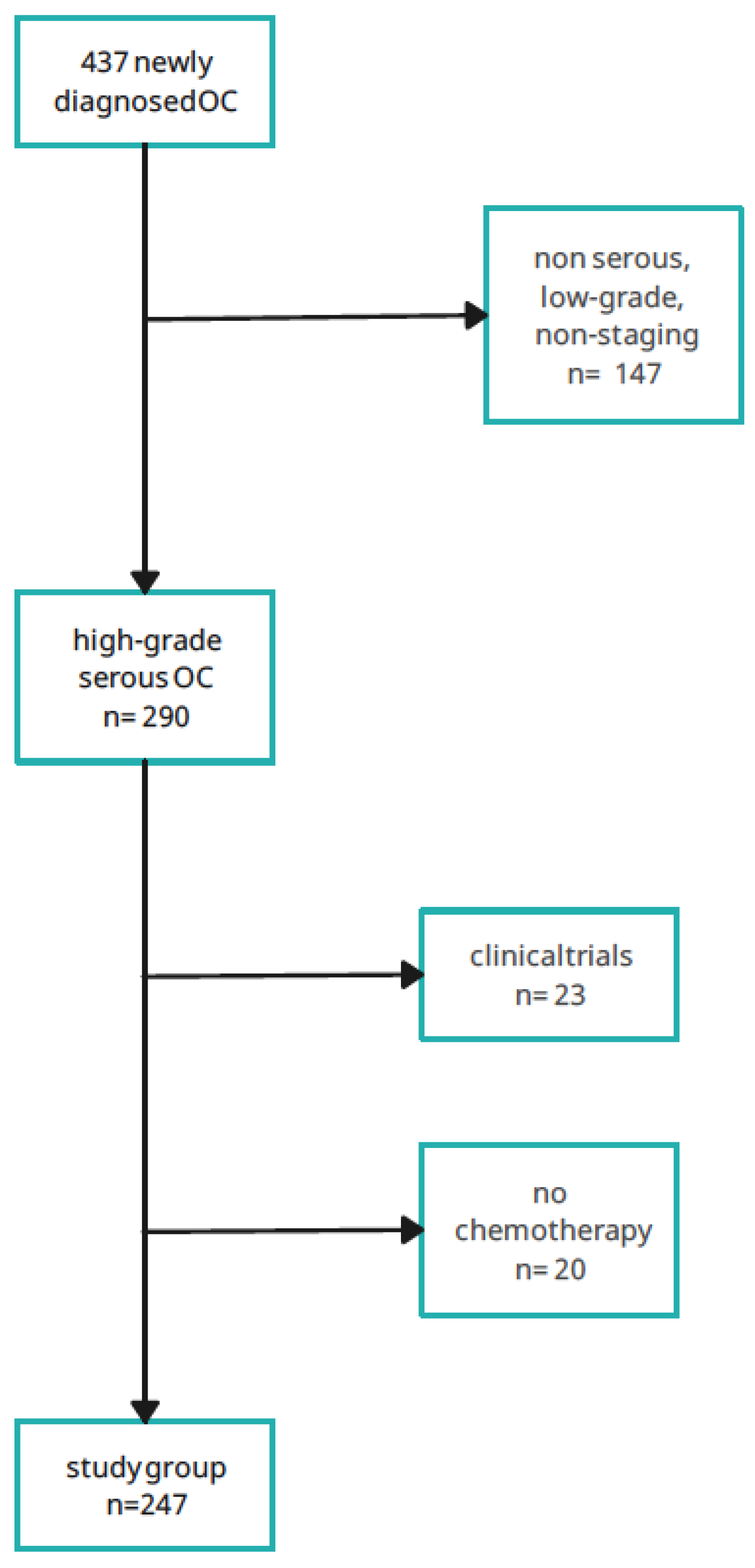
We observed patients who reported to the Department of Gyneacological Oncology between January 2018 and December 2023. We analyzed 437 women with a first diagnosis of ovarian, fallopian tube and primary peritoneal cancer, who had not been treated before. The inclusion criteria for the analysis were:
- (1)
high-grade ovarian, primary peritoneal and fallopian tube cancer,
- (2)
serous histological type,
- (3)
FIGO stage III, IV.
The exclusion criteria in the analysis were:
- (1)
lack of chemotherapy due to the patient's overall poor clinical condition or refusal of treatment,
- (2)
participation in clinical trials using another chemotherapeutics, e.g., pembrolizumab,
- (3)
incomplete documentation, including non-staging type of OC,
- (4)
lack of agreement for treatment.
The reaserch group is a real group of patients who administered to the department for diagnostics or therapy, not a selected group of patients. The
Scheme 1 presents a division into more homogenous cohorts including the possibility of receiving bevacizumab. Further divisions also included variables regarding the results of molecular tests that determined inclusion, in the absence of contraindications, in modern therapies using PARPi. For ease of description, patient groups will be marked BRCAmut, which means patients with
BRCA1/2 pathogenic or likely-pathogenic variant. Similarly, the patient group nonBRCAmut means patients with nonpathogenic or of no clinical significance variant.
The above work analyzes and divides patients into groups regarding the chemotherapy drugs they take. It is worth noting that during the study period, both new therapeutic programs appeared, and the inclusion criteria have changed. The availability of PARP inhibitors changed in subsequent years and the scope of use bevacizumab has extended.
4.2. Statistical Analysis
We conducted all analyses in the statistical software R, version 4.2.1 (R Core Team 2022, R: language and environment for statistical computing by the R Foundation for Statistical Computing, Vienna, Austria). Nominal variables are presented in the tables as the number and percentage of observations. Dependencies between two variables were analysed using a chi-squared test or Fisher’s exact test, depending on whether the assumption of expected counts of not less than five in at least 80% of the cells in the respective contingency table was satisfied. The strength of significant relationships between two categorical variables was measured using Cramer’s V coefficient.
Scheme 2.
Study design. OC- ovarian cancer, primary peritoneal and fallopian tube cancer; n- number.
Scheme 2.
Study design. OC- ovarian cancer, primary peritoneal and fallopian tube cancer; n- number.
5. Conclusions
To sum up, the introduction of modern treatment in our center very quickly changed the structure of patient treatment. Moreover, patients who were carriers of BRCA1/2 pathogenic or likely-pathogenic variant had previously received only chemotherapy, disappeared after the introduction of PARPi, in favor of new therapies. Additionally, the group of patients receiving PARPi in maintenance treatment, regardless of BRCA status, increased statistically significantly and this group remains stable. Then, most patients with no BRCA1/2 pathogenic or likely-pathogenic variant, who used PARPi in therapy in our center – received niraparib and therefore they are beneficiaries of molecular diagnostics in the field of HRD.
Author Contributions
Concept: S.M-K., R.M., M.S-N.; Methodology: S.M-K., R.M., M.S-N.; Study project: S.M-K., R.M.; Validation: G.P., K.J-N., M.P.; Analysis of data: S.M-K, M.S-N, R.M., D.P.; Conducting the study: S.M-K.; Data storing: L.T., G.P.; Writting a manuscript: S.M-K., D.P.; Editing and reviewing of manuscript: R.M., M.S-N.; Project administration: R.M., K.J-N.; Funding: S.M-K.
Funding
This research received no external funding.
Institutional Review Board Statement
The study received a positive opinion from the Poznań University of Medical Sciences's Bioethics Committee of the lack of a medical experiment and its retrospective nature.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data available at corresponding author.
Conflicts of Interest
The authors declare no conflicts of interests.
References
- The Global Cancer Observatory. GLOBOCAN 2020: International Agency Research on Cancer. International Agency for Research on Cancer. 2020;509.
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8). [CrossRef]
- McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and Cisplatin Compared with Paclitaxel and Cisplatin in Patients with Stage III and Stage IV Ovarian Cancer. New England Journal of Medicine. 1996;334(1). [CrossRef]
- du Bois A, Lück HJ, Meier W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95(17). [CrossRef]
- Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE, Boente M, Birrer MJ, Liang SX; Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011 Dec 29;365(26):2473-83. [CrossRef] [PubMed]
- Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16(8). [CrossRef]
- Tewari KS, Burger RA, Enserro D, Norquist BM, Swisher EM, Brady MF, Bookman MA, Fleming GF, Huang H, Homesley HD, Fowler JM, Greer BE, Boente M, Liang SX, Ye C, Bais C, Randall LM, Chan JK, Ferriss JS, Coleman RL, Aghajanian C, Herzog TJ, DiSaia PJ, Copeland LJ, Mannel RS, Birrer MJ, Monk BJ. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J Clin Oncol. 2019 Sep 10;37(26):2317-2328. Epub 2019 Jun 19. [CrossRef] [PubMed] [PubMed Central]
- du Bois A, Combe M, Rochon J, et al. Epirubicin/paclitaxel/carboplatin (TEC) vs paclitaxel/carboplatin (TC) in first-line treatment of ovarian cancer (OC) FIGO stages IIB–IV. An AGO-GINECO Intergroup phase III trial. Journal of Clinical Oncology. 2004;22(14_suppl). [CrossRef]
- Kristensen GB, Vergote I, Stuart G, et al. First-line treatment of ovarian/tubal/peritoneal cancer FIGO stage IIB-IV with paclitaxel/carboplatin with or without epirubicin (TEC vs TC). A gynecologic cancer intergroup study of the NSGO, EORTC GCG, and NCIC CTG. Results on progression-free survival. International Journal of Gynecological Cancer. 2005;15(6 SUPPL. 3). [CrossRef]
- Bookman MA, Brady MF, McGuire WP, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: A phase III trial of the gynecologic cancer intergroup. Journal of Clinical Oncology. 2009;27(9). [CrossRef]
- Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. Obstet Gynecol Surv. 2012;67(5). [CrossRef]
- Perren TJ, Swart AM, Pfisterer J, et al. A Phase 3 Trial of Bevacizumab in Ovarian Cancer. New England Journal of Medicine. 2011;365(26). [CrossRef]
- Verheijen RH, Massuger LF, Benigno BB, et al. Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission. Journal of Clinical Oncology. 2006;24(4). [CrossRef]
- Piccart MJ, Floquet A, Scarfone G, et al. Intraperitoneal cisplatin versus no further treatment: 8-Year results of EORTC 55875, a randomized phase III study in ovarian cancer patients with a pathologically complete remission after platinum-based intravenous chemotherapy. In: International Journal of Gynecological Cancer. Vol 13. ; 2003. [CrossRef]
- O'Malley DM, Krivak TC, Kabil N, Munley J, Moore KN. PARP Inhibitors in Ovarian Cancer: A Review. Target Oncol. 2023 Jul;18(4):471-503. Epub 2023 Jun 3. [CrossRef] [PubMed] [PubMed Central]
- Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035). [CrossRef]
- Farmer H, McCabe H, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035). [CrossRef]
- Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: A proof-of-concept trial. The Lancet. 2010;376(9737). [CrossRef]
- Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. Journal of Clinical Oncology. 2012;30(4). [CrossRef]
- Moore K, Colombo N, Scambia G, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. New England Journal of Medicine. 2018;379(26). [CrossRef]
- González-Martín A, Pothuri B, Vergote I, Graybill W, Lorusso D, McCormick CC, Freyer G, Backes F, Heitz F, Redondo A, Moore RG, Vulsteke C, O'Cearbhaill RE, Malinowska IA, Shtessel L, Compton N, Mirza MR, Monk BJ. Progression-free survival and safety at 3.5years of follow-up: results from the randomised phase 3 PRIMA/ENGOT-OV26/GOG-3012 trial of niraparib maintenance treatment in patients with newly diagnosed ovarian cancer. Eur J Cancer. 2023 Aug;189:112908. Epub 2023 May 3. [CrossRef] [PubMed Central]
- Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. New England Journal of Medicine. 2019;381(25). [CrossRef]
- Wang DF, Zhang J, Zhang C, Yu J, Shi Y, Xu SQ, Fan Y, Zhou FZ, Song SQ, Liu H, Zhang GN. [Real-world clinical data analysis of PARPi as first-line maintenance therapy in newly diagnosed epithelial ovarian cancer patients]. Zhonghua Fu Chan Ke Za Zhi. 2022 Sep 25;57(9):641-652. Chinese. [CrossRef] [PubMed]
- https://www.gov.pl/web/zdrowie/obwieszczenia-ministra-zdrowia-lista-lekow-refundowanych.
- Nguyen L, W. M. Martens J, Van Hoeck A, Cuppen E. Pan-cancer landscape of homologous recombination deficiency. Nat Commun. 2020;11(1). [CrossRef]
- Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69(4). [CrossRef]
- Alvarez Secord A, O’Malley DM, Sood AK, Westin SN, Liu JF. Rationale for combination PARP inhibitor and antiangiogenic treatment in advanced epithelial ovarian cancer: A review. Gynecol Oncol. 2021;162(2). [CrossRef]
- Vergote I, Ray-Coquard I, Anderson DM, et al. Population-adjusted indirect treatment comparison of the SOLO1 and PAOLA-1/ENGOT-ov25 trials evaluating maintenance olaparib or bevacizumab or the combination of both in newly diagnosed, advanced BRCA-mutated ovarian cancer. Eur J Cancer. 2021;157. [CrossRef]
Scheme 1.
– Analyzed periods in correlation with changes in the therapeutic program in Poland. OLA- olaparib; BEV- bevacizumab; NIRA- niraparib.
Scheme 1.
– Analyzed periods in correlation with changes in the therapeutic program in Poland. OLA- olaparib; BEV- bevacizumab; NIRA- niraparib.
Table 1.
Distribution of chemotherapy in patients.
Table 1.
Distribution of chemotherapy in patients.
| |
|
period |
|
I / January 2018 - April 2021 (40 months) |
II / May 2021 – October 2022 (17 months) |
III / November 2022 – December 2023 (13 months) |
| |
|
|
|
n |
% of concrete group |
% of group from whole period |
n |
% of concrete group |
% of group from whole period |
n |
% of concrete group |
% of group from whole period |
| |
|
BRCAmut |
|
|
|
|
|
|
|
|
|
|
| PC |
- |
- |
- |
7 |
24.1% |
6.6% |
0 |
0.0% |
0.0% |
0 |
0.0% |
0.0% |
| PC |
BEV |
- |
- |
13 |
44.8% |
12.3% |
1 |
4.8% |
1.2% |
3 |
15.0% |
5.0% |
| PC |
BEV |
OLA |
- |
5 |
17.2% |
4.7% |
13 |
61.9% |
16.0% |
10 |
50.0% |
16.7% |
| PC |
BEV |
- |
NIR |
0 |
0.0% |
0.0% |
0 |
0.0% |
0.0% |
1 |
5.0% |
1.7% |
| PC |
- |
OLA |
- |
4 |
13.9% |
3.8% |
7 |
33.3% |
8.7% |
5 |
25.0% |
8.3% |
| PC |
- |
- |
NIR |
0 |
0.0% |
0.0% |
0 |
0.0% |
0.0% |
1 |
5.0% |
1.7% |
| |
|
|
|
29 |
100.0% |
27.4% |
21 |
100.0% |
25.9% |
20 |
100.0% |
33.4% |
| |
|
nonBRCAmut |
|
|
|
|
|
|
|
|
|
|
| PC |
- |
- |
- |
21 |
42.9% |
19.9% |
6 |
10.5% |
7.4% |
7 |
20.0% |
11.7% |
| PC |
BEV |
- |
- |
27 |
55.1% |
25.5% |
27 |
47.4% |
33.3% |
13 |
37.1% |
21.7% |
| PC |
BEV |
OLA |
- |
0 |
0.0% |
0.0% |
0 |
0.0% |
0.0% |
4 |
11.4% |
6.6% |
| PC |
BEV |
- |
NIR |
0 |
0.0% |
0.0% |
9 |
15.8% |
11.1% |
3 |
8.6% |
5.0% |
| PC |
- |
OLA |
- |
1 |
2.0% |
0.9% |
0 |
0.0% |
0.0% |
0 |
0.0% |
0.0% |
| PC |
- |
- |
NIR |
0 |
0.0% |
0.0% |
15 |
26.3% |
18.6% |
8 |
22.9% |
13.3% |
| |
|
|
|
49 |
100.0% |
46.3% |
57 |
100.0% |
70.4% |
35 |
100.0% |
58.3% |
| |
|
no data |
|
|
|
|
|
|
|
|
|
|
| PC |
- |
- |
- |
15 |
53.6% |
14.1% |
2 |
66.7% |
2.5% |
2 |
40.0% |
3.3% |
| PC |
BEV |
- |
- |
12 |
42.9% |
11.3% |
1 |
33.3% |
1.2% |
3 |
60.0% |
5.0% |
| PC |
BEV |
OLA |
- |
0 |
0.0% |
0.0% |
0 |
0.0% |
0.0% |
0 |
0.0% |
0.0% |
| PC |
BEV |
- |
NIR |
0 |
0.0% |
0.0% |
0 |
0.0% |
0.0% |
0 |
0.0% |
0.0% |
| PC |
- |
OLA |
- |
1 |
3.5% |
0.9% |
0 |
0.0% |
0.0% |
0 |
0.0% |
0.0% |
| PC |
- |
- |
NIR |
0 |
0.0% |
0.0% |
0 |
0.0% |
0.0% |
0 |
0.0% |
0.0% |
| |
|
|
|
28 |
100.0% |
26.3% |
3 |
100.0% |
3.7% |
5 |
100.0% |
8.3% |
| |
|
total |
|
106 |
|
|
81 |
|
|
55 |
|
|
Table 2.
Distribution of chemotherapy in patients according to BRCAmut status.
Table 2.
Distribution of chemotherapy in patients according to BRCAmut status.
| |
|
Period I |
Period II |
Period III |
all |
|
|
|
| BRCA status |
|
N |
% |
N |
% |
N |
% |
N |
% |
χ² (df) |
p |
Vc |
| BRCAmut |
PC |
7 |
24.1% |
0 |
0.0% |
0 |
0.0% |
7 |
10.0% |
26.86* |
<0.001 |
0.45 |
| PC + BEV |
13 |
44.8% |
1 |
4.8% |
3 |
15.0% |
17 |
24.3% |
| PC + PARPi |
4 |
13.8% |
7 |
33.3% |
6 |
30.0% |
17 |
24.3% |
| PC + BEV + PARPi |
5 |
17.2% |
13 |
61.9% |
11 |
55.0% |
29 |
41.4% |
| all |
29 |
100.0% |
21 |
100.0% |
20 |
100.0% |
70 |
100.0% |
| |
PARPi together |
9 |
31.0% |
20 |
95.2% |
17 |
85.0% |
|
|
|
|
|
| nonBRCAmut |
PC |
21 |
42.9% |
6 |
10.5% |
7 |
20.0% |
34 |
24.1% |
36.13* |
<0.001 |
0.34 |
| PC + BEV |
27 |
55.1% |
27 |
47.4% |
13 |
37.1% |
67 |
47.5% |
| PC + PARPi |
1 |
2.0% |
15 |
26.3% |
8 |
22.9% |
24 |
17.0% |
| PC + BEV + PARPi |
0 |
0.0% |
9 |
15.8% |
7 |
20.0% |
16 |
11.3% |
| all |
49 |
100.0% |
57 |
100.0% |
35 |
100.0% |
141 |
100.0% |
| |
PARPi together |
1 |
2.0% |
24 |
42.1% |
15 |
42.9% |
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).