1. Introduction
Patients with tubo-ovarian cancers who are diagnosed with early stage disease have dramatically improved outcomes when compared to patients with advanced stage carcinoma [
1]. However, currently there are no effective screening tools in this disease site that enable earlier detection [
2,
3,
4,
5]. This work explores whether an optical imaging catheter can detect early-stage tubo-ovarian cancers or precursor lesions, focusing on high grade serous ovarian carcinoma (HGSOC) and serous tubal intraepithelial carcinoma (STIC) which originate in the fallopian tubes [
6,
7,
8]. The lack of adequate screening tools has led to opportunistic salpingectomy programs for primary prevention of tubo-ovarian cancers in the average-risk patients, encouraging patients to consider removal of the fallopian tubes at time of pelvic surgery for other indications [
9,
10]. High-risk patients, such as those with hereditary indications (BRCA1/2 mutations), are recommended risk-reducing bilateral salpingo-oophorectomy (RRBSO) after completion of childbearing or at the age of 35-40 causing early menopause with possible significant long-term health consequences [
11]. Novel early detection strategies that enable fallopian tube screening may support delay in risk-reducing definitive surgical procedures.
Proximally to distally, the fallopian tubes are divided into the intramural (connected to the uterine ostia), the isthmus, the ampulla, and the infundibulum. The infundibulum is funnel-shaped and opens into the peritoneal cavity, fringed with finger-like projections called fimbriae that extend towards the ovary. Microscopically, the fallopian tube consists of an innermost endosalpinx (mucosa) surrounded by muscularis (myosalpinx) which in turn is surrounded by serosa. The endosalpinx is folded longitudinally to form plicae which are most complex and pronounced in the distal fallopian tube. The epithelium of the fallopian tubes is a single layer of columnar epithelium which contains secretory epithelial cells in the isthmus, transitioning to ciliated epithelial cells in the ampulla and infundibulum. The myosalpinx consists of layers of muscle arranged in alternating directions. The serosa is mesothelium of visceral peritoneum and contains vasculature (supplied by the uterine and ovarian arteries), innervation, and lymphatics [
12,
13,
14,
15,
16]. The fallopian tubes are highly vascularized as previously visualized by confocal microtomography [
16,
17] and photoacoustic microscopy [
18,
19].
There is increasing evidence that HGSOC originates in the epithelium of the fallopian tube fimbriae [
8,
20,
21]. Precursor lesions may be microscopic in size and heterogeneously distributed. The diagnostic gold standard for tubo-ovarian cancers is a specialized histologic protocol (‘Sectioning and Extensively Examining the Fimbriated End’, SEE-FIM) that allows for detailed review of the entire fallopian tube and especially the fimbriae as classical methods have been shown to under-sample and underdiagnose small regions of invasion or STIC [
22,
23]. Conventional medical imaging techniques lack the resolution to examine the fallopian tubes for microscopic lesions: transvaginal ultrasound, even when combined with blood tests measuring cancer antigen 125 (CA-125), cannot detect disease early enough to reduce deaths due to tubo-ovarian cancers [
3,
5]. Endoscopic examination of the fallopian tubes (falloposcopy) was popular in the 1990s for fertility assessment prior to our understanding of the role of the fallopian tubes in tubo-ovarian cancers [
24]. Regardless, early falloposcopy approaches relied on fiber bundles which similarly lacked the resolution and contrast to identify small regions of lesion. Recently, advanced falloposcopy devices approaches have begun to be explored for potential tubo-ovarian cancer detection [
25,
26,
27,
28].
Optical imaging techniques use light to provide high-resolution visualizations of tissue at a limited depth of penetration. This can be delivered to luminal organs such as the fallopian tubes endoscopically through fiber-optics, allowing for detailed examination of inner lumen surfaces. We have previously developed a multimodal optical imaging catheter capable of co-registered optical coherence tomography (OCT) and autofluorescence imaging (AFI) which can image regions up to 16cm in length [
29,
30]. We hypothesize that OCT-AFI will provide utility in early tubo-ovarian cancer detection and present an imaging study demonstrating this technique in ex vivo fallopian tubes.
OCT produces volumetric images of tissue by scanning a low-coherence beam of light across a sample and interfering the collected backscattered light with a path-length-matched reference beam [
31]. This allows for the visualization of subsurface morphology, which has been precisely correlated to histology [
32], and has been explored in cancer applications for preoperative diagnosis and intraoperative detection of malignancies [
33]. While OCT has primarily found clinical adoption in ophthalmology, there is a growing body of work focused on endoscopic applications [
34,
35]. Endoscopic techniques have a reduced lateral resolution compared to microscopic OCT systems capable of sub-micron resolution [
36]; conventional endoscopic OCT has lateral resolutions on the order of 10-40 μm.
OCT has been identified as a potentially useful adjunct in many gynecologic applications including fertility assessment, investigation of chronic inflammatory conditions, and cancer screening [
37]. This has included laparoscopic or ex vivo imaging of the exterior of fallopian tubes [
38,
39,
40,
41] as well as falloposcopy [
25,
26,
28]. Recently, the first in vivo OCT falloposcopy imaging of healthy volunteers has been demonstrated, collecting long two-dimensional images via manual retraction of an imaging catheter [
28]. In OCT, the fallopian tubes appear largely homogenous, and the single cell layer of epithelium is indistinct. Plicae are visualized as folded and often overlapping structures [
26,
28]. Edema and fibrosis appear as regions of low and high intensity OCT respectively [
38]. Depending on the imaging depth of the OCT system, the mucosa, musculature, and even peritoneal tissue may be distinguishable [
25,
26]. External imaging of fallopian serosa has demonstrated vessel-like structures as regions of low intensity [
39] which appear similar in morphology to micro-focus computed tomography of fallopian vasculature [
12].
As the fallopian tubes and ovaries are complex in structure, distinguishing the most disease-relevant characteristics is challenging. Quantitative image processing extensions have been explored to improve tissue-specific contrast. One such approach is the depth-resolved optical attenuation coefficient which may capture changes in tissue composition [
14], where the attenuation coefficient has been demonstrated to be lower in regions of HGSOC compared to surrounding non-cancerous tissue [
40]. In addition to the intensity of OCT, subtle textural changes may encode information about the extracellular matrix (collagen remodeling) and other microstructural properties [
42]. Harlick texture features [
43] which have been used in various medical imaging applications have demonstrated promise in ex vivo ovarian cancer applications [
44,
45]. However, OCT texture is also subject to inherent speckle, which itself encodes sub-wavelength scattering information [
46]. Quantitative analysis of speckle distribution has demonstrated diagnostic potential in a cervical cancer mouse xenograft model [
47].
OCT lends itself well to combination with additional imaging modalities such as fluorescence imaging to improve diagnostic potential [
25,
27,
28,
29,
30]. Fluorescence imaging may examine the endogenous fluorophores (autofluorescence imaging, AFI) in tissue or may use contrast dyes. Blue-light AFI is used clinically in oral, bronchoscopic and colposcopic applications [
48,
49,
50,
51,
52]. It has been demonstrated ex vivo that clinically occult lesions can be detected via AFI in ovarian and fallopian tissues [
53,
54,
55,
56]. Blue-light AFI captures responses from a variety of biological sources, but predominantly is driven by characteristics of the extracellular matrix. In cancers, collagen remodeling and epithelial thickening result in less fluorescence response [
57]
.
Achieving multimodal imaging on a scale that allows for the cannulation of the fallopian tubes is challenging. Our approach using double-clad fiber (DCF) to allow for the co-registered collection of both OCT and AFI comes at a cost to each modality. The dopants in DCF contribute additional background fluorescence compared to pure-silica core single-mode fibers, which reduces AFI signal to background ratio [
58]. In DCF-based OCT, near infrared light may be coupled into higher-order modes which can introduce multipath artifacts. These artifacts appear as ghost images smeared in the A-line direction that may superimpose the fundamental image and cause a reduction in the usable ranging depth of the OCT [
59,
60,
61,
62]. However, the benefits of co-registered structural (OCT) and functional (AFI) examination of tissue may outweigh these costs.
2. Materials and Methods
2.1. Study Design
This work explores whether OCT-AFI will provide utility in early tubo-ovarian cancer detection and analyzes ex vivo imaging of the fallopian tubes to identify prospective image biomarkers that distinguish lesions. Due to the small sample size, this is a hypothesis-generating study intended to demonstrate feasibility, identify trends and provide future direction for diagnostic criteria. This work will explore the following questions:
Is there a statistical difference in these measurements between disease states, within an individual image or between images of patients with a lesion / without a lesion? Can these biomarkers be used to visualize areas of lesion?
In non-lesion cases, are there statistical differences in these measurements in different regions of the fallopian tube?
How repeatable are these measurements? Are there differences between the left and right fallopian tubes in patients when paired imaging is acquired?
Are there statistical correlations with age / other patient demographics that might be confounders?
Inclusion criteria:
Any patient undergoing salpingectomy at the Vancouver General Hospital was eligible for this study. Patients must have consented to the British Columbia Gynecologic Tissue Bank (UBC BCCA REB# H05-60119) as well as this study (UBC BCCA REB #H17-01716). Patients were recruited with a preference for those with known or suspected ovarian cancer (HGSOC) based on clinical history and CA125 levels although all samples were analyzed regardless of histotype.
Exclusion criteria:
Patients that had undergone chemotherapy prior to salpingectomy were excluded from this study. Samples that were imaged more than two hours after arrival at pathology were excluded as extended ischemic time results in changes in tissue properties and a reduction in autofluorescence response. Imaging deemed of insufficient quality (excessive bubbles or non-uniform rotational distortion; tissue contact for <50% of the image length; poor reference selection overlaying the desired image with multipath artifacts) was not included in this study.
2.2. Imaging System
Images were acquired with a previously described endoscopic OCT-autofluorescence imaging (OCT-AFI) system [
30]. Imaging catheters were fabricated in-house, comprised of a single double-clad fiber (DCF; SM-9/105/125-20A, Nufern, USA) surrounded by a torque cable to allow for multimodal imaging in a small catheter. A graded index fiber is used to focus the beam and provide a lateral resolution of approximately 26.5 μm in water.
AF is generated with blue (445 nm) excitation light transmitted in the DCF core. Emission is collected in the cladding (>480 nm) and detected by a photomultiplier tube (PMT, H9433-201, Hamamatsu, Japan). Infrared light (1310 ± 50nm; SSOCT-1310, Axsun Technologies Inc., Billerica, MA, USA) is transmitted and collected in the DCF core to provide OCT with an axial resolution of 7 μm in tissue. A custom DCF fiber optic rotary joint (Princetel, Hamilton Township, New Jersey, USA) and linear actuator allow for helical scan patterns to generate volumetric images of up to 16 cm in length. Light collected by the core and the cladding are separated using a double-clad fiber coupler (DCFC, DC530SEFA, Thorlabs, USA). Imaging catheters are housed in a 0.9 mm outer diameter window tube filled with sterile water for index matching, with pad printed sheath markings at 5 cm and 7 cm along the length. Seven unique catheters were fabricated and used throughout the course of this study.
2.3. Image Collection
The fallopian tubes are resected from the specimen and imaging is conducted as soon as possible after arrival at pathology; the average time between sample arrival and time of imaging was 70 minutes.
Prior to imaging, fluorescent positive and negative standards are imaged for calibration purposes. First, an image of fingertips is taken to set the OCT reference and assess AFI quality. As a negative (dark) control, the imaging catheter is inserted into a 15 mL test tube of water covered in matte black aluminum foil and an image is acquired. As a positive (bright) control, a 15 mL test tube of 0.98 μM fluorescein (selected for its similar intensity response to fingertips) is imaged; the catheter is positioned in the center of the test tube by two 3D printed holders. After imaging the standards, the catheter is wiped down to remove remaining fluorescein.
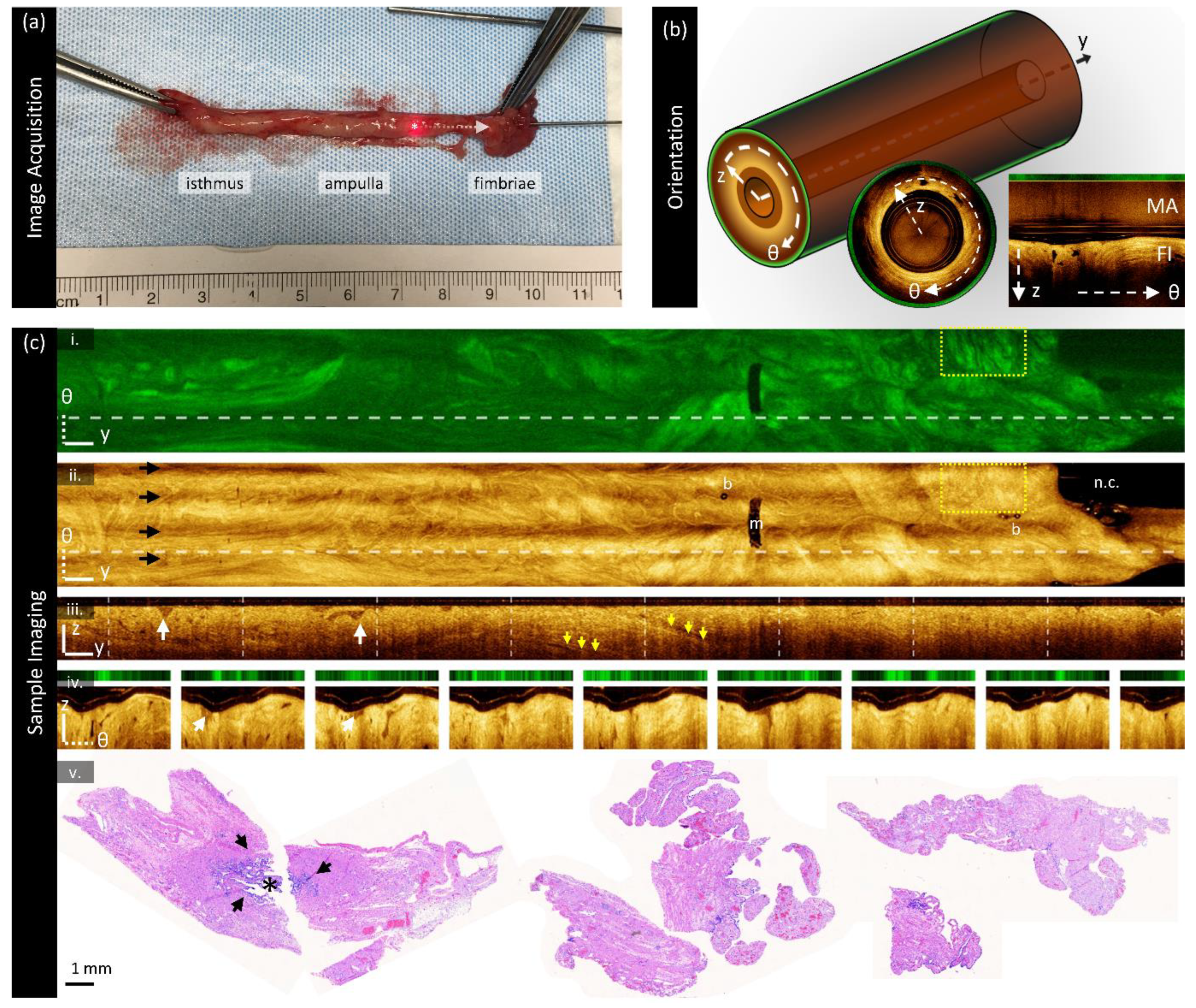
Image collection is shown in
Figure 1a. Once the fallopian tube is resected, it is challenging to cannulate the isthmus as one would for in vivo falloposcopy. Instead, a metal grossing probe is used to identify the abdominal ostia within the fimbriated end to assist in cannulation (top right of
Figure 1a). The imaging catheter is inserted into the fallopian tube until exit or until the sample is no longer catheterizable; the specimen is stabilized with forceps as needed. In
Figure 1a, part of the imaging catheter is visible exiting the infundibulum on the right side of the photograph; the end of the imaging core is denoted with a (‘*’). A ruler is included in the photograph for colocalization of the imaged region. Once in position, the optical core of the imaging catheter is retracted within the stationary window tube in a helical scan pattern with a retraction speed of 1 mm/s (1792 A-lines per B-frame) to acquire volumes. Following imaging, the SEE-FIM protocol [
22] is conducted: representative histological sections are taken approximately every 2 mm along the length of the sample and longitudinally at the fimbriae [
63].
The three-dimensional orientation system is described in
Figure 1b: y is the pullback dimension, θ is the angle around the pullback dimension, and z is the depth into the tissue. AFI is acquired en face (one intensity value collected per A-line). Most figures presented herein are enface (y-θ) mean-intensity projections or unwrapped cross-sectional sections (θ-z). Images are presented proximal (left) to distal (right). The inset center and right images in
Figure 1b show cross-sections (wrapped and unwrapped respectively). In the unwrapped cross-section, multipath artifacts (‘MA’) are indicated at the top of the frame above the fundamental image (‘FI’) generated from the fundamental mode (LP01). Occasionally there are additional multipath artifacts present below the fundamental image as well representing higher order modes coupled on both the forward and back-path; these are often lower in intensity and may not be present in all images.
Sample imaging is shown in
Figure 1c from a specimen containing no lesion or other abnormalities of note, cropped for viewing focusing on the distal region. AFI (
Figure 1c (i)) is acquired as one en face section (two-dimensional) and is presented on a scale from black (low fluorescence) to bright green (highly fluorescent). In this sample, some folding structures are visible with tendril-like darker regions interspersed with brighter areas.
OCT is presented in three views: mean en face projections (
Figure 1c (ii)), longitudinal sections (
Figure 1c (iii)) taken from the dashed line in the en face images, and cross-sections (
Figure 1c (iv)), also referred to as B-scans, taken from the dashed lines in the longitudinal section. The cross-sections presented in this paper are ‘unwrapped’ for display. The multipath artifact is masked out in the cross-sections for viewing purposes.
OCT is presented on a color scale from black (low intensity) to sepia (high intensity) corresponding to the magnitude of light returned from tissue. The en face OCT and AFI contain different features: for example, the yellow boxed region has many small, dark wrinkles in the AFI but appears largely homogenous in OCT. Examining the longitudinal section or cross-sections reveals many small gaps in tissue which we speculate are gaps between overlapping plicae (white arrows). There are also some longer structures (yellow arrows) visible in the longitudinal section which may represent ducts or vasculature as suggested by previous groups [
39].
Several imaging artifacts are present in this volume denoted on
Figure 1c (ii). There are small bubbles (‘b’) within the window tube and a sheath marker (‘m’) which obscure the image. There is a region with no tissue contact on the right-hand side of the image (‘n.c.’). There are also four bands spanning the length of the en face volume (black arrows): this is a birefringence artifact generated by this OCT-AFI system which could be overcome in future studies through polarization diverse detection [
64].
Sample histology from this specimen is demonstrated in
Figure 1c (v); stained with hematoxylin and eosin (H&E). This specimen contains no lesion, and the sections are largely representative of the fimbriae (longitudinal sections). The leftmost two sections appear to be a distal cross-section: they show a lumen (‘*’) with folded plicae bordered by dark purple epithelium (black arrows). Proximal cross-sections (not pictured) contain a smaller lumen with simpler plicae structures.
2.4. Image Preparation
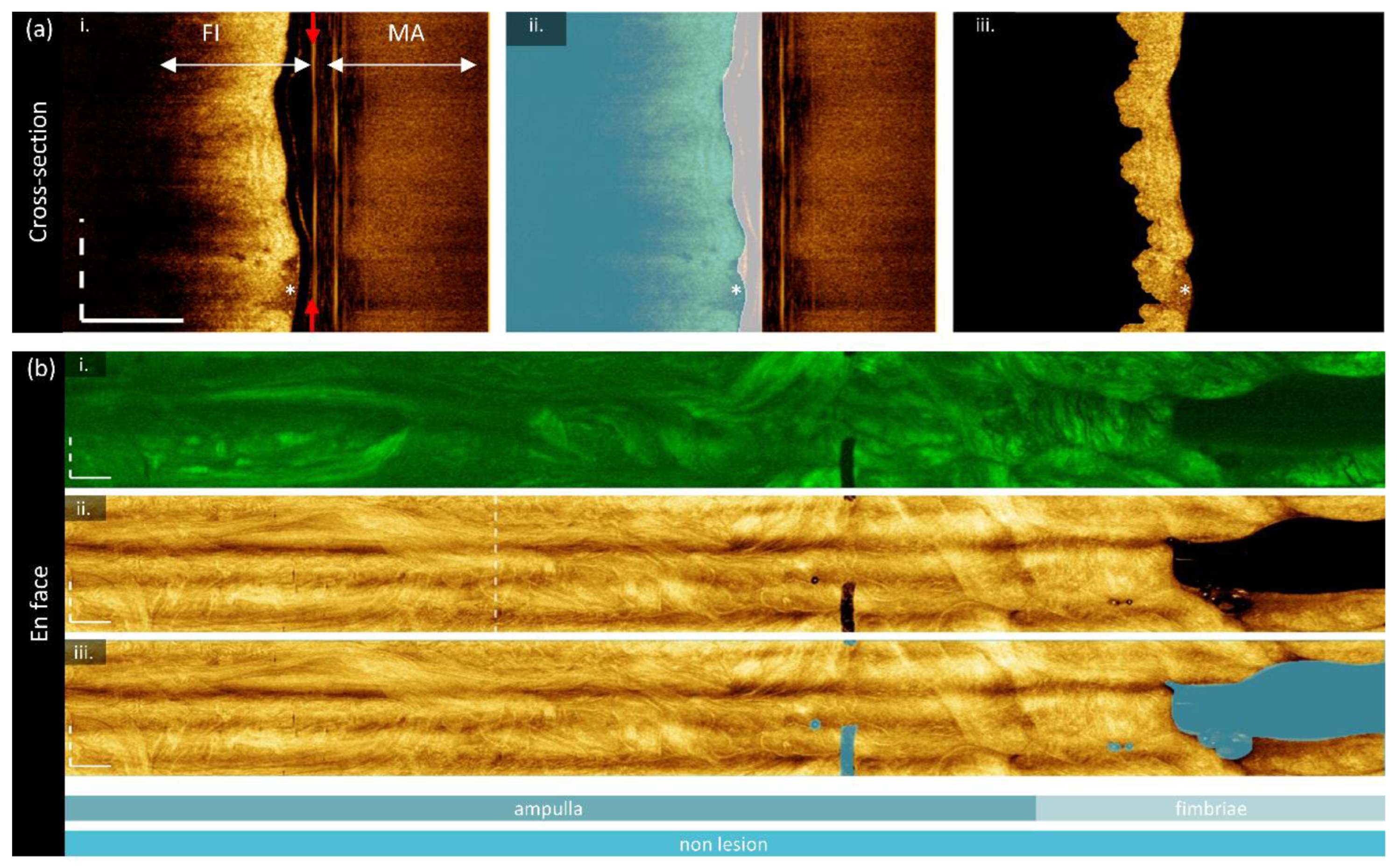
Image preparation is required to ensure the correct region is measured before imaging biomarkers are calculated. Two groups of masks are generated: cross-sectional masks to identify the luminal surface (
Figure 2a), and en face masks to remove imaging artifacts (
Figure 2b). All image processing is conducted in MATLAB 2024a; deep learning predictions are generated using Python 3.10 with a PyTorch framework. All experiments were performed on a Windows 10 operating system, with Intel Core i7-12700K 3.60 GHz CPU, NVIDIA GeForce GTX 3080Ti GPU, and 32 GB of RAM.
Cross-sectional lumen segmentation:
As the OCT volumes in this dataset comprise several thousand cross-sections manual segmentation is intractable. The complexity of tissue and variety of presentation preclude classical segmentation approaches; thus, we use a previously developed deep learning segmentation tool [
65].
Each cross-section is saved as an unwrapped .tif (
Figure 2a (i)), resampled such that pixels at the window tube are 10 μm square (index of refraction of water is assumed in the a-line direction) and smoothed with out-of-plane averaging with 5 adjacent sections in each direction. These cross-sections are interpreted with a previously trained luminal segmentation deep learning network: a four-layer U-Net trained with 532 manually-segmented cross-sections of endobronchial OCT from 39 lung transplant patients. This posed two domain transfer concerns: first, endobronchial tissue presents differently than fallopian tissue, and secondly, the OCT quality in OCT-AFI is lower and impeded by multipath artifacts. While future work may benefit from retraining a model to this use-case, we were able to generate sufficient quality segmentations through pre- and post-processing.
Before prediction, multipath artifacts (‘MA’,
Figure 2a (i)) were masked to prevent spurious segmentations. The lowest point of the multipath artifact, and an estimated lowest point of the fundamental image (‘FI’) were selected for each volume and all values outside of the fundamental image region were set to zero. Cross-sections were tiled horizontally to prevent discontinuities in the azimuthal direction, zero padded at the top of the frame to generate square tiles, and downsampled (192 x 192 pixel) for input into the network. Predictions were noisy and discontinuous, though they did identify the luminal surface. Post-processing (morphologic linking, combination with adjacent frames, thresholding) was required to generate a single continuous line from predictions. An example of the quality of segmentations produced by this approach is demonstrated in the blue region in
Figure 2a (ii) (region filled below luminal segmentation). The result is imperfect but sufficient for our purposes: there are occasional gaps that are excluded from calculations, and this approach is biased towards including air or mucous (‘*’) rather than closely segmenting the plicae.
Cross-sectional depth segmentation:
To allow for AFI calibration, the distance from the imaging probe to the tissue is required. A reflection from the outer diameter of the polyethelyne terephthalate (PET) tube attached to the optical core is used as a reference point (red arrows,
Figure 2a (i)). This location is consistent across the volume as the reference arm is not adjusted during acquisition; thus, it is calculated over the first 100 cross-sections to prevent outliers and an average location is taken for the whole volume. First, all values below the luminal surface are masked. All A-lines in the cross-section are compressed into a sum projection along the azimuthal axis. The two most prominent peaks are identified, and the peak with a lower coordinate (outer diameter) is taken to be the desired location. This prevents erroneous identification of the multipath artifact of the optical core packaging reflection which may appear similarly bright. The resulting region between the optical core and the luminal surface is presented as the white overlay in
Figure 2a (ii).
This same procedure was applied to the positive and negative standards for AFI calibration; however, as those samples contain no tissue, the outer surface of the sheath was used instead of the luminal surface. The optical core location was identified first, without tissue masking, as the low-scattering fluorescein and water did not produce intensity peaks comparable to the plastic reflections. The outer surface of the sheath was identified by fitting a continuous line to a binary threshold mask of the image set at 3 dB above the noisefloor. Pixels within this mask which appeared more than 750 μm away from the optical core were set to zero to exclude the walls of the test tube.
A-line truncation:
Lastly, all values that are not at least 6dB above the noisefloor are excluded from the bottom of the cross-section. If there is no multipath artifact at the bottom of the frame, the noisefloor is calculated from the lowermost 25 pixels (250 μm); otherwise, the noisefloor region is selected to start at the lowermost point above the multipath artifact. The selected region is smoothed with a 5-pixel Gaussian kernel and the noisefloor is taken to be the mean value of this region. The resulting region (‘visualized tissue’) for this cross-section is shown in
Figure 2a (iii).
En face segmentations:
The mean en face projection of OCT was segmented manually with in-house annotation software [
66]. The region of tissue to retain was segmented in one mask, and regions to remove (bubbles, sheath markers, other artifacts) were segmented in a second mask. A sample en face segmentation is visible in
Figure 2a (ii), where the blue regions will be masked out. This process was also conducted on the positive and negative standards for AFI calibration, and a region containing only air (background) in the tissue volume was selected for use as a background fluorescence value.
Diagnostic and regional labels:
We co-register imaging against pathology to provide diagnosis and region labels for each cross-section. These are best estimates of locations: diagnostic labels cover approximately 2 mm long cylindrical volumes and regional labels are estimated retrospectively. A pathologist assesses each histologic section produced through the SEE-FIM protocol as a diagnostic gold standard. The coordinates of the ends of the specimen in the image are identified manually on the en face OCT and compared against the recorded length from the pathology report and photographs taken during imaging. If the imaged area is longer than the pathology report, we scale pathology lengths linearly to the imaged region. If the entire sample was not imaged (i.e., could not be cannulated fully), we use the pathology-measured lengths directly.
We measure the longest axis of the fimbriae from the histology slides and use the area from the end of the tissue to this length as the fimbriated region. The remainder of the specimen is divided into equal spaced regions by the number of cross-sections. Each region is assigned a diagnostic label (lesion / no lesion) from its according histologic section. The ampulla region is estimated as 50% the length of the specimen starting at the beginning of the fimbriae region. Any other tissue is included in the isthmus region.
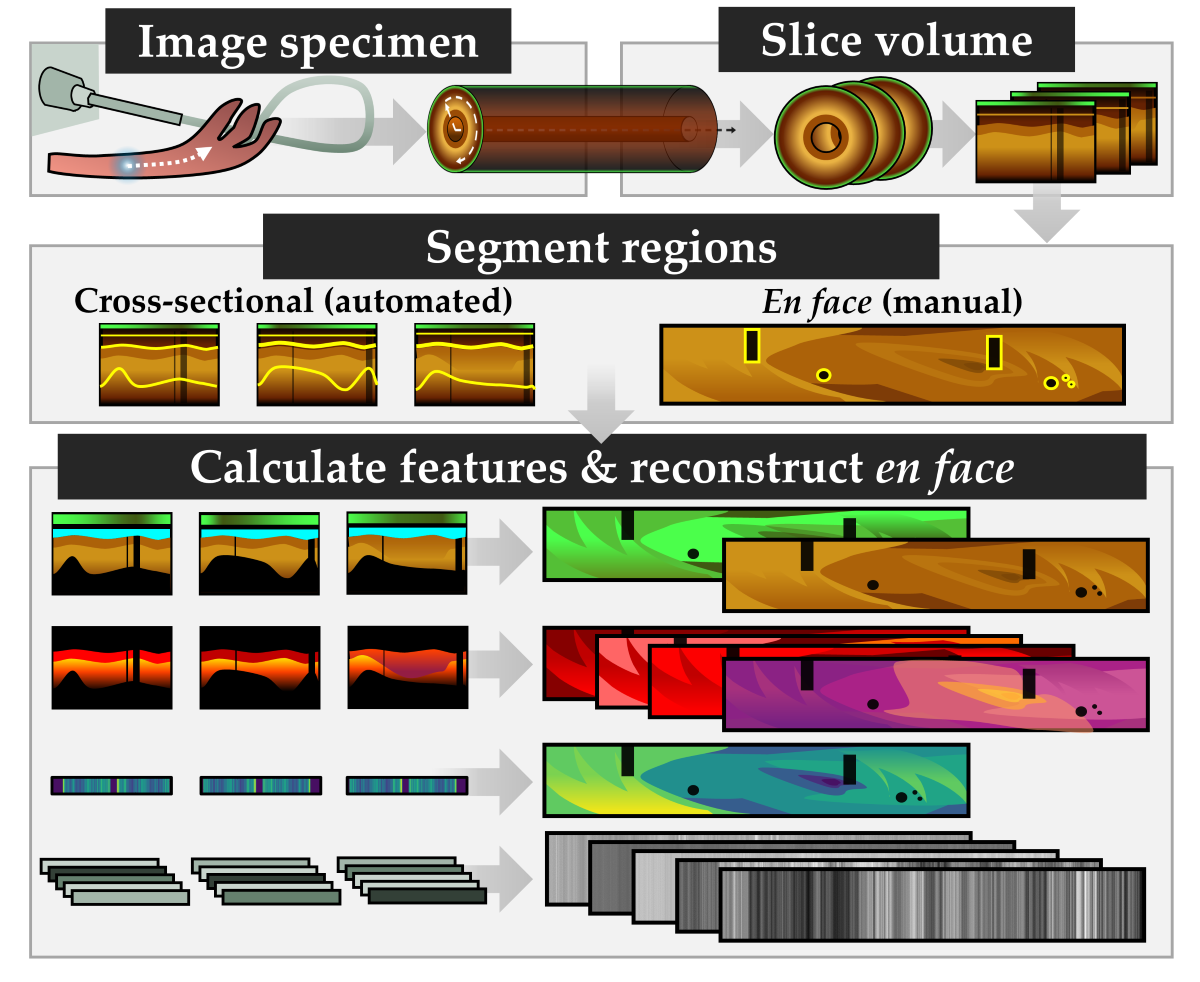
2.5. Biomarkers
After image preparation, each cross-section has a mask for the tissue region, the depth between the imaging core and tissue, and labels for diagnostic state and region. Eleven biomarkers are selected for investigation as described in
Table 1 and demonstrated in
Figure 3. This includes features related to functional characteristics (AFI), optical attenuation (OCT), and texture (OCT). After calculation, each measurement is rescaled such that all pixels are 10 μm square and reinterpreted as 2D en face view (through mean projection for three-dimensional features) for comparison against diagnostic and region labels. The median value of each feature over the region of interest is used as the measurement for later statistical analysis.
Where is intensity; is the distance from the optical core; is the optical attenuation coefficient; is the depth in pixels in the A-Line direction; is the pixel size; is the last value in z sufficiently above the noisefloor; is the shape parameter of the gamma distribution, is the scale parameter of the gamma distribution; is the value at cell in the gray level co-occurrence matrix; is the standard deviation and overbars represent the mean value of a feature.
Functional features:
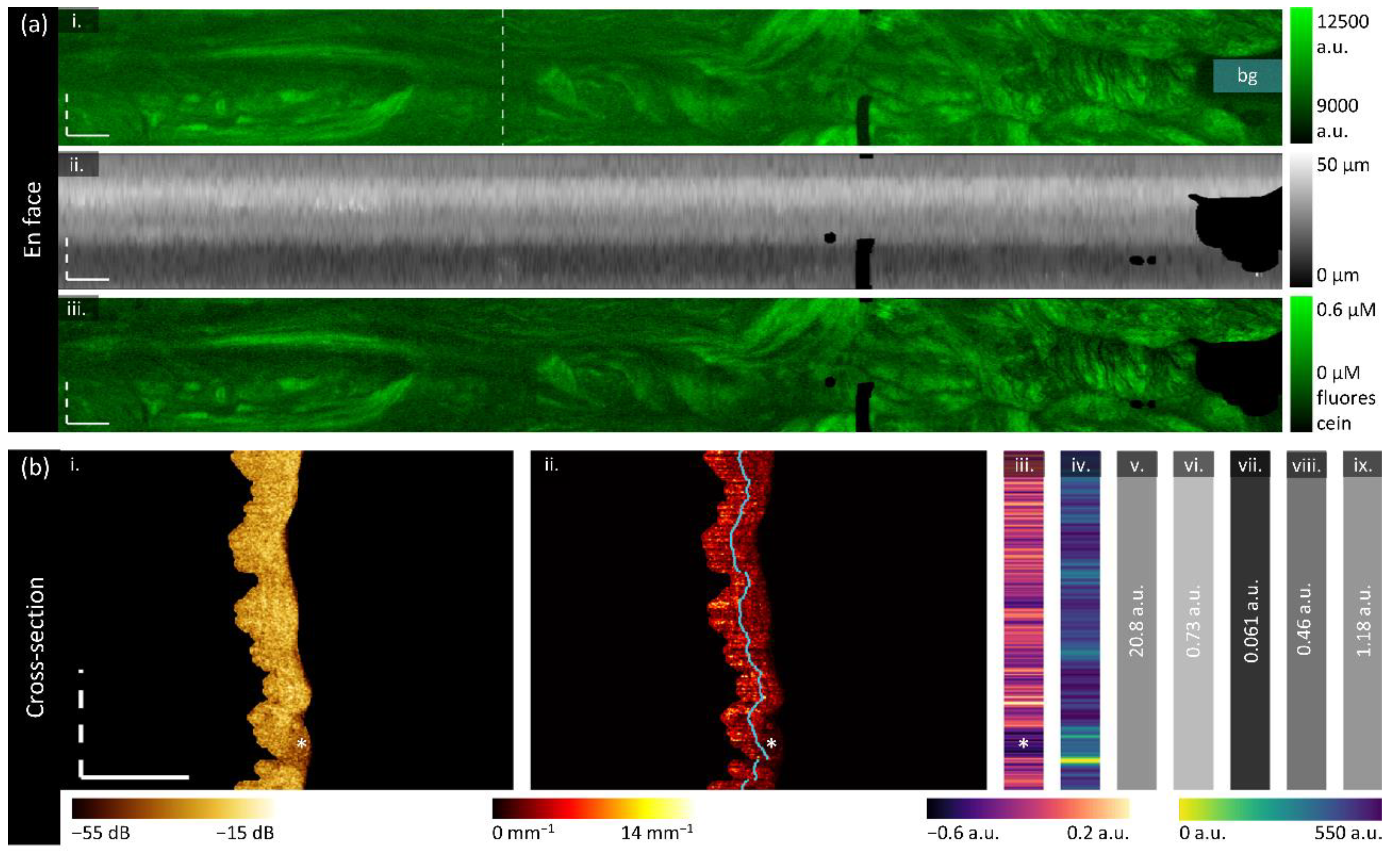
Raw autofluorescence intensity is subject to variations in the OCT-AFI system including but not limited to laser intensity, detector sensitivity, changes in the coupling fluid within the fiber optic rotary joint and coupling ability of differences between imaging catheters. To improve the comparability of measurements presented in this study, a calibration approach was implemented to rescale intensity values with respect to a positive and negative standard, the background intensity within the volume containing tissue, and the depth from the optical core as demonstrated in
Figure 3.
Using the optical core reflection and sheath or tissue segmentations, an en face depth map is constructed for the tissue volume and the two standard volumes (tissue depth map shown in
Figure 3a (ii)). A simple model was fit to estimate intensity as a function of depth for both standards. In the tissue volume, the mean background intensity is calculated over the manually selected region of air at the exit of the fallopian tube (‘bg’ in
Figure 3a (i)). For each pixel in the original en face AFI (
Figure 3a (i)), the calibrated intensity
Figure 3a (iii) is taken to be the intensity of the raw tissue pixel with the mean background intensity subtracted off divided by the difference between the bright and dark calibration curves at the corresponding depth of that pixel, multiplied by the fluorescein concentration of the positive standard. This approach allows AFI to be reported in μM of fluorescein.
Attenuation features:
The optical attenuation coefficient (
) describes the scattering and absorptive properties of tissue by characterizing the exponential decay of light in the A-line direction. It can be estimated in a depth-resolved manner from OCT data, producing a new three-dimensional volume of estimated attenuation at each voxel [
69]. This is more quantitative than OCT intensity alone, and compensates for variations from the power source, catheter quality, reference position, and/or user handling. The tissue-specific contrast provided by the optical attenuation has been explored in a variety of cancer detection applications [
70]. While attenuation coefficient values for tubo-ovarian cancers measured with falloposcopy have not been reported, measurements from the fallopian tube exterior with a micromotor catheter have demonstrated a decrease in the attenuation coefficient in cancer [
39].
We implement the algorithm described by Liu et al. [
67] over the segmented tissue region, resulting in the cross-section demonstrated in
Figure 3b (ii). We assess the overall attenuation coefficient (mean projection over the entire depth of tissue), the superficial attenuation coefficient (mean projection over the upper 50% of tissue depth, above the blue line in
Figure 3b (ii)), and deep attenuation coefficient (mean projection over the lower 50%). Generally, we visualize < 500 μm of tissue, and so we anticipate that the superficial region will contain endosalpinx and some myosalpinx, whereas the deep region will contain primarily myosalpinx, though this is complicated due to the folded nature of the plicae. Examining the example in
Figure 3b (ii), there appears to be a subtle stratification with a layer of lower attenuation coefficients close to the luminal surface underlain with a layer of higher attenuation values; calculating the mean projection of the attenuation coefficient over individual depth regions will allow us to examine these changes.
We calculate a ratiometric stratification biomarker to further examine the differences between the superficial and deep regions (
Figure 3b (iii)). This is calculated through the difference between superficial and deep projections divided by their sum. Negative values correspond to a higher value deep attenuation coefficient, and positive values correspond to a higher value superficial attenuation coefficient. From the example cross-section we see that generally the attenuation coefficient is lower in the superficial region, and the region containing mucous rather than tissue (‘*’) has very low attenuation coefficient values in the superficial region. We anticipate that carcinoma may appear as a loss of stratification, wherein the superficial and deep regions become homogenous with invasion.
Speckle features:
Speckle is inherent to low coherence imaging methods, and may contain sub-resolution characteristics [
46]. We estimate the speckle distribution by fitting the intensity data (no out-of-plane averaging) to a gamma distribution as described by Lindenmaier et al. [
47]. To achieve an estimation of this distribution per A-line while ensuring enough datapoints for a reasonable fit, we combine 5 adjacent A-lines in each direction and include the entire region of tissue depth. A-lines with a tissue depth of less than 10 pixels are excluded. The mean of the gamma distribution is described by
, where
is the shape parameter of the gamma distribution and
is the scale parameter. We use the mean of the gamma distribution as our biomarker, which is presented in
Figure 3b (iv). In a cervical cancer mouse xenograft study, a lower
value was found in tumor when compared to normal tissue [
47] even when changes were not visually distinguishable in OCT, so we anticipate this feature may appear similar in our application.
Gray Level Co-occurrence Matrix (GLCM) features:
GLCM features have been proposed in many medical imaging applications to describe textural changes [
43,
71]. We calculate these features on the log-transformed intensity data without out-of-plane averaging but after resampling each pixel to be 10 μm square.
First, the 5
th and 95
th quantiles of the entire volume are calculated as the minima and maxima for normalization. OCT data is masked to only include the tissue region, normalized to [0,1] using the identified minima and maxima, and binned to 32 gray levels. A GLCM is generated using the MATLAB
graycomatrix function with a 1-pixel shift in the azimuthal (fast axis) direction. Five Haralick features are calculated on the GLCM as described in
Table 1, resulting in one value per cross-section (
Figure 3b (v-ix)).
This study does not seek to report all possible GLCM features nor optimize normalization methods, binning, or directionality approaches. We elect to limit the number of calculated features to common radiomic feature descriptions with clear definitions of texture behaviors. Previous work has demonstrated textural features in combination with a classifier can distinguish tumor in transgenic mouse models of ovarian cancer [
44]. This study found energy, correlation, contrast and homogeneity to be their most statistically significant for differentiating treatment groups in two-dimensions, and entropy demonstrated the best ability to discriminate groups in three-dimensions. While we are implementing this in a two-dimensional approach in human fallopian tissue, we anticipate that we may be able to detect similar changes with the same approach.
2.6. Statistical Analysis
Statistical analysis is conducted in TIBCO Statistica 14. A single median value is used for each biomarker over the desired region in each volume for statistical analysis. The cross-sectional tissue masks and en face artifact masks described in
Section 2.4 are used to exclude measurements in regions of artifacts, bubbles, low-signal, or non-tissue contact.
As our sample size is small and limited in volumes containing lesions of interest, we do not correct for multiple comparisons and are not able to control for confounding covariates. This is a hypothesis-generating study intended to provide future direction and to examine potential diagnostic features; additional study is required to confirm their utility. We have selected a significance level of p<0.05 for all tests.
We explore several statistical questions described in
Table 2 comparing the measured biomarkers to various dependent variables. The Shapiro-Wilk W test [
72] is used to test biomarkers for normality and assess which features required parametric or non-parametric tests. For paired tests, missing data is excluded in a pairwise fashion. For unpaired tests, assumptions of homogeneity of variances was assessed with Levene’s test [
73], and the presence of outliers was assessed with Grubb’s test [
74].
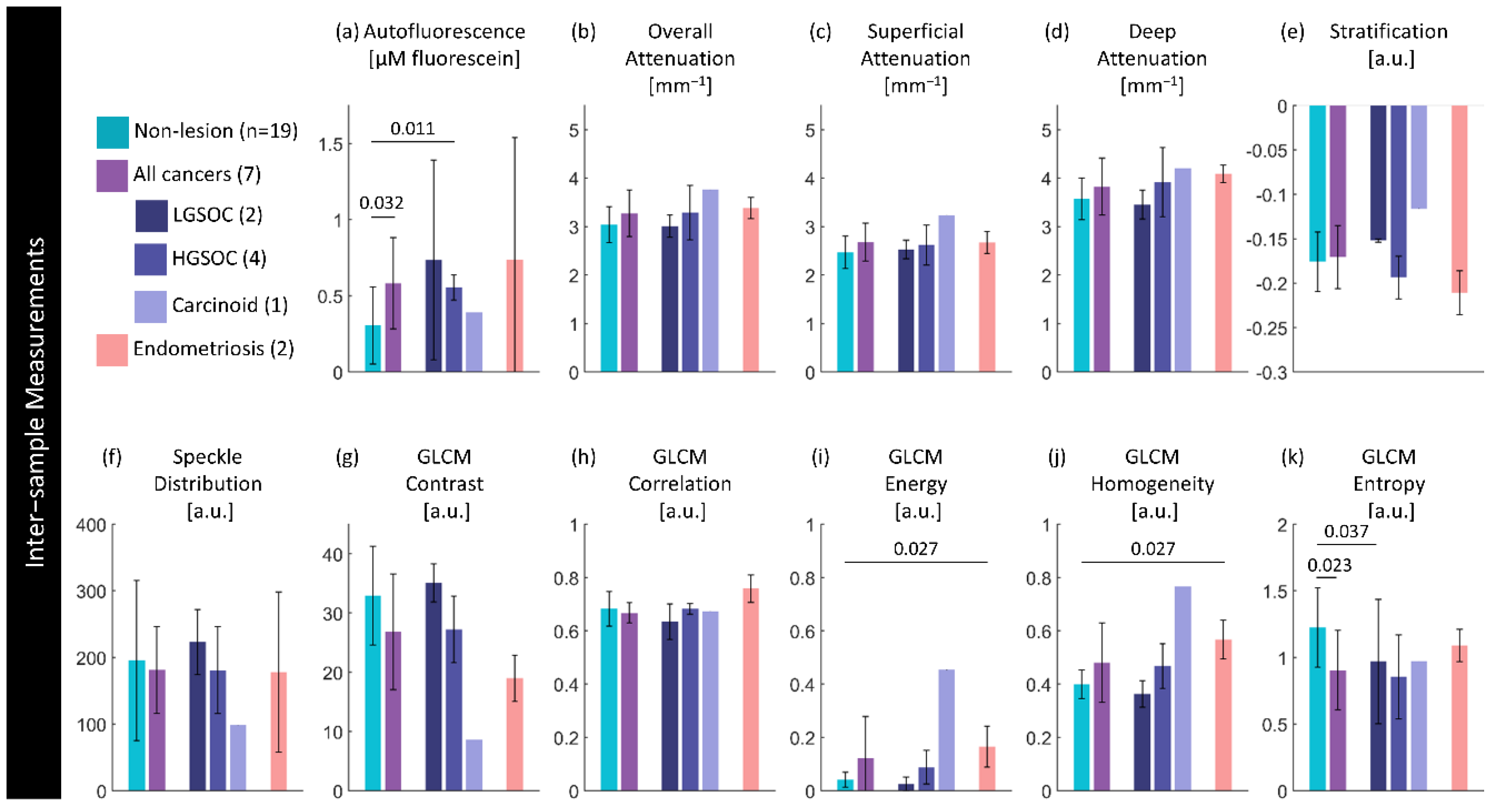
4. Discussion
4.1. Functional Biomarkers
This work replicates our previous findings that HGSOC and STIC appear as a loss of fluorescence when examined with widefield blue excitation [
53]. The AFI appears similar to other organs with a thin layer of epithelium such as the small airways of the lung. The findings here are limited as they are ex vivo specimens that have undergone on average more than an hour of ischemic time, but we anticipate that in vivo AFI would have improved contrast.
The overall increase in fluorescence intensity of specimens containing a lesion was unexpected. We hypothesize that this could be capturing broader changes related collagen remodelling or inflammation. If changes in the extracellular matrix detected with AFI are useful in tubo-ovarian cancer detection, other modalities that also examine collagen and fibrillar structures such as polarization-sensitive OCT (PS-OCT) may provide similar diagnostic utility. PS-OCT does not require double-clad fiber like OCT-AFI and provides higher OCT quality which may provide better examination of structural features.
Our findings are limited by the poor signal-to-background ratio of the AFI in this system, which may result in inconsistencies in measurement regardless of calibration as indicated by the high percentage error between the paired non-cancerous specimens. The background fluorescence contribution from the imaging system itself is comparable in scale to that of the tissue, and it varies over time – which is why we incorporate the background (air) fluorescence measurement into our calibration. Unfortunately, the background fluorescence contribution may change throughout the (sometimes minutes-long) acquisition and taking a mean measurement at the distal exit of the specimen may not be sufficient. Reducing the background system contributions and/or introducing a standard present in all cross-sections (ex. embedding fluorescent guide-stars on the catheter) may improve consistency. This approach may be preferred as following similar calibration approaches (imaging liquid standards) may not be possible in an in vivo imaging setting as the imaging catheter will need to be sterile.
4.2. Attenuation Biomarkers
Previous studies have reported lower optical attenuation of non-cancerous fallopian tubes (2.5 mm
-1) compared to our findings (3.13 mm
-1) [
40]. However, these are not measurements of the same tissue: Li et al. collected data from the exterior and serosa of the fallopian infundibula and fimbriae, whereas we collected data from within the lumen. They demonstrate similar trends of decreasing attenuation values in cancerous cases compared to non-cancerous cases; we found this to hold in our visual examination of the biomarkers though there was minimal difference in the median value of the whole region labelled as containing lesion.
An additional concern when comparing attenuation coefficients to literature is that they may be impacted by the confocal gate of the imaging catheter. Li et al. use a 3.8 mm diameter probe with a GRIN lens (Thorlabs GRIN2313A) with a NA of 0.23; the NA of our catheter is much lower (~0.03). Characterizing and correcting for the confocal effect of the custom imaging catheters used in this imaging study is not possible retrospectively, but in future work this would improve the reliability and comparability of attenuation measurements.
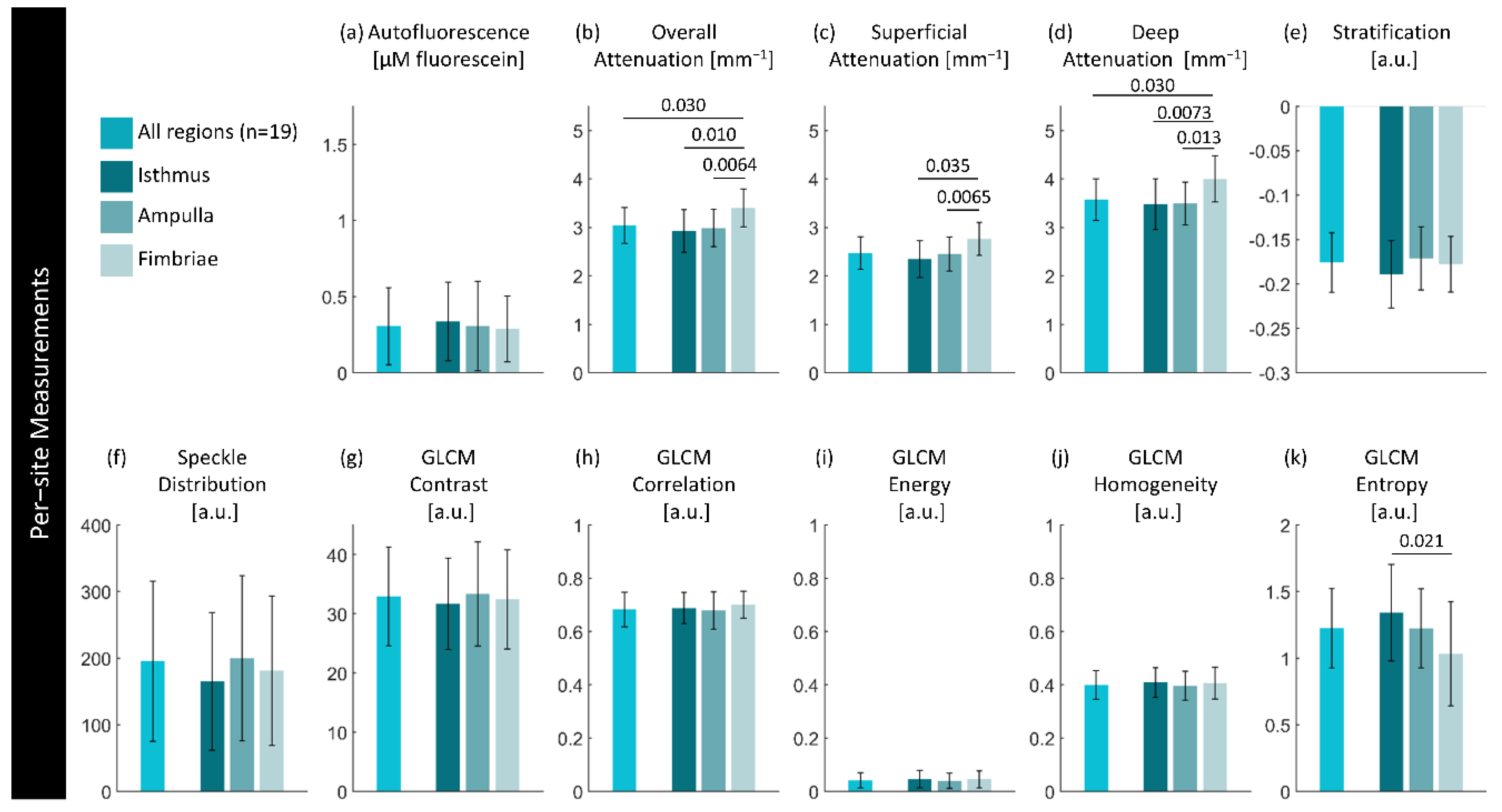
The change in attenuation in the fimbriae was unexpected, though does follow what Li et al. report when comparing non-cancerous infundibula to fimbriae. Given that HGSOC appears lower in attenuation, this should result in better contrast between lesion/non-lesion in the fimbriae where the earliest lesions originate. Depth resolved optical attenuation may be uniquely suited as a form of tissue-specific contrast in this application.
The stratification feature seems to capture similar vessel-like structures as previously reported [
39]. It also appears to capture changing tissue layering when the endosalpinx/myosalpinx and/or myosalpinx/serosa can be visualized.
4.3. Texture Biomarkers
We expected the mean speckle distribution to be able to extract sub-resolution changes, and while it does appear to capture some changes such as the vessel-like structures, it is largely impeded by the birefringence artifacts and folds in tissue. There are some specimen-wide trends with respect to disease state and anatomical location, but they are hard to assess visually. While there may be future application for this feature, further work is needed to isolate diagnostically-relevant contrast.
The GLCM features demonstrate reasonable potential, particularly entropy, in distinguishing HGSOC from non-lesion specimens and fimbriae from isthmus. This study only explores the barebones of what is possible here – for example, utilizing three-dimensional GLCM features may improve their ability to distinguish lesion [
44].
4.3. Study Limitations
This represents a small study (n=28) with few positive cases (n=7 cancers), which impacts the ability to draw firm conclusions about the biomarkers described within this paper. Instead, we present trends and potential directions for future investigation.
Recruitment and data collection took place from 2019-2023. Surgical delays during the Covid-19 pandemic resulted in an increase in neoadjuvant chemotherapy prior to surgery (exclusion criteria for this study) and advanced cancers that occluded the fallopian tubes preventing cannulation. Only one out of the four HGSOC specimens in this dataset includes STIC.
Data collection was conducted with a single OCT-AFI system that was not modified throughout the collection process. Retrospectively, we see several potential improvements for this device. There are methods to improve the signal-to-background ratio of the autofluorescence images (reducing plastics in the optical path, replacing components with low-fluorescence versions) which would likely improve detection of subtle features. The birefringence artifacts could be corrected through polarization diversity detection [
64]. The multipath artifacts could be removed through W-type rather than double-clad fiber catheters [
83], or perhaps may be leveraged to uncover additional tissue properties [
84].
The segmentation approaches applied in this work are functional but could be further optimized. The deep learning tool used to identify the luminal surface would benefit from retraining on OCT-AFI from this dataset to reduce the domain transfer issues. The surface identification was imperfect and often included regions of mucus as tissue. Similarly, the en face segmentation could be automated with its own network. Another challenge is the precision with which diagnostic and anatomical region labels can be assessed. While we can identify lesions within the labelled regions, they are not specific which limits our quantitative findings. Anatomical regions were estimated retrospectively and could instead have been measured during the imaging process to improve accuracy.
4.4. Translation & Future Directions
The eventual aim of this work is to develop a falloposcopic tool that can detect early and occult tubo-ovarian cancers in vivo prior to salpingectomy. This would be achieved through hysteroscopic cannulation of the fallopian tubes with a sterile imaging catheter under sedation. This approach has been recently demonstrated with a novel falloposcopy device of a similar size to our imaging catheter [
28], though this device has additional steering and rotation capabilities which may be required for cannulation.
There are several image quality improvements that could be made to the imaging device used in this study as previously described. The imaging catheters themselves are largely ready for in vivo translation apart from a validated sterilization protocol. They are 0.9 mm in outer diameter which fits within the working channel of a flexible hysteroscope (Olympus HYF-V). Images are acquired at a minimum pullback rate of 1 mm/s for distances up to 16 cm, allowing for imaging of the entire fallopian tube with real-time viewing of OCT and raw AFI during collection.
The imaging biomarkers described are calculated retrospectively on data. Improved automation of segmentations and translation from MATLAB to a faster language (e.x. C++) could perhaps allow for viewing in the order of minutes. Additionally, not all features presented within this work may be necessary; classification approaches could be explored to identify the most diagnostically-relevant features. While the features described here provide a wealth of information about the specimen, simplification may be preferable.
Novel early detection strategies that enable fallopian tube screening may support delay in risk-reducing definitive surgical procedures causing early menopause in high-risk patients. This requires confidence in diagnostic ability to rule out early or occult lesions, necessitating further study. Successful identification of early or occult disease via fallopian tube imaging in vivo would enable improved decision making around the timing of definitive surgery (e.g., delay for fertility preservation), and/or direct surgical procedure (e.g. more encompassing surgical staging) in patients at general population risk or with genetic predisposition.
Author Contributions
Conceptualization, JM, PML, JNM; methodology, JM; image collection, JM, AT, CH; software, JM, AT, CH, AZC, PML; validation, JM; formal analysis, JM; investigation, JM; resources, LH, JNM, CM and PML; data curation, JM; writing—original draft preparation, JM.; writing—review and editing, all authors; visualization, JM, CH; supervision, CM, JNM and PML; project administration, JM; funding acquisition, CM, JNM and PML. All authors have read and agreed to the published version of the manuscript.
Figure 1.
OCT orientation and sample imaging of a fallopian tube containing no lesion. (a) Endoscopic OCT is collected in a cylindrical volume (left); directions (‘z’, ‘θ’, ‘y’) are indicated. A sample cross-section (center) and an ‘unwrapped’ cross section (right) are demonstrated. The unwrapped cross section includes the fundamental image (‘FI’) and multipath artifact (‘MA’). (b) Image acquisition methods. The imaging catheter is inserted into the infundibulum and positioned for image acquisition. The catheter is retracted in the distal direction (arrow) within the stationary window tube (tip indicated by ‘*’). (c) Sample imaging from a fallopian tube containing no lesions: (i) en face autofluorescence image, (ii) OCT mean en face projection; (iii) longitudinal section taken from the dashed line in the en face images; (iv) cross-sections taken from the dashed lines in the longitudinal section. (v) Representative histology sections. All scale bars 1 mm.
Figure 1.
OCT orientation and sample imaging of a fallopian tube containing no lesion. (a) Endoscopic OCT is collected in a cylindrical volume (left); directions (‘z’, ‘θ’, ‘y’) are indicated. A sample cross-section (center) and an ‘unwrapped’ cross section (right) are demonstrated. The unwrapped cross section includes the fundamental image (‘FI’) and multipath artifact (‘MA’). (b) Image acquisition methods. The imaging catheter is inserted into the infundibulum and positioned for image acquisition. The catheter is retracted in the distal direction (arrow) within the stationary window tube (tip indicated by ‘*’). (c) Sample imaging from a fallopian tube containing no lesions: (i) en face autofluorescence image, (ii) OCT mean en face projection; (iii) longitudinal section taken from the dashed line in the en face images; (iv) cross-sections taken from the dashed lines in the longitudinal section. (v) Representative histology sections. All scale bars 1 mm.
Figure 2.
Image preparation for biomarker calculation. (a) Cross-sections are segmented to identify the luminal surface and imaging core (yellow arrow): (i) raw image demonstrating the multipath artifact (‘MA’) and fundamental image (‘FI’); (ii) segmentations; luminal region blue and optical core to luminal surface (white); (iii) final tissue segmentation excluding values < 6 dB above the noisefloor. (b) En face segmentation demonstrating the original AFI (i) and OCT (ii), and the masked en face OCT (iii). The cross-section presented in (a) is taken from the dashed vertical line in (ii). Sites are co-localized below the en face image: the isthmus is cropped out for presentation and this sample contains no lesion. All scale bars are 1 mm.
Figure 2.
Image preparation for biomarker calculation. (a) Cross-sections are segmented to identify the luminal surface and imaging core (yellow arrow): (i) raw image demonstrating the multipath artifact (‘MA’) and fundamental image (‘FI’); (ii) segmentations; luminal region blue and optical core to luminal surface (white); (iii) final tissue segmentation excluding values < 6 dB above the noisefloor. (b) En face segmentation demonstrating the original AFI (i) and OCT (ii), and the masked en face OCT (iii). The cross-section presented in (a) is taken from the dashed vertical line in (ii). Sites are co-localized below the en face image: the isthmus is cropped out for presentation and this sample contains no lesion. All scale bars are 1 mm.
Figure 3.
Biomarker calculation. (a) Calculation of en face functional biomarkers: (i) the original AFI with the background region (‘bg’) manually identified; (ii) the depth map constructed as the distance from the optical core reflection to the tissue surface for each A-line; (iii) the final calibrated AFI in units of fluorescein. (b) Calculation of cross-sectional (attenuation and texture) biomarkers from the dashed line in panel a (i): (i) the segmented OCT section; (ii) the depth-resolved attenuation coefficient cross-section with the regions of superficial and deep attenuation coefficient divided by the blue line; two-dimensional feature that results in one value per A-line: (iii) stratification and (iv) speckle contrast; and one-dimensional GLCM features: (v) contrast, (vi) correlation, (vii) energy, (viii) homogeneity, and (ix) entropy. All scale bars are 1 mm.
Figure 3.
Biomarker calculation. (a) Calculation of en face functional biomarkers: (i) the original AFI with the background region (‘bg’) manually identified; (ii) the depth map constructed as the distance from the optical core reflection to the tissue surface for each A-line; (iii) the final calibrated AFI in units of fluorescein. (b) Calculation of cross-sectional (attenuation and texture) biomarkers from the dashed line in panel a (i): (i) the segmented OCT section; (ii) the depth-resolved attenuation coefficient cross-section with the regions of superficial and deep attenuation coefficient divided by the blue line; two-dimensional feature that results in one value per A-line: (iii) stratification and (iv) speckle contrast; and one-dimensional GLCM features: (v) contrast, (vi) correlation, (vii) energy, (viii) homogeneity, and (ix) entropy. All scale bars are 1 mm.
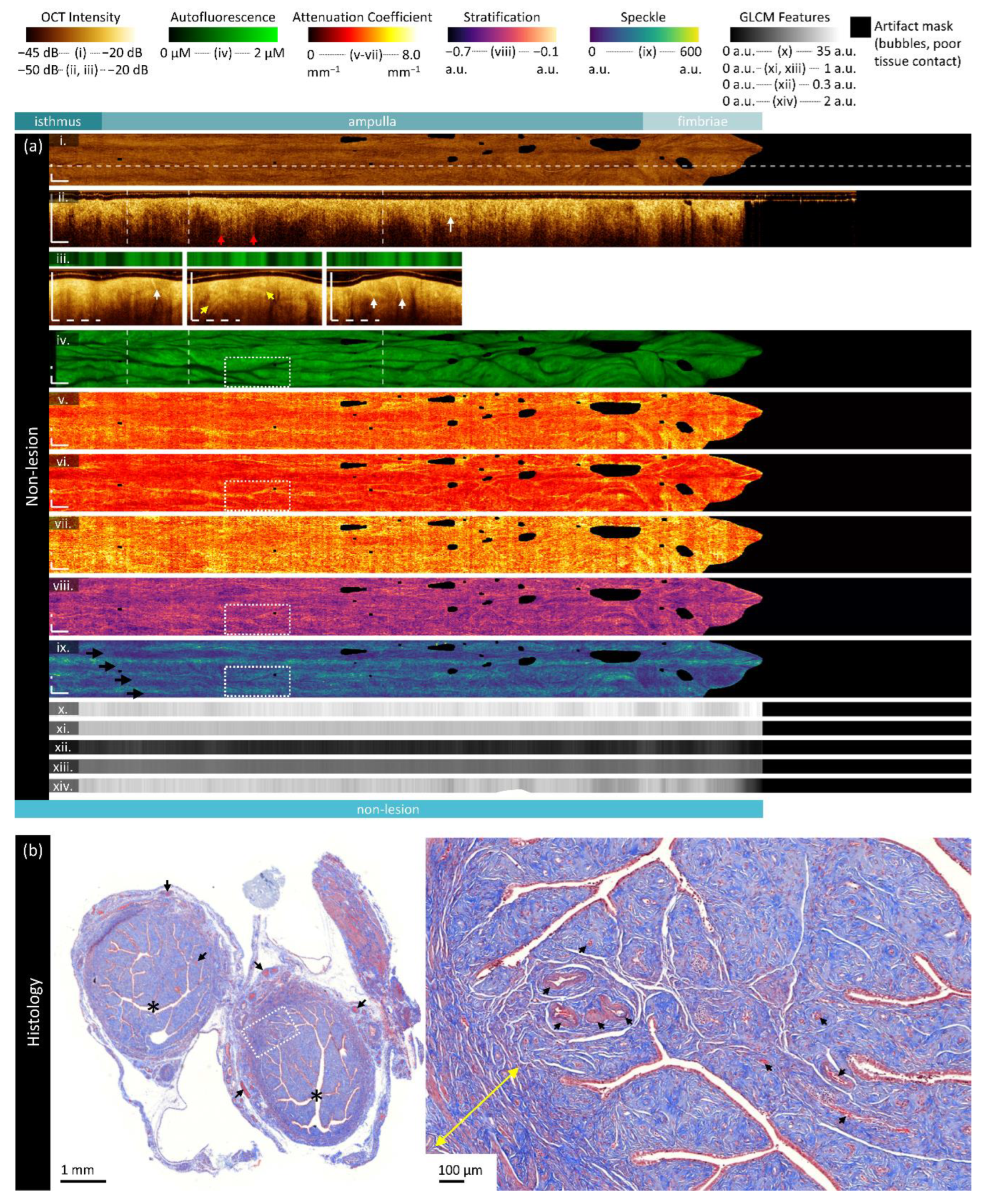
Figure 4.
Sample imaging of a fallopian tube containing no lesion. Panel (a) demonstrates the biomarkers: (i) mean OCT en face projection; (ii) longitudinal OCT section from the dashed line in panel (i); and three sample OCT cross-sections from dashed lines in the longitudinal section demonstrated in wrapped and unwrapped views alongside co-registered AFI. Longitudinal and depth sections are cropped to the region containing the fundamental image. The biomarkers are presented below: (iv) autofluorescence; (v) overall attenuation coefficient; (vi) superficial attenuation coefficient; (vii) deep attenuation coefficient; (viii) stratification; (ix) speckle distribution; (x) GLCM contrast; (xi) GLCM correlation; (xii) GLCM energy; (xiii) GLCM homogeneity; (xiv) GLCM entropy. Panel (b) demonstrates representative histology (Masson’s Trichrome); the right panel is inset from the boxed region on the left panel. All scale bars are 1 mm unless otherwise noted.
Figure 4.
Sample imaging of a fallopian tube containing no lesion. Panel (a) demonstrates the biomarkers: (i) mean OCT en face projection; (ii) longitudinal OCT section from the dashed line in panel (i); and three sample OCT cross-sections from dashed lines in the longitudinal section demonstrated in wrapped and unwrapped views alongside co-registered AFI. Longitudinal and depth sections are cropped to the region containing the fundamental image. The biomarkers are presented below: (iv) autofluorescence; (v) overall attenuation coefficient; (vi) superficial attenuation coefficient; (vii) deep attenuation coefficient; (viii) stratification; (ix) speckle distribution; (x) GLCM contrast; (xi) GLCM correlation; (xii) GLCM energy; (xiii) GLCM homogeneity; (xiv) GLCM entropy. Panel (b) demonstrates representative histology (Masson’s Trichrome); the right panel is inset from the boxed region on the left panel. All scale bars are 1 mm unless otherwise noted.
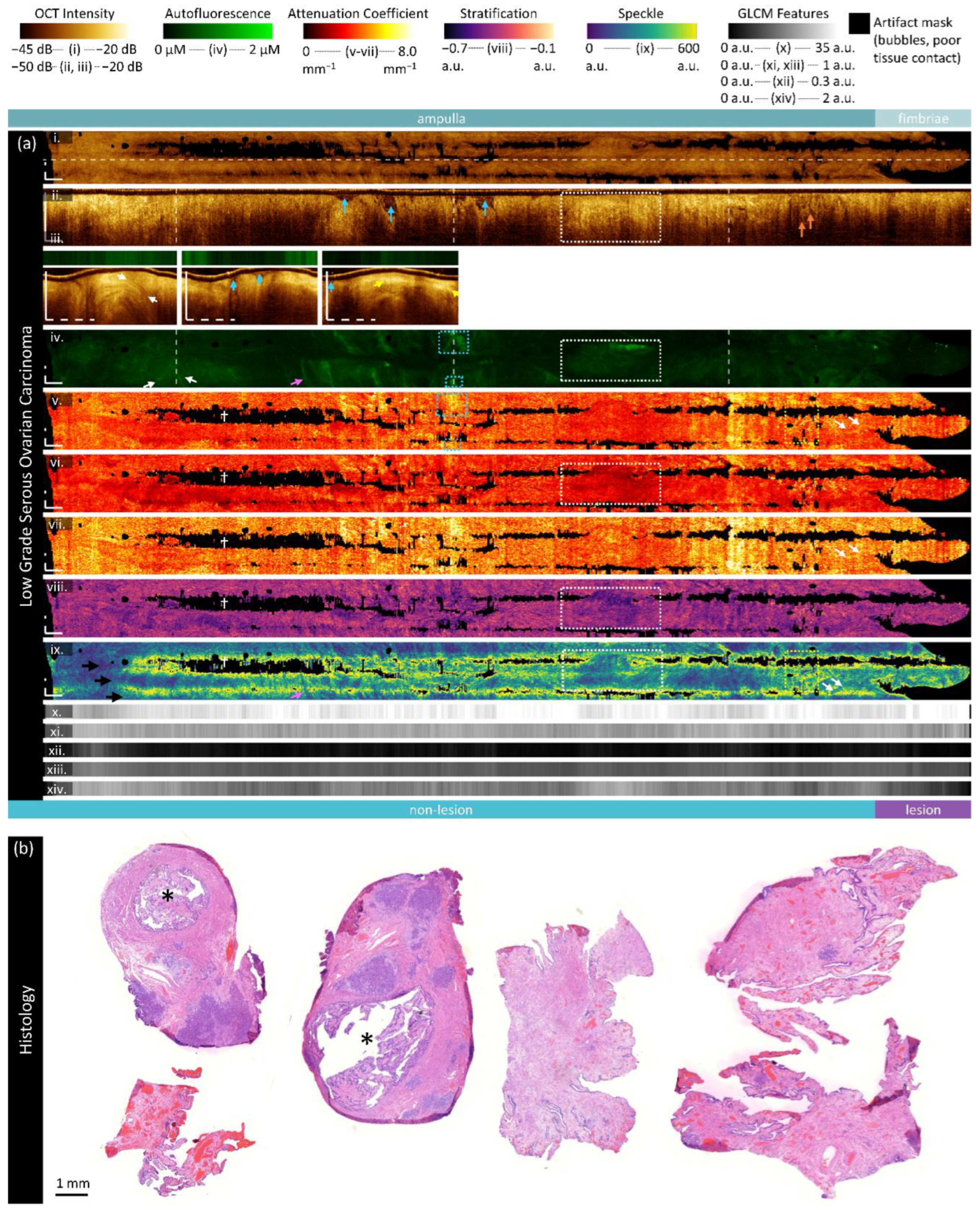
Figure 5.
Sample imaging of a fallopian tube containing LGSOC. Panel (a) demonstrates the biomarkers: (i) mean OCT en face projection; (ii) longitudinal OCT section from the dashed line in panel (i); and three sample OCT cross-sections from dashed lines in the longitudinal section demonstrated in wrapped and unwrapped views alongside co-registered AFI. Longitudinal and depth sections are cropped to the region containing the fundamental image. The biomarkers are presented below: (iv) autofluorescence; (v) overall attenuation coefficient; (vi) superficial attenuation coefficient; (vii) deep attenuation coefficient; (viii) stratification; (ix) speckle distribution; (x) GLCM contrast; (xi) GLCM correlation; (xii) GLCM energy; (xiii) GLCM homogeneity; (xiv) GLCM entropy. Panel (b) demonstrates representative histology. All scale bars are 1 mm.
Figure 5.
Sample imaging of a fallopian tube containing LGSOC. Panel (a) demonstrates the biomarkers: (i) mean OCT en face projection; (ii) longitudinal OCT section from the dashed line in panel (i); and three sample OCT cross-sections from dashed lines in the longitudinal section demonstrated in wrapped and unwrapped views alongside co-registered AFI. Longitudinal and depth sections are cropped to the region containing the fundamental image. The biomarkers are presented below: (iv) autofluorescence; (v) overall attenuation coefficient; (vi) superficial attenuation coefficient; (vii) deep attenuation coefficient; (viii) stratification; (ix) speckle distribution; (x) GLCM contrast; (xi) GLCM correlation; (xii) GLCM energy; (xiii) GLCM homogeneity; (xiv) GLCM entropy. Panel (b) demonstrates representative histology. All scale bars are 1 mm.
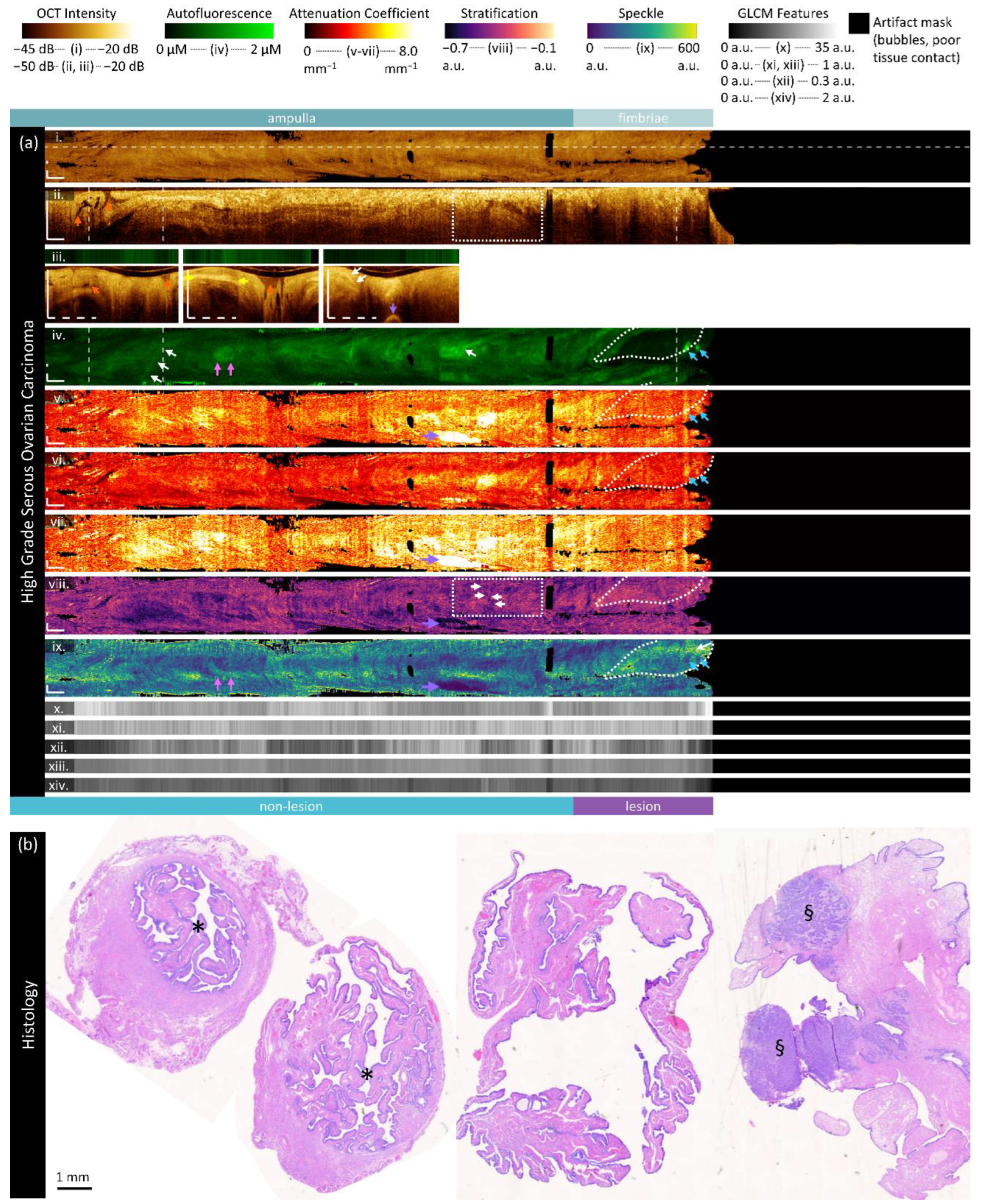
Figure 6.
Sample imaging of a fallopian tube containing HGSOC. Panel (a) demonstrates the biomarkers: (i) mean OCT en face projection; (ii) longitudinal OCT section from the dashed line in panel (i); and three sample OCT cross-sections from dashed lines in the longitudinal section demonstrated in wrapped and unwrapped views alongside co-registered AFI. Longitudinal and depth sections are cropped to the region containing the fundamental image. The bi-omarkers are presented below: (iv) autofluorescence; (v) overall attenuation coefficient; (vi) superficial attenuation coefficient; (vii) deep attenuation coefficient; (viii) stratification; (ix) speckle distribution; (x) GLCM contrast; (xi) GLCM correlation; (xii) GLCM energy; (xiii) GLCM homogeneity; (xiv) GLCM entropy. Panel (b) demonstrates representative histology. All scale bars are 1 mm.
Figure 6.
Sample imaging of a fallopian tube containing HGSOC. Panel (a) demonstrates the biomarkers: (i) mean OCT en face projection; (ii) longitudinal OCT section from the dashed line in panel (i); and three sample OCT cross-sections from dashed lines in the longitudinal section demonstrated in wrapped and unwrapped views alongside co-registered AFI. Longitudinal and depth sections are cropped to the region containing the fundamental image. The bi-omarkers are presented below: (iv) autofluorescence; (v) overall attenuation coefficient; (vi) superficial attenuation coefficient; (vii) deep attenuation coefficient; (viii) stratification; (ix) speckle distribution; (x) GLCM contrast; (xi) GLCM correlation; (xii) GLCM energy; (xiii) GLCM homogeneity; (xiv) GLCM entropy. Panel (b) demonstrates representative histology. All scale bars are 1 mm.
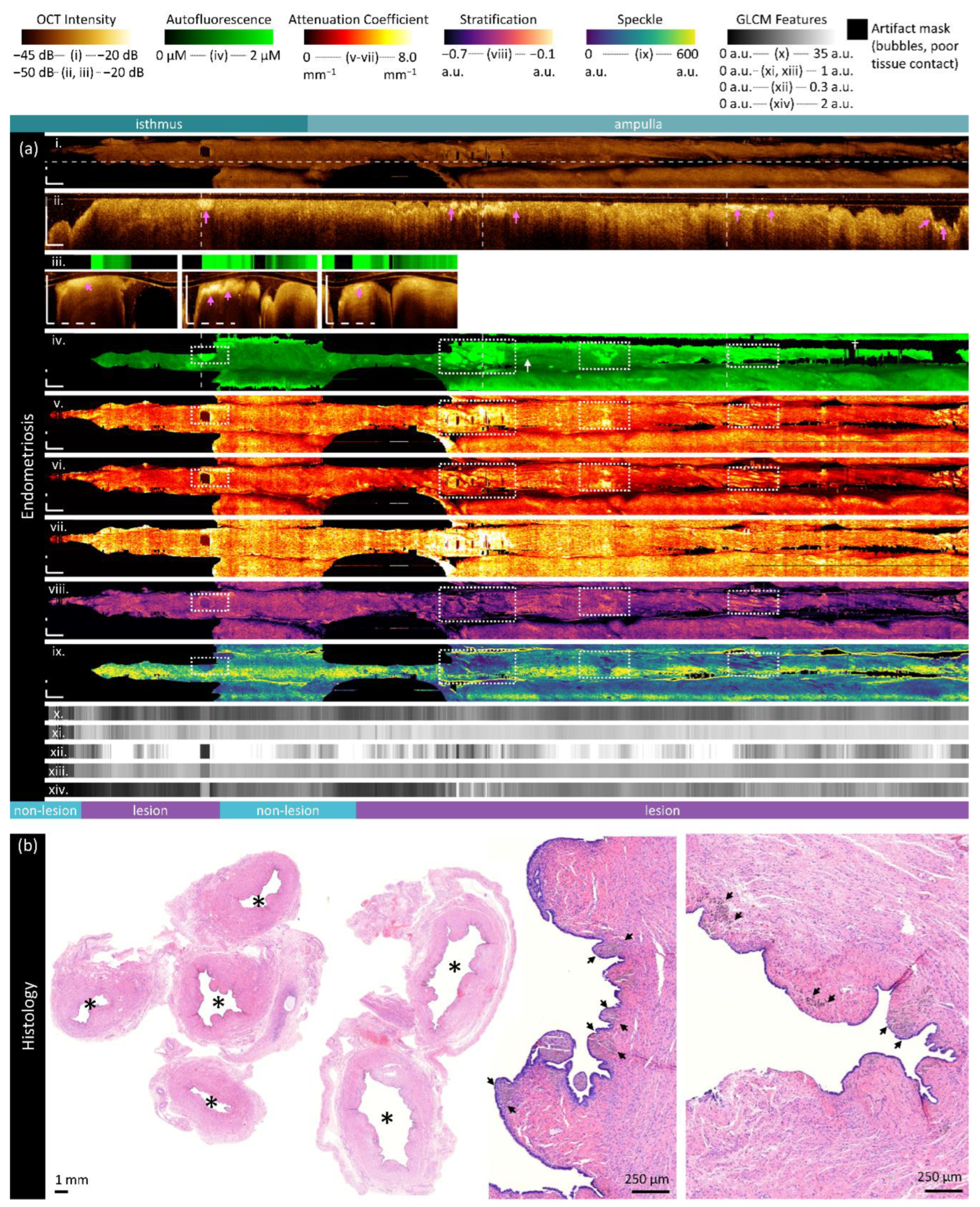
Figure 7.
Sample imaging of a fallopian tube containing endometriosis. Panel (a) demonstrates the biomarkers: (i) mean OCT en face projection; (ii) longitudinal OCT section from the dashed line in panel (i); and three sample OCT cross-sections from dashed lines in the longitudinal section demonstrated in wrapped and unwrapped views alongside co-registered AFI. Longitudinal and depth sections are cropped to the region containing the fundamental image. The biomarkers are presented below: (iv) autofluorescence; (v) overall attenuation coefficient; (vi) superficial attenuation coefficient; (vii) deep attenuation coefficient; (viii) stratification; (ix) speckle distribution; (x) GLCM contrast; (xi) GLCM correlation; (xii) GLCM energy; (xiii) GLCM homogeneity; (xiv) GLCM entropy. Panel (b) demonstrates representative histology. All scale bars are 1 mm unless otherwise noted.
Figure 7.
Sample imaging of a fallopian tube containing endometriosis. Panel (a) demonstrates the biomarkers: (i) mean OCT en face projection; (ii) longitudinal OCT section from the dashed line in panel (i); and three sample OCT cross-sections from dashed lines in the longitudinal section demonstrated in wrapped and unwrapped views alongside co-registered AFI. Longitudinal and depth sections are cropped to the region containing the fundamental image. The biomarkers are presented below: (iv) autofluorescence; (v) overall attenuation coefficient; (vi) superficial attenuation coefficient; (vii) deep attenuation coefficient; (viii) stratification; (ix) speckle distribution; (x) GLCM contrast; (xi) GLCM correlation; (xii) GLCM energy; (xiii) GLCM homogeneity; (xiv) GLCM entropy. Panel (b) demonstrates representative histology. All scale bars are 1 mm unless otherwise noted.
Figure 8.
Inter-sample measurements of each feature per diagnosis. Values are calculated using the median measurement for each biomarker per volume. The height of bars represents the mean value of specimens in that group; error bars are standard deviation. P-values for significant differences (p< 0.05) between disease states are indicated.
Figure 8.
Inter-sample measurements of each feature per diagnosis. Values are calculated using the median measurement for each biomarker per volume. The height of bars represents the mean value of specimens in that group; error bars are standard deviation. P-values for significant differences (p< 0.05) between disease states are indicated.
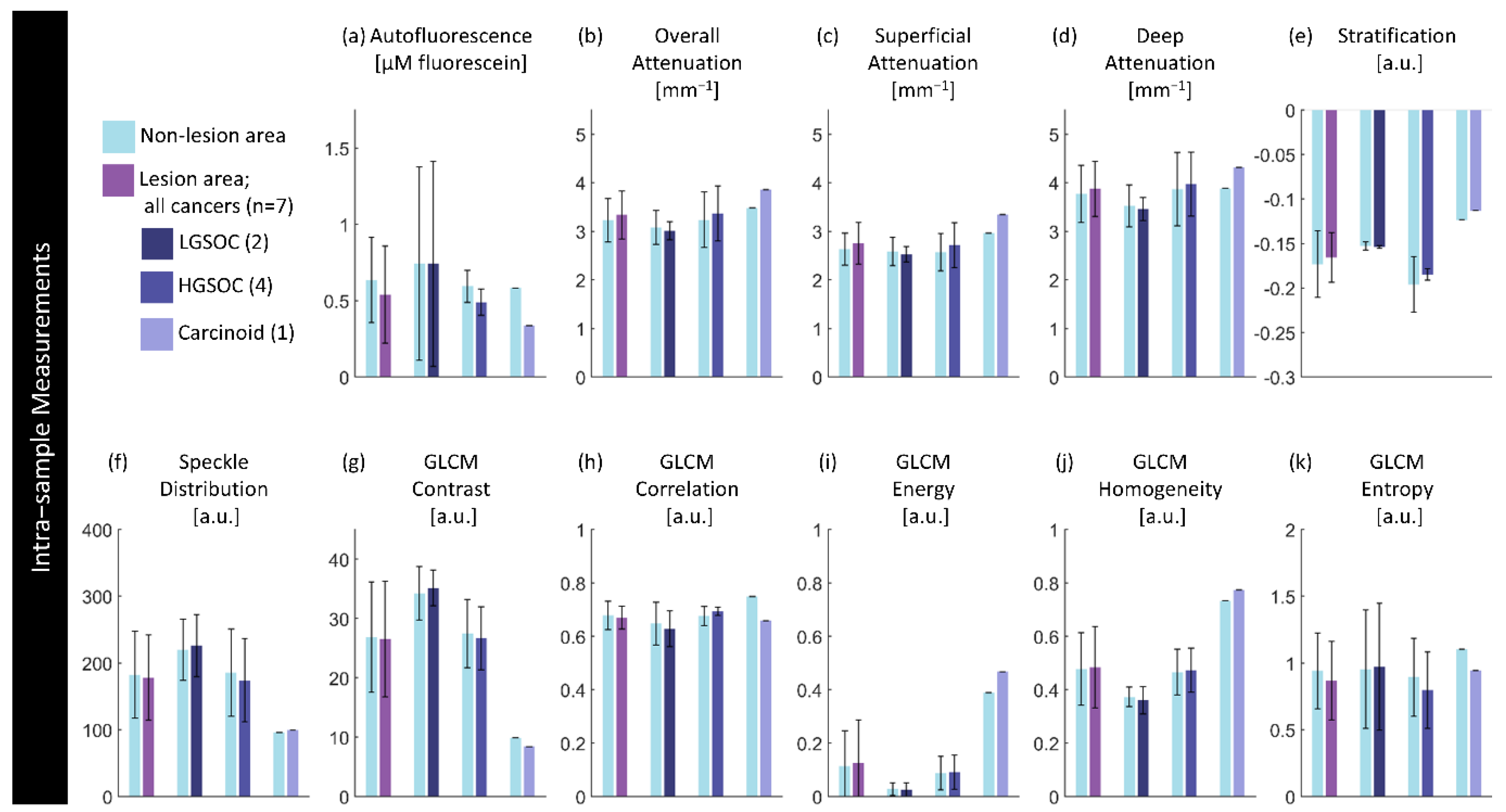
Figure 9.
Intra-sample measurements of each feature per diagnosis. Values are calculated using the median measurement for each biomarker per region for specimens containing a lesion of interest. The height of bars represents the mean value of specimens in that group; error bars are standard deviation. There are no significant differences (p<0.05) between paired lesion/non-lesion groups.
Figure 9.
Intra-sample measurements of each feature per diagnosis. Values are calculated using the median measurement for each biomarker per region for specimens containing a lesion of interest. The height of bars represents the mean value of specimens in that group; error bars are standard deviation. There are no significant differences (p<0.05) between paired lesion/non-lesion groups.
Figure 10.
Per-site measurements of each feature per region. Values are calculated using the median measurement for each biomarker per region of interest. The height of bars represents the mean value of specimens in that group; error bars are standard deviation. P-values for significant differences (p< 0.05) between disease states are indicated.
Figure 10.
Per-site measurements of each feature per region. Values are calculated using the median measurement for each biomarker per region of interest. The height of bars represents the mean value of specimens in that group; error bars are standard deviation. P-values for significant differences (p< 0.05) between disease states are indicated.
Table 1.
Summary of biomarkers.
Table 1.
Summary of biomarkers.
| Category |
Feature [units] |
Description |
Calculation |
| Functional |
Autofluorescence
[µM fluorescein] |
Intensity after calibration with respect to distance between the optical core and tissue using positive (0.98 µM fluorescein) and negative (water) standards. |
|
| Attenuation |
Overall Attenuation Coefficient
[mm-1] |
Mean optical attenuation coefficient over entire visualized tissue depth. |
Depth resolved method for estimating optical attenuation coefficient from OCT from Jian Liu et al. [67]:
|
Superficial Attenuation Coefficient
[mm-1] |
Mean optical attenuation coefficient over upper 50% of visualized tissue depth. |
Deep Attenuation
Coefficient
[mm-1] |
Mean optical attenuation coefficient over lower 50% of visualized tissue depth. |
Stratification
[a.u.] |
Ratiometric comparison of mean attenuation coefficient of superficial and deep regions. Ranges from -1 (higher deep attenuation) to +1 (higher superficial attenuation). |
|
| Texture |
Speckle Distribution |
Mean of the gamma distribution found by fitting all A-lines in the OCT cross-section. |
|
| GLCM Contrast |
Sum of squares variance or inertia; local variations between a pixel and its adjacent neighbours in the azimuthal direction. 0 represents no variation. |
Haralick features calculated from the gray level co-occurance matrix (GLCM) via MATLAB function graycoprops [43]:
|
| GLCM Correlation |
Joint probability of occurrence of intensity pairs between a pixel and its neighbor. Measured from -1 (perfect negative correlation) to +1 (perfect positive correlation). |
…via graycoprops [43]:
|
| GLCM Energy |
Angular second moment; uniformity of gray level distribution. Measured from 0 (no uniformity) to 1 (complete uniformity). |
…via graycoprops [43]:
|
| GLCM Homogeneity |
Inverse difference moment; similarity between a pixel and its adjacent neighbours in the azimuthal direction. 0 represents strong similarity. |
…via graycoprops [43]:
|
| GLCM Entropy |
Randomness of the image. 0 represents a completely uniform image. |
MATLAB function entropy [68]:
|
Table 2.
Summary of statistical tests.
Table 2.
Summary of statistical tests.
| Statistical question |
Parametric Test |
Non-parametric test |
| Is there a difference in measurements of biomarkers in volumes of different disease states? |
Unpaired t-test [75] |
Mann Whitney U test [76] |
| In volumes containing a lesion, is there a difference in measurements of biomarkers within the area of lesion compared to the area of non-lesion? |
Welch’s paired t-test [77,78] |
Wilcoxon rank sum [79] |
| In volumes without a lesion, is there a difference in measurements of biomarkers in different regions (isthmus, ampulla, fimbriae)? |
Welch’s paired t-test [77,78] |
Wilcoxon rank sum [79] |
| Are there differences between the left and right fallopian tubes in patients when paired imaging is acquired? |
Welch’s paired t-test [77,78] |
Wilcoxon rank sum [79] |
| In volumes without a lesion, are there statistical correlations between measurements and patient age? |
Spearman’s rank order [80] |
| In volumes without a lesion, are there statistical correlations between measurements and time difference between arrival and imaging of the specimen? |
Spearman’s rank order [80] |
Table 3.
Dataset demographics. Values for continuous measurements are described as mean, standard error, (range).
Table 3.
Dataset demographics. Values for continuous measurements are described as mean, standard error, (range).
| Diagnosis |
Sample Size |
Age |
Time to Imaging |
| Left |
Right |
Total |
| [#] |
[#] |
[#] |
[years] |
[minutes] |
| No lesion |
10 |
9 |
19 |
61
3
(42 – 81) |
69
5.4
(23-128) |
| Cancerous lesions |
3 |
4 |
7 |
65
3
(51 – 77) |
75
14.7
(30 – 127) |
| |
LGSOC |
0 |
2 |
2 |
65
4
(61 – 69) |
69
6.0
(63 – 75) |
| |
HGSOC |
3 |
1 |
4 |
69
3
(60 – 77) |
80
27
(30 – 127) |
| |
Carcinoid |
0 |
1 |
1 |
66
-
- |
66
-
- |
| Endometriosis |
1 |
1 |
2 |
62
18
(44 – 80) |
88
2.5
(85 – 90) |
| Total |
14 |
14 |
28 |
62 |
72 |
Table 4.
Dataset demographics for paired sets of imaging. Values for continuous measurements are described as mean, standard error, (range).
Table 4.
Dataset demographics for paired sets of imaging. Values for continuous measurements are described as mean, standard error, (range).
| |
Sample size |
Age |
Time to Imaging |
| Diagnosis |
[#] |
[years] |
[minutes] |
| Paired non-lesion |
5 |
57
6
(44 – 80) |
73
7.3
(49 – 90) |
Table 5.
Mean percentage differences in biomarker measurements between left and right fallopian tubes in paired specimens containing no lesion. Values reported are percentages.
Table 5.
Mean percentage differences in biomarker measurements between left and right fallopian tubes in paired specimens containing no lesion. Values reported are percentages.
| |
|
Functional |
Attenuation |
Texture |
| Diagnosis |
Sample size |
Auto- fluorescence |
Overall Attenuation |
Superficial Attenuation |
Deep Attenuation |
Stratification |
Speckle Distribution |
GLCM Contrast |
GLCM Correlation |
GLCM Energy |
GLCM Homogeneity |
GLCM Entropy |
| No lesion |
5 |
39.6 |
1.3 |
3.0 |
2.3 |
9.7 |
4.5 |
11.3 |
1.8 |
26.7 |
5.7 |
10.0 |