1. Introduction
The SARS-CoV-2 infection has generated one of the most critical and unprecedented public health crises in the modern world, having caused more than 776 million cases and 7 million deaths globally by August 18, 2024 [
1]. The main pathophysiological mechanism incriminated in the occurrence of the severe forms described in COVID-19 is immune hyperreactivity, i.e. the uncontrolled release of pro-inflammatory cytokines [
2]. In an attempt to withstand this ‘cytokine storm’, treatment protocols for COVID-19 include systemic corticosteroids, interleukins (IL) 1 and 6 receptor antagonists, and Janus kinase inhibitors [
3]. However, these drugs pose a risk of hepatitis B virus (HBV) reactivation in patients co-infected with SARS-CoV-2 and HBV.
To date, there are only a few studies [
4,
5,
6,
7] with small samples that have followed the occurrence of HBV reactivation (HBVr) in COVID-19 patients receiving immunosuppressive treatment. In addition, the different design of these studies makes it difficult to draw a conclusion. Thus, there is no consensus regarding the management of HBV-infected patients receiving COVID-19 immunosuppressants. This topic deserves attention all the more since the majority of the world's population lives in regions with intermediate or high HBV endemicity [
8].
Thus, we aimed to bring more data to fill these research gaps by conducting a prospective study to evaluate the risk of HBVr in COVID-19 patients receiving immunosuppressive treatment. In addition, we set out to find a common denominator for all studies related to HBVr in COVID-19 patients, in order to have an overview of the results to date and formulate management recommendations. As a secondary objective, we aimed to evaluate the seroprevalence of HBV infection in COVID-19 patients to clarify the importance of assessing the risk of HBVr in these patients.
2. Materials and Methods
2.1. Study Design and Participants
We performed a prospective observational cohort study in which we included patients co-infected with SARS-CoV-2 and HBV admitted to the National Institute of Infectious Diseases ‘Matei Bals’ (NIIDMB) in Bucharest, Romania.
We requested hepatitis B surface antigen (HBsAg), hepatitis B surface antibody (anti-HBs), hepatitis B core antibody (anti-HBc), hepatitis C virus antibody (anti-HCV) and human immunodeficiency virus (HIV) testing for all Romanian patients aged ≥ 18 years diagnosed with SARS-CoV-2 infection (by polymerase chain reaction (PCR) and/or rapid antigen test) who were hospitalized in 4 of the NIIDMB wards (III, IV, XI and XIII) between October 2021 and September 2022.
Patients who had positive HBsAg or negative HBsAg/positive anti-HBc were considered for inclusion in the study. Subsequently, we excluded patients with positive anti-HBs, including borderline values (≥ 8 IU/mL), those with antiviral treatment for HBV, patients with immunosuppressive diseases (e.g. HIV, leukaemia), as well as those receiving immunosuppressive treatment for other conditions. Patients who met these criteria were asked to participate in the study. Those who accepted enrolment signed an informed consent. We included both patients who received immunosuppressive treatment for COVID-19 and those without such therapy (the latter represented the control group).
A blood sample was collected in a tube without anticoagulant from all patients at the time of enrolment. The sample was centrifuged at 3,700 revolutions per minute (rpm) for 6 minutes at 24°C, and the serum was stored at -20°C for the subsequent determination of HBV viral load. For patients in whom HBV DNA was undetectable at follow-up, determination of baseline viral load was not considered necessary and was no longer performed.
Patients received the nationally recommended treatment for SARS-CoV-2 infection, depending on the severity of the disease, the availability of drugs and the decision of the attending physician.
Enrolled patients were called for follow-up (clinical examination and blood collection) 3 months after the last dose of immunosuppressive treatment and respectively after discharge if they did not receive such treatment.
The study was approved by the Bioethics Committee of NIIDMB (C11454/24.09.2021).
2.2. Data Collection
We entered patient data into two Microsoft Office Excel databases. The first included the age, sex and the result of viral serological markers of all COVID-19 patients who were screened for HBV as above-mentioned. The second database concerned the data of enrolled patients: demographics, comorbidities, body mass index (BMI), severity of COVID-19, immunosuppressive treatment received during hospitalization and laboratory data (including those from follow-up). We included the number of lymphocytes (determined using Beckman Coulter DxH Haematology Analyser, Siemens ADVIA 2120i Haematology System or Celltac G Nihon Kohden Haematology Analyser), prothrombin concentration (determined using Siemens Sysmex CS-2500i Coagulation Analyser or Instrumentation Laboratory ACL Top 550 Coagulation Analyser), serum level of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT) and total bilirubin (determined using Ortho Clinical Diagnostics VITROS 4600/5.1 FS Chemistry System), serological markers for HBV, HCV and hepatitis D virus (HDV) infections (determined using Ortho Clinical Diagnostics VITROS 3602 Immunodiagnostic System), and HBV viral load (determined by real-time quantitative PCR using Roche Cobas 6800 Systems, with the lower limit of quantification of 10 IU/mL and the lower limit of detection of 3 IU/mL).
2.3. Definitions:
2.3.1. The Following Drugs Were Considered Immunosuppressive Treatment: Systemic Corticosteroids, IL-1 and IL-6 Receptor Antagonists (Anakinra and Tocilizumab, Respectively), and/or Janus Kinase Inhibitors (Baricitinib), in Any Dose or Duration.
2.3.2.1. For HBsAg-Positive Patients, HBVr Was Defined as: (1) ≥ 2 log (100-fold) Increase in HBV DNA Level, If Previously Detectable; or (2) HBV DNA Level > 100 UI/mL, if Previously Undetectable.
2.3.2.2. For HBsAg-Negative/Anti-HBc-Positive Patients, HBVr Was Defined as: (1) Detectable HBV DNA, If Previously Undetectable; or (2) Reverse HBsAg Seroconversion (Reappearance of HBsAg).
2.3.3. The Severity of COVID-19 Was Defined as: (1) Mild Illness, If the Patient Had Symptoms of Acute Upper Respiratory Tract Infection but No Lung Involvement; (2) Moderate Illness, If There Was Lung Involvement without Oxygen Requirement or with low-Flow Oxygen Requirement (Below 6 L/min); (3) Severe Illness, If There Was Lung Involvement and High-Flow Oxygen Requirement (≥ 6 L/min); (4) Critical Illness, If the Patient Was Admitted to the Intensive Care Unit, Requiring Non-Invasive or Invasive Ventilatory Support.
2.4. Statistical Analysis
The collected data were analysed using IBM® SPSS® Statistics, Version 23.0, New York, USA (released 2015). We used the Shapiro-Wilk test to evaluate the distribution of quantitative variables, which proved to be non-Gaussian. Consequently, we presented the median and the interquartile range (IQR) for quantitative variables and the frequencies for nominal and ordinal variables. We used the Mann-Whitney test for bivariate analysis of the variables, except for the dichotomous variables for which we applied the Chi-square test (or Fisher’s exact test, when the expected frequencies were under 5). A p value below 0.05 was required for statistical significance.
3. Results
3.1. Seroprevalence of HBV Infection in COVID-19 Patients
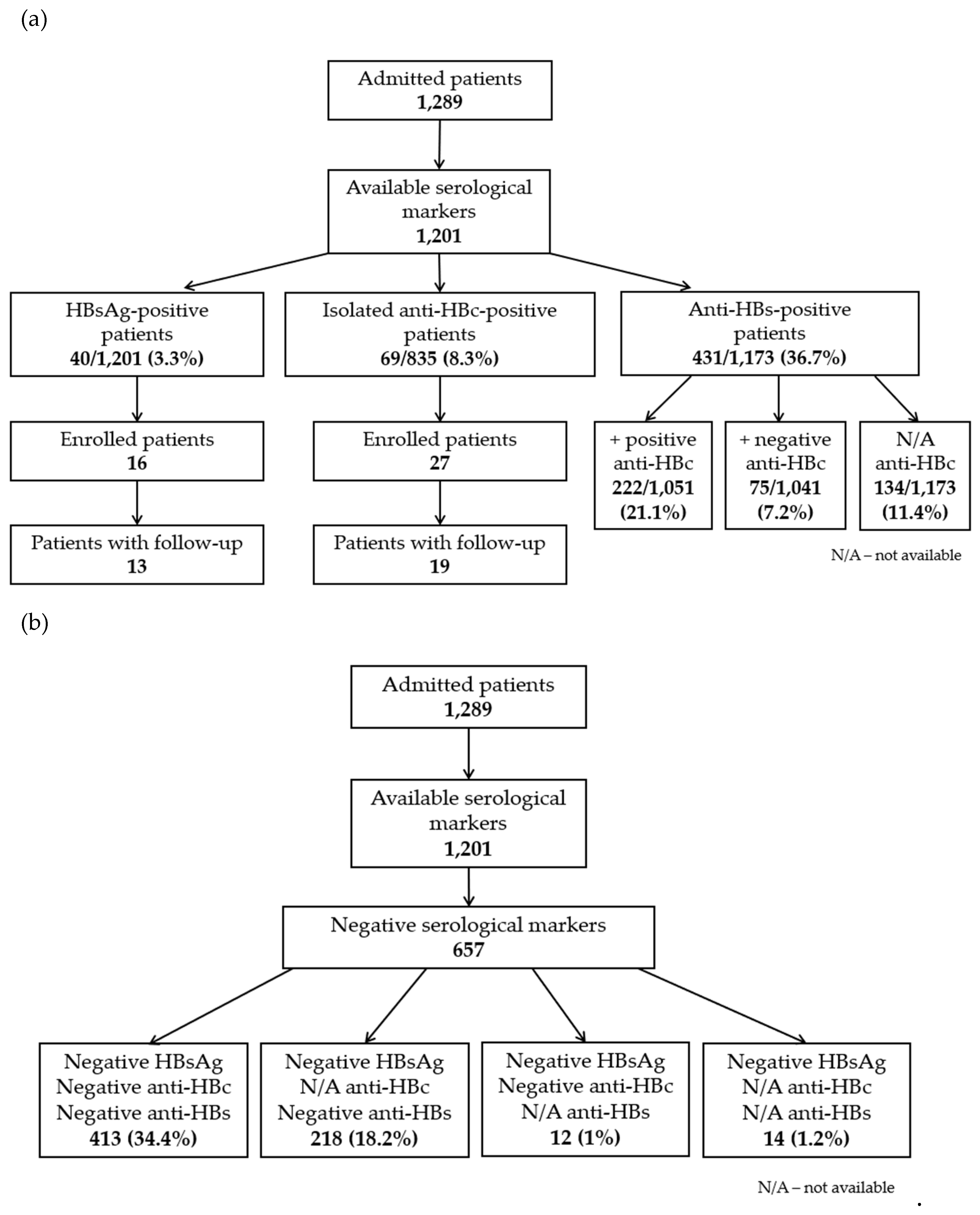
Between October 2021 and September 2022, 1,289 adults were admitted to 4 COVID-19 wards of NIIDMB. Among them, 1,201 patients were screened for HBV. The median age of these patients was 68 years (IQR 54-78), with a range of 18-97 years. More than half of the patients (55%) were female. For 816 (68%) of the patients, the result of all viral markers was available. For the other 32% of patients, some viral markers (especially anti-HBc) were unavailable. We considered positive hepatitis B e-antibody (anti-HBe) and positive hepatitis D virus antibody (anti-HDV) to be equivalent to positive anti-HBc in 2 and 1 patient, respectively, in whom anti-HBc was not available. Negative anti-HBe was not considered equivalent to negative anti-HBc, given that anti-HBe may disappear over time in patients with resolved infection.
We identified 333/835 (39.9%) anti-HBc-positive patients: 40/1,201 (3.3%) HBsAg-positive patients, 69/835 (8.3%) isolated anti-HBc-positive patients, 222/1,051 (21.1%) anti-HBs/anti-HBc-positive patients and 2 HBsAg-negative/anti-HBc-positive patients with unknown anti-HBs (
Figure 1A). Positive anti-HBc was more frequently found in men (178, 53.5%) than in women (155, 46.5%) (p<0.001). The median age of these patients was 72 years (IQR 64-80), with a range of 26-94 years. Four hundred and thirteen patients (34.4%) showed all negative viral markers (
Figure 1B). In addition, 50 anti-HCV-positive patients (4.2%) were identified. Eighteen out of 835 patients (2.2%) were co-infected with HBV and HCV (they concurrently had positive anti-HBc and anti-HCV).
3.2. General Characteristics of Study Participants
Forty-four patients accepted enrolment in this study, meeting the inclusion/exclusion criteria. Of these, only 32 patients presented for follow-up. The final cohort included 19 males (59.4%) with a median age of 67 years (IQR 61.5–74.5) and 13 females (40.6%) with a median age of 63 years (IQR 56–69). Most patients (n=28, 87.5%) had associated comorbidities. The most common was hypertension (n=20, 62.5%). The Charlson comorbidity index ranged from 1 to 5 (median 3, IQR 2-4). Fifty-six percent of patients were diagnosed with COVID-19 during the Omicron variant circulation (starting with the end of December 2021). Moderate forms of COVID-19 were predominant. Summary characteristics of study participants are presented in
Table 1. More detailed characteristics of each study participant including the administered treatment and viral loads are provided in
Table 2.
3.3. Hepatitis B Virus Status of the Study Participants
3.3.1. HBsAg-Positive Patients
Thirteen patients were HBsAg-positive. Of these, 9 had known chronic HBV infection. The other 4 patients were newly diagnosed, following the screening performed at admission. One of these patients (no. 12) had concurrently positive anti-HBs (titer 164 IU/mL), and both HBsAg and anti-HBs remained positive at subsequent follow-ups. She was probably co-infected with a pre-S/S mutated variant of HBV. All HBsAg-positive patients had negative hepatitis B e-antigen (HBeAg), positive anti-HBe and negative serological markers for HDV (HDVAg and anti-HDV). In addition, one patient was anti-HCV-positive but HCV RNA-negative.
3.3.2. HBsAg-Negative/Anti-HBc-Positive Patients
Nineteen patients were HBsAg-negative/anti-HBc-positive. All these patients had negative anti-HBs. Among them, one patient (no. 14) was known to have chronic HBV infection (the last medical evaluation 10 years ago). Thus, we documented spontaneous seroclearance of HBsAg. Patient no. 30 was positive for anti-HDV IgG and patient no. 17 for anti-HCV (HCV RNA was 1,927,058 IU/mL).
A patient (no. 15) who initially had isolated anti-HBc showed positive anti-HBs at follow-up (titer 16 IU/mL). It should be noted that this patient presented severe lymphopenia (lymphocyte count nadir was 450/μL) during hospitalization, which resolved by the time of follow-up. All other patients showed no change in the serological profile of viral markers.
3.4. Biochemical and Haematological Parameters of Study Participants
During hospitalization, 19 patients (59.4%) had elevated aminotransferases. For most patients (n=15, 78.9%), their levels were between 1 and 3 times the upper limit of normal (ULN), with only 1 patient (5.3%) presenting ALT above 5 times ULN. On follow-up, slightly elevated levels of aminotransferases persisted in 5 patients (15.6%). In a single patient (no. 17, co-infected with HCV), we detected levels of ALT (6 x ULN) and AST (4 x ULN) higher than during hospitalization. This patient received interferon-free antiviral treatment for HCV infection and achieved a sustained virological response. He did not experience HBVr during therapy.
Elevated levels of GGT were detected in 15 patients (56.9%). Most patients (n=11, 73.3%) showed values between 1 and 3 times ULN. Levels above 5 times ULN were observed in only one patient. On follow-up, 5 patients (15.6%) still had slightly elevated GGT. In only one patient we detected a GGT level above 3 times the ULN. The median of total bilirubin was 0.7 mg/dL (IQR 0.5-0.9), with only 2 values above the ULN (the highest was 1.6 mg/dL).
No patient showed significant alterations in standard coagulation tests. The median of prothrombin concentration was 86.5% (IQR 77.1-91).
Lymphopenia was detected in 24 patients (75%). The median of lymphocytes count nadir was 900/µL (IQR 570-1,200). On follow-up, 2 patients still had mild lymphopenia.
3.5. Immunosuppressive COVID-19 Treatment Administered during Hospitalization
The majority of the enrolled patients received corticosteroids (n=23, 71.9%), mostly intravenous (IV) dexamethasone. Only one patient (no. 18) received a single IV dose (150 mg) of methylprednisolone. Another 2 patients (no. 6 and no. 23) received oral methylprednisolone at discharge for gradual tapering of corticosteroid therapy. The duration of treatment as well as the cumulative doses of dexamethasone or equivalent are specified for each patient in
Table 2.
Moreover, 12 patients (37.5%) received treatment with tocilizumab, anakinra and/or baricitinib in combination with corticosteroids (
Table 1 and
Table 2).
3.6. Patients with HBVr
Patients were reassessed after a median period of 13 weeks (IQR 12-15) from the last dose of immunosuppressive treatment or from discharge if they did not receive such treatment.
According to the criteria we mentioned in the Materials and Methods section, 4 out of 23 patients (17.4%) who received immunosuppressive treatment had HBVr: 1 out of 8 HBsAg-positive patients (12.5%) and 3 out of 15 HBsAg-negative/anti-HBc-positive patients (20%) (
Table 2). These are:
1. Patient no. 8, aged 56, with positive HBsAg and baseline HBV DNA level of 179 (2.25 log) IU/mL, was treated with IV dexamethasone 8 mg/day for 5 days, then 4 mg/day for 2 days. The patient was initially re-evaluated 1 month after discharge and at that time HBV DNA was 1,660 (3.22 log) IU/mL. At 3 months, a viral load of 17,378 (4.24 log) IU/mL was detected. FibroMax was also performed, which revealed F1/A0-A1/S3/N2/H0. At the decision of her attending physician, treatment with entecavir 0.5 mg/day was then initiated.
2. Patient no. 14, aged 75, was known to have chronic HBV infection. However, we found isolated anti-HBc and undetectable HBV DNA at the time of admission. He received a single dose (100 mg) of subcutaneous anakinra, a single dose (400 mg) of IV tocilizumab and IV dexamethasone 8 mg/day for 3 days, then 6 mg/day for 5 days, followed by of 4 mg/day for 7 days. At follow-up, detectable viral load was found (HBV DNA < 10 IU/mL).
3. Patient no. 20, aged 63, with isolated anti-HBc and initially undetectable HBV DNA, developed a critical form of COVID-19, requiring admission to Intensive Care Unit and non-invasive ventilation. During hospitalization, she received a single dose (800 mg) of IV tocilizumab, oral baricitinib 4 mg/day for 3 days and IV dexamethasone in gradually decreasing doses: initially 8 mg every 12 hours for 1 day, then 12 mg/day for 3 days, then 8 mg/day for 7 days, 6 mg/day for 4 days, 4 mg/day for 1 day and 2 mg/day for 2 days. At follow-up, detectable viral load was found (HBV DNA < 10 IU/mL).
4. Patient no. 28, aged 80, with isolated anti-HBc and initially undetectable HBV DNA, was treated with IV dexamethasone 6 mg/day for 7 days, then 4 mg/day for 2 days. At follow-up, detectable HBV viral load (10 IU/mL) was found.
None of the HBVr cases had clinical, serological or biochemical complications.
Three of the 4 patients with HBVr (nos. 8, 20 and 28) were diagnosed with COVID-19 during the Omicron variant circulation.
HBVr did not correlate with sex, age, type of immunosuppressive treatment, severity of COVID-19 or viral variant.
4. Discussion
4.1. Seroprevalence of HBV Infection in COVID-19 Patients
Although the overall prevalence of HBV infection in Europe is low, there are still countries in Southern and Eastern Europe with intermediate endemicity [
9]. In our study, we identified an HBV prevalence (positive anti-HBc) of 39.9% in hospitalized patients with SARS-CoV-2 infection. The presence of anti-HBc was found more frequently in men and in patients over 64 years of age. Positive anti-HBc was also identified in younger age groups, starting with the age of 26, despite the implementation of universal childhood vaccination. Although the majority of anti-HBc-positive patients also presented anti-HBs, the degree of protection conferred by them may decrease over time. In addition, it is noteworthy that 8.3% of patients had isolated anti-HBc, while only 3.3% had positive HBsAg. This emphasizes the importance of performing a triple test panel for HBV screening, consisting of HBsAg, anti-HBc and anti-HBs, as the Centers for Disease Control and Prevention (CDC) also stated in its recent recommendations [
10]. Unfortunately, screening is often limited to HBsAg testing. This may result in misidentifying patients at risk of HBVr.
The high HBV exposure in COVID-19 patients identified in this study justifies the concern related to the risk of HBVr in patients receiving immunosuppressants.
4.2. Definitions of HBVr
HBVr is broadly defined as a sudden increase in HBV DNA levels or the reappearance of HBsAg in patients with resolved infection [
11]. However, to date, there is no consensus in establishing clear limits for HBVr. In
Table 3 we summarized the definitions of HBVr proposed by the medical associations dedicated to the management of liver diseases. In our study, we considered the definitions of HBVr that prevailed.
4.3. Risk of HBVr in Patients Receiving Immunosuppressive Treatment for COVID-19
In our study, we assessed the risk of HBVr in hospitalized patients co-infected with SARS-CoV-2 and HBV who received immunosuppressive treatment. We included both HBsAg-positive and HBsAg-negative/anti-HBc-positive patients, in whom we did a virological follow-up, by determining HBV DNA level at baseline and after a median period of 13 weeks from the end of the immunosuppressive treatment. We excluded patients with protective factors for HBVr (those with antiviral treatment for HBV and/or with positive anti-HBs). Of the 32 patients who were followed, 4 had HBVr. However, none of these reactivations were clinically manifest. Only in one case it was necessary to initiate antiviral treatment with entecavir. All patients with HBVr received at least one immunosuppressive treatment (tocilizumab, anakinra, baricitinib and/or systemic corticosteroids). Given that SARS-CoV-2 may itself represent a trigger for HBVr [
15,
16], we also included a control group (patients with SARS-CoV-2 infection without immunosuppressive treatment). Still, we have not documented any case of HBVr among them.
To date, there are few studies that have evaluated the occurrence of HBVr in patients with SARS-CoV-2 infection receiving immunosuppressive treatment. Additionally, these studies included only a small number of patients without protective factors for HBVr.
A retrospective study conducted in 2020 by Liu et al. included 20 patients with COVID-19 and chronic HBV infection (positive HBsAg), who were monitored virologically during hospitalization. Only 5 of them received immunosuppressive treatment (methylprednisolone). The authors considered HBVr a 2log increase in HBV DNA level in patients with previously detectable viremia and the reappearance of HBV DNA in patients with previously undetectable viremia. According to these criteria, 3 patients with HBVr were identified. Of these, only 2 received methylprednisolone for 4 days (dose not specified). The third, who had only inhaled interferon alfa-1b, had a small increase in HBV DNA level (initially undetectable, 20 IU/mL at follow-up) [
4]. This last situation is not considered HBVr by most guidelines [
12,
13,
14].
A prospective study conducted by Tajez et al. in 2020 included 61 HBsAg-negative/anti-HBc-positive patients with severe COVID-19, of which 38 patients received entecavir prophylaxis. Among the 23 patients without antiviral prophylaxis, 17 patients had positive anti-HBs. Thus, there were only 6 patients with isolated anti-HBc and without prophylactic treatment. Viral serological markers and HBV DNA level were performed in all these patients 1-2 months after the last dose of immunosuppressive treatment. No case of reverse seroconversion was identified, though 2 of the 6 patients with isolated anti-HBc had detectable HBV DNA level (<10 IU/mL). However, it should be mentioned that no baseline viral load was performed. Both patients received treatment with IL-6 receptor antagonists (siltuximab and tocilizumab, respectively). One of them additionally received methylprednisolone 250 mg/day for 3 days, followed by treatment with prednisone 0.5 mg/kg (duration was not specified – inferred to be at least 1 month) [
5].
Another prospective study conducted by Camarero et al. in the same year followed 11 patients with SARS-CoV-2 infection and positive HBsAg. Only in 4 of the patients who received immunosuppressive treatment the HBV DNA level was determined during hospitalization. Of these, one received entecavir. Among those without prophylaxis, one patient, who received methylprednisolone 250 mg/day for 3 days, had a 1.48 log increase in HBV DNA level compared to a previous viral load. This was considered HBVr [
6].
A recently published study conducted by Foo et al. in 2021-2022, included 54 HBsAg-negative/anti-HBc-positive patients who received tocilizumab or baricitinib for SARS-CoV-2 infection. Of these, only 4 patients had isolated anti-HBc. The others had protective anti-HBs. All patients were monitored for 3 months, but only serological tests were performed. No reverse seroconversion was identified [
7].
Another study, conducted by Yang et al. in 2022, aimed to assess the risk of death/critical COVID-19 in patients with different stages of HBV infection. They also followed 6 patients with HBVr (defined by reappearance of HBsAg) but did not report details of these cases (e.g. whether they received immunosuppressive treatment for COVID-19) [
17].
Studies related to the risk of HBVr in patients with SARS-CoV-2 infection receiving anakinra are almost non-existent. Only Mastroianni et al. included 30 patients who received anakinra. However, all these patients were anti-HBs-positive/anti-HBc-positive and received tenofovir prophylaxis [
18]. Thus, it is not surprising that no case of HBVr has been identified.
In addition to these studies, several case reports of HBVr in COVID-19 patients who received corticosteroid therapy [
19,
20,
21] or tocilizumab [
22] have been published.
As each previously mentioned study has considered different definitions for HBVr and had different inclusion criteria, it was necessary to find a common denominator. Therefore, in
Table 4 we presented the number of patients with HBV–SARS-CoV-2 co-infection and immunosuppressive treatment, without protective factors for HBVr, who were followed virologically (by HBV DNA) and experienced HBVr according to the definitions proposed by the medical associations dedicated to the management of liver diseases (see also
Table 3). We also included the results from our study.
4.4. Risk of HBVr in Patients Receiving Immunosuppressive Treatment for Non-COVID-19 Diseases
Most of the data related to the risk of HBVr associated with immunosuppressive drugs used for COVID-19 treatment (corticosteroids, tocilizumab, baricitinib) come from previous studies, conducted in patients with other diseases.
Regarding corticosteroids, a study published by Wong et al. in 2019 reported an increase in HBV DNA level of at least 1 log in 303 of 678 HBsAg-positive patients (44.7%) monitored for 1 year, most receiving a short course of corticosteroid treatment (less than 7 days) [
23]. In another study, published in 2020, Wong et al. followed 970 patients with isolated anti-HBc who received corticosteroid therapy, showing an annual risk of reverse HBsAg seroconversion of 1.8%, regardless of dose or duration of treatment [
24]. It is worth mentioning that, in particular, corticosteroids determine a risk of HBVr due to the presence of a steroid-responsive area in the viral genome [
9].
Data on tocilizumab therapy come mainly from patients with rheumatoid arthritis. Although a small number of HBsAg-positive patients without antiviral prophylaxis were included (a total of 10 patients), the 3 studies performed to date show a high risk of HBVr (60%) [
25,
26,
27]. In addition, several reports of severe cases, some fatal, in HBsAg-positive patients receiving tocilizumab have been published [
28,
29]. A systematic review conducted by Campbell et al. in 2021 identified 8 observational cohort studies that included 192 HBsAg-negative/anti-HBc-positive patients who received tocilizumab but no antiviral prophylaxis. In these patients, the pooled rate of HBVr was 2.6% (5/192) [
30,
31].
Regarding baricitinib, there is a study published by Harigai et al. in 2020, which included 215 HBsAg-negative/anti-HBc-positive patients with rheumatoid arthritis (201 with positive anti-HBs; 14 with isolated anti-HBc and undetectable HBV DNA at baseline). At follow-up, 32 patients (14.9%) had detectable HBV DNA level. Seven of these patients received concomitant corticosteroids [
32]. To the best of our knowledge, there is no study following HBsAg-positive patients on baricitinib.
To date, no cases of HBVr have been reported in patients receiving anakinra [
33]. However, there are almost no studies that have specifically followed this adverse event. We identified a single study that included 3 HBsAg-negative/anti-HBc-positive patients with rheumatoid arthritis who received anakinra and did not experience HBVr [
34].
4.5. Management of HBV–SARS-CoV-2 Co-Infected Patients Receiving Immunosuppressive Treatment
To date, there are no recommendations regarding the management of patients co-infected with SARS-CoV-2 and HBV who receive immunosuppressive treatment issued by a medical association dedicated to liver diseases. Thus, currently, the decision to monitor the patient or to administer antiviral prophylaxis depends on the internal protocol of the institution where the patient is admitted and/or on the decision of the attending physician.
From some clinicians’ point of view, it is not necessary to administer HBV prophylaxis in COVID-19 patients receiving immunosuppressants. Regarding corticosteroid therapy, the argument made is the short duration of treatment, generally less than 4 weeks (time limit beyond which prophylaxis would have been recommended) [
11]. As for the other immunosuppressive treatments used for SARS-CoV-2 infection (tocilizumab, anakinra, baricitinib), viral prophylaxis is not taken into account as they are not on the list of drugs associated with high or moderate risk of HBVr [
13]. These are also the reasons why the patients included in this study did not receive HBV prophylaxis (at the decision of their attending physicians).
On the other hand, given that high doses of corticosteroids are used in the treatment of COVID-19, even if for a short period of time, some authors recommend the administration of HBV prophylaxis in all HBsAg-positive patients, as well as in those HBsAg-negative/anti-HBc-positive with detectable viral load [
31,
35,
36]. HBV prophylaxis could also be considered in HBsAg-negative/anti-HBc-positive patients with undetectable viral load [
35,
37]. The same recommendations are made for other immunosuppressive drugs, such as tocilizumab and baricitinib [
31,
36]. However, no recommendations have been made for COVID-19 patients receiving anakinra, as this drug is less commonly used.
The data in
Table 4, although comprising a small number of studies and patients, show a significant risk of HBVr in patients with SARS-CoV-2 infection receiving immunosuppressive treatment. It is reassuring that most cases of HBVr had no clinical implications. However, there were also case reports of clinically manifest reactivations, some resulting in patients’ death [
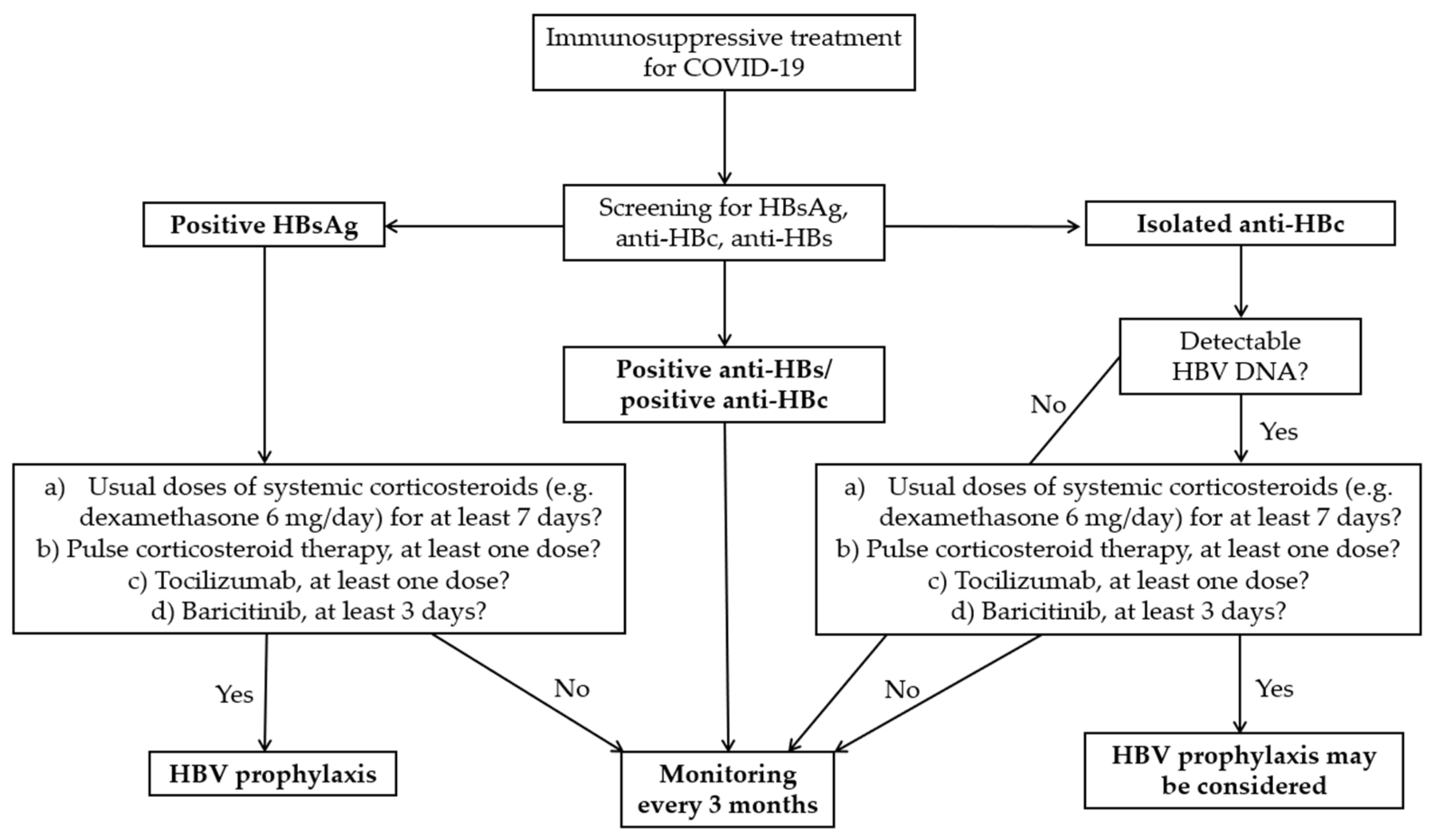
20]. Most of these severe cases were in HBsAg-positive patients. Larger studies are needed in order to establish an appropriate and cost-effective management in these patients. In the meantime, analysing the existing data, both from COVID-19 patients and from those with other diseases, we propose the algorithm illustrated in
Figure 2.
First, we recommend performing an HBV triple screen panel that includes HBsAg, anti-HBs and anti-HBc in all patients with SARS-CoV-2 infection for whom immunosuppressive treatment (e.g. tocilizumab, baricitinib, systemic corticosteroids) is indicated. It would be prudent to perform the same screening in patients receiving anakinra, given the limited data on the risk of HBVr related to this drug.
For HBsAg-positive patients, we suggest antiviral prophylaxis if they receive any of the following: a) usual doses of systemic corticosteroids (e.g. dexamethasone 6 mg/day) for at least 7 days; b) pulse corticosteroid therapy, at least one dose; c) tocilizumab, at least one dose; d) baricitinib, at least 3 days. We established these limits empirically, based on the observations made in our study, as well as in other studies performed to date. In addition, unlike other authors, who encourage prophylaxis in all HBsAg-positive patients, we recommend a higher threshold, considering that there is also the possibility of HBVr after stopping HBV prophylaxis [
38]. Obviously, patients who meet the criteria for HBV treatment even in the absence of immunosuppressants will receive antiviral treatment according to current guidelines.
In patients with isolated anti-HBc, we suggest performing an HBV viral load. If this is detectable and the criteria described in HBsAg-positive patients are met, HBV prophylaxis may be considered (
Figure 2). However, given that reactivations are not clinically significant in these patients, monitoring may be sufficient.
During the decision-making process it is important to consider the possibility of subsequent monitoring of patients. For example, in this study, 11 of 44 patients (25%) were lost to follow-up, although they initially committed to come for re-evaluation. To these patients are added those who, from the start, refused enrolment in the study due to the need for a follow-up. However, the addressability of patients could be higher in other countries. Another criterion that could be considered is the degree of liver fibrosis. Existing data suggest that patients with more advanced liver fibrosis have higher morbidity and mortality associated with HBVr [
13]. Therefore, we recommend that HBV prophylaxis be administered to these patients. Still, an impediment could be the long length of time until receiving the results of serological tests (such as FibroTest) or the unavailability of performing an elastography in a timely manner.
In anti-HBs-positive/anti-HBc-positive patients, we do not consider prophylaxis necessary, given the available data. Ideally, they should still be monitored after discharge.
Regarding anakinra treatment, there is no evidence related to the risk of HBVr in patients with COVID-19 or other diseases. However, data are limited, so we suggest screening for HBV and monitoring patients with positive markers for active or previous infection.
When HBV prophylaxis is decided, it is preferable to use nucleoside analogues with high genetic barrier (entecavir or tenofovir) for at least 6 months after the last dose of immunosuppressant [
13]. However, the use of lamivudine is also supported if the viral load is below 2,000 IU/mL and it is anticipated that the prophylaxis will be of short duration [
36]. If monitoring is decided, this ideally consists of repeating HBV DNA level, viral serological markers and liver enzymes every 3 months. An alternative is to monitor liver enzymes and perform HBV viral load in case of increased aminotransferases [
13,
31].
4.6. Strengths and Limitations of the Study
This study is one of the few that offers an in-depth analysis of the risk of HBVr in patients co-infected with HBV and SARS-CoV-2 receiving immunosuppressive treatment. Moreover, the study had a prospective design, and the patients were monitored not only clinically and serologically but also virologically (by HBV DNA). Also, as far as we know, this is the only study that followed virologically patients diagnosed with COVID-19 during the Omicron variant circulation. This is important, given that its subvariants continue to be dominant worldwide. Furthermore, considering that SARS-CoV-2 could itself represent a trigger for HBVr, another strength of the study is that it also included a control group, represented by COVID-19 patients who did not receive immunosuppressive treatment. Not least, this study also evaluated HBV seroprevalence in COVID-19 patients, in order to clarify the importance of assessing the risk of HBVr in patients receiving immunosuppressants.
This study is limited by the small number of participants. However, compared to other studies, this one included a larger number of patients without protective factors for HBVr (antiviral treatment and/or positive anti-HBs). In addition, we analysed under a common denominator all studies related to HBVr in COVID-19 patients receiving immunosuppressive treatment, allowing an overview of the results we have to date.
5. Conclusions
In conclusion, administration of immunosuppressants for COVID-19 treatment may result in a significant risk of HBVr in co-infected patients. We recommend performing an HBV triple screen panel that includes HBsAg, anti-HBs and anti-HBc in all patients with SARS-CoV-2 infection for whom immunosuppressive treatment is indicated. We suggest HBV prophylaxis in HBsAg-positive patients receiving any of the following: a) usual doses of systemic corticosteroids for at least 7 days; b) pulse corticosteroid therapy, at least one dose; c) tocilizumab, at least one dose; d) baricitinib, at least 3 days. The fact that 3 of the 4 patients with HBVr in this study were diagnosed with COVID-19 during the Omicron variant circulation highlights that the risk assessment of HBVr in COVID-19 patients remains a current issue. Larger studies are needed in order to establish an appropriate and cost-effective management in these patients. Also, we emphasize the need for an explicit and uniform definition of HBVr.
Author Contributions
Conceptualization, N.M., M.C.O., O-A.G., A-I.A., V.M., S.S.A., C.T. and V.A.; Methodology, N.M., M.C.O., C.T. and V.A.; Software, C.T.; Validation, N.M., S.S.A. and V.A.; Formal Analysis, O-A.G., A-I.A.; Investigation, O-A.G., A-I.A. and V.M.; Data curation, O-A.G., A-I.A., V.M. and S.S.A.; Writing-original draft preparation, N.M., M.C.O., C.T., S.S.A. and V.A.; Writing-review and editing, N.M., M.C.O., O-A.G., A-I.A., V.M., S.S.A., C.T. and V.A.; Supervision, N.M., M.C.O., C.T. and V.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee of the National Institute of Infectious Diseases 'Matei Bals' from Bucharest, Romania (C11454/24.09.2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We would like to acknowledge the invaluable help of the department heads, doctors and nurses of wards III, IV, XI and XIII, where the study took place, as well as the staff of the genetics laboratory, within National Institute of Infectious Diseases ‘Matei Bals’ from Bucharest, Romania. Publication of this paper was supported by the University of Medicine and Pharmacy ‘Carol Davila’, through the institutional program Publish not Perish.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. WHO COVID-19 Dashboard. Available online: https://covid19.who.int/ (accessed on 10 September 2024).
- Montazersaheb S, Hosseiniyan Khatibi SM, Hejazi MS, Tarhriz V, Farjami A, Ghasemian Sorbeni F, et al. COVID-19 infection: an overview on cytokine storm and related interventions. Virol J 2022;19(1):92. [CrossRef]
- National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf (accessed on 8 August 2024).
- Liu J, Wang T, Cai Q, Sun L, Huang D, Zhou G, et al. Longitudinal changes of liver function and hepatitis B reactivation in COVID-19 patients with pre-existing chronic hepatitis B virus infection. Hepatol Res 2020;50(11):1211-21. [CrossRef]
- Rodríguez-Tajes S, Miralpeix A, Costa J, López-Suñé E, Laguno M, Pocurull A, et al. Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy. J Viral Hepat 2021;28(1):89-94. [CrossRef]
- Gómez Camarero J, Badia Aranda E, Quiñones Castro R, Saiz Chumillas RM, Alcoba Vega L, Díez Ruiz S, et al. Hepatitis B and C screening in hospitalized patients with SARS-CoV-2 infection. Gastroenterol Hepatol 2022;45(4):256-64. [CrossRef]
- Foo H, Phan F, Bagatella M, Petrovski I, Nagendra V, Acharya P, et al. Risk of hepatitis B reactivation following baricitinib or tocilizumab for treatment of COVID-19. Eur J Clin Microbiol Infect Dis 2023;42(6):799-801. [CrossRef]
- MacLachlan JH, Cowie BC. Hepatitis B virus epidemiology. CSH Perspect Med 2015;5(5):a021410. [CrossRef]
- Trickey A, Bivegete S, Duffell E, McNaughton AL, Nerlander L, Walker JG, et al. Estimating hepatitis B virus prevalence among key population groups for European Union and European Economic Area countries and the United Kingdom: a modelling study. BMC Infect Dis 2023;23(1):457. [CrossRef]
- Conners EE, Panagiotakopoulos L, Hofmeister MG, Spradling PR, Hagan LM, Harris AM, et al. Screening and testing for hepatitis B virus infection: CDC recommendations — United States, 2023. MMWR Recomm Rep 2023;72(1):1–25. http://doi.org/10.15585/mmwr.rr7201a1.
- Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015;148(1):221-44.e3. [CrossRef]
- Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol 2019;25(2):93-159. [CrossRef]
- Lau G, Yu ML, Wong G, Thompson A, Ghazinian H, Hou JL, et al. APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy. Hepatol Int 2021;15(5):1031-48. [CrossRef]
- Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67(4):1560-99. [CrossRef]
- Librero Jiménez M, López Garrido MÁ, Fernández Cano MC. Letter to the editor: Reactivation of HBV triggered by SARS-CoV-2 in a patient with cirrhosis. Hepatology 2022;75(3):765-6. [CrossRef]
- Giugliano L, Pinon M, Calvo PL. COVID-19 as a trigger of acute-on-chronic hepatitis B presenting with undetectable INR due to hypercoagulability in a 16-year-old girl. Pediatr Infect Dis J 2023;42(2):143-5. [CrossRef]
- Yang S, Wang S, Du M, Liu M, Liu Y, He Y. Patients with COVID-19 and HBV coinfection are at risk of poor prognosis. Infect Dis Ther 2022;11(3):1229-42. [CrossRef]
- Mastroianni A, Greco S, Chidichimo L, Mauro MV, Urso F, Vangeli V. Antiviral prophylaxis for hepatitis B virus in COVID-19 patients treated with immunosuppressive drug therapy. Antiviral Therapy 2022;27(2). [CrossRef]
- Wu YF, Yu WJ, Jiang YH, Chen Y, Zhang B, Zhen RB, et al. COVID-19 or treatment associated immunosuppression may trigger hepatitis B virus reactivation: a case report. World J Clin Cases 2021;9(19):5266-9. [CrossRef]
- Sagnelli C, Montella L, Grimaldi P, Pisaturo M, Alessio L, De Pascalis S, et al. COVID-19 as another trigger for HBV reactivation: clinical case and review of literature. Pathogens 2022;11(7):816. [CrossRef]
- Braimakis I, Vasileiadi S, Trifylli EM, Papadopoulos N, Deutsch M. Can hepatitis B virus (HBV) reactivation result from a mild COVID-19 infection? Livers 2023;3(3):347-53. [CrossRef]
- Figueredo C, Haider T, Guddati H, Massoumi H. A case of hepatitis B reactivation associated hepatitis after tocilizumab therapy in a patient with SARS-CoV-2 infection. Am J Gastroenterol 2021;116:S1205-6. [CrossRef]
- Wong GL, Yuen BW, Chan HL, Tse YK, Yip TC, Lam KL, et al. Impact of dose and duration of corticosteroid on the risk of hepatitis flare in patients with chronic hepatitis B. Liver Int 2019;39(2):271-9. [CrossRef]
- Wong GL, Wong VW, Yuen BW, Tse YK, Yip TC, Luk HW, et al. Risk of hepatitis B surface antigen seroreversion after corticosteroid treatment in patients with previous hepatitis B virus exposure. J Hepatol 2020;72(1):57–66. [CrossRef]
- Chen LF, Mo YQ, Jing J, Ma JD, Zheng DH, Dai L. Short-course tocilizumab increases risk of hepatitis B virus reactivation in patients with rheumatoid arthritis: a prospective clinical observation. Int J Rheum Dis 2017;20(7):859-69. [CrossRef]
- Kuo MH, Tseng CW, Lu MC, Tung CH, Tseng KC, Huang KY, et al. Risk of hepatitis B virus reactivation in rheumatoid arthritis patients undergoing tocilizumab-containing treatment. Dig Dis Sci 2021;66(11):4026-34. [CrossRef]
- Chen MH, Chen MH, Liu CY, Tsai CY, Huang DF, Lin HY, et al. Hepatitis B virus reactivation in rheumatoid arthritis patients undergoing biologics treatment. J Infect Dis 2016;215(4):566–73. [CrossRef]
- Sonneveld MJ, Murad SD, van der Eijk AA, de Man RA. Fulminant liver failure due to hepatitis B reactivation during treatment with tocilizumab. ACG Case Rep J 2019;6(12):e00243. [CrossRef]
- Biehl A, Harinstein L, Brinker A, Glaser R, Muñoz M, Avigan M. A case series analysis of serious exacerbations of viral hepatitis and non-viral hepatic injuries in tocilizumab-treated patients. Liver Int 2021;41(3):515-28. [CrossRef]
- Campbell C, Andersson MI, Ansari MA, Moswela O, Misbah SA, Klenerman P, et al. Risk of reactivation of hepatitis B virus (HBV) and tuberculosis (TB) and complications of hepatitis C virus (HCV) following tocilizumab therapy: a systematic review to inform risk assessment in the COVID-19 era. Front Med 2021;8:706482. [CrossRef]
- Yip TC, Gill M, Wong GL, Liu K. Management of hepatitis B virus reactivation due to treatment of COVID-19. Hepatol Int 2022;16(2):257-68. [CrossRef]
- Harigai M, Winthrop K, Takeuchi T, Hsieh TY, Chen YM, Smolen JS, et al. Evaluation of hepatitis B virus in clinical trials of baricitinib in rheumatoid arthritis. RMD Open 2020; 6(1):e001095. [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury - Anakinra. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548615/ (accessed on 8 august 2024).
- Barone M, Notarnicola A, Lopalco G, Viggiani MT, Sebastiani F, Covelli M, et al. Safety of long-term biologic therapy in rheumatologic patients with a previously resolved hepatitis B viral infection. Hepatology 2015;62(1):40-6. [CrossRef]
- Varona Pérez J, Rodriguez Chinesta JM. Risk of hepatitis B reactivation associated with treatment against SARS-CoV-2 (COVID-19) with corticosteroids. Rev Clin Esp 2020;220(8):535-6. [CrossRef]
- Spera AM. Hepatitis B virus infection reactivation in patients under immunosuppressive therapies: pathogenesis, screening, prevention and treatment. World J Virol 2022;11(5):275-82. [CrossRef]
- Satsangi S, Gupta N, Kodan P. Current and new drugs for COVID-19 treatment and its effects on the liver. J Clin Transl Hepatol 2021;9(3):436-46. [CrossRef]
- Wong GL, Chan HL, Yuen BW, Tse YK, Luk HW, Yip TC, et al. The safety of stopping nucleos(t)ide analogue treatment in patients with HBeAg-negative chronic hepatitis B. Liver Int 2020;40(3):549-57. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).