1. Introduction
The cells of fungi, like some other organisms (bacteria, plants, etc.), are covered with the cell wall. A common property of organisms with the cell wall is the presence of intracellular hydrostatic pressure (turgor pressure or simply turgor). Turgor occurs because the overall osmolarity of the cytoplasm is usually higher than the extracellular environment, and water, entering the cell (or moving from other parts of the colony, if, for example, it is talking about aerial hyphae), creates pressure from the plasma membrane/protoplast on the cell wall (Lew, 2019). Fungi can relatively quickly regulate turgor pressure in their cells. There are many regulatory mechanisms: osmolyte synthesis (one of the main synthesis pathways is the high-osmolar glycerol (HOG) pathway); active transport of ions across the plasma membrane; the use of certain types of intrahyphal transport; hyphal growth and other mechanisms (for example, regulation of membrane permeability to water through aquaporins, changes in the hydrophobicity of cell covers, etc.; Lew, Nasserifar, 2009; Lew, 2010; Lew, 2019; de Oliveira et al., 2021; Herman, Bleichrodt, 2022). Turgor is of great importance for fungi: it regulates the size and shape of cells, as well as the tension of the plasma membrane; participates in the mechanism of apical growth and transport within hyphae; ensures the penetration of hyphae or haustoria into the substrate/tissue of the host; takes part in the active release of spores/conidia, in the capture of prey from predatory fungi, etc.. (Weber, 2002; Lew et al., 2004; Lew, Nasserifar, 2009; Abadeh, Lew, 2013; Steinberg et al., 2018; Herman, Bleichrodt, 2022; Chen et al., 2023; Panstruga et al., 2023).
It is known that in specialized fungal cells turgor pressure can reach high values (up to 8 MPa in the appressorium of Magnaporthe grisea, for example; Ryder et al, 2019; Gow, Lenardon, 2023). It was previously believed that turgor pressure in unspecialized cells of filamentous fungi under normal conditions (on standard nutrient media, for example) is also quite high (1-1.5 MPa and higher). However, modern studies indicate more modest values of average turgor pressure in vegetative hyphae. This divergence is due to different ways of assessing turgor. Thus, the previously used method of primary plasmolysis gives an overestimated result in fungi due to the strong adhesion of the plasma membrane to the cell wall (Robertson, Rizvi, 1968; Heath, Steinberg, 1999; Walker, White, 2017). For example, Lew and colleagues have shown in a number of studies using the pressure probe method that the turgor in Neurospora crassa cells averages about 0.5 MPa, which is approximately three times lower than the values given in earlier works (Robertson, Rizvi, 1968; Lew et al., 2004; Lew, Nasserifar, 2009; Municio-Diaz et al., 2022). Chevalier and coworkers (2024), using the deflation assay, showed that even in the apical cells of several species of ascomycetes and Mucor circinelloides, the turgor value is in the range of only 0.3-0.7 MPa (only in Aspergillus nidulans it exceeds 1 MPa). This is despite the fact that the average pressure in the apical cell (at least in the growing one) usually exceeds the pressure in the rest of the hypha. Moreover, the same authors showed that even such a canonical function of turgor as participation in the elongation of the apical cell during fungal growth is not so clear – they did not find a positive correlation between the value of turgor pressure in the apices and growth rate in a number of species of filamentous fungi (Chevalier et al., 2024).
It is distinguished two groups of fungi and fungi-like organisms based on their relationship to maintaining constant turgor values (unfortunately, too few organisms have been studied in this regard to be able to draw a complete picture for different systematic groups of fungi). Thus, in N. crassa, after placing its mycelium in a moderate hyperosmotic environment, first the turgor decreases significantly, then due to the uptake of ions from the outside and the synthesis of osmolytes, within about an hour the fungus almost restores the initial pressure (Lew et al., 2004; Lew, Nasserifar, 2009). The oomycete Achlia bisexualis under the same conditions also loses turgor, but does not at all strive to restore it (Lew et al., 2004). It is known that oomycetes, in principle, can live without turgor pressure; even their apical growth is possible at zero turgor (Harold et al., 1996; Lew et al., 2004). Moreover, their strategy for penetrating the substrate is different – the tip of the penetrating hypha not only may not be under pressure, but even the actin cap at the tip disassembles so that the apex becomes soft and flattened. (Walker et al., 2006). Halotolerant fungi living in environments with high salt concentrations also have zero or low turgor values (Lew, 2019).
Our recent studies have shown that basidiomycetes (at least the xylotroph Stereum hirsutum, the coprotroph Coprinus comatus and the plant pathogen Rhizoctonia solani) probably also do not always spend resources on maintaining constant turgor. Focusing on the change in the diameter of the hyphae (which has certain correlations with the value of turgor pressure) under moderate hyperosmotic conditions, it was found that these fungi do not have a tendency to quickly restore their original size. It has also been shown that in some cases the actin cytoskeleton affects the diameter of hyphae – with depolymerization of F-actin, the thickness of the vegetative hyphae of S. hirsutum changes (Mazheika et al., 2024 preprint). It was previously obtained that the destruction of F-actin in basidiomycetes (probably in other fungi) leads to increased formation of large invaginations (macroinvaginations) of the plasma membrane, to an increase in their size, to stimulation of the formation of tubular invaginations (Mazheika et al., 2020a, 2022). Such results evidently contradict established ideas about the fungal cell, which has been compared to a bicycle tire, in which an elastic rubber chamber (plasma membrane) is pressed tightly against an elastic but reinforced tire (the cell wall; Lew, 2019). In such a cell, even endocytosis with pin sizes up to 100 nm requires a lot of energy and full-fledged cross-linked F-actin elements, and the formation of macroinvaginations, the size of which is more than 200 nm, which in xylotrophic basidiomycetes can be in the form of thick tubes up to several tens of microns long, would be simply impossible, especially with disassembled F-actin.
All of the above indicates that turgor pressure is important in fungal physiology, but it is not the only factor determining the shape and size (their rapid change) of fungal cells, influencing plasma membrane tension and other vital functions, at least for certain groups of fungi. On the other hand, much indicates that the actin cytoskeleton of fungi is the number one candidate for supplementing or replacing turgor functions. Of course, the idea is not new: for example, Heath and Steinberg, in their 1999 work, discussed an alternative turgor physiological model for fungi, in which the contractile actin cytoskeleton and focal adhesion of the plasma membrane to the cell wall played an important role. However, over the past quarter century, new data have emerged in mycology that can be used to develop concepts alternative (complementary) to the turgor physiological model.
An important idea underlying this work can be formulated in other words: a basidiomycete fungus, especially a mycorrhiza-former or xylotroph, having colonies extending hundreds of meters in the soil litter, connecting living or dead trees, their remains, constantly undergoing rapid changes in environmental conditions, aggressiveness of other living things organisms with frequent nitrogen starvation, which have high-speed and very complex long-distance transport along hyphae and cords, simply cannot afford the luxury of significantly depending on turgor pressure. This review reconsiders and expands the previously proposed curtain model, which describes certain aspects of the organization and physiology of a non-apical and unspecialized fungal cell (Mazheika et al., 2020a). The model is focused on basidiomycetes, but it is likely that, in one variation or another, it can be applied to a wide range of fungi. In addition to considering the main components of the curtain model, this review also discusses different strategies for fungi responding to rapidly changing external conditions.
2. The Curtain Model
The curtain model describes the equilibrium physiological state of the fungal hyphae. In an equilibrium state, osmotic and other parameters of the external environment change either slightly or slowly, allowing the fungal cell to adapt to the change without a non-equilibrium lag. In non-equilibrium conditions, with rapid and significant changes in external conditions – stress, the curtain model is not suitable for describing the state of the fungal cell (for example, for many fungi and oomycetes, disassembly of F-actin, the main element of the curtain model, is shown during hyperosmotic shock; Slaninova et al., 2000; Walker et al., 2006; Bitsikas et al., 2011; Elhasi, Blomberg, 2019). Under stressful conditions, fungi trigger fast/instantaneous structural-physiological defense mechanisms – airbags (similar to the defense mechanisms in cars), which preventing cell damage in the first minutes of a shock. One example of airbag is the complex system of macroinvaginations in xylotrophic basidiomycetes (Mazheika et al., 2022); other examples of airbags will be given later in the text as the material is presented.
In addition to the properties of the curtain model, in which it describes only the equilibrium physiological states of the mycelium, the model is also characterized by the following: i. it works only in cells with relatively low or medium turgor, therefore not suitable for apical and various specialized fungal cells. ii. It is optimal for filamentous basidiomycetes, although it can also work on other groups of fungi. iii. The model is an open system – new components can be added to it as new scientific knowledge emerges.
The essence of the curtain model is briefly as follows: the actin cytoskeleton, together with other components of the curtain model (see below), can replace or supplement turgor pressure in the fungal cell. This is accomplished due to attaching of the actin cables from inside the cell to sites of focal adhesion of the plasma membrane to the cell wall. These actin driver cables regulate both the tension of the plasma membrane and create force acting on the elastic cell wall, maintaining and changing the shape and size of the fungal cell. In cells without turgor or with low turgor values (but have already reached an equilibrium state), the curtain system completely takes over the functions of turgor. In cells with turgor pressure (but not high), the curtain system corrects/compensates for the membrane tension, elastic strain of the cell wall, cell form/size and other parameters when small changes in turgor pressure both in the cell as a whole, and with uneven pressure distribution in different parts of one and the same cell/hypha. The curtain system allows the fungal hypha to continue its normal, uninterrupted life activity and functioning under equilibrium changes in external and internal conditions.
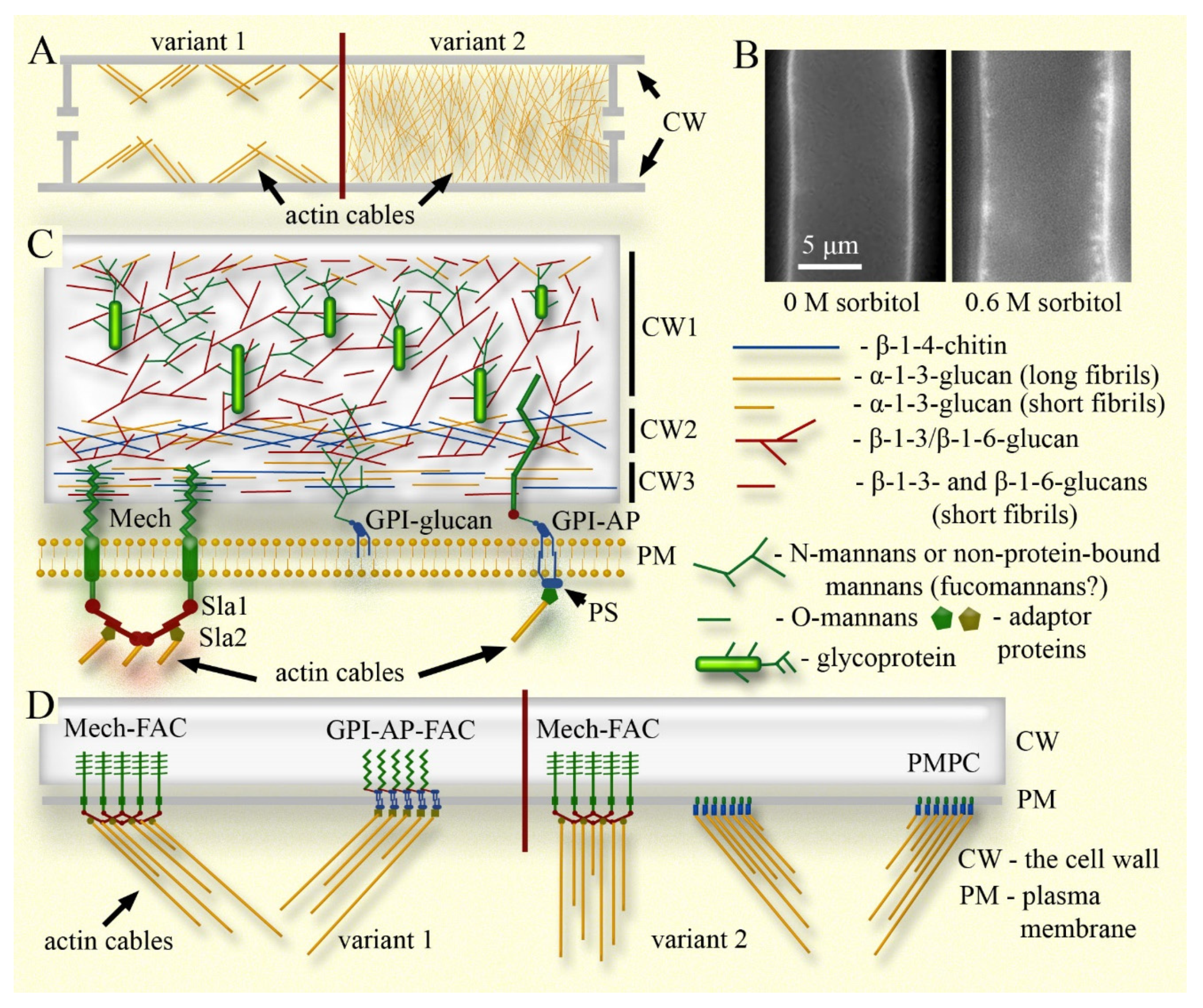
The curtain model includes the following four components (see
Figure 1 and
Figure 2): i) actin cables connected to focal adhesion complexes and sites (FACs/FASs) on the cytoplasmic side (possibly to other elements of the plasma membrane), which in cells with not high turgor take on a number of important functions, primarily, regulating the tension of the plasma membrane and regulating the size and shape of the hyphae. ii) The elastic cell wall that stretches or shrinks along with the whole cell, capable of quickly changing its elasticity if necessary. iii) Plasma membrane tightly adherent to the cell wall (preserving the periplasmic space) through FACs/FASs and other connections. iv) Systems of macroinvagination of the plasma membrane, which provide a membrane pool, a rapid change in the working surface of the plasma membrane and prevent damage and dysfunction of the membrane during both equilibrium and sudden changes in the size of the fungal cell. It should be noted that although the curtain model works in conjunction with turgor, supplementing or replacing it, turgor itself is not a component of the model, since the model can also work in cells with zero turgor.
If to combine all the listed components into a single system, it can be given the following analogy: a curtain hanging on a window is the plasma membrane; curtain folds – macroinvaginations; a curtain rod – the cell wall; rings securing the curtain to the rod – FACs/FASs; curtain drivers (sticks that can be used to push high curtains from below) – actin cables. Of course, such an analogy is not complete; it does not take into account, for example, the elasticity of the cell wall, changes in cell size, the influence of turgor, the participation of actin not only in plasma membrane tension, but also in changes in cell size and many other parameters of the fungal cell. However, it gives a superficial idea of what the curtain model describes and why it has such a name. Next, the components of the curtain model will be examined in detail.
3. Component I: Actin Cytoskeleton
3.1. Fungal Actin Cables
As is known, fungi have three main types of intracellular structures formed by F-actin: Arp2/3-dependent patches (in fact, small areas of actin cortex, which is not present in a complete form in fungi, but usually lining the entire internal surface of an animal cell), formin-dependent contractile rings, and formin-dependent cables (Berepiki et al., 2011; Lichius et al., 2011). Fungi have been shown to have other, but more specific, actin structures (Dagdas et al., 2012; Kopecka et al., 2013; Xu et al., 2021). The cables are bundles of parallel-displaced F-actin filaments, nucleated by formin and cross-linked by tropomyosin and other proteins. Cables can be quite long, usually a few micrometers in length, but can reach 25 μm (Berepiki et al., 2011). At the same time, they are a very dynamic structure, usually assembled and disassembled within a few seconds (Bergs et al., 2016; Takeshita, 2019). In filamentous fungi, actin cables perform very important functions at the apical cells. It is the cables that serve as rails for active transport (with the participation of myosin V motors) of secretory vesicles into the apical body (Spitzenkörper), intercepting cargo from microtubules. Actin cables not only ensure the concentration of enzymes and structural materials at the hyphal tip, necessary for apical growth, but are also in a loop of mutual regulation with microtubules, which supply protein polar markers to the apex that regulate the assembly of actin cables, in turn regulating the convergence of the ends of microtubules into the apex and enhancing the polarity of growth (Takeshita et al., 2014; Peñalva et al., 2017; Steinberg et al., 2018; Takeshita, 2019). However, the individual mechanisms of apical growth may vary among different fungi and fungi-like organisms (Lilje, Lilje, 2006; Wernet et al., 2021).
3.2. Functions of Actin Cables in the Curtain Model
Actin cables in the curtain model are assigned a control-power role and they perform several functions. There are the two most important ones (some secondary features will be mentioned in the following sections). First function: actin cables regulate plasma membrane tension in cells with not-high turgor pressure. By connecting to FACs/FASs proteins through adapter proteins (see next sections; another variant of the curtain model will also be described there, with regulation of membrane tension by actin not through FACs/FASs), the cables move them along the inner layer of the cell wall, providing tension on the plasma membrane. In this case, a complex system of cables can not only, as on a wire frame, tension the entire plasma membrane of the cell, replacing turgor pressure, but also provide a local change in the tension of the membrane. For example, a cable system can relax a local region of the plasma membrane without changing the tension of the entire membrane, for example, to accelerate endocytosis in this segment, or for another purpose. Such a local change in plasma membrane tension is probably possible even with high turgor pressure, but requires large energy costs (Mazheika et al., 2020a). The described function of actin cables in the curtain model is confirmed by the behavior of the plasma membrane when fungal mycelium is treated with actin polymerization inhibitors. In a number of publications, photographs of hyphae of various fungal species treated with the actin inhibitors show a large number of parietal invaginations of the plasma membrane (not always taken into account by the authors; Yamashita and May, 1998; Lee et al., 2007; Upadhyay and Shaw, 2008; Lewis et al., 2009). The deassembling of F-actin leads to an increase in the number and size of plasma membrane macroinvaginations, including tubular, in individual cells in
R. solani (Mazheika et al., 2020a). A similar result was obtained for
S. hirsutum (
Figure 2). In this fungus, latrunculin A (actin depolymerizer) increases the size of lomasomes (one of macroinvagination type, see next sections) and their number in hyphal cells by an average of 2–3 times (Mazheika et al., 2022). These results indicate that in fungi, plasma membrane tension in many cells is provided not by turgor, but by the actin cytoskeleton. Its depolymerization leads to a sharp relaxation of the membrane and the activation of a protective mechanism during sudden changes in conditions (airbag) – the formation of macroinvaginations of the plasma membrane.
The second function of actin cables in the curtain model is to participate in changing or maintaining the size (shape) of cells. It has been shown that depolymerization of F-actin can lead to changes in the diameter of S. hirsutum hyphae (Mazheika et al., 2024 preprint). Such changes are not large – on average no more than 7% of the hyphal diameter, and hyphae can both shrink and expand under the influence of latrunculin A. However, the destruction of F-actin does not affect the size of R. solani hyphae (Mazheika et al., 2024 preprint). This probably indicates that the shape-restraining function of actin in fungal cells is expressed differently in different species, in contrast to the function of regulating plasma membrane tension. It is also known that depolymerization of actin often leads to swelling of the hyphal tips. This is shown for Allomyces macrogynus, a number of oomycetes, A. nidulans, R. solani, etc. (Heath, 1990; Heath et al., 2003; Gupta, Heath, 1997; Srinivasan et al., 1996; Torralba et al., 1998; Heath, Steinberg, 1999; Walker et al., 2006; Lichius et al., 2011; Mazheika et al., 2020a). For oomycetes, it was shown that immediately after treatment of the mycelium with the actin polymerisation inhibitors, the apical cells, under pressure, first accelerate their growth, then stop it and often swell (in oomycetes, it is not the tip itself that often swells, but the subapex). Apical cells without turgor do not accelerate in growth before growth stops and rarely swell (Gupta, Heath, 1997). Often, swelling of the apices is explained by a failure of growth polarity – without actin cables, polar markers are distributed throughout the periphery of the apex, which leads to softening and radial synthesis of the cell wall (Lichius et al., 2011). However, judging by the rapidity and uniformity of the swelling of the apices, the swelling occurs primarily due to actin cables holding and shaping the hyphal tip with a thin apical cell wall. Without actin cables, turgor pressure in the growing apex easily inflates it. This can also explain the sharp acceleration of growth at the very beginning of treatment of mycelium with the actin inhibitors. Therefore, the function of actin cables in the hyphal tip is not only to transport vesicles to the apical body and regulate growth polarity, but also to create a rigid frame for the soft cell wall of the apex. A similar mechanism probably operates in non-apical cells, but it is less obvious there (the hypha changes only slightly in diameter as actin is disassembled) and is expressed differently in different fungi (for example S. hirsutum vs. R. solani). In Botrytis cinerea, in mutants with a deletion of one of the actin genes, the hyphae grow more slowly compared to the wild type, and they are more compact, as the authors point out, which may be associated with a reduction of the cell shape-restraining function of actin (Li et al., 2020). Perhaps, in study with the germination of fungal hyphae through a thin channel of a microfluidic device (Fukuda et al., 2021), growth cessation and swelling of apices in certain species of fungi is precisely associated with the deassembling of actin (the authors and other researchers offer another explanation; Fukuda et al., 2021; Wernet et al., 2021). In those fungi in which, during growth through a thin channel, mechanoreceptors are triggered and the CWI pathway is launched, the actin cables are disassembled and the apices swell, growth stops. In fungi with a lower growth rate and hyphal diameter, such actin disassembly does not occur, apparently due to differences in the sensitivity of triggering the CWI pathway.
It cannot be ruled out that the actin cytoskeleton performs its form-restraining function not only by creating mechanical resistance to cell shrinkage or expansion, but also by having contractility. Contractility of not only actomyosin rings in septa, but also the cytoplasm as a whole has been shown for a number of fungi, although this issue remains open and poorly studied (Heath, Steinberg, 1999; Reynaga-Peña, Bartnicki-García, 2005; Steinberg et al., 2017). Budding yeast has been shown to have a cell wall that is constantly vibrating at a frequency of approximately four oscillations per ms. The authors suggest that such oscillation is associated with the work of molecular motors (myosin, among others; Pelling et al., 2004). If actin cables can actively shrink/expand the fungal cell, then the actin cytoskeleton can even more fully replace turgor pressure: both buffering the change in cell size during osmotic fluctuation and substituting the turgor gradient to create mass flow transport inside the hyphae. There is data that disassembly of F-actin leads to a decrease in the rate of cytosolic streaming in A. niger, which can be used as confirmation of the actin contractile hypothesis (although the authors explain this effect differently; Bleichrodt et al., 2015).
It should be noted once again that the curtain model describes the equilibrium physiological state of the mycelium. Accordingly, actin performs its functions in a certain small range of fluctuations in osmotic and other conditions. In the case of a non-equilibrium process – a rapid and strong change in the osmolarity of the medium, for example, and a sharp drop in turgor – disassembly of F-actin occurs in many fungi. This is indicated for yeasts, some oomycetes, A. nidulans (Slaninova et al., 2000; Walker et al., 2006; Bitsikas et al., 2011). Probably, a comparison can be made here with a sailing ship in a storm – sailors remove the sails, otherwise both the sails and the ship itself may be damaged.
3.3. Microscopic Visualization of the Actin Cable System as a Component of the Curtain Model
If one looks at classical work on oomycetes with fluorescent labeling, for example in the review by Heath (1990), one can see that not only the hyphal apices, which have a fairly powerful cap of actin cables, but also the hyphae themselves are permeated with a dense matrix of actin cables/filaments (Gupta, Heath, 1997; Walker et al., 2006).
In filamentous fungi, the actin cable system is not as well visualized as in oomycetes. It is shown that the cables are well developed in the germ tubes of some fungi and in conidial anastomosis tubes; there is an actin cap in the apical cells of
Magnaporthe grisea also (Lichius et al., 2011; Li et al., 2020). A network of actin cables is shown in some fine penetration hyphae and in individual areas of vegetative hyphae; during the formation of septa and anastomoses; in the distribution of nuclei during the clamp connection formation; after damage to the hypha and the formation of a new one at the site of damage (Salo et al., 1989; Roberson, 1992; Timsonen et al., 1993; Gorfer et a., 2001; Timonen, Peterson, 2002; Berg et al., 2016; Jung et al., 2018; Raudaskoski, 2019; Li et al., 2020; Garduño-Rosales et al., 2022; Wernet et al., 2023). However, according to the curtain model, the actin cable system should be well developed, present in most cells, and not be occasionally visualized in certain areas of hyphae. It is possible that the lack of systematic visualization of the actin system is due to imperfections in our methods for fluorescent labeling of actin. So, phalloidin in many fungi does not work or labels actin very poor; and the expression of actin in the same ORF with a fluorescent protein leads to artifacts and poorly identifies actin cables (Heath et al., 2003; Takeshita, Fisher, 2019). Mycologists more often use either lifeact fused with a fluorescent protein or expression of tropomyosin with a fluorescent tag (Delgado-Alvarez et al., 2010; Lichius et al., 2011; Berg et al., 2016; Takeshita, Fisher, 2019; Garduño-Rosales et al., 2022). However, labeled lifeact can also affect the assembly of actin cables and there is evidence that in animals it does not label all types of actin structures (Lichius et al., 2011); and instead of tropomyosin, other crosslinkers may be included in the cables involved in the curtain model. It is possible that the actin cables in question are even more dynamic than the cables in the apices – and their rapid assembly/disassembly makes visualization even more difficult. It is also possible that we are not looking for exactly what we need – the regulation of plasma membrane tension and the form-restraining function may not be performed by powerful actin cables, which are easy to visualize microscopically, but rather by a network of very thin cables/filaments (
Figure 1A). Indeed, in the work of Garduño-Rosales et al. (2022) the authors indicate that in the hyphae of
Trichoderma atroviride there are individual cables that are part of “a very fine network of actin filaments that run the entire length of the hyphal tube” (also see the supplementary videos for this work). By the way, assumptions about the presence in the cytoplasm of hyphae of a dense meshwork of actin, not detectable by available means, were proposed back in the 80s of the last century (McKerracher, Heath, 1987).
4. Component II: The Cell Wall
The fungal cell wall is another important component of the curtain model. The fungal cell wall paradigm has undergone some changes recently. It became obvious that the cell wall is not a permanently rigid and low-permeability outer shell of the fungal cell. It is a dynamic complex structure that quickly changes its elasticity, easily changes its size and internal structure, and is permeable, if necessary, not only for small molecules, but also for larger particles, extracellular vesicles and even bacteria (Casadevall, Gow, 2022; Gow et al., 2023). Casadevall and Gow (2022) even proposed to abandon the usual term and rename the cell wall (to cell plegma or reticulum, for example).
4.1. The Structure of the Yeast Cell Wall
The molecular composition of the cell wall varies in different groups of fungi, but the main components in ascomycetes and basidiomycetes are generally similar: these are the glucans, chitin and glycoproteins (Kang et al., 2018; Panstruga et al., 2023). In Candida albicans, and other model yeasts, the cell wall consists of two main layers. The inner layer contains long fibrils of chitin and β-1-3-glucans cross-linked with each other. This layer is responsible for the degree of elasticity of the cell wall. The higher the level of fibril cross-linking here and the longer the chitin fibrils, the rigider the cell wall will be (Lenardon et al., 2020; Gow, Lenardon, 2023). It is this layer of the cell wall that thickens in response to hyperosmotic shock: it is assumed that crosslinks between fibrils are destroyed, and β-1-3-glucan fibrils are compressed along their axis. As a result, the chitin-glucan layer expands and creeps onto the mannanoprotein outer layer of the cell wall (Morris et al., 1986; Slaninova et al., 2000; Elhasi, Blomberg, 2019; Gow, Lenardon, 2023). If the chitin-glucan layer is relatively weakly cross-linked (in the case of C. albicans this will depend, for example, on the carbon source in the nutrient medium), then it itself will expand less during hyperosmotic shock and will practically not lead to an increase in the overall thickness of the cell wall (more precisely, the wall thickens in any case during osmotic shock, since the cell shrinks, but not so much). If the inner layer is relatively rigid, then in the hyperphase it can expand several times and lead to a significant thickening of the wall as a whole (Ene et al., 2015). It is known that yeast with such a more cross-linked chitin-glucan layer tolerates hyperosmotic shock better. It is believed that this is due to a lower rate of cell shrinkage under hypertonic conditions due to the greater rigidity of the cell wall (Ene et al., 2015; Gow, Lenardon, 2023). However, perhaps, here is more important the greater expansion of the wall – another version of the airbag – the expanding cell wall in a shrinking cell, follows the protoplast faster, does not allow the protoplast to tear away from the wall, and reduces the damageability of the plasma membrane.
The outer layer of the yeast cell wall is represented by mannanoproteins. The proteins are anchored in the chitin-glucan layer or less often in plasma membrane, and their long N-mannan branched chains form the negative charged (due to phosphomannans – individual N-mannan side chains are linked to other chains via phosphate) hydrophobic outer layer of the wall. The mannanoprotein layer is responsible for the relative hydrophobicity of the cell surface (partly replacing hydrophobins, which are absent in yeast), limited permeability of the cell wall for large molecules, and protects the inner rigid layer from lytic enzymes (Gow, Lenardon, 2023; Lopatukhin et al., 2024).
4.2. The Structure of the Cell Wall in Filamentous Fungi
The cell wall of filamentous fungi has been studied some less than that of yeast. Among the filamentous ascomycetes,
A. fumigatus, to a lesser extent
N. crassa, and other aspergilli have been mainly studied (Kang et al., 2018; Patel, Free, 2019; Chihara et al., 2020; Garcia-Rubio et al., 2020; Chakraborty et al., 2021; Latge, Wang, 2022; Lamon et al., 2023; Fernando et al., 2023). The following differences can be distinguished between ascomycete yeasts and filamentous ascomycetes, but it must be borne in mind that many questions are not completely resolved and pluralistic: (i) in the cell wall of filamentous ascomycetes, α-1-3-glucans are of great importance, which is little or absent in yeast. Based on the ssNMR (solid-state nuclear magnetic resonance) method, Kang and colleagues (2018) showed that in the inner rigid layer of the cell wall of
A. fumigatus, α-1-3-glucans are closely associated with chitin, and not β-1-3-glucans (but β-1-3-glucans are also present in significant amounts). This ssNMR model has been further developed, and now a number of authors believe that α-1-3-glucan is the most rigid molecule in the aspergillus cell wall, even more rigid than chitin. However, in the outer layer of the wall the α-1-3-glucan fibrils are also found, but here they are shorter and less rigid than in the inner layer and serve a different function. Modern ssNMR model assumes that α-1-3-glucan-chitin complexes are embedded in a soft, hydrogenated matrix of frequently branched β-1-3/β-1-6-glucans and short linear β-1-3/β-1-4-glucan filaments. These same glucans, together with mannans, form the mobile outer layer of the cell wall (Latge, Wang, 2022; Fernando et al., 2023). Other authors also note the presence of α-1-3-glucans in the cell wall of
A. fumigatus and
N. crassa, although they attribute less structural significance to it (Patel, Free, 2019; Chihara et al., 2020; Garcia-Rubio et al., 2020). Using fluorescent probes, it was shown that in
A. orizae the outside of the cell wall is labeled with the α-1-3-glucan probe, while in
Pleurotus ostreatus the outside is labeled with the β-glucan one, which may indicate that in ascomycetes α-1-3-glucan, at least in the outer layer of the wall more than in basidiomycetes (Nakazawa et al., 2024). Fernando and colleagues (2023) showed that in several halotolerant aspergillus species, at optimal growth concentrations of sodium chloride, there is no α-1-3-glucan in the cell wall. It appears only when the nutrient medium is highly salinized. This suggests that which glucan, α-1-3 or β-1-3, plays a role of rigid core in the cell wall is species- and condition-specific. (ii) It is possible that yeast and filamentous ascomycetes have different cross-linking glucans. It is traditionally believed that in yeast, both mannanoproteins and non-protein mannans (they are less common than mannanoproteins in yeast) bind to the β-1-3-glucan-chitin core through β-1-6-glucans. Actually, β-1-3-glucans and chitin themselves are cross-linked by β-1-6-glucans (Kang et al., 2018; Gow, Lenardon, 2023). Some of the authors believe that filamentous ascomycetes do not have β-1-6-glucans, and their function is performed by β-1-3/β-1-4-glucans (lichenin; Patel, Free, 2019; Chihara et al., 2020; Garcia-Rubio et al., 2020). On the other hand, Kang and colleagues (2018) attributed the cross-linking functions to β-1-4- and β-1-6-glucans in
A. fumigatus. It is possible that cross-linking is carried out not only by linear β-1-3/β-1-4-glucan fibrils, but also by branched β-1-3/β-1-6- glucans (Chakraborty et al., 2021; Fernando et al., 2023). (iii) The mannans of yeasts and filamentous ascomycetes also differ. First, yeast N-mannans can be very long (more than 100 mannose residues) and form, as mentioned above, the outer mannanoprotein layer of the yeast cell wall. Filamentous ascomycetes also have many N-mannans, including in the outer layer of the cell wall, but they are not so long (Tefsen et al., 2012; Patel, Free, 2019). However, non-protein-bound filamentous fungal mannans (FTGM – fungal-type galactomannans) can also be very long (the main chain can consist of 9-10 repeats of a module of several mannose quartets with an α-1-3 linkage, with an α-1-6-connection between the quartets; Chihara et al., 2020; Garcia-Rubio et al., 2020). Second, unlike yeast, in filamentous ascomycetes, mannans are represented by galactomannans. Both short N- and O-galactomannans and long branched non-protein-associated FTGMs are composed of mannose backbones with α-1-3- and α-1-6-linkages, and galactofuranose side chains with β-1-5- and β-1-6 bonds. In short N-mannans, galactofuranose can be represented by single terminal molecules (Tefsen et al., 2012; Patel, Free, 2019; Chihara et al., 2020). Also in
A. fumigatus, in addition to galactomannans, the outer layer of cell wall contains galactosaminogalactan (a polymer of galactose with an α-1-4 bond and N-acetylgalactosamine; Fontaine et al., 2011; Chihara et al., 2020; Garcia-Rubio et al., 2020). It has been shown that the amount of galactosaminogalactan in the cell wall increases with increasing salinity of the external environment in halotolerant aspergillus, and it is also greater in the germinating than in the dormant conidia of
A. fumigatus, indicating important functions of amino sugars in the cell wall remodeling (Lamon et al., 2023; Fernando et al., 2023). (iv) Mannans bind differently to glucans and the glucan-chitin complex in yeasts and filamentous ascomycetes. It is assumed that most yeast mannanoproteins are GPI-APs (glycophosphatidylinositol anchored proteins). GPI anchors can have various modifications, but in fungi they most often represent inositolphosphoceramide with two chains of fatty acids, connected through glucosamine and short mannan with either the C-terminus of the protein through phosphoethanolamide, or without ethanolamide with galactomannan (in filamentous fungi; Tefsen et al., 2012; Komath et al., 2022; Guo, Kundu, 2024). The main function of the GPI anchor is to bind a glycoprotein or galactomannan to the plasma membrane by inserting ceramide fatty chains into the outer lipid layer of the membrane (
Figure 1C). However, many yeast GPI anchors serve to attach mannanoproteins through β-1-6-glucan to the β-1-3-glucan-chitin complex (Patel, Free, 2019; Gow, Lenardon, 2023). It is assumed that such a bond is formed through the binding of the first mannose (closest to glucosamine and phosphatidylinositol) with β-1-6-glucan (De Groot et al., 2005; Komath et al., 2022). It is not clear whether the glucosamine-inositol phosphoceramide residue is lost or remains in the mannanoprotein-glucan complex. Also in yeast, mannanoproteins can be PIR proteins and covalently bind through glutamine in their PIR repeats to β-1-3-glucans or through disulfide bridges in their cysteine rich domains to other cell wall proteins (De Groot et al., 2005; Montano-Silva et al., 2024). In filamentous ascomycetes, GPI most often serves to anchor glycoproteins and non-protein-bound galactomannans/galactosamineglucans to the plasma membrane. If glycoproteins are associated with the glucan-chitin complex, it is rarely through a PIR or GPI connection. Perhaps such a connection occurs through the terminal galactofuranose residues of short N- or O-galactomannans, which bind to the crosslinking glucan, and through it to the glucan-chitin complex (Tefsen et al., 2012; Patel, Free, 2019; Gow, Lenardon, 2023).
The cell wall of filamentous basidiomycetes has been studied even less well than the wall of ascomycetes (
Figure 1B,C). There are classical and modern (ssNMR based) works devoted to the structure of the cell wall of
Schizophyllum commune, and some data regarding the structure of the wall of
P. ostreatus (Sietsma, Wessels, 1977; Ehren et al., 2020; Safeer et al., 2023; Nakazawa et al., 2024). No fundamental differences from the cell wall of filamentous ascomycetes are shown but there is still some dissimilarity. ssNMR analysis showed that the internal rigid layer of the cell wall in
S. commune, like in ascomycetes, is represented by the α-1-3-glucan-chitin complex (Ehren et al., 2020; Safeer et al., 2023). The complex is crosslinked and embedded in a matrix of β-1-3/β-1-6-glucans (schizophyllan) and short linear β-1-3- and β-1-6-glucans and mannans (
Figure 1C). These same cross-linking glucans form the outer mobile layer of the cell wall. In addition, short cross-linking α-glucans and branched mannans are present in the outer layer. N-acetylgalactosamine and N-galactosamine were detected, which may indicate that the mobility and hydrophilicity of the outer layer of the wall, as in ascomycetes, can be regulated, among other things, by changes in the content of aminopolysaccharides. Perhaps an important feature of the cell wall of
S. commune (and other basidiomycetes) is the presence of fucans in the inner layer (Ehren et al., 2020; Safeer et al., 2023). It is possible that fucose replaces galactofuranose in basidiomycetes, but further research is needed. There is evidence that there is also a lot of fucose in the cell walls of zygomycete fungi (Yugueros et al., 2024).
4.3. The Curtain Model and Mechanics of the Cell Wall
There is the concept of elastic strain of the cell wall (D). D is the degree of rapid/instantaneous deformation of the cell wall under the influence of a certain force/pressure (Zhao et al., 2005; Municio-Diaz et al., 2022). For filamentous fungi, D is most often roughly estimated through the change in hyphal radius, expressed as a percentage: 100x(R1-R0)/R0, where R0 is the initial radius of the hypha (before applying/changing force), R1 is the final radius (after applying/changing force; Atilgan et al., 2015; Davi et al., 2019; Municio-Diaz et al., 2022; Chevalier et al., 2023, 2024). ∆R is convenient to use to estimate D, first, because turgor is used simplistically as tensile pressure, neglecting other forces acting on the fungal hyphae, and turgor acts perpendicular to the cell surface, that is, the pressure vector coincides with the radius of the cell. Second, stretching of the cell wall along the long axis of the hypha can be neglected. It is assumed that the cell wall of fungi is isotropic (its elasticity is the same along and across), therefore the shrinkage (extension) of the hypha in length is always two times less than the change in the diameter of the hypha (Atilgan et al., 2015; Municio-Diaz et al., 2022). In addition, the real hypha is most often adhered to the substrate, which means that there is significant resistance to shrinkage-extension of the hypha along the long, but not transverse, axis. If to take the radius of the hypha Rr as R0, the cell wall of which is in a completely relaxed state (zero turgor, no other acting forces), then D = 100x(R1-Rr)/Rr ~ (PxRr)/(ExT). Here P is turgor pressure (more precisely ∆P = P1-Pr, but Pr = 0), E – Young’s modulus or elastic modulus, T – cell wall thickness. In other words, how much the hypha expands and what radius it reaches (R1) will be directly proportional to the magnitude of turgor and the thickness of the hypha itself, and inversely proportional to the elasticity of the cell wall. Of course, this dependence is relatively linear only in a certain range of P values. At high P values, the resistance of the cell wall material will slow down the elongation; at values close to zero, perhaps, too. In addition, at least T (and maybe E) is not, strictly speaking, a constant and depends on the degree of rapid/instantaneous stretching of the wall (the more the elastic material is stretched, the thinner it becomes). Therefore, it is better to present the formula in a simplified form: D = 100x(R1-Rr)/Rr ~ (PxRr)/(ErxTr), where Er and Tr correspond to the elasticity of the relaxed wall. It should also be taken into account that R1 may be less than Rr – under conditions of high hyperosmoticity, the protoplast tightly adhered to the cell wall compresses the cell wall (something like negative turgor is obtained; Atilgan et al., 2015; Chevalier et al., 2023, 2024).
The elasticity of the cell wall is an intrinsic property of the wall itself; it depends on the elastic modulus and wall thickness. Physical meaning E – the potential ability of the cell wall to elastically deform, depending on the structure, composition, degree of cross-linking and melanization, and other properties of the cell wall, calculated per unit cell wall thickness (Municio-Diaz et al., 2022; Chevalier et al., 2023, 2024). The higher E, the stiffer the cell wall and the more difficult it is to deform (Lew, Nasserifar, 2009; Lew, 2019; Tsugawa et al., 2022). However, E can be compensated for by wall thickness, but with certain limitations – a soft but thick cell wall will lose its elasticity, but a rigid and thin wall may become brittle rather than more elastic. E in fungi can vary from several MPa to several hundred MPa (Lew, 2019; Municio-Diaz et al., 2022). It should be taken also into account that both E and T values can change dynamically even within a few minutes, especially in the apical cells of the hyphae (Chevalier et al., 2023). However, these changes are not associated with the physical expand/shrinkage of the hyphae, which were mentioned just above, but with fast physiological remodeling of the cell wall. This is exactly how the yeast cell wall thickens under hyperosmotic conditions – not just physical thickening of the wall occurs due to the removal of its stretching, but internal rearrangements of the glucan-chitin and mannan layers (Ene et al., 2015; Gow, Lenardon, 2023).
Let's go back to the top of the formula (PxRr)/(ErxTr). The dependence of D on the size of the hypha itself (Rr) has not been unambiguously confirmed in real experiments. In our experiments, C. comatus hyphae shrinkaged in Triton X-100 (cells are permeabilized and protoplasts are detached from the cell wall – approximately, a relaxed state of the cell wall) by about 13%. In R. solani, in which the hyphae are on average twice as thick as the hyphae of C. comatus, under the same conditions by 10-11%. At the same time, the shrinkage in 0.6 M sorbitol in R. solani was slightly higher than the shrinkage in Triton X-100, but in C. comatus they did not differ, which suggests that on average, the turgor of R. solani is slightly lower than that of C. comatus (Mazheika et al., 2024 preprint). However, it is doubtful that increasing turgor in R. solani to the level of C. comatus will lead to a significant increase in R1. In the work of Chevalier and colleagues (2024), in the apical cells of hyphae using a deflation assay, there is also no correlation between D and Rr. On the other hand, in the above examples, it is possible there is leveling the effect of hyphal size on strain by the difference in the elasticity of the cell wall in different species of fungi.
Finally, the curtain model allows making an important amendment to the formula. Due to the shape-restraining function of actin, D will depend not only on turgor, but also on the total force (A) applied by the actin cytoskeleton to the cell wall. In contrast to turgor, A can be either positive or negative when R>Rr, depending on whether the actin cytoskeleton restrains the expansion of the hypha or, conversely, prevents it from shrinkage. Also, unlike turgor, A is a force, not pressure, its vector can be directed at different angles to the cell wall, therefore, in addition to the projection perpendicular to the surface of the cell wall, expanding/shrinkaging the hypha as turgor, A has projections of vectors directed parallel surface of the cell wall. Such vector projections stretch/compress the cell wall unevenly and in different directions. The effect of A on the cell wall will depend on the number of FACs/FASs. If, for simplicity, we take into account only the perpendicular projections A to the cell wall and do not take into account the discreteness of FACs/FASs, then formula D will take the following form: D ~ ((P + AxS)xRrxk)/(ErxTr). Here S is the area of the inner surface of the cell wall, and k is a reduction factor for Rr (since the actual effect of hyphal size on D is not so significant). A can be either positive or negative; if at A<0, |A|>P, the cell will shrink.
4.4. Is Rapid Thickening of the Cell Wall the Airbag Like in Yeast?
For yeast cells, changes in the thickness of the cell wall during cell shrinkage/expansion play an important role; perhaps, as mentioned above, it is one of the airbag variant in response to environmental variability (Ene et al., 2015; Gow, Lenardon, 2023). It is not known exactly whether the thickness and structure of the cell wall changes in the first minutes of hyperosmotic shock in filamentous fungi. Due to the relatively small thickness of the cell wall compared to the diameter and size of the hyphae, such changes are difficult to detect using conventional light microscopy. There is only information that the inner side of the cell wall, in contact with the periplasmic space, is loosened (
Figure 1B; Mazheika et al., 2020a). In addition, the work of Chevalier and colleagues (2023), using a “sandwich” of two fluorophores, showed that the wall thickness in the apical cell of
A. nidulans can change rapidly, but there is no data on the relationship between hyperosmotic shock and wall thickness. TEM study of
A. nidulans showed that in a hypertonic environment, thickening of the cell wall does not occur (Zhao et al., 2005). However, the authors grew mycelium in a medium with high osmolarity, and did not add an osmotic agent immediately before analysis – the fungus could have time to adapt to hypertonic conditions. On the contrary, the work of Fernando and colleagues (2023) showed that in halotolerant aspergillus the thickness of the cell wall during mycelial growth on 2 M sodium chloride increases almost 1.5 times relative to growth on 0.5 M salt (largely due to the outer mobile layer of the wall). However, here it is too not talking about instant changes in the fungal cell when the osmolarity of the external environment changes, but about gradual adjustment to conditions during growth. In any case, halotolerant aspergilli, like ascomycete yeasts, do not have α-1-3-glucans in the rigid layer of the cell wall. It is possible that in fungi with β-1-3-glucans in the inner layer, as the main linear polymer forming a complex with chitin, the cell wall is capable of greater rapid thickening than in fungi with α-1-3-glucans, due to the greater rigidity of the latter compared to β-1-3-glucans. Accordingly, we hypothesize that in filamentous fungi the rapid wall thickening in response to hyperosmotic shock is not as pronounced as in yeast. Some changes in thickness and composition occur; as already mentioned, cell expansion/shrinkage is in any case accompanied by a change in wall thickness, but the airbags in filamentous fungi are different. These will be discussed in the following sections.
4.5. Cell Wall as a Curtain Rod of the Curtain Model
The next section will discuss two variants for the curtain model. In one case, FACs/FASs are universal elements and they, like rings on a curtain rod, should move comparably freely along the inner surface of the cell wall under actin cables control. For such a mechanism to work, not only certain properties of FACs are required (discussed in the next section), but also an appropriate cell wall structure. It can be assumed that basidiomycetes and other filamentous fungi have an additional innermost sublayer of the cell wall. It is this that is loosened under hyperosmotic conditions, carried away by the adhesive bonds of the membrane and the wall during shrinkage of the hypha. It is possible that in this sublayer, glucan fibrils have a small number of crosslinks and branches, which facilitates the sliding between them of protein domains responsible for adhesion (see CW3 in
Figure 1C).
5. Component III: Plasma Membrane Adhesion to the Cell Wall
5.1. Plasmolysis and Changes in Cell Size with Changes in Osmotic and Other Conditions
Mycological works indicate that the fungal plasma membrane is firmly adhered to the cell wall and plasmolysis (detachment of the plasma membrane from the cell wall, for example in a hyperosmotic environment) is more difficult to achieve than in plants (Walker, White, 2017). This is confirmed by the overestimation of the results of measuring turgor by the method of primary (incipient) plasmolysis in comparison with direct measurement methods (Heath, Steinberg, 1999; Lew et al., 2004). In addition, indicative in this regard are turgor measurements using a deflation assay. The shrinkage of a fungal cell at high osmolarity levels of the medium is often twice as high (in radius) as the shrinkage of a perforated cell (microdamaged by laser; Atilgan et al., 2015; Chevalier et al., 2024). This may mean that the protoplast tightly adhered to the cell wall, shrinkaging under hyperosmotic conditions, drags the cell wall with it, compressing it further relative to the relaxed state. However, other interpretations of this effect are possible. For example, the hydrophobic polysaccharide matrix of the cell wall can itself compact under hyperosmotic conditions.
In the cells of higher plants during hyperosmotic shock, various types of plasmolysis are possible (see Oparka, 1994). In any case, the protoplast begins to gradually detach from the cell wall, shrinking, but remaining connected to the wall through thin Hechtian strands and wider connections – the Hechtian reticulum. These connections to the cell wall are permeated with actin cables (Hechtian strands) and microtubules (Hechtian reticulum), the connection involves the cortical endoplasmic reticulum, and the connection with the cell wall occurs through FACs/FASs. Shrinkage of the protoplast can take several tens of minutes. The cell wall usually does not change its size (Morris et al., 1986; Oparka, 1994; Cheng et al., 2017).
In fungi under hyperosmotic conditions, some things happen differently. First, the fungal cell begins to shrink entirely, along with the cell wall, and this happens quite quickly, within seconds, a maximum of several minutes (Morris et al., 1986; Lew, Nasserifar, 2009; Mazheika et al., 2024 preprint). A yeast cell, depending on the osmolarity of the external environment, can lose up to 70% in volume (which corresponds to a decrease in cell diameter by about 30-40%). At the same time, the thickness of the cell wall of the yeast cell can rapidly increase as described earlier (Morris et al., 1986; Slaninova et al., 2000; Atilgan et al., 2015; Ene et al., 2015; Elhasi, Blomberg, 2019; Gow, Lenardon, 2023). In filamentous fungi, the diameter of hyphae under moderate osmotic shock decreases by 10-25% (values are given from two publications, for several species of fungi and for certain concentrations of osmolytics – for other species of fungi and for other hyperosmotic conditions, other shrinkage values are possible; Lew, Nasserifar, 2009; Mazheika et al., 2024 preprint). There are studies that measured the shrinkage of apical cells in several species of filamentous fungi (mainly ascomycetes) at different osmolarity levels of the medium (Chevalier et al., 2023, 2024). There the reduction in radius reaches about 40%, but it is difficult to say whether this result will work for non-apical hyphal cells.
Second, plasmolysis in fungi, under moderate osmotic shock, occurs rarely, only in individual cells (but if it happens, it is usually faster than in plants). Fungi have no analogues of Hechtian strands, although if concave-type plasmolysis occurs (see Oparka, 1994), the plasma membrane remains connected to the cell wall at separate points (some FACs/FASs, probably), more or less evenly distributed along the perimeter of the cell. Between these points, the membrane detaches from the cell wall (Mazheika et al., 2020a; Mazheika et al., 2024 preprint). However, fungal plasmolysis strongly depends on environmental conditions, the state of the cell and can be species-specific. So, for example, in S. hirsutum, if the mycelium grown on an agarized nutrient medium is not immediately placed in a hyperosmotic liquid medium (with 0.6 M sorbitol), but after preincubation in the same growth medium, but without agar, then the probability of concave plasmolysis increases (Mazheika et al., 2024 preprint). This is probably due to the fact that the pressure in aerial hyphae coated with hydrophobins, forming part of a colony growing on a solid medium, is higher than in hyphae immersed in liquid or in agar gel, even if the composition of solid and liquid nutrient media is the same (the difference only in agar). Another example, it turned out that R. solani has its own mechanism for quickly responding to a nonequilibrium change in conditions – in this fungus, under moderate hyperosmotic stress, rapid concave plasmolysis occurs in many cells, but within ten minutes after being placed in a hyperosmotic environment, the protoplast restores its shape (Mazheika et al., 2024 preprint). This is another airbag variant.
5.2. What is the Mechanism of Tight Adhesion between the Plasma Membrane and the Cell Wall in Fungi?
In animal cells, the plasma membrane adheres to the ECM (extracellular matrix) using receptor proteins collectively called integrins. These are heterodimeric transmembrane proteins, the outer domains of which bind with affinity to various ECM ligands, and the cytoplasmic domains are associated with actin through adapter proteins (talin, for example). In the activated state, integrins cluster in the plasma membrane in focal adhesion complexes (FACs) and form focal adhesion sites (FASs). Depending on the adhesion force, the size and frequency of adhesive complexes will vary, up to fibrillar adhesion (Elhasi, Blomberg, 2019; Kechagia et al., 2019). In fungi, homologs of integrins are not found (there are only short regions of homology), but it is assumed that there are integrin-like proteins that also provide FACs/FASs. Confirmation of the existence of integrin-like proteins is indirect – it has been shown that in some fungi and oomycetes antibodies to animal integrins give positive signal; RGD peptides can act on fungi (they inhibit signal transduction and adhesion in animal cells, since RGD is a common motif in ligands to which integrins bind), etc. (Kaminskyj, Heath, 1995; Heath, Steinberg, 1999; Chitcholtan, Garrill, 2005; Elhasi, Blomberg, 2019).
It is assumed that in fungi, the functions of integrins can be performed by various mechanosensors, which are the initial step of the CWI pathway (Elhasi, Blomberg, 2019). Several types of cell wall mechanosensors are known in yeasts and filamentous ascomycetes. The main ones are Wsc- and Mid-type mechanosensors and signaling mucins (Yoshimi et al., 2022). Most often, the mechanosensors consist of an extracellular domain embedded in the cell wall, a transmembrane domain, and a short intracellular domain. The extracellular domain is highly O-glucanosylated, and in some sensors, it is also N-glucanosylated. In other words, the sensory domain is similar to a brush inserted between cell wall fibrils, in which branched bristles (O- and N-glucans) interact with glucans and mannanoproteins of the cell wall (see Mech and Mech-FAC in
Figure 1C,D). Typically, extracellular domains contain serine and threonine rich regions, which act as nanosprings, compressing and stretching under the influence of forces arising from perturbations in the cell wall. The signal is transmitted to the cytoplasmic tail of the sensor, which changes its conformation, then Rho1 GTFase is activated and various CWI pathways are launched (Kock et al., 2015; Elhasi, Blomberg, 2019; Yoshimi et al., 2022; Gow, Lenardon, 2023). Elhasi and Blomberg (2019) indicate that yeast Wsc1 is associated with F-actin through a protein complex Sla1 and Sla2. It is likely that Wsc1 is responsible for the disassembly of actin cables during osmotic shock or destruction of the cell wall. The Sla1/Sla2 complex is a functional analogue of human talin (by the way, chytrid fungi have real homologues of talin; Prostak et al., 2021). Hinze and Boucrot (2018) believe that Sla1 and Sla2 are homologues of the human F-BAR proteins intersectin and Hip1/R, respectively. In clathrin-dependent endocytosis in animals and yeast, they regulate actin assembly in pits/patches. In
S. pombe, Sla2 is involved in the polar organization of actin during cytokinesis (Castagnetti et al., 2005). Fungal Sla2, animal Hip1/R, and talin belong to the same superfamily of actin-binding proteins that have an I/LWEQ module at the C-terminus. It is this module in talin that is responsible for binding with F-actin; without this module, focal adhesion is impossible (Smith, McCann, 2007). It is likely that Sla1 mimics the N-terminus of talin, bearing in talin domains that bind to the inner side of the plasma membrane and integrins. Accordingly, actin, through adapter proteins like Sla1/Sla2, can cluster mechanosensors in the plasma membrane into FACs, ensuring focal adhesion at specific membrane sites (indeed, in yeast it has been shown that Wsc1 is often clustered in the plasma membrane;
Figure 1C,D; Heinisch et al., 2010). Moreover, due to the transmembrane domains of mechanosensors (or other proteins) clustered in the plasma membrane, when such a cluster is moved by actin along the cell wall, lateral resistance of the lipid bilayer is created, which makes it possible to change the tension of the membrane. There is evidence that in
A. nidulans strains deficient in WscA (a homolog of yeast Wsc1), cells swell more often under hyperosmotic conditions (Futagami et al., 2011). This result may indicate that actin in
A. nidulans performs a shape-restraining function through WscA-FACs. Unfortunately, cell wall mechanosensors in filamentous basidiomycetes have been practically not studied. In
Ustilago maydis and
Cryptococcus neoformans, only signaling mucins are known as mechanosensors (Yoshimi et al., 2022).
5.3. Different Variants of the Curtain Model, Differing in the Mechanism of Interaction between Actin, the Plasma Membrane and the Cell Wall
In accordance with the curtain model (with one of its variants), FACs/FASs should slide along the inner surface of the cell wall. This means that the variant with animal integrins, which affinity bound to ligands ECM is not suitable for fungi because the strength of the affinity bond is difficult to regulate, a strong bond will not allow FACs/FASs to be motile and in any case, even a weak affinity bond is specific to its ligand – having become detached from it, the protein will lose contact with the cell wall. Mechanoreceptors or other adhesive proteins have to be connected to the inner layer of the cell wall or the special sublayer, which was described in the previous sections, by bonds similar to hydrophobic ones – not strong and of little specificity. In principle, if the brush of O- and N-glucans, described above for fungal mechanosensors, does not form covalent bonds with glucan fibrils of the cell wall, then it may well provide a strong (in the perpendicular direction to the wall fibrils), but mobile (in the parallel direction) fixation of the sensor in the cell wall.
A different variant for organizing FACs/FASs is possible (
Figure 1D). As already described above, in filamentous fungi, some cell wall proteins, as well as some non-protein-bound mannans, are associated with the plasma membrane through GPI anchors (Tefsen et al., 2012; Komath et al., 2022; Guo, Kundu, 2024). For animal cells, there is a model for the formation of lipid rafts in which actin, through adapter proteins, interacts with phosphatidylserine located in the inner leaflet of the plasma membrane. Actin clusters phosphatidylserine, which in turn interact in the middle of the lipid bilayer through its long saturated fatty acid chains with chains of GPI-APs. As a result, GPI-APs and glycosphingolipids cluster in the outer leaflet of the membrane, and lipid rafts are formed (Saha et al., 2016; Komath et al., 2022). In the case of animal cells, such clustering of GPI-APs does not have mechanical functions – it regulates various properties of the plasma membrane and is involved in the transmission of signals into the cell (Saha et al., 2016). However, in fungi, it is possible to form GPI-FACs and they correspond to the characteristics of the curtain model if the lateral resistance of the lipid bilayer to the movement of the GPI-APs cluster by actin is sufficient to regulate membrane tension. Interestingly,
N. crassa has a specific cell wall mechanosensor, HAM-7, which lacks the typical transmembrane and intracellular domains but is inserted into the plasma membrane via a GPI anchor (Maddi et al., 2012).
Finally, else another variant for the curtain model is possible. Until now, we have accepted that actin performs both the regulation of membrane tension and the shape-restraining function through the same FACs/FASs. However, this may not be the case. It is possible that actin changes or maintains cell size by interacting with the cell wall through the proteins FACs/FASs (mechanoreceptors or GPI-APs). In this case, FACs/FASs proteins may not be mobile and may even be anchored in the cell wall by covalent bonds. Actin can regulate the tension of the plasma membrane by interacting with plasma membrane proteins not associated with the cell wall. Most likely, such proteins also cluster in the plasma membrane to create sufficient resistance to the lipid bilayer and ensure its tension under the action of actin (
Figure 1D, right side).
However, strong adhesion of the plasma membrane to the cell wall and the rarity of plasmolysis in fungi may be ensured not only by FACs/FASs. Single adhesion proteins can be distributed between the clusters of actin-associated mechanosensors and GPI-APs. They are not necessarily actin-associated and not directly involved in regulating membrane tension or maintaining cell shape. They provide additional adhesion that prevents membrane detachment from the cell wall between FASs under moderate osmotic stress (see GPI-glucan in
Figure 1C).
6. Component IV: Plasma Membrane Macroinvagination Systems
6.1. Macroinvaginations and Endocytosis
In both plants and yeast and filamentous fungi, invaginations of the plasma membrane are formed during osmotic shock and other stresses, and in a number of fungi, the invaginations are resident of cells (Morris et al., 1986; Oparka, 1994; Slaninova et al., 2000; Bitsikas et al., 2011; Ene et al., 2015; Elhasi, Blomberg, 2019; Mazheika, Kamzolkina, 2021; Mazheika et al., 2022). It does not matter whether the entire cell shrinks, as in fungi, or just the protoplast, as in plants, but when the cell/protoplast volume rapidly decreases, the excess plasma membrane must be structurally packaged to avoid membrane damage and maintain the functionality of the remaining unpacked membrane. Such packaging is provided by special structures (in some cases having a rather complex organization) – plasma membrane invaginations, or, more correctly – macroinvaginations, since their sizes can range from a couple of hundred nanometers to tens of micrometers, which differentiates them from fungal endocytic pins, the size of which rarely exceeds 100 nm (Mazheika, Kamzolkina, 2021). Macroinvaginations also differ from endocytic pins in their negative rather than positive connection with actin (depolymerization of actin often stimulates formation of macroinvaginations rather than blocking them), and their ambiguous connection with endocytosis (see below; Oparka, 1994; Yamashita and May, 1998; Lee et al., 2007; Upadhyay and Shaw, 2008; Lewis et al., 2009; Mazheika et al., 2020a, 2022). Many authors on plants and fungi have found that the lumen of macroinvaginations is labeled with calcofluor or primulin, and on TEM macroinvaginations may contain material similar to the cell wall material (Oparka, 1994; Slaninova et al., 2000; Elhasi, Blomberg, 2019). In this regard, it was previously assumed that macroinvaginations are invaginations of the cell wall itself into the cell (Morris et al., 1986). In fact, as discussed earlier, this phenomenon is a consequence of the tight adhesion of the plasma membrane to the cell wall (the shrinking protoplast pulls in the loosening inner layer of the cell wall to macroinvagination). However, it cannot be ruled out that during a strong hyperosmotic shock, when the cell wall passes the point of its relaxed state and begins to shrink further along with the protoplast, the cell wall deforms and its excess actually can protrudes into the cell.
The question of the relationship between endocytosis and macroinvaginations does not have a clear answer (Mazheika, Kamzolkina, 2021). In early studies on plants, it was assumed that macroinvaginations, after restoration of turgor and deplasmolysis, are scissored from the plasma membrane but, perhaps, do not follow the classical endocytotic path, merging with endosomes and vacuoles (Oparka, 1994). It was even proposed a separate term for the described process – osmocytosis (Robinson, Milliner, 1990). Using time-lapse video and labeling of the mycelium of S. hirsutum and other xylotrophic basidiomycetes by AM4-64 (a styryl fluorophore, an analogue of FM4-64, which embedds in the lipid bilayer of the plasma membrane), it was shown that macroendocytosis (the mechanism of which is obviously different from macropinocytosis in animals, which justifies the introduction of a separate term) takes place. However, this process is not frequent, it occurs only with certain types of macroinvaginations, and the further fate of the detached membrane structure is not known (Mazheika et al., 2022).
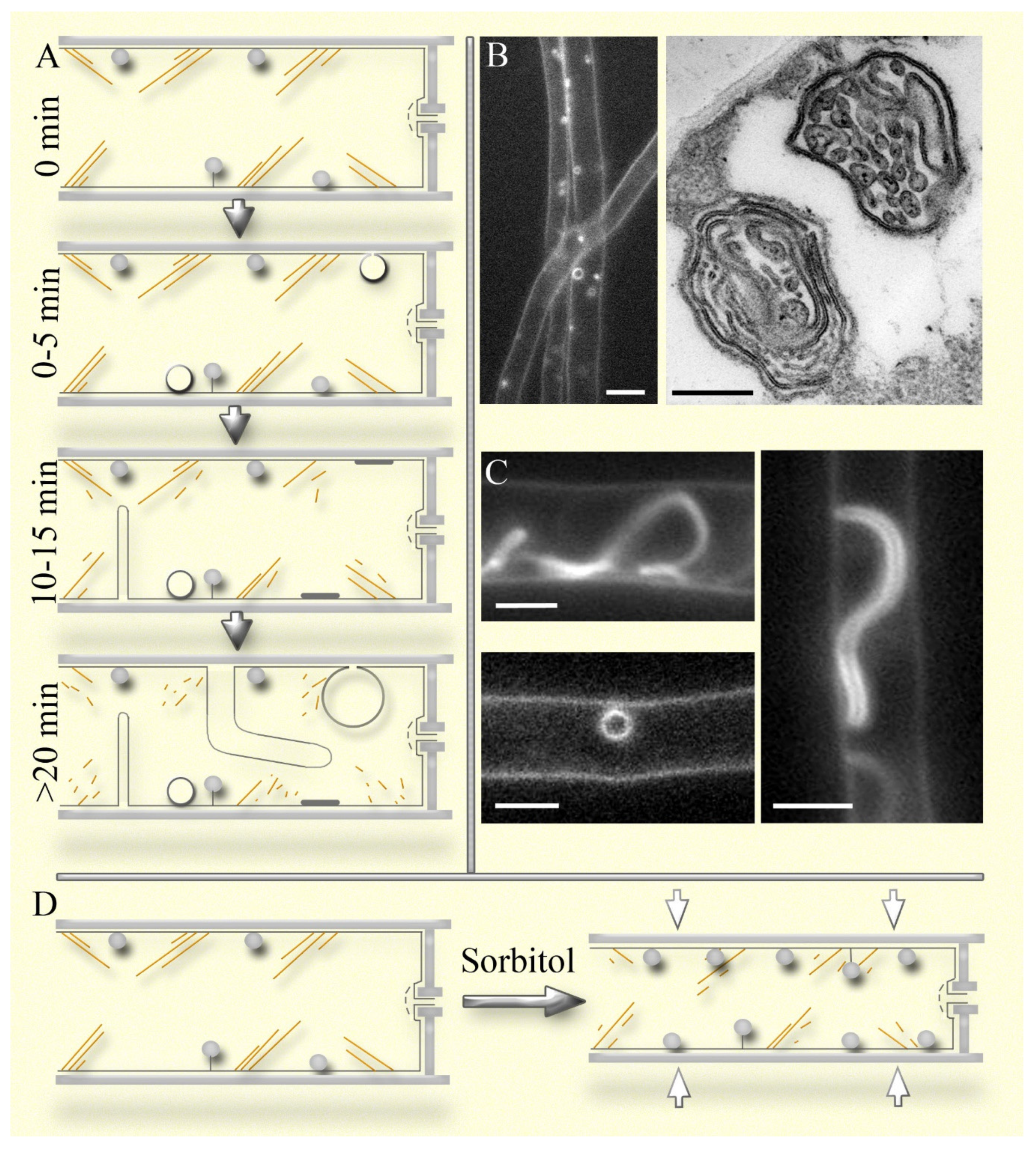
In our earlier works, we to some extent mistook different types of AM4-64-labeled macroinvaginations as structures of different steps in the endocytic pathway (Kamzolkina et al., 2017; Mazheika et al., 2020a, b). Small parietal dense lomasomes resemble endocytic pins, small vesicles and focal sections through transverse thin tubes – endosomes, large vesicular macroinvaginations and focal sections through thick tubes – vacuoles, into which endosomes delivered AM4-64. The similarity with the endocytic pathway is enhanced by the gradual appearance of the listed types of macroinvaginations in a microscopic slide being observed: for example, lomasomes are labeled in the slide immediately, and thick tubular and large vesicular macroinvaginations appear about twenty minutes after preparation of the microscopic sample (
Figure 2A; Mazheika et al., 2022). This phenomenon is due to the stressful conditions in which the microscopic sample is located: a thin layer of liquid, mechanical impact of the cover glass, gradual drying, etc. Most likely, disassembly or remodeling of the actin cytoskeleton begins in the microscopic specimen mycelium (
Figure 2A). This is why the use of latrunculin A does not affect the formation of the late large macroinvaginations (F-actin is already disassembled at this stage), but significantly affects early ones, as previously described. However, large macroinvaginations should not be classified as artifacts. The stressful conditions of the microscopic specimen are not unique; most likely, the fungus encounters similar conditions in nature, which means it forms large macroinvaginations not only in a laboratory. Moreover, the presence of macroinvaginations and their imitation of the endocytic pathway does not mean that fungi lack true endocytosis in non-apical cells. It was shown that among the large vacuole-like macroinvaginations labeled with AM4-64, VLCs (vacuole-lysosome candidates) were found (Mazheika et al., 2022). They have a weaker AM4-64 signal than macroinvaginations, and some of them are co-labeled with CFDA (vacuolar probe). There are 13 times fewer such VLCs than large macroinvaginations, and 60 times fewer than large vacuoles in the hyphae of
S. hirsutum. This result indicates that the vacuolar system of fungi, under normal conditions, is only to a small extent involved in the process of degradation of substances coming from the outside and from the plasma membrane by endocytosis (but the situation probably changes if mass autophagy is triggered in the hyphae).
6.2. Two Types of Macroinvagination Systems in Fungi
Plasma membrane macroinvaginations in fungi can be different, but during formation, they all go through the stage of a tube or lamella of varying thickness (from a thin filament or lamella of several tens of nanometers to a tube more than a micrometers thick; Mazheika et al., 2022). We distinguish two types of macroinvagination systems in fungi: simple and complex ones. Previously, we did not separate the concepts of two systems of macroinvaginations and two types of fungal endocytosis – classical one and macroendocytosis (Mazheika et al., 2020a, b). The simple macroinvagination system is characteristic of yeasts, mycelial ascomycetes, and some basidiomycetes, for example,
R. solani. In the simple system, macroinvaginations are not large: tubes or lamellae usually do not exceed five µm in length; rounded macroinvaginations rarely exceed half a micrometer in diameter (Mazheika et al., 2020a). Secondary invagination in macroinvaginations (formation of true lomasomes) is rare. In xylotrophic basidiomycetes (most likely also in mycorrhizal basidiomycetes, but no data) the system of macroinvaginations is complex. They have up to seven different types of macroinvaginations (all of them are described in Mazheika et al., 2022; also see
Figure 2A–C). The most common type is small parietal macroinvaginations that do not have a lumen when fluorescently labeled (they look like small dense balls pressed against the plasma membrane or attached to the membrane by a filamentous tube or lamella;
Figure 2A,B). At the electron microscopic level, they have a complex internal structure, most often consisting of secondary invaginations in the form of lamellae and tubes that fill the lumen of the primary invagination (
Figure 2B, right side). These are classic fungal lomasomes (Moore, McAlear, 1961; Brushaber, Jenkins, 1971; Mazheika, Kamzolkina, 2021). Lomasomes are always present in xylotroph cells, at least when the hypha is in an aquatic environment (in an airy or low-moisture environment, they may be absent or few, but this is an open question). During moderate hyperosmotic shock or actin depolymerization, their number increases 2-3 times, under certain conditions up to 5 times, and can reach up to fifty lomasomes per cell (
Figure 2D; Mazheika et al., 2022). Probably, in xylotrophs, it is the lomasomes (other macroinvaginations to a lesser extent) that are the main components of the curtain model, instantly forming new lomasomes and increasing the size and complexity of the existing ones with slight cell shrinkage, and disbanding lomasomes during the equilibrium expansion of the hypha. The disbandment occurs as follows: lomasomes are stretched into flat plaques – the primary invagination turns inside out, as it were, exposing the internal secondary invaginations into the periplasmic space (Mazheika et al., 2022). Lomasomes are also elements of the airbags during strong osmotic changes, but here, apparently, other types of macroinvaginations are also involved, which are structurally not as complex as lomasomes, but due to their size they are able to quickly deposit or release a significant surface area of the plasma membrane.
6.3. What is the Mechanism of Formation of Macroinvaginations?
A separate question that has not yet been studied at all is: what is the molecular mechanism of the formation of fungal macroinvaginations? Obviously, actin does not directly participate in the generation of macroinvaginations, since its depolymerization enhances the formation of most of them (Mazheika et al., 2020a; Mazheika et al., 2022). Actin regulates the formation of macroinvaginations indirectly through the regulation of plasma membrane tension and cell size. Macroinvaginations formed from thin tubes and lamellae (lomasomes, thin tubes, etc.) are probably formed with the participation of dynein-like proteins and/or BAR proteins. It is known that these proteins not only form the neck of tubular membrane invaginations, but with low membrane tension they can also form the invagination itself without the forceful participation of actin (Mooren et al., 2012; Hinze and Boucrot, 2018; Renard et al., 2018; Mazheika et al., 2020a). At the same time, if for a simple thin tube/lamella it may be sufficient to form the protein scaffold for neck of invagination, then for the assembly of a complex lomasome, with many secondary invaginations, lamellar or tubular, or a mixture of them (
Figure 2B, right side), complex regulation and a certain topology of proteins are needed. It is not known whether dynamin-like and BAR proteins can participate in the formation of thick macroinvagination tubes, the thickness of which in
S. hirsutum reaches two or more micrometers (
Figure 2A,C). It is believed that these proteins are associated with nano-scale membrane curvatures, but septins can perform similar functions, but be associated with micron-scale curvatures. Septins are conserved cytoskeletal proteins, often serving as a scaffold for actin structures in fungi. Unlike actin, septins can bind directly to the lipid membrane without adapter proteins; can form long filaments and recognize large curvatures in the plasma membrane (Bridges et al., 2016). It has been shown that in liposomes, septins can cause the formation of relatively thick tubes (about 0.5 μm thick; Tanaka-Takiguchi et al., 2009). Further research is needed, but septins are a perspective candidate for involvement in the formation of large macroinvaginations.
7. Non-Equilibrium States Not Controlled by the Curtain Model, Examples of the Airbags
As already mentioned, the curtain model describes an equilibrium state: in cells with low or moderate turgor, actin takes on the functions of regulating of plasma membrane tension and the frameworking function that prevents the cell from quickly changing its size during small shifts in osmolarity. In addition, the actin cytoskeleton with other components of the curtain model probably aligns the hypha along its axis if there are different turgor pressures in different parts of it. Maybe actin even takes part in creating the mass flow inside the hyphae. However, everything alters with a non-equilibrium change in external conditions: a sharp shift in the osmolarity of the substrate, rapid drying/watering of the substrate, the transition of the hypha from the aqueous to the air phase, or vice versa, mechanical stress, aggressive influence of the host or competitor, etc. Since such changes can occur frequently, and all of them can lead to a sharp change in osmotic pressure, the fungus not only needs to avoid cellular damage, but also minimize the period of time in which it loses physiological functionality. In this regard, all fungi from ascomycete yeast to basidiomycetes have chosen a single general strategy: with osmotic changes or changes in the permeability of the plasma membrane, they instantly change the diameter of the hyphae (or yeast cell); herewith the cell changes its volume as a single whole – the cell wall along with the protoplast (Morris et al., 1986; Lew, Nasserifar, 2009; Atilgan et al., 2015; Chevalier et al., 2024; Mazheika et al., 2020a, 2024 preprint). In conjunction with all this, the plasma membrane forms macroinvaginations, trying to maintain its integrity and functionality (Mazheika et al., 2022). However, with the general strategy, different groups of fungi have their own private protective mechanisms: as we called them above – airbags – structural-physiological defense mechanisms that are the first to respond to stress and prevent the cell from damage in the first seconds or minutes of shock exposure. Thus, in yeast, an important element of the airbag is the cell wall itself, which can quickly be structurally rearranged and significantly change its thickness (more than with just physical compression of the wall), reducing the risk of damage to the protoplast during cell shrinkage/expansion (Morris et al., 1986; Slaninova et al., 2000; Atilgan et al., 2015; Ene et al., 2015; Elhasi, Blomberg, 2019; Gow, Lenardon, 2023).
In basidiomycete xylotrophs, an important component of the airbag is the complex system of macroinvaginations of the plasma membrane. Lomasome-type macroinvaginations are not only constantly present in xylotroph cells, instantly responding to changes in plasma membrane tension, including significant, but also have a complex secondary structure, allowing more membrane to be deposited in a small-volume lomasome. In addition, if lomasomes fail to maintain the integrity and functionality of the plasma membrane under severe stress, larger, although less complex, macroinvaginations of the plasma membrane are formed (Mazheika et al., 2022).
R. solani is a phytopathogenic/endophytic fungus. The lifestyle in the tissues of living plants apparently means greater stability of osmotic conditions than that of the xylotroph in soil or dead wood. Hence, there is another principle of the airbag operation. The form-restaining function of actin in this fungus is less pronounced, and the macroinvagination system is simpler than in xylotrophic basidiomycetes. On the other hand, it was shown that in the case of a sharp change in osmotic conditions, rapid reversible concave plasmolysis occurs in R. solani cells (Mazheika et al., 2024 preprint). The fungus loses functionality for several minutes (in a medium with 0.6 M sorbitol, such plasmolysis lasts no more than 8-10 minutes), but maintains the integrity of the membrane. In fact, such concave plasmolysis is a primitive analogue of large macroinvaginations in xylotrophs (it is even possible that large macroinvaginations evolved evolutionarily on the basis of the rapid concave plasmolysis); it protects the plasma membrane from damage in the first minutes of hyperosmotic shock.
Of course, there are many mechanisms for the instantaneous physiological-structural response of a fungus to severe stress (airbags); only a few examples are given above. Extensive research is needed among different ecological-trophic groups of fungi in order to elucidate strategies for immediate stress physiological-structural resistance.
8. Conclusion and Perspectives
The core idea voiced in the Introduction that fungi, especially xylotrophic basidiomycetes, which are the most important components of the forest ecological communities of our planet, cannot afford to depend heavily on turgor, is appropriate to repeat at the end of this paper. The idea of a fungal hypha as a tightly inflated bicycle tube inserted into a tire is becoming outdated. The physiology of the filamentous fungus is very complex, and, in fact, we are only on the threshold of understanding the mechanisms of intrahyphal transport, signal transmission, oscillatory phenomena, stress resistance, aging and others. The curtain model is an attempt at a holistic approach to one of the many physiological aspects in mycology.
Many elements of the curtain model are speculative and require experimental research. First, it is necessary to obtain the highest quality fluorescence microscopic images of the actin cytoskeleton in non-apical basidiomycete cells. It is necessary to understand what the structural nature of the control actin elements is: large actin cables or a thin dense mesh, dynamic filaments or a relatively stable structure, etc. It is important to understand what is the fundamental difference between fungi such as R. solani and S. hirsutum – why disassembly of F-actin in R. solani does not affect the size of hyphae, but in S. hirsutum it does. Secondly, it needs to find out what the mechanism of interaction between actin, the plasma membrane and the cell wall is. Are mechanoreceptors or GPI-APs or other elements involved here; whether membrane tension and cell size support are controlled separately, or jointly, etc.? Third, it is important to continue studying the cell wall of basidiomycetes. Many problems here have not yet been resolved. In particular, research is needed into the inner layer of the cell wall adjacent to the periplasmic space – whether it can serve as a curtain rod or, conversely, whether focal complexes are stationary in it. Finally, it is necessary to elucidate the molecular mechanisms of the plasma membrane macroinvagination formation. For decades, mycologists have been observing lomasomes and other macroinvaginations in fungi. Only now is the nature and function of these structures beginning to become clearer. However, which proteins are involved in their formation and how all this is regulated remains unclear.
Paradigm shifts in fungal physiology have a major impact on applied mycology, plant pathology and medicine. There is a change in approaches and new prospects are opening up in the protection of forests and the fight against diseases of plants, animals and humans.
Acknowledgements
The authors dedicate this work to the memory of the remarkable Russian cytogeneticist, teacher and friend, Prof. Oksana Kolomiets. The research was carried out as part of the Scientific Project of the State Order of the Government of Russian Federation to Lomonosov Moscow State University No 121032300079–4 and VIGG RAS No 22022600162–0. The Moscow University Development Program (UDP-10) provided part of the equipment for this study.
Abbreviations
CWI – cell wall integrity (pathway), ECM – extracellular matrix, FACs/FASs – focal adhesion complexes/focal adhesion sites, FTGM – fungal-type galactomannans, GPI-APs – glycophosphatidylinositol anchored proteins, ssNMR – solid-state nuclear magnetic resonance, TEM – transmission electron microscopy
References
- Abadeh, A. and Lew, R.R., 2013. Mass flow and velocity profiles in Neurospora hyphae: partial plug flow dominates intra-hyphal transport. Microbiology, 159(Pt_11), pp.2386-2394. [CrossRef]
- Atilgan, E., Magidson, V., Khodjakov, A. and Chang, F., 2015. Morphogenesis of the fission yeast cell through cell wall expansion. Current Biology, 25(16), pp.2150-2157.
- Berepiki, A., Lichius, A., Read, N.D., 2011. Actin organization and dynamics in filamentous fungi. Nat. Rev. Microbiol. 9, 876–887.
- Bergs, A., Ishitsuka, Y., Evangelinos, M., Nienhaus, G.U. and Takeshita, N., 2016. Dynamics of actin cables in polarized growth of the filamentous fungus Aspergillus nidulans. Frontiers in microbiology, 7, p.682.
- Bitsikas, V., Karachaliou, M., Gournas, C. and Diallinas, G. 2011. Hypertonic conditions trigger transient plasmolysis, growth arrest and blockage of transporter endocytosis in Aspergillus nidulans and Saccharomyces cerevisiae. Mol. Membr. Biol. 28, 54–68.
- Bleichrodt, R.J., Hulsman, M., Wösten, H.A. and Reinders, M.J., 2015. Switching from a unicellular to multicellular organization in an Aspergillus niger hypha. MBio, 6(2), pp.10-1128.
- Bleichrodt, R.J., Hulsman, M., Wösten, H.A. and Reinders, M.J., 2015. Switching from a unicellular to multicellular organization in an Aspergillus niger hypha. MBio, 6(2), pp.10-1128.
- Bridges, A.A., Jentzsch, M.S., Oakes, P.W., Occhipinti, P. and Gladfelter, A.S., 2016. Micron-scale plasma membrane curvature is recognized by the septin cytoskeleton. The Journal of cell biology, 213(1), p.23.
- Brushaber, J.A. and Jenkins Jr, S.F., 1971. Lomasomes and vesicles in Poria monticola. Canadian Journal of Botany, 49(12), pp.2075-2079.
- Casadevall, A. and Gow, N.A., 2022. Ending the (Cell) wall metaphor in microbiology. The Cell Surface, 8, pp.100087.
- Castagnetti, S., Behrens, R. and Nurse, P., 2005. End4/Sla2 is involved in establishment of a new growth zone in Schizosaccharomyces pombe. Journal of cell science, 118(9), pp.1843-1850.
- Chakraborty, A., Fernando, L.D., Fang, W., Dickwella Widanage, M.C., Wei, P., Jin, C., Fontaine, T., Latgé, J.P. and Wang, T., 2021. A molecular vision of fungal cell wall organization by functional genomics and solid-state NMR. Nature communications, 12(1), p.6346.
- Chen Y, Liu J, Kang S, Wei D, Fan Y, Xiang M and Liu X, 2023. A palisade-shaped membrane reservoir is required for rapid ring cell inflation in Drechslerella dactyloides. Nat. Commun. 14: 7376.
- Chevalier, L., Klingelschmitt, F., Mousseron, L. and Minc, N., 2024. Mechanical Strategies Supporting Growth and Size Diversity in Filamentous Fungi. Molecular Biology of the Cell, pp.mbc-E24.
- Chevalier, L., Pinar, M., Le Borgne, R., Durieu, C., Peñalva, M.A., Boudaoud, A. and Minc, N., 2023. Cell wall dynamics stabilize tip growth in a filamentous fungus. PLoS Biology, 21(1), p.e3001981.
- Chihara, Y., Tanaka, Y., Izumi, M., Hagiwara, D., Watanabe, A., Takegawa, K., Kamei, K., Shibata, N., Ohta, K. and Oka, T., 2020. Biosynthesis of β-(1→ 5)-galactofuranosyl chains of fungal-type and O-mannose-type galactomannans within the invasive pathogen aspergillus fumigatus. Msphere, 5(1), pp.10-1128.
- Chitcholtan, K. and Garrill, A., 2005. A β4 integrin-like protein co-localises with a phosphotyrosine containing protein in the oomycete Achlya bisexualis: inhibition of tyrosine phosphorylation slows tip growth. Fungal genetics and biology, 42(6), pp.534-545. [CrossRef]
- Dagdas, Y.F., Yoshino, K., Dagdas, G., Ryder, L.S., Bielska, E., Steinberg, G. et al. 2012. Septin- mediated plant cell invasion by the rice blast fungus, Magnaporthe oryzae. Science, 336, 1590– 1595.
- Davì, V., Chevalier, L., Guo, H., Tanimoto, H., Barrett, K., Couturier, E., Boudaoud, A. and Minc, N., 2019. Systematic mapping of cell wall mechanics in the regulation of cell morphogenesis. Proceedings of the National Academy of Sciences, 116(28), pp.13833-13838.
- De Groot, P.W., Ram, A.F. and Klis, F.M., 2005. Features and functions of covalently linked proteins in fungal cell walls. Fungal genetics and biology, 42(8), pp.657-675.
- de Oliveira HC, Rossi SA, García-Barbazán I, Zaragoza Ó and Trevijano-Contador N, 2021. Cell wall integrity pathway involved in morphogenesis, virulence and antifungal susceptibility in Cryptococcus neoformans. J. Fungi. 7: 831.
- Delgado-Alvarez, D.L., Callejas-Negrete, O.A., Gómez, N., Freitag, M., Roberson, R.W., Smith, L.G. and Mouriño-Pérez, R.R., 2010. Visualization of F-actin localization and dynamics with live cell markers in Neurospora crassa. Fungal Genetics and Biology, 47(7), pp.573-586.
- Ehren, H.L., Appels, F.V., Houben, K., Renault, M.A., Wösten, H.A. and Baldus, M., 2020. Characterization of the cell wall of a mushroom forming fungus at atomic resolution using solid-state NMR spectroscopy. The Cell Surface, 6, p.100046.
- Elhasi, T. and Blomberg, A., 2019. Integrins in disguise-mechanosensors in Saccharomyces cerevisiae as functional integrin analogues. Microbial cell, 6(8), p.335.
- Ene, I.V., Walker, L.A., Schiavone, M., Lee, K.K., Martin-Yken, H., Dague, E., Gow, N.A., Munro, C.A. and Brown, A.J., 2015. Cell wall remodeling enzymes modulate fungal cell wall elasticity and osmotic stress resistance. MBio, 6(4), pp.10-1128.
- Fernando, L.D., Pérez-Llano, Y., Dickwella Widanage, M.C., Jacob, A., Martínez-Ávila, L., Lipton, A.S., Gunde-Cimerman, N., Latgé, J.P., Batista-García, R.A. and Wang, T., 2023. Structural adaptation of fungal cell wall in hypersaline environment. Nature Communications, 14(1), p.7082.
- Fontaine, T., Delangle, A., Simenel, C., Coddeville, B., van Vliet, S.J., Van Kooyk, Y., Bozza, S., Moretti, S., Schwarz, F., Trichot, C. and Aebi, M., 2011. Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS pathogens, 7(11), p.e1002372.
- Fukuda, S., Yamamoto, R., Yanagisawa, N., Takaya, N., Sato, Y., Riquelme, M. and Takeshita, N., 2021. Trade-off between plasticity and velocity in mycelial growth. MBio, 12(2), pp.10-1128.
- Futagami, T., Nakao, S., Kido, Y., Oka, T., Kajiwara, Y., Takashita, H., Omori, T., Furukawa, K. and Goto, M., 2011. Putative stress sensors WscA and WscB are involved in hypo-osmotic and acidic pH stress tolerance in Aspergillus nidulans. Eukaryotic cell, 10(11), pp.1504-1515.
- Garcia-Rubio, R., de Oliveira, H.C., Rivera, J. and Trevijano-Contador, N., 2020. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Frontiers in microbiology, 10, p.2993.
- Garduño-Rosales, M., Callejas-Negrete, O.A., Medina-Castellanos, E., Bartnicki-García, S., Herrera-Estrella, A. and Mouriño-Pérez, R.R., 2022. F-actin dynamics following mechanical injury of Trichoderma atroviride and Neurospora crassa hyphae. Fungal Genetics and Biology, 159, p.103672. [CrossRef]
- Gorfer, M., Tarkka, M.T., Hanif, M., Pardo, A.G., Laitiainen, E. and Raudaskoski, M., 2001. Characterization of small GTPases Cdc42 and Rac and the relationship between Cdc42 and actin cytoskeleton in vegetative and ectomycorrhizal hyphae of Suillus bovinus. Molecular plant-microbe interactions, 14(2), pp.135-144.
- Gow, N.A. and Lenardon, M.D., 2023. Architecture of the dynamic fungal cell wall. Nature Reviews Microbiology, 21(4), pp.248-259.
- Gow, N.A., Casadevall, A. and Fang, W., 2023. Top five unanswered questions in fungal cell surface research. The Cell Surface, 10, p.100114.
- Guo, Z. and Kundu, S., 2024. Recent research progress in glycosylphosphatidylinositol-anchored protein biosynthesis, chemical/chemoenzymatic synthesis, and interaction with the cell membrane. Current opinion in chemical biology, 78, p.102421.
- Gupta, G.D. and Heath, I.B. 1997. Actin disruption by latrunculin B causes turgor-related changes in tip growth of Saprolegnia ferax hyphae. Fungal Genet. Biol. 21, 64–75.
- Harold, R.L., Money, N.P. and Harold, F.M., 1996. Growth and morphogenesis in Saprolegnia ferax: is turgor required?. Protoplasma, 191, pp.105-114.
- Heath, I. and Steinberg, G. 1999. Mechanisms of hyphal tip growth: tube dwelling amebae revisited. Fungal Genet. Biol. 28: 79–93.
- Heath, I.B., 1990. The roles of actin in tip growth of fungi. In International Review of Cytology (Vol. 123, pp. 95-127). Academic Press.
- Heath, I.B., Bonham, M., Akram, A. and Gupta, G.D., 2003. The interrelationships of actin and hyphal tip growth in the ascomycete Geotrichum candidum. Fungal Genetics and Biology, 38(1), pp.85-97.
- Heinisch, J.J., Dupres, V., Wilk, S., Jendretzki, A. and Dufrêne, Y.F., 2010. Single-molecule atomic force microscopy reveals clustering of the yeast plasma-membrane sensor Wsc1. PLoS One, 5(6), p.e11104.
- Herman, K.C. and Bleichrodt, R., 2022. Go with the flow: mechanisms driving water transport during vegetative growth and fruiting. Fungal Biology Reviews, 41, pp.10-23.
- Hinze, C. and Boucrot, E., 2018. Local actin polymerization during endocytic carrier formation. Biochemical Society Transactions, 46(3), pp.565-576.
- Jung, E.M., Kothe, E. and Raudaskoski, M., 2018. The making of a mushroom: Mitosis, nuclear migration and the actin network. Fungal Genetics and Biology, 111, pp.85-91.
- Kaminskyj, S.G. and Heath, I.B., 1995. Integrin and spectrin homologues, and cytoplasm-wall adhesion in tip growth. Journal of cell science, 108(2), pp.849-856.
- Kamzolkina, O.V., Kiselica, M.A., Kudryavtseva, O.A., Shtaer, O.V. and Mazheika, I.S., 2017. Endocytosis and its inhibitors in basidiomycetous fungus Rhizoctonia solani. Moscow University Biological Sciences Bulletin, 72, pp.128-136.
- Kang, X., Kirui, A., Muszyński, A., Widanage, M.C.D., Chen, A., Azadi, P., Wang, P., Mentink-Vigier, F. and Wang, T., 2018. Molecular architecture of fungal cell walls revealed by solid-state NMR. Nature communications, 9(1), p.2747.
- Kang, X., Kirui, A., Muszyński, A., Widanage, M.C.D., Chen, A., Azadi, P., Wang, P., Mentink-Vigier, F. and Wang, T., 2018. Molecular architecture of fungal cell walls revealed by solid-state NMR. Nature communications, 9(1), p.2747.
- Kechagia, J.Z., Ivaska, J. and Roca-Cusachs, P., 2019. Integrins as biomechanical sensors of the microenvironment. Nature reviews Molecular cell biology, 20(8), pp.457-473.
- Kock, C., Dufrêne, Y.F. and Heinisch, J.J., 2015. Up against the wall: is yeast cell wall integrity ensured by mechanosensing in plasma membrane microdomains?. Applied and environmental microbiology, 81(3), pp.806-811.
- Kopecká, M., Kawamoto, S. and Yamaguchi, M., 2013. A new F-actin structure in fungi: actin ring formation around the cell nucleus of Cryptococcus neoformans. Microscopy, 62(2), pp.295-301.
- Lamon, G., Lends, A., Valsecchi, I., Wong, S.S.W., Duprès, V., Lafont, F., Tolchard, J., Schmitt, C., Mallet, A., Grélard, A. and Morvan, E., 2023. Solid-state NMR molecular snapshots of Aspergillus fumigatus cell wall architecture during a conidial morphotype transition. Proceedings of the National Academy of Sciences, 120(6), p.e2212003120.
- Latge, J.P. and Wang, T., 2022. Modern biophysics redefines our understanding of fungal cell wall structure, complexity, and dynamics. Mbio, 13(3), pp.e01145-22.
- Lee, M.T., Szeto, C.Y.Y., Ng, T.P. and Kwan, H.S. 2007. Endocytosis in the shiitake mushroom Lentinula edodes and involvement of GTPase LeRAB7. Eukaryot. Cell. 6, 2406–2418.
- Lenardon, M.D., Sood, P., Dorfmueller, H.C., Brown, A.J. and Gow, N.A., 2020. Scalar nanostructure of the Candida albicans cell wall; a molecular, cellular and ultrastructural analysis and interpretation. The Cell Surface, 6, p.100047. [CrossRef]
- Lew, R.R. 2019. Biomechanics of Hyphal Growth. In: Hoffmeister, D., Gressler, M. (eds) Biology of the Fungal Cell. The Mycota, vol 8. Springer, Cham., pp.83-94.
- Lew, R.R. and Nasserifar, S., 2009. Transient responses during hyperosmotic shock in the filamentous fungus Neurospora crassa. Microbiology, 155(3), pp.903-911.
- Lew, R.R., 2010. Turgor and net ion flux responses to activation of the osmotic MAP kinase cascade by fludioxonil in the filamentous fungus Neurospora crassa. Fungal Genetics and Biology, 47(8), pp.721-726.
- Lew, R.R., Levina, N.N., Walker, S.K. and Garrill, A. 2004. Turgor regulation in hyphal organisms. Fungal Genet. Biol. 41, 1007–1015.
- Lewis, M.W., Robalino, I.V. and Keyhani, N.O. 2009. Uptake of the fluorescent probe FM4-64 by hyphae and haemolymph-derived in vivo hyphal bodies of the entomopathogenic fungus Beauveria bassiana. Microbiology 155, 3110–3120.
- Li, H., Zhang, Z., Qin, G., He, C., Li, B. and Tian, S., 2020. Actin is required for cellular development and virulence of Botrytis cinerea via the mediation of secretory proteins. Msystems, 5(1), pp.10-1128.
- Lichius, A., Berepiki, A. and Read, N.D., 2011. Form follows function–the versatile fungal cytoskeleton. Fungal biology, 115(6), pp.518-540.
- Lilje, O. and Lilje, E., 2006. Comparative imaging of the vacuolar reticulum of Saprolegnia ferax. In Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues IV, SPIE. Vol. 6088, pp. 401-411.
- Lopatukhin, E.V., Ihalainen, Y.A., Markelova, N.N., Kuvarina, A.E. and Sadykova, V.S., 2024. Fungal Hydrophobins: Biosynthesis, Properties, and Possibilities of Application in Biotechnology. Applied Biochemistry and Microbiology, 60(3), pp.372-382.
- Maddi, A., Dettman, A., Fu, C., Seiler, S. and Free, S.J., 2012. WSC-1 and HAM-7 are MAK-1 MAP kinase pathway sensors required for cell wall integrity and hyphal fusion in Neurospora crassa.
- Mazheika, I., Voronko, O., Kamzolkina, O. 2020a. Early endocytosis as a key to understanding mechanisms of plasma membrane tension regulation in filamentous fungi. Biology of the Cell. 112:409–426.
- Mazheika, I., Voronko, O., Kudryavtseva, O., Novoselova, D., Pozdnyakov, L., Mukhin, V., Kolomiets, O. and Kamzolkina, O., 2020b. Nitrogen-obtaining and-conserving strategies in xylotrophic basidiomycetes. Mycologia, 112(3), pp.455-473.
- Mazheika, I.S. and Kamzolkina, O.V., 2021. Does macrovesicular endocytosis occur in fungal hyphae?. Fungal Biology Reviews, 38, pp.1-8.
- Mazheika, I.S., Psurtseva, N.V. and Kamzolkina, O.V., 2022. Lomasomes and other fungal plasma membrane macroinvaginations have a tubular and lamellar genesis. Journal of Fungi, 8(12), p.1316.
- Mazheika, I.S., Voronko, O.V., Kolomiets, O.L. and Kamzolkina, O.V., 2024. Instantaneous change in hyphal diameter in basidiomycete fungi. bioRxiv (preprint), pp.2024-02.
- McKerracher, L.J. and Heath, I.B., 1987. Cytoplasmic migration and intracellular organelle movements during tip growth of fungal hyphae. Experimental Mycology, 11(2), pp.79-100.
- Montano-Silva, P., Callejas-Negrete, O.A., Pereira-Santana, A. and Verdin, J., 2024. Cell wall-resident PIR proteins show an inverted architecture in Neurospora crassa, but keep their role as wall stabilizers. bioRxiv, pp.2024-07.
- Moore, R.T. and McAlear, J.H., 1961. Fine structure of Mycota. 5. Lomasomes – previously uncharacterized hyphal structures. Mycologia, 53(2), pp.194-200.
- Mooren, O.L., Galletta, B.J. and Cooper, J.A. (2012) Roles for actin assembly in endocytosis. Annu. Rev. Biochem. 81, 661–686.
- Morris, G.J., Winters, L., Coulson, G.E. and Clarke, K.J., 1986. Effect of osmotic stress on the ultrastructure and viability of the yeast Saccharomyces cerevisiae. Microbiology, 132(7), pp.2023-2034.
- Municio-Diaz, C., Muller, E., Drevensek, S., Fruleux, A., Lorenzetti, E., Boudaoud, A. and Minc, N., 2022. Mechanobiology of the cell wall–insights from tip-growing plant and fungal cells. Journal of Cell Science, 135(21), p.jcs259208.
- Nakazawa, T., Kawauchi, M., Otsuka, Y., Han, J., Koshi, D., Schiphof, K., Ramírez, L., Pisabarro, A.G. and Honda, Y., 2024. Pleurotus ostreatus as a model mushroom in genetics, cell biology, and material sciences. Applied Microbiology and Biotechnology, 108(1), p.217.
- Panstruga, R., Antonin, W. and Lichius, A., 2023. Looking outside the box: A comparative cross-kingdom view on the cell biology of the three major lineages of eukaryotic multicellular life. Cellular and Molecular Life Sciences, 80(8), p.198.
- Patel, P.K. and Free, S.J., 2019. The genetics and biochemistry of cell wall structure and synthesis in Neurospora crassa, a model filamentous fungus. Frontiers in microbiology, 10, p.2294.
- Pelling, A.E., Sehati, S., Gralla, E.B., Valentine, J.S. and Gimzewski, J.K., 2004. Local nanomechanical motion of the cell wall of Saccharomyces cerevisiae. Science, 305(5687), pp.1147-1150.
- Peñalva, M.A., Zhang, J., Xiang, X. and Pantazopoulou, A., 2017. Transport of fungal RAB11 secretory vesicles involves myosin-5, dynein/dynactin/p25, and kinesin-1 and is independent of kinesin-3. Molecular biology of the cell, 28(7), pp.947-961.
- Prostak, S.M., Robinson, K.A., Titus, M.A. and Fritz-Laylin, L.K., 2021. The actin networks of chytrid fungi reveal evolutionary loss of cytoskeletal complexity in the fungal kingdom. Current Biology, 31(6), pp.1192-1205.
- Raudaskoski, M., 2019. The central role of septa in the basidiomycete Schizophyllum commune hyphal morphogenesis. Fungal biology, 123(9), pp.638-649.
- Renard, H-F., Johannes, L. and Morsomme, P. (2018) Increasing diversity of biological membrane fission mechanisms. Trends. Cell Biol. 28, 274–286.
- Reynaga-Peña, C.G. and Bartnicki-García, S. (2005) Cytoplasmic contractions in growing fungal hyphae and their morphogenetic consequences. Arch. Microbiol. 183, 292–300.
- Roberson, R.W., 1992. The actin cytoskeleton in hyphal cells of Sclerotium rolfsii. Mycologia, 84(1), pp.41-51. [CrossRef]
- Robertson, N.F. and Rizvi, S.R.H., 1968. Some observations on the water-relations of the hyphae of Neurospora crassa. Annals of Botany, 32(2), pp.279-291.
- Robinson, D.G. and Milliner, S., 1990. Endocytosis in plants. Physiologia Plantarum, 79(1), pp.96-104.
- Ryder, L.S., Dagdas, Y.F., Kershaw, M.J., Venkataraman, C., Madzvamuse, A., Yan, X., Cruz-Mireles, N., Soanes, D.M., Oses-Ruiz, M., Styles, V. and Sklenar, J., 2019. A sensor kinase controls turgor-driven plant infection by the rice blast fungus. Nature, 574(7778), pp.423-427.
- Safeer, A., Kleijburg, F., Bahri, S., Beriashvili, D., Veldhuizen, E.J., van Neer, J., Tegelaar, M., de Cock, H., Wösten, H.A. and Baldus, M., 2023. Probing Cell-Surface Interactions in Fungal Cell Walls by High-Resolution 1H-Detected Solid-State NMR Spectroscopy. Chemistry–A European Journal, 29(1), p.e202202616.
- Saha, S., Anilkumar, A.A. and Mayor, S., 2016. GPI-anchored protein organization and dynamics at the cell surface. Journal of lipid research, 57(2), pp.159-175.
- Salo, V., Niini, S.S., Virtanen, I. and Raudaskoski, M., 1989. Comparative immunocytochemistry of the cytoskeleton in filamentous fungi with dikaryotic and multinucleate hyphae. Journal of Cell Science, 94(1), pp.11-24.
- Sietsma, J.H. and Wessels, J.G.H., 1977. Chemical analysis of the hyphal walls of Schizophyllum commune. Biochimica et Biophysica Acta (BBA)-General Subjects, 496(1), pp.225-239.
- Slaninova, I., Šesták, S., Svoboda, A. and Farkaš, V., 2000. Cell wall and cytoskeleton reorganization as the response to hyperosmotic shock in Saccharomyces cerevisiae. Archives of microbiology, 173, pp.245-252.
- Smith, S.J. and McCann, R.O., 2007. A C-terminal dimerization motif is required for focal adhesion targeting of Talin1 and the interaction of the Talin1 I/LWEQ module with F-actin. Biochemistry, 46(38), pp.10886-10898.
- Srinivasan, S., Vargas, M.M. and Roberson, R.W., 1996. Functional, organizational, and biochemical analysis of actin in hyphal tip cells of Allomyces macrogynus. Mycologia, 88(1), pp.57-70.
- Steinberg, G., Penalva, M.A., Riquelme, M., Wosten, H.A., Harris, S.D., 2017. Cell biology of hyphal growth. Microbiol. Spectr. 5, 1–34.
- Takeshita, N. 2019. Control of Actin and Calcium for Chitin Synthase Delivery to the Hyphal Tip of Aspergillus. In: Latgé, JP. (eds) The Fungal Cell Wall. Current Topics in Microbiology and Immunology, vol 425. Springer, Cham.
- Takeshita, N. and Fischer, R., 2019. The cytoskeleton and polarity markers during polarized growth of filamentous fungi. Biology of the Fungal Cell, pp.43-62.
- Takeshita, N., 2020. Control of actin and calcium for chitin synthase delivery to the hyphal tip of aspergillus. The Fungal Cell Wall: An Armour and a Weapon for Human Fungal Pathogens, pp.113-129. [CrossRef]
- Takeshita, N., Manck, R., Grün, N., de Vega, S.H. and Fischer, R., 2014. Interdependence of the actin and the microtubule cytoskeleton during fungal growth. Current Opinion in Microbiology, 20, pp.34-41. [CrossRef]
- Tanaka-Takiguchi, Y., Kinoshita, M. and Takiguchi, K., 2009. Septin-mediated uniform bracing of phospholipid membranes. Current Biology, 19(2), pp.140-145.
- Tefsen, B., Ram, A.F., Van Die, I. and Routier, F.H., 2012. Galactofuranose in eukaryotes: aspects of biosynthesis and functional impact. Glycobiology, 22(4), pp.456-469.
- Timonen, S., Peterson, R.L. 2002. Cytoskeleton in mycorrhizal symbiosis. In: Smith, S.E., Smith, F.A. (eds) Diversity and Integration in Mycorrhizas. Developments in Plant and Soil Sciences, vol 94. Springer, Dordrecht.
- TIMSONEN, S., FINAY, R.D., Söderström, B. and Raudaskoski, M., 1993. Identification of cytoskeletal components in pine ectomycorrhizas. New Phytologist, 124(1), pp.83-92.
- Torralba, S., Raudaskoski, M., Pedregosa, A.M. and Laborda, F., 1998. Effect of cytochalasin A on apical growth, actin cytoskeleton organization and enzyme secretion in Aspergillus nidulans. Microbiology, 144(1), pp.45-53.
- Tsugawa, S., Yamasaki, Y., Horiguchi, S., Zhang, T., Muto, T., Nakaso, Y., Ito, K., Takebayashi, R., Okano, K., Akita, E. and Yasukuni, R., 2022. Elastic shell theory for plant cell wall stiffness reveals contributions of cell wall elasticity and turgor pressure in AFM measurement. Scientific reports, 12(1), p.13044. [CrossRef]
- Upadhyay, S. and Shaw, B.D. 2008. The role of actin, fimbrin and endocytosis in growth of hyphae in Aspergillus nidulans. Mol. Microbiol. 68, 690–705.
- Walker, G.M. and White, N.A. 2017. Introduction to fungal physiology. In Fungi: Biology and Applications (Ed K. Kavanagh), John Wiley & Sons Inc. pp. 1–35.
- Walker, S.K., Chitcholtan, K., Christenhusz, G.M., Garrill, A., 2006. Invasive hyphal growth: an F-actin depleted zone is associated with invasive hyphae of the oomycetes Achlya bisexualis and Phytophthora cinnamomi. Fungal Genet. Biol. 43, 357–365.
- Wernet V, Kriegler M, Kumpost V, Mikut R, Hilbert L and Fischer R, 2023. Synchronization of oscillatory growth prepares fungal hyphae for fusion. Elife 12: e83310.
- Wernet, V., Herrero, S. and Fischer, R., 2021. Soft but not too soft—how a rigid tube expands without breaking. Mbio, 12(3), pp.10-1128.
- Xu, R., Li, Y.B., Liu, C., Shen, N., Zhang, Q., Cao, T., Qin, M., Han, L.B. and Tang, D., 2021. Twinfilin regulates actin assembly and Hexagonal peroxisome 1 (Hex1) localization in the pathogenesis of rice blast fungus Magnaporthe oryzae. Molecular Plant Pathology, 22(12), pp.1641-1655.
- Yamashita, R.A. and May, G.S. 1998. Constitutive activation of endocytosis by mutation of myoA, the myosin I gene of Aspergillus nidulans. J. Biol. Chem. 273, 14644–14648.
- Yoshimi, A., Miyazawa, K., Kawauchi, M. and Abe, K., 2022. Cell wall integrity and its industrial applications in filamentous fungi. Journal of fungi, 8(5), p.435.
- Yugueros, S.I., Peláez, J., Stajich, J.E., Fuertes-Rabanal, M., Sánchez-Vallet, A., Largo-Gosens, A. and Mélida, H., 2024. Study of fungal cell wall evolution through its monosaccharide composition: An insight into fungal species interacting with plants. The Cell Surface, p.100127.
- Zhao, L., Schaefer, D., Xu, H., Modi, S.J., LaCourse, W.R. and Marten, M.R., 2005. Elastic properties of the cell wall of Aspergillus nidulans studied with atomic force microscopy. Biotechnology progress, 21(1), pp.292-299. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).