Introduction
The knee joint is a complex structure comprising bones, cartilage, ligaments, tendons, and synovial fluid, each playing a crucial role in its overall function and health. The knee joint is formed by the articulation of the femur (thigh bone), tibia (shin bone), and patella (kneecap), which work together to facilitate movement and bear the body’s weight. The articular cartilage covers the ends of these bones, providing a smooth, frictionless surface for movement and acting as a shock absorber to distribute loads during activities such as walking, running, and jumping. This cartilage is essential for the smooth gliding motion of the joint and protecting the bones from wear and tear.
Ligaments and tendons are critical for stabilizing the knee joint and enabling its motion. The major ligaments include the anterior cruciate ligament (ACL), posterior cruciate ligament (PCL), medial collateral ligament (MCL), and lateral collateral ligament (LCL). These ligaments function to prevent excessive movement and maintain the joint’s stability by resisting forces that could dislocate or damage the joint. Tendons, such as the quadriceps and patellar tendons, connect muscles to bones and play a key role in facilitating the knee’s extension and flexion, allowing for a wide range of motion necessary for various physical activities.
The synovial fluid, produced by the synovium, is another critical component of the knee joint. This fluid lubricates the joint and nourishes the cartilage, reducing friction and wear during movement. The health of the synovium and the quality of synovial fluid are vital for maintaining joint function and preventing degenerative conditions. Synovial fluid acts as a shock absorber and a medium for nutrient and waste exchange for the avascular cartilage.
Mechanical loading, which refers to the application of force or pressure to the knee joint during various activities, significantly affects its health and functionality. Everyday activities, from walking and climbing stairs to engaging in sports, subject the knee to different types of mechanical loads, including compression, tension, shear, and hydrostatic pressure. These loads are essential for maintaining healthy joint tissues by stimulating cellular activities that promote repair and regeneration. However, excessive or abnormal mechanical loading can lead to tissue damage, inflammation, and degenerative conditions such as osteoarthritis. Understanding the optimal loading conditions that support joint health without causing damage is a critical aspect of knee joint biomechanics.
Mechanotransduction is a key process through which cells within the knee joint sense and respond to mechanical stimuli. This process involves the conversion of mechanical signals into biochemical responses, enabling cells to adapt to changes in their mechanical environment. Mechanoreceptors on the cell surface detect mechanical forces, triggering intracellular signaling pathways that regulate gene expression, protein synthesis, and cellular behavior. These pathways include the MAPK, NF-κB, and Wnt signaling cascades, which play critical roles in maintaining tissue homeostasis and facilitating repair processes. For instance, in cartilage, mechanotransduction influences the production of extracellular matrix components, which are essential for maintaining the structural integrity of the tissue.
Understanding the intricacies of mechanotransduction is crucial for developing targeted rehabilitation protocols aimed at enhancing recovery and preventing degeneration of the knee joint. Insights into how mechanical loading influences cellular responses can inform the design of therapeutic interventions that optimize loading conditions to promote tissue repair, reduce inflammation, and restore joint function. Rehabilitation strategies, such as controlled loading exercises, manual therapy, and the use of orthotics, can be tailored to harness the benefits of mechanotransduction, ensuring effective treatment of knee joint injuries and conditions.
Controlled loading exercises, for example, can help in modulating the mechanical environment of the joint to stimulate healing without causing further damage. Manual therapy techniques can assist in maintaining joint mobility and reducing pain by influencing mechanotransductive pathways. Orthotic devices can alter the distribution of mechanical loads across the joint, helping to protect damaged areas while allowing other parts to bear more load and adapt positively.
By integrating knowledge of mechanotransduction into clinical practice, healthcare providers can develop more precise and effective rehabilitation programs. These programs can help patients recover from injuries, manage chronic conditions, and improve their overall quality of life by maintaining healthy knee joint function. For instance, tailored exercise regimens that consider the principles of mechanotransduction can enhance the repair of damaged cartilage, improve synovial fluid dynamics, and strengthen the supportive structures around the knee.
In conclusion, a deep understanding of the knee joint’s response to mechanical loading and the mechanisms of mechanotransduction is essential for advancing rehabilitation practices and achieving optimal patient outcomes. This knowledge not only aids in the development of better therapeutic strategies but also provides a scientific basis for customizing rehabilitation programs to individual patient needs. Such personalized approaches are likely to result in more effective treatment, quicker recovery times, and a reduction in the incidence of chronic knee problems, thereby enhancing the overall quality of life for patients.
Mechanotransduction in the Knee Joint
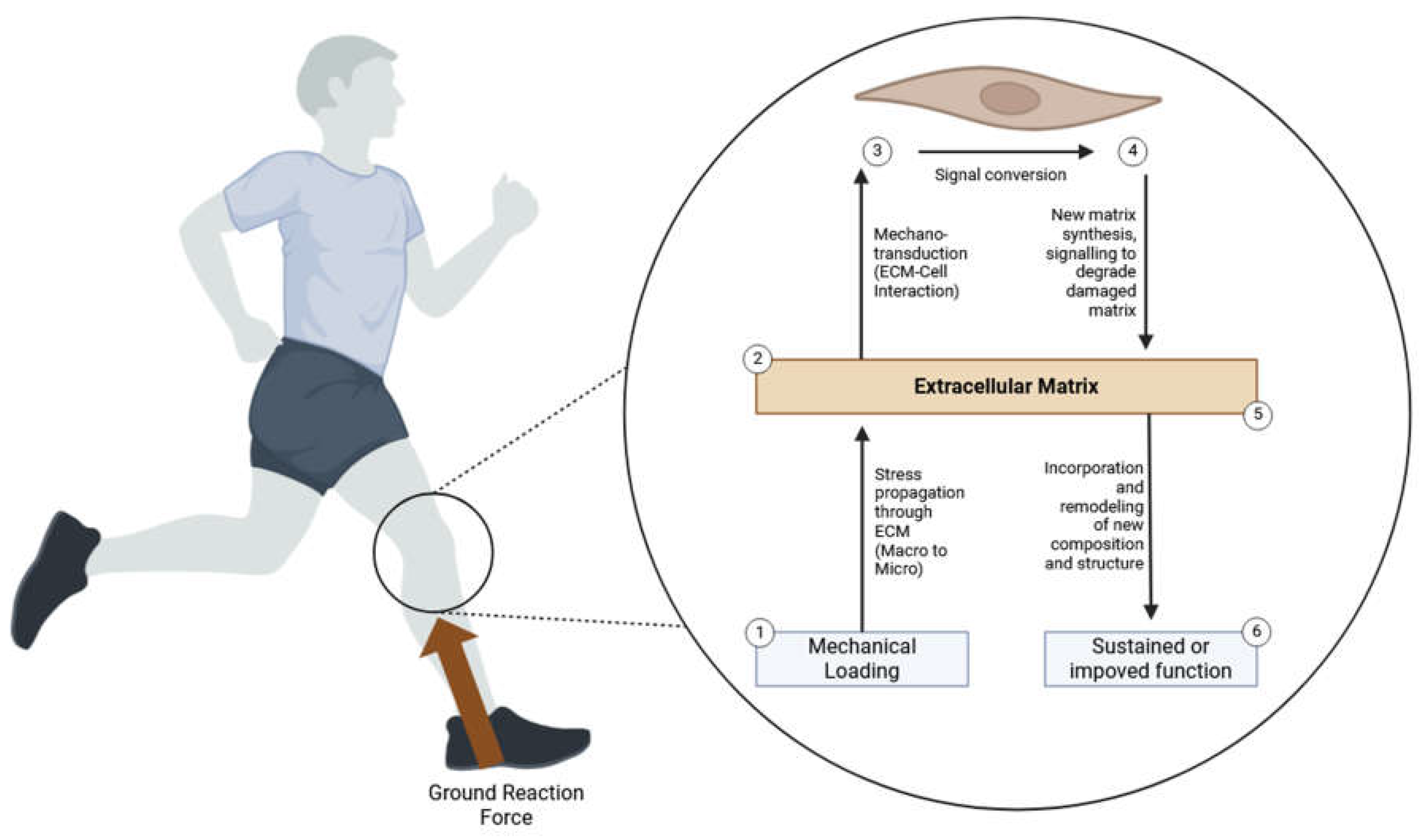
Mechanotransduction in the knee joint involves several cellular components, including mechanoreceptors, ion channels, and signaling pathways. This intricate process enables the knee joint to convert mechanical stimuli into biochemical responses, which are crucial for maintaining joint health, facilitating repair processes, and adapting to mechanical loads (
Figure 1). The key cells involved in this process are chondrocytes (cartilage cells), synoviocytes (synovial cells), and fibroblasts (ligament and tendon cells).
Chondrocytes are the primary cell type found in cartilage, the smooth, resilient tissue that covers the ends of bones in the knee joint. These cells play a critical role in maintaining cartilage integrity and functionality. Since cartilage is avascular, meaning it lacks its own blood supply, chondrocytes rely heavily on mechanical loading to facilitate the diffusion of nutrients and the removal of waste products. Chondrocytes sense mechanical stimuli through specialized cell surface receptors known as integrins and mechanosensitive ion channels. When activated by mechanical forces, these receptors initiate a series of intracellular signaling cascades, including the Mitogen-Activated Protein Kinase (MAPK), Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB), and Wnt signaling pathways. These pathways regulate various cellular processes, such as gene expression and protein synthesis, which are essential for the synthesis and degradation of the extracellular matrix (ECM) components like collagen and proteoglycans. By balancing the production and breakdown of ECM components, chondrocytes help maintain tissue homeostasis and facilitate cartilage repair and regeneration.
On a molecular level, the MAPK pathway in chondrocytes involves a sequence of phosphorylation events that transmit the signal from the cell surface to the nucleus. This pathway includes the activation of extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p38 MAPKs. These kinases ultimately lead to the activation of transcription factors such as Activator Protein 1 (AP-1), which regulates the expression of genes involved in cell proliferation, differentiation, and ECM production. Similarly, the NF-κB pathway is critical for the inflammatory response and cell survival, involving the translocation of NF-κB dimers into the nucleus to modulate gene expression related to inflammation and stress responses. The Wnt signaling pathway, particularly the canonical Wnt/β-catenin pathway, is essential for chondrocyte differentiation and the maintenance of cartilage homeostasis. Activation of this pathway leads to the accumulation of β-catenin in the cytoplasm and its subsequent translocation into the nucleus, where it influences the transcription of target genes crucial for cartilage repair and regeneration.
Synoviocytes are specialized cells located in the synovium, the thin membrane that lines the inner surface of the joint capsule. These cells are responsible for producing synovial fluid, a viscous liquid that lubricates the joint, reduces friction, and provides essential nutrients to the avascular cartilage. Mechanical loading stimulates synoviocytes through mechanosensitive receptors, such as integrins and stretch-activated ion channels. Upon activation, these receptors trigger intracellular signaling pathways that enhance the production of key synovial fluid components, including hyaluronic acid and lubricin. Hyaluronic acid contributes to the viscosity and lubricating properties of synovial fluid, while lubricin plays a crucial role in reducing friction between the cartilage surfaces during movement. These adaptations help protect the joint from mechanical wear and support overall knee joint health. Additionally, synoviocytes secrete anti-inflammatory cytokines that modulate the local immune response, thereby preventing excessive inflammation that could damage the joint tissues.
On a deeper molecular level, the synthesis of hyaluronic acid by synoviocytes is regulated by the enzyme hyaluronan synthase, which is upregulated in response to mechanical stress. The production of lubricin is controlled by mechanosensitive pathways involving the mechanistic target of rapamycin (mTOR) and the Hippo signaling pathway. These pathways regulate cellular growth, proliferation, and the synthesis of ECM components, ensuring that synovial fluid maintains its protective functions. Moreover, synoviocytes play a role in the immune modulation of the joint environment through the secretion of cytokines like interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α), which are involved in inflammatory responses. The balance between pro-inflammatory and anti-inflammatory signals is crucial for maintaining joint health and preventing chronic inflammatory conditions such as osteoarthritis.
Fibroblasts are the primary cells found in ligaments and tendons, the connective tissues that link bones and muscles, respectively. These cells are essential for maintaining the structural integrity and function of these tissues. Ligaments provide stability to the knee joint by preventing excessive movement, while tendons facilitate joint movement by transmitting forces from muscles to bones. Fibroblasts respond to mechanical loading by modulating collagen synthesis and ECM remodeling. Mechanotransduction in fibroblasts involves integrins, focal adhesion complexes, and mechanosensitive ion channels, which activate various intracellular signaling pathways. Notable pathways include the MAPK and Transforming Growth Factor-beta (TGF-β) pathways, which regulate the production and organization of collagen fibers. Proper mechanical loading ensures that fibroblasts maintain the strength and elasticity of ligaments and tendons, supporting joint stability and efficient force transmission. Excessive or abnormal mechanical loading, however, can lead to microtears, inflammation, and degenerative conditions such as tendinopathy, highlighting the need for balanced mechanical stimuli for ligament and tendon health. Furthermore, fibroblasts play a role in the healing process of injured ligaments and tendons by migrating to the injury site, proliferating, and synthesizing new ECM components to replace the damaged tissue.
On a molecular level, the MAPK pathway in fibroblasts involves the activation of ERKs, JNKs, and p38 MAPKs, leading to the regulation of genes involved in collagen synthesis and ECM remodeling. The TGF-β pathway is particularly important in fibroblasts, as it regulates the production of collagen and other ECM components through the activation of Smad proteins. These proteins translocate to the nucleus and modulate the expression of target genes involved in tissue repair and fibrosis. Additionally, fibroblasts express matrix metalloproteinases (MMPs), which are enzymes that degrade ECM components and facilitate tissue remodeling. The activity of MMPs is tightly regulated by tissue inhibitors of metalloproteinases (TIMPs), ensuring a balanced turnover of ECM components necessary for maintaining the structural integrity of ligaments and tendons.
In summary, mechanotransduction in the knee joint is a fundamental process through which chondrocytes, synoviocytes, and fibroblasts sense and respond to mechanical stimuli. This process involves complex interactions between mechanoreceptors, ion channels, and signaling pathways, enabling these cells to adapt to their mechanical environment. Understanding the mechanisms of mechanotransduction is essential for developing targeted rehabilitation protocols that optimize mechanical loading conditions to promote tissue repair, reduce inflammation, and restore joint function. By leveraging insights into mechanotransduction, clinicians can design more effective treatments for knee joint injuries and degenerative conditions, ultimately improving patient outcomes. Additionally, advances in mechanobiology research may lead to the development of biomimetic materials and tissue engineering approaches that replicate the natural mechanical environment of the knee joint, further enhancing the efficacy of therapeutic interventions. The integration of molecular biology with clinical practices promises to revolutionize the management of knee joint disorders, providing personalized and precise treatments that address the underlying cellular and molecular mechanisms driving these conditions (
Table 1).
1. Chondrocytes and Cartilage: Cartilage is an avascular tissue, meaning it lacks its own blood supply and thus relies heavily on mechanical loading for nutrient diffusion and waste removal. Chondrocytes, the only type of cells found in healthy cartilage, play a crucial role in maintaining cartilage integrity. These cells sense mechanical stimuli through mechanoreceptors such as integrins and mechanosensitive ion channels. Upon detecting mechanical load, these receptors activate intracellular signaling pathways, including MAPK (Mitogen-Activated Protein Kinase), NF-κB (Nuclear Factor kappa-light-chain-enhancer of activated B cells), and Wnt pathways.
The MAPK pathway is essential for transmitting signals from the cell surface to the DNA in the cell nucleus, leading to the expression of genes that control cell proliferation, differentiation, and survival. This pathway includes several sub-pathways such as ERK (extracellular signal-regulated kinases), JNK (c-Jun N-terminal kinases), and p38 MAPKs, each of which is activated by different stress signals and plays distinct roles in chondrocyte function. For instance, ERK activation is typically associated with cell growth and differentiation, while JNK and p38 are often linked to inflammatory responses and stress-induced apoptosis.
In chondrocytes, the activation of MAPK pathways results in the increased synthesis of extracellular matrix components, including type II collagen and proteoglycans. These components are crucial for the structural integrity and biomechanical properties of cartilage. Additionally, MAPK signaling influences the production of aggrecan, a proteoglycan that provides compressive resistance by attracting and retaining water molecules within the cartilage matrix.
The NF-κB pathway plays a pivotal role in inflammatory responses and is involved in the regulation of genes responsible for cell survival, apoptosis, and matrix remodeling. Activation of NF-κB in chondrocytes can lead to the production of catabolic enzymes such as matrix metalloproteinases (MMPs) and aggrecanases, which degrade the cartilage matrix. This catabolic activity is counterbalanced by tissue inhibitors of metalloproteinases (TIMPs), which help to maintain the structural integrity of the cartilage. Dysregulation of this balance can contribute to cartilage degeneration and the development of osteoarthritis.
The Wnt signaling pathway is another critical regulator of cartilage homeostasis. It influences chondrocyte proliferation, differentiation, and matrix synthesis. The Wnt pathway interacts with other signaling pathways, such as TGF-β and BMP pathways, to ensure a coordinated cellular response to mechanical stimuli. Wnt signaling also plays a role in the regulation of chondrocyte hypertrophy, a process that can lead to cartilage calcification and degradation if not properly controlled.
The cartilage matrix itself is primarily composed of type II collagen and proteoglycans. Type II collagen fibers form a robust network that provides tensile strength and structural support. Proteoglycans, such as aggrecan, are large molecules that attract and retain water, giving cartilage its unique ability to resist compressive forces. The interactions between collagen and proteoglycans are crucial for the biomechanical properties of cartilage. Furthermore, the ECM of cartilage contains other minor collagens (e.g., type IX and type XI collagen) and non-collagenous proteins (e.g., cartilage oligomeric matrix protein, or COMP) that contribute to the overall stability and function of the cartilage.
The balance between matrix synthesis and degradation is vital for tissue homeostasis and repair. An optimal mechanical environment promotes the production of extracellular matrix components, ensuring the cartilage remains resilient and functional. Conversely, excessive or insufficient loading can disrupt this balance, leading to cartilage breakdown and conditions such as osteoarthritis. In osteoarthritis, the degradation of the cartilage matrix surpasses its synthesis, leading to joint pain, inflammation, and loss of function.
In addition to the MAPK, NF-κB, and Wnt pathways, other molecular mechanisms are involved in chondrocyte mechanotransduction. For instance, the influx of calcium ions (Ca²⁺) through mechanosensitive channels acts as a secondary messenger in various intracellular signaling cascades. This Ca²⁺ signaling can activate various kinases and phosphatases, further modulating the activity of transcription factors and gene expression involved in cartilage maintenance.
The role of hypoxia in cartilage biology is also significant. Cartilage exists in a relatively low-oxygen environment, and chondrocytes have adapted to this condition by utilizing hypoxia-inducible factors (HIFs). HIFs regulate the expression of genes involved in energy metabolism, matrix production, and cell survival, enabling chondrocytes to function effectively under hypoxic conditions. HIF-1α, in particular, plays a crucial role in maintaining the chondrocyte phenotype and promoting the synthesis of key matrix components under hypoxic conditions.
Furthermore, the extracellular matrix (ECM) of cartilage is rich in signaling molecules such as growth factors, cytokines, and chemokines. These molecules interact with cell surface receptors on chondrocytes, influencing their behavior and matrix production. For example, transforming growth factor-beta (TGF-β) and bone morphogenetic proteins (BMPs) are potent anabolic factors that stimulate collagen and proteoglycan synthesis. Additionally, insulin-like growth factor 1 (IGF-1) and fibroblast growth factors (FGFs) play significant roles in promoting chondrocyte proliferation and matrix production.
The regulation of chondrocyte activity also involves epigenetic mechanisms, including DNA methylation, histone modifications, and non-coding RNAs such as microRNAs (miRNAs). These epigenetic factors can modulate the expression of genes involved in cartilage development, maintenance, and repair. For instance, specific miRNAs have been shown to regulate the expression of key matrix-degrading enzymes and inflammatory mediators in chondrocytes, highlighting their potential as therapeutic targets for cartilage-related diseases.
Overall, the intricate network of signaling pathways and molecular mechanisms underscores the complexity of cartilage biology and highlights the importance of maintaining a balanced mechanical environment to preserve cartilage function and prevent degenerative diseases. Understanding these processes at the molecular level provides valuable insights into potential therapeutic targets for cartilage repair and regeneration, offering hope for treating conditions such as osteoarthritis. Advances in molecular biology techniques, such as gene editing, tissue engineering, and regenerative medicine, hold promise for developing novel treatments to restore cartilage function and improve joint health.
2. Synoviocytes and Synovial Fluid: Synoviocytes are specialized cells found in the synovium, a membrane that lines the joint capsule and produces synovial fluid. This fluid serves several essential functions, including lubricating the joint, reducing friction, and providing nutrients to the avascular cartilage. Mechanical loading stimulates synoviocytes through mechanosensitive receptors, such as integrins and stretch-activated ion channels. When activated, these receptors enhance the production of key synovial fluid components like hyaluronic acid and lubricin.
Hyaluronic acid is a high molecular weight glycosaminoglycan that significantly increases the viscosity of synovial fluid, thereby improving its lubricating properties. It forms a viscoelastic network that can absorb mechanical shocks, thus protecting the cartilage from excessive stress and damage. The production of hyaluronic acid by synoviocytes is regulated by various signaling pathways, including those mediated by cytokines and growth factors. Transforming growth factor-beta (TGF-β) and platelet-derived growth factor (PDGF) are known to stimulate the synthesis of hyaluronic acid, while pro-inflammatory cytokines such as interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α) can decrease its production, leading to reduced joint lubrication and increased susceptibility to wear and tear.
Lubricin, also known as proteoglycan 4 (PRG4), is another critical component of synovial fluid produced by synoviocytes. Lubricin is a mucin-like glycoprotein that reduces friction between the cartilage surfaces during movement. It achieves this by forming a thin, slippery layer on the articular cartilage, which minimizes mechanical wear and prevents adhesion of cartilage surfaces. The expression of lubricin is regulated by mechanical stimuli and various biochemical signals. Mechanical loading can upregulate the synthesis of lubricin through the activation of integrins and stretch-activated ion channels, while factors like TGF-β and IL-4 can enhance its production at the molecular level.
Synoviocytes are classified into two main types: fibroblast-like synoviocytes (FLS) and macrophage-like synoviocytes (MLS). FLS are primarily responsible for the production of synovial fluid components, including hyaluronic acid and lubricin. They exhibit a high degree of plasticity and can respond dynamically to mechanical and biochemical cues. MLS, on the other hand, play a crucial role in immune surveillance and maintaining joint homeostasis. They produce cytokines and chemokines that regulate inflammation and tissue repair processes within the joint.
Mechanical loading activates several intracellular signaling pathways in synoviocytes, including the MAPK, NF-κB, and PI3K/Akt pathways. The MAPK pathway, involving ERK, JNK, and p38 kinases, mediates cellular responses to mechanical stress and regulates the expression of genes involved in the synthesis of synovial fluid components. The NF-κB pathway is primarily associated with inflammatory responses and can modulate the production of cytokines and matrix-degrading enzymes. The PI3K/Akt pathway plays a role in cell survival, proliferation, and metabolism, influencing the overall function of synoviocytes.
In addition to mechanical stimuli, synoviocytes respond to various biochemical signals present in the joint microenvironment. Cytokines, growth factors, and matrix metalloproteinases (MMPs) released by chondrocytes, immune cells, and other joint tissues can influence synoviocyte activity. For example, IL-1 and TNF-α are potent pro-inflammatory cytokines that can induce the production of MMPs and other catabolic factors by synoviocytes, contributing to cartilage degradation in conditions such as osteoarthritis and rheumatoid arthritis.
The interaction between synoviocytes and the extracellular matrix (ECM) is also crucial for maintaining joint health. The ECM of the synovium contains various components such as collagen, fibronectin, and laminin, which provide structural support and biochemical signals to synoviocytes. Integrins, which are transmembrane receptors on synoviocytes, mediate the attachment of these cells to the ECM and transduce signals that regulate cell adhesion, migration, and differentiation. Disruption of these interactions can lead to altered synoviocyte function and joint pathology.
Epigenetic regulation also plays a significant role in synoviocyte function. DNA methylation, histone modifications, and non-coding RNAs, such as microRNAs (miRNAs), influence the expression of genes involved in synovial fluid production and inflammatory responses. For instance, specific miRNAs have been shown to modulate the expression of key enzymes involved in hyaluronic acid synthesis and degradation, thereby affecting synovial fluid viscosity and joint lubrication.
Recent research has highlighted the role of extracellular vesicles (EVs) released by synoviocytes in intercellular communication within the joint. These EVs, which include exosomes and microvesicles, carry bioactive molecules such as proteins, lipids, and nucleic acids that can influence the behavior of neighboring cells. EVs derived from synoviocytes have been shown to modulate inflammatory responses, cartilage metabolism, and tissue repair processes, suggesting their potential as therapeutic agents for joint diseases.
Synoviocytes also play a role in the immune response within the joint. MLS, in particular, are involved in antigen presentation and the production of cytokines and chemokines that recruit immune cells to the joint. This immune activity is crucial for defending against infections and resolving inflammation. However, dysregulated immune responses by synoviocytes can contribute to chronic inflammation and joint damage in autoimmune conditions such as rheumatoid arthritis.
Overall, the intricate network of signaling pathways, molecular mechanisms, and cellular interactions highlights the complexity of synoviocyte function and synovial fluid regulation. Understanding these processes at the molecular level provides valuable insights into the pathophysiology of joint diseases and offers potential therapeutic targets for enhancing joint lubrication, reducing inflammation, and promoting cartilage repair in conditions such as osteoarthritis and rheumatoid arthritis. Advances in molecular biology and regenerative medicine hold promise for developing novel treatments aimed at restoring joint function and improving the quality of life for individuals with joint disorders.
3. Fibrochondrocytes and Meniscus: The menisci are fibrocartilaginous structures within the knee joint that play a vital role in load distribution, shock absorption, and joint stability. They are composed of a dense extracellular matrix (ECM) rich in collagen and proteoglycans, which provide both strength and flexibility. Mechanotransduction in meniscal cells, including fibrochondrocytes, is crucial for maintaining the health and function of the meniscus. These cells respond to mechanical loading by adjusting the synthesis of ECM components, ensuring the menisci can withstand and adapt to varying mechanical stresses.
The ECM of the meniscus primarily contains type I collagen, which provides tensile strength, and type II collagen, which contributes to compressive resistance. Proteoglycans, particularly aggrecan, are also abundant and help retain water, thus enhancing the shock-absorbing properties of the meniscus. The ECM components are organized in a unique, anisotropic pattern that reflects the complex mechanical environment of the knee joint, with collagen fibers aligning in different orientations to resist multidirectional loads.
Mechanotransduction in fibrochondrocytes involves the detection of mechanical stimuli through mechanoreceptors such as integrins and mechanosensitive ion channels. These receptors activate several intracellular signaling pathways, including MAPK (Mitogen-Activated Protein Kinase), NF-κB (Nuclear Factor kappa-light-chain-enhancer of activated B cells), and Wnt pathways. The activation of these pathways leads to the regulation of gene expression and protein synthesis, directly influencing the production and organization of ECM components.
The MAPK pathway, particularly the ERK, JNK, and p38 MAPKs, plays a significant role in transducing mechanical signals into cellular responses. ERK activation is generally associated with cell proliferation and differentiation, while JNK and p38 pathways are involved in stress responses and apoptosis. In the meniscus, MAPK signaling can modulate the synthesis of collagens and proteoglycans, thus maintaining the structural integrity and biomechanical properties of the tissue.
NF-κB signaling in fibrochondrocytes is primarily associated with inflammatory responses. Mechanical loading can modulate NF-κB activity, influencing the expression of cytokines and matrix metalloproteinases (MMPs). These enzymes play a crucial role in ECM remodeling by degrading collagen and other matrix components. Proper regulation of NF-κB activity ensures a balance between ECM synthesis and degradation, which is essential for the maintenance and repair of the meniscus.
The Wnt signaling pathway is another critical regulator of meniscal cell function. It influences cell proliferation, differentiation, and matrix production. Wnt signaling also interacts with other pathways, such as TGF-β and BMP pathways, to coordinate cellular responses to mechanical loading. This cross-talk between signaling pathways ensures that fibrochondrocytes can adapt to changes in mechanical stress, maintaining the health and functionality of the meniscus.
In addition to these pathways, fibrochondrocytes rely on intracellular calcium (Ca²⁺) signaling for mechanotransduction. Mechanosensitive ion channels allow the influx of Ca²⁺ in response to mechanical stimuli, acting as secondary messengers in various signaling cascades. Ca²⁺ signaling can activate kinases and phosphatases, further modulating the activity of transcription factors and gene expression related to ECM synthesis and organization.
Epigenetic regulation also plays a role in the response of fibrochondrocytes to mechanical loading. DNA methylation, histone modifications, and non-coding RNAs, such as microRNAs (miRNAs), can influence the expression of genes involved in ECM production and degradation. For instance, specific miRNAs have been shown to regulate the expression of collagens, proteoglycans, and MMPs, highlighting their potential as therapeutic targets for meniscal repair and regeneration.
The unique structure of the meniscus is supported by a network of blood vessels and nerves in the peripheral regions, which provide nutrients and sensory feedback. The central regions of the meniscus are avascular, relying on the diffusion of nutrients from the synovial fluid. This vascularization pattern influences the healing capacity of the meniscus, with peripheral tears having a better prognosis for repair compared to central tears.
The meniscus ECM also contains other important proteins such as fibronectin, elastin, and decorin, which contribute to its biomechanical properties and structural integrity. Fibronectin, for example, facilitates cell adhesion and migration, playing a role in tissue repair and maintenance. Elastin provides elasticity, allowing the meniscus to return to its original shape after deformation. Decorin is involved in collagen fibrillogenesis and interacts with growth factors to regulate ECM assembly and cell signaling.
Understanding the molecular mechanisms of meniscal degeneration and repair is crucial for developing effective treatments. In degenerative conditions such as osteoarthritis, the balance between ECM synthesis and degradation is disrupted, leading to the breakdown of meniscal tissue. This process involves increased activity of catabolic enzymes such as MMPs and aggrecanases, which degrade collagen and proteoglycans. Inflammatory cytokines such as IL-1β and TNF-α further exacerbate this degradation by upregulating catabolic pathways and downregulating anabolic pathways.
Recent advances in molecular biology and regenerative medicine offer promising strategies for meniscal repair and regeneration. Gene therapy approaches aim to enhance the expression of anabolic factors or inhibit catabolic factors in fibrochondrocytes. For example, gene delivery of growth factors such as TGF-β or IGF-1 can promote ECM synthesis and repair. Conversely, silencing genes that encode for catabolic enzymes using techniques like RNA interference (RNAi) can reduce ECM degradation.
Stem cell therapy is another promising approach for meniscal regeneration. Mesenchymal stem cells (MSCs) can differentiate into fibrochondrocytes and produce ECM components, promoting tissue repair. Bioengineering techniques are also being explored to create scaffolds that mimic the native meniscal ECM, providing a supportive environment for cell attachment, proliferation, and differentiation. These scaffolds can be combined with growth factors and stem cells to enhance meniscal regeneration.
Overall, the intricate network of signaling pathways, molecular mechanisms, and cellular interactions highlights the complexity of meniscal biology and the importance of maintaining ECM homeostasis. Advances in understanding these processes at the molecular level provide valuable insights into potential therapeutic strategies for meniscal injuries and degenerative conditions. By leveraging knowledge of signaling pathways and molecular mechanisms, these approaches aim to restore the structure and function of the meniscus, contributing to overall knee joint stability and longevity.
4. Fibroblasts and Ligaments/Tendons: Fibroblasts are the primary cells found in ligaments and tendons, tissues that connect bones and muscles, respectively, and play critical roles in stabilizing the knee joint and facilitating movement. These cells respond to mechanical loading by modulating collagen synthesis and matrix remodeling. Mechanotransduction in fibroblasts involves integrins, focal adhesion complexes, and mechanosensitive ion channels, which activate various intracellular signaling pathways. These pathways, including the MAPK and TGF-β (Transforming Growth Factor-beta) pathways, regulate the production and organization of collagen fibers, which are essential for the strength and elasticity of ligaments and tendons.
Fibroblasts produce type I collagen, which forms the bulk of the ECM in ligaments and tendons. This collagen type is arranged in parallel bundles, providing tensile strength and allowing these tissues to withstand significant mechanical loads. The synthesis of type I collagen is tightly regulated by mechanical stimuli. Integrins, transmembrane receptors that mediate cell-ECM interactions, play a crucial role in sensing mechanical stress and initiating intracellular signaling cascades. When fibroblasts experience mechanical loading, integrins cluster together, forming focal adhesions. These focal adhesions serve as sites where mechanical signals are transduced into biochemical signals, involving the recruitment and activation of various signaling proteins such as focal adhesion kinase (FAK) and Src family kinases.
One of the key signaling pathways activated by mechanical loading in fibroblasts is the MAPK pathway. This pathway includes several sub-pathways such as ERK (extracellular signal-regulated kinase), JNK (c-Jun N-terminal kinase), and p38 MAPK, each of which plays distinct roles in cellular responses. ERK activation promotes fibroblast proliferation and collagen synthesis, while JNK and p38 MAPKs are involved in the cellular response to stress and inflammation. Activation of these MAPK pathways results in the phosphorylation of transcription factors that regulate the expression of genes involved in ECM production and remodeling.
The TGF-β signaling pathway is another critical regulator of fibroblast function in ligaments and tendons. TGF-β is a cytokine that plays a central role in tissue repair and fibrosis. It signals through a receptor complex that phosphorylates SMAD proteins, which then translocate to the nucleus to regulate gene expression. In fibroblasts, TGF-β signaling enhances the synthesis of collagen and other ECM proteins, promoting tissue strength and resilience. Additionally, TGF-β modulates the expression of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs), balancing ECM synthesis and degradation to maintain tissue homeostasis.
Mechanosensitive ion channels, such as Piezo1 and Piezo2, also contribute to mechanotransduction in fibroblasts. These channels respond to mechanical deformation by allowing the influx of ions, particularly calcium (Ca²⁺), which acts as a secondary messenger in various signaling pathways. Ca²⁺ signaling can activate several kinases, including Ca²⁺/calmodulin-dependent protein kinase (CaMK) and protein kinase C (PKC), which further modulate the activity of transcription factors involved in collagen synthesis and fibroblast proliferation.
Epigenetic mechanisms, including DNA methylation, histone modifications, and non-coding RNAs (e.g., microRNAs), also play a role in regulating fibroblast function in response to mechanical stimuli. These epigenetic changes can influence the expression of genes involved in ECM production, degradation, and cell signaling, highlighting their importance in maintaining ligament and tendon health.
Fibroblasts in ligaments and tendons also interact with other cell types, such as tenocytes (specialized tendon cells) and ligamentocytes (specialized ligament cells), as well as immune cells. These interactions are mediated by cytokines, growth factors, and extracellular vesicles (EVs), which facilitate intercellular communication and coordinate tissue repair processes. For instance, EVs released by fibroblasts can carry proteins, lipids, and nucleic acids that influence the behavior of neighboring cells, contributing to the overall maintenance and repair of ligaments and tendons.
Proper mechanical loading helps maintain the structural integrity and function of these tissues, supporting joint stability and efficient force transmission during movement. However, excessive or abnormal loading can lead to microtears, inflammation, and conditions such as tendinopathy, highlighting the importance of balanced mechanical stimuli for ligament and tendon health. In conditions like tendinopathy, there is an imbalance between collagen synthesis and degradation, leading to a weakened ECM and increased susceptibility to injury.
Further molecular mechanisms involve the response to hypoxia, a condition often present in the dense and relatively avascular regions of ligaments and tendons. Hypoxia-inducible factors (HIFs), particularly HIF-1α, play a crucial role in adapting fibroblasts to low oxygen conditions. HIF-1α can upregulate the expression of genes involved in angiogenesis, ECM production, and metabolic adaptation, ensuring cell survival and function under hypoxic conditions.
Recent studies have also identified the role of extracellular matrix (ECM) stiffness in regulating fibroblast behavior. The mechanical properties of the ECM can influence cell fate decisions, including differentiation, proliferation, and apoptosis. Integrins and focal adhesions sense ECM stiffness and transmit signals to the nucleus through cytoskeletal elements and signaling pathways such as RhoA/ROCK and YAP/TAZ. These pathways modulate the expression of genes that control cell mechanics and matrix remodeling, ensuring that fibroblasts can adapt to changes in their mechanical environment.
Moreover, fibroblasts produce and respond to a variety of growth factors and cytokines, including fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and interleukins. These factors can autocrinely and paracrinely influence fibroblast activity, promoting cell proliferation, migration, and matrix synthesis. The interplay between these signaling molecules and mechanical cues is essential for coordinating the complex processes of tissue repair and regeneration.
Understanding the molecular biology of fibroblasts in ligaments and tendons provides valuable insights into the mechanisms underlying tissue maintenance and repair. Advances in molecular biology and regenerative medicine offer promising strategies for enhancing the repair and regeneration of these tissues. Gene therapy approaches aim to modulate the expression of key signaling molecules involved in mechanotransduction. For example, overexpression of growth factors like TGF-β or IGF-1 (insulin-like growth factor-1) can enhance collagen synthesis and tissue repair, while RNA interference (RNAi) techniques can be used to silence genes that encode for catabolic enzymes, reducing ECM degradation.
Stem cell therapy is another promising approach, with mesenchymal stem cells (MSCs) showing potential for differentiating into fibroblast-like cells and contributing to ECM production. Tissue engineering techniques, such as the development of scaffolds that mimic the native ECM of ligaments and tendons, provide a supportive environment for cell attachment, proliferation, and differentiation. These scaffolds can be combined with growth factors and stem cells to enhance tissue regeneration.
Overall, the intricate network of signaling pathways, molecular mechanisms, and cellular interactions highlights the complexity of ligament and tendon biology and the importance of maintaining ECM homeostasis. Advances in understanding these processes at the molecular level provide valuable insights into potential therapeutic strategies for ligament and tendon injuries and degenerative conditions. By leveraging knowledge of mechanotransduction and molecular biology, these approaches aim to restore the structure and function of ligaments and tendons, contributing to overall joint stability and mobility.
Mechanical Loading Modalities and Their Effects in Molecular Biology Context
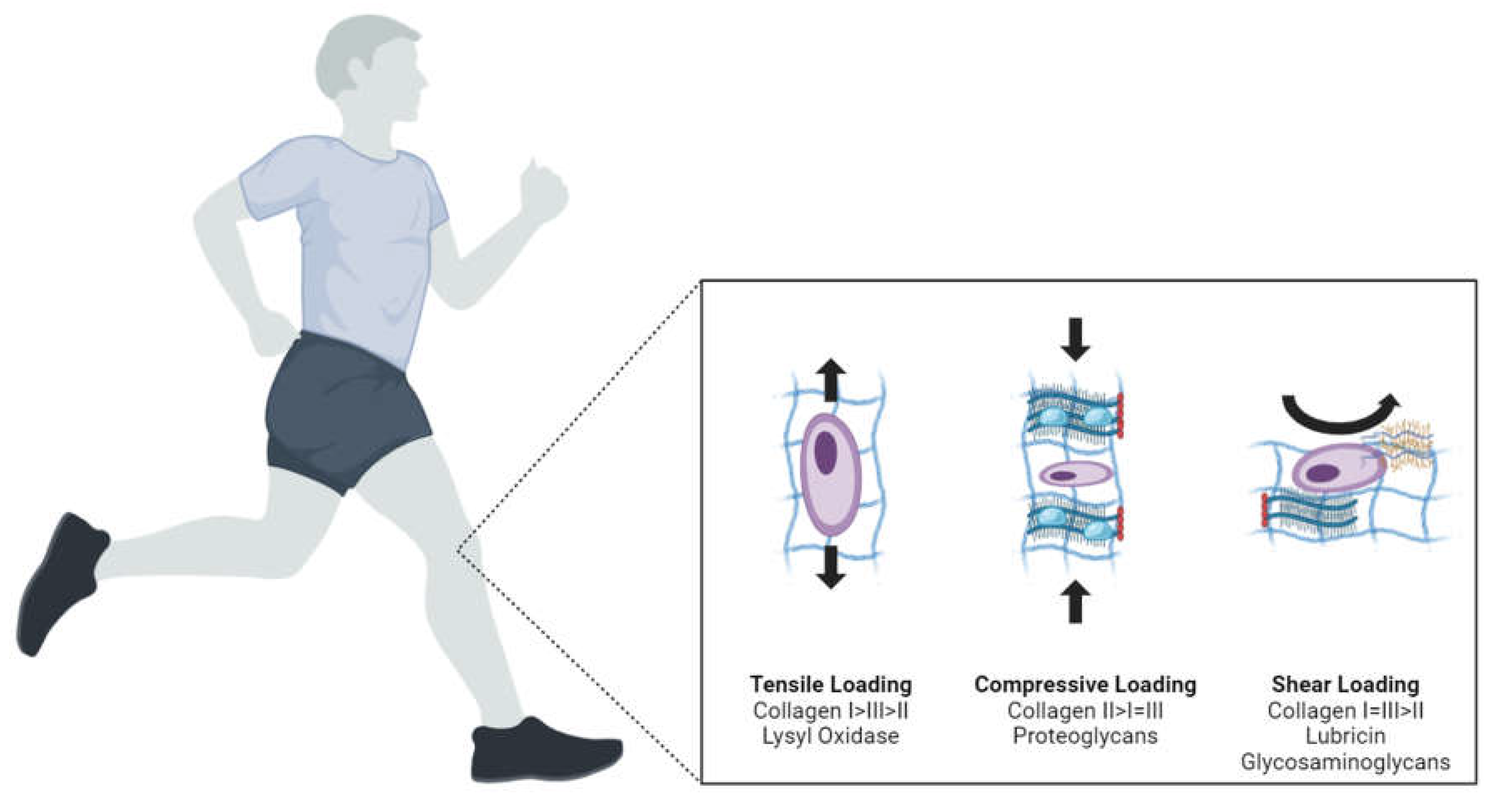
Different types of mechanical loading—compression, tension, shear, and hydrostatic pressure—have distinct effects on knee joint tissues at both macroscopic and molecular levels. Understanding these effects is crucial for designing effective rehabilitation protocols (
Figure 2).
On a molecular level, mechanical loading influences gene expression, protein synthesis, and cellular signaling pathways within knee joint tissues. For instance, compression loading typically stimulates chondrocytes in cartilage to produce extracellular matrix components such as collagen and proteoglycans, which are essential for maintaining tissue integrity and function. This type of loading can activate mechanotransduction pathways involving integrins and the cytoskeleton, leading to alterations in gene expression mediated by transcription factors such as NF-kB and AP-1. Additionally, compression can increase the expression of anabolic factors like insulin-like growth factor 1 (IGF-1) and transforming growth factor-beta (TGF-β), which promote cartilage repair and maintenance.
Tension loading, often experienced by tendons and ligaments, promotes the synthesis of collagen fibers, thereby enhancing the tensile strength of these tissues. This mechanical stimulus can activate the mechanosensitive ion channels and the MAPK signaling pathway, resulting in increased production of structural proteins and enzymes that remodel the extracellular matrix. Furthermore, tension loading can influence the expression of genes related to matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), which play crucial roles in the remodeling and turnover of the extracellular matrix.
Shear stress, which occurs during sliding movements of the knee joint, affects the endothelial cells lining the blood vessels, influencing nitric oxide production and inflammatory responses. This type of stress can modulate the expression of shear-responsive genes such as eNOS and COX-2, which play roles in vascular tone and inflammation. Shear stress can also impact the production of vascular endothelial growth factor (VEGF), which is critical for angiogenesis and the maintenance of blood supply to the joint tissues.
Hydrostatic pressure, experienced during joint loading and unloading, influences the behavior of synoviocytes, the cells that produce synovial fluid. This pressure can regulate the expression of genes involved in fluid secretion and composition, affecting the lubrication and nutrient supply within the joint. Hydrostatic pressure can also modulate the activity of ion channels and transporters, such as aquaporins, which are essential for maintaining the osmotic balance and fluid homeostasis in the joint cavity.
Moreover, mechanical loading can influence the inflammatory responses within the knee joint. For example, different loading modalities can alter the expression of cytokines and chemokines, which are critical mediators of inflammation. Compression loading can reduce the expression of pro-inflammatory cytokines such as interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α), while promoting the release of anti-inflammatory cytokines like interleukin-10 (IL-10). These changes can help mitigate inflammation and promote a favorable environment for tissue repair and regeneration.
Mechanical loading also impacts the production of matrix-degrading enzymes and their inhibitors. For example, compression and tension can regulate the activity of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs), which are involved in the degradation and remodeling of the extracellular matrix. The balance between MMPs and TIMPs is crucial for maintaining the structural integrity of the joint tissues and preventing excessive matrix degradation.
By comprehensively understanding these molecular mechanisms, researchers and clinicians can develop targeted rehabilitation protocols that optimize tissue repair and regeneration, ultimately improving knee joint function and reducing the risk of injury. These protocols can be tailored to modulate specific molecular pathways and cellular responses, enhancing the efficacy of rehabilitation and promoting long-term joint health (
Table 2).
1. Compression
Compression loading is essential for cartilage health, stimulating proteoglycan synthesis and inhibiting catabolic enzymes. Chondrocytes within the cartilage sense compressive forces through mechanoreceptors like integrins and mechanosensitive ion channels. These receptors activate intracellular signaling pathways such as MAPK and NF-κB, leading to the upregulation of anabolic processes. Proteoglycans, primarily aggrecan, are synthesized and integrate with the collagen network to provide compressive resistance and maintain cartilage elasticity. However, excessive compression can lead to cartilage degeneration and osteoarthritis. Overloading can cause increased expression of matrix metalloproteinases (MMPs) and aggrecanases, enzymes that degrade the extracellular matrix, leading to a breakdown of cartilage structure and function.
On a molecular level, the mechanotransduction process begins when compressive forces are applied to the cartilage. Integrins, which are transmembrane receptors, connect the extracellular matrix to the intracellular cytoskeleton. When activated by compression, integrins facilitate the clustering and recruitment of focal adhesion complexes that link to actin filaments, leading to the activation of focal adhesion kinase (FAK). This activation triggers downstream signaling cascades such as the MAPK pathway, which includes ERK1/2, p38, and JNK, all of which play roles in regulating gene expression related to anabolic and catabolic processes.
Additionally, mechanosensitive ion channels such as PIEZO1 and TRPV4 are critical for chondrocyte response to compression. These channels respond to mechanical stimuli by altering their conformation and allowing the influx of ions like calcium (Ca2+), which acts as a second messenger in various signaling pathways. The increase in intracellular Ca2+ can activate Ca2+-dependent kinases and phosphatases, further influencing gene transcription and protein synthesis.
The NF-κB pathway, another crucial signaling route, is activated under compressive loading and regulates the expression of genes involved in inflammatory and stress responses. Activation of NF-κB leads to the transcription of genes that encode for anti-inflammatory cytokines and proteins that inhibit the activity of catabolic enzymes. This helps to maintain a balance between anabolic and catabolic activities within the cartilage, ensuring tissue homeostasis and integrity.
Proteoglycan synthesis, particularly of aggrecan, is a key anabolic process promoted by compression. Aggrecan is a large proteoglycan that, together with hyaluronic acid and link proteins, forms a complex that traps water molecules, providing the cartilage with its load-bearing properties and resilience. This complex integrates into the collagen network, primarily composed of type II collagen, enhancing the tissue’s ability to withstand compressive forces and maintain its elasticity.
The synthesis of these extracellular matrix components is tightly regulated by various signaling molecules and growth factors. For instance, transforming growth factor-beta (TGF-β) and insulin-like growth factor 1 (IGF-1) are upregulated in response to compressive loading. These growth factors bind to their respective receptors on chondrocytes, activating SMAD and PI3K/Akt pathways, which further enhance the transcription of genes involved in matrix production and cell survival.
However, when compression loading exceeds physiological levels, it can have detrimental effects. Excessive mechanical stress can disrupt the homeostatic balance, leading to increased production of MMPs and aggrecanases. These enzymes degrade collagen and proteoglycan components of the extracellular matrix, resulting in the loss of cartilage structure and function. Prolonged overloading and the subsequent breakdown of the extracellular matrix can initiate and exacerbate degenerative conditions such as osteoarthritis. In osteoarthritis, the persistent degradation of cartilage leads to the exposure of subchondral bone, inflammation, pain, and reduced joint mobility.
Excessive compression also induces the production of reactive oxygen species (ROS) within chondrocytes, which can cause oxidative stress and damage cellular components, including lipids, proteins, and DNA. This oxidative stress can further activate catabolic pathways and inflammatory responses, exacerbating cartilage degradation. Additionally, pro-inflammatory cytokines such as interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) are often upregulated under excessive compressive stress, contributing to the inflammatory milieu that drives cartilage breakdown and osteoarthritis progression.
Furthermore, the altered mechanical environment can influence the behavior of other joint tissues, including the subchondral bone, synovium, and menisci. For example, excessive compression can lead to subchondral bone sclerosis and the formation of osteophytes, further compromising joint function and exacerbating pain.
In summary, while moderate compression is beneficial for cartilage health by promoting anabolic activities and maintaining tissue integrity, excessive compression triggers catabolic pathways that can lead to cartilage degeneration and osteoarthritis. Understanding the molecular biology underlying these responses is crucial for developing therapeutic strategies to prevent and treat cartilage-related disorders. This knowledge can inform the design of biomechanical interventions, pharmacological treatments targeting specific molecular pathways, and regenerative medicine approaches to restore cartilage function.
2. Tension
Tensile loading primarily affects ligaments and tendons, promoting collagen synthesis and alignment. Fibroblasts and tenocytes, the primary cells in these tissues, respond to tensile forces by activating integrin-mediated signaling pathways. These pathways include the focal adhesion kinase (FAK) and MAPK cascades, which lead to the production of type I collagen and other extracellular matrix components. Proper tensile loading facilitates the alignment of collagen fibers along the direction of force, enhancing the tensile strength of the tissue. Controlled tensile loading can therefore enhance tissue repair and strength. However, excessive tension may cause microtears, inflammation, and tendinopathy. Inflammatory cytokines such as IL-1β and TNF-α can be upregulated in response to overloading, leading to increased MMP activity and subsequent matrix degradation.
On a molecular level, the response of fibroblasts and tenocytes to tensile loading involves several key signaling pathways and molecular events. Integrins, which are transmembrane receptors that link the extracellular matrix to the cytoskeleton, play a critical role in sensing tensile forces. Upon activation by mechanical stretch, integrins cluster and recruit focal adhesion proteins, leading to the activation of FAK. This kinase phosphorylates various downstream targets, initiating signaling cascades such as the MAPK pathway, which includes ERK1/2, p38, and JNK. These pathways are crucial for regulating gene expression related to extracellular matrix production and cell survival.
One of the primary responses to tensile loading is the upregulation of type I collagen synthesis. Type I collagen is the main structural protein in tendons and ligaments, providing tensile strength and rigidity. The synthesis of collagen is regulated at the transcriptional level by factors such as TGF-β and connective tissue growth factor (CTGF), which are upregulated in response to mechanical stretch. These growth factors bind to their respective receptors, activating SMAD and PI3K/Akt signaling pathways, which enhance the transcription of collagen genes.
Tensile loading also influences the organization and alignment of collagen fibers. The mechanical stretch directs the alignment of newly synthesized collagen fibrils along the axis of the tensile force, which is essential for the functional properties of tendons and ligaments. This process involves the activity of enzymes such as lysyl oxidase, which cross-links collagen molecules, thereby stabilizing the extracellular matrix and increasing tissue tensile strength.
However, excessive tensile loading can lead to tissue damage and pathology. Overstretching can cause microtears in the collagen fibers, triggering an inflammatory response. The damaged tissue releases danger-associated molecular patterns (DAMPs), which activate inflammatory pathways and recruit immune cells to the site of injury. Inflammatory cytokines such as IL-1β and TNF-α are upregulated, further amplifying the inflammatory response.
These cytokines stimulate the production of matrix metalloproteinases (MMPs), which degrade extracellular matrix components. MMP-1 and MMP-13, in particular, are upregulated in response to excessive tensile loading and are responsible for the breakdown of collagen. This degradation weakens the structural integrity of tendons and ligaments, leading to tendinopathy and other degenerative conditions.
Furthermore, excessive tensile loading can disrupt cellular homeostasis, leading to increased production of reactive oxygen species (ROS) and oxidative stress. ROS can damage cellular components, including DNA, proteins, and lipids, exacerbating the inflammatory response and further promoting matrix degradation. Oxidative stress also activates signaling pathways such as NF-κB, which enhances the expression of pro-inflammatory cytokines and MMPs, creating a vicious cycle of inflammation and tissue breakdown.
Another critical aspect of tensile loading is its effect on mechanosensitive transcription factors, such as yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ). These transcription factors are part of the Hippo signaling pathway and are sensitive to mechanical cues. Upon mechanical stretch, YAP/TAZ translocate to the nucleus where they regulate the expression of genes involved in cell proliferation, differentiation, and extracellular matrix production. The activation of YAP/TAZ signaling is essential for the adaptive response of tendons and ligaments to mechanical loading, promoting tissue growth and repair.
In the context of tendon and ligament repair, controlled tensile loading is used therapeutically to promote healing and restore function. Mechanical loading protocols are designed to apply appropriate levels of tension that stimulate collagen synthesis and alignment without causing excessive damage. These protocols are often combined with pharmacological interventions targeting specific molecular pathways to enhance tissue regeneration and reduce inflammation.
Emerging therapies such as the use of mesenchymal stem cells (MSCs) and gene therapy are also being explored to enhance tendon and ligament repair. MSCs can differentiate into tenocytes and produce extracellular matrix components in response to mechanical loading. Gene therapy approaches aim to modulate the expression of key regulatory genes involved in the mechanotransduction pathways, enhancing the regenerative capacity of tendons and ligaments.
In summary, tensile loading plays a crucial role in the maintenance and repair of tendons and ligaments by promoting collagen synthesis and alignment. However, excessive tension can lead to tissue damage, inflammation, and degenerative conditions. Understanding the molecular biology underlying these processes is essential for developing effective therapeutic strategies to enhance tissue repair and prevent overuse injuries. This knowledge can inform the design of biomechanical interventions, pharmacological treatments targeting specific molecular pathways, and regenerative medicine approaches to restore tendon and ligament function.
3. Shear
Shear stress influences synovial fluid dynamics and cartilage health. Chondrocytes subjected to moderate shear stress can experience an anabolic response, characterized by increased synthesis of proteoglycans and type II collagen. This response is mediated by mechanotransduction pathways involving integrins and the cytoskeleton, which activate intracellular signaling cascades such as the Wnt and MAPK pathways. However, excessive shear stress can disrupt the integrity of the cartilage surface, leading to cell apoptosis and increased expression of catabolic enzymes. The overproduction of pro-inflammatory mediators like prostaglandin E2 (PGE2) and nitric oxide (NO) can exacerbate inflammatory conditions and contribute to cartilage degradation.
On a molecular level, shear stress exerts its effects on chondrocytes through the activation of integrins, which are transmembrane receptors that connect the extracellular matrix to the actin cytoskeleton. When shear forces are applied, integrins undergo conformational changes and cluster together, forming focal adhesions. These focal adhesions act as mechanotransducers that link mechanical stimuli to biochemical signals within the cell. The activation of integrins leads to the recruitment and phosphorylation of focal adhesion kinase (FAK), which subsequently triggers downstream signaling pathways such as the MAPK cascade, including ERK1/2, p38, and JNK.
In addition to integrin-mediated signaling, shear stress also influences the Wnt signaling pathway, which plays a crucial role in regulating cartilage homeostasis. The activation of Wnt signaling by shear stress promotes the stabilization and nuclear translocation of β-catenin, a key transcriptional coactivator. In the nucleus, β-catenin interacts with TCF/LEF transcription factors to induce the expression of genes involved in chondrocyte proliferation and matrix production, including those encoding for proteoglycans and type II collagen.
Shear stress also affects the production of nitric oxide (NO) and prostaglandin E2 (PGE2), which are important mediators of chondrocyte function and inflammation. Moderate shear stress can upregulate the expression of endothelial nitric oxide synthase (eNOS), leading to increased NO production. NO acts as a signaling molecule that can enhance the synthesis of extracellular matrix components and protect against oxidative stress. However, excessive shear stress can induce the expression of inducible nitric oxide synthase (iNOS), resulting in high levels of NO that contribute to inflammation and cartilage degradation.
Prostaglandin E2 (PGE2) is another mediator that is regulated by shear stress. Under moderate shear stress, PGE2 can play a protective role by promoting the synthesis of matrix components and inhibiting the activity of catabolic enzymes. However, excessive shear stress can lead to overproduction of PGE2, which can have pro-inflammatory effects. PGE2 is synthesized from arachidonic acid through the cyclooxygenase (COX) pathway, and its overproduction can enhance the expression of matrix metalloproteinases (MMPs) and aggrecanases, enzymes that degrade the extracellular matrix.
Excessive shear stress can disrupt the homeostasis of chondrocytes and lead to cell apoptosis. Mechanistically, this process involves the activation of stress-activated protein kinases (SAPKs) such as JNK and p38, which are part of the MAPK signaling pathway. These kinases can induce the expression of pro-apoptotic genes and inhibit the expression of anti-apoptotic genes, leading to programmed cell death. Additionally, excessive shear stress can cause the release of reactive oxygen species (ROS), which can further damage cellular components and exacerbate apoptosis.
The inflammatory response triggered by excessive shear stress involves the upregulation of pro-inflammatory cytokines such as interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α). These cytokines can activate nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling, leading to the transcription of genes involved in inflammation and catabolism. NF-κB activation enhances the production of MMPs and aggrecanases, contributing to the breakdown of the extracellular matrix and cartilage degradation.
Shear stress also impacts the behavior of synovial cells and the composition of synovial fluid. Moderate shear stress can stimulate synoviocytes to produce hyaluronic acid, a key component of synovial fluid that provides lubrication and shock absorption in the joint. This process is regulated by the activation of hyaluronan synthases and is crucial for maintaining joint homeostasis. However, excessive shear stress can alter synovial fluid composition, reducing its lubricating properties and increasing friction within the joint, which can exacerbate cartilage wear and inflammation.
Additionally, shear stress can influence the metabolic activity of chondrocytes and synoviocytes. Under moderate shear stress, these cells can increase the uptake of glucose and amino acids, enhancing their anabolic activity and supporting matrix synthesis. This metabolic shift is mediated by signaling pathways such as AMPK and mTOR, which regulate cellular energy homeostasis and protein synthesis. Conversely, excessive shear stress can lead to metabolic dysfunction, reducing the availability of essential nutrients and impairing cell function.
The extracellular matrix (ECM) remodeling in response to shear stress involves various proteolytic enzymes and their inhibitors. The balance between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) is critical for maintaining ECM integrity. Shear stress can regulate the expression and activity of these enzymes, influencing the turnover and repair of the cartilage matrix. In conditions of excessive shear stress, the upregulation of MMPs and downregulation of TIMPs can lead to uncontrolled matrix degradation, contributing to cartilage erosion and joint dysfunction.
Furthermore, shear stress affects the communication between chondrocytes and other joint cells through the release of extracellular vesicles (EVs). These vesicles contain bioactive molecules such as proteins, lipids, and nucleic acids that can modulate cellular responses and influence tissue homeostasis. Shear stress-induced changes in EV composition and release can alter intercellular signaling, impacting processes such as inflammation, matrix synthesis, and cell survival.
In summary, shear stress plays a complex role in cartilage health by regulating chondrocyte function, matrix production, and synovial fluid dynamics through various mechanotransduction pathways. Moderate shear stress induces anabolic responses that promote cartilage maintenance, while excessive shear stress triggers catabolic and inflammatory responses that lead to cartilage degradation. Understanding the molecular biology underlying these processes is essential for developing therapeutic strategies to prevent and treat cartilage-related disorders, such as osteoarthritis. These strategies may include targeted modulation of signaling pathways, anti-inflammatory treatments, and the use of mechanical loading protocols to optimize cartilage health.
4. Hydrostatic Pressure
Hydrostatic pressure, such as that experienced during aquatic therapy, can promote chondrocyte metabolism and matrix synthesis. This type of loading is particularly beneficial in reducing joint load while providing therapeutic benefits. Chondrocytes under hydrostatic pressure exhibit enhanced anabolic activity, including increased production of proteoglycans and type II collagen. The pressure stimulates mechanoreceptors and ion channels that activate signaling pathways like the PI3K/Akt and ERK pathways, which support cell survival and matrix production. Hydrostatic pressure also helps maintain a favorable environment for chondrocytes by reducing inflammation and oxidative stress, making it a valuable modality in therapeutic settings for individuals with joint issues such as osteoarthritis.
On a molecular level, the response of chondrocytes to hydrostatic pressure involves the activation of various mechanoreceptors and ion channels. One key mechanoreceptor involved in this process is the integrin complex, which connects the extracellular matrix to the cytoskeleton. Hydrostatic pressure can induce conformational changes in integrins, leading to the activation of focal adhesion kinase (FAK). This activation triggers downstream signaling cascades, including the PI3K/Akt and ERK pathways.
The PI3K/Akt pathway plays a crucial role in promoting cell survival and growth. Upon activation by hydrostatic pressure, PI3K (phosphoinositide 3-kinase) generates phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which recruits and activates Akt (protein kinase B). Activated Akt phosphorylates various substrates involved in promoting protein synthesis, cell survival, and growth. For example, Akt can inhibit pro-apoptotic factors such as BAD and caspase-9, while activating mTOR (mechanistic target of rapamycin), which enhances protein synthesis and cell growth.
Similarly, the ERK (extracellular signal-regulated kinase) pathway is activated in response to hydrostatic pressure. ERK is a part of the MAPK (mitogen-activated protein kinase) signaling cascade and is crucial for regulating cell proliferation, differentiation, and matrix production. Hydrostatic pressure stimulates the phosphorylation of ERK1/2, which then translocates to the nucleus to activate transcription factors such as ELK1 and AP-1. These transcription factors induce the expression of genes involved in extracellular matrix synthesis, including those encoding for proteoglycans and type II collagen.
Hydrostatic pressure also influences the activity of mechanosensitive ion channels such as TRPV4 and PIEZO1. These channels respond to mechanical stimuli by allowing the influx of calcium ions (Ca2+) into the chondrocytes. The increase in intracellular Ca2+ acts as a second messenger, activating various Ca2+-dependent signaling pathways. For instance, Ca2+/calmodulin-dependent protein kinase II (CaMKII) can be activated, leading to the phosphorylation of CREB (cAMP response element-binding protein) and the subsequent transcription of genes involved in matrix production and cell survival.
In addition to promoting anabolic activities, hydrostatic pressure helps maintain a favorable environment for chondrocytes by reducing inflammation and oxidative stress. This is partly achieved by modulating the production of reactive oxygen species (ROS) and enhancing the antioxidant defense mechanisms within chondrocytes. Hydrostatic pressure can upregulate the expression of antioxidant enzymes such as superoxide dismutase (SOD) and catalase, which neutralize ROS and protect cellular components from oxidative damage. This reduction in oxidative stress is crucial for maintaining chondrocyte viability and function, particularly in inflammatory conditions such as osteoarthritis.
Hydrostatic pressure also modulates the inflammatory response in chondrocytes. It can downregulate the expression of pro-inflammatory cytokines such as interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α), which are typically upregulated in osteoarthritis and contribute to cartilage degradation. By reducing the levels of these cytokines, hydrostatic pressure helps to create an anti-inflammatory environment that supports cartilage repair and regeneration. Additionally, hydrostatic pressure can inhibit the activation of NF-κB, a key transcription factor involved in inflammatory responses, further reducing inflammation and catabolic activity in chondrocytes.
Moreover, hydrostatic pressure has been shown to influence the synthesis of extracellular matrix components beyond proteoglycans and type II collagen. For instance, it can enhance the production of hyaluronic acid, a critical component of the synovial fluid that provides lubrication and shock absorption in the joint. This is mediated by the upregulation of hyaluronan synthase enzymes, which are responsible for the synthesis of hyaluronic acid.
The application of hydrostatic pressure in therapeutic settings, such as aquatic therapy, leverages these molecular responses to promote joint health and function. Aquatic therapy provides a controlled environment where hydrostatic pressure can be precisely applied to joints, reducing the mechanical load while enhancing the beneficial effects of pressure on chondrocyte metabolism and matrix synthesis. This makes hydrostatic pressure an effective modality for managing joint disorders like osteoarthritis, where reducing joint stress and promoting tissue repair are critical for improving patient outcomes.
Hydrostatic pressure also impacts the behavior of synoviocytes, the cells lining the synovial membrane that produce synovial fluid. Under the influence of hydrostatic pressure, synoviocytes can increase the production of lubricin and other glycoproteins that enhance the lubricating properties of synovial fluid. This helps to reduce friction within the joint, protecting cartilage surfaces from wear and tear.
Furthermore, hydrostatic pressure influences the expression of growth factors and cytokines that regulate cartilage homeostasis. For instance, it can enhance the expression of transforming growth factor-beta (TGF-β) and insulin-like growth factor 1 (IGF-1), which are critical for cartilage repair and regeneration. TGF-β, in particular, plays a key role in promoting the synthesis of extracellular matrix components and inhibiting the activity of matrix metalloproteinases (MMPs) that degrade the matrix. IGF-1 supports chondrocyte proliferation and survival, further contributing to cartilage maintenance.
Hydrostatic pressure also affects the metabolic activity of chondrocytes. Under hydrostatic pressure, chondrocytes can enhance the uptake of glucose and amino acids, which are essential for energy production and protein synthesis. This metabolic shift supports the increased anabolic activity required for matrix synthesis and cell proliferation. Additionally, hydrostatic pressure can influence the mitochondrial function of chondrocytes, enhancing ATP production and reducing the generation of reactive oxygen species (ROS).
The anti-inflammatory effects of hydrostatic pressure are also mediated by the modulation of signaling pathways such as the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway. Nrf2 is a transcription factor that regulates the expression of antioxidant and cytoprotective genes. Under hydrostatic pressure, Nrf2 can be activated, leading to the upregulation of genes that encode for antioxidant enzymes and proteins that protect cells from oxidative stress and inflammation.
In summary, hydrostatic pressure plays a significant role in promoting chondrocyte metabolism and matrix synthesis through the activation of mechanotransduction pathways involving integrins, ion channels, and signaling cascades such as PI3K/Akt and ERK. By enhancing anabolic activity, reducing inflammation, and mitigating oxidative stress, hydrostatic pressure supports cartilage health and provides therapeutic benefits in conditions like osteoarthritis. Understanding these molecular mechanisms is essential for optimizing the use of hydrostatic pressure in clinical and therapeutic settings to enhance joint function and repair.
Rehabilitation and Mechanotransduction
Effective rehabilitation strategies leverage the principles of mechanotransduction to optimize knee joint recovery. Mechanotransduction refers to the process by which cells convert mechanical stimuli into biochemical signals, leading to cellular responses that contribute to tissue repair and adaptation. This intricate process is essential for maintaining and restoring the function of musculoskeletal tissues, particularly following injury or surgery. Understanding and utilizing mechanotransduction is critical for developing effective rehabilitation protocols that promote optimal healing and functional recovery.
Mechanotransduction involves a complex interplay between mechanical forces and cellular responses. When mechanical forces are applied to tissues, they cause deformation at the cellular level, which is detected by mechanoreceptors on the cell surface. These receptors then initiate a cascade of intracellular signaling pathways that influence gene expression, protein synthesis, and cellular behavior. This results in various biological responses, such as the proliferation of cells, production of extracellular matrix components, and remodeling of tissues. For instance, in the knee joint, mechanical loading can stimulate the production of collagen and other structural proteins by chondrocytes (cartilage cells), enhancing the repair and strength of damaged cartilage.
At the molecular level, mechanotransduction is mediated by several key molecules and signaling pathways. Integrins, which are transmembrane receptors, play a pivotal role in sensing mechanical forces and transmitting signals into the cell. When integrins bind to extracellular matrix (ECM) components, they form focal adhesions, which are complexes that link the ECM to the cytoskeleton. This connection allows mechanical forces to be transmitted across the cell membrane, leading to the activation of intracellular signaling pathways, such as the mitogen-activated protein kinase (MAPK) pathway and the focal adhesion kinase (FAK) pathway. These pathways regulate various cellular processes, including gene expression, cell proliferation, and apoptosis, which are critical for tissue repair and adaptation.
Another important aspect of mechanotransduction is the role of ion channels, such as stretch-activated ion channels and piezo channels, which respond to mechanical stimuli by altering ion flux across the cell membrane. These channels can modulate intracellular calcium levels, which serve as a secondary messenger in various signaling pathways. Increased intracellular calcium can activate calcium-dependent proteins, such as calmodulin and calcineurin, which further influence cellular responses to mechanical loading.
In the context of knee rehabilitation, mechanotransduction can be harnessed through carefully designed exercise programs and therapeutic interventions. Controlled loading, for example, involves applying specific mechanical forces in a gradual and progressive manner to stimulate cellular responses without causing additional harm. This approach is fundamental in guiding tissue repair processes, promoting the formation of healthy tissue, and preventing the development of excessive scar tissue or fibrosis.
Additionally, mechanotransduction is influenced by the type, magnitude, frequency, and duration of mechanical stimuli. Different tissues and cells respond uniquely to various mechanical environments. Therefore, rehabilitation protocols must be tailored to the specific needs of the patient and the nature of the injury. For example, weight-bearing exercises can be beneficial for bone and cartilage health, while non-weight-bearing exercises might be more suitable during the early stages of recovery when minimizing joint stress is crucial.
Moreover, the timing of mechanical loading is crucial. Early mechanical loading, introduced soon after injury or surgery, can enhance tissue repair and functional recovery. This approach, however, must be carefully managed to avoid overloading and potential re-injury. Gradual progression in mechanical loading ensures that tissues adapt appropriately, enhancing their resilience and function over time.
At a molecular level, early mechanical loading can activate growth factors such as transforming growth factor-beta (TGF-β) and insulin-like growth factor 1 (IGF-1), which are vital for tissue regeneration and repair. TGF-β, for instance, plays a crucial role in ECM production and remodeling, while IGF-1 promotes cellular proliferation and differentiation. The activation of these growth factors through mechanotransduction pathways underscores the importance of mechanical stimuli in driving effective rehabilitation outcomes.
By leveraging the principles of mechanotransduction, rehabilitation strategies can be optimized to promote effective knee joint recovery. This involves a comprehensive understanding of the biological responses to mechanical stimuli and the careful application of controlled mechanical forces to stimulate beneficial cellular responses. Such strategies not only facilitate tissue repair and adaptation but also contribute to the overall functional recovery of the knee joint, improving patient outcomes and quality of life.
Understanding the molecular biology underpinning mechanotransduction allows for more targeted and effective rehabilitation interventions. For instance, identifying specific signaling molecules and pathways involved in mechanotransduction can lead to the development of novel therapeutic agents or techniques that enhance the body’s natural repair mechanisms. Furthermore, advancements in molecular biology, such as gene editing and stem cell therapy, hold promise for improving rehabilitation outcomes by directly influencing the cellular processes involved in tissue repair and regeneration.
In conclusion, the integration of molecular biology into rehabilitation strategies provides a deeper understanding of mechanotransduction and its role in tissue repair and adaptation. This knowledge is crucial for designing effective rehabilitation protocols that optimize knee joint recovery, ultimately leading to improved patient outcomes and quality of life.
1. Controlled Loading
Gradual and controlled mechanical loading is essential for enhancing tissue repair and strength. This approach involves applying mechanical forces in a progressive manner to stimulate cellular responses without causing further damage. At the molecular level, controlled loading activates a series of mechanotransductive pathways that are critical for tissue repair and adaptation. When mechanical forces are applied, mechanoreceptors on the cell surface, such as integrins, sense these forces and initiate signaling cascades within the cell.
In the context of knee rehabilitation, controlled loading can help in the formation of new collagen fibers and the realignment of existing ones, thereby improving the structural integrity of the joint. Collagen, the primary structural protein in connective tissues, responds to mechanical loading through a process called collagen fibrillogenesis. This involves the synthesis of procollagen molecules within fibroblasts, which are then secreted into the extracellular matrix (ECM) and assembled into mature collagen fibers. These fibers undergo cross-linking, which enhances their tensile strength and stability, contributing to the overall integrity of the joint.
Controlled loading also influences the activity of growth factors such as transforming growth factor-beta (TGF-β) and vascular endothelial growth factor (VEGF). TGF-β plays a pivotal role in the synthesis and deposition of ECM components, while VEGF promotes angiogenesis, the formation of new blood vessels, which is crucial for delivering nutrients and oxygen to the healing tissues. The mechanical stimulation provided by controlled loading enhances the expression and activity of these growth factors, facilitating tissue repair and regeneration.
In addition to integrins, other cellular structures such as primary cilia and stretch-activated ion channels play significant roles in mechanotransduction. Primary cilia are small, hair-like organelles that extend from the surface of most cell types and act as sensory antennae. They can detect mechanical changes in the environment and relay these signals into the cell, influencing processes like cell division and differentiation. Stretch-activated ion channels, such as piezo channels, respond to mechanical deformation by allowing ions to flow into the cell, which can trigger downstream signaling events essential for cellular adaptation and tissue repair.
Protocols for controlled loading should be tailored to the specific injury and patient condition, gradually increasing load intensity to avoid overloading. This might involve starting with low-impact activities such as stationary cycling or swimming before progressing to more weight-bearing exercises like walking or jogging. These low-impact activities provide a gentle introduction to mechanical loading, reducing the risk of re-injury while promoting initial stages of tissue repair.
As the rehabilitation progresses, the intensity and type of mechanical loading can be adjusted based on the patient’s response. For instance, stationary cycling provides a controlled and consistent load on the knee joint, promoting joint mobility and muscle strength without excessive impact. Swimming, particularly in a warm water environment, not only reduces joint stress due to buoyancy but also enhances muscle relaxation and circulation, further aiding the healing process.
Weight-bearing exercises such as walking and jogging introduce higher mechanical loads, which are beneficial for bone density and joint stability. These exercises stimulate osteoblasts, the cells responsible for bone formation, through mechanotransductive signaling pathways. This enhances bone remodeling and increases bone mineral density, which is particularly important for preventing osteoporosis and improving overall joint health.
In addition to these physical activities, resistance training using elastic bands or light weights can be incorporated to further enhance muscle strength and endurance. Resistance training provides targeted mechanical loading to specific muscle groups, promoting hypertrophy and improving functional capacity. The gradual increase in resistance ensures that muscles and joints adapt appropriately, minimizing the risk of overuse injuries.
Further molecular insights into controlled loading reveal the involvement of other key signaling pathways such as the Wnt/β-catenin pathway and the Hippo pathway. The Wnt/β-catenin pathway is crucial for regulating cell proliferation and differentiation, particularly in response to mechanical stress. Activation of this pathway can lead to increased expression of genes involved in tissue repair and regeneration. The Hippo pathway, on the other hand, regulates organ size and tissue homeostasis by controlling cell growth and apoptosis. Mechanical loading can modulate the activity of the Hippo pathway, influencing cell fate decisions and tissue remodeling.
Controlled loading also affects the production of matrix metalloproteinases (MMPs), which are enzymes that degrade various components of the ECM. MMPs play a vital role in tissue remodeling by breaking down damaged ECM components and allowing for the deposition of new, healthy matrix. The balance between MMP activity and ECM synthesis is critical for maintaining tissue integrity during the repair process.
In conclusion, controlled loading is a fundamental component of knee rehabilitation that leverages the principles of mechanotransduction to stimulate tissue repair and strength. By understanding the molecular mechanisms involved and tailoring protocols to individual needs, controlled loading can optimize the healing process and restore joint function effectively. This approach not only enhances the structural integrity of the knee joint but also contributes to overall functional recovery, improving the patient’s quality of life. The integration of molecular biology into rehabilitation strategies provides a comprehensive framework for developing targeted and effective interventions that promote optimal tissue repair and regeneration.
2. Exercise Therapy
Structured exercise programs play a pivotal role in stimulating mechanotransductive responses that promote joint health. Mechanotransduction, the process by which cells convert mechanical stimuli into biochemical signals, is fundamental to the benefits derived from exercise therapy. By strategically combining weight-bearing and non-weight-bearing exercises, rehabilitation protocols can optimize knee recovery through targeted cellular responses that enhance tissue repair, muscle strength, and joint stability.
Weight-bearing exercises, such as squats and lunges, help in rebuilding muscle strength and joint stability. These exercises exert mechanical forces on the bones and joints, activating mechanoreceptors on the surface of osteocytes (bone cells) and chondrocytes (cartilage cells). This activation initiates intracellular signaling cascades involving molecules such as integrins and focal adhesion kinases (FAKs), which lead to the activation of pathways like the MAPK and Wnt/β-catenin pathways. These pathways promote the synthesis of collagen and other extracellular matrix (ECM) components, enhancing the structural integrity and resilience of the joint.
Integrins are transmembrane receptors that connect the extracellular matrix to the cytoskeleton inside the cell. When mechanical forces are applied to the joint during weight-bearing exercises, integrins cluster together and recruit focal adhesion proteins, including talin, paxillin, and vinculin, forming focal adhesion complexes. These complexes serve as signaling hubs that activate various downstream pathways, such as the Rho/ROCK pathway, which regulates cytoskeletal dynamics and cell contraction, and the PI3K/Akt pathway, which promotes cell survival and growth. The combined activation of these pathways enhances the production of ECM components like collagen and proteoglycans, which are essential for maintaining joint integrity.
In addition to strengthening muscles and joints, weight-bearing exercises stimulate the production of bone morphogenetic proteins (BMPs) and transforming growth factor-beta (TGF-β). BMPs are critical for bone formation and remodeling, while TGF-β regulates ECM production and tissue repair. The combined effect of these growth factors is an increase in bone density and improved joint stability, which are essential for preventing injuries and maintaining joint health. BMPs, in particular, activate the Smad signaling pathway, which translocates to the nucleus and promotes the expression of osteogenic genes such as RUNX2 and Osterix, driving the differentiation of mesenchymal stem cells into osteoblasts and enhancing bone formation.
Non-weight-bearing exercises, such as leg lifts and stretches, focus on improving flexibility and range of motion without placing undue stress on the joint. These exercises are particularly beneficial during the early stages of rehabilitation when minimizing joint load is crucial. Non-weight-bearing exercises primarily stimulate mechanotransductive responses in the soft tissues surrounding the joint, including muscles, tendons, and ligaments. These tissues contain mechanoreceptors that detect mechanical changes and initiate signaling pathways that promote cellular repair and adaptation. For instance, cyclic stretching of tendons can activate the ERK1/2 and JNK pathways, which are involved in promoting tenocyte proliferation and collagen synthesis, aiding in tendon repair and strengthening.
Aquatic therapy is particularly effective as it provides resistance while minimizing joint load, allowing patients to perform exercises that might be too painful on land. The buoyancy of water reduces the impact on the joints, making it an ideal environment for early rehabilitation exercises. The resistance provided by water enhances muscle activation and endurance, promoting the synthesis of ECM components and improving joint function. At the molecular level, aquatic therapy can increase the expression of anti-inflammatory cytokines and reduce the levels of pro-inflammatory mediators, thereby accelerating the healing process and reducing pain and swelling. Water resistance also provides a continuous, multidirectional mechanical load that stimulates proprioceptive pathways, enhancing neuromuscular coordination and balance.
Resistance training, through the use of bands or light weights, further enhances muscle strength and endurance, contributing to the overall rehabilitation process. Resistance training induces mechanical strain on muscle fibers, leading to the activation of satellite cells, which are muscle stem cells responsible for muscle repair and growth. This process involves the release of growth factors such as insulin-like growth factor 1 (IGF-1) and fibroblast growth factor (FGF), which stimulate muscle protein synthesis and hypertrophy. IGF-1, in particular, activates the PI3K/Akt/mTOR pathway, which is crucial for protein synthesis and muscle growth. Resistance training also upregulates the expression of myogenic regulatory factors (MRFs) such as MyoD and myogenin, which are essential for satellite cell differentiation and muscle fiber formation.
Moreover, resistance training modulates the expression of mechanosensitive genes and proteins, such as myosin heavy chain (MHC) and actin, which are essential for muscle contraction and strength. The increased muscle strength and endurance resulting from resistance training enhance the support and stability of the knee joint, reducing the risk of re-injury and promoting long-term joint health. Resistance training also induces the production of ECM-degrading enzymes such as matrix metalloproteinases (MMPs), which help remodel the ECM and facilitate the removal of damaged tissue, allowing for the deposition of new, healthy matrix components.
Further molecular insights into exercise therapy reveal the involvement of other key signaling molecules and pathways, such as nitric oxide (NO) and reactive oxygen species (ROS). NO is a signaling molecule produced by endothelial cells in response to mechanical shear stress, and it plays a crucial role in vasodilation, enhancing blood flow and nutrient delivery to the tissues. ROS, which are byproducts of cellular metabolism, can act as signaling molecules to promote adaptive responses to mechanical loading, such as the activation of antioxidant defenses and the upregulation of protective proteins like heat shock proteins (HSPs).
In conclusion, exercise therapy leverages the principles of mechanotransduction to stimulate beneficial cellular responses that promote knee joint health and recovery. By combining weight-bearing and non-weight-bearing exercises, incorporating aquatic therapy, and utilizing resistance training, structured exercise programs can optimize the rehabilitation process. Understanding the molecular mechanisms underlying these exercises allows for the development of targeted interventions that enhance tissue repair, muscle strength, and joint stability, ultimately improving patient outcomes and quality of life. The integration of molecular biology into rehabilitation strategies provides a comprehensive framework for developing targeted and effective interventions that promote optimal tissue repair and regeneration.
3. Manual Therapy
Manual therapy encompasses a range of techniques such as joint mobilization and manipulation, which modulate mechanical stimuli to enhance mechanotransduction and tissue repair. These hands-on methods involve applying pressure and movement to the knee joint, aiding in pain alleviation, improving joint mobility, and stimulating mechanotransductive pathways. Mechanotransduction is the process by which cells convert mechanical stimuli into biochemical signals, initiating a cascade of molecular events that promote tissue repair and regeneration. Integrins, which are cell surface receptors, are crucial in this process as they link the extracellular matrix (ECM) to the cell’s cytoskeleton. When mechanical pressure is applied during manual therapy, integrins cluster and activate intracellular signaling pathways, such as the MAPK and PI3K-Akt pathways. This activation leads to the production of proteins that aid in tissue repair, facilitating recovery and healing.
At the molecular level, the application of mechanical forces through manual therapy initiates a series of intracellular events. Integrins, upon activation by mechanical stress, undergo conformational changes that enable them to bind to ECM proteins such as fibronectin, collagen, and laminin. This binding triggers the recruitment of focal adhesion kinase (FAK) to the integrin complexes. FAK, once activated, phosphorylates downstream signaling molecules, including Src family kinases, leading to the activation of multiple signaling cascades such as the Ras-Raf-MEK-ERK pathway and the PI3K-Akt pathway. These pathways collectively contribute to cellular responses that include increased protein synthesis, cell proliferation, and survival, which are essential for tissue repair and regeneration.
Furthermore, gentle mobilization techniques used in manual therapy can increase the production of synovial fluid, which nourishes the cartilage and promotes smoother joint movements. Synovial fluid contains hyaluronic acid and lubricin, essential for reducing friction and wear within the joint. The mechanical stimulation from manual therapy enhances the activity of synoviocytes, the cells responsible for producing synovial fluid, thereby improving joint lubrication and overall joint health. This fluid also delivers essential nutrients to chondrocytes, the cells in cartilage, supporting their function and viability, which is vital for maintaining healthy joint cartilage.
In addition to these mechanical and biochemical effects, manual therapy also influences the local inflammatory environment. The mechanical forces applied during therapy can modulate the expression of cytokines and growth factors. For instance, mechanical loading has been shown to upregulate anti-inflammatory cytokines like IL-10, which suppresses pro-inflammatory pathways and mitigates inflammation. Concurrently, growth factors such as TGF-β are upregulated, promoting tissue remodeling and healing. TGF-β, in particular, plays a pivotal role in the synthesis of ECM components and the regulation of cellular differentiation and proliferation, essential processes for effective tissue repair.
When manual therapy is combined with exercise therapy, the benefits are even more pronounced. Exercise therapy induces additional mechanical stimuli, sustaining and enhancing the mechanotransductive effects initiated by manual therapy. This combination ensures that the joint becomes both strong and flexible, significantly improving rehabilitation outcomes and reducing the risk of re-injury. Exercise further promotes muscle support around the joint, leading to greater joint stability and enhanced overall function. The integration of manual therapy and exercise therapy leverages the body’s molecular biology to optimize healing and rehabilitation processes, offering a comprehensive approach to joint health and recovery.
At the cellular level, exercise stimulates similar mechanotransductive pathways as manual therapy, further enhancing the cellular responses involved in tissue repair. The mechanical load from exercise activates integrins and associated signaling pathways, leading to increased production of ECM proteins and growth factors that support tissue integrity and function. Additionally, exercise induces the expression of genes involved in muscle hypertrophy and strength, such as those regulated by the mechanistic target of rapamycin (mTOR) pathway, which is critical for muscle protein synthesis and growth.
Overall, the integration of manual therapy and exercise therapy provides a synergistic effect, harnessing the body’s innate molecular mechanisms to promote optimal healing, reduce pain, and improve functional outcomes. This combined approach ensures a more effective and comprehensive rehabilitation strategy, addressing both the mechanical and biochemical aspects of joint health and recovery.
4. Early Mechanical Loading
Early mechanical loading, initiated soon after injury, can profoundly impact the rehabilitation process, influencing molecular and cellular mechanisms that underpin tissue repair and regeneration. Research indicates that introducing controlled mechanical forces at an early stage can prevent the formation of excessive scar tissue and promote more organized tissue repair. The principle behind early mechanical loading is to stimulate mechanotransductive pathways, which play a critical role in cellular responses to mechanical stimuli. By applying these forces, cells in the injured tissue are encouraged to produce a more organized extracellular matrix, leading to stronger and more functional tissue repair.
Mechanotransduction begins with the activation of integrins, cell surface receptors that link the extracellular matrix (ECM) to the cell’s cytoskeleton. When mechanical forces are applied, integrins undergo conformational changes, clustering together and initiating a cascade of intracellular signaling events. This clustering recruits focal adhesion kinase (FAK), which phosphorylates and activates Src family kinases. This activation leads to the initiation of multiple signaling pathways, including the MAPK (mitogen-activated protein kinase) and PI3K-Akt (phosphoinositide 3-kinase-protein kinase B) pathways.
The MAPK pathway, involving ERK (extracellular signal-regulated kinase), JNK (c-Jun N-terminal kinase), and p38 kinases, regulates gene expression, cellular growth, and differentiation. Activation of this pathway leads to the transcription of genes encoding ECM proteins, such as collagen and fibronectin, critical for tissue repair. Simultaneously, the PI3K-Akt pathway promotes cell survival and growth by inhibiting apoptosis (programmed cell death) and stimulating protein synthesis through mTOR (mechanistic target of rapamycin) activation.
Early mechanical loading should be carefully monitored and adjusted based on the patient’s pain levels and healing progress. This approach might include gentle movements and partial weight-bearing activities that progressively increase in intensity. For instance, patients can start with isometric exercises, which involve contracting muscles without joint movement, and gradually progress to isotonic exercises that involve movement and load. The goal is to provide enough mechanical stimulus to activate mechanotransductive pathways without exacerbating the injury, thereby facilitating a quicker and more effective recovery.
One of the key benefits of early mechanical loading is the prevention of excessive scar tissue formation. Scar tissue, which is composed mainly of disorganized collagen fibers, can limit joint mobility and function if it becomes too abundant. By applying controlled mechanical forces, the alignment of collagen fibers can be improved, resulting in a more organized and functional repair tissue. The TGF-β (transforming growth factor-beta) signaling pathway plays a pivotal role here, as it regulates the synthesis of ECM components and modulates the activity of fibroblasts, the cells responsible for collagen production.
Moreover, early mechanical loading has been shown to influence the inflammatory response to injury. Mechanical forces can modulate the expression of cytokines and growth factors involved in inflammation and healing. For example, early loading can increase levels of anti-inflammatory cytokines such as IL-10, which suppress pro-inflammatory pathways and mitigate inflammation. Concurrently, mechanical loading enhances the expression of growth factors like TGF-β, which promote tissue regeneration and repair. These molecular changes help create a more favorable environment for healing, reducing pain and swelling, and promoting faster recovery.
In conclusion, leveraging the principles of mechanotransduction in rehabilitation strategies is crucial for optimizing knee joint recovery. Controlled loading, structured exercise therapy, manual therapy, and early mechanical loading are key approaches that, when appropriately combined and tailored to individual needs, can significantly enhance the healing process and restore joint function. Early mechanical loading, in particular, plays a vital role in preventing excessive scar tissue formation, promoting organized tissue repair, and modulating the inflammatory response. By carefully managing the intensity and progression of mechanical forces, clinicians can harness the body’s natural healing mechanisms to achieve better rehabilitation outcomes and quicker recovery times.
Rehabilitation Strategies Based on Musculoskeletal Healing Stages: Early Mechanical Loading
Rehabilitation strategies for musculoskeletal injuries must be meticulously tailored to align with the distinct stages of healing, which include inflammation, proliferation, and remodeling. Each of these stages necessitates specific interventions to optimize tissue repair, restore function, and prevent further injury. From a molecular biology perspective, understanding the underlying cellular and molecular mechanisms during these healing stages can significantly enhance the effectiveness of rehabilitation strategies.
In the inflammation stage, the body initiates a defense response to injury, characterized by the release of pro-inflammatory cytokines such as interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α). These molecules promote the recruitment of immune cells to the injury site, which helps clear debris and pathogens. Rehabilitation during this stage focuses on protecting the injured area, reducing inflammation and pain, and promoting the initial healing process. Gentle range-of-motion exercises and isometric contractions can be introduced to maintain some level of muscle activation without exacerbating the injury, which can also help modulate the inflammatory response and prevent excessive tissue degradation.
During the proliferation stage, fibroblasts and other repair cells are activated to synthesize extracellular matrix components, primarily collagen, which form the scaffold for new tissue. Growth factors such as transforming growth factor-beta (TGF-β) and vascular endothelial growth factor (VEGF) play critical roles in this process, promoting cell proliferation, angiogenesis, and collagen deposition. Controlled mechanical loading becomes crucial in this phase to stimulate the production of new collagen fibers and facilitate the organization of these fibers in a functional manner. Activities such as low-intensity resistance exercises, balance training, and gradual reintroduction of functional movements are beneficial. These exercises can enhance the expression of mechanical growth factors like insulin-like growth factor-1 (IGF-1), which further supports tissue regeneration.
In the remodeling stage, the newly formed tissue undergoes maturation and reorganization to enhance its mechanical properties. This stage is regulated by matrix metalloproteinases (MMPs), which remodel the extracellular matrix, and by the continuous production and cross-linking of collagen fibers. The objective during this phase is to enhance the strength and flexibility of the repaired tissues and to ensure they are adequately prepared to handle normal daily activities and sports-specific demands. Progressive overload principles should be applied, with a gradual increase in the intensity and complexity of exercises. Plyometric exercises, sport-specific drills, and functional training become integral components of the rehabilitation program during this stage. These activities help stimulate the final alignment of collagen fibers along the lines of mechanical stress, improving the overall strength and functionality of the tissue.
By closely adhering to these stage-specific rehabilitation strategies, practitioners can significantly enhance the healing process, minimize the risk of re-injury, and ensure a more efficient and effective return to full function. The careful implementation of early mechanical loading, adjusted appropriately for each stage of healing, plays a critical role in achieving these outcomes. Moreover, leveraging insights from molecular biology can inform the timing and type of interventions, ensuring that rehabilitation not only supports but also accelerates the natural healing processes at the cellular and molecular levels (
Table 3).
1. Inflammation Stage.
The inflammation stage is the initial and critical response to musculoskeletal injury. It is characterized by vasodilation, invasion of platelets, and recruitment of inflammatory cells such as neutrophils, monocytes, and macrophages. These processes are regulated by a complex network of chemical mediators, including histamine, bradykinin, and prostaglandin E2 (PGE2), each contributing to specific aspects of the inflammatory response. At the molecular level, various signaling pathways and cellular mechanisms are activated to ensure a coordinated response that promotes tissue repair and recovery.
Vasodilation is the process of widening blood vessels, which increases blood flow to the injured area, thereby allowing essential nutrients and immune cells to reach the site of damage. This is facilitated by the relaxation of smooth muscle cells within the vessel walls and is primarily mediated by histamine, bradykinin, and PGE2. Histamine, released from mast cells, basophils, and platelets upon injury, binds to H1 receptors on endothelial cells. This binding leads to their contraction and increases vascular permeability, allowing immune cells and proteins to exit the bloodstream and enter the site of injury. Histamine also stimulates endothelial nitric oxide synthase (eNOS) to produce nitric oxide (NO), which diffuses into adjacent smooth muscle cells, causing them to relax and leading to vasodilation. This process helps deliver immune cells and nutrients to the site of injury and clears cellular debris. At a molecular level, the binding of histamine to its receptors triggers intracellular signaling cascades involving secondary messengers such as cyclic AMP (cAMP) and calcium ions, which further propagate the signal and amplify the inflammatory response.
Bradykinin, formed from kininogen through the action of the enzyme kallikrein during tissue injury, binds to B2 receptors on endothelial cells, promoting the release of NO and prostacyclin (PGI2), both potent vasodilators. Additionally, bradykinin increases the permeability of the vascular endothelium, allowing plasma proteins and immune cells to enter the tissue, further supporting the inflammatory response and aiding in tissue repair. Bradykinin sensitizes nociceptors, contributing to pain signaling, serving as a protective mechanism by encouraging the individual to limit movement and prevent further injury. The binding of bradykinin to its receptors activates G-protein-coupled receptor pathways, leading to the activation of phospholipase C (PLC), which in turn generates inositol triphosphate (IP3) and diacylglycerol (DAG), resulting in calcium release and protein kinase C (PKC) activation, key players in the inflammatory signaling network.
PGE2 is synthesized from arachidonic acid via the cyclooxygenase (COX) pathway, with COX-2 being particularly active in response to inflammatory signals. PGE2 acts on EP2 and EP4 receptors on smooth muscle cells, increasing intracellular cyclic AMP (cAMP) levels, which leads to muscle relaxation and vasodilation. PGE2 also sensitizes sensory nerves, contributing to the sensation of pain and amplifying the inflammatory response. The synthesis of PGE2 involves the activation of phospholipase A2 (PLA2), which releases arachidonic acid from membrane phospholipids. Arachidonic acid is then converted to prostaglandin H2 (PGH2) by COX enzymes, and finally to PGE2 by specific synthases. This cascade is tightly regulated by various signaling molecules and feedback mechanisms to ensure an appropriate inflammatory response.
Platelets are crucial for initiating hemostasis and facilitating subsequent tissue repair through several mechanisms. Platelet activation is triggered by the exposure of subendothelial collagen and von Willebrand factor (vWF) in the damaged endothelium. These molecules bind to glycoprotein receptors (GPVI and GPIb) on platelets, causing them to adhere to the site of injury. Once activated, platelets release adenosine diphosphate (ADP) and thromboxane A2 (TXA2), which further amplify platelet activation and aggregation, forming a platelet plug that prevents further blood loss and stabilizes the initial injury site. The formation of the platelet plug is crucial for preventing excessive blood loss and providing a temporary scaffold for subsequent tissue repair. Activated platelets release a variety of growth factors, including platelet-derived growth factor (PDGF) and transforming growth factor-beta (TGF-β), which recruit and activate fibroblasts and smooth muscle cells. These growth factors are crucial for initiating the subsequent stages of tissue repair and regeneration by promoting cell proliferation and matrix synthesis. The intracellular signaling pathways involved in platelet activation and aggregation include the activation of phosphoinositide 3-kinase (PI3K), protein kinase B (Akt), and small GTPases like Rap1, which coordinate cytoskeletal reorganization and integrin activation, essential for stable platelet adhesion and thrombus formation.
The recruitment and activation of inflammatory cells are essential for clearing debris and orchestrating tissue repair. Neutrophils are attracted to the injury site by chemotactic agents such as interleukin-8 (IL-8), complement component C5a, and leukotriene B4 (LTB4). Neutrophils perform phagocytosis to engulf and destroy pathogens, release proteolytic enzymes like elastase and collagenase to degrade damaged tissue, and generate reactive oxygen species (ROS) to kill microbes. These actions are critical for clearing cellular debris and preventing infection, helping create a clean environment conducive to tissue repair. Monocytes are attracted to the injury site by chemokines like monocyte chemoattractant protein-1 (MCP-1 or CCL2) and differentiate into macrophages upon entering the tissue. M1 macrophages produce pro-inflammatory cytokines (IL-1, IL-6, TNF-α) and ROS, enhancing pathogen clearance and sustaining the inflammatory response, while M2 macrophages secrete anti-inflammatory cytokines (IL-10, TGF-β) and growth factors, resolving inflammation and promoting tissue repair and remodeling. The balance between M1 and M2 macrophages is crucial for the progression from inflammation to healing, with the shift from M1 to M2 macrophages being critical for transitioning from the inflammatory phase to the healing phase. The differentiation and function of macrophages are regulated by transcription factors such as NF-κB and STAT3, which integrate signals from cytokines and growth factors to modulate gene expression and cellular behavior.
The chemical mediators orchestrating these responses include histamine, which induces vasodilation and increases vascular permeability, facilitating the influx of immune cells and nutrients to the injury site. This helps to clear debris and supports the initial stages of healing. Bradykinin promotes vasodilation, increases vascular permeability, and sensitizes nociceptors, contributing to pain signaling and enhancing the inflammatory response. PGE2 induces vasodilation, enhances vascular permeability, and sensitizes sensory nerves to pain. PGE2 also modulates inflammatory responses by influencing the behavior of immune cells, supporting the transition from acute inflammation to tissue repair. Additional mediators like leukotrienes (e.g., LTB4), produced from arachidonic acid by the lipoxygenase pathway, act as potent chemotactic agents for neutrophils and other leukocytes, supporting their recruitment to the injury site. Nitric oxide (NO), produced by endothelial cells (via eNOS) and macrophages (via iNOS), induces smooth muscle relaxation, leading to vasodilation and possesses antimicrobial properties, aiding in the defense against infection at the injury site. These additional mediators support various aspects of the inflammatory response, including the recruitment of immune cells, vasodilation, and pathogen clearance, with their coordinated action being essential for effective tissue repair.
At a molecular level, the inflammation stage is governed by intricate signaling networks that involve the activation of various receptors, transcription factors, and secondary messengers. For instance, the NF-κB pathway is pivotal in regulating the expression of pro-inflammatory cytokines and adhesion molecules that facilitate leukocyte recruitment and activation. The MAPK and JAK-STAT pathways also play crucial roles in transducing signals from cytokines and growth factors, leading to the transcriptional activation of genes involved in inflammation, cell proliferation, and survival. These molecular pathways ensure that the inflammatory response is tightly regulated and can effectively transition to the proliferative phase of healing. Understanding these molecular mechanisms provides valuable insights into potential therapeutic targets for modulating the inflammatory response and enhancing tissue repair.
2. Fibroblastic Stage.
The fibroblastic stage follows the initial inflammatory response and involves the activation and proliferation of fibroblasts, which are responsible for synthesizing and organizing the extracellular matrix (ECM) components necessary for tissue repair. Key growth factors, such as Transforming Growth Factor-beta 1 (TGF-β1), Bone Morphogenetic Proteins (BMPs), and Connective Tissue Growth Factor (CTGF), play pivotal roles in this process. TGF-β1 binds to TGF-β receptors (TGF-βRI and TGF-βRII) on fibroblasts, initiating the phosphorylation of Smad2/3 proteins. These phosphorylated Smads form complexes with Smad4, which then translocate to the nucleus to regulate the transcription of ECM genes, promoting the production of ECM components. This pathway is crucial for activating fibroblasts and promoting their proliferation and ECM production. The Smad complexes act as transcription factors, driving the expression of genes necessary for ECM synthesis and organization. By promoting the production of ECM components, TGF-β1 supports the formation of a stable matrix for tissue repair, providing structural support and facilitating the healing process.
TGF-β1 enhances the production of type I and type III collagen, fibronectin, and integrins, contributing to the assembly and stability of the ECM. It also inhibits the expression of matrix metalloproteinases (MMPs), reducing ECM degradation and supporting the formation of a stable matrix for tissue repair. The increased production of collagen provides the necessary scaffold for tissue repair, ensuring mechanical strength and stability. By inhibiting MMPs, TGF-β1 helps maintain the integrity of the newly formed ECM, preventing its premature degradation and ensuring effective tissue repair. BMPs bind to BMP receptors (BMPR-I and BMPR-II), leading to the activation of Smad1/5/8 proteins. These proteins form complexes with Smad4 and translocate to the nucleus to influence gene expression, promoting the synthesis of ECM components and differentiation of fibroblasts. The activation of Smad1/5/8 proteins by BMPs drives the expression of genes involved in ECM production and fibroblast differentiation, essential for orchestrating the activities of fibroblasts during tissue repair. BMPs promote the differentiation of fibroblasts into myofibroblasts, which are specialized for ECM production and wound contraction. This differentiation is crucial for effective tissue repair and remodeling.
BMPs not only stimulate fibroblast differentiation but also enhance the production of ECM components. They play a critical role in the synthesis of collagen and other structural proteins, contributing to the robustness of the ECM. BMP signaling involves the phosphorylation and activation of receptor-regulated Smads (R-Smads), which then partner with co-Smad (Smad4) and translocate to the nucleus to regulate target gene expression. This regulation ensures that fibroblasts produce adequate amounts of ECM proteins to support tissue repair. The differentiation of fibroblasts into myofibroblasts, driven by BMPs, is marked by the expression of alpha-smooth muscle actin (α-SMA), which is essential for wound contraction and the mechanical strength of the tissue.
CTGF interacts with cell surface receptors such as integrins and heparan sulfate proteoglycans, activating downstream signaling pathways like the MAPK/ERK pathway. These pathways promote cell proliferation, migration, and ECM synthesis. The activation of the MAPK/ERK pathway by CTGF drives the expression of genes involved in cell proliferation, migration, and ECM production, essential for coordinating the activities of fibroblasts during tissue repair. CTGF promotes fibroblast migration to the injury site, ensuring that sufficient cells are present to produce the ECM necessary for tissue repair. CTGF also enhances the expression of collagen, fibronectin, and proteoglycans, and improves fibroblast adhesion and migration, facilitating ECM deposition and remodeling, contributing to the structural and functional recovery of the injured tissue.
The proliferation of activated fibroblasts is a critical aspect of the fibroblastic stage. These cells extensively proliferate in response to growth factors, increasing the number of cells capable of synthesizing ECM components. This cellular proliferation ensures that a sufficient number of fibroblasts are available to produce the ECM, supporting effective tissue repair. Fibroblasts produce large quantities of type I and type III collagen, which are essential for the structural integrity of the newly formed tissue. This collagen forms a scaffold that provides mechanical strength and supports further cellular activities. The production of collagen by fibroblasts is regulated by various signaling pathways, including the TGF-β/Smad, MAPK, and PI3K/Akt pathways, which ensure the precise control of collagen synthesis and deposition.
Initially, the collagen fibers are laid down in a random, haphazard manner, forming a provisional matrix that fills the wound space and provides temporary mechanical strength. This initial disorganized collagen network provides immediate mechanical support, allowing for the continuation of the healing process. Over time, this disorganized collagen network becomes the foundation of scar tissue. The random orientation of collagen fibers leads to the characteristic stiffness and reduced functionality of scar tissue compared to normal tissue. The development of scar tissue is a critical aspect of wound healing, providing structural support but often resulting in impaired tissue function. The formation of scar tissue, while necessary for immediate structural support, can result in reduced tissue functionality and flexibility, underscoring the importance of effective rehabilitation strategies to minimize scar tissue formation and promote optimal healing.
Enzymes such as lysyl oxidase (LOX) mediate the cross-linking of collagen fibers, increasing the tensile strength of the ECM. This cross-linking process is essential for stabilizing the newly formed tissue and ensuring its mechanical integrity. The cross-linking of collagen fibers enhances the tensile strength of the ECM, providing stability and durability to the repaired tissue. Despite the initial random organization, subsequent remodeling processes involve the reorganization of collagen fibers. Fibroblasts and myofibroblasts exert mechanical forces that attempt to align collagen fibers along the lines of tension, although this reorganization is often incomplete in scar tissue. This remodeling improves the mechanical properties of the tissue but may not fully restore its original functionality. The reorganization of collagen fibers along lines of tension improves the mechanical properties of the tissue, enhancing its functionality and resilience.
Other molecules, such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), also contribute to the wound healing process by promoting angiogenesis and further fibroblast recruitment and activation. VEGF stimulates the formation of new blood vessels from pre-existing vasculature, ensuring an adequate supply of oxygen and nutrients to the healing tissue. This angiogenesis is critical for sustaining the metabolic needs of proliferating fibroblasts and other cells involved in tissue repair. PDGF attracts fibroblasts to the wound site and stimulates their proliferation and ECM production. The signaling pathways activated by VEGF and PDGF involve receptor tyrosine kinases that trigger downstream cascades, such as the PI3K/Akt and Ras/MAPK pathways, promoting cellular activities essential for tissue repair and regeneration. The formation of new blood vessels ensures an adequate supply of oxygen and nutrients to the healing tissue, supporting effective tissue repair and regeneration.
At a molecular level, the fibroblastic stage is governed by a complex interplay of signaling pathways and transcriptional regulators that coordinate the activities of fibroblasts and other cells involved in tissue repair. The precise regulation of these pathways ensures that ECM production, cell proliferation, and tissue remodeling occur in a controlled and efficient manner. Understanding these molecular mechanisms provides valuable insights into potential therapeutic targets for enhancing tissue repair and minimizing fibrosis, ultimately improving clinical outcomes for patients with musculoskeletal injuries.
3. Remodeling Stage.
The remodeling stage involves the improvement of the organization and mechanical properties of the extracellular matrix (ECM) through a dynamic process involving the coordinated activity of various cells, enzymes, and signaling pathways. Fibroblasts and myofibroblasts play key roles in this process by synthesizing and remodeling collagen and other ECM components. At the molecular level, this stage is driven by intricate interactions among cellular mechanisms, enzymatic activities, and growth factor signaling, all of which contribute to the effective remodeling and strengthening of the tissue.
Collagen remodeling is a critical aspect of the remodeling stage, involving several molecular processes. Enzymes such as lysyl oxidase (LOX) catalyze the formation of covalent cross-links between collagen molecules, enhancing the tensile strength and stability of the ECM. This process is crucial for ensuring the durability and mechanical integrity of the remodeled tissue. LOX-mediated cross-linking involves the oxidative deamination of lysine residues in collagen and elastin, resulting in the formation of aldehyde groups that spontaneously react to form covalent bonds, thereby stabilizing the ECM structure. Additionally, matrix metalloproteinases (MMPs), particularly MMP-1 (collagenase) and MMP-9 (gelatinase), are involved in the degradation of disorganized collagen fibers. MMPs are zinc-dependent endopeptidases that degrade various ECM components, and their activity is tightly regulated by tissue inhibitors of metalloproteinases (TIMPs). The balance between MMPs and TIMPs is essential for maintaining ECM homeostasis, as it ensures the removal of damaged matrix components while allowing new matrix synthesis. Myofibroblasts exert contractile forces on the ECM, aligning collagen fibers along the lines of mechanical stress, which improves the structural integrity and functional properties of the tissue, making it more resilient and capable of withstanding mechanical loads. This alignment is facilitated by the integrin-mediated attachment of myofibroblasts to the ECM, which triggers intracellular signaling pathways such as focal adhesion kinase (FAK) and RhoA/ROCK, promoting cytoskeletal reorganization and force generation. The reorganization and cross-linking of collagen fibers enhance the tensile strength and mechanical properties of the tissue, supporting its long-term functionality.
Cellular mechanisms are also pivotal in the remodeling stage. Fibroblasts and myofibroblasts continue to proliferate and produce ECM components. Myofibroblasts, characterized by the expression of alpha-smooth muscle actin (α-SMA), generate contractile forces that facilitate tissue contraction and collagen fiber alignment. This cellular activity is crucial for the reorganization and strengthening of the remodeled tissue. The differentiation of fibroblasts into myofibroblasts is driven by TGF-β signaling, which activates the SMAD and non-SMAD pathways, leading to the expression of α-SMA and other contractile proteins. Cell-ECM interactions mediated by integrins trigger intracellular signaling pathways, such as the FAK and the MAPK/ERK pathways, promoting cell migration, survival, and ECM production, supporting the dynamic remodeling of the tissue. Integrins are transmembrane receptors that link the ECM to the cytoskeleton, facilitating bidirectional signaling that coordinates cellular responses to mechanical and biochemical cues. The interaction between cells and the ECM is essential for coordinating cellular activities during tissue repair, ensuring effective remodeling and functional recovery.
Growth factors and cytokines play significant roles in the remodeling stage. Transforming Growth Factor-beta (TGF-β) is a pivotal regulator of ECM remodeling, stimulating fibroblast proliferation, myofibroblast differentiation, and the synthesis of collagen and other ECM proteins. TGF-β signaling involves the activation of receptor serine/threonine kinases, which phosphorylate SMAD2/3 proteins. These phosphorylated SMADs form complexes with SMAD4 and translocate to the nucleus, where they regulate the transcription of target genes involved in ECM production and cell differentiation. TGF-β also modulates the expression of MMPs and TIMPs, influencing ECM turnover and ensuring a balanced remodeling process. Connective Tissue Growth Factor (CTGF) enhances the fibrotic response by promoting collagen synthesis and fibroblast adhesion to the ECM. CTGF is induced by TGF-β and functions through its interaction with cell surface receptors such as integrins and heparan sulfate proteoglycans, activating downstream signaling pathways like the MAPK/ERK and PI3K/Akt pathways, which promote cell proliferation, migration, and ECM synthesis. Platelet-Derived Growth Factor (PDGF) attracts fibroblasts to the wound site and stimulates their proliferation and ECM production, essential for sustaining the remodeling process and ensuring adequate cellular activity. PDGF signaling involves the activation of receptor tyrosine kinases, leading to the activation of the Ras/MAPK and PI3K/Akt pathways, which regulate gene expression, cell survival, and cytoskeletal reorganization. These growth factors and cytokines are crucial for orchestrating the activities of fibroblasts and myofibroblasts, supporting the dynamic remodeling of the tissue and ensuring its long-term functionality.
Extracellular matrix components such as fibronectin and elastin contribute to the structural integrity and elasticity of the tissue. Fibronectin is a high-molecular-weight glycoprotein that facilitates cell adhesion and migration by binding to integrins and other ECM components, forming a scaffold that supports cellular activities during tissue repair. Elastin provides resilience to the ECM, allowing it to withstand mechanical stress and maintain its functional properties. Elastin is synthesized as a soluble precursor, tropoelastin, which undergoes extensive cross-linking by LOX to form insoluble elastin fibers. Proteoglycans and glycosaminoglycans (GAGs) retain water within the ECM, maintaining its viscoelastic properties and providing a hydrated environment conducive to cellular activities. Proteoglycans consist of a core protein with covalently attached GAG chains, which interact with other ECM components and cell surface receptors to modulate cell behavior and ECM organization. The coordinated production of these ECM components ensures the structural integrity and functional resilience of the remodeled tissue, supporting its long-term performance.
Continuous collagen synthesis can lead to several consequences, including scar formation and tendon adhesions. Persistent activation of fibroblasts and continuous collagen synthesis result in excessive ECM deposition, leading to fibrosis. The resulting scar tissue is often stiffer and less elastic than the original tissue due to the dense collagen network. This fibrosis can impair the functional recovery of the tissue and lead to long-term complications. Although the scar tissue provides immediate tensile strength, it lacks the intricate organization and biomechanical properties of the native tissue, impairing function and leading to reduced flexibility, strength, and overall tissue performance. The formation of scar tissue, while necessary for immediate structural support, often results in impaired tissue functionality and flexibility, highlighting the importance of effective rehabilitation strategies to minimize scar tissue formation and promote optimal healing.
Continuous collagen deposition around tendons can lead to adhesions, where the tendons adhere to surrounding tissues, restricting tendon gliding and impairing joint mobility. This leads to functional limitations and discomfort, significantly affecting the functional recovery of the affected limb and hindering the rehabilitation process. Tendon adhesions pose significant challenges for rehabilitation, often requiring interventions to restore mobility and function. Addressing these adhesions is crucial for achieving successful long-term recovery. Molecular pathways in adhesion formation include the involvement of inflammatory mediators. Persistent inflammation, characterized by elevated levels of pro-inflammatory cytokines (e.g., IL-1, TNF-α), promotes fibrosis and adhesion formation. These cytokines activate signaling pathways such as NF-κB and JAK/STAT, which upregulate the expression of fibrotic genes and promote the recruitment and activation of fibroblasts and myofibroblasts. The chronic inflammatory state sustains the activation of fibroblasts and myofibroblasts, leading to excessive ECM deposition and adhesion development.
The fibroblast-to-myofibroblast transition, induced by TGF-β and other growth factors, produces large amounts of collagen and contracts the ECM, contributing to adhesion development. This transition is mediated by the activation of SMAD and non-SMAD pathways, which regulate the expression of contractile proteins and ECM components. Understanding the molecular pathways involved in adhesion formation is crucial for developing strategies to prevent and treat these adhesions, ensuring effective rehabilitation and functional recovery. Research into molecular inhibitors of TGF-β signaling, MMPs, and inflammatory cytokines holds promise for therapeutic interventions aimed at reducing fibrosis and improving tissue remodeling outcomes. By targeting these molecular pathways, it may be possible to modulate the remodeling process, minimize scar formation, and enhance the functional recovery of injured tissues.
Future Directions
Further research is needed to elucidate the specific signaling pathways involved in knee joint mechanotransduction and explore novel therapeutic interventions to enhance knee joint health. Understanding the precise molecular mechanisms through which mechanical forces translate into biochemical signals is crucial. This knowledge can lead to the development of targeted therapies that improve rehabilitation outcomes by precisely modulating these pathways to enhance tissue repair and regeneration.
One promising area of research involves identifying the key molecules and cellular components that mediate mechanotransduction in knee joint tissues. For example, further study into the role of integrins and their associated proteins, such as focal adhesion kinase (FAK) and paxillin, could provide deeper insights into how mechanical signals are transduced into cellular responses. Advanced imaging techniques and molecular biology tools, such as CRISPR-Cas9 gene editing and RNA sequencing, could be employed to dissect the interactions and modifications of these proteins under different mechanical loads.
Additionally, research should focus on the downstream signaling pathways activated by mechanotransduction, including the MAPK and PI3K-Akt pathways. Investigating how these pathways are modulated during different stages of tissue repair and in response to varying intensities of mechanical loading can reveal critical windows for therapeutic intervention. For instance, understanding how the timing and magnitude of mechanical stimuli influence the activation of these pathways could inform the design of rehabilitation protocols that maximize healing while minimizing the risk of re-injury.
Another future direction involves the exploration of biomaterials and engineered tissues that can enhance mechanotransduction. These materials could be designed to mimic the natural extracellular matrix and provide optimal mechanical cues to support tissue repair. Hydrogel scaffolds, for example, can be engineered to deliver controlled mechanical stimuli and release bioactive molecules that promote healing. Combining these advanced materials with traditional rehabilitation techniques could lead to more effective treatments for knee joint injuries.
Moreover, personalized medicine approaches should be considered in future research. Individual variability in mechanotransductive responses suggests that therapies could be tailored to the specific needs and biological characteristics of each patient. Biomarkers that indicate the state of mechanotransductive signaling pathways in the knee joint could be identified and used to customize rehabilitation protocols. For example, patients with lower baseline activity of the PI3K-Akt pathway might benefit from different mechanical loading regimens or adjunctive pharmacological treatments that enhance this pathway’s activity.
Emerging technologies, such as wearable devices and smart rehabilitation equipment, could also play a significant role in future research and clinical practice. These technologies can provide real-time feedback on joint loading and movement patterns, allowing for precise control and adjustment of therapeutic interventions. Integrating data from these devices with advanced analytics and machine learning algorithms could optimize rehabilitation strategies and improve patient outcomes.
In conclusion, future research in knee joint mechanotransduction should focus on elucidating the specific signaling pathways involved, exploring novel biomaterials and engineered tissues, and developing personalized medicine approaches. By advancing our understanding of the molecular mechanisms underlying mechanotransduction and leveraging new technologies, we can create targeted therapies that significantly enhance rehabilitation outcomes and promote long-term knee joint health.
Discussion
The review underscores the importance of understanding mechanotransduction in developing effective rehabilitation strategies. Controlled mechanical loading can stimulate beneficial cellular responses, enhancing tissue repair and preventing further degeneration. Rehabilitation protocols must be tailored to individual patient needs, considering the type, magnitude, frequency, and duration of mechanical stimuli.
Mechanotransduction involves a complex interplay between mechanical forces and cellular responses. When mechanical forces are applied to tissues, they cause deformation at the cellular level, which is detected by mechanoreceptors on the cell surface. These receptors then initiate a cascade of intracellular signaling pathways that influence gene expression, protein synthesis, and cellular behavior. This results in various biological responses, such as the proliferation of cells, production of extracellular matrix components, and remodeling of tissues. For instance, in the knee joint, mechanical loading can stimulate the production of collagen and other structural proteins by chondrocytes, enhancing the repair and strength of damaged cartilage.
At the molecular level, mechanotransduction is mediated by several key molecules and signaling pathways. Integrins, which are transmembrane receptors, play a pivotal role in sensing mechanical forces and transmitting signals into the cell. When integrins bind to extracellular matrix (ECM) components, they form focal adhesions, which are complexes that link the ECM to the cytoskeleton. This connection allows mechanical forces to be transmitted across the cell membrane, leading to the activation of intracellular signaling pathways such as the mitogen-activated protein kinase (MAPK) pathway and the focal adhesion kinase (FAK) pathway. These pathways regulate various cellular processes, including gene expression, cell proliferation, and apoptosis, which are critical for tissue repair and adaptation.
Another important aspect of mechanotransduction is the role of ion channels, such as stretch-activated ion channels and piezo channels, which respond to mechanical stimuli by altering ion flux across the cell membrane. These channels can modulate intracellular calcium levels, which serve as a secondary messenger in various signaling pathways. Increased intracellular calcium can activate calcium-dependent proteins such as calmodulin and calcineurin, which further influence cellular responses to mechanical loading.
In the context of knee rehabilitation, mechanotransduction can be harnessed through carefully designed exercise programs and therapeutic interventions. Controlled loading, for example, involves applying specific mechanical forces in a gradual and progressive manner to stimulate cellular responses without causing additional harm. This approach is fundamental in guiding tissue repair processes, promoting the formation of healthy tissue, and preventing the development of excessive scar tissue or fibrosis.
Additionally, mechanotransduction is influenced by the type, magnitude, frequency, and duration of mechanical stimuli. Different tissues and cells respond uniquely to various mechanical environments. Therefore, rehabilitation protocols must be tailored to the specific needs of the patient and the nature of the injury. For example, weight-bearing exercises can be beneficial for bone and cartilage health, while non-weight-bearing exercises might be more suitable during the early stages of recovery when minimizing joint stress is crucial.
Moreover, the timing of mechanical loading is crucial. Early mechanical loading, introduced soon after injury or surgery, can enhance tissue repair and functional recovery. This approach, however, must be carefully managed to avoid overloading and potential re-injury. Gradual progression in mechanical loading ensures that tissues adapt appropriately, enhancing their resilience and function over time.
At a molecular level, early mechanical loading can activate growth factors such as transforming growth factor-beta (TGF-β) and insulin-like growth factor 1 (IGF-1), which are vital for tissue regeneration and repair. TGF-β, for instance, plays a crucial role in ECM production and remodeling, while IGF-1 promotes cellular proliferation and differentiation. The activation of these growth factors through mechanotransduction pathways underscores the importance of mechanical stimuli in driving effective rehabilitation outcomes.
By leveraging the principles of mechanotransduction, rehabilitation strategies can be optimized to promote effective knee joint recovery. This involves a comprehensive understanding of the biological responses to mechanical stimuli and the careful application of controlled mechanical forces to stimulate beneficial cellular responses. Such strategies not only facilitate tissue repair and adaptation but also contribute to the overall functional recovery of the knee joint, improving patient outcomes and quality of life.
Understanding the molecular biology underpinning mechanotransduction allows for more targeted and effective rehabilitation interventions. For instance, identifying specific signaling molecules and pathways involved in mechanotransduction can lead to the development of novel therapeutic agents or techniques that enhance the body’s natural repair mechanisms. Furthermore, advancements in molecular biology, such as gene editing and stem cell therapy, hold promise for improving rehabilitation outcomes by directly influencing the cellular processes involved in tissue repair and regeneration.
Conclusion
Mechanotransduction plays a pivotal role in the knee joint’s response to mechanical loading, serving as a fundamental mechanism by which cells sense and respond to mechanical stimuli. Understanding the cellular and molecular mechanisms underlying this process is essential for developing effective rehabilitation strategies. This knowledge allows clinicians to harness the body’s natural healing processes, facilitating tissue repair and regeneration while minimizing the risk of further injury.
At the molecular level, mechanotransduction involves a complex network of signaling pathways that are activated in response to mechanical stimuli. Integrins, which are transmembrane receptors, play a crucial role in this process by connecting the extracellular matrix (ECM) to the cytoskeleton inside the cell. When mechanical forces are applied to the knee joint, integrins undergo conformational changes, clustering together and recruiting various intracellular proteins such as focal adhesion kinase (FAK) and paxillin. This clustering leads to the activation of multiple downstream signaling cascades, including the MAPK (mitogen-activated protein kinase) and PI3K-Akt (phosphoinositide 3-kinase-protein kinase B) pathways.
The MAPK pathway, which includes ERK (extracellular signal-regulated kinase), JNK (c-Jun N-terminal kinase), and p38 kinases, regulates gene expression, cellular growth, and differentiation. Activation of this pathway leads to the transcription of genes encoding ECM proteins, such as collagen and fibronectin, which are critical for tissue repair and structural integrity. Simultaneously, the PI3K-Akt pathway promotes cell survival and growth by inhibiting apoptosis (programmed cell death) and stimulating protein synthesis through mTOR (mechanistic target of rapamycin) activation. These pathways collectively contribute to the production of a more organized and functional extracellular matrix, enhancing tissue repair and regeneration.
By leveraging controlled loading, exercise therapy, manual therapy, and orthotic devices, clinicians can optimize knee joint recovery and prevent long-term degeneration. Controlled mechanical loading, applied at appropriate stages of rehabilitation, can stimulate the production of ECM components, enhance tissue organization, and prevent the formation of excessive scar tissue. Exercise therapy, tailored to the patient’s specific needs, can further reinforce these effects by promoting muscle strength, joint stability, and overall functional improvement. Exercise-induced mechanical stimuli continue to activate mechanotransductive pathways, sustaining and enhancing the initial benefits achieved through controlled loading.
Manual therapy complements these approaches by providing targeted mechanical stimuli that activate mechanotransductive pathways, reduce inflammation, and enhance synovial fluid production, thereby improving joint lubrication and mobility. The mechanical forces applied during manual therapy can modulate the expression of cytokines and growth factors involved in inflammation and healing. For instance, anti-inflammatory cytokines such as IL-10 and growth factors like TGF-β are upregulated, promoting tissue regeneration and repair. The integration of manual therapy with exercise regimens ensures a comprehensive approach to rehabilitation, addressing both the mechanical and biochemical aspects of knee joint health.
Orthotic devices, such as braces and insoles, play a crucial role in supporting and stabilizing the knee joint during the recovery process. These devices can be designed to provide optimal mechanical alignment, reduce undue stress on the joint, and facilitate controlled loading. By ensuring proper joint mechanics, orthotic devices help prevent further injury and promote a more effective healing environment.
In conclusion, a thorough understanding of mechanotransduction and its impact on knee joint physiology is essential for developing advanced rehabilitation protocols. By combining controlled loading, exercise therapy, manual therapy, and orthotic devices, clinicians can create a multifaceted approach that maximizes the potential for recovery and minimizes the risk of long-term joint degeneration. These strategies, grounded in molecular and cellular biology, offer a promising pathway to improving outcomes for individuals recovering from knee injuries and enhancing their overall quality of life. The future of knee rehabilitation lies in the integration of these approaches, supported by ongoing research into the molecular mechanisms that drive tissue repair and regeneration.
References
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J Cell Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, L.; Wang, H. Mechanobiology of chondrocytes and articular cartilage. J Biomech. 2017, 50, 59–70. [Google Scholar]
- Wang, P.; Zhu, F.; Konstantopoulos, K. Probing the molecular mechanisms of mechanotransduction using single-cell techniques. Trends Biotechnol. 2018, 36, 347–359. [Google Scholar]
- Mow, V.C.; Huiskes, R. Basic Orthopaedic Biomechanics & Mechano-biology. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- Mak, A.F. Unraveling the puzzles of mechanobiology in bone and cartilage: how much do we know? Biorheology. 2004, 41, 401–415. [Google Scholar]
- Lee, D.A.; Noguchi, T.; Knight, M.M. Response of chondrocyte subpopulations cultured within unloaded and loaded agarose. J Orthop Res. 2000, 18, 725–732. [Google Scholar] [CrossRef]
- Fitzgerald, J.B.; Jin, M.; Grodzinsky, A.J. Shear and compression differentially regulate clusters of functionally related temporal transcription patterns in cartilage tissue. J Biol Chem. 2006, 281, 24095–24103. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Platsoucas, C.D. The role of T cells in the pathogenesis of osteoarthritis. Arthritis Rheum. 2007, 56, 409–424. [Google Scholar] [CrossRef]
- Lee, A.S.; Ellman, M.B.; Yan, D. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013, 527, 440–447. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Shen, S. The inflammatory response in the pathogenesis of osteoarthritis. J Cell Physiol. 2017, 232, 3157–3165. [Google Scholar]
- Rhee, D.K.; Marcelino, J.; Baker, M. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005, 115, 622–631. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Mankin, H.J. Articular cartilage: Tissue design and chondrocyte-matrix interactions. Instr Course Lect. 1998, 47, 477–486. [Google Scholar] [PubMed]
- Wang, J.H.C.; Thampatty, B.P. An introductory review of cell mechanobiology. Biomech Model Mechanobiol. 2006, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.R. Collagen of articular cartilage. Arthritis Res. 2002, 4, 30–35. [Google Scholar] [CrossRef]
- Knudson, C.B.; Knudson, W. Cartilage proteoglycans. Semin Cell Dev Biol. 2001, 12, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M. Osteoarthritis. Nat Rev Dis Primers. 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Butler, D.L.; Goldstein, S.A. Biomechanics and mechanobiology in functional tissue engineering. J Biomech. 2014, 47, 1933–1940. [Google Scholar] [CrossRef]
- Little, C.B.; Smith, M.M. Animal models of osteoarthritis. Curr Rheumatol Rev. 2008, 4, 232–240. [Google Scholar] [CrossRef]
- Findlay, D.M.; Kuliwaba, J.S. Bone-cartilage crosstalk: a conversation for understanding osteoarthritis. Bone Res. 2016, 4, 16028. [Google Scholar] [CrossRef]
- Andarawis-Puri, N.; Flatow, E.L. Tendon fatigue in response to mechanical loading. J Musculoskelet Neuronal Interact. 2011, 11, 106–114. [Google Scholar]
- Screen, H.R.C.; Berk, D.E.; Kadler, K.E. Tendon functional extracellular matrix. J Orthop Res. 2015, 33, 793–799. [Google Scholar] [CrossRef]
- Docking, S.I.; Cook, J. Pathological tendons maintain sufficient aligned fibrillar structure on ultrasound tissue characterization (UTC). Scand J Med Sci Sports. 2015, 25. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Klein, J.D.; Luo, Q. Treadmill exercise counteracts the suppression of bone formation and osteocyte Wnt/β-catenin signaling in a mouse model of type 1 diabetes. Bone. 2016, 82, 30–38. [Google Scholar]
- Arno, S.; Hadley, S.; Campbell, K.A. The effect of shear stress on the mechanobiology of the meniscus. J Biomech. 2017, 55, 80–87. [Google Scholar]
- Nugent, G.E.; Schmidt, T.A.; Schumacher, B.L. Static and dynamic compression regulate cartilage metabolism of proteoglycans and prostaglandin E2. J Orthop Res. 2006, 24, 116–125. [Google Scholar]
- Jang, K.W.; Kim, S.W.; Shin, D.H. Effect of hydrostatic pressure on human chondrocytes and rat chondrosarcoma cells. Integr Biol (Camb). 2014, 6, 70–80. [Google Scholar]
- Silbernagel, K.G.; Thomeé, R.; Eriksson, B.I. Full symptomatic recovery does not ensure full recovery of muscle-tendon function in patients with Achilles tendinopathy. Br J Sports Med. 2007, 41, 276–280. [Google Scholar] [CrossRef]
- Gomez-Barrena, E.; Rosset, P.; Lozano, D. Bone fracture healing: cell therapy in delayed unions and nonunions. Bone. 2015, 70, 93–101. [Google Scholar] [CrossRef]
- O’Neill, T.W.; McCabe, P.S.; McBeth, J. Update on the epidemiology, risk factors and disease outcomes of osteoarthritis. Best Pract Res Clin Rheumatol. 2018, 32, 312–326. [Google Scholar] [CrossRef]
- Hurley, M.V.; Scott, D.L. Improvements in quadriceps sensorimotor function and disability of patients with knee osteoarthritis following a clinically practicable exercise regime. Br J Rheumatol. 1998, 37, 1181–1187. [Google Scholar] [CrossRef]
- Roos, E.M.; Juhl, C.B. Osteoarthritis 2012 year in review: rehabilitation and outcomes. Osteoarthritis Cartilage. 2012, 20, 1477–1483. [Google Scholar] [CrossRef]
- Bennell, K.L.; Hunter, D.J.; Hinman, R.S. Management of osteoarthritis of the knee. BMJ. 2012, 345. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; McConnell, S.; Harmer, A.R. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.L.; Persson, M.S.M.; Stocks, J. Relative efficacy of different types of exercise for treatment of knee and hip osteoarthritis: network meta-analysis. Ann Rheum Dis. 2019, 78, 810–819. [Google Scholar]
- Hermann, W.; Lambova, S.; Muller-Ladner, U. Current treatment options for osteoarthritis. Curr Rheumatol Rev. 2018, 14, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Page, P. Current concepts in muscle stretching for exercise and rehabilitation. Int J Sports Phys Ther. 2012, 7, 109–119. [Google Scholar]
- Bialosky, J.E.; George, S.Z.; Horn, M.E. The influence of noxious stimulation and spinal manipulation on the central processing of pain-related information: a systematic review of functional brain imaging studies. J Pain. 2009, 10, 441–456. [Google Scholar]
- Brantingham, J.W.; Cassa, T.K.; Bonnefin, D. Manipulative therapy for lower extremity conditions: expansion of literature review. J Manipulative Physiol Ther. 2012, 35, 127–166. [Google Scholar] [CrossRef]
- Collins, N.J.; Misra, D.; Felson, D.T. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Ou. Arthritis Care Res (Hoboken). 2011, 63 (Suppl 11). [Google Scholar] [CrossRef]
- Hunter, D.J.; Schofield, D.; Callander, E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014, 10, 437–441. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Altman, R.D.; April, K.T. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012, 64, 465–474. [Google Scholar] [CrossRef]
- Bennell, K.L.; Kyriakides, M.; Metcalf, B. Neuromuscular versus quadriceps strengthening exercise in patients with medial knee osteoarthritis and varus malalignment: a randomized controlled trial. Arthritis Rheumatol. 2014, 66, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Crossley, K.M.; Stefanik, J.J.; Selfe, J. Patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester. Part 1: Terminology, definitions, clinical examination, natural history, patellofemoral osteoarthritis and patient-reported outcome measures. Br J Sports Med. 2016, 50, 839–843. [Google Scholar] [PubMed]
- Thorp, A.A.; Sumner, D.R.; Wimmer, M.A. The biomechanical role of the patella and implications for total knee arthroplasty without patellar resurfacing. J Bone Joint Surg Am. 2006, 88 (Suppl 4), 112–117. [Google Scholar]
- Rönn, K.; Reischl, N.; Gautier, E. Current surgical treatment of knee osteoarthritis. Arthritis. 2011, 2011, 454873. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Bejarano, T.; Novo, M. Current interventions in the management of knee osteoarthritis. J Pharm Bioallied Sci. 2013, 5, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L. Osteoarthritis year in review 2015: clinical. Osteoarthritis Cartilage. 2016, 24, 36–48. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med Clin North Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Alentorn-Geli, E.; Cugat, R.; Álvarez-Díaz, P. Return to play after anterior cruciate ligament injuries in elite soccer players. Knee Surg Sports Traumatol Arthrosc. 2009, 17, 812–820. [Google Scholar]
- Felson, D.T. Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage. 2013, 21, 10–15. [Google Scholar] [CrossRef]
- Simão, A.P.; Mendonça, V.A.; Santos, J.M. Inflammatory and angiogenic biomarkers in patients with ischemic stroke. J Stroke Cerebrovasc Dis. 2013, 22, 340–346. [Google Scholar]
- Tonge, D.P.; Pearson, M.J.; Jones, S.W. The hallmarks of osteoarthritis and the potential to develop personalised disease-modifying pharmacological therapeutics. Osteoarthritis Cartilage. 2014, 22, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Adams, J.; Leddy, H.A.; McNulty, A.L. The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Curr Rheumatol Rep. 2014, 16, 451. [Google Scholar] [CrossRef] [PubMed]
- Nuki, G.; Salter, D.M. The impact of mechanical stress on the pathophysiology of osteoarthritis. In: Brandt KD, Lohmander LS, Doherty M, eds. Osteoarthritis. 2nd ed. Oxford: Oxford University Press; 2003; 49–62. [Google Scholar]
- Orozco, R.; Soler, R.; Morera, C. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011, 92, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Uitterlinden, E.J.; Jahr, H.; Koevoet, J.L. Glucosamine increases hyaluronic acid production in human synovium explants. Osteoarthritis Cartilage. 2006, 14, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Henrotin, Y.; Biesalski, H.K. Scientific evidence and rationale for the development of curcumin and resveratrol as nutraceuticals for joint health. Int J Mol Sci. 2012, 13, 4202–4232. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Guermazi, A.; Guehring, H. Intra-articular sprifermin in symptomatic radiographic knee osteoarthritis: Results of the 2-year randomised controlled FORWARD trial. Ann Rheum Dis. 2019, 78, 1022–1031. [Google Scholar]
- Loeser, R.F. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009, 17, 971–979. [Google Scholar] [CrossRef]
- Pelletier, J.P.; Martel-Pelletier, J.; Abramson, S.B. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001, 44, 1237–1247. [Google Scholar] [CrossRef]
- Sandell, L.J. Etiology of osteoarthritis: genetics and synovial joint development. Nat Rev Rheumatol. 2012, 8, 77–89. [Google Scholar] [CrossRef]
- Calvet, J.; Orellana, C.; Larrosa, M. Assessing changes in bone microstructure in patients with knee osteoarthritis using calcaneal quantitative ultrasound. Clin Exp Rheumatol. 2014, 32, 209–215. [Google Scholar]
- Lane NE, Nevitt MC, Arden NK. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage. 2011, 19, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Goldring, M.B. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004, 427. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B. Pathogenesis and treatment of osteoarthritis. Med Clin North Am. 2010, 94, 455–492. [Google Scholar] [CrossRef]
- Cowin AJ, Holmes TM, Brosnan P, Ferguson MW. “Expression of TGF-beta and its receptors in murine fetal and adult dermal wounds.” Eur J Dermatol. 2001, 11, 424–31.
- Wild T, Rahbarnia A, Kellner M, Sobotka L, Eberlein T. “Basics in nutrition and wound healing.” Nutrition. 2010, 26, 862–6.
- Agren MS, Steenfos HH, Dabelsteen S, et al. “Proliferation and mitogenic response to PDGF-BB of fibroblasts isolated from chronic venous leg ulcers is ulcer-age dependent.” J Invest Dermatol. 1999, 112, 463–9.
- Baum CL, Arpey C. “Normal cutaneous wound healing: clinical correlation with cellular and molecular events.” Dermatol Surg. 2005, 31, 674–86.
- Bello YM, Phillips TJ. “Recent advances in wound healing.” JAMA. 2000, 283, 716–8.
- Braun Falco O, Plewig G, Wolff HH, Burgdorf WHC, Landthaler M. “Dermatologie und venerologie.” 5th ed. Heidelberg: Springer; 2005; 13.
- Bucalo B, Eaglestein WH, Falanga V. “Inhibition of cellular proliferation by chronic wound fluid.” Wound Rep Reg. 1993, 1, 181–6.
- Bullen EC, Longaker MT, Updike DL, et al. “TIMP-1 is decreased and activated gelatinases are increased in chronic wounds.” J Invest Dermatol. 1995, 104, 236–40.
- Clark RAF. “Cutaneous tissue repair. Basic biologic considerations.” J Am Acad Dermatol. 1985, 13, 701–25. [Google Scholar]
- Cowin AJ, Hatzirodos N, Holding CA, et al. “Effect of healing on the expression of transforming growth factor βs and their receptors in chronic venous leg ulcers.” J Invest Dermatol. 2001, 117, 1282–9.
- Desmoulière A, Chaponnier C, Gabbiani G. “Tissue repair, contraction, and the myofibroblast.” Wound Rep Reg. 2005, 13, 7–12.
- Dissemond, J. “Wann ist eine Wunde chronisch? ” Hautarzt. 2006, 57, 55. [Google Scholar] [CrossRef]
- Enoch S, Price P. “Cellular, molecular and biochemical differences in the pathophysiology of healing between acute wounds, chronic wounds and wounds in the aged.” World Wide Wounds. 2004.
- Falanga, V. “Mechanisms of cutaneous wound repair.” In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Fitzpatrick’s dermatology in general medicine. 6th ed. New York: McGraw-Hill Medical Publishing Division; 2003; 236–246. [Google Scholar]
- Falanga V, Shen J. “Growth factors, signal transduction and cellular responses.” In: Falanga V, editor. Cutaneous wound healing. London: Martin Dunitz; 2001; 81–93.
- Biomedicines. “Cellular and Molecular Processes in Wound Healing.” MDPI.
- Scar Formation: Cellular Mechanisms. SpringerLink.
- “From Wound to Scar: Scarring Explained—Pathophysiology of Wound Healing.” SpringerLink.
- Goldring, M.B. Update on the biology of the chondrocyte and new approaches to treating joint diseases. Best Pract Res Clin Rheumatol. 2006, 20, 1003–1025. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Boileau, C.; Pelletier, J.P.; Roughley, P.J. Cartilage biology, pathology, and repair. Best Pract Res Clin Rheumatol. 2008, 22, 351–384. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Bais M, McLean J, Sebastiani P, et al. Transcriptional analysis of fracture healing and the induction of embryonic stem cell-related genes. PLoS One. 2009, 4. [Google Scholar] [CrossRef]
- Takahata Y, Hagino H, Kimura A, et al. Regulatory Mechanisms of Prg4 and Gdf5 Expression in Articular Cartilage and Functions in Osteoarthritis. Int J Mol Sci. 2022, 23, 4672. [Google Scholar] [CrossRef]
- Tramś, E.; Kamiński, R. Molecular Biology of Meniscal Healing: A Narrative Review. Int J Mol Sci. 2024, 25, 768. [Google Scholar] [CrossRef]
- Derfoul, A.; Miyoshi, A.D.; Freeman, D.E.; Tuan, R.S. Glucosamine promotes chondrogenic phenotype in both chondrocytes and mesenchymal stem cells and inhibits MMP-13 expression and matrix degradation. Osteoarthritis Cartilage. 2007, 15, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Xiao, R.; Li, J.B.; Zhu, Q. Regenerative approaches for cartilage repair in the treatment of osteoarthritis. Osteoarthritis Cartilage. 2017, 25, 1577–1587. [Google Scholar] [CrossRef]
- Skazny AV, Aschner M, Zhang F, et al. Molecular mechanisms of environmental pollutant-induced cartilage damage: from developmental disorders to osteoarthritis. Arch Toxicol. [CrossRef]
- Browne, J.E.; Branch, T.P. Surgical alternatives for treatment of articular cartilage lesions. J Am Acad Orthop Surg. 2000, 8, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer P, Albrecht D, Andereya S, et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: a guideline by the working group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Trauma (DGOU). Knee. 2016, 23, 426–435. [Google Scholar] [CrossRef]
- Zhang Z, Zhong X, Ji H, et al. Matrix-induced autologous chondrocyte implantation for the treatment of chondral defects of the knees in Chinese patients. Drug Des Dev Ther. 2014, 8, 2439–2448. [Google Scholar] [CrossRef]
- Mandelbaum B, Browne JE, Fu F, et al. Treatment outcomes of autologous chondrocyte implantation for full-thickness articular cartilage defects of the trochlea. Am J Sports Med. 2007, 35, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, R.M.; Gibson, J.; Xu, R.H.; Lee, F.Y.; Drissi, H. Efficient differentiation of human iPSC-derived mesenchymal stem cells to chondroprogenitor cells. J Cell Biochem. 2013, 114, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Ohnuki, M.; Takahashi, K. Present and future challenges of induced pluripotent stem cells. Philos Trans R Soc B. 2015, 370, 20140367. [Google Scholar] [CrossRef]
- Scott, A.; Backman, L.J.; Speed, C. Tendinopathy: Update on Pathophysiology. J Orthop Sports Phys Ther. 2015, 45, 833–841. [Google Scholar] [CrossRef]
- Andarawis-Puri, N.; Flatow, E.L.; Soslowsky, L.J. Tendon basic science: development, repair, regeneration, and healing. J Orthop Res. 2015, 33, 780–784. [Google Scholar] [CrossRef]
- Millar, N.L.; Murrell, G.A.; McInnes, I.B. Inflammatory mechanisms in tendinopathy: informing treatment strategies. J Orthop Sports Phys Ther. 2017, 47, 163–171. [Google Scholar] [CrossRef]
- Kjaer, M.; Langberg, H.; Heinemeier, K.; Bayer, M.L.; Hansen, M.; Holm, L. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports. 2009, 19, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.D.; Stride, M.; Scott, A. Tendons–time to revisit inflammation. Br J Sports Med. 2014, 48, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, L.A.; van der Meulen, M.C.; Bertram, J.E.; Starrak, G.S.; Nixon, A.J. Insulin-like growth factor-I improves cellular and molecular aspects of healing in a collagenase-induced model of flexor tendinitis. J Orthop Res. 2002, 20, 910–919. [Google Scholar] [CrossRef]
- Wang, J.H.; Thampatty, B.P.; Lin, J.S.; Im, H.J. Mechanoregulation of gene expression in fibroblasts. Gene. 2007, 391, 1–15. [Google Scholar] [CrossRef]
- Langberg, H.; Skovgaard, D.; Asp, S.; Kjær, M. Time pattern of exercise-induced changes in Type I collagen turnover after prolonged endurance exercise in humans. Calcif Tissue Int. 2000, 67, 41–44. [Google Scholar] [CrossRef]
- Svoboda, S.J.; Williams, R.N.; Patel, N.B.; Noonan, T.J. Partial medial collateral ligament injury: nonoperative management and return to play. Sports Health. 2012, 4, 349–354. [Google Scholar] [CrossRef]
- Griffin LY, Agel J, Albohm MJ, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000, 8, 141–150. [Google Scholar] [CrossRef]
- Wright, R.W.; Preston, E.; Fleming, B.C.; et al. A systematic review of anterior cruciate ligament reconstruction rehabilitation: part, I.I. Open chain exercises versus closed chain exercises. J Knee Surg. 2008, 21, 233–240. [Google Scholar] [CrossRef]
- Wilk, K.E.; Romaniello, W.T.; Soscia, S.M.; Arrigo, C.A.; Andrews, J.R. The relationship between subjective knee scores, isokinetic testing, and functional testing in the ACL-reconstructed knee. J Orthop Sports Phys Ther. 1994, 20, 60–73. [Google Scholar] [CrossRef]
- Howard, J.S.; Fazio, M.A.; Mattacola, C.G.; Uhl, T.L.; Jacobs, C.A. Structure, sex, and strength and knee and hip kinematics during landing. J Athl Train. 2011, 46, 376–385. [Google Scholar] [CrossRef]
- Hart, J.M.; Pietrosimone, B.G.; Hertel, J.; Ingersoll, C.D. Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010, 45, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Lepley, L.K.; Gribble, P.A.; Thomas, A.C.; Tevald, M.A.; Sohn, D.H.; Buckthorpe, M.W. Comprehensive impairment-based exercise testing following ACL reconstruction: A systematic review. Sports Med. 2015, 45, 1057–1085. [Google Scholar]
- Ulasli, A. M.; Ozcakar, L.; Murrel, W.D. ( Ultrasound imaging and guidance in the management of knee osteoarthritis in regenerative medicine field. Journal of Clinical Orthopaedics and Trauma 2019, 10, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Andia, I. , Maffulli, N. New biotechnologies for musculoskeletal injuries. The Surgeon 2019, 17, 244–255. [Google Scholar] [CrossRef]
- Roato, I. , et al. Concentrated adipose tissue infusion for the treatment of knee osteoarthritis: clinical and histological observations. International Orthopaedics 2019, 43, 15–23. [Google Scholar] [CrossRef]
- Hernigou, P. , et al. Subchondral stem cell therapy versus contralateral total knee arthroplasty for osteoarthritis following secondary osteonecrosis of the knee. International Orthopaedics 2018, 42, 2563–2571. [Google Scholar] [CrossRef]
- Bowman, E. N. , et al. Hyaluronic acid injections for osteoarthritis of the knee: predictors of successful treatment. International Orthopaedics 2018, 42, 733–740. [Google Scholar] [CrossRef]
- Norland, R. , et al. Opportunities for regenerative rehabilitation and advanced technologies in physical therapy: perspective from academia. Physical Therapy 2016, 96, 550–557. [Google Scholar] [CrossRef]
- Melo, S. N. S. , et al. Gold-induced cytokine (GOLDIC®) injection therapy in patient with plantar fasciosis: a case report. Indian Journal of Orthopaedics 2020, 54, 348–351. [Google Scholar] [CrossRef]
- Starinski, J. Biologics in wound healing: repair versus regeneration. Current Orthopaedic Practice 2016, 27, 490. [Google Scholar] [CrossRef]
- Ingber, D. E. Mechanotransduction: cells, matrices, and machines. ACS Biomaterials Science & Engineering 2018, 4, 2094–2107. [Google Scholar] [CrossRef]
- Wang, J. H. C. , et al. Mechanobiology of tendon and developmental mechanobiology of the Achilles tendon. Journal of Orthopaedic Research 2019, 37, 1969–1980. [Google Scholar] [CrossRef]
- Basak, S. , Gopinath, P. Mechanome-guided strategies in regenerative rehabilitation. Current Opinion in Biomedical Engineering 2023, 26, 100449. [Google Scholar] [CrossRef]
- Glatt, V. , et al. Regenerative rehabilitation: The role of mechanotransduction in orthopaedic regenerative medicine. Journal of Orthopaedic Research. [CrossRef]
- Duan, Y. , Li, C. The mechanotransduction signaling pathways in the regulation of osteogenesis. International Journal of Molecular Sciences 2023, 24, 14326. [Google Scholar] [CrossRef]
- Chiquet, M. , et al. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2009, 1793, 911–920. [Google Scholar] [CrossRef]
- Eyckmans, J. , et al. Nature and nurture: influence of biophysical and biochemical cues on lineage specification. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef]
- Hynes, R. O. Integrins: bidirectional, allosteric signaling machines. Cell 2009, 110, 673–687. [Google Scholar] [CrossRef]
- Geiger, B. , et al. Transmembrane extracellular matrix-cytoskeleton crosstalk. Nature Reviews Molecular Cell Biology 2009, 2, 793–805. [Google Scholar] [CrossRef]
- Rozario, T. , DeSimone, D. W. The extracellular matrix in development and morphogenesis: a dynamic view. Developmental Biology 2010, 341, 126–140. [Google Scholar] [CrossRef]
- Wang, H. B. , et al. Force-induced focal adhesion translocation: a mechanism for sensing and responding to mechanical signals. Journal of Cell Biology 2010, 133, 931–943. [Google Scholar] [CrossRef]
- Vogel, V. , Sheetz, M. Local force and geometry sensing regulate cell functions. Nature Reviews Molecular Cell Biology 2006, 7, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. H.-C. , et al. The influence of hydrogel matrix density on chondrocyte and MSC mechanotransduction in 3D culture. Journal of the Mechanical Behavior of Biomedical Materials 2011, 4, 602–609. [Google Scholar] [CrossRef]
- Maheshwari, G. , Ingber, D. E. Mechanobiology and tissue engineering: the role of the cytoskeleton in heart tissue development. Journal of Cardiovascular Pharmacology 2009, 33, S16–S20. [Google Scholar] [CrossRef]
- Martin, P. Wound healing—aiming for perfect skin regeneration. Science 2008, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. , Sharpless, D. S. Cellular and molecular mechanisms of mechanotransduction in chondrocytes. Clinical Orthopaedics and Related Research 2008, 427, S14–S24. [Google Scholar] [CrossRef]
- Ingber, D. E. Tensegrity: the architectural basis of cellular mechanotransduction. Annual Review of Physiology 2008, 59, 575–599. [Google Scholar] [CrossRef]
- Burridge, K. , Chrzanowska-Wodnicka, M. Focal adhesions, contractility, and signaling. Annual Review of Cell and Developmental Biology 2009, 12, 463–519. [Google Scholar] [CrossRef]
- Jaalouk, D. E. , Lammerding, J. Mechanotransduction gone awry. Nature Reviews Molecular Cell Biology 2009, 10, 63–73. [Google Scholar] [CrossRef]
- Discher, D. E. , et al. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Puklin-Faucher, E. , Sheetz, M. P. The mechanical integrin cycle. Journal of Cell Science 2009, 122, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Humphries, M. J. , et al. Integrin-ligand binding and activation: what is the molecular mechanism? ACS Chemical Biology 2006, 1, 311–315. [Google Scholar] [CrossRef]
- Curtis, A. , Wilkinson, C. Nantotechniques and approaches in biotechnology. Trends in Biotechnology 2001, 19, 97–101. [Google Scholar] [CrossRef]
- Immunomodulatory therapy in children with paediatric inflammatory multisystem syndrome related to SARS-CoV-2 (PIMS-TS): a randomized, controlled, open-label trial. Lancet Child Adolesc Health. 2024.
- Methylprednisolone versus intravenous immunoglobulins in PIMS-TS: a multicenter randomized trial. Lancet Child Adolesc Health. 2023.
- Cardiac assessment and inflammatory markers in children with PIMS-TS treated with steroids vs. immunoglobulins. EClinicalMedicine. 2023.
- Effect of anakinra, vs. usual care in mild-to-moderate COVID-19 pneumonia: a randomized trial. Lancet Respir Med. 2021. [Google Scholar]
- National consensus on managing paediatric inflammatory multisystem syndrome related to COVID-19: Delphi results. Lancet Child Adolesc Health. 2021.
- PIMS-TS as a new pediatric systemic inflammatory disease post-SARS-CoV-2. Int J Mol Sci. 2021.
- Metal nanoparticle hybrid hydrogels promoting wound healing: state-of-the-art. Theranostics. 2024.
- Novel therapies in tissue repair and inflammation management: Focus on chronic conditions. Ther Adv Chronic Dis. 2023.
- Role of cytokines in wound healing and tissue regeneration. Cell Mol Life Sci. 2022.
- Advances in fibroblast activation and ECM remodeling in wound healing. Matrix Biol. 2021.
- The impact of therapeutic exercises on the remodeling stage post-surgery. J Orthop Res. 2020.
- The role of laser therapy in inflammation and tissue repair. Lasers Med Sci. 2019.
Figure 1.
The figure illustrates the process of mechanotransduction in a runner’s leg, depicting how mechanical loading leads to changes in the extracellular matrix (ECM) and ultimately results in sustained or improved function. The sequence begins with mechanical loading (1) due to ground reaction force during running. This mechanical load propagates stress through the ECM from macro to micro scales (2). The ECM then interacts with cells through mechanotransduction (3), converting the mechanical signals into cellular responses. These signals induce new matrix synthesis and the degradation of damaged matrix components (4). The ECM undergoes incorporation and remodeling of new composition and structure (5), leading to sustained or improved function (6) of the tissue. The diagram highlights the dynamic interplay between mechanical forces and cellular responses in maintaining tissue health and function.
Figure 1.
The figure illustrates the process of mechanotransduction in a runner’s leg, depicting how mechanical loading leads to changes in the extracellular matrix (ECM) and ultimately results in sustained or improved function. The sequence begins with mechanical loading (1) due to ground reaction force during running. This mechanical load propagates stress through the ECM from macro to micro scales (2). The ECM then interacts with cells through mechanotransduction (3), converting the mechanical signals into cellular responses. These signals induce new matrix synthesis and the degradation of damaged matrix components (4). The ECM undergoes incorporation and remodeling of new composition and structure (5), leading to sustained or improved function (6) of the tissue. The diagram highlights the dynamic interplay between mechanical forces and cellular responses in maintaining tissue health and function.
Figure 2.
Knee joints a remarkable capacity to adapt to different types of mechanical loads, with the most well-documented changes occurring in response to tensile and compressive stresses. The musculoskeletal system experiences three primary types of mechanical loads: tension (where the tissue is stretched in one direction), compression (where the tissue is pressed in one or more directions), and shear (where the tissue is compressed while sliding). Knee soft tissues developing under tensile load show a dense, aligned matrix predominantly composed of type I collagen fibers. In contrast, musculoskeletal tissues subjected to compressive forces display a fibrocartilaginous phenotype characterized by sparsely connected, unaligned, and smaller type I collagen fibers along with larger proteoglycans. Knee joint tissues exposed to shear stress develop a partially aligned matrix and produce high levels of surface lubricating proteins such as lubricin, proteoglycan 4, and hyaluronic acid.
Figure 2.
Knee joints a remarkable capacity to adapt to different types of mechanical loads, with the most well-documented changes occurring in response to tensile and compressive stresses. The musculoskeletal system experiences three primary types of mechanical loads: tension (where the tissue is stretched in one direction), compression (where the tissue is pressed in one or more directions), and shear (where the tissue is compressed while sliding). Knee soft tissues developing under tensile load show a dense, aligned matrix predominantly composed of type I collagen fibers. In contrast, musculoskeletal tissues subjected to compressive forces display a fibrocartilaginous phenotype characterized by sparsely connected, unaligned, and smaller type I collagen fibers along with larger proteoglycans. Knee joint tissues exposed to shear stress develop a partially aligned matrix and produce high levels of surface lubricating proteins such as lubricin, proteoglycan 4, and hyaluronic acid.
Table 1.
This table outlines key components, functions, mechanotransduction and signaling pathways, molecular mechanisms, and clinical relevance of various cell types and tissues involved in joint health, including chondrocytes in cartilage, synoviocytes in synovial fluid, fibrochondrocytes in the meniscus, and fibroblasts in ligaments and tendons. Each cell type is associated with specific ECM components and signaling pathways that regulate their functions and responses to mechanical stress, highlighting their roles in maintaining tissue integrity, enabling nutrient exchange, and their potential as therapeutic targets for conditions like osteoarthritis, joint inflammation, and tissue injuries.
Table 1.
This table outlines key components, functions, mechanotransduction and signaling pathways, molecular mechanisms, and clinical relevance of various cell types and tissues involved in joint health, including chondrocytes in cartilage, synoviocytes in synovial fluid, fibrochondrocytes in the meniscus, and fibroblasts in ligaments and tendons. Each cell type is associated with specific ECM components and signaling pathways that regulate their functions and responses to mechanical stress, highlighting their roles in maintaining tissue integrity, enabling nutrient exchange, and their potential as therapeutic targets for conditions like osteoarthritis, joint inflammation, and tissue injuries.
| Topic |
Key Components |
Functions |
Mechanotransduction and Signaling Pathways |
Molecular Mechanisms |
Clinical Relevance |
| Chondrocytes and Cartilage |
Chondrocytes, ECM (Type II Collagen, Proteoglycans), Aggrecan, Minor Collagens (Type IX and XI), Non-collagenous Proteins (COMP) |
Cartilage integrity, nutrient diffusion, waste removal, structural support, tensile strength, compressive resistance |
Mechanoreceptors (Integrins, Mechanosensitive Ion Channels), MAPK Pathway (ERK, JNK, p38), NF-κB Pathway, Wnt Pathway, Ca²⁺ Signaling, HIFs (Hypoxia-inducible factors) |
Gene expression regulation, synthesis of ECM components, production of catabolic enzymes, inflammatory responses, epigenetic mechanisms (DNA methylation, histone modifications, miRNAs) |
Osteoarthritis, cartilage degeneration, potential therapeutic targets for cartilage repair |
| Synoviocytes and Synovial Fluid |
Synoviocytes (FLS and MLS), Synovial Fluid, Hyaluronic Acid, Lubricin |
Joint lubrication, friction reduction, nutrient provision, immune surveillance |
Mechanosensitive Receptors (Integrins, Stretch-activated Ion Channels), MAPK Pathway, NF-κB Pathway, PI3K/Akt Pathway |
Regulation of synovial fluid production, cytokine and growth factor signaling, ECM interaction, epigenetic regulation, EVs (Extracellular Vesicles) |
Joint lubrication, inflammation, cartilage degradation, therapeutic targets for enhancing joint lubrication and reducing inflammation |
| Fibrochondrocytes and Meniscus |
Fibrochondrocytes, ECM (Type I and II Collagen, Proteoglycans, Fibronectin, Elastin, Decorin) |
Load distribution, shock absorption, joint stability, tensile strength, compressive resistance |
Mechanoreceptors (Integrins, Mechanosensitive Ion Channels), MAPK Pathway (ERK, JNK, p38), NF-κB Pathway, Wnt Pathway, Ca²⁺ Signaling |
ECM synthesis and organization, gene expression regulation, inflammatory responses, epigenetic mechanisms (DNA methylation, histone modifications, miRNAs) |
Meniscal injuries, degenerative conditions, potential therapeutic targets for meniscal repair and regeneration |
| Fibroblasts and Ligaments/Tendons |
Fibroblasts, ECM (Type I Collagen), Growth Factors (FGF, PDGF), Cytokines |
Tissue strength, elasticity, joint stability, force transmission |
Integrins, Focal Adhesion Complexes, Mechanosensitive Ion Channels, MAPK Pathway (ERK, JNK, p38), TGF-β Pathway, Ca²⁺ Signaling |
Collagen synthesis and matrix remodeling, gene expression regulation, response to hypoxia, ECM stiffness, epigenetic mechanisms (DNA methylation, histone modifications, miRNAs) |
Tendinopathy, ligament injuries, potential therapeutic targets for enhancing tissue repair and regeneration |
Table 2.
The table summarizes the impact of mechanical forces on cartilage health and repair, detailing key components, functions, signaling pathways, molecular mechanisms, and clinical relevance for compression, tension, shear, and hydrostatic pressure. Each force type influences chondrocytes, ECM, and related pathways to promote tissue synthesis, reduce inflammation, and maintain cartilage elasticity, with clinical applications in osteoarthritis prevention, tendon and ligament repair, and regenerative medicine strategies.
Table 2.
The table summarizes the impact of mechanical forces on cartilage health and repair, detailing key components, functions, signaling pathways, molecular mechanisms, and clinical relevance for compression, tension, shear, and hydrostatic pressure. Each force type influences chondrocytes, ECM, and related pathways to promote tissue synthesis, reduce inflammation, and maintain cartilage elasticity, with clinical applications in osteoarthritis prevention, tendon and ligament repair, and regenerative medicine strategies.
| Topic |
Key Components |
Functions |
Mechanotransduction and Signaling Pathways |
Molecular Mechanisms |
Clinical Relevance |
| Compression |
Chondrocytes, ECM (Proteoglycans, Aggrecan, Type II Collagen), Integrins, Mechanosensitive Ion Channels |
Promotes proteoglycan synthesis, inhibits catabolic enzymes, maintains cartilage elasticity |
Integrins, Mechanosensitive Ion Channels (PIEZO1, TRPV4), MAPK Pathway (ERK, p38, JNK), NF-κB Pathway, Ca2+ Signaling |
Proteoglycan and collagen synthesis, gene expression regulation, anabolic and catabolic balance, oxidative stress reduction, inflammatory response modulation, matrix degradation prevention |
Osteoarthritis prevention and treatment, cartilage health maintenance, therapeutic interventions for cartilage repair |
| Tension |
Fibroblasts, Tenocytes, ECM (Type I Collagen), Integrins, Focal Adhesion Complexes |
Promotes collagen synthesis and alignment, enhances tissue strength and repair |
Integrins, Focal Adhesion Complexes, MAPK Pathway (ERK, p38, JNK), FAK Signaling, TGF-β Pathway, Ca2+ Signaling |
Collagen synthesis and alignment, matrix remodeling, gene expression regulation, inflammatory response modulation, oxidative stress response, mechanosensitive transcription factors (YAP/TAZ), metabolic adaptation |
Tendon and ligament repair, tendinopathy prevention, therapeutic loading protocols, regenerative medicine strategies |
| Shear |
Chondrocytes, Synoviocytes, ECM (Proteoglycans, Type II Collagen), Integrins, Mechanosensitive Ion Channels |
Regulates synovial fluid dynamics, promotes matrix synthesis, maintains cartilage health |
Integrins, Cytoskeleton, FAK Signaling, MAPK Pathway (ERK, p38, JNK), Wnt Pathway, Nitric Oxide (NO) Signaling |
Gene expression regulation, proteoglycan and collagen synthesis, NO production, inflammatory mediator regulation (PGE2, NO), apoptotic response modulation, extracellular matrix (ECM) remodeling, intercellular communication via extracellular vesicles (EVs) |
Osteoarthritis prevention and treatment, cartilage health maintenance, therapeutic interventions for cartilage repair, synovial fluid enhancement |
| Hydrostatic Pressure |
Chondrocytes, ECM (Proteoglycans, Type II Collagen), Synoviocytes, Integrins, Ion Channels |
Promotes chondrocyte metabolism and matrix synthesis, reduces inflammation and oxidative stress |
Integrins, Ion Channels (TRPV4, PIEZO1), PI3K/Akt Pathway, ERK Pathway, Ca2+ Signaling, Nrf2 Pathway |
Anabolic activity promotion, inflammation reduction, oxidative stress mitigation, extracellular matrix (ECM) synthesis, metabolic activity enhancement, mitochondrial function improvement, antioxidant enzyme upregulation, anti-inflammatory cytokine regulation |
Aquatic therapy, osteoarthritis management, cartilage repair and regeneration, joint function improvement, synovial fluid enhancement |
Table 3.
The table outlines the healing stages of inflammation, fibroblastic, and remodeling, detailing cellular processes, biophysical characteristics, and therapeutic interventions. During inflammation, vasodilation and inflammatory cell invasion cause swelling and pain, treated with cryotherapy and NSAIDs; the fibroblastic stage involves growth factor-driven ECM synthesis, managed with manual therapy and therapeutic exercises; and the remodeling stage focuses on ECM organization and mechanical property enhancement, requiring tailored manual therapy and exercises to restore function and strength.
Table 3.
The table outlines the healing stages of inflammation, fibroblastic, and remodeling, detailing cellular processes, biophysical characteristics, and therapeutic interventions. During inflammation, vasodilation and inflammatory cell invasion cause swelling and pain, treated with cryotherapy and NSAIDs; the fibroblastic stage involves growth factor-driven ECM synthesis, managed with manual therapy and therapeutic exercises; and the remodeling stage focuses on ECM organization and mechanical property enhancement, requiring tailored manual therapy and exercises to restore function and strength.
| Healing stage |
Cellular phase |
Biophysical characteristics |
Therapeutic intervention |
| Inflammation Stage |
Vasodilation, invasion of platelets, and inflammatory cells (neutrophils, monocytes, and macrophages) are crucial processes in the body’s response to injury [145,146]. These events are orchestrated by a complex interplay of chemical mediators, including histamine, bradykinin, and PGE2, each playing specific roles at the molecular level to injury, facilitating effective tissue repair and restoration of function [147,148].
|
Swelling, erythema, warmth, pain |
Cryotherapy, preferably with compression
NSAIDs (unless contraindicated)
Manual therapy |
| The strength of the scar depends on the temporary clot and stitches |
Methods: electrical stimulation, laser therapy, ultrasound, PEMF, ESWT, isometric and BFR training, electroacupuncture, EPTE |
Fibroblastic stage.
|
Growth factors such as Transforming Growth Factor-beta 1 (TGF-β1), Bone Morphogenetic Proteins (BMP), and Connective Tissue Growth Factor (CTGF) play critical roles in wound healing by activating fibroblastic cells [149,150]. Upon activation, these fibroblastic cells undergo proliferation and upregulate the synthesis of extracellular matrix (ECM) components including collagen, fibronectin, and proteoglycans [151,152].
|
Expression of inflammatory markers |
Manual therapy: passive range of motion, soft tissue mobilization, joint mobilization |
| The scar begins to gain tensile strength |
Methods: electrical stimulation, laser therapy, ultrasound, PEMF, ESWT, electroacupuncture, EPTE
Therapeutic exercises: prescribed to achieve the goal of full weight bearing on the surgical limb while protecting the tissues (slow eccentric tempo), BFR training |
Remodelling stage.
|
The remodeling of the scar improves the organization and mechanical properties of the extracellular matrix (ECM) through a dynamic process involving the coordinated activity of various cells, enzymes, and signaling pathways [153,154]. Fibroblasts and myofibroblasts play key roles in this process by synthesizing and remodeling collagen and other ECM components [155,156].
|
The inflammation should subside; pain, if present, may be due to osteoarthritis, DOMS, re-damage to healing tissue |
Manual therapy depending on needs, based on the patient’s assessment of the operated limb and the rest of the body; passive and active range of motion, soft tissue mobilization, including scar mobilization, joint mobilization |
Methods: Typically discontinued at this stage unless patient assessment indicates special requirements for the surgical limb or rest of the body
Therapeutic exercises: prescribed to increase active ROM and flexibility, build muscle strength and endurance, improve proprioception, motor control, and improve cardiovascular fitness |
| Abbreviations: BMP, bone morphogenetic protein; CTGF, connective tissue growth factor; DOMS, delayed onset muscle soreness; ECM, extracellular matrix; ESWT, extracorporeal shock wave therapy; NSAIDs, non-steroidal anti-inflammatory drugs; PEMF, pulsed electromagnetic field therapy; BFR, blood flow restriciton; EPTE, percutaneous electrolysis therapy; PGE2, prostaglandin E2; ROM, range of motion; TGF-β1, transforming growth factor-β1. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).