1. Introduction
Calves are born without gammaglobulin, and the immune system of newborns is immature (Cortese 2009), and contracting a disease affects growth retardation and later productivity (Abe et al. 1995; Kimura et al. 1983; Takino et al. 2018). Therefore, on-farm disease prevention measures are taken by improving the rearing environment and vaccination programmes. Probiotics are one of the most widely used preventive measures against infectious diseases in animal production. In 1989, Fuller defined probiotics as "live, orally available microorganisms that have a beneficial effect on the host by improving the intestinal microflora" (Fuller 1989). In 1998, Salminen et al. redefined probiotics as "live microorganisms or foods containing them that exert a health effect when administered to the host in appropriate amounts" (Salminen et al. 1998). In addition, the effects of probiotics vary depending on the strain administered and, in particular, the immunostimulatory effects of Lactobacillus probiotics vary depending on the strain (Fang et al. 2000). Lacticaseitobacillus casei activates Th1-type immune responses (Perdigón et al. 2002) and Limosilactobacillus reuteri activates Th2-type immune responses (Mustafa et al., 2019), and some strains of bacteria themselves induce Th1-type immune responses and others induce Th2-type immune responses. Lactiplantibacillus plantarum has been isolated from fermented foods such as pickles, and examples of its functional properties include preventing obesity and insulin resistance (Okubo et al. 2013), improving immune activity and reducing stress (Nishimura et al. 2016), as well as anti-inflammatory effects (Park et al. 2013).
The Lactiplantibacillus plantarum RGU-LP1 strain (LP1) used in this study has been reported to control inflammation in adult cattle (Chida et al. 2021), and it has also been shown to have anti-inflammatory properties (Kishida et al. 2022).
It is expected to support health management by improving the immune balance and gut microbiota of newborn calves with immature immunomodulatory mechanisms. Therefore, the aim of this study was to evaluate the effects of feeding LP1 to newborn calves on growth and immune function and to compare the economic costs of calf rearing.
2. Materials and Methods
2.1. Experimental Animals
Holstein calves (male, 1 week old) were obtained from a cattle market in Hokkaido, Japan, and were housed individually in calf pens to avoid direct contact. All calves were clinically observed for a 5-day acclimation period, and healthy calves were used for the experiment. The study was divided into 13 calves each in LP1-treated (LP1) and LP1-non-treated (CN) groups. All experiments were conducted in accordance with the animal care guidelines of the Scientific Feed Laboratory co. Ltd. in accordance with the Basic Guidelines for the Conduct of Animal Experiments in Research Institutions, etc., issued by the Ministry of Education, Culture, Sports, Science and Technology. (Approval number: 078-2021-1).
2.2. Probiotics
Calves were fed milk replacer (Mildash, National Federation of Agricultural Cooperative Associations), and in the LP1 group, the milk replacer was supplemented with lyophilised granules of Lactiplantibacillus plantarum RGU LP1 strain (LP1) (Patent No. 5610472), a strain stored in our laboratory. This feed was prepared by Scientific Feed Laboratory co., ltd (Tokyo, Japan) and the bacterial count was adjusted to 10⁸ CFU/g. The dose was 10 g per calf per day and fed in the milk replacer. As a control, the same milk replacer was fed to the control group at the same time.
2.3. Study protocol for Probiotic Feeding
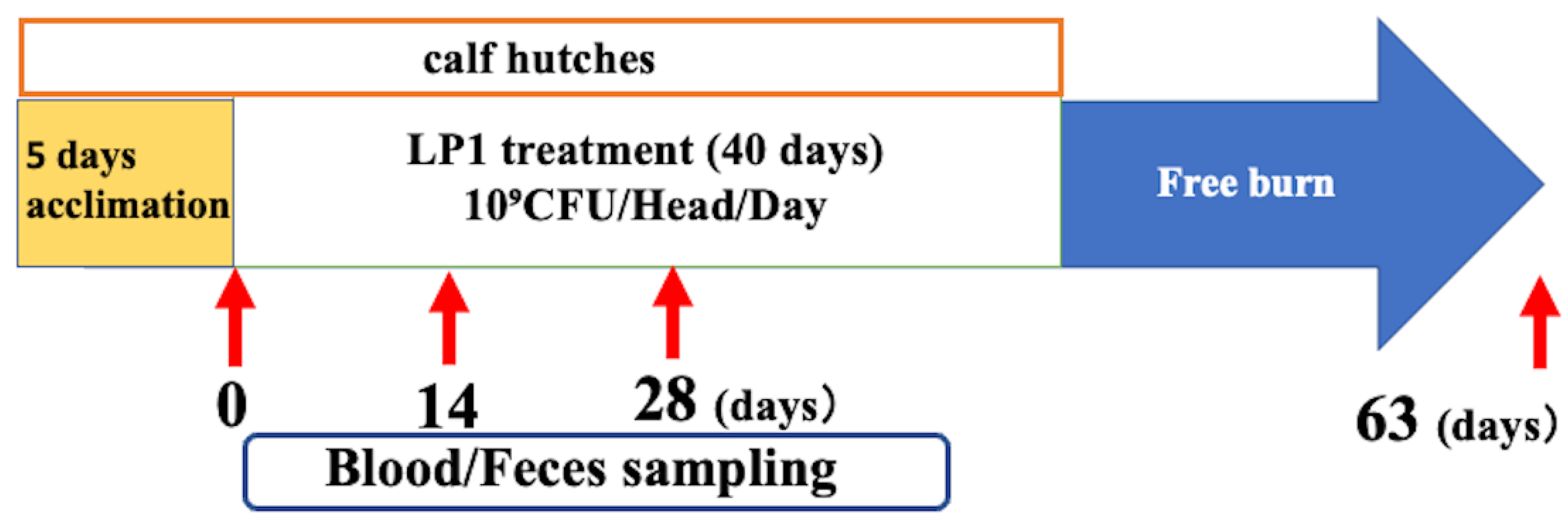
LP1 was administered after a 5-day acclimatisation period following the arrival of the calves from the livestock market. The probiotic treatment period lasted 40 days and all calves were housed in calf pens. After the dosing period, the calves were housed in open pens and observed for 63 days from the start of LP1 feeding (
Figure 1).
2.4. Faecal and Blood Samples
Faecal samples were collected at 0, 14 and 28 days after the start of LP1 administration for analysis of gut microflora. Blood samples were taken at 0, 14, 28, 49 and 63 days after the start of treatment to measure the health check, serum cytokines and to assess vaccine antibody titres. Peripheral blood mononuclear cells (PBMCs) were analysed for cytokine gene expression on days 28 and 63.
2.5. Clinical Observation
At follow-up, faecal scores, daily weight gain, number of treatments and total treatment costs were evaluated. Faecal scores were performed during the rearing period of the calves. Faecal score was defined as score 1 for normal faeces, score 2 for soft faeces and score 3 for watery faeces. Daily gain was calculated as the average daily gain per animal based on body weight at the time of initiation and at the end of the treatment period. The number of treatments was performed for 63 days from the start of dosing to the end of observation, and the number of treatments and total treatment cost (¥) per animal in each group were calculated based on the number of treatments received per individual during this period and the number of treatment points, excluding the cost of home visits.
2.6. Intestinal Bacteria Culture
Intestinal bacteria were analysed on selective media for four genera (Lactobacillus sp., Bifidobacterium sp., Clostridium sp., and Coliform bacteria) and total anaerobic bacteria. Faecal samples were diluted 10-fold in sterile phosphate-buffered saline (PBS) and then added to BBL™ LBS agar (Becton, Dickinson, Franklin Lakes, NJ, USA), BS agar, DHL agar (Nissui Pharmaceuticals, Osaka, Japan) and modified GAM agar. BS agar medium was prepared by adding BS additive to BL agar medium (Nissui Pharmaceutical Co., Ltd.) and sterilising it at 121℃ for 15 min according to the manufacturer's instructions, and then adding horse defibrinated blood to reach a final concentration of 10%. GAM agar medium was prepared by adding Bacto™ Agar (Becton, Dickinson, Franklin Lakes, NJ, USA) to GAM liquid medium (Nissui) to a final concentration of 1.5% and sterilised at 115℃ for 15 min. The DHL agar medium was incubated under aerobic conditions at 37℃ for 24 hours, and the LBS agar, BS agar, CW agar and GAM agar medium were incubated under anaerobic conditions at 37℃ for 48 hours. The genera and species of bacteria identified on the selective media used in this study were Lactobacillus for LBS agar, Bifidobacterium for BS agar, E. coli and hydrogen sulfide producing bacteria for DHL agar, and Clostridium for CW agar. The CFU/g of each bacterial species in 1 g of faeces was calculated by measuring the colonies detected after incubation on selective media.
2.7. Serum Cytokine
Bovine serum interleukin-1β (IL1β) and interleukin-6 (IL6) levels were measured on days 0, 14, 28 and 63 after LP1 administration. Serum IL1β levels were determined using the Bovine IL1β ELISA Kit (Invitrogen, Carlsbad, CA. USA) and serum IL6 levels were determined using the Bovine IL6 ELISA Reagent Kit (Invitrogen, Carlsbad, CA. USA). The assay was performed according to the kit protocol. Quantitative values for each cytokine were determined by calculation based on the kit standards.
2.8. Peripheral Blood Mononuclear Cell (PBMC) Isolation
EDTA supplemented blood was separated from the PBMC layer by Ficoll-Conrey (Specific gravity 1.086) density gradient centrifugation. Centrifugation was performed at 2,500 rpm for 20 minutes. After collection of the PBMC layer, the cells were washed with PBS and resuspended in serum-free RPMI 1640 with 5% FCS to adjust the cell count to 2x106 cells/mL. The PBMCs were used for the following cytokine gene expression assay.
2.9. Measurement of Cytokine Gene Expression
PBMCs from each cow were stimulated with lipopolysaccharide (LPS O111: B4; Sigma-Aldrich Japan G.K., Osaka, Japan) and incubated with LPS (final concentration 5 μg/mL in RPMI 1640 medium) for 6 hours at 37°C, 5% CO2 gas incubator. Unstimulated PBMCs were used as controls. After the reaction, samples were subjected to RNA extraction using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the instructions. The extracted RNA was cDNA synthesized using ReverTra Ace (Toyobo, Gifu, Japan) oligo(dT)20 primers according to the kit manual.
Interleukin-2 (IL2), interleukin-5 (IL5), interleukin-10 (IL10), interleukin-12 (IL12), interferon-γ (IFNγ), transforming growth factor-β (TGFβ) and
housekeeping genes (GAPDH) cytokine gene expression was quantified by Rotor-Gene (Qiagen, Hilden, Germany) using KOD SYBR qPCR Mix (Toyobo, Shiga, Japan) (
Supplementary Table S1). The gene expression was normalized to
GAPDH expression and calculated by ΔΔCT analysis.
2.10. Statistical Analysis
Statistical significance was determined using R Studio (R version 4.0.3) with Student's t-test, Welch's t-test, Mann-Whitney u-test, Steel-Dwass method and Turkey Honest Significant Differences (Turkey HSD) as multiple comparison tests and those showing p<0.05 were considered as significant differences. Significant differences are indicated in the figure as follows: *: p<0.05, **: p<0.01, ***: p<0.001. The numerical values of the results are presented as mean + standard error. For parametric tests, 95% confidence intervals for the difference in means between the LP1 group and the CN group were calculated using Excel (Microsoft® Excel® for Microsoft 365 MSO).
3. Results
3.1. Clinical Follow-Up and Medical Costs
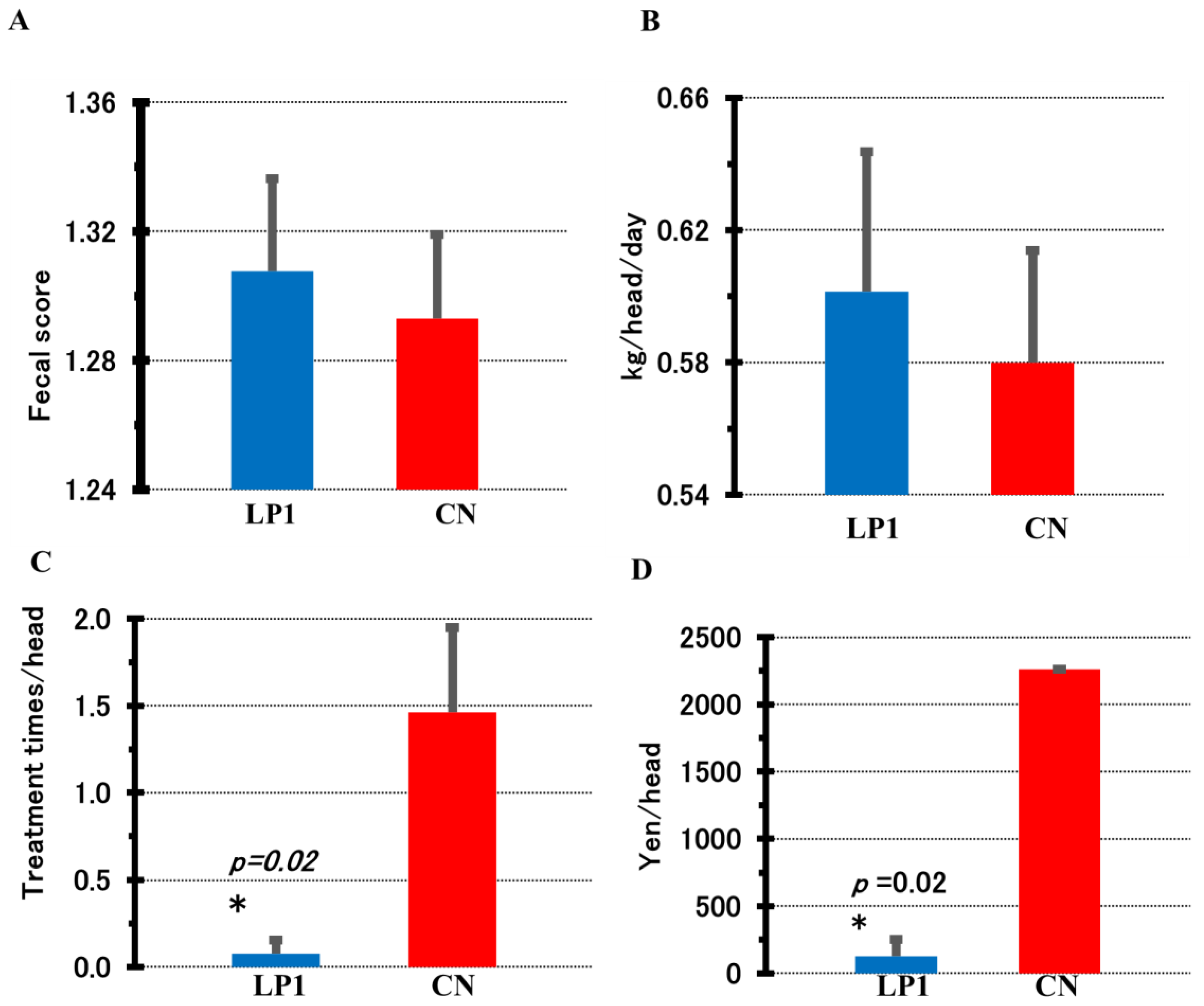
No calves died during the observation period, but there were individuals who became ill during their stay in the calf pens. Some animals in the LP1 group had transient soft stools during the administration period, but none had diarrhoea. The faecal score was 1.31±0.03 for the LP1 group and 1.29±0.03 for the CN group, with no significant difference in faecal scores (p = 0.918). (
Figure 2A) Daily gain was also 0.60 kg/head/day in the LP1 group and 0.58 kg/head/day in the CN group, with slightly better gain in the LP1 group (p = 0.518,
Figure 2B) In terms of number of treatments, one animal with pneumonia in the LP1 group received a treatment on day 13 of lactation. In contrast, 6 cows in the CN group were treated (1 with pneumonia and 5 with diarrhoea) (
Supplementary Table S2). The total number of treatments, converted per animal, is shown in (
Figure 2C). The number of treatments was 0.07 per cow in the LP1 group and 1.4 per cow in the CN group, significantly lower in the LP1 group (p = 0.02), and the total treatment cost per cow was 126.6 yen in the LP1 group and 2,259.2 yen in the CN group, indicating that LP1 treatment reduced total medical costs by 2,133 yen (about 15.1USD, 13.6 EUR, p = 0.02,
Figure 2D).
3.2. Comparison of Gut Bacteria Counts
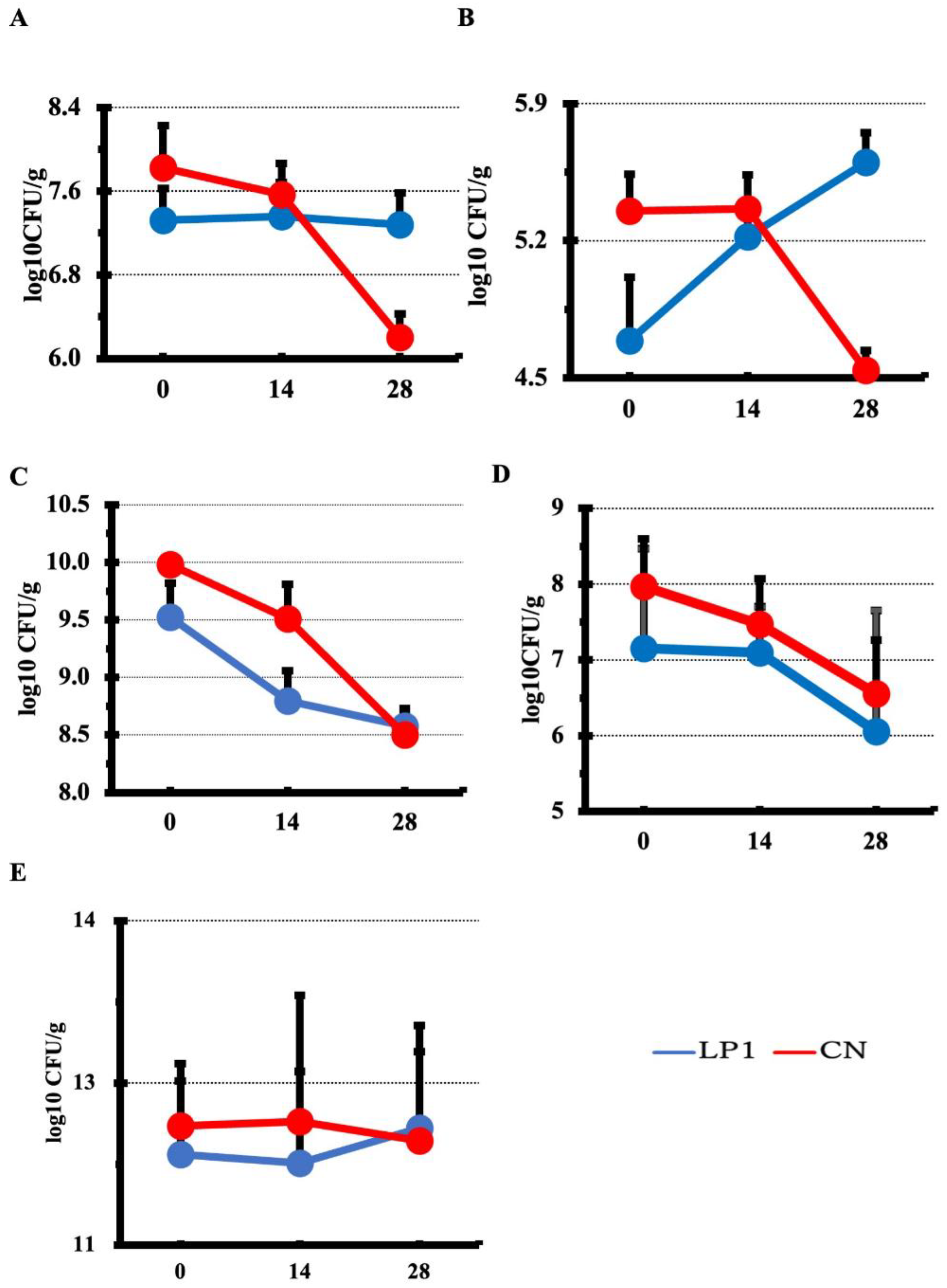
The number of bacteria of each genus detected in the faeces on days 0, 14 and 28 after treatment is expressed as colony forming units/g (CFU/g,
Figure 3).
For the Lactobacillus species, the number of bacteria in the LP1 group remained at log₁₀ 7.32 to log₁₀ 7.28 CFU/g from day 0 to 28 after treatment, while in the CN group it decreased from log₁₀ 7.83 to log₁₀ 6.20 CFU/g from day 0 to 28 after treatment. The decrease was significantly lower at day 28 compared to the LP1 group (p = 0.009,
Figure 3A). For Clostridium species, the LP1 group showed an increase from log₁₀ 4.69 CFU/g to log₁₀ 5.60 CFU/g from day 0 to 28 after treatment, while the CN group showed a decrease from log₁₀ 5.36 to log₁₀ 4.54 CFU/g from day 14 to 28 after treatment. And the difference between the two groups changed significantly on day 28 (p = 0.00005,
Figure 3B). Other Bifidobacterium species, coliform group and total anaerobic bacteria counts did not differ significantly between groups during the observation period (
Figure 3C–E).
This figure shows the results of warship comparisons with and without LP1 administration for four items: LP1, LP1-treated group; CN, LP1-untreated control group. The comparison items are as follows: A: fecal score, B: daily increase in body weight, C: number of treatments, and D: total treatment cost. a Mann-Whitney u-test was performed for statistical analysis between the two groups, LP1 and CN (n=13 each). Values are presented as mean + standard error, indicating a decrease in the number of treatments and total medical costs with LP1 treatment. *p<0.05.
3.3. ILβ and IL6 in Serum
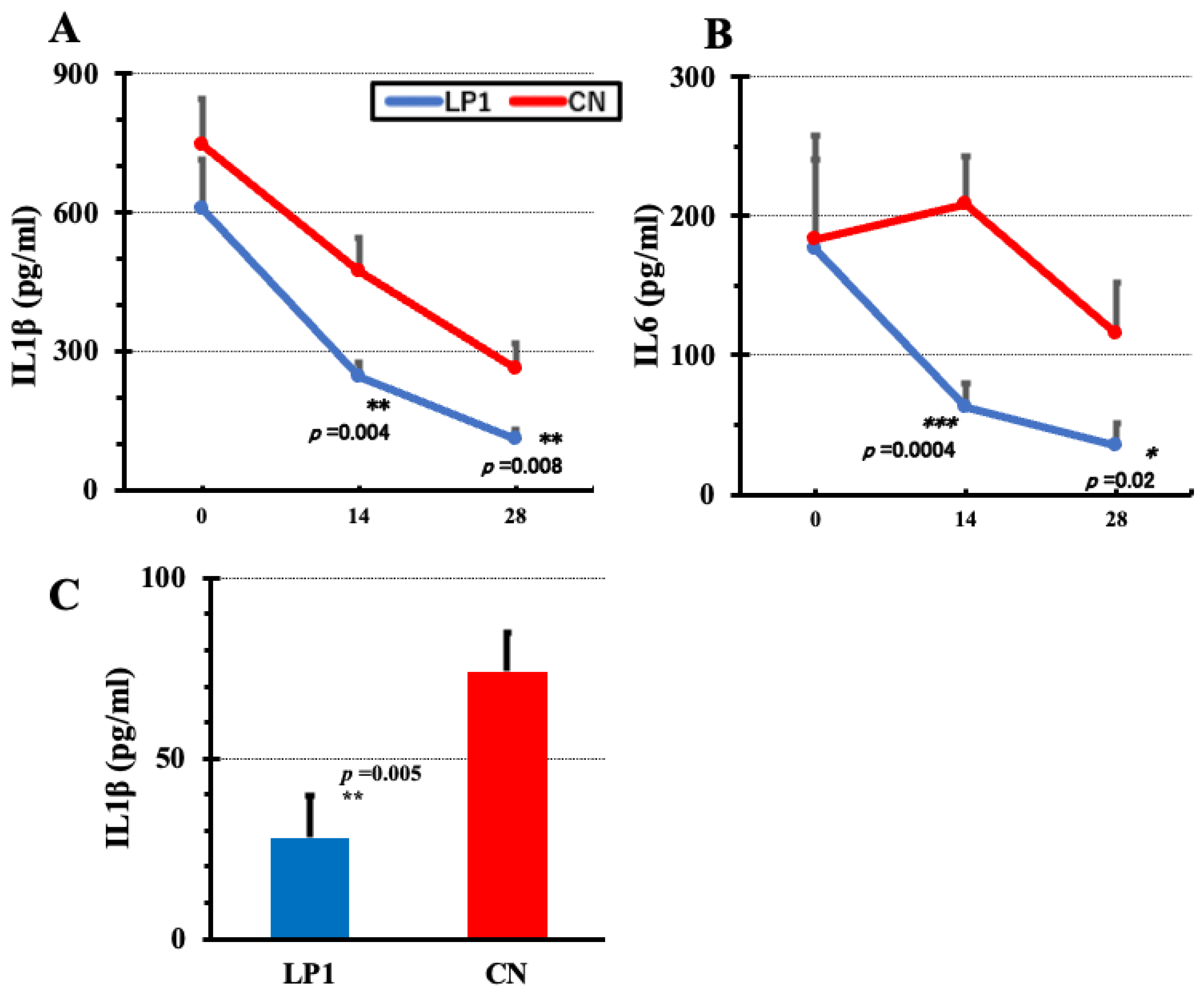
Serum IL1β and IL6 concentrations were measured by indirect sandwich ELISA. At 0, 14 and 28 days after LP1 treatment, IL1β concentration trends were significantly decreased in the LP1 group compared to the CN group at 14 and 28 days after treatment (p =0.004) and at 14 days (p =0.008) (
Figure 4A).The trend of IL6 concentration was not significantly different between groups before treatment, but was significantly lower in the LP1 group compared to the CN group at 14 and 28 days after treatment (p =0.0004) at 14 and 28 days (p =0.02,
Figure 4B).
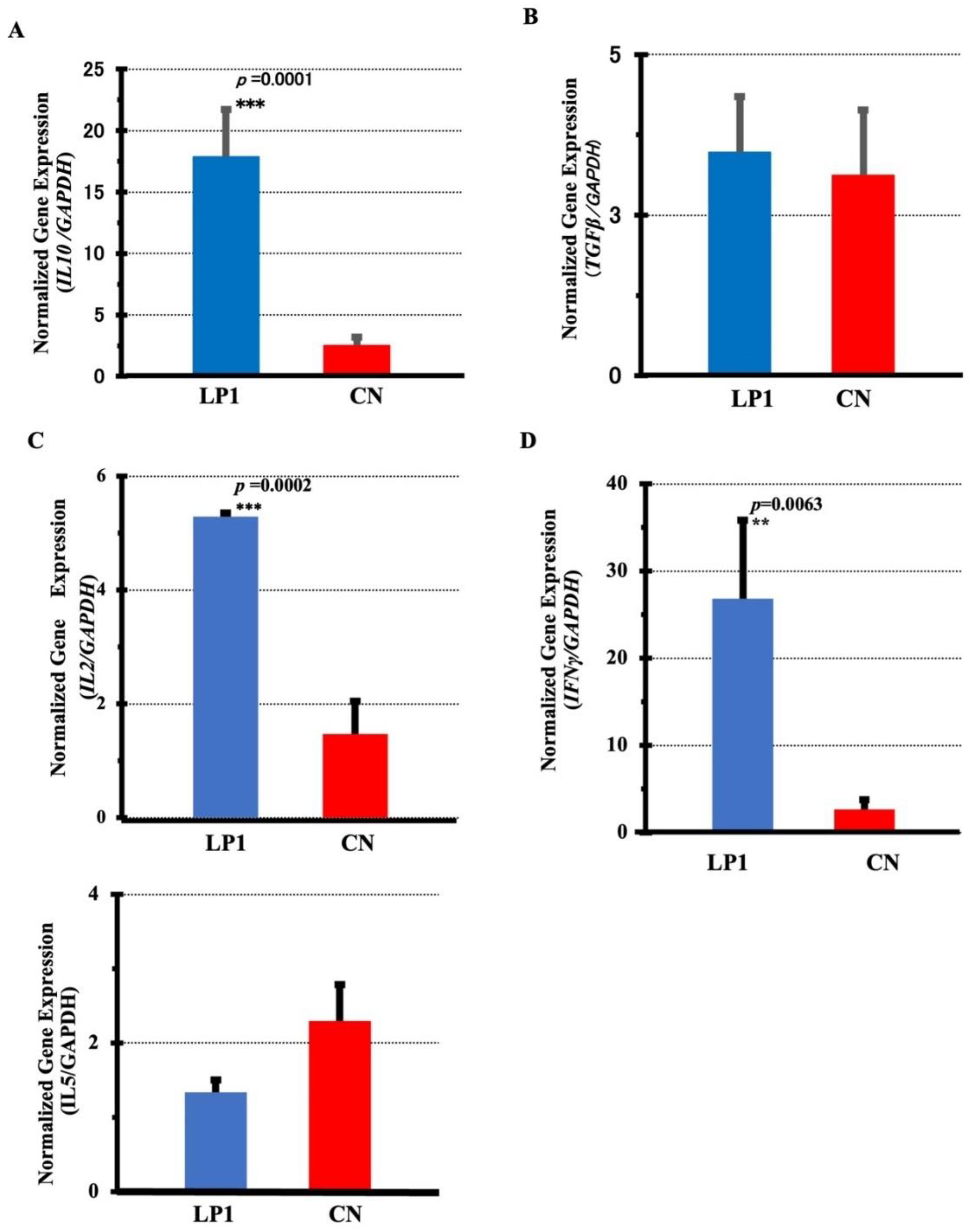
3.4. Cytokine Gene Expression in PBMC 28 Days after LP1 Treatment
Gene expression of each cytokine was examined in LPS-stimulated PBMC 28 days after treatment. The cytokines examined were
IL2, IL5, IL10, TGFβ and
IFNγ. GAPDH expression was normalised as a housekeeping gene.
IL10 expression was significantly higher in the LP1 group (17.88 vs. 2.51 in the CN group (p=0.0001, 95CI (7.326, 23.402)), while
TGFβ showed a difference in mean values, but not significant (p=0.396; 95CI (-2.406, 3.106),
Figure 5A,B). Furthermore, in the LP1 and CN groups,
IL2 expression was significantly higher in the LP1 group, 5.29 versus 1.47 in the CN group (p = 0.0002, 95CI (1.877, 5.763). Similarly,
IFNγ expression was significantly higher in the LP1 group, 26.85 versus 2.65 in the CN group (p = 0.0063; 95CI (4.657, 43.743),
Figure 5C,D). On the other hand,
IL5 expression was 2.3 in the CN group versus 1.34 in the LP1 group, showing a trend towards higher gene expression in the CN group, but no significant difference (p = 0.084; 95CI (-0.108, 2.028),
Figure 5E).
3.5. Serum Cytokine Levels on Day 63 after Lp1 Feeding
To evaluate the effect of LP1 treatment after completion of treatment, serum IL1β and IL6 were measured on day 63 after the start of treatment. Serum IL1β was significantly lower in the LP1 group at 28.12 pg/mL compared to 74.04 pg/mL in the CN group (p=0.005;
Figure 4C) when the concentrations of both groups were compared. In addition, serum IL6 was below the detection limit in each group on the same day (data not shown).
3.6. Comparison of Cytokine Expression in PBMCs 3 Weeks after the End of LP1 Treatment
Gene expression of each cytokine was examined using PBMCs obtained from calves 63 days after LP1 administration (3 weeks after the end of LP1 treatment). The cytokines analysed were
IL2, IL10, IL12, TGFβ and
IFNγ. GAPDH expression was standardised as a housekeeping gene.
IL10 expression was significantly higher (p=0.01, 95CI (0.288, 2.700)) in the CN group (0.65 vs. 2.14 in the LP1 group);
TGFβ expression was significantly higher (0.358 in the CN group vs. 0.991 in the LP1 group), a difference in means (p=0.09,
Figure 6A,B).
IL2 expression was 1.418 in the LP1 group and 1.413 in the CN group with no difference between groups (p=0.5);
IL12 expression was significantly higher in the LP1 group at 1.89 compared to 0.46 in the CN group (p=0.02, 95CI (0.053, 2.807)).
IFNγ expression was 0.13 in the CN group versus 0.36 in the LP1 group, a difference in the mean but not significant (p = 0.07,
Figure 6C–E).
4. Discussion
Newborns' immune systems are immature against pathogens and vulnerable to disease as maternal antibodies decline. It has been previously reported that functional Lactobacillus LP1 regulates inflammation in adult cattle (Chida et al. 2021), and this study showed that it enhances immune function in calves and confers resistance to infections such as pneumonia and diarrhoea. The efficacy of treatment with LP1 was evaluated by examining changes in gut microbes and cytokine expression in PBMC, and by comparing growth rate, disease incidence and cost of treatment between groups.
Diarrhoea is one of the leading causes of mortality in newborn calves, most of which occur in the first month of life (Urie et al. 2018). Diarrhoea is often caused by rotavirus, coronavirus, and together with another bacteria sp. Diarrhoea and pneumonia are the most costly diseases in calf management, caused by a combination of viral and bacterial infections, often exacerbated by inappropriate housing conditions and stress, resulting in a poor prognosis. These diseases are common during the lactation period and cause significant economic losses by affecting subsequent growth (Cho and Yoon 2014; Peel 2020). Preventing disease and reducing the number of treatments for these newborn calves will reduce medical costs and provide economic benefits.
The gut microbiota is closely related to a calf's immune function and stimulates the development of immune function and control of allergies (Hua et al. 2016). The gut microbiota changes significantly with disease and antibiotic use and has been reported to be affected in calves (Keijser et al. 2019; Mitsuoka 2014; Takino et al. 2018). In our study, LP1 treatment of calves maintained stable Lactobacillus spp. and increased Clostridium spp. The increase in Clostridium spp. may be partly due to the LP1 strain stabilising bacterial diversity by maintaining calf health. Lactobacillus spp. produce lactic acid, a type of volatile fatty acid, and volatile fatty acids have functions in the intestinal tract such as immune regulation, increasing peristalsis and aiding nutrient absorption (Sivieri et al. 2013).
Butyric acid bacteria, such as Clostridium butyricum, are selectively cultured on the CW agar medium used in this study (Klopp et al. 2022), and it has been reported that butyric acid-producing bacteria induce regulatory T cells via the production of butyrate, a volatile fatty acid, in the intestinal tract. These regulatory T cells (hereafter referred to as Treg) produce IL10 and are involved in immune regulation (Kanai et al. 2015). It is therefore suggested that the increase in butyrate-producing bacteria increased Tregs and regulated immune function. The anti-inflammatory and immunomodulatory effects of lactic acid bacteria-derived short-chain fatty acids (SCFAs) In the periphery, particularly in the gut-pulmonary axis, a reduction in SCFA-induced lung inflammation may also be a factor in the results of this study (Maslowski et al. 2009; Vaughan et al. 2019). These findings suggest that LP1 administration may improve the gut microbiota and act on gut and lung immune function. Some Lactobacillus spp. produce SCFA, which induce regulatory T cells (Arpaia et al. 2013), suggesting that they play a role in controlling inflammatory immunity. In this study, the direct effect of LP1 and changes in the bacterial flora that act to control inflammation may also be a factor.
Comparison of serum cytokine levels showed that LP1 treatment significantly reduced both IL1β and IL6, pro-inflammatory cytokines. On the other hand, significant upregulation of IL2, IL10 and IFNγ gene expression was observed in LPS-stimulated PBMCs. Excessive production of pro-inflammatory cytokines causes biological damage such as fever and anorexia, whereas anti-inflammatory cytokines such as IL10 regulate inflammatory responses by controlling the production of pro-inflammatory cytokines (Beheshtipour and Raeeszadeh 2020; Saraiva et al. 2020; Sheil et al. 2006). While previous studies have shown that LP1 suppresses inflammatory cytokines and increases IL10 expression in adult cattle (Chida et al., 2021), the present study showed that LP1 also has an inflammation-modulating effect in calves.
The results of this study suggest that LP1 regulates the expression of inflammatory cytokines in response to LPS stimulation, accompanied by IL10 expression, to low levels appropriate for immune activation in calves, thereby controlling excessive inflammation. Thus, LP1 is expected to regulate exaggerated immune responses to antigenic stimuli, such as pathogens, and promote appropriate cytokine expression by modulating the calf's exaggerated immune response to infection. This is expected to reduce disease exacerbation in newborn calves and shorten treatment time. In addition, this effect is sustained for approximately 3 weeks after cessation of feeding, making it a valuable probiotic for livestock management.
This study showed that feeding LP1 to calves stabilises gut microbiota diversity and regulates immune function to control excessive inflammatory response, resulting in economic benefits for disease management. In addition, LP1 can induce appropriate cytokine induction in response to microbial antigens such as LPS, which is expected to suppress disease progression in newborn calves and reduce treatment time. Although the functional components derived from LP1 could not be elucidated in this study, we will investigate the biological effects of cell wall components such as peptidoglycan and fatty acids, extracellular polysaccharides, lactic acid bacteria metabolites, SCFA and nucleic acids on immunoregulatory functions.
5. Patents
The L. plantarum RGU-LP1 strain used in this study is a laboratory stock patented by the authors (LP1, Patent# 35610472).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Figure S1: title; Table S1: title; Video S1: title.
Author Contributions
K.O. Sample collection and preliminary processing, investigation, molecular biology analysis, writing - original draft, data curation, software application; S.T. Sample collection and preliminary processing, investigation, molecular biology analysis, methodology, investigation, data curation, software application and formal analysis; C.I.: Sample collection and preliminary processing, Methodology, formal analysis, preliminary processing; T.T.: Methodology, visualization, investigation, formal analysis, software, validation, sample collection and preliminary processing; K.H: conceptualization, methodology, validation, supervision, writing - review and editing, project administration, funding acquisition.
Funding
This work was partly supported by a Scientific Feed Laboratory Research Grant (2020-2021).
Acknowledgments
We would like to thank Dr. T. Sakai (Scientific Feed Laboratory), Dr. J. Nagatsuka (Jun clinic) for supporting the livestock management in this study. We would like to express our deep gratitude to Dohoh Farm Co. for the livestock management in this study, their support was critical to our research success.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cortese, V.S. Neonatal Immunology. Veterinary Clinics of North America - Food Animal Practice 2009, 25, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.; Ishibashi, N.; Shimamura, S. Effect of Administration of Bifidobacteria and Lactic Acid Bacteria to Newborn Calves and Piglets. Journal of Dairy Science 1995, 78, 2838–2846. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Yoshikane, M.; Kobayashi, A.; Mitsuoka, T. An Application of Dried Bifidobacteria Preparation to Scouring Animals. Bifidobacteria and Microflora 1983, 2, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Takino, T.; Kato-mori, Y.; Orihashi, T.; Hagiwara, k. Rotavirus Infection Transiently Affects Intestinal Microbiome Composition in Newborn Calves. Journal of Veterinary Medicine and Research 2018, 5. [Google Scholar]
- Fuller, R. Probiotics in man and animals. Journal of Applied Bacteriology 1989, 66, 365–378. [Google Scholar]
- Salminen, S.; Bouley, C.; Boutron-Ruault, M.C.; Cummings, J.H.; Franck, A.; Gibson, G.R.; Isolauri, E.; Moreau, M.C.; Roberfroid, M.; Rowland, I. Functional food science and gastrointestinal physiology and function. The British Journal of Nutrition 1998, 80 (Suppl. S1), S147–S171. [Google Scholar] [CrossRef]
- Fang, H.; Elina, T.; Heikki, A.; Seppo, S. Modulation of humoral immune response through probiotic intake. FEMS Immunology and Medical Microbiology 2000, 29, 47–52. [Google Scholar] [CrossRef]
- Perdigón, G.; Maldonado Galdeano, C.; Valdez, J.C.; Medici, M. Interaction of lactic acid bacteria with the gut immune system. European Journal of Clinical Nutrition 2002, 56, S21–S26. [Google Scholar] [CrossRef]
- Mustafa, S.M.; Chua, L.S.; El-Enshasy, H.A.; Majid, F.A.A.; Hanapi, S.Z.; Malik, R.A. Effect of temperature and pH on the probiotication of Punica granatum juice using Lactobacillus species. J. Food Biochem. 2019, 43, e12805. [Google Scholar] [CrossRef]
- Okubo, T.; Takemura, N.; Yoshida, A.; Sonoyama, K. KK/Ta Mice Administered Lactobacillus plantarum Strain No. 14 Have Lower Adiposity and Higher Insulin Sensitivity. Bioscience of Microbiota, Food and Health 2013, 32, 93–100. [Google Scholar] [CrossRef]
- Nishimura, M.; Ohkawara, T.; Tetsuka, K.; Kawasaki, Y.; Nakagawa, R.; Satoh, H.; Sato, Y.; Nishihira, J. Effects of yogurt containing Lactobacillus plantarum HOKKAIDO on immune function and stress markers. Journal of Traditional and Complementary Medicine 2016, 6, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; NGO, V.; Kwon, Y.M.; Lee, Y.T.; Yoo, S.; Cho, Y.H.; Hong, S.M.; Hwang, H.S.; Ko, E.J.; Jung, Y.J.; Moon, D.W.; Jeong, E.J.; Kim, M.C.; Lee, Y.N.; Jang, J.H.; Oh, J.S.; Kim, C.H.; Kang, S.M. Lactobacillus plantarum DK119 as a Probiotic Confers Protection against Influenza Virus by Modulating Innate Immunity. PLoS ONE 2013, 8, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Chida, S.; Sakamoto, M.; Takino, T.; Kawamoto, S.; Hagiwara, K. Changes in immune system and intestinal bacteria of cows during the transition period. Veterinary and Animal Science, 2021, 14, 100222. [Google Scholar] [CrossRef] [PubMed]
- Kishida, S.; Kato-Mori, Y.; Okamoto, M.; Hagiwara, K. Anti-inflammatory effect a specific Lactiplantibacillus plantarum in an ovalbumin-induced asthma model. Microbiol Immunol. 2022, 66, 442–452. [Google Scholar] [CrossRef]
- Urie, N.J.; Lombard, J.E.; Shivley, C.B.; Kopral, C.A.; Adams, A.E.; Earleywine, T.J.; Olson, J.D.; Garry, F.B. Preweaned heifer management on US dairy operations: Part V. Factors associated with morbidity and mortality in preweaned dairy heifer calves. Journal of Dairy Research 2018, 101, 9229–9244. [Google Scholar] [CrossRef]
- Cho, Y. il, Yoon, K.J. An overview of calf diarrhea - infectious etiology, diagnosis, and intervention. Journal of Veterinary Science 2014, 15, 1–17. [Google Scholar] [CrossRef]
- Peel, D.S. The Effect of Market Forces on Bovine Respiratory Disease. Veterinary Clinics of North America - Food Animal Practice 2020, 36, 497–508. [Google Scholar] [CrossRef]
- Hua, X.; Goedert, J.J.; Pu, A.; Yu, G.; Shi, J. Allergy associations with the adult fecal microbiota: Analysis of the American Gut Project. EBioMedicine, 2016, 3, 172–179. [Google Scholar] [CrossRef]
- Keijser, B.J.F.; Agamennone, V.; Van Den Broek, T.J.; Caspers, M.; van de Braak, A.; Bomers, R.; Havekes, M.; Schoen, E.; van Baak, M.; Mioch, D.; Bomers, L.; Montijn, R.C. Dose-dependent impact of oxytetracycline on the veal calf microbiome and resistome. BMC Genomics 2019, 20, 1–14. [Google Scholar] [CrossRef]
- Mitsuoka, T. Establishment of intestinal bacteriology. Bioscience of Microbiota, Food and Health 2014, 33, 99–116. [Google Scholar] [CrossRef]
- Sivieri, K.; Morales, M.L.V.; Adorno, M.A.T.; Sakamoto, I.K.; Saad, S.M.I.; Rossi, E.A. Lactobacillus acidophilus CRL 1014 improved “ gut health” in the SHIME® reactor. BMC Gastroenterology 2013, 13, 1. [Google Scholar] [CrossRef]
- Klopp, R.N.; Hernandez Franco, J.F.; Hogenesch, H.; Dennis, T.S.; Cowles, K.E.; Boerman, J.P. Effect of medium-chain fatty acids on growth, health, and immune response of dairy calves. Journal of Dairy Science 2022, 105, 7738–7749. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Mikami, Y.; Hayashi, A. A breakthrough in probiotics: Clostridium butyricum regulates gut homeostasis and anti-inflammatory response in inflammatory bowel disease. Journal of Gastroenterology 2015, 50, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; Xavier, R.J.; Teixeira, M.M.; Mackay, C. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.; Frazer, Z.A.; Hansbro, P.M.; Yang, I.A. COPD and the gut-lung axis: The therapeutic potential of fibre. Journal of Thoracic Disease 2019, 11, S2173–S2180. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; Rudensky, A.Y. Metabolites produced by commensal bacteria promote peripheral regulatory T cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Beheshtipour, J.; Raeeszadeh, M. Evaluation of interleukin-10 and pro-inflammatory cytokine profile in calves naturally infected with neonatal calf diarrhea syndrome. Archives of Razi Institute 2020, 75, 213–218. [Google Scholar]
- Saraiva, M.; Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. Journal of Experimental Medicine 2020, 217, 1–19. [Google Scholar] [CrossRef]
- Sheil, B.; MacSharry, J.; O’Callaghan, L.; O'Riordan, A.; Waters, A.; Morgan, J.; Collins.
Figure 1.
Overview of the LP1 feeding schedule. The calves were clinically healthy at one week of age, and the calves were housed individually in calf hatches and kept acclimatised for 5 days. Calves were divided into LP1-treated (LP1) and control groups (CN) for the study. Calves received LP1 (109 CFU/head/day) with milk replacer for 40 days, while the control group received equal amounts of the same milk replacer. After completion of treatment, each calf was moved to a free barn and kept under the same feeding management. Red arrows indicate blood or fecal sampling.
Figure 1.
Overview of the LP1 feeding schedule. The calves were clinically healthy at one week of age, and the calves were housed individually in calf hatches and kept acclimatised for 5 days. Calves were divided into LP1-treated (LP1) and control groups (CN) for the study. Calves received LP1 (109 CFU/head/day) with milk replacer for 40 days, while the control group received equal amounts of the same milk replacer. After completion of treatment, each calf was moved to a free barn and kept under the same feeding management. Red arrows indicate blood or fecal sampling.
Figure 2.
Comparison of health status, number of treatments, and cost of treatment with and without LP1 feeding.
Figure 2.
Comparison of health status, number of treatments, and cost of treatment with and without LP1 feeding.
Figure 3.
Intestinal bacterial changes by LP1 feeding. Fecal samples from calves in each group were cultured on selective media; A: Lactobacillus sp., B: Clostridium sp., C: Bifidobacterium sp., D: Coliform group, E: total anaerobic bacteria counts. Horizontal axis indicates days after LP1 administration (0, 14, 28 days). Number of calves in each group: LP1 (n= 13) and CN (n=13), Welch's t test was used for intergroup analysis.
Figure 3.
Intestinal bacterial changes by LP1 feeding. Fecal samples from calves in each group were cultured on selective media; A: Lactobacillus sp., B: Clostridium sp., C: Bifidobacterium sp., D: Coliform group, E: total anaerobic bacteria counts. Horizontal axis indicates days after LP1 administration (0, 14, 28 days). Number of calves in each group: LP1 (n= 13) and CN (n=13), Welch's t test was used for intergroup analysis.
Figure 4.
Comparison of serum IL1β and IL6 concentrations. Serum IL1β and IL6 concentrations quantified by ELISA are shown; A: serum IL1β, B: serum IL6. Horizontal axis indicates number of days after LP1 feeding (0, 14, 28 days) Number of calves per group: LP1 (n=13) CN (n=13) Student's t test was used to analyze between two groups at each sampling point.
Figure 4C, Serum IL1β concentration 3 weeks after the end of LP1 feeding; Concentration of IL1β in serum is shown; statistical analysis between the two groups confirmed a significant difference by Welch's t test (**p<0.01).
Figure 4.
Comparison of serum IL1β and IL6 concentrations. Serum IL1β and IL6 concentrations quantified by ELISA are shown; A: serum IL1β, B: serum IL6. Horizontal axis indicates number of days after LP1 feeding (0, 14, 28 days) Number of calves per group: LP1 (n=13) CN (n=13) Student's t test was used to analyze between two groups at each sampling point.
Figure 4C, Serum IL1β concentration 3 weeks after the end of LP1 feeding; Concentration of IL1β in serum is shown; statistical analysis between the two groups confirmed a significant difference by Welch's t test (**p<0.01).
Figure 5.
Cytokine gene expression in LPS-stimulated PBMCs 28 days after LP1 feeding. Cytokine gene expression in LPS-stimulated PBMCs on day 28 post-LP1 treatment normalized by GAPDH is shown A: IL10, B: TGFβ, C: IL2, D: IFNγ, and E: IL5. LP1 (LP1-treated group, n=13), CN (control group, n=13), Statistical analysis between the two groups was based on F test, Student's t test and Welch's t test.
Figure 5.
Cytokine gene expression in LPS-stimulated PBMCs 28 days after LP1 feeding. Cytokine gene expression in LPS-stimulated PBMCs on day 28 post-LP1 treatment normalized by GAPDH is shown A: IL10, B: TGFβ, C: IL2, D: IFNγ, and E: IL5. LP1 (LP1-treated group, n=13), CN (control group, n=13), Statistical analysis between the two groups was based on F test, Student's t test and Welch's t test.
Figure 6.
Cytokine expressions in PBMC at 3 weeks after the end of LP1 feeding. Cytokine gene expression in PBMC at 3 weeks after LP1 treatment are shown. Each gene expression was normalized to GAPDH expression, A: IL10, B: TGFβ, C: IL2, D: IL12, and E: IFNγ. Statistical analysis between LP1 (LP1 group, n=13) CN (control group, n=12) 2 groups was based on F test, Student's t test and Welch's t test. Significance is indicated by *p<0.05.
Figure 6.
Cytokine expressions in PBMC at 3 weeks after the end of LP1 feeding. Cytokine gene expression in PBMC at 3 weeks after LP1 treatment are shown. Each gene expression was normalized to GAPDH expression, A: IL10, B: TGFβ, C: IL2, D: IL12, and E: IFNγ. Statistical analysis between LP1 (LP1 group, n=13) CN (control group, n=12) 2 groups was based on F test, Student's t test and Welch's t test. Significance is indicated by *p<0.05.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).