1. Introduction
Plastic is one of the most widely used materials in the world today, which is used as a construction material, automotive, packaging and among other applications that can be given because these are synthetic polymers that are durable, malleable, economical and versatile [
1], this has made them a fundamental part of our lives in the last century. The indiscriminate use of plastic has brought with it a series of problems to ecosystems, as of 2015 a total of 8300 million tons (mt) had been generated [
2] of which only 2500 mt were in use, 500 mt were recycled and the rest were disposed of in landfills and in the natural environment affecting the different ecosystems [
3]. The effect of these polymers on the environment has produced an increase in research in the area during the last 4 years, which has led to the design of various remediation technologies to avoid and decrease the impact generated by these wastes [
4].
There are a multitude of methods designed for the elimination or removal of plastic materials in different environments, among which chemical, physical and biological methods are recognized, the latter being the technologies most investigated by biotechnologists, since they include the use of living organisms to remediate contaminating compounds, in addition to being environmentally friendly and more economical in their application [
5].
The biodegradation of plastic fragments is considered one of the imminent solutions to mitigate the environmental threat [
6]. The difficult biodegradability of petroleum-based synthetic polymers hinders the use of living organisms in their management; however, during the last few years, potential microorganisms have been discovered for their decomposition, which use the plastic molecules as a carbon source [
7], where microbes use their enzymatic complexes that allow them to degrade the polymers in several stages and thus obtain energy from them [
8]. This method produces modifications in the physicochemical properties of the plastic, converting it into by-products that are less polluting for the environment.
Currently, research on the biodegradation of plastics has focused on higher animal species such as insects, specifically on holometabolous larvae, such as coleoptera and lepidoptera, which have been shown to be able to consume plastics and mineralize them thanks to their intestinal microbiota [
9]
The darkling beetle
Zophobas morio of the family Tenebrionidae belongs to the order Coleoptera and is an insect of high industrial potential due to its varied applications, since the larval stage of this organism has several characteristics that make it a potential focus of study [
10]. The larvae of
Zophobas morio have a high content of nutrients such as proteins and fats, which has allowed them to be used to formulate food for livestock, however, these insects have shown to be able to feed on some synthetic polymers and survive for long periods of time, with which it has been recognized that in their intestines there are microorganisms with enzymes that have the ability to degrade synthetic polymers allowing the superworms to obtain nutrients and energy [
11].
Superworms have been able to chew and feed on polystyrene foam, these polymers are biodegraded in their intestines allowing them to feed and continue their life cycle, until they reach their adult stage of beetle that does not consume plastic [
12]. However, their ability to degrade other synthetic materials present in nature has not yet been investigated. Therefore, in this work, for the first time, reused plastics that are commonly used by the population, such as polyisoprene rubber, low density polyethylene bags and butyl rubber, are used as a diet for superworms.
Zophobas morio larvae have demonstrated that they can consume many types of substrates, so it is expected that they are able to feed and survive by basing their diet on these synthetic polymers, in addition, the objective of this work is to evaluate the metabolic capacity and recognize the intestinal bacteria of Zophobas morio larvae when fed with different synthetic polymers. The results obtained will have an important value for understanding the biological complex of superworms that allows them to metabolize the plastic material, allowing the development of new research associated with bioremediation.
2. Materials and Methods
2.1. Experimental Design and Feeding Treatments
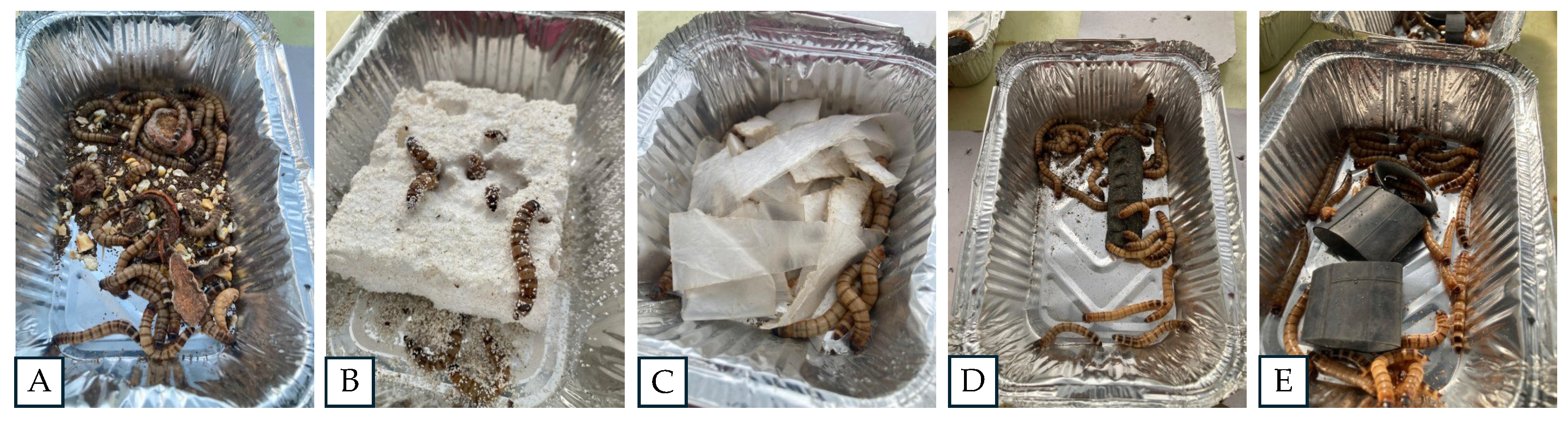
Zophobas morio larvae were provided by the company (Grillos y más SpA, Pitrufquén, Chile) and experiments were conducted in the laboratories and greenhouse of the Center of Biotechnology in Natural Resources (CENBio), University Catholic of Maule, Chile. The feeding treatments of Zophobas morio larvae based on plastics derived from petroleum were the following: 5 g of PS- expanded polystyrene or better known as Styrofoam; 5 g of PBD- low density polyethylene from plastic bags; 10 g of PI- polyisoprene; 10 g of BR- butyl rubber; and 10 g of a control- organic matter (mix of potato, carrot and apple). Each treatment had three replicates using aluminum containers (Length x Width x Height = 20 x 13 x 4 cm) with 30 larvae of ten days old per replicate. The treatments were incubated for 30 days in a microclimate chamber (RGX-400EF, FAITHFUL), which was regulated at 25°C temperature and 40% relative humidity. The variables determined were the following: number of live larvae after 30 days; survival rate (%); substrate consumption (%) and consumption rate (mg/d/larvae). The climatic chamber was in a double-door greenhouse to contain escape risks. The experiment was monitored every three days to hydrate larvae and removing died maggots.
2.2. Statistical Analysis
The data measured in the feeding treatments were processed and analyzed using IBM SPSS Statistics with a predetermined significance level of p=0.05 for all statistical evaluations. Normality tests were performed to determine if the data followed a normal distribution and homogeneity. Subsequently, ANOVA analysis followed by a Tukey’s test was used to determine statistical significance among treatments.
2.3. Isolation of Bacteria from Gut of Zophobas morio Larvae
After 30 days of feeding with the different diets, a random sample of five larvae was selected for each treatment. The larvae of each group were washed separately 3 times with sterile distilled water to control major contaminants. They were subsequently dissected under a binocular magnifying glass, making a cut on the lateral side from the head to the end of the abdomen to extract the intestine. Intestinal samples were stored in 15 mL centrifuge tubes. The larval intestine samples were mixed with 10 mL of 0.9% saline, to homogenize the samples sterile beads were added during the vortex shaker for 10 min. Serial dilutions were performed with reference to the 0.5 McFarland standard. After this, 300 μl aliquots of the dilutions were taken to dispense into Petri dishes with LB Agar. The plates were incubated for 24 h at 30°C. After 24 h, microbial colonies were isolated according to diverse morphologies and higher growth. For each isolate, three successive subcultures were performed.
2.4. Identification of Culturable Intestinal Bacteria of Zophobas morio
All DNA extractions of the bacteria isolated from the larvae of each feeding treatment were performed with a total DNA extraction kit (NucleoSpin Plasmid) following the protocol suggested by the manufacturer. PCR was performed to amplify the 16S gene with universal primers (27F/1492R). PCR products were purified with an EXO+SAP enzymatic clean-up kit (Thermo Fisher Scientific). Amplicons were quantified by agarose gel electrophoresis with a molecular weight marker. A sequencing reaction for the study of the V3-V4 regions of the 16S rRNA gene was performed using a kit (BigDye) with the primers (314F: 5’-CCTACGGGGGNGGCWGCAG-3’; 805R: 5’-GACTACHVGGGTATCTAATCC-3’). A final purification was performed with the kit (BigDye X-terminator). Samples were sequenced with the 3130XL platform (Applied Biosys-tems) by the Sanger method at the Central Analítica of the Instituto de Química of Universidade de São Paulo, Brasil. The sequences obtained were cleaned with Bioedit 7.7 (Nucleics Co., Sydney, Australia) and aligned with Mafft alignment software. The sequences obtained were deposited in the BLAST (Basic Local Alignment Search Tool) database of the National Center for Biotechnology Information (NCBI) to identify the bacterial species according to the established parameters.
2.5. Microbial Role in the Ingestion of Plastics by Zophobas morio
In order to determine the microbial role in the ingestion of plastics by Zophobas morio. The susceptibility to antibiotics of the culturable strains isolated from the intestines of Zophobas morio was evaluated by antibiogram analysis. Streptomycin (STR), kanamycin (KAN), ampicillin (AMP) and neomycin (NEO) antibiotics at 100 ug/mL were utilized. The diameter of the hale of inhibition (mm) was used as a selection criterion for the best treatment. Subsequently, the Z.morio larvae were fed with a diet supplemented with antibiotics to control major intestinal bacteria. In this pretreatment stage, a total of sixty larvae were fed with a mixture of organic substrate with 500 mg Ampicillin. After 10 days of organic feeding with the antibiotic supplement, the larvae were divided into two separate groups to be fed during additional 15 days with the synthetic polymers that previously showed the best ingestion results (PS and PBD). Each treatment had two replicates and the samples were maintained under controlled conditions as explained previously in 2.1.
2.6. Physicochemical Analysis of Frass
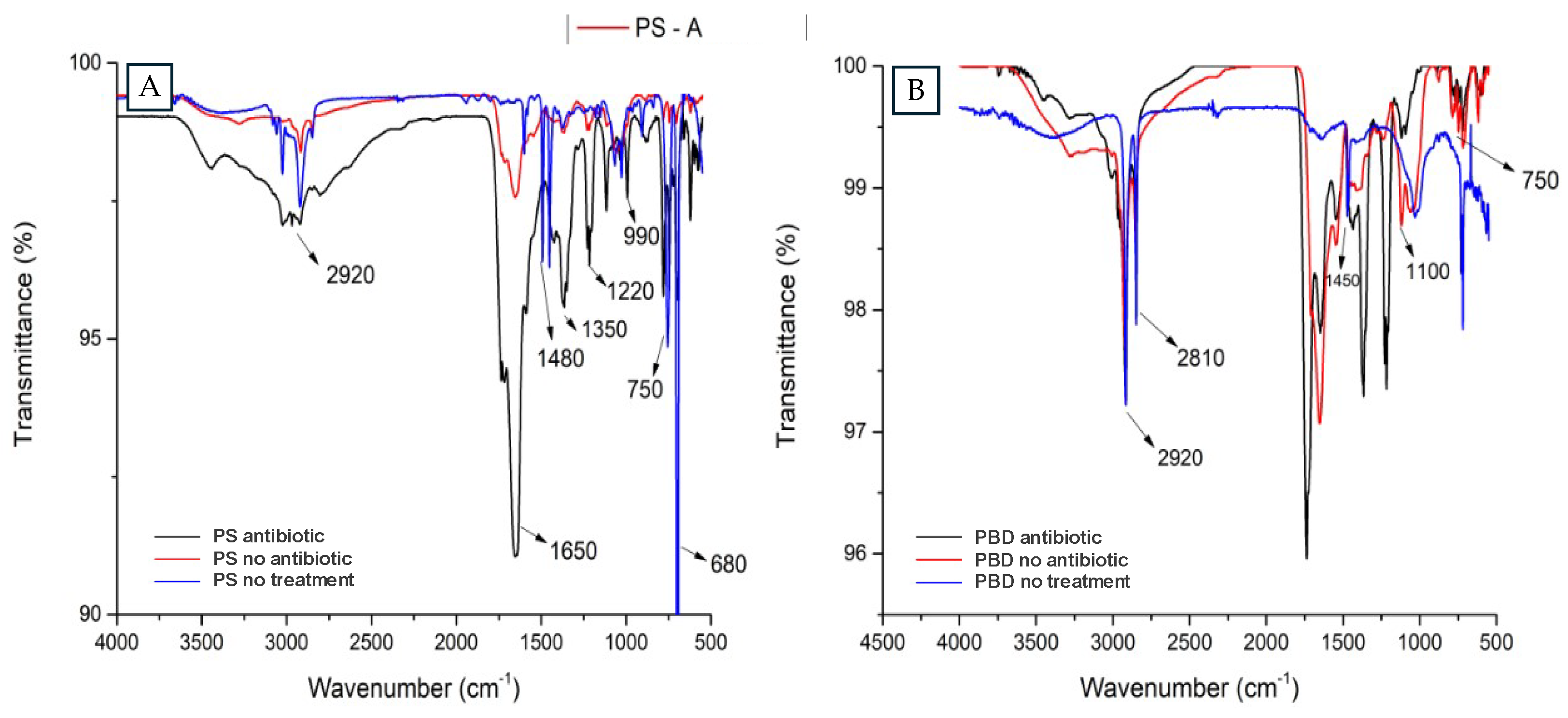
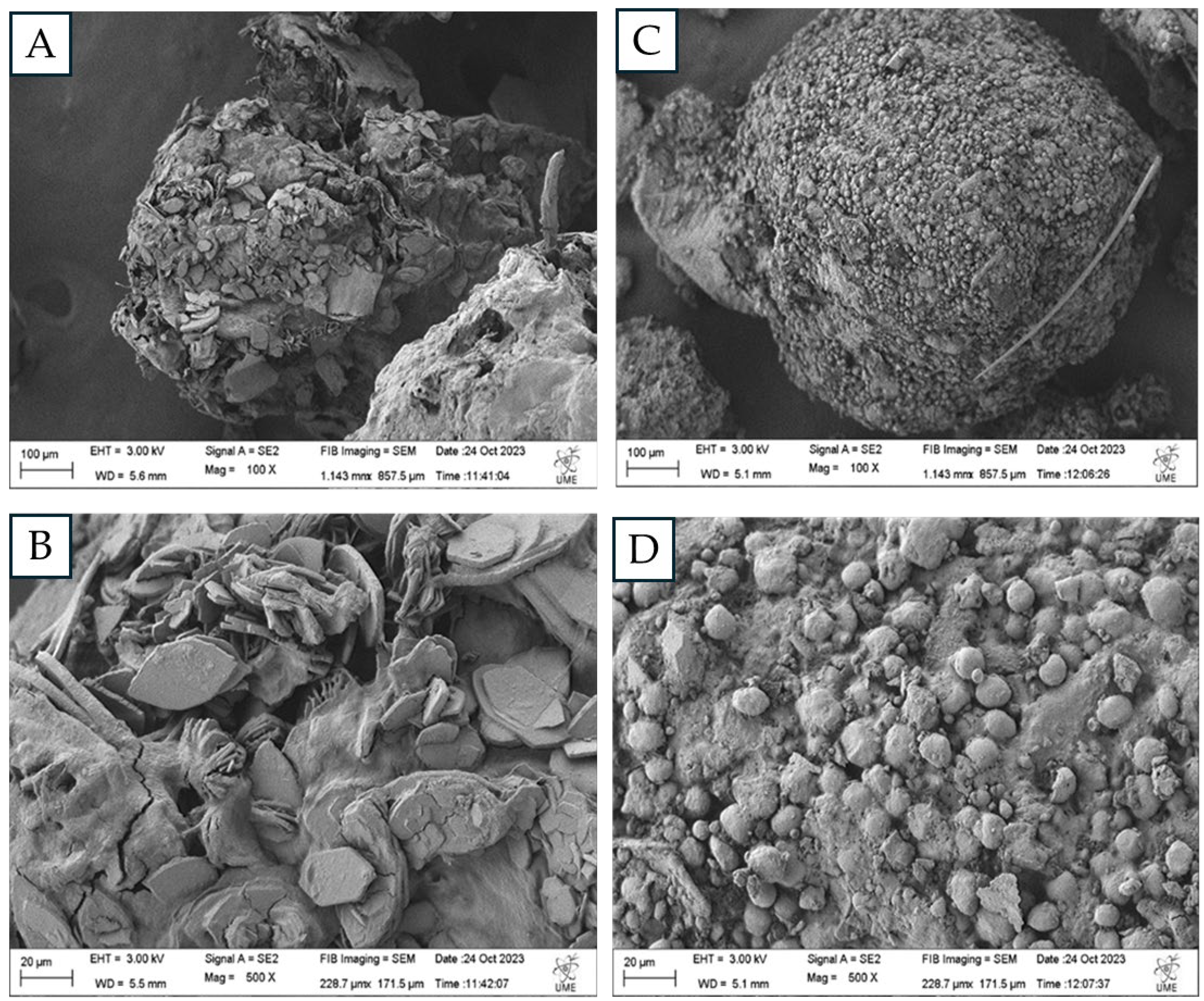
In order to identify the changes in the chemical structure and morphology of the polymers after the larvae digestion, 10 mg of frass were collected from the plastic based diet treatments with polystyrene foam (PS), and polyethylene bags (PBD) including Zophobas morio feces treated with and without antibiotics and a polymer control with no treatment were analyzed by the FTIR with Attenuated Total Reflectance-ATR (PerkinElmer) available at the Bioprocess laboratory-CENBio of the Universidad Católica del Maule. In addition to verify differences in the frass surfaces, samples of the feces of PS treatment and organic control were studied using the Scanning Electron Microscope (SEM, Zeiss EVO MA10) of the electron microscopy unit (UME) at the Universidad Austral de Chile.
4. Discussion
Plastic materials have been a significant challenge for the community, so finding strategies to mitigate the negative effects of their recalcitrant degradation is essential. In this study, relevant information on the adaptation of superworms was obtained by subjecting them to a diet based on commonly used synthetic polymers. The larvae of
Zophobas morio were able to survive feeding on synthetic polymers thanks to their digestive tract and the microbes present in it, which allow the plastics to be degraded and mineralized. These insects had considerably good survival rates, with BR being the plastic that had the lowest performance with 10% survival, which is why it was discarded for the following analyses. The PS obtained 53.5% survival after 30 days of feeding trial, a result considered lower than in the case of other previous reports [
12]. The average PS consumption rate was calculated to be 3.2 mg/d/larvae, a result considerably higher than the 0.43 mg/d/larva obtained previously [
13]. In the PBD-based diet treatment, a survival rate of 67.4% and a consumption rate of 0.2 mg/d/larvae was obtained. Comparable results had previously been obtained, where with similar conditions they managed to obtain a survival rate of 94, and a consumption rate of 0.29 mg/d/larvae[
14]. In the case of PI, positive results were obtained, despite being a treatment never tested before, the superworms managed to survive with a value of 60.9% and a consumption rate of 0.2 mg/d/larvae. These results show the adaptive plasticity that these insects have achieved over time, which has allowed them to persist by consuming plastic materials that may be present in nature, confirming part of the hypothesis that these animals can survive by feeding plastics.
There is a wide variety of insects that have the ability to feed on synthetic polymers such as expanded polystyrene, however, the larvae of
Zophobas morio have proven to be the most voracious when it comes to consuming PS, eating 2 times more than other insects of the Coleoptera order such as
Tenebrio molitor and 3 times more than the larvae of
Galleria mellonella of the Lepidoptera order under similar conditions [
15]. The mealworm or
Tenebrio molitor, has the capacity to consume PBD in low quantities, with a consumption rate of 0.05 mg/d/larvae [
16], unlike the results of this work where the superworms obtained an average of 0.25 mg/d/larvae.
In the identification of microbes, it was possible to isolate a greater number of bacteria in the larvae subjected to the PI diet, an unusual result, since these were the ones with the lowest consumption of the plastic substrate (6 mg/larvae), but with high survival percentage (60.9%), which suggests that the larvae can feed on this carbon source, however, it may be that its structure is difficult for the superworm to chew, therefore using the rubber with another texture may give better results.
Mitra & Das (2023) suggests that the intestinal bacteria that proliferate are dependent on the substrate consumed by the insect, which shows that the results obtained in this work are consistent with the information described, since the microorganisms identified in each of the diets were different, with the exception of
Bacillus cereus, which was found in PBD and PI diet-based treatments [
17].
There are difficulties to investigate the degradation of PI by microorganisms, since it has been documented that it is a very slow process that requires a large number of bacteria to degrade small amounts of polyisoprene, so it has been little considered in research [
18], however, superworms were able to survive feeding on this material. Larvae that consumed a diet based on polyisoprene were identified as having intestinal bacteria such as
Citrobacter sp,
Bacillus cereus,
Bacillus wiedmannii and
Kliebsella aerogenes. Although there are no reports on the behavior and gut microbiota of
Zophobas morio when consuming this polimer, identified bacteria such as
Bacillus cereus and
Bacillus wiedmannii may be closely related to polymer degradation in the gut, since in other studies it was recognized that bacteria of the bacillus group are able to adhere and form a biofilm on the rubber [
19]. Brandon et al. (2018) identified
Citrobacter sp as a polyethylene degrading bacterium, therefore it may be related to polymer degradation in the intestine [
20]. In thi study, it was determined that gut bacteria of superworms play an important role in the decomposition of synthetic polymers. recognizing that different bacterial groups proliferate in the intestines to degrade specific types of polymers, meaning that the bacteria identified in the intestines of superworms were dependent on the plastic used in each treatment. Gut bacteria such as Enterobacter sp and
Kluyvera ascorbata were recognized from the styrofoam-fed superworms. Jiang et al. (2021) [
15] reported that the Kluyvera family is involved in the degradation of PS in the intestines of
Zophobas morio upon consumption of this plastic material. Luo et al. (2021) [
21] recognized that the amount of Enterobacteriaceae increases significantly in the gut of larvae when their diet is based on PS, so it is considered a crucial bacterium in the degradation of this synthetic polymer.
In the treatment with low density polyethylene (PBD), 3 species were identified, such as Enterobacteriaceae,
Kluyvera sp and
Bacillus cereus, these bacteria have been studied for their degradative potential [
22], since Enterobacteriaceae and Bacillus have managed to form colonies on polyethylene sheets using it as the only carbon source, in addition, other studies show
Bacillus cereus has managed to reduce up to 35.7% the size of a 30 micron polyethylene sheet [
23].
Pivato et al. (2022) demonstrated that
Kluyvera sp remains in the gut of superworms when fed inorganic polymers, such as PBD and PS, as well as being identified in more insects with the ability to consume these materials [
24].
The FTIR spectra suggests that PS was mostly degraded in the intestines of the larvae when the antibiotic was not present. The sample of larvae treated with antibiotic, where much similarity with the control is appreciated, identifying the C-H stretching bond at 3000 cm
-1, the C=C stretching bond at 1450 cm
-1 and the vibrational bands in the 700 to 800 cm
-1 range characteristic of PS [
25].
The characteristic bonds of PBD were recognized in the fecal samples of the larvae when they were not treated with antibiotics, recognizing tension peaks of the C-H bond at 2920 cm
-1 and C-C at 1450 cm
-1 [
26], in addition to the CH
2 bending movement associated to 750 cm-1, however, a degradation feature was recognized, since, the spectrum associated to the C-O bond at 1100 cm-1 was incorporated, indicating oxygen incorporation at the structure [
26]. Peng et al. (2020) demonstrates that the plastics ingested by the larvae were metabolized being oxidized during the biodegradation process [
27]. Brandon et al. (2018) also mentions that the feces of
Tenebrio molitor larvae were shown to have oxygen incorporation forming O-C bonds and alcoholic groups after ingestion of these plastics [
20]. The working samples were compared with the organic matter control, and this shows to have a strong C-H bond signal corresponding to the aliphatic structures of the organic matter [
28].
The scanning electron microscopy images showed noticeable differences between the feces of insects fed with PS and those fed with organic matter. Different microbial groups were observed on the surface of the two samples, in addition, degradation products with hexagonal shapes were recognized in the case of PS, different from the images reported in the study of the feces of
Galleria melleonella larvae fed with PS [
29], where only a breakage of the polymer structure was observed in the feces. In the control, a homogeneous structure was observed on the entire surface, with spheres completely surrounding the frass fragments.
The degradation of synthetic polymers in the gut of superworms is still an unknown subject of study, therefore, the results obtained in this research are promising. The hypothesis was confirmed and related work is expected to continue in order to further investigate the metabolism of insects that have adapted to survive by consuming plastics.
Future related studies may focus on the functional and structural knowledge of microbial genes through transcriptome, metagenomics or proteomics studies, which will facilitate the identification of enzymes associated with the plastic degradation process.