Submitted:
10 September 2024
Posted:
11 September 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Results
Designated Reference Laboratories
EURLs for IVD Assessment under Regulation (EU) 2017/746 (IVDR), Art. 100
EURLs for Public Health under Regulation (EU) 2022/2371
NRLs under Regulation (EU) 2022/2371
Reference Laboratories Designated by Other Competent Organisations
Calibration (Reference) Laboratories
Referral Laboratories
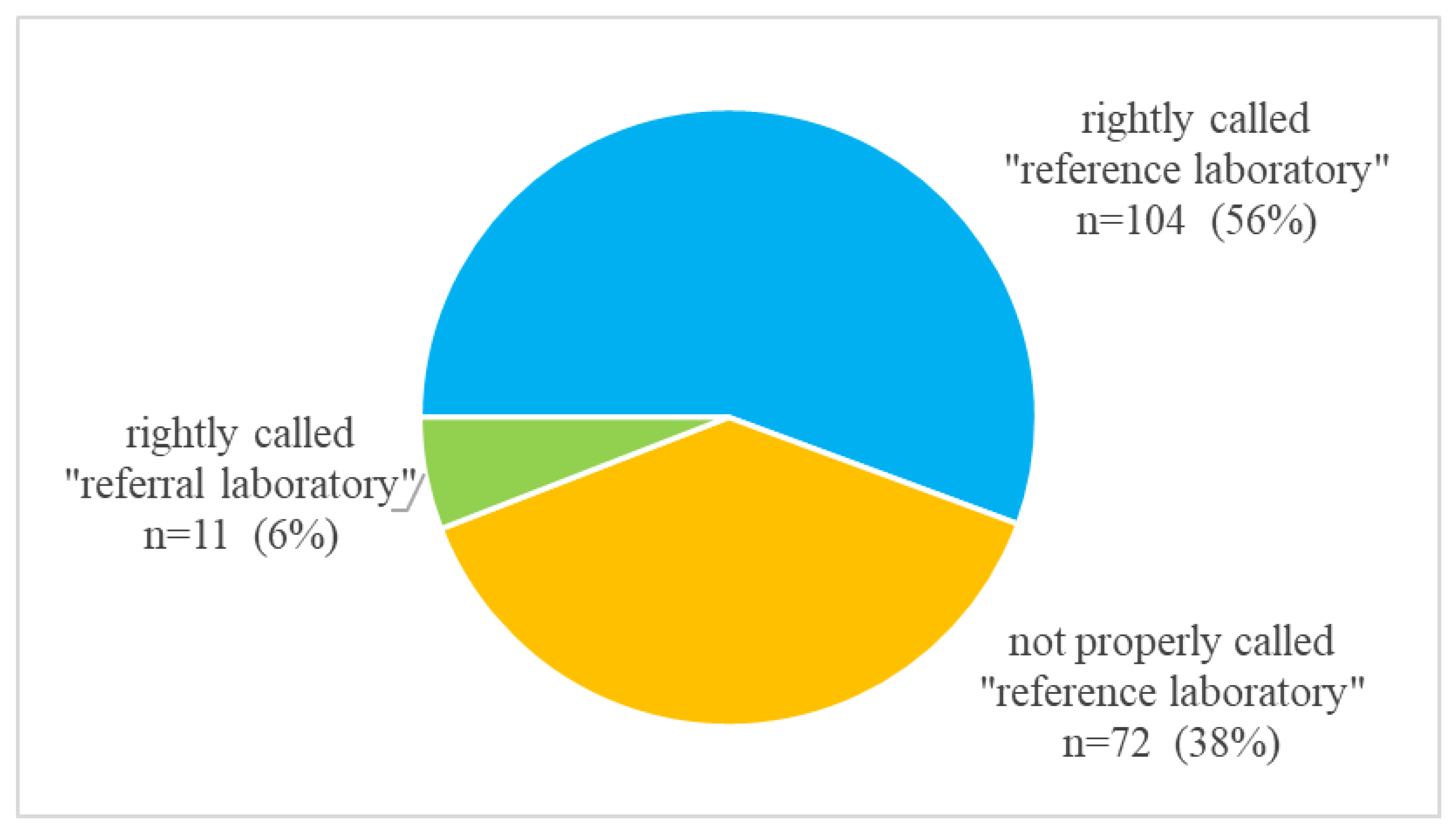
“Reference” and “Referral” Laboratories in Recent Scientific Publications
Discussion
Conclusion
Author contributions
Funding sources
Competing interests
Statement
References
- European Parliament and Council. Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU (Text with EEA relevance). Available online: http://data.europa.eu/eli/reg/2017/746/oj (accessed on 4 September 2024).
- European Parliament and Council. Regulation (EU) 2022/2371 of the European Parliament and of the Council of 23 November 2022 on serious cross-border threats to health and repealing Decision No 1082/2013/EU. Available online: http://data.europa.eu/eli/reg/2022/2371/oj (accessed on 4 September 2024).
- European Parliament and Council. Decision No 2119/98/EC of the European Parliament and of the Council of 24 September 1998 setting up a network for the epidemiological surveillance and control of communicable diseases in the Community. Available online: http://data.europa.eu/eli/dec/1998/2119/oj (accessed on 4 September 2024).
- European Parliament and Council. Decision No 1082/2013/EU of the European Parliament and of the Council of 22 October 2013 on serious cross-border threats to health and repealing Decision No 2119/98/EC. Available online: http://data.europa.eu/eli/dec/2013/1082/oj (accessed on 4 September 2024).
- European Centre for Disease Prevention and Control (ECDC). Disease and Laboratory Networks. Available online: https://www.ecdc.europa.eu/en/about-ecdc/what-we-do/partners-and-networks/disease-and-laboratory-networks (accessed on 4 September 2024).
- European Parliament and Council. Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products, amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC, and repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/EC and Council Decision 92/438/EEC (Official Controls Regulation) (Text with EEA relevance). Available online: http://data.europa.eu/eli/reg/2017/625/oj (accessed on 4 September 2024).
- European Commission. European Union Reference Laboratories for Animal Health, Food and Feed, and Plant Health. Available online: https://food.ec.europa.eu/horizontal-topics/european-union-reference-laboratories_en (accessed on 4 September 2024).
- European Commission. Commission Implementing Regulation (EU) 2023/2713 of 5 December 2023 designating European Union reference laboratories in the field of in vitro diagnostic medical devices. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L_202302713 (accessed on 4 September 2024).
- Biomedical Alliance in Europe. Statement: Urgent action needed to prevent widespread shortage of diagnostic tests. Available online: https://www.biomedeurope.org/images/news/2024/Statement_prevention_shortage_IVDs_final_16.01.pdf (accessed on 4 September 2024).
- Medical Devices Coordination Group (MDCG). Guidance on Classification Rules for in vitro Diagnostic Medical Devices under Regulation (EU) 2017/746. Available online: https://health.ec.europa.eu/system/files/2023-02/md_mdcg_2020_guidance_classification_ivd-md_en.pdf (accessed on 4 September 2024).
- The Borderline and Classification Working Group (BCWG). Manual on borderline and classification for medical devices under Regulation (EU) 2017/745 on medical devices and Regulation (EU) 2017/746 on in vitro diagnostic medical devices. Available online: https://health.ec.europa.eu/system/files/2023-09/md_borderline_manual_en.pdf (accessed on 4 September 2024).
- European Commission. Commission Implementing Regulation (EU) 2022/944 of 17 June 2022 laying down rules for the application of Regulation (EU) 2017/746 of the European Parliament and of the Council as regards the tasks of and criteria for European Union reference laboratories in the field of in vitro diagnostic medical devices. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022R0944 (accessed on 4 September 2024).
- ISO/IEC 17025:2017 General requirements for the competence of testing and calibration laboratories. Geneva, Switzerland: International Organization for Standardization (ISO); 2017.
- European Commission, Commission Implementing Regulation (EU) 2022/1107 of 4 July 2022 laying down common specifications for certain class D in vitro diagnostic medical devices in accordance with Regulation (EU) 2017/746 of the European Parliament and of the Council (Text with EEA relevance). Available online: http://data.europa.eu/eli/reg_impl/2022/1107/oj (accessed on 4 September 2024).
- Bretthauer M, Gerke S, Hassan C, Ahmad OF, Mori Y. The New European Medical Device Regulation: Balancing Innovation and Patient Safety. Ann Intern Med 2023;176:844-8. [CrossRef]
- Lubbers BR, Schilhabel A, Cobbaert CM, Gonzalez D, Dombrink I, Brüggemann M, et al. The New EU Regulation on In Vitro Diagnostic Medical Devices: Implications and Preparatory Actions for Diagnostic Laboratories. Hemasphere 2021;5:e568. [CrossRef]
- European Parliament and Council. Regulation (EU) 2024/1860 of the European Parliament and of the Council of 13 June 2024 amending Regulations (EU) 2017/745 and (EU) 2017/746 as regards a gradual roll-out of Eudamed, the obligation to inform in case of interruption or discontinuation of supply, and transitional provisions for certain in vitro diagnostic medical devices (Text with EEA relevance). Available online: http://data.europa.eu/eli/reg/2024/1860/oj (accessed on 4 September 2024).
- Covington & Burling LLP. European Commission Proposes to Extend Transitional Periods for In-Vitro-Diagnostic Medical Devices. Available online: www.insideeulifesciences.com/2024/01/25/european-commission-proposes-to-extend-transitional-periods-for-in-vitro-diagnostic-medical-devices/ (accessed on 4 September 2024).
- European Commission. Commission Implementing Regulation (EU) 2024/892 of 22 March 2024 designating European Union reference laboratories for certain specific areas of public health. Available online: http://data.europa.eu/eli/reg_impl/2024/892/oj (accessed on 4 September 2024).
- European Commission. EU Reference Laboratories for public health. Available online: https://health.ec.europa.eu/health-security-and-infectious-diseases/surveillance-and-early-warning/eu-reference-laboratories-public-health_en (accessed on 4 September 2024).
- European Parliament and Council. Regulation (EU) 2021/522 of the European Parliament and of the Council of 24 March 2021 establishing a Programme for the Union’s action in the field of health (‘EU4Health Programme’) for the period 2021-2027, and repealing Regulation (EU) No 282/2014 (Text with EEA relevance). Available online: http://data.europa.eu/eli/reg/2021/522/oj (accessed on 4 September 2024).
- European Centre for Disease Prevention and Control (ECDC). Disease and laboratory networks. Available online: https://www.ecdc.europa.eu/en/about-ecdc/what-we-do/partners-and-networks/disease-and-laboratory-networks (accessed on 4 September 2024).
- European Commission. Call for Applications for the Designation of an EU Reference Laboratory for Public Health in the field of Food- and water- borne bacteria. Available online: https://health.ec.europa.eu/document/download/79f4172a-ba0b-426a-a672-6cd9de5e9c5c_en?filename=security_2024-eurl-call_fwb-bacteria_call_en.pdf (accessed on 4 September 2024).
- Robert Koch Institut. National Reference Centers and Consultant Laboratories. Available online: https://www.rki.de/EN/Content/infections/Diagnostics/NatRefCentresConsultantLab/natRefCentresConsultantLab_node.html (accessed on 4 September 2024).
- Institut Pasteur. Centres Nationaux de Référence. Available online: https://www.pasteur.fr/fr/sante-publique/CNR/les-cnr (accessed on 4 September 2024).
- UK Health Security Agency. Specialist and reference microbiology: laboratory tests and services. Available online: https://www.gov.uk/guidance/specialist-and-reference-microbiology-laboratory-tests-and-services (accessed on 4 September 2024).
- Austrian Agency for Health and Food Safety. National Reference Centres & Laboratories. Available online: https://www.ages.at/en/ages/reference-centres-laboratories (accessed on 4 September 2024).
- The Infectious Diseases Toolkit. Available online: https://www.infectious-diseases-toolkit.org/national-resources/ (accessed on 4 September 2024).
- European Centre for Disease Prevention and Control (ECDC). Core functions of microbiology reference laboratories for communicable diseases. Stockholm: ECDC; 2010. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/1006_TER_Core_functions_of_reference_labs.pdf (accessed on 4 September 2024).
- European Parliament and Council. Decision No 1082/2013/EU of the European Parliament and of the Council of 22 October 2013 on serious cross-border threats to health and repealing Decision No 2119/98/EC. Available online: http://data.europa.eu/eli/dec/2013/1082/oj (accessed on 4 September 2024).
- Albiger B, Revez J, Leitmeyer KC, Struelens MJ. Networking of Public Health Microbiology Laboratories Bolsters Europe's Defenses against Infectious Diseases. Front Public Health 2018;6:46. [CrossRef]
- European Centre for Disease Prevention and Control. Core functions of microbiology reference laboratories for communicable diseases. Stockholm: ECDC; 2010. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/1006_TER_Core_functions_of_reference_labs.pdf (accessed on 4 September 2024).
- European Centre for Disease Prevention and Control. ECDC public health microbiology strategy 2018-2022. Stockholm: ECDC; 2018. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/ECDC-public-health-microbiology-strategy-2018-2022.pdf (accessed on 4 September 2024).
- European Commission. Surveillance and early warning. Available online: https://health.ec.europa.eu/health-security-and-infectious-diseases/surveillance-and-early-warning_en (accessed on 4 September 2024).
- European Commission. Commission Implementing Decision (EU) 2018/945 of 22 June 2018 on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D0945 (accessed on 4 September 2024).
- World Health Organization. WHO H5 Reference Laboratories and the Terms of Reference. Available online: https://www.who.int/initiatives/global-influenza-surveillance-and-response-system/h5-reference-laboratories (accessed on 4 September 2024).
- World Health Organization. Global HIV Programme Laboratory Network. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/treatment/hiv-drug-resistance/laboratory-network (accessed on 4 September 2024).
- World Health Organization, Measles and Rubella Laboratory Network. Available online: https://www.who.int/europe/initiatives/measles-and-rubella-laboratory-network (accessed on 4 September 2024).
- World Health Organization. Available online: https://polioeradication.org/polio-today/polio-now/surveillance-indicators/the-global-polio-laboratory-network-gpln/ (accessed on 4 September 2024).
- World Health Organization, TB Supranational Reference Laboratory Network (SRLN). Available online: https://www.who.int/groups/tb-supranational-reference-laboratory-network (accessed on 4 September 2024).
- World Health Organization, Prequalification of Medical Products. Available online: https://extranet.who.int/prequal/ (accessed on 4 September 2024).
- European Centre for Disease Prevention and Control, Disease and laboratory networks. Available online: https://www.ecdc.europa.eu/en/about-ecdc/what-we-do/partners-and-networks/disease-and-laboratory-networks (accessed on 4 September 2024).
- European Centre for Disease Prevention and Control, European COVID-19 reference laboratory network (ECOVID-LabNet). Available online: https://www.ecdc.europa.eu/en/about-ecdc/what-we-do/partners-and-networks/disease-and-laboratory-networks/european-covid-19 (accessed on 4 September 2024).
- European Centre for Disease Prevention and Control, European Reference Laboratory Network for TB (ERLTB-Net). Available online: https://www.ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/erltb-net (accessed on 4 September 2024).
- European Centre for Disease Prevention and Control, European Reference Laboratory Network for Human Influenza (ERLI-Net). Available online: https://www.ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/erlinet (accessed on 4 September 2024).
- The World Anti-Doping Agency (WADA). Available online: https://www.wada-ama.org/en/anti-doping-partners/laboratories (accessed on 4 September 2024).
- The World Anti-Doping Agency (WADA), World Anti-Doping Code International Standard Laboratories 2021. Montreal, Quebec, Canada, 2021. Available online: https://www.wada-ama.org/sites/default/files/resources/files/isl_2021.pdf (accessed on 4 September 2024).
- The World Anti-Doping Agency (WADA), List of WADA-Accredited Laboratories. Available online: https://www.wada-ama.org/en/resources/lab-documents/list-wada-accredited-laboratories (accessed on 4 September 2024).
- Eurotransplant Reference Laboratory (ETRL). Available online: https://etrl.eurotransplant.org/about-eurotransplant/about-the-etrl/ (accessed on 4 September 2024).
- European Federation for Immunogenetics (EFI). Available online: https://efi-web.org/ (accessed on 4 September 2024).
- European Federation for Immunogenetics (EFI) - Constitution of the European Federation for Immunogenetics. Available online: https://efi-web.org/fileadmin/Efi_web/About_EFI/Constitution/2023-04-28_Constitution_EFI_English.pdf (accessed on 4 September 2024).
- Harmer A, Mascaretti L, Petershofen E. Accreditation of histocompatibility and immunogenetics laboratories: Achievements and future prospects from the European Federation for Immunogenetics Accreditation Programme. HLA. 2018 May 2. Epub ahead of print. [CrossRef] [PubMed]
- European Federation for Immunogenetics (EFI) - EFI accredited laboratories. Available online: https://efi-web.org/accreditation/efi-accredited-laboratories (accessed on 4 September 2024).
- Association for the Advancement of Blood & Biotherapies. Available online: https://www.aabb.org/standards-accreditation/accreditation/accredited-facilities/aabb-accredited-blood-banks-transfusion-services-and-blood-centers (accessed on 4 September 2024).
- College of American Pathologists Accreditation. Available online: https://www.cap.org/laboratory-improvement/accreditation (accessed on 4 September 2024).
- U.S. FDA Clinical Laboratory Improvement Amendments (CLIA). Available online: https://www.fda.gov/medical-devices/ivd-regulatory-assistance/clinical-laboratory-improvement-amendments-clia (accessed on 4 September 2024).
- Revelli N, Villa MA, Paccapelo C, Manera MC, Erba E, Truglio F, et al. The immunohaematology reference laboratory: the experience of the Policlinico Maggiore Hospital, Mangiagalli and Regina Elena Foundation, Milan. Blood Transfus. 2009;7:94-9. [CrossRef] [PubMed] [PubMed Central]
- ISO 21151:2020 In vitro diagnostic medical devices - Requirements for international harmonisation protocols establishing metrological traceability of values assigned to calibrators and human samples. Geneva, Switzerland: International Organization for Standardization (ISO); 2020.
- Bureau International des Poids et Mesures (BIPM). Available online: https://www.bipm.org/en/ (accessed on 4 September 2024).
- Miller WG, Jones GR, Horowitz GL, Weykamp C. Proficiency Testing/External Quality Assessment: Current Challenges and Future Directions. Clinical Chemistry 2011;57:1670–80. [CrossRef]
- ISO 15195:2018 - Laboratory medicine - Requirements for the competence of calibration laboratories using reference measurement procedures. Geneva, Switzerland: International Organization for Standardization (ISO); 2018.
- ISO 17511:2020 - In vitro diagnostic medical devices - Requirements for establishing metrological traceability of values assigned to calibrators, trueness control materials and human samples. Geneva, Switzerland: International Organization for Standardization (ISO); 2020.
- ISO 15193:2009 - In vitro diagnostic medical devices - Measurement of quantities in samples of biological origin - Requirements for content and presentation of reference measurement procedures. Geneva, Switzerland: International Organization for Standardization (ISO); 2009.
- ISO 15194:2009 - In vitro diagnostic medical devices - Measurement of quantities in samples of biological origin - Requirements for certified reference materials and the content of supporting documentation. Geneva, Switzerland: International Organization for Standardization (ISO); 2009.
- JCTLM Accurate results for patient care [Internet]. Home - JCTLM. Available online: https://jctlm.org/ (accessed on 4 September 2024).
- JCTLM Accurate results for patient care [Internet]. JCTLM Database. Available online: https://www.jctlmdb.org/#/app/home (accessed on 4 September 2024).
- Siekmann L. Requirements for reference (calibration) laboratories in laboratory medicine. Clin Biochem Rev 2007;28:149-54.
- RELA - IFCC External Quality assessment scheme for Reference Laboratories in Laboratory Medicine. Available online: http://dgkl-rfb.de:81 (accessed on 4 September 2024).
- ISO 15189:2022. Medical laboratories – requirements for quality and competence. Geneva, Switzerland: International Organization for Standardization (ISO); 2022.
- Isaacs D, Fitzgerald D. Seven alternatives to evidence based medicine. BMJ 1999;319:1618. [CrossRef]
- Directorate-General for Health and Food Safety. Expression of interest open – Possible second call for EU reference laboratories for high-risk in vitro diagnostic medical devices. Available online: https://health.ec.europa.eu/latest-updates/expression-interest-open-possible-second-call-eu-reference-laboratories-high-risk-vitro-diagnostic-2024-02-22_en (accessed on 4 September 2024).
- International Laboratory Accreditation Cooperation. Available online: https://ilac.org/ (accessed on 4 September 2024).
| Type of laboratory | Designated reference laboratories | Referral laboratories | ||||
|---|---|---|---|---|---|---|
|
Name |
European Union Reference Laboratories for | National Reference Laboratories [3] |
Reference laboratories designated by other organisations | Calibration (reference) laboratories |
Medical laboratories |
|
| IVD assessment [1] | public health [2] | |||||
| designated by | European Commission | European Commission | National government | Competent organisation | Endorsement by IFCC, listing by the JCTLM |
n/a |
| current number | 5 (as of 5 December 2023) [8] |
6 (as of 22 March 2024) [19] |
(not collected) | (not collected) | 27 (listed by JCTLM) [65] |
(not collected) |
| Service | ||||||
| area | Performance evaluation of class D IVDs | Public health surveillance and epidemiology | Public health surveillance and epidemiology | As agreed with designator | Operation of RMPs compliant to ISO 15195 and ISO 17025 | Routine laboratory diagnostics, area of expertise |
| aim | Only IVDs compliant with IVDR on the market |
Provision of continuous, robust monitoring and early warning and response mechanisms | Provision of continuous, robust monitoring and early warning and response mechanisms; routine diagnostics |
As agreed with designator | Metrological traceability and harmonisation of measurement results | Support of referring laboratory |
| applicable standard | ISO 17025 | ISO 17025 | ISO 17025 (in addition ISO 15189 if analyses also for patient diagnostics are carried out) |
As agreed with designator | ISO 17025 | ISO 15189 |
| prerequisites | Designation by European Commission | Designation by European Commission | Designation by national government | As specified by designator | Accreditation according to ISO 17025 | Operating licence under national law |
| examination procedures used | Methods according to common specifications and harmonised in the subnetworks of the EURLs | Methods according to common specifications and harmonised in the subnetworks of the EURLs | Methods selected by the NRL | As agreed with designator | RMPs | Routine examination procedures |
| materials analysed | Harmonised between the EURLs and according to the common specifications | Clinical specimens, specimens of non-human origin | Clinical specimens, specimens of non-human origin | As agreed with designator | Materials intended to become RMs or CRMs, but also quality control materials | Clinical specimens |
| report type provided and recipient | Evaluation report to NB | ??? | Medical or micro- biological report to referrer | As agreed with designator | Calibration certificates | Medical report to referrer |
| in operation from | October 1, 2024 | January 2025 | Individually different | n/a | 1998 | as long as laboratory diagnostics |
| costs for services borne by | customer (NB) |
EU4Health programme |
Regulated differently from state to state | to be determined by the designator |
customer (IVD manufacturer, EQA provider) |
Patients, their health insurance company or the referrer |
| non-financial benefit from service | none recognisable | Epidemiological surveillance data | Epidemiological surveillance data | none recognisable | standardisation of clinical measurements | none recognisable |
| Referral laboratory according to ISO 15189:2022 | no | no | can be | can be | no | yes |
| Referrer / customer / user of services | ||||||
| is / are | NBs | ??? | Referring diagnostic laboratory, national and European public health authorities | Designator or their eligible organisations | Manufacturers of IVD and CRM, EQA providers |
Patient and physician |
| may select laboratory | yes | ??? | no | yes | yes | yes |
| selects measurands to be determined | no | ??? | no | As agreed with designator | yes | yes |
| receives result/report | yes | yes | yes | As agreed with designator | yes | yes |
| regarding IVD compliance assessment [1,8] | regarding public health surveillance [2,19] |
|---|---|
|
|
Devices intended to be used for the following purposes are classified as class D (1):
|
| Function | Description | Activities |
|---|---|---|
| Reference diagnostics | The reference laboratory has state-of-the-art validated laboratory methods in operation and the ability to deliver accurate confirmation of diagnostic results within its field of expertise. This may include the analysis of samples in a variety of areas, such as the verification of results (e.g. detection or confirmation) reported by external laboratories, the detection of specific microbial markers and the investigation of atypical samples. |
|
| Reference material resources | If necessary, the reference laboratory develops and maintains - in accordance with international standards and procedures - a collection of relevant reference material that is to be shared with laboratories and organisations that request such materials. These materials can include reference laboratory strains and cultures, clinical isolates, sera, genetic materials, etc. These resources are important for the varied purposes of quality assurance systems, method evaluation and validation. |
|
| Scientific advice | The reference laboratory is a resource and coordination point for expertise within its specific area and shares information and advice with relevant stakeholders. This can include technical advice on methods and procedures, scientific support and advice on the interpretation and relevance of laboratory findings on pathogens to relevant public health authorities (policy makers and public health professionals). |
|
| Collaboration and research | The reference laboratory is at the forefront of technological and scientific development in its field of expertise, particularly in areas relevant to public health action. Contacts with regional and international laboratory networks as well as related initiatives should be established and maintained. Examples of collaboration are involvement in EU and other international disease-specific networks, network activities of regional laboratories, or global initiatives via WHO or the US CDC. |
|
| Monitoring, alert and response | The reference laboratory performs or contributes to surveillance activities, or has established channels of communication with the national surveillance body to regularly report incidence data and provide an ‘alert function’ for unusual occurrences. These can include failure of a diagnostic test, detection of changes in incidence, virulence, drug resistance, emergence of a possibly infectious disease of unknown aetiology, etc. In the case of an outbreak, the reference laboratory supports outbreak investigations, e.g. by offering diagnostic services, advice and technical expertise, and, upon request, provides surge capacity for diagnostics. |
|
| WHO reference, accredited, or merely designated laboratories | ||
| H5 Reference Laboratories (Influenza A(H5N1)) | [36] | |
| Global HIV, hepatitis and sexually transmitted infections (STIs) and resistance programmes | [37] | |
| measles and rubella laboratory network | [38] | |
| for poliomyelitis | [39] | |
| for tuberculosis | [40] | |
| for prequalification of medical products (IVDs, medicines, vaccines and immunisation devices, vector control) |
[41] | |
| ECDC reference laboratory networks | ||
| European COVID-19 reference laboratory network (ECOVID-LabNet) | [43] | |
| European Reference Laboratory Network for TB (ERLTB-Net) | [44] | |
| European Reference Laboratory Network for Human Influenza (ERLI-Net) | [45] | |
| WADA accredited laboratories | ||
| for doping control analysis | [48] | |
| ETRL reference laboratory | ||
| EPT provider for histocompatibility related assays for laboratories accredited or not accredited by the European Federation for Immunogenetics (EFI) | [49] | |
| EFI accredited laboratories | ||
| for immunogenetics, tissue typing and transplantation | [50] | |
| Immunohematology Reference Laboratories | ||
| Organisations designating immunohematology Reference Laboratories: | ||
| Association for the Advancement of Blood & Biotherapies (formerly American Association of Blood Banks) (AABB) |
[54] | |
| College of American Pathologists (CAP) | [55] | |
| according to the standard ISO 15189, if required by accreditation organisations with authorization according to the Clinical Laboratory Improvement Amendments (CLIA) |
[56,68] | |
| Analyte category | Reference measurement procedures (RMP) |
Reference measurement services(4) (RMS) |
Reference materials (RM) |
|---|---|---|---|
| Blood cell counting | 3(1) | 0 | 0 |
| Blood grouping | 0 | 0 | 3 |
| Coagulation factors | 0 | 0 | 1 |
| Drugs | 29 | 5 | 23 |
| Electrolytes | 46 | 40 | 36 |
| Enzymes | 7 | 111 | 10ju |
| Metabolites and substrates | 52 | 72 | 80 |
| Non-electrolyte metals | 15 | 0 | 41 |
| Non-peptide hormones | 40 | 41 | 32 |
| Nucleic acids | 9(2) | 0 | 24 |
| Proteins | 27(3) | 14 | 38 |
| Vitamins and micronutrients | 10 | 2 | 2 |
| total | 238 | 285 | 290 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





