Submitted:
10 September 2024
Posted:
11 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Experimental Reagents and Instruments
2.2. Materials Preparation
2.2.1. Synthesis of Metal Organic Frameworks (MOFs)
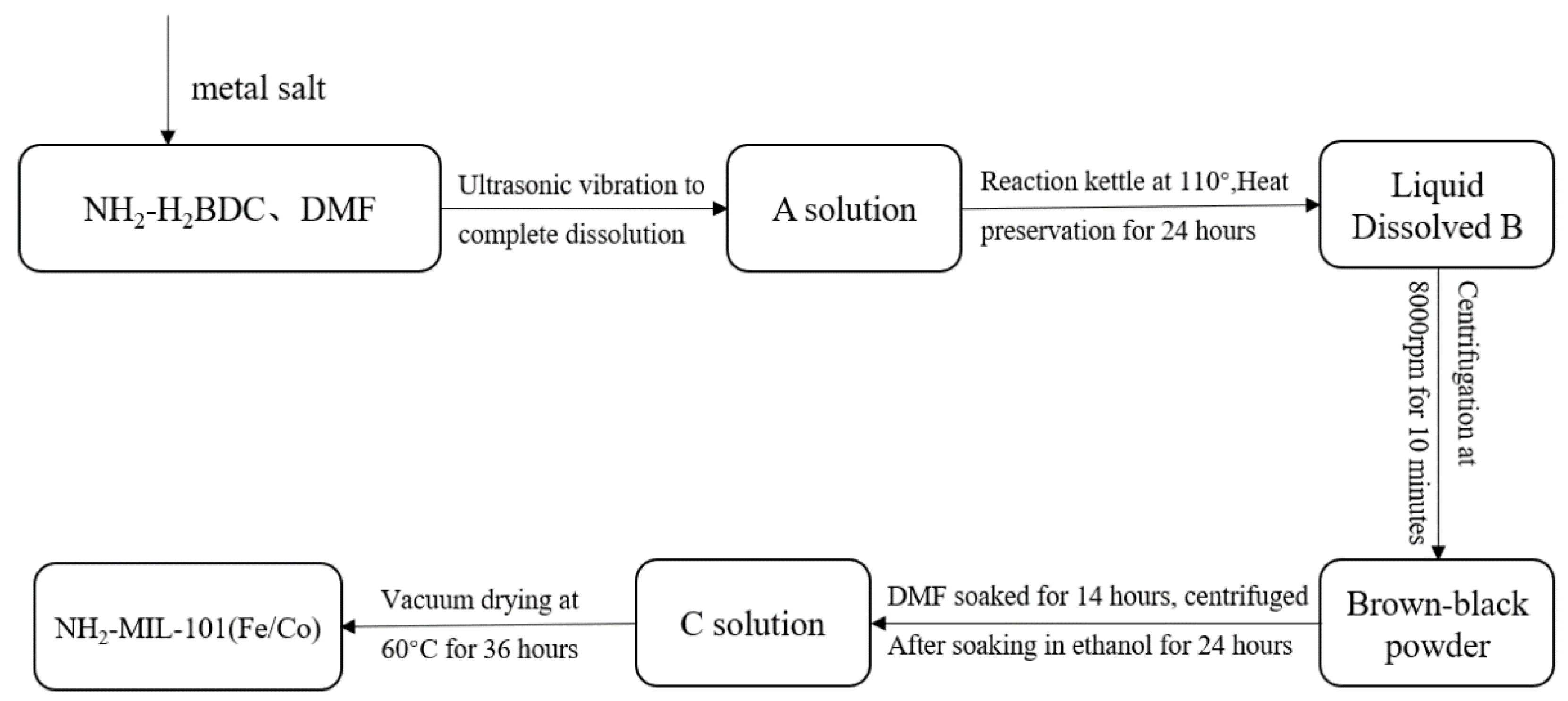
2.2.2. Synthesis of Metal Organic Framework-Derived Carbon Materials
2.3. Activation of Fe/Co-CNs for Degradation of Organic Pollutants by Peroxydisulfate
3. Results and Discussion
3.1. Structural and Morphological Characterization
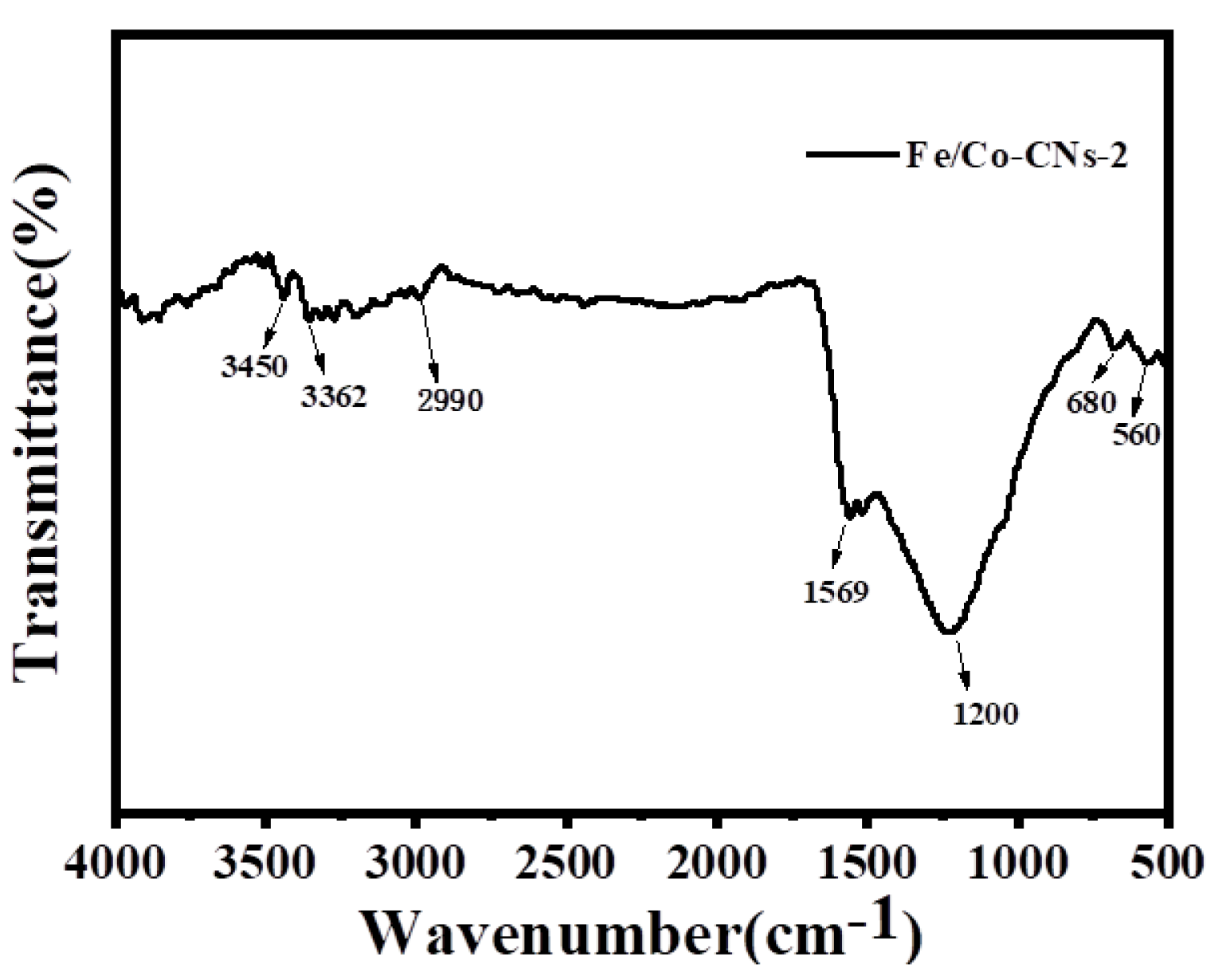
3.1.1. Structural Characterization
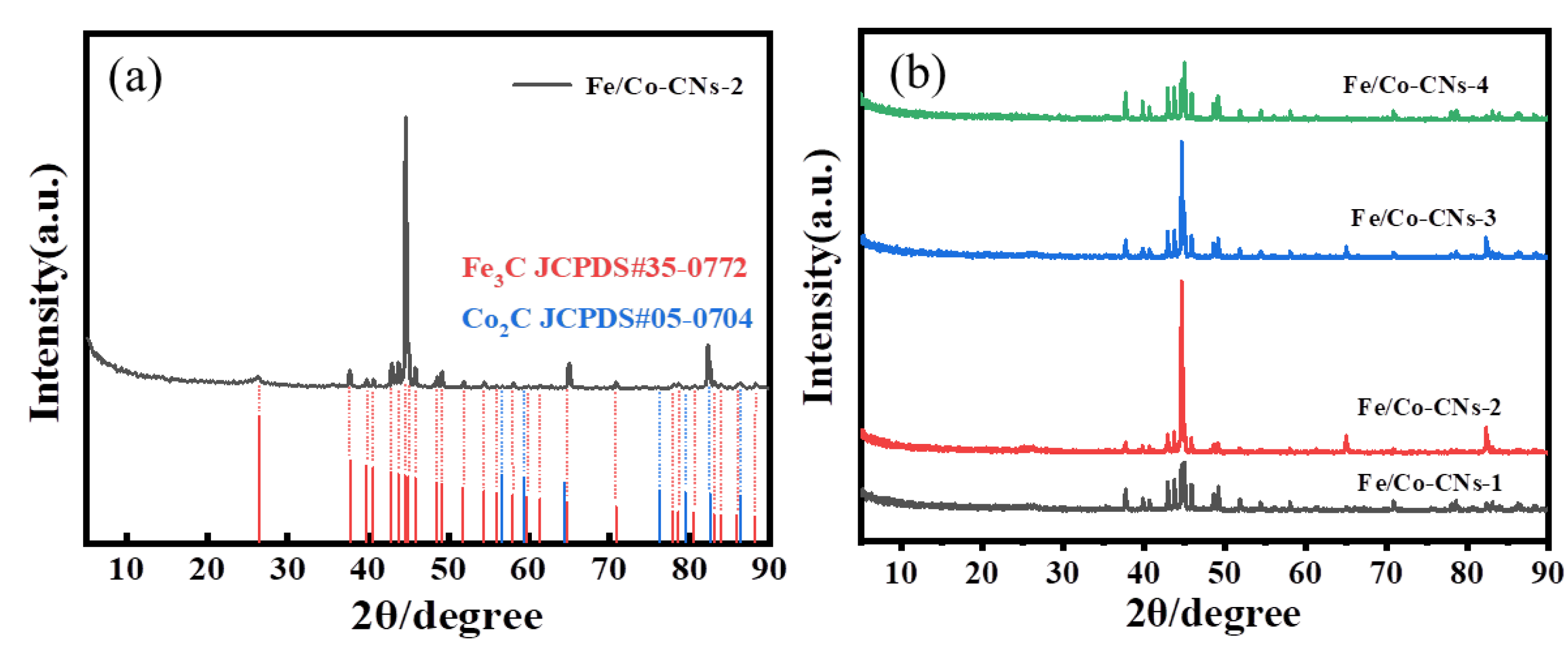
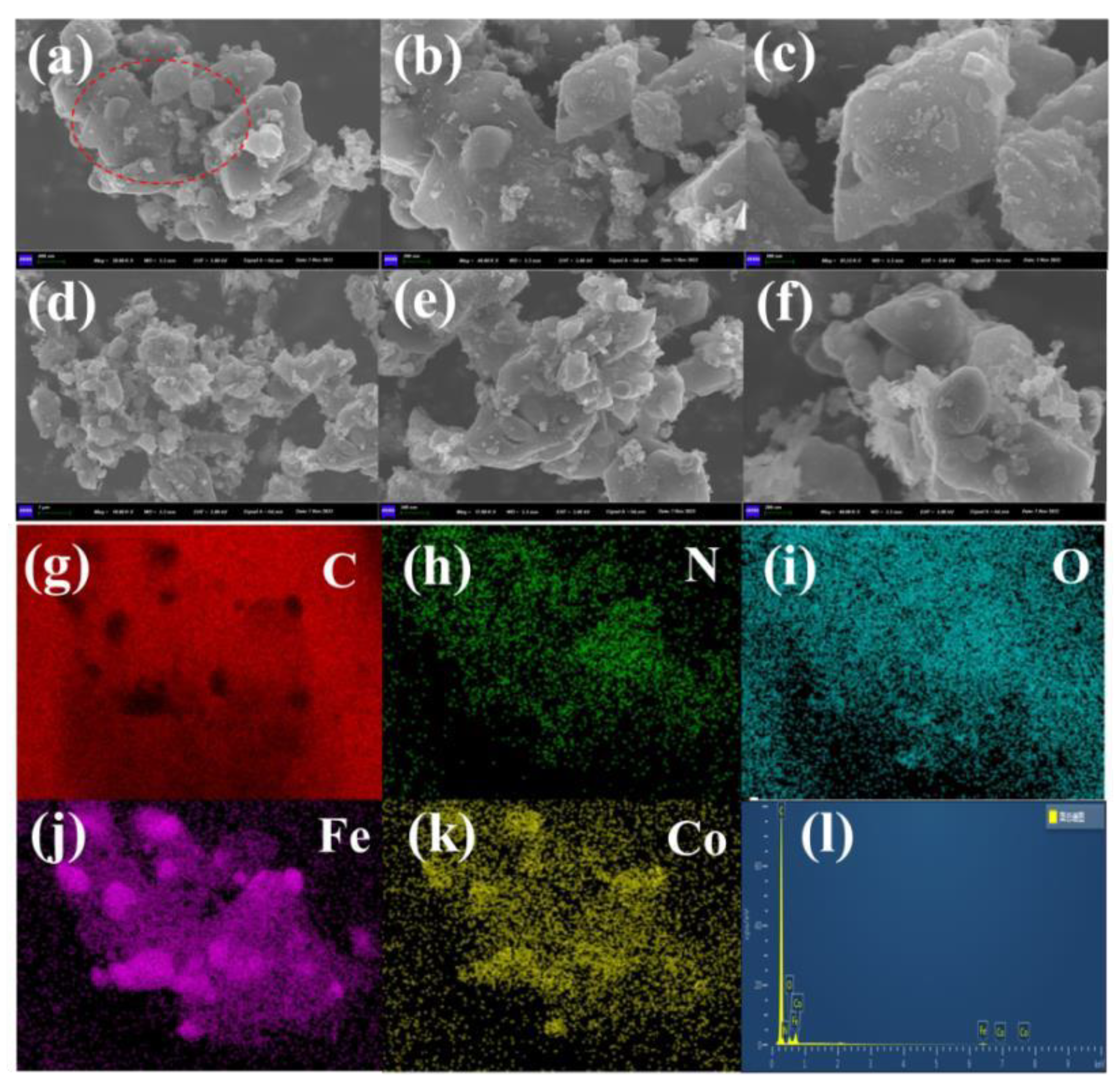
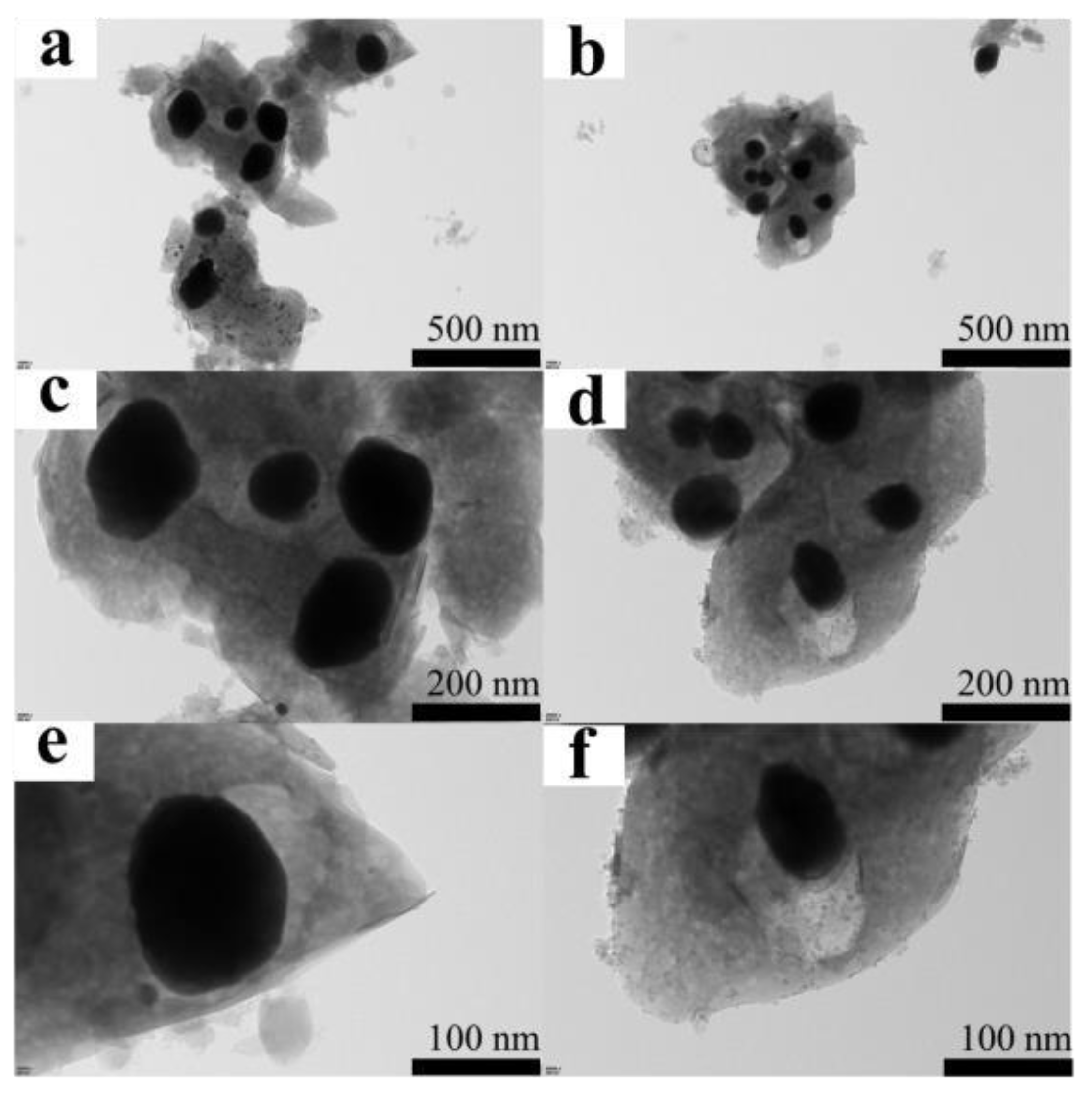
3.1.2. Morphology and Structural Characterization
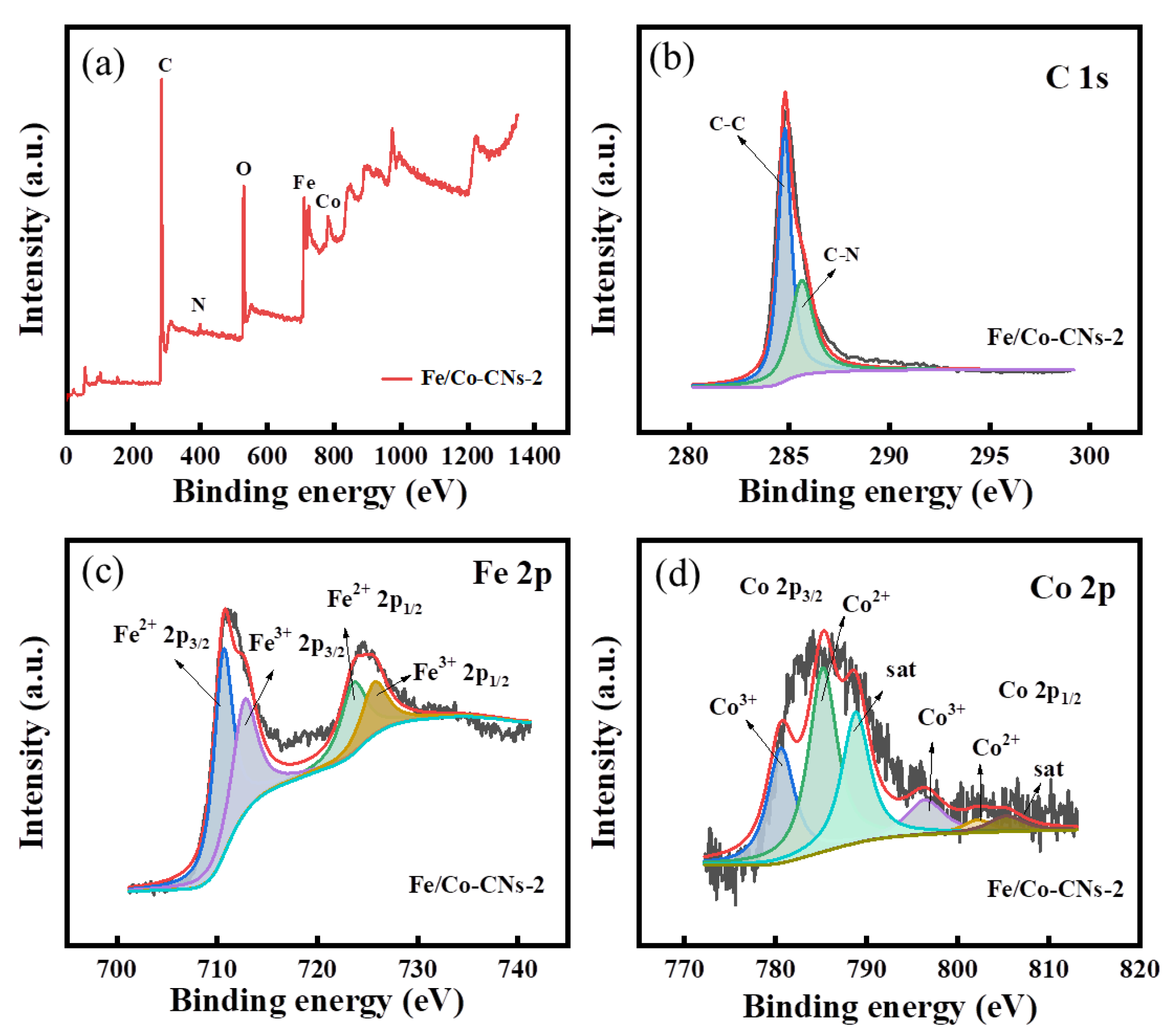
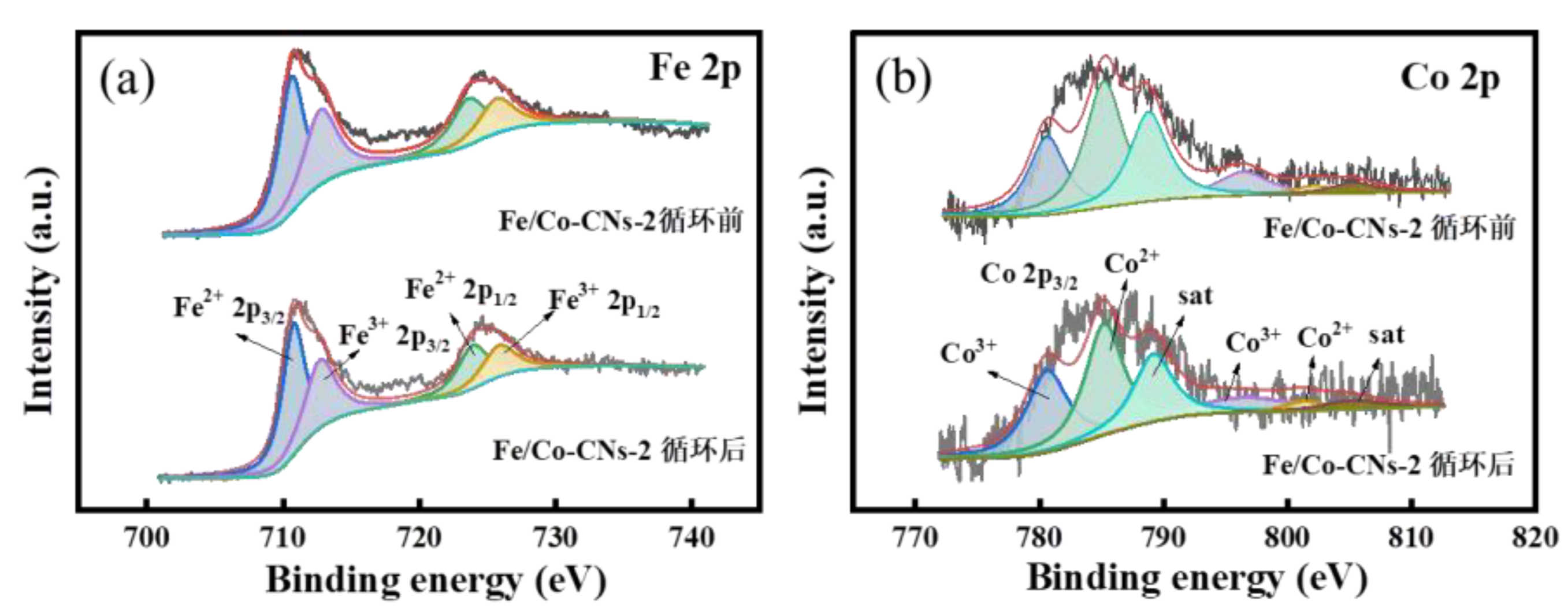
3.1.4. XPS Analysis
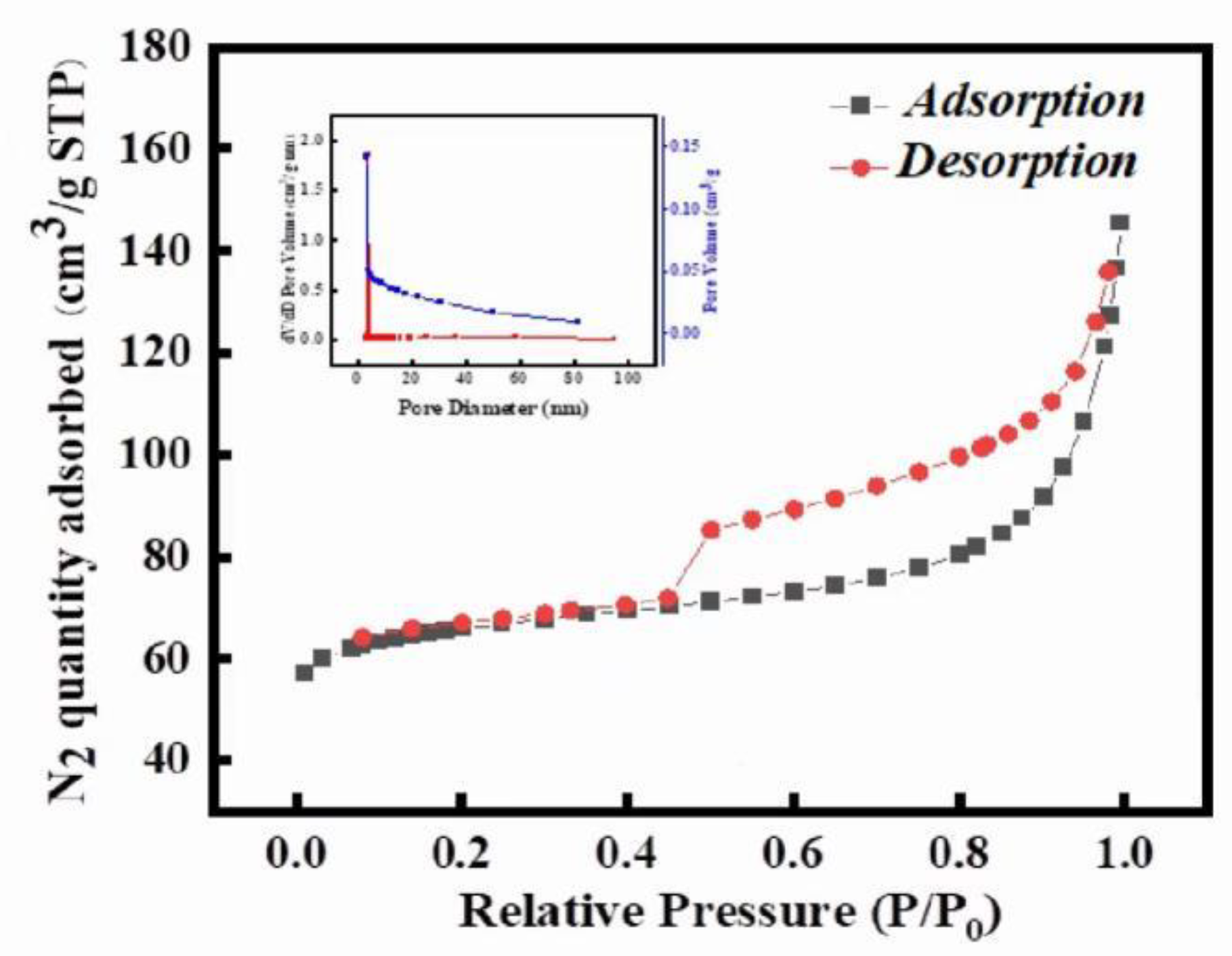
3.1.6. Calculation of Surface Areas
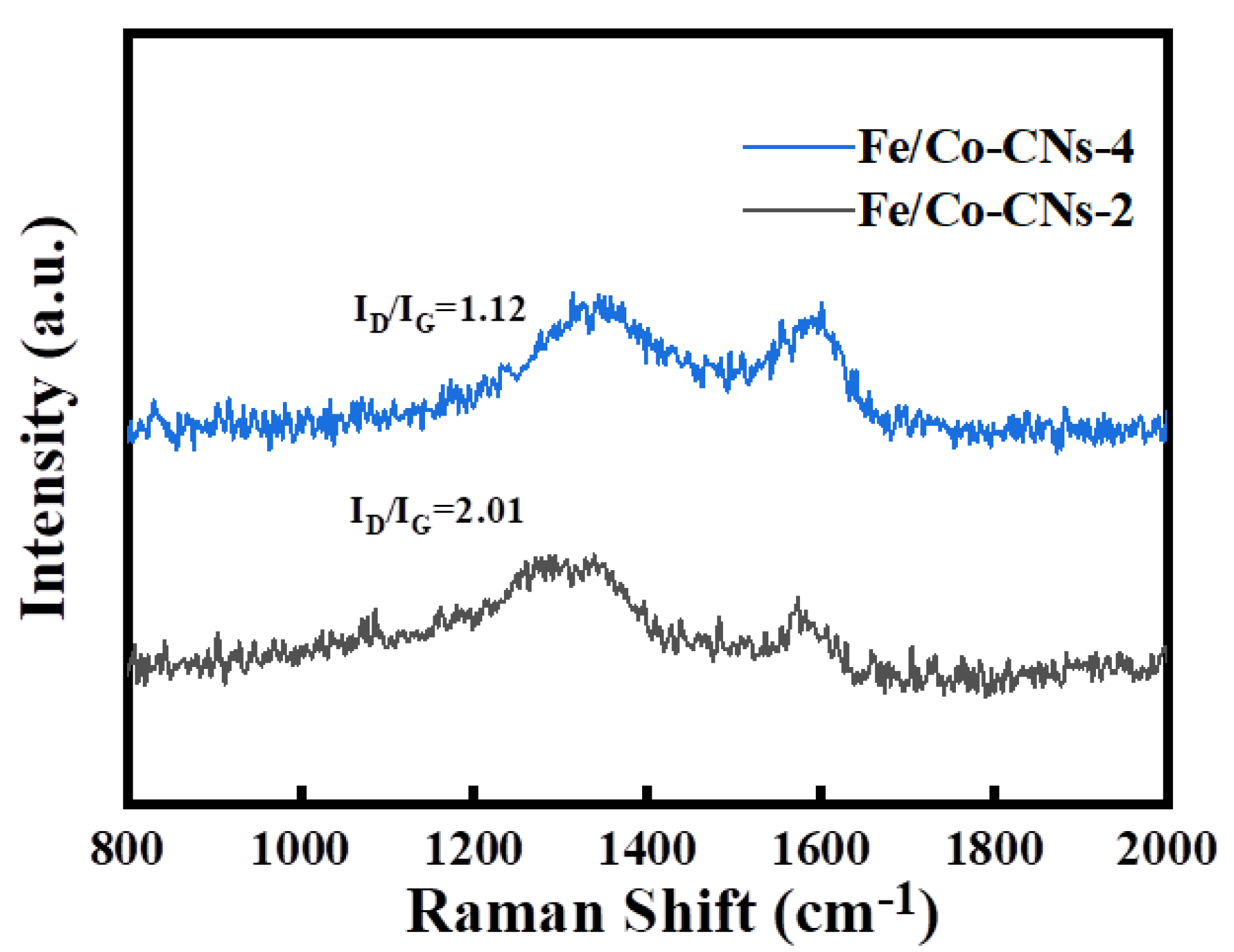
3.1.7. Raman Analysis
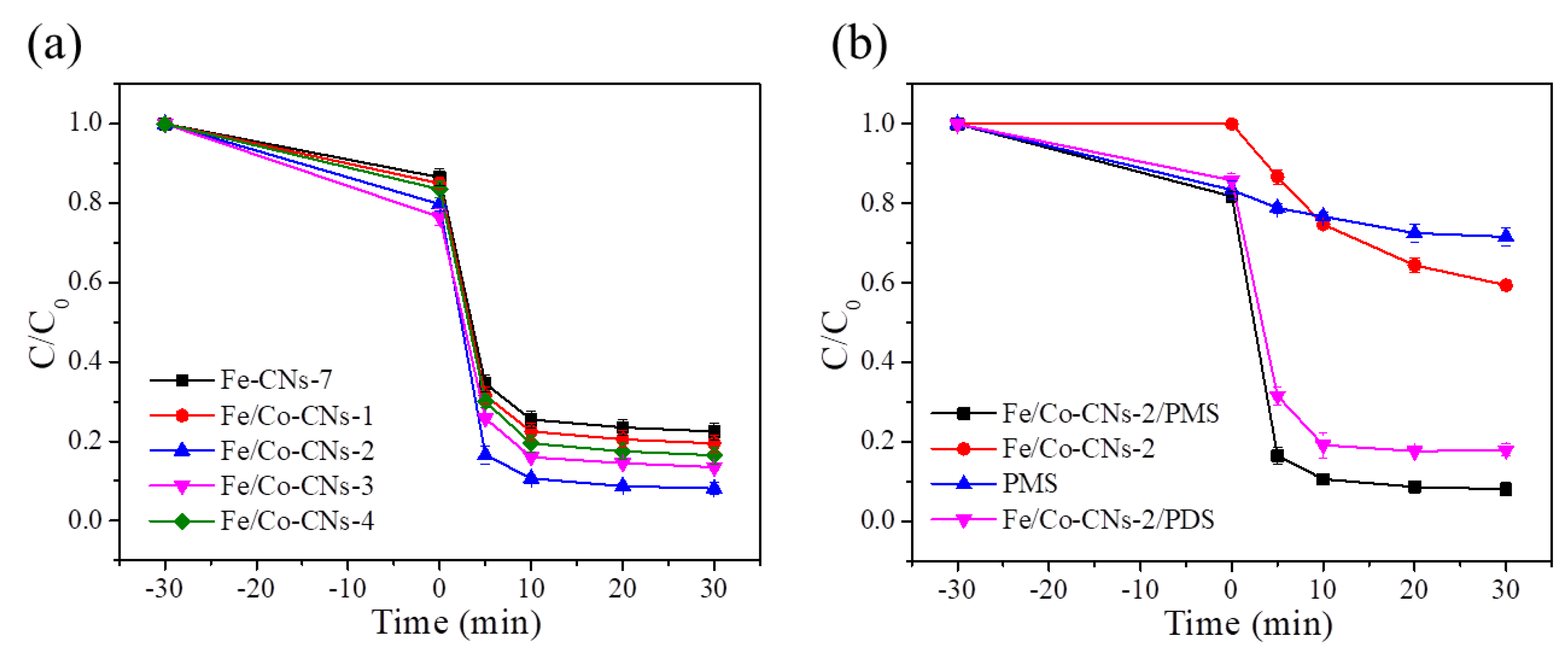
3.2. Evaluation of Fe/Co-CNs' Performance in Pollutant Degradation
4. Factors Affecting Pollutant Degradation by Fe/Co-CNs-2
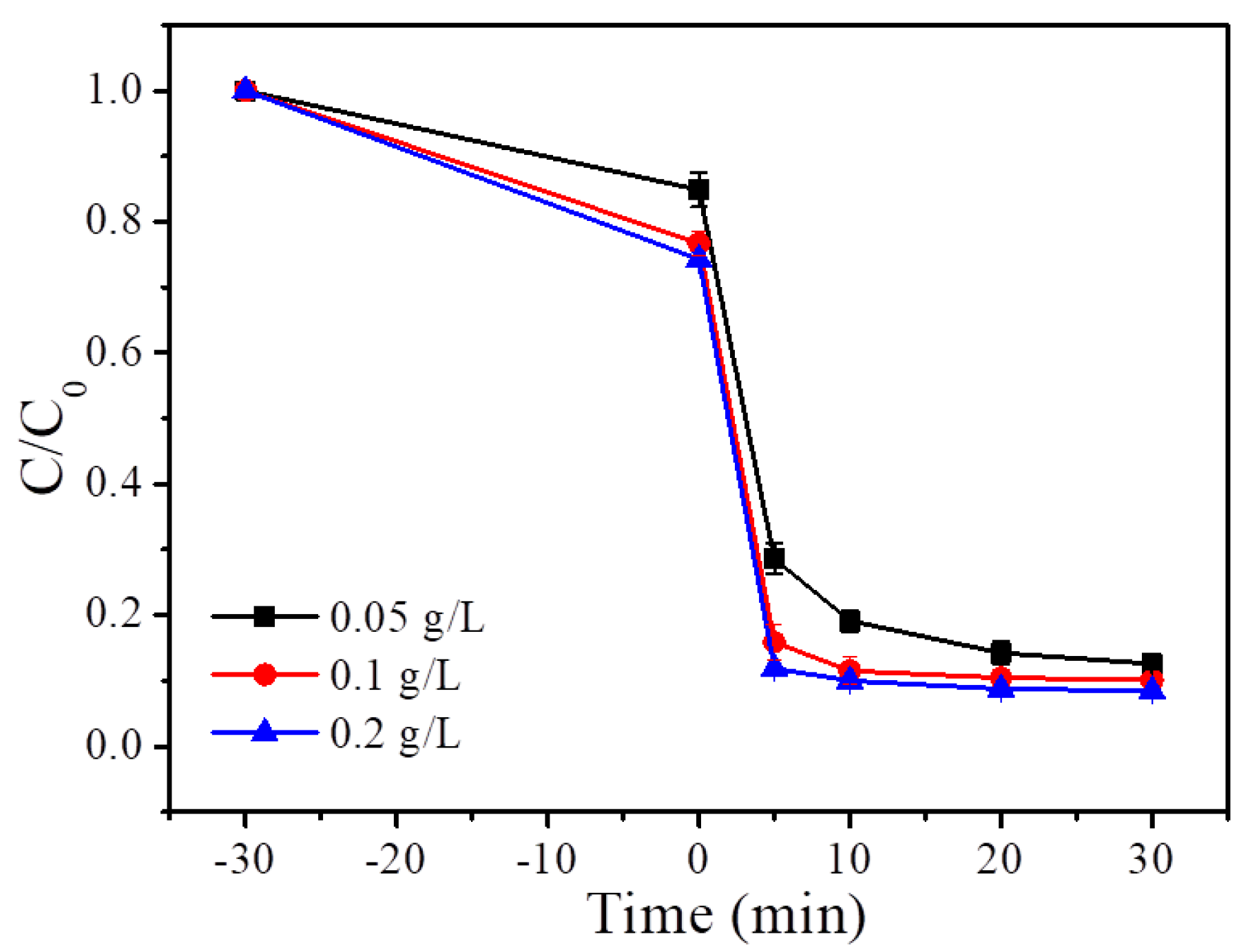
4.1. Catalyst Dosage
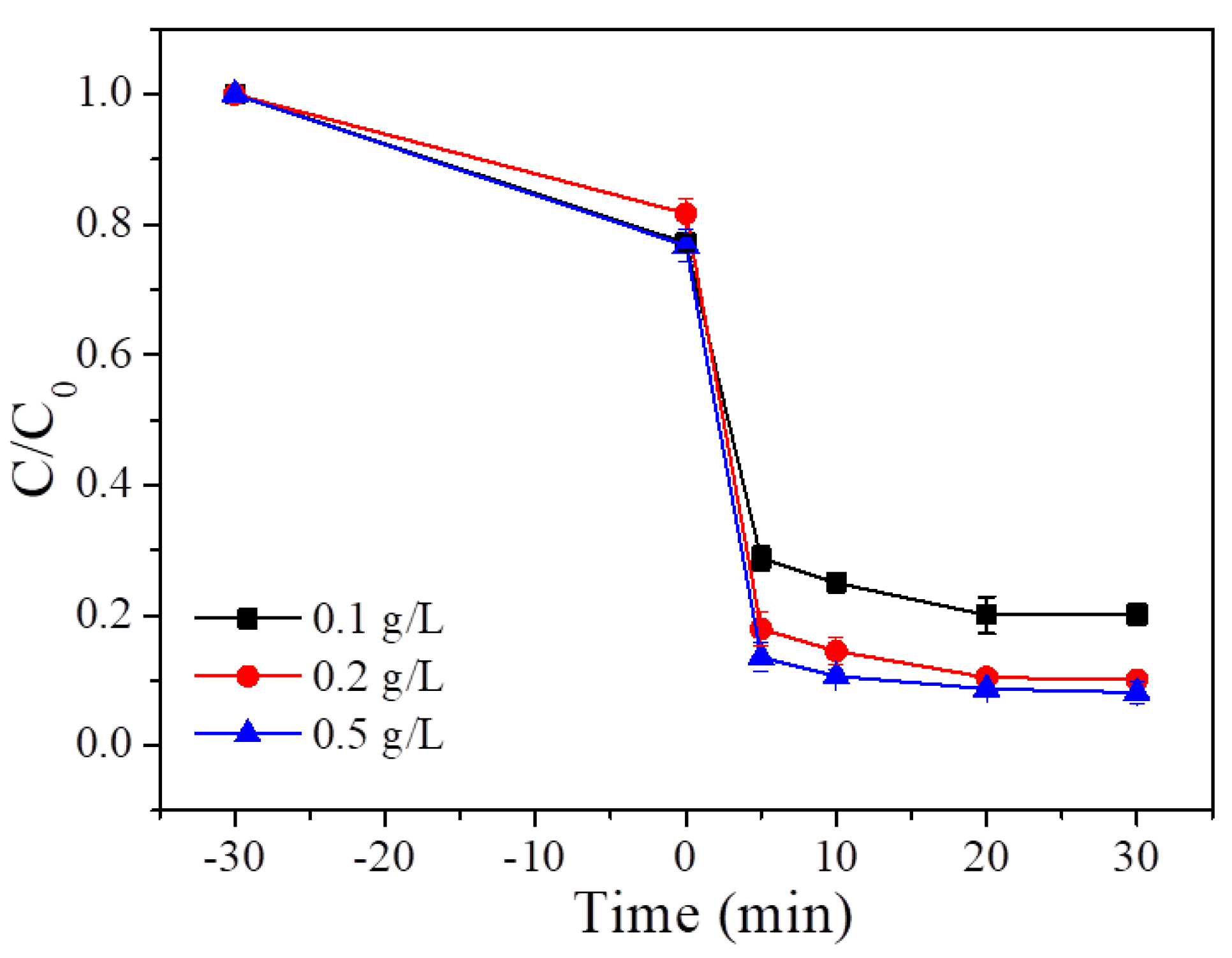
4.2. PMS Dosage
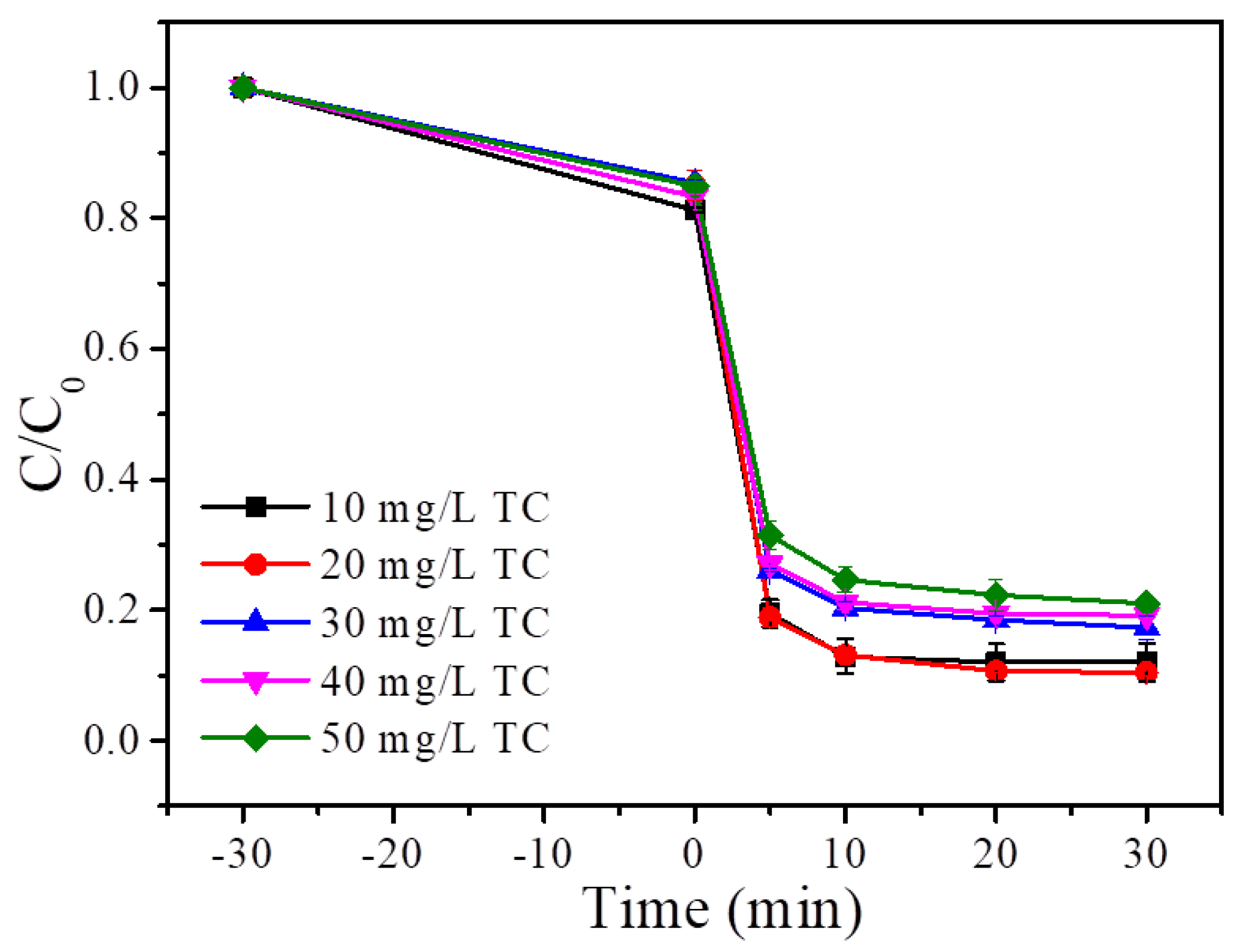
4.3. TC Concentration
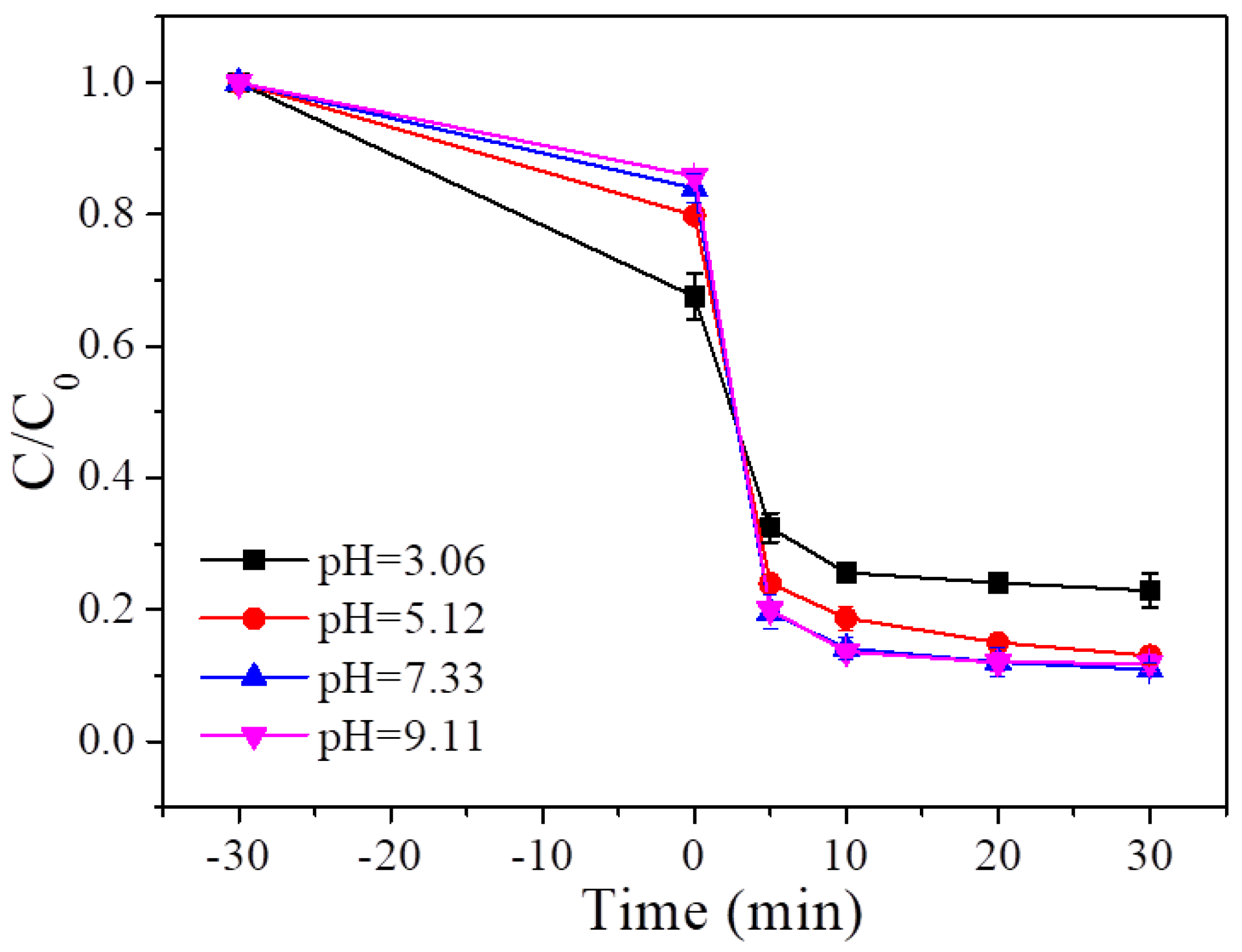
4.4. Initial pH of the Solution
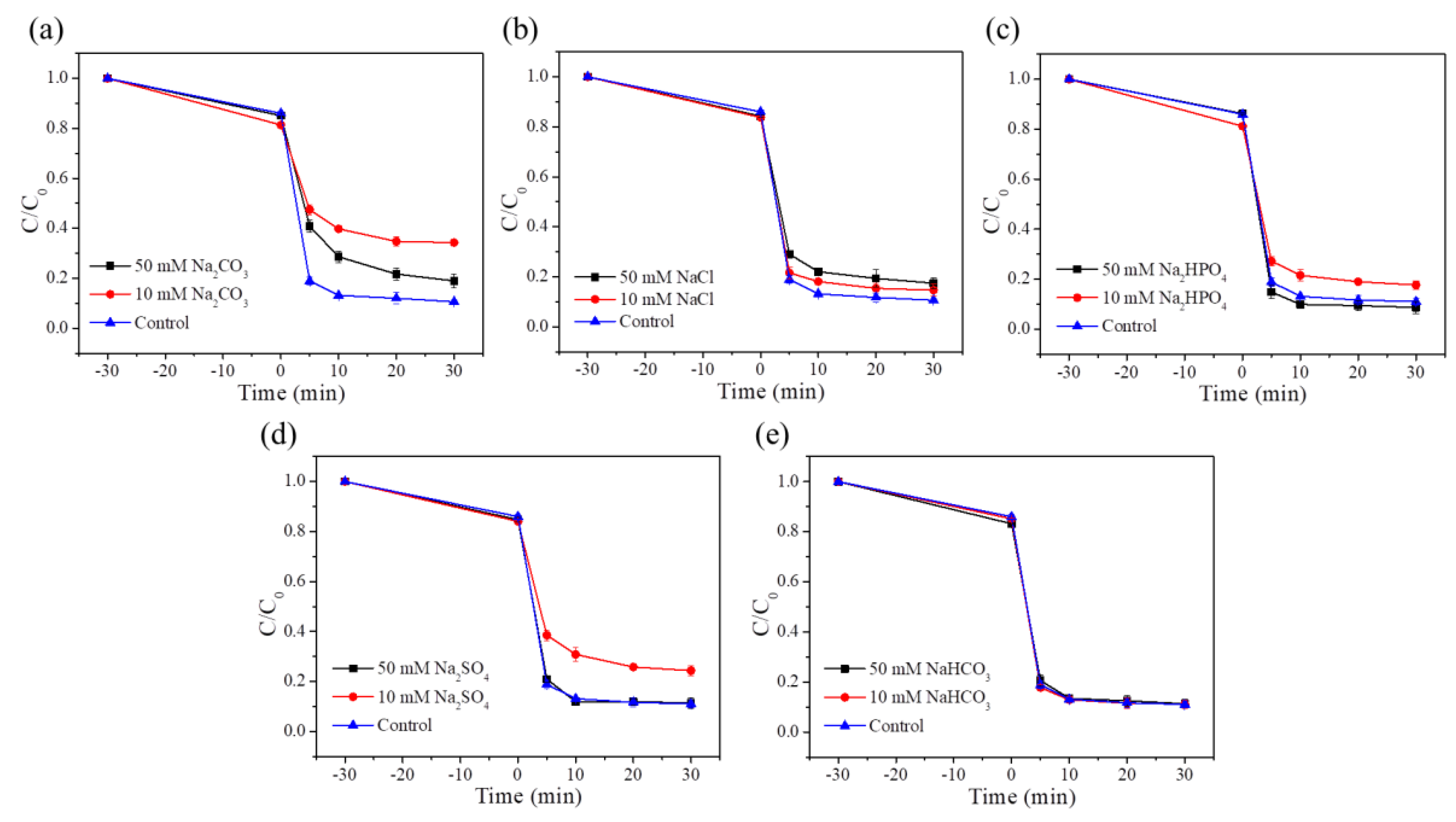
4.5. Anions
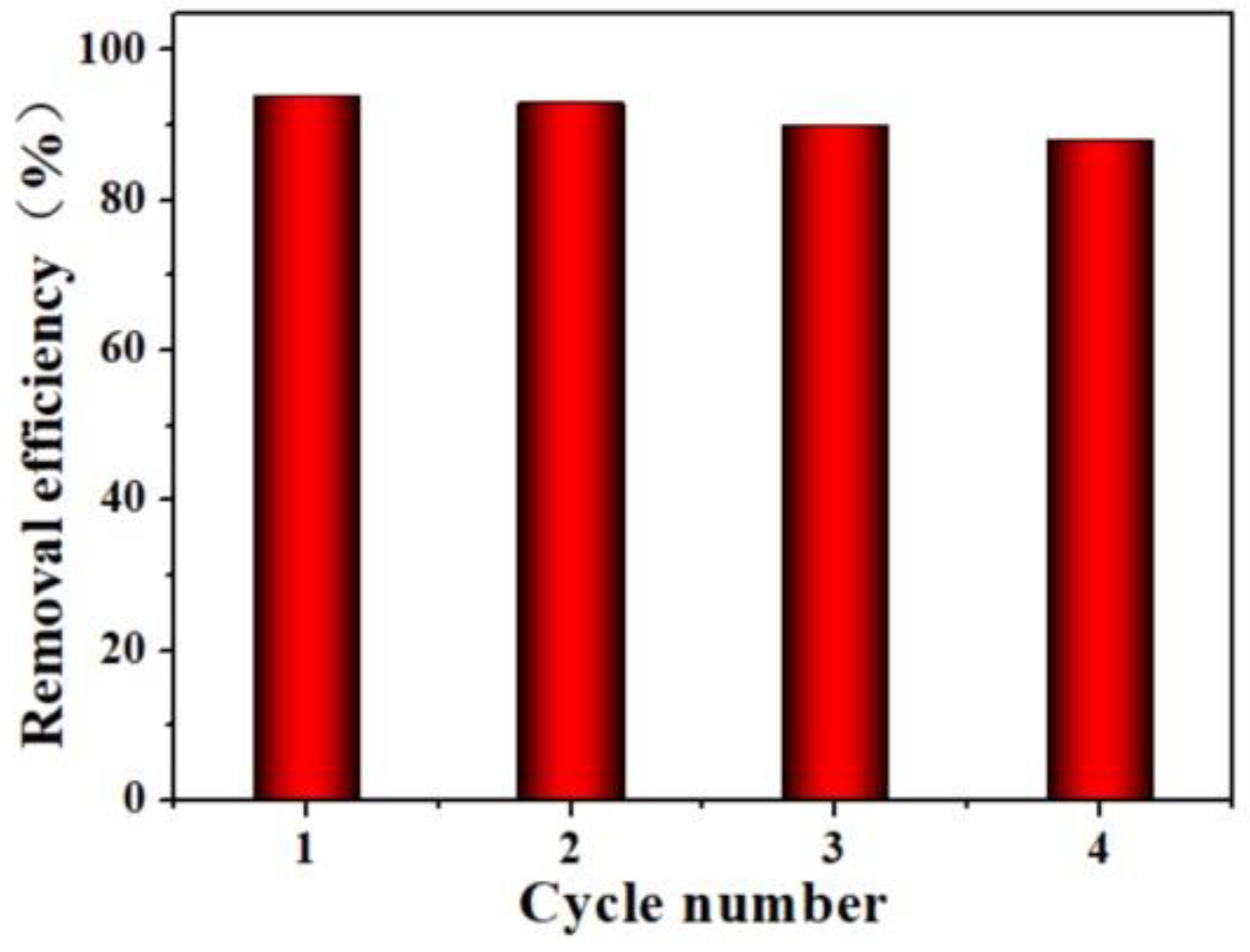
5. Stability of Fe/Co-CNs-2 in Pollutant Degradation
6. Mechanism Analysis of Pollutant Degradation by Fe/Co-CNs-2
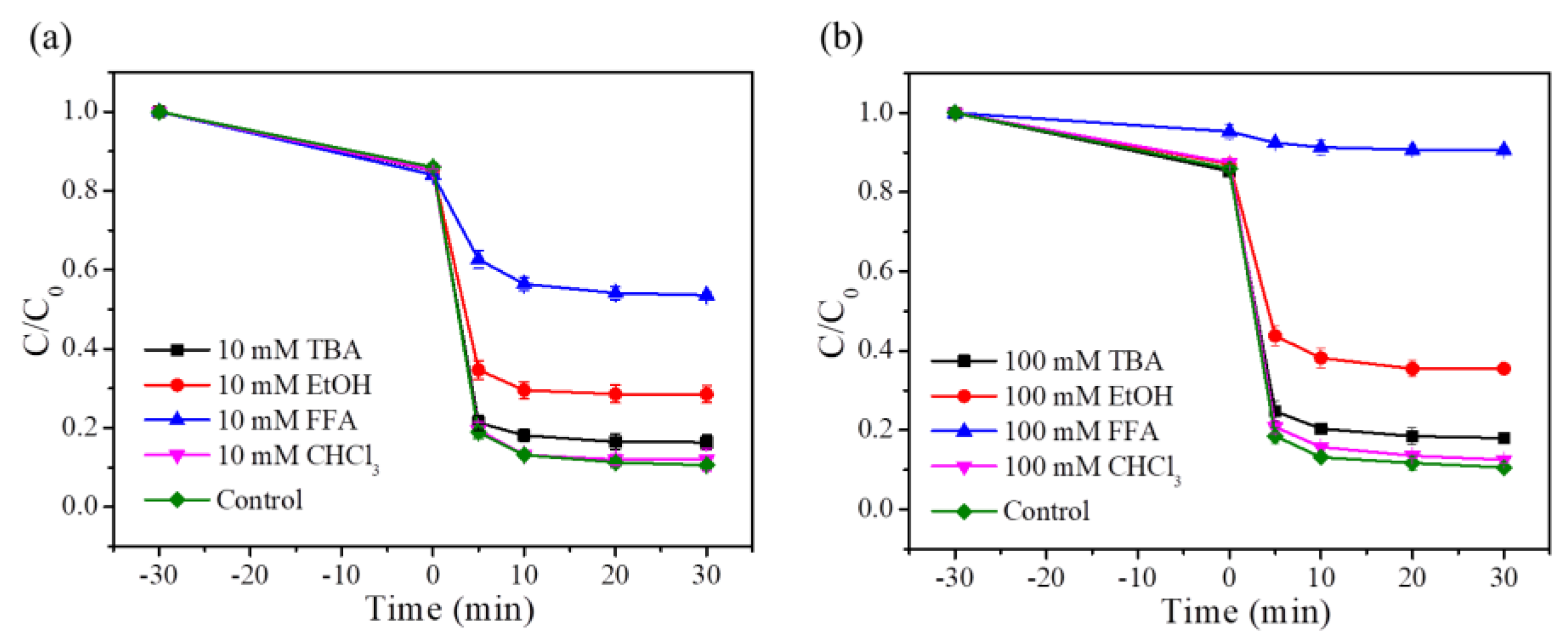
6.1. Free Radical Quenching Experiment of Fe/Co-CNs-2
6.2. XPS Spectra of Fe/Co-CNs-2 after Degradation
7. Conclusion
Acknowledgments
Conflicts of Interest
References
- Kumwimba M N, Meng F, Iseyemi O, et al. Removal of non-point source pollutants from domestic sewage and agricultural runoff by vegetated drainage ditches (VDDs): Design, mechanism, management strategies, and future directions[J]. Science of the Total Environment, 2018, 639: 742-759. [CrossRef]
- Launay M A, Dittmer U, Steinmetz H. Organic micropollutants discharged by combined sewer overflows–characterisation of pollutant sources and stormwater-related processes[J]. Water Research, 2016, 104: 82-92. [CrossRef]
- Li X, Wang S, Xu B, et al. MOF etching-induced Co-doped hollow carbon nitride catalyst for efficient removal of antibiotic contaminants by enhanced perxymonosulfate activation[J]. Chemical Engineering Journal,2022, 441: 136074. 10.1016/j.cej.2022.136074.
- Zhang M, Luo R, Wang C, et al. Confined pyrolysis of metal–organic frameworks to N-doped hierarchical carbon for non-radical dominated advanced oxidation processes[J]. Journal of Materials ChemistryA, 2019, 7(20): 12547-12555. 10.1039/C9TA02931A.
- Wang L, Zhu J, ZhangY, et al. MOFs-derived Mn/C composites activating peroxymonosulfate for efficient degradation of sulfamethazine: Kinetics, pathways, and mechanism[J]. Surfaces and Interfaces, 2023, 36:102551. [CrossRef]
- LIU W J, PARK Y K, BUI H M, et al. Hofmann-MOF-derived CoFeNi nanoalloy@CNT as a magnetic activator for peroxymonosulfate to degrade benzophenone-1 in water[J/OL]. Journal of Alloys and Compounds, 2023, 937: 165189. [CrossRef]
- Duan X, Su C, Miao J, et al. Insights into perovskite-catalyzed peroxymonosulfate activation: maneuverable cobalt sites for promoted evolution of sulfate radicals[J]. Applied Catalysis B: Environmental, 2018, 220: 626-634.
- Lin K Y A, Chen B J. Magnetic carbon-supported cobalt derived from a Prussian blue analogue as a heterogeneous catalyst to activate peroxymonosulfate for efficient degradation of caffeine in water[J]. Journal of colloid and interface science, 2017, 486: 255-264. [CrossRef]
- M. Kohantorabi, G. Moussavi, S. Giannakis, A review of the innovations in metaland carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants[J].Chem. Eng, 2021,411:127957. [CrossRef]
- D. Du, W. Chen, C. Tian, Y. Li, C. Chen, N. Liang, Hollow multi-dimension CoCeS/C as peroxymonosulfate activator toward ofloxacin degradation via coexisting free radical and nonradical pathways[J]. Alloy. Compd,2022,905 :164218. [CrossRef]
- Y. Mei, Y. Zhang, J. Li, X. Deng, Y. Yang, Q. Yang, B. Jiang, B. Xin, T. Yao, J. Wu,Synthesis of Co-doped CeO2 nanoflower: enhanced adsorption and degradation performance toward tetracycline in Fenton-like reaction[J].Alloy. Compd,2022,904:163879. [CrossRef]
- Z. Li, J. Kang, Y. Tang, C. Jin, H. Luo, S. Li, J. Liu, M. Wang, C. Lv, The enhanced Pnitrophenol degradation with Fe/Co3O4 mesoporous nanosheets via peroxymonosulfate activation and its mechanism insight[J]. Alloy. Compd,2021,858:157739.
- Y. Deng, X. Zou, Z. Liu, J. Wang, Z. Wang, J. Tang, Co7Fe3/CoFe2O4@C Lamellar composites derived from Co–Fe LDH/PVA as an effective heterogeneous activator of peroxymonosulfate[J]. Alloy. Compd,2021, 854 : 157244.
- Zhang H, Cui H, Li J, et al. Frogspawn inspired hollow Fe3C@ N–C as an efficient sulfur host for high-rate lithium–sulfur batteries[J]. Nanoscale, 2019, 11(44): 21532-21541. [CrossRef]
- Li Z, Lin T, Yu F, et al. Mechanism of the Mn promoter via CoMn spinel for morphology control: formation of Co2C nanoprisms for Fischer–Tropsch to olefins reaction[J]. Acs Catalysis, 2017, 7(12): 8023-8032. [CrossRef]
- Gu A, Wang P, Chen K, et al. Core-shell bimetallic Fe-Co MOFs to activated peroxymonosulfate for efficient degradation of 2-chlorophenol[J]. Separation and Purification Technology, 2022, 298: 121461. [CrossRef]
- Wang J, Liao Z, Ifthikar J, et al. Treatment of refractory contaminants by sludge-derived biochar/persulfate system via both adsorption and advanced oxidation process[J]. Chemosphere, 2017, 185: 754-763. [CrossRef]
- Li H, Zhang J, Yao Y, et al. Nanoporous bimetallic metal-organic framework (FeCo-BDC) as a novel catalyst for efficient removal of organic contaminants[J]. Environmental Pollution, 2019, 255: 113337. [CrossRef]
- Lai L, Potts J R, Zhan D, et al. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction[J]. Energy & Environmental Science, 2012, 5(7): 7936-7942.
- Long Y, Bu S, Huang Y, et al. N-doped hierarchically porous carbon for highly efficient metal-free catalytic activation of peroxymonosulfate in water: A non-radical mechanism[J]. Chemosphere, 2019, 216: 545-555. [CrossRef]
- Liu C, Wang Y, Zhang Y, et al. Enhancement of Fe@ porous carbon to be an efficient mediator for peroxymonosulfate activation for oxidation of organic contaminants: incorporation NH2-group into structure of its MOF precursor[J]. Chemical Engineering Journal, 2018, 354: 835-848. [CrossRef]
- Mengelizadeh N, Mohseni E, Dehghani M H. Heterogeneous activation of peroxymonosulfate by GO-CoFe2O4 for degradation of reactive black 5 from aqueous solutions: Optimization, mechanism, degradation intermediates and toxicity[J]. Journal of Molecular Liquids, 2021, 327: 114838. [CrossRef]
- Tang L, Liu Y, Wang J, et al. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism[J]. Applied Catalysis B: Environmental, 2018, 231: 1-10. [CrossRef]
- Lei Y, Chen C S, Ai J, et al. Selective decolorization of cationic dyes by peroxymonosulfate: non-radical mechanism and effect of chloride[J]. Rsc Advances, 2016, 6(2): 866-871. [CrossRef]
- Wang J, Liao Z, Ifthikar J, et al. One-step preparation and application of magnetic sludge-derived biochar on acid orange 7 removal via both adsorption and persulfate based oxidation[J]. RSC advances, 2017, 7(30): 18696-18706. [CrossRef]
- Zhang M, Luo R, Wang C, et al. Confined pyrolysis of metal– organic frameworks to N-doped hierarchical carbon for non-radical dominated advanced oxidation processes[J]. Journal of Materials Chemistry A, 2019, 7(20): 12547-12555.
- Wang C, Kim J, Kim M, et al. Nanoarchitectured metal–organic framework-derived hollow carbon nanofiber filters for advanced oxidation processes[J]. Journal of Materials Chemistry A, 2019, 7(22): 13743-13750.
- Yang Y, Banerjee G, Brudvig G W, et al. Oxidation of organic compounds in water by unactivated peroxymonosulfate[J]. Environmental science & technology, 2018, 52(10): 5911-5919. [CrossRef]
- Li H, Yao Y, Chen J, et al. Heterogeneous activation of peroxymonosulfate by bimetallic MOFs for efficient degradation of phenanthrene: Synthesis, performance, kinetics, and mechanisms[J]. Separation and Purification Technology, 2021, 259: 118217.
- Hou C, Chen W, Fu L, et al. Facile synthesis of a Co/Fe bi-MOFs/CNF membrane nanocomposite and its application in the degradation of tetrabromobisphenol A[J]. Carbohydrate polymers, 2020, 247: 116731. [CrossRef]
- Gong C, Chen F, Yang Q, et al. Heterogeneous activation of peroxymonosulfate by Fe-Co layered doubled hydroxide for efficient catalytic degradation of Rhoadmine B[J]. Chemical engineering journal, 2017, 321: 222-232. [CrossRef]
- Liu F, Cao J, Yang Z, et al. Heterogeneous activation of peroxymonosulfate by cobalt-doped MIL-53 (Al) for efficient tetracycline degradation in water: Coexistence of radical and non-radical reactions[J]. Journal of colloid and interface science, 2021, 581: 195-204. [CrossRef]
| Co doping amount | NH2-H2BDC | FeCl3·6H2O | Co(NO3)2·6H2O | Labeled as |
| 10% | 1.24 mmol | 2.25 mmol | 0.25 mmol | Fe/Co-CNs-1 |
| 20% | 1.24 mmol | 2 mmol | 0.5 mmol | Fe/Co-CNs-2 |
| 30% | 1.24 mmol | 1.75 mmol | 0.75 mmol | Fe/Co-CNs-3 |
| 40% | 1.24 mmol | 1.5 mmol | 1 mmol | Fe/Co-CNs-4 |
| 50% | 1.24 mmol | 1.25 mmol | 1.25 mmol | Fe/Co-CNs-5 |
| 70% | 1.24 mmol | 0.75mol | 1.75 mmol | Fe/Co-CNs-7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





