You are currently viewing a beta version of our website. If you spot anything unusual, kindly let us know.
Preprint
Article
Comparative Evaluation of Lipid Profile, C-reactive Protein and Paraoxonase-1 Activity in Dogs with Inflammatory Protein-Losing Enteropathy and Healthy Dogs
Altmetrics
Downloads
67
Views
31
Comments
0
This version is not peer-reviewed
Abstract
Chronic inflammation alters lipoprotein metabolism and causes changes in serum concentrations of lipids, C-reactive protein (CRP), and paraoxonase-1 activity (PON-1), an enzyme, which in the gastrointestinal tract may act as a local detoxifier, antioxidant, and immunomodulator. However, unlike in people with inflammatory bowel disease, scarce information is available in dogs with protein-losing enteropathy secondary to chronic inflammatory enteropathy (iPLE). The first aim was to describe and compare the lipid profile, CRP concentrations and PON-1 activity in healthy dogs and in dogs with iPLE. The second aim was to evaluate correlations among clinicopathological, histologic data, and lipid profiles in dogs with iPLE. Serum samples from 51 iPLE and 40 healthy dogs were used to study albumin, total protein, CRP, PON-1 activity, cholesterol, triglycerides, and lipoprotein classes obtained by electrophoretic separation. There was a significant difference of all serum analytes between iPLE and healthy dogs. Significant correlations between the lipid profile and the existing chronic enteropathy activity index were not found. Some lipoprotein classes correlated with CRP and PON-1. Triglycerides were significantly higher in dogs with both inflammation and lymphangiectasia. Lipoproteins, CRP and PON-1 activity are altered in dogs with iPLE. Results need to be confirmed in further studies.
Keywords:
Subject: Medicine and Pharmacology - Veterinary Medicine
1. Introduction
Canine protein-losing enteropathy (PLE) is a syndrome characterized by an abnormal loss of serum proteins through the gastrointestinal mucosa [1,2,3]. Numerous gastrointestinal diseases such as intestinal lymphangiectasia, lymphoma, regional fungal infections, and chronic inflammatory enteropathy, if severe enough, can lead to PLE [1,2,3]. In people, PLE is usually associated with primary intestinal lymphangiectasia (IL) [4]. In dogs, although a genetic susceptibility to the development of primary IL has been reported for some breeds, PLE is more commonly associated with secondary IL resulting from chronic inflammatory enteritis (CIE) [1,2,3,5,6,7,8,9]. Since lymphatics are the primary transporter of lipids, lipid-soluble vitamins, food antigens, bacteria-derived lipopolysaccharides, and gut hormones from the intestine to the blood, their dysfunction can contribute to the pathogenesis and progression of the intestinal inflammation [10]. The diagnostic workup of dogs with PLE is similar to that of dogs with CIE [2]. Although dietary therapy (often with low-fat or ultra low-fat formulations) alone is associated with a positive outcome, glucocorticoids (anti-inflammatory or immunosuppressive dosages), immunosuppressive agents, and supportive therapies are needed in some dogs with PLE [2,7,9,11,12,13,14,15,16]. Although prolonged survival can occur, PLE is often characterized by guarded prognosis and high rate of relapse [1,2,17,18,19].
Various lipid profile changes have been described in human patients with inflammatory bowel disease and could be mainly summarized by decreased levels of total cholesterol (Chol) and low-density lipoproteins (LDLs), variable levels of high-density lipoproteins (HDLs), and normal or increased levels of triglycerides (TGs) [20,21,22,23]. These changes are thought to be the result of a complex interaction of inflammatory cytokines with down-regulation of the lipolytic enzyme activity, malnutrition, and lipid malabsorption [24,25]. In addition, total and HDL-cholesterol levels are correlated with the systemic inflammatory status [26]; indeed, interleukin-6 and C-reactive protein (CRP), a biomarker of systemic inflammation, are closely related and together play a role in general lipid metabolism, inhibiting adipocyte lipoprotein lipase activity [27]. Finally, additional mediators such as paraoxonase-1 (PON-1), an HDL-bound antioxidant enzyme, which in the gastrointestinal tract may act as a local detoxifier, antioxidant, and immunomodulator, may be responsible for some changes within the lipid profile [28,29].
In veterinary medicine, serum and plasma lipoprotein profiles have been sporadically investigated both in healthy and diseased dogs [30,31,32,33,34,35]. Among healthy dogs, HDLs is commonly the predominant lipoproteins fraction, while lower percentages of LDLs and very low-density lipoproteins (VLDLs) are observed [36]. Among diseased dogs, the percentage of HDLs decreases in chronic kidney disease, nephrotic syndrome, babesiosis, leishmaniasis, and pancreatitis; while the percentage of VLDLs, LDLs and chylomicrons increases in brachycephalic syndrome, chronic kidney disease, nephrotic syndrome, diabetes mellitus, sepsis, and pancreatitis [31,32,33,34,37]. However, to the best of the authors’ knowledge, no information exists on the lipid profile in dogs with PLE secondary to chronic inflammatory enteropathy (iPLE). CRP, a nonspecific marker of inflammation, has been used in CIE and iPLE to select the best clinical approach at the onset of treatment, and to document disease progression and response to treatment [38,39,40,41]. Low serum PON-1 activity concentrations have already been demonstrated in some dogs with both acute and chronic inflammation, likely because an intense oxidation occurs [42,43]. However, to the best of the authors’ knowledge, no information exists on PON-1 activity in dogs with iPLE.
Based on these premises, the study aimed (i) to describe and compare the lipid profile, CRP e PON-1 activity between healthy dogs and dogs with iPLE, and (ii) to evaluate associations among clinicopathological data, histopathological findings, and lipid profile in dogs with iPLE.
2. Materials and Methods
2.1. Animals
This study was conducted on 51 left-over serum samples from privately-owned dogs that received a diagnosis of iPLE from January 2021 to March 2022 at the Veterinary Teaching Hospital, Department of Veterinary Sciences, University of Turin, Italy, and other referral clinics in northern and central Italy, and 40 left-over serum samples from healthy dogs. The study received the official approval of the Institutional Ethics and Animal Welfare Committee (protocol number 42/2021).
The criteria for the diagnosis of iPLE were: chronic gastrointestinal signs lasting for more than 3 weeks, hypoalbuminemia of gastrointestinal origin (≤ 2.8 g/dL), and histopathological evidence of benign gastrointestinal inflammation with or without lymphangiectasia on multiple biopsies collected by endoscopy [1,2,3,5,8]. The histopathologic examination, made in accordance with the histopathological standards of the World Small Animal Veterinary Association Gastrointestinal Standardization Group, was required [44]. In order to rule out infectious, parasitic, liver and pancreatic diseases, along with intestinal diseases of other etiology and extraintestinal diseases, the following diagnostic investigations were required: abdominal ultrasound examination, fecal flotation and giardia antigen-test, complete blood count, biochemistry, pre- and post-prandial bile acids, urinalysis, urinary protein to creatinine ratio, serum basal cortisol or ACTH stimulation test (if basal cortisol ≤ 2µg/dl), trypsin-like-immunoreactivity, pancreas specific lipase levels, serum folate and cobalamin concentrations. In order to confirm the gastrointestinal origin of hypoalbuminemia, dogs were also required to have no clinically relevant proteinuria (negative urine dipstick test result or urine protein to creatinine ratio ≤ 0.5) and no evidence of clinically relevant hepatic disease (normal pre- and post-prandial bile acid concentrations or normal synthetic liver function and enzyme activity). Exclusion criteria were complete and sustained response to dietary trials and gut microbiota manipulation (i.e., pre-, pro-, syn- and postbiotics, fecal microbiota transplantation and antibiotics), and a histopathologic diagnosis of neoplasia. All dogs underwent gastroduodenoscopy. Colonoscopy with ileal intubation was performed when possible. Biopsies from stomach, duodenum, and, when available, ileum and colon, were collected for histologic examination. The severity of morphologic and inflammatory lesions in the duodenum, ileum, and colon were recorded as follows: 0= normal, 1= mild, 2= moderate, 3= marked [44]. The mean cumulative lesion score calculated as the sum of individual lesion scores from each segment was considered for statistical analysis. Each dog was further assigned to group 1 or group 2 based on the presence of inflammation with only mild or no lacteal dilation, and inflammation with moderate or severe lacteal dilation, respectively.
Information gathered from the medical records on signalment, body weight (kg), and canine chronic enteropathy clinical activity index (CCECAI) score [45] was studied, in addition to the type of diet and ongoing anti-inflammatory or immunosuppressive therapies at admission. The CCECAI score was calculated using the serum albumin concentration, presence or absence of peripheral edema and peritoneal effusion on ultrasound examination, and the owner’s scores on appetite, activity level, vomiting, fecal consistency and frequency, weight loss and pruritus. Four disease severity groups were identified based on the CCECAI scores: mild disease (CCECAI 4-5; MI), moderate disease (CCECAI 6-8; MO), severe disease (CCECAI 9-11; S) and very severe disease (CCECAI ≥12; VS) [45].
The control group included healthy owned-dogs, regularly vaccinated, and receiving appropriate ecto-and endo-parasite preventive treatment. All healthy dogs were belonging to staff at the Veterinary Teaching Hospital, Department of Veterinary Sciences, University of Turin, Italy, or were presented at the same facilities of the study group for their annual check-up and vaccination. Dogs were considered healthy based on unremarkable history and physical examination, complete blood count and serum biochemistry, negative fecal flotation, and absence of any gastrointestinal sign within one year prior to enrollment. In addition, there was no history drug administration in the 6 months before.
2.2. Sample Collection, Lipid Profile, CRP, and PON-1 Activity
The serum left-over samples obtained from centrifugation of blood collected from each dog at admission and after 12h of fasting were separated and immediately stored at -80°C until analysis. Sample storage varied from 3 to 18 months.
All analyses were performed at the clinical pathology laboratory of the Veterinary Teaching Hospital of the University of Milan, Lodi, Italy. The evaluation of the lipid profile included Chol, TGs and lipoprotein classes. Frozen serum samples were thawed and used to measure serum concentrations of albumin (Alb), total proteins (TP), Chol, TGs, and CRP. All these analytes were measured on the automated chemistry analyzer BT 3500 (Biotecnica Instruments SPA, Rome, Italy) using reagents and methods provided by Futurlab Srl (Limena, PD, Italy). Specifically, Chol and TGs were measured with the colorimetric enzymatic CHOD-PAP and GPO-PAP methods, respectively. PON-1 activity was also measured on the same instrument, using the reagent and method validated in dogs, as previously described [42]. Based on serum albumin concentration, dogs were further assigned to group A (2.2 to 2.8 g/dL), B (1.5 to 2.19 g/dL), C (1.2 to 1.49 g/dL), and D (<1.2 g/dL). Lipoprotein analysis was carried out on the same serum samples on buffered (pH 8.5) agarose gel with a semi-automated instrument (Hydrasis, Sebia Italia S.r.l.), using kits produced by the manufacturer (Hydragel 15 lipoproteins). After migration (160 V, 25 min), agarose gels (8 g/L) were stained with Sudan black, washed with ethanol (45%), dried, and placed on the gel scanner for the densitometric analysis. Scanned images were analyzed using the software Phoresis (Sebia Italia S.r.l., Bagno a Ripoli, Italy) that calculates the area under the peaks corresponding to HDLs, VLDLs, LDLs and chylomicrons and expresses the results as a percentage of the total area (HDL%, VLDL%, LDL%, chylomicrons%).
2.3. Statistical Analysis
All data were analyzed with the software GraphPad Prism 9.5.1 (Dotmatics). Significance was set at p<0.05. Data were tested for normality by the Shapiro Wilk test. Data were reported as median, minimum, and maximum. Data were compared between healthy and diseased dogs by use of a Student t-test in case of normal distribution and Mann-Whitney U-test in case of non-normal distribution. All comparisons among groups were performed by use of One-way Anova test or Kruskal-Wallis test dependent on normality test. P-values were adjusted with Dunn’s multiple comparison test. Due to the low numerosity, data from groups C and D were merged in a single group (C+D). Correlations were assessed by use of Spearman correlation test.
3. Results
3.1. Patient Data
Among healthy control dogs, 24 (13 spayed) were female and 16 (7 neutered) were male. Seven dogs were mixed breed (17.5%), and 33 were purebred (82.5%). Median age was 48 months (range 12-208), median body weight was 15.2 kg (range 2.5-42). Most of the healthy control dogs were fed commercial nutritionally complete and balanced canine diets of different brands.
Among dogs with iPLE, 20 (39.2%) were female (15 spayed) and 31 (60.8%) were male (2 neutered). Eight dogs were mixed-breed (15.7%), 43 dogs were purebred (84.3%) represented as follows: German Shepherd (9 dogs), Golden Retriever, English Setter, and Yorkshire Terrier (3 dogs each breed), Australian Shepherd, Border Collie, Chihuahua, Labrador Retriever, Maltese Dog, and Spanish greyhound (2 dogs each breed), American Staffordshire Terrier, Belgian Shepherd, Boston Terrier, Cavalier King Charles, Cesky Terrier, Cocker Spaniel, Dachshund, Doberman Pinscher, Jack Russell Terrier, Pitbull, Podenco ibicenco, Pug, and Rottweiler (1 dog each breed). Median age was 84 months (range 19-171), median body weight was 15 kg (range 2.4-47.5), and median CCECAI score was 9 (range 3-17). Six, 19, 13, and 13 dogs were assigned to group MI, MO, S, and vs. severity subgroup, respectively. Sex and body weight did not significantly differ between healthy and diseased dogs. Age was significantly higher in the iPLE dogs compared to the healthy dogs (p < 0.0001). All dogs had gastrointestinal duodenoscopy performed. Thirty-four dogs (66.7%) had concurrent lower GI endoscopy in which the ileum was successfully intubated in 11 dogs (21.6%). On histopathology, a predominantly lymphoplasmacytic infiltration of the intestinal mucosa was found in all dogs. With regard to the histologic lesion severity, mild (grade 1) duodenal, ileal and colonic histologic lesions were found in 0, 0, and 4 dogs, respectively; moderate (grade 2) duodenal, ileal, and colonic histologic lesions were found in 25, 9, and 29 dogs, respectively; marked (grade 3) duodenal, ileal, and colonic histologic lesions were found in 26, 2, and 1 dog, respectively. Dilated crypts with proteinaceous material and cellular debris (crypt abscesses) were identified in 10 dogs (19.6%), and they were further classified as mild (n=4; 7.8%), moderate (n=3; 5.9%), and marked (n=3; 5.9%). Lacteal dilation was identified in 40 dogs (78.4%), and it was further classified as mild (n=17, 42.5%), moderate (n=22, 55%), and marked (n=1, 2.5%). Twenty-seven dogs were further assigned to group 1 (inflammation with only mild or no lacteal dilation), 24 to group 2 (inflammation with moderate or severe lacteal dilation). At admission, 12 dogs were on highly digestible gastrointestinal commercial diets, 3 on highly digestible low-fat commercial diets, 15 on limited ingredient commercial diets, 9 on home-cooked low-fat diets, and 8 on hydrolyzed diets. Four dogs were fed different diet types. In addition to the diet, 13 dogs were on prednisolone. Doses (0.5-1 mg/kg SID) and length of administration (some days to 3 weeks) were depending on the case.
3.2. Comparative Evaluation of the Lipid Profile and Other Laboratory Parameters between Healthy Control Dogs and iPLE Dogs
Results recorded in healthy control and iPLE dogs are reported in Table 1 and, as regards the lipid profile, in Figure 1. Serum concentrations of Alb, TP, Chol, HDLs, VLDLs, and PON-1 activity were significantly lower in iPLE dogs compared to healthy control dogs. Serum concentration of TGs, LDLs, chylomicrons and CRP were significantly higher in iPLE dogs compared to healthy control dogs. Among iPLE dogs, 30 and 39 showed decreased Chol, and PON-1 activity, respectively, while 26 and 13 showed increased CRP and TGs, respectively. Based on the concentrations of serum albumin, 6 dogs (11.8%) were assigned to group A, 29 (56.9%) to group B, 12 (23.5%) to group C, and 4 (7.8%) to group D.
3.3. Correlations of the CRP with CCECAI Scores, Histopathological Findings, and Prednisolone Therapy
A positive weak correlation between CCECAI scores and CRP (r = 0.28, p = 0.042) was found. The mean cumulative lesion score did not correlate with CRP. No significant difference in CRP was found between group 1 (inflammation with only mild or no lacteal dilation) and 2 (inflammation with moderate or severe lacteal dilation), nor between dogs that were on prednisolone at admission and dogs that were not.
3.4. Correlations of the Lipid Profile with Age, Gender, Body Weight, CCECAI Score, Alb, CRP, and PON-1 Activity Concentrations
Significant correlations of the lipid profile and age, body weight, PON-1 activity, and CRP are summarized in Table 2. Significant correlations or differences among groups between the lipid profile and the CCECAI score, or the CCECAI disease severity groups were not found. Significant correlations between the lipid profile and Alb were not found. However, when considering the hypoalbuminemia groups, some significant differences regarding Chol (p = 0.015), HDLs (p = 0.001), VLDLs (p = 0.026), and LDLs (p = 0.048) were found. More specifically, Chol and HDLs were significantly lower in group C+D compared to B (adjusted p = 0.015 and adjusted p = 0.0008, respectively), and VLDLs were significantly higher in group C+D compared to A (adjusted p = 0.045). LDLs didn’t significantly differ at Dunn’s multiple comparison test. Serum concentrations of Chol, HDL, LDL and VLDL lipoprotein classes in dogs classified by hypoalbuminemia groups are reported in Figure 2.
3.5. Correlations of the Lipid Profile with Histopathological Findings, Prednisolone Therapy and Diet
No significant correlations between the lipid profile and the mean cumulative lesion score were found. By the comparison of the lipid profile between dogs of the group 1 (inflammation with only or no lacteal dilation) and group 2 (inflammation with moderate or severe lacteal dilation), TGs were significantly higher in dogs of the group 2 (p = 0.0045).
No significant differences in the lipid profile between dogs that were on prednisolone at admission and dogs that were not were found. No significant differences in the lipid profile among dogs classified based on the type of diet upon admission were found.
4. Discussion
We described and compared the lipid profile, CRP and PON-1 activity between healthy control dogs and dogs with iPLE, and explored associations among clinicopathological data, histopathological findings, and lipid profile in dogs with iPLE.
Hypoalbuminemia is the hallmark of the PLE syndrome in dogs; however, hypocholesterolemia can be observed and it can help in define the prognosis [1,7,46]. To the authors’ knowledge and unlike in people, scarce or no information is available on serum TGs and lipoprotein changes in dogs with CIE [47]. Recently, the lipoprotein profile of cavitary effusions was investigated in some dogs with PLE, but the same analysis was not performed on serum samples [47]. Transudates of dogs with PLE were found to be poor in protein, cholesterol, and HDL contents and high in VLDL and LDL contents [47]. To date, the diagnosis of canine dyslipidemia is based on the measurement of pre-prandial serum cholesterol and triglyceride concentrations, while the lipoprotein analysis is not routinely used. Some explanations can be attempted. A gold standard validated method for the evaluation of canine lipoproteins is currently lacking; different techniques can be used, some of them developed for human use, expensive, time and labor consuming or not suitable for canine lipoprotein analysis [30,32,36,37,47,48,49,50]. Accordingly, information concerning reliable reference intervals for the different lipoprotein classes is lacking, results are poorly reproducible and not always comparable. Agarose gel electrophoresis is used to classify lipoproteins by the nomenclature beta, pre-beta, and alpha based on the mobility of LDLs, VLDLs, and HDLs, respectively [51]. This method was selected here because it provides accurate separation of canine lipoproteins, and it might be superior to the wet chemistry method for identifying some lipoprotein classes, especially LDLs which are substantial in some dogs [30,35,48,52]. However, it has not been extensively investigated for use in canine gastroenterology.
In both healthy control dogs and iPLE dogs, the HDL lipoprotein class was predominant, followed by the LDL and VLDL lipoprotein classes, respectively. According to the available information, these results are not surprising since the HDL is the predominant lipoprotein class in dogs, whereas in humans, LDL class predominates [53]. On the other hand, there are neither reports regarding lipoprotein profiles in dogs with iPLE, nor published solid reference intervals obtained by agarose gel electrophoresis to be used here for comparison. Hypocholesterolemia, variable levels of HDLs and LDLs, and normal or increased levels of TGs are common findings in humans with active IBD [20,21,22,23,26,54,55]. Moreover, some of these findings are independently associated with more severe disease [23]. Similarly, our iPLE dogs showed significantly decreased serum concentrations of Chol, and percentage of HDLs and VLDLs compared to healthy controls, while concentrations of TGs were normal or increased [26]. However, it cannot be ruled out that some dogs with normal TGs have had hypotriglyceridemia, since there is not a lower reference limit for TGs. Hypocholesterolemia, presumably secondary to inflammation, lymphangiectasia, fat malabsorption and malnutrition, has been already documented among dogs with iPLE, while scattered information is available for serum TGs [7,11,56]. Hypocholesterolemia is a common feature in human patients with acute diseases, and it has been related to surrogate markers of disease severity including IBD-related surgeries and number of hospitalizations [57,58].
In this study, no significant correlations were found between disease severity, as assessed by the CCECAI score, and the lipid profile. Some hypotheses may be attempted. The CCECAI score, although routinely used to assess the clinical severity of canine CIE, might not be appropriate to identify subsets of dogs with severe intestinal inflammation and lipid malabsorption. Moreover, the severity of lipid malabsorption could not play a pivotal role in the clinical manifestation of some dog.
Similar to previous results, CRP was significantly increased in iPLE dogs when compared with healthy dogs [59,60]. Our results were also in accordance with previously published results showing a correlation between CRP and clinical severity scores in dogs with CIE [59]. However, no correlations or differences among groups between CRP concentration and histopathological finding or ongoing prednisolone therapy at admission in iPLE dogs were found. Therefore, in some dogs with iPLE, CRP might be not useful for assessing histopathologic severity of inflammation and lacteal dilation, nor for assessing the response to treatment, in contrast with previous observations [59]. This discrepancy, however, might be because of differences in population such as number of dogs that had ileal and colonic evaluation, number of dogs that were on prednisolone at admission, dosages and length of prednisolone administration). PON-1 activity was significantly decreased in iPLE dogs when compared with healthy dogs. Since the decrease of PON-1 activity seems to couple with inflammation, our results might suggest that oxidation and inflammation might occur in some dogs with iPLE [61]. During inflammation, one of the most consistent alterations is the reduced serum concentration of HDLs [62]. In our study, a negative correlation between serum CRP concentration and percentage of HDLs has been found, as previously observed [63]. Indeed, HDLs are thought to have anti-inflammatory properties [64]. One additional property of HDLs is to prevent the LDLs from oxidation, and several HDL-related proteins, such as ApoA1, PON-1 and transferrin could affect this important function [65,66]. The anti-oxidative function of HDLs is strongly associated with disease severity, while the use of anti-inflammatory treatments significantly restores the antioxidant functions of HDLs towards normal [67,68]. In this study, positive correlations were found between PON-1 activity concentrations and HDLs; while negative correlations were found between PON-1 activity concentrations and LDLs, and between CRP concentrations and HDLs. Taken together, these results might support the hypothesis that changes in HDLs depend on oxidative stress likely associated with inflammation. Therefore, similarly to human medicine, canine HDLs might have anti-inflammatory and anti-oxidative properties, while LDLs might play a pro-inflammatory role [63]. Furthermore, positive correlations between age and Chol, and body weight and Chol were found in iPLE dogs, suggesting that biochemical characteristics of lipid metabolism disorders may be affected by aging and weight [69,70].
Significant associations between the lipid profile and Alb were not found. However, when considering the hypoalbuminemia groups, Chol and HDLs were significantly lower in group C+D compared to B, while VLDLs were significantly higher in group C+D compared to group B. These results are likely explained by the lipoprotein lipid content and function, along with inflammation, lymphangiectasia, fat malabsorption and malnutrition leading to hypocholesterolemia in dogs with iPLE. Indeed, HDLs is a major carrier of the total circulating lipid, especially free and esterified cholesterol in dogs, while TGs account for a significant proportion of the lipids in VLDLs and LDLs, but not in HDLs [71].
No significant associations between the lipid profile and the mean cumulative lesion score were found. This result is not surprising since the mean cumulative lesion score might have been influenced by the lack of ileal and colonic mucosa evaluation in some iPLE dogs. However, since iPLE is a heterogeneous disorder and affected dogs might show different magnitude of intestinal inflammation and lymphangiectasia, we compared the lipid profile between dogs of the group 1 (inflammation with only mild or no lacteal dilation) and group 2 (inflammation with moderate or severe lacteal dilation). By this comparison, TGs were significantly higher in dogs of the group 2. It can be hypothesized that these dogs with both inflammation and moderate or severe lacteal dilation, similarly to some patients with IBD, are characterized by a severe mucosal immune system dysregulation. This dysregulation leads to an increase of inflammatory cytokines that, in turn, may result in a decrease in lipoprotein lipase enzyme activity, leading to a characteristic lipoprotein profile with increased serum triglycerides and decreased HDLs [72].
In human patients with IBD, the administration of glucocorticoids increases total Chol, HDLs and LDLs [20]. The increase in HDLs and LDLs can be explained by an increase of VLDLs synthesis and lipoprotein lipase activity [73]. To date, glucocorticoids are a commonly prescribed treatment for dogs with CIE. Indeed, glucocorticoids in addition to a dietary change, seem to be appropriate for some dogs with iPLE [11,72]. For these reasons, dogs that were on prednisolone at admission were not excluded from this study. Percentages of different lipoprotein classes of dogs receiving prednisolone did not significantly differ from those not receiving prednisolone. Moreover, neither Chol nor TGs concentrations were significantly different among dogs assigned to these 2 therapeutic subgroups. Although not expected, these results might have been influenced by the low number of dogs receiving prednisolone prior to the admission, low doses and different length of prednisolone administration.
For dogs with iPLE and evidence of lymphangiectasia, low-fat diets alone or combined with prednisolone are proven to be useful [1,2,3,14,15,74,75]. However, to the best of the authors’ knowledge, no information exists on the influence of diets on lipoprotein profiles in dogs with iPLE. At the time of admission, the dogs of this study were fed different commercial and home-cooked diets, some of which were low-fat in content, that had previously been selected on a case-by-case basis. Some canine limited ingredient diets are fish- or vegetal-based diets, while highly digestible gastrointestinal diets are usually meat-based diets. Fish- and vegetal-based diets have been demonstrated to lower LDLs in dogs and humans, respectively [76,77]. No significant differences in the lipid profile among our iPLE dogs assigned to different diet groups were found. However, these results might have been influenced by the type of grouping. Indeed, the diet subgroups were by diet type and not by content of fat. Diet types have wide ranging amounts of fat, and there is also wide variation in fat among limited ingredient diets and highly digestible gastrointestinal commercial diets.
This study included some limitations. First, the lack of information about the gold standard method for lipoprotein evaluation. Without this information, the clinical utility of some data presented here cannot be interpreted reliably. Second, the results of the lipoprotein agarose gel electrophoresis are expressed as a percentage of the total area. Therefore, the increase of a lipoprotein class determines the reduction of another class, thus limiting the possibility of demonstrating an absolute increase of a lipoprotein class. Third, the diet subgroups were by diet type and not by content of fat, as already described above. This mainly because the exact information about the brand of diets prescribed before admission was missing or not precise. Fourth, not all iPLE dogs had ileal and colonic mucosa evaluated. This influenced the mean cumulative lesion score and potentially also the associations with the lipid profile. Finally, the storage stability of serum samples at -80°C was not assessed. However, it was recently observed that canine serum lipoproteins are stable for several months when stored at −80°C, similarly to the storage of our samples [78].
5. Conclusions
Our study is the first to describe the lipid profile in dogs with iPLE. Overall, significant differences in percentages of serum lipoprotein classes, CRP and PON-1 activity between dogs with iPLE and healthy control dogs were found. Moreover, triglycerides were significantly higher in dogs with both intestinal inflammation and lymphangiectasia. Some of these findings are consistent with previous studies in humans with IBD, suggesting that lipid metabolism is also affected in dogs with iPLE. Additional investigation into the lipid metabolism of dogs with iPLE is warranted.
Author Contributions
Conceptualization and methodology, A.G., P.G., S.P.; formal analysis, F.C.; investigation, A.B., B.B., E.B., F.B., F.C., R.F., S.M., D.S.; writing—original draft preparation, P.G.; writing-review and editing, F.C., A.G., P.G., S.P. All authors have read and agreed to the published version of the manuscript.
Funding
The analyses were performed at the laboratory of the Department of Veterinary Medicine, University of Milan, Italy, at the laboratory’s own costs.
Institutional Review Board Statement
The study was approved by the Institutional Ethics and Animal Welfare Committee (protocol number 42/2021) of the Department of Veterinary Sciences, Turin University.
Informed Consent Statement
The owners gave written informed consent to their dog’s enrollment in the study. The work involved the use of non-experimental animals only, and followed high standards (“best practice”) of individual veterinary clinical patient care. All samples (blood) were obtained during standard veterinary diagnostic procedures.
Data Availability Statement
All data analyzed during this study are included in this published article.
Conflicts of Interest
Alessia Giordano, Sara Meazzi, Saverio Paltrinieri and Donatella Scavone are employed at the laboratory were the analyses were performed. The remaining authors declare no conflicts of interest.
References
- Dossin, O.; Lavoué, R. Protein-losing enteropathies in dogs. Vet. Clin. North Am. Small Anim. Pract. 2011, 41, 399–418. [Google Scholar] [CrossRef]
- Craven, M.D.; Washabau, R.J. Comparative pathophysiology and management of protein-losing enteropathy. J. Vet. Intern. Med. 2018, 33, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, K.; Iennarella-Servantez, C. Canine Protein Losing Enteropathies and Systemic Complications. Vet. Clin. North Am. Small Anim. Pract. 2021, 51, 11–122. [Google Scholar] [CrossRef] [PubMed]
- Rovenská, E.; Rovenský, J. Lymphatic vessels: structure and function. Isr. Med. Assoc. J. 2011, 13, 762–768. [Google Scholar] [PubMed]
- Craven, M.; Simpson, J.W.; Ridyard, A.E.; Chandler, M.L. Canine inflammatory bowel disease: retrospective analysis of diagnosis and outcome in 80 cases (1995-2002). J. Small Anim. Pract. 2004, 45, 336–342. [Google Scholar] [CrossRef]
- Kull, P.A.; Hess, R.S.; Craig, L.E.; Saunders, H.M.; Washabau, R.J. Clinical, clinicopathologic, radiographic, and ultrasonographic characteristics of intestinal lymphangiectasia in dogs: 17 cases (1996-1998). J. Am. Vet. Med. Assoc. 2001, 219, 197–202. [Google Scholar] [CrossRef]
- Gianella, P.; Lotti, U.; Bellino, C.; Bresciani, F.; Cagnasso, A.; Fracassi, F.; D’Angelo, A.; Pietra, M. Clinicopathologic and prognostic factors in short- and long-term surviving dogs with protein-losing enteropathy. Schweiz. Arch. Tierheilkd. 2017, 159, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.W. , Jergens, A.E. Pitfalls and progress in the diagnosis and management of canine inflammatory bowel disease. Vet. Clin. North Am. Small Anim. Pract. 2011, 41, 381–398. [Google Scholar] [CrossRef]
- Simmerson, S.M.; Armstrong, P.J.; Wünschmann, A.; Jessen, C.R.; Crews, L.J.; Washabau, R.J. Clinical features, intestinal histopathology, and outcome in protein-losing enteropathy in Yorkshire Terrier dogs. J. Vet. Intern. Med. 2014, 28, 331–337. [Google Scholar] [CrossRef]
- Alexander, J.S.; Ganta, V.C.; Grisham, M.B. Emerging roles of lymphatics in inflammatory bowel disease. Ann. N. Y. Acad. Sci. 2010, Suppl 1, E75–85. [Google Scholar] [CrossRef]
- Salavati Schmitz, S.; Gow, A.; Bommer, N.; Morrison, L.; Mellanby, R. Diagnostic features, treatment, and outcome of dogs with inflammatory protein-losing enteropathy. J. Vet. Intern. Med. 2019, 33, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Dandrieux, J.R.S.; Noble, P.J.M.; Scase, T.J.; Cripps, P.J.; German, A.J. Comparison of a chlorambucil-prednisolone combination with an azathioprine-prednisolone combination for treatment of chronic enteropathy with concurrent protein-losing enteropathy in dogs: 27 cases (2007–2010). J. Am. Vet. Med. Assoc. 2013, 242, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Hiyoshi, S.; Ohno, K.; Uchida, K.; Goto-Koshino, Y.; Maeda, S.; Mizutani, N.; Takeuchi, A.; Tsujimoto, H. Prognostic factors in dogs with protein-losing enteropathy. Vet. J. 2015, 205, 28–32. [Google Scholar] [CrossRef]
- Okanishi, H.; Yoshioka, R.; Kagawa, Y.; Watari, T. The clinical efficacy of dietary fat restriction in treatment of dogs with intestinal lymphangiectasia. J. Vet. Intern. Med. 2014, 28, 809–81715. [Google Scholar] [CrossRef]
- Rudinsky, A.J.; Howard, J.P.; Bishop, M.A.; Sherding, R.G.; Parker, V.J.; Gilor, C. Dietary management of presumptive protein-losing enteropathy in Yorkshire terriers. J. Small Anim. Pract. 2017, 58, 103–108. [Google Scholar] [CrossRef]
- Jablonski, S.A. Pathophysiology, diagnosis, and management of canine intestinal lymphangiectasia: a comparative review. Animals (Basel). 2022, 12, 2791. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Kathrani, A. Incidence of relapse of inflammatory protein-losing enteropathy in dogs and associated risk factors. J. Vet. Intern. Med. 2022, 36, 1981–1988. [Google Scholar] [CrossRef]
- Ohta, H.; Nagata, N.; Yokoyama, N.; Osuga, T.; Sasaki, N.; Morishita, K.; Takiguchi, M. Prognostic value of small intestinal dilatation in dogs with protein-losing enteropathy. J. Vet. Med. Sci. 2021, 11, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, K.; Rizzo, J.; Jergens, A.E.; Chang, Y.M. Hypovitaminosis D is associated with negative outcome in dogs with protein losing enteropathy: a retrospective study of 43 cases. BMC Vet. Res. 2017, 13, 96. [Google Scholar] [CrossRef]
- Sleutjes, J.A.M.; Roeters van Lennep, J.E.; van der Woude, C.J.; de Vries, A.C. Lipid Changes After Induction Therapy in Patients with Inflammatory Bowel Disease: Effect of Different Drug Classes and Inflammation. Inflamm. Bowel Dis. 2023, 29, 531–538. [Google Scholar] [CrossRef]
- Agouridis, A.P.; Elisaf, M.; Milionis, H.J. An overview of lipid abnormalities in patients with inflammatory bowel disease. Ann. Gastroenterol. 2011, 24, 181–187. [Google Scholar] [PubMed]
- Ripollés-Piquer, B.R.; Nazih, H.; Bourreille, A.; Segain, J.P.; Huvelin, J.M.; Galmiche, J.P.; Bard, J.M. Altered lipid, apolipoprotein, and lipoprotein profiles in inflammatory bowel disease: consequences on the cholesterol efflux capacity of serum using Fu5AH cell system. Metabolism 2006, 55, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Koutroumpakis, E.; Ramos-Rivers, C.; Regueiro, M.; Hashash, J.G.; Barrie, A.; Swoger, J.; Baidoo, L.; Schwartz, M.; Dunn, M.A.; Koutroubakis, J.E.; Binion, D.G. Association Between Long-Term Lipid Profiles and Disease Severity in a Large Cohort of Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 2016, 61, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kullo, I.J.; Pardi, D.S.; Loftus, E.V. Epidemiology, risk factors and management of cardiovascular diseases in IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 26–35. [Google Scholar] [CrossRef]
- Sammalkorpi, K.T.; Valtonen, V.V.; Maury, CP. Lipoproteins and acute phase response during acute infection. Interrelationships between C-reactive protein and serum amyloid-A protein and lipoproteins. Ann. Med. 1990, 22, 397–401. [Google Scholar] [CrossRef]
- Romanato, G.; Scarpa, M.; Angriman, I.; Faggian, D.; Ruffolo, C.; Marin, R.; Zambon, S.; Basato, S.; Zanoni, S.; Filosa, T.; Pilon, F.; Manzato, E. Plasma lipids and inflammation in active inflammatory bowel disease. Aliment. Pharmacol. Ther. 2009, 29, 298–307. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Nordan, R.P.; McIntosh, J.; Calvo, J.C.; Scow, R.O.; Jablons, D. Interleukin 6 reduces lipoprotein lipase activity in adipose tissue of mice in vivo and in 3T3-L1 adipocytes: a possible role for interleukin 6 in cancer cachexia. Cancer Res. 1992, 52, 4113–4116. [Google Scholar] [PubMed]
- Szczeklik, K.; Mach, T.; Cibor, D.; Owczarek, D.; Sapa, J.; Papież, M.; Pytko-Polończyk, J.; Krzyściak, W. Correlation of Paraoxonase-1 with the Severity of Crohn’s Disease. Molecules. 2018, 23, 2603. [Google Scholar] [CrossRef]
- Boehm, D.; Krzystek-Korpacka, M.; Neubauer, K.; Matusiewicz, M.; Berdowska, I.; Zielinski, B.; Paradowski, L.; Gamian, A. Paraoxonase-1 status in Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2009, 15, 93–99. [Google Scholar] [CrossRef]
- Xenoulis, P.G.; Cammarata, P.J.; Walzem, R.L.; Macfarlane, R.D.; Suchodolski, J.S.; Steiner, J.M. Novel lipoprotein density profiling in healthy dogs of various breeds, healthy Miniature Schnauzers, and Miniature Schnauzers with hyperlipidemia. BMC Vet. Res. 2013, 9, 47. [Google Scholar] [CrossRef]
- Whitney, M.S.; Boon, G.D.; Rebar, A.H.; Ford, R.B. Effects of acute pancreatitis on circulating lipids in dogs. Am. J. Vet. Res. 1987, 48, 1492–1497. [Google Scholar] [PubMed]
- Gianella, P.; Caccamo, R.; Bellino, C.; Bottero, E.; Fietta, F.; Roncone, S.; Ostanello, F.; Pietra, M.; Buracco, P. Evaluation of metabolic profile and C-reactive protein concentrations in brachycephalic dogs with upper airway obstructive syndrome. J. Vet. Intern. Med. 2019, 33, 2183–2192. [Google Scholar] [CrossRef] [PubMed]
- Behling-Kelly, E. Serum Lipoprotein Changes in Dogs with Renal Disease. J. Vet. Intern. Med. 2014, 28, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Ibba, F.; Rossi, G.; Meazzi, S.; Giordano, A.; Paltrinieri, S. Serum concentration of high-density lipoproteins (HDLs) in leishmaniotic dogs. Res. Vet. Sci. 2015, 98, 89–91. [Google Scholar] [CrossRef]
- Rogers, W.A.; Donovan, E.F.; Kociba, G.J. Lipids and lipoproteins in normal dogs and in dogs with secondary hyperlipoproteinemia. J. Am. Vet. Med. Assoc. 1975, 166, 1092–1100. [Google Scholar] [PubMed]
- Mizutani, H.; Sako, T.; Arai, N.; Kuriyama, K.; Yoshimura, I.; Mori, A.; Iwase, K.; Hirose, H. Application of gel permeation HPLC for lipoprotein profiling in dogs. J. Vet. Med. Sci. 2010, 72, 813–817. [Google Scholar] [CrossRef]
- Rossi, G.; Kuleš, J.; Rafaj, R.B.; Mrljak, V.; Lauzi, S.; Giordano, A.; Paltrinieri, S. Relationship between paraoxonase 1 activity and high-density lipoprotein concentration during naturally occurring babesiosis in dogs. Res. Vet. Sci. 2014, 97, 318–324. [Google Scholar] [CrossRef]
- Jergens, A.E.; Schreiner, C.A.; Frank, D.E.; Niyo, Y.; Ahrens, F.E.; Eckersall, P.D.; Benson, T.J.; Evans, R. A scoring index for disease activity in canine inflammatory bowel disease. J. Vet. Intern. Med. 2003, 17, 291–297. [Google Scholar] [CrossRef]
- Heilmann, R.M.; Berghoff, N.; Mansell, J.; Grützner, N.; Parnell, N.K.; Gurtner, C.; Suchodolski, J.S.; Steiner, J.M. Association of fecal calprotectin concentrations with disease severity, response to treatment, and other biomarkers in dogs with chronic inflammatory enteropathies. J. Vet. Intern. Med. 2018, 32, 679–692. [Google Scholar] [CrossRef]
- Tamura, Y.U.; Ohta, H.; Kagawa, Y.; Osuga, T.; Morishita, K.; Sasaki, N.; Takiguchi, M. Plasma amino acid profiles in dogs with inflammatory bowel disease. J. Vet. Intern. Med. 2019, 33, 1602–1607. [Google Scholar] [CrossRef]
- Equilino, M.; Théodoloz, V.; Gorgas, D.; Doherr, M.G.; Heilmann, R.M.; Suchodolski, J.S.; Steiner, J.M.; Burgener, I.A. Evaluation of serum biochemical marker concentrations and survival time in dogs with protein-losing enteropathy. J. Am. Vet. Med. Assoc. 2015, 246, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Giordano, A.; Pezzia, F.; Kjelgaard-Hansen, M.; Paltrinieri, S. ; Serum paraoxonase 1 activity in dogs: Preanalytical and analytical factors and correlation with C-reactive protein and alpha-2-globulin. Vet. Clin. Pathol. 2013, 42, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Carretón, E.; Cerón, J.J.; Martínez-Subiela, S.; Tvarijonaviciute, A.; Caro-Vadillo, A.; Montoya-Alonso, J.A. Acute phase proteins and markers of oxidative stress to assess the severity of the pulmonary hypertension in heartworm-infected dogs. Parasit. Vectors. 2017, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Washabau, R.J.; Day, M.J.; Willard, M.D.; Hall, E.J.; Jergens, A.E.; Mansell, J.; Minami, T.; Bilzer, T.W.; WSAVA International Gastrointestinal Standardization Group; et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J. Vet. Intern. Med. 2010, 24, 10–26. [Google Scholar] [CrossRef]
- Allenspach, K; Wieland, B.; Gröne, A.; Gaschen, F. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 2007, 21, 700–708. [Google Scholar] [CrossRef]
- Kathrani, A.; Parkes, G. A Preliminary Study of Modulen IBD Liquid Diet in Hospitalized Dogs with Protein-Losing Enteropathy. Animals. 2022, 12, 1594. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.H.; Behling-Kelly, E.; Borjesson, D.L. Lipoprotein profile of pleural and peritoneal transudates in dogs and cats. J. Vet. Intern. Med. 2022, 36, 464–472. [Google Scholar] [CrossRef]
- Behling-Kelly, E. Comparison of 2 electrophoretic methods and a wet-chemistry method in the analysis of canine lipoproteins. Vet. Clin. Pathol. 2016, 45, 124–134. [Google Scholar] [CrossRef]
- Suto, A.; Yamasaki, M.; Takasaki, Y.; Fujita, Y.; Abe, R.; Shimizu, H.; Ohta, H.; Takiguchi, M. Lc-ms/ms analysis of canine lipoproteins fractionated using the ultracentrifugation-precipitation method. J. Vet. Med. Sci. 2013, 75, 1471–1477. [Google Scholar] [CrossRef]
- Xenoulis, P.G.; Cammarata, P.J.; Walzem, R.L.; Suchodolsky, J.S.; Steiner, J.M. Serum triglyceride and cholesterol concentrations and lipoprotein profiles in dogs with naturally occurring pancreatitis and healthy control dogs. J. Vet. Intern. Med. 2020, 34, 644–652. [Google Scholar] [CrossRef]
- Noble, R.P. Electrophoretic separation of plasma lipoproteins in agarose gel. J. Lipid Res. 1968, 9, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Whitney, M.S.; Boon, G.D.; Rebar, A.H.; Story, J.A.; Bottoms, G.D. Ultracentrifugal and electrophoretic characteristics of the plasma lipoproteins of miniature schnauzer dogs with idiopathic hyperlipoproteinemia. J. Vet. Intern. Med. 1993, 7, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.D.G. Lipoprotein metabolism in dogs and cats. Comp. Haematol, Int, 1996, 6, 17–23. [Google Scholar] [CrossRef]
- Fan, F.; Mundra, P.A.; Fang, L.; Galvin, A.; Moore, X.L.; Weir, J.M.; Wong, G.; White, D.A.; Chin-Dusting, J.; Sparrow, M.P.; Meikle, P.J.; Dart, A.M. Lipidomic profiling in inflammatory bowel disease: Comparison between ulcerative colitis and crohn’s disease. Inflamm. Bowel Dis. 2015, 21, 1511–1518. [Google Scholar] [CrossRef]
- Sappati Biyyani, R.S.; Putka, B.S.; Mullen, K.D. Dyslipidemia and lipoprotein profiles in patients with inflammatory bowel disease. J. Clin. Lipidol. 2010, 4, 478–482. [Google Scholar] [CrossRef]
- Wennogle, S.A.; Stockman, J.; Webb, C.B. Prospective evaluation of a change in dietary therapy in dogs with steroid-resistant protein-losing enteropathy. J. Small Anim. Pract. 2021, 62, 756–764. [Google Scholar] [CrossRef]
- Giovannini, I.; Boldrini, G.; Chiarla, C.; Giuliante; Vellone, M.; Nuzzo, G. Pathophysiologic correlates of hypocholesterolemia in critically ill surgical patients. Intensive Care Med, 1999, 25, 748–751. [Google Scholar] [CrossRef]
- Fraunberger, P.; Nagel, D.; Walli, A.K.; Seidel, D. Serum cholesterol and mortality in patients with multiple organ failure. Crit. Care Med. 2000, 28, 3574–3575. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.S.; Lee, D.; Yun, T.; Koo, Y.; Chae, Y.; Kang, J.H.; Kang, B.T.; Yang, M.P.; Kim, H. Clinical signs, duodenal histopathological grades, and serum high-mobility group box 1 concentrations in dogs with inflammatory bowel disease. J. Vet. Intern. Med. 2021, 35, 2205–2214. [Google Scholar] [CrossRef]
- Otoni, C.O.; Heilmann, R.M.; García-Sancho, M.; Sainz, A.; Ackermann, N.R.; Suchodolski, J.S.; Steiner, J.M.; Jergens, A.E. Serologic and fecal markers to predict response to induction therapy in dogs with idiopathic inflammatory bowel disease. J. Vet. Intern. Med. 2018, 32, 999–1008. [Google Scholar] [CrossRef]
- Ruggerone, B.; Scavone, D.; Troìa, R.; Giunti, M.; Dondi, F.; Paltrinieri, S. Comparison of Protein Carbonyl (PCO), Paraoxonase-1 (PON1) and C-Reactive Protein (CRP) as Diagnostic and Prognostic Markers of Septic Inflammation in Dogs. Vet. Sci. 2021, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Feillet-Coudray, C.; Fouret, G.; Vigor, C.; Bonafos, B.; Jover, B.; Blachnio-Zabielska, A.; Rieusset, J.; Casas, F.; Gaillet, S.; Landrier, J.F.; Durand, T.; Coudray, C. Long-term measures of dyslipidemia, inflammation, and oxidative stress in rats fed a high-fat/high-fructose diet. Lipids. 2019, 54, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Khovidhunkit, W.; Kim, M.S.; Memon, R.A.; Shigenaga, J.K.; Moser, A.H.; Feingold, K.R.; Grunfeld, C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 2004, 45, 1169–1196. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Yu, R.; Gharavi, N.; Huang, W.; Ezra, N.; Lotfizadeh, A.; Anantharamaiah, G.M.; Alipour, N.; Van Lenten, B.J.; Reddy, S.T.; Marelli, D. High-density lipoprotein: Antioxidant and anti-inflammatory properties. Curr. Atheroscler. Rep. 2007, 9, 244–248. [Google Scholar] [CrossRef]
- Liu, D.; Ding, Z.; Wu, M.; Xu, W.; Qian, M.; Du, Q.; Zhang, L.; Cui, Y.; Zheng, J.; Chang, H.; Huang, C.; Lin, D.; Wang, Y. The apolipoprotein A-I mimetic peptide, D-4F, alleviates ox-LDL-induced oxidative stress and promotes endothelial repair through the eNOS/HO-1 pathway. J. Mol. Cell. Cardiol. 2017, 105, 77–88. [Google Scholar] [CrossRef]
- Lioudaki, S.; Verikokos, C.; Kouraklis, G.; Ioannou, C.; Chatziioannou, E.; Perrea, D.; Klonaris, C. Paraoxonase-1: characteristics and role in atherosclerosis and carotid artery disease. Curr. Vasc. Pharmacol. 2019, 17, 141–146. [Google Scholar] [CrossRef]
- Gomez Rosso, L.; Lhomme, M.; Merono, T.; Sorroche, P.; Catoggio, L.; Soriano, E.; Saucedo, C.; Malah, V.; Dauteuille, C.; Boero, L.; Lesnik, P.; Robillard, P.; Chapman, M.J.; Brites, F.; Kontush, A. Altered lipidome and antioxidative activity of small, dense HDL in normolipidemic rheumatoid arthritis: relevance of inflammation. Atherosclerosis. 2014, 237, 652–660. [Google Scholar] [CrossRef]
- Popa, C.; van Tits, L.J.; Barrera, P.; Lemmers, H.L.M.; van den Hoogen, F.H.J.; van Riel, P.L.C.M.; Radstake, T.R.D.J.; Netea, M.G.; Roest, M.; Stalenhoef, A.F.H. Anti-infammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann. Rheum. Dis. 2009, 68, 868–872. [Google Scholar] [CrossRef]
- Kawasumi, K.; Kashiwado, N.; Okada, Y.; Sawamura, M.; Sasaki, Y.; Iwazaki, E.; Mori, N.; Yamamoto, I.; Arai, T.; et al. Age effects on plasma cholesterol and triglyceride profiles and metabolite concentrations in dogs. BMC Vet. Res. 2014, 10, 57. [Google Scholar] [CrossRef]
- Shiho, U.; Yasuda, H.; Koketsu, Y. Lipoprotein cholesterol and triglyceride concentrations associated with dog body condition score; effect of recommended fasting duration on sample concentrations in Japanese private clinics. J. Vet. Med. Sci. 2015, 77, 1063–1069. [Google Scholar] [CrossRef]
- Maldonado, E.N.; Romero, J.R.; Ochoa, B.; Aveldaño, M.I. Lipid and fatty acid composition of canine lipoproteins. Comp. Biochem. Physiol. 2001, 128, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, R.R.; Sappati Biyyani, S.; Putka, B.s.; Mullen, K.D. Dyslipidemia and lipoprotein profiles in patients with inflammatory bowel disease J. Clin. Lipidol. 2010, 4, 478–82. [Google Scholar] [CrossRef]
- Ettinger, W.H.; Klinefelter, H.F.; Kwiterovitch, P.O. Effect of short-term, low-dose corticosteroids on plasma lipoprotein lipids. Atherosclerosis 1987, 63, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.; Martinez, S.A.; Shiroma, J.T.; Warson, A.T.; Hostutler, R.A. Prospective Evaluation of Low-Fat Diet Monotherapy in Dogs with Presumptive Protein-Losing Enteropathy. J. Am. Anim. Hosp. Assoc. 2023, 59, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Ohta, H.; Yokoyama, N.; Teoh, Y.B.; Nisa, K.; Sasaki, N.; Osuga, T.; Morishita, K.; Takiguchi, M. Clinical characteristics of dogs with food-responsive protein-losing enteropathy. J. Vet. Intern. Med. 2020, 34, 659–668. [Google Scholar] [CrossRef]
- Pasquini, A.; Luchetti, E.; Cardini, G. Plasma lipoprotein concentrations in the dog: The effects of gender, age, breed and diet. J. Anim. Physiol. Anim. Nutr. 2008, 92, 718–722. [Google Scholar] [CrossRef]
- Koch, C.A.; Kjeldsen, E.W.; Frikke-Schmidt, R. Vegetarian or vegan diets and blood lipids: a meta-analysis of randomized trials. Eur. Heart. J. 2023, 44, 2609–2622. [Google Scholar] [CrossRef]
- Minamoto, T.; Parambeth, J.C.; Walzem, R.L.; Payne, H.R.; Lidbury, J.A.; Suchodolski, J.S.; Steiner, J.M. Evaluation of density gradient ultracentrifugation serum lipoprotein profiles in healthy dogs and dogs with exocrine pancreatic insufficiency. J. Vet. Diagn. Invest. 2018, 30, 878–886. [Google Scholar] [CrossRef]
Figure 1.
Serum concentrations of cholesterol, triglycerides, HDL, VLDL, LDL, and chylomicron lipoprotein classes in healthy control dogs and iPLE dogs. Red lines indicate the median value. iPLE=inflammatory protein-losing enteropathy; HDLs=high-density lipoproteins; LDLs=low-density lipoproteins; VLDLs=very low-density lipoproteins.
Figure 1.
Serum concentrations of cholesterol, triglycerides, HDL, VLDL, LDL, and chylomicron lipoprotein classes in healthy control dogs and iPLE dogs. Red lines indicate the median value. iPLE=inflammatory protein-losing enteropathy; HDLs=high-density lipoproteins; LDLs=low-density lipoproteins; VLDLs=very low-density lipoproteins.

Figure 2.
Serum concentrations of cholesterol, HDL, LDL and VLDL lipoprotein classes in iPLE dogs assigned to hypoalbuminemia groups. Group A (2.2 to 2.8 d/dL), group B (1.5 to 2.19 g/dL), group C (1.2 to 1.49 g/dL), group D (<1.2 d/dL). Red lines indicate the median value. Chol=cholesterol; iPLE=inflammatory protein-losing enteropathy; HDLs=high-density lipoproteins; LDLs=low-density lipoproteins; VLDLs=very low-density lipoproteins.
Figure 2.
Serum concentrations of cholesterol, HDL, LDL and VLDL lipoprotein classes in iPLE dogs assigned to hypoalbuminemia groups. Group A (2.2 to 2.8 d/dL), group B (1.5 to 2.19 g/dL), group C (1.2 to 1.49 g/dL), group D (<1.2 d/dL). Red lines indicate the median value. Chol=cholesterol; iPLE=inflammatory protein-losing enteropathy; HDLs=high-density lipoproteins; LDLs=low-density lipoproteins; VLDLs=very low-density lipoproteins.
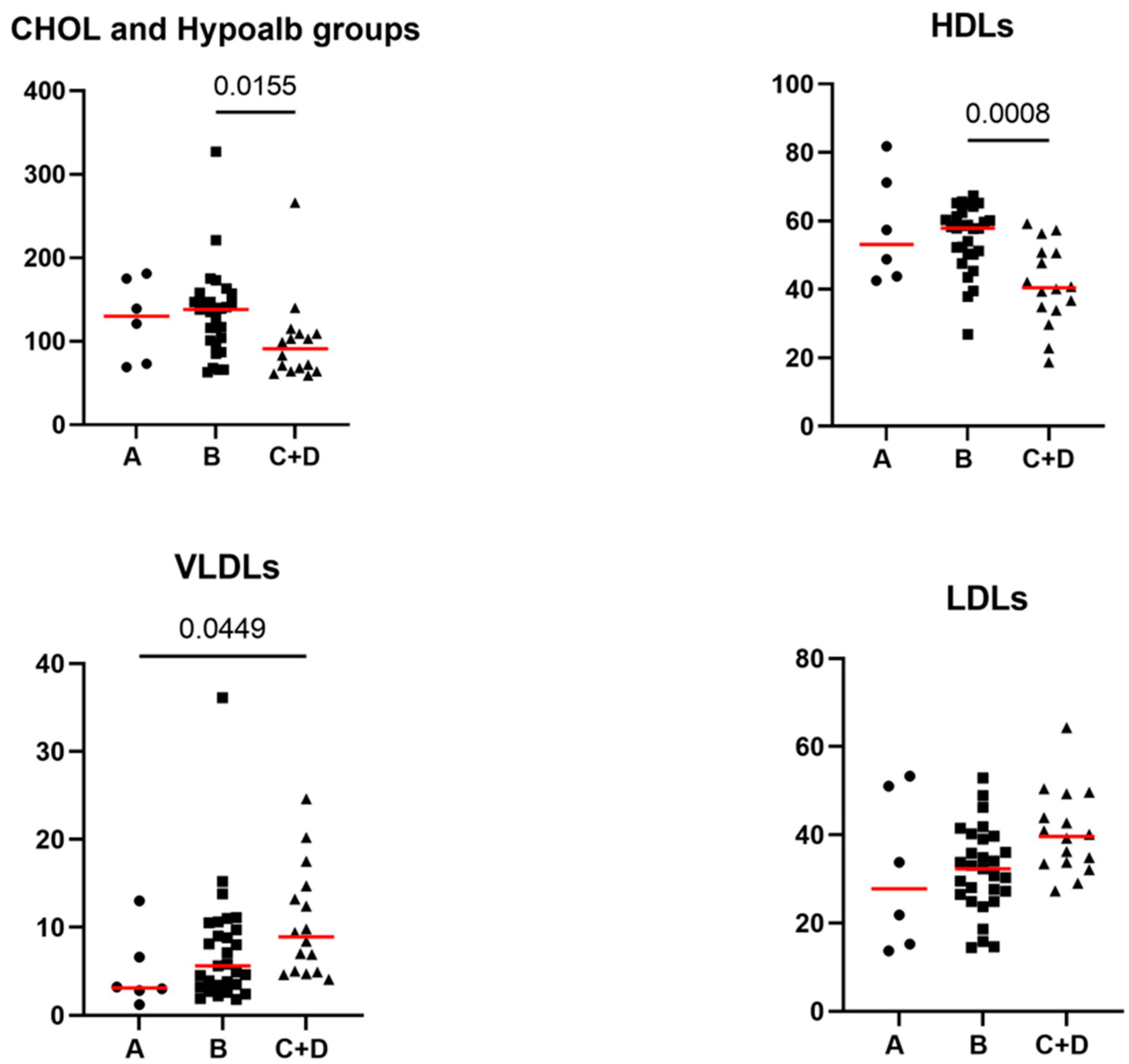
Table 1.
Comparative evaluation of the lipid profile, Alb, TP, CRP, and PON-1 activity concentrations between healthy control dogs and iPLE dogs.
Table 1.
Comparative evaluation of the lipid profile, Alb, TP, CRP, and PON-1 activity concentrations between healthy control dogs and iPLE dogs.
| Parameter | Control dogs | iPLE dogs | Reference interval | P-value |
|---|---|---|---|---|
| Alb (g/dL) | 3.6 (2.9-4.1) | 1.7 (0.8-2.7) | 2.8-4 | <0.0001 |
| Chol (mg/dL) | 209.5 (118-289) | 116 (59-327) | 135-270 | <0.0001 |
| Chylomicrons (%) | 2.1 (0.3-8.4) | 5.6 (0.5-22.8) | - | <0.0001 |
| CRP (mg/L) | 0.9 (0-14.6) | 5.4 (0-48.4) | ≤5 | =0.009 |
| HDLs (%) | 65.5 (44.4-79.5) | 52.3 (18.7-81.8) | - | <0.0001 |
| LDLs (%) | 15.8 (6.5-39.1) | 33.8 (13.7-64.3) | - | <0.0001 |
| PON-1 activity (U/mL) | 199 (129-303) | 77 (13.3-223) | ≥116 | <0.0001 |
| VLDLs (%) | 15.6 (8.1-30.4) | 6.6 (1.2-36.1) | - | <0.0001 |
| TGs (mg/dL) | 55 (11-122) | 83 (30-172) | <104 | <0.0001 |
| TP (g/dL) | 6.4 (4.8-8.9) | 4.1 (2.0-7.4) | 5.4-7.5 | <0.0001 |
Data are reported as median (minimum-maximum). Alb=albumin; Chol=total cholesterol; CRP=C-reactive protein; HDLs=high-density lipoproteins; iPLE=inflammatory protein-losing enteropathy; LDLs=low-density lipoproteins; PON-1=paraoxonase-1; VLDLs=very low-density lipoproteins; TGs=triglycerides; TP=total proteins.
Table 2.
Significant correlations between the lipid profile and selected study variables.
| Pair of variables tested for correlation |
Correlation coefficient (r) | Significance level (p) |
|---|---|---|
| HDLs and PON-1 | 0.60 | <0.0001 |
| HDLs and CRP | -0.28 | =0.048 |
| LDLs and body weight | -0.36 | =0.009 |
| LDLs and PON-1 | -0.68 | <0.0001 |
| Chol and age | 0.30 | =0.034 |
| Chol and body weight | 0.30 | =0.039 |
| Chol and PON-1 | 0.83 | <0.0001 |
| CRP and VLDLs | 0.46 | =0.001 |
Chol=total cholesterol; CRP=C-reactive protein; HDLs=high-density lipoproteins; LDLs=low-density lipoproteins; PON-1=paraoxonase-1. p < 0.05 indicates a significant correlation. The strength of the correlations is categorized as follows: 0.00–0.39 weak correlation; 0.40-0.59 moderate correlation; 0.60-0.79 strong correlation; 0.80-1.00 very strong correlation.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Submitted:
12 September 2024
Posted:
13 September 2024
You are already at the latest version
Alerts
This version is not peer-reviewed
Submitted:
12 September 2024
Posted:
13 September 2024
You are already at the latest version
Alerts
Abstract
Chronic inflammation alters lipoprotein metabolism and causes changes in serum concentrations of lipids, C-reactive protein (CRP), and paraoxonase-1 activity (PON-1), an enzyme, which in the gastrointestinal tract may act as a local detoxifier, antioxidant, and immunomodulator. However, unlike in people with inflammatory bowel disease, scarce information is available in dogs with protein-losing enteropathy secondary to chronic inflammatory enteropathy (iPLE). The first aim was to describe and compare the lipid profile, CRP concentrations and PON-1 activity in healthy dogs and in dogs with iPLE. The second aim was to evaluate correlations among clinicopathological, histologic data, and lipid profiles in dogs with iPLE. Serum samples from 51 iPLE and 40 healthy dogs were used to study albumin, total protein, CRP, PON-1 activity, cholesterol, triglycerides, and lipoprotein classes obtained by electrophoretic separation. There was a significant difference of all serum analytes between iPLE and healthy dogs. Significant correlations between the lipid profile and the existing chronic enteropathy activity index were not found. Some lipoprotein classes correlated with CRP and PON-1. Triglycerides were significantly higher in dogs with both inflammation and lymphangiectasia. Lipoproteins, CRP and PON-1 activity are altered in dogs with iPLE. Results need to be confirmed in further studies.
Keywords:
Subject: Medicine and Pharmacology - Veterinary Medicine
1. Introduction
Canine protein-losing enteropathy (PLE) is a syndrome characterized by an abnormal loss of serum proteins through the gastrointestinal mucosa [1,2,3]. Numerous gastrointestinal diseases such as intestinal lymphangiectasia, lymphoma, regional fungal infections, and chronic inflammatory enteropathy, if severe enough, can lead to PLE [1,2,3]. In people, PLE is usually associated with primary intestinal lymphangiectasia (IL) [4]. In dogs, although a genetic susceptibility to the development of primary IL has been reported for some breeds, PLE is more commonly associated with secondary IL resulting from chronic inflammatory enteritis (CIE) [1,2,3,5,6,7,8,9]. Since lymphatics are the primary transporter of lipids, lipid-soluble vitamins, food antigens, bacteria-derived lipopolysaccharides, and gut hormones from the intestine to the blood, their dysfunction can contribute to the pathogenesis and progression of the intestinal inflammation [10]. The diagnostic workup of dogs with PLE is similar to that of dogs with CIE [2]. Although dietary therapy (often with low-fat or ultra low-fat formulations) alone is associated with a positive outcome, glucocorticoids (anti-inflammatory or immunosuppressive dosages), immunosuppressive agents, and supportive therapies are needed in some dogs with PLE [2,7,9,11,12,13,14,15,16]. Although prolonged survival can occur, PLE is often characterized by guarded prognosis and high rate of relapse [1,2,17,18,19].
Various lipid profile changes have been described in human patients with inflammatory bowel disease and could be mainly summarized by decreased levels of total cholesterol (Chol) and low-density lipoproteins (LDLs), variable levels of high-density lipoproteins (HDLs), and normal or increased levels of triglycerides (TGs) [20,21,22,23]. These changes are thought to be the result of a complex interaction of inflammatory cytokines with down-regulation of the lipolytic enzyme activity, malnutrition, and lipid malabsorption [24,25]. In addition, total and HDL-cholesterol levels are correlated with the systemic inflammatory status [26]; indeed, interleukin-6 and C-reactive protein (CRP), a biomarker of systemic inflammation, are closely related and together play a role in general lipid metabolism, inhibiting adipocyte lipoprotein lipase activity [27]. Finally, additional mediators such as paraoxonase-1 (PON-1), an HDL-bound antioxidant enzyme, which in the gastrointestinal tract may act as a local detoxifier, antioxidant, and immunomodulator, may be responsible for some changes within the lipid profile [28,29].
In veterinary medicine, serum and plasma lipoprotein profiles have been sporadically investigated both in healthy and diseased dogs [30,31,32,33,34,35]. Among healthy dogs, HDLs is commonly the predominant lipoproteins fraction, while lower percentages of LDLs and very low-density lipoproteins (VLDLs) are observed [36]. Among diseased dogs, the percentage of HDLs decreases in chronic kidney disease, nephrotic syndrome, babesiosis, leishmaniasis, and pancreatitis; while the percentage of VLDLs, LDLs and chylomicrons increases in brachycephalic syndrome, chronic kidney disease, nephrotic syndrome, diabetes mellitus, sepsis, and pancreatitis [31,32,33,34,37]. However, to the best of the authors’ knowledge, no information exists on the lipid profile in dogs with PLE secondary to chronic inflammatory enteropathy (iPLE). CRP, a nonspecific marker of inflammation, has been used in CIE and iPLE to select the best clinical approach at the onset of treatment, and to document disease progression and response to treatment [38,39,40,41]. Low serum PON-1 activity concentrations have already been demonstrated in some dogs with both acute and chronic inflammation, likely because an intense oxidation occurs [42,43]. However, to the best of the authors’ knowledge, no information exists on PON-1 activity in dogs with iPLE.
Based on these premises, the study aimed (i) to describe and compare the lipid profile, CRP e PON-1 activity between healthy dogs and dogs with iPLE, and (ii) to evaluate associations among clinicopathological data, histopathological findings, and lipid profile in dogs with iPLE.
2. Materials and Methods
2.1. Animals
This study was conducted on 51 left-over serum samples from privately-owned dogs that received a diagnosis of iPLE from January 2021 to March 2022 at the Veterinary Teaching Hospital, Department of Veterinary Sciences, University of Turin, Italy, and other referral clinics in northern and central Italy, and 40 left-over serum samples from healthy dogs. The study received the official approval of the Institutional Ethics and Animal Welfare Committee (protocol number 42/2021).
The criteria for the diagnosis of iPLE were: chronic gastrointestinal signs lasting for more than 3 weeks, hypoalbuminemia of gastrointestinal origin (≤ 2.8 g/dL), and histopathological evidence of benign gastrointestinal inflammation with or without lymphangiectasia on multiple biopsies collected by endoscopy [1,2,3,5,8]. The histopathologic examination, made in accordance with the histopathological standards of the World Small Animal Veterinary Association Gastrointestinal Standardization Group, was required [44]. In order to rule out infectious, parasitic, liver and pancreatic diseases, along with intestinal diseases of other etiology and extraintestinal diseases, the following diagnostic investigations were required: abdominal ultrasound examination, fecal flotation and giardia antigen-test, complete blood count, biochemistry, pre- and post-prandial bile acids, urinalysis, urinary protein to creatinine ratio, serum basal cortisol or ACTH stimulation test (if basal cortisol ≤ 2µg/dl), trypsin-like-immunoreactivity, pancreas specific lipase levels, serum folate and cobalamin concentrations. In order to confirm the gastrointestinal origin of hypoalbuminemia, dogs were also required to have no clinically relevant proteinuria (negative urine dipstick test result or urine protein to creatinine ratio ≤ 0.5) and no evidence of clinically relevant hepatic disease (normal pre- and post-prandial bile acid concentrations or normal synthetic liver function and enzyme activity). Exclusion criteria were complete and sustained response to dietary trials and gut microbiota manipulation (i.e., pre-, pro-, syn- and postbiotics, fecal microbiota transplantation and antibiotics), and a histopathologic diagnosis of neoplasia. All dogs underwent gastroduodenoscopy. Colonoscopy with ileal intubation was performed when possible. Biopsies from stomach, duodenum, and, when available, ileum and colon, were collected for histologic examination. The severity of morphologic and inflammatory lesions in the duodenum, ileum, and colon were recorded as follows: 0= normal, 1= mild, 2= moderate, 3= marked [44]. The mean cumulative lesion score calculated as the sum of individual lesion scores from each segment was considered for statistical analysis. Each dog was further assigned to group 1 or group 2 based on the presence of inflammation with only mild or no lacteal dilation, and inflammation with moderate or severe lacteal dilation, respectively.
Information gathered from the medical records on signalment, body weight (kg), and canine chronic enteropathy clinical activity index (CCECAI) score [45] was studied, in addition to the type of diet and ongoing anti-inflammatory or immunosuppressive therapies at admission. The CCECAI score was calculated using the serum albumin concentration, presence or absence of peripheral edema and peritoneal effusion on ultrasound examination, and the owner’s scores on appetite, activity level, vomiting, fecal consistency and frequency, weight loss and pruritus. Four disease severity groups were identified based on the CCECAI scores: mild disease (CCECAI 4-5; MI), moderate disease (CCECAI 6-8; MO), severe disease (CCECAI 9-11; S) and very severe disease (CCECAI ≥12; VS) [45].
The control group included healthy owned-dogs, regularly vaccinated, and receiving appropriate ecto-and endo-parasite preventive treatment. All healthy dogs were belonging to staff at the Veterinary Teaching Hospital, Department of Veterinary Sciences, University of Turin, Italy, or were presented at the same facilities of the study group for their annual check-up and vaccination. Dogs were considered healthy based on unremarkable history and physical examination, complete blood count and serum biochemistry, negative fecal flotation, and absence of any gastrointestinal sign within one year prior to enrollment. In addition, there was no history drug administration in the 6 months before.
2.2. Sample Collection, Lipid Profile, CRP, and PON-1 Activity
The serum left-over samples obtained from centrifugation of blood collected from each dog at admission and after 12h of fasting were separated and immediately stored at -80°C until analysis. Sample storage varied from 3 to 18 months.
All analyses were performed at the clinical pathology laboratory of the Veterinary Teaching Hospital of the University of Milan, Lodi, Italy. The evaluation of the lipid profile included Chol, TGs and lipoprotein classes. Frozen serum samples were thawed and used to measure serum concentrations of albumin (Alb), total proteins (TP), Chol, TGs, and CRP. All these analytes were measured on the automated chemistry analyzer BT 3500 (Biotecnica Instruments SPA, Rome, Italy) using reagents and methods provided by Futurlab Srl (Limena, PD, Italy). Specifically, Chol and TGs were measured with the colorimetric enzymatic CHOD-PAP and GPO-PAP methods, respectively. PON-1 activity was also measured on the same instrument, using the reagent and method validated in dogs, as previously described [42]. Based on serum albumin concentration, dogs were further assigned to group A (2.2 to 2.8 g/dL), B (1.5 to 2.19 g/dL), C (1.2 to 1.49 g/dL), and D (<1.2 g/dL). Lipoprotein analysis was carried out on the same serum samples on buffered (pH 8.5) agarose gel with a semi-automated instrument (Hydrasis, Sebia Italia S.r.l.), using kits produced by the manufacturer (Hydragel 15 lipoproteins). After migration (160 V, 25 min), agarose gels (8 g/L) were stained with Sudan black, washed with ethanol (45%), dried, and placed on the gel scanner for the densitometric analysis. Scanned images were analyzed using the software Phoresis (Sebia Italia S.r.l., Bagno a Ripoli, Italy) that calculates the area under the peaks corresponding to HDLs, VLDLs, LDLs and chylomicrons and expresses the results as a percentage of the total area (HDL%, VLDL%, LDL%, chylomicrons%).
2.3. Statistical Analysis
All data were analyzed with the software GraphPad Prism 9.5.1 (Dotmatics). Significance was set at p<0.05. Data were tested for normality by the Shapiro Wilk test. Data were reported as median, minimum, and maximum. Data were compared between healthy and diseased dogs by use of a Student t-test in case of normal distribution and Mann-Whitney U-test in case of non-normal distribution. All comparisons among groups were performed by use of One-way Anova test or Kruskal-Wallis test dependent on normality test. P-values were adjusted with Dunn’s multiple comparison test. Due to the low numerosity, data from groups C and D were merged in a single group (C+D). Correlations were assessed by use of Spearman correlation test.
3. Results
3.1. Patient Data
Among healthy control dogs, 24 (13 spayed) were female and 16 (7 neutered) were male. Seven dogs were mixed breed (17.5%), and 33 were purebred (82.5%). Median age was 48 months (range 12-208), median body weight was 15.2 kg (range 2.5-42). Most of the healthy control dogs were fed commercial nutritionally complete and balanced canine diets of different brands.
Among dogs with iPLE, 20 (39.2%) were female (15 spayed) and 31 (60.8%) were male (2 neutered). Eight dogs were mixed-breed (15.7%), 43 dogs were purebred (84.3%) represented as follows: German Shepherd (9 dogs), Golden Retriever, English Setter, and Yorkshire Terrier (3 dogs each breed), Australian Shepherd, Border Collie, Chihuahua, Labrador Retriever, Maltese Dog, and Spanish greyhound (2 dogs each breed), American Staffordshire Terrier, Belgian Shepherd, Boston Terrier, Cavalier King Charles, Cesky Terrier, Cocker Spaniel, Dachshund, Doberman Pinscher, Jack Russell Terrier, Pitbull, Podenco ibicenco, Pug, and Rottweiler (1 dog each breed). Median age was 84 months (range 19-171), median body weight was 15 kg (range 2.4-47.5), and median CCECAI score was 9 (range 3-17). Six, 19, 13, and 13 dogs were assigned to group MI, MO, S, and vs. severity subgroup, respectively. Sex and body weight did not significantly differ between healthy and diseased dogs. Age was significantly higher in the iPLE dogs compared to the healthy dogs (p < 0.0001). All dogs had gastrointestinal duodenoscopy performed. Thirty-four dogs (66.7%) had concurrent lower GI endoscopy in which the ileum was successfully intubated in 11 dogs (21.6%). On histopathology, a predominantly lymphoplasmacytic infiltration of the intestinal mucosa was found in all dogs. With regard to the histologic lesion severity, mild (grade 1) duodenal, ileal and colonic histologic lesions were found in 0, 0, and 4 dogs, respectively; moderate (grade 2) duodenal, ileal, and colonic histologic lesions were found in 25, 9, and 29 dogs, respectively; marked (grade 3) duodenal, ileal, and colonic histologic lesions were found in 26, 2, and 1 dog, respectively. Dilated crypts with proteinaceous material and cellular debris (crypt abscesses) were identified in 10 dogs (19.6%), and they were further classified as mild (n=4; 7.8%), moderate (n=3; 5.9%), and marked (n=3; 5.9%). Lacteal dilation was identified in 40 dogs (78.4%), and it was further classified as mild (n=17, 42.5%), moderate (n=22, 55%), and marked (n=1, 2.5%). Twenty-seven dogs were further assigned to group 1 (inflammation with only mild or no lacteal dilation), 24 to group 2 (inflammation with moderate or severe lacteal dilation). At admission, 12 dogs were on highly digestible gastrointestinal commercial diets, 3 on highly digestible low-fat commercial diets, 15 on limited ingredient commercial diets, 9 on home-cooked low-fat diets, and 8 on hydrolyzed diets. Four dogs were fed different diet types. In addition to the diet, 13 dogs were on prednisolone. Doses (0.5-1 mg/kg SID) and length of administration (some days to 3 weeks) were depending on the case.
3.2. Comparative Evaluation of the Lipid Profile and Other Laboratory Parameters between Healthy Control Dogs and iPLE Dogs
Results recorded in healthy control and iPLE dogs are reported in Table 1 and, as regards the lipid profile, in Figure 1. Serum concentrations of Alb, TP, Chol, HDLs, VLDLs, and PON-1 activity were significantly lower in iPLE dogs compared to healthy control dogs. Serum concentration of TGs, LDLs, chylomicrons and CRP were significantly higher in iPLE dogs compared to healthy control dogs. Among iPLE dogs, 30 and 39 showed decreased Chol, and PON-1 activity, respectively, while 26 and 13 showed increased CRP and TGs, respectively. Based on the concentrations of serum albumin, 6 dogs (11.8%) were assigned to group A, 29 (56.9%) to group B, 12 (23.5%) to group C, and 4 (7.8%) to group D.
3.3. Correlations of the CRP with CCECAI Scores, Histopathological Findings, and Prednisolone Therapy
A positive weak correlation between CCECAI scores and CRP (r = 0.28, p = 0.042) was found. The mean cumulative lesion score did not correlate with CRP. No significant difference in CRP was found between group 1 (inflammation with only mild or no lacteal dilation) and 2 (inflammation with moderate or severe lacteal dilation), nor between dogs that were on prednisolone at admission and dogs that were not.
3.4. Correlations of the Lipid Profile with Age, Gender, Body Weight, CCECAI Score, Alb, CRP, and PON-1 Activity Concentrations
Significant correlations of the lipid profile and age, body weight, PON-1 activity, and CRP are summarized in Table 2. Significant correlations or differences among groups between the lipid profile and the CCECAI score, or the CCECAI disease severity groups were not found. Significant correlations between the lipid profile and Alb were not found. However, when considering the hypoalbuminemia groups, some significant differences regarding Chol (p = 0.015), HDLs (p = 0.001), VLDLs (p = 0.026), and LDLs (p = 0.048) were found. More specifically, Chol and HDLs were significantly lower in group C+D compared to B (adjusted p = 0.015 and adjusted p = 0.0008, respectively), and VLDLs were significantly higher in group C+D compared to A (adjusted p = 0.045). LDLs didn’t significantly differ at Dunn’s multiple comparison test. Serum concentrations of Chol, HDL, LDL and VLDL lipoprotein classes in dogs classified by hypoalbuminemia groups are reported in Figure 2.
3.5. Correlations of the Lipid Profile with Histopathological Findings, Prednisolone Therapy and Diet
No significant correlations between the lipid profile and the mean cumulative lesion score were found. By the comparison of the lipid profile between dogs of the group 1 (inflammation with only or no lacteal dilation) and group 2 (inflammation with moderate or severe lacteal dilation), TGs were significantly higher in dogs of the group 2 (p = 0.0045).
No significant differences in the lipid profile between dogs that were on prednisolone at admission and dogs that were not were found. No significant differences in the lipid profile among dogs classified based on the type of diet upon admission were found.
4. Discussion
We described and compared the lipid profile, CRP and PON-1 activity between healthy control dogs and dogs with iPLE, and explored associations among clinicopathological data, histopathological findings, and lipid profile in dogs with iPLE.
Hypoalbuminemia is the hallmark of the PLE syndrome in dogs; however, hypocholesterolemia can be observed and it can help in define the prognosis [1,7,46]. To the authors’ knowledge and unlike in people, scarce or no information is available on serum TGs and lipoprotein changes in dogs with CIE [47]. Recently, the lipoprotein profile of cavitary effusions was investigated in some dogs with PLE, but the same analysis was not performed on serum samples [47]. Transudates of dogs with PLE were found to be poor in protein, cholesterol, and HDL contents and high in VLDL and LDL contents [47]. To date, the diagnosis of canine dyslipidemia is based on the measurement of pre-prandial serum cholesterol and triglyceride concentrations, while the lipoprotein analysis is not routinely used. Some explanations can be attempted. A gold standard validated method for the evaluation of canine lipoproteins is currently lacking; different techniques can be used, some of them developed for human use, expensive, time and labor consuming or not suitable for canine lipoprotein analysis [30,32,36,37,47,48,49,50]. Accordingly, information concerning reliable reference intervals for the different lipoprotein classes is lacking, results are poorly reproducible and not always comparable. Agarose gel electrophoresis is used to classify lipoproteins by the nomenclature beta, pre-beta, and alpha based on the mobility of LDLs, VLDLs, and HDLs, respectively [51]. This method was selected here because it provides accurate separation of canine lipoproteins, and it might be superior to the wet chemistry method for identifying some lipoprotein classes, especially LDLs which are substantial in some dogs [30,35,48,52]. However, it has not been extensively investigated for use in canine gastroenterology.
In both healthy control dogs and iPLE dogs, the HDL lipoprotein class was predominant, followed by the LDL and VLDL lipoprotein classes, respectively. According to the available information, these results are not surprising since the HDL is the predominant lipoprotein class in dogs, whereas in humans, LDL class predominates [53]. On the other hand, there are neither reports regarding lipoprotein profiles in dogs with iPLE, nor published solid reference intervals obtained by agarose gel electrophoresis to be used here for comparison. Hypocholesterolemia, variable levels of HDLs and LDLs, and normal or increased levels of TGs are common findings in humans with active IBD [20,21,22,23,26,54,55]. Moreover, some of these findings are independently associated with more severe disease [23]. Similarly, our iPLE dogs showed significantly decreased serum concentrations of Chol, and percentage of HDLs and VLDLs compared to healthy controls, while concentrations of TGs were normal or increased [26]. However, it cannot be ruled out that some dogs with normal TGs have had hypotriglyceridemia, since there is not a lower reference limit for TGs. Hypocholesterolemia, presumably secondary to inflammation, lymphangiectasia, fat malabsorption and malnutrition, has been already documented among dogs with iPLE, while scattered information is available for serum TGs [7,11,56]. Hypocholesterolemia is a common feature in human patients with acute diseases, and it has been related to surrogate markers of disease severity including IBD-related surgeries and number of hospitalizations [57,58].
In this study, no significant correlations were found between disease severity, as assessed by the CCECAI score, and the lipid profile. Some hypotheses may be attempted. The CCECAI score, although routinely used to assess the clinical severity of canine CIE, might not be appropriate to identify subsets of dogs with severe intestinal inflammation and lipid malabsorption. Moreover, the severity of lipid malabsorption could not play a pivotal role in the clinical manifestation of some dog.
Similar to previous results, CRP was significantly increased in iPLE dogs when compared with healthy dogs [59,60]. Our results were also in accordance with previously published results showing a correlation between CRP and clinical severity scores in dogs with CIE [59]. However, no correlations or differences among groups between CRP concentration and histopathological finding or ongoing prednisolone therapy at admission in iPLE dogs were found. Therefore, in some dogs with iPLE, CRP might be not useful for assessing histopathologic severity of inflammation and lacteal dilation, nor for assessing the response to treatment, in contrast with previous observations [59]. This discrepancy, however, might be because of differences in population such as number of dogs that had ileal and colonic evaluation, number of dogs that were on prednisolone at admission, dosages and length of prednisolone administration). PON-1 activity was significantly decreased in iPLE dogs when compared with healthy dogs. Since the decrease of PON-1 activity seems to couple with inflammation, our results might suggest that oxidation and inflammation might occur in some dogs with iPLE [61]. During inflammation, one of the most consistent alterations is the reduced serum concentration of HDLs [62]. In our study, a negative correlation between serum CRP concentration and percentage of HDLs has been found, as previously observed [63]. Indeed, HDLs are thought to have anti-inflammatory properties [64]. One additional property of HDLs is to prevent the LDLs from oxidation, and several HDL-related proteins, such as ApoA1, PON-1 and transferrin could affect this important function [65,66]. The anti-oxidative function of HDLs is strongly associated with disease severity, while the use of anti-inflammatory treatments significantly restores the antioxidant functions of HDLs towards normal [67,68]. In this study, positive correlations were found between PON-1 activity concentrations and HDLs; while negative correlations were found between PON-1 activity concentrations and LDLs, and between CRP concentrations and HDLs. Taken together, these results might support the hypothesis that changes in HDLs depend on oxidative stress likely associated with inflammation. Therefore, similarly to human medicine, canine HDLs might have anti-inflammatory and anti-oxidative properties, while LDLs might play a pro-inflammatory role [63]. Furthermore, positive correlations between age and Chol, and body weight and Chol were found in iPLE dogs, suggesting that biochemical characteristics of lipid metabolism disorders may be affected by aging and weight [69,70].
Significant associations between the lipid profile and Alb were not found. However, when considering the hypoalbuminemia groups, Chol and HDLs were significantly lower in group C+D compared to B, while VLDLs were significantly higher in group C+D compared to group B. These results are likely explained by the lipoprotein lipid content and function, along with inflammation, lymphangiectasia, fat malabsorption and malnutrition leading to hypocholesterolemia in dogs with iPLE. Indeed, HDLs is a major carrier of the total circulating lipid, especially free and esterified cholesterol in dogs, while TGs account for a significant proportion of the lipids in VLDLs and LDLs, but not in HDLs [71].
No significant associations between the lipid profile and the mean cumulative lesion score were found. This result is not surprising since the mean cumulative lesion score might have been influenced by the lack of ileal and colonic mucosa evaluation in some iPLE dogs. However, since iPLE is a heterogeneous disorder and affected dogs might show different magnitude of intestinal inflammation and lymphangiectasia, we compared the lipid profile between dogs of the group 1 (inflammation with only mild or no lacteal dilation) and group 2 (inflammation with moderate or severe lacteal dilation). By this comparison, TGs were significantly higher in dogs of the group 2. It can be hypothesized that these dogs with both inflammation and moderate or severe lacteal dilation, similarly to some patients with IBD, are characterized by a severe mucosal immune system dysregulation. This dysregulation leads to an increase of inflammatory cytokines that, in turn, may result in a decrease in lipoprotein lipase enzyme activity, leading to a characteristic lipoprotein profile with increased serum triglycerides and decreased HDLs [72].
In human patients with IBD, the administration of glucocorticoids increases total Chol, HDLs and LDLs [20]. The increase in HDLs and LDLs can be explained by an increase of VLDLs synthesis and lipoprotein lipase activity [73]. To date, glucocorticoids are a commonly prescribed treatment for dogs with CIE. Indeed, glucocorticoids in addition to a dietary change, seem to be appropriate for some dogs with iPLE [11,72]. For these reasons, dogs that were on prednisolone at admission were not excluded from this study. Percentages of different lipoprotein classes of dogs receiving prednisolone did not significantly differ from those not receiving prednisolone. Moreover, neither Chol nor TGs concentrations were significantly different among dogs assigned to these 2 therapeutic subgroups. Although not expected, these results might have been influenced by the low number of dogs receiving prednisolone prior to the admission, low doses and different length of prednisolone administration.
For dogs with iPLE and evidence of lymphangiectasia, low-fat diets alone or combined with prednisolone are proven to be useful [1,2,3,14,15,74,75]. However, to the best of the authors’ knowledge, no information exists on the influence of diets on lipoprotein profiles in dogs with iPLE. At the time of admission, the dogs of this study were fed different commercial and home-cooked diets, some of which were low-fat in content, that had previously been selected on a case-by-case basis. Some canine limited ingredient diets are fish- or vegetal-based diets, while highly digestible gastrointestinal diets are usually meat-based diets. Fish- and vegetal-based diets have been demonstrated to lower LDLs in dogs and humans, respectively [76,77]. No significant differences in the lipid profile among our iPLE dogs assigned to different diet groups were found. However, these results might have been influenced by the type of grouping. Indeed, the diet subgroups were by diet type and not by content of fat. Diet types have wide ranging amounts of fat, and there is also wide variation in fat among limited ingredient diets and highly digestible gastrointestinal commercial diets.
This study included some limitations. First, the lack of information about the gold standard method for lipoprotein evaluation. Without this information, the clinical utility of some data presented here cannot be interpreted reliably. Second, the results of the lipoprotein agarose gel electrophoresis are expressed as a percentage of the total area. Therefore, the increase of a lipoprotein class determines the reduction of another class, thus limiting the possibility of demonstrating an absolute increase of a lipoprotein class. Third, the diet subgroups were by diet type and not by content of fat, as already described above. This mainly because the exact information about the brand of diets prescribed before admission was missing or not precise. Fourth, not all iPLE dogs had ileal and colonic mucosa evaluated. This influenced the mean cumulative lesion score and potentially also the associations with the lipid profile. Finally, the storage stability of serum samples at -80°C was not assessed. However, it was recently observed that canine serum lipoproteins are stable for several months when stored at −80°C, similarly to the storage of our samples [78].
5. Conclusions
Our study is the first to describe the lipid profile in dogs with iPLE. Overall, significant differences in percentages of serum lipoprotein classes, CRP and PON-1 activity between dogs with iPLE and healthy control dogs were found. Moreover, triglycerides were significantly higher in dogs with both intestinal inflammation and lymphangiectasia. Some of these findings are consistent with previous studies in humans with IBD, suggesting that lipid metabolism is also affected in dogs with iPLE. Additional investigation into the lipid metabolism of dogs with iPLE is warranted.
Author Contributions
Conceptualization and methodology, A.G., P.G., S.P.; formal analysis, F.C.; investigation, A.B., B.B., E.B., F.B., F.C., R.F., S.M., D.S.; writing—original draft preparation, P.G.; writing-review and editing, F.C., A.G., P.G., S.P. All authors have read and agreed to the published version of the manuscript.
Funding
The analyses were performed at the laboratory of the Department of Veterinary Medicine, University of Milan, Italy, at the laboratory’s own costs.
Institutional Review Board Statement
The study was approved by the Institutional Ethics and Animal Welfare Committee (protocol number 42/2021) of the Department of Veterinary Sciences, Turin University.
Informed Consent Statement
The owners gave written informed consent to their dog’s enrollment in the study. The work involved the use of non-experimental animals only, and followed high standards (“best practice”) of individual veterinary clinical patient care. All samples (blood) were obtained during standard veterinary diagnostic procedures.
Data Availability Statement
All data analyzed during this study are included in this published article.
Conflicts of Interest
Alessia Giordano, Sara Meazzi, Saverio Paltrinieri and Donatella Scavone are employed at the laboratory were the analyses were performed. The remaining authors declare no conflicts of interest.
References
- Dossin, O.; Lavoué, R. Protein-losing enteropathies in dogs. Vet. Clin. North Am. Small Anim. Pract. 2011, 41, 399–418. [Google Scholar] [CrossRef]
- Craven, M.D.; Washabau, R.J. Comparative pathophysiology and management of protein-losing enteropathy. J. Vet. Intern. Med. 2018, 33, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, K.; Iennarella-Servantez, C. Canine Protein Losing Enteropathies and Systemic Complications. Vet. Clin. North Am. Small Anim. Pract. 2021, 51, 11–122. [Google Scholar] [CrossRef] [PubMed]
- Rovenská, E.; Rovenský, J. Lymphatic vessels: structure and function. Isr. Med. Assoc. J. 2011, 13, 762–768. [Google Scholar] [PubMed]
- Craven, M.; Simpson, J.W.; Ridyard, A.E.; Chandler, M.L. Canine inflammatory bowel disease: retrospective analysis of diagnosis and outcome in 80 cases (1995-2002). J. Small Anim. Pract. 2004, 45, 336–342. [Google Scholar] [CrossRef]
- Kull, P.A.; Hess, R.S.; Craig, L.E.; Saunders, H.M.; Washabau, R.J. Clinical, clinicopathologic, radiographic, and ultrasonographic characteristics of intestinal lymphangiectasia in dogs: 17 cases (1996-1998). J. Am. Vet. Med. Assoc. 2001, 219, 197–202. [Google Scholar] [CrossRef]
- Gianella, P.; Lotti, U.; Bellino, C.; Bresciani, F.; Cagnasso, A.; Fracassi, F.; D’Angelo, A.; Pietra, M. Clinicopathologic and prognostic factors in short- and long-term surviving dogs with protein-losing enteropathy. Schweiz. Arch. Tierheilkd. 2017, 159, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.W. , Jergens, A.E. Pitfalls and progress in the diagnosis and management of canine inflammatory bowel disease. Vet. Clin. North Am. Small Anim. Pract. 2011, 41, 381–398. [Google Scholar] [CrossRef]
- Simmerson, S.M.; Armstrong, P.J.; Wünschmann, A.; Jessen, C.R.; Crews, L.J.; Washabau, R.J. Clinical features, intestinal histopathology, and outcome in protein-losing enteropathy in Yorkshire Terrier dogs. J. Vet. Intern. Med. 2014, 28, 331–337. [Google Scholar] [CrossRef]
- Alexander, J.S.; Ganta, V.C.; Grisham, M.B. Emerging roles of lymphatics in inflammatory bowel disease. Ann. N. Y. Acad. Sci. 2010, Suppl 1, E75–85. [Google Scholar] [CrossRef]
- Salavati Schmitz, S.; Gow, A.; Bommer, N.; Morrison, L.; Mellanby, R. Diagnostic features, treatment, and outcome of dogs with inflammatory protein-losing enteropathy. J. Vet. Intern. Med. 2019, 33, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Dandrieux, J.R.S.; Noble, P.J.M.; Scase, T.J.; Cripps, P.J.; German, A.J. Comparison of a chlorambucil-prednisolone combination with an azathioprine-prednisolone combination for treatment of chronic enteropathy with concurrent protein-losing enteropathy in dogs: 27 cases (2007–2010). J. Am. Vet. Med. Assoc. 2013, 242, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Hiyoshi, S.; Ohno, K.; Uchida, K.; Goto-Koshino, Y.; Maeda, S.; Mizutani, N.; Takeuchi, A.; Tsujimoto, H. Prognostic factors in dogs with protein-losing enteropathy. Vet. J. 2015, 205, 28–32. [Google Scholar] [CrossRef]
- Okanishi, H.; Yoshioka, R.; Kagawa, Y.; Watari, T. The clinical efficacy of dietary fat restriction in treatment of dogs with intestinal lymphangiectasia. J. Vet. Intern. Med. 2014, 28, 809–81715. [Google Scholar] [CrossRef]
- Rudinsky, A.J.; Howard, J.P.; Bishop, M.A.; Sherding, R.G.; Parker, V.J.; Gilor, C. Dietary management of presumptive protein-losing enteropathy in Yorkshire terriers. J. Small Anim. Pract. 2017, 58, 103–108. [Google Scholar] [CrossRef]
- Jablonski, S.A. Pathophysiology, diagnosis, and management of canine intestinal lymphangiectasia: a comparative review. Animals (Basel). 2022, 12, 2791. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Kathrani, A. Incidence of relapse of inflammatory protein-losing enteropathy in dogs and associated risk factors. J. Vet. Intern. Med. 2022, 36, 1981–1988. [Google Scholar] [CrossRef]
- Ohta, H.; Nagata, N.; Yokoyama, N.; Osuga, T.; Sasaki, N.; Morishita, K.; Takiguchi, M. Prognostic value of small intestinal dilatation in dogs with protein-losing enteropathy. J. Vet. Med. Sci. 2021, 11, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, K.; Rizzo, J.; Jergens, A.E.; Chang, Y.M. Hypovitaminosis D is associated with negative outcome in dogs with protein losing enteropathy: a retrospective study of 43 cases. BMC Vet. Res. 2017, 13, 96. [Google Scholar] [CrossRef]
- Sleutjes, J.A.M.; Roeters van Lennep, J.E.; van der Woude, C.J.; de Vries, A.C. Lipid Changes After Induction Therapy in Patients with Inflammatory Bowel Disease: Effect of Different Drug Classes and Inflammation. Inflamm. Bowel Dis. 2023, 29, 531–538. [Google Scholar] [CrossRef]
- Agouridis, A.P.; Elisaf, M.; Milionis, H.J. An overview of lipid abnormalities in patients with inflammatory bowel disease. Ann. Gastroenterol. 2011, 24, 181–187. [Google Scholar] [PubMed]
- Ripollés-Piquer, B.R.; Nazih, H.; Bourreille, A.; Segain, J.P.; Huvelin, J.M.; Galmiche, J.P.; Bard, J.M. Altered lipid, apolipoprotein, and lipoprotein profiles in inflammatory bowel disease: consequences on the cholesterol efflux capacity of serum using Fu5AH cell system. Metabolism 2006, 55, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Koutroumpakis, E.; Ramos-Rivers, C.; Regueiro, M.; Hashash, J.G.; Barrie, A.; Swoger, J.; Baidoo, L.; Schwartz, M.; Dunn, M.A.; Koutroubakis, J.E.; Binion, D.G. Association Between Long-Term Lipid Profiles and Disease Severity in a Large Cohort of Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 2016, 61, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kullo, I.J.; Pardi, D.S.; Loftus, E.V. Epidemiology, risk factors and management of cardiovascular diseases in IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 26–35. [Google Scholar] [CrossRef]
- Sammalkorpi, K.T.; Valtonen, V.V.; Maury, CP. Lipoproteins and acute phase response during acute infection. Interrelationships between C-reactive protein and serum amyloid-A protein and lipoproteins. Ann. Med. 1990, 22, 397–401. [Google Scholar] [CrossRef]
- Romanato, G.; Scarpa, M.; Angriman, I.; Faggian, D.; Ruffolo, C.; Marin, R.; Zambon, S.; Basato, S.; Zanoni, S.; Filosa, T.; Pilon, F.; Manzato, E. Plasma lipids and inflammation in active inflammatory bowel disease. Aliment. Pharmacol. Ther. 2009, 29, 298–307. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Nordan, R.P.; McIntosh, J.; Calvo, J.C.; Scow, R.O.; Jablons, D. Interleukin 6 reduces lipoprotein lipase activity in adipose tissue of mice in vivo and in 3T3-L1 adipocytes: a possible role for interleukin 6 in cancer cachexia. Cancer Res. 1992, 52, 4113–4116. [Google Scholar] [PubMed]
- Szczeklik, K.; Mach, T.; Cibor, D.; Owczarek, D.; Sapa, J.; Papież, M.; Pytko-Polończyk, J.; Krzyściak, W. Correlation of Paraoxonase-1 with the Severity of Crohn’s Disease. Molecules. 2018, 23, 2603. [Google Scholar] [CrossRef]
- Boehm, D.; Krzystek-Korpacka, M.; Neubauer, K.; Matusiewicz, M.; Berdowska, I.; Zielinski, B.; Paradowski, L.; Gamian, A. Paraoxonase-1 status in Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2009, 15, 93–99. [Google Scholar] [CrossRef]
- Xenoulis, P.G.; Cammarata, P.J.; Walzem, R.L.; Macfarlane, R.D.; Suchodolski, J.S.; Steiner, J.M. Novel lipoprotein density profiling in healthy dogs of various breeds, healthy Miniature Schnauzers, and Miniature Schnauzers with hyperlipidemia. BMC Vet. Res. 2013, 9, 47. [Google Scholar] [CrossRef]
- Whitney, M.S.; Boon, G.D.; Rebar, A.H.; Ford, R.B. Effects of acute pancreatitis on circulating lipids in dogs. Am. J. Vet. Res. 1987, 48, 1492–1497. [Google Scholar] [PubMed]
- Gianella, P.; Caccamo, R.; Bellino, C.; Bottero, E.; Fietta, F.; Roncone, S.; Ostanello, F.; Pietra, M.; Buracco, P. Evaluation of metabolic profile and C-reactive protein concentrations in brachycephalic dogs with upper airway obstructive syndrome. J. Vet. Intern. Med. 2019, 33, 2183–2192. [Google Scholar] [CrossRef] [PubMed]
- Behling-Kelly, E. Serum Lipoprotein Changes in Dogs with Renal Disease. J. Vet. Intern. Med. 2014, 28, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Ibba, F.; Rossi, G.; Meazzi, S.; Giordano, A.; Paltrinieri, S. Serum concentration of high-density lipoproteins (HDLs) in leishmaniotic dogs. Res. Vet. Sci. 2015, 98, 89–91. [Google Scholar] [CrossRef]
- Rogers, W.A.; Donovan, E.F.; Kociba, G.J. Lipids and lipoproteins in normal dogs and in dogs with secondary hyperlipoproteinemia. J. Am. Vet. Med. Assoc. 1975, 166, 1092–1100. [Google Scholar] [PubMed]
- Mizutani, H.; Sako, T.; Arai, N.; Kuriyama, K.; Yoshimura, I.; Mori, A.; Iwase, K.; Hirose, H. Application of gel permeation HPLC for lipoprotein profiling in dogs. J. Vet. Med. Sci. 2010, 72, 813–817. [Google Scholar] [CrossRef]
- Rossi, G.; Kuleš, J.; Rafaj, R.B.; Mrljak, V.; Lauzi, S.; Giordano, A.; Paltrinieri, S. Relationship between paraoxonase 1 activity and high-density lipoprotein concentration during naturally occurring babesiosis in dogs. Res. Vet. Sci. 2014, 97, 318–324. [Google Scholar] [CrossRef]
- Jergens, A.E.; Schreiner, C.A.; Frank, D.E.; Niyo, Y.; Ahrens, F.E.; Eckersall, P.D.; Benson, T.J.; Evans, R. A scoring index for disease activity in canine inflammatory bowel disease. J. Vet. Intern. Med. 2003, 17, 291–297. [Google Scholar] [CrossRef]
- Heilmann, R.M.; Berghoff, N.; Mansell, J.; Grützner, N.; Parnell, N.K.; Gurtner, C.; Suchodolski, J.S.; Steiner, J.M. Association of fecal calprotectin concentrations with disease severity, response to treatment, and other biomarkers in dogs with chronic inflammatory enteropathies. J. Vet. Intern. Med. 2018, 32, 679–692. [Google Scholar] [CrossRef]
- Tamura, Y.U.; Ohta, H.; Kagawa, Y.; Osuga, T.; Morishita, K.; Sasaki, N.; Takiguchi, M. Plasma amino acid profiles in dogs with inflammatory bowel disease. J. Vet. Intern. Med. 2019, 33, 1602–1607. [Google Scholar] [CrossRef]
- Equilino, M.; Théodoloz, V.; Gorgas, D.; Doherr, M.G.; Heilmann, R.M.; Suchodolski, J.S.; Steiner, J.M.; Burgener, I.A. Evaluation of serum biochemical marker concentrations and survival time in dogs with protein-losing enteropathy. J. Am. Vet. Med. Assoc. 2015, 246, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Giordano, A.; Pezzia, F.; Kjelgaard-Hansen, M.; Paltrinieri, S. ; Serum paraoxonase 1 activity in dogs: Preanalytical and analytical factors and correlation with C-reactive protein and alpha-2-globulin. Vet. Clin. Pathol. 2013, 42, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Carretón, E.; Cerón, J.J.; Martínez-Subiela, S.; Tvarijonaviciute, A.; Caro-Vadillo, A.; Montoya-Alonso, J.A. Acute phase proteins and markers of oxidative stress to assess the severity of the pulmonary hypertension in heartworm-infected dogs. Parasit. Vectors. 2017, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Washabau, R.J.; Day, M.J.; Willard, M.D.; Hall, E.J.; Jergens, A.E.; Mansell, J.; Minami, T.; Bilzer, T.W.; WSAVA International Gastrointestinal Standardization Group; et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J. Vet. Intern. Med. 2010, 24, 10–26. [Google Scholar] [CrossRef]
- Allenspach, K; Wieland, B.; Gröne, A.; Gaschen, F. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 2007, 21, 700–708. [Google Scholar] [CrossRef]
- Kathrani, A.; Parkes, G. A Preliminary Study of Modulen IBD Liquid Diet in Hospitalized Dogs with Protein-Losing Enteropathy. Animals. 2022, 12, 1594. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.H.; Behling-Kelly, E.; Borjesson, D.L. Lipoprotein profile of pleural and peritoneal transudates in dogs and cats. J. Vet. Intern. Med. 2022, 36, 464–472. [Google Scholar] [CrossRef]
- Behling-Kelly, E. Comparison of 2 electrophoretic methods and a wet-chemistry method in the analysis of canine lipoproteins. Vet. Clin. Pathol. 2016, 45, 124–134. [Google Scholar] [CrossRef]
- Suto, A.; Yamasaki, M.; Takasaki, Y.; Fujita, Y.; Abe, R.; Shimizu, H.; Ohta, H.; Takiguchi, M. Lc-ms/ms analysis of canine lipoproteins fractionated using the ultracentrifugation-precipitation method. J. Vet. Med. Sci. 2013, 75, 1471–1477. [Google Scholar] [CrossRef]
- Xenoulis, P.G.; Cammarata, P.J.; Walzem, R.L.; Suchodolsky, J.S.; Steiner, J.M. Serum triglyceride and cholesterol concentrations and lipoprotein profiles in dogs with naturally occurring pancreatitis and healthy control dogs. J. Vet. Intern. Med. 2020, 34, 644–652. [Google Scholar] [CrossRef]
- Noble, R.P. Electrophoretic separation of plasma lipoproteins in agarose gel. J. Lipid Res. 1968, 9, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Whitney, M.S.; Boon, G.D.; Rebar, A.H.; Story, J.A.; Bottoms, G.D. Ultracentrifugal and electrophoretic characteristics of the plasma lipoproteins of miniature schnauzer dogs with idiopathic hyperlipoproteinemia. J. Vet. Intern. Med. 1993, 7, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.D.G. Lipoprotein metabolism in dogs and cats. Comp. Haematol, Int, 1996, 6, 17–23. [Google Scholar] [CrossRef]
- Fan, F.; Mundra, P.A.; Fang, L.; Galvin, A.; Moore, X.L.; Weir, J.M.; Wong, G.; White, D.A.; Chin-Dusting, J.; Sparrow, M.P.; Meikle, P.J.; Dart, A.M. Lipidomic profiling in inflammatory bowel disease: Comparison between ulcerative colitis and crohn’s disease. Inflamm. Bowel Dis. 2015, 21, 1511–1518. [Google Scholar] [CrossRef]
- Sappati Biyyani, R.S.; Putka, B.S.; Mullen, K.D. Dyslipidemia and lipoprotein profiles in patients with inflammatory bowel disease. J. Clin. Lipidol. 2010, 4, 478–482. [Google Scholar] [CrossRef]
- Wennogle, S.A.; Stockman, J.; Webb, C.B. Prospective evaluation of a change in dietary therapy in dogs with steroid-resistant protein-losing enteropathy. J. Small Anim. Pract. 2021, 62, 756–764. [Google Scholar] [CrossRef]
- Giovannini, I.; Boldrini, G.; Chiarla, C.; Giuliante; Vellone, M.; Nuzzo, G. Pathophysiologic correlates of hypocholesterolemia in critically ill surgical patients. Intensive Care Med, 1999, 25, 748–751. [Google Scholar] [CrossRef]
- Fraunberger, P.; Nagel, D.; Walli, A.K.; Seidel, D. Serum cholesterol and mortality in patients with multiple organ failure. Crit. Care Med. 2000, 28, 3574–3575. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.S.; Lee, D.; Yun, T.; Koo, Y.; Chae, Y.; Kang, J.H.; Kang, B.T.; Yang, M.P.; Kim, H. Clinical signs, duodenal histopathological grades, and serum high-mobility group box 1 concentrations in dogs with inflammatory bowel disease. J. Vet. Intern. Med. 2021, 35, 2205–2214. [Google Scholar] [CrossRef]
- Otoni, C.O.; Heilmann, R.M.; García-Sancho, M.; Sainz, A.; Ackermann, N.R.; Suchodolski, J.S.; Steiner, J.M.; Jergens, A.E. Serologic and fecal markers to predict response to induction therapy in dogs with idiopathic inflammatory bowel disease. J. Vet. Intern. Med. 2018, 32, 999–1008. [Google Scholar] [CrossRef]
- Ruggerone, B.; Scavone, D.; Troìa, R.; Giunti, M.; Dondi, F.; Paltrinieri, S. Comparison of Protein Carbonyl (PCO), Paraoxonase-1 (PON1) and C-Reactive Protein (CRP) as Diagnostic and Prognostic Markers of Septic Inflammation in Dogs. Vet. Sci. 2021, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Feillet-Coudray, C.; Fouret, G.; Vigor, C.; Bonafos, B.; Jover, B.; Blachnio-Zabielska, A.; Rieusset, J.; Casas, F.; Gaillet, S.; Landrier, J.F.; Durand, T.; Coudray, C. Long-term measures of dyslipidemia, inflammation, and oxidative stress in rats fed a high-fat/high-fructose diet. Lipids. 2019, 54, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Khovidhunkit, W.; Kim, M.S.; Memon, R.A.; Shigenaga, J.K.; Moser, A.H.; Feingold, K.R.; Grunfeld, C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 2004, 45, 1169–1196. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Yu, R.; Gharavi, N.; Huang, W.; Ezra, N.; Lotfizadeh, A.; Anantharamaiah, G.M.; Alipour, N.; Van Lenten, B.J.; Reddy, S.T.; Marelli, D. High-density lipoprotein: Antioxidant and anti-inflammatory properties. Curr. Atheroscler. Rep. 2007, 9, 244–248. [Google Scholar] [CrossRef]
- Liu, D.; Ding, Z.; Wu, M.; Xu, W.; Qian, M.; Du, Q.; Zhang, L.; Cui, Y.; Zheng, J.; Chang, H.; Huang, C.; Lin, D.; Wang, Y. The apolipoprotein A-I mimetic peptide, D-4F, alleviates ox-LDL-induced oxidative stress and promotes endothelial repair through the eNOS/HO-1 pathway. J. Mol. Cell. Cardiol. 2017, 105, 77–88. [Google Scholar] [CrossRef]
- Lioudaki, S.; Verikokos, C.; Kouraklis, G.; Ioannou, C.; Chatziioannou, E.; Perrea, D.; Klonaris, C. Paraoxonase-1: characteristics and role in atherosclerosis and carotid artery disease. Curr. Vasc. Pharmacol. 2019, 17, 141–146. [Google Scholar] [CrossRef]
- Gomez Rosso, L.; Lhomme, M.; Merono, T.; Sorroche, P.; Catoggio, L.; Soriano, E.; Saucedo, C.; Malah, V.; Dauteuille, C.; Boero, L.; Lesnik, P.; Robillard, P.; Chapman, M.J.; Brites, F.; Kontush, A. Altered lipidome and antioxidative activity of small, dense HDL in normolipidemic rheumatoid arthritis: relevance of inflammation. Atherosclerosis. 2014, 237, 652–660. [Google Scholar] [CrossRef]
- Popa, C.; van Tits, L.J.; Barrera, P.; Lemmers, H.L.M.; van den Hoogen, F.H.J.; van Riel, P.L.C.M.; Radstake, T.R.D.J.; Netea, M.G.; Roest, M.; Stalenhoef, A.F.H. Anti-infammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann. Rheum. Dis. 2009, 68, 868–872. [Google Scholar] [CrossRef]
- Kawasumi, K.; Kashiwado, N.; Okada, Y.; Sawamura, M.; Sasaki, Y.; Iwazaki, E.; Mori, N.; Yamamoto, I.; Arai, T.; et al. Age effects on plasma cholesterol and triglyceride profiles and metabolite concentrations in dogs. BMC Vet. Res. 2014, 10, 57. [Google Scholar] [CrossRef]
- Shiho, U.; Yasuda, H.; Koketsu, Y. Lipoprotein cholesterol and triglyceride concentrations associated with dog body condition score; effect of recommended fasting duration on sample concentrations in Japanese private clinics. J. Vet. Med. Sci. 2015, 77, 1063–1069. [Google Scholar] [CrossRef]
- Maldonado, E.N.; Romero, J.R.; Ochoa, B.; Aveldaño, M.I. Lipid and fatty acid composition of canine lipoproteins. Comp. Biochem. Physiol. 2001, 128, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, R.R.; Sappati Biyyani, S.; Putka, B.s.; Mullen, K.D. Dyslipidemia and lipoprotein profiles in patients with inflammatory bowel disease J. Clin. Lipidol. 2010, 4, 478–82. [Google Scholar] [CrossRef]
- Ettinger, W.H.; Klinefelter, H.F.; Kwiterovitch, P.O. Effect of short-term, low-dose corticosteroids on plasma lipoprotein lipids. Atherosclerosis 1987, 63, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.; Martinez, S.A.; Shiroma, J.T.; Warson, A.T.; Hostutler, R.A. Prospective Evaluation of Low-Fat Diet Monotherapy in Dogs with Presumptive Protein-Losing Enteropathy. J. Am. Anim. Hosp. Assoc. 2023, 59, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Ohta, H.; Yokoyama, N.; Teoh, Y.B.; Nisa, K.; Sasaki, N.; Osuga, T.; Morishita, K.; Takiguchi, M. Clinical characteristics of dogs with food-responsive protein-losing enteropathy. J. Vet. Intern. Med. 2020, 34, 659–668. [Google Scholar] [CrossRef]
- Pasquini, A.; Luchetti, E.; Cardini, G. Plasma lipoprotein concentrations in the dog: The effects of gender, age, breed and diet. J. Anim. Physiol. Anim. Nutr. 2008, 92, 718–722. [Google Scholar] [CrossRef]
- Koch, C.A.; Kjeldsen, E.W.; Frikke-Schmidt, R. Vegetarian or vegan diets and blood lipids: a meta-analysis of randomized trials. Eur. Heart. J. 2023, 44, 2609–2622. [Google Scholar] [CrossRef]
- Minamoto, T.; Parambeth, J.C.; Walzem, R.L.; Payne, H.R.; Lidbury, J.A.; Suchodolski, J.S.; Steiner, J.M. Evaluation of density gradient ultracentrifugation serum lipoprotein profiles in healthy dogs and dogs with exocrine pancreatic insufficiency. J. Vet. Diagn. Invest. 2018, 30, 878–886. [Google Scholar] [CrossRef]
Figure 1.
Serum concentrations of cholesterol, triglycerides, HDL, VLDL, LDL, and chylomicron lipoprotein classes in healthy control dogs and iPLE dogs. Red lines indicate the median value. iPLE=inflammatory protein-losing enteropathy; HDLs=high-density lipoproteins; LDLs=low-density lipoproteins; VLDLs=very low-density lipoproteins.
Figure 1.
Serum concentrations of cholesterol, triglycerides, HDL, VLDL, LDL, and chylomicron lipoprotein classes in healthy control dogs and iPLE dogs. Red lines indicate the median value. iPLE=inflammatory protein-losing enteropathy; HDLs=high-density lipoproteins; LDLs=low-density lipoproteins; VLDLs=very low-density lipoproteins.

Figure 2.
Serum concentrations of cholesterol, HDL, LDL and VLDL lipoprotein classes in iPLE dogs assigned to hypoalbuminemia groups. Group A (2.2 to 2.8 d/dL), group B (1.5 to 2.19 g/dL), group C (1.2 to 1.49 g/dL), group D (<1.2 d/dL). Red lines indicate the median value. Chol=cholesterol; iPLE=inflammatory protein-losing enteropathy; HDLs=high-density lipoproteins; LDLs=low-density lipoproteins; VLDLs=very low-density lipoproteins.
Figure 2.
Serum concentrations of cholesterol, HDL, LDL and VLDL lipoprotein classes in iPLE dogs assigned to hypoalbuminemia groups. Group A (2.2 to 2.8 d/dL), group B (1.5 to 2.19 g/dL), group C (1.2 to 1.49 g/dL), group D (<1.2 d/dL). Red lines indicate the median value. Chol=cholesterol; iPLE=inflammatory protein-losing enteropathy; HDLs=high-density lipoproteins; LDLs=low-density lipoproteins; VLDLs=very low-density lipoproteins.

Table 1.
Comparative evaluation of the lipid profile, Alb, TP, CRP, and PON-1 activity concentrations between healthy control dogs and iPLE dogs.
Table 1.
Comparative evaluation of the lipid profile, Alb, TP, CRP, and PON-1 activity concentrations between healthy control dogs and iPLE dogs.
| Parameter | Control dogs | iPLE dogs | Reference interval | P-value |
|---|---|---|---|---|
| Alb (g/dL) | 3.6 (2.9-4.1) | 1.7 (0.8-2.7) | 2.8-4 | <0.0001 |
| Chol (mg/dL) | 209.5 (118-289) | 116 (59-327) | 135-270 | <0.0001 |
| Chylomicrons (%) | 2.1 (0.3-8.4) | 5.6 (0.5-22.8) | - | <0.0001 |
| CRP (mg/L) | 0.9 (0-14.6) | 5.4 (0-48.4) | ≤5 | =0.009 |
| HDLs (%) | 65.5 (44.4-79.5) | 52.3 (18.7-81.8) | - | <0.0001 |
| LDLs (%) | 15.8 (6.5-39.1) | 33.8 (13.7-64.3) | - | <0.0001 |
| PON-1 activity (U/mL) | 199 (129-303) | 77 (13.3-223) | ≥116 | <0.0001 |
| VLDLs (%) | 15.6 (8.1-30.4) | 6.6 (1.2-36.1) | - | <0.0001 |
| TGs (mg/dL) | 55 (11-122) | 83 (30-172) | <104 | <0.0001 |
| TP (g/dL) | 6.4 (4.8-8.9) | 4.1 (2.0-7.4) | 5.4-7.5 | <0.0001 |
Data are reported as median (minimum-maximum). Alb=albumin; Chol=total cholesterol; CRP=C-reactive protein; HDLs=high-density lipoproteins; iPLE=inflammatory protein-losing enteropathy; LDLs=low-density lipoproteins; PON-1=paraoxonase-1; VLDLs=very low-density lipoproteins; TGs=triglycerides; TP=total proteins.
Table 2.
Significant correlations between the lipid profile and selected study variables.
| Pair of variables tested for correlation |
Correlation coefficient (r) | Significance level (p) |
|---|---|---|
| HDLs and PON-1 | 0.60 | <0.0001 |
| HDLs and CRP | -0.28 | =0.048 |
| LDLs and body weight | -0.36 | =0.009 |
| LDLs and PON-1 | -0.68 | <0.0001 |
| Chol and age | 0.30 | =0.034 |
| Chol and body weight | 0.30 | =0.039 |
| Chol and PON-1 | 0.83 | <0.0001 |
| CRP and VLDLs | 0.46 | =0.001 |
Chol=total cholesterol; CRP=C-reactive protein; HDLs=high-density lipoproteins; LDLs=low-density lipoproteins; PON-1=paraoxonase-1. p < 0.05 indicates a significant correlation. The strength of the correlations is categorized as follows: 0.00–0.39 weak correlation; 0.40-0.59 moderate correlation; 0.60-0.79 strong correlation; 0.80-1.00 very strong correlation.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Comparative Evaluation of Peripheral Blood Neutrophil to Lymphocyte Ratio, Serum Albumin to Globulin Ratio and Serum C-Reactive Protein to Albumin Ratio in Dogs with Inflammatory Protein-Losing Enteropathy and Healthy Dogs
Federica Cagnasso
et al.
Animals,
2023
Clinicopathological and Fecal Proteome Evaluations in 16 Dogs Presenting Chronic Diarrhea Associated with Lymphangiectasia
Giacomo Rossi
et al.
Veterinary Sciences,
2021
The Fatty Acid-Based Erythrocyte Membrane Lipidome in Dogs with Chronic Enteropathy
Paolo Crisi
et al.
Animals,
2021
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






