1. Introduction
Erectile dysfunction (ED) is the inability to attain or maintain an erection sufficient for performing sexual intercourse [
1]. The prevalence worldwide is variable between the different geographic regions, but is high and growing worlwide, reaching 42% in the US and 42.6–48.6% in Europe [
1]. A previous study estimated that the worldwide population affected by ED may reach 322 million people by 2025, meaning an increase of 115% since 1995 [
2]. Up to 50% of patients do not seek treatment, resulting in the infradiagnosis of the disease [
3]. Apart from the medical aspect, this disease has an economic impact, with a loss of productivity estimated to be
$7270 per worker per year in the US [
4].
Several diseases have been related to ED, with COVID-19 being one of the most recent. Since its description [
5], COVID-19 has been known to be an infectious disease that has led to a great variety of complications [
6,
7,
8] in affected individuals, with a strong socioeconomic impact [
9]. Some of them will last for a long time, which has led to the description of a condition known as long COVID [
10]. SARS-CoV-2 vaccination strategies have been implemented to try to change the natural history of the disease, but a number of serious adverse effects have been described [
11]. Since Sansone et al. described a relationship between COVID-19 and ED for the first time [
12], other investigations [
13,
14] reported similar findings. Concerning the duration of ED following COVID-19, a study by Gök et al. showed that ED seems to last for at least one year after COVID-19 recovery [
15].
Nevertheless, to the date no predictive tool has been created to assess the risk of developing post-COVID-19 ED. COVID-19 is a disease with a complex pathogenesis [
5] that has shown an unequal recovery of health-related quality of life after the acute phase [
16]. Therefore, it is important to recognise those patients who are at risk of having long-lasting ED after COVID-19 recovery. The preservation of erectile function is critical because, apart from its sexual aspect, it is a marker of cardiovascular [
12,
17] and general health [
17]. Thus, ED patients could be screened for cardiovascular diseases and eventually be treated in order to avoid the associated complications.
Despite knowing that ED following COVID-19 can persist even 12 months after recovery, no strategies have been designed to perform the early detection of this condition using recovered COVID-19 patients. Our work aimed to develop a risk predictive model for the identification of features involved in the prediction of ED 12 months after COVID-19 recovery. The main contribution of our research is the creation of a clinical tool to determine the key issues that influence most ED at 12 months following COVID-19 recovery.
2. Materials and Methods
2.1. Study Design
We carried out a prospective observational multicentre investigation in the following institutions: Clinic University Hospital of Valladolid, the Río Hortega University Hospital and the Santa Barbara Hospital. This study was approved by the ethics committees of all three institutions: CEIC Área de Salud Valladolid Este (cod: PI-GR-21-2405), CEIC Área de Salud Valladolid Oeste (cod: 21-PI211) and CEIC Área de Salud Burgos y Soria (cod: 2873). We performed the study in accordance with the ethical standards of the Declaration of Helsinki. Patients signed written informed consent prior to being enrolled in the study and were classified according to their history of COVID-19. For the cohort of patients with a past history of COVID-19, we enrolled men aged 40–70 years hospitalised for COVID-19 and discharged from hospital between 1 January 2021 and 31 March 2022. In these patients, hospital admission decisions were based on a COVID-19 diagnosis (established on RT-PCR from nasopharyngeal swab) in addition to clinical or radiological findings [fever resistant to medical treatment; pneumonia diagnosed by X-ray or CT] and followed the institutional protocols concerning COVID-19 management of each of the participant centres at the time of the study. For the cohort of patients without a past history of COVID-19, we enrolled men aged 40–70 years with neither a past history of microbiologic diagnosis of COVID-19 nor a past history of hospital admission in the last 12 months who were recruited in a Urology outpatient consultation between 1 January 2022 and 31 March 2023. Convenience sampling was used due to the limited availability of cases of interest. Exclusion criteria were a past history of rectal, urethral, prostate or penile prosthesis surgery and a past history of ED. We declared those individuals who had not experienced sexual intercourse in the month before the assessment to be non-sexually active, and they were also excluded.
2.2. Outcomes
We assessed ED in both groups using the 5-item International Index of Erectile Function (IIEF-5) [
18], which is a self-reporting instrument for the evaluation of male sexual function using five questions focused on erectile function. Designed for easy use by physicians in the clinical settings [
18], it is a powerful tool as it allows the diagnosis of ED to be stablished [
18], and it has been used in a wide variety of scientific works [
19,
20,
21]. According to the standardised use of the instrument, ED is diagnosed when a score of 5-21 is obtained, while a score of 22-25 dismisses the diagnosis of ED [
18]. We collected data concerning epidemiological variables (age, BMI, highest level of education, occupation, physical activity, civil status, living arrangements, SARS-CoV-2 vaccination), habits (smoking, alcohol, coffee, cannabis), comorbidities (ischemic heart disease, high blood pressure [hypertension], atrial fibrillation, heart failure, stroke, peripheral arterial disease [PAD], diabetes, obstructive sleep apnoea syndrome [OSAS], chronic obstructive pulmonary disease [COPD], asthma, hypothyroidism, chronic kidney failure, chronic active hepatitis, Parkinson disease, cancer, anxiety/depression, lower urinary tract symptoms [LUTS], autoimmune disease) and active treatments (beta blockers, statins, non-thiazide diuretics, spironolactone, corticosteroids, antiplatelet therapy, anticoagulation therapy [acenocumarol or new oral anticoagulants], low-molecular-weight heparin [LMWH], benzodiazepines, antidepressants, antipsychotics, anticholinergics, alpha-1 receptor antagonists or 5-alpha reductase inhibitors). Intensive Care Unit (ICU) transfer data during hospitalisation for the worsening of symptoms was collected for the past history of COVID-19 cohort. We defined smokers as every patient who was an active (regular cigarette smoking for a duration >6 months) or former smoker (no history of smoking for >6 months) at the time of the assessment and quantified this through the use of the pack-year measure. Cannabis consumption was declared for patients that reported having smoked cannabis ≥3 times/week in the month before the assessment. Coffee consumption was defined on a daily intake of at least one cup of regular coffee/day at the time of assessment.
2.3. Data Collection
We designed a questionnaire with questions regarding the outcomes described previously. Patients completed the questionnaire through a telephone interview or filled in it using Google Forms between 1 January 2022 and 31 March 2023.
2.4. Data Analysis
First, we performed a descriptive analysis of the previously described variables. Results were reported as medians (interquartile range) for continuous variables and as frequencies and percentages for categorical variables. Empty records or those filled with null data were eliminated.
2.4.1. Test of Proportions
We calculated the prevalence of ED in each of the cohorts and performed a test of proportions in order to compare the differences in the proportions of ED for both cohorts. The null hypothesis stated that the proportions of positive cases of ED were equal in the two cohorts but the alternative hypothesis stated that they were different. The level of confidence in our
z-hypothesis test was 0.95 and the equation for the test of proportions (
z-test) carried out was as follows:
p = proportion of the sample
x1 = success in the No past history of COVID-19 cohort
x2 = success in the Past history of COVID-19 cohort
n1 = observations in the No past history of COVID-19 cohort
n2 = observations in the Past history of COVID-19 cohort
2.4.2. Regression Model
A logistic regression model for the selection of the variables involved in the prediction of ED was used in our sample, the following methodology was applied (
Figure 1).
ED is a dichotomous variable, with 0 indicating a non-success and 1 denoting a success in a set of Bernoulli experiments following a binomial distribution. Therefore, we opted for a statistical model that fitted the intrinsic properties of this variable: a binary logistic regression model.
At first, we used Pearson’s correlation to eliminate highly correlated variables. The correlation coefficient is a number that expresses the degree of dependence of one set with respect to another. In mathematical terms, we can consider the Pearson’s correlation coefficient to be the normalised covariance; this is denoted by the Greek letter ρ (ro) as shown in the following equation,
where
is the covariance and
are the respective standard deviations of the groups of variables. This value is in the interval [-1,1] and can be interpreted as follows: When ρ=1 the two sets maintain a linear dependence, when ρ=-1 the two sets maintain an inverse linear dependence and when ρ=0 they are considered orthogonal signals. The coefficient threshold used for the dropout process in our study was 0.8.
Binary logistic regression is a statistical technique that aims to test hypotheses or causal relationships when the dependent variable, or variable to be predicted, is a binary variable which has only two categories (success or failure). Although its reading resembles multiple linear regression, which is used when the dependent variable is ordinal or scalar, logistic regression is based on probabilities. In this technique, the independent variables attempt to predict the probability that something will occur over the probability that it will not occur. The logistic regression model can be described as shown in equation Eq.3:
By subtracting the probability
from Eq.2, we obtain the equation Eq.4:
By using this model, we can use an optimisation algorithm to minimise the error between the desired probabilities, adjusting the vector of weights
. Each of the variables is related to an associated weight (
), which determines its importance. These weights are the input for an RFE algorithm, which trains the model recursively until the desired number of variables is obtained. To measure the performance of the model obtained, the standard metrics of Area Under the Curve (AUC) and the Receiver Operator Characteristic (ROC) curve are used. We then performed a two-factor factorial design of experiments, as shown in
Table 1, in order to collect the necessary data.
We used the programming language Python (from Python Software Foundation) version 3.8 and the Sklearn (Version 1.1) package libraries [
22] to perform the descriptive analysis and the regression model.
3. Results
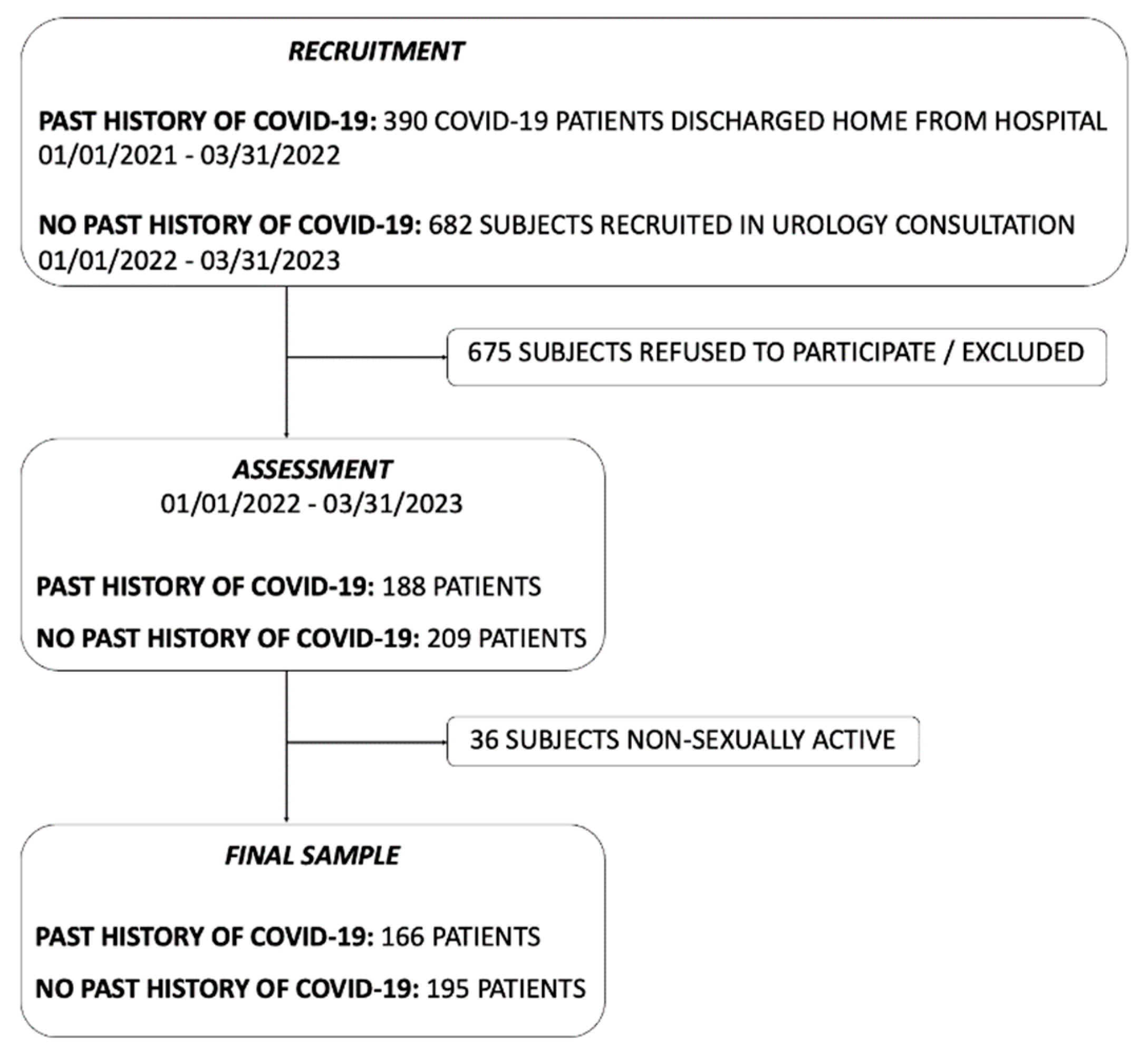
We identified 1072 eligible patients. Following the application of exclusion criteria and the exclusion of non-sexually active individuals, we obtained the final sample for our study (
Figure 2).
3.1. Descriptive Analysis
The individuals with a past history of COVID-19 completed the questionnaire a median of 374 days (interquartile range 20) after discharge from hospital. The median age was 55 years in both cohorts. The descriptive analysis of the most relevant variables is shown in
Table 2. The detailed descriptive analysis of all of the variables investigated is shown in
Table S1 (Supplementary Material).
3.2. Test of Proportions
The prevalence of ED was higher in the COVID-19 group (55.9%) than in the control group (44.1%). The test of proportions carried out (shown in
Table 3) rejected the null hypothesis (
p = 7.4 x 10
-5).
3.3. Regression Model
Following the application of Pearson’s correlation test, we eliminated the following variables: non-thiazide diuretics (correlation coefficient = 0.9), benzodiazepines (correlation coefficient = 0.9) and antipsychotics (correlation coefficient = 0.8).
The best AUC was achieved for the model with 40 variables (AUC = 0.8). The top 15 variables in the model are shown in
Table 4. A complete description of all variables of the model is shown in
Table S2 (Supplementary Material).
4. Discussion
To the best of our knowledge, this is the first time a predictive model has been developed for the prediction of ED at 12 months following COVID-19 recovery. A past history of COVID-19 was shown to be an independent predictor of ED in our sample.
Given that ED is detected in recovered COVID-19 patients, even 12 months after the disease, one question remains unsolved: Can we predict who will be affected by ED? Both COVID-19 and ED are complex diseases whose aetiology might be challenging to analyse. In our study, we opted for a binary logistic regression approach, which allowed us to create a predictive model for ED. The model developed showed adequate performance in our population, with an AUC of 0.8. The use of predictive models in COVID-19 recovered patients could help to identify men who might be at risk of developing ED. The mechanism of post-COVID-19 ED might be multifactorial, as described by Zhang [
14], with the impairment of the endothelial function being one of the described causes [
23,
24]. We know that ED might be the first symptom of a silent cardiovascular disease [
17,
21], and it has been proposed that post-COVID-19 ED patients should undergo a comprehensive cardiovascular assessment [
14]. In this way, the implementation of our predictive model could allow the early detection of these individuals.
A past history of COVID-19 behaved as an independent predictor of ED (weight
1.3). This is a pivotal finding because it demonstrates the relationship between COVID-19 and ED, even 12 months after the disease. The selection of a past history of COVID-19 among the features of our predictive model, which is actually one of the most important, reveals the long-lasting impact and influence of COVID-19 on general health. As such, our findings would take COVID-19 to the level of other well-known determinants of ED [
25]. SARS-CoV-2 vaccination was also one of the features selected in our model. Although a link between SARS-CoV-2 vaccination and long COVID has been described [
26], there are few works that have specifically investigated the link between SARS-CoV-2 vaccination and ED to date. Mehta et al. reported that the SARS-CoV-2 vaccination had no adverse effect on erectile function [
27], but the sample did not include recovered COVID-19 patients. Given the known adverse effects of COVID-19 on ED, it would be interesting to assess how SARS-CoV-2 vaccination interacts with erectile function in recovered patients, who might already have some level of ED. Diaz et al. concluded that erectile function between vaccinated and unvaccinated individuals was not statistically different [
28]. If we look into their work, although recovered COVID-19 patients were enrolled, no specific analysis regarding their relationship with SARS-CoV-2 vaccination and ED was described [
28]. Our findings regarding SARS-CoV-2 vaccination and the prediction of ED are purely clinical and not supported by molecular methods. We could not collect information about the number of vaccination doses or the time from COVID-19 recovery to vaccination, which are interesting facts. Therefore, they should be interpreted cautiously.
We found that the prevalence of ED was higher in the past history of COVID-19 cohort (55.9% vs. 44.1%), while the proportions test carried out demonstrated statistical significance (
p = 7.4 x 10
-5). A relationship between COVID-19 and ED was described for the first time in 2020 [
12] and later in other works [
13,
14]. Moreover, Gök et al. reported an impairment of erectile function outcomes even 12 months after COVID-19 recovery [
15]. Our findings support the results obtained by these authors. We were not able to collect baseline erectile function data prior to COVID-19 due to the design of our study, but we selected a cohort of patients with no past history of COVID-19 in order to balance this. The ED rate in that cohort (44.1%) was similar to the described Spanish ED prevalence rates (43.5%) [
1]. In addition, the individuals with previously diagnosed ED were excluded from the study.
Erectile function is a marker of general health [
12,
17], while ED is considered a strong predictor of cardiovascular events [
21,
29]. Prior to 3 October 2023, there have been more than 600,000,000 known COVID-19 cases worldwide [
30]. Given the high global COVID-19 incidence and the unlikely eradication of the disease in the future [
31,
32], more complications and sequels are expected in the coming months and years. Our predictive model could be decisive in the prediction of ED following COVID-19 in community settings. The application of our predictive tool in other recovered COVID-19 patients would lead to the prompt identification of individuals at risk of developing ED. They could be then subjected to a comprehensive cardiovascular assessment which could avoid the onset of cardiovascular diseases and, consequently, the adverse impact on healthcare systems.
Some limitations must be described: Data of interest were collected through a survey, trusting the facts described by participants. Our predictive model should be externally validated in other populations. Future investigations should include specific analysis about the effect of SARS-CoV-2 vaccination on the erectile function of recovered COVID-19 patients, a statistical analysis to evaluate the influence of the comorbidities of COVID-19 recovered patients on ED and sharing datasets about ED in recovered COVID-19 patients. This would allow the creation of large multicentric databases, leading to the application of predictive tools involved in ED screening. A reduction in the utilisation of health resources and the socioeconomic impact of the disease could then be seen.
5. Conclusions
In conclusion, we developed a regression model for the prediction of ED at 12 months following COVID-19 recovery. The application of our predictive tool in recovered COVID-19 patients in a community setting could eventually lead to avoidance of the adverse effects of ED and the associated unfavourable economic impact. A future line of investigation should include the specific assessment of the effect of SARS-CoV-2 vaccination and other comorbidities on the erectile function of recovered COVID-19 patients.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Descriptive analysis of the characteristics of the patients enrolled in the study; Table S2: Variables included in the predictive model for ED.
Author Contributions
Conceptualization, Fernando Natal Alvarez, Maria Consuelo Conde Redondo and Eduardo Tamayo Gomez; Data curation, Fernando Natal Alvarez, Alfonso Bahillo Martinez and Mario Jojoa; Formal analysis, Alfonso Bahillo Martinez and Mario Jojoa; Funding acquisition, Eduardo Tamayo Gomez; Investigation, Fernando Natal Alvarez, Nicolas Sierrasesumaga Martin, Alejandro Garcia Viña and Carmen Marfil Peña; Methodology, Fernando Natal Alvarez, Alfonso Bahillo Martinez, Mario Jojoa and Eduardo Tamayo Gomez; Project administration, Fernando Natal Alvarez and Eduardo Tamayo Gomez; Resources, Alfonso Bahillo Martinez, Mario Jojoa and Eduardo Tamayo Gomez; Software, Mario Jojoa; Supervision, Eduardo Tamayo Gomez; Validation, Fernando Natal Alvarez and Eduardo Tamayo Gomez; Visualization, Maria Consuelo Conde Redondo, Nicolas Sierrasesumaga Martin, Alejandro Garcia Viña and Carmen Marfil Peña; Writing – original draft, Fernando Natal Alvarez and Mario Jojoa; Writing – review & editing, Alfonso Bahillo Martinez and Eduardo Tamayo Gomez.
Funding
This work was funded by Instituto de Salud Carlos III (grant number PI21/00917, PI18/01238, CIBERINFEC CB21/13/00051), Junta de Castilla y León (grant number GRS 2546/A/22, GRS 2425/A/ 21, GRS 1922/A/19, GRS 2057/A/19), Consejería de Educación de Castilla y León (grant number VA256P20) and Fundación Ramón Areces (grant number CIVP19A5953).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committees of all three institutions: CEIC Área de Salud Valladolid Este (cod: PI-GR-21-2405), CEIC Área de Salud Valladolid Oeste (cod: 21-PI211) and CEIC Área de Salud Burgos y Soria (cod: 2873).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We appreciate the collaboration of the Research Unit of the Clinic University Hospital of Valladolid, Spain.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Goldstein I, Goren A, Li VW, Tang WY, Hassan TA. Epidemiology update of erectile dysfunction in eight countries with high burden. Sex Med Rev. 2020 Jan;8(1):48-58. [CrossRef]
- Aytac IA, Mckinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU International (1999), 84, 50-56. [CrossRef]
- Lin H, Zhao L, Wu H, Cao M, Jiang H. Sexual life and medication taking behaviours in young men: an online survey of 92620 respondents in China. Int J Clin Pract. 2020 Jan;74(1):e13417. [CrossRef]
- Rojanasarot S, Bhattacharyya SK, Burnett AL. Cost of lost productivity due to erectile dysfunction and impact of employer benefit exclusion of penile prosthesis implantation treatment. J Occup Environ Med. 2022 May 1;64(5):403-408. [CrossRef]
- Lamers MM, Haagmans BL. SARS-CoV-2 pathogenesis. Nat Rev Microbiol. 2022 May;20(5):270-284. [CrossRef]
- Qu G, Zhen Q, Wang W, Fan S, Wu Q, Zhang C et al. Health-related quality of life of COVID-19 patients after discharge: A multicentre follow-up study. J Clin Nurs. 2021;30(11-12):1742-1750. [CrossRef]
- Tamayo-Velasco Á, Bombín-Canal C, Cebeira MJ, Sánchez-De Prada L, Miramontes-González JP, Martin-Fernández M et al. Full characterisation of thrombotic events in all hospitalised COVID-19 patients in a Spanish tertiary hospital during the first 18 months of the pandemic. J Clin Med. 2022;11(12):3443. [CrossRef]
- Tamayo-Velasco Á, Martínez-Paz P, Peñarrubia-Ponce MJ, de la Fuente I, Pérez-Gonzalez S, Fernandez I et al. HGF, IL-1α, and IL-27 are robust biomarkers in early severity stratification of COVID-19 patients. J Clin Med. 2021;10(9):2017. [CrossRef]
- Stylianou T, Ntelas K. Impact of COVID-19 pandemic on mental health and socioeconomic aspects in Greece. Int J Environ Res Public Health. 2023 Jan 19;20(3):1843. [CrossRef]
- Yong SJ. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect Dis (Lond). 2021;53(10):737-754. [CrossRef]
- Iba T, Levy JH. Thrombosis and thrombocytopenia in COVID-19 and after COVID-19 vaccination. Trends Cardiovasc Med. 2022 Jul;32(5):249-256. [CrossRef]
- Sansone A, Mollaioli D, Ciocca G, Colonnello E, Limoncin E, Balercia G et al. “Mask up to keep it up”: Preliminary evidence of the association between erectile dysfunction and COVID-19. Andrology. 2021;9(4):1053-1059. [CrossRef]
- Hebert KJ, Matta R, Horns JJ, Paudel N, Das R, McCormick BJ et al. Prior COVID-19 infection associated with increased risk of newly diagnosed erectile dysfunction. Int J Impot Res. 2023 Mar 15:1-5. [CrossRef]
- Zhang J, Shi W, Zou M, Zeng Q, Feng Y, Luo Z et al. Prevalence and risk factors of erectile dysfunction in COVID-19 patients: A systematic review and meta-analysis. J Endocrinol Invest. 2023;46:795-804. [CrossRef]
- Gök A, Altan M, Dogan AE, Eraslan A, Uysal FS, Öztürk U et al. Does post-COVID-19 erectile dysfunction improve over time? J Clin Med. 2023;12(3):1241. [CrossRef]
- Maestre-Muñiz MM, Arias A, Mata-Vázquez E, Martin-Toledano M, Lopez-Larramona G, Ruiz-Chicote AM, et al. Long-term outcomes of patients with Coronavirus Disease 2019 at one year after hospital discharge. J Clin Med. 2021;10(13):2945. [CrossRef]
- Jannini EA. SM = SM: The interface of systems medicine and sexual medicine for facing non-communicable diseases in a gender-dependent manner. Sex Med Rev. 2017 Jul;5(3):349-364. [CrossRef]
- Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999; 11(6):319-326. [CrossRef]
- Zhang K, Stricker P, Löhr M, Stehling M, Suberville M, Cussenot O, et al. A multi-center international study to evaluate the safety, functional and oncological outcomes of irreversible electroporation for the ablation of prostate cancer. Prostate Cancer Prostatic Dis. 2024 Jan 9;27(3):525–30. [CrossRef]
- Kim JK, Lee YJ, Kim H, Song SH, Jeong SJ, Byun SS. A prospectively collected observational study of pelvic floor muscle strength and erectile function using a novel personalized extracorporeal perineometer. Sci Rep. 2021 Sep 15;11(1):18389. [CrossRef]
- Inman BA, St. Sauver JL, Jacobson DJ, Mcgree ME, Nehra A, Lieber MM, et al. A Population-Based, Longitudinal Study of Erectile Dysfunction and Future Coronary Artery Disease. Mayo Clin Proc. 2009 Feb;84(2):108-13. [CrossRef]
- Hao J, Ho TK. Machine learning made easy: A review of scikit-learn package in python programming language. J Educ Behav Stat. 2019;44(3): 348-361. [CrossRef]
- Kresch E, Achua J, Saltzman R, Khodamoradi K, Arora H, Ibrahim E, et al. COVID-19 Endothelial Dysfunction Can Cause Erectile Dysfunction: Histopathological, Immunohistochemical, and Ultrastructural Study of the Human Penis. World J Mens Health. 2021 Jul 1;39(3):466–9. [CrossRef]
- Sansone A, Mollaioli D, Ciocca G, Limoncin E, Colonnello E, Vena W, et al. Addressing male sexual and reproductive health in the wake of COVID-19 outbreak. J Endocrinol Invest. 2021 Feb 1;44(2):223–31. [CrossRef]
- McCabe MP, Sharlip ID, Lewis R, Atalla E, Balon R, Fisher AD et al. Risk factors for sexual dysfunction among women and men: A consensus statement form the Fourth International Consultation on Sexual Medicine 2015. J Sex Med. 2016;13(2):153-167. [CrossRef]
- Romero-Rodríguez E, Pérula-de Torres LÁ, Castro-Jiménez R, Gonzalez-Lama J, Jimenez-Garcia C, Gonzalez-Bernal JJ et al. Hospital admission and vaccination as predictive factors of long COVID-19 symptoms. Front Med (Lausanne). 2022 Nov 11:9:1016013. [CrossRef]
- Mehta P, Chakraborty A, Andrabi SW, Sharma B, Kumar R, Bhaskar LVKS et al. COVID-19 vaccination does not affect male sexual functions. Reprod Biol Endocrinol. 2023; 21(1):3. [CrossRef]
- Diaz P, Zizzo J, Blachman-Braun R, Gandhi DA, Reddy R, Zucker IJ et al. COVID-19 vaccination not associated with increased risk of erectile dysfunction. Andrologia. 2022; 54(10):e14563. [CrossRef]
- Yannas D, Frizza F, Vignozzi L, Corona G, Maggi M, Rastrelli G. Erectile dysfunction is a hallmark of cardiovascular disease: Unavoidable matter of fact or opportunity to improve men's health? J Clin Med. 2021;10(10):2221. [CrossRef]
- COVID-19 Map - Johns Hopkins Coronavirus Resource Centre [Internet]. Available online: https://coronavirus.jhu.edu/map.html (accessed on 21 May 2024).
- Ghasemiyeh P, Mohammadi-Samani S. Lessons we learned during the past four challenging years in the COVID-19 era: pharmacotherapy, long COVID complications, and vaccine development. Virol J. 2024 Apr 26;21(1):98. [CrossRef]
- Hu X, Hu Z, Xu T, Zhang K, Lu HH, Zhao J et al. Equilibrium points and their stability of COVID-19 in US. Sci Rep. 2024; 14:1628. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).