Submitted:
11 September 2024
Posted:
12 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. ELISA Assay for Human MUC5AC Detection from Human Serum
2.3. Statistical Considerations
3. Results
3.1. Role of sMUC5AC in Patients Receiving NAT
3.1.1. sMUC5AC Level Is Associated with Clinicopathological Features in the Resected Sample
3.1.2. Association of sMUC5AC Level on Outcome
3.1.3. Pre-Surgery Model to Predict PFS
3.1.4. High vs. Low MUC5AC Groups
3.2. sMUC5AC as a Predictor for Recurrence Post-Surgery
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J Clin 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O'Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. New England Journal of Medicine 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Groot, V.P.; Daamen, L.A.; He, J.; Wolfgang, C.L.; Molenaar, I.Q. Patterns of Recurrence After Surgery for Pancreatic Cancer. In Textbook of Pancreatic Cancer; Springer International Publishing: 2021; pp. 1153–1168.
- Paniccia, A.; Hosokawa, P.; Henderson, W.; Schulick, R.D.; Edil, B.H.; Mccarter, M.D.; Gajdos, C. Characteristics of 10-Year Survivors of Pancreatic Ductal Adenocarcinoma. JAMA Surgery 2015, 150, 701. [Google Scholar] [CrossRef] [PubMed]
- Oettle, H.; Post, S.; Neuhaus, P.; Gellert, K.; Langrehr, J.; Ridwelski, K.; Schramm, H.; Fahlke, J.; Zuelke, C.; Burkart, C.; et al. Adjuvant Chemotherapy With Gemcitabine vs Observation in Patients Undergoing Curative-Intent Resection of Pancreatic Cancer. JAMA 2007, 297, 267. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Stocken, D.D.; Bassi, C.; Ghaneh, P.; Cunningham, D.; Goldstein, D.; Padbury, R.; Moore, M.J.; Gallinger, S.; Mariette, C.; et al. Adjuvant Chemotherapy With Fluorouracil Plus Folinic Acid vs Gemcitabine Following Pancreatic Cancer Resection. JAMA 2010, 304, 1073. [Google Scholar] [CrossRef]
- Sinn, M.; Bahra, M.; Liersch, T.; Gellert, K.; Messmann, H.; Bechstein, W.; Waldschmidt, D.; Jacobasch, L.; Wilhelm, M.; Rau, B.M.; et al. CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. Journal of Clinical Oncology 2017, 35, 3330–3337. [Google Scholar] [CrossRef]
- Jones, R.P.; Psarelli, E.-E.; Jackson, R.; Ghaneh, P.; Halloran, C.M.; Palmer, D.H.; Campbell, F.; Valle, J.W.; Faluyi, O.; O’Reilly, D.A.; et al. Patterns of Recurrence After Resection of Pancreatic Ductal Adenocarcinoma. JAMA Surgery 2019, 154, 1038. [Google Scholar] [CrossRef]
- Izumo, W.; Higuchi, R.; Furukawa, T.; Yazawa, T.; Uemura, S.; Shiihara, M.; Yamamoto, M. Evaluation of preoperative prognostic factors in patients with resectable pancreatic ductal adenocarcinoma. Scandinavian Journal of Gastroenterology 2019, 54, 780–786. [Google Scholar] [CrossRef]
- Munir, R.; Soreide, K.; Ravindran, R.; Powell, J.J.; Harrison, E.M.; Adair, A.; Wigmore, S.J.; Parks, R.W.; Garden, O.J.; Kirkpatrick, L.; et al. Patterns of recurrence after curative-intent surgery for pancreas cancer reinforce the importance of locoregional control and adjuvant chemotherapy. 2018. [CrossRef]
- Labori, K.J.; Bratlie, S.O.; Andersson, B.; Angelsen, J.-H.; Biörserud, C.; Björnsson, B.; Bringeland, E.A.; Elander, N.; Garresori, H.; Grønbech, J.E.; et al. Neoadjuvant FOLFIRINOX versus upfront surgery for resectable pancreatic head cancer (NORPACT-1): a multicentre, randomised, phase 2 trial. The Lancet Gastroenterology & Hepatology 2024, 9, 205–217. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Duong, M.; Ahmad, S.A.; Gandhi, N.S.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L.; Chiorean, E.G.; Guthrie, K.A.; Lowy, A.M.; et al. Efficacy of Perioperative Chemotherapy for Resectable Pancreatic Adenocarcinoma. JAMA Oncology 2021, 7, 421. [Google Scholar] [CrossRef] [PubMed]
- Fietkau, R.; Ghadimi, M.; Grützmann, R.; Wittel, U.A.; Jacobasch, L.; Uhl, W.; Croner, R.S.; Bechstein, W.O.; Neumann, U.P.; Waldschmidt, D.; et al. Randomized phase III trial of induction chemotherapy followed by chemoradiotherapy or chemotherapy alone for nonresectable locally advanced pancreatic cancer: First results of the CONKO-007 trial. Journal of Clinical Oncology 2022, 40, 4008. [Google Scholar] [CrossRef]
- Ghaneh, P.; Palmer, D.; Cicconi, S.; Jackson, R.; Halloran, C.M.; Rawcliffe, C.; Sripadam, R.; Mukherjee, S.; Soonawalla, Z.; Wadsley, J.; et al. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol Hepatol 2023, 8, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Groot Koerkamp, B.; Janssen, Q.P.; van Dam, J.L.; Bonsing, B.A.; Bos, H.; Bosscha, K.P.; Haberkorn, B.C.M.; de Hingh, I.H.J.T.; Karsten, T.M.; Van der Kolk, M.B.; et al. LBA83 Neoadjuvant chemotherapy with FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy for borderline resectable and resectable pancreatic cancer (PREOPANC-2): A multicenter randomized controlled trial. Ann Oncol 2023, 34, S1323. [Google Scholar] [CrossRef]
- Katz, M.H.G.; Shi, Q.; Meyers, J.; Herman, J.M.; Chuong, M.; Wolpin, B.M.; Ahmad, S.; Marsh, R.; Schwartz, L.; Behr, S.; et al. Efficacy of Preoperative mFOLFIRINOX vs mFOLFIRINOX Plus Hypofractionated Radiotherapy for Borderline Resectable Adenocarcinoma of the Pancreas: The A021501 Phase 2 Randomized Clinical Trial. JAMA Oncol 2022, 8, 1263–1270. [Google Scholar] [CrossRef]
- Versteijne, E.; van Dam, J.L.; Suker, M.; Janssen, Q.P.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; et al. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J Clin Oncol 2022, 40, 1220–1230. [Google Scholar] [CrossRef]
- Poruk, K.E.; Gay, D.Z.; Brown, K.; Mulvihill, J.D.; Boucher, K.M.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. The clinical utility of CA 19-9 in pancreatic adenocarcinoma: diagnostic and prognostic updates. Curr Mol Med 2013, 13, 340–351. [Google Scholar] [CrossRef]
- Elbanna, K.Y.; Jang, H.J.; Kim, T.K. Imaging diagnosis and staging of pancreatic ductal adenocarcinoma: a comprehensive review. Insights Imaging 2020, 11, 58. [Google Scholar] [CrossRef]
- Bauer, A.K.; Umer, M.; Richardson, V.L.; Cumpian, A.M.; Harder, A.Q.; Khosravi, N.; Azzegagh, Z.; Hara, N.M.; Ehre, C.; Mohebnasab, M.; et al. Requirement for MUC5AC in KRAS-dependent lung carcinogenesis. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Rose, M.C.; Voynow, J.A. Respiratory Tract Mucin Genes and Mucin Glycoproteins in Health and Disease. Physiological Reviews 2006, 86, 245–278. [Google Scholar] [CrossRef]
- López-Ferrer, A.; de Bolós, C.; Barranco, C.; Garrido, M.; Isern, J.; Carlstedt, I.; Reis, C.A.; Torrado, J.; Real, F.X. Role of fucosyltransferases in the association between apomucin and Lewis antigen expression in normal and malignant gastric epithelium. Gut 2000, 47, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Current Opinion in Colloid & Interface Science 2006, 11, 164–170. [Google Scholar] [CrossRef]
- Kebouchi, M.; Hafeez, Z.; Le Roux, Y.; Dary-Mourot, A.; Genay, M. Importance of digestive mucus and mucins for designing new functional food ingredients. Food Research International 2020, 131, 108906. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, S.; Tanaka, H.; Iwauchi, T.; Yoshii, M.; Ito, G.; Amano, R.; Yamada, N.; Sawada, T.; Ohira, M.; Hirakawa, K. Identification of HLA-A*0201- and A*2402-restricted epitopes of mucin 5AC expressed in advanced pancreatic cancer. Pancreas 2011, 40, 896–904. [Google Scholar] [CrossRef]
- Nagata, K.; Horinouchi, M.; Saitou, M.; Higashi, M.; Nomoto, M.; Goto, M.; Yonezawa, S. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg 2007, 14, 243–254. [Google Scholar] [CrossRef]
- Kim, G.E.; Bae, H.I.; Park, H.U.; Kuan, S.F.; Crawley, S.C.; Ho, J.J.; Kim, Y.S. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology 2002, 123, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kumar, S.; Momi, N.; Sasson, A.R.; Batra, S.K. Mucins in pancreatic cancer and its microenvironment. Nature Reviews Gastroenterology & Hepatology 2013, 10, 607–620. [Google Scholar] [CrossRef]
- Krishn, S.R.; Ganguly, K.; Kaur, S.; Batra, S.K. Ramifications of secreted mucin MUC5AC in malignant journey: a holistic view. Carcinogenesis 2018, 39, 633–651. [Google Scholar] [CrossRef]
- Matsuyama, M.; Kondo, F.; Ishihara, T.; Yamaguchi, T.; Ito, R.; Tsuyuguchi, T.; Tawada, K.; Yokosuka, O. Evaluation of pancreatic intraepithelial neoplasia and mucin expression in normal pancreata. J Hepatobiliary Pancreat Sci 2012, 19, 242–248. [Google Scholar] [CrossRef]
- Yamasaki, H.; Ikeda, S.; Okajima, M.; Miura, Y.; Asahara, T.; Kohno, N.; Shimamoto, F. Expression and localization of MUC1, MUC2, MUC5AC and small intestinal mucin antigen in pancreatic tumors. Int J Oncol 2004, 24, 107–113. [Google Scholar] [CrossRef]
- Manne, A.; Esnakula, A.; Sheel, A.; Sara, A.; Manne, U.; Paluri, R.K.; He, K.; Yang, W.; Sohal, D.; Kasi, A.; et al. Mature MUC5AC Expression in Resected Pancreatic Ductal Adenocarcinoma Predicts Treatment Response and Outcomes. International Journal of Molecular Sciences 2024, 25, 9041. [Google Scholar] [CrossRef] [PubMed]
- Manne, A.; Esnakula, A.; Abushahin, L.; Tsung, A. Understanding the Clinical Impact of MUC5AC Expression on Pancreatic Ductal Adenocarcinoma. Cancers 2021, 13, 3059. [Google Scholar] [CrossRef]
- Manne, A.; Kasi, A.; Esnakula, A.K.; Paluri, R.K. Predictive Value of MUC5AC Signature in Pancreatic Ductal Adenocarcinoma: A Hypothesis Based on Preclinical Evidence. International Journal of Molecular Sciences 2023, 24, 8087. [Google Scholar] [CrossRef] [PubMed]
- Manne, A.; Mneimneh, W.; Elkadi, O.; Escobar, D.E.; Coley, J.; Guzman, G.B.; Fnu, S.M.d.; Alkharabsheh, O.; Khushman, M.d.M. The pattern of mucin 5AC (MUC5AC) expression using immunohistochemistry and its prognostic significance in patients with pancreatic ductal adenocarcinoma. Journal of Clinical Oncology 2020, 38, e16756. [Google Scholar] [CrossRef]
- Manne, A.; Yu, L.; Hart, P.A.; Tsung, A.; Esnakula, A. Differential Expression and Diagnostic Value of MUC5AC Glycoforms in Pancreatic Ductal Adenocarcinoma. Cancers 2023, 15, 4832. [Google Scholar] [CrossRef]
- Kaur, S.; Smith, L.M.; Patel, A.; Menning, M.; Watley, D.C.; Malik, S.S.; Krishn, S.R.; Mallya, K.; Aithal, A.; Sasson, A.R.; et al. A Combination of MUC5AC and CA19-9 Improves the Diagnosis of Pancreatic Cancer: A Multicenter Study. Am J Gastroenterol 2017, 112, 172–183. [Google Scholar] [CrossRef]
- Yue, T.; Maupin, K.A.; Fallon, B.; Li, L.; Partyka, K.; Anderson, M.A.; Brenner, D.E.; Kaul, K.; Zeh, H.; Moser, A.J.; et al. Enhanced discrimination of malignant from benign pancreatic disease by measuring the CA 19-9 antigen on specific protein carriers. PLoS One 2011, 6, e29180. [Google Scholar] [CrossRef]
- Yang, K.S.; Ciprani, D.; O’Shea, A.; Liss, A.S.; Yang, R.; Fletcher-Mercaldo, S.; Mino-Kenudson, M.; Fernández-Del Castillo, C.; Weissleder, R. Extracellular Vesicle Analysis Allows for Identification of Invasive IPMN. Gastroenterology 2021, 160, 1345–1358. [Google Scholar] [CrossRef]
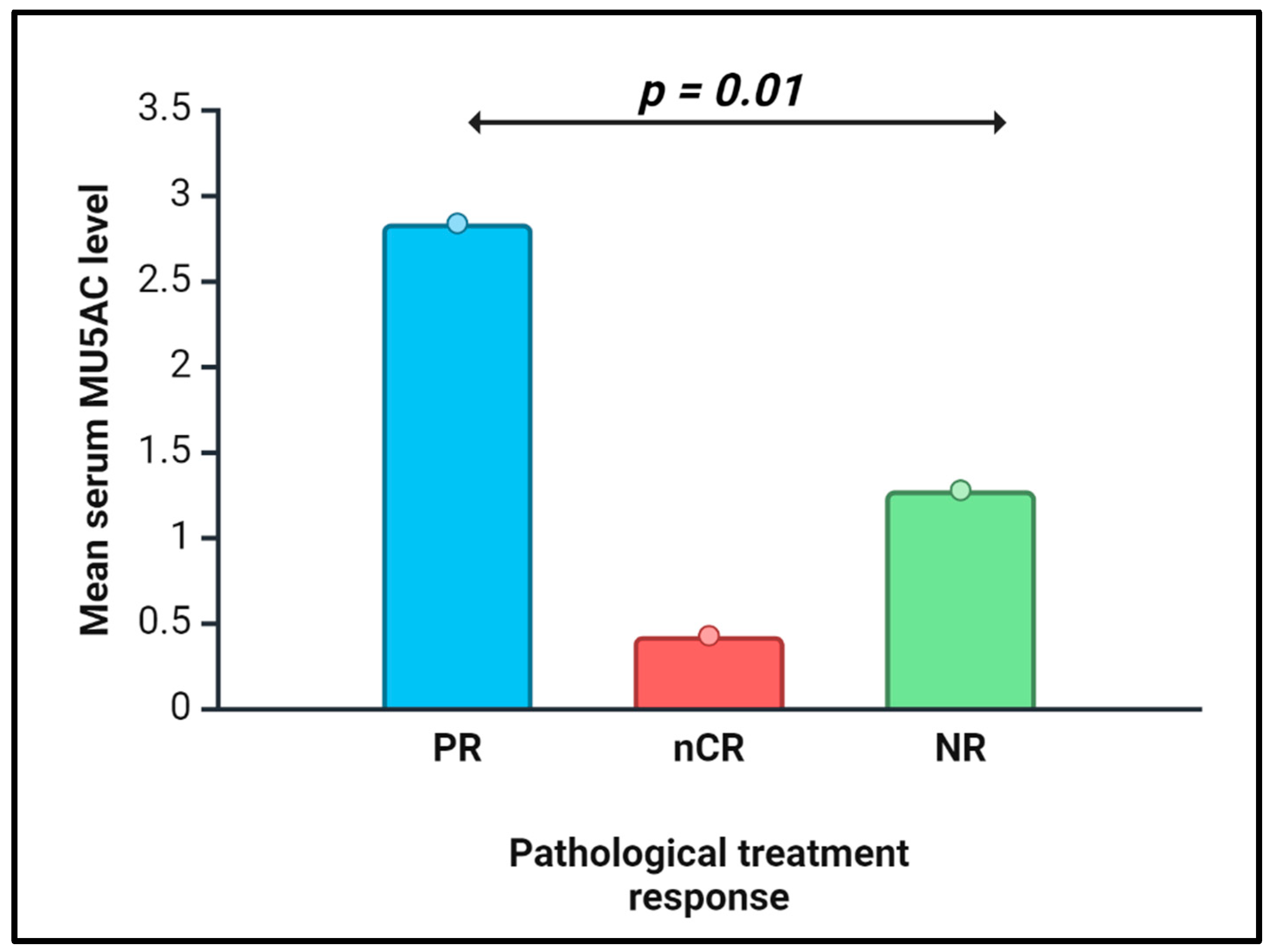
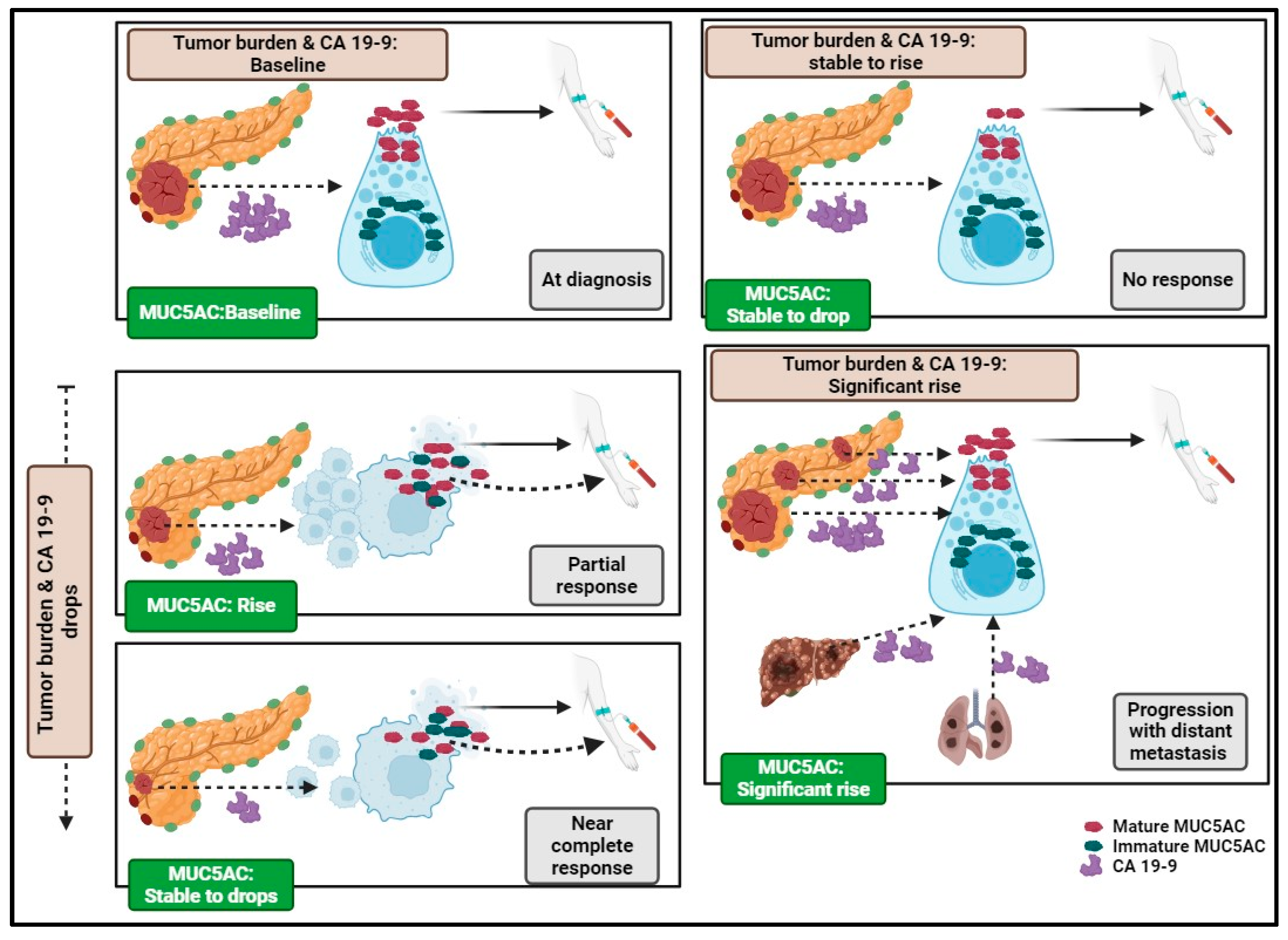
| Pathological feature | In all NAT (N=23) P-value* |
FOLFIRNOX (N=19) P-value* |
|---|---|---|
| Pathological differentiation, G1-2 vs G3 | Not significant | Not significant |
| Peripancreatic extension | Not significant | Not significant |
| Treatment effect, nCR vs. PR vs. NR | 0.01 | 0.0013 |
| Treatment effect, OR vs. NR | Not significant | 0.005 |
| Lymphovascular invasion | Not significant | Not significant |
| Perineural invasion | Not significant | Not significant |
| Margins-status, positive vs. negative | 0.0119 | 0.03 |
| Residual disease, R0 vs. R1-R2 | 0.002 | 0.007 |
| Tumor size (≤ 2 cms vs. > 2cm) | Not significant | Not significant |
| Node-status (N0 vs. N1-N2) | Not significant | Not significant |
| Premalignant lesion#, yes vs. no | Not significant | Not significant |
| Neoadjuvant CRT | Not significant | Not significant |
| Multivariate analysis | Univariate analysis | |||
|---|---|---|---|---|
| Factor tested | PFS | OS | PFS | OS |
| Serum MUC5AC level | 0.0029 | 0.1371 | 0.01 | 0.09 |
| CA19-9 on the same day* | 0.0013 | 0.2926 | 0.3 | 0.1 |
| Pathological differentiation, G1-2 vs. G3 | 0.0020 | 0.2619 | 0.01 | 0.01 |
| Lymph vascular invasion | 0.0011 | 0.0954 | 0.2 | 0.9 |
| Perineural invasion | 0.0404 | 0.0605 | 0.8 | 0.8 |
| Margins | 0.0060 | 0.0171 | 0.1 | 0.01 |
| Residual disease (R0 vs R1/R2) | 0.0040 | 0.0855 | 0.2 | 0.4 |
| Tumor size, ≤ 2cms vs. 2 cms | 0.0091 | 0.1698 | 0.5 | 0.8 |
| Node status (N0 vs. N1-N2) | 0.0163 | 0.9336 | 0.8 | 0.7 |
| Association with premalignant lesions | 0.1692 | 0.3048 | 0.4 | 0.9 |
| Peripancreatic invasion | 0.7750 | 0.7744 | 0.4 | 0.5 |
| NAT CRT, Yes vs. No | 0.0076 | 0.1435 | 0.8 | 0.4 |
| Pathological treatment response | 0.0072 | 0.3427 | 0.8 | 0.7 |
| Adjuvant therapy received (5FU-based vs. Gem based vs. none) | 0.0112 | 0.0192 | 0.09 | 0.01 |
| Extracellular MUC5AC composite score | 0.1058 | 0.9590 | 0.3 | 0.8 |
| NAT combination received# | 0.1458 | 0.1946 | 0.1 | 0.4 |
| Factor | Prob>ChiSq | Level1 | Level2 | Hazard Ratio | Lower | Upper |
|---|---|---|---|---|---|---|
| Pre-surgery Model-1 for NAT (n=23) | ||||||
| sMUC5AC | 0.0122 | 1.446621 | 1.063369 | 1.93764 | ||
| CA19-9 on the same day | 0.1039 | 1.000247 | 0.999892 | 1.000518 | ||
| NAT received | 0.3767 | 1.446621 | 1.063369 | 1.93764 | ||
| 0.1630 | FOLFOX | FOLFIRINOX | 7.3548982 | 0.1630 | 0.4457831 | |
| 0.9611 | GEM NP | FOLFIRINOX | 0.9688818 | 0.9611 | 0.2718774 | |
| 0.1930 | GEM NP | FOLFOX | 0.1317329 | 0.1930 | 0.006226 | |
| Pre-surgery Model-2 for NAT (n=23) | ||||||
| sMUC5AC | 0.0040 | 1.476825 | 1.120562 | 1.938447 | ||
| CA19-9 on the same day* | 0.0885 | 1.000254 | 0.999908 | 1.00052 | ||
| Pre-surgery Model-3 for NAT (n=23) | ||||||
| sMUC5AC | 0.0077 | 1.520411 | 1.103173 | 2.091129 | ||
| CA19-9 on the same day* | 0.8292 | 1.000046 | 0.99955 | 1.000435 | ||
| CA19-9 at diagnosis | 0.0998 | 1.000209 | 0.999901 | 1.000452 | ||
| NAT received | 0.3792 | |||||
| 0.1658 | FOLFOX | FOLFIRINOX | 7.299006 | 0.4388868 | 121.38777 | |
| 0.8624 | GEM NP | FOLFIRINOX | 1.1210998 | 0.3076474 | 4.0854067 | |
| 0.2307 | GEM NP | FOLFOX | 0.1535962 | 0.0071775 | 3.2868913 | |
| Pre-surgery Model-4 for NAT (n=23) | ||||||
| sMUC5AC | 0.0027 | 1.543962 | 1.157315 | 2.077201 | ||
| CA19-9 on the same day* | 0.7725 | 1.00006 | 0.99958 | 1.000439 | ||
| CA19-9 at diagnosis | 0.1000 | 1.0002 | 0.999906 | 1.00043 | ||
| Pre-surgery Model-1 for FOLFIRINOX (n=19) | ||||||
| sMUC5AC | 0.0192 | 1.413824 | 1.037685 | 1.898663 | ||
| CA19-9 on the same day* | 0.1588 | 1.000212 | 0.999856 | 1.000479 | ||
| Pre-surgery Model-2 for FOLFIRINOX (n=19) | ||||||
| sMUC5AC | 0.0125 | 1.487112 | 1.076844 | 2.059867 | ||
| CA19-9 on the same day* | 0.9629 | 1.00001 | 0.999487 | 1.000414 | ||
| CA19-9 at diagnosis | 0.1288 | 1.0002 | 0.999878 | 1.000454 | ||
| Factors tested | NAT-group (p-value)* | FOLFIRINOX (p-value)* |
|---|---|---|
| Threshold MUC5AC in ng/mL (N) | ≤ 1.82 (n=16) vs. >1.82 (n=7) | ≤ 1.74 (N=14) vs. > 1.74 (n=5) |
| CA19-9 mean (ng/mL) | 831 vs. 361 | 817 vs. 358 |
| nCR vs. PR vs. NR (%) | 12.5/25/62.5 vs. 0/71/29 (0.09) | 14/21/65 vs. 0/80/20 (0.06) |
| OR (%) | 38 vs. 71 (0.1) | 36 vs. 80 (0.08) |
| Mature MUC5AC expression (H-score) | 118 vs. 180 (0.1) | 131 vs. 162 |
| Immature MUC5AC expression (H-score) | 119 vs. 163 (0.1) | 133 vs. 150 |
| EC-mature MUC5AC-detection % | 50 vs. 86 (0.1) | 50 vs. 80 |
| EC-M CS | 85 vs. 176 (0.08) | 96 vs. 156 |
| Pathological differentiation# | 69% vs. 71% | 70 vs. 67 |
|
Tumor size, ≤ 2 cm vs. > 2 cm, % of patients with > 2 cm |
88 vs. 43 (0.02) | 86 vs. 40 (0.04) |
| Residual disease, R0 vs R1/R2, R1/R2% | 38 vs. 100 (0.005) | 36 vs. 100 (0.01) |
| Margin-positive % | 30 vs. 86 (0.03) | 36 vs. 80 (0.08) |
| Node positive % | 82 vs. 57 | 79 vs. 40 (0.1) |
| Perineural invasion-positive % | 94 vs. 71 | 93 vs. 60 (0.08) |
| Peripancreatic extension | 56 vs. 57 | 65 vs. 60 |
| Lymph vascular invasion- positive % | 63 vs. 49 | 57 vs. 20 (0.1) |
| Progression-free survival (in months) | 8 vs. 4 (0.04) | 8 vs. 4 (p=0.07) |
| Overall survival (in months) | 22 vs. 8 (0.08) | 17 vs. 8 |
| Term | P-value | HR | Lower 95% | Upper 95% |
|---|---|---|---|---|
| Post-surgery model for PFS – 1 | ||||
| Serum MUC5AC level | 0.0277 | 3.088526 | 1.137139 | 9.489153 |
| CA19-9 on the same day | 0.0236 | 1.000781 | 1.000114 | 1.00153 |
| Postoperative therapy | 0.09 | 3.088526 | ||
| Post-surgery model for OS – 1 | ||||
| Serum MUC5AC level | 0.0354 | 2.088487 | 0.974643 | 4.058677 |
| CA19-9 on the same day | 0.6035 | 0.999879 | 0.999356 | 1.000302 |
| CA19-9 at diagnosis | 0.0363 | 1.000531 | 0.999937 | 1.001009 |
| Post-surgery model for OS – 2 | ||||
| Serum MUC5AC level | 0.06 | 1.859388 | 0.881738 | 3.476052 |
| CA19-9 on the same day | 0.9 | 1.000004 | 0.999497 | 1.000404 |
| Post-surgery model for OS – 3 | ||||
| Serum MUC5AC level | 0.0288 | 3.340325 | 1.224686 | 12.52107 |
| CA19-9 on the same day | 0.4142 | 0.999796 | 0.999234 | 1.000254 |
| Postoperative therapy | 0.5903 | |||
| Mean* sMUC5AC |
Median# sMUC5AC | Abnormal sCA19-9^ |
Mean sMUC5AC + sCA19-9 | Median sMUC5AC+ sCA19-9 | |
|---|---|---|---|---|---|
| Sensitivity (%) | 25 | 50 | 44 | 63 | 82 |
| Specificity (%) | 100 | 67 | 100 | 100 | 67 |
| PPV (%) | 100 | 89 | 100 | 100 | 93 |
| NPV (%) | 20 | 20 | 25 | 33 | 40 |
| Accuracy (%) | 37 | 53 | 53 | 68 | 79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





