Submitted:
12 September 2024
Posted:
12 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Summary of Abbreviations
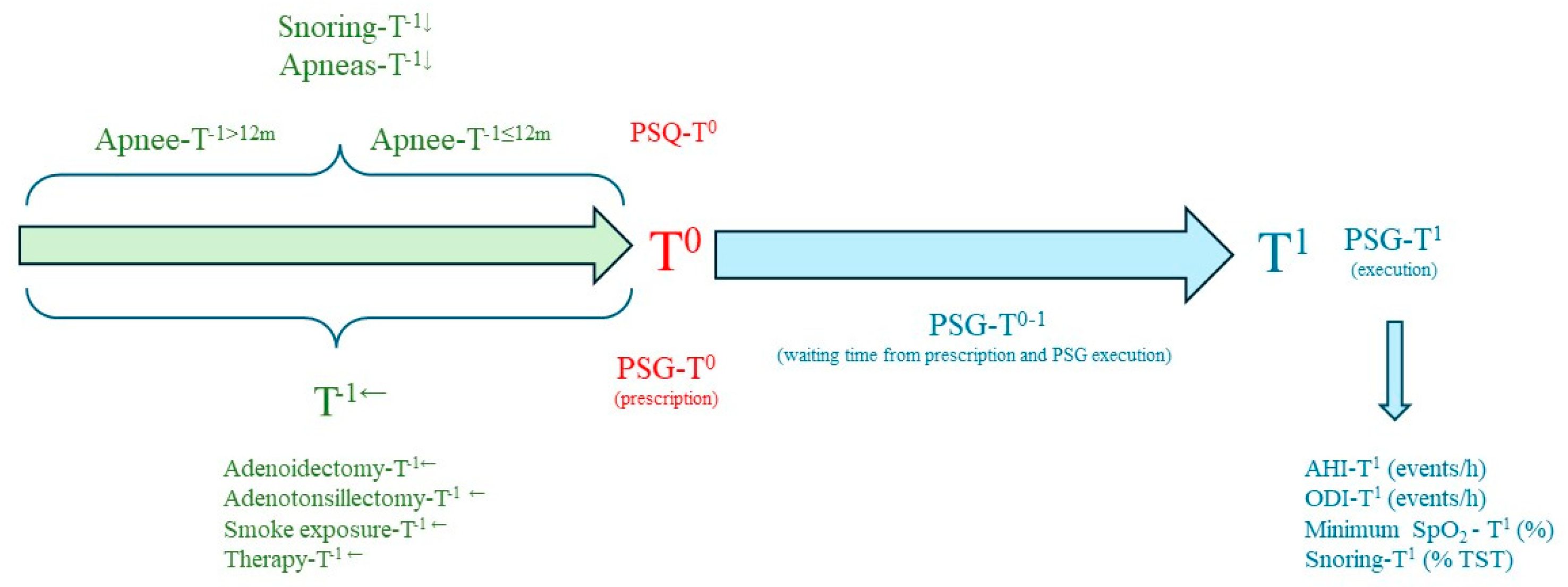
2.2. Polysomnography
2.3. Pediatric Sleep Questionnaire
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gipson, K.; Lu, M.; Kinane, T.B. Sleep-Disordered Breathing in Children. Pediatr Rev 2019, 40, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Sateia, M.J. International classification of sleep disorders-third edition: highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Kaditis, A.G.; Alonso Alvarez, M.L.; Boudewyns, A.; Alexopoulos, E.I.; Ersu, R.; Joosten, K.; Larramona, H.; Miano, S.; Narang, I.; Trang, H.; et al. Obstructive sleep disordered breathing in 2- to 18-year-old children: diagnosis and management. Eur Respir J 2016, 47, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.P.; Pietropaoli, N.; Supino, M.C.; Vitelli, O.; Rabasco, J.; Evangelisti, M.; Del Pozzo, M.; Kaditis, A.G. Diagnosis of Pediatric Obstructive Sleep Apnea Syndrome in Settings With Limited Resources. JAMA Otolaryngol Head Neck Surg 2015, 141, 990–996. [Google Scholar] [CrossRef]
- Chervin, R.D.; Weatherly, R.A.; Garetz, S.L.; Ruzicka, D.L.; Giordani, B.J.; Hodges, E.K.; Dillon, J.E.; Guire, K.E. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg 2007, 133, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Bitners, A.C.; Arens, R. Evaluation and Management of Children with Obstructive Sleep Apnea Syndrome. Lung 2020, 198, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Zaffanello, M.; Franchini, M.; Piacentini, G. Pediatric Sleep-Disordered Breathing and Long-Term Complications: Clinical and Health Implications. In J Clin Med; Switzerland, 2022; Volume 11.
- Piotto, M.; Gambadauro, A.; Rocchi, A.; Lelii, M.; Madini, B.; Cerrato, L.; Chironi, F.; Belhaj, Y.; Patria, M.F. Pediatric Sleep Respiratory Disorders: A Narrative Review of Epidemiology and Risk Factors. Children (Basel) 2023, 10. [Google Scholar] [CrossRef]
- Savini, S.; Ciorba, A.; Bianchini, C.; Stomeo, F.; Corazzi, V.; Vicini, C.; Pelucchi, S. Assessment of obstructive sleep apnoea (OSA) in children: an update. Acta Otorhinolaryngol Ital 2019, 39, 289–297. [Google Scholar] [CrossRef]
- Lee, C.F.; Lee, C.H.; Hsueh, W.Y.; Lin, M.T.; Kang, K.T. Prevalence of Obstructive Sleep Apnea in Children With Down Syndrome: A Meta-Analysis. J Clin Sleep Med 2018, 14, 867–875. [Google Scholar] [CrossRef]
- Zaffanello, M.; Antoniazzi, F.; Tenero, L.; Nosetti, L.; Piazza, M.; Piacentini, G. Sleep-disordered breathing in paediatric setting: existing and upcoming of the genetic disorders. Ann Transl Med 2018, 6, 343. [Google Scholar] [CrossRef]
- Zaffanello, M.; Pietrobelli, A.; Piacentini, G.; Guzzo, A.; Antoniazzi, F. The Impact of Growth Hormone Therapy on Sleep-Related Health Outcomes in Children with Prader-Willi Syndrome: A Review and Clinical Analysis. J Clin Med 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Lu, C.-H.; Lin, H.-C.; Chen, P.-C.; Chou, K.-H.; Lin, W.-M.; Tsai, N.-W.; Su, Y.-J.; Friedman, M.; Lin, C.-P.; et al. White matter damage and systemic inflammation in obstructive sleep apnea. Sleep 2015, 38, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Orrù, G.; Storari, M.; Scano, A.; Piras, V.; Taibi, R.; Viscuso, D. Obstructive Sleep Apnea, oxidative stress, inflammation and endothelial dysfunction-An overview of predictive laboratory biomarkers. Eur Rev Med Pharmacol Sci 2020, 24, 6939–6948. [Google Scholar] [CrossRef] [PubMed]
- Tagetti, A.; Bonafini, S.; Zaffanello, M.; Benetti, M.V.; Vedove, F.D.; Gasperi, E.; Cavarzere, P.; Gaudino, R.; Piacentini, G.; Minuz, P.; et al. Sleep-disordered breathing is associated with blood pressure and carotid arterial stiffness in obese children. J Hypertens 2016. [Google Scholar] [CrossRef]
- Burns, A.T.; Hansen, S.L.; Turner, Z.S.; Aden, J.K.; Black, A.B.; Hsu, D.P. Prevalence of Pulmonary Hypertension in Pediatric Patients With Obstructive Sleep Apnea and a Cardiology Evaluation: A Retrospective Analysis. J Clin Sleep Med 2019, 15, 1081–1087. [Google Scholar] [CrossRef]
- Agarwal, N.; Sharma, B.C. Insulin resistance and clinical aspects of non-alcoholic steatohepatitis (NASH). Hepatol Res 2005, 33, 92–96. [Google Scholar] [CrossRef]
- Zaffanello, M.; Piacentini, G.; La Grutta, S. Beyond the growth delay in children with sleep-related breathing disorders: a systematic review. Panminerva Med 2020, 62, 164–175. [Google Scholar] [CrossRef]
- Zaffanello, M.; Pietrobelli, A.; Zoccante, L.; Ferrante, G.; Tenero, L.; Piazza, M.; Ciceri, M.L.; Nosetti, L.; Piacentini, G. Mental Health and Cognitive Development in Symptomatic Children and Adolescents Scoring High on Habitual Snoring: Role of Obesity and Allergy. Children (Basel) 2023, 10. [Google Scholar] [CrossRef]
- Marcus, C.L.; Brooks, L.J.; Ward, S.D.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Lehmann, C.; Schechter, M.S.; Sheldon, S.; et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012, 130. [Google Scholar] [CrossRef]
- Borrelli, M.; Corcione, A.; Cimbalo, C.; Annunziata, A.; Basilicata, S.; Fiorentino, G.; Santamaria, F. Diagnosis of Paediatric Obstructive Sleep-Disordered Breathing beyond Polysomnography. Children (Basel) 2023, 10. [Google Scholar] [CrossRef]
- Baker-Smith, C.M.; Isaiah, A.; Melendres, M.C.; Mahgerefteh, J.; Lasso-Pirot, A.; Mayo, S.; Gooding, H.; Zachariah, J. Sleep-Disordered Breathing and Cardiovascular Disease in Children and Adolescents: A Scientific Statement From the American Heart Association. J Am Heart Assoc 2021, 10, e022427. [Google Scholar] [CrossRef] [PubMed]
- Getnet, F.; Demissie, M.; Assefa, N.; Mengistie, B.; Worku, A. Delay in diagnosis of pulmonary tuberculosis in low-and middle-income settings: systematic review and meta-analysis. BMC Pulm Med 2017, 17, 202. [Google Scholar] [CrossRef] [PubMed]
- Muttamba, W.; Kyobe, S.; Komuhangi, A.; Lakony, J.; Buregyeya, E.; Mabumba, E.; Basaza, R.K. Delays in diagnosis and treatment of pulmonary tuberculosis in patients seeking care at a regional referral hospital, Uganda: a cross sectional study. BMC Res Notes 2019, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, C.; Zhang, X.; Yu, H.; Yan, X.; Wang, L.; Tong, T.; Zhang, H.; Dai, H.; Tong, H. Factors influencing patient delay in individuals with obstructive sleep apnoea: a study based on an integrated model. Ann Med 2022, 54, 2828–2840. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.S.; Tsai, W.H.; Santana, M.J.; Penz, E.D.; Flemons, W.W.; Fraser, K.L.; Hanly, P.J.; Pendharkar, S.R. Effects of Wait Times on Treatment Adherence and Clinical Outcomes in Patients With Severe Sleep-Disordered Breathing: A Secondary Analysis of a Noninferiority Randomized Clinical Trial. JAMA Netw Open 2020, 3, e203088. [Google Scholar] [CrossRef]
- Thornton, C.S.; Povitz, M.; Tsai, W.H.; Loewen, A.H.; Ip-Buting, A.; Kendzerska, T.; Flemons, W.W.; Fraser, K.L.; Hanly, P.J.; Pendharkar, S.R. Impact of wait times for treatment on clinical outcomes in patients with obstructive sleep apnoea: protocol for a randomised controlled trial. ERJ Open Res 2022, 8. [Google Scholar] [CrossRef]
- Flemons, W.W.; Douglas, N.J.; Kuna, S.T.; Rodenstein, D.O.; Wheatley, J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med 2004, 169, 668–672. [Google Scholar] [CrossRef]
- McIntyre, D.; Chow, C.K. Waiting Time as an Indicator for Health Services Under Strain: A Narrative Review. Inquiry 2020, 57, 46958020910305. [Google Scholar] [CrossRef]
- Chang, S.J.; Chae, K.Y. Obstructive sleep apnea syndrome in children: Epidemiology, pathophysiology, diagnosis and sequelae. Korean J Pediatr 2010, 53, 863–871. [Google Scholar] [CrossRef]
- Nosetti, L.; Zaffanello, M.; Katz, E.S.; Vitali, M.; Agosti, M.; Ferrante, G.; Cilluffo, G.; Piacentini, G.; La Grutta, S. Twenty-year follow-up of children with obstructive sleep apnea. J Clin Sleep Med 2022, 18, 1573–1581. [Google Scholar] [CrossRef]
- Chervin, R.D.; Hedger, K.; Dillon, J.E.; Pituch, K.J. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med 2000, 1, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Dekking, F.M. A Modern Introduction to Probability and Statistics: Understanding Why and How, Springer, 2005.
- Greenland, S.; Senn, S.J.; Rothman, K.J.; Carlin, J.B.; Poole, C.; Goodman, S.N.; Altman, D.G. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol 2016, 31, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Chai-Coetzer, C.L.; Antic, N.A.; McEvoy, R.D. Ambulatory models of care for obstructive sleep apnoea: Diagnosis and management. Respirology 2013, 18, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Corral-Peñafiel, J.; Pepin, J.L.; Barbe, F. Ambulatory monitoring in the diagnosis and management of obstructive sleep apnoea syndrome. Eur Respir Rev 2013, 22, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, P.; Tolonen, U.; Löppönen, H. Snoring and obstructive sleep apnea in children: a 6-month follow-up study. Arch Otolaryngol Head Neck Surg 2000, 126, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Phua, C.Q.; Jang, I.J.; Tan, K.B.; Hao, Y.; Senin, S.R.B.; Song, P.R.; Soh, R.Y.; Toh, S.T. Reducing cost and time to diagnosis and treatment of obstructive sleep apnea using ambulatory sleep study: a Singapore sleep centre experience. Sleep Breath 2021, 25, 281–288. [Google Scholar] [CrossRef]
- Hung, C.J.; Kang, B.H.; Lin, Y.S.; Su, H.H. Comparison of a home sleep test with in-laboratory polysomnography in the diagnosis of obstructive sleep apnea syndrome. J Chin Med Assoc 2022, 85, 788–792. [Google Scholar] [CrossRef]
- Halbower, A.C.; Degaonkar, M.; Barker, P.B.; Earley, C.J.; Marcus, C.L.; Smith, P.L.; Prahme, M.C.; Mahone, E.M. Childhood obstructive sleep apnea associates with neuropsychological deficits and neuronal brain injury. PLoS Med 2006, 3, e301. [Google Scholar] [CrossRef]
- Ferry, A.M.; Wright, A.E.; Ohlstein, J.F.; Khoo, K.; Pine, H.S. Efficacy of a Pediatric Sleep Questionnaire for the Diagnosis of Obstructive Sleep Apnea in Children. Cureus 2020, 12, e12244. [Google Scholar] [CrossRef]
- Zhai, F.; Li, Y.; Chen, J. Comparison of polysomnography, sleep apnea screening test and cardiopulmonary coupling in the diagnosis of pediatric obstructive sleep apnea syndrome. Int J Pediatr Otorhinolaryngol 2021, 149, 110867. [Google Scholar] [CrossRef]
- Corbo, G.M.; Fuciarelli, F.; Foresi, A.; De Benedetto, F. Snoring in children: association with respiratory symptoms and passive smoking. Bmj 1989, 299, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Au, C.T.; Leung, T.F.; Wing, Y.K.; Lam, C.W.; Li, A.M. Effects of passive smoking on snoring in preschool children. J Pediatr 2013, 163, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Jara, S.M.; Benke, J.R.; Lin, S.Y.; Ishman, S.L. The association between secondhand smoke and sleep-disordered breathing in children: a systematic review. Laryngoscope 2015, 125, 241–247. [Google Scholar] [CrossRef]
- Nosetti, L.; Paglietti, M.G.; Brunetti, L.; Masini, L.; La Grutta, S.; Cilluffo, G.; Ferrante, G.; Zaffanello, M.; Verrillo, E.; Pavone, M.; et al. Application of latent class analysis in assessing the awareness, attitude, practice and satisfaction of paediatricians on sleep disorder management in children in Italy. PLoS ONE 2020, 15, e0228377. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Ewing, C.; Gnanapragasam, J.; Majaesic, C.; MacLean, J.; Mandhane, P.J. Changes to a pediatric sleep disordered breathing clinic improve wait-times and clinic efficiency. Pediatr Pulmonol 2016, 51, 1234–1241. [Google Scholar] [CrossRef]
- Burke, B.L., Jr.; Hall, R.W. Telemedicine: Pediatric Applications. Pediatrics 2015, 136, e293–e308. [Google Scholar] [CrossRef] [PubMed]
- Sisk, B.; Alexander, J.; Bodnar, C.; Curfman, A.; Garber, K.; McSwain, S.D.; Perrin, J.M. Pediatrician Attitudes Toward and Experiences With Telehealth Use: Results From a National Survey. Acad Pediatr 2020, 20, 628–635. [Google Scholar] [CrossRef]
- Rizzo, L.; Barbetta, E.; Ruberti, F.; Petz, M.; Tornesello, M.; Deolmi, M.; Fainardi, V.; Esposito, S. The Role of Telemedicine in Children with Obstructive Sleep Apnea Syndrome (OSAS): A Review of the Literature. J Clin Med 2024, 13. [Google Scholar] [CrossRef]
- Heath, D.S.; El-Hakim, H.; Al-Rahji, Y.; Eksteen, E.; Uwiera, T.C.; Isaac, A.; Castro-Codesal, M.; Gerdung, C.; Maclean, J.; Mandhane, P.J. Development of a pediatric obstructive sleep apnea triage algorithm. J Otolaryngol Head Neck Surg 2021, 50, 48. [Google Scholar] [CrossRef]
- Esposito, S.; Ricci, G.; Gobbi, R.; Vicini, C.; Caramelli, F.; Pizzi, S.; Fadda, A.; Ferro, S.; Plazzi, G. Diagnostic and Therapeutic Approach to Children and Adolescents with Obstructive Sleep Apnea Syndrome (OSA): Recommendations in Emilia-Romagna Region, Italy. Life (Basel) 2022, 12. [Google Scholar] [CrossRef]
- Locci, C.; Cenere, C.; Sotgiu, G.; Puci, M.V.; Saderi, L.; Rizzo, D.; Bussu, F.; Antonucci, R. Adenotonsillectomy in Children with Obstructive Sleep Apnea Syndrome: Clinical and Functional Outcomes. J Clin Med 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Connolly, H.V.; Tomaselli, L.T.; McKenna Benoit, M.K. Adenotonsillectomy for pediatric obstructive sleep apnea: how to predict those at risk for postoperative complications. J Clin Sleep Med 2020, 16, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Tauman, R.; Gulliver, T.E.; Krishna, J.; Montgomery-Downs, H.E.; O'Brien, L.M.; Ivanenko, A.; Gozal, D. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr 2006, 149, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Emani, J.; Suskind, D.L.; Baroody, F.M. Predictors of persistent sleep apnea after surgery in children younger than 3 years. JAMA Otolaryngol Head Neck Surg 2013, 139, 1002–1008. [Google Scholar] [CrossRef]
| Continuous Variable | Mean (SD) | 25° pc | 50° pc | 75° pc | Kolmogorov-Smirnov test* |
|---|---|---|---|---|---|
| Age PSG-T0 (years) | 5.2 (2.8) | 3.3 | 4.6 | 6.4 | <0.001 |
| PSQ-T0 (score) | 0.34 (0.19) | 0.22 | 0.36 | 0.45 | 0.012 |
| PSG-T0-1 (months) | 5.2 (5.0) | 2.1 | 4.2 | 8.2 | <0.001 |
| Age PSG-T1 (years) | 5.6 (2.8) | 3.8 | 5.0 | 6.8 | <0.001 |
| Weight-T1 (kg) | 22.5 (13.7) | 15 | 18 | 25 | <0.001 |
| Height-T1 (cm) | 113 (19) | 99 | 110 | 125 | 0.005 |
| PSG-T1 | |||||
| AHI-T1 (Events/h) | 7.0 (7.9) | 2.3 | 4.1 | 8.0 | <0.001 |
| ODI-T1 (Events/h) | 6.0 (7.4) | 2 | 3.5 | 6.3 | <0.001 |
| SpO2-T1 (%) | 96.8 (1.0) | 96.1 | 96.9 | 97.5 | 0.001 |
| SpO2 minimum-T1 (%) | 89.1 (4.4) | 88 | 90 | 92 | <0.001 |
| Snoring-T1 (%) | 4.5 (4.6) | 9 | 3.5 | 6.6 | <0.001 |
| Categorical variable | Subcategories | N (%) |
|---|---|---|
| Sex | Male | 133 (62.1) |
| Snoring-T-1← (minimum, months; missing=don't know) | I do not know | 21 (9.8) |
| 0-6 | 15 (7) | |
| 6-12 | 38 (17.8) | |
| 12-18 | 4 (1.9) | |
| 18-24 | 109 (50.9) | |
| > 24 | 27 (12.6) | |
| Apnee-T-1← (minimum, months; missing=don't know) | I do not know | 71 (33.1) |
| 0-6 | 18 (8.4) | |
| 6-12 | 30 (14) | |
| 12-18 | 29 (13.6) | |
| 18-24 | 5 (2.3) | |
| > 24 | 66 (30.8) | |
| Drugs-T-1← Taken orally | No | 186 (86.9) |
| Antihistamines | 10 (4.7) | |
| Corticosteroids | 5 (2.3) | |
| Antileukotrienes | 4 (1.9) | |
| Other | 4 (1.9) | |
| Drugs-T-1← spray, inhalant | No | 162 (75.7) |
| Steroids | 51 (23.8) | |
| Salbutamol | 0 | |
| Steroids, salbutamol | 1 (0.5) | |
| Otorhinolaryngology surgery-T-1← | Adenotonsillectomy | 15 (7.5) |
| Adenoidectomy | 9 (4.2) | |
| Tonsillectomy | 1 (0.5) | |
| Exposure to secondhand smoke-T-1← | Yes | 42 (19.6) |
| Allergy test (prick test) | Yes | 38 (17.8) |
| Not executed | 154 (72.0) | |
| Result of the PSG-T1 (Categories) | Primary snoring | 28 (13.1) |
| Mild | 100 (46.7) | |
| Moderate | 43 (20.1) | |
| Severe | 43 (20.1) |
| Apnee-T-1≤12m (n.72), Mean (SD) |
Apnee-T-1>12m (n. 75), Mean (SD) |
Test U (Mann-Whitney), P | |
|---|---|---|---|
| Apnee-T-1 (months) | 7.35 (3.74) | 23.2 (2.5) | 0.001 |
| Snoring-T-1 (months) | 15.2 (12.7) | 30.3 (10.8) | 0.001 |
| PSG-T0 (years) | 4.6 (2.6) | 5.5 (2.7) | 0.027 |
| PSQ-T0 (score) | 0.37 (0.2) | 0.34 (0.21) | 0.466 |
| PSG-T0-1 (months) | 4.1 (3.8) | 5.9 (3.8) | 0.001 |
| PSG-T1 (years) | 4.9 (2.7) | 6.0 (2.7) | 0.006 |
| AHI-T1 (events/h) | 7.9 (8.2) | 6.8 (7.5) | 0.224 |
| ODI-T1 (events/h) | 6.7 (7.6) | 6.0 (7.0) | 0.316 |
| SpO2-T1 minimum (%) | 87.9 (5.3) | 89.3 (4.3) | 0.127 |
| Snoring-T1 (% TST) | 4.3 (4.2) | 4.3 (4.4) | 0.946 |
| Categorical variables | Subcategories | Apnee-T-1≤ 12m (n. 72); % | Apnee-T-1>12m (n.75); % |
Pearson Chi-Square | Fisher's Exact Test | Contingency coefficient |
|---|---|---|---|---|---|---|
| Sex | Males | 63.9 | 60.0 | 0.647 | - | 0.040 |
| Adenoidectomy-T-1← | Yes | 9.7 | 1.3 | 0.027 | 0.182 | |
| Tonsillectomy-T-1← | Yes | 0 | 0 | - | - | - |
| Adenotonsillectomy-T-1← | Yes | 6.9 | 13.3 | - | 0.157 | 0.105 |
| 0.012 | - | 0.238 | ||||
| Secondhand smoke-T-1← | Yes | 16.7 | 18.7 | 0.751 | - | 0.026 |
| Allergy | Yes | 50.0 | 75.0 | - | 0.189 | 0.227 |
| Drugs-T-1← taken orally | Antihistamines | 2.8 | 2.7 | |||
| Steroids | 2.8 | 4.0 | ||||
| Antileukotrienes | 1.4 | 4.0 | ||||
| Other | 2.8 | 0.0 | 0.530 | - | 0.145 | |
| Drugs-T-1← spray or inhalants | Steroids | 29.2 | 25.3 | |||
| Salbutamol | 0 | 0 | ||||
| Steroids+salbutamol | 0 | 1.3 | 0.550 | 0.090 | ||
| PSG result-T1 (Categories, %) | Snoring | 9.7 | 10.7 | |||
| Mild OSA | 41.7 | 46.7 | ||||
| Moderate OSA | 22.2 | 21.3 | ||||
| Severe OSA | 26.4 | 21.3 | 0.885 | - | 0.066 |
| PSG-T0-1≤ 3m (n. 78); Mean (SD) | PSG-T0-1>3m (n.136); Mean (SD) | Test U (Mann-Whitney) P | |
|---|---|---|---|
| Snoring-T-1← (minimum, months; missing=don't know) | 20.5 (14.2) [Total Responses n. 74] |
23.4 (13.8) [Total Responses n. 120] |
0.084 |
| Apnee-T-1← (minimum, months; missing=don't know) | 11.6 (8.0) [Total Responses n. 55] |
17.7 (8.1) [Total Responses n. 92] |
< 0.001 |
| PSG-T0 (years) | 5.0 (2.6) | 5.3 (2.9) |
0.688 |
| PSG-T0-1 | 1.4 (1.0) | 7.4 (5.1) | < 0.001 |
| PSQ-T0 (score) | 0.35 (0.18) | 0.34 (0.20) | 0.313 |
| Age PSG-T1 (years) | 5.1 (2.6) | 5.8 (2.9) | 0.053 |
| AHI-T1 (Events/h) | 7.7 (8.5) | 6.6 (7.6) | 0.226 |
| ODI-T1 (Events/h) | 6.2 (7.4) | 5.8 (7.4) | 0.361 |
| Snoring-T1 (% TST) | 4.6 (4.7) | 4.5 (4.5) | 0.751 |
| Categorical variables | Subcategories | PSG-T0-1≤ 3m, % | PSG-T0-1>3m; % | Pearson Square Chi | Fisher's Exact Test | Contingency coefficient |
|---|---|---|---|---|---|---|
| N. | - | 78 | 136 | - | - | - |
| Sex | Males | 64.1 | 61 | 0.656 | 0.383 | 0.030 |
| PSG result-T1 (Categories) | Snoring | 7.7 | 16.2 | |||
| Mild OSA | 42.3 | 49.3 | ||||
| Moderate OSA | 26.9 | 16.2 | ||||
| Severe OSA | 23.1 | 18.4 | 0.085 | - | 0.173 | |
| Medications taken orally-T-1← | Antihistamines | 7.7 | 2.9 | |||
| Steroids | 3.8 | 1.5 | ||||
| Antileukotrienes | 0 | 2.9 | ||||
| Other | 2.6 | 1.5 | 0.174 | - | 0.170 | |
| Pharmacy Spray Nasal-T-1← | Steroids | 26.6 | 22.8 | |||
| Salbutamol | 0 | 0 | ||||
| Steroids+salbutamol | 0 | 0.7 | 0.680 | - | 0.060 | |
| Adenotonsillectomy-T-1← | Yes | 10.3 | 5.9 | 0.242 | 0.183 | 0.080 |
| Adenoidectomy- T-1← | Yes | 3.8 | 4.4 | 0.843 | 0.573 | 0.014 |
| Tonsillectomy-T-1← | No | 100 | 99.3 | 0.448 | 0.636 | 0.052 |
| 0.588 | 0.094 | |||||
| Exposure to secondhand smoke-T-1← | Yes | 17.9 | 20.6 | 0.640 | 0.390 | 0.032 |
| Allergy | Yes | 68.4 | 61 | 0.578 | 0.398 | 0.072 |
| N | Age PSG-T0, years (SD) | Age PSG-T1, years (SD) | PSG-T0-1, Months (SD) | |
|---|---|---|---|---|
| Age, years (SD) | 5.2 (2.8) | 5.6 (2.8) | 5.2 (5.0) | |
| Spearman's rho correlation coefficient, r (Sign. a due code) | ||||
| Snoring-T-1← (minimum, months; missing=don't know) | 194 | 0.216 (0.003) | 0.233 (0.001) | 0.119 (0.098) |
| Apnee-T-1← (minimum, months; missing=don't know) | 147 | 0.187 (0.023) | 0.237 (0.004) | 0.327 (<0.001) |
| PSQ-T0 (score) | 214 | 0.167 (0.015) | 0.143 (0.037) | -0.091 (0.186) |
| AHI-T1 (events/hour) | 214 | -0.116 (0.091) | -0.127 (0.064) | -0.097 (0.157) |
| ODI-T1 (eventi/ora) | 214 | -0.152 (0.026) | -0.162 (0.018) | -0.089 (0.195) |
| Snoring-T1 (% TST) | 194 | 0.086 (0.210) | -0.075 (0.273) | -0.045 (0.512) |
| Spearman's rho correlation coefficient, r (Sign. Two-tailed); adjusted for age at the PSG-T0 | 214 | |||
| Snoring-T-1← (minimum, months; missing=don't know) | 214 | - | 0.189 (0.027) | 0.195 (0.023) |
| Apnee-T-1← (minimum, months; missing=don't know) | 214 | - | 0.308 (<0.001) | 0.259 (0.002) |
| PSQ-T0 (score) | 214 | - | -0.103 (0.230) | -0.172 (0.045) |
| AHI-T1 (events/hour) | 214 | - | -0.005 (0.957) | -0.052 (0.549) |
| ODI-T1 (events/hour) | 214 | - | -0.004 (0.959) | -0.041 (0.634) |
| Russamento-T1 (% TST) | 214 | - | -0.009 (0.916) | -0.004 (0.962) |
| Correlation coefficient rho of Spearman, r (Sign. a two-tailed); adjusted for age at the PSG-T1 | 214 | |||
| Snoring-T-1← (minimum, months; missing=don't know) | 214 | -0.158 (0.066) | - | 0.166 (0.052) |
| Apnee-T-1← (minimum, months; missing=don't know) | 214 | -0.290 (0.001) | - | 0.241 (0.004) |
| PSQ-T0 (score) | 214 | 0.111 (0.196) | - | -0.179 (0.036) |
| AHI-T1 (events/hour) | 214 | -0.019 (0.830) | - | -0.032 (0.714) |
| ODI-T1 (events/hour) | 214 | -0.019 (0.822) | - | -0.020 (0.813) |
| Snoring-T1 (% TST) | 214 | 0.024 (0.781) | - | -0.017 (0.841) |
| Dependent variable: PSG-T0-1, Months (SD). Predictors: Age at PSQ-T0, years (SD); snoring-T-1←, apnee-T-1←; AHI-T1 (events/hour); ODI-T1 (events/hour); snoring-T1 (%); PSQ-T0 |
Non-standardised coefficients, T | S.E. | Standardised coefficients, Beta | t | p | C.I. per B 95 % (lower-upper) |
|---|---|---|---|---|---|---|
| Variables Entered in the Model | ||||||
| Apnee-T-1← (Months) | 0.118 | 0.037 | 0.264 | 3.225 | 0.002 | 0.046 – 0.191 |
| PSQ-T0 | -3.163 | 1.554 | -0.167 | -2.040 | 0.043 | -6.321 – (-0.096) |
| Constant | 4.174 | 0.852 | 4.9 | 0.001 | 2.489 – 5.858 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





