1. Introduction
Endocrine disrupting chemicals (EDCs) are substances of either natural or artificial origin that mimic or modify hormonal actions. EDCs are found in numerous widely used products including plastics, detergents, building materials, cosmetics, and pesticides. Organisms can be exposed to these hazards via inhalation and feeding, through the skin, the placenta, and the maternal milk during lactation [
1]. Due to their rudimentary detoxification mechanisms, embryos and developing organisms are more vulnerable to the effects of these hormone-mimicking chemicals. Accumulating evidence shows that EDC exposures during critical time windows can modify the developmental process of certain systems, leading to long lasting and even transgenerational aberrations [
2].
The mandible or lower jaw differs from other bones in terms of biomechanics, morphology, physiology and morphogenesis. Mandible is the only mobile bone of the skull and has important roles in supporting dentition and in the outfit of the temporo-mandibular joint. Mandible formation is an early developmental process taking place between gestational weeks 7-14 in humans [
3] and embryonic days E13.5-16.5 in mice [
4]. During craniofacial development, neuroectoderm-derived neural crest cells interact with cells of ectoderm and paraxial mesoderm and migrate towards maxillary and mandibular prominences where they contribute to the formation of Meckel’s cartilage. The latter provides the template for mandibles, teeth, and other craniofacial structures [
5]. These early cellular interactions require tight synchronization between cell proliferation and differentiation to avoid jaw aberrations that may permanently impact craniofacial shaping and function. The process of oral bone formation and odontogenesis is sensitive to external interventions including chemicals and hormones. Rodents, in utero exposed to ethanol [
6], nicotine [
7], or thyroxin [
8] exhibit impaired mandible morphology at later life stages. Estrogens, apart from being significant contributors to the formation of skeletal bones [
9], participate in craniofacial bone development [
10,
11]. Evidence from ERαKO C57BL/6 mice shows that estrogens, via estrogen receptor alpha (ERα), mediate the maturation of mandibular condylar cartilage in males [
12].
Consequently, estrogen-mimicking EDCs have been investigated for their effects on bone development. Perinatal exposure to Bisphenol A (BPA) or phthalates leads to impaired skeletal bone development in several species [
13,
14]. Regarding mandibular development, BPA and phthalates have been reported to affect the craniofacial [
15] and skeletal growth [
16] in zebrafish. In rats, perinatal exposure to BPA disturbed amelogenesis in male offspring by altering estrogenic signaling [
17,
18]. Exposure of adult mice to di-(2-ethylhexyl) phthalate (DEHP) caused enamel defects in their continuously growing incisors [
19]. In humans, the developmental impact of phenols and phthalates has been examined only on skeletal bones, where maternal levels of these chemicals during pregnancy have been associated with decreased bone mineral density in their progeny [
20,
21,
22].
Humans are exposed to chemical mixtures rather than single compounds [
23]. In previous studies, we have composed human-relevant mixtures based on epidemiological data from the Swedish Environmental, Longitudinal, Mother and child, Asthma and allergy (SELMA) pregnancy cohort study [
24]. Using Weighted Quantile Sum (WQS) regression, we identified EDC mixtures measured in blood or urine of SELMA women around pregnancy week 10 associated with health outcomes in their children. Further analyses of these mixtures in different cell and animal models revealed significant effects on reproduction [
25], metabolic growth [
26,
27,
28] and neurodevelopment [
28,
29,
30]. We have previously reported neurodevelopmental effects of Mixture N1, an EDC mixture associated with language delay at 30 months, by identifying that in utero exposure to Mixture N1 altered the brain transcriptome and epigenome and, consequently, the behavior of mice offspring in adulthood [
29,
30].
In light of the impact of Mixture N1 on neural tissue development, we sought to examine the effects of this mixture in the formation of mouse mandible, a structure that partially originates from neural crest cells. For this reason, pregnant mice were exposed daily throughout gestation to Mixture N1 at doses of 0.5x, 10x, 100x and 500x (representing times of human serum concentrations measured or estimated in pregnant women of the SELMA cohort study) or the vehicle. We then performed micro-Computer Tomography (micro-CT) based analysis in the mandibles of adult male offspring to accurately quantify potential changes in mandibular morphometry and bone composition. Histological staining was applied to reveal the areas of pre-hypertrophic and mature hypertrophic chondrocytes in the condylar head.
2. Results
2.1. Mixture N1 Exposure Affects Mandibular Morphometry.
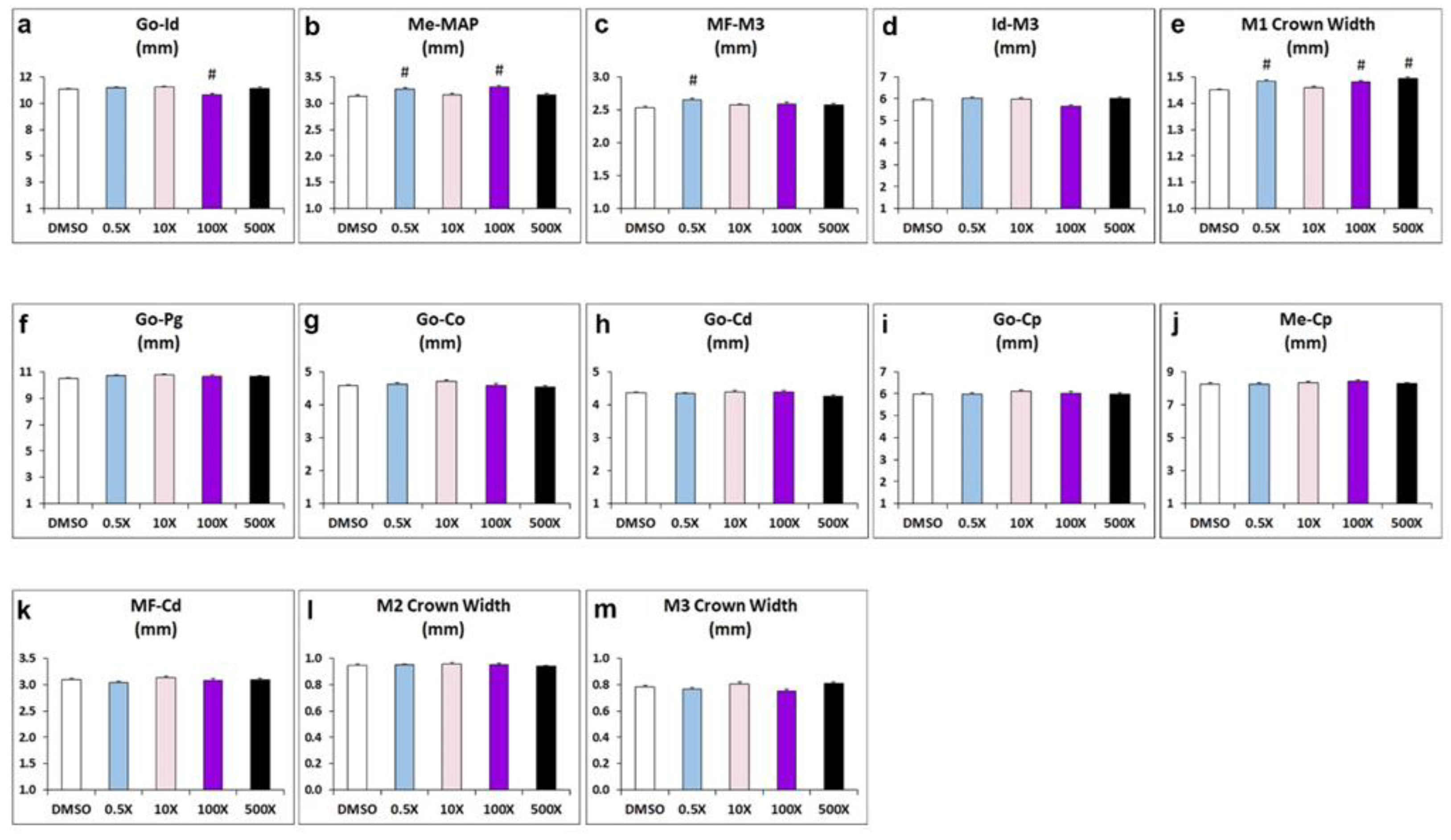
Prenatal exposure to Mixture N1 resulted in significant alterations in multiple landmarks of the offspring’s mandible in adulthood (Figure 1 & Table S1). A statistically significant reduction in the distance from Gonion (Go) to Infradental was evident in the 100x group vs. DMSO (Figure 1a). Furthermore, the width-related distance from Menton to Mandibular alveolar point (MAP) was significantly increased in the groups 0.5x and 100x, compared to DMSO (Figure 1b). The distance from M3 molar to mandibular foramen (MF) was increased in the 0.5 x group (Figure 1c), while M3 to Infradental distance was not significantly changed (Figure 1d). Regarding the size of mandibles, a significant increase in the Μ1 crown width was prominent in the 0.5x, 100x and 500x groups compared to DMSO group (Figure 1e). No significant effects were observed in the distances between Gonion (Go) to Pogonion, Go to Condyle dorsal part (Co), Go to Condyle ventral part (Cd), Go to Coronoid process (Cp), Menton to Cp, MF to Cd, and in the crown width of molars M2 and M3 (Figure 1f-m).
Collectively, we observed increased distance from Menton to MAP in mandibles of mice treated with 0.5x and 100x, but not in mice treated with 10x or 500x Mixture N1. The reduced distance from Gonion to Infradental was detected only in the 100x exposed mice. The width of M1 molar crown was significantly increased in all experimental groups except for 10x. These findings suggest that prenatal exposure to increasing quantities of Mixture N1 leads to morphometric alternations in mandibles in a non-monotonic manner.
Figure 1.
Morphometric analysis of distance measurements in mandibles from adult mice prenatally exposed to 0.5x, 10x, 100x and 500x of Mixture N1 or the vehicle (DMSO). Quantification of the measurements. Data represent estimated marginal means+/- SEM. Generalized linear models (GLM) and Bonferroni post hoc tests were performed for statistical analysis. (a) Distance from Go to Id was decreased (W4=26.548; P<0.001) in the 100x group vs. DMSO (p=0.004). (b) Distance from Me to MAP was increased (W4=27.826; P<0.001) in the groups 0.5x (p=0.004) and 100x (p<0.001), compared to DMSO. (c) The distance from M3 molar to MF was increased (W4=20.497; P<0.001) in the 0.5 x group (p<0.001). (d) The distance from M3 to Id was not significantly changed. (e). The Μ1 crown width was increased (W4=66.279; P<0.001) in the 0.5x, 100x and 500x groups (p<0.001 for all). No significant effects were observed in the distances from Go to Pg (f), Go to Co (g), Go to Cd (h), Go to Cp (i), Me to Cp (j), MF to Cd (k), and in the crown width of molars M2 (l) and M3 (m). Go: Gonion; Id: Infradental; Me: Menton; MAP: Mandibular Alveolar Point; MF: Mandibular Foramen; Pg: Pogonion; Co: dorsal condylar process; Cd: ventral condylar process; Cp: Coronoid process; M: Molar. # Statistically significant vs. DMSO group.
Figure 1.
Morphometric analysis of distance measurements in mandibles from adult mice prenatally exposed to 0.5x, 10x, 100x and 500x of Mixture N1 or the vehicle (DMSO). Quantification of the measurements. Data represent estimated marginal means+/- SEM. Generalized linear models (GLM) and Bonferroni post hoc tests were performed for statistical analysis. (a) Distance from Go to Id was decreased (W4=26.548; P<0.001) in the 100x group vs. DMSO (p=0.004). (b) Distance from Me to MAP was increased (W4=27.826; P<0.001) in the groups 0.5x (p=0.004) and 100x (p<0.001), compared to DMSO. (c) The distance from M3 molar to MF was increased (W4=20.497; P<0.001) in the 0.5 x group (p<0.001). (d) The distance from M3 to Id was not significantly changed. (e). The Μ1 crown width was increased (W4=66.279; P<0.001) in the 0.5x, 100x and 500x groups (p<0.001 for all). No significant effects were observed in the distances from Go to Pg (f), Go to Co (g), Go to Cd (h), Go to Cp (i), Me to Cp (j), MF to Cd (k), and in the crown width of molars M2 (l) and M3 (m). Go: Gonion; Id: Infradental; Me: Menton; MAP: Mandibular Alveolar Point; MF: Mandibular Foramen; Pg: Pogonion; Co: dorsal condylar process; Cd: ventral condylar process; Cp: Coronoid process; M: Molar. # Statistically significant vs. DMSO group.
2.2. Mixture N1 Exposure Affects Mandibular Bone Composition.
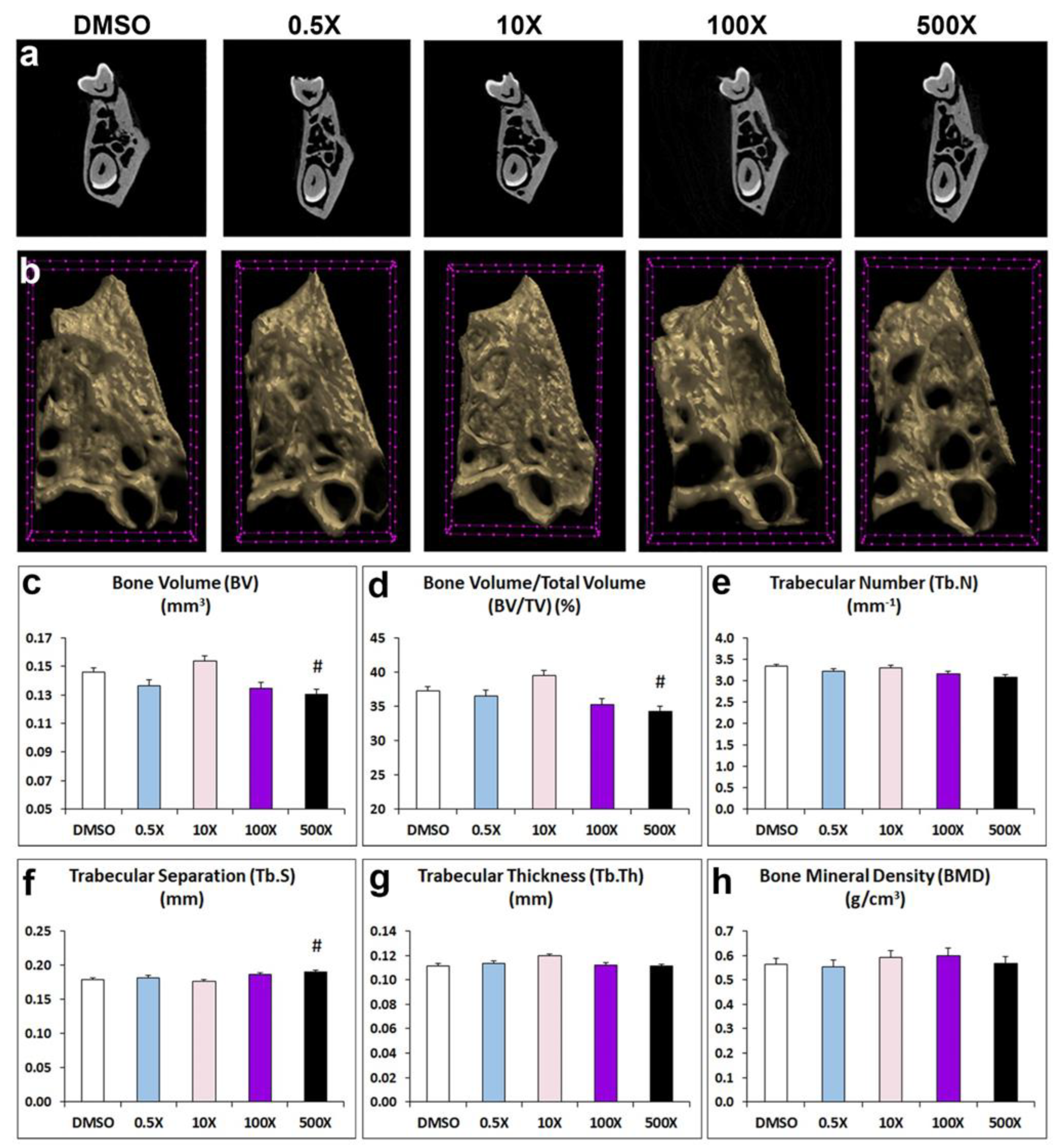
To explore the effects of Mixture N1 on adult structural bone parameters of the prenatally exposed offspring, we analyzed the microarchitecture of alveolar bone, condylar cancellus bone and cortical bone (Figure 2 & Table S2). Mixture N1 treatment had significant effects on the alveolar trabecular bone of the 500x exposed mice. Specifically, alveolar bone volume (BV, Figure 2c) and bone volume fraction (BV/TV, Figure 2d) were decreased, while trabecular separation (Tb.S, Figure 2f) was increased, compared to DMSO-treated offspring. No significant changes were observed in the other parameters of the alveolar bone: Trabecular Number (Tb.N, Figure 2e), Thickness,(Tb.Th Figure 2g) and Bone Mineral Density, (BMD, Figure 2h) as compared to DMSO group.
Figure 2.
Representative 2D (a) and 3D (b) micro-CT images of trabecular alveolar bone from adult mice prenatally exposed to DMSO, 0.5x, 10x, 100x and 500x of Mixture N1. (c-h) Quantification of the measurements. Data represent estimated marginal means+/- SEM. Generalized linear models (GLM) and Bonferroni post hoc tests were performed for statistical analysis. c) BV was decreased (W4=27.966, P<0.001) in 500X vs. DMSO (p=0.008). d) BV/TV was decreased (W4=29.316 P<0.001) in 500X vs. DMSO (p=0.019). f) Tb.S was increased (W4=18.371, P=0.001) in 500X vs. DMSO (p=0.009). Trabecular Number (Tb.N), (e), Thickness (Tb.Th) (g) and Bone Mineral Density (BMD) (h) did not differ significantly from DMSO group. # Statistically significant vs. DMSO group.
Figure 2.
Representative 2D (a) and 3D (b) micro-CT images of trabecular alveolar bone from adult mice prenatally exposed to DMSO, 0.5x, 10x, 100x and 500x of Mixture N1. (c-h) Quantification of the measurements. Data represent estimated marginal means+/- SEM. Generalized linear models (GLM) and Bonferroni post hoc tests were performed for statistical analysis. c) BV was decreased (W4=27.966, P<0.001) in 500X vs. DMSO (p=0.008). d) BV/TV was decreased (W4=29.316 P<0.001) in 500X vs. DMSO (p=0.019). f) Tb.S was increased (W4=18.371, P=0.001) in 500X vs. DMSO (p=0.009). Trabecular Number (Tb.N), (e), Thickness (Tb.Th) (g) and Bone Mineral Density (BMD) (h) did not differ significantly from DMSO group. # Statistically significant vs. DMSO group.
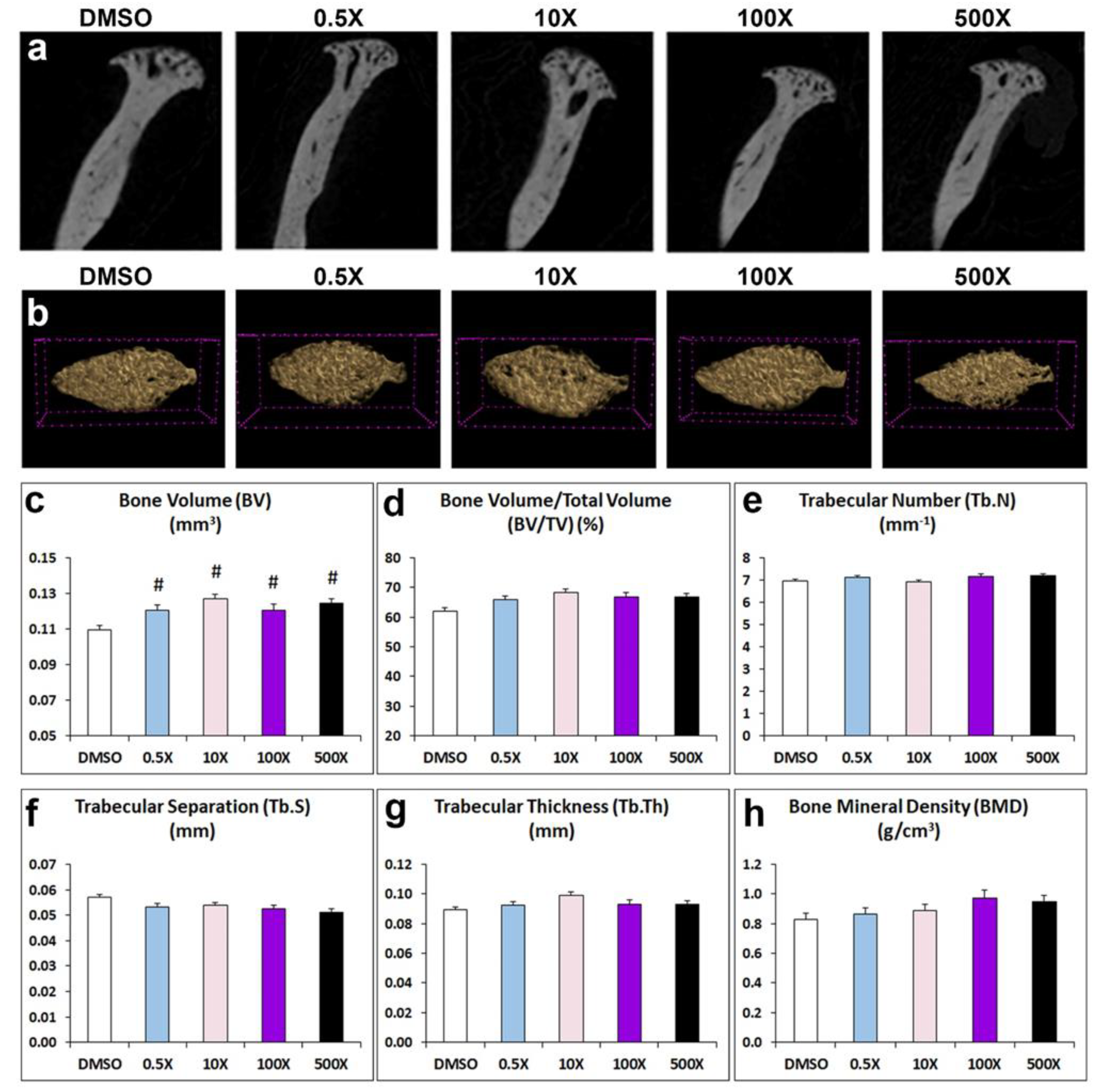
The micro-CT analysis of the cancellous bone in the condylar head showed significant increase of the BV in all experimental groups treated with Mixture N1 compared to the DMSO group. (Figure 3c). However, other important condylar bone parameters such as BV/TV, Tb.N, Tb.S, Tb.Th and BMD were not significantly affected by prenatal treatment with Mixture N1 (Figure 3d-h & Table S2).
Figure 3.
Representative 2D (a) and 3D (b) micro-CT images of cancellous bone in condylar head from adult mice prenatally exposed to DMSO, 0.5x, 10x, 100x and 500x of Mixture N1. Scale bar 100μm. Quantification of the measurements (c-h). Data represent estimated marginal means+/- SEM. Generalized linear models (GLM) and Bonferroni post hoc tests were performed for statistical analysis. c) BV was significantly increased in all treated groups (W4=29.733, P<0.001) as compared to DMSO (p= 0.017, p<0.001, p=0.033, and p<0.001 for groups 0.5x, 10x, 100x and 500x, respectively). # denotes statistical significance vs. DMSO treated group.
Figure 3.
Representative 2D (a) and 3D (b) micro-CT images of cancellous bone in condylar head from adult mice prenatally exposed to DMSO, 0.5x, 10x, 100x and 500x of Mixture N1. Scale bar 100μm. Quantification of the measurements (c-h). Data represent estimated marginal means+/- SEM. Generalized linear models (GLM) and Bonferroni post hoc tests were performed for statistical analysis. c) BV was significantly increased in all treated groups (W4=29.733, P<0.001) as compared to DMSO (p= 0.017, p<0.001, p=0.033, and p<0.001 for groups 0.5x, 10x, 100x and 500x, respectively). # denotes statistical significance vs. DMSO treated group.
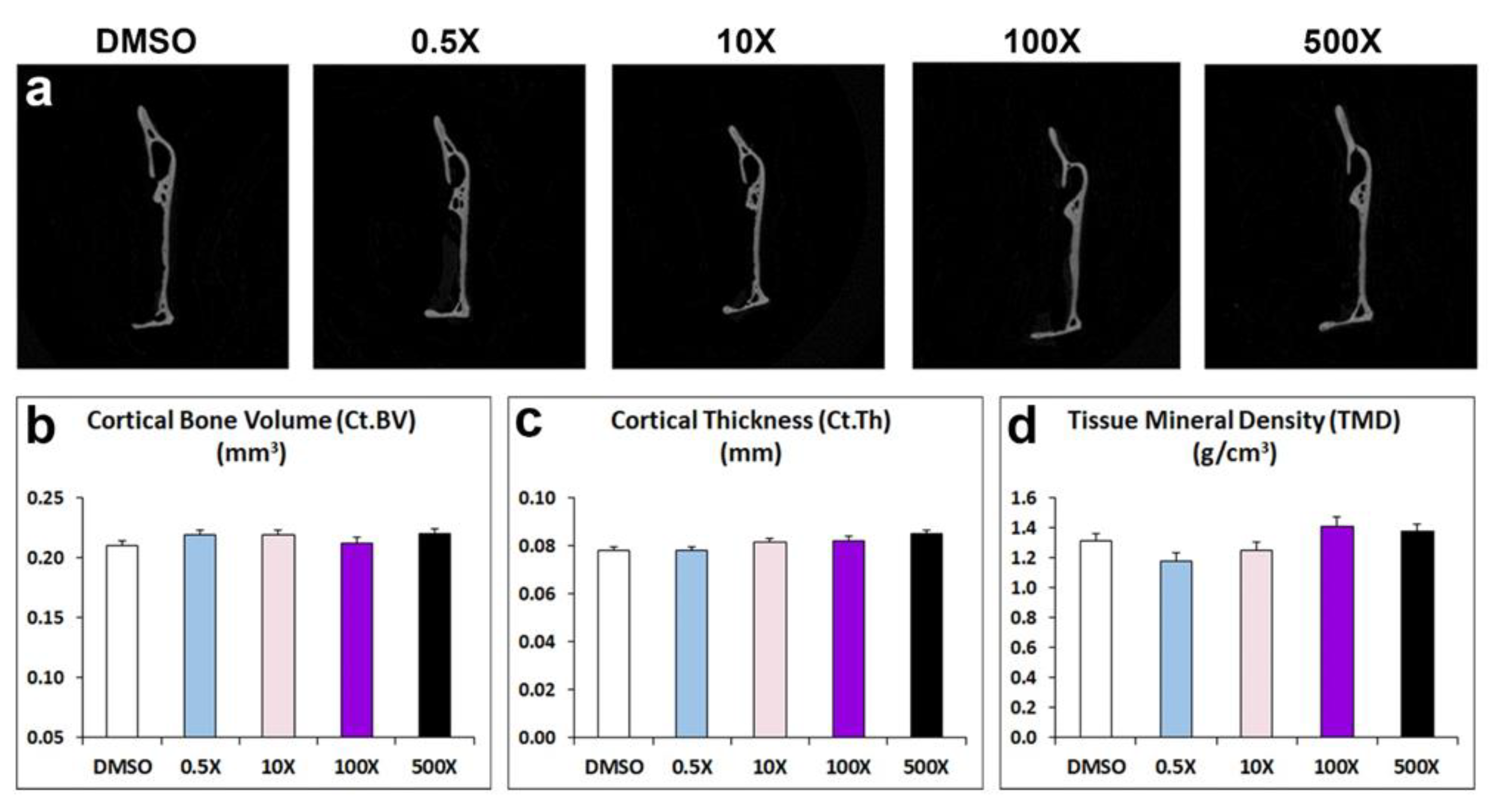
In contrast to the detected changes in the alveolar and condylar bone, the architecture of mandibular cortical bone of adult male offspring prenatally exposed to Mixture N1 was not affected in terms of cortical bone volume, thickness and tissue mineral density (Figure 4, &Table S2).
Figure 4.
a) Representative micro-CT images of cortical bone of the mandible from adult mice prenatally exposed to DMSO, 0.5x, 10x, 100x and 500x of Mixture N1. Quantification of the measurements for Ct.BV (b), Ct.Th (c) and TMD (d). Data represent Estimated Marginal Means+/- SEM. Generalized linear models (GLM) were performed for statistical analysis.
Figure 4.
a) Representative micro-CT images of cortical bone of the mandible from adult mice prenatally exposed to DMSO, 0.5x, 10x, 100x and 500x of Mixture N1. Quantification of the measurements for Ct.BV (b), Ct.Th (c) and TMD (d). Data represent Estimated Marginal Means+/- SEM. Generalized linear models (GLM) were performed for statistical analysis.
2.3. Mixture N1 Exposure Affects Condylar Cartilage.
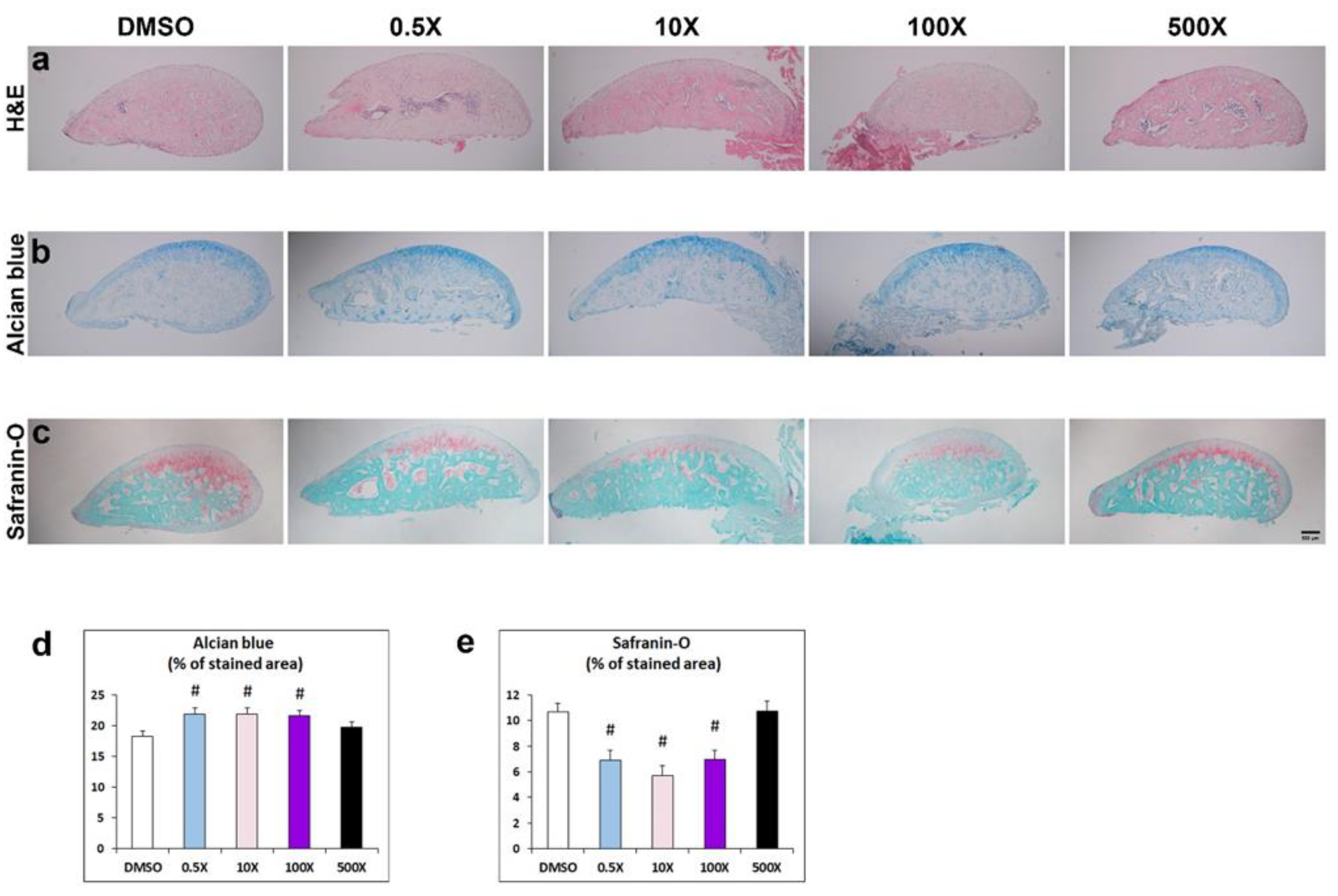
Following the detected changes in the cancellous bone of the condyle, we pursued to detect corresponding histological alterations of the condylar head. Hematoxylin and Eosin (H&E) staining provided a first indication of the characteristic zonal cartilage organization of this area (Figure 5a). Alcian blue staining revealed the areas of progenitors and flattened pre-hypertrophic chondrocytes in zonal organization of the cartilage (Figure 5b), whereas Safranin-O / Methylene green was utilized to stain the glycosaminoglycans- and proteoglycans-positive hypertrophic chondrocytes (Figure 5c). We detected a significant increase of the Alcian Blue positive area at the cancellous bone of condylar head of mice exposed to 0.5x, 10x and 100x Mixture N1 (Figure 5d) whereas the Safranin-O-stained area of the same groups (0.5x, 10x and 100x) was significantly decreased (Figure 5e). These findings suggest that prenatal exposure to Mixture N1 alters the ratio of progenitor and pre-hypertrophic chondrocytes to mature hypertrophic chondrocytes leading to altered cartilage architecture in the offspring.
Figure 5.
(a-c) Representative photomicrographs of condylar head from adult mice prenatally exposed to DMSO, 0.5x, 10x, 100x and 500x of Mixture N1, stained with H&E (a), Alcian blue (b) and Safranin-O-red/ Methylene green (c). Scale bar 100μm. (d-e) Quantification of % condyle head area stained with Alcian blue and Safranin-O. The Alcian blue stained area was significantly modified by Mixture N1 (W4=56.060; P<0.001). The % of Alcian blue stained area was significantly increased vs. DMSO in the groups of 0.5x (p=0.007), 10x (p=0.007) and 100x (p<0.001) (d). The Safranin-O-stained area was modified by the Mixture exposure (W4=39.939; P<0.001). The % of Safranin-stained area was significantly reduced vs. DMSO in the groups of 0.5x (p=0.002), 10x (p<0.001) and 100x (p=0.002) (e). Data represent estimated marginal means +/- SEM. Generalized linear models (GLM) of the measurements and Bonferroni post hoc tests were performed for statistical analysis. # denotes statistical significance vs. DMSO treated group.
Figure 5.
(a-c) Representative photomicrographs of condylar head from adult mice prenatally exposed to DMSO, 0.5x, 10x, 100x and 500x of Mixture N1, stained with H&E (a), Alcian blue (b) and Safranin-O-red/ Methylene green (c). Scale bar 100μm. (d-e) Quantification of % condyle head area stained with Alcian blue and Safranin-O. The Alcian blue stained area was significantly modified by Mixture N1 (W4=56.060; P<0.001). The % of Alcian blue stained area was significantly increased vs. DMSO in the groups of 0.5x (p=0.007), 10x (p=0.007) and 100x (p<0.001) (d). The Safranin-O-stained area was modified by the Mixture exposure (W4=39.939; P<0.001). The % of Safranin-stained area was significantly reduced vs. DMSO in the groups of 0.5x (p=0.002), 10x (p<0.001) and 100x (p=0.002) (e). Data represent estimated marginal means +/- SEM. Generalized linear models (GLM) of the measurements and Bonferroni post hoc tests were performed for statistical analysis. # denotes statistical significance vs. DMSO treated group.
3. Discussion
Mixture N1 composition (DEP, DBP, DBzP, DIDP/DPHP) BPA, TCP, 3-PBA and p,p’-DDE), was defined by associating maternal levels of exposure to chemicals during pregnancy with the neurodevelopmental progress of their children in the SELMA study [
24]. We have previously demonstrated the significant impact of Mixture N1 on neurodevelopment upon gestational exposure in mice [
29,
30]. The present study showed a developmental effect of Mixture N1 on structural parameters of the murine mandible, which exhibits a neural crest origin. Specifically, in utero N1 exposure modified the distances between several landmarks of the mandible and the crown width of M1 molar, it reduced the trabecular alveolar bone volume (BV), whereas it increased the BV in the cancellous bone and altered the composition of the chondrocyte zone of the condylar head.
Mandibular shape and size are critical for proper functioning of the temporomandibular junction and mastication. Consequently, changes in its shape and size may lead to malocclusion. Our results showed altered distances between cephalometric landmarks of mandibular length (Gonion to Infradental, Infradental to M3 molar and Mandibular Foramen to M3) and width (Menton to MAP) in Mixture N1 exposed mice. This suggests a potential deformity of mandibular shape that could have influenced the increase in the width of the M1 molar, also witnessed in several of the experimental groups. Many of the detected effects exhibited a non-monotonic pattern that has often been reported to characterize the action of EDCs [
31,
32].
Mandibular composition provides an ideal microenvironment to support dentition and at the same time to endure multidirectional forces during chewing. Accordingly, the ramus and the angular process consist of cortical bone, while the alveolar region that hosts the teeth, and the condylar process consist of trabecular bone. In our study, the impact of Mixture N1 was detected in the trabecular areas of the alveolar bone and the condylar head. The reduced alveolar bone volume in teeth sockets contributes to periodontium loosening and potential teeth loss. Although studies linking phthalates with periodontitis are scarce, a recent epidemiological study associated urinary phthalate metabolites with increased odds of periodontitis in adult subjects [
33]. The differential impact of Mixture N1 on the alveolar bone volume fraction (reduced) and the cancellous bone of the condyle (increased) may reflect the different ossification mode of these structures. The alveolar bone at the intermediate part of the mandible (in between the molar roots) is formed by intramembranous ossification, while the condyle follows endochondral ossification [
34]. The condylar cartilage serves as a site of condylar growth through the hypertrophic chondrocytes that transdifferentiate into osteoblasts in the deeper layers of the condyle [
35].
Our results showed increased area of proliferating chondrocytes and reduced area of hypertrophic chondrocytes in the condyle of groups exposed to 0.5x, 10x and 100x Mixture N1. Given that the hypertrophic chondrocytes contribute to condylar ossification, this implies an enhanced potential for endochondral ossification that coincides with the increased bone volume fraction detected in the same groups. It has been shown that condylar cartilage growth in both sexes of mice is supported by gonadal steroids, especially by estrogens via ERa [
12,
36]. We suggest that in utero exposure to components of Mixture N1 interfering with estrogen signaling, such as BPA and phthalates [
37,
38], could have contributed to the detected changes in the rate of chondrocyte differentiation towards ossification.
Although direct comparisons cannot be made between the effects of single EDCs and EDC mixtures, evidence from mice perinatally exposed to BPA (200μg BPA/kg BW) show reduced bone volume in the trabecular femoral bones of adult male offspring [
39]. In our study, prenatal exposure to a lower dose of BPA (74.99 μg/kg/kg BW in the 500x group of Mixture N1 [
26]) had similar effects in the mandibular alveolar bone. Furthermore, BPA could have also acted as an androgen receptor (AR) antagonist [
40], counteracting the protective effects of androgens on trabecular bone mass exerted via AR signaling in osteoblasts [
36]. Nevertheless, the effects of EDC mixtures cannot be directly extrapolated to those of single compounds due to the potential mutual interactions among the contributing chemicals [
41]. These interactions could account for the lack of effects on cortical bone in our study, although it has been shown that developmental BPA exposure increases the cortical thickness of femoral bones in adult male rat offspring [
42] that exhibit the same ossification pattern with mandibular cortical bone.
Collectively, our results provide the first evidence regarding the impact of gestational exposure to an epidemiologically-defined mixture in the mandible of adult mouse offspring. The effects of Mixture N1 exhibited a non-monotonic pattern and were detected in the alveolar and condylar bone. More research is required to reveal the potential mechanisms, taking into consideration that single EDCs probably have different outcomes from their mixtures due to their synergistic, additive, or opposing interactions which eventually lead to variable non- monotonic actions [
32].
Strengths and limitations
To our knowledge this is the first study to report impaired development of mandibular components in a mammalian model upon prenatal exposure to a human-relevant EDC mixture. Further studies are required to delineate the mechanisms of in utero effects of EDC mixtures in oral osteogenesis and dentition, since developmental alterations in mandible/condyle growth may lead to inadequate morphology and function in later life, leading to malocclusion and other complications [
43]. Given that mandibular alterations were detected in mice offspring even at low doses corresponding to the 0.5x and 10x (times) of the exposure concentrations measured in pregnant women of the SELMA cohort study, it would be relevant to include dental examinations in the follow up of children from such cohorts.
4. Methodology
4.1. Mixture N1
A detailed description of the establishment of Mixture N1 can be found in our previous publications [
29,
30]. In brief, the urine and serum levels of 26 suspected or known EDCs or their metabolites detected in 2,354 women of the SELMA study at median pregnancy week 10, where subjected to weighted quantile sum (WQS) regression analysis to select those chemicals that were associated with language delay in children at 2.5 years of age. The daily intake (DI) of the selected chemicals, the plasma concentrations from the DI estimates, and the geometric means of both urinary and serum of these chemicals were then used to determine their mixing proportions. Mixture N1 composition is shown in
Table 1.
4.2. Experimental Protocol and Tissue Preparation
In the present study we examined the effect of prenatal exposure to Mixture N1 on the mandibles of three months old male offspring. The animal tissues and data were collected in a previous study for Mixture N1 [
29]. The study’s protocol was approved by the Ethical Committee of the Prefecture of Attica-Veterinary department (#4783) and performed in accordance with the European Communities Council Directive of 22 September 2010 (2010/63/EU). In brief, two-month-old C57/BL6 mice, purchased from the Hellenic Pasteur Institute (Athens, Greece), were used for breeding. The animals were housed under standard conditions of temperature and illumination and were offered a phytoestrogen-deficient pellet food (Altromin 1324P, Lage, Germany) and tap water
ad libitum. From gestational day 1 to parturition, pregnant mice received daily via food the vehicle (DMSO) or the N1 mixture at doses of 0.5x, 10x, 100x and 500x hsc (
times of human serum concentration represents the exposure concentrations relative to the geometric mean of the concentrations measured in pregnant women of the SELMA cohort study). Accordingly, the daily exposure of pregnant dams throughout pregnancy was 0.001, 0.22, 2.2 and 11 mg/kg bw of Mixture N1, respectively. Offspring were euthanized at three months of age under isoflurane anesthesia. The mandibles were removed and fixed in neutral formalin. One hemimandible was dehydrated and kept for micro-CT analysis. The respective condyles from the other half were decalcified in 10% EDTA solution, washed in tap water, dehydrated, and embedded in paraffin. The number of samples per group used in the analyses was: DMSO: n=10, 0.5x: n=8, 10x: n=10, 100x: n=7, 500x: n=11.
4.3. Micro-CT Analysis
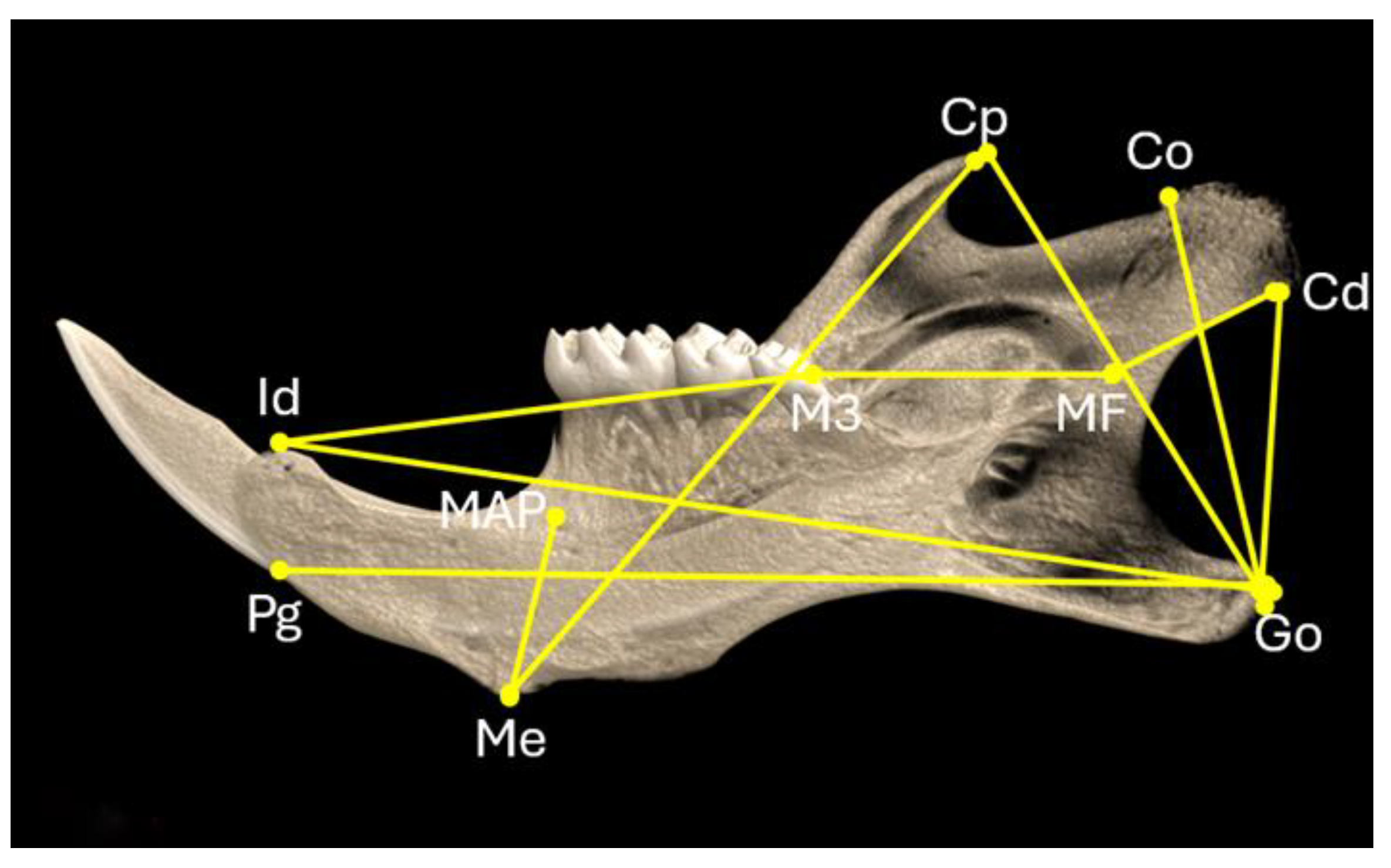
The microarchitecture of the mandibular bones was evaluated using a high-resolution SkyScan1172 microtomographic (micro-CT) imaging system (Bruker). Images were acquired at 60 KeV, 100 µA, pixel size 10μm, with a 0.5mm aluminum filter. Two-dimensional reconstruction images were generated using Nrecon software (Bruker) and analyzed using Ctan software while 3D reconstructed images of alveolar bone were generated using Ctvol software (Bruker). Morphometric measurements were performed with Data Viewer software (Bruker micro-CT). Analyses were performed between the following reference points: Gonion (Go) to dorsal point of the condylar process (Co); Go to ventral point of the condylar process (Cd); Go to Coronoid process (Cp); Go to the most antero-dorsal point on mandibular symphysis (Pogonion, Pg); Go to the most postero-dorsal point on mandibular symphysis (Infradental, Id); Menton (Me) to Mandibular alveolar point (MAP); Me to Cp, Mandibular foramen (MF) to Co; MF to M3 molar; Id to the most posterior part of M3 molar (Figure 6). The crown width of M1, M2 and M3 molars was also measured.
Figure 6.
Landmarks used in micro-CT analysis. Go, Gonion; Co. dorsal point of the condylar process; Cd, ventral point of the condylar process; Cp, Coronoid process; Pg, Pogonion; Id, Infradental; Me, Menton; MAP, Mandibular alveolar point; MF, Mandibular foramen.
Figure 6.
Landmarks used in micro-CT analysis. Go, Gonion; Co. dorsal point of the condylar process; Cd, ventral point of the condylar process; Cp, Coronoid process; Pg, Pogonion; Id, Infradental; Me, Menton; MAP, Mandibular alveolar point; MF, Mandibular foramen.
Alveolar, trabecular, and cortical bones were analyzed from transaxial sections, respectively, between the roots of the first molar, at the mandibular condyle, and at the posterior edge of the ramus. The parameters of bone volume (BV, mm3), tissue volume (TV, mm3), bone volume fraction (BV/TV, %), trabecular number (Tb.N, mm-1), trabecular separation (Tb.S, mm), trabecular thickness (Tb.Th, mm) and bone mineral density (BMD, g/cm3) were measured for alveolar trabecular bone and condyle head. Cortical bone was assessed through cortical bone volume (Ct.BV, mm3), tissue volume (TV, mm3), cortical thickness (Ct.Th, mm), and tissue mineral density (TMD, g/cm3).
4.4. Histomorphometry of Condylar Head
Basic histology of the condylar head was examined in 4μm paraffin sections (Leica RM 2125 microtome) stained with haematoxylin and eosin (H&E). Alcian blue and Safranin-O / Methylene green staining was used to locate the different zones of chondrocytes in the condylar head. Digital images of stained sections, corresponding to the mid-coronal part of the condylar head, were obtained by Nikon Eclipse E400 and the stained areas were quantified as % of total condylar head area using Image J (v.1.54f) by two independent observers blindly. Animal samples used in quantification of staining: DMSO: n=6; 0.5x: n=5; 10x: n=4; 100x: n=6; 500x: n= 4. For each sample, an average of 3-5 sections was used in the analysis.
4.5. Statistical Evaluation
Data were analyzed by generalized linear models (GLM), with the dose of Mixture N1 (treatment) as predictor factor and the treatment (litter) as a build nested predictor factor (IBM SPSS v. 26). In the case of statistically significant dose effects, Bonferroni post hoc tests were used to determine specific group differences compared to the DMSO-treated group. To control for type I error due to multiple statistical comparisons, an adjusted P-value threshold (P<0.0015) has been calculated dividing p=0.05 by the total number of statistical comparisons performed.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Micro-CT measured landmark distances in mandibles from adult male mice in utero exposed to different doses of Mixture N1 or the vehicle (DMSO). Table S2: Micro-CT measured mandibular alveolar, condylar and cortical bone components in samples of adult mice in utero exposed to different doses of Mixture N1 or the vehicle (DMSO).
Author Contributions
V.R: Investigation, Imaging and micro-CT analyses, Validation, Writing & editing original draft. A.S: Supervision of the animal study, Data curation, Formal analysis, Validation, Writing & editing original draft. Ath.S: Investigation, Methodology, Data processing, Reading – review original draft. C-G.B: Conceptualization of EDC-MixRisk project, Review & editing original draft. J.R: Conceptualization of EDC-MixRisk project, Review & editing original draft. M.A: Supervision of micro-CT analyses, Validation, Editing original draft Reading, Funding acquisition. E.K: Conceptualization of the study, Data curation, Formal analysis, Investigation, Validation, Writing review & editing original draft, Funding acquisition.
Funding
Research reported in this publication was supported by FOREUM grant (StroPHe, #061 to M.A) and by the EU H2020 Project EDC-MixRisk (grant agreement No. 634880).
Institutional Review Board Statement
The animal study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethical Committee of the Prefecture of Attica-Veterinary department (#4783) and performed in accordance with the European Communities Council Directive of 22 September 2010 (2010/63/EU).
Data Availability Statement
Acknowledgements
We are grateful to T. Meletakos for excellent technical assistance. The authors also wish to acknowledge the support from the InfrafrontierGR infrastructure for providing micro-CT facilities.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Gore A.C, Chappell V.A, Fenton S.E, Flaws J.A, Nadal A, Prins G.S, et al. Executive summary to EDC-2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 2015 Dec;36(6):593-602. [CrossRef]
- Alavian-Ghavanini A, Rüegg J. Understanding Epigenetic effects of endocrine disrupting chemicals: From mechanisms to novel test methods. Basic Clin Pharmacol Toxicol. 2018 Jan;122(1):38-45. [CrossRef]
- Lee S.K, Kim Y.S, Oh H.S, Yang K.H, Kim E.C, Chi J.G. Prenatal development of the human mandible. Anat. Rec. 2001, Jul 1;263(3):314-25. [CrossRef]
- Ramaesh T, Bard J.B.L. The growth and morphogenesis of the early mouse mandible: a quantitative analysis. J. of Anatomy 2003, Aug;203(2):213-22. [CrossRef]
- Chai Y, Jiang X, Ito Y, Bringas P Jr, Han J, Rowitch D.H, et al. Fate of the mammalian cranial neural crest (CNC) during tooth and mandibular morphogenesis, Development 2000 Apr;127(8):1671-9. [CrossRef]
- Carvalho I, Martinelli C, Milhan N, Marchini A, Dutra T, de Souza D, et al. Prenatal alcohol exposure reduces mandibular calcium and phosphorus concentrations in newborn rats. J Oral Sci. 2016;58(3):439-44. [CrossRef]
- Durham E.L, Balog C, Howie R.N, Boyce M.A, Arand J.R, Warren G, et al. Effects of nicotine exposure on murine mandibular development PLOS ONE 2019 . [CrossRef]
- Kesterke M.J, Judd M.A, Mooney M.P, Siegel M.I, Elsalanty M, Howie R.N, et al. Maternal environment and craniofacial growth: geometric morphometric analysis of mandibular shape changes with in utero thyroxine overexposure in mice. J Anat. 2018 July; 233(1): 46–54. [CrossRef]
- Noirrit-Esclassan E., Valera M.C, Tremollieres F, Arnal J.F, Lenfant F, Fontaine C, Vinel A. Critical role of estrogens on bone homeostasis in both male and female: from physiology to medical Implications. Int. J. Mol. Sci. 2021, 22(4), 1568; [CrossRef]
- Cohen S.P, LaChappelle A.R, Walker B.S, Lassiter C.S. Modulation of estrogen causes disruption of craniofacial chondrogenesis in Danio rerio, Aquatic Toxicology 2014, Vol. 152, pages 113-120. [CrossRef]
- Omori M.A, Matsumoto M.A.N, Segato R.A.B, da Silva L.A.B, Filho P.N, Kuchler E.C. The effect of estrogen on craniofacial dimensions: a systematic review. Dentistry 3000. (2019). [CrossRef]
- Robinson J.L, Gupta V, Soria P, Clanaman E, Gurbarg S, Xu M et al. Estrogen receptor alpha mediates mandibular condylar cartilage growth in male mice. Orthod Craniofac Res. 2017 Jun;20 Suppl 1(Suppl 1):167-171. [CrossRef]
- Chin K-Y, Pang K-L, Mark-Lee W.F. A review on the effects of bisphenol A and Its derivatives on skeletal health. Int J Med Sci. 2018; 15(10): 1043–1050. [CrossRef]
- Iwobi N, Nicole R, Sparks N.R. Endocrine Disruptor-induced bone damage due to hormone dysregulation: A review. Int J Mol Sci. 2023 May5;24(9):8263. [CrossRef]
- Wenlong H, Xin W, Shukai Z, Ruotong W, Caixia L, Kusheng W. Effect of bisphenol A on craniofacial cartilage development in zebrafish (Danio rerio) embryos: A morphological study. Ecotoxicol Environ Saf. 2021 Apr 1:212:111991. [CrossRef]
- Pu S-Y, Hamid N, Yi-Wei Ren Y-W, Pei D-S. Effects of phthalate acid esters on zebrafish larvae: Development and skeletal morphogenesis. Chemosphere 2020 May:246:125808. [CrossRef]
- Jedeon K, De la Dure-Molla M, Brookes S.J, Loiodice S, Marciano C, Kirkham J, et al. Enamel defects reflect perinatal exposure to bisphenol A. Am J Pathol. 2013 Jul; 183(1): 108118. [CrossRef]
- Jedeon K, Loiodice S, Marciano C, Vinel A, Canivenc Lavier M-C, Berdal A, Babajko S. Estrogen and bisphenol A affect male rat enamel formation and promote ameloblast proliferation. Endocrinology, 2014, Vol. 155. [CrossRef]
- Bui A.T, Houari S, Loiodice S, Bazin D, Sadoine J, Roubier N, Vennat E, et al. Use of dental defects associated with low-dose di(2-Ethylhexyl) phthalate as an early marker of exposure to environmental toxicants. Environ Health Perspect. 2022 Jun; 130(6): 067003. [CrossRef]
- Van Zwol-Janssens C, Trasande L, Asimakopoulos A.G, Martinez-Moral M-P, Kannan K, Philips E.M, et al. Fetal exposure to bisphenols and phthalates and childhood bone mass: a population-based prospective cohort study Environ. Res., 186 (2020). [CrossRef]
- Kuiper J.R, Braun J.M, Calafat A.M, Lanphear B.P, Cecil K.M, Chenet A, et al. Associations of pregnancy phthalate concentrations and their mixture with early adolescent bone mineral content and density: The Health Outcomes and Measures of the Environment (HOME) Study. Bone. 2022 Jan; 154: 116251. [CrossRef]
- Kuiper J.R, Pan S, Lanphear B.P, Calafat A.M, Chen A, Kim M Cecil K.M, et al. Associations of maternal gestational urinary environmental phenols concentrations with bone mineral density among 12-year-old children in the HOME Study. Int J Hyg Environ Health 2023; Mar 248: 114104. [CrossRef]
- Kortenkamp A. Ten years of mixing cocktails: A review of combination effects of Endocrine-Disrupting Chemicals. Environmental Health Perspectives 2007; Volume 115, Issue Suppl 1, Pages 98 – 105 . [CrossRef]
- Bornehag C-G, Moniruzzaman S, Larsson M, Boman Lindström C, Mikael Hasselgren M, Bodin A, et al. The SELMA study: A birth cohort study in Sweden following more than 2000 mother-child pairs. Paediatr. Perinat. Epidemiol. 2012; 26:456–467. [CrossRef]
- Repouskou A, Panagiotidou E, Panagopoulou L, Bisting PL, Tuck AR, Sjödin MOD, Lindberg J, Bozas E, Rüegg J, Gennings C, Bornehag CG, Damdimopoulou P, Stamatakis A, Kitraki E. Gestational exposure to an epidemiologically defined mixture of phthalates leads to gonadal dysfunction in mouse offspring of both sexes. Sci Rep. 2019 Apr 23;9(1):6424. [CrossRef]
- Smirnova A, Mentor A, Ranefall P, Bornehag CG, Brunström B, Mattsson A, Jönsson M. Increased apoptosis, reduced Wnt/β-catenin signaling, and altered tail development in zebrafish embryos exposed to a human-relevant chemical mixture. Chemosphere. 2021 Feb;264(Pt 1):128467. [CrossRef]
- Lizunkova P, Engdahl E, Borbély G, Gennings C, Lindh C, Bornehag CG, Rüegg J.A Mixture of Endocrine Disrupting Chemicals Associated with Lower Birth Weight in Children Induces Adipogenesis and DNA Methylation Changes in Human Mesenchymal Stem Cells. Int J Mol Sci. 2022 Feb 19;23(4):2320. [CrossRef]
- Caporale N, Leemans M, Birgersson L, Pierre-Luc Germain P-L, Cristina Cheroni C, Borbély G, et al. From cohorts to molecules: Adverse impacts of endocrine disrupting mixtures, Science 2022; Feb 18;375(6582): eabe8244. [CrossRef]
- Repouskou A, Papadopoulou AK, Panagiotidou E, Trichas P, Lindh C, Bergman A, et al. Long term transcriptional and behavioral effects in mice developmentally exposed to a mixture of endocrine disruptors associated with delayed human neurodevelopment. Sci Rep. 2020 Jun 9;10(1):9367. [CrossRef]
- Di Criscio M, Lodahl J, Stamatakis A, Kitraki E, Bakoyiannis I, Repouskou A, et al. A human-relevant mixture of endocrine disrupting chemicals induces changes in hippocampal DNA methylation correlating with hyperactive behavior in male mice. Chemosphere 2023; 313, p. 137633. [CrossRef]
- Vandenberg L.N. non-monotonic dose responses in studies of endocrine disrupting chemicals: Bisphenol A as a case study. Dose Response. 2014; 12:259-76.
- Zoeller R.T, Vandenberg L.N. Assessing dose–response relationships for endocrine disrupting chemicals (EDCs): a focus on non-monotonicity. Environ Health 2015; 14(1):1–5. PMID 10.1186/s12940-015-0029-4.
- Bian M, Jiang W, Wang M, Shi Y, Wu Z. Association of phthalate metabolites with periodontitis: a population-based study. BMC Oral Health 2024; 24, 541. [CrossRef]
- Yuan Y, Chai Y. Regulatory mechanisms of jaw bone and tooth development, Curr Top Dev Biol. Curr Top Dev Biol. 2019; 133: 91–118. [CrossRef]
- Jing Y, Zhou X, Han X, Jing J, von der Mark K, Wang J, et al. Chondrocytes directly transform into bone cells in mandibular condyle growth. J Dent Res. 2015 Dec; 94(12): 1668–1675. [CrossRef]
- Robinson J.L, Soria P, Xu M, Vrana M, Luchetti J, Lu H.H, Chen J, et al. Estrogen promotes mandibular condylar fibrocartilage chondrogenesis and inhibits degeneration via estrogen receptor alpha in female mice. Scientific Reports 2018, vol. 8, article number: 8527. https://www.nature.com/articles/s41598-018-26937-w.
- Alonso-Magdalena P., Ropero AB., Soriano S., García-Arévalo M., Ripoll C., Fuentes E. et al. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol Cell Endocrinol. 2012;355(2):201–207. [CrossRef]
- Engel A., Buhrke T., Imber F., Jessel S., Seidel A., Völkel W., Lampen A. Agonistic and antagonistic effects of phthalates and their urinary metabolites on the steroid hormone receptors ERα, ERβ, and AR. Toxicol Lett. 2017 Aug 5;277:54-63. [CrossRef]
- Dirkes R.K, Welly R.J, Mao J, Kinkade J, Vieira-Potter V.J, Rosenfeld C.S, et al. Gestational and lactational exposure to BPA, but not BPS, negatively impacts trabecular microarchitecture and cortical geometry in adult male offspring. Bone Rep. 2021 Nov 3:15:101147. [CrossRef]
- Teng C, Goodwin B, Shockley K, Xia M, Huang R, Norris J, et al. Bisphenol A affects androgen receptor function via multiple mechanisms. Chem Biol Interact. 2013 May 25;203(3):556-64. [CrossRef]
- Martin O, Scholze M, Ermler S, McPhie J, Bopp SK, Kienzler A, et al. A Ten years of research on synergisms and antagonisms in chemical mixtures: A systematic review and quantitative reappraisal of mixture studies. .Environ Int. 2021 Jan;146:106206. [CrossRef]
- Lejonklou M.H, Christiansen S, Örberg J, Shen L, Larsson S, Boberg J, et al. Low-dose developmental exposure to bisphenol A alters the femoral bone geometry in wistar rats. Chemosphere. 2016 Dec:164:339-346. [CrossRef]
- Pirttiniemi P, Peltomaki T, Muller L, Luder H.U. Abnormal mandibular growth and the condylar cartilage. European Journal of Orthodontics. 2009; 31(1):1–11. [CrossRef]
Table 1.
Mixture N1 composition.
Table 1.
Mixture N1 composition.
| Compound |
GM (nmol/mL) |
Mixing percentages(% of urine + serum) |
| DEP |
0.03204 |
44.80 |
| DBP |
0.02855 |
39.92 |
| BBzP |
0.00568 |
7.94 |
| DIDP/DPHP |
0.00352 |
4.92 |
| BPA |
0.00047 |
0.66 |
| TCP |
0.00056 |
0.78 |
| 3-PBA |
0.00011 |
0.15 |
| p,p’-DDE |
0.00059 |
0.82 |
| Total |
0.0715 |
100 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).