1. Introduction
Coffee is a popular brewed beverage enjoyed worldwide due to its pleasant taste and complex aroma. Cold brew (CB) coffee has become the fastest-growing hot spot in the ready-to-drink coffee market in recent years. It is predicted that global revenues in coffee will reach US
$585 billion by 2025, especially due to the appearance of specialty coffees [
1]. CB coffee is typically prepared by steeping coffee grounds in cold water for 12-24 hours at a temperature range of 4-15℃. Compared to hot brew coffee, CB coffee has different physical, chemical, and sensory characteristics. Most substances in CB coffee, such as furans, pyrazines, aldehydes, and esters, have a higher content, lower acidity and softer taste [
2,
3]. Although CB coffee has a good market prospect, its long preparation time has limited its development. To address this, several researchers have explored ways to speed up the extraction process, including ultrasonic-assisted [
4], stirring-assisted ,[
5] and vacuum cyclic extraction [
6] CB coffee.
Ultra-high pressure (UHP) technology has gained wide attention in the food industry, particularly in tea and traditional Chinese medicine extraction, due to its little impact on nutritional value and sensory quality [
7,
8,
9]. However, this technology has not been extensively studied in coffee extraction. Two studies by Zhang [
10] and Chen [
11] showed that UHP technology could accelerate coffee extraction, substituting the process of grinding. Zhang et al. [
10] soaked whole coffee beans for 12 hours, while Chen et al. [
11] used different UHP pressures ranging from 100 MPa to 500 MPa to extract CB coffee. The results showed that the extraction yield, total dissolved solids, total phenol content, and trigonelline and chlorogenic acid contents were more susceptible to pressure and holding time. However, factors that affect the quality of coffee as a beverage, such as the degree of roasting, have not been addressed.
The degree to which coffee is roasted can have a significant impact on its quality. The roasting process can eliminate unpleasant aromas and play a crucial role in determining its color and taste [
12]. Complex reactions during the roasting process, such as the Maillard reaction, Caramelization, and Strecker Degradation [
13,
14], contribute to the unique flavor of coffee. Previous research on coffee roast level has focused on comparing extraction conditions, physicochemical characteristics, and sensory properties of hot and CB coffee [
15,
16,
17]. Studies have shown that, under the same roasting degree, CB coffee has lower titratable acid (TA), total phenol content (TPC), and antioxidant activity than hot brew coffee. The extraction rate and total sugar (TS) of CB coffee are higher than those of hot brew coffee. Additionally, research [
18] has shown that the roasting degree has a greater impact on CB coffee compared with hot brew coffee. However, the effect of roasting degree on UHP CB coffee is still unclear.
In this study, we investigated the impact of different roasting degrees on the quality of coffee beans after undergoing ultra-high pressure treatment. We also evaluated the impact of pressure and roasting degree on the non-volatile and volatile components and sensory evaluation of UHP CB coffee. Understanding the impact of roasting degree on UHP CB coffee would offer a theoretical basis for identifying the most suitable roasting beans for UHP CB coffee. It also offers a new exploration for using UHP technology to enhance the quality of CB coffee. This study is valuable for marketing the UHP CB coffee.
2. Materials and Methods
2.1. Chemicals and Coffee Samples
Caffeine, chlorogenic acids (CGAs), trigonelline, and 1,1-diphenyl-2-picrylhydrazyl radical (DPPH), Yuanye Biotechnology Co. Ltd (Shanghai, China). 2-octanol and 2,2'-azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS), Titan Technology Co., Ltd (Shanghai, China). Folin-phenol (2M), Sigma–Aldrich Chemical Co. Ltd (Shanghai, China).
2.2. Coffee Sample Preparation
Arabica coffee beans from Ethiopia were selected and purchased from Yanbei Coffee Co. Ltd (Shanghai, China). The classification of different roasting degrees (light, medium, dark) corresponds to the Agtron color value zone (73, 62, 53).
The preparation of UHP CB coffee:
Fifteen grams of ground coffee were added to 210 mL of water in a bottle at 4℃. The bottle was then placed in a high hydrostatic pressure unit (UHP-600 ultra-high, Baotou Kefa High Voltage Technology Co., Ltd) and treated for 20 min at 300 MPa, as previously described [
11]. When the UHP voltage equipment was complete and atmospheric pressure was achieved, a filter paper was used to filter the coffee samples and stored in a 4℃ refrigerator.
2.3. Total Dissolved Solids and Extraction Yield
The total dissolved solids (TDS) were measured using a TDS refractometer (Pal-Coffee, ATAGO, Japan), the surface of which was cleaned with a paper towel after each use [
19].
The extraction yield (EY) represented the proportion of the extracted coffee substance to the total weight of the coffee beans, and was calculated by Equation 1:
Where Mb indicates the total weight of the extract and Mgc represents the quality of the ground coffee used during the extraction process.
2.4. Total Titratable Acidity, Total Sugars, and Total Phenolic Compounds
The methods devised by Rao et al., Wang et al., Chow et al., and BILGE et al. [
12,
20,
21] were used to measure the total titratable acidity (TA), total sugars (TS), and total phenol compounds (TPC). TA was measured by titrating 0.1 M NaOH until pH reached 6.5 in 50 mL coffee. TS concentration was measured by the phenol-sulfuric acid method. TPC concentration was measured by the Folin–Ciocalteu colorimetric method [
22], using gallic acid as the standard.
2.5. Antioxidant Capacity and Melanoidins
Melanoidins were measured using the method of Mori [
23] et al. with slight revisions. The coffee was diluted to a concentration of 1:19, and its absorbance was determined at 420 nm.
The antioxidant activities were measured using the methods of Dong et al. and Gorecki et al. [
24,
25], based on the scavenging capability of ABTS and DPPH. The units of absorbance were mmol/L Trolox.
2.6. Caffeine, Trigonelline, and CGAs
High-performance liquid chromatography (HPLC) (LC-20A HPLC, Shimadzu, Japan) was used to measure the CGA, trigonelline, and caffeine contents [
26].
Chromatographic column and conditions used:
Caffeine contents: WodaSilTM C-18 column (150 mm × 4.6 mm × 5 µm, Shimadzu, Japan). Mobile phase: 24% methanol and 76% water. Temperature: 30 ℃. Flow rate: 1.0 mL/min. Injection volume: 10.0 µL. Detection wavelength: 272 nm.
Trigonelline contents: WondaCract ODS-2 column (150 mm × 4.6 mm × 5 µm, Shimadzu, Japan). Mobile phase: 12% methanol and 88% water. Temperature: 30 ℃. Flow rate: 1.0 mL/min. Injection volume: 10.0 µL. Detection wavelength: 260 nm.
CGA contents: WondaCract ODS-2 column (150 mm × 4.6 mm × 5 µm, Shimadzu, Japan). Mobile phase: acetonitrile and 1% acetic acid (the ratio is 15:85 (v/v)). Temperature: 30 ℃. Flow rate: 1.0 mL/min. Injection volume: 10.0 µL. Detection wavelength: 260 nm.
2.7. Volatile Compounds
The volatile compounds present in coffee were measured using headspace solid-phase microextraction (HS-SPME) and gas chromatography/mass spectrometry (GC–MS) (Shimadzu, TQ-80, Japan) following the method of Yu [
16] with minor modifications.
Qualitative analysis: The volatile compounds were identified by the retention indices (RIs) and the NIST.17 database.
Quantitative analysis: 2-Octanol (44 μg/mL) was used as the internal standard. The remaining volatile compounds in coffee were determined based on the ratio of peak area of internal standard to concentration. For the calculation of RI, a C6-C30 n alkane series was used.
The odorant activity value (OAV) is the ratio of the concentration of an aromatic compound to the threshold, and can be used to evaluate the extent to which aromatic compounds contribute to the flavor. The OAV was calculated using Equation 2:
Where C represents the concentration of the aromatic compound and Ct indicates the threshold of the aroma compound.
2.8. Sensory Evaluation
As per the guidelines laid out by the Specialty Coffee Association (SCA), a group of 12 trained professionals conducted a sensory evaluation of coffee samples in a specialized room. All the members involved in the cupping process had undergone a rigorous coffee quality protocol laid out by the SCA and had achieved Q grader certificates. The evaluation was conducted in professional cupping glasses at a temperature of 20°C ± 3°C. The members trained tasted each sample and rated the intensity of the smell perceived retronasally. Prior to the evaluation, all referees underwent additional training to ensure that they understood the specific meaning of the sensory vocabulary. The flavor attributes included nutty/cocoa, fruity, floral, caramel, sweetness, sourness, astringency, bitterness, flavor, body, aftertaste, and overall [
22].
2.9. Statistical Analysis
All statistical analyses, including comparisons of the concentrations of volatile and non-volatile compounds, were conducted using analysis of variance (ANOVA) with p < 0.05 considered statistically significant (p < 0.05). GraphPad Prism 10 and SIMCA 14 were used for the construction of the heatmap, principal component analysis (PCA), and the orthogonal partial least squares-discriminant analysis (OPLS-DA) plot.
3. Results
3.1. Physicochemical Characteristics
Table 1 presents the physicochemical characteristics of ultra-high pressure (UHP) CB coffee at different roast levels, including measurements of TDS, EY, TA, TPC, and TS. The TDS and EY values for UHP CB coffee and the control group (traditional CB coffee) were not significantly different for the same roast level. However, TDS and EY values increased significantly (p<0.05) as the roast level increased. Michael et al. [
27] showed that the decomposition of compounds during roasting might lead to the loss of soluble solids, along with the formation of new compounds. Additionally, high-temperature roasting destroys the cellular matrix, making compounds easier to extract. This means that regardless of the extraction method used, TDS and EY values continue to increase. The UHP method quickly releases a large number of water-soluble compounds under high pressure and short holding times, achieving TDS and EY values similar to conventional CB coffee [
28].
The value of TA and TPC in UHP CB coffee decreases as the roast level increases. This trend is also observed in conventional CB coffee. During the roasting process, soluble protonated acidic compounds are lost due to decomposition or synthesis reactions, leading to a decrease in TA concentration. Additionally, some organic acids like citric acid and malic acid present in raw beans decompose during roasting [
29]. The concentration of TA in coffee is the lowest at the highest degree of roasting. The trend of TA is more obvious in UHP CB coffee than in conventional CB coffee due to its shorter extraction time. The influence of roast level on the TPC of UHP CB coffee is greater than that of conventional CB coffee. This is due to the poor stability of phenolic substances that are sensitive to temperature changes during roasting. The increase in temperature causes rapid decomposition of phenolic substances and increases the size/number of pores between coffee bean tissue structures, leading to rapid release of some phenolic substances like CGAs during the initial extraction time. However, achieving equilibrium requires a longer extraction time. This effect is more significant in light roasting than in medium and dark roasting. The TS content in UHP CB coffee is higher than that in conventional CB coffee. However, the TS content of coffee gradually decreases with the increase in roast level. The effect of roasting degree on melanoidin of UHP CB coffee was shown to be less than that of conventional CB coffee, probably because the static extraction system of CB coffee was not conducive to the release of some insoluble substances. The coffee bean pores during dark roasting were larger compared with those of coffee beans during light roasting, enhancing the release of melanoidin content in CB coffee [
30].
3.2. Non-Volatile Components
Table 2 presents the bioactive ingredients of UHP and CB coffee at various roast levels. Regardless of the extraction method used, the concentration of three common non-volatile substances, namely caffeine, trigonelline, and chlorogenic acid group, de-creased significantly with an increase in the roasting degree (P<0.05). When compared with CB coffee, the impact of UHP coffee on chlorogenic acids (CGA) content was more responsive to increased roasting. It is evident that the influence of the roasting degree on the extraction of non-volatile components of CB coffee was higher than that of UHP CB coffee.
There is no significant difference in caffeine content between UHP coffee and CB coffee. Caffeine is a heat-stable alkaloid that may lose a portion of its content during roasting, with a minority lost during sublimation [
31]. Furthermore, the structure of coffee beans changes when they are roasted, with the stomata closing and inorganic gases accumulating inside the bean. This increase in pressure causes the beans to crack with a characteristic popping sound, and small amounts of caffeine may be released. As a result, caffeine loss may be greater at higher roasting temperatures [
16]. However, when it comes to the extraction of caffeine, the extraction method does not affect the extraction concentration; rather, the bean itself has a greater impact.
Trigonelline is a derivative of pyridine that indirectly promotes the formation of desired flavor compounds during coffee roasting, such as furan, pyrazine, pyridine, and pyrrole. It is important as a precursor of flavor and aroma compounds as well as a beneficial nutritional factor, as documented in previous studies [
34]. As the coffee roasting degree increases, the content of trigonelline decreases significantly (P < 0.05). During roasting, the content of trigonelline, which is high in green coffee beans, continuously decreases, while N-methylpyridine and niacin, two thermal decomposition products of trigonelline, increase continuously. N-methylpyridine and niacin are positively correlated with the roasting degree [
30]. Therefore, the UHP extraction method is useful in rapidly extracting trigonelline in a short time, and UHP coffee possesses a similar concentration of trigonelline as conventional CB coffee under dark roasting conditions. Moreover, the CGA content in medium and light-roasted coffee was significantly higher compared with that of dark-roasted coffee (P<0.05), consistent with the findings of Trugo et al. [
33]. This result was attributed to the fact that the high roasting temperatures decomposed CGA, causing lower extraction concentrations.
3.3. Antioxidant Capacity
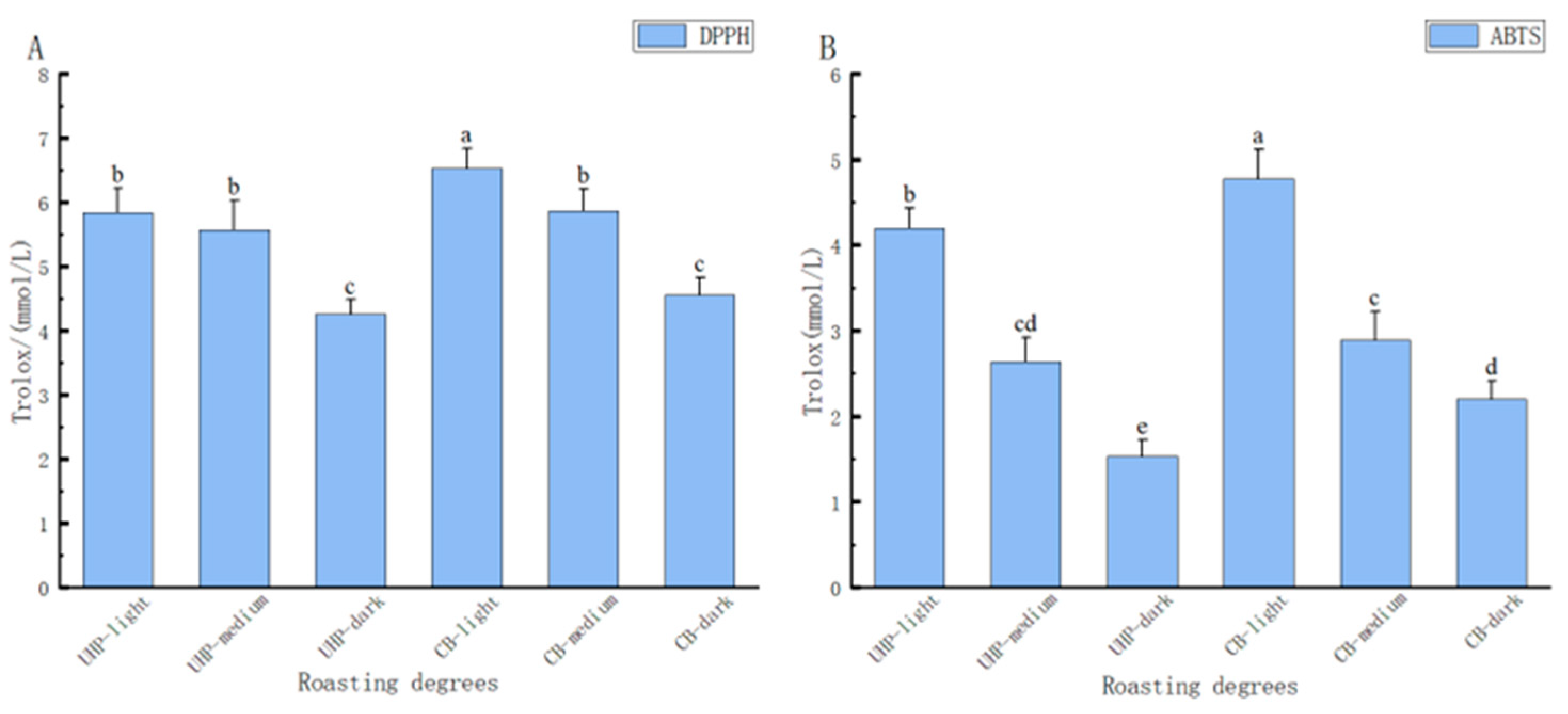
Figure 1 presents the antioxidant capacity of CB coffee after UHP treatment in terms of DPPH and ABTS free radical scavenging capacity. The study revealed that the antioxidant capacity of both UHP coffee and conventional CB coffee decreased significantly with an increase in roasting degree (P < 0.05). There was a significantly higher decrease in the DPPH free radical scavenging ability of UHP coffee during medium and dark roasting, while the ABTS scavenging capacity of conventional CB coffee had a more significant fall during light to medium roasting. Furthermore, the research demonstrated that the antioxidant capacity of coffee was affected by various factors such as the CGA content, total phenol content, and melanoid substances. These factors determine the strength of coffee's antioxidant [
31] properties. Dark roasting had the highest effect on these antioxidant compounds as melanoidins and other antioxidant compounds produced during roasting were not soluble in low-temperature water, in turn reducing the extraction efficiency of the cold extraction process [
18]. Therefore, under dark roasting conditions, the gap in antioxidant capacity between UHP coffee and CB coffee was smaller. Substances that are difficult to dissolve in low-temperature water get easily dissolved at high pressure, making up for the time effect of conventional CB coffee extraction.
3.4. Volatile Composition
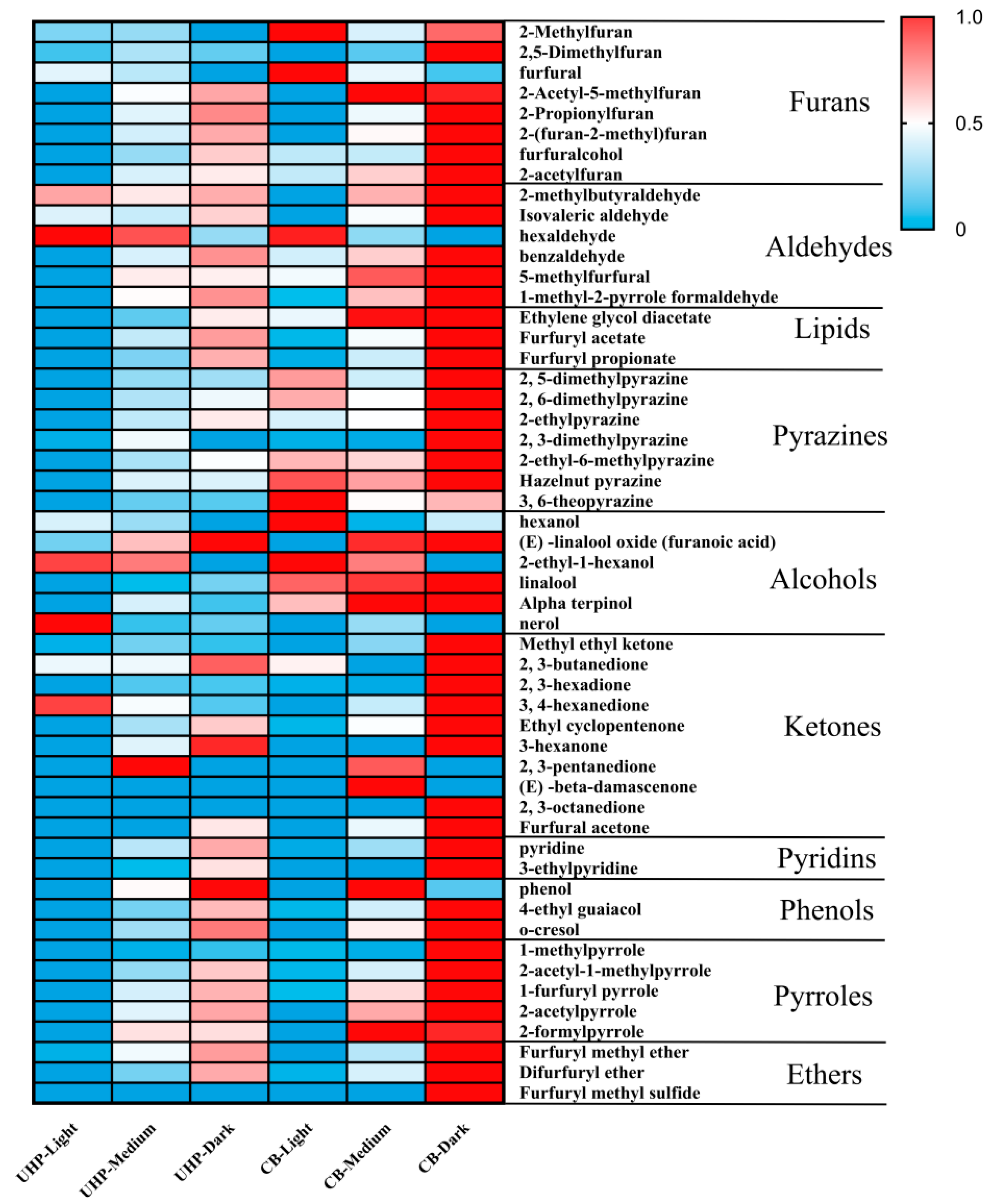
Figure 2 displays heat maps of volatile compounds found in UHP and CB coffee. The study aimed to investigate the effect of different roasting degrees on the volatile components of UHP CB coffee, and it detected 53 volatile compounds. The most prominent compounds found were furan, pyrazine, esters, and aldehydes. In general, the overall volatile components of both UHP and CB coffee increased as the roasting degree intensified. Among them, the contents of volatile components, such as pyrazines, furans (except furfural), esters, pyrrole, pyridine, and most aldehydes, increased significantly between light and medium roasting (P<0.05). Moreover, the impact of roasting degree on UHP coffee was comparatively less than that of CB coffee in medium to dark roasting.
Furfural is a substance that is most commonly found in light roasting. It has a sweet wood, almond, and toasted bread aroma. Unlike other furans, its volatile com-ponents decrease as the roasting degree deepens, which is consistent with previous studies [
16]. This may be due to the production of furan from furfural or furfurol [
35]. Additionally, aromatic compounds, such as pyrrole, are formed in coffee beans via the Maillard reaction occurring at dark roasting [
36]. This study also found an increase in the content of pyrroles, which are described as musty, smoky, and herbal negative flavors. It is worth noting that furfuryl acetate, which has a floral, fruity, and sweet scent, is the most significant change among all volatile compounds. Its concentration was lowest in light roasting and highest in dark roasting. However, despite its higher content, UHP and CB coffee showed significantly lower sensory scores in floral and fruity aroma than light and medium roasting in the sensory evaluation of UHP CB coffee under different roasting degrees.
Pyrazine is the primary aroma-active compound found in medium and dark-roasted coffee. It is formed by the Maillard reaction that occurs during coffee roasting [
37]. The pyrazine compounds are often responsible for producing roasted and nutty odors in various heat-treated foods, such as coffee and cocoa aromas [
38]. However, despite the similar proportion of pyrazine volatile components in UHP coffee and CB coffee, the nutty aroma intensity in UHP coffee is lower than that of CB, and the sensory evaluation score is also lower. Dark roasting coffee samples contain Furol iso-valerate, ethyl guaiacol, cocoa crotonal, 2-acetylpyrrole, phenol, o-cresol, and 2-formylpyrrole, most of which provide negative odors such as musty and smoky odor. Previous literature has also reported that guaiacol, which is detected only in dark roasting coffee, makes an important contribution to coffee aroma [
39]. The guaiacol content increases significantly during the roasting process, which may be responsible for the increased smoky taste of the coffee samples.
In addition, the effects of different degrees of roasting and treatments on the coffee samples were examined using OPLS-DA, calculating the variable influence on projection (VIP), which reflects the magnitude of the contribution of aromatic compounds to the overall fit and classification power of the sample. Usually, only compounds with OAV>1 and VIP>1 are recognized as major contributors to aroma. As shown in the
Table 4, 15 compounds were detected and arranged from high to low according to the magnitude of the OAV. Hazelnut pyrazine, linalool, butane-2, 3-dione, 3-methyl butanal, and furfuryl methyl sulfide, described as hazelnut, floral, sweet, and fruity, were the top five compounds contributing the most to the aroma, with all having OAVs greater than 100. Furthermore, the OAVs were higher as the degree of roasting increased. These results thus explain not only the contribution of these compounds to the coffee aroma in relation to different roasting degrees but also the difference between UHP and CB coffees.
3.5. Sensory Evaluation
As the degree of roasting increases, the intensity of nutty flavor, astringency, bitterness, body, and aftertaste also increases. In contrast, the intensity of floral, fruity and sourness decreases with the increase of roasting degree. Additionally, medium roasting results in a higher intensity [
36,
40] of caramel flavor. As previous studies have also shown, CB coffee at medium roasting has a stronger caramel aroma, a more balanced flavor, and a higher overall rating than CB coffee at dark roasting. This is due to medium-roasting coffee being more integrated with other coffee-like characteristics, such as bitter and roasty flavors, while dark-roasting coffee has lower non-coffee-like characteristics, such as sweet, fruity, and coffee-like characteristics, such as bitter and roasty flavors. Moreover, at all roasting degrees, CB coffee has a higher intensity of nutty, astringent, bitter flavor and aftertaste. The content of aldehydes, esters, and pyrazines in CB coffee was also higher than that in UHP coffee. These representative volatile components may be one of the reasons for the difference in sensory evaluation between the two different extraction methods.
Table 3.
Sensory evaluation associated with different roasting degrees and extraction conditions.
Table 3.
Sensory evaluation associated with different roasting degrees and extraction conditions.
| Specific attributes |
UHP-L |
UHP-M |
UHP-D |
CB-L |
CB-M |
CB-D |
| Flavor-Nutty/Cocoa |
4.25±0.28d
|
5.63±0.37c
|
7.00±0.52b
|
4.50±0.40d
|
6.38±0.33b
|
8.50±0.65a
|
| Flavor-Fruity |
6.50±0.40b
|
4.25±0.20c
|
3.00±0.18d
|
7.50±0.39a
|
4.50±0.52c
|
2.00±0.15c
|
| Flavor-Floral |
4.50±0.30a
|
2.80±0.20b
|
1.00±0.22c
|
4.50±0.38a
|
2.50±0.25b
|
1.00±0.10c
|
| Flavor-Caramelly |
4.00±0.22c
|
5.25±0.35b
|
5.00±0.30b
|
5.00±0.42b
|
6.50±0.48a
|
5.00±0.33b
|
| Sweetness |
4.00±0.32b
|
5.20±0.29a
|
3.00±0.20c
|
5.00±0.43a
|
5.25±0.37a
|
2.00±0.25d
|
| Sourness |
5.50±0.33a
|
4.20±0.45b
|
3.00±0.22c
|
5.75±0.40a
|
4.50±0.39b
|
2.50±0.20d |
| Astringency |
2.00±0.15e
|
3.13±0.28d
|
5.00±0.35b
|
3.00±0.20d
|
4.75±0.38c
|
6.50±0.42a
|
| Bitterness |
2.00±0.10e
|
3.35±0.30d
|
5.50±0.40b
|
3.00±0.28d
|
4.30±0.42c
|
6.50±0.42a
|
| Flavor |
4.00±0.35d
|
5.33±0.45c
|
7.00±0.60ab
|
5.00±0.47c
|
6.85±0.50b
|
8.00±0.65a
|
| Body |
3.50±0.20d
|
4.50±0.42c
|
7.00±0.50a
|
5.00±0.44bc
|
5.50±0.30b
|
7.50±0.62a
|
| Aftertaste |
4.00±0.33d
|
5.50±0.39c
|
7.00±0.50a
|
5.50±0.44bc
|
6.50±0.35b
|
7.50±0.40a
|
| Overall |
7.00±0.35b
|
6.50±0.42b
|
5.00±0.33c
|
8.00±0.47a
|
7.80±0.67a
|
4.00±0.38d
|
Table 4.
Odor thresholds and OAVs of the major contributors to the odor of coffee samples.
Table 4.
Odor thresholds and OAVs of the major contributors to the odor of coffee samples.
| Compounds |
odor description |
Retention index |
Reference Retention index |
M/Z |
Threshold value(ug/kg) |
OAVs |
| UHP-Light |
UHP-Medium |
UHP-Dark |
CB-Light |
CB-Medium |
CB-Dark |
| Hazelnut pyrazine |
nutty meaty roasted hazelnut |
1489 |
1494 |
150,135,149 |
0.084 |
3660.26 |
5911.55 |
5913.94 |
8061.77 |
8563.89 |
8886.86 |
| linalool |
floral sweet rose woody blueberry |
1048 |
1098 |
71,93,55 |
0.22 |
1511.96 |
2628.95 |
2746.82 |
746.49 |
3522.20 |
3734.97 |
| butane-2,3-dione |
strong butter sweet creamy pungent |
613 |
589 |
41,68 |
0.059 |
2765.71 |
2780.95 |
4069.59 |
1071.06 |
2992.19 |
4736.33 |
| 3-methylbutanal |
ethereal aldehydic chocolate peach |
632 |
654 |
44,43,41 |
1.1 |
657.62 |
619.01 |
815.10 |
226.50 |
708.74 |
1221.61 |
| Furfuryl methyl sulfide |
onion garlic pungent vegetable horseradish |
984 |
979 |
81,53,128 |
0.4 |
251.22 |
584.52 |
774.63 |
187.91 |
496.11 |
1029.28 |
| 1-Methylpyrrole-2-carboxaldehyde; |
roasted nutty |
991 |
971 |
109,53,80 |
37 |
8.81 |
27.57 |
35.57 |
14.58 |
32.02 |
45.96 |
| hexan-1-ol |
ethereal oil fruity alcoholic sweet |
859 |
864 |
56,43,41 |
5.6 |
16.48 |
15.11 |
10.97 |
23.96 |
12.26 |
6.12 |
| hexanal |
fruity fatty leafy sweaty |
807 |
832 |
44,56,41 |
5 |
26.21 |
22.15 |
8.12 |
25.00 |
7.82 |
3.70 |
| 2-methylbutanal |
cocoa coffee nutty malty fermented alcoholic |
704 |
695 |
41,29,57 |
84.3 |
7.66 |
9.00 |
10.95 |
4.92 |
10.76 |
16.48 |
| 1-Furfurylpyrrole |
plastic green waxy fruity coffee vegetable |
1128 |
1170 |
81,147,53 |
100 |
2.76 |
7.56 |
10.24 |
4.46 |
9.33 |
14.24 |
| 5-methylfurfural |
spice caramel maple |
959 |
953 |
110,53,27 |
1110 |
3.74 |
6.00 |
5.98 |
5.74 |
7.23 |
7.95 |
| 2-ethyl-6-methylpyrazine |
nutty peanut musty corn raw earthy bread |
1011 |
1005 |
121,67,39 |
500 |
1.74 |
2.59 |
2.95 |
3.33 |
3.18 |
4.20 |
| 4-Ethyl-2-methoxyphenol |
spicy smoky bacon phenolic clove |
1257 |
1288 |
137,152,15 |
69.5 |
<1 |
1.80 |
4.39 |
<1 |
2.85 |
6.92 |
| o-cresol |
phenolic plastic medicinal herbal leathery |
1048 |
1059 |
108,79,77 |
25 |
<1 |
1.96 |
4.64 |
<1 |
3.22 |
6.04 |
| 2, 6-dimethylpyrazine |
cocoa roasted nuts roast beef coffee |
925 |
928 |
108,42,40 |
718 |
<1 |
1.00 |
1.08 |
1.21 |
1.10 |
1.44 |
3.6. Principal Component Analysis
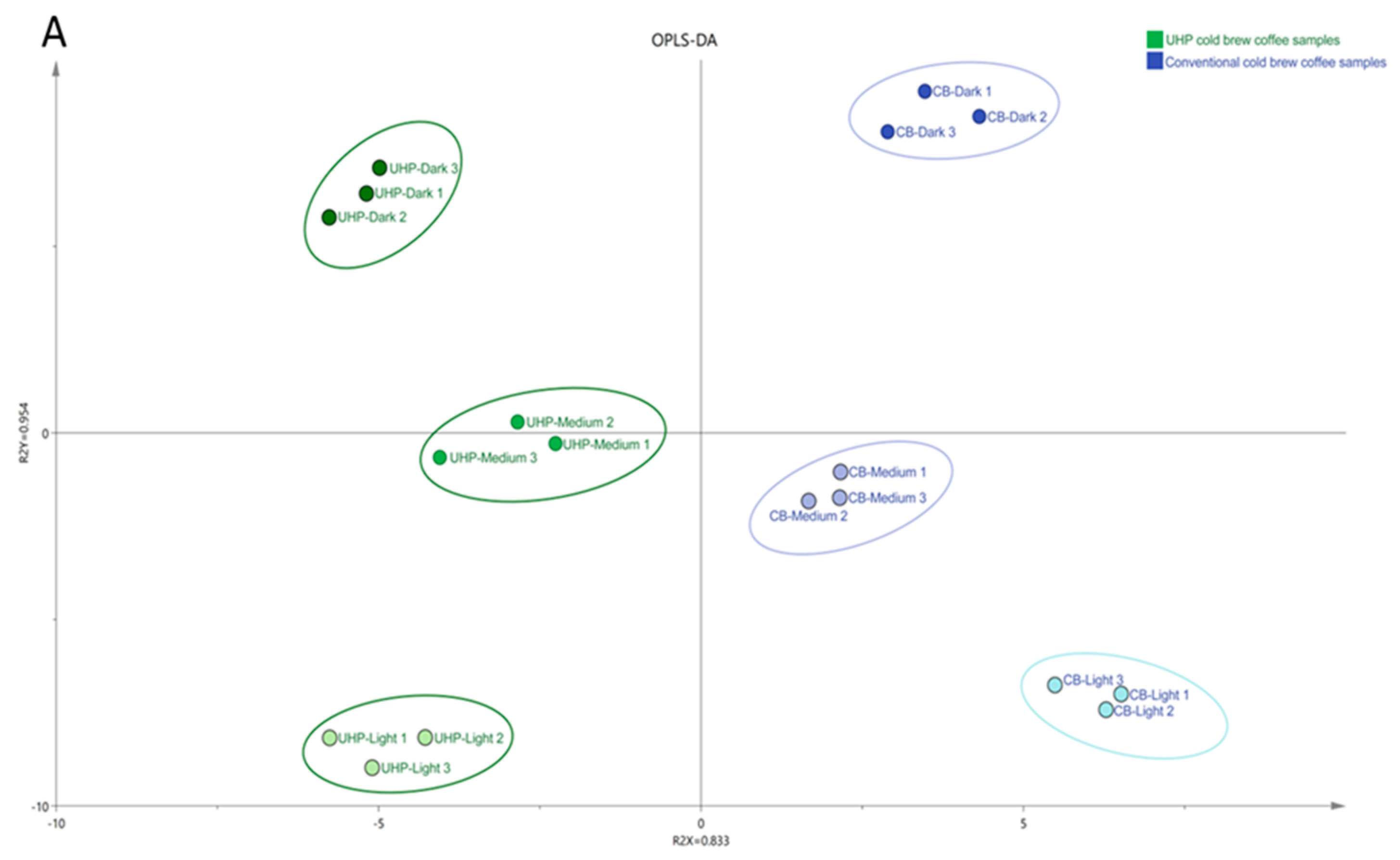
Figure 3A shows an OPLS-DA plot of UHP and CB coffee samples at different roasting degrees. The six types of coffee samples are well-separated. Among them, the distance between UHP-medium coffee and CB-medium coffee is the closest.
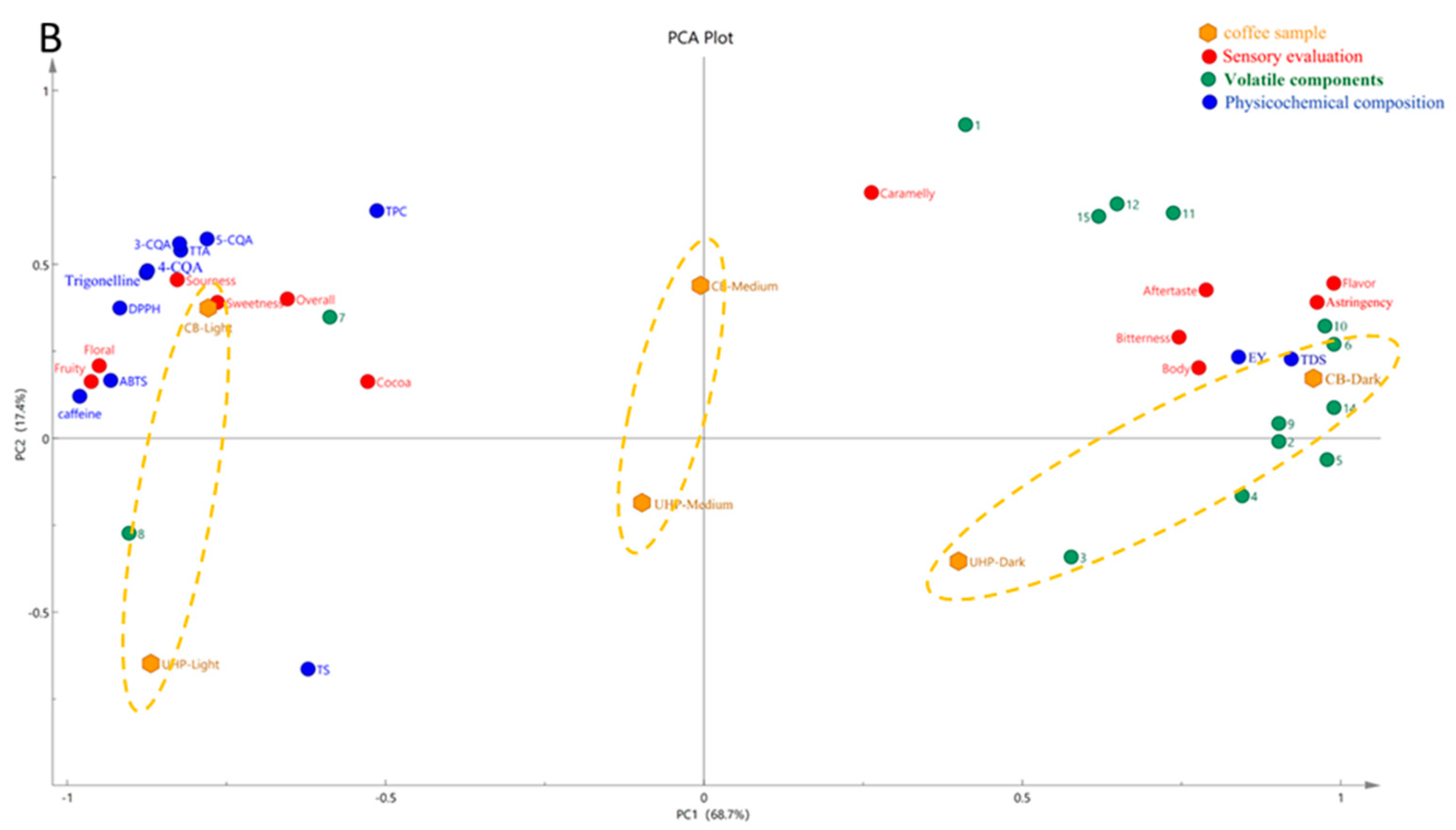
Figure 3B shows the PCA of physicochemical characteristics, non-volatile components, sensory evaluation, and the major contributors to coffee odor. The PCA components explain variances of 68.7% and 17.4%, resulting in a total variance explained of 86.7%. CB coffee was found in the positive PC2 axis while UHP coffee was located in the negative PC2 axis, which clearly separated the two. Additionally, there was a significant difference between the two coffees due to their different degrees of roasting. Lightly-roasted coffees were distributed on the left side of the PC1 axis. Medium-roasted coffees were located near the origin, while dark-roasted coffees were on the right side of the PC1 axis. The difference between UHP and CB coffee at medium roasting was smaller compared to light and dark roasting coffees. This indicates that the influence of roasting degree on UHP coffee was less than that of CB coffee. In other words, medium-roasting coffee beans are more suitable for ultra-high pressure extraction. In addition, the distance between the three in UHP coffee was much smaller than that in CB coffee, and corresponded with the positions of the different coffee samples in the OPLS-DA plot (
Figure 3 A) and the magnitude of change in the OAV (
Table 3).
CB Coffee, when dark roasted, is characterized by relatively richer volatile compounds. The positive flavors of pyrazines, furans, esters, and ketones, which represent the volatile substances of nuts and caramel, and the negative flavors of pyrrole, pyridine, and ethers, which represent the herbal, smoky, and plastic aroma components, are all clustered around the deep-roasted CB coffee. Although they had a higher volatile concentration, the sensory scores of the floral and fruity flavors were significantly lower compared with those of the lightly baked sample group, consistent with the results of previous studies [
41]. Light-roasted CB coffee is characterized by a higher concentration of non-volatile components and antioxidant capacity. Regarding the sensory evaluation, they contain sour, sweet, and floral flavors, so the overall evaluation score was also higher. Light-roasted UHP coffee had a higher TS, which facilitated the release of sugar substances. Longer holding times (in the CB) have been shown to improve the retention of flavor compounds and, thus, have richer aroma components [
21]. Although the UHP coffee had a lower flavor intensity than that of conventional CB coffee, it had similar flavor substances to CB coffee in a shorter time and had a lower negative aroma, which effectively resolved the issue of long extraction time in CB coffee.
4. Discussion
In this study, physicochemical and flavor characteristics were compared between coffee samples obtained using different extraction methods and degrees of roasting. The study found that some physiochemical indices, such as total dissolved solids (TDS), extraction yield (EY), total titratable acidity (TTA), and caffeine content, had similar trends and variations regardless of the extraction method. However, more physio-chemical indices in UHP coffee were affected by the high pressure, although they showed the same trend but a smaller range, such as total solids (TS), melanoidin, chlorogenic acids (CGAs), and antioxidant capacity measured by DPPH and ABTS as-says. Additionally, the influence of roasting degree on the total phenolic content (TPC) of UHP coffee was greater than that of CB coffee.
HS-SPME-GC-MS analysis revealed that odor-active compounds of Ultra-High Pressure (UHP) coffee and CB coffee increased with the level of roasting. This increase was observed in a variety of compounds, including aldehydes, esters, pyrazines, alcohols, pyridines, phenols, pyrrole, and ethers. Furthermore, there were significant differences in these volatile components between light and medium roasting. However, the influence of roasting on UHP coffee was less than that of CB coffee. OPLS-DA combined the calculation of OAVs found out and listed the major contribution compounds of aroma in coffee, such as Hazelnut pyrazine, Linalool, Butane-2,3-dione, 3-methylbutanal and Furfuryl methyl sulfide. The OAVs were higher as the degree of roasting increased. However, the OAVs in CB coffee had more drastic change than those in UHP coffee. Sensory evaluation indicated that CB coffee has a richer nutty-like flavor, astringency, bitterness, flavor, body, and aftertaste than UHP coffee. The Principal Component Analysis (PCA) demonstrated that high pressure narrowed the coffee samples at different roasting levels. The distance between medium-roasted coffee with two extraction methods was much smaller than the other two roast levels. Consequently, the effect of roasting on the volatile components of different CB coffee was different between the UHP and CB methods.
However, the difference between the two extraction methods is relatively small when it comes to medium-roasted coffee beans. Therefore, medium-roasted coffee beans may be more suitable for UHP coffee than light and dark-roasted coffee beans. The application of ultra-high pressure technology in the coffee industry is yet to be fully explored. In the future, other factors that affect coffee beans, such as water quality and coffee species, should be investigated. This study also provides a basis for determining the appropriate degree of roasting for UHP CB coffee. Additionally, it paves the way for the future marketization of ultra-high pressure CB coffee.
Author Contributions
Conceptualization, Q.S. and Y.X.; methodology, Q.S and W.T.; software, Y.X.; validation, Q.S., F.J. and Y.Z.; formal analysis, Q.S.; investigation, Q.S.; resources, Y.X., X.Z. and Y.Z.; data curation, W.T.; writing—original draft preparation, Q.S.; writing—review and ed-iting, Y.X. and Q.S.; visualization, Q.S.; supervision, Y.X., X.Z. and Y.Z.; project administration, Y.X., X.Z. and Y.Z.; funding acquisition, Y.X. and H.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Shanghai Institute of technology. (Project identification code: SIT-2024-LL24)
Informed Consent Statement
All subjects gave their informed consent for inclusion before they participated in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors, upon reasonable request.
Acknowledgments
This work was supported by the “Alliance Program” of the Shanghai Asso-ciation for Promotion of Transformation of Scientific and Technological Achievements (no. LM201956).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ridder, M. Cold brew coffee market value in the U.S. 2015-2025. 2022. Retrieved from: https://www.statista.com/statistics/659724/cold-brew-coffee-sales-us/.
- Cai, Y.; Xu, Z.; Pan, X.; et al. Comparative Profiling of Hot and Cold Brew Coffee Flavor Using Chromatographic and Sensory Approaches. Foods 2022, 2968. [Google Scholar] [CrossRef] [PubMed]
- Stanek, Natalia; Zarębska, Magdalena; Biłos, Łukasz; et al. Influence of coffee brewing methods on the chromatographic and spectroscopic profiles, antioxidant and sensory properties. Scientific reports 2021, 21377. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Yang, M.; Zhang, J.; et al. Feasibility of Ultrasound-Assisted Extraction for Accelerated Cold Brew Coffee Processing: Characterization and Comparison with Conventional Brewing Methods. Frontiers in Nutrition 2022, 849811. [Google Scholar] [CrossRef] [PubMed]
- Maruf, A.; Gui-Hun, J.; Su, P.J.; et al. Effects of ultrasonication, agitation and stirring extraction techniques on the physicochemical properties, health-promoting phytochemicals, and structure of cold brewed coffee. Sci Food Agric 2018, 290–301. [Google Scholar]
- Kyroglou, S.; Laskari, R.; Vareltzis, P. Optimization of sensory properties of cold brew coffee produced by reduced pressure cycles and its physicochemical characteristics. Molecules 2022, 2971. [Google Scholar] [CrossRef]
- Hu, Wen; Guo, Ting; Jiang, Wj; et al. Effects of ultrahigh pressure extraction on yield and antioxidant activity of chlorogenic acid and cymaroside extracted from flower buds of Lonicera japonica. Chinese Journal of Natural Medicines 2015, 445–453. [Google Scholar] [CrossRef]
- Nan, J.; Zou, M.; Wang, H.; et al. Effect of ultra-high pressure on molecular structure and properties of bullfrog skin collagen. International journal of biological macromolecules 2018, 200–207. [Google Scholar] [CrossRef]
- Huang, H.W.; Chen, B.Y.; et al. Extraction of bioactive ingredients from fr uiting bodies of Antrodia cinnamomea assisted by high hydrostatic pressure[J]. Journal of Food Science and Technology 2019, 3988–3997. [Google Scholar]
- Zhang, L.; Wang, X.; Manickavasagan, A.; et al. Extraction and Physicochemical Characteristics of High Pressure-Assisted Cold Brew Coffee. Future Foods 2022, 5, 100–113. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, Y.; Tang, W.; et al. Evaluation of Physicochemical Characteristics and Sensory Properties of Cold Brew Coffees Prepared Using Ultrahigh Pressure under Different Extraction Conditions. Foods 2023, 12, 3857. [Google Scholar] [CrossRef]
- Rao, Niny Z; Fuller, Megan; et al. Physiochemical characteristics of hot and cold brew coffee chemistry: The effects of roast level and brewing temperature on compound extraction. Foods 2020, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Fasano, E.; de Vivo, A.; et al. Processing effects on acrylamide content in roasted coffee production [J]. Food Chem 2020, 319, 126550. [Google Scholar] [PubMed]
- Elmacı, İlkay; Gok, Ilkay. Effect of three post-harvest methods and roasting degree on sensory profile of Turkish coffee assessed by Turkish and Brazilian panelists [J]. Journal of the Science of Food and Agriculture 2021, 101, 5368–5377. [Google Scholar] [PubMed]
- Fuller, M.; Rao, N.Z. The Effect of Time, Roasting Temperature, and Grind Size on Caffeine and Chlorogenic Acid Concentrations in Cold Brew Coffee [J]. Sci Rep 2017, 7, 17979. [Google Scholar] [PubMed]
- Yu, J.M.; Chu, M.; Park, H.; Park, J.; Lee, K.G. Analysis of Volatile Compounds in Coffee Prepared by Various Brewing and Roasting Methods. Foods 2021, 10, 1347. [Google Scholar] [CrossRef]
- Muzykiewicz-Szymańska, A.; Nowak, A.; Wira, D.; et al. The effect of brewing process parameters on antioxidant activity and caffeine content in infusions of roasted and unroasted Arabica coffee beans originated from different countries. Molecules 2021, 26, 3681. [Google Scholar] [CrossRef]
- Pan, L.; Xiao, Y.; Jiang, F.; et al. Comparison of Characterization of Cold Brew and Hot Brew Coffee Prepared at Various Roasting Degrees[J]. Journal of Food Processing and Preservation 2023, 2023. [Google Scholar]
- Gloess, A.N.; Schönbächler, B.; Klopprogge, B.; D`Ambrosio, L.; Chatelain, K.; Bongartz, A.; Strittmatter, A.; Rast, M.; Yeretzian, C. Comparison of Nine Common Coffee Extraction Methods: Instrumental and Sensory Analysis. European Food Research and Technology 2013, 236, 607–627. [Google Scholar] [CrossRef]
- Wang, X.; William, J.; Fu, Y.; Lim, L.T. Effects of Capsule Parameters on Coffee Extraction in Single-Serve Brewer. Food Res Int 2016, 89, 797–805. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhausser, S.M. A Method for Routine Measurements of Total Sugar and Starch Content in Woody Plant Tissues. Tree Physiol 2004, 24, 1129–36. [Google Scholar] [CrossRef]
- Bilge, G. Investigating the Effects of Geographical Origin, Roasting Degree, Particle Size and Brewing Method on the Physicochemical and Spectral Properties of Arabica Coffee by Pca Analysis. J Food Sci Technol 2020, 57, 3345–3354. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.L.B.; Viegas, M.C.; Ferrão, M.A.G.; et al. Coffee Brews Composition from Coffea Canephora Cultivars with Different Fruit-Ripening Seasons. British Food Journal 2020, 122, 827–840. [Google Scholar] [CrossRef]
- Dong, W.; Hu, R.; Chu, Z.; et al. Effect of Different Drying Techniques on Bioactive Components, Fatty Acid Composition, and Volatile Profile of Robusta Coffee Beans. Food Chem 2017, 234, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Gorecki, M.; Hallmann, E. The Antioxidant Content of Coffee and Its in Vitro Activity as an Effect of Its Production Method and Roasting and Brewing Time. Antioxidants (Basel) 2020, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, N.; Moreno, F.L.; Osorio, C.; et al. Specialty and regular coffee bean quality for cold and hot brewing: Evaluation of sensory profile and physicochemical characteristics [J]. Lwt 2021, 145, 111363. [Google Scholar]
- Ginz, M.; Balzer, H.H.; et al. Formation of aliphatic acids by carbohydrate degradation during roasting of coffee[J]. European Food Research and Technology 2000, 211, 404–410. [Google Scholar]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innovative Food Science & Emerging Technologies 2008, 9, 85–91. [Google Scholar]
- Kenichi, Yanagimoto; Ochi, Hirotomo; Lee, Kwang-Geun; et al. Antioxidative activities of fractions obtained from brewed coffee [J]. Journal of agricultural and food chemistry 2004, 52, 592–596. [Google Scholar]
- Wei, Feifei. Chemical Changes in the Components of Coffee Beans during Roasting [M]. Coffee in health and disease prevention 2015, 83–91. [Google Scholar]
- Crozier, Thomas WM; Stalmach, Angelique; et al. Espresso coffees, caffeine and chlorogenic acid intake: potential health implications. Food & function 2012, 30–33. [Google Scholar]
- Herawati, Dian; Giriwono, Puspo Edi; et al. Critical roasting level determines bioactive content and antioxidant activity of Robusta coffee beans. Food science and biotechnology 2019, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Trugo, L.C.; Macrae, R. A study of the effect of roasting on the chlorogenic acid composition of coffee using HPLC. Food Chemistry 1984, 219–227. [Google Scholar] [CrossRef]
- Vorotnikov, Vassili; Mpourmpakis, Giannis; Vlachos, Dionisios, G. DFT study of furfural conversion to furan, furfuryl alcohol, and 2-methylfuran on Pd (111). Acs Catalysis 2012, 2, 2496–2504. [Google Scholar]
- Moon, Joon-Kwan; Shibamoto, Takayuki. Role of roasting conditions in the profile of volatile flavor chemicals formed from coffee beans. Journal of Agricultural and Food Chemistry 2009, 5823–5831. [Google Scholar] [CrossRef] [PubMed]
- Poisson, Luigi; et al. New insight into the role of sucrose in the generation of α-diketones upon coffee roasting. Journal of agricultural and food chemistry 2016, 2422–2431. [Google Scholar] [CrossRef] [PubMed]
- Pickard, Stephanie; et al. Determination of the alkylpyrazine composition of coffee using stable isotope dilution–gas chromatography–mass spectrometry (SIDA-GC-MS). Journal of agricultural and food chemistry 2013, 6274–6281. [Google Scholar] [CrossRef] [PubMed]
- Münchow, Morten; Alstrup, Jesper; et al. Roasting conditions and coffee flavor: A multi-study empirical investigation. Beverages 2020, 6–29. [Google Scholar] [CrossRef]
- Anastasiou, Evilena; et al. Prehistoric schistosomiasis parasite found in the Middle East. The Lancet Infectious Diseases 2014, 553–554. [Google Scholar] [CrossRef]
- Córdoba, Nancy; et al. Specialty and regular coffee bean quality for cold and hot brewing: Evaluation of sensory profile and physicochemical characteristics. LWT 2021, 111363. [Google Scholar] [CrossRef]
- Seninde, Denis Richard; Chambers IV, Edgar; Chambers, Delores. Determining the impact of roasting degree, coffee to water ratio and brewing method on the sensory characteristics of cold brew Ugandan coffee. Food Research International 2020, 137–109667. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).