1. Introduction
The treatment and storage of nuclear waste is a prominent problem in current nuclear energy applications [
1]. The tritium-containing wastewater generated during reactor operation needs to be treated before it can be discharged into the environment. As hydrogen isotope, tritium has small atomic radius and high chemical activity, its permeation and retention in the reactor material is almost unavoidable. The tritium in the reactor is mainly derived from three sources: the fission reaction of
235U, the neutron reaction of the antimony-beryllium pellet in the secondary neutron source rod, the neutron reaction of boron and lithium in the primary coolant [
2,
3]. In order to reduce tritium leakage in reactors, an effective way is to keep tritium trapped in the fuel cladding material or structural components of the reactor, and the most economical and feasible method is to apply a tritium barrier coating to the surface of the material.
There are many types of tritium resistant coatings, among which ceramic coatings are one of the key research and development directions because of their excellent tritium inhibitory properties, good corrosion resistance and mechanical properties. Ceramic coatings include nitride coatings, silicide coatings, oxide coatings, etc. Nitride coatings (TiN, CrN, etc.) have a permeability reduction factor (PRF) of up to 10
3 [
4,
5], but they are susceptible to oxidation at high temperatures. Silicide coatings, such as SiC, have excellent corrosion resistance and PRF is about 10
2 [
6]. Oxide coating is the earliest type of tritium inhibitor coating that has been studied and has a wide range of applications and research bases. Typical oxide coatings include Cr
2O
3 [
7], Er
2O
3 [
8], Al
2O
3 [
9,
10,
11,
12,
13], and PRF can reach 10
2~10
3. Among them, Al
2O
3 has the best anti-tritium effect, and the PRF can reach more than 10
3 [
14,
15]. But it has the problem of easy peeling, and difficult manufacturing process of the preparation of α- Al
2O
3 phase which has the best tritium resistance performance [
12].
In this study, in order to avoid the peeling of the Al
2O
3 coating, the FeAl/Al
2O
3 coating was studied [
16,
17]. A FeAl transition layer was formed between the Al
2O
3 and the metal matrix, which could alleviate the thermal mismatch between Al
2O
3 coating and the substrate, and makes the coating have a certain self-healing ability. FeAl/Al
2O
3 coatings have excellant mechanical properties (such as hardness, wear resistance, and thermal shock properties), can alleviate down material oxidation and vulcanization [
18], can inhibit material corrosion and carburizing [
19,
20,
21], and can effectively block tritium penetration [
22,
23], which makes it have good potential for high-temperature applications.
In the actual manufacturing process and application process in the nuclear reactor, the tritium resistant coating may cause mechanical damage due to collision or thermal shock, which will affect the tritium penetration resistance of the coating. Therefore, it is necessary to study the influence of coating damage degree on its tritium penetration resistance to guide the engineering application. In this paper, based on the FeAl/Al2O3 coating on the surface of stainless steel, the different degrees of mechanical damage on the coating and thermal shock are simulated, and the changes of tritium permeability performance of the coating under different conditions are tested and evaluated by a gas-phase hydrogen isotope permeation test platform.
2. Experimental Methods
2.1. Coating Preparation
The FeAl/Al
2O
3 coating was prepared on 316L stainless steel substrate by RF magnetron sputtering technology. First, by adjusting the ratio of Fe and Al in the target, a FeAl transition layer was deposited on the substrate, and the ratio of the area of the Fe target to the Al target used in the sputtering process was about 3:2 [
24]. Subsequently, the Al
2O
3 ceramic coating was deposited by reactive sputtering in an Ar+O
2 atmosphere using an Al target. To exclude the influence of impurities on the coating, all targets and gases are 99.999% pure. The structure of coating deposition is shown in
Figure 1. The coating sample is a disc with a thickness of 0.5 mm and a diameter of 12 mm.
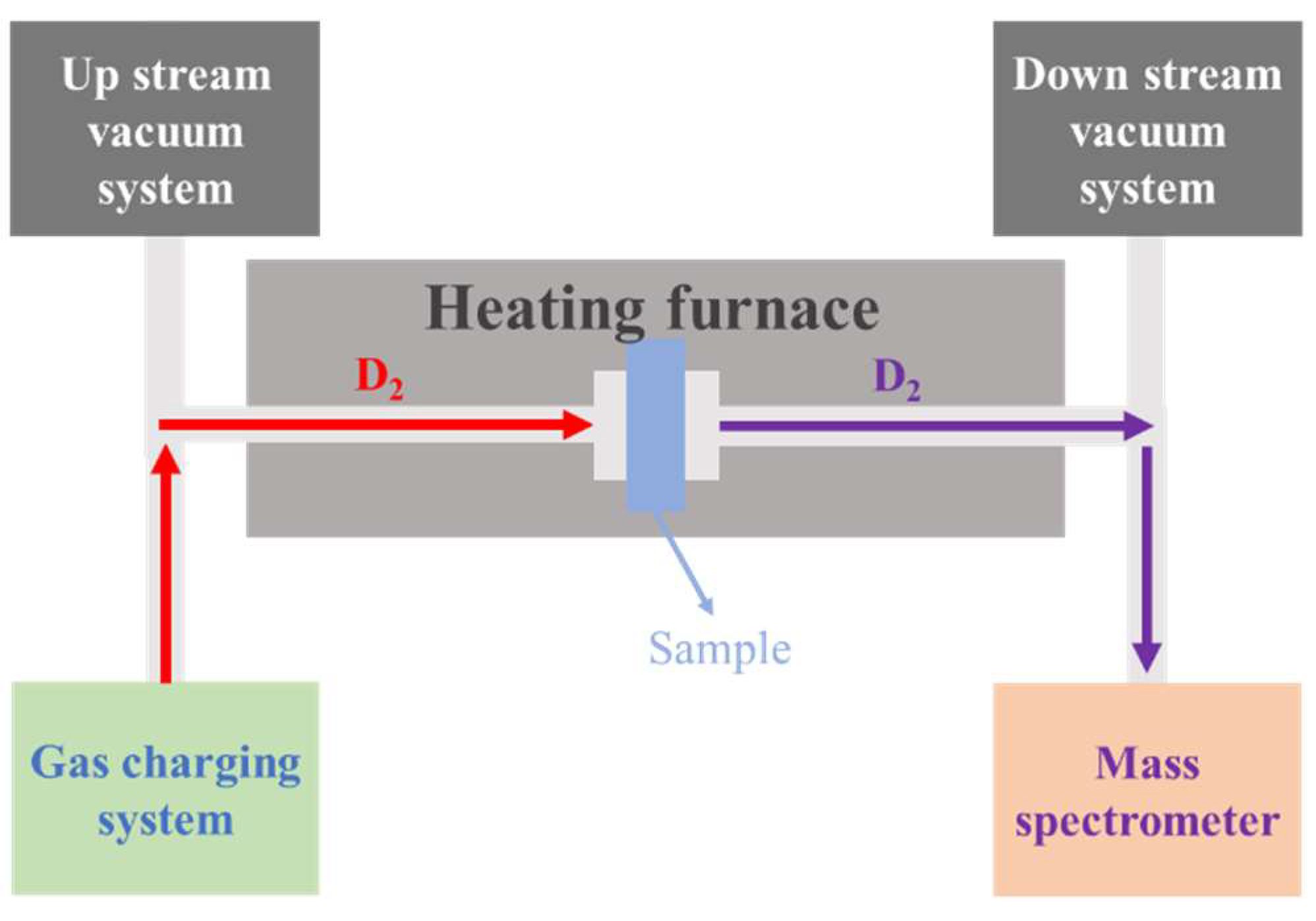
2.2. Gas hydrogen Isotope Permeation (GDP) Test
Since tritium is radioactive, its isotope deuterium is used for permeation experiments [
25]. The structure of the experimental device used in this experiment is shown in
Figure 2. The sample is clamped between the upstream and downstream by means of a VCR (Vacuum Coupling Radius) seal with the coating side facing upstream. In order to eliminate interference, the vacuum in the device was evacuated before the experiment, and the vacuum degree of the upstream pipeline was 1⨯10
-4Pa, and the downstream pipeline could reach 1⨯10
-6Pa. During the experiment, deuterium gas was charged into the upstream pipeline at a certain pressure (100 kPa and 500 kPa), the temperature was adjusted through a high-temperature furnace, and the measurements were carried out at 500°C, 450°C and 400°C. The deuterium permeation current signal is measured using a mass spectrometer at the downstream detection end.
2.3. Microstructure Inspection
After the deuterium penetration test, the microstructure and topography of the sample surface and cross-section were characterized using a field emission scanning electron microscope (FEEM) model Thermo Scientific Apreo 2c.
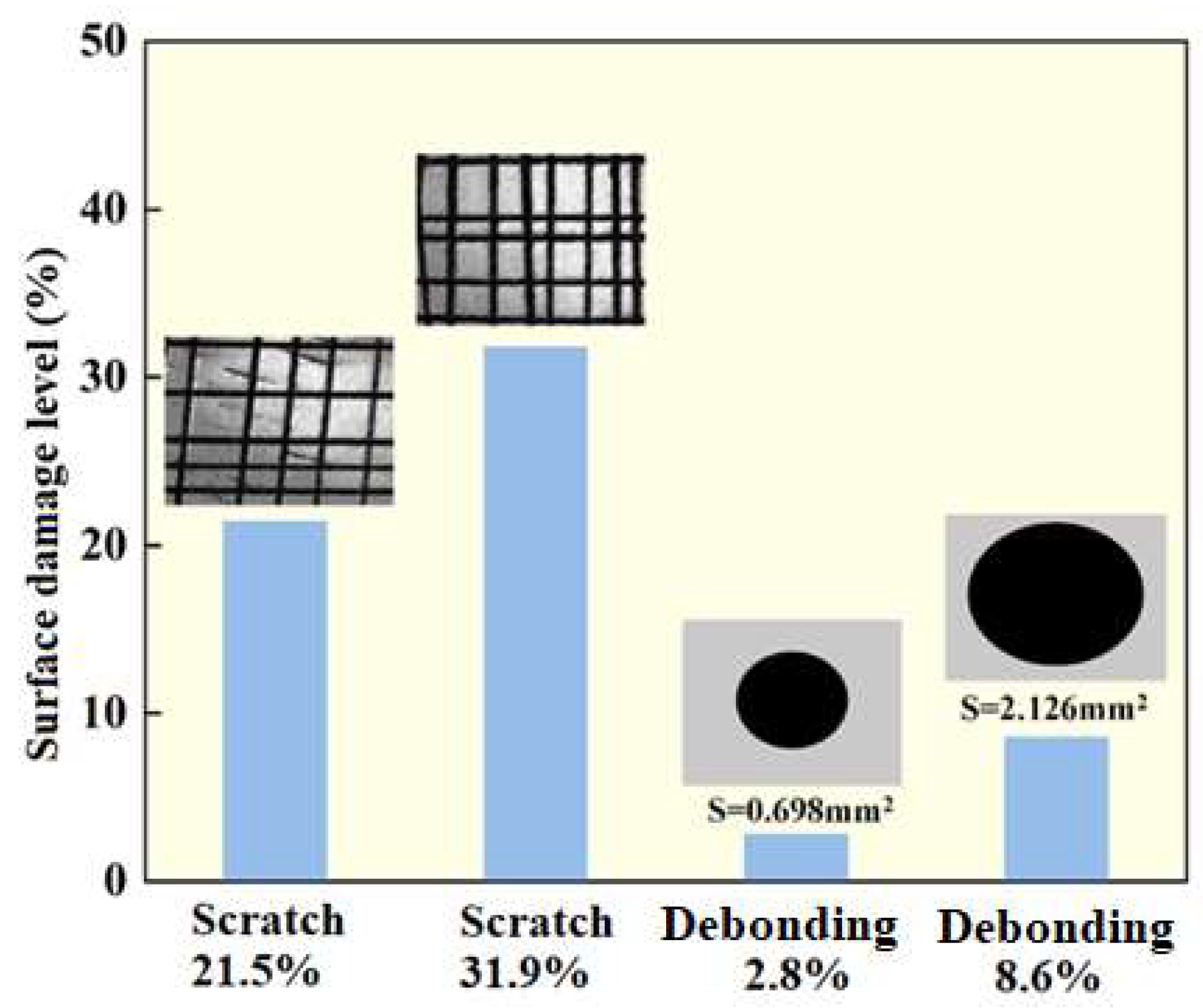
2.4. Simulation of Mechanical Damage of Coating Surface
In order to simulate the effects of different damage mechanisms and different damage degrees on the permeability of FeAl/Al2O3 coatings, three damage modes were simulated:
scratch damage: scratch meter was used to create the linear groove-like damage;
debonding (or spalling) damage: spatula was used to create the regional loss of coating on the surface;
damage caused by thermal cycling: 30 times thermal shock experiments were carried out on the samples with the temperature between root temperature and 450°C. The heating rate and cooling rate were both more than 500°C/s.
The damage ratios simulated are shown in the
Figure 3.
3. Results and Analysis
3.1. Hydrogen Isotope Permeation
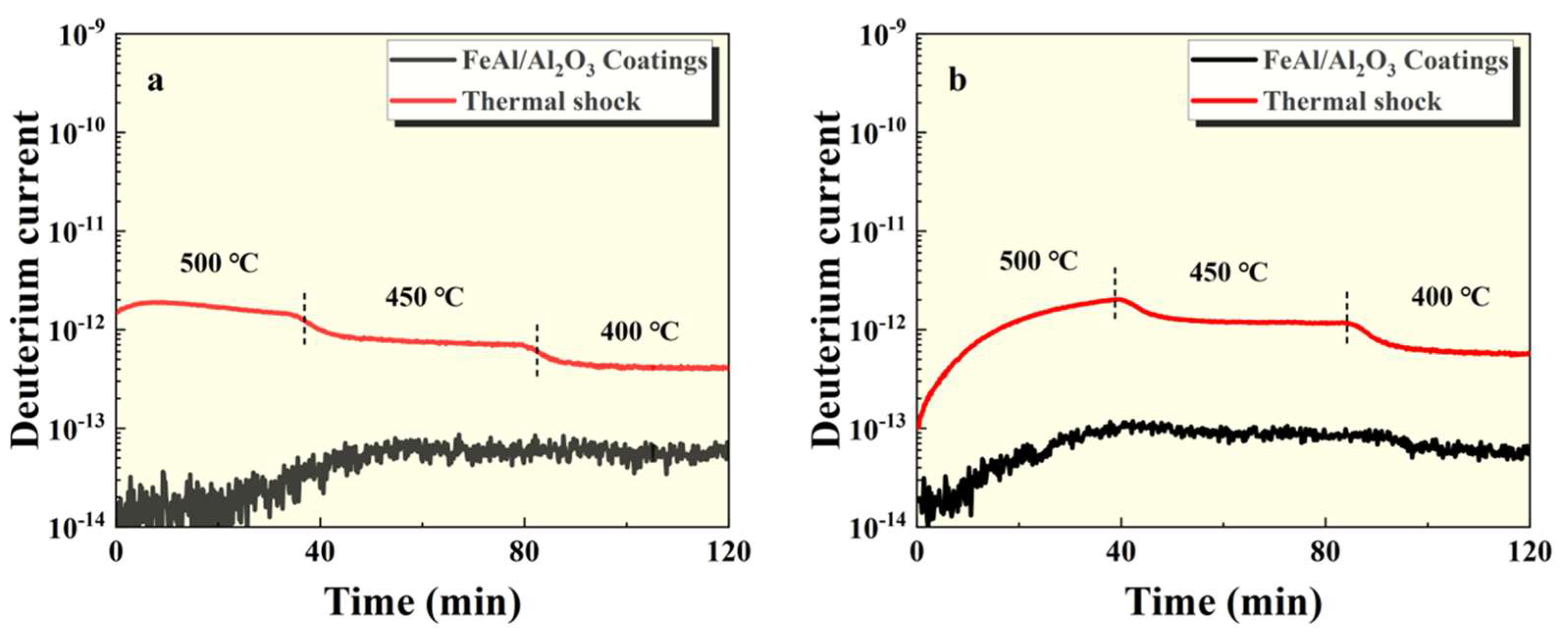
The GDP test results of the intact FeAl/Al
2O
3 coating sample and the damaged sample at 100kPa and 500kPa pressures are shown in
Figure 4~
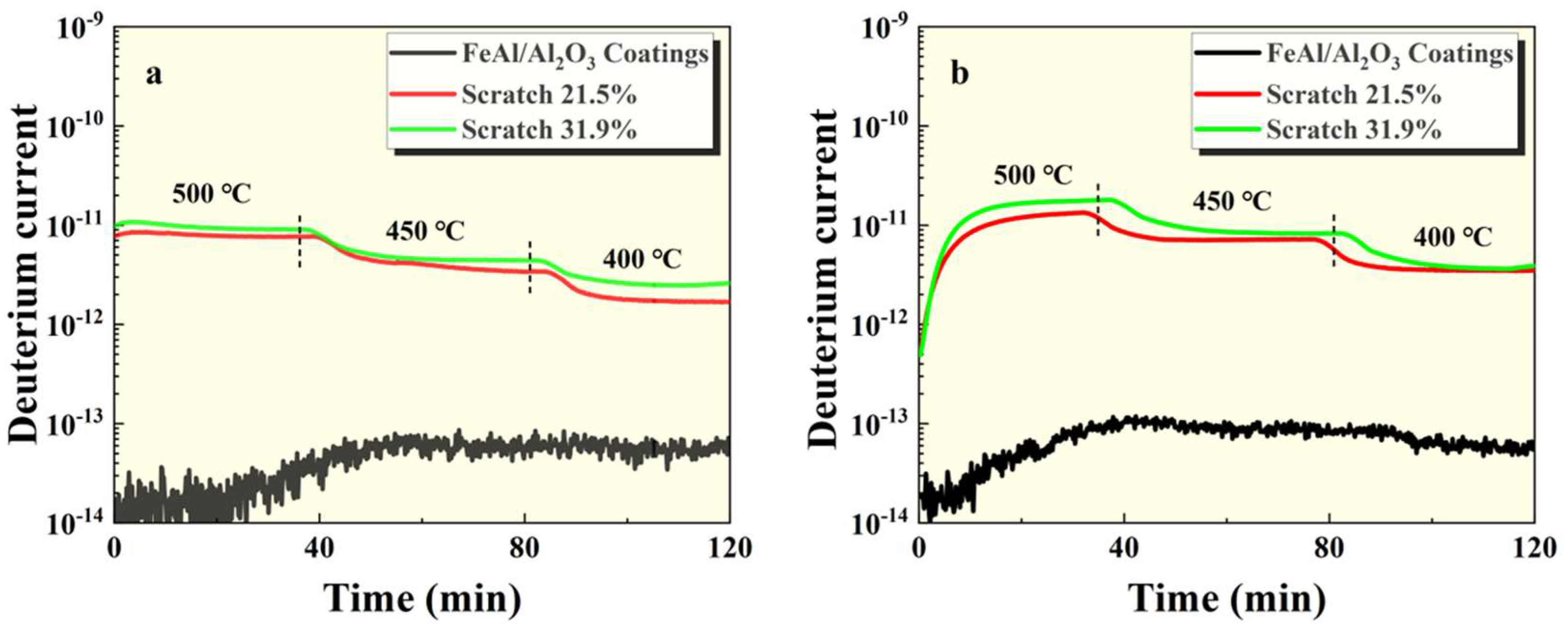
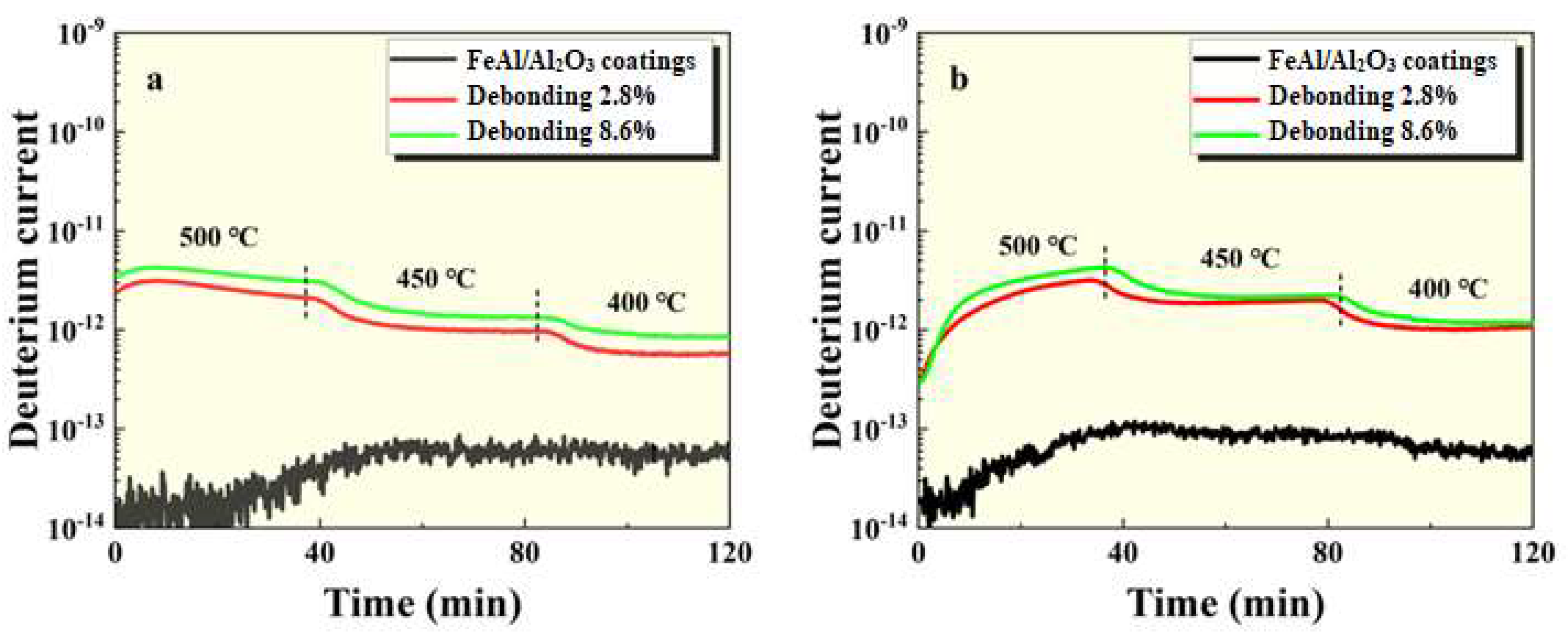
Figure 6. It can be seen that the permeation signal of the intact sample was maintained at the order of 1⨯10
-13, indicating that it had excellent tritium permeation resistance. Compared with the intact sample, the permeation signal of the thermal shock, scratch and debonding samples increased by 2 orders of magnitude, 3 orders of magnitude and 2 orders of magnitude, respectively. It can be seen that any mechanical damage has a negative impact on the tritium penetration resistance of coating. Among them, scratches are particularly serious impact on the coating. In addition, as can be seen from
Figure 5 and
Figure 6, the greater the degree of damage to the coating surface, the worse the tritium resistance effect, which is in line with expectations.
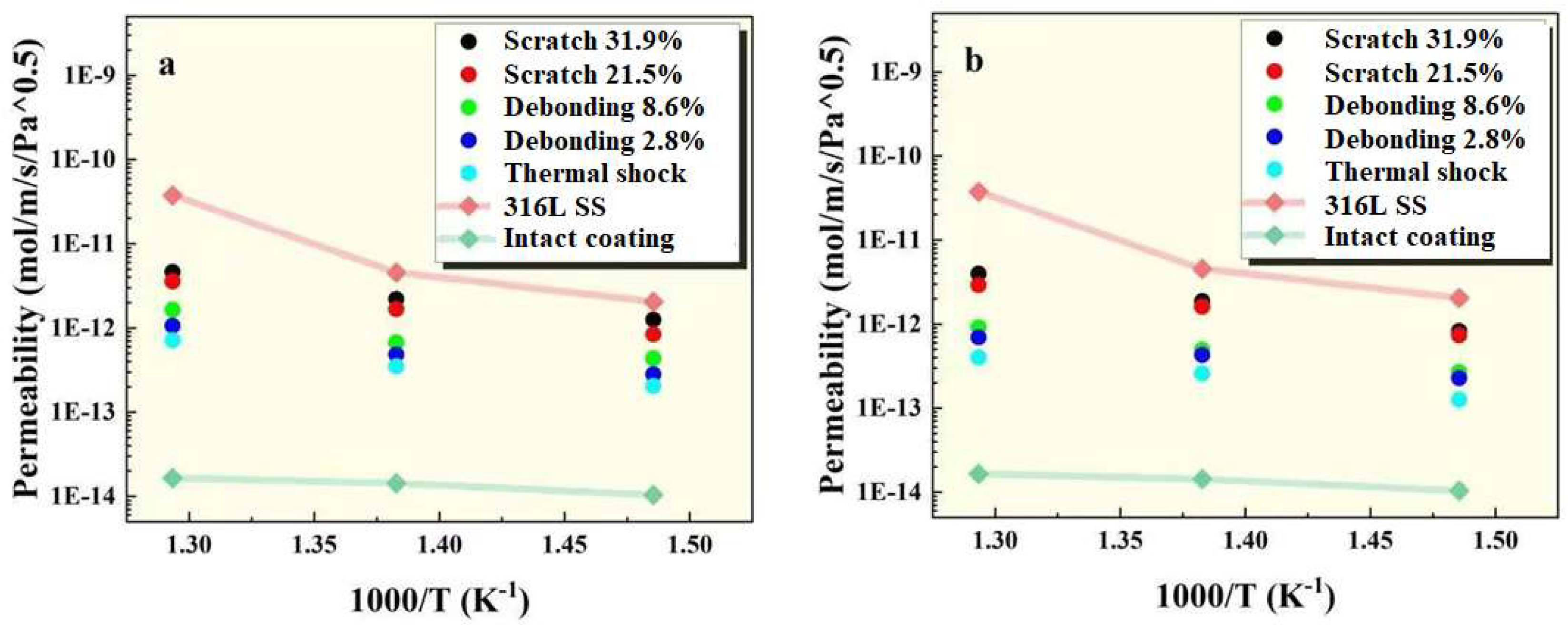
Based on the permeation signal measurements, the permeability of the sample at different temperatures was calculated and compared with 316L stainless steel without coating and with intact coating, as shown in
Figure 7. The results show the intact coating can reduce the permeability of tritium by 2~3 orders of magnitude compared to the 316L stainless steel without coating, and coatings with missing integrity still retain certain tritium penetration resistance. Furthermore, it can be seen that the permeability at 100 kPa is slightly higher than that of 500 kPa, which may be due to the presence of the coating makes the surface reaction process of deuterium atoms lower than that of bulk diffusion.
3.2. Microstructure Examination Results
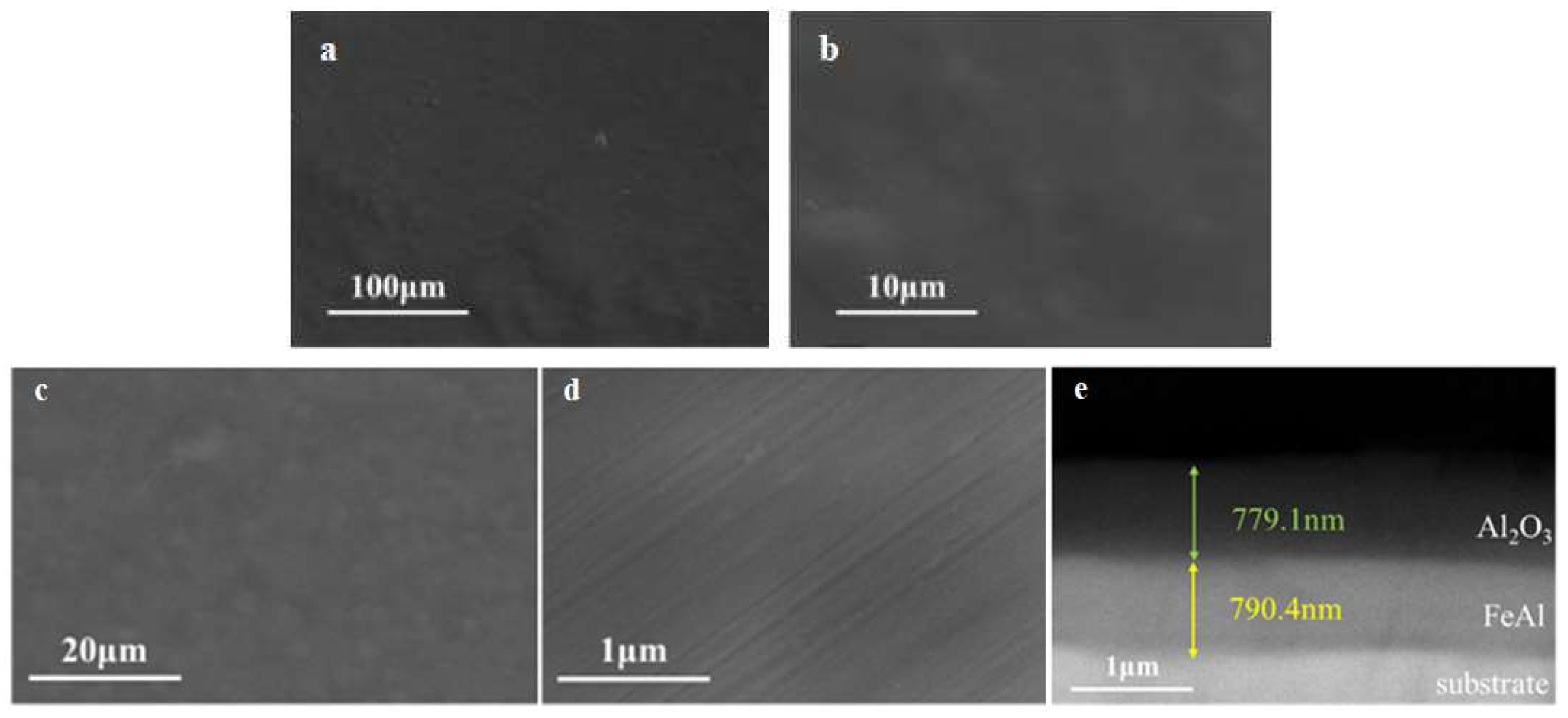
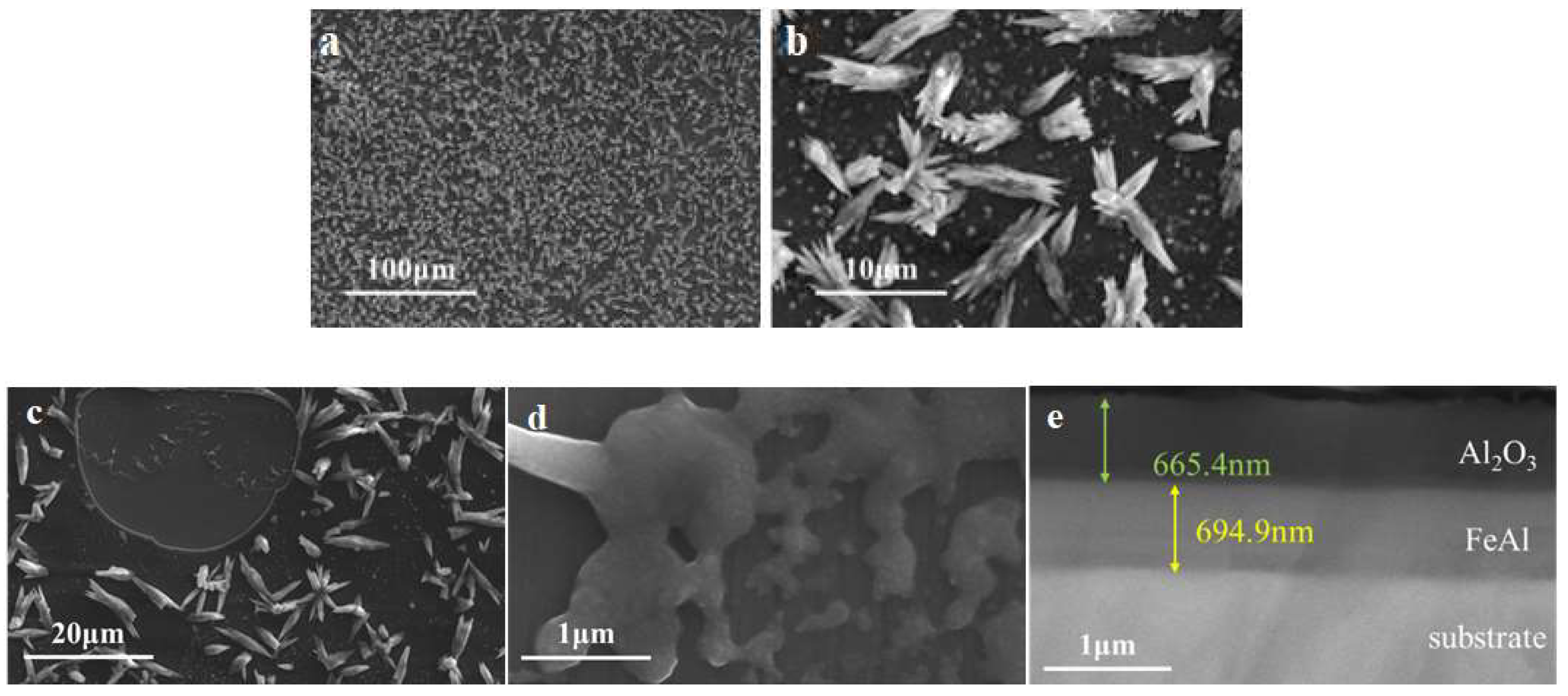
3.2.1. Intact Coating
Microstructure examination results of the intact FeAl/Al
2O
3 coating are shown in
Figure 8. It shown that the FeAl/Al
2O
3 coating prepared had high surface flatness and coating integrity. And after deuterium gas penetration test at high temperature, the surface of the coating remained flat and no obvious peeling and cracking. The cross-sectional image showed that the coating is clearly layered and the interface is intact, indicating that the coating has good high-temperature stability.
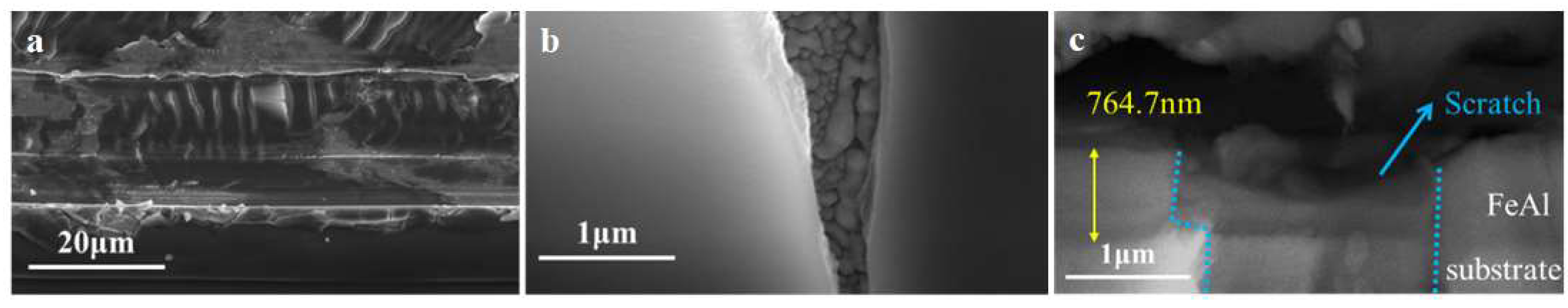
3.2.2. Coating after Thermal Shock
Microstructure examination results of the FeAl/Al
2O
3 coating after thermal shock are shown in
Figure 9. It shown that there is no obvious cracking on the surface of the coating after thermal shock, but some dendritic-like crystals were produced on the surface. And after deuterium penetration, the dendritic-like crystals remained on the surface, but the coating on the surface had peeled off. The spalling area is flat and a white lamellar structure can be seen inside, which is formed by cluster-like aggregation and could be produced after the coating is peeled off. The cross-sectional results showed that the coating structure after thermal shock is intact and the interior is not damaged. Based on the above results, it is judged that the spalling of Al
2O
3 layer on the outermost surface after thermal shock is due to the difference in the thermal expansion coefficient of the coating and the substrate. And since it is not affected by mechanical forces, the spalling area of the coating is relatively flat.
According to the results of microstructure examination, the increase in deuterium permeability after thermal shock is caused by the spalling of the Al2O3 layer on the surface. In addition, the dendritic -like crystals formed on the surface of the coating also increase the surface area of deuterium gas adsorption, which promotes the deuterium penetration process.
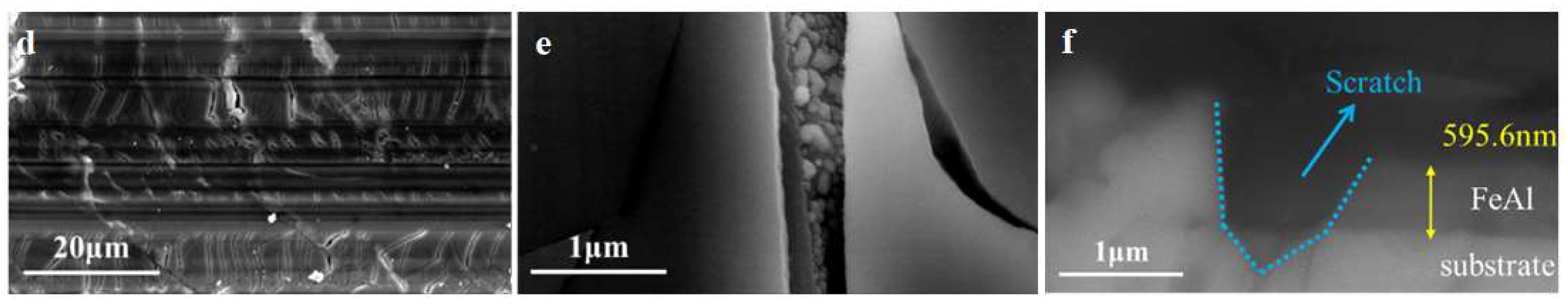
3.2.3. Coating with Scratch
SEM results of the FeAl/Al
2O
3 coating with scratch after GDP test are shown in
Figure 10. It could be seen that there are a large number of cracks in the scratch area. The internal morphology of the crack was uneven, with a roughness obviously greater than that of the coating surface. Such a structure will be more conducive to the attachment and dissociation of deuterium molecules, thus promoting the permeation. In addition, the cross-sectional results showed that the scratches have seriously damaged the coating. Both the Al
2O
3 layer and the internal FeAl layer have been damaged. The scratch depth had reached the surface of the substrate, which will expose the substrate and allow deuterium to penetrate directly from this area. As a result, the tritium resistance of the coating was reduced.
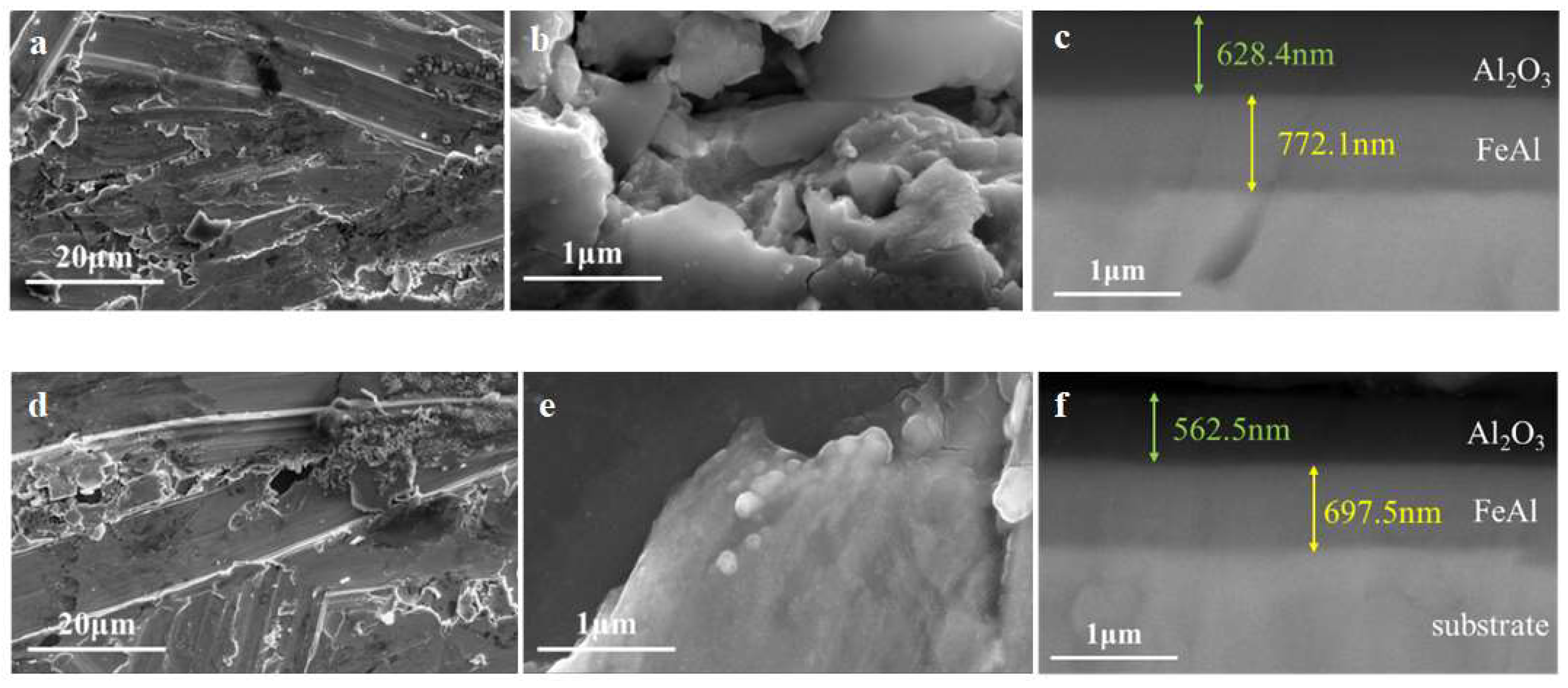
3.2.4. Coating with Debonding
Figure 11 showed the SEM results of the FeAl/Al
2O
3 coating with debonding after GDP test. It can be seen that the topography of the coating surface at the debonding place was uneven. There were some tiny potholes and the edges of the holes were broken. The cross-sectional inspection results showed that the interface structure of the coating was intact and there was no exposure of the substrate. Combined with the results of permeation experiments, it shows that the FeAl layer retained inside can still play a role in preventing deuterium penetration.
4. Summary
In this paper, the tritium resistance performance of FeAl/Al2O3 coatings with different mechanical damage was evaluated by carrying out coating damage simulation and gas hydrogen isotope permeation (GDP) test. And the potential causes of the change in tritium resistance performance after coating damage were analyzed through microscopic examination.
The test results verify the correlation between the tritium permeation resistance of the FeAl/Al2O3 coating and the integrity of the coating. The intact FeAl/Al2O3 coating has excellent tritium resistance performance, about 3 orders of magnitude. But when the coating integrity is missing, the tritium resistance performance is significantly reduced. The greater the damage degree of the coating, the worse the tritium resistance performance. In the most severe case, the tritium penetration resistance of the coating is almost completely lost.
The microstructure results show that the tritium resistance performance of FeAl/Al2O3 coating is related to the surface and cross-sectional state of the coating. When the surface of the coating is uneven or there are dendritic-like crystals or rough cracks inside, the surface area for tritium adsorption and dissociation increases, which is beneficial to tritium penetration process. If the Al2O3 layer on the outer surface of the coating peels off, the tritium penetration barrier effect in the peeling area is greatly reduced. When the integrity of the internal FeAl layer is also damaged, the substrate is directly exposed, tritium will penetrate directly from this area, and the coating will basically lose the function of tritium barrier.
Therefore, in order to ensure the tritium resistance performance of FeAl/Al2O3 coating, the integrity of the coating should be ensured as much as possible in the actual manufacturing and application process.
References
- Jean-Baptiste P., Baumier D., Fourré E., Dapoigny A., Clavel B. The distribution of tritium in the terrestrial and aquatic environments of the Creys-Malville nuclear power plant (2002–2005) [J]. Journal of Environmental Radioactivity, 2007, 94(2): 107-118. [CrossRef]
- Haiying Chen, Chunming Zhang, Shaowei Wang, Huaibin Li. Computational analysis of ~3H source term for pressurized water reactor [J]. Atomic Energy Science and Technology, 2016, 50(03): 459-463.
- Ye Ping, Yang Xiaoyong. Sources and effects of tritium in high-temperature gas-cooled reactor closed Brayton indirect circulation [J]. Atomic Energy Science and Technology, 2009, (S2): 4.
- Zhang Shuting, Huang Xiangmei, Zhao Dongye, et al. Experimental study on deuterium permeation of the first wall of a new type of TiN-containing tritium barrier transition layer [J]. Materials Reports, 2022, 36(S1): 38-42.
- Liu Liangliang, Ruan Qingdong, Xiao Shu, et al. Fabrication and hydrogen permeation resistance of dense CrN coatings [J]. Surface and Coatings Technology, 2022, 437: 128326. [CrossRef]
- Yao Z., Suzuki A., Levchuk D., Terai T. Sic coating by rf sputtering as tritium permeation barrier for fusion blanket [J]. Fusion Science and Technology, 2007, 52(4): 865-869. [CrossRef]
- Di Jiao, Wang Ya, Zhang Hang, et al. Preparation of Cr2O3 Tritium Inhibitor Coatings by Electroplating and High Temperature Oxidation [J]. Journal of Rare Metal Materials and Engineering, 2019, 48(02): 656-661.
- Chikada T., Suzuki A., Terai T., Muroga T., Koch F. Compatibility of erbium oxide coating with liquid lithium-lead alloy and corrosion protection effect of iron layer [J]. Fusion Engineering and Design, 2013, 88(6-8): 640-643. [CrossRef]
- Feng Jun, Chen Meiyan, Tong Honghui, Jin Fanya, Dan Min, Xu Zejin, Shen Liru, Zhang Guikai. Preparation of Alumina Coatings as Tritium Permeation Barrier by a Composite Treatment of Low Temperature Plasma [J]. Rare Metal Materials and Engineering, 2017, 46(10): 2837-2841. [CrossRef]
- Yang F. L., Xiang X., Lu G. D., Zhang G. K., Tang T., Shi Y., Wang X. L. Tritium permeation characterization of Al2O3/FeAl coatings as tritium permeation barriers on 321 type stainless steel containers [J]. Journal of Nuclear Materials, 2016, 478: 144-148. [CrossRef]
- Wang L. L., Wu Y. Y., Luo X. F., Ning Z. E., Wang J. H., Yang J. J., Feng Y. J., Liao J. L., Yang Y. Y., Feng K. M., Liu N., Gong M. Effects of Ar/O2 ratio on preparation and properties of multilayer Cr2O3/α-Al2O3 tritium permeation barrier [J]. Surface & Coatings Technology, 2018, 339: 132-138. [CrossRef]
- Zhang Guikai. Theoretical study on hydrogen behavior in α-Al2O3 tritium inhibitor coating materials[D]; University of Science and Technology of China, 2014.
- Hu Li, Zhang Guikai, Tang Tao. Research Progress on the Formation Mechanism and Low Temperature Preparation Technology of Al2O3 Thin Films on FeAl/Al2O3 Tritium Barrier Coatings [J]. Materials for Mechanical Engineering, 2019, 43(06): 1-7.
- Shao Zongming. Preparation and properties of Fe-Al/Al2O3 tritium inhibitor coating[D]; University of Science and Technology of China, 2023.
- Wang Wenxuan. Preparation of anti-tritium permeable coating layer of Al2O3/FeAl on the surface of CLAM steel[D]; China Academy of Engineering Physics, 2016.
- Song B., Dong S. J., Liao H. L., Coddet C. Microstructure and wear resistance of FeAl/Al2O3 intermetallic composite coating prepared by atmospheric plasma spraying [J]. Surface & Coatings Technology, 2015, 268: 24-29. [CrossRef]
- Magnee A., Offergeld E., Leroy M., Lefort A. Fe-AI Intermetallic Coating Applications to Thermal Energy Conversion Advanced Systems; proceedings of the ITSC 1998, F, 1998 [C].
- Tortorelli P. F., DeVan J. H. Behavior of iron aluminides in oxidizing and oxidizing/sulfldizing environments [J]. 1992: 573-577. [CrossRef]
- Sanjib Majumdar, Bhaskar Paul, Poulami Chakraborty, Jugal Kishor, Vivekanand Kain, Gautam Kumar Dey. Formation of Al2O3/FeAl coatings on a 9Cr-1Mo steel, and corrosion evaluation in flowing Pb-17Li loop [J]. Journal of Nuclear Materials, 2017, 486: 60-65. [CrossRef]
- Gang Ji, Omar Elkedim, Thierry Grosdidier. Deposition and corrosion resistance of HVOF sprayed nanocrystalline iron aluminide coatings [J]. Surface and Coatings Technology, 2005, 190(2): 406-416. [CrossRef]
- Knowles Shawn D, Senor David J, Forbes Steven V, Johnson Roger N, Hollenberg Glenn W. Method of coating the interior surface of hollow objects with a diffusion coating [Z]. Google Patents. 2005.
- Huang J., Xie H., Luo L. M., Zan X., Liu D. G., Wu Y. C. Preparation and properties of FeAl/Al2O3 composite tritium permeation barrier coating on surface of 316L stainless steel [J]. Surface & Coatings Technology, 2020, 383. [CrossRef]
- Shao Z. M., Yang H., Zhang S. W., Liu W. P., Xiao Z. Q., Zheng M. J. A dense Fe-Al/Al2O3 coating as tritium permeation barrier on CLAM steel by hot-dipping aluminizing [J]. Surface & Coatings Technology, 2022, 440. [CrossRef]
- Yilong Zhong, Changda Zhu, Wei Zhang, Jian Yang, Jiuguo Deng, Qingyu Li, Hao Liu, Yi Zhou, Huifang Yue, Xi Qiu, Jijun Yang. Exploring the application potential of FexAl/Al2O3 coatings for lead-cooled fast reactors [J]. Journal of Nuclear Materials, 2024, 588: 154807. [CrossRef]
- Zhou Xiaokai, Tong Lili. Simulation of tritium diffusion behavior in zirconium alloy cladding [J]. High Power Laser and Particle Beams, 2021, 33(03): 132-137.
Figure 1.
The FeAl/Al2O3 coating samples prepared.
Figure 1.
The FeAl/Al2O3 coating samples prepared.
Figure 2.
Structure of gas hydrogen isotope permeation test device.
Figure 2.
Structure of gas hydrogen isotope permeation test device.
Figure 3.
Scratch and debonding damage simulation and surface damage ratios.
Figure 3.
Scratch and debonding damage simulation and surface damage ratios.
Figure 4.
GDP results of FeAl/Al2O3 coating after thermal shock at (a) 100kPa and (b) 500kPa pressures.
Figure 4.
GDP results of FeAl/Al2O3 coating after thermal shock at (a) 100kPa and (b) 500kPa pressures.
Figure 5.
GDP results of FeAl/Al2O3 coating with scratch at (a) 100kPa and (b) 500kPa pressures.
Figure 5.
GDP results of FeAl/Al2O3 coating with scratch at (a) 100kPa and (b) 500kPa pressures.
Figure 6.
GDP results of FeAl/Al2O3 coating with debonding at (a) 100kPa and (b) 500kPa pressures.
Figure 6.
GDP results of FeAl/Al2O3 coating with debonding at (a) 100kPa and (b) 500kPa pressures.
Figure 7.
Permeability of FeAl/Al2O3 coatings at (a) 100kPa and (b) 500kPa pressures.
Figure 7.
Permeability of FeAl/Al2O3 coatings at (a) 100kPa and (b) 500kPa pressures.
Figure 8.
SEM results of the intact FeAl/Al2O3 coating, (a, b) before GDP test, (c, d, e) after GDP test.
Figure 8.
SEM results of the intact FeAl/Al2O3 coating, (a, b) before GDP test, (c, d, e) after GDP test.
Figure 9.
SEM results of FeAl/Al2O3 coating after thermal shock, (a, b) before GDP test, (c, d, e) after GDP test.
Figure 9.
SEM results of FeAl/Al2O3 coating after thermal shock, (a, b) before GDP test, (c, d, e) after GDP test.
Figure 10.
SEM results of FeAl/Al2O3 coating with (a, b, c) scratch 21.5% and (d, e, f) scratch 31.9% after GDP test.
Figure 10.
SEM results of FeAl/Al2O3 coating with (a, b, c) scratch 21.5% and (d, e, f) scratch 31.9% after GDP test.
Figure 11.
SEM results of FeAl/Al2O3 coating with (a, b, c) debonding 2.8% and (d, e, f) debonding 8.6% after GDP test.
Figure 11.
SEM results of FeAl/Al2O3 coating with (a, b, c) debonding 2.8% and (d, e, f) debonding 8.6% after GDP test.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).