Introduction
In late 2019, an outbreak of coronavirus disease 2019 (COVID-19) emerged and spread rapidly worldwide. As of May 5th, 2020, where we began this study, there were more than 3.6M confirmed cases and >250,000 deaths attributed to COVID-19 worldwide; with over 69,000 deaths alone in the United States. These numbers have reached 7M deaths worldwide including 1.2M in United States only (1,2). Despite the development of multiple vaccines, the virus and its new variants continue to wreak havoc in the public health sector. While the mortality rate for COVID-19 was reported to be around 2%, the number was significantly increased among older patients with underlying coexisting conditions. Notably, in hospitalized patients, the death rate approached 15%. Individuals who were at mild to moderate risk, such as those with cancer, could demonstrate significant clinical deterioration within 24-48 hours.
In recent years during COVID-19 pandemic, we observed the implementation of policies such as “shelter in place” aimed at preventing the unnecessary strain on the healthcare system, measures that may become necessary in the face of another pandemic. Some high-risk patients were instructed to “weather the storm” at home without adequate monitoring, despite the potential for rapid deterioration in their health (3). Over the past decade, the emergence of commercially available, affordable, and lightweight sensors has significantly accelerated the adoption of remote patient monitoring systems within the healthcare system for continuous and comprehensive patient tracking. Even prior to the COVID-19 pandemic, substantial evidence indicated that continuous monitoring of vital signs, such as pulse oximetry and heart rate, was associated with reduced mortality (4,5). This aligns with numerous studies conducted during the pandemic, which explored the use of pulse oximetry and other wearable devices for continuous vital sign monitoring (4,6,7). During the COVID-19 pandemic, numerous studies evaluated the use of technology for patient management and assessed its effectiveness in reducing hospitalizations (3,6,8–11). While some initiatives were labeled as Remote Patient Monitoring, they often focused more on telehealth, e-visits, and the utilization of patients’ existing technologies, such as smartphones to connect to healthcare professionals. These studies demonstrated encouraging results, indicating that technology can significantly enhance patient management (6,8,9).
In a series of studies, we had introduced and documented the development and implementation of our remote patient monitoring system and its clinical validation within older patient populations at-risk of various health conditions (12–15). This platform, known as Sensing At-Risk Population (SARP), encompasses activity monitoring through smartwatches, indoor localization using stationary beacons, and the collection of additional physiological data via wireless sensors. Data is securely and automatically transmitted to a HIPAA-compliant cloud infrastructure. For this study, we expanded the capabilities of our system to serve as a monitoring hub and to support additional devices tailored to the needs of COVID+ patients. We incorporated commercially available Bluetooth-enabled thermometers, pulse oximeters, and respiratory distress monitors. These additions enabled us to capture relevant information for monitoring and assessing COVID+ cases. The primary objective of this study was to enhance existing outpatient COVID+ clinical trials, which incorporates the variables that our system captures as secondary or exploratory endpoints. By leveraging our platform, we aimed to minimize COVID+ patients’ exposure while collecting crucial vital information. Our intention was to gather this data within a natural environment, shedding light on the diverse array of symptoms presented by COVID+ patients, thus contributing to a better understanding of this disease and the ongoing pandemic using the emerging remote patient monitoring systems.
Methods
Design, Setting and Participants
Study design and participants Patient recruitment occurred from November 2020 to March 2021 at University of California, Los Angeles (UCLA) Health center. We emphasized that participation in the study would not affect their care at UCLA and was entirely separate from their medical treatment. Participation would conclude either after a two-week period or if the patient was admitted to the hospital during that timeframe.
Eligible participants were individuals aged 18 or older with a COVID-19 diagnosis who were not hospitalized or had known exposure to a COVID-19 positive case. They required the ability to manage their condition at home, access to home WiFi, proficiency in either English or Spanish, and a willingness to provide informed consent by signing the approved form (IRB #20-001565) from the University of California, Los Angeles, titled “Early Detection of Health Improvement and Decline through Remote Health Monitoring in COVID-19 Positive Patients.
Inclusion and Exclusion Criteria
Wearable device compatibility (ability to wear a watch), willingness to host the remote monitoring system for a 2-week period, age 18 or older, current UCLA Health patient, confirmed positive COVID-19 lab result or known exposure to COVID-19.
To ensure the meaningfulness of the activity data, we imposed an additional inclusion criterion requiring a minimum daily wear time of the smartwatch of at least 4 hours. However, the utilization of the Remote Patient Monitoring (RPM) kit was not limited solely to the smartwatch; it also included a thermometer and pulse oximeter for spot checks, along with a respiratory monitor capable of continuous data capture. Data collection was conducted either continuously or at specific intervals from these devices. For analysis, we included individuals with measurements taken on more than 7 distinct days and calculated the mean value of the metric for each day.
Exclusion Criteria: Clinical diagnosis of movement disorders (e.g., Parkinson’s Disease), failure to meet inclusion criteria.
Sensing At-Risk Population (SARP)
Sensing At-Risk Population (SARP) is a Remote Patient Monitoring system (RPM) developed by UCLA’s Center for SMART Health, designed to cater to patients beyond the confines of healthcare institutions. Its primary purpose is to monitor vulnerable, at-risk populations by simulating the measurement of Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) (16) through cost-effective sensor technology. SARP has been employed to generate prognostic data and predictive models for mortality and functional decline (12–15).
The core components of SARP encompass hardware, including an Android Smartwatch and readily available proximity BLE Beacons, and clinically validated software featuring activity recognition and indoor localization algorithms. Additionally, SARP incorporates a remotely triggered adaptive smart questionnaire mechanism, data visualization tools, and algorithms for assessing frailty, all within a HIPAA-compliant infrastructure (17).
Activity features were derived from three groups of parameters using smartwatches and BLE beacons: (1) activity recognition (e.g., sitting time, standing time), (2) indoor localization (e.g., time in bed, time in the bathroom), and (3) raw acceleration quantification (Mean Absolute Deviation in Accelerometer signal: MAD). By combining these attributes, we created features such as sitting time in bed and energy expenditure during walking or while in bed (12,13).
To ensure fair comparisons among patients with different watch wear times, we normalized features by dividing the time spent on activities or in locations by the total wear time (uptime). Energy-related features were also normalized by uptime to yield energy intensity and by the total daily value to calculate energy percentage.
Bluetooth Low Energy (BLE) beacons are used in the SARP system to estimate indoor patient locations by measuring the Received Signal Strength Indicator (RSSI) values via smartwatches (18–20). Patients were instructed to place a BLE beacon in each designated indoor location: the kitchen, bathroom, bedroom, dining room, and TV/sitting room. If the system does not detect any beacons, it infers that the patient is outside, indicating they are not at home.
Data Collection
For this study, SARP was modified to integrate a series of FDA-cleared Bluetooth-enabled devices, including pulse oximeters, thermometers, and Ultra-Wideband (UWB) radar technology for monitoring respiratory characteristics. Numerous studies have demonstrated the utility of such metrics for patient assessment, with subsequent research validating the accuracy of Bluetooth-enabled devices for home or remote monitoring (5,7,9,10,21–24). However, discussing the efficacy of these devices is beyond the scope of this paper; we assume their outputs are reliable given that all the devices used have FDA clearance. Nevertheless, it is worth noting that some studies suggest UWB radar may overestimate respiratory rates at low levels and underestimate them at high levels, as indicated in (25,26).
Bluetooth-enabled devices were used to augment an established system, gathering extra patient data remotely. This information was securely uploaded to a HIPAA-compliant cloud system, enhancing the infrastructure for remote patient monitoring. Bluetooth-enabled devices used for capturing vital signs complemented the existing SARP infrastructure by remotely gathering additional vital data from patients. The data from these devices were collected and uploaded to a HIPAA-compliant Azure IoT cloud infrastructure. By incorporating additional Bluetooth devices, we dynamically expanded the capabilities of our remote monitoring system to accommodate varying patient needs. Specifically, for this project, pulse oximeters and thermometers were used to detect COVID-19 symptoms, such as shortness of breath and fever, enabling us to make testing recommendations for patients. Additionally, SARP integrated an FDA-cleared Bluetooth device equipped with radar, a microphone, and a light sensor to monitor respiration and detect conditions such as hypoventilation, hyperventilation, and impaired lung function. The respiration monitoring device was recommended to be positioned adjacent to the patient’s bed to observe breathing patterns, primarily during nighttime or when the patient was present in the room.
We recorded the day-to-day changes in the following sensor measurements and compiled the assessments shown in the
Table 1:
Position: Lying down, Sitting, Standing, Walking
Active: Active (Walking), Active (Not Walking), Non-Active
Steps: Total daily steps. Total distance traveled
Indoor Localization: Bedroom, Bathroom, Kitchen, Dining Room, Family Room, Office
Sleep Quality: Duration and Toss and Turn
Heart Rate: ever hour + during walking speed measurements
Temperature with Infrared technology
SpO2 Oxygen Saturation with pulse oximetry
Breath per Min (BrPM)with UWB radar Technology
Respiration Waveform (Inhalation/Exhalation Patterns) with UWB radar Technology
As described in (12), active/non-active is determined by an empirical threshold of 0.02 m/s2 (2 cm/s2) imposed on the MAD value of an accelerometer signal in every 10 seconds. The threshold translates to 1 meter of displacement of the hand in 10 seconds (hand wearing the smartwatch with accelerometer). Furthermore, the energy expenditure is estimated to be proportional to the Mean Absolute Deviation (MAD) of accelerometer magnitude signal.
Exploratory Analysis
The primary objective of this study was to conduct an observational analysis aimed at developing predictive models for forecasting adverse COVID-19 outcomes, including hospitalization within 24 or 48 hours. Given the absence of adverse events among the participants, the research emphasis shifted towards an exploratory analysis, concentrating on the delineation of characteristics and behaviors of COVID-19 patients as captured by the remote patient monitoring system.
All the analysis were performed using Python Programming Language (version 3.11.3) libraries Pandas (version 2.2.1), Numpy (version 1.25.2), Scipy (version 1.11.1), Scikit-learn (version 1.3) and Searborn library (version 0.12.2) was used for statistical data visualization.
Activity and Energy
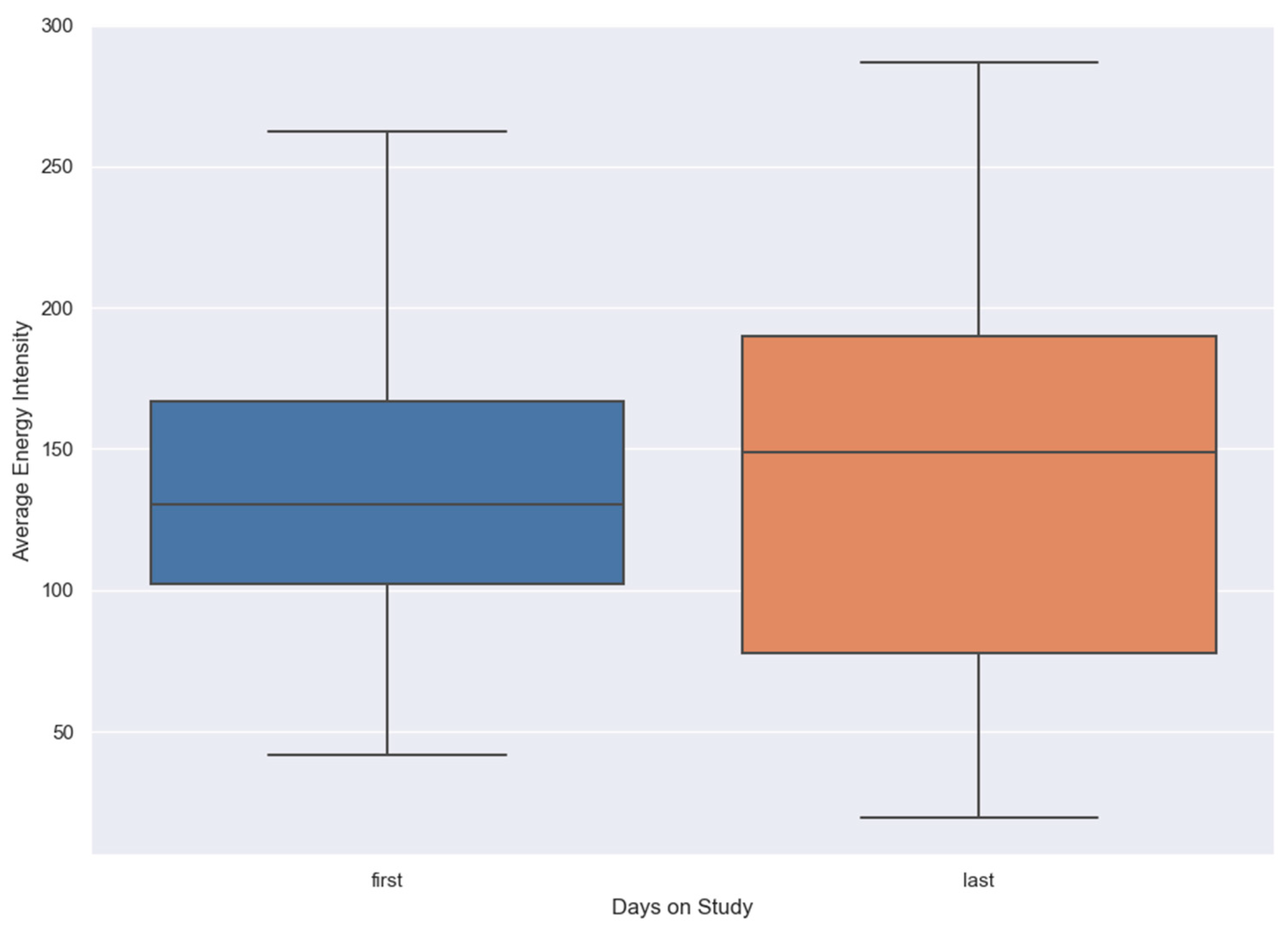
Energy intensity, as detailed in the SARP section, was analyzed for all individuals on their first day of the study (baseline) and their last day using a box plot. The plot displays the median line, representing the central tendency, and the overall distribution of energy intensity values. It is important to note that these observations were made after applying the inclusion criteria, which only considered days where patients had more than 4 hours of watch wear time.
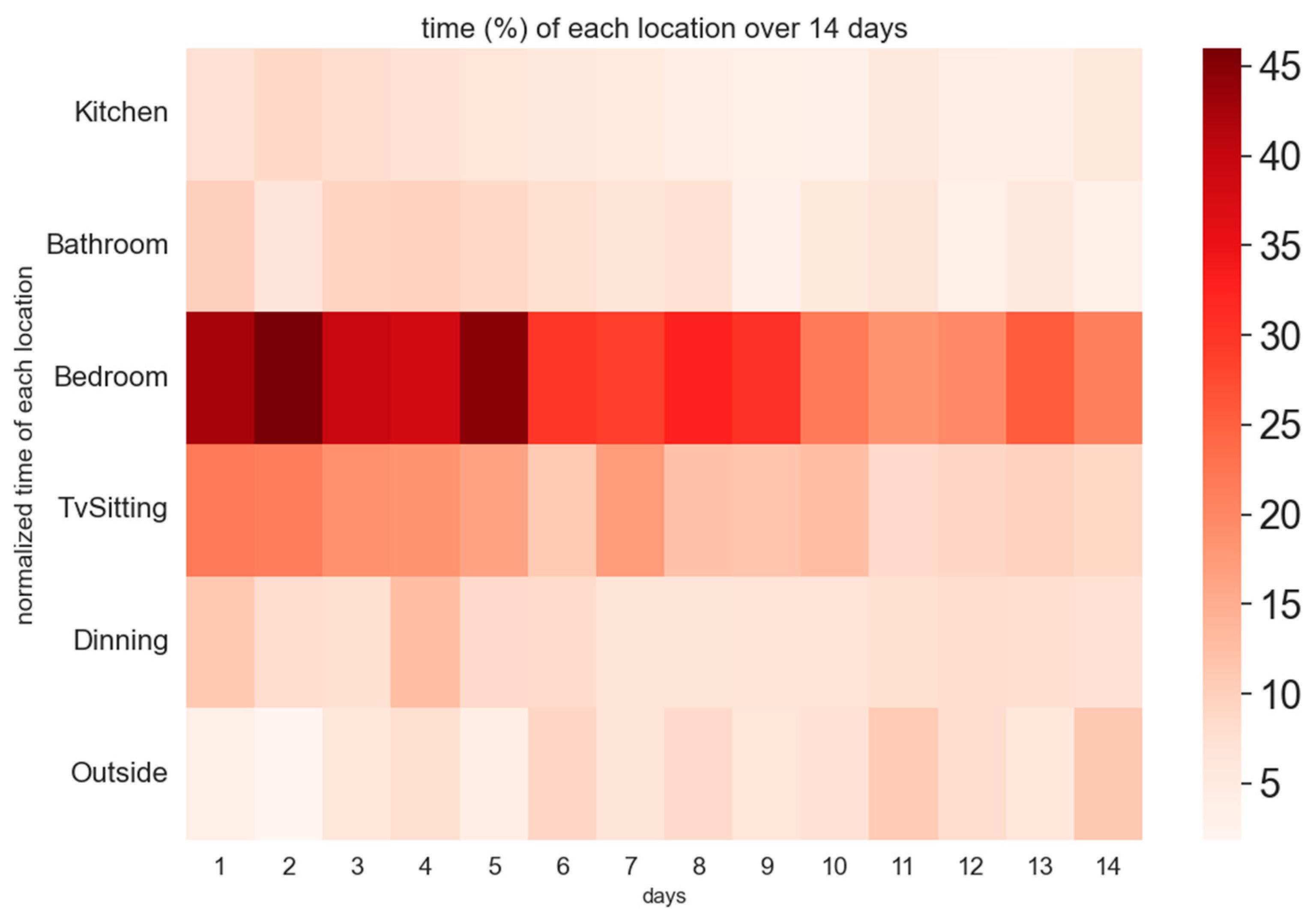
Using Bluetooth beacons, the time each patient spent in specified locations within their home was calculated to better form a daily storyline of their activity patterns. The goal was to assess whether patients spent less time in the bedroom as the study progressed and whether they spent more time outside their residence. The percentage of time spent in each location was calculated by dividing the duration in that location by the total uptime (watch wear time) for that day. A heatmap was generated to visualize the average percentage of time spent in each location longitudinally over a 2-week period.
It is important to note that data was analyzed after applying inclusion criteria, and some participants were in the study for fewer than 2 weeks. For each day of the study, the time spent in each location was averaged across the number of patients observed on that particular day. Given that the start and end dates varied across patients, the analysis was conducted by calculating each patient’s individual day. For example, “Day 1” represents the average time percentage spent in each location for all patients on their respective first day, which may differ chronologically between patients.
Vital Signs
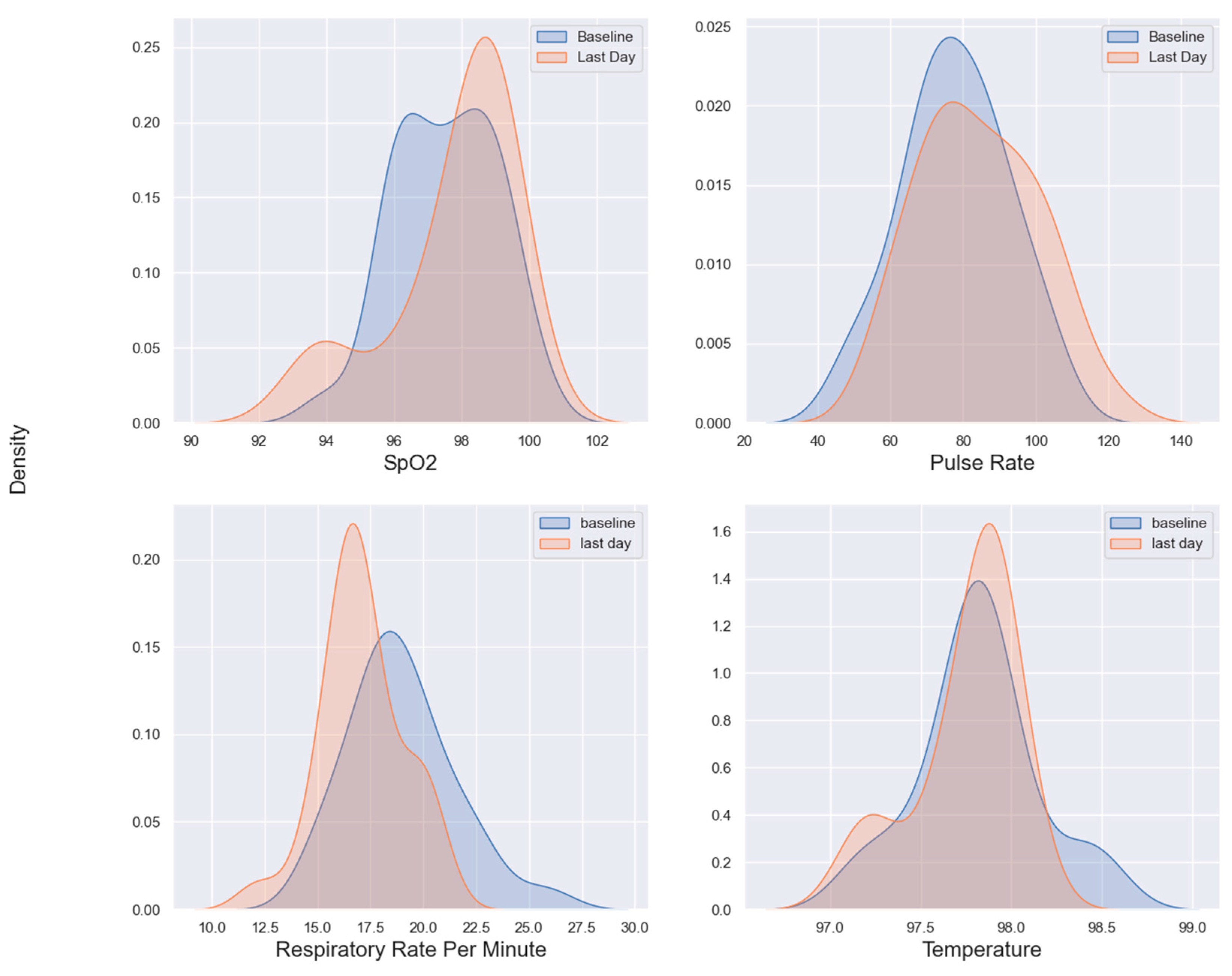
The distributions of SpO2, pulse rate, respiratory rate, and temperature were visualized using kernel density estimate (KDE) plots. The KDE plots depict patients’ baseline (the first day of data collection) and their final day (the last available day of the study). For each patient, the mean value of observations was used for both the baseline and the final day. A minimum interval of 7 days between the baseline and final day was enforced to exclude patients with only a few days of data, as their differences could not accurately represent longitudinal trends. It is important to note that the KDE visualization may include out-of-bounds values due to the edge effect, where Gaussian distribution-based estimation extends beyond the actual data range. When the observations are close to the edges of the data range, the KDE which is formed by centering around each data point, may extend beyond the boundary. This can be addressed by truncating the graph and clipping x-axis out of bound values, although this is merely a visualization adjustment. Alternatively, the edge effect can be mitigated by smoothing the KDE curve through bandwidth adjustment. In the Seaborn library, the default bandwidth value is determined by Scott’s rule (set to 1) (27) and automatically adapts to the data characteristics (28). While increasing the bandwidth reduces sensitivity to individual observations and produces a smoother curve, the authors opted not to adjust the bandwidth due to the limited number of observations and the need to maintain the graph’s sensitivity to changes.
Self-Reported Survey
Daily self-reported surveys, collected using the SARP app provided to patients on tablets, were integrated with sensor data. The purpose of this integration was to later align the data with potential complications, including cardiovascular issues or hospitalizations, to retrospectively investigate whether any early indications could have been inferred from the observational data. This analysis aimed to identify potential early warning signs and develop timely interventions.
Results
Demographic Characteristics
Out of 176 interested patients who were approached by our team, 73 met the eligibility criteria and were enrolled to utilize our remote patient monitoring kit, SARP as detailed in
Table 2. Of these, 41 patients successfully connected to and complied with the system.
To reiterate, the SARP RPM included a smartwatch, thermometer, pulse oximeter and a respiratory rate monitoring device. We established an additional inclusion criterion that mandated a minimum daily wear time for the smartwatch of at least 4 hours.
Activity and Energy
There were 22 patients with substantial watch data (> 4 hours per day) with average usage (SD) of 10.1 (4.3) days of watch wear time. 39 patients used Pulse Oximeter to check the SpO2 and Heart Rate with average usage (SD) of 10.1 (4.7) days of utilizing the device. The number of patients who used thermometer decreases to 37 with average (SD) usage period of 9.7 (4.8) days. The number of patients with respiratory data is 36 with average (SD) of 11.4 (4.7) nights of continuous monitoring of breathing rate per minute.
Box plot in
Figure 1 shows that the overall energy levels of patients increased on the last day of the study compared to the first day. Both the mean intensity and the variability of energy levels showed an upward trend. The figure indicates an overall improvement in the physical activity of COVID-19 patients over time, though with varying degrees of recovery across the patients.
By analyzing the heatmap of patient activities shown in
Figure 2, it is evident that towards the end of the study, patients were expending less energy in the bedroom and more in other areas outside the home. The fading color in the bedroom area towards the end of the study suggests a decrease energy expended in that location. Similarly, there is a noticeable decline in activity within other home settings, such as the TV/sitting area, as captured by indoor localization beacons. This trend likely reflects patients’ gradual recovery and increased mobility, allowing them to engage more in activities outside their homes.
Vital Signs
Figure 3.
Biomarkers: First Day vs. Last Day.
Figure 3.
Biomarkers: First Day vs. Last Day.
Investigating
Figure 4 kernel density estimation graphs for four vital signs shows that the distribution for SpO2 has shifted slightly to the right from baseline to the last day, indicating an overall improvement in oxygen saturation levels by the end of the study. The of the last day’s SpO2 distribution is higher than the baseline, suggesting more patients reached higher SpO2 levels on the last day.
The pulse rate distribution on the last day shows a slight shift to the right compared to the baseline. This suggests that patients’ pulse rates tended to increase slightly by the end of the study. The distribution also appears to broaden slightly, indicating greater variability in pulse rates among patients on the last day.
On the respiratory rate subgraph, there is a noticeable shift in distribution towards lower rates on the last day compared to the baseline. This may indicate that, on average, patients had a lower respiratory rate by the end of the study, potentially reflecting improved respiratory function. The last day’s distribution is narrower, suggesting reduced variability in respiratory rates, with most patients converging around healthier respiratory measures.
The temperature distributions however remain relatively similar in both baseline and the last day, with only slight difference.
Self-Reported Survey
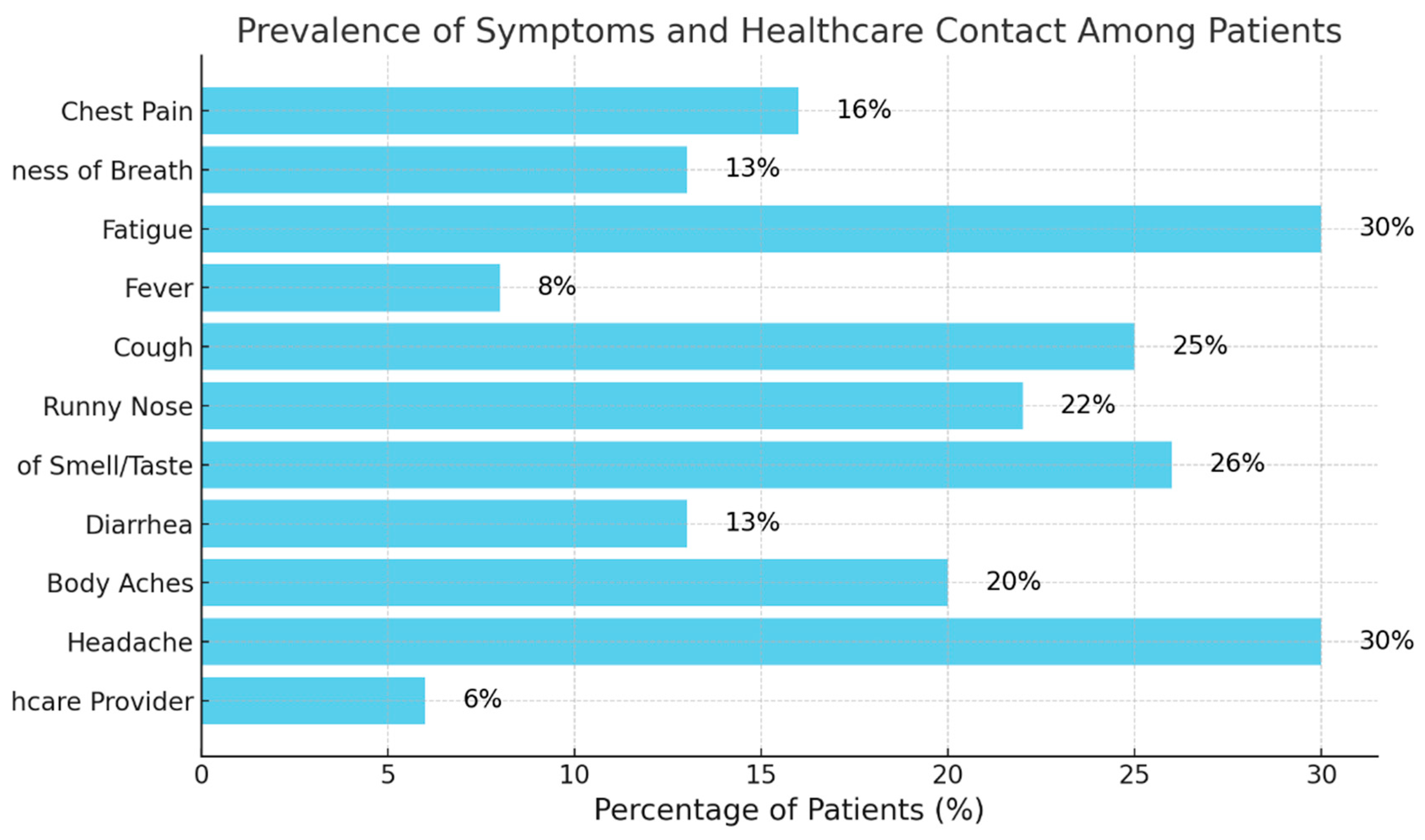
Figure 4 illustrates the prevalence of symptoms reported by patients at least once during the two-week observation period.
Figure 4.
prevalence of symptoms.
Figure 4.
prevalence of symptoms.
Figure 4 indicates that fatigue and headache were the most prevalent symptoms amongst COVID-19 patients followed by cough and loss of smell. Despite these symptoms, only 6% of patients contacted a healthcare provider, suggesting either mild severity or potential difficulties to seeking medical advice. Since there was a direct contact number for the patients in the study and the ease of contacting them, it would be safe to assume the mild severity was the main reason. This underscores the need for careful monitoring of common COVID-19 symptoms.
Discussion
Our analysis of activity and indoor location suggests that a positive trend in data from wearable and remote monitoring devices is aligned with recovery of COVID-19 patients over the study period.
Figure 1 shows an increase in overall energy levels, with both the mean energy intensity and variability rising from the first to the last day, indicating an overall improvement in physical activity.
Figure 2 offers additional insights, revealing that patients progressively expended less energy in the bedroom and other indoor locations, such as the TV/sitting area, as the study progressed. This decline in indoor activity suggests that patients were recovering and becoming more mobile, with greater energy expenditure in locations outside their homes. The shift in activity distribution likely reflects improved physical health, as indicated by the higher intensity color in the heatmap corresponding to outdoor locations toward the end of the study.
Our study results are consistent with a systemic scoping review of studies published between 2019 and 2022, in which the smartwatches or fitness trackers were reported as the preferred type of wearable technologies for early and pre-symptomatic detection (29–32). Much of the published literature is focused on using wearables for early detection (33,34). These studies do show that wearables can track heart rate and heart rate variability with improvement over the course of 7 days (35). Moreover, SpO2 and activity tracking from wearable device might be able to help identify COVID-19 patients at risk for sudden death (36) based on a systematic review. However, the best results are looking at physiological features in a multi-modal approach where one can achieve sensitivity as high as 90% and specificity as high as 80% (37). Our study asked about self-reported symptoms and the importance of combining symptoms with sensor data that has been shown to allow for better predictive models (38). The use of wearables and remote monitoring has also been explored in managed Long COVID (39).
The prevalence of fever among COVID-19 patients varies across studies and cohorts, with a high percentage observed in hospitalized patients (40). However, in non-hospitalized patients, fever is less frequently observed or may be entirely absent. In studies such as (41), fever was recorded for fewer than 2 days throughout the course of illness, primarily in frail patients, even after lowering the temperature threshold from 100.4°F to 100°F. Body temperature in our cohort never exceeded 98.6°F.
Figure 3 indicates that patients’ body temperatures were relatively stable throughout the study with a marginal increase by the last day. Both distributions of body temperatures in baselines and last day are tightly clustered indicating consistent body temperatures across patients. This suggests that either the cohort did not exhibit fever, or the Non-contact Infrared Thermometers (NCIT) used were not sufficiently sensitive, despite being FDA-cleared. This aligns with an FDA study (42) highlighting misleading labeling of FDA-cleared devices during COVID-19, showing that NCITs may fail to reliably detect fevers when used on adults and may not meet the accuracy specifications advertised in manufacturers’ instructions for use. Notably, 8% of patients in the study reported fever in the self-reported surveys (
Figure 4), indicating that the subjective experience of fever was prevalent in at least 8% of the cohort.
Figure 3 of vital signs in overall, suggests that by the end of the study, patients generally showed signs of physiological improvement, with higher SpO2 levels, slightly elevated pulse rates, and lower respiratory rates. Such trends indicate a recovery or improvement in the monitored patients’ vital signs.
Study Limitations
Patient compliance with wearing a smartwatch and using the Bluetooth-enabled thermometer and pulse oximeters was one of the main challenges of this study, and we anticipate this to be a common obstacle in similar studies that aim to utilize wearable technology for patient monitoring. In contrast, the UWB respiration monitoring device required no patient interaction and could monitor respiration passively. However, the accuracy of this device could be compromised if more than one person was in the bedroom or if the patient left the room. To mitigate this, we ensured that only the results from patients who lived alone in the monitored bedroom were included in the analysis. While setting up the devices was designed to be straightforward—requiring only a tablet and a simple app we provided to connect all devices to the patients’ home internet in one step—it is reasonable to assume that certain patients, particularly those less familiar with technology, may have found the setup process challenging.
Conclusions
The COVID-19 pandemic demonstrated the importance of telehealth and remote monitoring of at-risk patients. A recent cost-utility analysis estimates that daily pulse oximetry use with a follow-up after three weeks could reduce the mortality rate to 6 per 1,000 patients, compared to 26 per 1,000 without at-home monitoring. Various studies suggest that remote patient monitoring in COVID-19 patients could potentially reduce hospitalizations and deaths by as much as 80% and yield cost savings of around $12,000 per patient (10,43). This underscores the growing importance of utilizing technology for continuous at-home monitoring, which can enhance the quality of care while significantly reducing costs.
In this study, we demonstrated that affordable remote monitoring devices can effectively track individuals over time, revealing trends in their health status. However, a significant challenge in remote and home monitoring systems is ensuring participant adherence. Our study found that only 41 out of 73 individuals consistently used all the provided devices. This finding aligns with previous research, which suggests that patients are more likely to engage in remote monitoring or mHealth when they perceive an immediate personal benefit. Additionally, patients tend to prefer passive sensing methods over more demanding active monitoring that requires their ongoing participation. This study also generated novel data specific to COVID-19, comparing self-reported symptoms with objective monitoring data.
Acknowledgments
The authors would like to express their gratitude to the researchers cited here and apologize to those whose work, because of page restrictions, could not be mentioned. This research was mainly funded by W. M. Keck Foundation COVID-19 research fund at UCLA.
Conflicts of Interest
The SARP system is protected by a patent (US Patent 10937547) (15) in which RR and AN are listed as co-inventors. RR and AN are cofounders of InvistaHealth LLC. Other authors have declared no potential conflict of interest regarding the publication of this paper.
Abbreviations
| RPM |
Remote Patient Monitoring |
| SARP |
Sensing At-Risk Patients |
| MAD |
Mean Absolute Deviation |
References
- The true death toll of COVID-19 estimating global excess mortality [Internet]. [cited 2024 Sep 7]. Available from: https://www.who.int/data/stories/the-true-death-toll-of-covid-19-estimating-global-excess-mortality.
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 May;20(5):533–4.
- KLCC | NPR for Oregonians [Internet]. 2020 [cited 2024 Sep 7]. Rapid COVID-19 Testing Underway For All Patient Admittance To PeaceHealth Hospitals. Available from: https://www.klcc.org/health-medicine/2020-04-21/rapid-covid-19-testing-underway-for-all-patient-admittance-to-peacehealth-hospitals.
- Greenhalgh T, Knight M, Inda-Kim M, Fulop NJ, Leach J, Vindrola-Padros C. Remote management of covid-19 using home pulse oximetry and virtual ward support. BMJ. 2021 Mar 25;372:n677.
- McGrath SP, McGovern KM, Perreard IM, Huang V, Moss LB, Blike GT. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2021 Dec 1;17(8):557–61.
- Seshadri DR, Davies EV, Harlow ER, Hsu JJ, Knighton SC, Walker TA, et al. Wearable Sensors for COVID-19: A Call to Action to Harness Our Digital Infrastructure for Remote Patient Monitoring and Virtual Assessments. Front Digit Health [Internet]. 2020 Jun 23 [cited 2024 Sep 7];2. Available from: https://www.frontiersin.org/journals/digital-health/articles/10.3389/fdgth.2020.00008/full.
- Shah S, Majmudar K, Stein A, Gupta N, Suppes S, Karamanis M, et al. Novel Use of Home Pulse Oximetry Monitoring in COVID-19 Patients Discharged From the Emergency Department Identifies Need for Hospitalization. Acad Emerg Med [Internet]. 2020 Aug [cited 2024 Sep 7];27(8):681–92. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7323027/.
- Crotty BH, Dong Y, Laud P, Hanson RJ, Gershkowitz B, Penlesky AC, et al. Hospitalization Outcomes Among Patients With COVID-19 Undergoing Remote Monitoring. JAMA Netw Open. 2022 Jul 1;5(7):e2221050.
- Wurzer D, Spielhagen P, Siegmann A, Gercekcioglu A, Gorgass J, Henze S, et al. Remote monitoring of COVID-19 positive high-risk patients in domestic isolation: A feasibility study. PLoS One. 2021;16(9):e0257095.
- Pronovost PJ, Cole MD, Hughes RM. Remote Patient Monitoring During COVID-19: An Unexpected Patient Safety Benefit. JAMA [Internet]. 2022 Mar 22 [cited 2024 Sep 7];327(12):1125–6. Available from. [CrossRef]
- Annis T, Pleasants S, Hultman G, Lindemann E, Thompson JA, Billecke S, et al. Rapid implementation of a COVID-19 remote patient monitoring program. J Am Med Inform Assoc [Internet]. 2020 Jul 27 [cited 2024 Sep 7];27(8):1326–30. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7239139/.
- Ramezani R, Zhang W, Xie Z, Shen J, Elashoff D, Roberts P, et al. A Combination of Indoor Localization and Wearable Sensor–Based Physical Activity Recognition to Assess Older Patients Undergoing Subacute Rehabilitation: Baseline Study Results. JMIR mHealth and uHealth [Internet]. 2019 Jul 10 [cited 2024 Sep 7];7(7):e14090. Available from: https://mhealth.jmir.org/2019/7/e14090.
- Moatamed B, Arjun, Shahmohammadi F, Ramezani R, Naeim A, Sarrafzadeh M. Low-cost indoor health monitoring system. In: 2016 IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN) [Internet]. 2016 [cited 2024 Sep 7]. p. 159–64. Available from: https://ieeexplore.ieee.org/document/7516252.
- Wong K, Shen J, Ramezani R, Zhang W, Xie Z, Naeim A, et al. A pilot study of a wearable monitoring system as an adjunct to geriatric assessment in older adults with cancer. JCO [Internet]. 2020 May 20 [cited 2024 Sep 7];38(15_suppl):2062–2062. Available from: https://ascopubs.org/doi/10.1200/JCO.2020.38.15_suppl.2062.
- RAMEZANI R, MOATAMED B, Arjun, NAEIM A, Sarrafzadeh M. Subject assessment using localization, activity recognition and a smart questionnaire [Internet]. US10937547B2, 2021 [cited 2024 Sep 7]. Available from: https://patents.google.com/patent/US10937547B2/en.
- Mlinac ME, Feng MC. Assessment of Activities of Daily Living, Self-Care, and Independence. Arch Clin Neuropsychol [Internet]. 2016 Sep [cited 2024 Sep 7];31(6):506–16. Available from. [CrossRef]
- Ramezani R, Cao M, Earthperson A, Naeim A. Developing a Smartwatch-Based Healthcare Application: Notes to Consider. Sensors [Internet]. 2023 Jan [cited 2024 Sep 7];23(15):6652. Available from: https://www.mdpi.com/1424-8220/23/15/6652.
- Bouchard K, Ramezani R, Naeim A. Features based proximity localization with Bluetooth emitters. In: 2016 IEEE 7th Annual Ubiquitous Computing, Electronics & Mobile Communication Conference (UEMCON) [Internet]. 2016 [cited 2024 Sep 7]. p. 1–5. Available from: https://ieeexplore.ieee.org/document/7777845.
- Bouchard K, Ramezani R, Arjun, Naeim A. Evaluation of Bluetooth beacons behavior. In: 2016 IEEE 7th Annual Ubiquitous Computing, Electronics & Mobile Communication Conference (UEMCON) [Internet]. 2016 [cited 2024 Sep 7]. p. 1–3. Available from: https://ieeexplore.ieee.org/document/7777846.
- Bouchard K, Eusufzai MR, Ramezani R, Naeim A. Generalizable spatial feature for human positioning based on Bluetooth beacons. In: 2016 IEEE 7th Annual Ubiquitous Computing, Electronics & Mobile Communication Conference (UEMCON) [Internet]. 2016 [cited 2024 Sep 7]. p. 1–5. Available from: https://ieeexplore.ieee.org/document/7777884.
- Ishikawa M, Sakamoto A. Patient SafetyNet for the Evaluation of Postoperative Respiratory Status by Nurses: A Presurvey and Postsurvey Study. Journal of PeriAnesthesia Nursing [Internet]. 2021 Feb 1 [cited 2024 Sep 7];36(1):14–7. Available from: https://www.jopan.org/article/S1089-9472(20)30098-8/abstract.
- Nicolò A, Massaroni C, Schena E, Sacchetti M. The Importance of Respiratory Rate Monitoring: From Healthcare to Sport and Exercise. Sensors (Basel). 2020 Nov 9;20(21):6396.
- McFadden JP, Price RC, Eastwood HD, Briggs RS. Raised respiratory rate in elderly patients: a valuable physical sign. Br Med J (Clin Res Ed). 1982 Feb 27;284(6316):626–7.
- Goldfine CE, Oshim MFT, Chapman BP, Ganesan D, Rahman T, Carreiro SP. Contactless Monitoring System Versus Gold Standard for Respiratory Rate Monitoring in Emergency Department Patients: Pilot Comparison Study. JMIR Formative Research [Internet]. 2024 Feb 16 [cited 2024 Sep 7];8(1):e44717. Available from: https://formative.jmir.org/2024/1/e44717.
- Lazaro A, Girbau D, Villarino R. ANALYSIS OF VITAL SIGNS MONITORING USING AN IR-UWB RADAR. PIER [Internet]. 2010 [cited 2024 Sep 7];100:265–84. Available from: http://www.jpier.org/PIER/pier.php?paper=09120302.
- Immoreev I, Tao TH. UWB radar for patient monitoring. IEEE Aerospace and Electronic Systems Magazine [Internet]. 2008 Nov [cited 2024 Sep 7];23(11):11–8. Available from: https://ieeexplore.ieee.org/document/4693985.
- Scott DW. Multivariate Density Estimation and Visualization. In: Gentle JE, Härdle WK, Mori Y, editors. Handbook of Computational Statistics [Internet]. Berlin, Heidelberg: Springer Berlin Heidelberg; 2012 [cited 2024 Sep 9]. p. 549–69. Available from: http://link.springer.com/10.1007/978-3-642-21551-3_19.
- Waskom M. seaborn: statistical data visualization. JOSS [Internet]. 2021 Apr 6 [cited 2024 Sep 8];6(60):3021. Available from: https://joss.theoj.org/papers/10.21105/joss.03021.
- Cheong SHR, Ng YJX, Lau Y, Lau ST. Wearable technology for early detection of COVID-19: A systematic scoping review. Preventive Medicine [Internet]. 2022 Sep 1 [cited 2024 Sep 11];162:107170. Available from: https://www.sciencedirect.com/science/article/pii/S0091743522002195.
- Mishra T, Wang M, Metwally AA, Bogu GK, Brooks AW, Bahmani A, et al. Pre-symptomatic detection of COVID-19 from smartwatch data. Nat Biomed Eng. 2020 Dec;4(12):1208–20.
- Mirjalali S, Peng S, Fang Z, Wang CH, Wu S. Wearable Sensors for Remote Health Monitoring: Potential Applications for Early Diagnosis of Covid-19. Adv Mater Technol. 2022 Jan;7(1):2100545.
- Channa A, Popescu N, Skibinska J, Burget R. The Rise of Wearable Devices during the COVID-19 Pandemic: A Systematic Review. Sensors [Internet]. 2021 Jan [cited 2024 Sep 11];21(17):5787. Available from: https://www.mdpi.com/1424-8220/21/17/5787.
- Cleary JL, Fang Y, Sen S, Wu Z. A caveat to using wearable sensor data for COVID-19 detection: The role of behavioral change after receipt of test results. PLOS ONE [Internet]. 2022 Dec 30 [cited 2024 Sep 11];17(12):e0277350. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0277350.
- Sanches CA, Silva GA, Librantz AFH, Sampaio LMM, Belan PA. Wearable Devices to Diagnose and Monitor the Progression of COVID-19 Through Heart Rate Variability Measurement: Systematic Review and Meta-Analysis. J Med Internet Res [Internet]. 2023 Nov 14 [cited 2024 Sep 11];25:e47112. Available from: https://www.jmir.org/2023/1/e47112.
- Erratum to “COVID-19 surveillance based on consumer wearable devices”, 2024 [Internet]. [cited 2024 Sep 11]. [CrossRef]
- Takahashi S, Nakazawa E, Ichinohe S, Akabayashi A, Akabayashi A. Wearable Technology for Monitoring Respiratory Rate and SpO2 of COVID-19 Patients: A Systematic Review. Diagnostics [Internet]. 2022 Oct [cited 2024 Sep 11];12(10):2563. Available from: https://www.mdpi.com/2075-4418/12/10/2563.
- Mason AE, Hecht FM, Davis SK, Natale JL, Hartogensis W, Damaso N, et al. Author Correction: Detection of COVID-19 using multimodal data from a wearable device: results from the first TemPredict Study. Sci Rep [Internet]. 2022 Mar 16 [cited 2024 Sep 11];12(1):4568. Available from: https://www.nature.com/articles/s41598-022-08723-x.
- Quer G, Radin JM, Gadaleta M, Baca-Motes K, Ariniello L, Ramos E, et al. Wearable sensor data and self-reported symptoms for COVID-19 detection. Nat Med [Internet]. 2021 Jan [cited 2024 Sep 11];27(1):73–7. Available from: https://www.nature.com/articles/s41591-020-1123-x.
- Khondakar KR, Kaushik A. Role of Wearable Sensing Technology to Manage Long COVID. Biosensors (Basel) [Internet]. 2022 Dec 31 [cited 2024 Sep 11];13(1):62. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9855989/.
- Islam MA, Kundu S, Alam SS, Hossan T, Kamal MA, Hassan R. Prevalence and characteristics of fever in adult and paediatric patients with coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis of 17515 patients. PLOS ONE [Internet]. 2021 Apr 6 [cited 2024 Sep 9];16(4):e0249788. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0249788.
- Shi SM, Bakaev I, Chen H, Travison TG, Berry SD. Risk Factors, Presentation, and Course of Coronavirus Disease 2019 in a Large, Academic Long-Term Care Facility. Journal of the American Medical Directors Association [Internet]. 2020 Oct 1 [cited 2024 Sep 9];21(10):1378-1383.e1. Available from: https://www.sciencedirect.com/science/article/pii/S1525861020307337.
- Sullivan SJL, Rinaldi JE, Hariharan P, Casamento JP, Baek S, Seay N, et al. Clinical evaluation of non-contact infrared thermometers. Sci Rep [Internet]. 2021 Nov 11 [cited 2024 Sep 9];11(1):22079. Available from: https://www.nature.com/articles/s41598-021-99300-1.
- Iuliano AD, Chang HH, Patel NN, Threlkel R, Kniss K, Reich J, et al. Estimating under-recognized COVID-19 deaths, United States, march 2020-may 2021 using an excess mortality modelling approach. Lancet Reg Health Am. 2021 Sep;1:100019.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).