Submitted:
11 September 2024
Posted:
12 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Heterogeneous Catalytic Systems
2.1. Cu-Based Catalysts in Direct CO2 Hydrogenation
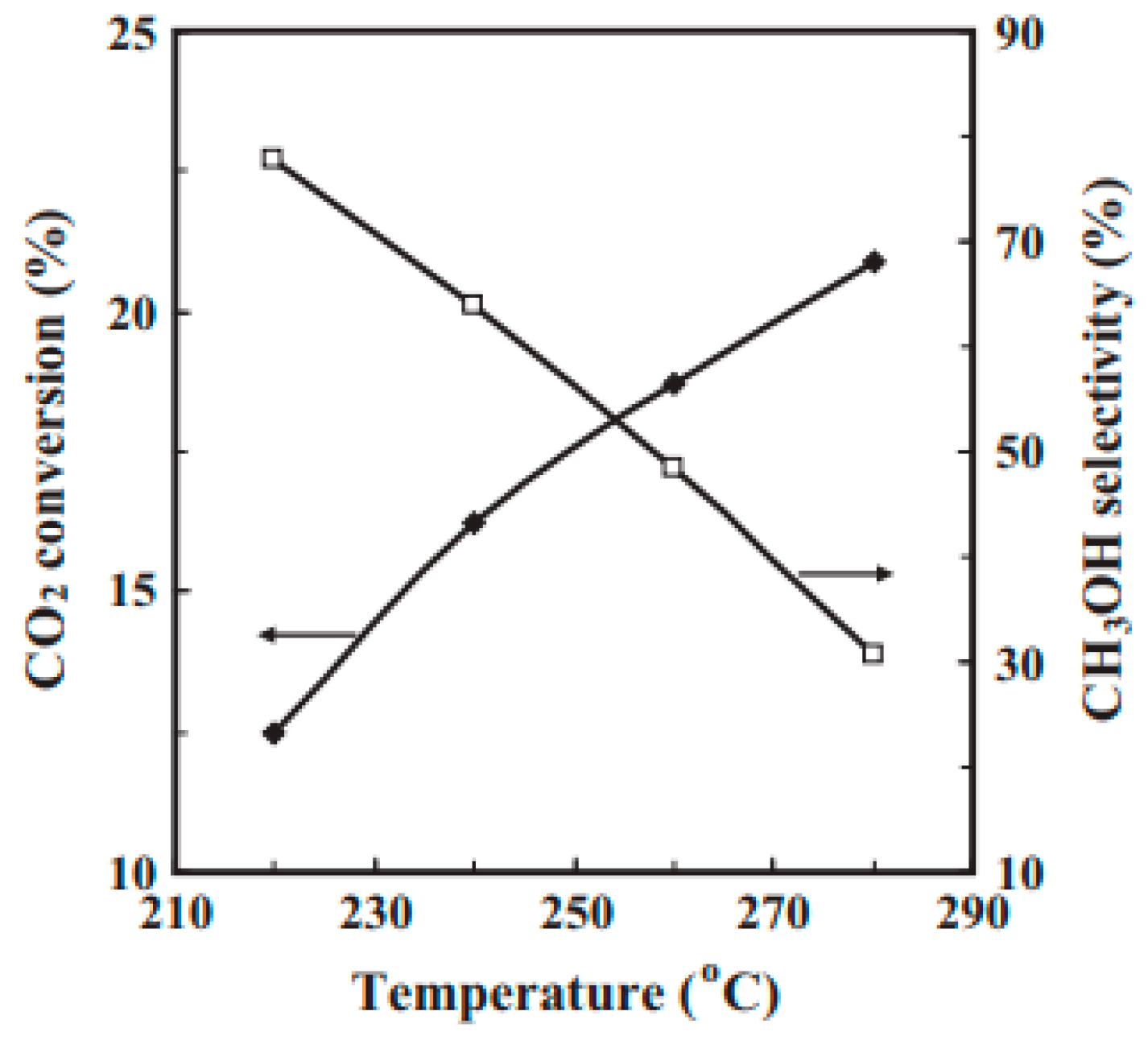
2.1.1. Cu/ZnO/Al2O3 Ternary Industrial Catalysts
2.1.2. Cu-Based Catalysts
2.1.3. Novel Cu-Based Catalyst Formulation
2.2. Noble Metal-Based Catalysts
2.3. Transitional Metal Carbides Catalysts
3. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, S.A.; John, V.M.; Pacca, S.A.; Horvath, A. Carbon Dioxide Reduction Potential in the Global Cement Industry by 2050. Cem Concr Res 2018, 114, 115–124. [CrossRef]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A. V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem Rev 2017, 117, 9804–9838. [CrossRef]
- Bansode, A.; Urakawa, A. Towards Full One-Pass Conversion of Carbon Dioxide to Methanol and Methanol-Derived Products. J Catal 2014, 309, 66–70. [CrossRef]
- Jadhav, S.G.; Vaidya, P.D.; Bhanage, B.M.; Joshi, J.B. Catalytic Carbon Dioxide Hydrogenation to Methanol: A Review of Recent Studies. Chemical Engineering Research and Design 2014, 92, 2557–2567. [CrossRef]
- Aversano, S.D.; Villante, C.; Gallucci, K.; Vanga, G. E-Fuels : A Comprehensive Review of the Most Promising Technological Alternatives towards an Energy Transition. 2024, 1–43.
- Schwiderowski, P.; Ruland, H.; Muhler, M. Current Developments in CO2 Hydrogenation towards Methanol: A Review Related to Industrial Application. Curr Opin Green Sustain Chem 2022, 38, 100688. [CrossRef]
- Https://Cdnpetrochemicalsummit.Com/ Available online: https://cdnpetrochemicalsummit.com/wp-content/uploads/2023/06/3-Mark-Berggren-for-CPS-2023-Global-Methanol.pdf (accessed on 12 August 2024).
- Biswal, T.; Shadangi, K.P.; Sarangi, P.K.; Srivastava, R.K. Conversion of Carbon Dioxide to Methanol: A Comprehensive Review. Chemosphere 2022, 298, 134299. [CrossRef]
- Nemmour, A.; Inayat, A.; Janajreh, I.; Ghenai, C. Green Hydrogen-Based E-Fuels (E-Methane, E-Methanol, E-Ammonia) to Support Clean Energy Transition: A Literature Review. Int J Hydrogen Energy 2023, 48, 29011–29033. [CrossRef]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-Liquid via Synthesis of Methanol, DME or Fischer–Tropsch-Fuels: A Review. Energy Environ Sci 2020, 13, 3207–3252. [CrossRef]
- Farfan, J.; Fasihi, M.; Breyer, C. Trends in the Global Cement Industry and Opportunities for Long-Term Sustainable CCU Potential for Power-to-X. J Clean Prod 2019, 217, 821–835. [CrossRef]
- Von Der Assen, N.; Jung, J.; Bardow, A. Life-Cycle Assessment of Carbon Dioxide Capture and Utilization: Avoiding the Pitfalls. Energy Environ Sci 2013, 6, 2721–2734. [CrossRef]
- Murthy, P.S.; Liang, W.; Jiang, Y.; Huang, J. Cu-Based Nanocatalysts for CO2Hydrogenation to Methanol. Energy and Fuels 2021, 35, 8558–8584. [CrossRef]
- Bonetto, R.; Crisanti, F.; Sartorel, A. Carbon Dioxide Reduction Mediated by Iron Catalysts: Mechanism and Intermediates That Guide Selectivity. ACS Omega 2020, 5, 21309–21319. [CrossRef]
- Wang, J.; Zhang, G.; Zhu, J.; Zhang, X.; Ding, F.; Zhang, A.; Guo, X.; Song, C. CO2Hydrogenation to Methanol over In2O3-Based Catalysts: From Mechanism to Catalyst Development. ACS Catal 2021, 11, 1406–1423. [CrossRef]
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent Advances in Carbon Dioxide Hydrogenation to Methanol via Heterogeneous Catalysis. Chem Rev 2020, 120, 7984–8034. [CrossRef]
- Stangeland, K.; Li, H.; Yu, Z. CO2 Hydrogenation to Methanol: The Structure–Activity Relationships of Different Catalyst Systems. Energy Ecol Environ 2020, 5, 272–285. [CrossRef]
- Bozzano, G.; Manenti, F. Efficient Methanol Synthesis: Perspectives, Technologies and Optimization Strategies. Prog Energy Combust Sci 2016, 56, 71–105. [CrossRef]
- Ye, R.P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 Hydrogenation to High-Value Products via Heterogeneous Catalysis. Nat Commun 2019, 10. [CrossRef]
- Etim, U.J.; Song, Y.; Zhong, Z. Improving the Cu/ZnO-Based Catalysts for Carbon Dioxide Hydrogenation to Methanol, and the Use of Methanol As a Renewable Energy Storage Media. Front Earth Sci (Lausanne) 2020, 8, 1–26. [CrossRef]
- Darji, H.R.; Kale, H.B.; Shaikh, F.F.; Gawande, M.B. Advancement and State-of-Art of Heterogeneous Catalysis for Selective CO2 Hydrogenation to Methanol. Coord Chem Rev 2023, 497, 215409. [CrossRef]
- Xu, L.; Chen, X.; Deng, C.; Hu, K.; Gao, R.; Zhang, L.; Wang, L.; Zhang, C. Hydrogenation of Carbon Dioxide to Methanol over Non-Noble Catalysts: A State-of-the-Art Review. Atmosphere (Basel) 2023, 14. [CrossRef]
- Ojelade, O.A.; Zaman, S.F. A Review on Pd Based Catalysts for CO2 Hydrogenation to Methanol: In-Depth Activity and DRIFTS Mechanistic Study. Catalysis Surveys from Asia 2020, 24, 11–37. [CrossRef]
- Pacchioni, G. From CO 2 to Methanol on Cu / ZnO / Al 2 O 3 Industrial Catalyst . What Do We Know about the Active Phase and the Reaction Mechanism ? 2024. [CrossRef]
- Miller, J.E. Initial Case for Splitting Carbon Dioxide to Carbon Monoxide and Oxygen. 2007. [CrossRef]
- Liu, X.M.; Lu, G.Q.; Yan, Z.F.; Beltramini, J. Recent Advances in Catalysts for Methanol Synthesis via Hydrogenation of CO and CO2. Ind Eng Chem Res 2003, 42, 6518–6530. [CrossRef]
- Tijm, P.J.A.; Waller, F.J.; Brown, D.M. Methanol Technology Developments for the New Millennium. Appl Catal A Gen 2001, 221, 275–282. [CrossRef]
- Galindo Cifre, P.; Badr, O. Renewable Hydrogen Utilisation for the Production of Methanol. Energy Convers Manag 2007, 48, 519–527. [CrossRef]
- Sollai, S.; Porcu, A.; Tola, V.; Ferrara, F.; Pettinau, A. Renewable Methanol Production from Green Hydrogen and Captured CO2: A Techno-Economic Assessment. Journal of CO2 Utilization 2023, 68, 102345. [CrossRef]
- Lee, B.; Lee, H.; Lim, D.; Brigljević, B.; Cho, W.; Cho, H.S.; Kim, C.H.; Lim, H. Renewable Methanol Synthesis from Renewable H2 and Captured CO2: How Can Power-to-Liquid Technology Be Economically Feasible? Appl Energy 2020, 279. [CrossRef]
- Rivarolo, M.; Bellotti, D.; Magistri, L.; Massardo, A.F. Feasibility Study of Methanol Production from Different Renewable Sources and Thermo-Economic Analysis. Int J Hydrogen Energy 2016, 41, 2105–2116. [CrossRef]
- Kanuri, S.; Roy, S.; Chakraborty, C.; Datta, S.P.; Singh, S.A.; Dinda, S. An Insight of CO2 Hydrogenation to Methanol Synthesis: Thermodynamics, Catalysts, Operating Parameters, and Reaction Mechanism. Int J Energy Res 2022, 46, 5503–5522. [CrossRef]
- Guil-López, R.; Mota, N.; Llorente, J.; Millán, E.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis. Materials 2019, 12. [CrossRef]
- Din, I.U.; Shaharun, M.S.; Alotaibi, M.A.; Alharthi, A.I.; Naeem, A. Recent Developments on Heterogeneous Catalytic CO2 Reduction to Methanol. Journal of CO2 Utilization 2019, 34, 20–33. [CrossRef]
- Zanne, M.; Počuča, M.; Bajec, P. Environmental and Economic Benefits of Slow Steaming. Transactions on Maritime Science 2013, 2, 123–127. [CrossRef]
- Behrens, M.; Stefan, K.; Girsgdies, F.; Kasatkin, I.; Hermerschmidt, F.; Mette, K.; Ruland, H.; Muhler, M.; Schlögl, R. Knowledge-Based Development of a Nitrate-Free Synthesis Route for Cu/ZnO Methanol Synthesis Catalysts via Formate Precursors. Chemical Communications 2011, 47, 1701–1703. [CrossRef]
- Prieto, G.; De Jong, K.P.; De Jongh, P.E. Towards “greener” Catalyst Manufacture: Reduction of Wastewater from the Preparation of Cu/ZnO/Al2O3 Methanol Synthesis Catalysts. Catal Today 2013, 215, 142–151. [CrossRef]
- Baltes, C.; Vukojević, S.; Schüth, F. Correlations between Synthesis, Precursor, and Catalyst Structure and Activity of a Large Set of CuO/ZnO/Al2O3 Catalysts for Methanol Synthesis. J Catal 2008, 258, 334–344. [CrossRef]
- Fan, H.; Zheng, H.; Li, Z. Preparation of Cu/ZnO/Al2O3 Catalyst under Microwave Irradiation for Slurry Methanol Synthesis. Frontiers of Chemical Engineering in China 2010, 4, 445–451. [CrossRef]
- Lei, H.; Hou, Z.; Xie, J. Hydrogenation of CO2 to CH3OH over CuO/ZnO/Al2O3 Catalysts Prepared via a Solvent-Free Routine. Fuel 2016, 164, 191–198. [CrossRef]
- Da Silva, R.J.; Pimentel, A.F.; Monteiro, R.S.; Mota, C.J.A. Synthesis of Methanol and Dimethyl Ether from the CO2 Hydrogenation over Cu·ZnO Supported on Al2 and Nb2. Journal of CO2 Utilization 2016, 15, 83–88. [CrossRef]
- Hong, Z.S.; Cao, Y.; Deng, J.F.; Fan, K.N. CO2 Hydrogenation to Methanol over Cu/ZnO/Al2O3 Catalysts Prepared by a Novel Gel-Network-Coprecipitation Method. Catal Letters 2002, 82, 37–44. [CrossRef]
- Lee, W.J.; Bordoloi, A.; Patel, J.; Bhatelia, T. The Effect of Metal Additives in Cu/Zn/Al2O3 as a Catalyst for Low-Pressure Methanol Synthesis in an Oil-Cooled Annulus Reactor. Catal Today 2020, 343, 183–190. [CrossRef]
- Li, C.; Yuan, X.; Fujimoto, K. Development of Highly Stable Catalyst for Methanol Synthesis from Carbon Dioxide. Appl Catal A Gen 2014, 469, 306–311. [CrossRef]
- Liu, Y.; Zhang, Y.; Wang, T.; Tsubaki, N. Efficient Conversion of Carbon Dioxide to Methanol Using Copper Catalyst by a New Low-Temperature Hydrogenation Process. Chem Lett 2007, 36, 1182–1183. [CrossRef]
- Gaikwad, R.; Bansode, A.; Urakawa, A. High-Pressure Advantages in Stoichiometric Hydrogenation of Carbon Dioxide to Methanol. J Catal 2016, 343, 127–132. [CrossRef]
- Angelo, L.; Kobl, K.; Tejada, L.M.M.; Zimmermann, Y.; Parkhomenko, K.; Roger, A.C. Study of CuZn MOx Oxides (M = Al, Zr, Ce, CeZr) for the Catalytic Hydrogenation of CO2 into Methanol. Comptes Rendus Chimie 2015, 18, 250–260. [CrossRef]
- Ahmed, H.E.; Ahmed, M.I. Thermal Performance of Annulus with Its Applications; A Review. Renewable and Sustainable Energy Reviews 2017, 71, 170–190. [CrossRef]
- Angelo, L.; Kobl, K.; Tejada, L.M.M.; Zimmermann, Y.; Parkhomenko, K.; Roger, A.C. Study of CuZn MOx Oxides (M = Al, Zr, Ce, CeZr) for the Catalytic Hydrogenation of CO2 into Methanol. Comptes Rendus Chimie 2015, 18, 250–260. [CrossRef]
- Lacerda de Oliveira Campos, B.; John, K.; Beeskow, P.; Herrera Delgado, K.; Pitter, S.; Dahmen, N.; Sauer, J. A Detailed Process and Techno-Economic Analysis of Methanol Synthesis from H2 and CO2 with Intermediate Condensation Steps. Processes 2022, 10. [CrossRef]
- Xiao, J.; Mao, D.; Guo, X.; Yu, J. Effect of TiO2, ZrO2, and TiO2–ZrO2 on the Performance of CuO–ZnO Catalyst for CO2 Hydrogenation to Methanol. Appl Surf Sci 2015, 338, 146–153. [CrossRef]
- Xiao, J.; Mao, D.; Wang, G.; Guo, X.; Yu, J. CO2 Hydrogenation to Methanol over CuO[Sbnd]ZnO[Sbnd]TiO2[Sbnd]ZrO2 Catalyst Prepared by a Facile Solid-State Route: The Significant Influence of Assistant Complexing Agents. Int J Hydrogen Energy 2019, 44, 14831–14841. [CrossRef]
- Li, L.; Mao, D.; Yu, J.; Guo, X. Highly Selective Hydrogenation of CO2 to Methanol over CuO-ZnO-ZrO2 Catalysts Prepared by a Surfactant-Assisted Co-Precipitation Method. J Power Sources 2015, 279, 394–404. [CrossRef]
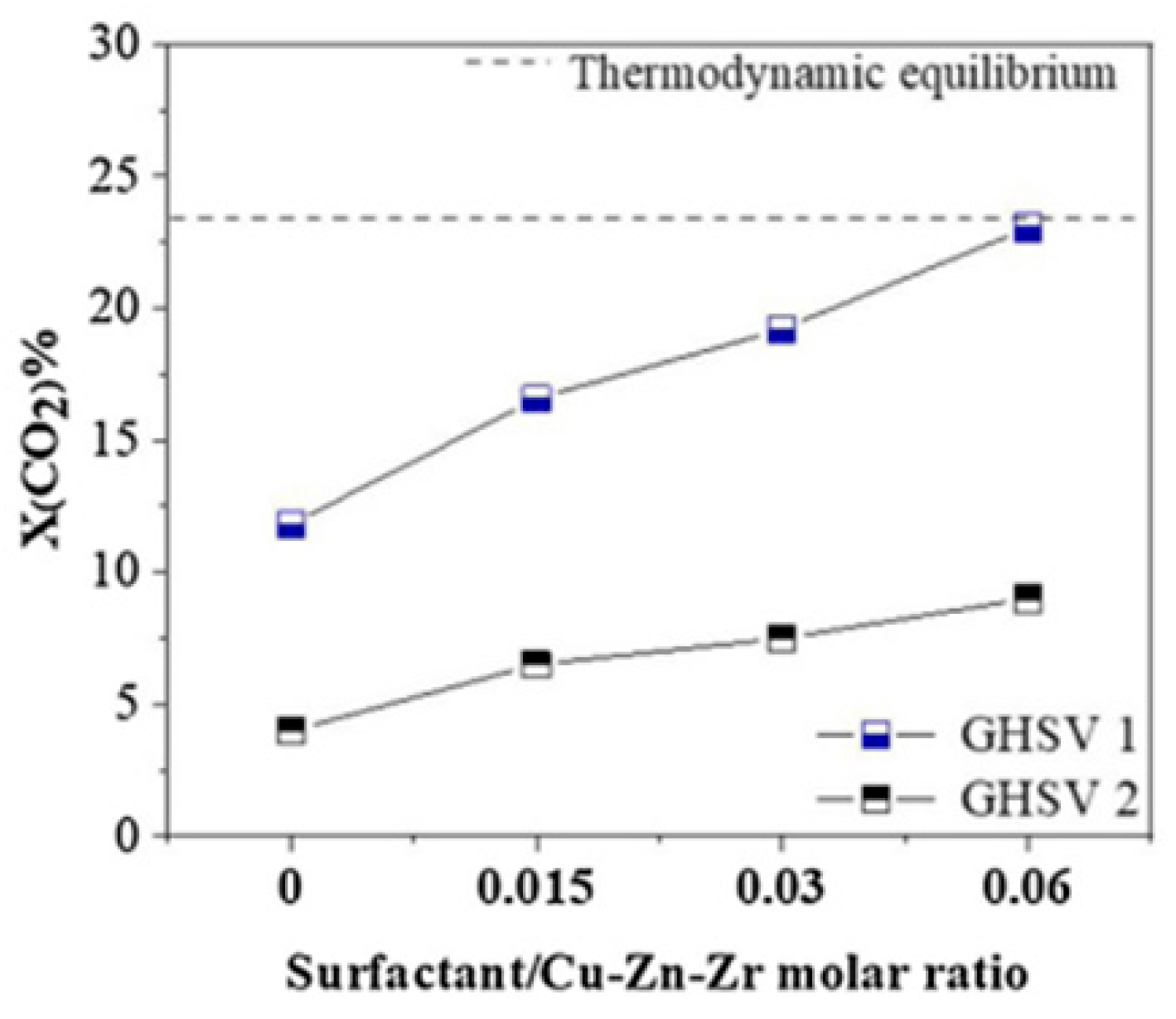
- Marcos, F.C.F.; Lin, L.; Betancourt, L.E.; Senanayake, S.D.; Rodriguez, J.A.; Assaf, J.M.; Giudici, R.; Assaf, E.M. Insights into the Methanol Synthesis Mechanism via CO2hydrogenation over Cu-ZnO-ZrO2catalysts: Effects of Surfactant/Cu-Zn-Zr Molar Ratio. Journal of CO2 Utilization 2020, 41. [CrossRef]
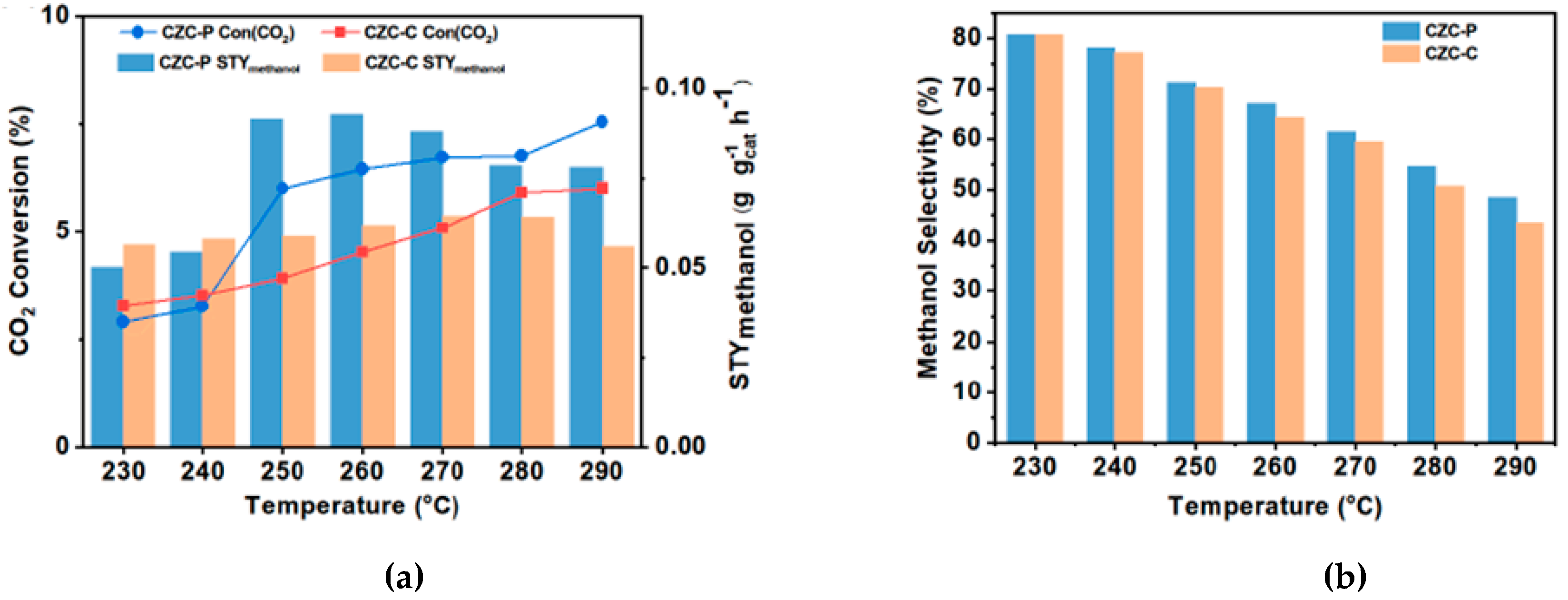
- Marcos, F.C.F.; Cavalcanti, F.M.; Petrolini, D.D.; Lin, L.; Betancourt, L.E.; Senanayake, S.D.; Rodriguez, J.A.; Assaf, J.M.; Giudici, R.; Assaf, E.M. Effect of Operating Parameters on H2/CO2 Conversion to Methanol over Cu-Zn Oxide Supported on ZrO2 Polymorph Catalysts: Characterization and Kinetics. Chemical Engineering Journal 2022, 427. [CrossRef]
- Zhan, H.; Li, F.; Gao, P.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Sun, Y. Methanol Synthesis from CO2 Hydrogenation over La-M-Cu-Zn-O (M = Y, Ce, Mg, Zr) Catalysts Derived from Perovskite-Type Precursors. J Power Sources 2014, 251, 113–121. [CrossRef]
- Xie, Z.; Hei, J.; Li, C.; Yin, X.; Wu, F.; Cheng, L.; Meng, S. Constructing Carbon Supported Copper-Based Catalysts for Efficient CO2 Hydrogenation to Methanol. RSC Adv 2023, 13, 14554–14564. [CrossRef]
- Fan, Y.J.; Wu, S.F. A Graphene-Supported Copper-Based Catalyst for the Hydrogenation of Carbon Dioxide to Form Methanol. Journal of CO2 Utilization 2016, 16, 150–156. [CrossRef]
- Verma, S.K.; Tripathi, P.; Bhatnagar, A. Carbon Nanotubes for CO2 Capture and Conversion. Nanomaterials for Carbon Dioxide Capture and Conversion Technologies 2023, 245–260. [CrossRef]
- Großmann, D.; Dreier, A.; Lehmann, C.; Grünert, W. Methanol Synthesis over Cu-ZnO Aggregates Supported on Carbon Nanotubes. Appl Catal A Gen 2015, 504, 351–360. [CrossRef]
- Gamboa, A.; Marques, L.M.; Fernandes, E.C. Electric Field Assisted Mass Production of Carbon Nanotubes on 303L Stainless Steel. Diam Relat Mater 2021, 113, 108274. [CrossRef]
- Deerattrakul, V.; Dittanet, P.; Sawangphruk, M.; Kongkachuichay, P. CO2 Hydrogenation to Methanol Using Cu-Zn Catalyst Supported on Reduced Graphene Oxide Nanosheets. Journal of CO2 Utilization 2016, 16, 104–113. [CrossRef]
- Witoon, T.; Numpilai, T.; Phongamwong, T.; Donphai, W.; Boonyuen, C.; Warakulwit, C.; Chareonpanich, M.; Limtrakul, J. Enhanced Activity, Selectivity and Stability of a CuO-ZnO-ZrO2 Catalyst by Adding Graphene Oxide for CO2 Hydrogenation to Methanol. Chemical Engineering Journal 2018, 334, 1781–1791. [CrossRef]
- Wang, G.; Chen, L.; Sun, Y.; Wu, J.; Fu, M.; Ye, D. Carbon Dioxide Hydrogenation to Methanol over Cu/ZrO2/CNTs: Effect of Carbon Surface Chemistry. RSC Adv 2015, 5, 45320–45330. [CrossRef]
- Sun, Y.; Chen, L.; Bao, Y.; Wang, G.; Zhang, Y.; Fu, M.; Wu, J.; Ye, D. Roles of Nitrogen Species on Nitrogen-Doped CNTs Supported Cu-ZrO2 System for Carbon Dioxide Hydrogenation to Methanol. Catal Today 2018, 307, 212–223. [CrossRef]
- Luo, Z.; Tian, S.; Wang, Z. Enhanced Activity of Cu/ZnO/C Catalysts Prepared by Cold Plasma for CO2 Hydrogenation to Methanol. Ind Eng Chem Res 2020, 59, 5657–5663. [CrossRef]
- Bahruji, H.; Bowker, M.; Hutchings, G.; Dimitratos, N.; Wells, P.; Gibson, E.; Jones, W.; Brookes, C.; Morgan, D.; Lalev, G. Pd/ZnO Catalysts for Direct CO2hydrogenation to Methanol. J Catal 2016, 343, 133–146. [CrossRef]
- Xu, J.; Su, X.; Liu, X.; Pan, X.; Pei, G.; Huang, Y.; Wang, X.; Zhang, T.; Geng, H. Methanol Synthesis from CO2 and H2 over Pd/ZnO/Al2O3: Catalyst Structure Dependence of Methanol Selectivity. Appl Catal A Gen 2016, 514, 51–59. [CrossRef]
- Collins, S.E.; Baltanás, M.A.; Delgado, J.J.; Borgna, A.; Bonivardi, A.L. CO2 Hydrogenation to Methanol on Ga2O3-Pd/SiO2 Catalysts: Dual Oxide-Metal Sites or (Bi)Metallic Surface Sites? Catal Today 2021, 381, 154–162. [CrossRef]
- Choi, E.J.; Lee, Y.H.; Lee, D.W.; Moon, D.J.; Lee, K.Y. Hydrogenation of CO2 to Methanol over Pd–Cu/CeO2 Catalysts. Molecular Catalysis 2017, 434, 146–153. [CrossRef]
- Fujitani, T.; Saito, M.; Kanai, Y.; Watanabe, T.; Nakamura, J.; Uchijima, T. Development of an Active Ga2O3 Supported Palladium Catalyst for the Synthesis of Methanol from Carbon Dioxide and Hydrogen. Appl Catal A Gen 1995, 125, L199–L202. [CrossRef]
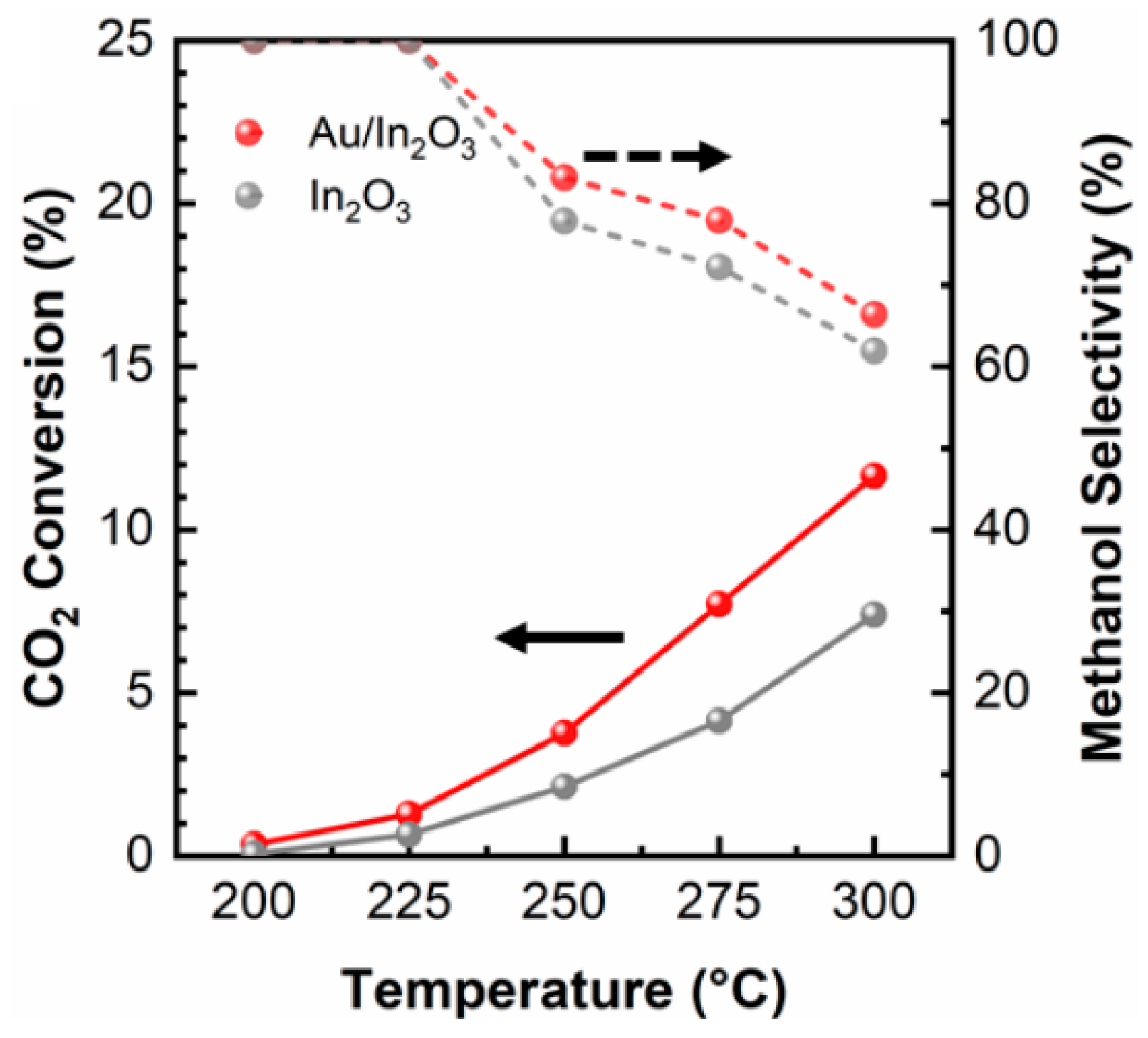
- Rui, N.; Zhang, F.; Sun, K.; Liu, Z.; Xu, W.; Stavitski, E.; Senanayake, S.D.; Rodriguez, J.A.; Liu, C.J. Hydrogenation of CO2to Methanol on a Auδ+-In2O3- XCatalyst. ACS Catal 2020, 10, 11307–11317. [CrossRef]
- Sagar, T.V.; Zavašnik, J.; Finšgar, M.; Novak Tušar, N.; Pintar, A. Evaluation of Au/ZrO2 Catalysts Prepared via Postsynthesis Methods in CO2 Hydrogenation to Methanol. Catalysts 2022, 12, 1–25. [CrossRef]
- Sun, K.; Rui, N.; Zhang, Z.; Sun, Z.; Ge, Q.; Liu, C.J. A Highly Active Pt/In2O3catalyst for CO2hydrogenation to Methanol with Enhanced Stability. Green Chemistry 2020, 22, 5059–5066. [CrossRef]
- Han, Z.; Tang, C.; Wang, J.; Li, L.; Li, C. Atomically Dispersed Ptn+ Species as Highly Active Sites in Pt/In2O3 Catalysts for Methanol Synthesis from CO2 Hydrogenation. J Catal 2021, 394, 236–244. [CrossRef]
- Dongil, A.B.; Zhang, Q.; Pastor-Pérez, L.; Ramírez-Reina, T.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Effect of Cu and Cs in the β-Mo2 c System for Co2 Hydrogenation to Methanol. Catalysts 2020, 10, 1–9. [CrossRef]
- Zhang, Y.; He, Y.; Cao, M.; Liu, B.; Li, J. High Selective Methanol Synthesis from CO2 Hydrogenation over Mo-Co-C-N Catalyst. Fuel 2022, 325, 124854. [CrossRef]
- Zhou, S.; Zeng, H.C. Boxlike Assemblages of Few-Layer MoS2Nanosheets with Edge Blockage for High-Efficiency Hydrogenation of CO2to Methanol. ACS Catal 2022, 12, 9872–9886. [CrossRef]
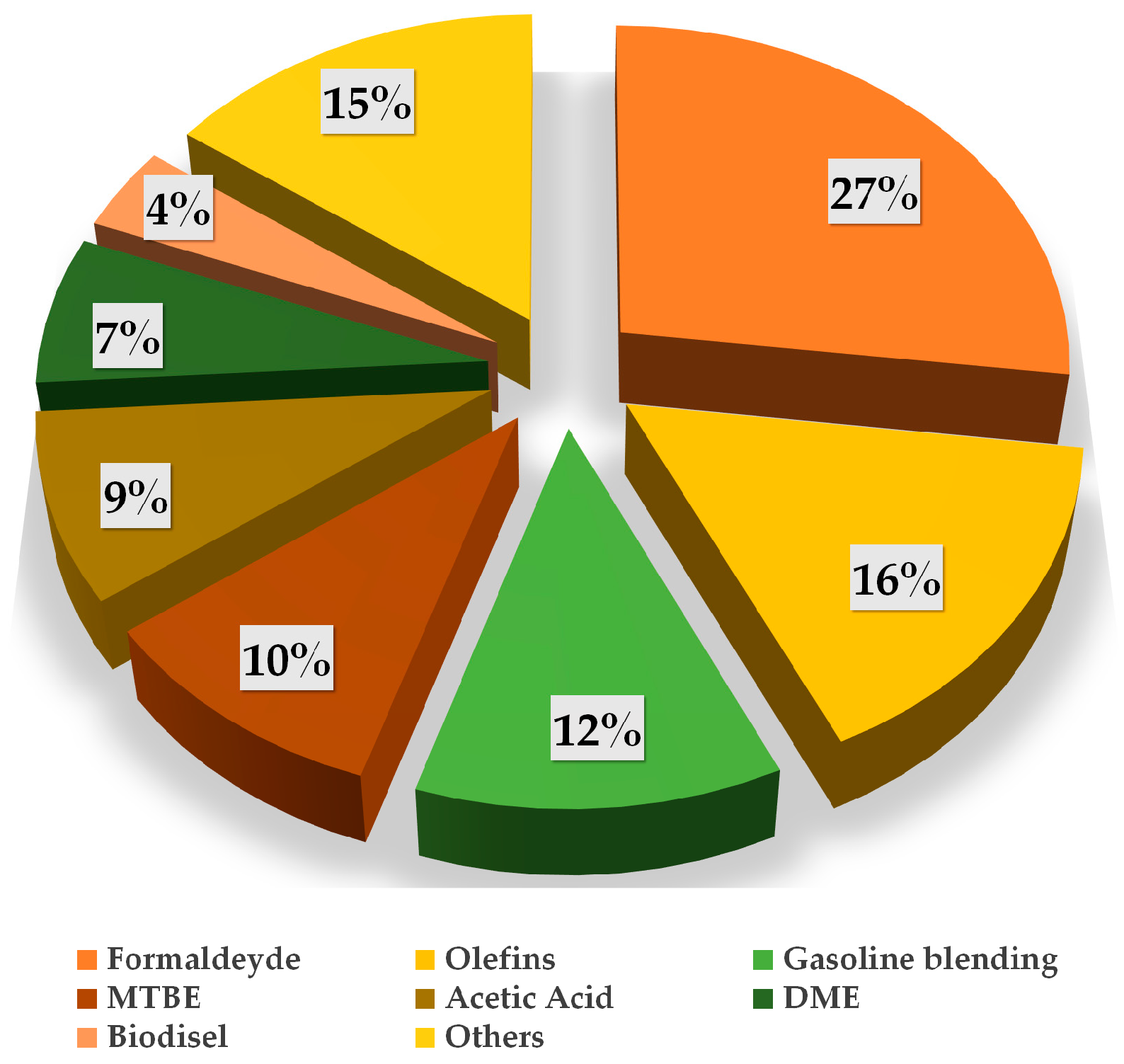
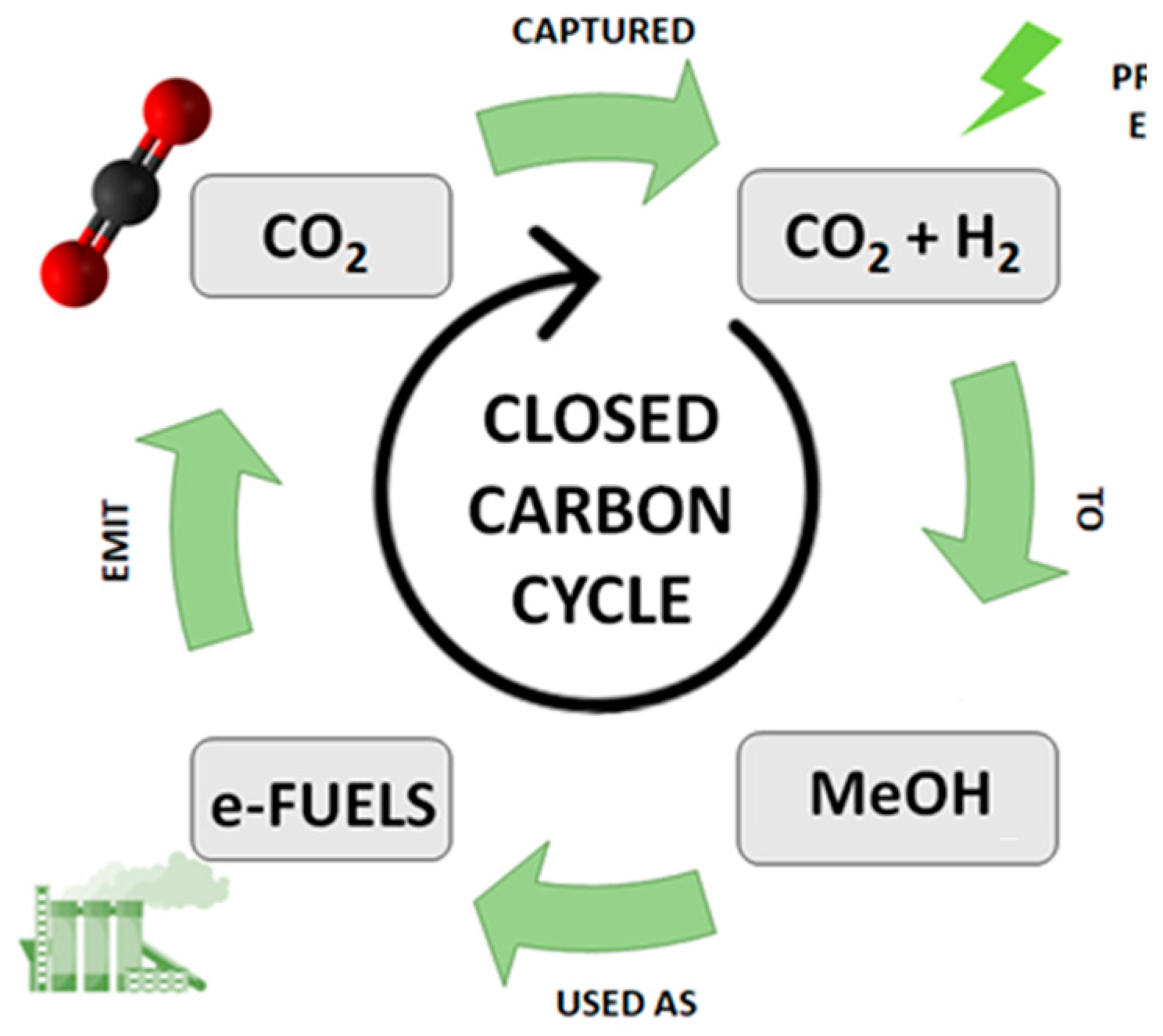
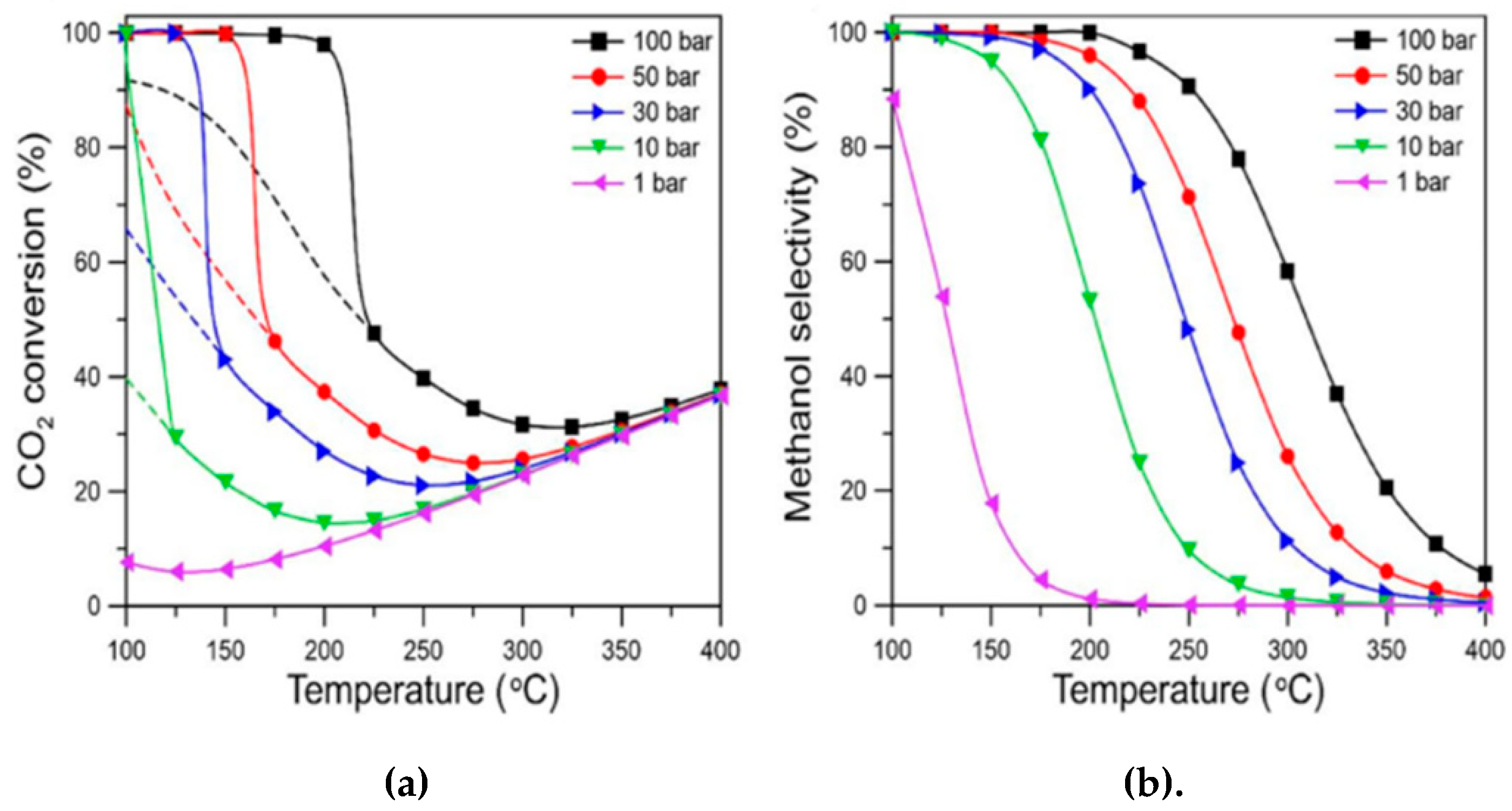
| Catalyst | Type of reactor | CO2/H2 ratio | GHSV / ml·min-1 g-1 | Conditions / bar; °C | Conversion / % | Selectivity / % | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu-based ternary industrial catalysts | |||||||||||
| Cu/ZnO/Al2O3 | Fixed-bed | 1:14 | 175 | 360; 260 | 95.7 | 98.2 | [3] | ||||
| Cu/ZnO/Al2O3 | Fixed-bed | 1:3 | 167 | 442; 280 | 65.3 | 91.9 | [46] | ||||
| Cu/ZnO/Al2O3 | Fixed-bed | 1:3 | 60 | 20; 240 | 20.1 | 31.3 | [42] | ||||
| Cu/ZnO/Al2O3 | Fixed-bed | No info | No info | 30; 230 | 18.7 | 43.0 | [44] | ||||
| CuO/ZnO/Al2O3 | Tubular | 1:3 | 60 | 30; 240 | 16.2 | 63.8 | [40] | ||||
| Cu/ZnO/Al2O3 | Annulus | 5:64 | 100 | 30; 250 | 7.0 | 98.5 | [43] | ||||
| Cu/ZnO/Al2O3 | Slurry | No info | No info | 50; 170 | 5.2 | 11.9 | [45] | ||||
| Cu/ZnO/Al2O3 | Fixed-bed | 1:4 | 167 | 50; 240 | 7.3 | 51.0 | [49] | ||||
| Cu/ZnO/Al2O3 | Fixed-bed | 1:4 | 167 | 50; 260 | 15.5 | 36.0 | [49] | ||||
| Cu/ZnO/Al2O3 | Tubular | 1:3 | No info | 50; 270 | 12.7 | 65.0 | [41] | ||||
| Cu/ZnO/Al2O3 | Flow model | 1:3 | 30-667 | 70; 100 | 53.9 | 99.8 | [50] | ||||
| Cu-based catalysts | |||||||||||
| Cu/ZnO/Al2O3/ZrO2 | Fixed-bed | 1:4 | 167 | 50; 280 | 23.2 | 33.0 | [49] | ||||
| Cu/ZnO/Al2O3/CeO2 | Fixed-bed | 1:4 | 167 | 50; 280 | 20.4 | 27.0 | [49] | ||||
| CuO/ZnO | SS tubular | 22:66 | 40 | 30; 270 | 16.1 | 36.5 | [51] | ||||
| CuO/ZnO/TiO2 | Tubular | 22:66 | 40 | 30; 270 | 16.4 | 38.8 | [51] | ||||
| CuO/ZnO/ZrO2 | Tubular | 22:66 | 40 | 30; 270 | 17.0 | 41.5 | [51] | ||||
| CuO/ZnO/TiO2/ZrO2 | Tubular | 22:66 | 40 | 30; 270 | 17.4 | 43.8 | [51] | ||||
| CuO/ZnO/TiO2/ZrO2 | Fixed-bed | 22:66 | 60 | 30; 270 | 8.1 | 47.1 | [52] | ||||
| CuO/ZnO/TiO2/ZrO2/citric acid | Fixed-bed | 22:66 | 60 | 30; 270 | 16.1 | 43.7 | [52] | ||||
| Catalyst | Type of reactor | CO2/H2 ratio |
GHSV / ml·min-1 g-1 |
Conditions / bar;°C |
Conversion / % | Selectivity / % | Reference | ||||
| Cu-based catalysts | |||||||||||
| CuO/ZnO/TiO2/ZrO2/oxalic acid | Fixed-bed | 22:66 | 60 | 30; 270 | 17.8 | 46.1 | [52] | ||||
| CuO/ZnO/ZrO2 | Quartz tubular | 1:3 | 60 | 30; 240 | 12.1 | 54.1 | [53] | ||||
| Cu/ZnO/ZrO2 | Quartz | 1:3 | 100 | 30; 250 | 23.0 | 75.0 | [54] | ||||
| Cu/ZnO/ZrO2 | Fixed-bed | No info | 62 | 30; 250 | 5.0 | 70.0 | [55] | ||||
| La/Cu/Mg/ZnO | Quartz | 1:3 | 60 | 50; 250 | 9.1 | 65.2 | [56] | ||||
| La/Cu/ZnO | Quartz | 1:3 | 60 | 50; 250 | 6.4 | 57.9 | [56] | ||||
| Novel Cu-based catalyst formulation | |||||||||||
| Cu/ZnO/Graphene | Fixed-bed | 1:3 | 40 | 15; 250 | 26.0 | 5.1 | [62] | ||||
| CuO/ZnO/ZrO2/Graphene | Fixed-bed | 3:9 | No info | 20; 200 | 4.5 | 75.9 | [63] | ||||
| CuO/ZnO/ZrO2/Al2O3/Graphene | Fixed-bed | 1:3 | 101 | 20; 240 | 14.7 | 74.0 | [58] | ||||
| Cu/ZrO2/CNTS | Fixed-bed | 23:69 | 60 | 30; 200 | 5.0 | 82.0 | [65] | ||||
| Cu/ZrO2/CNTS | Fixed-bed | 23:69 | 60 | 30; 260 | 16.3 | 68.5 | [64] | ||||
| Cu/ZnO/AC | Fixed-bed | 1:3 | 100 | 40; 230 | 2.7 | 80.0 | [66] | ||||
| Cu/ZnO/AC | Fixed-bed | 1:3 | 100 | 40; 290 | 7.5 | 50.0 | [66] | ||||
| Noble metal-based catalysts | |||||||||||
| Pd/ZnO | Fixed-bed | 1:3 | No info | 20; 250 | 11.0 | 60.0 | [67] | ||||
| Pd/ZnO/Al2O3 | Fixed-bed | 23:69 | 60 | 30; 180 | 2.9 | 79.4 | [68] | ||||
| Pd/Ga2O3/SiO2 | Glass micro | 1:3 | No info | 30; 250 | 1.9 | 65.0 | [69] | ||||
| Pd/Cu/CeO2 | Fixed-bed | 22:66 | No info | 30; 270 | 17.8 | 23.7 | [70] | ||||
| Catalyst | Type of reactor | CO2/H2 ratio | GHSV / ml·min-1 g-1 | Conditions / bar;°C | Conversion / % | Selectivity / % | Reference | ||||
| Pd/Al2O3 | Fixed-bed | 1:3 | No info | 50; 250 | 3.4 | 29.9 | [71] | ||||
| Pd/Cr2O3 | Fixed-bed | 1:3 | No info | 50; 250 | 2.1 | 22.4 | [71] | ||||
| Pd/Ga2O3 | Fixed-bed | 1:3 | No info | 50; 250 | 19.6 | 51.5 | [71] | ||||
| Pd/TiO2 | Fixed-bed | 1:3 | No info | 50; 250 | 15.5 | 3.0 | [71] | ||||
| Pd/ZnO | Fixed-bed | 1:3 | No info | 50; 250 | 13.8 | 37.5 | [71] | ||||
| Pd/ZrO2 | Fixed-bed | 1:3 | No info | 50; 250 | 0.4 | 4.3 | [71] | ||||
| Au/In2O3 | Fixed-bed | 19:76 | 350 | 50; 225 | 1.3 | 100.0 | [72] | ||||
| Au/In2O3 | Fixed-bed | 19:76 | 350 | 50; 250 | 3.8 | 83.2 | [72] | ||||
| Au/In2O3 | Fixed-bed | 19:76 | 350 | 50; 275 | 7.7 | 78.0 | [72] | ||||
| Au/In2O3 | Fixed-bed | 19:76 | 350 | 50; 300 | 11.7 | 67.8 | [72] | ||||
| Au/ZrO2 | SS tubular fixed-bed | No info | No info | 40; 240 | 6.8 | 75.0 | [73] | ||||
| Pt/In2O3 | Quartz-lined fixed-bed | 24:72 | No info | 20; 300 | 8.3 | 41.0 | [75] | ||||
| Pt/In2O3 | Vertical fixed-bed | 19:76 | 350 | 50; 300 | 17.3 | 54.0 | [74] | ||||
| Transitional metal carbides catalysts | |||||||||||
| Mo2C | Fixed-bed | 1:3 | 127 | 20; 150 | 3.3 | 60.0 | [76] | ||||
| Cs/Mo2C | Fixed-bed | 1:3 | 127 | 20; 150 | 3.0 | 50.0 | [76] | ||||
| Mo-Co-C-N | Fixed-bed | 23:68 | 100 | 20; 275 | 9.2 | 58.4 | [77] | ||||
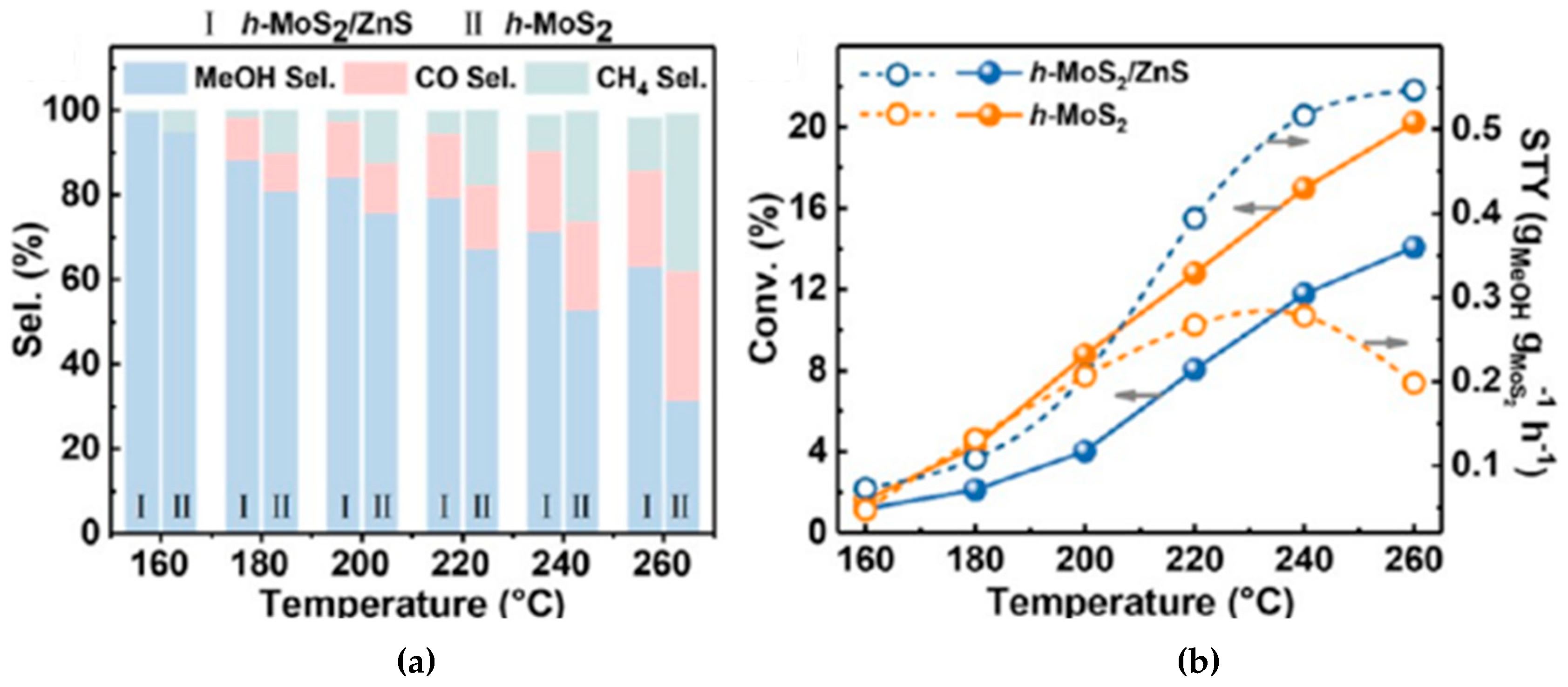
| h-MoS2/ZnS | Fixed-bed | 1:4 | 100 | 50; 260 | 13.0 | 67.3 | [78] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





