1. Introduction
Probiotic application is widespread in humans and animals for their anti-inflammatory and immunomodulatory response, as well as improved gut health [
1,
2,
3]. The definition of probiotic in the root sense means “for life,” and the critical expectation is that live and active cultures should be delivered in substantial amounts to observe health benefits [
4]. Probiotics are Gram-positive bacilli or cocci with oxygen requirements ranging from obligate to facultative anaerobes. Some genera, such as
Bacillus, are spore formers. Lactic acid bacteria (LAB) are non-spore formers, and some are probiotics and predominant residents of the gut [
5]. Probiotics have desirable attributes that benefit the host, such as producing short-chain fatty acids (SCFAs), immunomodulation, surviving acidic environments, pathogen exclusion, and influencing the microbiome composition [
6,
7]. Probiotics are now used as an additive to kibble and wet foods for companion animal gut health [
2,
8]. Their beneficial effects are either transient or ineffective [
2,
9] primarily due to their inability to colonize the inflamed bowel [
10,
11,
12,
13].
In a mammalian dysbiotic gut, pockets of inflammation and dysregulated epithelial cell tight junction permeability are evident [
14]. The inflamed state of the bowel propels the reconstruction of the enteric flora to that of a negative state, termed dysbiosis [
15]. Canine gut dysbiosis is marked by pockets of inflammation and an imbalance in the standard phyla of bacteria
Firmicutes,
Fusobacteria, Bacteroidetes, Proteobacteria, and
Actinobacteria [
16,
17]. Their metabolic byproducts, such as SCFAs, reduce intestinal inflammation and regulate the commensal community [
17].
Canines are exposed to numerous pathogenic and environmental insults that make them vulnerable to illness. Dog food has been linked to multiple outbreaks associated with
Salmonella enterica,
Listeria monocytogenes,
Escherichia coli, and other pathogens [
18,
19,
20]. Using probiotic bacteria with their beneficial properties as a canine food additive is one of the attractive methods of reducing pathogen exposure, as well as antibiotic use and promoting gut health [
13].
Probiotic bacteria have been used in human food or animal diets to prevent pathogen colonization and gut health through competitive exclusion [
21,
22,
23]. Probiotic
Bacillus lipopeptides have been used to eliminate
S. aureus colonization within a rural Thai population [
24,
25].
Lacticaseibacillus rhamnosus was shown to produce acetic acid and surfactants that reduce biofilm formation by
Acinetobacter baumannii [
26].
Lacticaseibacillus rhamnosus GG was also shown to reduce
Salmonella enterica Typhimurium colonization in a heat-stress cell culture model [
27]. Probiotics have also been used to sequester mycotoxin from feed [
28,
29,
30].
The addition of probiotics as a canine food additive has gained notoriety for promoting the overall health of the companion animal [
2]. Clinical trials for probiotics in pet food have been carried out, and several products on the market contain stable probiotics that may promote pathogen clearance and improve gut health while promoting overall animal fitness [
31].
Natural probiotics may have limited capabilities of colonizing the inflamed-damaged tissue; therefore, recombinant bioengineering has been used as a novel strategy to enhance probiotic epithelial adhesion, biofilm formation, immunomodulation, and pathogen colonization resistance [
10,
11,
32,
33,
34,
35]. Nevertheless, genetically engineered and native probiotics possess the same inherent properties for pathogen exclusion, such as nutrient partitioning, biofilm formation, and clusters of abundance within the gastrointestinal (GI) tract. This is an intrinsic property of the gut flora [
36].
Biofilm formation by pathogenic or beneficial bacteria aids in their adhesion and colonization to biotic and abiotic surfaces [
37,
38,
39]. Since probiotics are natural gut inhabitants, they can form a biofilm on the mucosal surface, often in mixed communities, which is a vital and desirable attribute [
39]. During biofilm formation, bacteria communicate via the messaging system termed quorum sensing [
39,
40,
41]. Probiotic biofilms promote gastrointestinal healing, community structure, and immunomodulatory response [
42]. Bacteria produce exopolysaccharides, proteins, and extracellular DNA to aid in biofilm formation [
43]. Probiotic-based biofilms are often localized in specific pockets within the GI tract. They can competitively inhibit pathogen colonization, such as seen with the
Listeria monocytogenes challenge in a mouse model [
10]. Furthermore, biosurfactants from
Lactobacillus jensenii and
Lacticaseibacillus rhamnosus have shown antimicrobial and antibiofilm formation properties against clinically relevant strains of
Acinetobacter baumannii,
Escherichia coli and
Staphylococcus aureus (MRSA) [
44]. A cocktail of
Lactobacillus fermentum and
Lactiplantibacillus plantarum could also reduce
S. aureus (MRSA) biofilm production [
45].
Therefore, this study evaluated the antimicrobial activities, epithelial cell adhesion, biofilm formation, and anti-inflammatory response of a canine probiotic blend (LabMAX-3). Madin-Darby Canine Kidney (MDCK) cell line was used as a gut epithelial cell model. MDCK cells are derived from the renal tubule of the canine kidney and display enterocyte-like absorbing properties [
46,
47]. Our data show that the canine kibble probiotic additive with biofilm-forming capabilities, robust antimicrobial activities, and anti-inflammatory response can potentially improve canine gastrointestinal health.
2. Materials and Methods
2.1. Probiotic Preparation
A commercial proprietary probiotic blend, LabMAX-3, containing three probiotic strains of
Enterococcus faecium,
Lactobacillus acidophilus, and
Lacticaseibacillus casei, was received from CH2 Animal Solutions (Ottumwa, IA, USA). The lyophilized powder was plated on DeMann Rogosa Sharpe (MRS, Thermo Fisher Scientific, Walthan, MA, USA) agar plates and incubated at 37°C for 48 h under anaerobic conditions using a Gaspak (Thermo Fisher Scientific). The colonies were picked based on phenotype, and each bacterial culture morphology was verified by microscopy (Leica, Deerfield, IL, USA).
L. acidophilus NRRL 31910,
Pediococcus acidilactici H and
E. faecium ATCC 8459, as controls, were propagated in MRS under anaerobic conditions.
Lacticaseibacillus casei ATCC 334 was cultivated in modified MRS agar (MMRS) containing 1% w/v proteose peptone, 0.5% w/v yeast extract, 0.1% v/v Tween 80, 0.2% w/v meat extract, 37 mM C
2H
3NaO
2, 8.8 mM C
6H
14N
2O
7 dissolved in 0.2 M potassium phosphate (dibasic, pH 7.0), 0.8 mM MgSO
4, 0.24 mM MnSO
4 and 1% w/v mannitol and supplemented with erythromycin (2 µg/mL, Thermo Fisher Scientific) and grown in anaerobic conditions [
10].
Table 1.
Bacterial cultures used in the study.
Table 1.
Bacterial cultures used in the study.
| Bacteria |
Identification |
Source |
Provided By |
| Enterotoxigenic Escherichia coli
|
F4 (K88) |
Swine rectal isolate |
Paul Ebner/Animal Sciences
Purdue University |
| Enterotoxigenic Escherichia coli
|
O78:H11 |
Human fecal isolate |
ATCC 35401 |
| Listeria monocytogenes |
F4244 |
Clinical isolate, Human Central Nervous System (CNS) isolate |
CDC, Our lab collection |
| Campylobacter jejuni |
ATCC 29428 |
Human fecal isolate |
ATCC |
|
Salmonella enterica serovar Typhimurium |
ST-1 |
|
Our collection |
| Lactobacillus acidophilus |
LabMAX-3 |
CH2 Animal Solutions |
CH2 Animal Solutions |
| Lacticaseibacillus casei |
LabMAX-3 |
CH2 Animal Solutions |
CH2 Animal Solutions |
| Enterococcus faecium |
LabMAX-3 |
CH2 Animal Solutions |
CH2 Animal Solutions |
| Lactobacillus acidophilus |
NRRL 31910 |
ATCC |
Our lab collection |
| Lacticaseibacillus casei |
ATCC 334 |
Cheese |
Our collection |
| Enterococcus faecium |
ATCC 8459 |
Cheese |
Dharmendra Mishra, Purdue University |
| Pediococcus acidilactici |
H |
Fermented sausage |
Our Lab collection [48] |
| Staphylococcus aureus |
ATCC 25923 (Rosenbach) |
Clinical Isolate |
Our Lab collection |
2.2. PCR Confirmation of Probiotic Strains
Probiotic culture isolates in LabMAX-3 were confirmed by PCR amplification of species-specific target genes listed in
Table 2. Target gene amplification was performed using PCR Master Mix (Platinum™ Hot Start, Thermo Fisher Cat# 130000). Equal volume of template (2 µL of resuspended colony) was added, and DNA was amplified via a thermocycler (Applied Biosystems, Carlsbad, CA, USA). Thermocycling conditions are as follows: 94°C for 10 min, 30 cycles at 94°C at 30s per cycle, 30 s at 60°C, 72°C at 30s and 5 min at 72°C. PCR products were analyzed by agarose gel electrophoresis (2%) and DNA bands were visualized under UV exposure using a Kodak EDAS 290 camera with ID LE 3.6.3 software.
2.3. Pathogen Propagation
Cultures of enterotoxigenic
Escherichia. coli (ETEC) strains F4 and O78:H11,
Staphylococcus aureus ATCC 25923
Salmonella enterica serovar Typhimurium ST1, and
Listeria monocytogenes F4244 from our culture collection (
Table 1) were cultivated in Tryptic Soy Broth supplemented with 0.6% yeast extract (TSBYE) at 37°C for 18-24 h.
Campylobacter jejuni ATCC 29428 was cultivated in microaerophilic conditions (5% CO
2, 37°C, 7 days) in Bolton Broth (Neogen, Lansing, MI, USA).
2.4. Antimicrobial Activity Testing on Agar Plates
Antimicrobial activity of individual probiotic cultures,
Enterococcus faecium,
Lactobacillus acidophilus, and
Lacticaseibacillus casei, mixture (1:1:1) of three designated, LabMAX-3,
P. acidilactici H and
L. casei ATCC334 was tested against pathogens (
Table 1) as before [
48]. Briefly, overnight-grown (37°C for 18 h) cultures of probiotic strains were inoculated (1 µL, stab-method) on a base layer of MRS agar and incubated for 18-20 h at 37°C, anaerobically. Pathogens were grown at 37°C for 24 h in TSBYE aerobically. Tryptic Soy Agar supplemented with 0.6% yeast extract (TSAYE) soft top agar (0.8% agar) was prepared, and 10 µL of the respective pathogens were inoculated and vortexed. The inoculated soft agar (5 mL) was then poured onto the base layer, swirled, air-dried, and incubated at 37°C for 24 h. The zones of inhibition around the stab culture were measured.
For the preparation of cell-free supernatants, probiotic bacterial culture (18-20 h grown as above) supernatants were collected after centrifugation (14,000x, 5 min), and passed through 0.45 µm membrane filters. A 20 µl aliquot was tested on a sterile blotting paper disc against the test pathogens that were overlaid on TSA agar plates [
48].
In separate experiments, each 18-20-h grown probiotic cultures were heat-treated (80°C for 10 min) and 10 µl of each culture was placed on the BHI agar surface and overlaid with the test pathogens as above [
48].
2.5. Biofilm Formation by Probiotics
Biofilm formation by probiotic blend LabMAX-3 was analyzed in multi-well polystyrene plates as before [
51]. Briefly, anaerobically grown probiotic bacteria were suspended in 1:1 ratio of MRS and MMRS and dispensed into 96-well tissue culture plates at 1.0 x 10
8/well (TPP, Switzerland) and incubated at 37°C for 24, 48, and 72 h anaerobically.
Lacticaseibacillus casei ATCC 334 and
Staphylococcus aureus were used as positive controls and grown in MRS and TSBYE, respectively, and inoculated at 1 x 10
8/well in 1:1 ratio of MRS: MMRS or MRS:TSB, respectively and incubated at 37°C for 24, 48, 72 h aerobically. Every 24 h interval, the old media was aseptically replaced with fresh media. Biofilms were washed three times in 0.2 mM phosphate buffered saline, pH 7.2 (PBS) to remove planktonic bacteria. Bacterial counts in the biofilm were enumerated after scraping the biofilms from the well, collecting the cells in an Eppendorf tube, sonication for 20 min using iSonic (Chicago, IL, USA), and serially diluted for plating on MRS or TSAYE agar plates [
38].
The formation of biofilms was also assayed by crystal violet staining. Briefly, washed biofilms were stained with 1% crystal violet (Sigma), washed three times in PBS, air-dried for 15 min at room temperature (~25°C, RT), treated with 95% ethanol, and the absorbance (OD590
nm) of supernatant was measured [
51].
In a separate experiment, bacterial biofilms were allowed to form in multiwall chambered glass slides (LabTek II, Cat# 154534, Thermo Fisher) that were UV-pretreated for 45 min. Wells were washed 3x in PBS, air-dried for 15 min at RT, heat fixed, and subjected to Gram staining using crystal violet without the counter staining and visualized under Leica Microscope (Deerfield, IL, USA) with 1000x magnification.
2.6. MDCK Cell Line Preparation
The Madin-Darby Canine Kidney (MDCK, NBL-2, CCL-34, ATCC, Manasas, VA, USA) cell line was cultivated in Dulbecco’s Modified Eagles Medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% Fetal Bovine Serum (FBS, Gibco), termed D10F. The canine cell line was grown at 37°C at 5% CO2, passaged from 4-15, and propagated in T-75 flasks (TPP, Switzerland), till 75% confluence. The cells were treated with Trypsin-EDTA (0.25%) (Gibco), centrifuged (800xg for 2.5 min), and resuspended in D10F. The MDCK cells were counted by 0.4% Trypan blue (Gibco) staining and seeded at densities of 104/well.
2.7. Treatment of MDCK with Lipopolysaccharide
Lipopolysaccharide (LPS, 1 mg/mL, Sigma, St Louis, MO, USA) was reconstituted in sterile deionized (d) H
2O and aliquoted for one-time usage. MDCK cells were treated with LPS at 1 µg/mL in D10F for 24 h at 37°C and 5% CO
2 to induce modest inflammation [
47]. The monolayers were washed three times in DMEM to remove the LPS present and treated with probiotics. Control wells (no LPS), received equivalent amounts of dH
2O to serve as a vehicle control.
2.8. Probiotic Adhesion to MDCK Cell Line by Plate Counting
MDCK cells were seeded at 1 x 104 cells/ml/well and cultured for 9 days to allow monolayer formation. Cell monolayers were washed three times in DMEM and treated without (no LPS groups) or with freshly prepared D10F containing 1 µg/mL LPS and incubated for 24 h (37°C, 5% CO2). Monolayers were then washed three times in DMEM to remove residual LPS, and cells were examined for monolayer integrity by microscope.
Probiotics were grown anaerobically for 24 h in MRS or MMRS as before and added to MDCK cell monolayers with a multiplicity of exposure (MOE) of 1000 and incubated at 37°C with 5% CO2 under humidified conditions for 24 h. Supernatants were collected for lactate dehydrogenase (LDH) and cytokine profiles (see below). MDCK cell monolayers were washed three times in DMEM to remove unattached bacteria. Cell monolayers were detached using Triton-X 100 (Sigma) treatment (0.1% for 5 min), vortexed, serially diluted, plated, and incubated at 37°C for 48 h, anaerobically. Colony counts were plotted to determine bacterial adhesion.
2.9. Adhesion and Biofilm Analysis by Giemsa Staining
MDCK cells were seeded (1x 104 cells/ml/chambered well) in UV-pretreated (45 min) cassettes (LabTek II, Cat# 154534, Thermo Fisher) and incubated at 37°C at 5% CO2 for 9 days and inoculated with bacteria at MOI, 1000 as above. The cell monolayers were fixed with 100% methanol for 10 min, air-dried, and stained with Giemsa stain (1:20 dilution in dH2O and methanol and stained for 45 min, air-dried, and visualized under the Leica microscope.
2.10. Lactate Dehydrogenase Assay
Lactate dehydrogenase (LDH) release was from MDCK cells and percent cytotoxicity was calculated as per the manufacturer’s instructions (Cayman Chemicals, Ann Arbor, MI, USA) [
10,
52].
2.11. Cytokine ELISA
Canine cytokines IL-10, TGFβ and TNFα was purchased from R&D Systems (Minneapolis, MN, USA), and cytokine levels in MDCK culture supernatants were quantified following the manufacturer's instruction.
2.12. Data Analysis
Data were analyzed with GraphPad Prism (La Jolla, CA, USA) with unpaired Mann-Whitney tests. All data sets are represented of at least three experimental/biological replicates. Data are presented with standard error of the mean (±) or box-whisker plots with an interquartile range.
3. Results and Discussion
3.1. Antimicrobial Activity of Probiotics Against Pathogens
Probiotic microbes have long been acknowledged to possess antimicrobial properties against human and animal pathogens [
1,
53,
54]. LabMAX-3, used in this study, contained three probiotic cultures of
Lactobacillus acidophilus,
Lacticaseibacillus casei, and
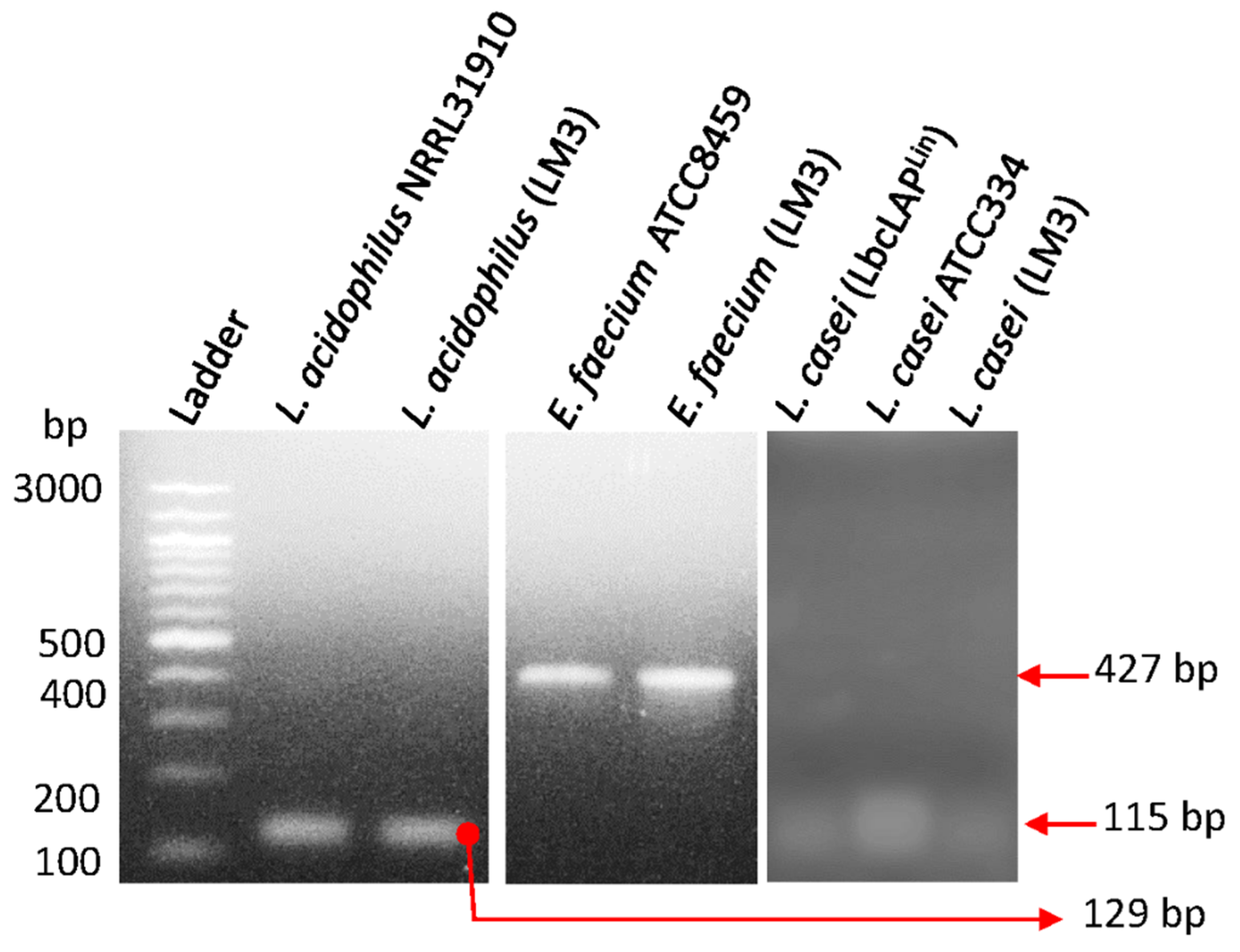
Enterococcus faecium. The commercial LabMAX-3 lyophilized powder blend could not be directly used in our proposed experiments due to the presence of some insoluble materials (part of formulations), which significantly interfered with our experiments (data not shown). Thus, before the experiment, we isolated each culture from the blend and mixed them at a 1:1:1 ratio, designated LabMAX-3. The identity of each culture was verified by PCR targeting 16S rRNA gene (
Figure 1), and by light microscopy (
Figure S1) to be
Lactobacillus acidophilus,
Lacticaseibacillus casei, and
Enterococcus faecium.
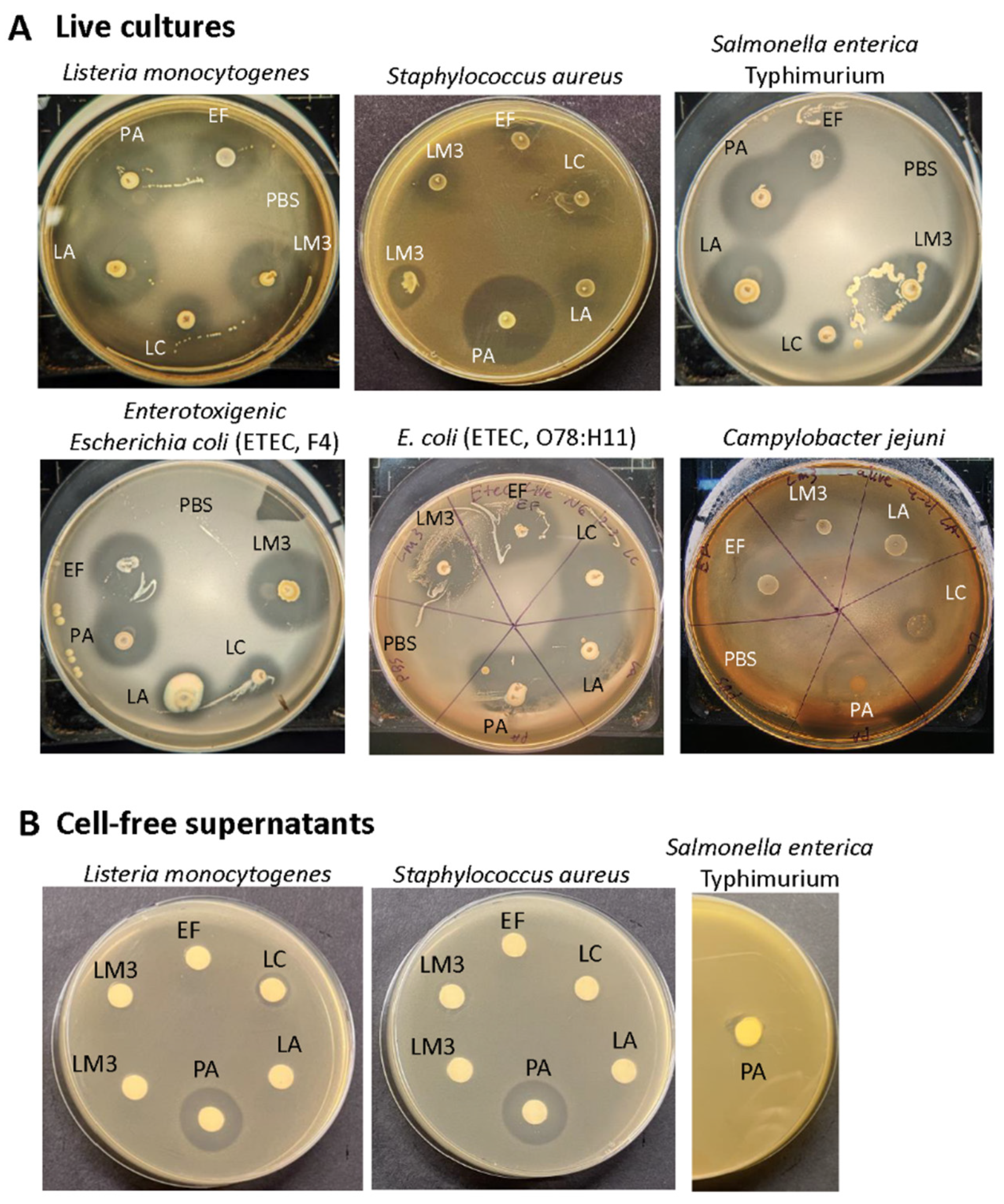
Antimicrobial activity of each live probiotic culture and the LabMAX-3 showed strong inhibitory zones against both Gram-negative
Salmonella enterica serovar Typhimurium, enterotoxigenic
Escherichia coli (ETEC) strains F4 and O78:H11, and
Campylobacter jejuni ATCC 29428 and Gram-positive,
L. monocytogenes (
Figure 2,
Table 3). The zone of inhibition produced by LabMAX-3 was comparable to zones produced by
L. acidophilus,
E. faecium, and
P. acidilactici.
L. casei showed the weakest antimicrobial response among the lactic acid bacterial cultures tested (
Table 3). Surprisingly, neither heat-killed probiotic bacteria nor cell-free culture supernatants of the probiotic bacteria showed any growth inhibition of test pathogens except for heat-killed
L. acidophilus cells, which showed a faint zone of inhibition against
Listeria,
Salmonella, and ETEC (
Figure S1). On the other hand, cell supernatant from
P. acidilactici, a known bacteriocin producer [
48] inhibited
L. monocytogenes and
S. aureus but not
Salmonella (
Figure 2) since bacteriocin (Pediocin AcH) produced by
P. acidilactici is effective only against Gram-positive bacteria. These results indicate that live probiotic bacteria produce inhibitory compounds that are effective against the Gram-positive and Gram-negative pathogens tested. Since heat treatment or cell-free culture supernatants of LabMAX-3 did not show an effect suggesting, interaction with live cultures is essential for suppressing pathogens' growth (
Figure S1).
Probiotic bacteria inhibit pathogens by competing with colonization sites, modulating microbial communities, and inhibiting pathogen growth by producing acids, hydrogen peroxide, bacteriocins, and other inhibitors [
1,
54]. Since cell-free culture supernatants from LabMAX-3 showed no inhibition, compared to
P. acidilactici supernatant, bacteriocin may not significantly contribute to the inhibitory effects. Previous studies have demonstrated that probiotic supplementation can reduce hemorrhagic diarrhea and inflammatory bowel disease-like symptoms in dogs [
2,
55,
56]. Panja et al. [
57] reported that feeding a probiotic-supplemented diet to dogs increased creatinine levels in the serum. Likewise, a probiotic blend of
Lacticaseibacillus casei Zhang,
Lactiplantibacillus plantarum P-8 and
Bifidobacterium animalis improved cytokine profiles and serum immunoglobulins and altered microbial community. Microbiota profile showed increased
Lactobacillus spp. and
Faecalibacterium prausnitzii presence and decreased
Sutterella stercoricanisn and
Escherichia coli in the feces [
58].
Lacticaseibacillus rhamnosus GG (LGG) was also reduced
Escherichia coli (EHEC) O157:H7-mediated attachment-effacement lesion and improved tight junction integrity by redistributing claudin-1 and Zo-1 on MDCK cell line [
59].
3.2. LabMAX-3 Forms Biofilm on Abiotic Surface
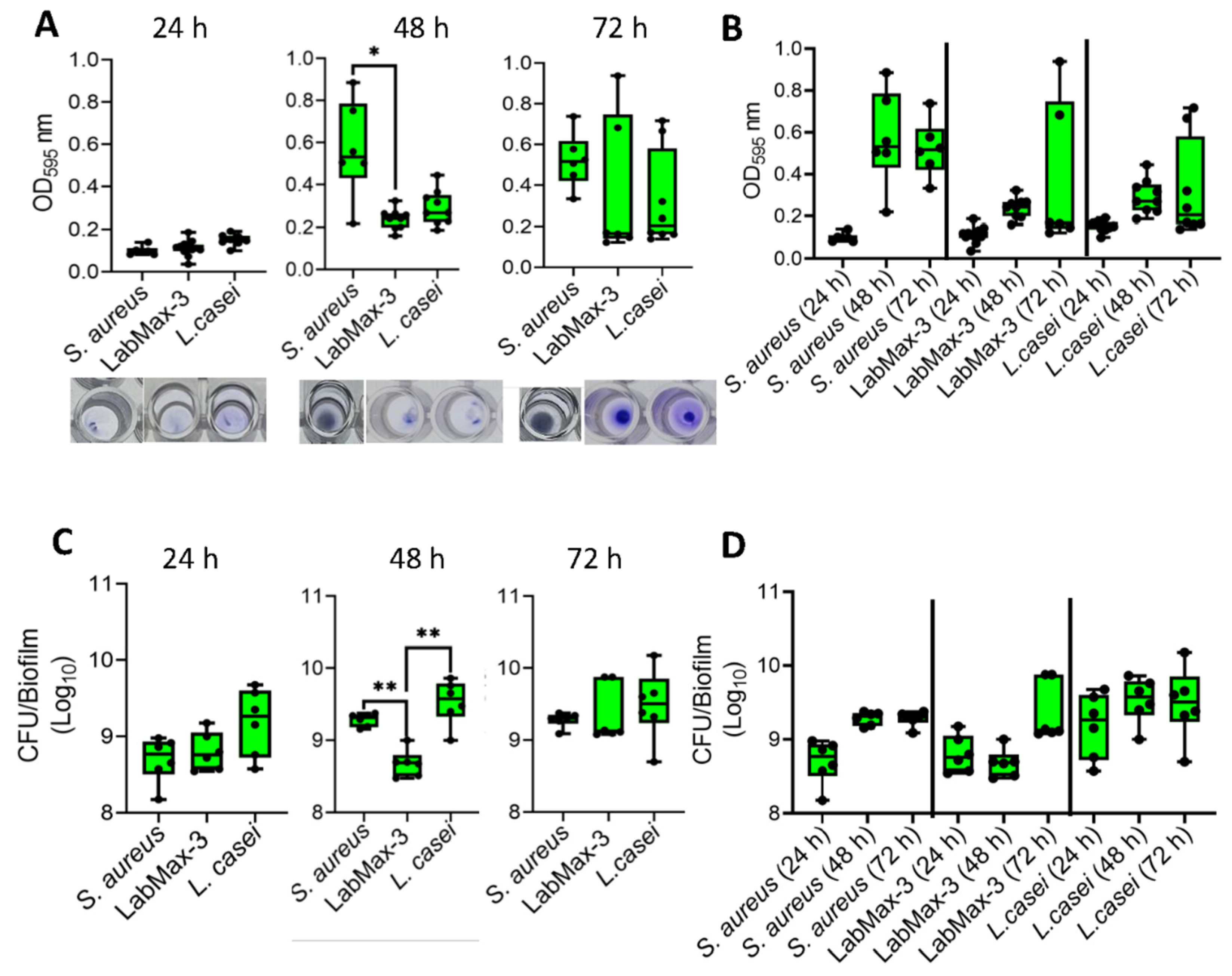
Analysis of biofilm formation by probiotic blend LabMAX-3 on polystyrene 96-well microtiter plate as measured by crystal violet staining showed a gradual increase in cell mass over 72 h (
Figure 3). LabMAX-3-mediated biofilm formation was compared with
S. aureus and
L. casei ATCC334. At 48 h, biofilm produced by
S. aureus was significantly higher than LabMAX-3 (
p < 0.05), while at 72 h, there were no statistical differences among the three cultures. Further analysis shows LabMAX-3 and
L. casei ATCC334 biofilm-mass grew over 72 h and followed similar growth kinetics (
Figure 3AB).
Biofilm formation was also quantified by counting cells in the biofilm. LabMAX-3 bacterial counts gradually increased from 24 h to 72 h, showing about 1 log increase (
Figure 3CD).
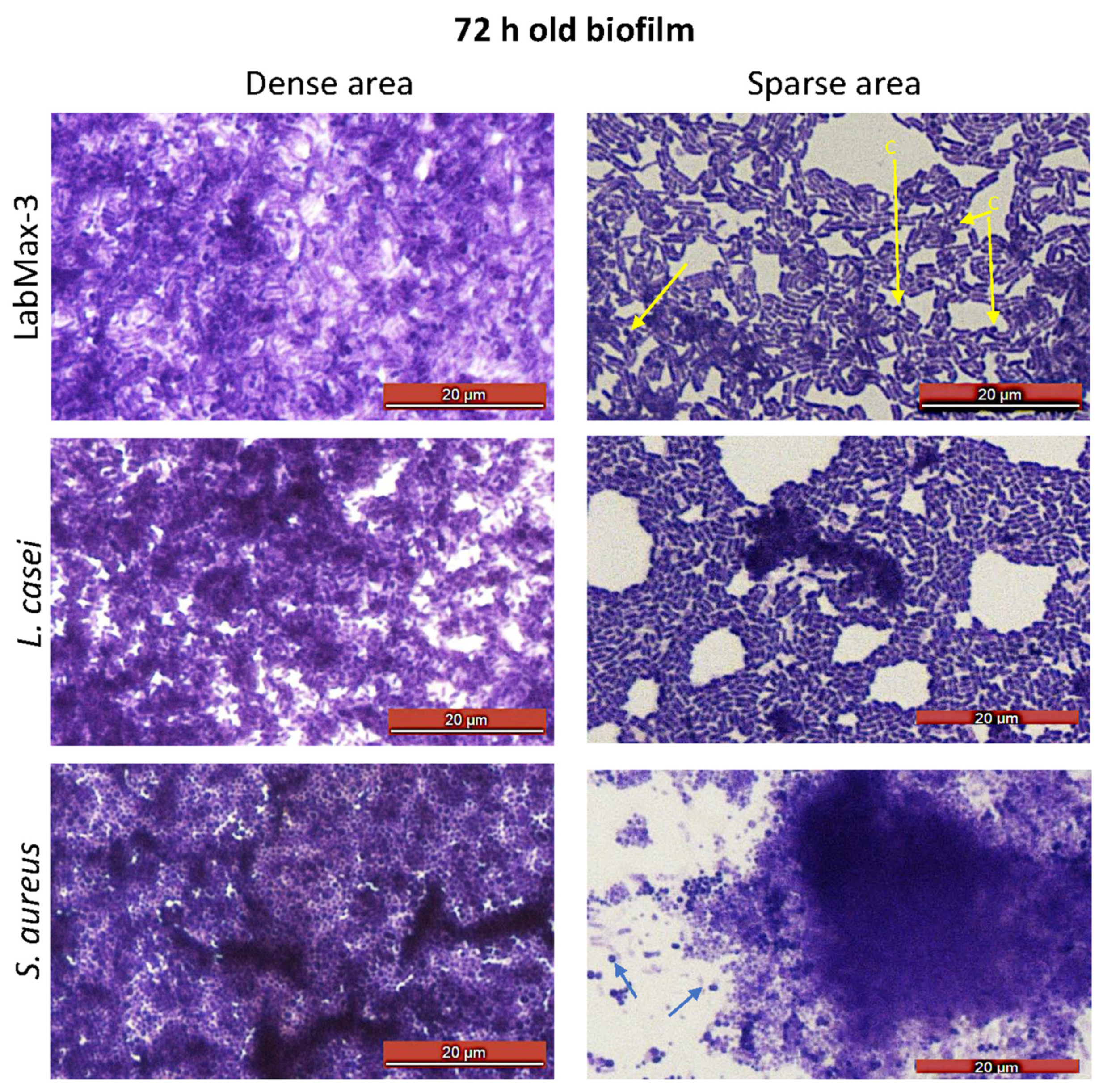
Light microscopic analysis of crystal violet-stained biofilms on glass slides shows spatial bacterial arrangement within the biofilms (
Figure 4). LabMAX-3 forms multilayered biofilms on glass surfaces with dense and sparse areas. Close examination of biofilm architecture in the sparsed area (
Figure 4, right panel) revealed the dominance of rod-shaped cells (lactobacilli) with a few embedded coccoid cells (
E. faceum). Furthermore, the LabMAX-3-mediated biofilm architecture appeared very similar to that of
L. casei ATCC334. In contrast,
S. aureus formed multilayered dense biofilms, and only a few dispersed individual cells (cocci) are visible near the dense cell mass of the biofilm (right panel). These data indicate that a probiotic LabMAX-3 blend produces multilayered mixed culture biofilms with dense and light areas comparable to biofilms produced by
L. casei ATCC334 and
S. aureus.
Probiotic biofilm formation is critical for ensuring colonization in the gut [
60]. Probiotics in the LabMAX-3 formed robust biofilms in 72 h on the abiotic surfaces, comparable to
L. casei and
S. aureus. These data indicate that
L. casei in the LabMAX-3 mix would likely contribute to biofilm cell mass by producing glycocalyx [
61]. Similarly, the contribution of
L. acidophilus and
E. faecium cannot be ruled out, akin to previous reports [
62,
63].
3.3. LabMAX-3 Probiotic Blend Adhesion and Biofilm Formation on MDCK Cells
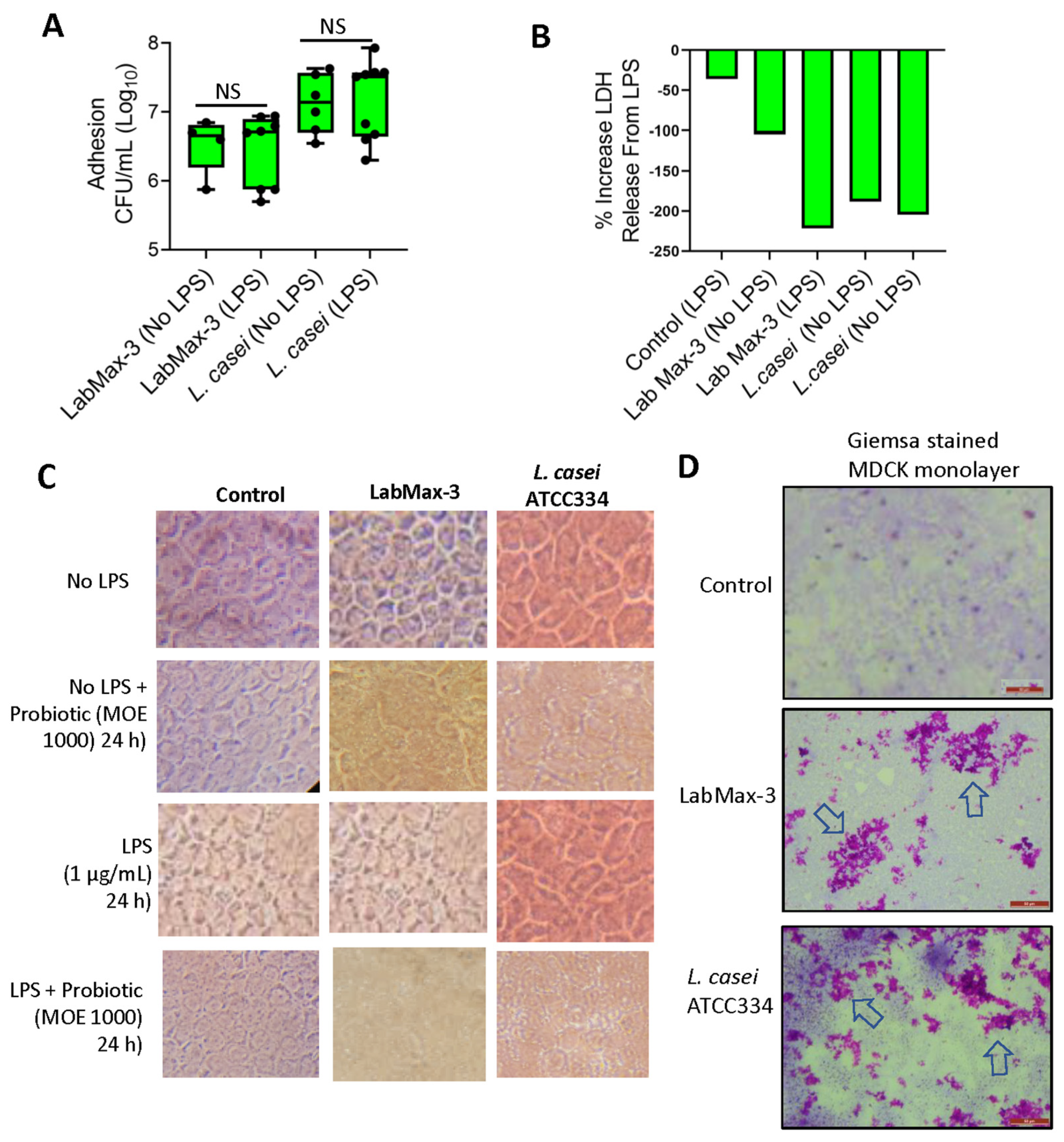
We also examined the adhesion and biofilm-forming characteristics of LabMAX-3 to MDCK cell monolayers pretreated with or without LPS. LPS was used to simulate inflamed conditions [
47]. LabMAX-3 adhesion to control MDCK cells (without LPS pre-treatment) was calculated to be about 6.7 log CFU/ml, and similar counts (~6.8 log CFU/ml) to MDCK was observed when MDCK cells were pre-treated with LPS (
Figure 5). In contrast,
L casei showed slightly higher adhesion (7.1 log CFU/ml) to MDCK cells with or without LPS treatment.
LDH release from MDCK cells during LabMAX-3 adhesion was analyzed, and values were below zero, indicating that LabMAX-3 treatment did not cause cytotoxicity (
Figure 5B). Further microscopic analysis of cell monolayers did not reveal any visible cell damage, and the monolayer integrity remained intact during LabMAX-3 exposure (
Figure 5C). These data indicate that the probiotic blend LabMAX-3 efficiently adheres to MDCK cells without causing any cell damage, and the adhesion is comparable to
L. casei ATCC334 [
10]. These data further suggest that LabMAX-3 can interact with healthy and LPS-treated inflamed tissue to promote canine gut health.
Next, we analyzed the adhesion patterns of LabMAX-3 to MDCK cells by Giemsa staining (
Figure 5D). Data show that LabMAX-3 attaches to MDCK cells, forming patchy biofilm-like structures throughout the monolayer.
L. casei ATCC334 also forms similar patchy biofilm-like structures on MDCK cells, slightly at a higher frequency. The biofilms produced by LabMAX-3 and
L. casei on MDCK follow a similar trend as the adhesion counts reported in
Figure 5A. These data indicate that adhesion of LabMAX-3 is facilitated by forming biofilms, ensuring prolonged persistence and potential health benefits to the host.
The ability of probiotic strains to produce biofilms on host epithelial cells is critical for ensuring colonization, pathogen exclusion, and promoting health [
10,
13,
60,
64]. In general, probiotic bacteria in the gut are transient, and their poor colonization in an inflamed gut environment may explain their inconsistent health benefits [
65]. Therefore, LPS pretreatment on the MDCK cell line was used to create an inflamed condition in the gut [
47]. LabMAX-3 could adhere and form biofilms on MDCK cell monolayers irrespective of LPS treatment.
3.4. Anti-inflammatory Response of LabMAX-3 to MDCK Cells
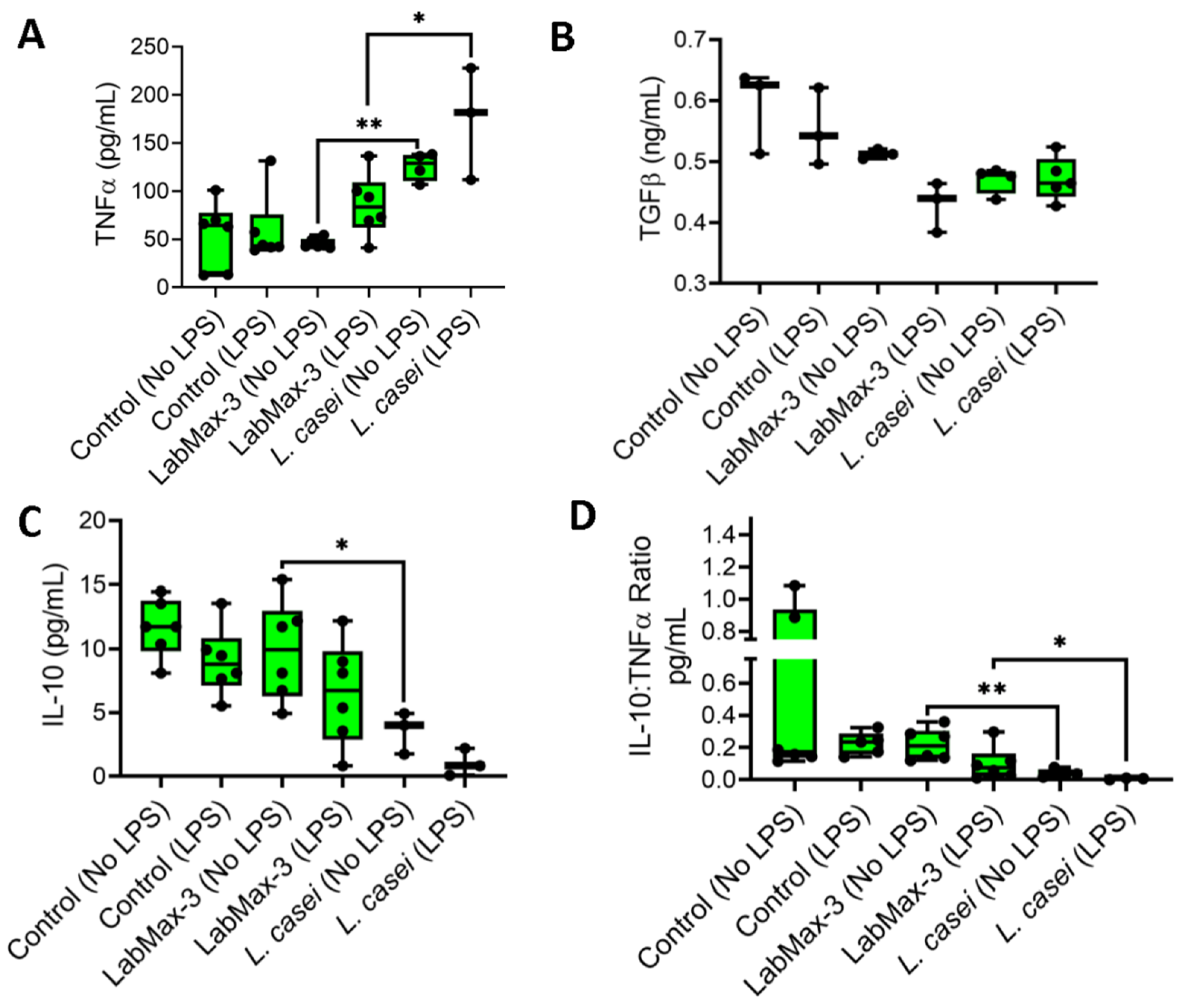
We quantified the proinflammatory (TNFα) and anti-inflammatory (IL-10 and TGFβ) cytokine profiles of LabMAX-3 on MDCK monolayers (
Figure 6). TNFα expression in LPS-pretreated MDCK cells exposed to LabMAX-3 was significantly higher (
p = 0.005) than in cells without LPS treatment. A similar result was also observed for
L. casei ATCC334. In contrast, a reduced IL-10 level (numerical difference but not a statistically significant difference) was noticed in MDCK cells pretreated with LPS compared to those without LPS treatment following LabMAX-3 exposure. A similar trend was observed with
L. casei ATCC334 treatment. IL-10 and TNFα ratio analysis demonstrated a significant (
p < 0.05) reduction in values in LPS pretreated MDCK cells than those with LPS treatment following LabMAX-3 exposure. TGFβ secretion showed no significant differences between LabMAX-3 and
L. casei ATCC334 treatment. Cytokine profile data indicate LabMAX-3 has an anti-inflammatory response towards MDCK cells consistent with probiotic functionality reported by others [
3,
66].
4. Conclusions
Probiotic supplementation for systemic health in canines is a growing market. Here, we explored LabMAX-3 as a novel kibble additive comprising three renowned probiotics: Lactobacillus acidophilus, Enterococcus faecium, and Lacticaseibacillus casei. LabMAX-3 successfully inhibited the growth of notable canine-associated pathogens (Salmonella enterica, Listeria monocytogenes, enterotoxigenic Escherichia coli). LabMAX-3 was shown to be a strong biofilm producer on both abiotic and biotic surfaces. The ability of LabMAX-3 to form biofilm-like structures and strong adhesion to epithelial MDCK cell monolayers suggests its potential to improve canine gut health. LabMAX-3 showed no cytotoxic effects on the MDCK cell line, with and without LPS stimulation. Anti-inflammatory properties of LabMAX-3 promote higher IL-10 and TNFα ratios when compared to L. casei strain ATCC334 alone. Together, these data indicate that LabMAX-3 is viable as a supplement to companion animal kibble for promoting systemic health.
The advantages of using a combination of three probiotic cultures in the LabMAX-3 are that they showed robust antimicrobial activity against pathogens, formed biofilms, and efficiently attached to MDCK cells, promoting an anti-inflammatory response. Another benefit of a multi-probiotic mixture is the chance of a positive effect on health in case one or more members fail to grow in an unfavorable host environment. Commercial probiotic supplements for human use often consist of multiple probiotic cultures, and a similar probiotic blend comprising multiple species and strains should be used to ensure pets' health.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.Figure S1.
Author Contributions
Conceptualization, Nicholas Gallina, Mandy Horn and Arun Bhunia; Data curation, Nicholas Gallina and Nicole Tardi; Formal analysis, Nicholas Gallina and Nicole Tardi; Funding acquisition, Lavanya Reddivari and Arun Bhunia; Investigation, Nicholas Gallina, Nicole Tardi and Arun Bhunia; Methodology, Nicholas Gallina, Nicole Tardi, Xilin Li and Alvin Cai; Resources, Bruce Applegate, Lavanya Reddivari and Arun Bhunia; Writing – original draft, Nicholas Gallina; Writing – review & editing, Bruce Applegate, Lavanya Reddivari and Arun Bhunia.
Funding
The research was supported in part by funds from CH2 Animal Solutions (Ottumwa, IA 52501, USA), US Department of Agriculture – National Institute of Food and Agriculture (NIFA) National Needs Fellowship grant number 2021-38420-34058, and the USDA-NIFA Hatch Grant # 1016249. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
Data Availability Statement
All data are presented in the manuscript.
Conflicts of Interest
The study was partly supported by funds provided by CH2 Animal Solutions, and Mrs. Mandy Horn, an employee of CH2 Animal Solutions is a co-author of the study. We declare that ”the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.”
References
- Amalaradjou, M. A. R.; Bhunia, A. K., Modern approaches in probiotics research to control foodborne pathogens. Adv. Food Nutr. Res. 2012, 67, 185-239. [CrossRef]
- Schmitz, S. S., Value of Probiotics in Canine and Feline Gastroenterology. Vet Clin North Am Small Anim Pract 2021, 51, (1), 171-217. [CrossRef]
- Cristofori, F.; Dargenio, V. N.; Dargenio, C.; Miniello, V. L.; Barone, M.; Francavilla, R., Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front. Immunol. 2021, 12, 578386. [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G. R.; Merenstein, D. J.; Pot, B.; Morelli, L.; Canani, R. B.; Flint, H. J.; Salminen, S.; Calder, P. C.; Sanders, M. E., Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, (8), 506-514. [CrossRef]
- Lee, J. Y.; Tsolis, R. M.; Bäumler, A. J., The microbiome and gut homeostasis. Science 2022, 377, (6601), eabp9960. [CrossRef]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J. M.; Dequenne, I.; de Timary, P.; Cani, P. D., How Probiotics Affect the Microbiota. Front Cell Infect Microbiol 2019, 9, 454. [CrossRef]
- Zou, J.; Reddivari, L.; Shi, Z.; Li, S.; Wang, Y.; Bretin, A.; Ngo, V. L.; Flythe, M.; Pellizzon, M.; Chassaing, B.; Gewirtz, A. T., Inulin Fermentable Fiber Ameliorates Type I Diabetes via IL22 and Short-Chain Fatty Acids in Experimental Models. Cell Mol Gastroenterol Hepatol 2021, 12, (3), 983-1000. [CrossRef]
- Pilla, R.; Suchodolski, J. S., The Gut Microbiome of Dogs and Cats, and the Influence of Diet. Vet Clin North Am Small Anim Pract 2021, 51, (3), 605-621. [CrossRef]
- White, R.; Atherly, T.; Guard, B.; Rossi, G.; Wang, C.; Mosher, C.; Webb, C.; Hill, S.; Ackermann, M.; Sciabarra, P.; Allenspach, K.; Suchodolski, J.; Jergens, A. E., Randomized, controlled trial evaluating the effect of multi-strain probiotic on the mucosal microbiota in canine idiopathic inflammatory bowel disease. Gut Microbes 2017, 8, (5), 451-466. [CrossRef]
- Drolia, R.; Amalaradjou, M. A. R.; Ryan, V.; Tenguria, S.; Liu, D.; Bai, X.; Xu, L.; Singh, A. K.; Cox, A. D.; Bernal-Crespo, V.; Schaber, J. A.; Applegate, B. M.; Vemulapalli, R.; Bhunia, A. K., Receptor-targeted engineered probiotics mitigate lethal Listeria infection. Nat. Commun. 2020, 11, (1), 6344. [CrossRef] [PubMed]
- Ryan, V. E.; Bailey, T. W.; Liu, D.; Vemulapalli, T.; Cooper, B.; Cox, A. D.; Bhunia, A. K., Listeria adhesion protein-expressing bioengineered probiotics prevent fetoplacental transmission of Listeria monocytogenes in a pregnant Guinea pig model. Microb Pathog 2021, 151, 104752. [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E., The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, (5), 716-729. [CrossRef]
- Bhunia, A. K., Microbes as a tool to defend against antibiotic resistance in food animal production. Indian J. Anim. Hlth 2019, 58, (2), 01-18. [CrossRef]
- Levy, M.; Kolodziejczyk, A. A.; Thaiss, C. A.; Elinav, E., Dysbiosis and the immune system. Nat Rev Immunol 2017, 17, (4), 219-232. [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H., Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175-184. [CrossRef]
- Suchodolski, J. S.; Camacho, J.; Steiner, J. M., Analysis of bacterial diversity in the canine duodenum, jejunum, ileum, and colon by comparative 16S rRNA gene analysis. FEMS Microbiol. Ecol. 2008, 66, (3), 567-578. [CrossRef]
- Pilla, R.; Suchodolski, J. S., The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front Vet Sci 2019, 6, 498. [CrossRef]
- Lambertini, E.; Buchanan, R. L.; Narrod, C.; Ford, R. M.; Baker, R. C.; Pradhan, A. K., Quantitative assessment of human and pet exposure to Salmonella associated with dry pet foods. Int. J. Food Microbiol. 2016, 216, 79-90. [CrossRef] [PubMed]
- Antunes, P.; Novais, C.; Peixe, L.; Freitas, A. R., Pet Food Safety: Emerging bacterial hazards and implications for Public Health. Curr. Opin. Food Sci. 2024, 101165. [CrossRef]
- Nemser, S. M.; Doran, T.; Grabenstein, M.; McConnell, T.; McGrath, T.; Pamboukian, R.; Smith, A. C.; Achen, M.; Danzeisen, G.; Kim, S., Investigation of Listeria, Salmonella, and toxigenic Escherichia coli in various pet foods. Foodborne Pathog. Dis. 2014, 11, (9), 706-709. [CrossRef]
- Sola-Oladokun, B.; Culligan, E. P.; Sleator, R. D., Engineered probiotics: Applications and biological containment. Annu. Rev. Food Sci. Technol. 2017, 8, (1), 353-370. [CrossRef]
- Tan, L.; Fu, J.; Feng, F.; Liu, X.; Cui, Z.; Li, B.; Han, Y.; Zheng, Y.; Yeung, K. W. K.; Li, Z.; Zhu, S.; Liang, Y.; Feng, X.; Wang, X.; Wu, S., Engineered probiotics biofilm enhances osseointegration via immunoregulation and anti-infection. Sci. Adv. 2020, 6, (46). [CrossRef]
- Zhou, J.; Li, M.; Chen, Q.; Li, X.; Chen, L.; Dong, Z.; Zhu, W.; Yang, Y.; Liu, Z.; Chen, Q., Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat Commun 2022, 13, (1), 3432. [CrossRef]
- Piewngam, P.; Zheng, Y.; Nguyen, T. H.; Dickey, S. W.; Joo, H. S.; Villaruz, A. E.; Glose, K. A.; Fisher, E. L.; Hunt, R. L.; Li, B.; Chiou, J.; Pharkjaksu, S.; Khongthong, S.; Cheung, G. Y. C.; Kiratisin, P.; Otto, M., Pathogen elimination by probiotic Bacillus via signalling interference. Nature 2018, 562, (7728), 532-537. [CrossRef]
- Piewngam, P.; Khongthong, S.; Roekngam, N.; Theapparat, Y.; Sunpaweravong, S.; Faroongsarng, D.; Otto, M., Probiotic for pathogen-specific Staphylococcus aureus decolonisation in Thailand: a phase 2, double-blind, randomised, placebo-controlled trial. Lancet Microbe 2023, 4, (2), e75-e83. [CrossRef]
- Al-Shamiri, M. M.; Wang, J.; Zhang, S.; Li, P.; Odhiambo, W. O.; Chen, Y.; Han, B.; Yang, E.; Xun, M.; Han, L.; Han, S., Probiotic Lactobacillus Species and Their Biosurfactants Eliminate Acinetobacter baumannii Biofilm in Various Manners. Microbiol Spectr 2023, 11, (2), e0461422. [CrossRef]
- Burkholder, K. M.; Bhunia, A. K., Salmonella enterica serovar Typhimurium adhesion and cytotoxicity during epithelial cell stress is reduced by Lactobacillus rhamnosus GG. Gut Pathog 2009, 1, (1), 14. [CrossRef]
- Nathan, V. B.; Lu, H.; Horn, N. L.; Drolia, R.; Bhunia, A. K., Sequestration of zearalenone using microorganisms blend in vitro. Lett Appl Microbiol 2023, 76, (2), ovad020. [CrossRef]
- Ragoubi, C.; Quintieri, L.; Greco, D.; Mehrez, A.; Maatouk, I.; D’Ascanio, V.; Landoulsi, A.; Avantaggiato, G., Mycotoxin removal by Lactobacillus spp. and their application in animal liquid feed. Toxins 2021, 13, (3), 185. [CrossRef]
- Liu, L.; Xie, M.; Wei, D., Biological detoxification of mycotoxins: Current status and future advances. Int. J. Mol. Sci. 2022, 23, (3), 1064. [CrossRef]
- Grześkowiak, Ł.; Endo, A.; Beasley, S.; Salminen, S., Microbiota and probiotics in canine and feline welfare. Anaerobe 2015, 34, 14-23. [CrossRef]
- Mathipa, M. G.; Bhunia, A. K.; Thantsha, M. S., Internalin AB-expressing recombinant Lactobacillus casei protects Caco-2 cells from Listeria monocytogenes-induced damages under simulated intestinal conditions. PLoS One 2019, 14, (7), e0220321. [CrossRef] [PubMed]
- Mathipa, M. G.; Thantsha, M. S.; Bhunia, A. K., Lactobacillus casei expressing Internalins A and B reduces Listeria monocytogenes interaction with Caco-2 cells in vitro. Microb. Biotechnol. 2019, 12, (4), 715-729. [CrossRef]
- Amalaradjou, M. A.; Bhunia, A. K., Bioengineered probiotics, a strategic approach to control enteric infections. Bioengineered 2013, 4, (6), 379-87. [CrossRef]
- Cruz, K. C. P.; Enekegho, L. O.; Stuart, D. T., Bioengineered Probiotics: Synthetic Biology Can Provide Live Cell Therapeutics for the Treatment of Foodborne Diseases. Front. Bioeng. Biotechnol. 2022, 10, 890479. [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F. J.; Gil-Campos, M.; Gil, A., Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, (suppl_1), S49-S66. [CrossRef]
- Bai, X.; Liu, D.; Xu, L.; Tenguria, S.; Drolia, R.; Gallina, N. L. F.; Cox, A. D.; Koo, O.-K.; Bhunia, A. K., Biofilm-isolated Listeria monocytogenes exhibits reduced systemic dissemination at the early (12–24 h) stage of infection in a mouse model. npj Biofilms and Microbiomes 2021, 7, (1), 1-16. [CrossRef] [PubMed]
- Zhu, X.; Liu, D.; Singh, A. K.; Drolia, R.; Bai, X.; Tenguria, S.; Bhunia, A. K., Tunicamycin mediated inhibition of wall teichoic acid affect Staphylococcus aureus and Listeria monocytogenes cell morphology, biofilm formation and virulence. Front. Microbiol. 2018, 9, (July), 1352. [CrossRef] [PubMed]
- Motta, J. P.; Wallace, J. L.; Buret, A. G.; Deraison, C.; Vergnolle, N., Gastrointestinal biofilms in health and disease. Nat Rev Gastroenterol Hepatol 2021, 18, (5), 314-334. [CrossRef]
- Azimi, S.; Klementiev, A. D.; Whiteley, M.; Diggle, S. P., Bacterial Quorum Sensing During Infection. Annu Rev Microbiol 2020, 74, 201-219. [CrossRef]
- Mukherjee, S.; Bassler, B. L., Bacterial quorum sensing in complex and dynamically changing environments. Nat Rev Microbiol 2019, 17, (6), 371-382. [CrossRef]
- Zhang, H.; Yeh, C.; Jin, Z.; Ding, L.; Liu, B. Y.; Zhang, L.; Dannelly, H. K., Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate. Synth Syst Biotechnol 2018, 3, (2), 113-120. [CrossRef]
- Bai, X.; Gallina, N. L. F.; Bhunia, A. K., Microbial Biofilms in Food Safety and Public Health Domains. In Reference Module in Food Science, Elsevier: 2023. [CrossRef]
- Sambanthamoorthy, K.; Feng, X.; Patel, R.; Patel, S.; Paranavitana, C., Antimicrobial and antibiofilm potential of biosurfactants isolated from lactobacilli against multi-drug-resistant pathogens. BMC Microbiol 2014, 14, 197. [CrossRef]
- Nataraj, B. H.; Ramesh, C.; Mallappa, R. H., Functional group characterization of lactic bacterial biosurfactants and evaluation of antagonistic actions against clinical isolates of methicillin-resistant Staphylococcus aureus. Lett Appl Microbiol 2021, 73, (3), 372-382. [CrossRef]
- Irvine, J. D.; Takahashi, L.; Lockhart, K.; Cheong, J.; Tolan, J. W.; Selick, H. E.; Grove, J. R., MDCK (Madin–Darby canine kidney) cells: a tool for membrane permeability screening. J. Pharmaceut. Sci. 1999, 88, (1), 28-33. [CrossRef] [PubMed]
- Capellini, F. M.; Vencia, W.; Amadori, M.; Mignone, G.; Parisi, E.; Masiello, L.; Vivaldi, B.; Ferrari, A.; Razzuoli, E., Characterization of MDCK cells and evaluation of their ability to respond to infectious and non-infectious stressors. Cytotechnology 2020, 72, 97-109. [CrossRef]
- Bhunia, A. K.; Johnson, M. C.; Ray, B., Purification, characterization and antimicrobial spectrum of a bacteriocin produced by Pediococcus acidilactici. J. Appl. Bacteriol. 1988, 65, (4), 261-268. [CrossRef]
- Kim, E.; Yang, S. M.; Lim, B.; Park, S. H.; Rackerby, B.; Kim, H. Y., Design of PCR assays to specifically detect and identify 37 Lactobacillus species in a single 96 well plate. BMC Microbiol 2020, 20, (1), 96. [CrossRef]
- Belloso Daza, M. V.; Almeida-Santos, A. C.; Novais, C.; Read, A.; Alves, V.; Cocconcelli, P. S.; Freitas, A. R.; Peixe, L., Distinction between Enterococcus faecium and Enterococcus lactis by a gluP PCR-Based Assay for Accurate Identification and Diagnostics. Microbiol Spectr 2022, 10, (6), e0326822. [CrossRef]
- Bai, X.; Xu, L.; Singh, A. K.; Qiu, X.; Liu, M.; Abuzeid, A.; El-Khateib, T.; Bhunia, A. K., Inactivation of Polymicrobial Biofilms of Foodborne Pathogens Using Epsilon Poly-L-Lysin Conjugated Chitosan Nanoparticles. Foods 2022, 11, (4), 569. [CrossRef]
- Roberts, P. H.; Davis, K. C.; Garstka, W. R.; Bhunia, A. K., Lactate dehydrogenase release assay from Vero cells to distinguish verotoxin producing Escherichia coli from non-verotoxin producing strains. J. Microbiol. Methods 2001, 43, (3), 171-181. [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A., Probiotic mechanisms of action. Annals Nutr. Metabol. 2012, 61, (2), 160-174. [CrossRef]
- Aleman, R. S.; Yadav, A., Systematic review of probiotics and their potential for developing functional nondairy foods. Appl. Microbiol. 2023, 4, (1), 47-69. [CrossRef]
- Rossi, G.; Pengo, G.; Caldin, M.; Palumbo Piccionello, A.; Steiner, J. M.; Cohen, N. D.; Jergens, A. E.; Suchodolski, J. S., Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL# 3 strains in dogs with idiopathic inflammatory bowel disease. Plos One 2014, 9, (4), e94699.
- Ziese, A.-L.; Suchodolski, J. S.; Hartmann, K.; Busch, K.; Anderson, A.; Sarwar, F.; Sindern, N.; Unterer, S., Effect of probiotic treatment on the clinical course, intestinal microbiome, and toxigenic Clostridium perfringens in dogs with acute hemorrhagic diarrhea. 2018, 13, (9), e0204691.
- Panja, K.; Areerat, S.; Chundang, P.; Palaseweenun, P.; Akrimajirachoote, N.; Sitdhipol, J.; Thaveethaptaikul, P.; Chonpathompikunlert, P.; Niwasabutra, K.; Phapugrangkul, P.; Kovitvadhi, A., Influence of dietary supplementation with new Lactobacillus strains on hematology, serum biochemistry, nutritional status, digestibility, enzyme activities, and immunity in dogs. Vet World 2023, 16, (4), 834-843. [CrossRef]
- Xu, H.; Huang, W.; Hou, Q.; Kwok, L.-Y.; Laga, W.; Wang, Y.; Ma, H.; Sun, Z.; Zhang, H., Oral administration of compound probiotics improved canine feed intake, weight gain, immunity and intestinal microbiota. Front. Immunol. 2019, 10, 394673. [CrossRef] [PubMed]
- Johnson-Henry, K. C.; Donato, K. A.; Shen-Tu, G.; Gordanpour, A.; Sherman, P. A., Lactobacillus rhamnosus strain GG prevents enterohemorrhagic Escherichia coli O157 : H7-Induced changes in epithelial barrier function. Infect. Immun. 2008, 76, (4), 1340-1348. [CrossRef]
- Gao, J.; Sadiq, F. A.; Zheng, Y.; Zhao, J.; He, G.; Sang, Y., Biofilm-based delivery approaches and specific enrichment strategies of probiotics in the human gut. Gut Microbes 2022, 14, (1), 2126274. [CrossRef]
- Freeman, D. J.; Falkiner, F. R.; Keane, C. T., New method for detecting slime production by coagulase negative staphylococci. J Clin Pathol 1989, 42, (8), 872-4. [CrossRef]
- Salas-Jara, M. J.; Ilabaca, A.; Vega, M.; García, A., Biofilm Forming Lactobacillus: New Challenges for the Development of Probiotics. Microorganisms 2016, 4, (3). [CrossRef]
- Popović, N.; Dinić, M.; Tolinački, M.; Mihajlović, S.; Terzić-Vidojević, A.; Bojić, S.; Djokić, J.; Golić, N.; Veljović, K., New Insight into Biofilm Formation Ability, the Presence of Virulence Genes and Probiotic Potential of Enterococcus sp. Dairy Isolates. front. Microbiol. 2018, 9. [CrossRef] [PubMed]
- Bustamante, M.; Oomah, B. D.; Oliveira, W. P.; Burgos-Díaz, C.; Rubilar, M.; Shene, C., Probiotics and prebiotics potential for the care of skin, female urogenital tract, and respiratory tract. Folia Microbiol 2020, 65, 245-264. [CrossRef]
- Gorreja, F.; Walker, W. A., The potential role of adherence factors in probiotic function in the gastrointestinal tract of adults and pediatrics: a narrative review of experimental and human studies. Gut Microbes 2022, 14, (1), 2149214. [CrossRef]
- Sebastián Domingo, J. J., Review of the role of probiotics in gastrointestinal diseases in adults. Gastroenterol. Hepatol. 2017, 40, (6), 417-429. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).