1. Introduction
Tablets are solid forms administered orally and they constitute 80% of the market because of its best stability and ease of large-scale production, adherence and treatment (Arshad et al. 2021). These dosage forms may vary in size, shape, weight, hardness, thickness, disintegration and solution characteristics, among other aspects, depending on their intended use and method of manufacture. Therefore, to ensure compliance and quality assurance, it is important to carry out tests on the finished product, which are important to verify the exact dose of the drug in the pharmaceutical form and to check if the packaging guarantees its stability, what guarantees efficacy and treatment of the patient (Krauser et al. 2020; Pinto et al. 2015, Machado et al. 2021).
The quality control is part of Good Manufacturing Practices and it ensures the quality of a drug before it is released to the market. Thus, pharmacopoeias present standard tests and equipments, along with basic characteristics that must be followed to maintain the drug quality control. In this sense, diabetes mellitus affect approximately 3% of the world population and it is among the most commom diseases in Brazil, the 5th country in incidence of diabetes in the world, in 2021, with 16.8 million adult patients (20 to 79 years old) and approximately 7.6% of the Brazilian population, thus having a large medicine dispensing for their treatment (Moisés, 2006; Guariguata et al. 2014; Abubakar et al. 2015; Coridiola; Pelegrini, 2016; SBD, 2019; Brasil, 2019). Thus, the high prevalence of diabetes mellitus and its complications demonstrate the need for investments in its prevention, on the disease control and the longitudinal care (Iser et al. 2015; Flor; Campos, 2017).
Thus, among the most used antidiabetics, Glibenclamide stands out, a second-generation hypoglycemic drug, administered in the form of a tablet, orally, for the treatment of type 2 diabetes mellitus. This drug belongs to the class of Sulphonylureas and its mechanism of action is related to the increase in insulin secretion, as they act on the pancreatic beta cells, which normalizes carbohydrate metabolism. This drug is available by the Brazilian Popular Pharmacy Program, accessible to diabetic patients who use the Unified Health System. Then, the registration of this generic drug, in 2001, resulted in 35% savings for the consumer when comparing its value with the reference drug (Brasil, 2001; Rang;Dale, 2016).
Thus, drugs can have three classifications: reference, similar and generic. In 2003, RDC nº 134 established criteria for the adequacy of similar drugs already marketed in Brazil, so that companies had to present studies that proved the therapeutic equivalence between the similar registered drug and its respective reference drug. Thus, the generic and similar equivalent are interchangeable with the innovator, when their patent protection period expires. This interchangeability is recognized through the performance of bioequivalence tests, carried out by laboratories accredited by ANVISA. The generics must have the same drug, same amount and pharmaceutical form as the reference drug, being a pharmaceutical equivalent of the innovative drug, while the similar equivalent differs only in the size of the product, packaging, labeling and excipients (Brasil, 1999; Messa et al. 2014; Medeiros et al. 2019).
In this way, quality control is an important part of Good Manufacturing Practices (GMP) and must ensure that a drug is approved only when its quality parameters are in accordance with the provisions of the Pharmacopoeias. Thus, it is essential to carry out physicochemical quality control tests of glibenclamide tablets in different pharmaceutical specialties to verify that the analyzed samples are in accordance with the Brazilian Pharmacopoeia to guarantee the expected pharmacological effect (Brasil, 2001).
Therefore, the present work aims to evaluate the reference, generic and similar tablets of glibenclamide through quality control tests, comparing the results with each other and relating the possible clinical impacts on the patient in the face of changes in the quality control of medicines.
2. Materials and Methods
2.1. Samples
Uncoated tablets containing 5 mg of glibenclamide were purchased at Pague Menos Drugstore, in Várzea Grande, Mato Grosso. To carry out the quality control tests, the samples were divided into three groups of 78 tablets each (
Table 1). All tests were performed in triplicate at the Quality Control laboratory of the Centro Universitário de Várzea Grande (UNIVAG).
Thus, the samples were separated, removing them from their primary and secondary packaging to place them in a beaker. Each beaker was identified with “reference”, “generic” and “similar”.
2.2. Quality Control of Glibenclamide Tablets
2.2.1. Determination of Average Weight
Twenty tablets of each pharmaceutical specialty (n=20) were individually weighed, in triplicate, on an analytical balance (Astral Científica) to calculate the mean weights and standard deviation, tolerating no more than two units outside the specified limits. For tablets weighing between 80 and 250 mg, the weight variation allowed is +/- 7.5% in relation to the average weight. However, no sample must contain unit weight above or below twice the percentages indicated by the Brazilian Pharmacopoeia, 6th Edition (de Oliveira Alves et al. 2017; Brasil, 2019)
2.2.2. Determination of Friability
Twenty tablets of each pharmaceutical specialty (n=20) were used and introduced into a friabilometer, which rotates around its axis at a speed of 25 ± 1 rotations per minute. With 100 rotations performed, that is, 4 minutes, the tablets were removed from the equipment and weighed again, after removing any powder residue from the tablets. No tablet, at the end of the test, can be broken, chipped, cracked or broken, and this evaluation is performed visually. Thus, friability was calculated as the difference between the initial weight and the final weight of the tablets, which is measured as a function of the percentage of powder lost. Samples with a loss equal to or less than 1.5% of their weight were considered approved in the test (Brasil, 2019).
2.2.3. Disintegration Test
This test was performed in triplicate, with the aid of an oscillating tube device (Disintegrator Mod. 301-AC), where 6 tablets of each pharmaceutical specialty (n=6) were distributed in transparent tubes contained in a basket of the equipment, adding the discs in each tube to ensure that the pills remained retained from the bottom of the basket. The basket with the respective samples was transferred to the device support and subjected to vertical movements in a liquid medium with 800 ml of distilled water maintained by the thermostat at 37º ± 1ºC, until the complete disintegration of the tablets or a maximum of 30 minutes each. At the end of the test, no residue from the tested units should remain on the metal screen of the disintegration device (Brasil, 2019).
3. Statistical Analysis
The results of the parametric tests were expressed in terms of mean ± standard error (S.E.M.). To compare more than two means, one-way analysis of variance (ANOVA) was used, and when there was statistical significance, it was followed by Student-Newman-Keuls multiple comparison test and column statistics, using the GraphPad Prism 5.0 software. Values of p < 0.05 were considered statistically significant.
4. Results
4.1. Determination of Average Weight
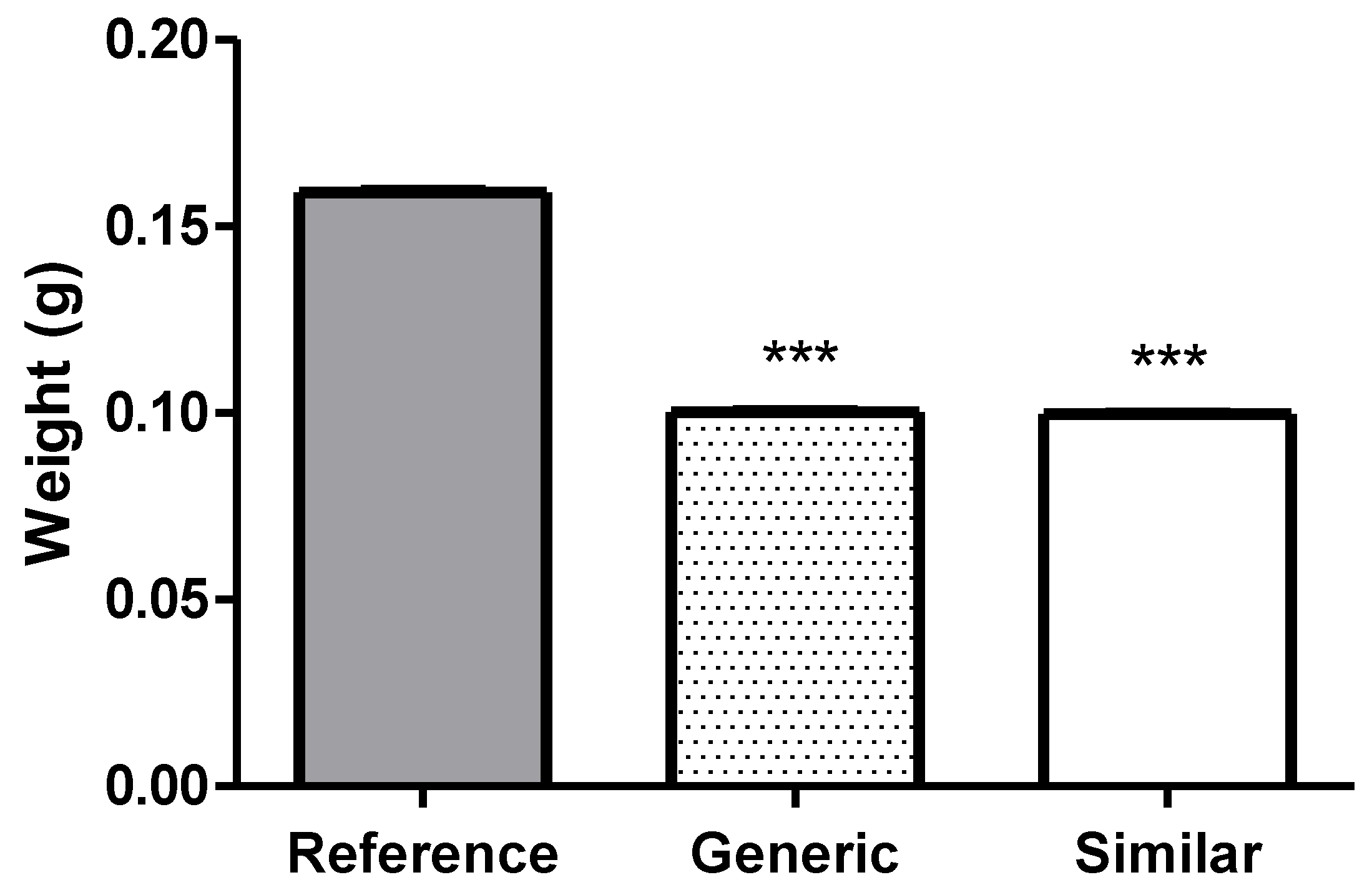
Thus, the average weight test of Reference, Generic and Similar tablets (n=20) were, respectively, 0,1591, 0,1002 and 0,0996g, and its results were presented by the mean of the values found (
Figure 1). It was possible to observe that the Generic and Similar tablets were lighter by 37.02% and 37.40% (
p < 0.001), respectively, when compared to the Reference drug.
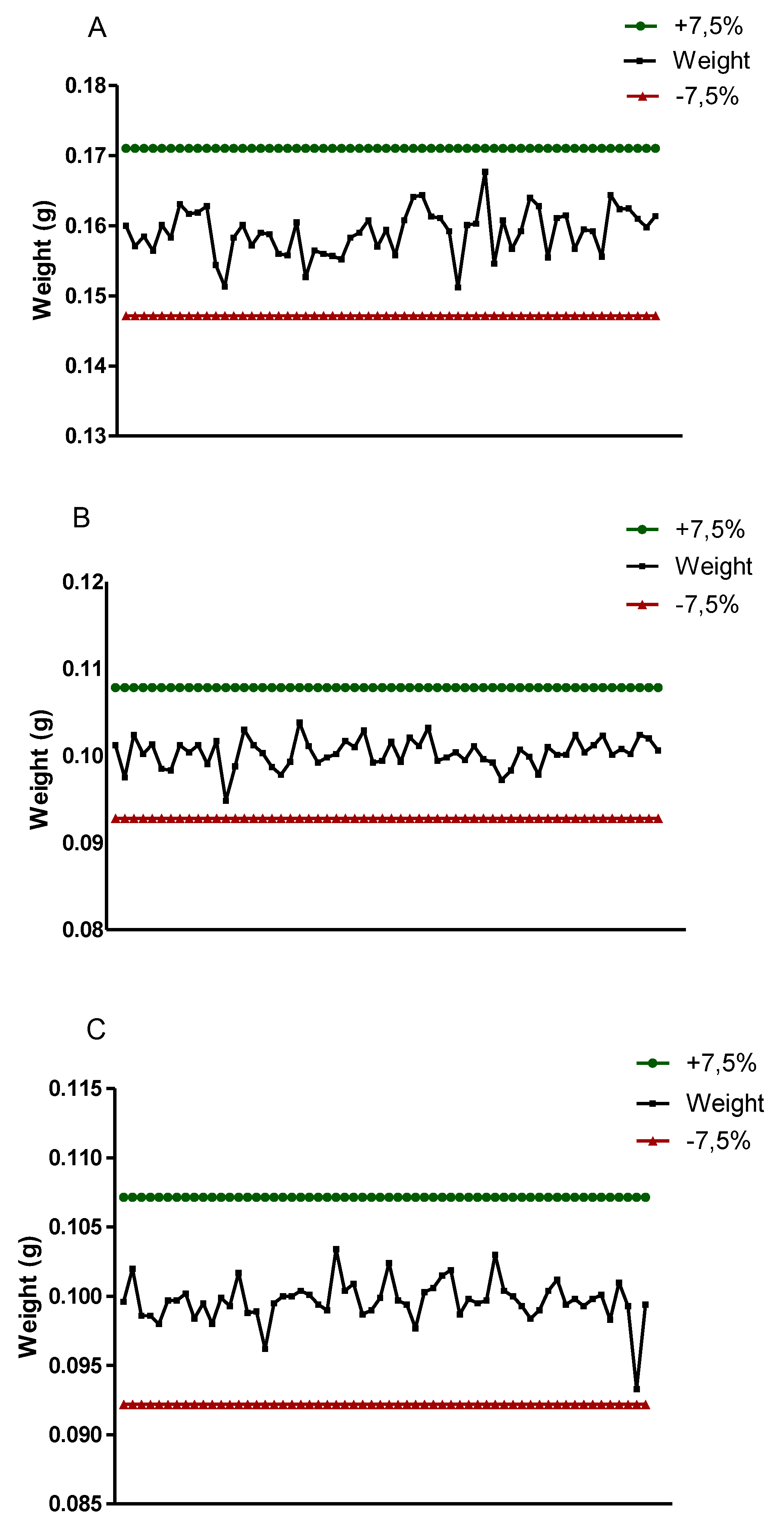
Thus, among the pills evaluated, it was observed that the highest weights found for the Reference (Daonil), Generic (Glibenclamide) and Similar (Glionil) pills were, respectively, 0.1644g, 0.1017g and 0.1034g, respectively, while the less weights obtained, in the same order, were 0.1512g, 0.0948g and 0.0983g (
Figure 2), thus, they are all in the range that correspond to tablets weighing 80 to 250 mg with a variation limit of ± 7.5% from the average weight, according to the Brazilian Pharmacopoeia, 6th Edition (Brasil, 2019, Pereira et.al. 2020).
4.2. Determination of Friability
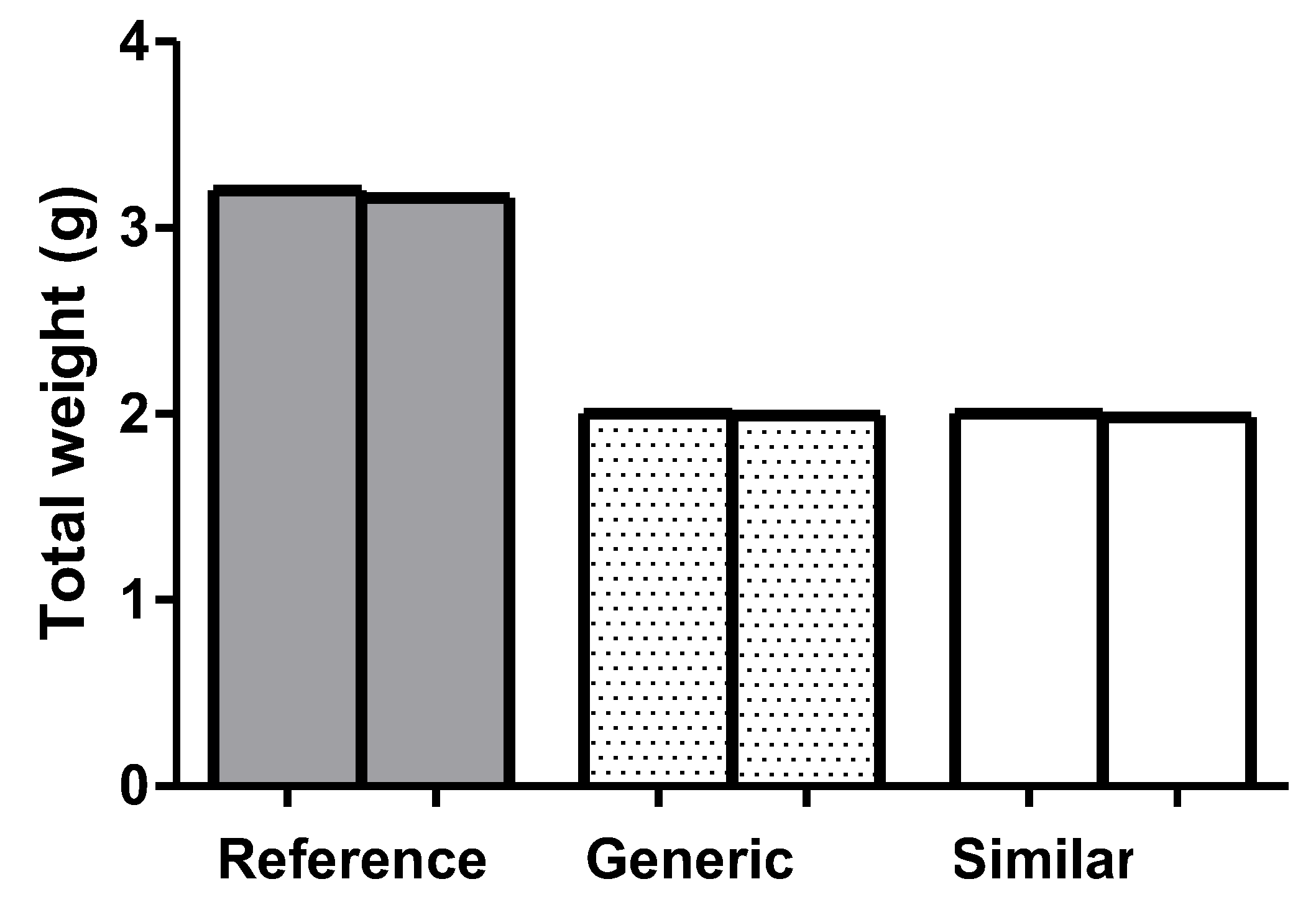
The Glibenclamide tablets (n=20) were submitted to a friabilometer and to recognize if there was a loss of mass of the tablets, they were weighed before and after submitting them to the friability test. The weight loss of the reference, generic and similar pills was, respectively, 0.54%, 0.49% and 0.28% (
Figure 3), with no statistical difference or standard deviation.
4.3. Disintegration Test
The disintegration time intervals required for the Reference, Generic and Similar Glibenclamide tablets were, respectively, 35 seconds, 29 seconds and 33 seconds (
Table 2). Thus, they remained within the 30-minute interval, as recommended in the Brazilian Pharmacopoeia, 6th edition.
5. Discussion
The quality of Glibenclamide 5 mg Reference (Daonil), Generic (Glibenclamide) and Similar Interchangeable (Glionil) medicines was analyzed. The three pharmaceutical specialties were subjected to tests of average weight, friability and tablet disintegration, and all presented results within the parameters established by the Brazilian Pharmacopoeia, 6th Edition (Brasil, 2019, Abebe et al. 2020).
According to RDC No. 301 of August 21, 2019, quality control is the execution of relevant and necessary tests, which guarantee the safety and effectiveness of the product. For these tests to be released for commercialization or distribution, their quality must be considered satisfactory (Brasil, 2019). The weight assessment test analyzes the uniformity of weight between the units of the same batch and proves whether the amount of the active ingredient declared by the laboratory is within the acceptable variation limit, determined by the Brazilian Pharmacopoeia (Costa; Gomes, 2017).
Thus, the determination of average weight was performed in triplicate with the Reference, Generic and Similar tablets (n=20) and their weights had a statistical difference, when compared to the reference drug.
The friability test, according to the Brazilian Pharmacopoeia, is applied only to uncoated tablets, and a loss of less than 1.5% of their weight are acceptable, while those that are chipped or that separate into layers are not considered (Brasil, 2019). Thus, none of the tablets evaluated at the end of the test was cracked or broken, however, two tablets (Reference) broke when removed from the blister, and were discarded from the tests. Therefore, none showed a loss of 1.5%, a limit established by the Brazilian Pharmacopoeia, 6th Edition (Brasil, 2019).
In the disintegration test, the time required for the tablets to completely disintegrate is evaluated. This test seeks to reproduce, in vitro, the release of the active ingredient from the tablets that occurs in the gastrointestinal medium and needs to disintegrate into small particles, increasing the contact surface with the medium and facilitating its absorption by the body, which is directly linked to the bioavailability and therapeutic action of the drug (Pereira et al. 2020).
Low quality medicines can result in treatment failure, which justifies the need for continuous evaluation of the effectiveness of medicines that circulate in the market, having main importance with the therapy used in the treatment of chronic diseases, such as diabetes, for which daily therapy long term is mandatory. According to Johnston and Holt (2014), the quality of medicines is not only commercial, but also legal, ethical and moral, because when there are failures to comply with quality specifications considered essential, there can be implications, such as lack of clinical response and, consequently, the loss of trust in the health system and in the regulatory authority (Abebe et al. 2020).
According to the studies by Nogueira et al. (2020), clinical and educational interventions related to pharmaceutical care have a significant impact on the type 2 diabetes mellitus control. Therefore, according to all the results obtained with the glibenclamide tablets tested, except the determination of average weight, there was no significant difference between the reference, generic and similar drugs, and all of them presented acceptable values for Brazilian legislation, which also corroborates the studies carried out by Magalhães et al. (2021), which resulted in similar values in relation to the Reference and Similar Glibenclamide tablets, which presented, respectively, the average weight of 0.159g and 0.100g, friability of 0.37% and 0.59%, and disintegration time of 34 seconds and 57 seconds.
6. Conclusion
In this study, it was possible to evaluate the quality of Glibenclamide tablets, through tests of average weight, friability and drug disintegration. Thus, the results of all analyzed samples were similar to the reference product and, thus, they were within the standard established by the Brazilian Pharmacopoeia, 6th edition, with the exception of two tablets of the reference drug that broke when removed from the blister, and were discarded.
Thus, the research carried out demonstrates the importance of quality control tests, as they guarantee the effectiveness and safety of medicines and it is ideal for the tablets to present the parameters determined by the literature to guarantee better results in the health and quality of life of the patient, in addition to improve the treatment adherence for type 2 diabetes mellitus. Therefore, this study ensures that the analyzed generic and similar drugs can be used interchangeably in clinical practice.
References
- Arshad, M. S., Zafar, S., Yousef, B., Alyassin, Y., Ali, R., AlAsiri, A., Chang, M. W., Ahmad, Z., Ali Elkordy, A., Faheem, A., & Pitt, K. (2021). A review of emerging technologies enabling improved solid oral dosage form manufacturing and processing. Advanced drug delivery reviews, 178, 113840. [CrossRef]
- Abebe, S.; Ketema, G.; Kassahun, H. In vitro Comparative Quality Assessment of Different Brands of Furosemide Tablets Marketed in Northwest Ethiopia. Drug Design, Development and Therapy, v. 14, p. 5119, 2020. [CrossRef]
- ANVISA. AGENCIA NACIONAL DE VIGILÂNCIA SANITÁRIA. Resolução RDC nº 134, 2003.
- Abubakar, I. I.; Tillmann, Taavi; Banerjee, A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet, v. 385, n. 9963, p. 117-171, 2015. [CrossRef]
- BRASIL, Agência Nacional de Vigilância Sanitária, RESOLUÇÃO - RDC Nº 134, de 29 de maio de 2003. Dispõe sobre a adequação dos medicamentos já registrados. Diário Oficial da União, Brasília, DF, 2003. Disponível em: http://antigo.anvisa.gov.br/documents/10181/2718376/RDC_134_2003_COMP.pdf/9e10dede-c5fa-4069-9b5e-8d6d000bed31 Acesso em: 05 set. 2021.
- BRASIL, Conselho Federal de Farmácia, Resolução nº 301, de 21 de agosto de 2019. Dispõe sobre as Diretrizes Gerais de Boas Práticas de Fabricação de Medicamentos. Diário Oficial da União, Brasília, DF, 21 ago. 2019. https://www.in.gov.br/web/dou/-/resolucao-rdc-n-301-de-21-de-agosto-de-2019-211914064. Acesso em: 05 set. 2021.
- BRASIL, Conselho Federal de Farmácia, Resolução Nº 357 de 20 de abril de 2001. Aprova o regulamento técnico das Boas Práticas de Farmácia. Diário Oficial da União, Brasília, DF, 2001. Disponível em: https://www.cff.org.br/userfiles/22%20-%20BRASIL_%20CONSELHO%20FEDERAL%20DE%20FARM%C3%81CIA%202001%20Resolucao_357_2001_CFF.pdf, acesso em: 05 set. 2021.
- BRASIL. Coordenação de estudos legislativos. Lei nº 9.787, de fevereiro de 1999. Dispõe sobre a vigilância sanitária, estabelece o medicamento genérico, dispõe sobre a utilização de nomes genéricos em produtos farmacêuticos e dá outras providências. Coleção de Leis da República Federativa do Brasil, Brasília, DF. 1999 fev. 10. Disponível em: <http://www.cff.org.br/userfiles/file/leis /9787.pdf>. Acesso em: 05 set. 2021.
- BRASIL. Farmacopeia Brasileira. vol. 1. 6ed ed. Brasília, 2019.
- BRASIL. Farmacopeia Brasileira, Insumos Farmacêuticos e Especialidades. vol. 2. 6ed ed. Brasília, 2019.
- BRASIL. Ministério da Saúde. Saúde aprova genérico para tratamento de gota e diabetes. Brasília, 2001b. Disponível em: <https://economia.estadao.com.br/noticias/geral,anvisa-aprova-genericos-para-gota-e-diabete,20010828p14576> Acesso em: 31 out. 2021.
- Costa, V. A. M.; Gomes, W. P. (2017). Determinação do Peso Médio e doseamento de medicamentos de referência, genéricos e similares contendo ácido acetilsalicílico (AAS). Revista Conexão Eletrônica, Três Lagoas, 14(1), 101-111.
- de Oliveira Alves, J., Órfão, M. K., Bonfilio, R., Ribeiro, E. B., Andrighett, C. R., & de Sousa Valladão, D. M. (2017). Avaliação da qualidade de medicamentos contendo clortalidona. O Mundo da Saúde, 41(03), 285-297. [CrossRef]
- Flor, L. S.; Campos, M. R., 2017. Prevalência de diabetes mellitus e fatores associados na população adulta brasileira: evidências de um inquérito de base populacional. Revista brasileira de epidemiologia, 20, 16-29. [CrossRef]
- Guariguata, L., Whiting, D. R., Hambleton, I., Beagley, J., Linnenkamp, U., & Shaw, J. E. (2014). Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes research and clinical practice, 103(2), 137–149. [CrossRef]
- Iser, B. P. M., Stopa, S. R., Chueiri, P. S., Szwarcwald, C. L., Malta, D. C., Monteiro, H. O. D. C., et al. 2015. Prevalência de diabetes autorreferido no Brasil: resultados da Pesquisa Nacional de Saúde 2013. Epidemiologia e Serviços de Saúde, 24(2), 305-314. [CrossRef]
- Krauser, D. C., Horn, R. C., Bonfanti-Azzolin, G., Norbert Deuschle, R. A., & Kessler Nunes Deuschle, V. C. (2020). AVALIAÇÃO DA QUALIDADE DE COMPRIMIDOS DISPENSADOS EM UMA FARMÁCIA PÚBLICA DO NOROESTE DO RIO GRANDE DO SUL. Revista Contexto &Amp; Saúde, 20(38), 94–100. [CrossRef]
- Machado, M.G. M.; Maior, J.F.A. S.; Ruaro, T. C.; al., E. Farmacotécnica e Tecnologia de Medicamentos Líquidos e Semissólidos. 2021. Disponível em: https://integrada.minhabiblioteca.com.br/#/books/9786556901985/. Acesso em: 05 set. 2021.
- Magalhães, R. D. C.; da Silva, V. A.; Pantoja, M. A. A.; Noleto, W. J. S.; Magalhães, E. A.; da Silva Rodrigues, T. (2021). Physical chemical control of the medicinal product glibenclamide reference and similar. Brazilian Journal of Health Review, 4(4), 16191-16194. [CrossRef]
- Medeiros, E. F. C.; Da Mota, L. V.; Alvim, H. G. O. Medicamentos de referência, genérico e similar: avaliação da qualidade dos comprimidos de captopril e enalapril. Revista de Divulgação Científica Sena Aires, v. 8, n. 1, p. 49-61, 2019.
- Messa, R.V., Farnelli, B.C.F., Menegati, C.D., Menegati, F. Avaliação da qualidade de comprimidos de hidroclorotiazida: medicamentos de referência, genérico e similar comercializados na cidade de Dourados - MS. Interbio.2014; 8(1): 72-8.
- Pereira, G. C., Barbosa, N. A., de Souza, V. O., de Lima, R. Q., da Silva, M. T. (2020). Avaliação da qualidade dos comprimidos de ibuprofeno vendidos irregularmente no centro de Manaus em comparação aos medicamentos comercializados em drogarias. Brazilian Journal of Technology, 3(4), 160-168. [CrossRef]
- Johnston, A.; Holt, D. W. (2014). Substandard drugs: a potential crisis for public health. British journal of clinical pharmacology, 78(2), 218–243. [CrossRef]
- Nogueira M, Otuyama LJ, Rocha PA, Pinto VB. Pharmaceutical care-based interventions in type 2 diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials. Einstein (Sao Paulo). 2020 Jan 31;18:eRW4686. PMID: 32022107; PMCID: PMC6986882. [CrossRef]
- Pinto, T.D.J. A.; Kaneko, T. M.; Pinto, A. F. Controle Biológico de Qualidade de Produtos Farmacêuticos, Correlatos e Cosméticos: Editora Manole, 2015. Disponível em: https://integrada.minhabiblioteca.com.br/#/books/9788520450062/. Acesso em: 2021 set. 05.
- RANG H. P.; DALE M. M. Farmacologia, 8ª edição.Rio de Janeiro: Elsevier, 2016.
- SBD - Sociedade Brasileira de Diabetes. Diretrizes da Sociedade Brasileira de Diabetes: 2019-2020. São Paulo: Clannad; 2017. SEABRA, A.L.R.
- Moisés, R.P. Tecnologia de produção de comprimidos. Fármacos & Medicamentos. São Paulo, v. 7, n. 38, p. 38-46, 2006.
- Coridiola, J. F. F. Pelegrini, D. D. Avaliação comparativa da qualidade de comprimidos de dipirona similar em relação ao de referência. SaBios: Revista de Saúde e Biologia, v.11, n.1, p.48-57, 2016.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).