Submitted:
09 September 2024
Posted:
10 September 2024
You are already at the latest version
Abstract
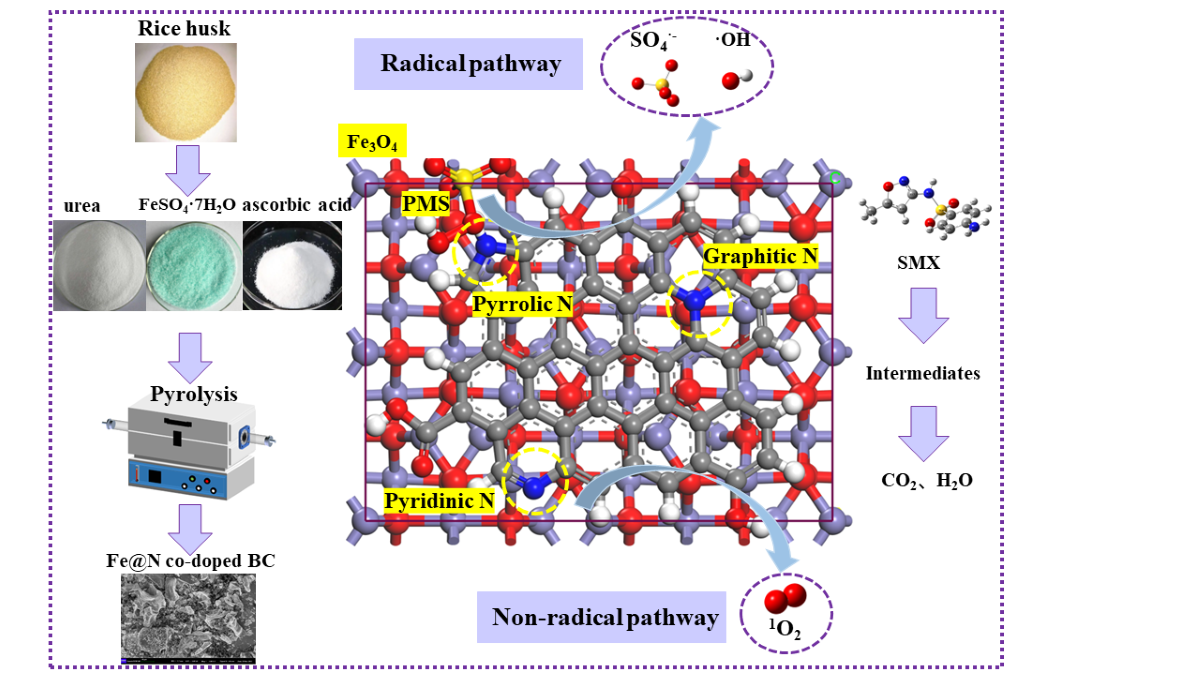
Keywords:
1. Introduction
2. Results and Discussion
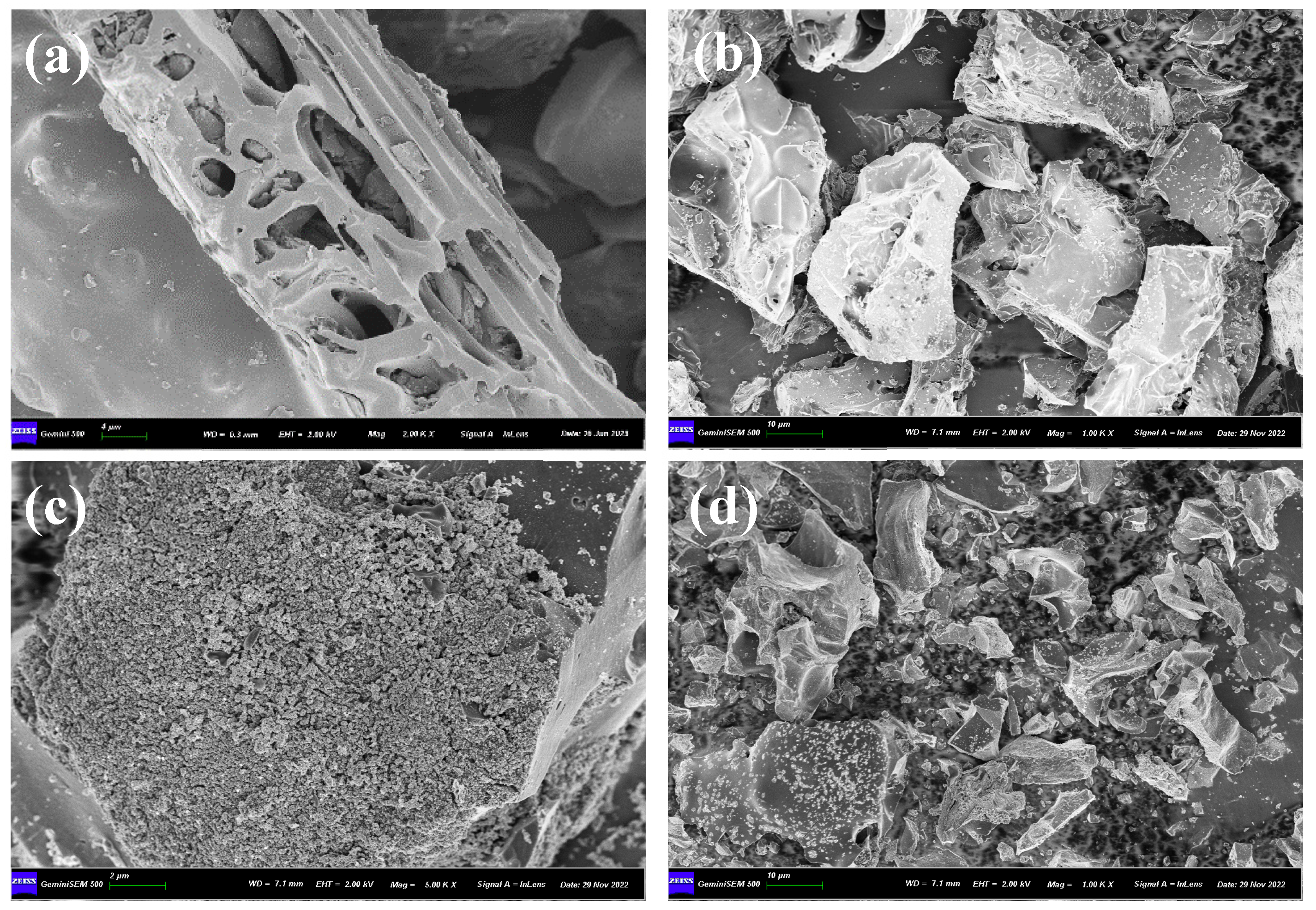
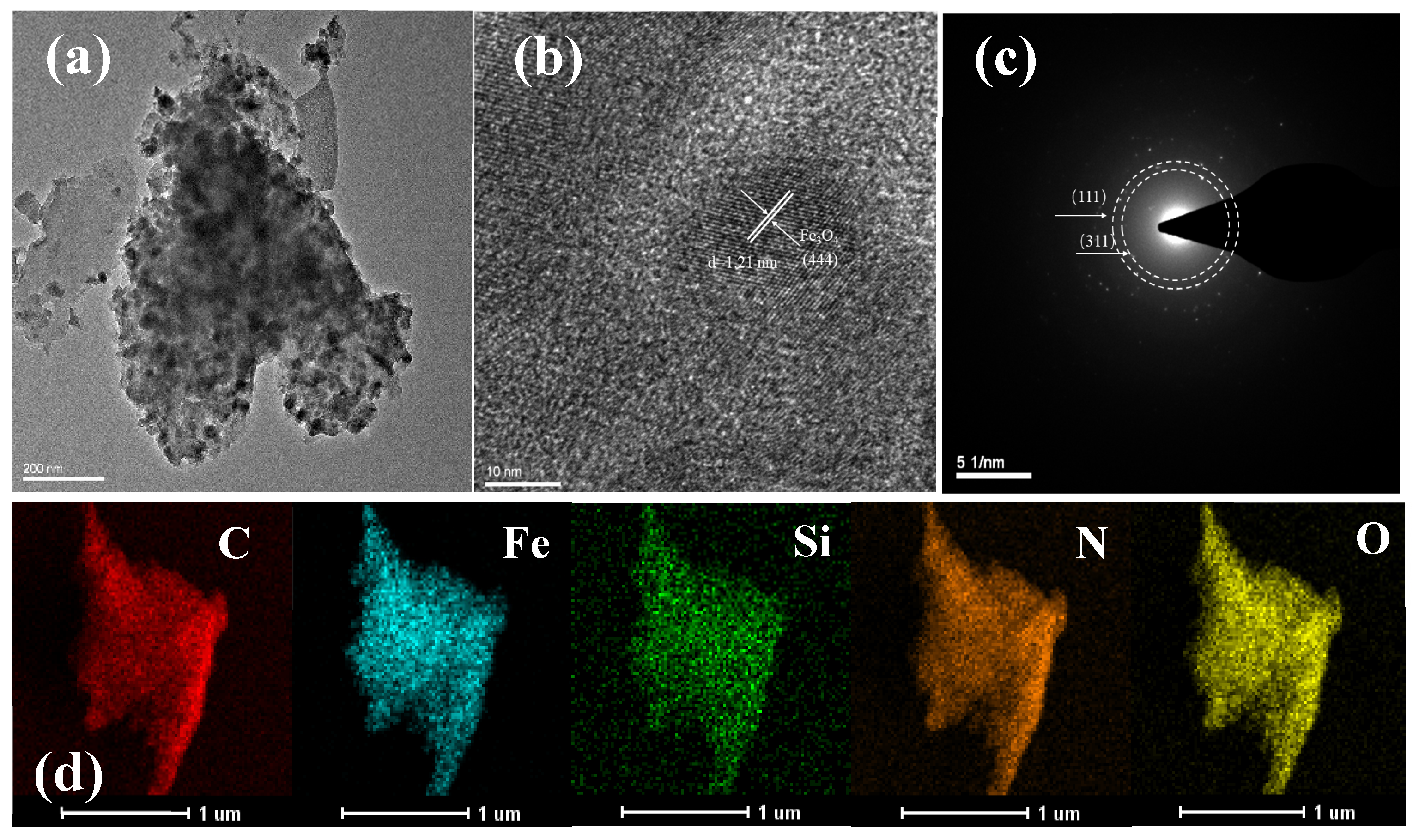
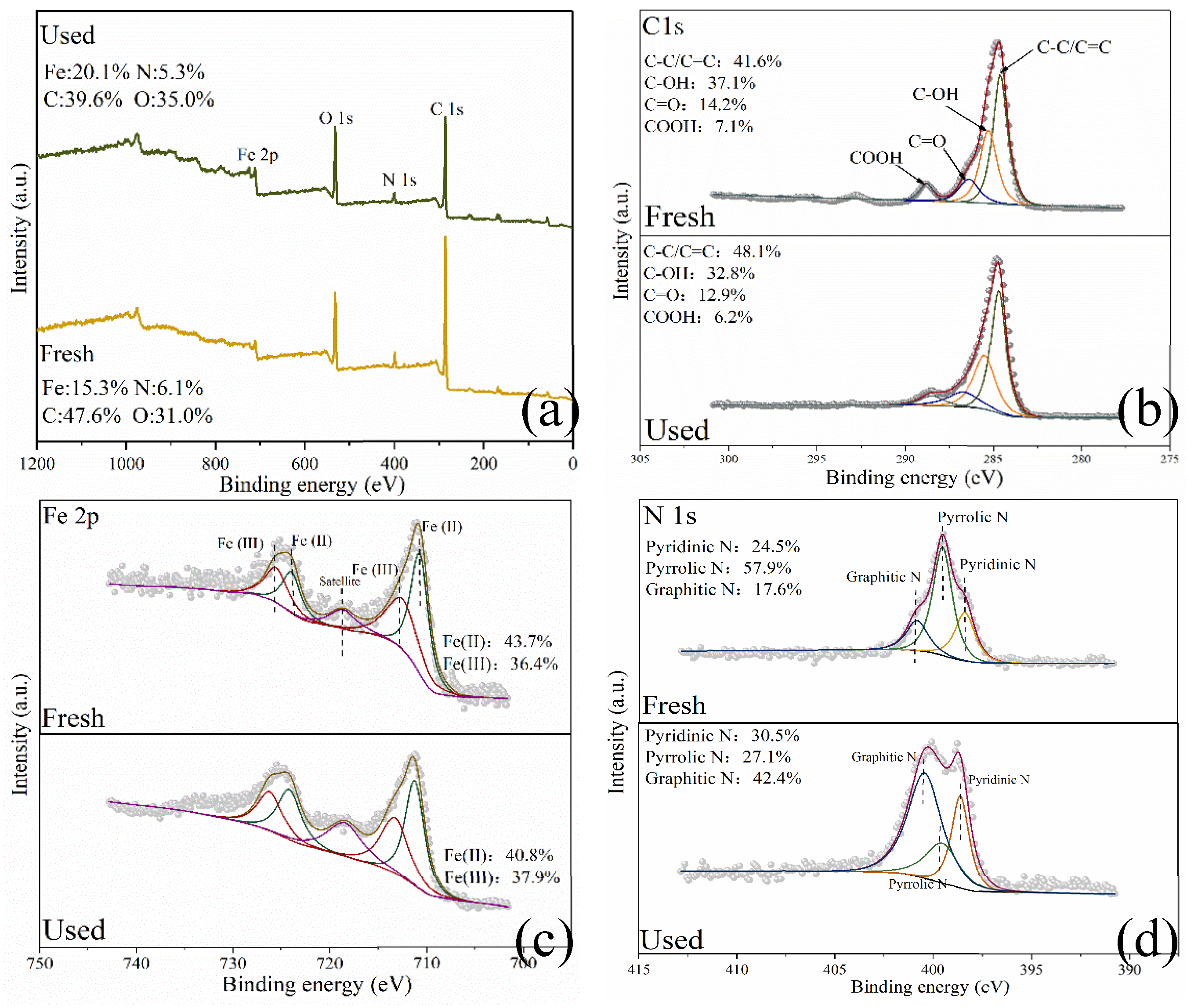
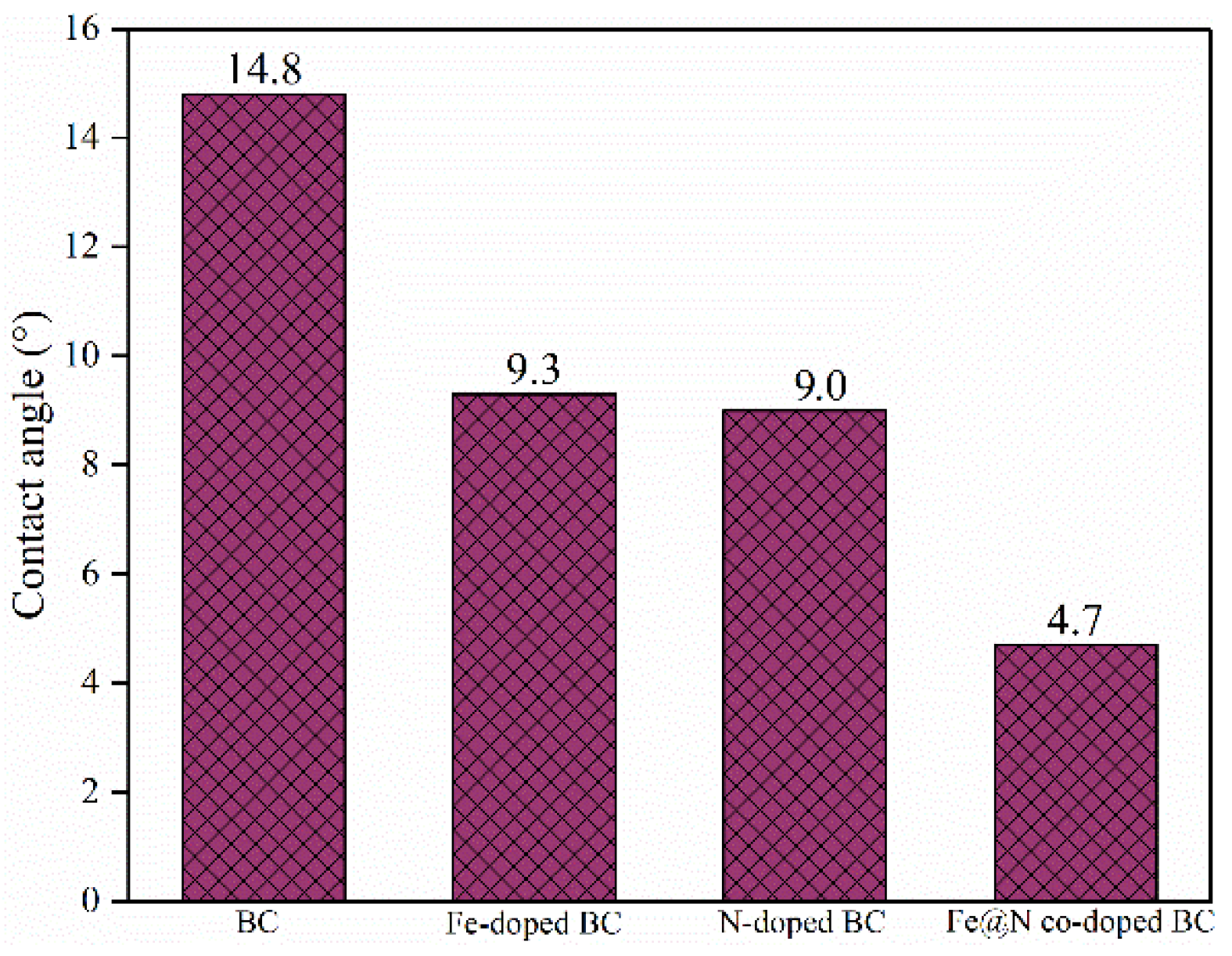
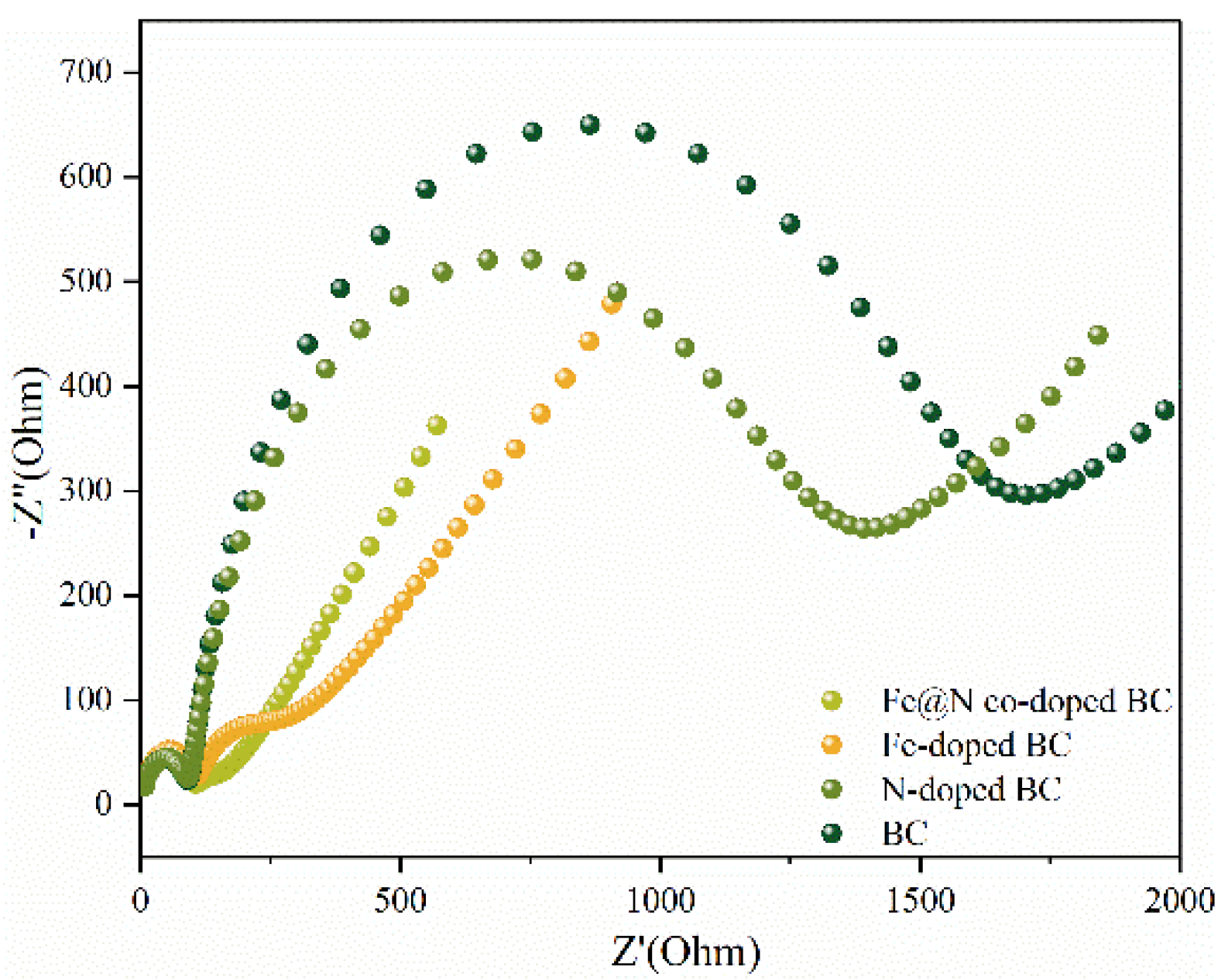
2.1. Characterization
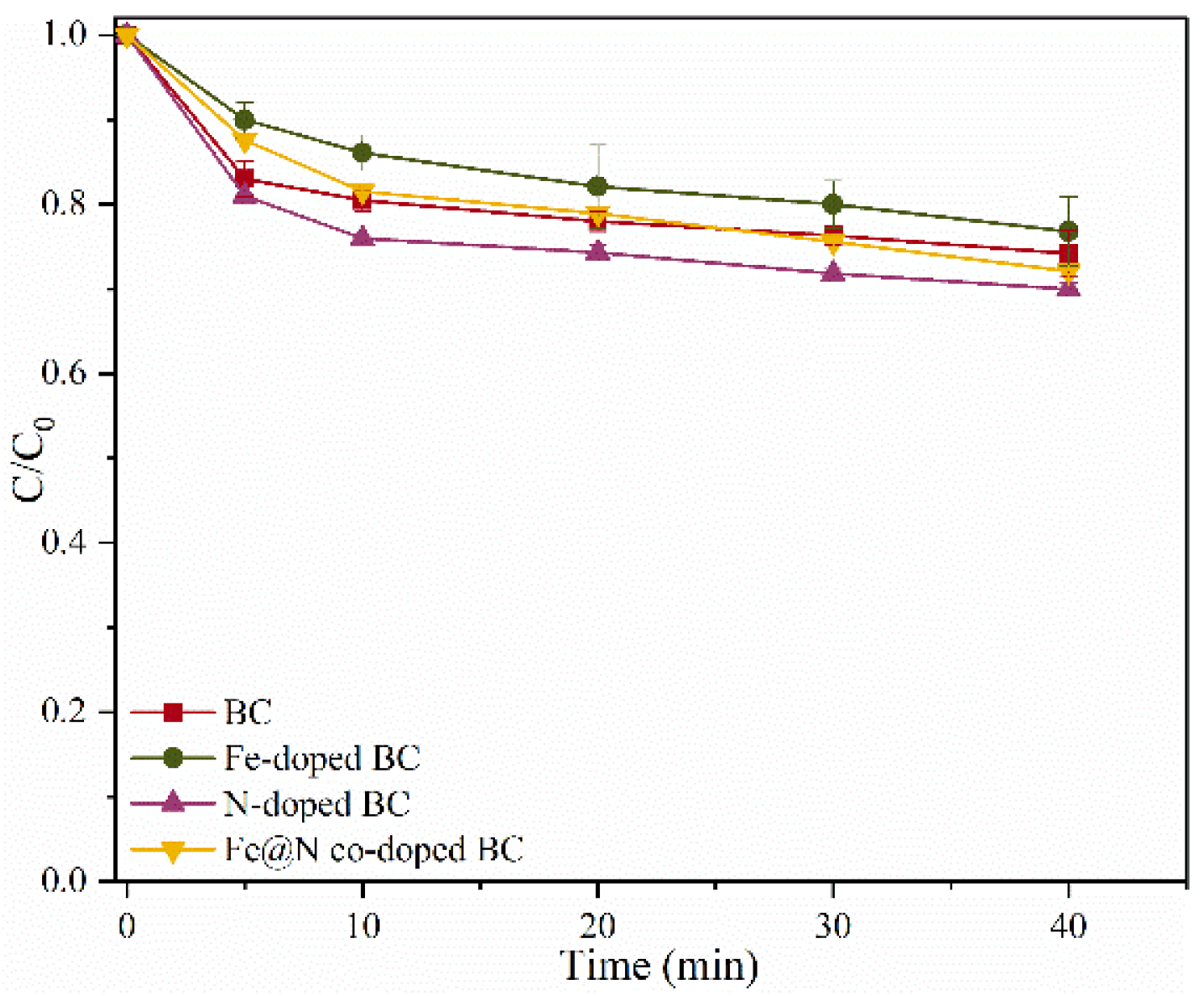
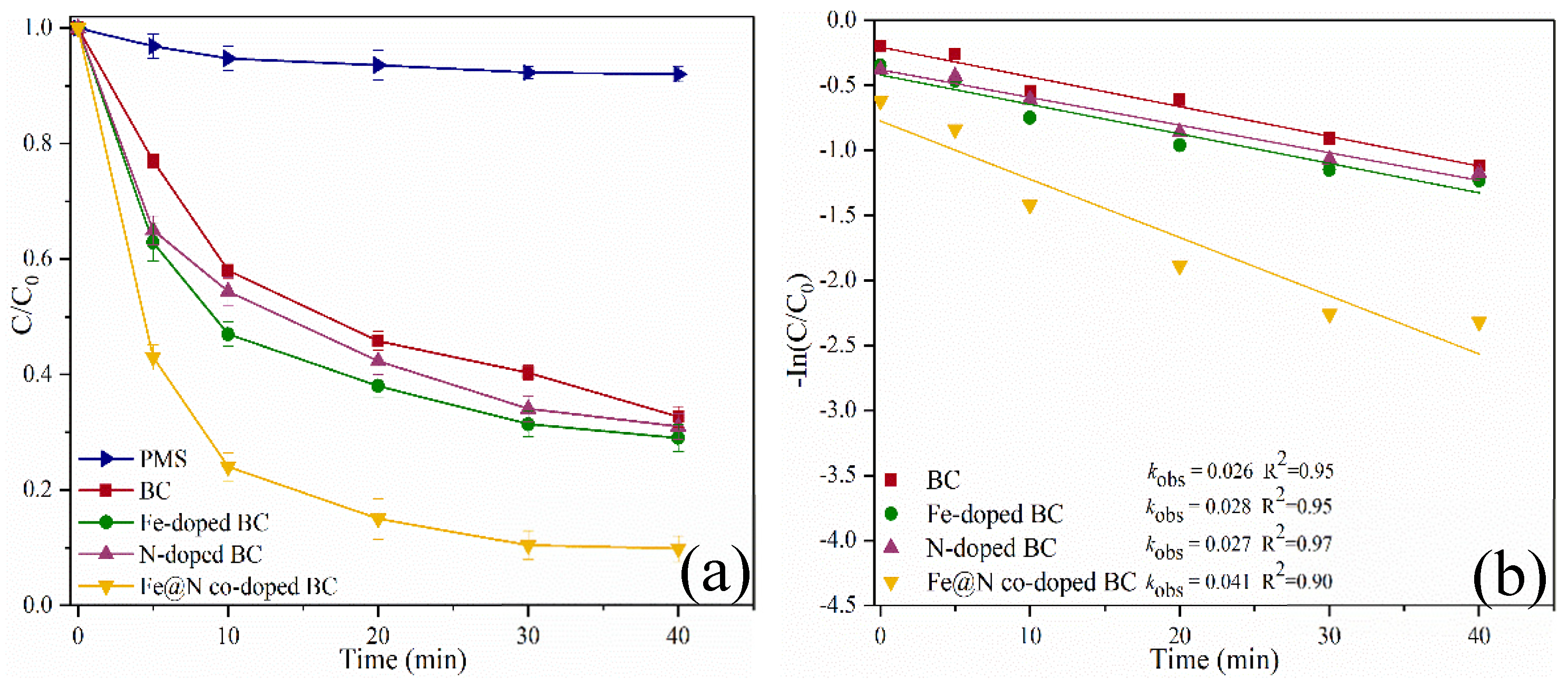
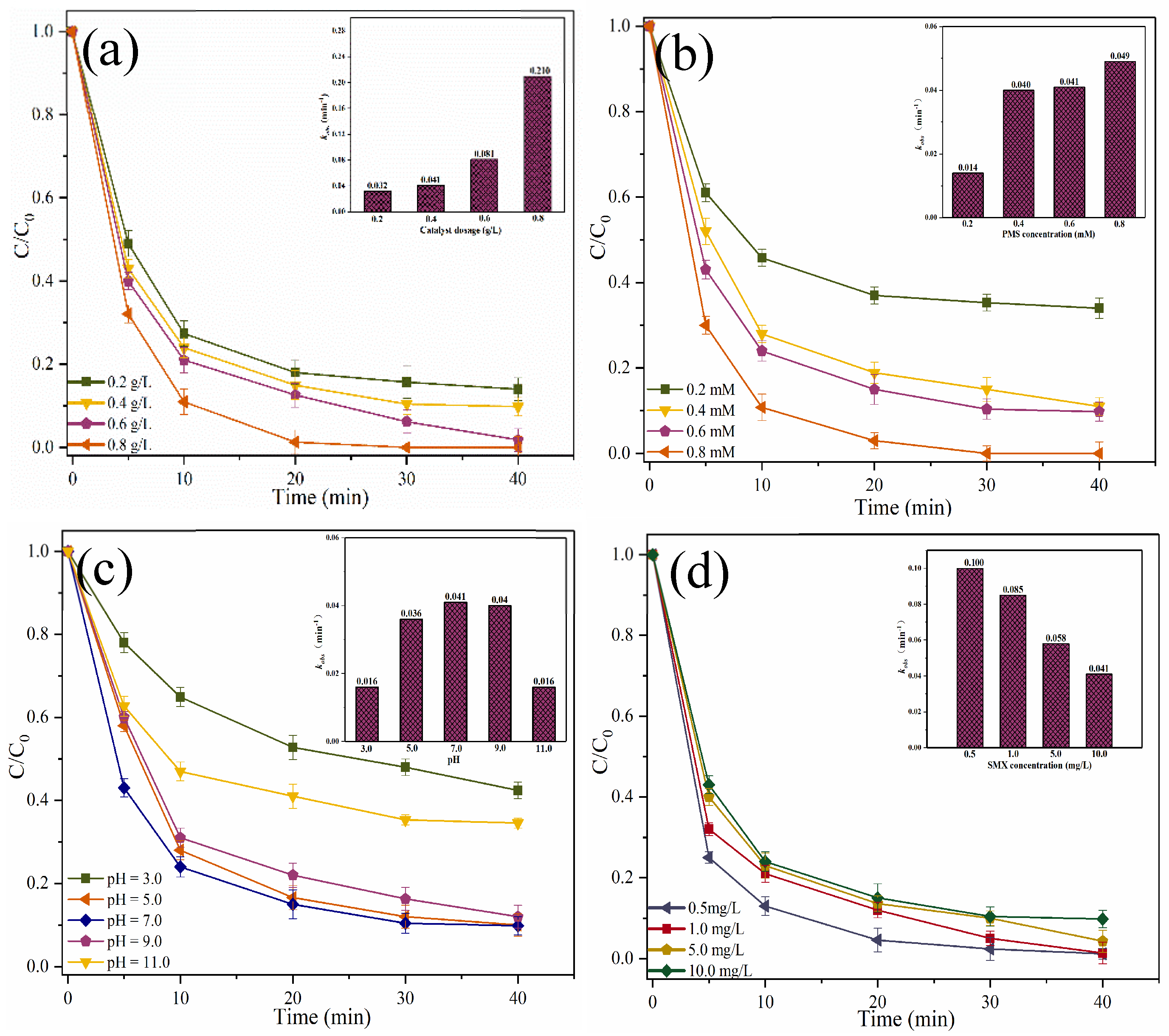
2.2. Catalytic Oxidation of SMX
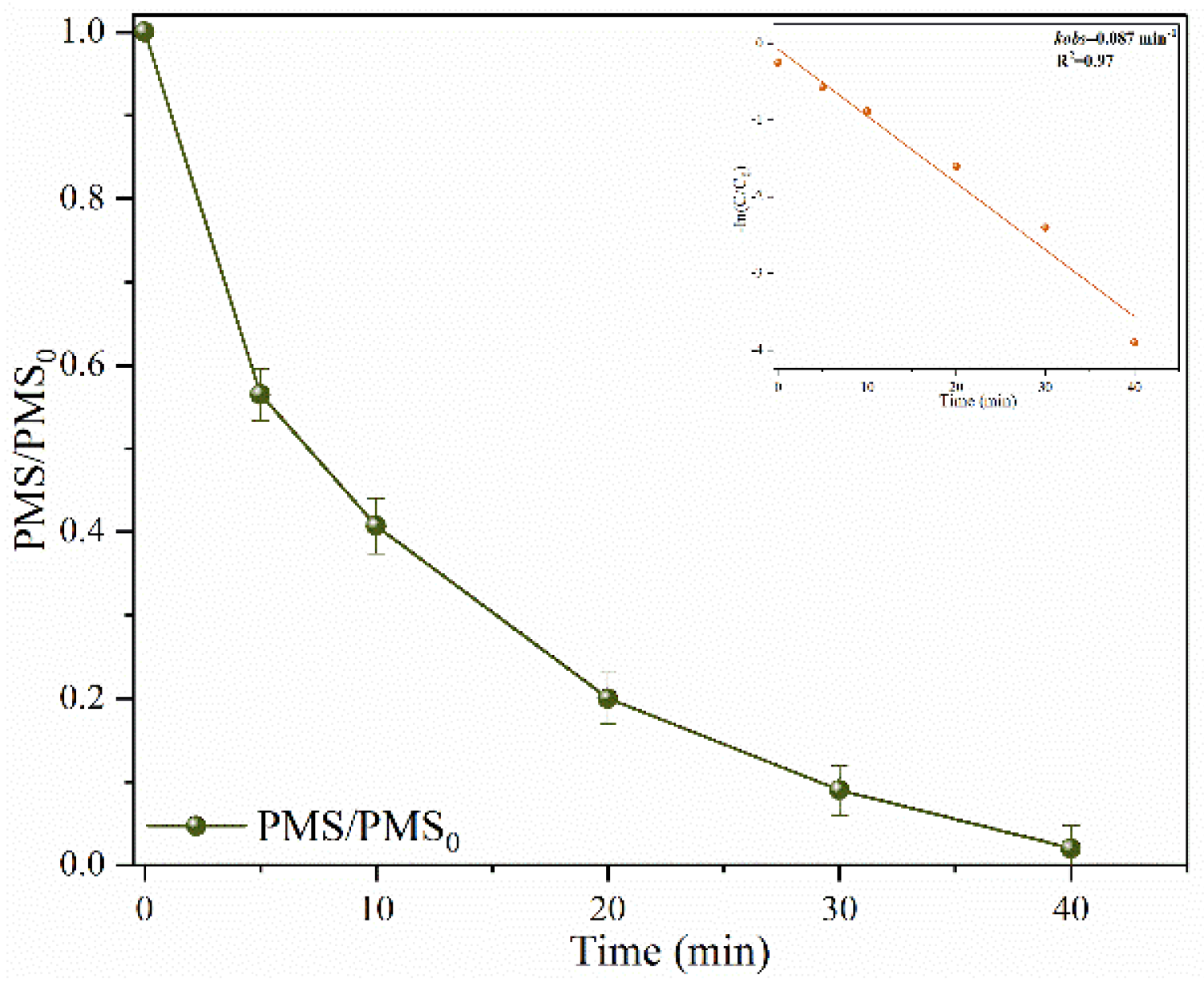
2.3. Mechanism Discussion
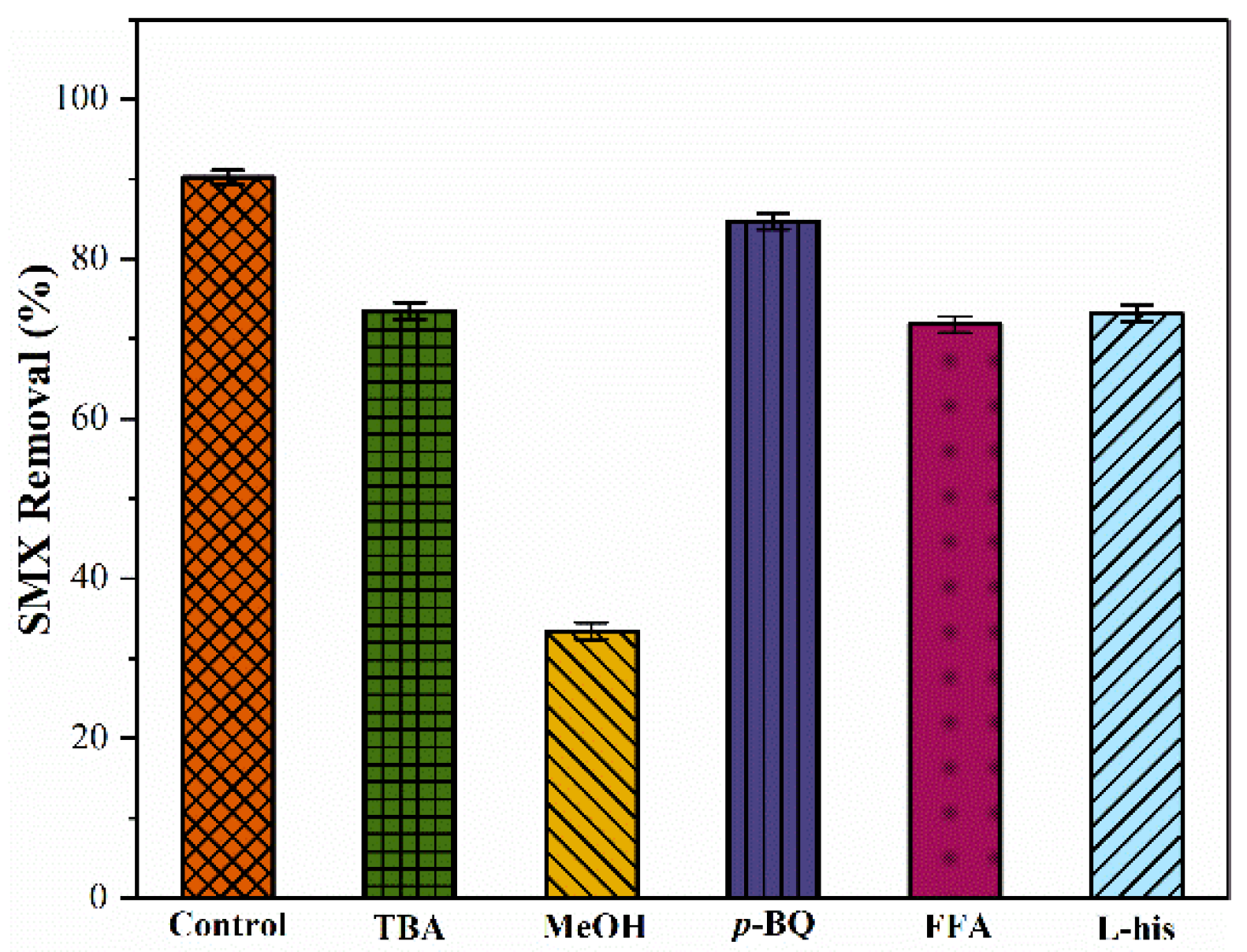
2.3.1. Identification of ROSs
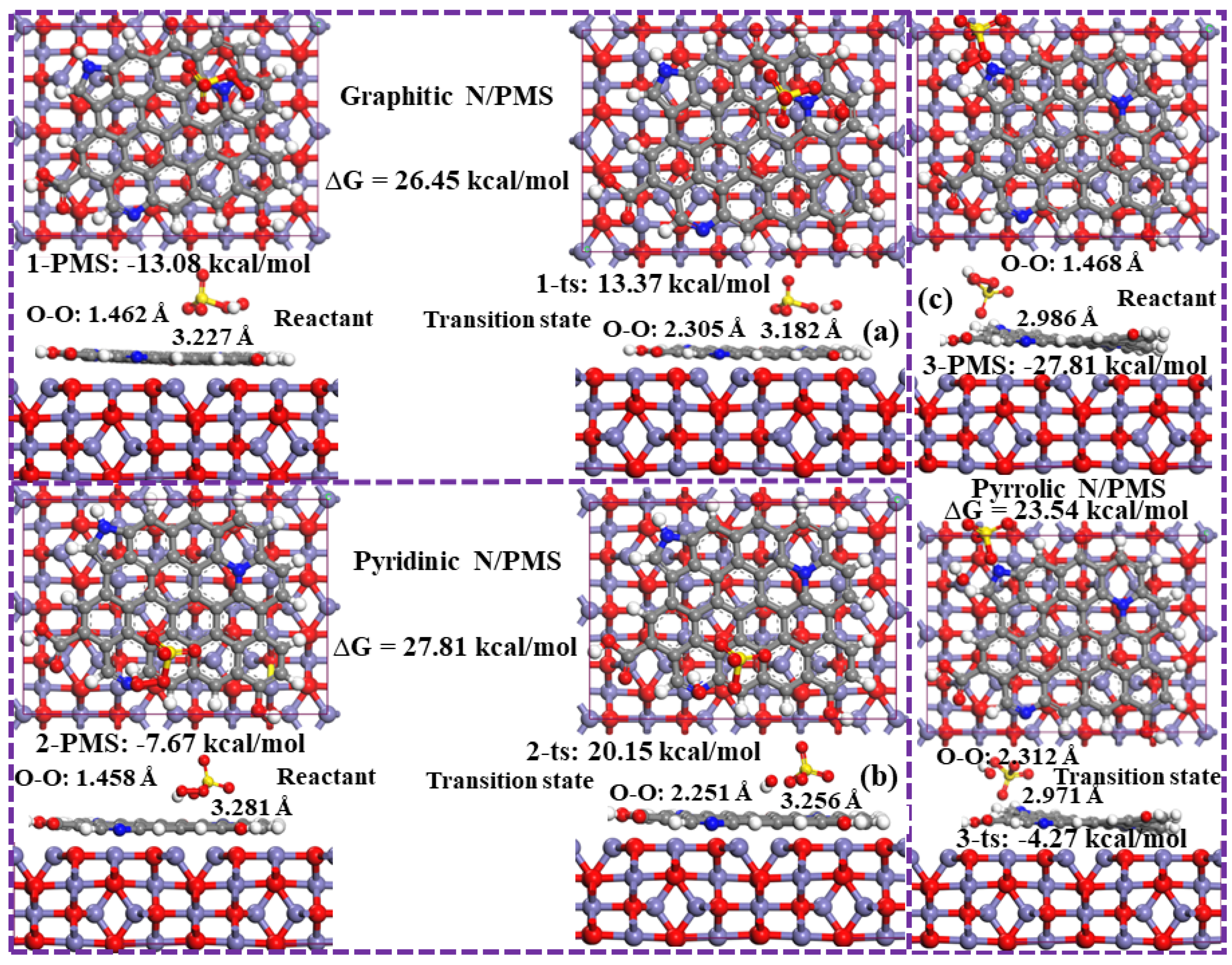
2.3.2. Reaction Mechanism
2.4. Degradation Pathways of SMX
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Reaction Procedures
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- J. Du, W. Guo, H. Wang, R. Yin, H. Zheng, X. Feng, D. Che, N. Ren, Hydroxyl radical dominated degradation of aquatic sulfamethoxazole by Fe(0)/bisulfite/O2: Kinetics, mechanisms, and pathways, Water Res, 138 (2018) 323-332.
- M. Xu, J. Li, Y. Yan, X. Zhao, J. Yan, Y. Zhang, B. Lai, X. Chen, L. Song, Catalytic degradation of sulfamethoxazole through peroxymonosulfate activated with expanded graphite loaded CoFe2O4 particles, Chemical Engineering Journal, 369 (2019) 403-413.
- Y. Xu, S. Liu, M. Wang, J. Zhang, H. Ding, Y. Song, Y. Zhu, Q. Pan, C. Zhao, H. Deng, Thiourea-assisted one-step fabrication of a novel nitrogen and sulfur co-doped biochar from nanocellulose as metal-free catalyst for efficient activation of peroxymonosulfate, J Hazard Mater, 416 (2021) 125796.
- L. Xu, B. Fu, Y. Sun, P. Jin, X. Bai, X. Jin, X. Shi, Y. Wang, S. Nie, Degradation of organic pollutants by Fe/N co-doped biochar via peroxymonosulfate activation: Synthesis, performance, mechanism and its potential for practical application, Chemical Engineering Journal, 400 (2020).
- J. Wang, S. Wang, Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants, Chemical Engineering Journal, 334 (2018) 1502-1517.
- C. Zhao, B. Shao, M. Yan, Z. Liu, Q. Liang, Q. He, T. Wu, Y. Liu, Y. Pan, J. Huang, J. Wang, J. Liang, L. Tang, Activation of peroxymonosulfate by biochar-based catalysts and applications in the degradation of organic contaminants: A review, Chemical Engineering Journal, 416 (2021).
- J. Zhen, S. Zhang, X. Zhuang, S. Ahmad, T. Lee, H. Si, C. Cao, S.-Q. Ni, Sulfate radicals based heterogeneous peroxymonosulfate system catalyzed by CuO-Fe3O4-Biochar nanocomposite for bisphenol A degradation, Journal of Water Process Engineering, 41 (2021).
- C. Wang, R. Huang, R. Sun, J. Yang, M. Sillanpää, A review on persulfates activation by functional biochar for organic contaminants removal: Synthesis, characterizations, radical determination, and mechanism, Journal of Environmental Chemical Engineering, 9 (2021).
- T. Liu, K. Cui, C.X. Li, Y. Chen, Q. Wang, X. Yuan, Y. Chen, J. Liu, Q. Zhang, Efficient peroxymonosulfate activation by biochar-based nanohybrids for the degradation of pharmaceutical and personal care products in aquatic environments, Chemosphere, 311 (2023) 137084.
- J. Yu, H. Feng, L. Tang, Y. Pang, G. Zeng, Y. Lu, H. Dong, J. Wang, Y. Liu, C. Feng, J. Wang, B. Peng, S. Ye, Metal-free carbon materials for persulfate-based advanced oxidation process: Microstructure, property and tailoring, Progress in Materials Science, 111 (2020).
- Y. Zhao, M. Song, Q. Cao, P. Sun, Y. Chen, F. Meng, The superoxide radicals’ production via persulfate activated with CuFe2O4@Biochar composites to promote the redox pairs cycling for efficient degradation of o-nitrochlorobenzene in soil, J Hazard Mater, 400 (2020) 122887.
- M. Xi, K. Cui, M. Cui, Y. Ding, Z. Guo, Y. Chen, C. Li, X. Li, Enhanced norfloxacin degradation by iron and nitrogen co-doped biochar: Revealing the radical and nonradical co-dominant mechanism of persulfate activation, Chemical Engineering Journal, 420 (2021).
- A.A. Azzaz, C. Matei Ghimbeu, S. Jellai, L. El-Bassi, M. Jeguirim, Olive Mill by-Products Thermochemical Conversion via Hydrothermal Carbonization and Slow Pyrolysis: Detailed Comparison between the Generated Hydrochars and Biochars Characteristics, Processes, 10 (2022).
- S. Wang, J. Wang, Activation of peroxymonosulfate by sludge-derived biochar for the degradation of triclosan in water and wastewater, Chemical Engineering Journal, 356 (2019) 350-358.
- L. Zhao, X. Cao, O. Masek, A. Zimmerman, Heterogeneity of biochar properties as a function of feedstock sources and production temperatures, J Hazard Mater, 256-257 (2013) 1-9.
- K. Zhu, Q. Bin, Y. Shen, J. Huang, D. He, W. Chen, In-situ formed N-doped bamboo-like carbon nanotubes encapsulated with Fe nanoparticles supported by biochar as highly efficient catalyst for activation of persulfate (PS) toward degradation of organic pollutants, Chemical Engineering Journal, 402 (2020).
- J. Shi, Y. Wang, W. Du, Z. Hou, Synthesis of graphene encapsulated Fe3C in carbon nanotubes from biomass and its catalysis application, Carbon, 99 (2016) 330-337.
- D.-G. Kim, S.-O. Ko, Effects of thermal modification of a biochar on persulfate activation and mechanisms of catalytic degradation of a pharmaceutical, Chemical Engineering Journal, 399 (2020).
- J. He, Y. Xiao, J. Tang, H. Chen, H. Sun, Persulfate activation with sawdust biochar in aqueous solution by enhanced electron donor-transfer effect, Sci Total Environ, 690 (2019) 768-777.
- Y. Shang, C. Chen, P. Zhang, Q. Yue, Y. Li, B. Gao, X. Xu, Removal of sulfamethoxazole from water via activation of persulfate by Fe3C@NCNTs including mechanism of radical and nonradical process, Chemical Engineering Journal, 375 (2019).
- X. Li, Y. Jia, M. Zhou, X. Su, J. Sun, High-efficiency degradation of organic pollutants with Fe, N co-doped biochar catalysts via persulfate activation, J Hazard Mater, 397 (2020) 122764.
- B. Yao, Z. Luo, S. Du, J. Yang, D. Zhi, Y. Zhou, Magnetic MgFe(2)O(4)/biochar derived from pomelo peel as a persulfate activator for levofloxacin degradation: Effects and mechanistic consideration, Bioresour Technol, 346 (2022) 126547.
- K. Xiao, F. Liang, J. Liang, W. Xu, Z. Liu, B. Chen, X. Jiang, X. Wu, J. Xu, J. Beiyuan, H. Wang, Magnetic bimetallic Fe, Ce-embedded N-enriched porous biochar for peroxymonosulfate activation in metronidazole degradation: Applications, mechanism insight and toxicity evaluation, Chemical Engineering Journal, 433 (2022).
- Y. Hu, D. Chen, R. Zhang, Y. Ding, Z. Ren, M. Fu, X. Cao, G. Zeng, Singlet oxygen-dominated activation of peroxymonosulfate by passion fruit shell derived biochar for catalytic degradation of tetracycline through a non-radical oxidation pathway, J Hazard Mater, 419 (2021) 126495.
- M. Wang, H. Xu, Q. Li, G. Zhou, Q. Ye, Q. Wang, J. Zhang, Panda manure biochar-based green catalyst to remove organic pollutants by activating peroxymonosulfate: Important role of non-free radical pathways, Journal of Environmental Chemical Engineering, 9 (2021).
- J. Huang, Y. Cao, B. Qin, G. Zhong, J. Zhang, H. Yu, H. Wang, F. Peng, Highly efficient and acid-corrosion resistant nitrogen doped magnetic carbon nanotubes for the hexavalent chromium removal with subsequent reutilization, Chemical Engineering Journal, 361 (2019) 547-558.
- Y. Gao, Y. Chen, T. Song, R. Su, J. Luo, Activated peroxymonosulfate with ferric chloride-modified biochar to degrade bisphenol A: Characteristics, influencing factors, reaction mechanism and reuse performance, Separation and Purification Technology, 300 (2022).
- M. Xiong, J. Yan, G. Fan, Y. Liu, B. Chai, C. Wang, G. Song, Built-in electric field mediated peroxymonosulfate activation over biochar supported-Co3O4 catalyst for tetracycline hydrochloride degradation, Chemical Engineering Journal, 444 (2022).
- H. Zhang, Y. Song, L.-c. Nengzi, J. Gou, B. Li, X. Cheng, Activation of persulfate by a novel magnetic CuFe2O4/Bi2O3 composite for lomefloxacin degradation, Chemical Engineering Journal, 379 (2020).
- R. Luo, M. Li, C. Wang, M. Zhang, M.A. Nasir Khan, X. Sun, J. Shen, W. Han, L. Wang, J. Li, Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol A under high salinity condition, Water Res, 148 (2019) 416-424.
- X. Li, Z. Yang, G. Wu, Y. Huang, Z. Zheng, H.F. Garces, K. Yan, Fabrication of ultrathin lily-like NiCo2O4 nanosheets via mooring NiCo bimetallic oxide on waste biomass-derived carbon for highly efficient removal of phenolic pollutants, Chemical Engineering Journal, 441 (2022).
- X. Huang, Z. Yu, Y. Shi, Q. Liu, S. Fang, Highly efficient activation of peroxymonosulfate by Co, S co-doped bamboo biochar for sulfamethoxazole degradation: Insights into the role of S, Journal of Environmental Chemical Engineering, 10 (2022).
- T. Liu, K. Cui, Y. Chen, C. Li, M. Cui, H. Yao, Y. Chen, S. Wang, Removal of chlorophenols in the aquatic environment by activation of peroxymonosulfate with nMnOx@Biochar hybrid composites: Performance and mechanism, Chemosphere, 283 (2021) 131188.
- W. Ren, G. Nie, P. Zhou, H. Zhang, X. Duan, S. Wang, The Intrinsic Nature of Persulfate Activation and N-Doping in Carbocatalysis, Environ Sci Technol, 54 (2020) 6438-6447.
- Y. Ding, X. Wang, L. Fu, X. Peng, C. Pan, Q. Mao, C. Wang, J. Yan, Nonradicals induced degradation of organic pollutants by peroxydisulfate (PDS) and peroxymonosulfate (PMS): Recent advances and perspective, Sci Total Environ, 765 (2021) 142794.
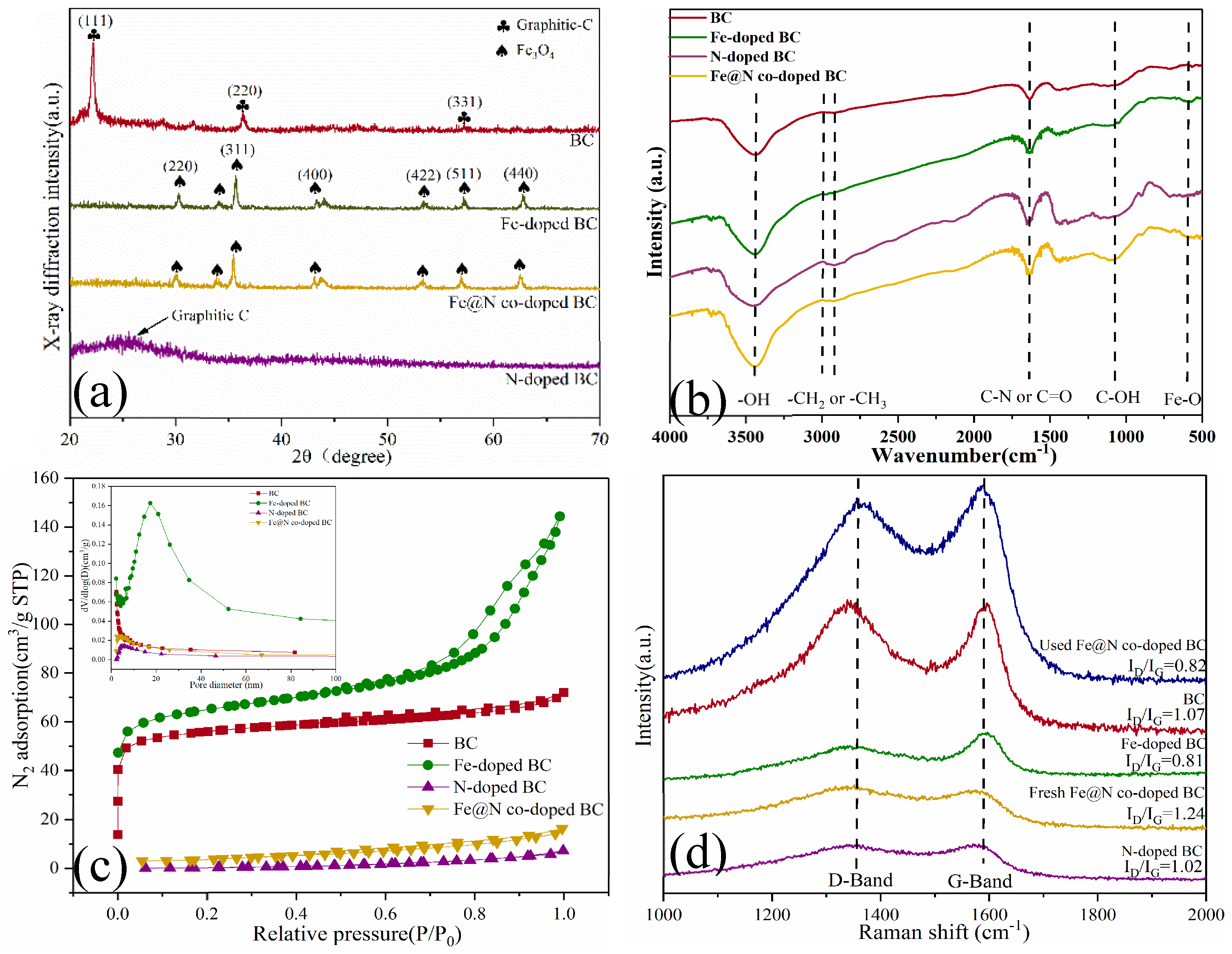


| Samples | SBET (m2/g) a | Pore volume (cm3/g) a | Average pore diameter (nm) a | ID/IG b |
|---|---|---|---|---|
| BC | 194.86 | 0.110 | 2.46 | 1.07 |
| Fe-doped BC | 216.38 | 0.220 | 3.64 | 0.81 |
| N-doped BC | 287.67 | 0.300 | 3.56 | 1.02 |
| Fe@N co-doped BC | 269.21 | 0.240 | 3.48 | 2.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





