Submitted:
11 September 2024
Posted:
12 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Sample Collection and Preparation
Sample Digestion and Mineral Elements Determination
Proximate Analysis
Quantitative Determination of Phytochemicals
Determination of Antioxidant Vitamins
Determination of Amino Acids Profile
Antioxidant Activity
- DPPH Radical Scavenging Activity:
- 2.
- Ferric Reducing Antioxidant Power (FRAP):
- 3.
- Total Phenolic Content (TPC):
Thiobarbituric Acid (TBA) Assay
Statistical Analysis
3. Results
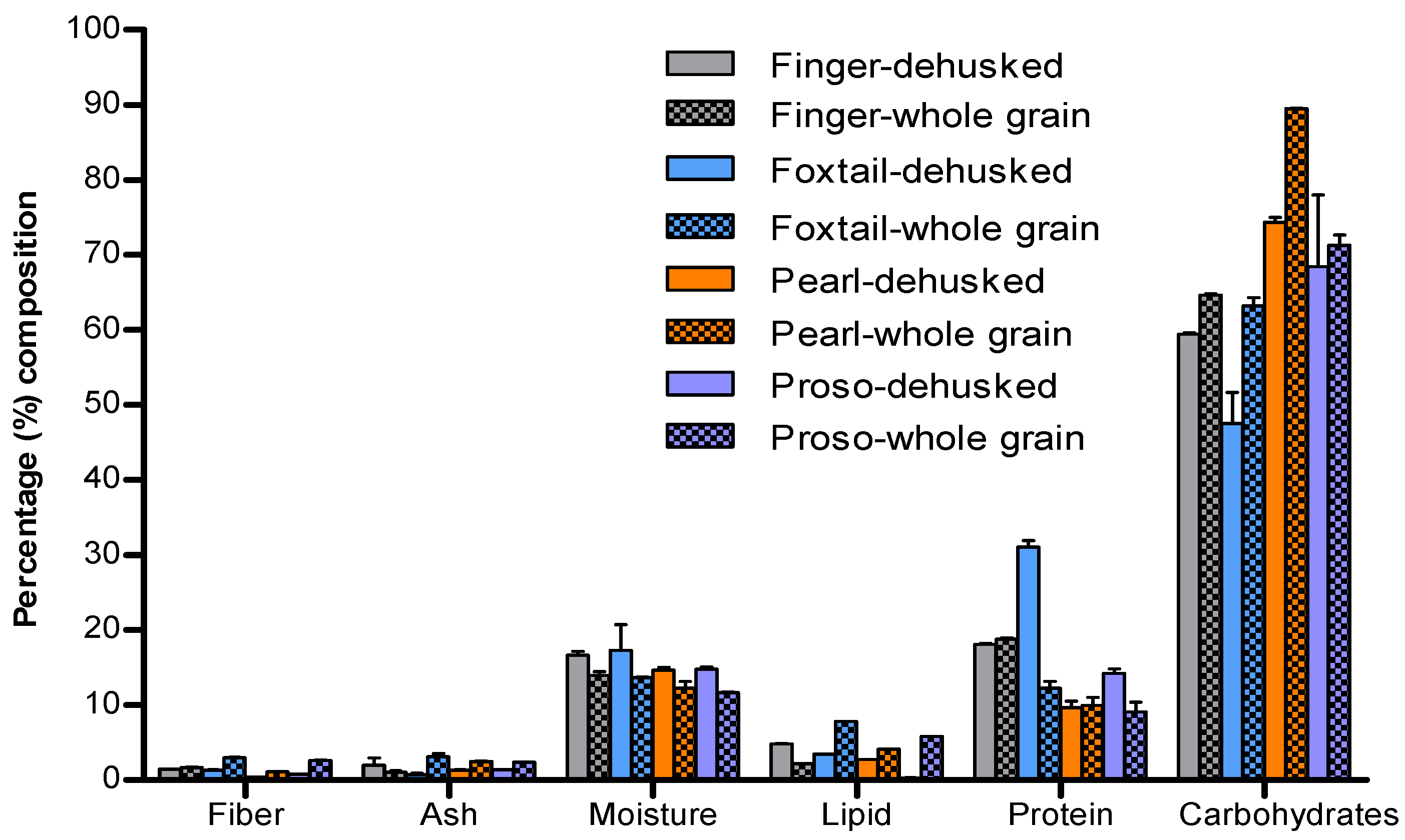
Proximate Composition of Millet Varieties
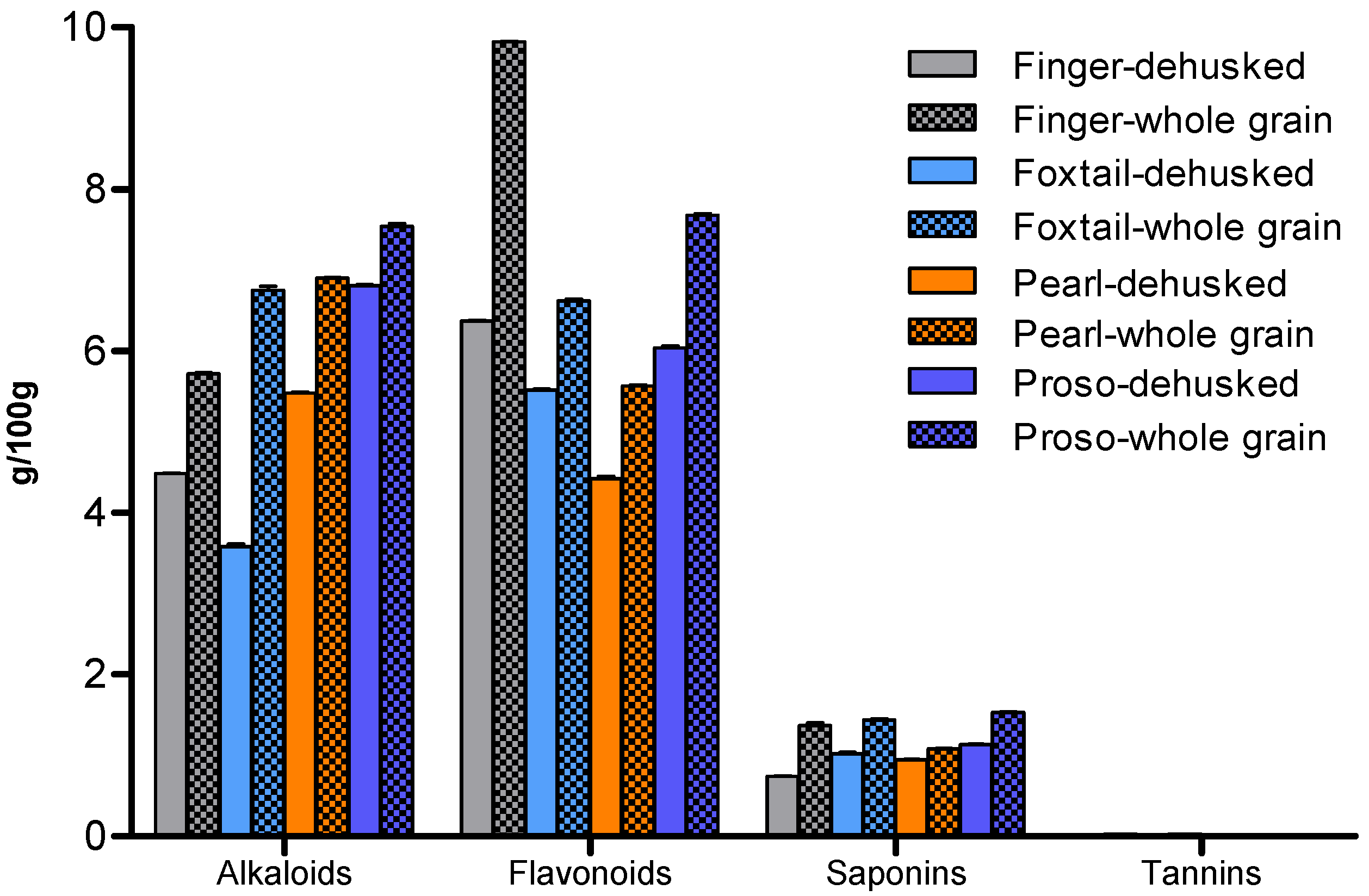
Phytochemical Composition of Millet Varieties
Amino Acid Composition of Millet Varieties
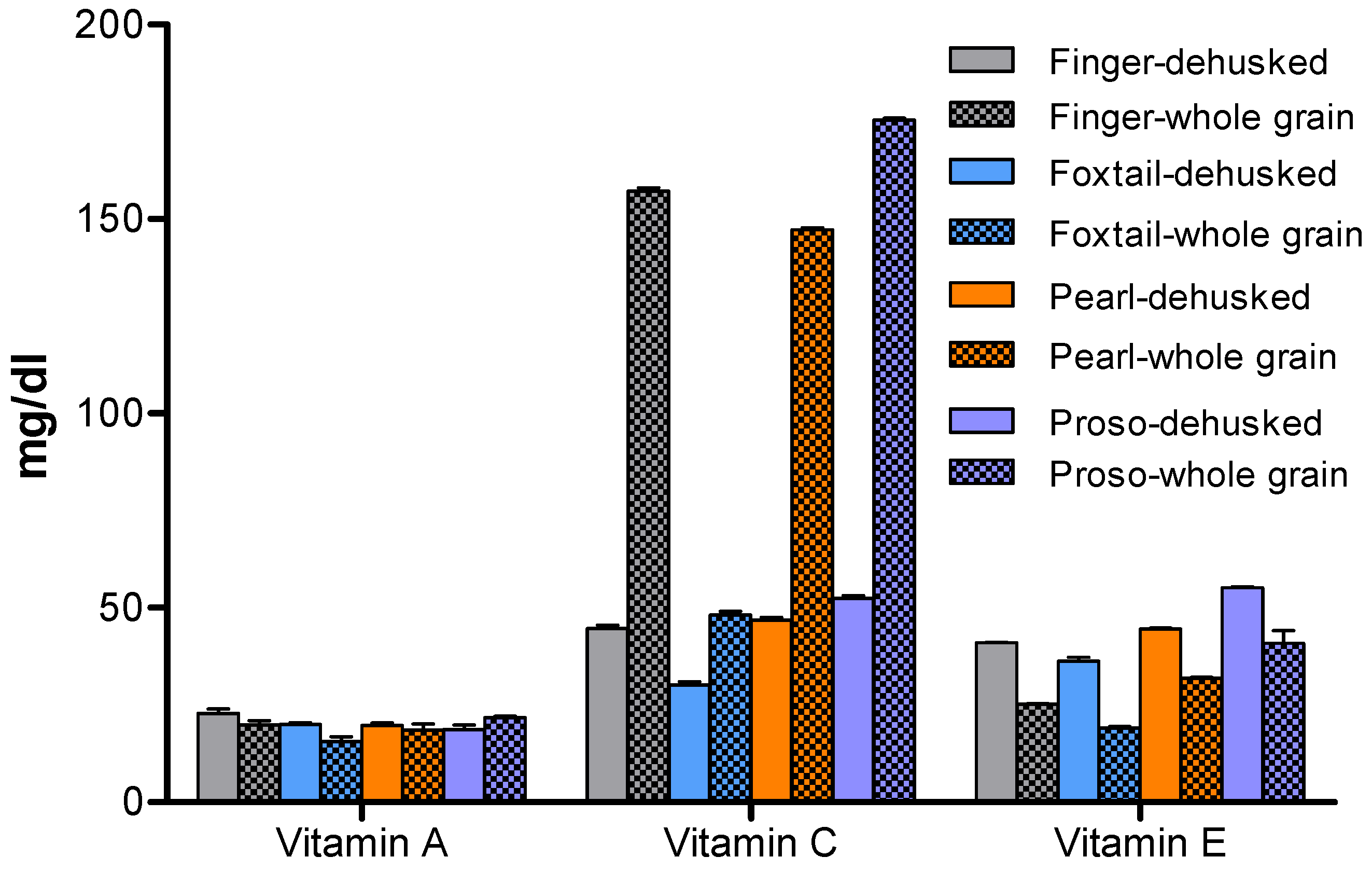
Vitamin Composition of Millet Varieties
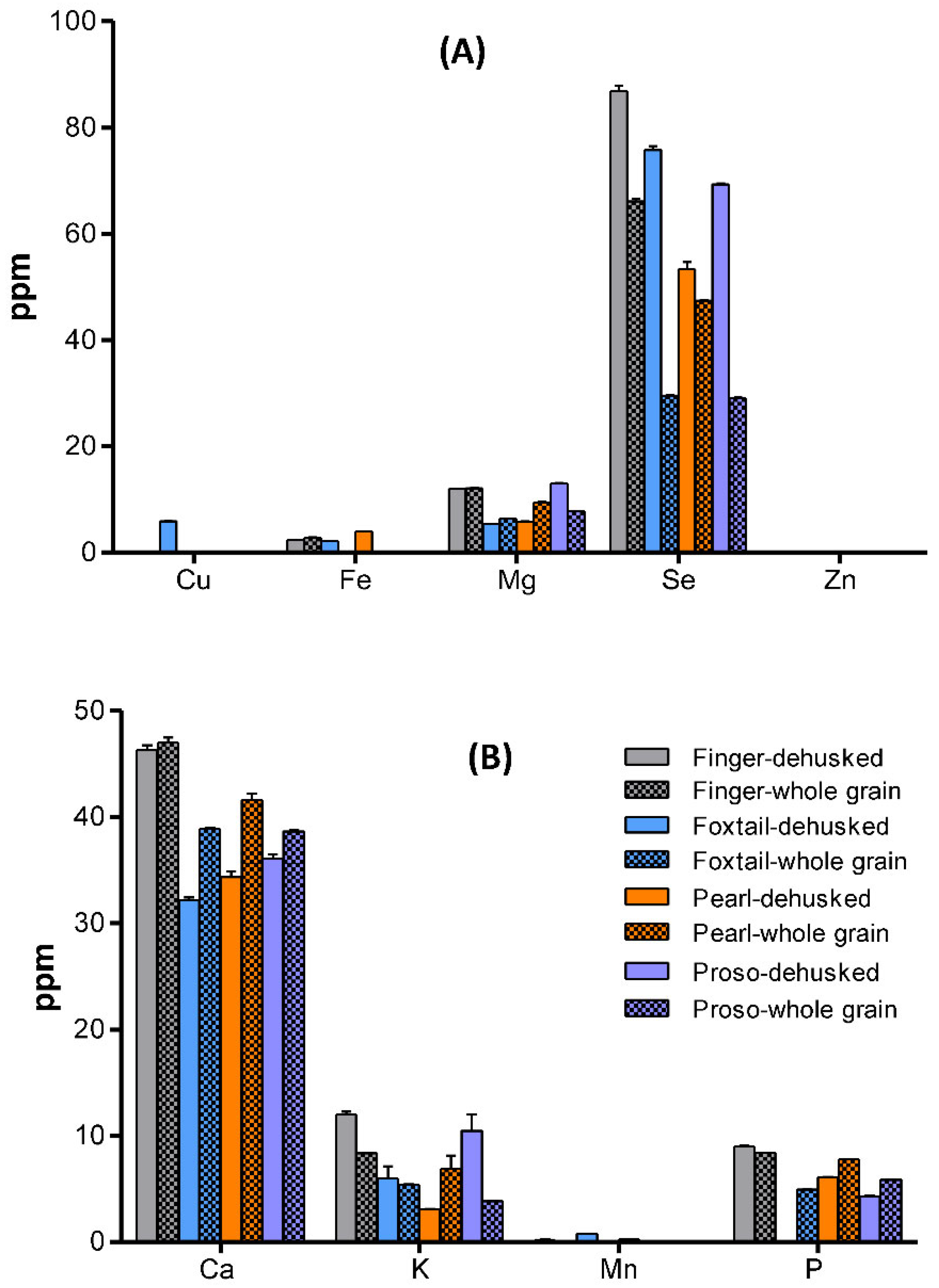
Mineral Composition of Millet Varieties
Antioxidant Activity and TBA Levels of Millet Varieties
4. Discussion
Proximate Composition
Phytochemical Composition
Amino Acid Profile
Vitamin Content
Mineral Composition
Antioxidant Activity
5. Conclusions
Author Contributions
Acknowledgements
Conflicts of Interest
References
- Abduljalil, M.M.; Umar, S.A.; Umar, R.A. Level of Fortificants in the “Mandatory Fortified” Wheat Flour Sold in Sokoto Metropolis, Sokoto State, Nigeria. Nigerian Journal of Biochemistry and Molecular Biology 2023, 38, 9–19. [Google Scholar]
- Alyaqoubi, S.; Abdullah, A.; Addai, Z.R. Antioxidant activity of goat's milk from three different locations in Malaysia; AIP Publishing, 2014; pp. 1–5. [Google Scholar] [CrossRef]
- Amadou, I.; Le, G. W.; Amza, T.; Sun, J.; Shi, Y. H. Purification and Characterization of Foxtail Millet-derived Peptides with Antioxidant and Antimicrobial Activities. Food Res. Int. 2013, 51, 422–428. [Google Scholar] [CrossRef]
- Aniket, S.B.; Heena, V.S.; Suchita, S.B. Proximate Composition of Finger Millet (Eleusine coracana) in Regional areas of Maharashtra. International Journal of Advanced Research in Biological Sciences 2020, 7, 193–199. [Google Scholar] [CrossRef]
- AOAC. Official method of analysis of the association of Analytical chemis, 15th ed; 1990. [Google Scholar]
- AOAC (Association of Official Analytical Chemicals). Official Method of Analysis of the AOAC, (W. Horwitz Editor), 18th ed.; AOAC: Washington, DC, 2006. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000; Volume 992, p. 16, Methods 925.10, 65.17, 974.24. [Google Scholar]
- Asharani, V.T.; Jayadeep, A.; Malleshi, N.G. Natural Antioxidants in Edible Flours of Selected Small Millets. Int. J. Food Prop. 2010, 13, 41–50. [Google Scholar] [CrossRef]
- Awuchi, C.; Igwe, V.; Echeta, C. The Functional Properties of Foods and Flours. International Journal of Advanced Academic Research Sciences 2019, 5, 2488–9849. [Google Scholar]
- Baker, H.; Frank, O. Determination of serum tocopherol. In: clinical biochemistry, 6th ed.; Eimememenn Medical Books: London, 1968; pp. 902–903. [Google Scholar]
- Bassey, O.A.; Lowry, O.H.; Brock, M.J.; Lopez, J.A. The determination of vitamin A and carotene in small quantities of blood serum. J. Biochem. 1946, 234, 177–188. [Google Scholar] [CrossRef]
- Bhatti, M.; Peter, S.J.; John, E. Determination of trace element using Unicam 969 Atomic absorption Spectrophotometer. Ann. Inter. Med. 2006, 205, 96–105. [Google Scholar]
- Boham, B.A.; Kocipai-Abyazan, R. Flavonoids and Condensed Tannins from Leaves of Hawaiian vaccinium vaticulatum and V. calycinium. Pacific Sci. 1974, 48, 458–463. [Google Scholar]
- Bot, M.H.; Bawa, G.S.; Omage, J.J.; Onimisi, P.A.; Bot, D.Y.; Udom, I.E. Proximate composition of red and black finger millet (Eleusine coracana) varieties. Nigerian Journal of Animal Production 2021, 47, 46–53. [Google Scholar] [CrossRef]
- Caligiuri, S.P.B.; Pierce, G.N. A review of the relative efficacy of dietary, nutritional supplements, lifestyle, and drug therapies in the management of hypertension. Critical Reviews in Food Science and Nutrition. 2017, 57, 3508–3527. [Google Scholar] [CrossRef]
- Chethan, S.; Malleshi, N.G. Finger millet polyphenols: optimization of extraction and the effect of pH on their stability. Food Chemistry 2007, 105, 862–870. [Google Scholar] [CrossRef]
- Dandare, S.U.; Ezeonwumelu, I.J.; Ezeh, C.P.; Auta, H. Determination of in vitro antioxidant and radical scavenging activities of different extracts of Allium sativum (Garlic). IOSR Journal of Pharmacy and Biological Sciences 2014, 9, 69–73. [Google Scholar]
- Dandare, S.U.; Ezeonwumelu, I.J.; Shinkafi, T.S.; Magaji, U.F.; Adio AA, I.; Ahmad, K. L-alanine supplementation improves blood glucose level and biochemical indices in alloxan-induced diabetic rats. Journal of Food Biochemistry 2021, 45, e13590. [Google Scholar] [CrossRef] [PubMed]
- Dhliwayo, T.C.; Chopera, P.; Matsungo, T.M.; Chidewe, C.; Mukanganyama, S.; Nyakudya, E.; Mtambanengwe, F.; Mapfumo, P.; Nyanga, L.K. Effect of germination and roasting on the proximate, mineral and anti-nutritional factors in finger millet. 2023, 23, 24346–24362. [Google Scholar]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J Agric Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Ezeonwumelu, I.J.; Mode, A.M.; Magaji, U.F.; Nzoniwu, N.A.; Tangaza, M.H.; Tanimu, F.I.; Dandare, S.U. Coadministration of L- alanine and L- glutamine ameliorate blood glucose levels, biochemical indices and histological features in alloxan-induced diabetic rats. Journal of Food Biochemistry 2022, 00, e14420. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization). . World food situation; 2017. 20 February. Available online: http://www.fao.org/world foods ituat ion/csdb/en/ (accessed on 25 February 2020).
- FAO. Millet Post-harvest Operations Post-harvest Compendium, 2024. 2001. accessed on 13th January. Available online: https://www.fao.org/fileadmin/user_upload/inpho/docs/Post_Harvest_Compendium_-_MILLET.pdf.
- FOA. International Year of Millets 2023. Inaugural Seminar Celebrating the International Year of Millets in Abuja, Nigeria. 2023. Available online: https://www.fao.org/millets-2023/events/detail/inaugural-seminar-celebrating-the-international-year-of-millets-in-abuja--nigeria/en (accessed on 30 December 2023).
- Gowda NA, N.; Siliveru, K.; Prasad PV, V.; Bhatt, Y.; Netravati, B.P.; Gurikar, C. Modern Processing of Indian Millets: A Perspective on Changes in Nutritional Properties. Foods 2022, 11, 499. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free Radicals and Antioxidants: Updating a Personal View. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef]
- Harbone, J.B. Phytochemicals Methods. A Guide to Modern Techniques of Plant Analysis, 3rd ed.; Chapman and Hall Publishing: London, UK, 1998; p. 286. [Google Scholar]
- Hassan, Z.M.; Sebola, N.A.; Mabelebele, M. The nutritional use of millet grain for food and feed: a review. Agriculture & food security 2021, 10, 16. [Google Scholar] [CrossRef]
- Hegde, P.S.; Anitha, B.; Chandra, T.S. In Vivo Effect of Whole Grain Flour of Finger Millet (eleucineCoracana) and Kodo Millet (paspalum Scrobiculatum) on Rat Dermal Wound Healing. Indian J. Exp. Biol. 2005, 43, 254–258. [Google Scholar]
- Ibrahim, A.; Bashir, M.; Idi, A.; Buhari, H.H. Proximate Analysis, Sensory Evaluation and Production of Bread from Finger Millet and Wheat Flour. Bayero Journal of Pure and Applied Sciences 2022, 14, 101–107. [Google Scholar] [CrossRef]
- Ikegwu, T.M.; Nkama, I.; Gabriel Okafor, I. Comparative Studies of the Proximate, Microscopic and Thermal Properties of Processed Maize, Wheat, Millet, Cassava and Bambara Nut Flours. Acta Scientifci Nutritional Health 2023, 7, 38–47. [Google Scholar] [CrossRef]
- Ismaila, F.; Obiangeli, O.; Bilkisu, A. Effects of Dehulling On the Mineral Elements Content of Some Cereals (Maize, Millet, And Guinea Corn). Journal of Agricultural Research 2022, 10, 1–12. [Google Scholar]
- Iwe, M.; Onyeukwu, U.; Agiriga, A.; Yildiz, F. Proximate, functional and pasting properties of FARO 44 rice, African yam bean and brown cowpea seeds composite flour. Cogent Food & Agriculture 2016, 2, 1142409. [Google Scholar] [CrossRef]
- Izge, A.U.; Song, I.M. Pearl millet breeding and production in Nigeria: problems and prospects. Journal of environmental issues and agriculture in developing countries 2013, 5. [Google Scholar]
- Joseph Ikwebe, M.; Mayel, H.; Silas, T.V.; Mamman, E.; Dennis, S.J. Effects of dehulling on the levels of micronutrients in Maize, millet and sorghum grains. FUW Trends in Science & Technology Journal. 2020. e-ISSN:24085162; p-ISSN: 20485170; April, 2021: Vol. 6 No. 1 pp. 220 – 222. Available online: www.ftstjournal.com.
- Kent, N.L. Technology of Cereal, 3rd Ed ed; Published by Pergamum Press: Oxford, New York, Toronto, Sydney, Paris, and Frankfurt, 2006; p. 127. [Google Scholar]
- Kumar, A.; Rani, M.; Mani, S.; Shah, P.; Singh, D.B.; Kudapa, H.; Varshney, R.K. Nutritional Significance and Antioxidant-Mediated Antiaging Effects of Finger Millet: Molecular Insights and Prospects. Front. Sustain. Food Syst. 2021, 5, 684318. [Google Scholar] [CrossRef]
- Kumar, S.K. The Importance of Antioxidant and Their Role in Pharmaceutical Science - a Review. Asian Journal of Research in Chemistry and Pharmaceutical Sciences 2014, 1, 27–44. [Google Scholar]
- Kumari, D.; Madhujith, T.; Chandrasekara, A. Comparison of phenolic content and antioxidant activities of millet varieties grown in different locations in Sri Lanka. Food Science and Nutrition 2017, 5, 474–485. [Google Scholar] [CrossRef]
- Lee, S.H.; Chung, I.M.; Cha, Y.S.; Park, Y. Millet Consumption Decreased Serum Concentration of Triglyceride and C-reactive Protein but Not Oxidative Status in Hyperlipidemic Rats. Nutr. Res. 2010, 30, 290–296. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, Y.; Shao, Y.; Zhao, Y. Environmental factors influence the phytochemical composition and bioactive properties of cereals and pulses: A review. Journal of the Science of Food and Agriculture 2018, 98, 4544–4552. [Google Scholar]
- Maharajan, T.; Antony Ceasar, S.; Ajeesh Krishna, T.P.; Ignacimuthu, S. Finger millet [Eleusine coracana (L.) Gaertn]: an orphan crop with a potential to alleviate the calcium deficiency in the semi-arid tropics of Asia and Africa. Frontiers in Sustainable Food Systems 2021, 5, 684447. [Google Scholar] [CrossRef]
- Mills, A.K.T. Stefanescu, J. He. The global epidemiology of hypertension. Nature Reviews Nephrology. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Mobolanle, E.; Olatunji, A.; Mercy Temitope, B. Comparative Study of Mineral Elements Distribution in Sorghum and Millet from Minna and Bida, North Central Nigeria. International Journal of Food Nutrition and Safety 2013, 3, 55–63. [Google Scholar]
- Mode, A.M.; Magaji, U.F.; Nzoniwu, N.A.; Wamakko, H.H.; Ahmad, I.; Dandare, S.U. Comparative analysis of the antioxidant capacity of milk fromdifferent breeds of cow in Nigeria. Int J Biol Chem Sci. 2023, 17, 600–617. [Google Scholar] [CrossRef]
- Mounika, D.; Sangeetha, U.; Sireesha, G. Estimation of phytochemicals in Millets and selected Millet products. Indian J. Applied & Pure Bio 2022, 37, 810–820. [Google Scholar]
- Nassarawa, S. Comparative of Proximate and Mineral Composition of Commercially-Available Millet Types in Katsina Metropolis, Nigeria. World Journal of Food Science and Technology 2019, 3, 14. [Google Scholar] [CrossRef]
- Nidhee, S.; Purnima, S. Analysis of the proximate composition, mineral content and antioxidant compound of pearl millet flour. International Journal of Home Science 2023, 9, 304–306. [Google Scholar]
- Sanusi, N.S. Comparative of Proximate and Mineral Composition of Commercially-Available Millet Types in Katsina Metropolis, Nigeria. World Journal of Food Science and Technology 2019, 3, 14. [Google Scholar] [CrossRef]
- Onwuka, G.I. Food Analysis and Instrumentation (Theory and Practice), 1st ed.; Napthali Prints: Surulere, Lagos, Nigeria, 2005; pp. 156–161. [Google Scholar]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: a comparative study. Journal of agricultural and food chemistry 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Owheruo, J.O.; Ifesan BO, T.; Kolawole, A.O. Physicochemical properties of malted finger millet (Eleusine coracana) and pearl millet (Pennisetum glaucum). Food Science & Nutrition 2019, 7, 476–482. [Google Scholar] [CrossRef]
- Pasha, K.V.; Ratnavathi, C.V.; Ajani, J.; Raju, D.; Kumar, S.M.; Beedu, S.R. Proximate, Mineral Composition and Antioxidant Activity of Traditional Small Millets Cultivated and Consumed in Rayalaseema Region of South India. J. Sci. Food Agr. 2018, 98, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Pearson, D. The Chemical Analysis of Foods, 7th ed.; Churchill Livingstone: Edinburgh, 1976. [Google Scholar]
- Pujari, S.N; Hoskeri, J.H. Minor Millet Phytochemicals and their Pharmacological Potentials. Pharmacognosy Reviews 2022, 16, 100–106. [Google Scholar] [CrossRef]
- Rajasekaran, N.S.; Nithya, M.; Rose, C.; Chandra, T.S. The Effect of Finger Millet Feeding on the EarlyResponses during the Process of Wound Healing in Diabetic Rats. Biochim. Biophys. Acta 2004, 1689, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Ramashia, S.; Mashau, M.; Onipe, O. Millets Cereal Grains: Nutritional Composition and Utilisation in Sub-Saharan Africa 2021. [CrossRef]
- Rosentrater, K.A.; Evers, A.D. Kent’s technology of cereals: An introduction for students of food science and agriculture; Woodhead Publishing, 2017. [Google Scholar]
- Royal Tropical Institute (KIT). All in on millet? Amsterdam. Authored by Bitzer, V.; Royal Tropical Institute (KIT). All in on millet? Amsterdam. 2023. Authored by Bitzer, V., Petrutiu, S., Huet, E. and Diallo, M.. Available online: https://www.kit.nl/wp-content/uploads/2023/08/allinMillet.pdf (accessed on 28 July 2024).
- Rudzińska, A.; Juchaniuk, P.; Oberda, J.; Wiśniewska, J.; Wojdan, W.; Szklener, K.; Mańdziuk, S. Phytochemicals in Cancer Treatment and Cancer Prevention—Review on Epidemiological Data and Clinical Trials. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Shah, P.; Kumar, A.; Kumar, V.; Tripathi, M.K.M. Phytochemicals and Their Health Attributes in Millet and Millet Technology; Springer Nature Singapore Pte Ltd.: Singapore, 2021. [Google Scholar] [CrossRef]
- Shobana, S.; Sreerama, Y.N.; Malleshi, N.G. Composition and Enzyme Inhibitory Properties of Finger Millet(eleusine Cora Cana L.) Seed Coat Phenolics: Mode of Inhibition of a Gluco Sidase and Pancreatic Amylase. Food Chem 2009, 115, 1268–1273. [Google Scholar] [CrossRef]
- Simonelli, C.; Galassi, L.; Cormegna, M.; Bianchi, P. Chemical, Physical, Textural and Sensory Evaluation on Italian Rice Varieties. Universal Journal of Agricultural Research 2017, 5, 104–112. [Google Scholar] [CrossRef]
- Szewczyk, A.; Pęczek, F. Furoquinoline Alkaloids: Insights into Chemistry, Occurrence, and Biological Properties. International Journal of Molecular Sciences 2023, 24. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Phosanam, A.; Stockmann, R. Perspectives on Saponins: Food Functionality and Applications. International Journal of Molecular Sciences 2023, 24, 13538. [Google Scholar] [CrossRef]
- Twinomuhwezi, H.; Godswill Awuchi, C.; Rachael, M. Comparative Study of the Proximate Composition and Functional Properties of Composite Flours of Amaranth, Rice, Millet, and Soybean. American Journal of Food Science and Nutrition 2020, 6, 6–19. [Google Scholar]
- Usman, S.; Kamalu, T.; Suwaibatu, M.; Abubakar, C.S. Proximate Analysis and Mineral Compositions of some Cereals commonly sold in Kafin Hausa Market, Jigawa State, Nigeria. Dutse Journal of Pure and Applied Sciences 2021, 7, 22–29. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin MT, D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell B. 2007, 39, 44–64. [Google Scholar] [CrossRef]
- Vandana, L. In-Vitro Study for Evaluation of Proximate Composition, Phytochemical & Neutraceutical Properties of Different Millet Samples. International Journal of Science and Research (IJSR) 2018, 7, 1889–1892. [Google Scholar]
- Yildirim, A.; Mavi, A.; Oktay, M.; Kara, A.A.; Algur, O.F.; Bilaloglu, V. Comparison of antioxidant and antimicrobial activities of tilia (Tilia argentea Desf ex DC), sage (Salvia triloba l.), and black tea (Camellia sinensis) extracts. Journal of Agriculture & Food Chemistry 2000, 48, 5030–4. [Google Scholar] [CrossRef]
- Zieliński, H.; Kozłowska, H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. Journal of agricultural and food chemistry 2000, 48, 2008–2016. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, Y.; Bao, J.; Beta, T. Phenolic compounds and antioxidant properties of sorghum grains of varying genotypes. Journal of Agricultural and Food Chemistry 2015, 63, 8909–8915. [Google Scholar]
| Amino Acid (g/100gprotein) | Finger-dehusked | Finger-whole grain | Foxtail-dehusked | Foxtail-whole grain | Pearl-dehusked | Pearl-whole grain | Proso-dehusked | Proso-whole grain |
| Alanine | 7.51 | 7.82 | 4.44 | 7.40 | 7.17 | 7.50 | 5.84 | 6.71 |
| Arginine | 5.16 | 5.42 | 4.04 | 5.08 | 4.30 | 5.33 | 4.30 | 4.82 |
| Aspartic acid | 7.56 | 7.91 | 5.30 | 6.98 | 7.38 | 7.82 | 7.17 | 7.51 |
| Cysteine | 2.12 | 2.48 | 2.06 | 2.36 | 2.30 | 2.48 | 1.64 | 1.82 |
| Glutamic acid | 7.78 | 19.46 | 10.37 | 19.00 | 18.32 | 19.23 | 18.03 | 18.63 |
| Glycine | 3.66 | 3.80 | 2.87 | 3.71 | 3.61 | 3.82 | 3.30 | 3.52 |
| Histidine | 2.17 | 3.53 | 1.92 | 2.72 | 2.36 | 2.46 | 2.21 | 2.40 |
| Isoleucine | 4.06 | 4.39 | 3.01 | 4.16 | 3.63 | 4.26 | 3.64 | 3.90 |
| Leucine | 8.93 | 9.81 | 7.41 | 11.21 | 9.22 | 10.30 | 9.81 | 10.09 |
| Lysine | 3.08 | 3.63 | 2.25 | 3.69 | 3.13 | 3.58 | 3.34 | 3.61 |
| Methionine | 2.08 | 2.49 | 1.23 | 1.98 | 2.16 | 2.40 | 2.17 | 2.24 |
| Phenylalanine | 4.79 | 5.15 | 2.84 | 5.23 | 4.52 | 4.88 | 4.44 | 5.06 |
| Proline | 5.99 | 6.50 | 3.55 | 6.19 | 2.13 | 5.89 | 5.49 | 5.79 |
| Serine | 3.97 | 4.62 | 3.05 | 4.16 | 4.19 | 4.46 | 3.54 | 3.92 |
| Threonine | 3.16 | 3.75 | 2.19 | 3.50 | 2.97 | 3.80 | 3.39 | 3.61 |
| Trytophan | 1.65 | 1.94 | 1.18 | 1.87 | 1.73 | 1.81 | 1.66 | 1.73 |
| Tyrosine | 3.27 | 3.61 | 2.58 | 3.44 | 3.10 | 3.61 | 3.61 | 3.96 |
| Valine | 5.14 | 5.50 | 3.02 | 5.61 | 5.44 | 5.55 | 5.00 | 5.20 |
| Antioxidant Capacity | Finger-dehusked | Finger-whole grain | Foxtail-dehusked | Foxtail-whole grain | Pearl-dehusked | Pearl-whole grain | Proso-dehusked | Proso-whole grain |
| TBA (µg) | 173.28±0.37a | 164.74±0.64a | 266.02±3.85c | 311.09±6.42b | 196.15±3.2d | 172.00±0.98a | 180.76±2.31a,d | 262.80±16.36c,e |
| TPC (mg/GA100 of FW) | 2.39±0.04b | 3.13±0.01a | 0.52±0.06d | 2.59±0.26b,c | 2.46±0.20b,c,e,f | 2.71±0.02b,c,e | 2.15±0.02b,f | 0.70±0.10 d,g |
| FRAP (mgTE/100g of FW) | 23.99±0.62b | 25.74±0.44a | 3.41±0.10c,d | 4.18±0.18c | 5.49±0.26e,f | 5.66±0.18e | 4.59±0.31c,f | 5.42±0.09e,f |
| DPPH (%) | 55.57±0.40b | 63.84±0.54a | 54.04±0.67b,d | 57.99±0.42c | 49.85±0.90f | 90.13±0.10e | 60.18±0.99g | 64.61±0.59a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





