Submitted:
10 September 2024
Posted:
11 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Physiological Characteristics of Exogenous MeJA in Alleviating Deep Sowing Stress in Maize Inbred Lines
2.1.1. Morphological Analysis of Exogenous MeJA Alleviating Deep Sowing Stress in Maize Inbred Lines
2.1.2. Morphological Analysis of Exogenous MeJA Alleviating Deep Sowing Stress in Maize Inbred Lines
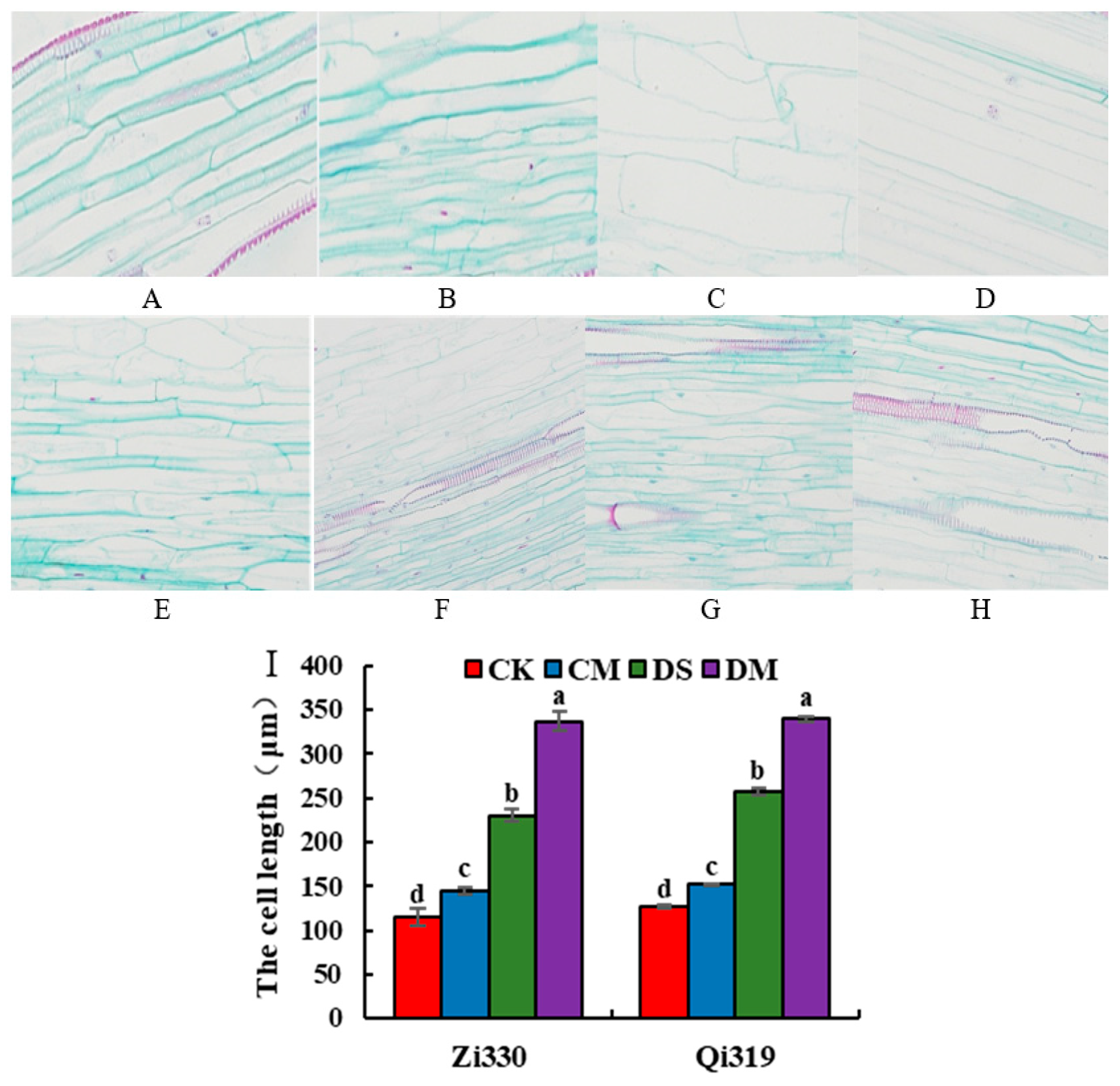
2.1.3. Cytological Observation of Exogenous MeJA Alleviating Deep Sowing Stress in Maize Inbred Lines
2.2. Transcriptome Analysis of Exogenous MeJA Alleviating Deep Sowing Stress under Deep Sowing Stress
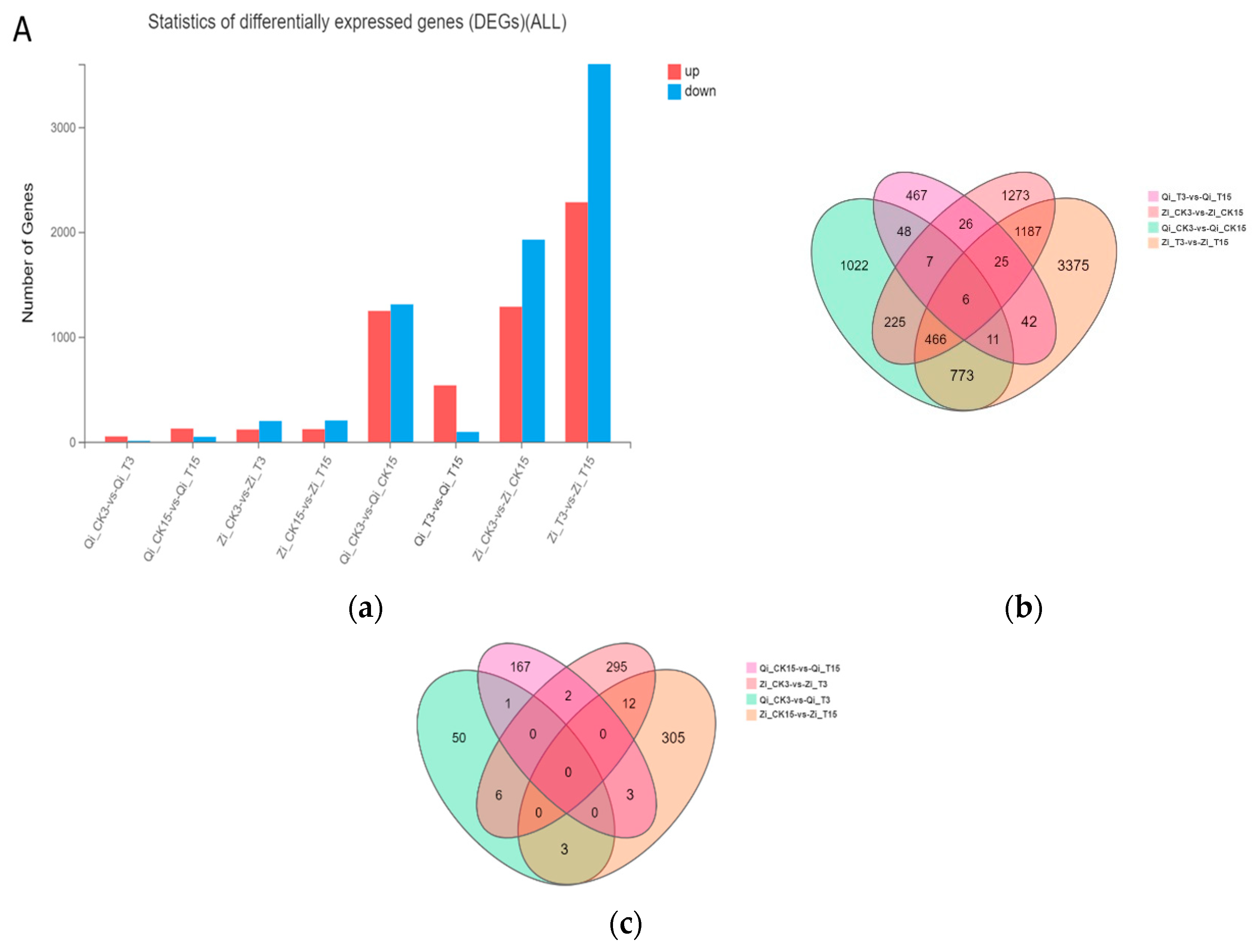
2.2.1. Analysis of Sequencing Results
2.2.2. Identification and Functional Analysis of Differentially Expressed Genes
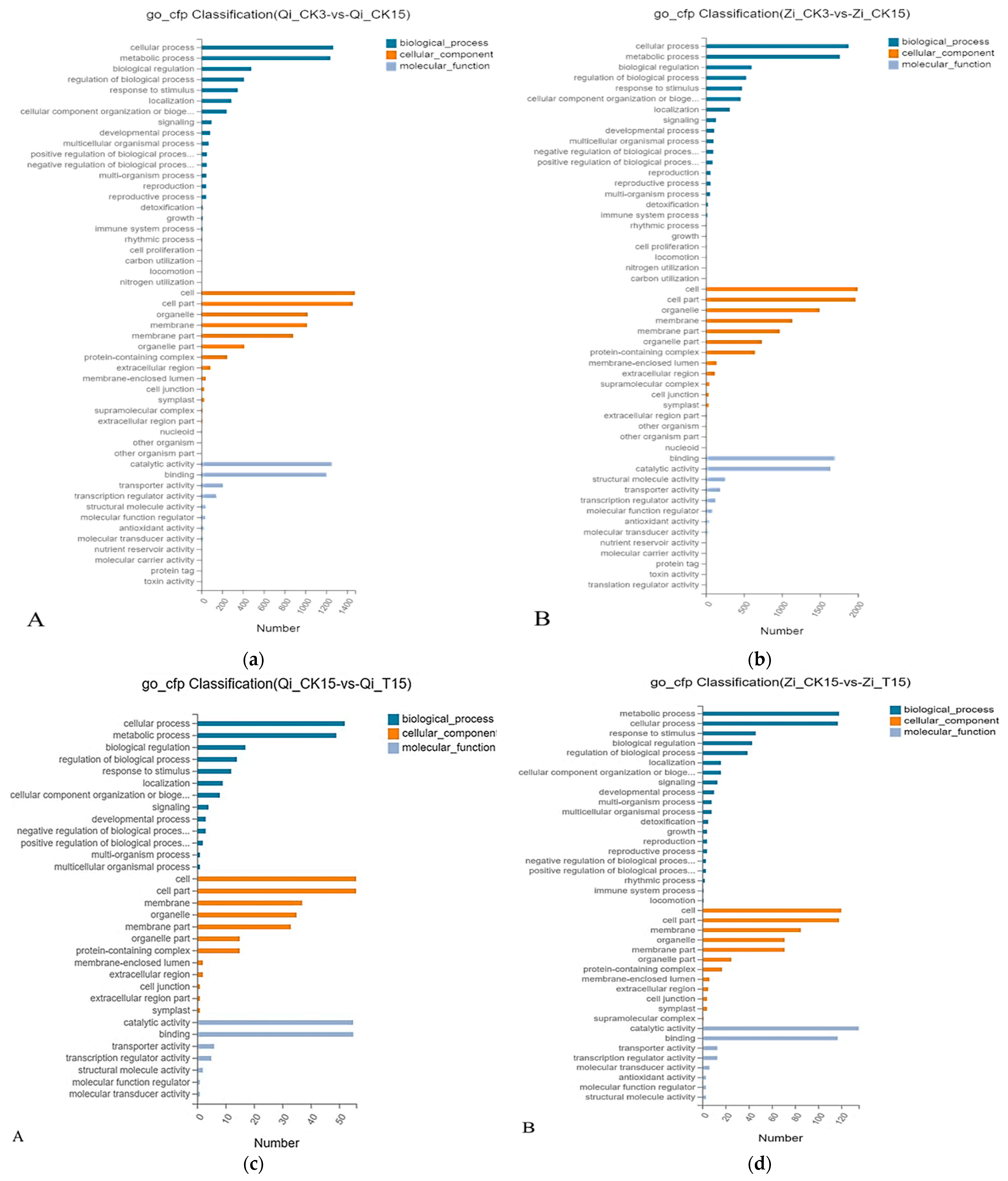
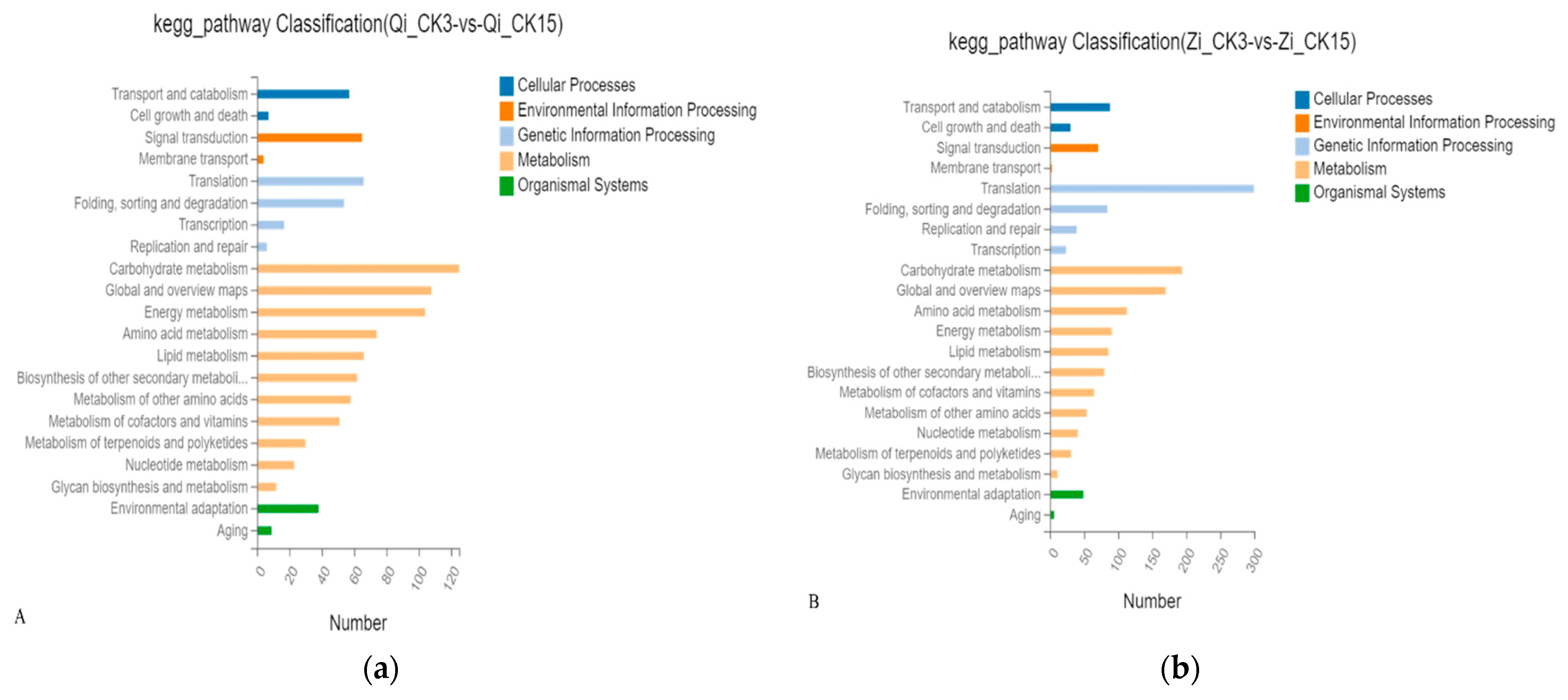
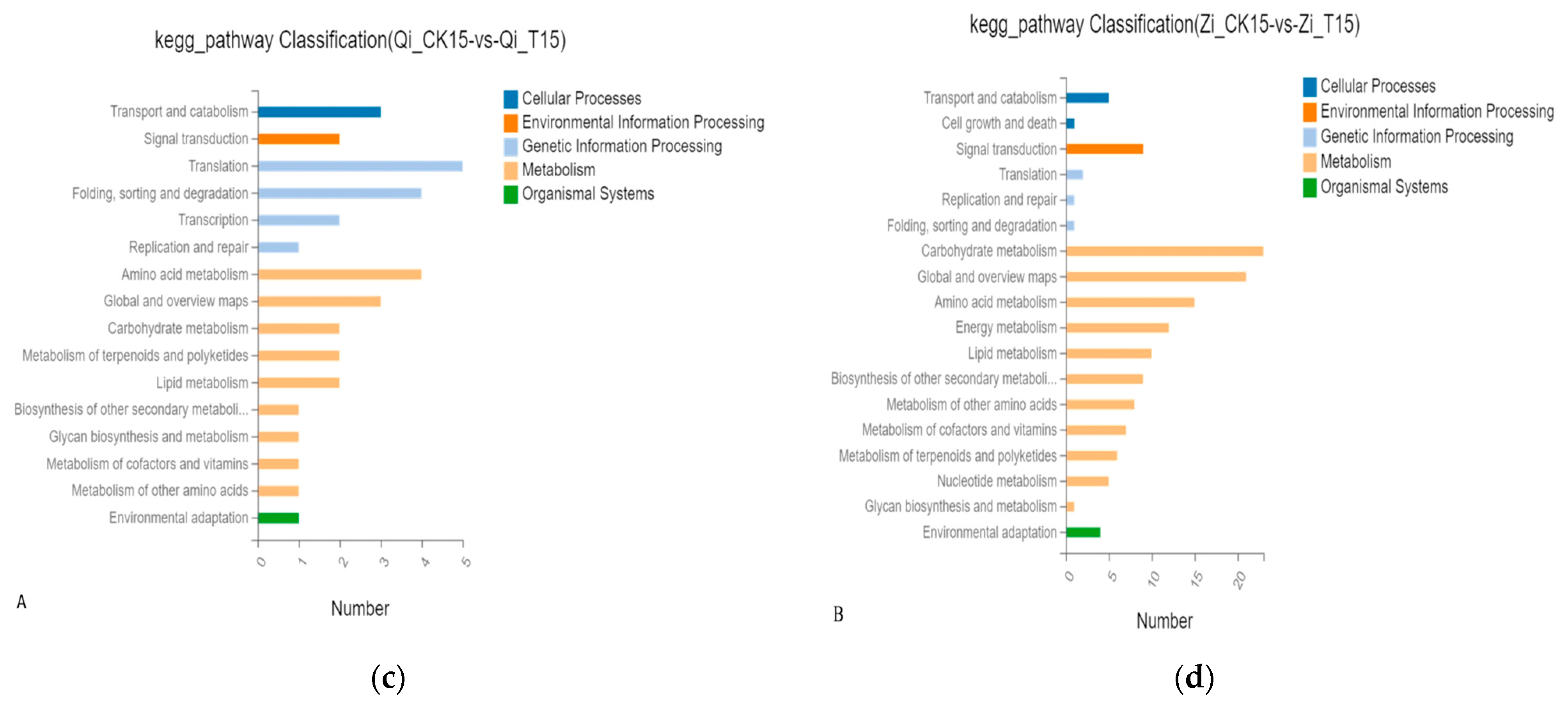
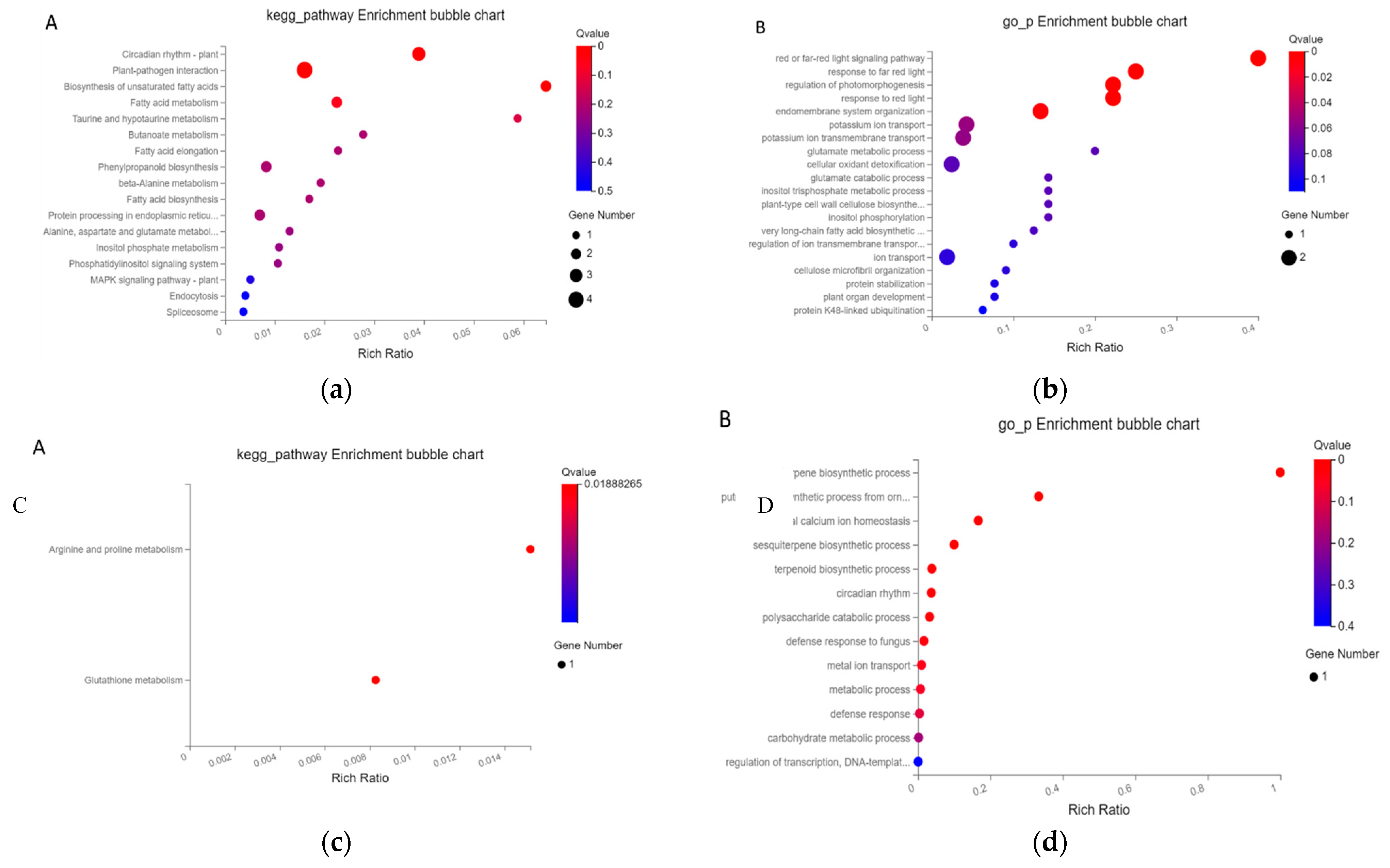
2.2.3. GO Analysis of Differentially Expressed Genes
2.2.4. Pathway Enrichment Analysis of Differentially Expressed Genes
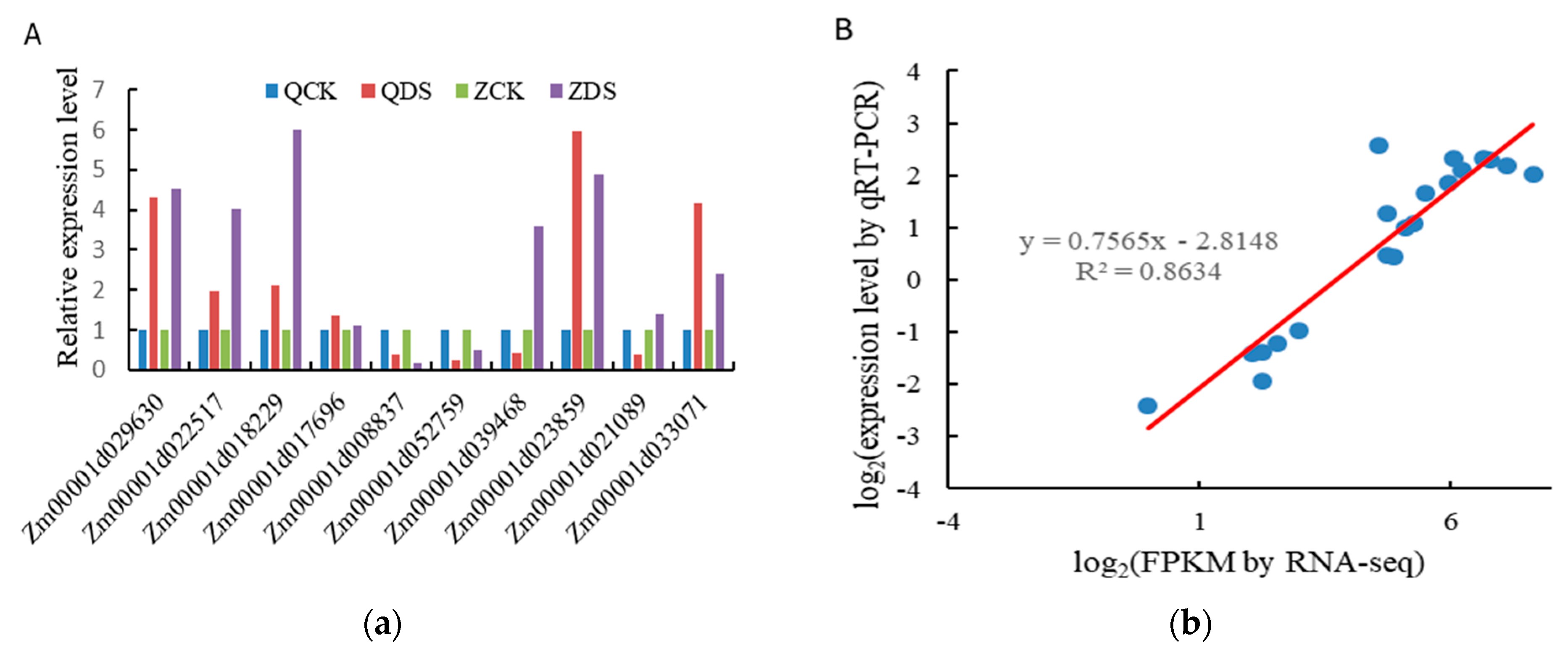
2.2.6. Validation of DEGs by qRT-PCR Analysis
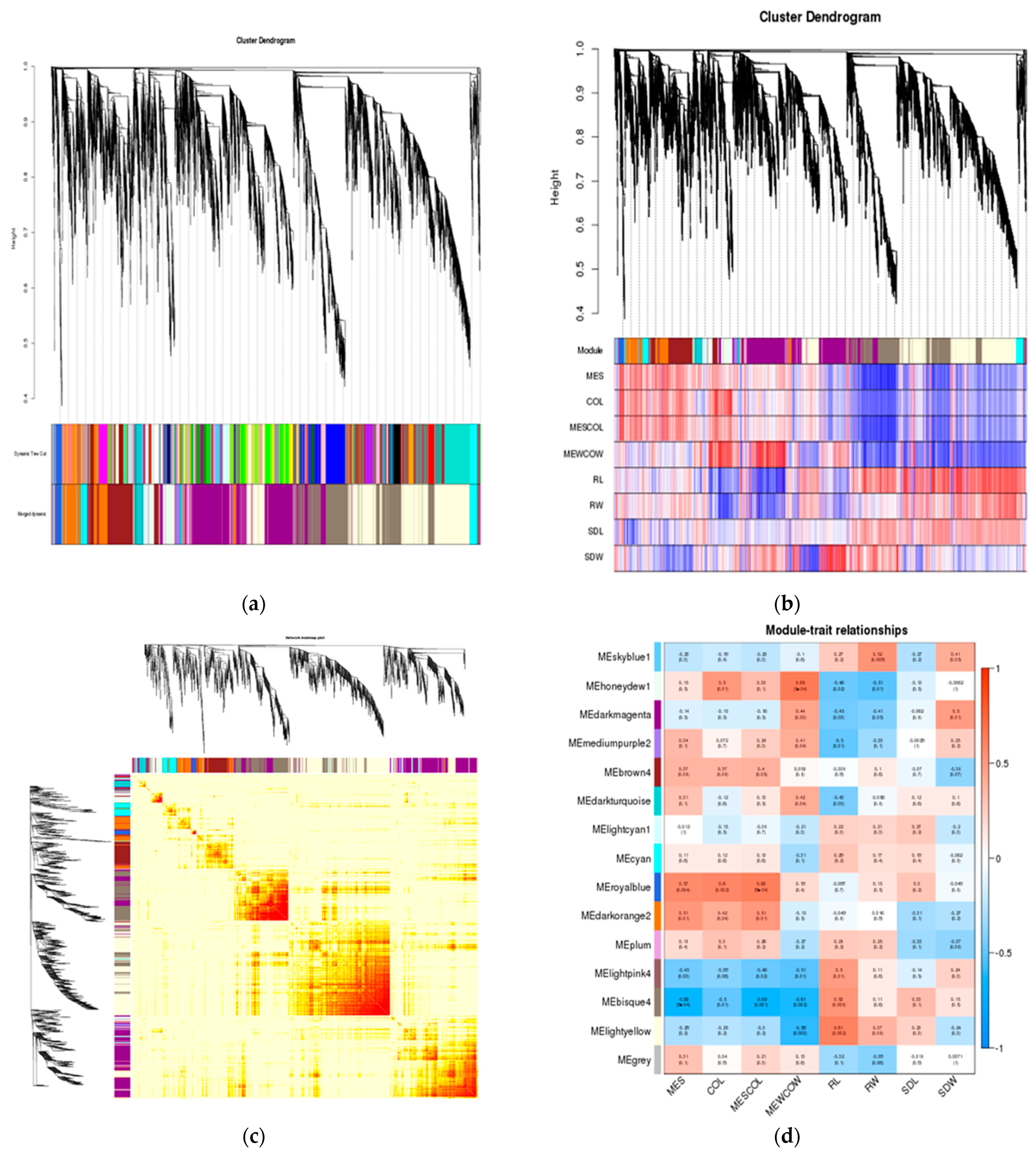
2.2.7. Gene Co-Expression Network Analysis
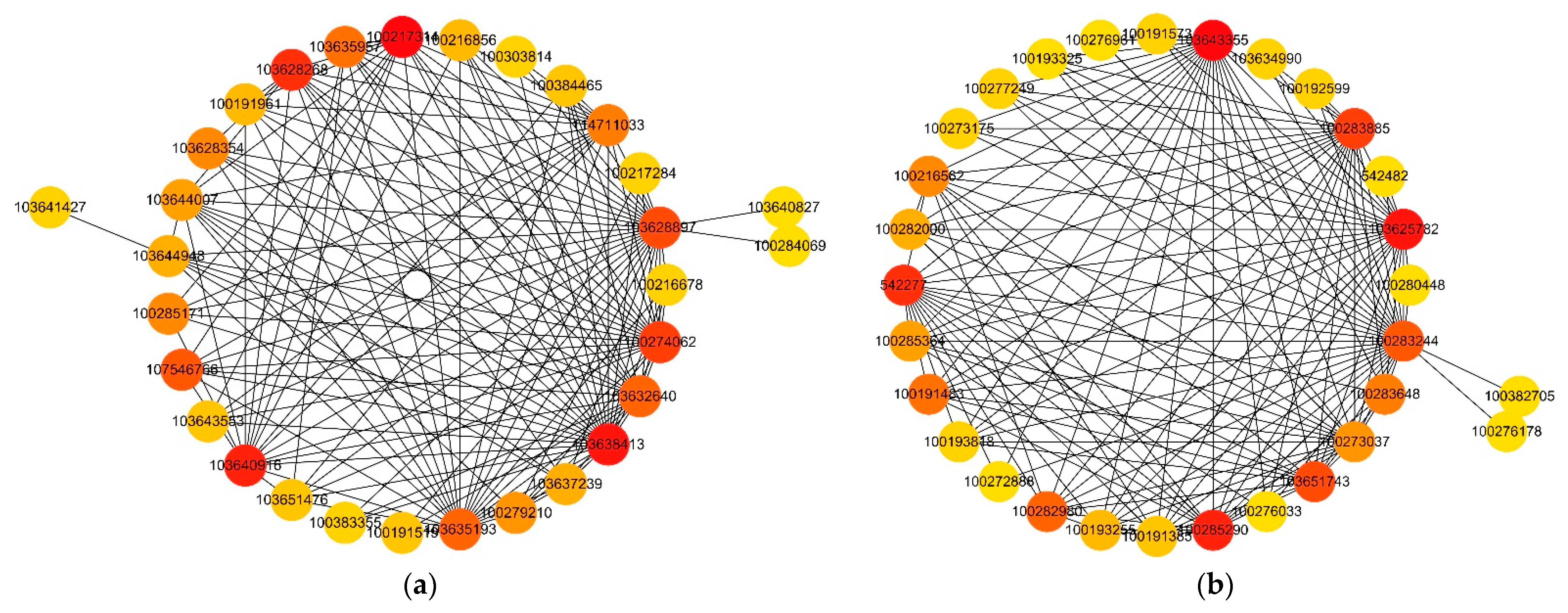
2.2.8. Analysis of Hub Genes Interaction Network in the Module
3. Discussion
3.1. Phenotypic Analysis of Exogenous MeJA to Alleviate Deep Sowing Stress
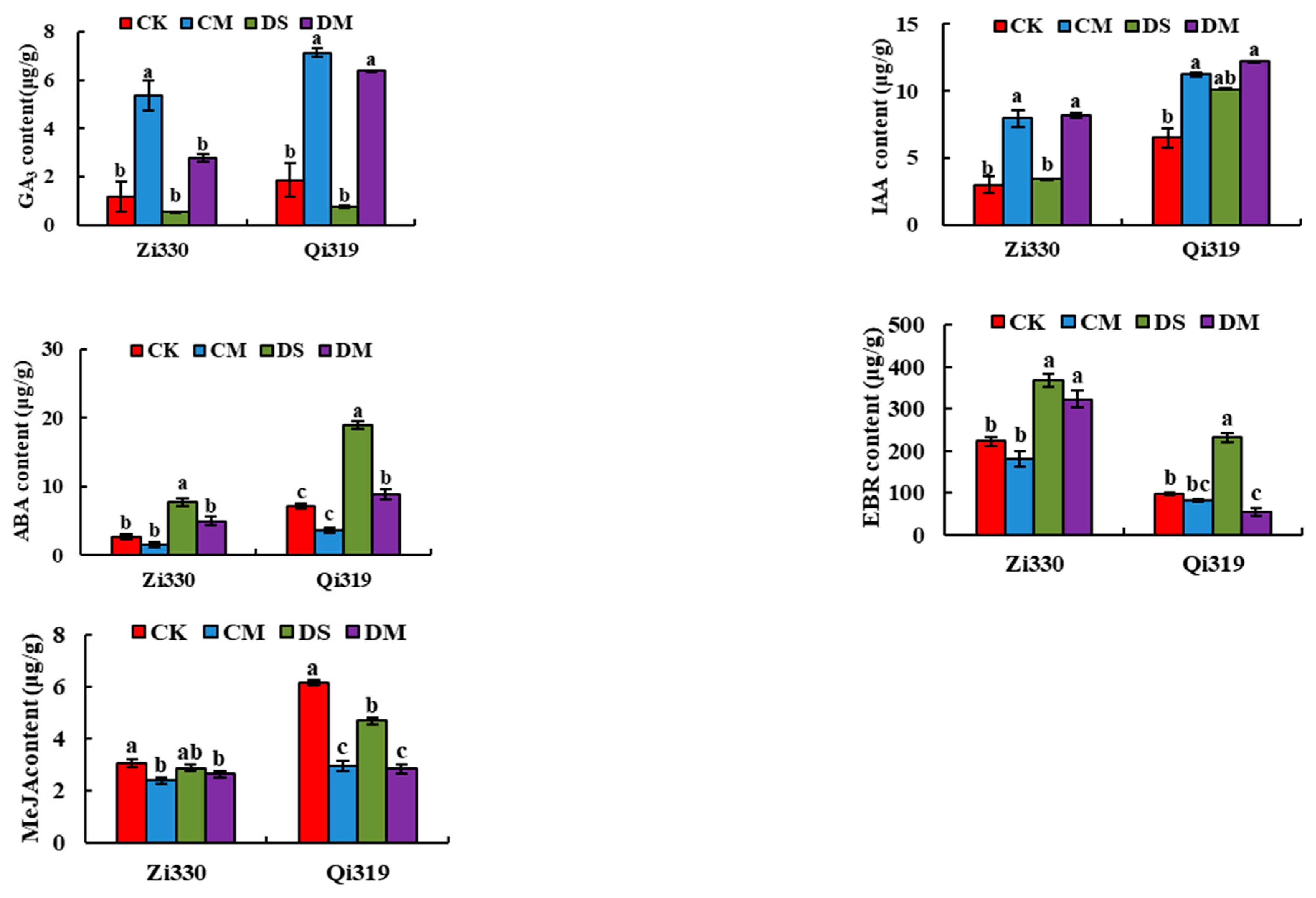
3.2. Analysis of Relative Hormone Content in Maize Mesocotyl Under Exogenous MeJA Treatment
3.3. Cytological Analysis of Maize Mesocotyl Under Exogenous MeJA Treatment
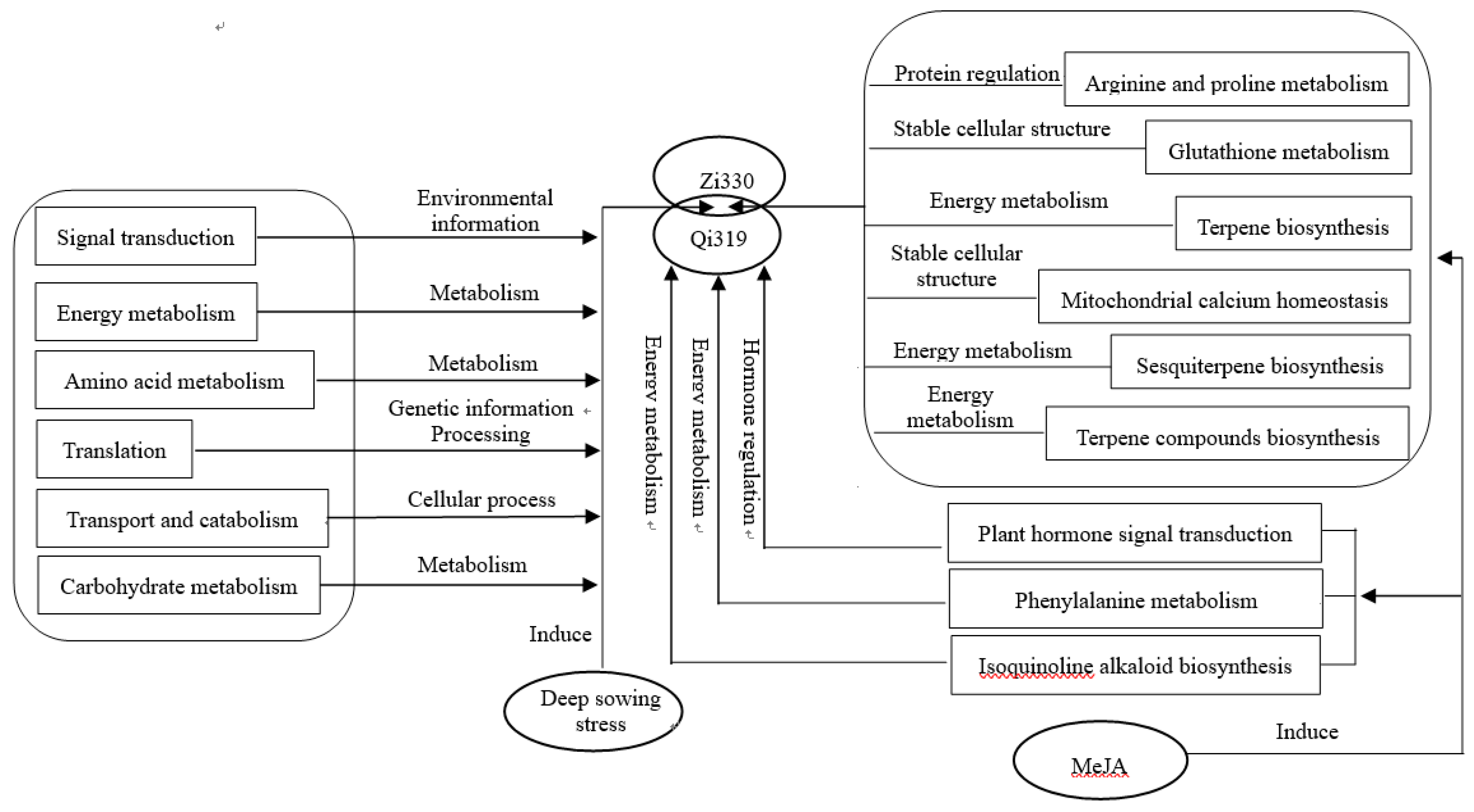
3.4. Analysis of Key Differential Expressed Genes Under Deep Sowing Stress and Exogenous MeJA Mitigation Treatment
4. Materials and Methods
4.1. Experiment Materials
4.2. Experiment method
4.2.1. Seedling culture and treatment
4.2.2. Determination of Related Indexes of Deep Sowing
4.2.3. Transcriptomic analysis of deep seeding stress in maize
4.2.4. Analysis of Differentially Expressed Genes
4.2.5. Weighted Gene Co-Expression Network Analysis (WGCNA)
4.3. qRT-PCR of Differentially Expressed Genes
4.4. Statistics Analysis of Data
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang C.T.; Li S.K. Assessment of limiting factors and techniques prioritization for maize production in China.Scientia Agricultura Sinica, 2010, 43(6): 1136-1146. [CrossRef]
- Sun, C.X.; Shen X.Y. Research progress on identification indicators and quantitative analysis methods for crop drought resistance.Chinese Journal of Agriculture, 2002, 18 (1): 49-51.
- Avramova, V.; Abdelgawad, H.; Vasileva, I.; Petrova, A.S.; Holek, A., Mariën, J., Asard, H., Beemster, G.T.S. High antioxidant activity facilitates maintenance of cell division in leaves of drought tolerant maize hybrids. Frontiers in Plant Science, 2017(8):84-99. [CrossRef]
- Ribaut, J.M.; Hoisington, D. A.; Deutsch, J. A.; Jiang, C.; Gonzalez-de-Leon, D. Identification of quantitative trait loci under drought conditions in tropical maize. 1. Flowering parameters and the anthesis-silking interval. Theoretical and Applied Genetics, 1996, 92: 905-914.
- Wang, Y.T.; Zhou, Y.F.; Li, F.X.; Yi B.; Bai, W.; Yan, T.; Xu, W.J.; Gao, M.C.; Huang, R.D. Identification and classification of drought resistance of sorghum varieties during germination period based on principal component analysis and SOM clustering analysis. Journal of Crops, 2014, 40 (01): 110-121. [CrossRef]
- Zhao, X.Q.; Zhong, Y. Research progress on the physiological response mechanism and molecular genetic mechanism of maize seeds to deep sowing stress. Molecular Plant Breeding, 2021, 19 (07): 2381-2390. [CrossRef]
- Li L. The regulatory mechanism of hypocotyl elongation and growth in weedy rice. Shenyang Agricultural University, 2013.
- Xue, R.H.; Jin, S.A. Methyl jasmonate: an important plant signal transduction molecule. Biotechnology Communications, 2006,17 (06): 985-988.
- Swiatck, A.; Dongen, W. V.; Onckelen, H.V. Metabolic fate of jasmonates in tobacco bright yellow-2 cells. Plant Physiol, 2004, 135(1): 161. DOI:10.2307/4281736.
- Shi, Y.M.; Tao, Y.W.; Qin, W.Y.; Fei, X.N. The effects of low temperature and plant growth regulators on the flowering of vanilla. Journal of Horticulture, 1997, 24 (2): 185-188.
- Du, Z.M.; Hao, J.S.; Zhou, J.; Li, H.X.; Gao, Q.Y.; Cui, H.B.; Zhen, X.B.; Ma, C.; Liang, J. N. Field in situ remediation of copper and cadmium compound polluted soil with four improvers. Journal of Soil Science, 2012, 49 (3): 508-517.
- Gao, Y. J.; Yang, J.L.; Chen, L.; Zhu, G.; Zhe, X.Y.; Liu, W.; Li, S.X. Soil water use status of dry land winter wheat under different cultivation modes, Nitrogen Application Rates, and Planting Density. Agricultural Research in Arid Areas, 2007, 25 (3): 45-50.
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell & Environment, 2010, 33(4): 453-467. [CrossRef]
- Kutschera, U.; Wang, Z.Y. Growth-limiting proteins in maize coleoptiles and the auxin brassinosteroid hypothesis of mesocotyl elongation. Protoplasma, 2016, 253(1): 3-14. [CrossRef]
- Hu, Z.; Yamauchi, T.; Yang, J.; Yusuke, J.; Tomoko, T.M.; Hiroaki, I.; Itsuro, T.; Yoshiaki, N.; Nobuhiro, T.; Shinjiro, Y. Strigolactone and cytokinin act antagonistically in regulating rice mesocotyl elongation in darkness[J]. Plant & cell physiology, 2014, 55(1): 30-41. [CrossRef]
- Liang, Q.; Wang, C.; Ma, D.R.; Li, L.; Cui, Z.B.; Wang, X.X.; Qian, Q.; Cai, B.D.; Feng, Y.Q.; Chen, W.F. Cortical microtubule disorganized related to an endogenous gibberellin increase plays an important role in rice mesocotyl elongation. Plant Biotechnology, 2016, 33(2): 59-69. [CrossRef]
- Wu, S.Q.; Ding, R.; Li X.S. Effects of gibberellin and Abscisic acid on the elongation and growth of mesocotyl in etiolated seedlings of black rice. Amino Acids and Biological Resources, 2002, (03): 44-56. [CrossRef]
- Zhao, G.W.; Ma P.; Wang J.H.; Wang, G.Y. Identification of tolerance to deep sowing and physiological response to deep sowing stress in different maize inbred lines. Journal of Maize Sciences, 2009, 17 (05): 9-13. [CrossRef]
- Zhou, D.B. Plant hormone and Cytoskeleton alignment. Communication of Plant physiology, 2005 (02): 224-228.
- Cosgrove, D.J. Expansive growth of plant cell walls. Plant physiology and biochemistry: PPB, 2000, 38(1-2): 109-124.
- Karpinska, B.; Karlsson, M.; Schinkel, H.; Streller, S.; Süss, K.H.; Melzer, M.; Wingsle, G. A novel superoxide dismutase with a high isoelectric point in higher plants. expression, regulation, and protein localization. Plant physiology, 2001, 126(4): 1668-1677. www.plantphysiol.org © 2001 American Society of Plant Biologists.
- Jiang, M.; Zhang, J. Involvement of plasma-membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta, 2002, 215(6):1022-1030. [CrossRef]
- Frahry, G.; Schopfer, P. NADH-stimulated, cyanide-resistant superoxide production in maize coleoptiles analyzed with a tetrazolium-based assay. Planta, 2001, 212(2): 175-183. [CrossRef]
- Yoda, H.; Yamaguchi, Y.; Sano, H. Induction of hypersensitive cell death by hydrogen peroxide produced through polyamine degradation in tobacco plants. Plant physiology, 2003, 132(4): 1973-1981. [CrossRef]
- Wang, L.J.; Fang, X.; Yang, C.Q.; Li, J.W.; Chen, X.Y. Secondary metabolism and regulation of terpenoids in plants. Chinese Science: Life Sciences, 2013, 43 (12): 1030-1046.
- Zhao,G.; Wang, J. Effect of auxin on mesocotyl elongation of dark-grown maize under different seeding depths. Russian Journal of Plant Physiology, 2010, 57(1): 79-86. [CrossRef]
- Kutschera, U.; Khanna, R. Auxin action in developing maize coleoptiles: challenges and open questions. Plant signaling & behavior, 2020,15(6). [CrossRef]
- Pan, B.; Zhong, T.; Zhao, G. Promoting deep-sowing germinability of corn (Zea mays) by seed soaking with gibberellic acid[J]. Archives of Agronomy and Soil Science, 2017, 63(9): 1314-1323. [CrossRef]
- Fan, M.; Bai, M.Y.; Kim, J.G.; Wang, T.; Eunkyoo, O.; Chen, L.; Ho Park, C.; Seung-Hyun, S.; Kim, S.K.; Mudgett, M. B.; Wang, Z.Y. The bHLH transcription factor HBI1 mediates the trade-off between growth and pathogen-associated molecular pattern-triggered immunityin Arabidopsis. The Plant cell, 2014, 26(2): 828-841. [CrossRef]
- Wan, F.X.; Ye, K.J.; Wei, Q.T.; Zhao, Z.J.; Zhang, Y.H.; Wang, X.H. Cloning and expression analysis of ERF transcription factor in eggplant. Molecular Plant Breeding, 2024. https://link.cnki.net/urlid/46.1068.S.20240518.2018.002.
- Zhang, Z.; Huang, R. Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol Biol, 2010, 73(3): 241-249. [CrossRef]
- Wang, S.J.; Fan, Y.; Du, S.H.; Zhao, K.; Liu, Q.; Yao, W.J.; Zheng, T.C.; Han, Y.Z. PtaERF194 inhibits plant growth and enhances drought tolerance in poplar. Tree Physiology, 2022, 42(8): 1678-1692. [CrossRef]
- Duan, Y.J.; Shang, X.G.; Tian, R.P.; Li, W.X.; Song, X.H.; Zhang, D.Y.; Guo, W.Z. Suppressing a mitochondrial calcium uniporter activates the calcium signaling pathway and promotes cell elongation in cotton. The Crop Journal, 2024, 12: 411-421. [CrossRef]
- Humphrey, T.V.; Haasen, K.E.; Aldea-Brydges, M.G.; Sun, H.; Zayed, Y.; Indriolo, E.; Goring, D.R. PERK-KIPK-KCBP signalling negatively regulates root growth in Arabidopsis thaliana. J Exp Bot, 2015, 66 (1): 71-83. [CrossRef]
- Peng, Y.L.; Yang, F.L.; Zhao, X.Q.; Ren, X.W. Analysis of differences in deep sowing tolerance among different maize inbred lines. Agricultural Research in Arid Areas, 2014, 32 (01): 25-33+51.
- Tian, C.X.; Zhang, Y.M.; Ma, H.L. Preliminary study on the method of making embryonic structure paraffin slices of poa pratensis. Grassland Science, 2013, 30 (12): 1980-1986.
- Wang, S.P.; Ruan, X.F. Isolation and purification of four plant hormone. Communication of Plant physiology, 1987, (05): 50-53. [CrossRef]
- Shahzad, A.; Gul, h.; Ahsan,M.; Wang, D.P.; Fahad, S. Comparative genetic evaluation of maize imported lines at seeding and maturity stages under drought stress. Journal of Plant Growth Regulation, 2023, (42): 989-1005. [CrossRef]
- Audio, S.; Claverie, J.M. The significance of digital gene expression profiles. Genome Research, 1997, 7 (10): 986-995.
- Ye, J.; Fang, L.; Zheng, H.K.; Zhang, Y.; Chen, J.; Zhang, Z.J.; Wang, J.; Li, S.T.; i, R.Q.; Bolund, L.; Wang, J. WEGO: a web tool for plotting GO annotations. Nuclear Acids Research, 2006, 34 (2): 293-297.
- Yao, Y.; Xiong, E.; Qu, X.; Li, J.; Liu, H.; Quan, L.; Lu, W.; Zhu, X.; Chen, M.; Li, K.; et al. WGCNA and transcriptome proffling reveal hub genes for key development stage seed size/oil content between wild and cultivated soybean. BMC Genom.2023, 24, 494. [CrossRef]
- Li, S.T.; Chen, H.; Hou, Z.; Li Y.; Yang C.L.; Wang D.J.; Song C.P. Screening of abiotic stress-responsive cotton genes using a cotton full length cDNA overexpressing Arabidopsis library. Journal of Integrative Plant Biology, 2020, 62(07): 998-1016. [CrossRef]
| Material | Treatment | SDL(cm) | RL(cm) | SDW(g) | RW(g) |
|---|---|---|---|---|---|
| Zi330 | CK | 26.60±0.81c | 33.10±0.64a | 1.57±0.06a | 1.38±0.12a |
| CM | 33.73±1.18a | 29.00±0.21b | 1.29±0.02b | 1.20±0.07ab | |
| DS | 29.23±0.86bc | 20.27±0.48c | 1.17±0.04b | 0.96±0.03b | |
| DM | 31.85±1.55ab | 18.80±0.30c | 1.26±0.05b | 1.28±0.01a | |
| Qi319 | CK | 34.10±2.17a | 34.93±0.64a | 1.21±0.09a | 1.32±0.03a |
| CM | 27.47±1.26ab | 34.67±0.79a | 0.86±0.04b | 1.33±0.06a | |
| DS | 25.25±5.05b | 23.40±1.20c | 1.07±0.10ab | 1.14±0.07b | |
| DM | 33.90±0.70a | 26.67±0.64b | 1.15±0.10ab | 1.34±0.03a |
| Material | Treatment | MES(cm) | COL(cm) | MES+COL(cm) | MESW+COLW(g) |
|---|---|---|---|---|---|
| Zi330 | CK | 2.67±0.17c | 2.57±0.20b | 5.23±0.35c | 0.33±0.04c |
| CM | 2.67±0.33c | 2.23±0.09b | 4.90±0.25c | 0.25±0.01c | |
| DS | 5.53±0.29b | 6.30±0.71a | 11.83±0.43a | 0.75±0.02a | |
| DM | 7.00±0.60a | 2.80±0.30b | 9.80±0.30b | 0.62±0.05b | |
| Qi319 | CK | 2.40±0.21d | 3.20±0.21b | 5.60±0.21c | 0.24±0.02c |
| CM | 3.13±0.09c | 2.50±0.40b | 5.63±0.32c | 0.23±0.03c | |
| DS | 8.00±0.20b | 3.53±1.90b | 13.30±1.00b | 0.37±0.06b | |
| DM | 8.60±0.12a | 7.10±0.35a | 15.70±0.26a | 0.52±0.03a |
| Sample | Total Raw Reads (M) | Total Clean Reads (M) | Total Clean Bases (Gb) | Clean Reads Q20 (%) | Clean Reads Q30 (%) | Clean Reads Ratio (%) |
|---|---|---|---|---|---|---|
| Zi_3_CK1 | 45.57 | 44.02 | 6.60 | 96.47 | 91.25 | 96.60 |
| Zi_3_CK2 | 45.57 | 44.01 | 6.60 | 96.50 | 91.33 | 96.56 |
| Zi_3_CK3 | 45.57 | 44.25 | 6.64 | 96.55 | 91.34 | 97.09 |
| Zi_3_T1 | 45.57 | 44.37 | 6.66 | 96.41 | 91.05 | 97.37 |
| Zi_3_T2 | 45.57 | 43.91 | 6.59 | 96.61 | 91.56 | 96.34 |
| Zi_3_T3 | 45.57 | 44.24 | 6.64 | 96.53 | 91.38 | 97.08 |
| Zi_15_CK1 | 45.57 | 44.51 | 6.68 | 96.42 | 91.08 | 97.66 |
| Zi_15_CK2 | 45.57 | 44.40 | 6.66 | 96.54 | 91.34 | 97.43 |
| Zi_15_CK3 | 45.57 | 44.29 | 6.64 | 96.53 | 91.36 | 97.17 |
| Zi_15_T1 | 45.57 | 44.26 | 6.64 | 96.59 | 91.50 | 97.13 |
| Zi_15_T2 | 45.57 | 44.1 | 6.62 | 96.55 | 91.35 | 96.77 |
| Zi_15_T3 | 45.57 | 44.38 | 6.66 | 96.51 | 91.31 | 97.37 |
| Qi_3_CK1 | 45.56 | 44.28 | 6.64 | 96.54 | 91.42 | 97.19 |
| Qi_3_CK2 | 45.57 | 44.18 | 6.63 | 96.56 | 91.45 | 96.95 |
| Qi_3_CK3 | 45.57 | 44.21 | 6.63 | 96.52 | 91.34 | 97.01 |
| Qi_3_T1 | 45.57 | 44.17 | 6.63 | 96.52 | 91.38 | 96.92 |
| Qi_3_T2 | 45.57 | 44.32 | 6.65 | 96.44 | 91.13 | 97.25 |
| Qi_3_T3 | 45.57 | 44.17 | 6.63 | 96.47 | 91.26 | 96.92 |
| Qi_15_CK1 | 45.57 | 44.15 | 6.62 | 96.44 | 91.17 | 96.88 |
| Qi_15_CK2 | 45.57 | 44.11 | 6.62 | 96.52 | 91.33 | 96.78 |
| Qi_15_CK3 | 47.33 | 44.12 | 6.62 | 96.65 | 91.72 | 93.23 |
| Qi_15_T1 | 45.57 | 44.07 | 6.61 | 96.59 | 91.55 | 96.7 |
| Qi_15_T2 | 45.57 | 43.95 | 6.59 | 96.64 | 91.65 | 96.43 |
| Qi_15_T3 | 45.57 | 43.93 | 6.59 | 96.62 | 91.65 | 96.40 |
| Gene ID | KEGG Pathway Desc | GO_P Desc |
|---|---|---|
| 100272950 | Plant hormone signal transduction | transcription, DNA-templated; regulation of transcription, DNA-templated |
| 100281647 | NA | systemic acquired resistance |
| 100284161 | NA | NA |
| 100284641 | NA | systemic acquired resistance |
| 103638673 | NA | NA |
| 103641531 | Glycine, serine and threonine metabolism; Tyrosine metabolism;Phenylalanine metabolism; beta-Alanine metabolism; Isoquinoline alkaloid biosynthesis; Tropane, piperidine and pyridine alkaloid biosynthesis | amine metabolic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





