Introduction
Mpox (Monkeypox) is an emerging zoonotic disease caused by the mpox virus, a member of the Orthopoxvirus genus, which also includes variola virus (the causative agent of smallpox), vaccinia virus, and cowpox virus (1,2). First identified in 1958 in laboratory monkeys, mpox has since been recognized as an important human pathogen, particularly in regions of Central and West Africa (1-3). The first human case was documented in 1970 in the Democratic Republic of Congo. Since then, sporadic outbreaks have occurred in endemic regions, with a significant resurgence noted in recent years, particularly outside traditional areas, raising global public health concerns which required the use of specific clinical diagnostic pathways (1,5). Mpox is a double-stranded DNA virus that shares considerable genetic similarity with variola virus. The virus has two genetic clades: the West African clade, which is associated with a lower mortality rate, and the Central African (Congo Basin) clade, which is more virulent and associated with higher mortality and human-to-human transmission. Mpox causes a disease in humans that resembles smallpox, characterized by fever, rash, vesicular lesions and lymphadenopathy (1,3). However, mpox is generally less severe than smallpox, with a lower case fatality rate (1,4,5).
Smallpox vaccination, which was discontinued after the global eradication of smallpox in 1980, has been shown to provide cross-protection against other orthopoxviruses, including mpox (6,7). Particularly vaccinated individuals maintained high levels of vaccinia-specific IgG and neutralizing antibodies for decades, with no significant decline observed several years post-vaccination (6,7). Individuals who had multiple vaccinations exhibited slightly higher antibody titers compared to those with a single vaccination, but the difference was not substantial (8).
This cross-protection is possibly and primary due to the antigenic similarities between the viruses, which allow antibodies generated against smallpox to recognize and neutralize mpox as well as several other immune factors (6,7,8). However, as immunity wanes over time and new generations are born without any exposure to smallpox vaccination, the global population is becoming increasingly susceptible to mpox and other orthopoxvirus infections.
Given the recent mpox outbreaks reported by the WHO (3), and that previously in non-endemic countries (9), there is an urgent need to explore the potential of smallpox antibodies in providing cross-protection against mpox. This study investigates the cross-reactivity of smallpox antibodies with mpox, focusing on their potential use in post-exposure prophylaxis (PEP) to prevent or mitigate mpox outbreaks.
Materials and Methods
Human Immunoglobulin Preparations
For this study, three different batches of human immunoglobulin preparations containing immunoglobulin class G (IgG) (IntratectTM and YimmugoTM, Biotest AG, Germany) were used, each with a protein concentration of 100 g/L, a purity of at least 98% IgG and a mean Fc activity of 98% (Intratect) and 97% (Yimmugo). The plasma used for these preparations was collected from several thousand donors, providing a broad representation of the human antibody repertoire. These preparations were tested for their binding activity against mpox and vaccinia virus (VACV), both of which are orthopoxviruses.
Immunoassays
The binding activities of the immunoglobulin preparations were measured using commercially available ELISA kits designed specifically for mpox and VACV. The Anti-Mpox Virus IgG ELISA Kit (FineTest, Wuhan, China) and the Human Anti-Vaccinia Virus IMV/Envelope Protein/H3L/p35 IgG ELISA Kit (Alpha Diagnostic International, San Antonio, TX, USA) were carried out according to the manufacturer´s instructions. The ELISA tests are based on the principle of antigen-antibody interaction, where immobilized antigens (from mpox or VACV) capture antibodies from the immunoglobulin preparations. Detection of bound IgG was performed using a horseradish peroxidase-conjugated anti-human IgG antibody, followed by colorimetric analysis. The implementation of the ELISA method consisted in the evaluation of the assay set up to perform the analyte detection. After the implementation of the analytical method some parameters were evaluated (such as accuracy, precision and sensitivity) to verify the method. To quantify the binding activity, an in-house standard, calibrated to 1000 U/mL, was used. Data were analyzed using Parallel Line Assay software (PLA 3.0, Stegman Systems) resulting in a potency that was converted to a binding activity in arbitrary units using the predefined standard activity. The results were further analyzed using GraphPad Prism 6 for statistical evaluation. A linear regression analysis was performed using the measured binding activities of various intravenous immunoglobulin preparations, including Intratect and Yimmugo.
Results
Binding Activity of Human Immunoglobulin Preparations
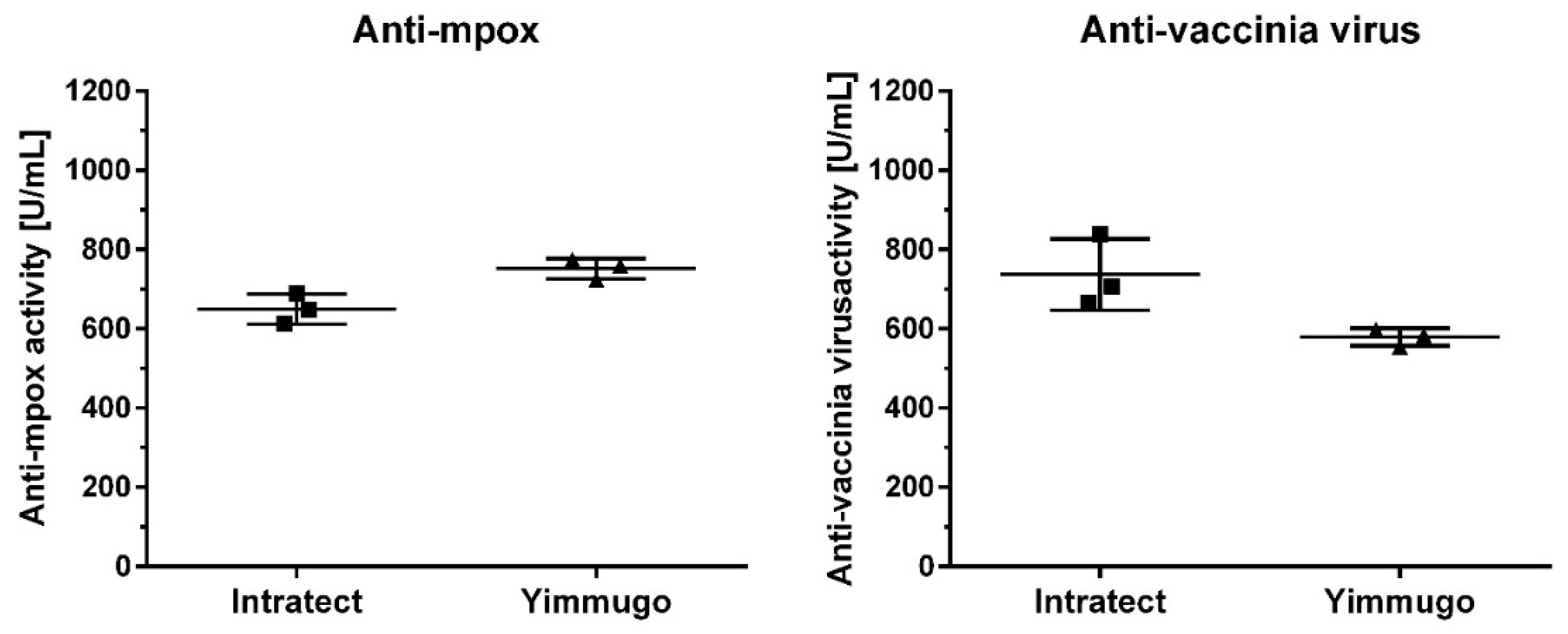
The binding activities of the three batches of human immunoglobulin preparations against mpox and VACV were determined using ELISA. The results, presented in
Figure 1, show that all tested batches exhibited significant binding activity against both MPXV and VACV. The mean binding activity against mpox in the two immunoglobulin preparations Intratect and Yimmugo was found to be 650 ± 39 U/mL and 752 ± 26 U/mL, respectively, while the binding activity against VACV was 737 ± 90 U/mL and 579 ± 22 U/mL.
Linear Relationship Between Binding Activities
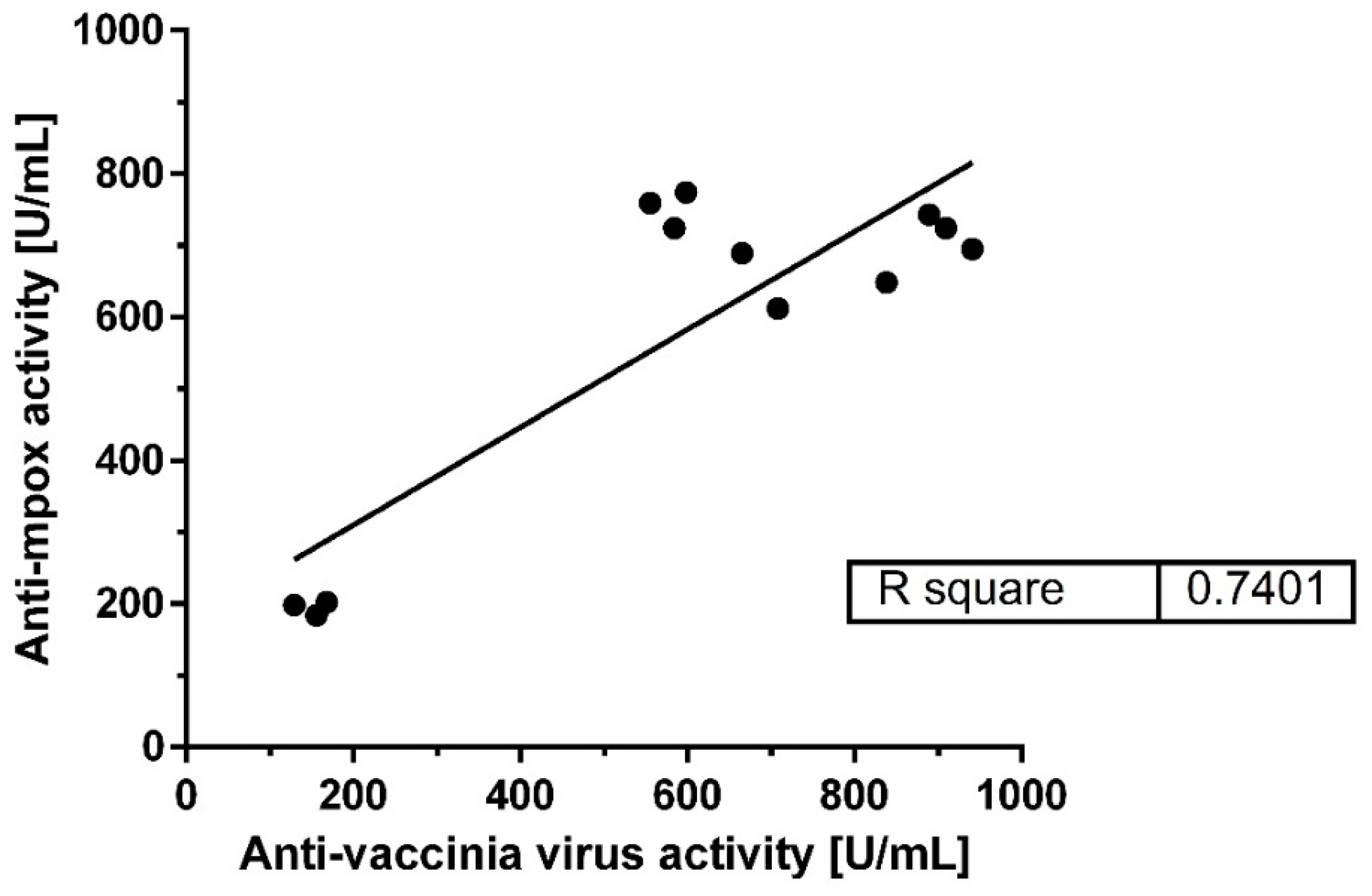
A strong linear relationship was observed between the binding activities against mpox virus and VACV across all batches, as depicted in
Figure 2. The linear regression analysis yielded an R-square value of 0.74, indicating a high degree of correlation between the antibody responses to the two viruses. This suggests that antibodies generated against smallpox (VACV) can effectively bind to mpox virus antigens, supporting the hypothesis of cross-reactivity.
Comparison of Binding Potency
The comparison of binding potency across the three batches of the immunoglobulin preparations is summarized in
Table 1. The results indicate that while there is some variation in binding potency between batches, all exhibit strong cross-reactivity with mpox virus, with potency values ranging from 612 to 689 U/mL and 724 to 774 U/mL for mpox virus and 665 to 838 U/mL and 555 to 598 U/mL for VACV. This consistency across batches highlights the reliability of using these preparations for potential therapeutic applications.
Discussion
The presence observed in this study of specific antibodies for smallpox and the mpox virus underscores the potential utility of smallpox vaccination and immunoglobulin preparations in combating mpox outbreaks. The strong linear relationship between binding activities against mpox virus and VACV suggests that smallpox antibodies can recognize and neutralize mpox virus, offering a possible mechanism for cross-protection. This cross-reactivity is particularly relevant in the context of recent mpox outbreaks reported by the WHO, which have occurred in both endemic and non-endemic regions. The re-emergence of mpox in non-endemic areas, coupled with the global cessation of smallpox vaccination, has left a significant portion of the population susceptible to orthopoxvirus infections.
Previously it has been found that antibodies induced by the smallpox vaccine (Dryvax) are both necessary and sufficient to protect against a lethal mpox virus challenge. B cells, which produce these antibodies, are crucial for this protection (5-8). In addition, the same paper showed that the passive transfer of vaccinia-neutralizing antibodies to non-immunized macaques provided protection against severe mpox disease, further underscoring the importance of antibodies in immunity.
Therefore, the use of smallpox vaccines, such as ACAM2000 and JYNNEOS, as post-exposure prophylaxis (PEP) for mpox is supported by the findings of this study. These vaccines, which induce strong antibody responses against VACV, could be repurposed to provide protection against mpox virus, particularly in the early stages of an outbreak. Additionally, the administration of immunoglobulin preparations with high titers of smallpox antibodies could serve as an immediate intervention for exposed individuals, offering passive immunity and potentially reducing the severity of the disease. Indeed a recent paper also suggests that laboratory workers who received first-generation smallpox vaccines exhibited higher levels of neutralizing antibodies against both VACV and mpox compared to those who received third-generation vaccines (10-12).
Recently it has been found that individuals who were previously vaccinated against smallpox or who had a natural mpox virus infection showed a stronger immune response, with higher levels of cross-reactive antibodies that could neutralize mpox virus (6-8). This indicates that prior exposure to vaccinia virus (through vaccination or infection) enhances the immune response to mpox virus. All these previous data on human volunteers or animal models are in accordance with our findings on intravenous immunoglobulin preparations where we found evidences of the presence not only of vaccinia antibodies but also mpox antibodies. Thus given the waning immunity in the post-smallpox vaccination era, there may be an urgent need not only to consider reintroducing smallpox vaccination or a modified previous smallpox vaccine in high-risk populations (4-6), particularly in areas where mpox is re-emerging (3), but also to focus on other prophylactic strategies. Indeed, public health strategies should focus on surveillance, rapid response and the development of targeted PEP protocols that leverage the cross-reactivity of orthopoxvirus antibodies. This could also be a new strategy also for future emerging viral or multi drug resistant bacterial infection too.
Conclusion
Antibodies have been pivotal in the evolutionary success of our species, serving as a fundamental component of the immune system's defense against pathogens. Over the past century, passive immunization—harnessing pre-formed antibodies—has been strategically employed to combat a wide range of infectious diseases. Recent non-human primate (NHP) studies have provided compelling evidence that post-exposure and therapeutic administration of antibodies can significantly influence the virus infection, as more recently proved for the progression of Ebola Virus Disease (EVD), highlighting their potential in managing this severe viral infection (14). The findings of this study demonstrate significant cross-reactivity between smallpox antibodies and the mpox virus, highlighting the potential of smallpox vaccination and immunoglobulin therapy as effective tools in managing mpox outbreaks. As the WHO continues to monitor the situation in Africa and other regions, the integration of these prophylactic strategies into public health responses is crucial (3,9). However further research is needed to optimize PEP protocols and ensure that populations at risk are adequately protected against the mpox contagiousness when undergoing to prophylaxis with intravenous immunoglobulin preparations.
References
- Upadhayay S, Arthur R, Soni D, Yadav P, Navik U, Singh R, Gurjeet Singh T, Kumar P. Monkeypox infection: The past, present, and future. Int Immunopharmacol. 2022 Dec;113(Pt A):109382. [CrossRef]
- Thornhill, J. P.; et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef] [PubMed]
- WHO. Mpox – African Region. 2024. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON528.
- Carannante N, Tiberio C, Bellopede R, Liguori M, Di Martino F, Maturo N, Di Sarno R, Scarica S, Fusco G, Cardillo L, de Martinis C, Atripaldi L, Perrella A. Monkeypox Clinical Features and Differential Diagnosis: First Case in Campania Region. Pathogens. 2022 Aug 1;11(8):869. [CrossRef]
- Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019 Oct 16;13(10):e0007791. [CrossRef] [PubMed] [PubMed Central]
- Taub DD, Ershler WB, Janowski M, Artz A, Key ML, McKelvey J, Muller D, Moss B, Ferrucci L, Duffey PL, Longo DL. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am J Med. 2008 Dec;121(12):1058-64. [CrossRef] [PubMed] [PubMed Central]
- Townsend, M. B.; et al. Humoral immunity to smallpox vaccines and monkeypox virus challenge: proteomic assessment and clinical correlations. J. Virol. 2013, 87, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Edghill-Smith, Y.; et al. Smallpox vaccine–induced antibodies are necessary and sufficient for protection against monkeypox virus. Nature Medicine 2005, 11, 740–747. [Google Scholar] [CrossRef] [PubMed]
- WHO. Multi-country monkeypox outbreak in non-endemic countries. 2023. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385.
- Pittman, P. R.; et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N. Engl. J. Med. 2019, 381, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Zaeck LM, Lamers MM, Verstrepen BE, Bestebroer TM, van Royen ME, Götz H, Shamier MC, van Leeuwen LPM, Schmitz KS, Alblas K, van Efferen S, Bogers S, Scherbeijn S, Rimmelzwaan GF, van Gorp ECM, Koopmans MPG, Haagmans BL, GeurtsvanKessel CH, de Vries RD. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat Med. 2023 Jan;29(1):270-278. [CrossRef] [PubMed] [PubMed Central]
- Schmidt KM, Jonsdottir HR. Third-generation smallpox vaccines induce low-level cross-protecting neutralizing antibodies against Monkeypox virus in laboratory workers. Heliyon. 2024 ;10(10):e31490. [CrossRef]
- Available online: https://www.who.int/news/item/14-08-2024-who-director-general-declares-mpox-outbreak-a-public-health-emergency-of-international-concern.
- Kupferschmidt, K. Scientists scramble to set up monkeypox vaccine trials. Science 2022, 377, 696–697. [Google Scholar] [CrossRef] [PubMed]
- 14 Roozendaal R, Hendriks J, van Effelterre T, et al. Nonhuman primate to human immunobridging to infer the protective effect of an Ebola virus vaccine candidate. NPJ Vaccines. 2020 Dec 17;5(1):112. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).