1. Introduction
Sleep disorders comprise a group of conditions that disrupt normal sleep patterns, including apnea, insomnia, sleep-related respiratory disorder, centralized somnolence, day-night rhythm sleep-wake disorder (including jet lag and shift work), sleep-associated motor disorder, and anomalous sleep [
1]. Besides directly impairing quality of life [
2], sleep disorders contributed to various physical and psychological complications, including metabolic disorders [
3,
4], cardiovascular disorders [
5,
6], cognitive decline [
7,
8] and depression [
9]. Previous studies demonstrated that oxidative stress exacerbates sleep disorders by altering neuronal activity and inflammatory responses [
10]. Conversely, consuming antioxidant-rich foods can significantly improve sleep disorders [
11].
Vitamin C is a potent antioxidant [
12] that reduces oxidative stress by absorbing and neutralizing free radicals produced by the body [
13,
14]. In previous research on rats, vitamin C was shown to reduce oxidative and carbonyl stress in obstructive sleep apnea and prevent metabolic, hormonal, and lipid peroxidation issues caused by sleep deprivation [
15]. Several cross-sectional studies have elevated the effect of vitamin C on sleep status. Mhaidat NM.et al found that dietary vitamin C reduced sleep deprivation-induced memory impairment [
16]. Moreover, individuals with lower vitamin C intake were more likely to experience shorter sleep durations [
17]. And a study based on NHANES data identified a significant negative association between serum vitamin C levels and sleep disorders [
18].
While numerous cross-sectional studies have investigated the relationship between vitamin C and sleep, there is a scarcity of longitudinal research on this topic. Moreover, most cross-sectional studies had relatively small sample sizes and lacked a clear definition of sleep disorders. Thus, this study utilized data from the UK Biobank to conduct a longitudinal analysis of the impact of dietary vitamin C on sleep disorders, as defined by ICD classification [
19].
2. Materials and Methods
2.1. Study Population
This study utilized data collected from the UK Biobank (
https://www.ukbiobank.ac.uk/). They were comprehensively assessed between 2006 and 2010 and their health status was tracked through the linked death and health records section. The UK Biobank represents a massive, population-based, prospective study. The evaluation visits encompassed electronic signature consent forms, self-administered touchscreen questionnaires, brief computer-assisted interviews, physical and functional measurements, as well as the collection of blood, urine, and saliva samples [
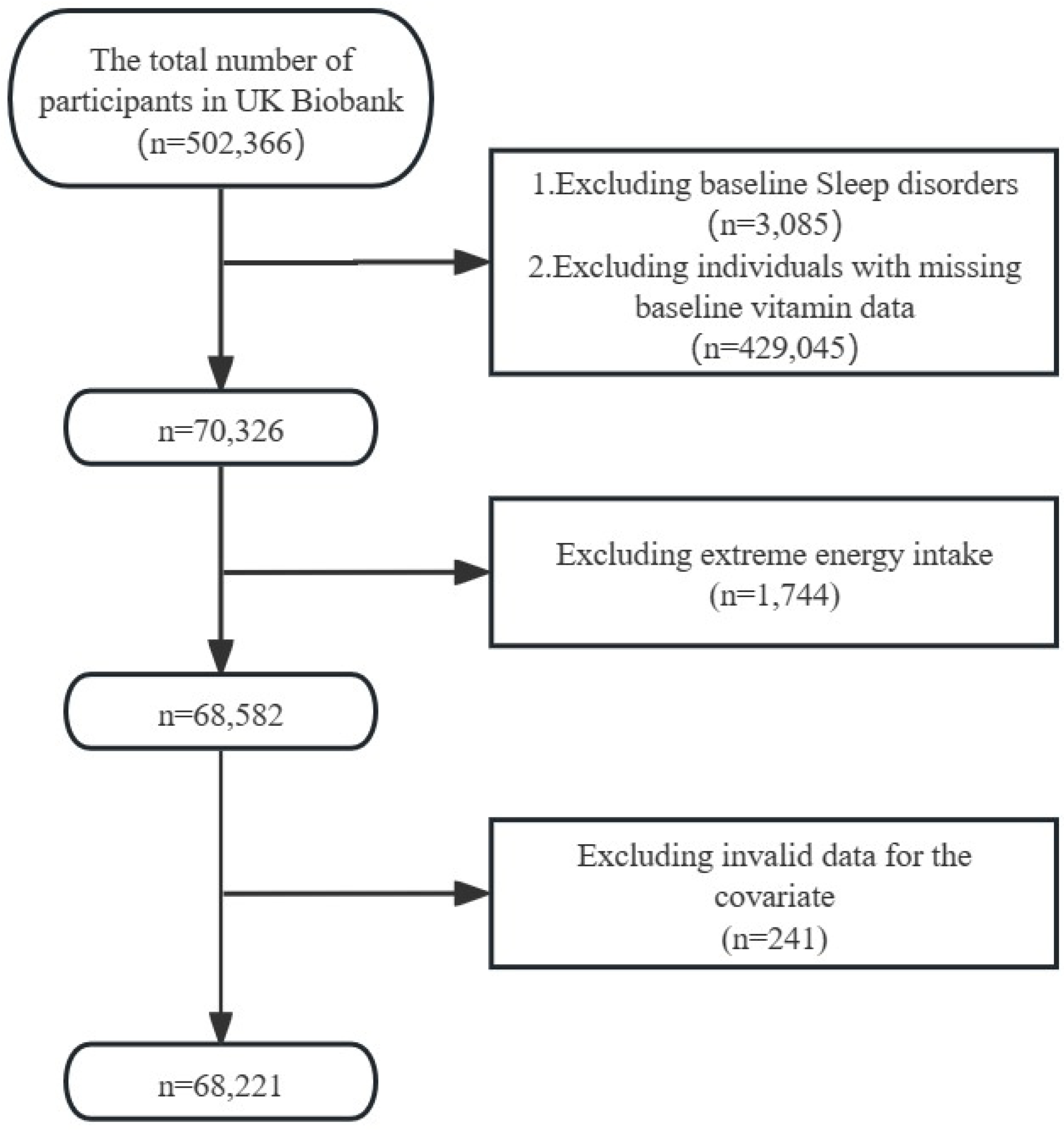
20]. The North West Multi-centre Research Ethics Committee (MREC) granted ethical approval after all participants gave their informed consent. The inclusion criteria for this study were as follows: (1) absence of sleep disorders at baseline; (2) complete dietary vitamin C data at baseline. The exclusion criteria were: (1) extreme energy intake; and (2) incomplete covariate data. Ultimately, a total of 68,221 participants were included in the analysis.
Figure 1 depicted the flowchart of study participants.
2.2. Assessment of Sleep Disorder
Our primary outcome variable is sleep disorders, identified using International Classification of Diseases (ICD) codes obtained through coordination with national registries. Specifically, instances of sleep disorders are classified using ICD-10 codes G47.0-9 (
Supplementary Tables S1). Participants’ hospitalisation data were available up to 30 September 2023. Participants who died were assigned the date of death as the review date. Sleep disorder onset date corresponded to the first reported diagnosis.
2.3. Assessment of Dietary Vitamin C Intake
We utilized data from the internet-based 24-hour dietary questionnaire (Oxford WebQ) of the UK Biobank to evaluate dietary vitamin C intake. Vitamin C was analyzed using baseline data from 2009-2010. This data was then categorized into five groups: Q1 (<51.27mg/d), Q2 (51.27-90.44mg/d), Q3 (90.44-132.88mg/d), Q4 (132.88-191.51mg/d), and Q5 (>191.51mg/d) based on quintiles.
2.4. Assessment of Covariates
Based on previous literature regarding diet intake and sleep [
21,
22,
23], we included a series of covariates in our study. The demographic variables included gender, age, education level, household income, Townsend Deprivation Index (TDI), and ethnicity. Additionally, we incorporated several health behavior variables such as Body Mass Index (BMI), physical activity, smoking status, alcohol drinker status, and further adjusted for total energy intake. In addition, we included the following health factors: vitamin C supplements, hypertension, diabetes, stroke, and malignant neoplasms.
2.5. Statistical Analysis
We compared the characteristics of individuals with and without sleep disorders, as well as the varying levels of dietary vitamin C intake. Normality of continuous variables was assessed using the Kolmogorov-Smirnov (K-S) test. Continuous variables following a normal distribution were expressed as mean ± standard deviation (SD) and compared using Student’s t-test, while non-normally distributed variables were reported as median and interquartile range (IQR) and analyzed using the Mann-Whitney U test. Categorical variables were presented as frequencies and percentages and analyzed using the χ² test.
To estimate the association between dietary vitamin C intake and the risk of sleep disorders, we used a Cox regression model to calculate the hazard ratio (HR) and its 95% confidence interval (CI). We conducted additional analyses on the impact of dietary vitamin C on sleep apnea, insomnia and other types of sleep disorders. Survival time was defined as the interval from the start of follow-up to the occurrence of the endpoint event. For participants who did not observe the endpoint event, survival time was calculated until their death or the end of the follow-up period. The lowest vitamin C intake group (Q1) served as the reference group. Multicollinearity was assessed using the variance inflation factor (VIF), and no signs of multicollinearity were detected in any of the models. We constructed three models:
Model 1 serves as the crude model.
Model 2 adjusts for age and gender (male, female).
Model 3 incorporates additional variables, including race(white, other) education level(college or university degree=1, a levels/ as levels or equivalent=2, o levels/ GCSEs or equivalent=3, CSEs or equivalent=4, NVQ or HND or HNC or equivalent=5, other professional qualifications eg: nursing, teaching=6, other=0), household income (less than 18,000=1, 18,000 to 30,999=2, 31,000 to 51,999=3, 52,000 to 100,000=4, greater than 100,000=5, other=0), TDI, BMI, physical activity, smoking status(never=0, previous=1, current=2), alcohol drinker status(never=0, previous=1, current=2), total energy intake, vitamin C supplements(yes=1, no=0), hypertension, diabetes, stroke, and malignant neoplasms.
Due to the differing prevalence of sleep disorders across genders and age groups, we performed stratified analyses based on these variables. Furthermore, as previous studies have demonstrated that sleep-related breathing disorders, insomnia, and insufficient sleep can contribute to elevated blood pressure, we specifically examined the effect of dietary vitamin C intake on sleep disorders in people with and without hypertension.
We used a restricted cubic spline model with four knots to assess the dose-response relationship between dietary vitamin C intake and the risk of sleep disorders, analyzing the effects of vitamin C under different dose conditions.
In performing sensitivity analyses to consider potential delays in disease onset, we excluded subjects who developed sleep disorders within two years of the investigation, and further analyzed associations between dietary vitamin C and sleep disorders, as well as sleep apnea, insomnia, and other types of sleep disorders. Results were considered statistically significant when the P-value on both sides was less than 0.05.
3. Results
This survey included a total of 68,221 participants who met the inclusion criteria. The average age was 56.24 ± 8.14 years old.
Table 1 showed the comparison of characteristics between the sleep disorders group and the non-sleep disorders group. Participants with sleep disorders tended to be older, more likely to be male, had a higher BMI, smoke more frequently, consumed more total energy, had higher rates of hypertension, stroke, diabetes and malignant neoplasms, and had lower family income, education level, Townsend deprivation index, MET scores and vitamin C intake.
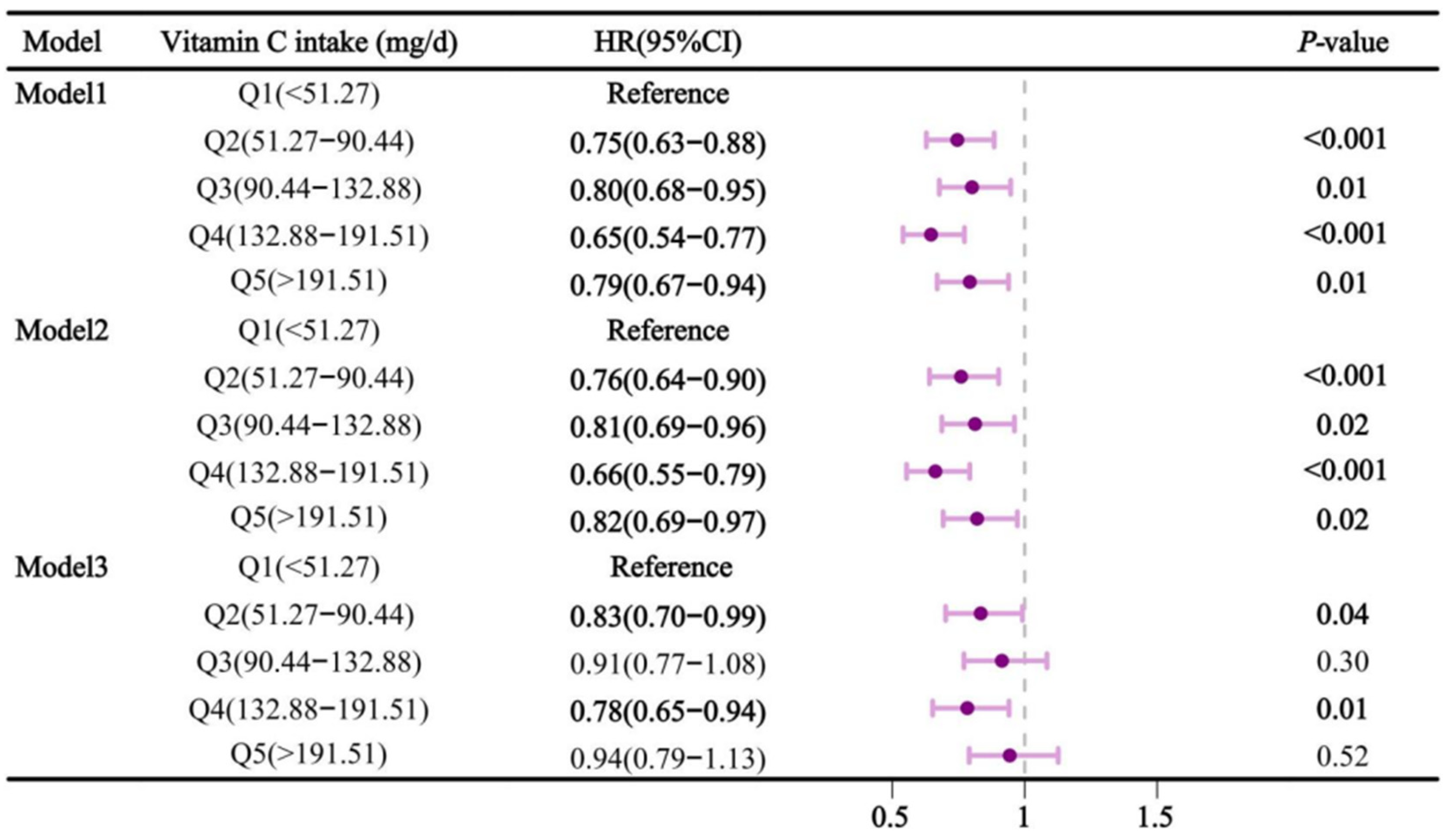
Figure 2 showed the relationship between dietary vitamin C intake and sleep disorders. In Model 1, higher vitamin C intake significantly reduced the risk of sleep disorders (
P < 0.05). Compared to Q1, the hazard ratios (HR) and 95% confidence intervals (CI) for Q2, Q3, Q4, and Q5 were 0.75 (0.63-0.88), 0.80 (0.68-0.95), 0.65 (0.54-0.77), and 0.79 (0.67-0.94), respectively. In model 2, this relationship remained, with HRs (95% CI) of 0.76 (0.64-0.90), 0.81 (0.69-0.96), 0.66 (0.55-0.79), and 0.82 (0.69-0.97) for Q2, Q3, Q4, and Q5, respectively, compared with Q1. However, in model 3, Q2 and Q4 showed significant protection against sleep disturbances compared to Q1, with HRs (95% ci) of 0.83 (0.70-0.99) and 0.78 (0.65-0.94), respectively.
We further conducted a stratified analysis by gender and age.
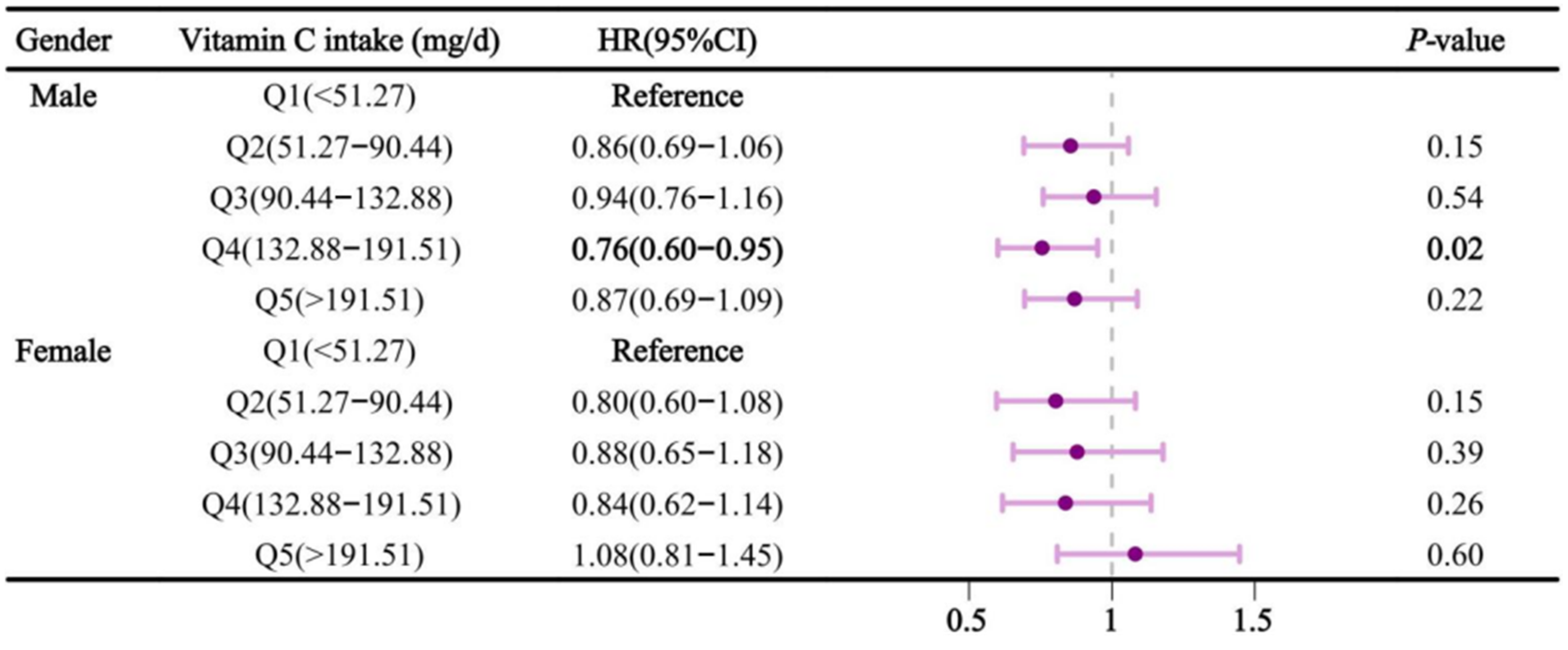
Figure 3 showed the association between vitamin C intake and sleep disorders after stratifying by gender. In the multivariable-adjusted model, compared to Q1, dietary vitamin C intake in Q4 was negatively associated with sleep disorders in males, with an HR (95% CI) of 0.76 (0.60-0.95). The results were not significant in females. Similarly, in the age-stratified analysis (
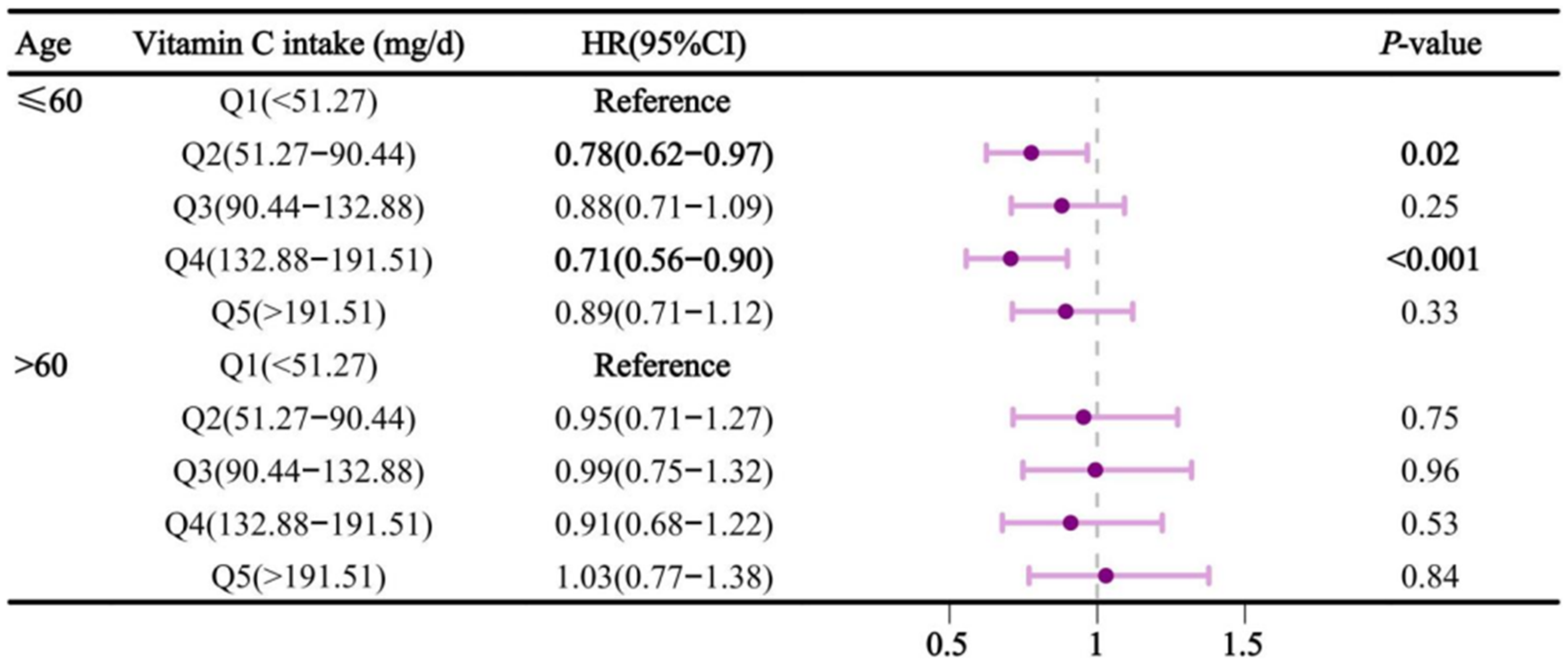
Figure 4), dietary vitamin C intake in participants aged ≤60 was negatively associated with sleep disorders in Q2 and Q4, with HRs (95% CI) of 0.78 (0.62-0.97) and 0.71 (0.56-0.90), respectively. The results of
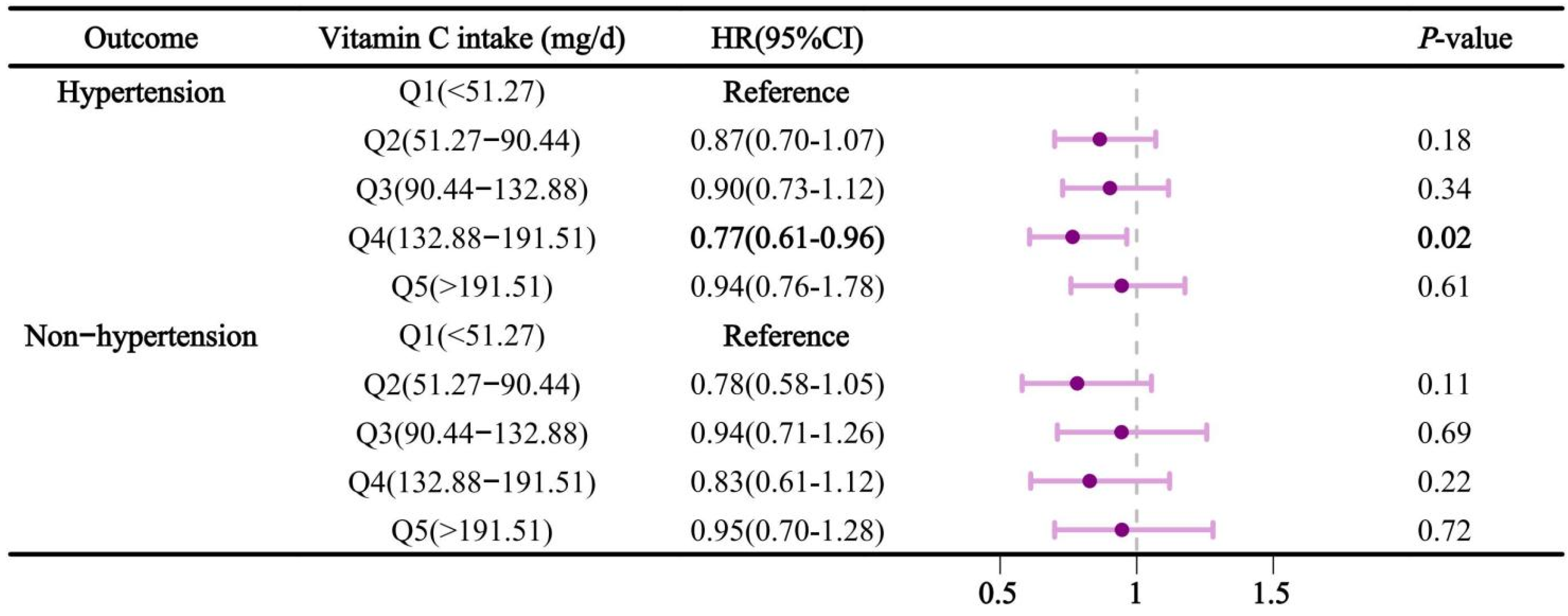
Figure 5, stratified by hypertension, revealed that dietary vitamin C intake in Q4 had a significant impact on sleep problems in hypertensive subjects, with an HR and 95% confidence interval of 0.77 (0.61-0.96). Similarly, in the further analysis of dietary vitamin C and sleep apnea, the results remained similar to the main findings (
Supplementary Tables S2-5). In model 3, dietary vitamin C was protective against sleep apnea in Q4, with HRs (95% CI) of 0.75 (0.62-0.92), compared with Q1. However, no significant effect of dietary vitamin C was found on insomnia and other types of sleep disorders (
Supplementary Tables S6–S12).
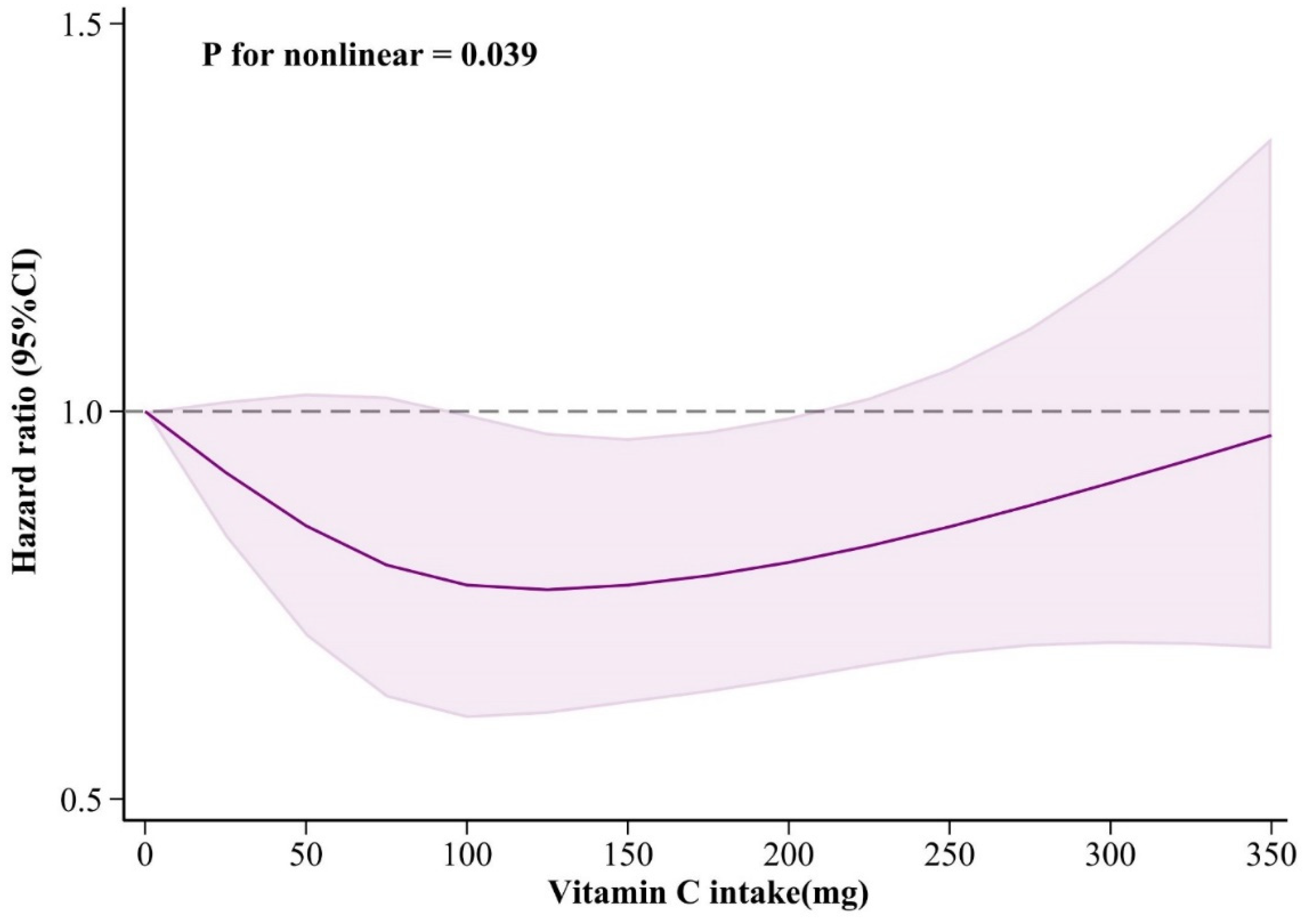
In the dose-response relationship, adjusting covariates were consistent with Model 3. The dose-response relationship was illustrated in
Figure 6. There is a nonlinear association between dietary vitamin C intake and sleep disorders (
P for nonlinear = 0.039). When vitamin C intake was between about 125 and 200 mg/day, the risk of sleep disorders reached a relatively low level. In
Supplementary Figures S1–S3, We also conducted additional analyses of the dose-response relationship in males, participants aged ≤60, and individuals with hypertension.
The results of the sensitivity analysis were shown in
Table 2. After excluding participants who developed sleep disorders within two years, the study results remained consistent with the main findings. Compared to Q1, dietary vitamin C intake in Q4 continued to show a protective effect against sleep disorders 0.80 (95% CI: 0.66-0.97), and for apnea 0.77 (95% CI: 0.62-0.95).
4. Discussion
Our results showed that higher dietary vitamin C intake was associated with a lower risk of sleep disorders, particularly in males, those aged ≤60 years, and hypertensive patients. This result remained consistent among patients with sleep apnea. This protective effect was not seen in cases of insomnia or other types of sleep disorders. Also in analyses that excluded participants who became ill after two years, the results remained robust. This suggests that vitamin C may be crucial in reducing the risk of sleep disorders.
A Japanese study showed that dietary vitamin C was associated with insomnia [
24], which is a sleep disorder [
25]. Other studies have also found a correlation between serum vitamin C and sleep disorders [
26]. The general population should ideally consume about 110 mg/d to achieve adequate 50 µmol/L circulating vitamin C concentrations [
27], so serum vitamin C may be an intrinsic expression of dietary vitamin C. This is all consistent with the protective effect of dietary vitamin C on sleep disorders found in our study.
Substantial associations with age stratification have been found in previous studies in individuals under 65 years of age, which is essentially similar to the significance we found in those under 60 years of age. This might be related to the fact that aging impacts vitamin absorption due to changing dietary requirements and deteriorating digestive systems [
28,
29]. Our study showed a significant effect of vitamin C intake on sleep disorders in males, but not in females. Simultaneously, we did not find an association between vitamin C and insomnia, even though another study found a relationship between vitamin C intake in the diet and sleeplessness [
24]. The cross-sectional study methodology, sample size selection, and regional variances could all be contributing factors to these discrepancies.
Vitamin C may be associated with sleep disorders through the following mechanisms. Firstly, vitamin C may reduce the incidence of sleep disorders by utilizing its anti-inflammatory and antioxidant properties [
11,
26]. Studies revealed that ascorbic acid has been shown to effectively improve vascular function in sleep apnea patients with endothelial dysfunction by interacting with tetrahydrobiopterin (BH4) and maintaining its reduced state, as well as through endothelial nitric oxide synthase [
30]. In addition, adrenocorticotropic hormone increased intravenous vitamin C concentrations [
31], while the synthetic hexapeptide growth hormone-releasing peptide (GHRP-6) enhanced sleep quality by stimulating the secretion of hypothalamic-pituitary-adrenal (HPA) hormones [
32].
There are several strengths in our study. Firstly, our study benefited from the large sample size of the cohort study, thus increasing the reliability of our results. Additionally, by defining the diagnosis based on ICD-10 results, we ensured consistency in identifying sleep disorders, which helps reduce misunderstandings and errors, and improves the accuracy and comparability of the data. Thirdly, we performed stratified analyses by age, gender, and hypertension status to control for confounding variables, enhance the precision and interpretability of the study, and more accurately assess the independent relationship between vitamin C and sleep disorders. Fourthly, in the sensitivity analysis, we also excluded individuals who developed the condition within two years of follow-up, helping to reduce the impact of reverse causality and thereby allowing a more accurate assessment of the long-term relationship between vitamin C and sleep disorders. Fifthly, we also used restricted cubic splines to analyze the relationship, which allowed us to quantify the association between vitamin C intake and sleep disorders, reveal the dose-response relationship, and help determine the optimal dose of vitamin C, providing scientific evidence for health interventions. Finally, in our multivariable analysis, we adjusted for dietary energy intake and other disease-related confounders. This reduced their effect on the results and allowed a more accurate assessment of the relationship between vitamin C and sleep problems.
There are some limitations to our study. Firstly, we used 24-hour dietary recalls, which might lead to memory biases and potentially affect the accuracy of our findings. Secondly, we did not consider the effect of cooking on vitamin C levels. Thirdly, due to limited data, we only explored dietary data rather than optimal blood indicators for vitamin C status. Lastly, the UK Biobank dataset includes half a million participants from the UK, which may introduce geographical limitations.
5. Conclusions
Adequate vitamin C intake reduced the risk of developing sleep disorders. This association remained consistent among male participants and those ≤60 years old, with similar results for sleep apnea. Therefore, it is important to consume sufficient dietary vitamin C and maintain nutritional balance in daily diet.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Table S1: Codes and names of sleep disorders defined according to ICD10. Table S2: Association between dietary vitamin C intake and sleep apnea. Table S3: Association between dietary vitamin C intake and sleep apnea after gender stratification. Table S4: Association between dietary vitamin C intake and sleep apnea after age stratification. Table S5: Association between dietary vitamin C and sleep apnea in participants with and without hypertension. Table S6: Association between dietary vitamin C intake and insomnia. Table S7: Association between dietary vitamin C intake and insomnia after gender stratification. Table S8: Association between dietary vitamin C intake and insomnia after age stratification. Table S9: Association between dietary vitamin C and insomnia in participants with and without hypertension. Table S10: Association between dietary vitamin C intake and other types of sleep disorders. Table S11: Association between dietary vitamin C intake and other types of sleep disorders after gender stratification. Table S12: Association between dietary vitamin C intake and other types of sleep disorders after age stratification. Table S13: Association between dietary vitamin C and other types of sleep disorders in participants with and without hypertension. Figure S1: The results of dose-response between dietary vitamin C intake and sleep disorders in males. Figure S2: The results of dose-response between dietary vitamin C intake and sleep disorders in individuals aged ≤60. Figure S3: The results of dose-response between dietary vitamin C intake and sleep disorders in individuals with hypertension.
Author Contributions
Qiuge Zhang: Conceptualization, Methodology, Data Curation, Formal analysis, Writing—Original Draft, Visualization. Xueting QI: Visualization, Investigation. Zhaoguo Wang: Writing—Review & Editing. Dongfeng Zhang: Resources, Writing—Review & Editing. Tong Wang: Supervision, Writing—Review & Editing, Project administration.
Funding
This research received no external funding.
Institutional Review Board Statement
This research has been conducted using the UK Biobank resource under application number 95715.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data are contained within the article. The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Acknowledgments
The authors sincerely appreciate the participation of all subjects in this study
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Karna, B., A. Sankari, and G. Tatikonda. “Sleep Disorder.” In Statpearls. Treasure Island (FL) ineligible companies. Disclosure: Abdulghani Sankari declares no relevant financial relationships with ineligible companies. Disclosure: Geethika Tatikonda declares no relevant financial relationships with ineligible companies.: StatPearls Publishing.
- Copyright © 2024, StatPearls Publishing LLC., 2024.
- Alanazi, M. T., N. T. Alanazi, M. A. Alfadeel, and B. A. Bugis. “Sleep Deprivation and Quality of Life among Uterine Cancer Survivors: Systematic Review.” Support Care Cancer 30, no. 3 (2022): 2891-900. [CrossRef]
- Cappuccio, F. P., and M. A. Miller. “Sleep and Cardio-Metabolic Disease.” Curr Cardiol Rep 19, no. 11 (2017): 110. [CrossRef]
- Smiley, A., D. King, and A. Bidulescu. “The Association between Sleep Duration and Metabolic Syndrome: The Nhanes 2013/2014.” Nutrients 11, no. 11 (2019). [CrossRef]
- Cappuccio, F. P., F. M. Taggart, N. B. Kandala, A. Currie, E. Peile, S. Stranges, and M. A. Miller. “Meta-Analysis of Short Sleep Duration and Obesity in Children and Adults.” Sleep 31, no. 5 (2008): 619-26. [CrossRef]
- Sánchez-de-la-Torre, M., and F. Barbé. “Sleep Disorders and Cardiovascular Disease.” Med Clin (Barc) 158, no. 2 (2022): 73-75. [CrossRef]
- Van Dongen, H. P., G. Maislin, J. M. Mullington, and D. F. Dinges. “The Cumulative Cost of Additional Wakefulness: Dose-Response Effects on Neurobehavioral Functions and Sleep Physiology from Chronic Sleep Restriction and Total Sleep Deprivation.” Sleep 26, no. 2 (2003): 117-26. [CrossRef]
- Ma, Y., L. Liang, F. Zheng, L. Shi, B. Zhong, and W. Xie. “Association between Sleep Duration and Cognitive Decline.” JAMA Netw Open 3, no. 9 (2020): e2013573. [CrossRef]
- Chen, M. C., H. W. Burley, and I. H. Gotlib. “Reduced Sleep Quality in Healthy Girls at Risk for Depression.” J Sleep Res 21, no. 1 (2012): 68-72. [CrossRef]
- Bin Heyat, M. B., F. Akhtar, A. Sultana, S. Tumrani, B. N. Teelhawod, R. Abbasi, M. Amjad Kamal, A. Y. Muaad, D. Lai, and K. Wu. “Role of Oxidative Stress and Inflammation in Insomnia Sleep Disorder and Cardiovascular Diseases: Herbal Antioxidants and Anti-Inflammatory Coupled with Insomnia Detection Using Machine Learning.” Curr Pharm Des 28, no. 45 (2022): 3618-36. [CrossRef]
- Lei, X., Z. Xu, and W. Chen. “Association of Oxidative Balance Score with Sleep Quality: Nhanes 2007-2014.” J Affect Disord 339 (2023): 435-42. [CrossRef]
- Caritá, A. C., B. Fonseca-Santos, J. D. Shultz, B. Michniak-Kohn, M. Chorilli, and G. R. Leonardi. “Vitamin C: One Compound, Several Uses. Advances for Delivery, Efficiency and Stability.” Nanomedicine 24 (2020): 102117. [CrossRef]
- Carr, A. C., and S. Maggini. “Vitamin C and Immune Function.” Nutrients 9, no. 11 (2017). [CrossRef]
- Buettner, G. R. “The Pecking Order of Free Radicals and Antioxidants: Lipid Peroxidation, Alpha-Tocopherol, and Ascorbate.” Arch Biochem Biophys 300, no. 2 (1993): 535-43. [CrossRef]
- Olayaki, L. A., S. O. Sulaiman, and N. B. Anoba. “Vitamin C Prevents Sleep Deprivation-Induced Elevation in Cortisol and Lipid Peroxidation in the Rat Plasma.” Niger J Physiol Sci 30, no. 1-2 (2015): 5-9.
- Mhaidat, N. M., K. H. Alzoubi, O. F. Khabour, N. H. Tashtoush, S. A. Banihani, and K. K. Abdul-razzak. “Exploring the Effect of Vitamin C on Sleep Deprivation Induced Memory Impairment.” Brain Res Bull 113 (2015): 41-7. [CrossRef]
- Grandner, M. A., N. Jackson, J. R. Gerstner, and K. L. Knutson. “Dietary Nutrients Associated with Short and Long Sleep Duration. Data from a Nationally Representative Sample.” Appetite 64 (2013): 71-80.
- Wang, S., F. Lai, L. Zhao, J. Zhou, D. Kong, H. Yu, and Y. Ding. “Association between Vitamin C in Serum and Trouble Sleeping Based on Nhanes 2017-2018.” Sci Rep 14, no. 1 (2024): 9727. [CrossRef]
- “Icd-10: There’s a Code for That.” Lancet 386, no. 10002 (2015): 1420.
- Sudlow, C., J. Gallacher, N. Allen, V. Beral, P. Burton, J. Danesh, P. Downey, P. Elliott, J. Green, M. Landray, B. Liu, P. Matthews, G. Ong, J. Pell, A. Silman, A. Young, T. Sprosen, T. Peakman, and R. Collins. “Uk Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age.” PLoS Med 12, no. 3 (2015): e1001779. [CrossRef]
- Godos, J., R. Ferri, F. Caraci, F. I. I. Cosentino, S. Castellano, F. Galvano, and G. Grosso. “Adherence to the Mediterranean Diet Is Associated with Better Sleep Quality in Italian Adults.” Nutrients 11, no. 5 (2019). [CrossRef]
- Sun, M., L. Wang, X. Wang, L. Tong, J. Fang, Y. Wang, Y. Yang, and B. Li. “Interaction between Sleep Quality and Dietary Inflammation on Frailty: Nhanes 2005-2008.” Food Funct 14, no. 2 (2023): 1003-10. [CrossRef]
- Zhang, Y., C. Chen, L. Lu, K. L. Knutson, M. R. Carnethon, A. D. Fly, J. Luo, D. M. Haas, J. M. Shikany, and K. Kahe. “Association of Magnesium Intake with Sleep Duration and Sleep Quality: Findings from the Cardia Study.” Sleep 45, no. 4 (2022). [CrossRef]
- Matsuura, N., A. Saito, O. Takahashi, M. Rahman, R. Tajima, H. Mabashi-Asazuma, and K. Iida. “Associations between Nutritional Adequacy and Insomnia Symptoms in Japanese Men and Women Aged 18-69 Years: A Cross-Sectional Study.” Sleep Health 6, no. 2 (2020): 197-204. [CrossRef]
- Sateia, M. J. “International Classification of Sleep Disorders-Third Edition: Highlights and Modifications.” Chest 146, no. 5 (2014): 1387-94. [CrossRef]
- Atrooz, F., and S. Salim. “Sleep Deprivation, Oxidative Stress and Inflammation.” Adv Protein Chem Struct Biol 119 (2020): 309-36. [CrossRef]
- Carr, A. C., and J. Lykkesfeldt. “Factors Affecting the Vitamin C Dose-Concentration Relationship: Implications for Global Vitamin C Dietary Recommendations.” Nutrients 15, no. 7 (2023). [CrossRef]
- Schlüter, N., and P. Groß. “ [Special Aspects of Nutrition in Elderly].” Swiss Dent J 129, no. 11 (2019): 929-36. [CrossRef]
- Saltzman, J. R., and R. M. Russell. “The Aging Gut. Nutritional Issues.” Gastroenterol Clin North Am 27, no. 2 (1998): 309-24. [CrossRef]
- Mortensen, A., and J. Lykkesfeldt. “Does Vitamin C Enhance Nitric Oxide Bioavailability in a Tetrahydrobiopterin-Dependent Manner? In Vitro, in Vivo and Clinical Studies.” Nitric Oxide 36 (2014): 51-7. [CrossRef]
- Padayatty, S. J., J. L. Doppman, R. Chang, Y. Wang, J. Gill, D. A. Papanicolaou, and M. Levine. “Human Adrenal Glands Secrete Vitamin C in Response to Adrenocorticotrophic Hormone.” Am J Clin Nutr 86, no. 1 (2007): 145-9. [CrossRef]
- Frieboes, R. M., H. Murck, P. Maier, T. Schier, F. Holsboer, and A. Steiger. “Growth Hormone-Releasing Peptide-6 Stimulates Sleep, Growth Hormone, Acth and Cortisol Release in Normal Man.” Neuroendocrinology 61, no. 5 (1995): 584-9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).