Introduction
Water is a natural resource that is critical to human survival (Adimall and Qian, 2022; Ugwu et al., 2017). It sustains all forms of life and generates jobs and wealth in the water, tourism, and recreation industries. Te global slogan “Water is Life” implies that water is one of the most basic human needs. Life as we know it on our planet would be impossible without water (Adimalla et al., 2022; Bekele et al., 2018).
Water is an essential natural resource for humans to live (Adimall, and Qian, 2022; Ugwu et al., 2017). Water nourishes all forms of life and contributes to the economic growth of several industries such as water, tourism, and recreation. The worldwide expression "Water is Life" demonstrates the idea that water is an essential and fundamental requirement for the continued existence of people. Water is essential for supporting life on our planet (Adimalla et al., 2022; Bekele et al., 2018).
The high clay content of the soil, which results in a slow rate of water percolation and a significant distance to the groundwater, will lead to a greater likelihood of bacterial survival compared to the well-aerated sand. Nevertheless, the extended duration of the flow permits ample time for the water to undergo filtration prior to its entry into the groundwater (Adetunde and Glover, 2010). A multitude of significant challenges that jeopardize the existence of humanity on Earth arise from the scarcity of potable water in numerous areas, along with the degradation of environmental aesthetics (Adimalla 2020; Bote and Desta, 2022).
Microorganisms have a significant impact on the quality of water, particularly in relation to waterborne diseases. The specific bacteria that are associated with these diseases include Salmonella spp., Shigella spp., Escherichia coli, and Vibrio cholera. These factors contribute to the occurrence of typhoid fever, diarrhea, dysentery, gastroenteritis, and cholera (Adetunde and Glover, 2010). Consequently, water is analyzed microbiologically in order to assess its hygienic condition and its appropriateness for common purposes (Ohanu et al., 2012). Yemen is classified as a developing country that does not have effective policies or programs in place to manage or prevent the spread of disease-causing microbes among its people (Al-Hadheq et al., 2023; Mengstie et al., 2020). The World Health Organization (WHO) reports that over 30% of the global population lacks access to potable water. Every year, 829,000 individuals suffer to diarrhea caused by a drink of contaminated drinking water, inadequate sanitation, and poor hand hygiene (WHO, 2019).
In order to provide a safe source of drinking water, it is essential to perform observation for the existence of pathogens. Nevertheless, doing a comprehensive examination of the water supply for each individual bacterium would be costly and time-consuming. Instead, an indicator organism is employed to signal the potential existence of harmful bacteria, which are capable of causing diseases (Charles et al., 2005). In this current research, we have performed microbiological analysis of drinking water samples from Taiz and Ibb governorates to assessment of the quality drinking water from bacterial side.
Materials and Methods
Study Area and Period
This cross-sectional study was carried out in different areas of water supply in the Taiz and Ibb governorates, Yemen, during the period from January to February 2023. In total, 99 water samples from different sources, distribution networks, and house tanks in Taiz and Ibb governorates were collected for bacterial analysis.
Sample Collection
Drinking water samples were aseptically collected in a sterilized 300-mL capacity and placed in an insulated cold box for transport to a water testing laboratory. Water samples are examined as soon as possible on arrival and always within 6 hours of collection (Lansing et al., 2005).
Microbiological Analysis
For the total bacterial count, about one mL of each water sample was separately inoculated onto a plate containing 25 mL of nutrient agar with 1 mL of water sample and incubated at 37°C for 72 hours. The total bacterial visible colonies were counted after the incubation period according to the following formula: CFU/mL = (Number of colonies × dilution factor) / volume of culture plate.
Furthermore, the detection of Enterococcus faecalis was performed by inculcating one mL of a water sample into 10 mL of Azide dextrose broth and incubating at 37°C for 24 hours. Investigation of Escherichia coli: One mL from the water sample was inculcated into 10 mL of MacConkey broth and incubated at 37°C as a presumptive test for 48 hr. In addition, the Eosin Methylene blue was inoculated by loop and incubated at 37°C for 24 h to check the positive presumptive test. The isolation of coliform was done by using MacConkey agar that differentiates between lactose and non-lactose fermenter organisms, where coliforms are lactose fermenters and produce pink colonies on this medium after the incubation period. The quality control of each process was done by incubating two control plates for checking the sterility of the samples.
Statistical Analysis
The data collected from the results of microbiology tests, were analyzed by using SPSS Version 16. P-values of less than 0.05 (P<0.05) were considered statistically significant.
Results
Results of Studied Samples from Taiz Government
The fecal bacteria were not present in all studied water sources, but the high total bacterial counts were in Al-Hoban, followed by Kalaba, and the low number was in Al-Dabab, as shown in
Table 1. Our finding showed that the relationship between the free chlorine ratio and the total bacterial count was variable in some regions, where the high ratio of free chlorine was in the distribution reservoir and the low ratio was in Bab Mosa, whereas in Al-Shamasi, Al-Markazi, and Kalaba, the ratio was variable. However, the total bacterial count was high in Bab Mosa, while in Kalaba, Al-Shmasi, and the distribution reserve, it was the same. It also shows no growth of the fecal bacteria in all studied regions.
The total bacterial count before and after sterilization and the effectiveness of sterilization showed that the effectiveness of sterilization was high in Al-Markazi, low in Bab Mosa, and variable in other regions, as shown in
Table 2.
Results of Studied Samples from Ibb Government
The number of bacteria in samples collected from different sources of water in the Ibb government was higher in Al-Sign Al-Markazi well and lower in Al-Salaba; however, there was no growth of fecal bacteria in all studied water samples, as shown in
Table 3.
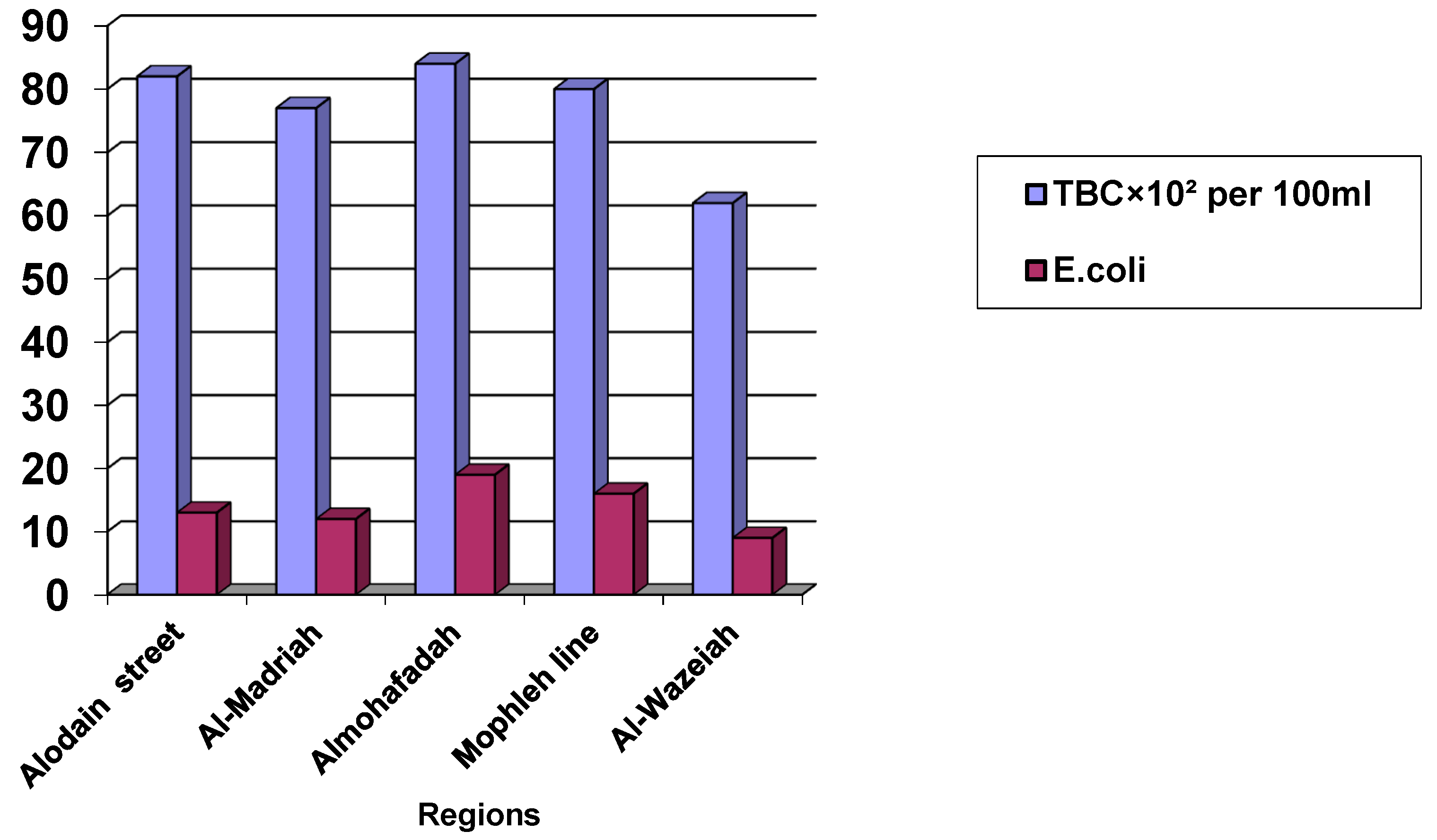
Our finding showed that the number of bacteria in samples taken from the distribution network of five regions supplied by Al-Salaba and Al-Mala'ab wells was higher in the Mophleh line, followed by Al-Madariah, Alodain Street, and Al-Wazeiah regions, but the lower number was in the Al-Mohafadah region. However, the fecal bacteria were not present in all regions, as shown in
Table 4.
Our results showed that there where the higher number of total bacteria and
E. coli were present in Al-Mohafadah region and the lower number were in Al-Wazeiah region, however, the
Enterococcus fecalis was not present, as showing as in
Figure 1.
The number of bacteria in samples taken from the distribution network of the two regions was supplied by Al-Signs Al-Markazi well, where a higher number was present in the Wadi Al-Dahab region and a lower number in the Al-Signs Al-Markazi region, and
Enterococcus faecalis was not present, as shown in
Table 5. Our findings showed that the number of bacteria in samples taken from the distribution network of two regions supplied by Al-Signs Al-Markazi well, where a higher number was present in the Wadi Al-Dahab region and a lower number in the Al-Signs Al-Markazi region and
Enterococcus faecalis was not present.
These results showed that the higher number was present in the Akamat Easa region, followed by the Haratha region, while the lower number was in the Dar Al-Sharaf region. However, the fecal bacteria were not present in all studied regions, as shown in
Table 6.
Finally, the number of bacteria in samples taken from houses in tanks of three regions was supplied by seven and eight wells, where the higher number of total bacteria was present in the Haratha region and the lower number was present in Akamat Easa; however, the number of
Escherichia coli was high in Haratha, Akamat Easa, and Dar Al-Sharaf, as shown in
Table 7.
Discussion
The occurrence of waterborne diseases in developed countries is generally low due to a generally good system of water treatment, distribution, and monitoring. Waterborne diseases are among the leading causes of morbidity and mortality in low- and middle-income countries, frequently called developing countries.
Developed countries typically have good systems for treating, distributing, and monitoring water, which helps to reduce the incidence of waterborne illnesses. Low- and middle-income nations, often known as developing countries, have one of the highest rates of morbidity and mortality from waterborne diseases.
In wealthy countries, the incidence of waterborne infections is often low due to an effective system of water treatment, distribution, and monitoring. Waterborne infections are a significant source of illness and death in poor and middle-income nations, sometimes referred to as developing countries (Adimall, and Qian, 2022). Yemen, such a developing country, suffers from a lot of water-related health problems. Contaminated or polluted water is water that contains poisonous chemicals, pathogenic organisms, industrial waste, or sewage. The majority of diseases in underdeveloped nations can be attributed to the absence of potable water, such as typhoid, cholera, and hepatitis (Ohanu et al., 2012; Penna et al., 2002).
In Taiz governorate, the results of the study showed no growth of indicator bacteria in all studied water samples. This means that these waters are free from pathogenic bacteria. Thus, these waters are drinkable and fit for human consumption from a bacteriological perspective. In addition, the total bacterial count was high in Bab Mosa, while in Kalaba, Al-Shmasi, and the distribution reserve, it was the same. It also shows no growth of the fecal bacteria in all studied regions. Similar findings were reported in Thailand (Mengstie et al., 2020) and Iran (Shahryari et al., 2020). Other studies have also reported the high bacteria count in water in Iran (Kouchesfahani et al., 2015; Moazeni et al., 2012).
In addition, the present study found that there was no growth of indicator bacteria in all samples taken from the Ibb Governorate wells and distribution network, but the samples taken from house tanks (10%) showed growth of E. coli, which indicates that the water is unacceptable for human consumption according to WHO (WHO, 2015). This could be attributed to the presence of gelatinous layers accumulating on the walls of the tank, resulting in turbidity (Lewandowski and Beyenal, 2007). Similar findings were reported in Iraq (Aldhamin, 2023) and Pakistan (Shafique et al., 2020). In addition, other reports revealed that most bacteria contaminating drinking water in Aden and Hadhramout governorates were E. coli (Hassan et al., 2008; Bin Hamed and Bubakr, 2019). A high rate of E. coli was reported in drinking water in Thailand (Yongyod et al., 2023) and Indonesia (Alfian et al., 2023).
Author Contributions
Al-Hadheq and Al-Abood Conceived and designed the experiments. Al-Hadheq performed the experiments: Al-Hadheq, Al-Aboud, and Al-Arabi analyzed the data and wrote the first draft of the manuscript. Al-Maktari contributed to the writing of the manuscript. Al-Hadheq, Al-Asbahi, and Edress agree with the manuscript results and conclusions. All authors have read, revised, and approved the final manuscript.
Data Availability
The original data source could be shared upon the request of the principal investigator.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
The study protocol was approved by the medical microbiology department, faculty of sciences, Ibb University.
References
- Adimalla, N.; Qian, H. Evaluation of non-carcinogenic causing health risks (NCHR) associated with exposure of fuoride and nitrate contaminated groundwater from semiarid region of south India. Enviro Sci Poll Resear. 2022, 3. [Google Scholar] [CrossRef]
- Ugwu, S.N.; Umuokoro, A.F.; Echiegu, E.A.; Ugwuishiwu, B.O.; Enweremadu, C.C. Comparative study of the use of natural and artifcial coagulants for the treatment of sullage (domestic wastewater). Cogent Engine. 2017, 4, 1365676–1365713. [Google Scholar] [CrossRef]
- Adimalla, N.; Manne, R.; Zhang, Y.; Xu, Y.; Qian, H. Evaluation of groundwater quality and its suitability for drinking purposes in semi-arid region of Southern India: an application of GIS. Geo Inter. 2022, 37, 10843–10854. [Google Scholar] [CrossRef]
- Bekele, M.; Dananto, M.; Tadele, D. International Institute for applied research article number: se-. Appl Res J Enviro Engin. 2018, 1, 26–38. [Google Scholar] [CrossRef]
- Adetunde, L.A.; Glover, R.L. Bacteriological Quality of Borehole Water Used by Students of University for Development Studies, Navrongo Campus in Upper-East Region of Ghana. Current Research Journal of Biological Sciences. 2010, 2, 361–364. [Google Scholar]
- Adimalla, N. Spatial distribution, exposure, and potential health risk assessment from nitrate in drinking water from semi-arid region of South India. Human and Ecological Risk Assessment: An Intern. J. 2020, 26, 310–334. [Google Scholar] [CrossRef]
- Bote, M.E.; Desta, W.M. Removal of turbidity from domestic wastewater using electrocoagulation: optimization with response surface methodology. Chemistry Africa. 2022, 5, 123–134. [Google Scholar] [CrossRef]
- Ohanu, M.E.; Udoh, I.P.; Eleazar, C.I. Microbiological Analysis of Sachet and Tap Water in Enugu State of Nigeria. Advances in Microbiology 2012, 2, 547–551. [Google Scholar] [CrossRef]
- Al-Hadheq, A.A.; Al-Ofairi, B.A.; Al-Helali, M.; Al-Mahbashi, A.; Al-Ghoury, A.; Al-Awar, M. The Incidence of Viral Hepatitis B and Hepatitis C Infections and Associated Risk Factors among Blood Donors in Amran Governorate, Yemen. Al-Razi Univ J Med Sci. 2022, 6, 22–28. [Google Scholar] [CrossRef]
- Mengstie, Y.A.; Desta, W.M.; Alemayehu, E. Assessment of Drinking Water Quality in Urban Water Supply Systems: The Case of Hawassa City, Ethio. Intern. J Anal. Chem. 3, 1–15. [CrossRef]
- World Health Organization. Water, sanitation, hygiene and health: a primer for health professionals., Geneva (WHO/CED/PHE/WSH/19.149). Licence: 2019; CC BY-NC-SA 3.0 IGO.
- Charles P, Jan I, Pepper I, Maier M. Environmental microbiology. California: Academic press. 2000:491-5.
- Lansing M, Prescott, John P, Harely, Donald A, Klein. Microbiology. United State: Mc Graw Hill. 2005. 6th edition.P400.
- Ohanu, M.E.; Udoh, I.P.; Eleazar, C.I. Microbiological Analysis of Sachet and Tap Water in Enugu State of Nigeria. Adva. Micro. 2012, 2, 547–558. [Google Scholar] [CrossRef]
- Penna, V.T.; Martins, S.A.; Mazzola, P.G. Identification of bacteria in drinking and purified water during the monitoring of a typical water purification system. 2002, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadheq, A.A.; Al-Eryan, M.A.; Edrees, W.H.; Al-Nosary, T.A. Prevalence of Intestinal Parasitic Infections among Children Attending Some School in Amran Governorate, Yemen. J. Amr. Uni. 2023, 3, 279–288. [Google Scholar] [CrossRef]
- Shahryari, A.; Smith, C.D.; Amini, A. Degradation of Bacterial Water Quality in Drinking Water after Bottling. The Open Micro. J. 2020, 14, 1–5. [Google Scholar] [CrossRef]
- Kouchesfahani, M.; Alimohammadi, M.; Nabizadeh, R.; Aslani, H.; Rezaie, S.; Asadian, S. Pseudomonas aeruginosa and Heterotrophic Bacteria Count in Bottled Waters in Iran. Iran J Public Health 2015, 44, 1514–9. [Google Scholar]
- Moazeni, M.; Atefi, M.; Ebrahimi, A.; Razmjoo, P.; Vahid Dastjerdi, M. Evaluation of chemical and microbiological quality in 21 brands of Iranian bottled drinking waters in 2012: a comparison study on label and real contents. J Environ Public Health 2013, 469–590. [Google Scholar] [CrossRef]
- World Health Organization and UNICEF. (2015); Update and MDG Assessment. Retrieved 22 February 22, from www.wssinfo.org.
- Lewandowski Z. and Beyenal H. Fundamentals of Biofilm Research. New York: CRC press. 2007; PP 103-109.
- Aldhamin, A. Evaluation of the quality of potable water in Al-Rusafa side, Baghdad. Iraq. Revis Bionat. 2023, 8, 53. [Google Scholar] [CrossRef]
- Shafique, N.; Mirza, A.I.; Hassan, M. Assessment of Drinking Water Quality Of Sheikhupura City. Intern. J Agricul. Susta. Develop. 2020, 2, 50–65. [Google Scholar]
- Hassan, N.A.; Mugbil, N.A.; Alballem, F.A. Biological Analysis of Drinking Water in some primary and secondary schools at Aden Governorate. Aden University Journal of Natural and Applied Sciences. 2008, 3, 509–516. [Google Scholar]
- Bin Hamed, A.; Bubakr, k. Assessment of Bacteriological Quality of Drinking Water in Some Primary and Secondary Schools in Mukalla City-Hadhramout/Yemen. Hadhramout University Journal of Natural & Applied Sciences. 2019, 16, 185–192. [Google Scholar]
- Yongyod, R.; et al. Microbiological Quality and Sanitation of Food Stalls and Drinking Water Vending Machines. Enviro. Natural Resour. J. 2023, 21, 20–31. [Google Scholar] [CrossRef]
- Alfian, A.R.; Firdani, F.; Sari, P.N. Risk of Disease due to Contamination of Refile Drinkind Water Using Quantitative Microbial Risk Assessment. Suranaree J. Sci. 2023, 30, 1–6. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).