Submitted:
11 September 2024
Posted:
12 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
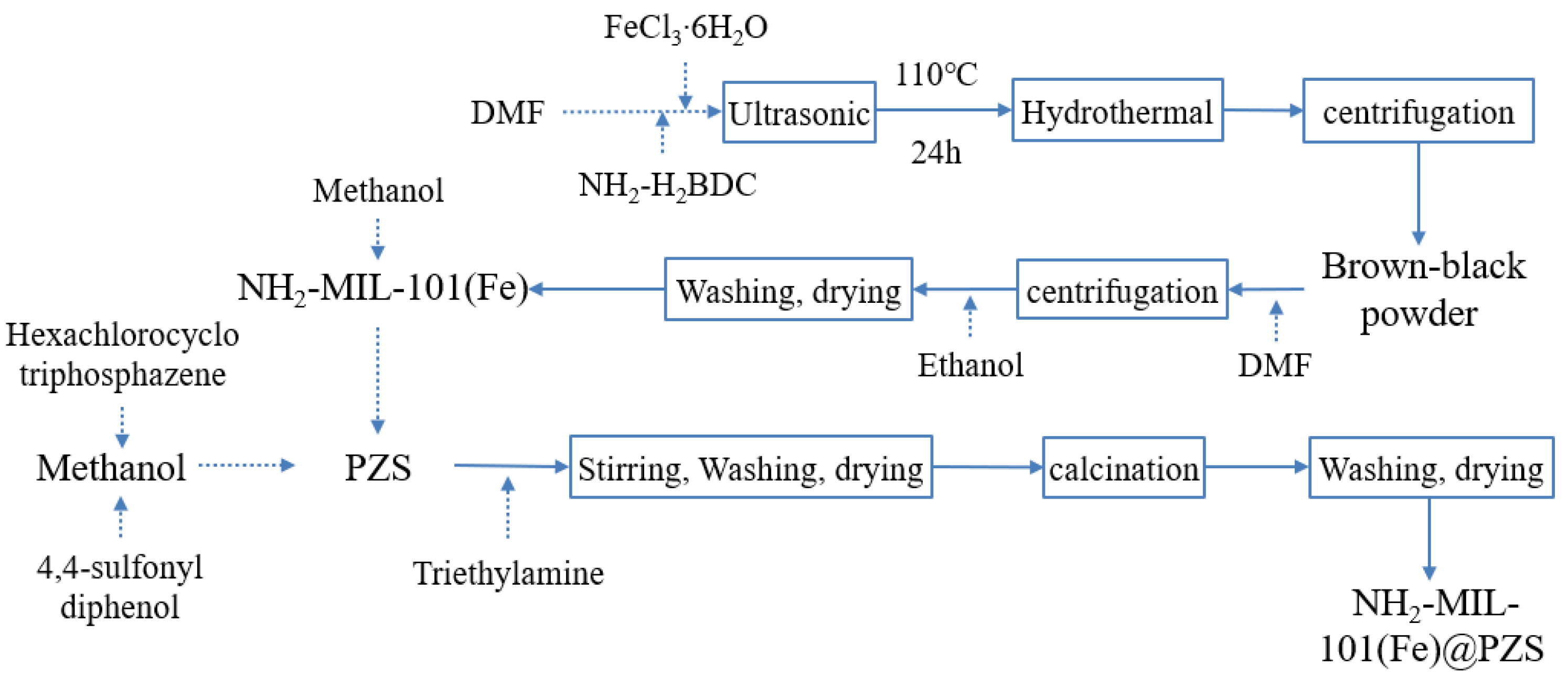
2.2. Preparation of NH2-MIL-101 (Fe)
2.3. Preparation of NH2-MIL-101 (Fe) @ PZS
2.4. Preparation of Fe-CNs-P/S
2.5. Material Characterization
2.6. Degradation of TC
3. Results
3.1. Characterization of Materials
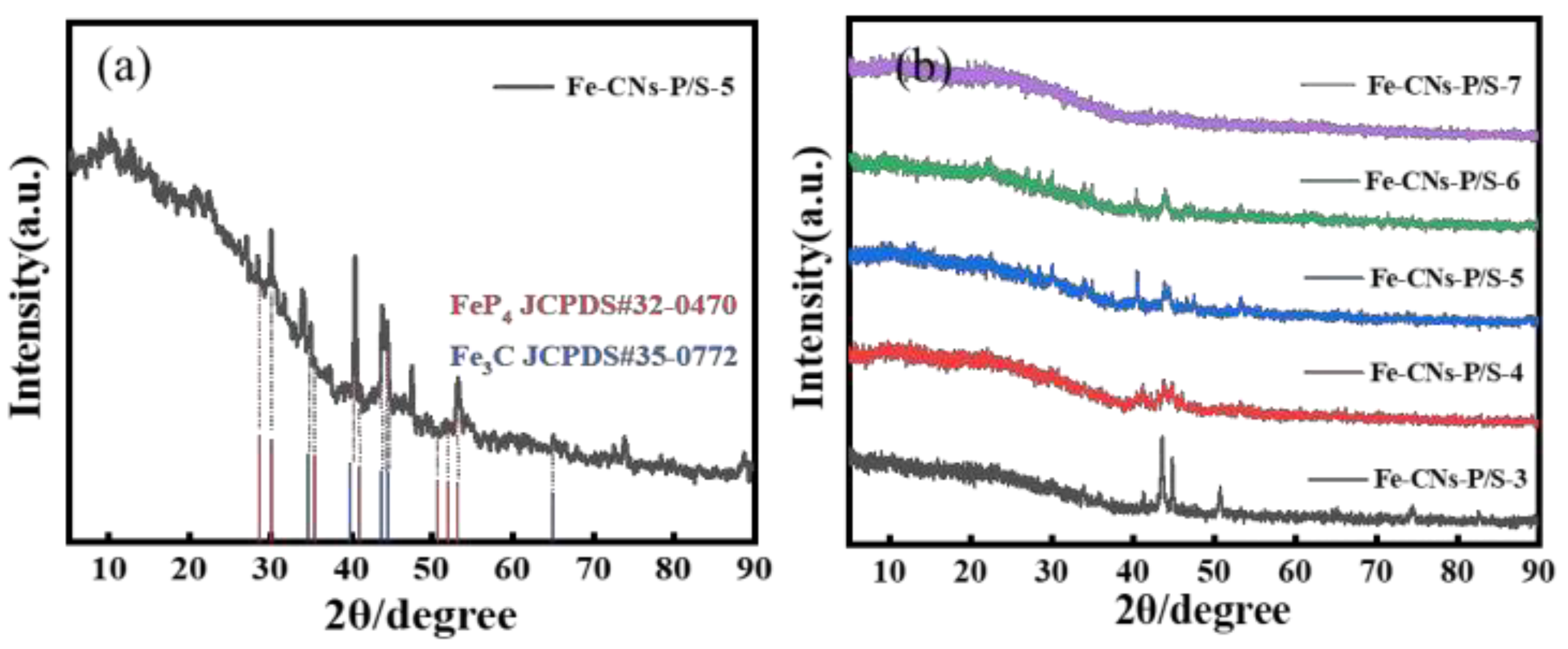
3.1.1. XRD Analysis
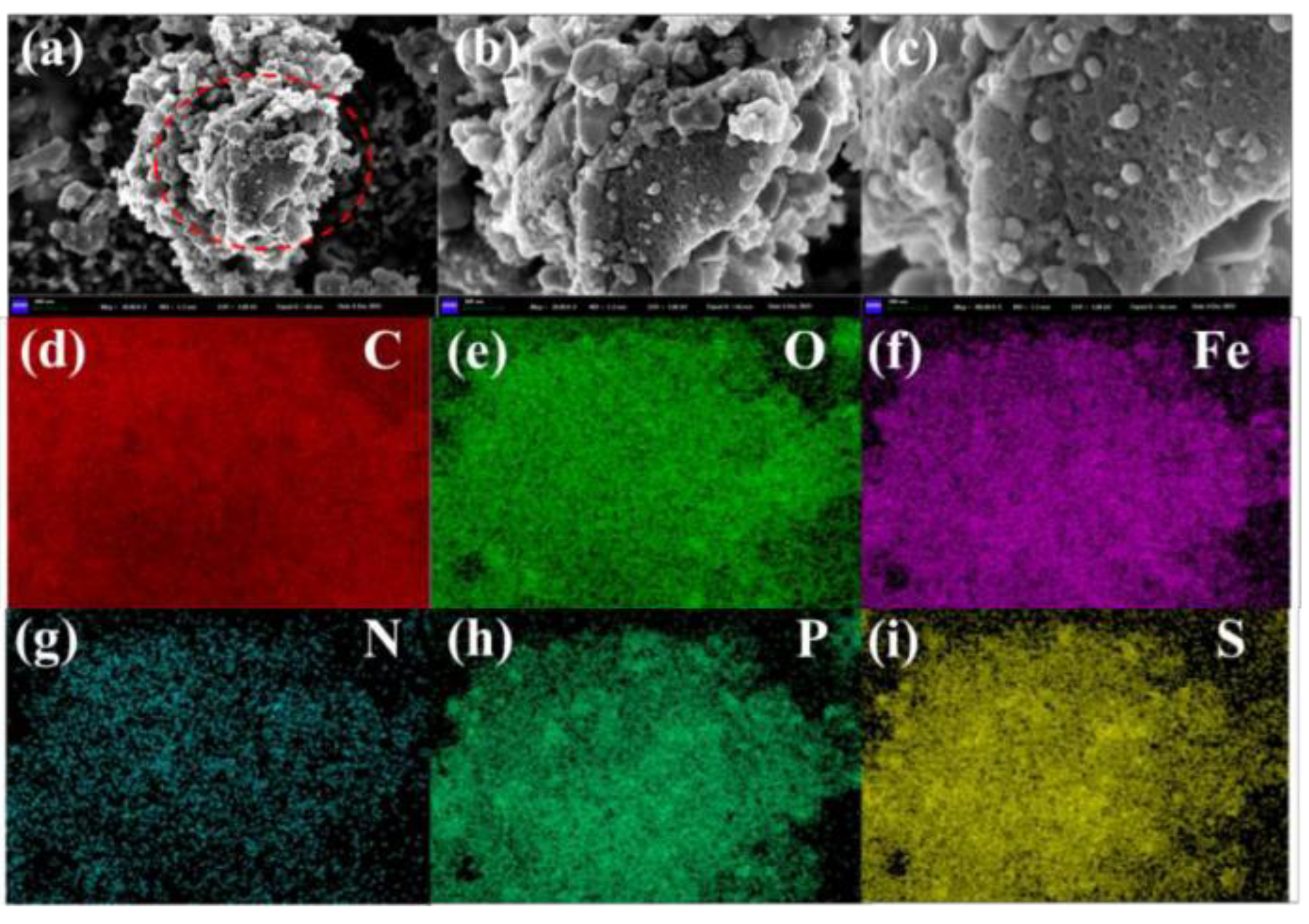
3.1.2. SEM Analysis
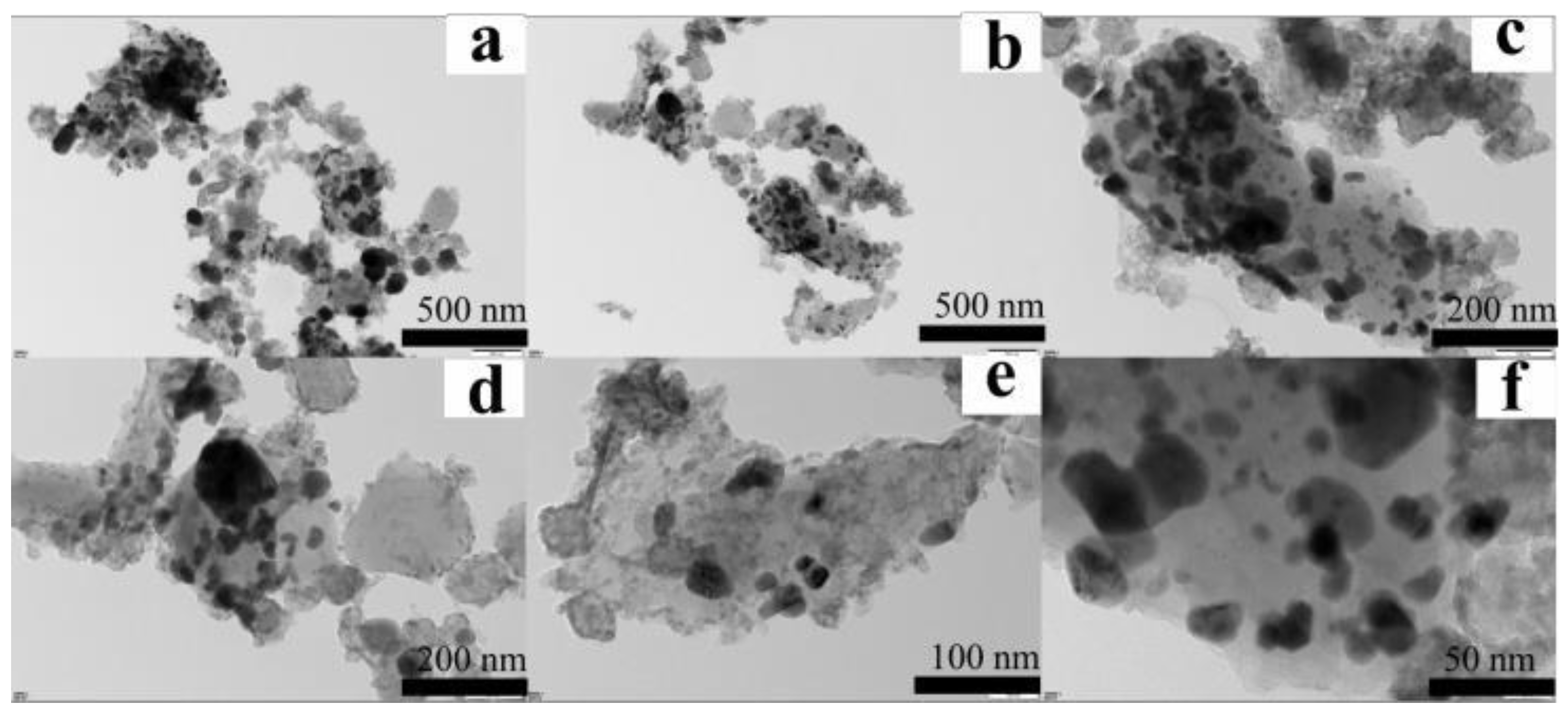
3.1.3. TEM Analysis
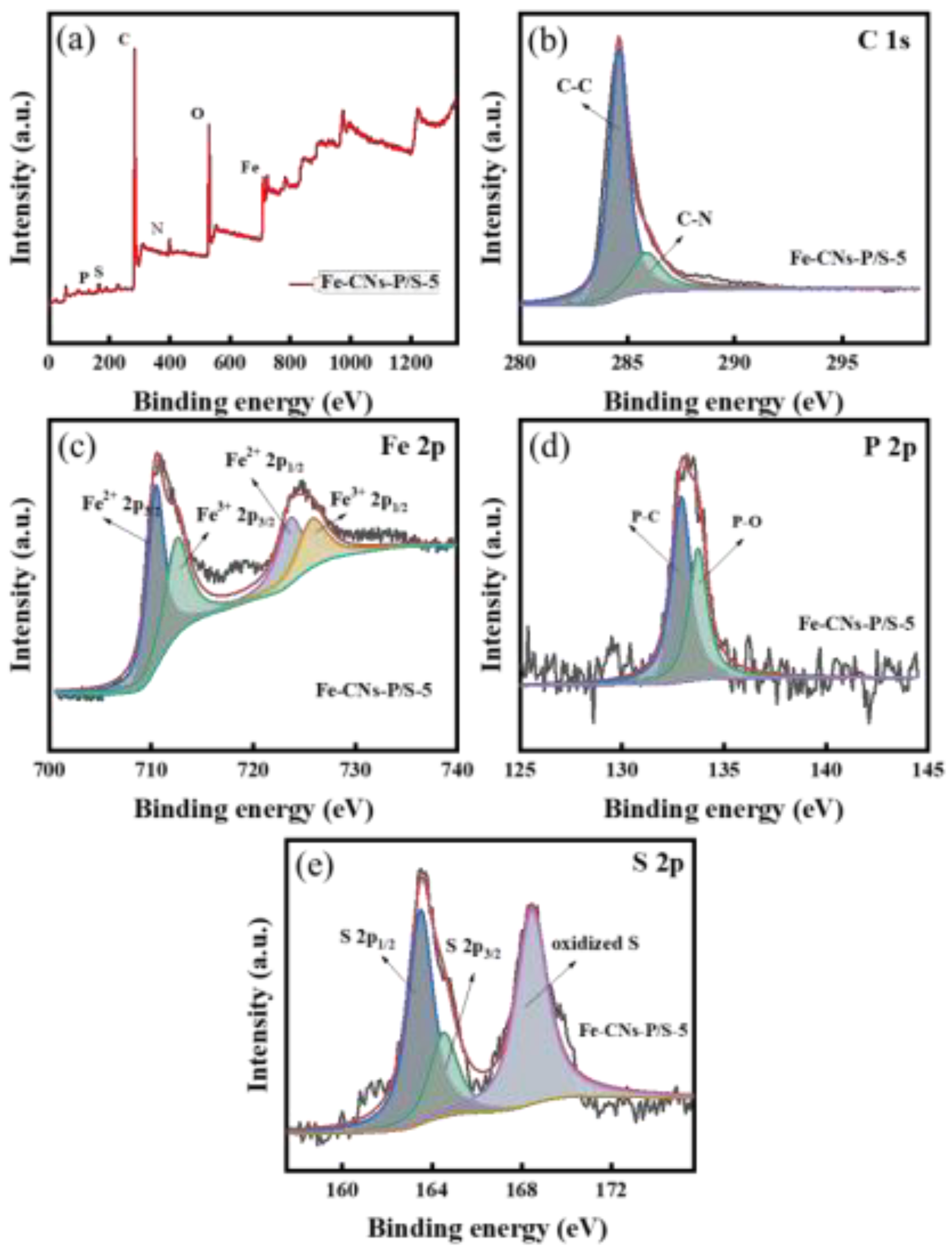
3.1.4. XPS Analysis
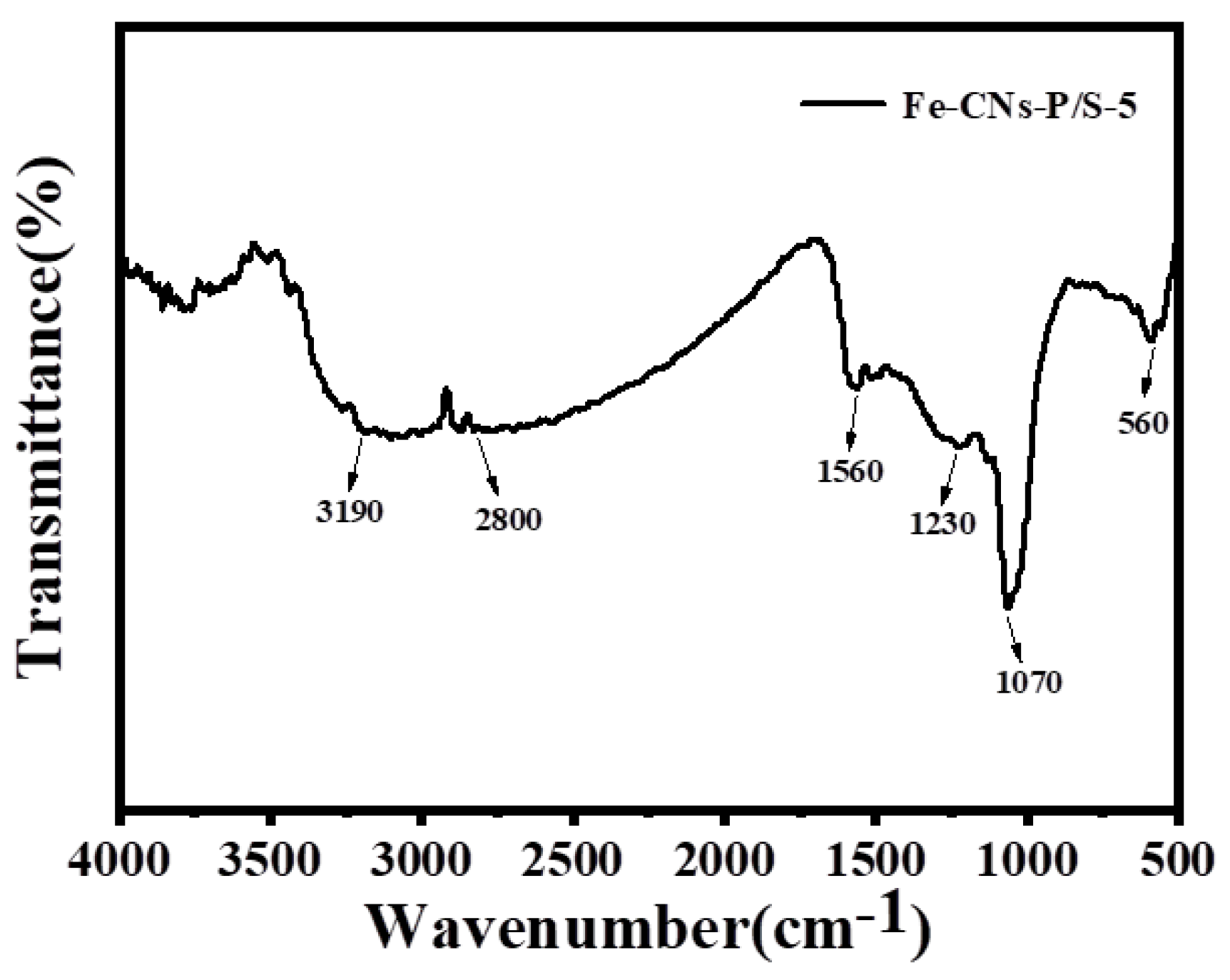
3.1.5. FTIR Analysis
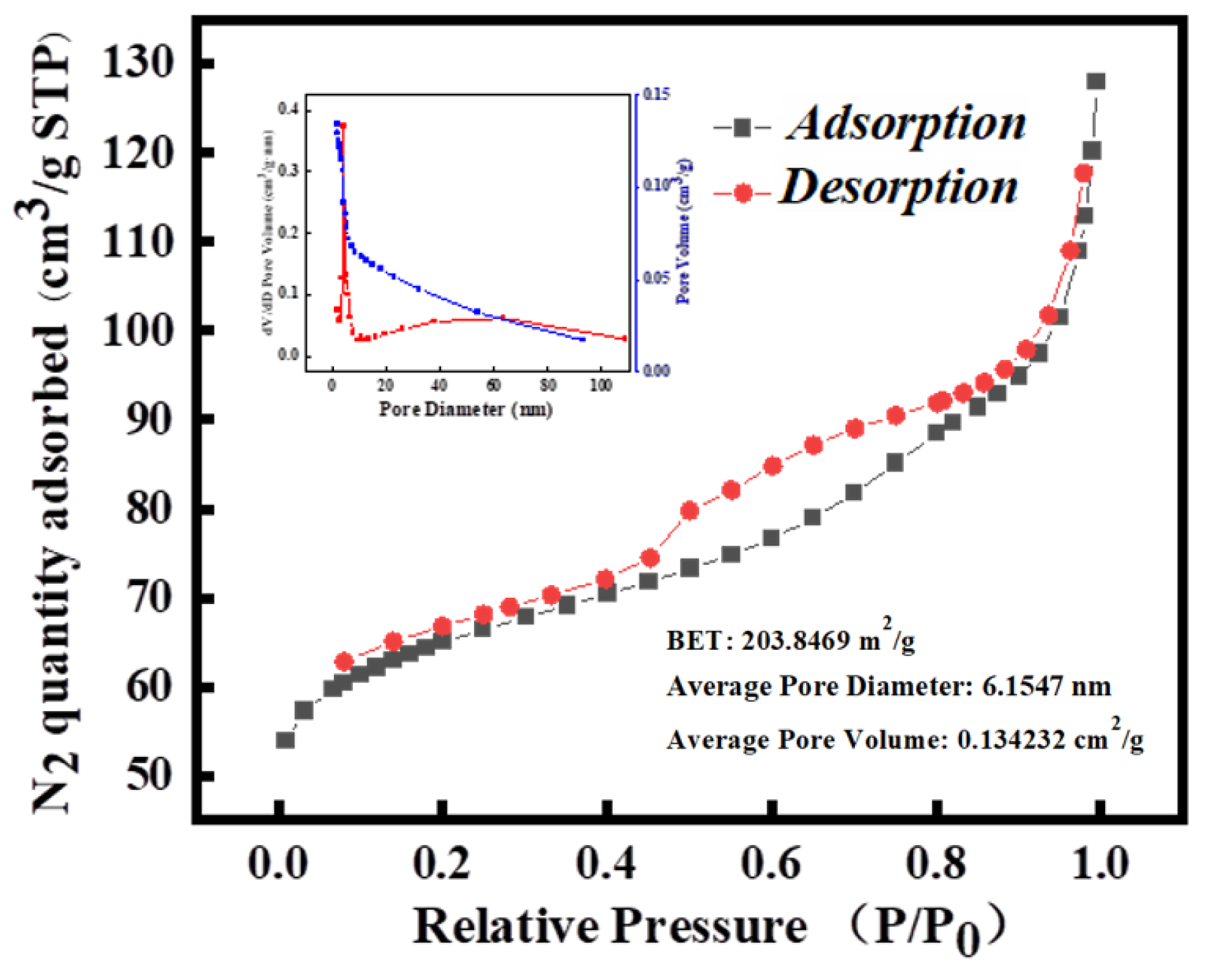
3.1.6. BET Analysis
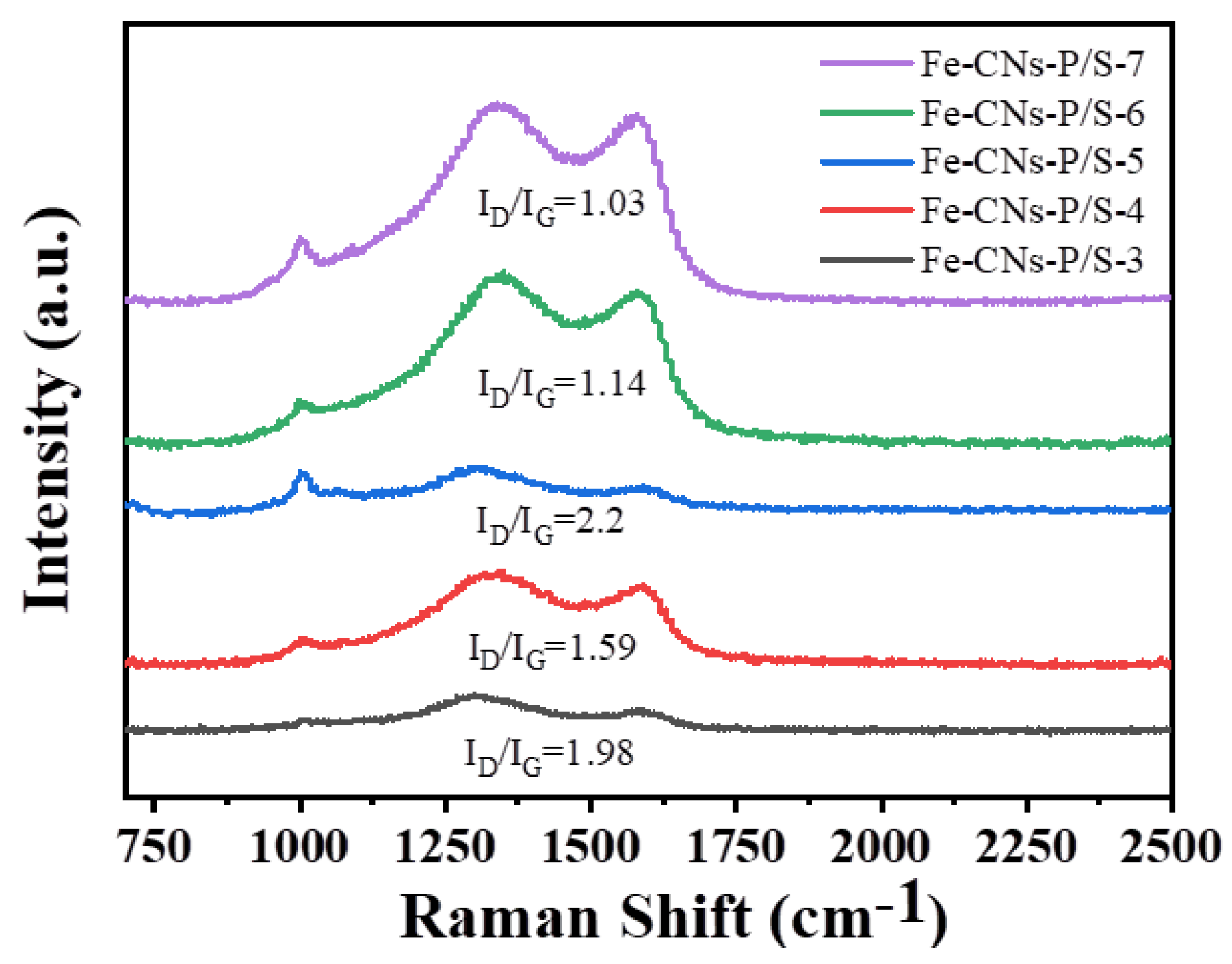
3.1.7. Raman Analysis
3.2. Degradation of TC
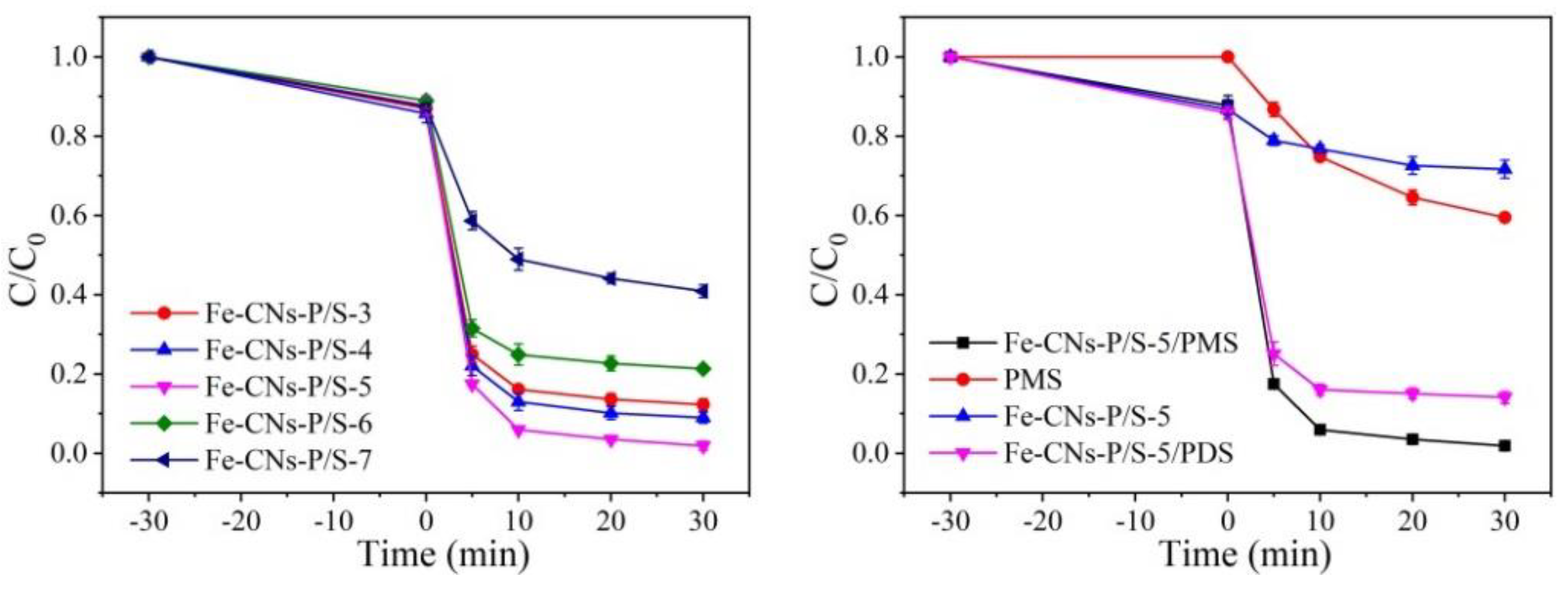
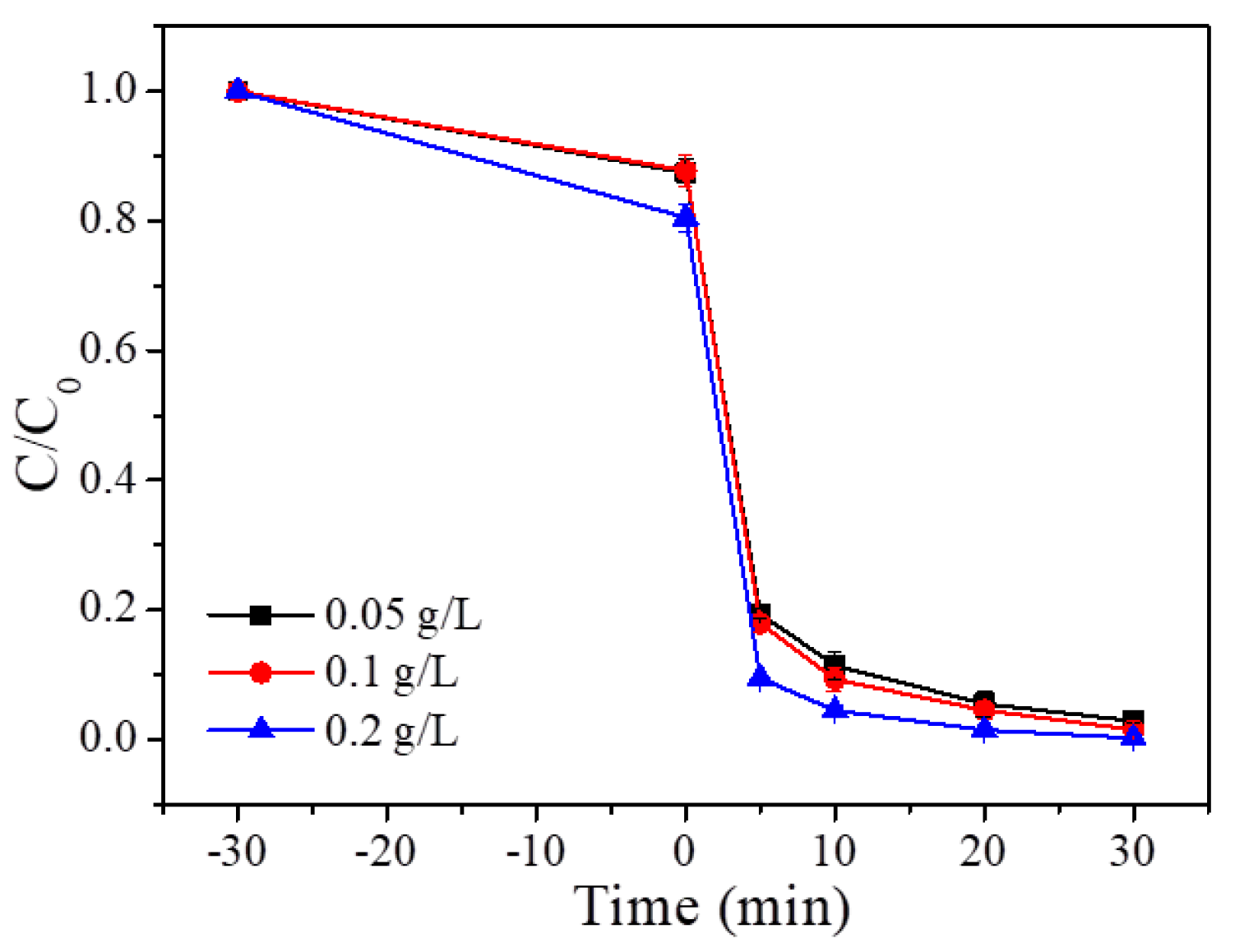
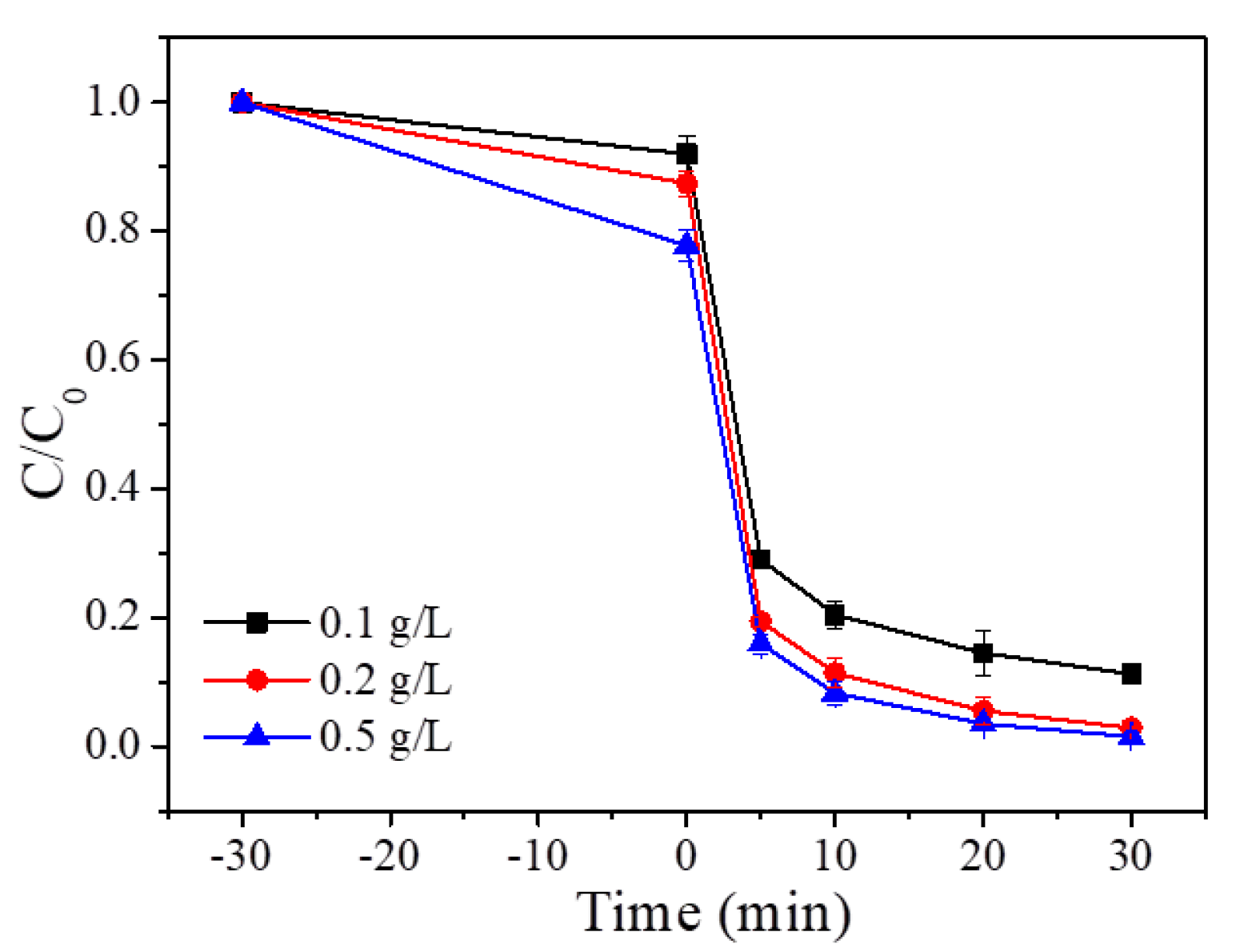
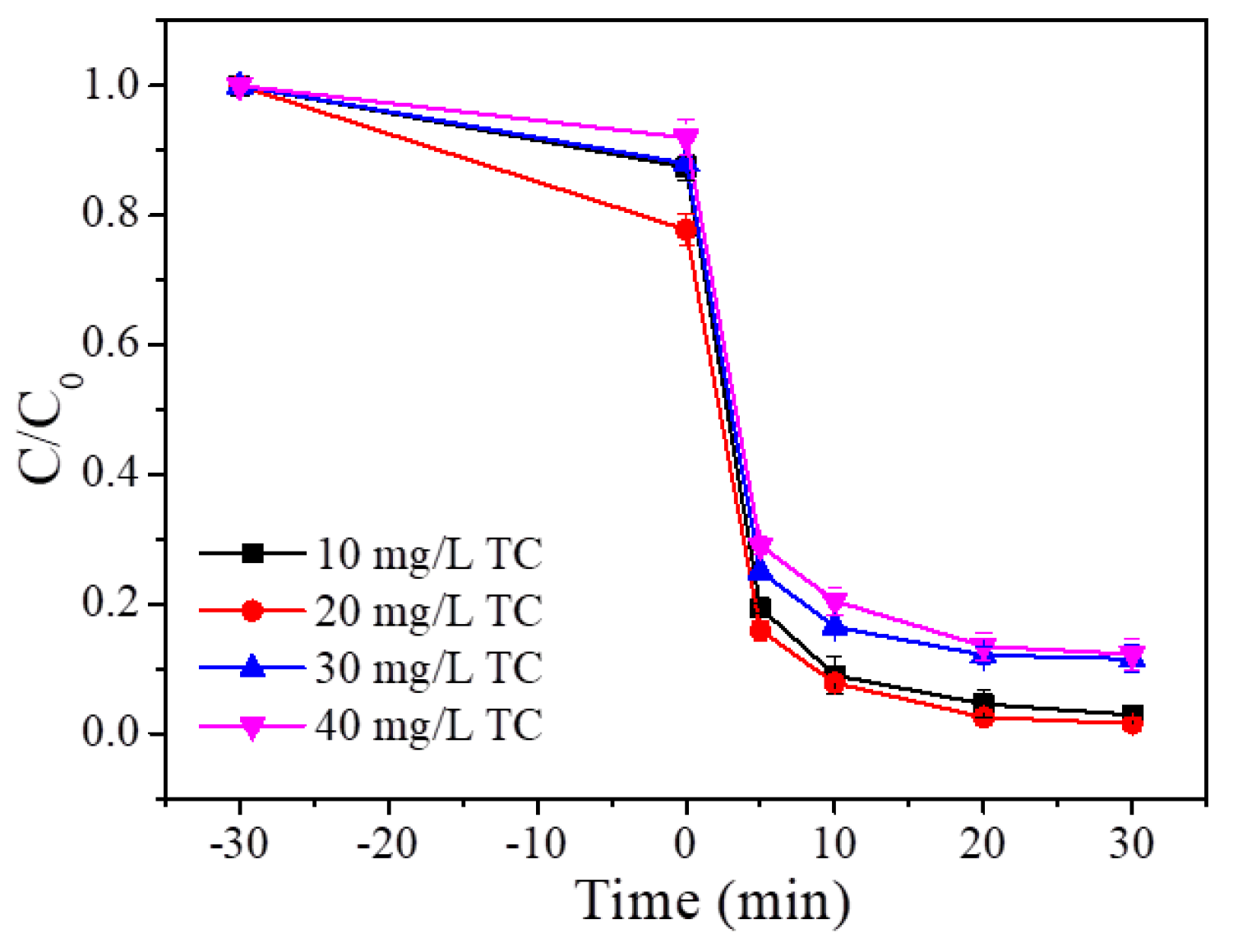
3.2.1. Effect of P/S Doping Amount
3.2.2. Effect of P/S Doping Amount
3.2.3. Effect of P/S Doping Amount
3.2.4. Effect of P/S Doping Amount
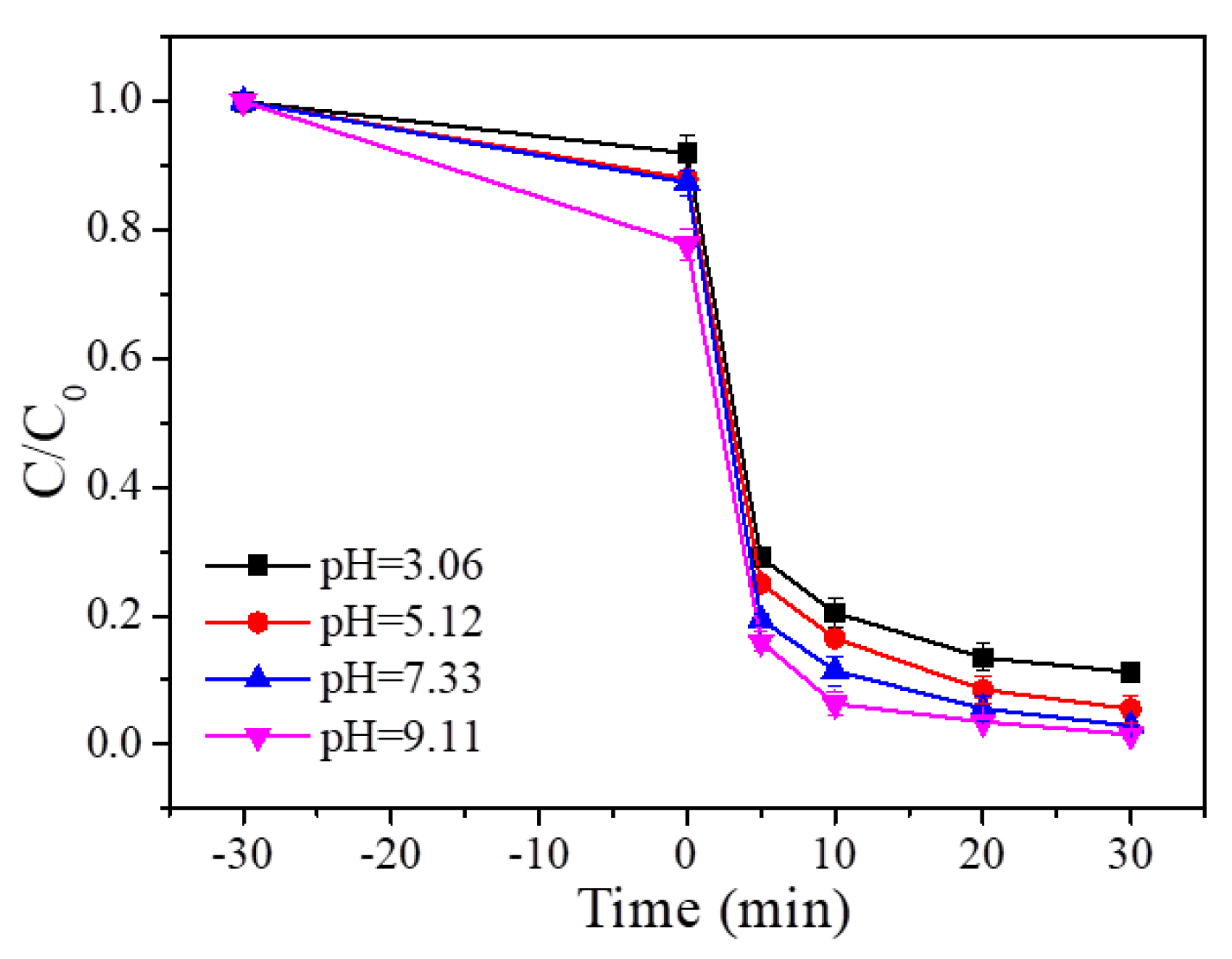
3.2.5. Effect of Initial pH of Solution
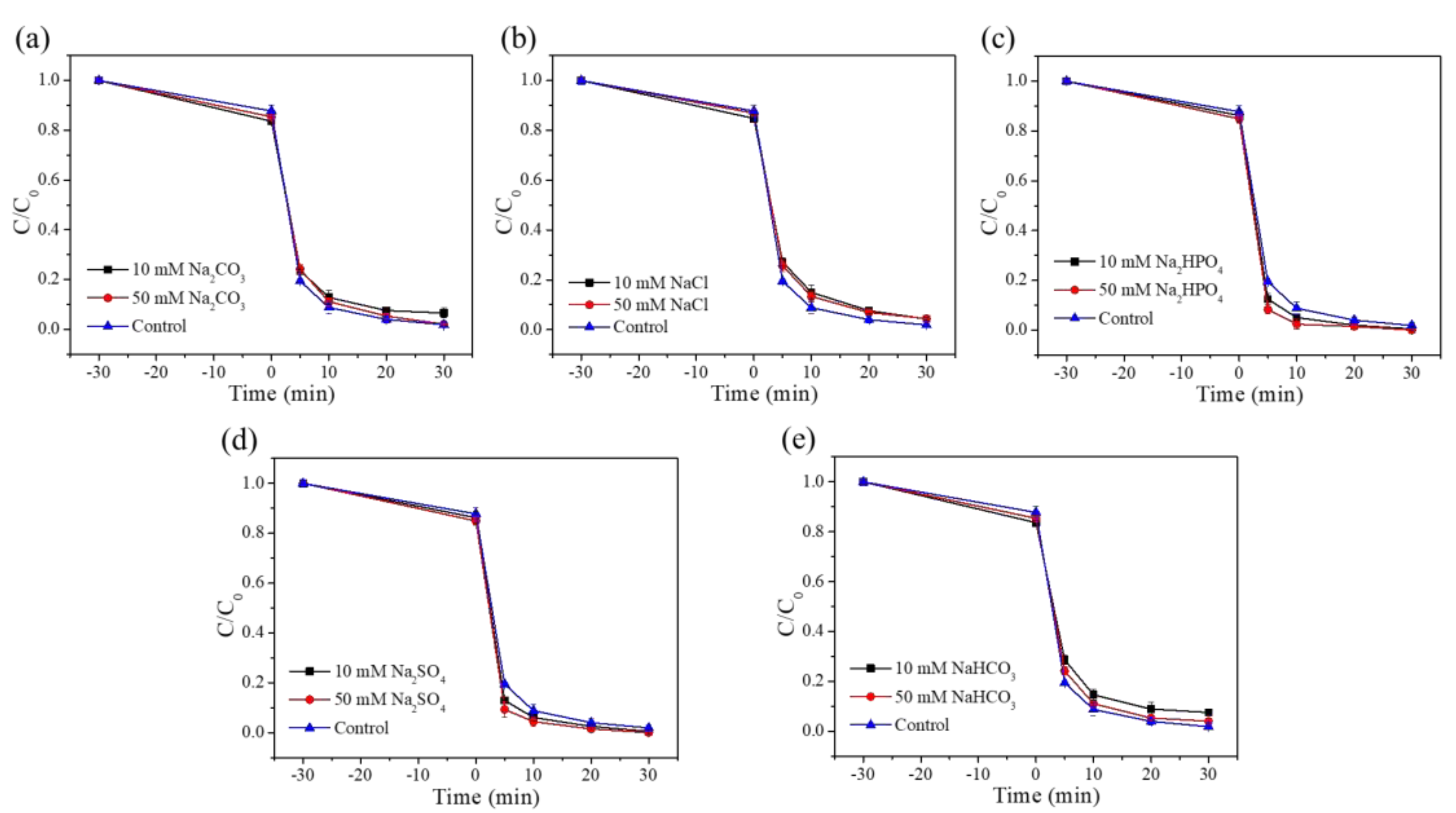
3.2.6. Effect of Inorganic Anions
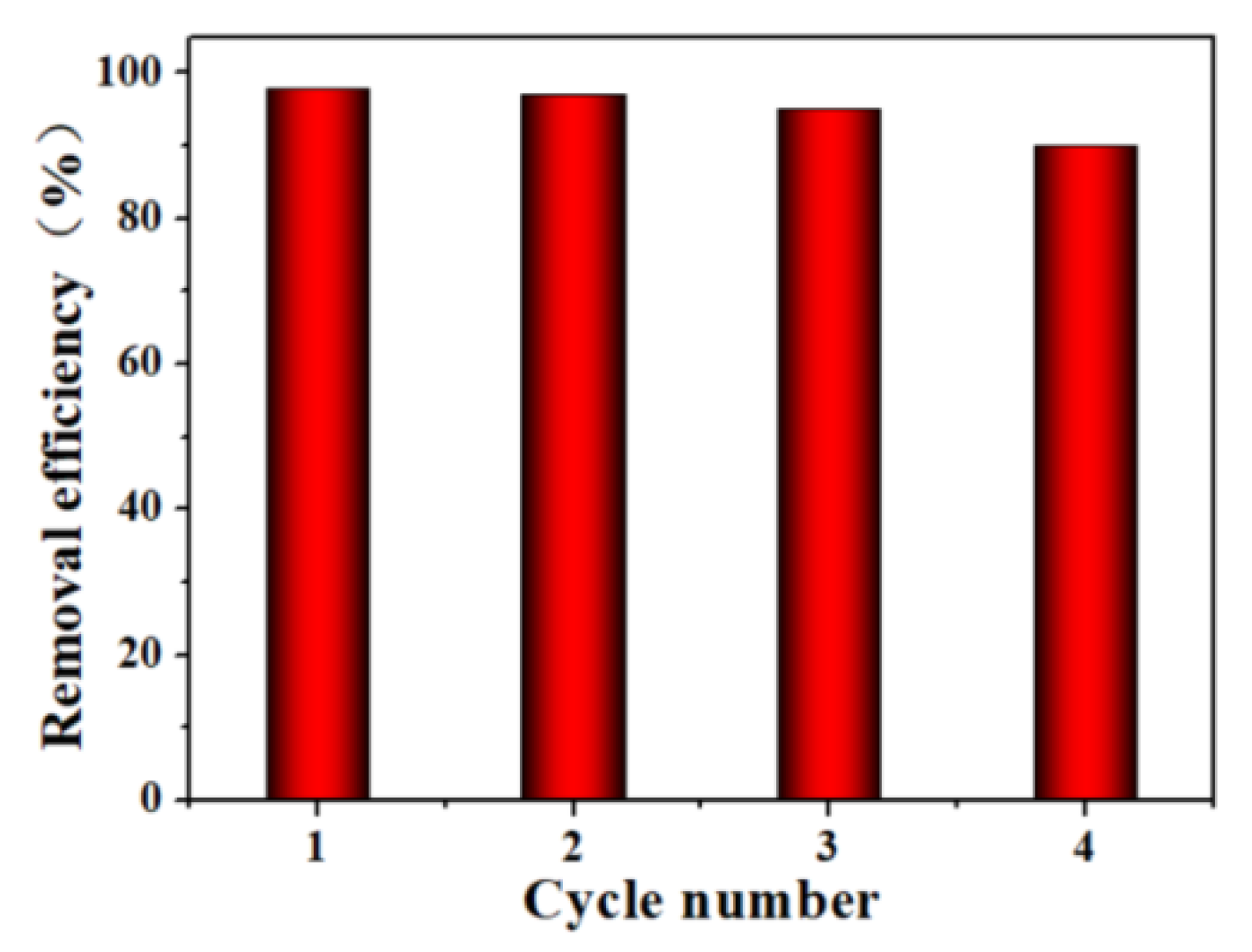
3.2.7. Cycle Efficiency
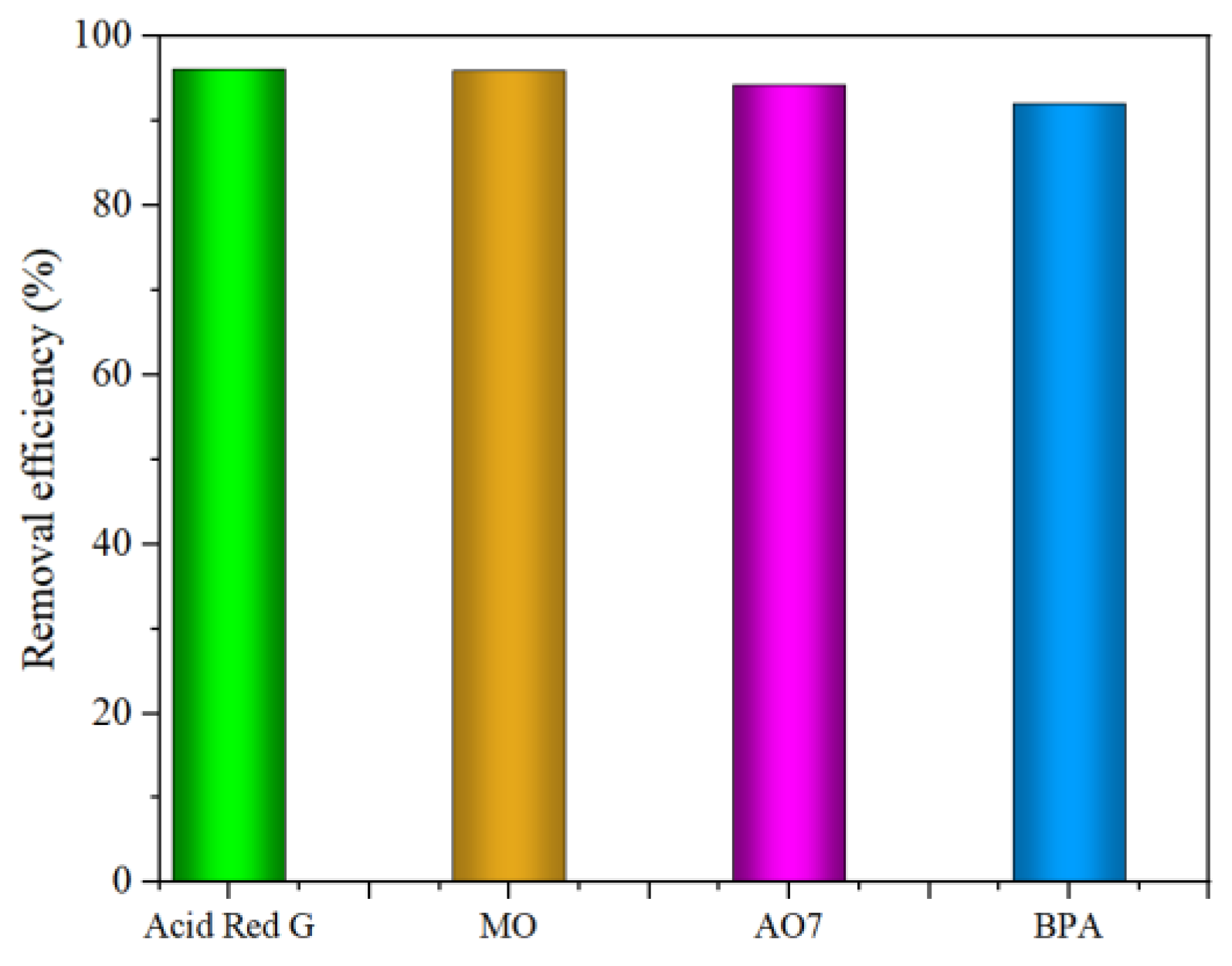
3.2.8. Universality
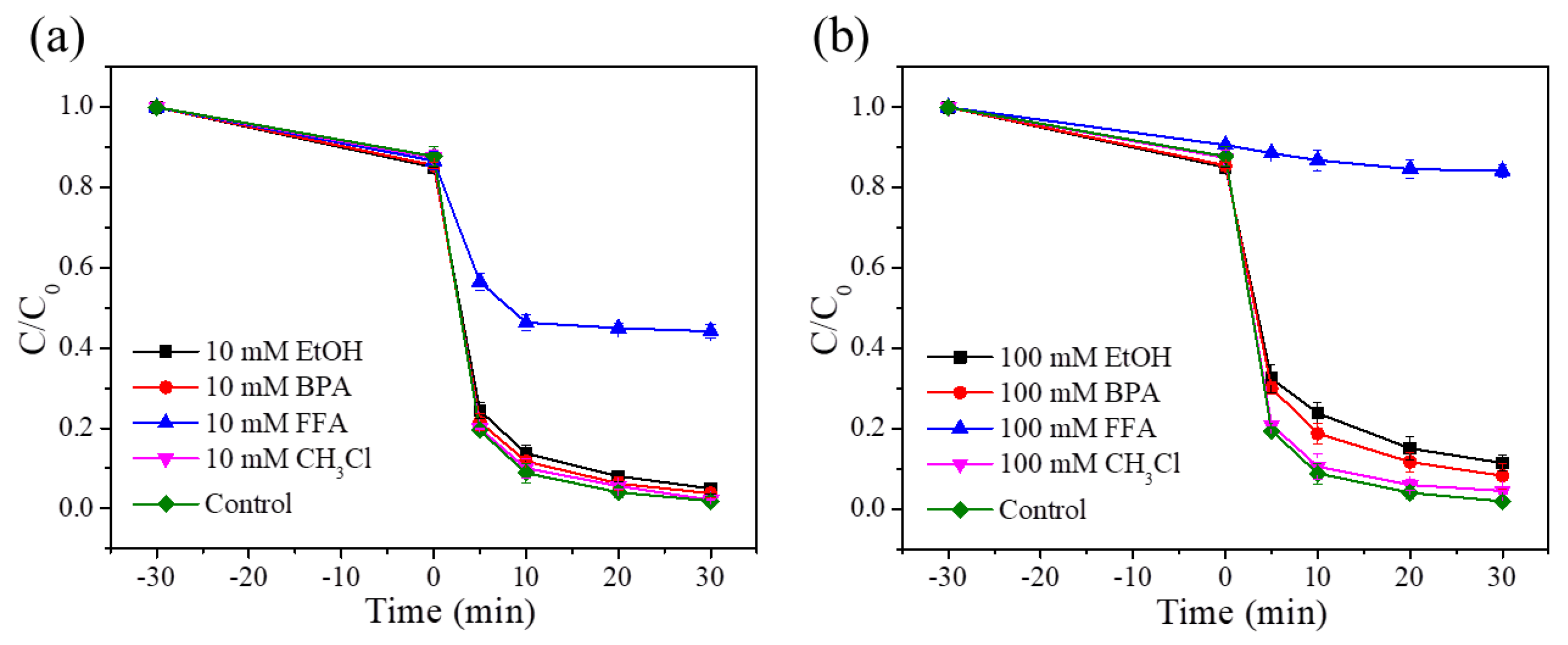
3.2.9. Free Radical Quenching Experiment
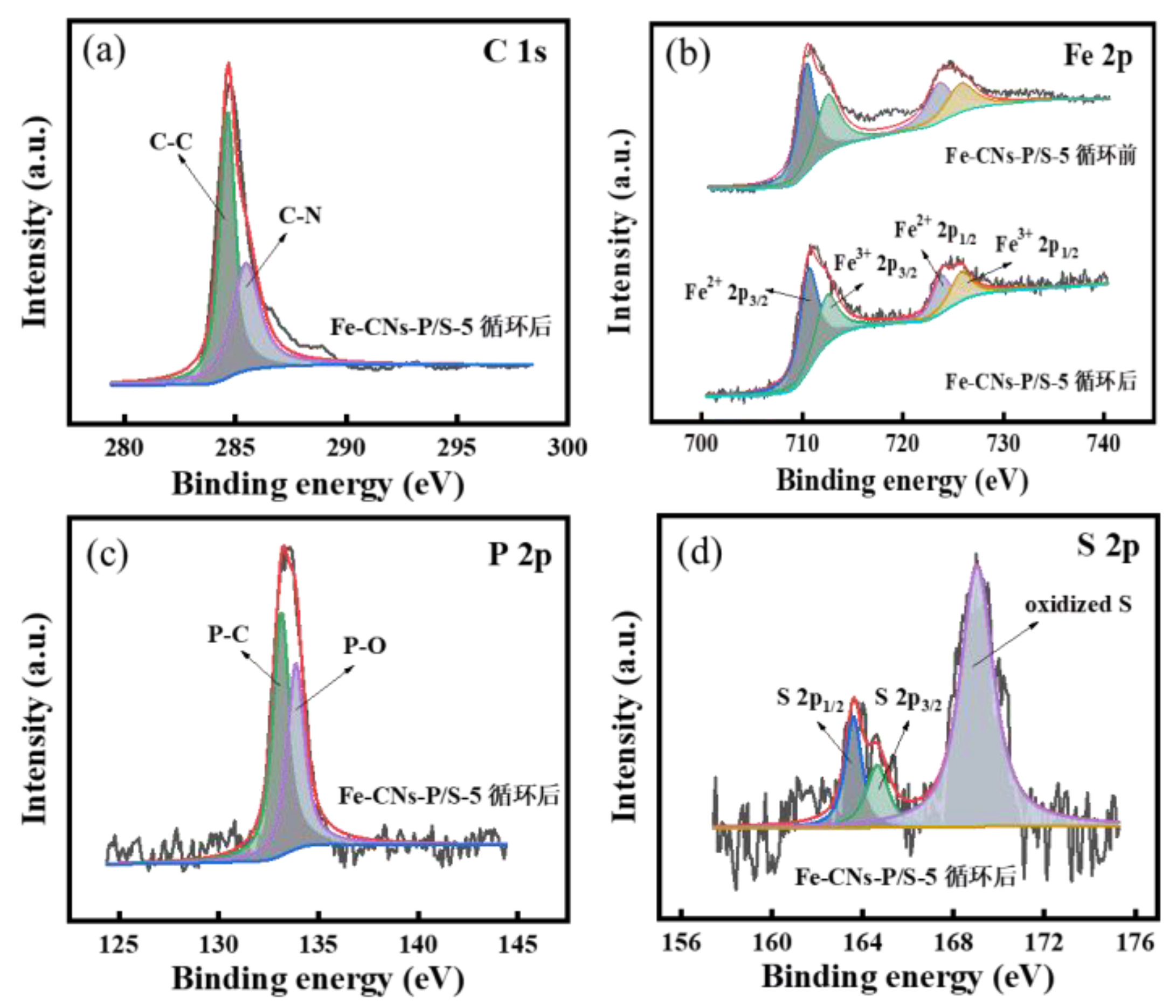
3.2.10. XPS Analysis before and after Degradation
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Hena, S.; Gutierrez, L.; Croué, J.-P. Removal of Pharmaceutical and Personal Care Products (PPCPs) from Wastewater Using Microalgae: A Review. J. Hazard. Mater. 2021, 403, 124041. [Google Scholar] [CrossRef]
- Xiang, Y.; Wu, H.; Li, L.; Ren, M.; Qie, H.; Lin, A. A Review of Distribution and Risk of Pharmaceuticals and Personal Care Products in the Aquatic Environment in China. Ecotoxicol. Environ. Saf. 2021, 213, 112044. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, J.; Chen, Y.; Li, X.; Xiong, W.; Zhou, Y.; Zhou, C.; Xu, R.; Zhang, Y. Mn-Doped Zirconium Metal-Organic Framework as an Effective Adsorbent for Removal of Tetracycline and Cr(VI) from Aqueous Solution. Microporous Mesoporous Mater. 2019, 277, 277–285. [Google Scholar] [CrossRef]
- Zhu, P.; Li, Y.; Chen, F.; Luo, X.; Zhou, Y.; Qiu, Q.; Xie, T. Construction of 3D Flower-like FeTiO3/MoS2Heterostructure Photocatalyst for Degradation of Tetracycline Hydrochloride. Journal of Alloys and Compounds 2023, 937, 168425. [Google Scholar] [CrossRef]
- Golrizkhatami, F.; Taghavi, L.; Nasseh, N.; Panahi, H.A. Synthesis of Novel MnFe2O4/BiOI Green Nanocomposite and Its Application to Photocatalytic Degradation of Tetracycline Hydrochloride: (LC-MS Analyses, Mechanism, Reusability, Kinetic, Radical Agents, Mineralization, Process Capability, and Purification of Actual Pharmaceutical Wastewater). Journal of Photochemistry and Photobiology A: Chemistry 2023, 444, 114989. [Google Scholar] [CrossRef]
- Van Tri, D.; Barcelo, D.; Le Luu, T. The Performances of Persulfate Activators to Degrade the Persistent Organic Pollutants in Industrial Wastewater. Case Studies in Chemical and Environmental Engineering 2023, 8, 100539. [Google Scholar] [CrossRef]
- Bautista, P.; Mohedano, A.F.; Casas, J.A.; Zazo, J.A.; Rodriguez, J.J. An Overview of the Application of Fenton Oxidation to Industrial Wastewaters Treatment. Journal of Chemical Technology & Biotechnology 2008, 83, 1323–1338. [Google Scholar] [CrossRef]
- Wang, X.; He, L.; Zhou, Y.; Wang, N.; Zhu, L.; Yan, J.; Ding, D.; Zhu, W.; Zuo, X.; Wang, J.; Wu, X. Deep Mineralization of Bisphenol A via Cu–Mn Spinel Oxide Nanospheres Anchored N-Doped Carbon Activated Peroxymonosulfate. Chem. Eng. J. 2024, 497, 154687. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, Y.; Zhang, J.; Song, L.; Shi, B. Pollutant Degradation Behaviors in a Heterogeneous Fenton System through Fe/S-Doped Aerogel. Science of The Total Environment 2020, 714, 136436. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, D. Activation of Peroxymonosulfate by FeOOH Modified Biochar for the Degradation of Tetracycline Hydrochloride. Materials Letters 2024, 375, 137218. [Google Scholar] [CrossRef]
- Zhang, G.; Xia, S.; Li, H.; Du, H.; Li, T.; Cao, Y.; Meng, Y.; Wang, J.; Zhong, C.; Lau, W.-M. Mediated Peroxydisulfate Activation at Oxygen Vacancy Sites: Synergistic Degradation of Norfloxacin by Radical Pathway and Non-Radical Pathway. Separation and Purification Technology 2025, 354, 129018. [Google Scholar] [CrossRef]
- Sun, H.; Kwan, C.; Suvorova, A.; Ang, H.M.; Tadé, M.O.; Wang, S. Catalytic Oxidation of Organic Pollutants on Pristine and Surface Nitrogen-Modified Carbon Nanotubes with Sulfate Radicals. Applied Catalysis B: Environmental 2014, 154–155, 134–141. [Google Scholar] [CrossRef]
- Cao, J.; Sun, S.; Li, X.; Yang, Z.; Xiong, W.; Wu, Y.; Jia, M.; Zhou, Y.; Zhou, C.; Zhang, Y. Efficient Charge Transfer in Aluminum-Cobalt Layered Double Hydroxide Derived from Co-ZIF for Enhanced Catalytic Degradation of Tetracycline through Peroxymonosulfate Activation. Chemical Engineering Journal 2020, 382, 122802. [Google Scholar] [CrossRef]
- Li, Y.; Pan, L.; Zhu, Y.; Yu, Y.; Wang, D.; Yang, G.; Yuan, X.; Liu, X.; Li, H.; Zhang, J. How Does Zero Valent Iron Activating Peroxydisulfate Improve the Dewatering of Anaerobically Digested Sludge? Water Res. 2019, 163, 114912. [Google Scholar] [CrossRef] [PubMed]
- Asif, B.M.; Ji, B.; Maqbool, T.; Zhang, Z. Algogenic Organic Matter Fouling Alleviation in Membrane Distillation by Peroxymonosulfate (PMS): Role of PMS Concentration and Activation Temperature. Desalination 2021, 516, 115225. [Google Scholar] [CrossRef]
- Khataee, A. Application of Central Composite Design for the Optimization of Photo-Destruction of a Textile Dye Using UV/SO Process. Polish Journal of Chemical Technology 2009, 11, 38–45. [Google Scholar] [CrossRef]
- Li, H.; Wan, J.; Ma, Y.; Huang, M.; Wang, Y.; Chen, Y. New Insights into the Role of Zero-Valent Iron Surface Oxidation Layers in Persulfate Oxidation of Dibutyl Phthalate Solutions. Chemical Engineering Journal 2014, 250, 137–147. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, F.; Pang, H. A Review of MOFs and Their Composites-Based Photocatalysts: Synthesis and Applications. Advanced Functional Materials 2021, 31, 2104231. [Google Scholar] [CrossRef]
- Du, X.; Zhou, M. Strategies to Enhance Catalytic Performance of Metal–Organic Frameworks in Sulfate Radical-Based Advanced Oxidation Processes for Organic Pollutants Removal. Chemical Engineering Journal 2021, 403, 126346. [Google Scholar] [CrossRef]
- Li, Z.; Tang, X.; Huang, G.; Luo, X.; He, D.; Peng, Q.; Huang, J.; Ao, M.; Liu, K. Bismuth MOFs Based Hierarchical Co3O4-Bi2O3 Composite: An Efficient Heterogeneous Peroxymonosulfate Activator for Azo Dyes Degradation. Separation and Purification Technology 2020, 242, 116825. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, J.; Yang, F.; Wang, M.; Zhang, T.; Hao, Y.; Yang, W. Sandwich-like Porous MXene/Ni3S4/CuS Derived from MOFs as Superior Supercapacitor Electrode. Journal of Alloys and Compounds 2022, 906, 163863. [Google Scholar] [CrossRef]
- Wang, R.; Dong, X.-Y.; Du, J.; Zhao, J.-Y.; Zang, S.-Q. MOF-Derived Bifunctional Cu3P Nanoparticles Coated by a N,P-Codoped Carbon Shell for Hydrogen Evolution and Oxygen Reduction. Advanced Materials 2018, 30, 1703711. [Google Scholar] [CrossRef] [PubMed]
- Behera, P.; Subudhi, S.; Tripathy, S.P.; Parida, K. MOF Derived Nano-Materials: A Recent Progress in Strategic Fabrication, Characterization and Mechanistic Insight towards Divergent Photocatalytic Applications. Coordination Chemistry Reviews 2022, 456, 214392. [Google Scholar] [CrossRef]
- Liu, D.; Gu, W.; Zhou, L.; Wang, L.; Zhang, J.; Liu, Y.; Lei, J. Recent Advances in MOF-Derived Carbon-Based Nanomaterials for Environmental Applications in Adsorption and Catalytic Degradation. Chemical Engineering Journal 2022, 427, 131503. [Google Scholar] [CrossRef]
- Cheng, Y.; Hao, Z.; Hao, C.; Deng, Y.; Li, X.; Li, K.; Zhao, Y. A Review of Modification of Carbon Electrode Material in Capacitive Deionization. RSC Adv. 2019, 9, 24401–24419. [Google Scholar] [CrossRef]
- Yu, X.; Wang, L.; Shen, X.; Wu, Y.; Xu, L.; Zhang, Y.; Shi, J.; Gan, L. New Insight into the S and N Co-Doped Poplar Biochar for Efficient BPA Removal via Peroxymonosulfate Activation: S for Adsorptive Removal and N for Catalytic Removal. Separation and Purification Technology 2025, 354, 128809. [Google Scholar] [CrossRef]
- He, Y.; Yang, Y.; Ye, X.; Lv, Y.; Liu, Y.; Liu, M. Enhanced Persulfate Activation for Sulfadiazine Degradation by N, S Self-Doped Biochar from Sludge and Sulfonated Lignin: Emphasizing the Roles of Graphitic Nitrogen and Thiophene Sulfur. Journal of Environmental Chemical Engineering 2024, 12, 112407. [Google Scholar] [CrossRef]
- Qu, G.; Jia, P.; Zhang, T.; Tang, S.; Pervez, Md. N.; Pang, Y.; Li, B.; Cao, C.; Zhao, Y. Synergistic Activation of Peroxymonosulfate by Intrinsic Defect and Graphitic N of N, P Co-Doped Carbon Microspheres for BPA Degradation. Chem. Eng. J. 2023, 475, 145888. [Google Scholar] [CrossRef]
- Chen, W.; Lei, L.; Zhu, K.; He, D.; He, H.; Li, X.; Wang, Y.; Huang, J.; Ai, Y. Peroxymonosulfate Activation by Fe-N-S Co-Doped Tremella-like Carbocatalyst for Degradation of Bisphenol A: Synergistic Effect of Pyridine N, Fe-Nx, Thiophene S. J. Environ. Sci. 2023, 129, 213–228. [Google Scholar] [CrossRef]
- Ma, W.; Wang, N.; Tong, T.; Zhang, L.; Lin, K.-Y. A.; Han, X.; Du, Y. Nitrogen, Phosphorus, and Sulfur Tri-Doped Hollow Carbon Shells Derived from ZIF-67@poly (Cyclotriphosphazene-Co-4, 4′-Sulfonyldiphenol) as a Robust Catalyst of Peroxymonosulfate Activation for Degradation of Bisphenol A. Carbon 2018, 137, 291–303. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, H.; Li, J.; Liu, Y.; Yang, Y.; Wang, M. Frogspawn Inspired Hollow Fe3C@N–C as an Efficient Sulfur Host for High-Rate Lithium–Sulfur Batteries. Nanoscale 2019, 11, 21532–21541. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Bu, S.; Huang, Y.; Shao, Y.; Xiao, L.; Shi, X. N-Doped Hierarchically Porous Carbon for Highly Efficient Metal-Free Catalytic Activation of Peroxymonosulfate in Water: A Non-Radical Mechanism. Chemosphere 2019, 216, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ye, L.; Ye, Z.; Xie, S.; Qiu, Y.; Liao, F.; Lin, C.; Liu, M. N, P Co-Doped Core/Shell Porous Carbon as a Highly Efficient Peroxymonosulfate Activator for Phenol Degradation. Sep. Purif. Technol. 2021, 276, 119286. [Google Scholar] [CrossRef]
- Lai, L.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.; Gong, H.; Shen, Z.; Lin, J.; Ruoff, R.S. Exploration of the Active Center Structure of Nitrogen-Doped Graphene-Based Catalysts for Oxygen Reduction Reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.; Kim, M.; Lim, H.; Zhang, M.; You, J.; Yun, J.-H.; Bando, Y.; Li, J.; Yamauchi, Y. Nanoarchitectured Metal–Organic Framework-Derived Hollow Carbon Nanofiber Filters for Advanced Oxidation Processes. J. Mater. Chem.a 2019, 7, 13743–13750. [Google Scholar] [CrossRef]
- Cui, Q.; Zhang, W.; Chai, S.; Zuo, Q.; Kim, K.-H. The Potential of Green Biochar Generated from Biogas Residue as a Heterogeneous Persulfate Activator and Its Non-Radical Degradation Pathways: Adsorption and Degradation of Tetracycline. Environ. Res. 2022, 204, 112335. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of Sulfate Radical through Heterogeneous Catalysis for Organic Contaminants Removal: Current Development, Challenges and Prospects. Appl. Catal. B 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Wu, Q.; Chang, J.-S.; Ren, N. Singlet Oxygen-Dominated Peroxydisulfate Activation by Sludge-Derived Biochar for Sulfamethoxazole Degradation through a Nonradical Oxidation Pathway: Performance and Mechanism. Chem. Eng. J. 2019, 357, 589–599. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, J.; Tan, J.; Yu, H.; Liu, X. Facile Route for Fabricating Co(OH)2@WO3 Microspheres from Scheelite and Its Environmental Application for High-Performance Peroxymonosulfate Activation. J. Cleaner Prod. 2022, 340, 130714. [Google Scholar] [CrossRef]
- Gu, A.; Wang, P.; Chen, K.; Djam Miensah, E.; Gong, C.; Jiao, Y.; Mao, P.; Chen, K.; Jiang, J.; Liu, Y.; Yang, Y. Core-Shell Bimetallic Fe-Co MOFs to Activated Peroxymonosulfate for Efficient Degradation of 2-Chlorophenol. Sep. Purif. Technol. 2022, 298, 121461. [Google Scholar] [CrossRef]
- Hu, P.; Su, H.; Chen, Z.; Yu, C.; Li, Q.; Zhou, B.; Alvarez, P.J.J.; Long, M. Selective Degradation of Organic Pollutants Using an Efficient Metal-Free Catalyst Derived from Carbonized Polypyrrole via Peroxymonosulfate Activation. Environ. Sci. Technol. 2017, 51, 11288–11296. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Chen, F.; Yang, Q.; Luo, K.; Yao, F.; Wang, S.; Wang, X.; Wu, J.; Li, X.; Wang, D.; Zeng, G. Heterogeneous Activation of Peroxymonosulfate by Fe-Co Layered Doubled Hydroxide for Efficient Catalytic Degradation of Rhoadmine B. Chem. Eng. J. 2017, 321, 222–232. [Google Scholar] [CrossRef]
- Liu, F.; Cao, J.; Yang, Z.; Xiong, W.; Xu, Z.; Song, P.; Jia, M.; Sun, S.; Zhang, Y.; Zhong, X. Heterogeneous Activation of Peroxymonosulfate by Cobalt-Doped MIL-53(Al) for Efficient Tetracycline Degradation in Water: Coexistence of Radical and Non-Radical Reactions. J. Colloid Interface Sci. 2021, 581, 195–204. [Google Scholar] [CrossRef] [PubMed]
| P/S Doping amount | NH2-MIL-101(Fe) | Hexachlorocyclotriphosphazene | 4,4-sulfonyl diphenol | Final product name |
|---|---|---|---|---|
| 30% | 0.6825 g | 0.09 g | 0.2025 g | Fe-CNs-P/S-3 |
| 40% | 0.585 g | 0.12 g | 0.27 g | Fe-CNs-P/S-4 |
| 50% | 0.4875 g | 0.15 g | 0.3375 g | Fe-CNs-P/S-5 |
| 60% | 0.39 g | 0.18 g | 0.405 g | Fe-CNs-P/S-6 |
| 70% | 0.2925 g | 0.21 g | 0.4725 g | Fe-CNs-P/S-7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





